Key Points
Question
To what extent does bempedoic acid lower low-density lipoprotein cholesterol (LDL-C) levels in patients at high cardiovascular risk who have ongoing hypercholesterolemia, despite the use of maximally tolerated lipid-lowering therapy?
Findings
In this clinical trial that included 779 randomized patients, the addition to stable background lipid-lowering therapy of bempedoic acid compared with placebo resulted in mean LDL-C levels of 97.6 mg/dL vs 122.8 mg/dL at 12 weeks, a difference that was statistically significant.
Meaning
Bempedoic acid provided additional LDL-C lowering in patients who did not achieve an adequate response to lipid-lowering therapy when compared with placebo.
Abstract
Importance
Additional treatment options are needed for patients who do not achieve sufficient reduction in low-density lipoprotein cholesterol (LDL-C) level with available lipid-lowering therapies.
Objective
To assess the efficacy of bempedoic acid vs placebo in patients at high cardiovascular risk receiving maximally tolerated lipid-lowering therapy.
Design, Setting, and Participants
Phase 3, randomized, double-blind, placebo-controlled clinical trial conducted at 91 clinical sites in North America and Europe from November 2016 to September 2018, with a final date of follow-up of September 22, 2018. A total of 779 patients with atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia, or both met randomization criteria, which included LDL-C level 70 mg/dL (1.8 mmol/L) or greater while receiving maximally tolerated lipid-lowering therapy.
Interventions
Patients were randomized 2:1 to treatment with bempedoic acid (180 mg) (n = 522) or placebo (n = 257) once daily for 52 weeks.
Main Outcomes and Measures
The primary end point was percent change from baseline in LDL-C level at week 12. Secondary measures included changes in levels of lipids, lipoproteins, and biomarkers.
Results
Among 779 randomized patients (mean age, 64.3 years; 283 women [36.3%]), 740 (95.0%) completed the trial. At baseline, mean LDL-C level was 120.4 (SD, 37.9) mg/dL. Bempedoic acid lowered LDL-C levels significantly more than placebo at week 12 (–15.1% vs 2.4%, respectively; difference, –17.4% [95% CI, –21.0% to –13.9%]; P < .001). Significant reductions with bempedoic acid vs placebo were observed at week 12 for non–high-density lipoprotein cholesterol (–10.8% vs 2.3%; difference, –13.0% [95% CI, –16.3% to –9.8%]; P < .001), total cholesterol (–9.9% vs 1.3%; difference, –11.2% [95% CI, –13.6% to –8.8%]; P < .001), apolipoprotein B (–9.3% vs 3.7%; difference, –13.0% [95% CI, –16.1% to –9.9%]; P < .001), and high-sensitivity C-reactive protein (median, –18.7% vs –9.4%; difference, –8.7% [asymptotic confidence limits, –17.2% to –0.4%]; P = .04). Common adverse events included nasopharyngitis (5.2% vs 5.1% with bempedoic acid and placebo, respectively), urinary tract infection (5.0% vs 1.9%), and hyperuricemia (4.2% vs 1.9%).
Conclusions and Relevance
Among patients at high risk for cardiovascular disease receiving maximally tolerated statins, the addition of bempedoic acid compared with placebo resulted in a significant lowering of LDL-C level over 12 weeks. Further research is needed to assess the durability and clinical effect as well as long-term safety.
Trial Registration
ClinicalTrials.gov Identifier: NCT02991118
This randomized clinical trial compares the efficacy of bempedoic acid, an experimental drug that inhibits an enzyme upstream of HMG-CoA reductase, vs placebo for lowering levels of low-density lipoprotein cholesterol (LDL-C) in patients at high cardiovascular risk with ongoing hypercholesterolemia receiving maximally tolerated lipid-lowering therapy.
Introduction
The 2018 multisociety guidelines on management of blood cholesterol recommend high-intensity or maximally tolerated statin therapy to lower cholesterol levels and reduce risk in patients with clinical atherosclerotic cardiovascular disease (ASCVD), with a primary goal of achieving a 50% or greater reduction in level of low-density lipoprotein cholesterol (LDL-C).1 For patients in whom statin therapy alone is insufficient, including those who are at very high cardiovascular risk or who have heterozygous familial hypercholesterolemia, the guidelines advocate addition of nonstatin agents. Despite treatment with available lipid-lowering therapies, many patients at high cardiovascular risk due to ASCVD, heterozygous familial hypercholesterolemia, or both do not achieve appropriate LDL-C levels.2,3,4,5,6
The consistent linear association between pharmacologic lowering of LDL-C levels and reduced cardiovascular risk7,8 supports the development of novel lipid-lowering therapies that can be used in conjunction with existing treatment options. Bempedoic acid (Esperion Therapeutics Inc) is an oral, once-daily, first-in-class drug being developed for the treatment of hyperlipidemia. Bempedoic acid is a prodrug activated in the liver to bempedoyl-CoA, which subsequently inhibits ATP-citrate lyase, an enzyme upstream of 3-hydroxy-3-methylglutaryl-CoA reductase, the target of statins, in the cholesterol biosynthesis pathway.9,10 Inhibition of cholesterol synthesis triggers up-regulation of hepatic LDL receptor expression, thus increasing clearance of LDL particles and lowering circulating LDL-C levels.11 Bempedoic acid and statins both inhibit cholesterol synthesis in the liver, but bempedoic acid is not activated in skeletal muscle.9
In clinical trials, bempedoic acid administered alone or in addition to ezetimibe or statins significantly lowered levels of LDL-C as well as other atherogenic lipoproteins and inflammatory biomarkers and had a tolerability profile similar to that of placebo.12,13,14 This phase 3 study evaluated 12-week efficacy of bempedoic acid (180 mg once daily) vs placebo for lowering LDL-C levels in patients with ASCVD, heterozygous familial hypercholesterolemia, or both who had persistent hypercholesterolemia despite maximally tolerated lipid-lowering therapy.
Methods
The study protocol (Supplement 1) and informed consent documents were approved by an institutional review board or independent ethics committee at each study site. All study participants provided written informed consent. The statistical analysis plan for the study is available in Supplement 2.
Patients
The CLEAR Wisdom trial enrolled adults at high cardiovascular risk because of ASCVD (coronary heart disease [CHD] or CHD risk equivalents), heterozygous familial hypercholesterolemia, or both. Documented CHD included acute myocardial infarction, silent myocardial infarction, unstable angina, coronary revascularization, or clinically significant CHD diagnosed by invasive or noninvasive testing. Cerebrovascular atherosclerotic disease and symptomatic peripheral arterial disease, but not type 2 diabetes mellitus, were considered CHD risk equivalents. Participants were required to be receiving stable, maximally tolerated lipid-lowering therapy and to have a fasting LDL-C level of 100 mg/dL (2.6 mmol/L) or higher at the first screening visit and 70 mg/dL (1.8 mmol/L) or higher 1 week before randomization. Maximally tolerated lipid-lowering therapy was determined by the investigator and included a maximally tolerated statin dose alone or in combination with other approved lipid-lowering therapies, excluding simvastatin at an average daily dose of 40 mg or greater, mipomersen, lomitapide, lipoprotein apheresis, or gemfibrozil. Cholestin was prohibited. Patients were excluded from the study if they had a total fasting triglyceride level of 500 mg/dL (5.6 mmol/L) or higher, body mass index 50 or greater (calculated as weight in kilograms divided by height in meters squared), severe renal impairment (estimated glomerular filtration rate <30 mL/min/1.73 m2), recent (within 3 months of screening) CHD event, or clinically significant disease that could interfere with study participation.
Study Design
This phase 3, randomized, double-blind, placebo-controlled clinical trial was conducted from November 2016 to September 2018. Patients were screened at 91 clinical sites in North America and Europe; of these, 86 sites randomized patients to study treatment. After completing a 1-week screening period and 4-week placebo run-in phase, patients were randomized 2:1 to treatment with bempedoic acid (180 mg) or placebo once daily for 52 weeks. Randomization was performed centrally using an interactive web response system, with stratification by presence of heterozygous familial hypercholesterolemia and baseline statin intensity (low-, moderate-, or high-intensity15). Patients not receiving statin therapy (maximal tolerated dose = 0) were included in the low-intensity group for the purposes of stratification. Patients were asked to self-identify their race and ethnicity according to protocol-defined fixed categories to evaluate potential differences in response to therapy.
Patients continued stable background lipid-lowering therapy throughout the study. However, beginning at week 24, investigators were notified by the central laboratory when a patient’s LDL-C level was both higher than 170 mg/dL (4.4 mmol/L) and elevated by 25% or more from baseline. After confirmation, the investigator was permitted to adjust background lipid-lowering therapy, including addition of a new medication or dose adjustment of existing medications. The sponsor, clinical site personnel, and patients were blinded to study treatment and lipid measures for 52 weeks.
Assessments
Blood samples for assessment of fasting lipids, including levels of LDL-C, high-density lipoprotein cholesterol (HDL-C), non–HDL-C, total cholesterol, and triglycerides were collected at baseline and at weeks 4, 12, 24, and 52 (to convert LDL-C, HDL-C, and total cholesterol values to mmol/L, multiply by 0.0259; to convert triglyceride values to mmol/L, multiply by 0.0113). LDL-C values were calculated using the Friedewald formula except when triglyceride level was greater than 400 mg/dL or LDL-C level was 50 mg/dL or less, in which case direct measurement of LDL-C was performed. Levels of apolipoprotein B (apoB) and high-sensitivity C-reactive protein (hsCRP) were measured at baseline and at weeks 12, 24, and 52. Quantification of lipids and biomarkers was performed at a central laboratory (Q2 Solutions).
Safety and tolerability were assessed by treatment-emergent adverse events, laboratory findings, physical examination findings, vital sign measurements, and electrocardiogram readings. Adverse events of special interest included hepatic events, muscle-related events, metabolic acidosis, hypoglycemia, new-onset or worsening diabetes, hyperuricemia, gout, renal events, and neurocognitive disorders. A blinded independent committee adjudicated designated cardiovascular and noncardiovascular clinical end points.
End Points
The primary efficacy end point was the percent change from baseline to week 12 in LDL-C level. Secondary efficacy end points were the percent change from baseline to week 24 in LDL-C and to week 12 in levels of non–HDL-C, total cholesterol, apoB, and hsCRP as well as absolute change from baseline to weeks 12 and 24 in LDL-C. Tertiary efficacy end points included week 24 and 52 data for efficacy measures and percent changes from baseline in levels of triglycerides and HDL-C.
Statistical Analysis
A planned sample size of 750 randomized patients (500 assigned to receive bempedoic acid and 250 assigned to receive placebo) was estimated to provide greater than 95% power to detect a between-group difference of 15% in LDL-C level percent change from baseline to week 12, a threshold established using historic benchmarks for lipid-lowering therapies. This calculation was based on a 2-sided t test at the 5% level of significance, with a standard deviation of 15%.
For efficacy analyses, patients were analyzed according to their randomization group. Safety analyses were performed using the safety population, which included all patients who received 1 or more doses of study drug. Percent changes from baseline in efficacy measures (other than hsCRP) were analyzed using analysis of covariance with treatment group and randomization stratification parameters as factors and baseline value as a covariate. Missing data were imputed using a pattern-mixture model (see statistical analysis plan in Supplement 2). For hsCRP, nonparametric analyses (Wilcoxon rank-sum test) with Hodges-Lehmann estimates of location shift and 95% asymptotic confidence limits were performed, without imputation for missing values. Efficacy end points were analyzed using a stepdown approach in which the primary and secondary end points were tested sequentially to preserve the family-wise type I error rate using the following order: LDL-C at week 12 (primary end point), LDL-C at week 24, non–HDL-C at week 12, total cholesterol at week 12, apoB at week 12, and hsCRP at week 12. Each hypothesis was tested at a significance level of .05 (2-sided). Statistical significance at each step was required to test the next hypothesis. Other lipid parameters (triglycerides, HDL-C) and measurement time points as well as safety measures were described using descriptive statistics. No imputation was performed for tertiary efficacy end points. Baseline LDL-C, non–HDL-C, total cholesterol, triglycerides, and HDL-C values were defined as the mean of the last 2 nonmissing values on or before day 1; for other parameters, baseline was defined as the last value prior to the first dose of study drug.
To explore the effect of patients who discontinued study treatment, an on-treatment analysis was performed for primary and key secondary end points using data collected during the on-treatment period (ie, collected from patients still receiving study treatment within 7 days of the efficacy measurement). Subgroup analyses for the primary end point and for safety assessments were performed in the following groups: cardiovascular disease risk category (ASCVD vs heterozygous familial hypercholesterolemia), baseline statin intensity (low/moderate [including no statin] vs high), baseline LDL-C category (<130 mg/dL, ≥130 and <160 mg/dL, ≥160 mg/dL), history of diabetes, age (<65 years, ≥65 to <75 years, ≥75 years), race, sex, body mass index category (<25, ≥25 and <30, ≥30), and region.
Post Hoc Analyses
Post hoc analyses for the primary end point were performed in subgroups according to background statin intensity (with “no statin” comprising its own group) and type of lipid-lowering therapy, and in patients who maintained stable background lipid-lowering therapy. Additional post hoc analyses were performed to assess changes in glycemic control in patients with diabetes, impaired fasting glucose, and normoglycemia at baseline. A post hoc analysis using a mixed-effects model with site as a random effect was performed to evaluate the influence of study site on the primary outcome.
No imputation was performed for missing data in sensitivity, subgroup, or post hoc analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc); all tests were 2-sided, with a significance level of .05.
Results
Patients
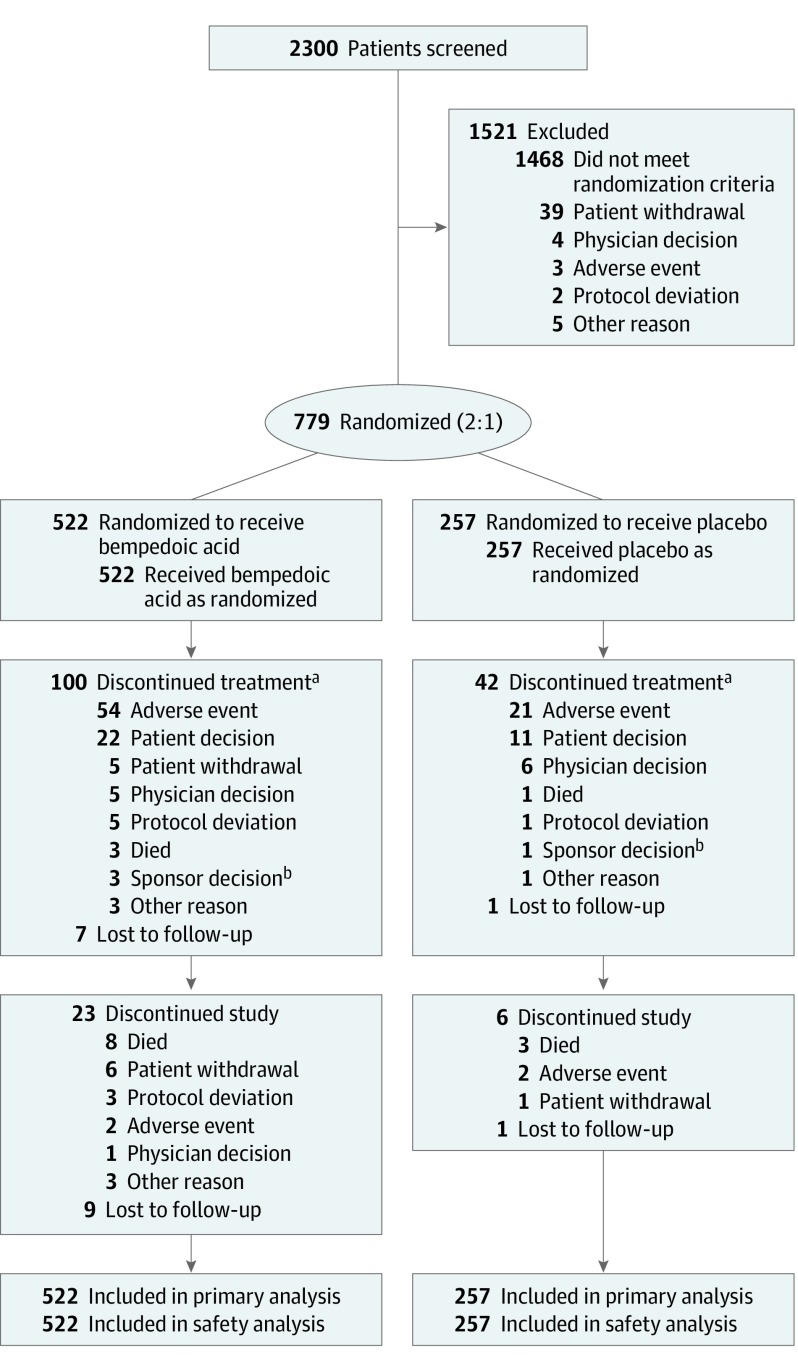
A total of 2300 patients were screened, and 779 were randomized to receive bempedoic acid (n = 522) or placebo (n = 257) (Figure 1). Seven hundred forty randomized patients (95.0%) completed the study, and 629 (80.7%) completed treatment. Median study drug exposure was similar in the bempedoic acid (363 days) and placebo (364 days) groups.
Figure 1. Participant Flow in the CLEAR Wisdom Randomized Clinical Trial.
Discontinuations are categorized by the primary reason for discontinuation. If a patient had a fatal adverse event and the death occurred at the end of treatment or study, then “death” was reported as the primary reason for both discontinuation of study treatment and discontinuation from the study. If a patient had a fatal adverse event and the death occurred after the end of treatment, “adverse event” was reported as the primary reason for discontinuation of study treatment, and “death” as the primary reason for discontinuation from the study.
aComprising patients who discontinued study drug but continued with study visits and assessments.
bFour patients (3 bempedoic acid, 1 placebo) discontinued study drug after implementation of a protocol amendment that made them no longer eligible for study treatment because of use of simvastatin at an average daily dose of 40 mg or greater.
The patient population was predominantly men (496/779 [63.7%]) and white (735/779 [94.4%]) and had a mean age of 64.3 (SD, 8.8) years. Most patients (736/779 [94.5%]) had ASCVD without heterozygous familial hypercholesterolemia. A history of diabetes was reported by 236 patients (30.3%) and a history of impaired fasting glucose by 14 patients (1.8%). Mean baseline LDL-C level was 120.4 (37.9) mg/dL. The majority of patients (698/779 [89.6%]) were receiving background statin therapy (53.0% high-intensity). Five percent of patients (41/779) were not taking lipid-lowering therapy at baseline. Demographics and baseline characteristics were generally balanced between treatment groups (Table 1).
Table 1. Patient Demographics and Baseline Characteristicsa.
| Parameter | Bempedoic Acidb (n = 522) | Placebob (n = 257) |
|---|---|---|
| Age, mean (SD), y | 64.1 (8.8) | 64.7 (8.7) |
| Sex, No. (%) | ||
| Men | 328 (62.8) | 168 (65.4) |
| Women | 194 (37.2) | 89 (34.6) |
| Race, No. (%) | ||
| White | 491 (94.1) | 244 (94.9) |
| Black or African American | 24 (4.6) | 12 (4.7) |
| Otherc | 7 (1.3) | 1 (0.4) |
| Hispanic or Latino, No. (%) | 43 (8.2) | 19 (7.4) |
| Cardiovascular disease risk category, No. (%) | ||
| ASCVD only | 495 (94.8) | 241 (93.8) |
| Heterozygous familial hypercholesterolemia with or without ASCVD | 27 (5.2) | 16 (6.2) |
| Disease history, No. (%)d | ||
| Hypertension | 438 (83.9) | 224 (87.2) |
| Coronary heart disease | 432 (82.8) | 205 (79.8) |
| Diabetes | 155 (29.7) | 81 (31.5) |
| Impaired fasting glucose | 9 (1.7) | 5 (1.9) |
| Body mass index, mean (SD)e | 30.0 (5.2) | 30.6 (5.0) |
| eGFR category, mL/min/m2, No. (%) | ||
| ≥90 | 107 (20.5) | 56 (21.8) |
| ≥60 to <90 | 338 (64.8) | 164 (63.8) |
| <60 | 77 (14.8) | 37 (14.4) |
| Background lipid-lowering therapy, No. (%)f,g | ||
| Statin without other nonstatin therapy | 416 (79.7) | 196 (76.3) |
| Statin with other nonstatin therapy | 54 (10.3) | 32 (12.5) |
| Nonstatin(s) alone | 22 (4.2) | 15 (5.8) |
| None | 30 (5.7) | 14 (5.4) |
| Statin intensity, No. (%)h | ||
| Low or no statin | 78 (14.9) | 40 (15.6) |
| Moderate | 166 (31.8) | 82 (31.9) |
| High | 278 (53.3) | 135 (52.5) |
| Receiving ezetimibe, No. (%) | 38 (7.3) | 24 (9.3) |
| Lipids, mean (SD), mg/dL | ||
| Total cholesterol | 202.1 (42.7) | 204.8 (46.1) |
| HDL-C | 51.4 (12.9) | 51.1 (13.1) |
| Non–HDL-C | 150.7 (42.7) | 153.7 (44.4) |
| LDL-C | 119.4 (37.7) | 122.4 (38.3) |
| LDL-C category, No. (%) | ||
| <130 | 365 (69.9) | 173 (67.3) |
| ≥130 and <160 | 89 (17.0) | 45 (17.5) |
| ≥160 | 68 (13.0) | 39 (15.2) |
| Triglycerides, median (IQR) | 139.3 (102.5-190.0) | 143.0 (106.0-189.0) |
| Apolipoprotein B, mean (SD), mg/dL | 116.2 (29.6) | 118.6 (30.5) |
| hsCRP, median (IQR), mg/L | 1.61 (0.87-3.46) | 1.88 (0.92-3.79) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: To convert total cholesterol, HDL-C, and LDL-C levels to mmol/L, multiply by 0.0259; triglyceride values to mmol/L, multiply by 0.0113; hsCRP values to nmol/L, multiply by 9.524.
Baseline for LDL-C, HDL-C, non-HDL-C, triglycerides, and total cholesterol was defined as the mean of the last 2 nonmissing values on or before day 1. Baseline for apolipoprotein B and hsCRP was defined as the last nonmissing value on or before day 1. Baseline for all other parameters was defined as last measurement before the first dose of study drug.
Entries are mean (SD) unless otherwise indicated.
Including American Indian or Alaska Native (n = 1), Asian (n = 4), Native Hawaiian or other Pacific Islander (n = 1), and multiple (n = 2).
Presence of these conditions was determined by patient-reported medical history.
Calculated as weight in kilograms divided by height in meters squared.
Changes in background lipid-lowering therapy during the study were reported by 61 patients (11.7%) in the bempedoic acid group and 33 patients (12.8%) in the placebo group.
Five patients (2 bempedoic acid, 3 placebo) were receiving background treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
Statin intensity classification is based on the 2013 American College of Cardiology/American Heart Association guidelines.15
Primary Outcome
At week 12, bempedoic acid lowered LDL-C levels significantly more than placebo (–15.1% vs 2.4%, respectively; P < .001), for a placebo-corrected least-squares mean difference of –17.4% (95% CI, –21.0% to –13.9%). Mean LDL-C level at week 12 in the bempedoic acid group was 97.6 mg/dL, compared with 122.8 mg/dL in the placebo group (Figure 2; eTable 1 in Supplement 3).
Figure 2. Effect of Bempedoic Acid on Low-Density Lipoprotein Cholesterol (LDL-C) Level.
A, Error bars indicate 95% CIs. Numbers of patients at each time point are those with evaluable data per treatment group; no imputation was performed for missing data. See eTable 1 in Supplement 3 for data. B, Boxes indicate 25th and 75th percentiles; horizontal lines within boxes indicate median LDL-C values; error bars indicate Tukey-style upper and lower bounds; points beyond the error bars indicate individual patient values outside of this range.
Secondary and Other Efficacy Outcomes
At week 24, change in LDL-C level from baseline was –12.1% in the bempedoic acid group and 2.7% in the placebo group (difference, –14.8% [95% CI, –19.5% to –10.0%]; P < .001). Significant reductions at week 12 with bempedoic acid vs placebo were observed for levels of non–HDL-C, total cholesterol, apoB, and hsCRP (P < .05) (Table 2). Improvements in lipid parameters and biomarkers were maintained through week 52 (Figure 2 and Table 2). In the bempedoic acid treatment group, mean absolute LDL-C level was less than 100 mg/dL at all postbaseline assessments. Changes from baseline in triglyceride levels were comparable between treatment groups, and statistically significant reductions in HDL-C levels were observed in the bempedoic acid group (P < .001) (eTable 2 in Supplement 3).
Table 2. Percent Change From Baseline in Secondary Efficacy Variables.
| Parameter, wk | Bempedoic Acid | Placebo | LS Mean Difference (95% CI)b | P Value | ||
|---|---|---|---|---|---|---|
| No. | Change From Baseline, LS Mean (SE), %a | No. | Change From Baseline, LS Mean (SE), %a | |||
| Non–HDL-C | ||||||
| 12 | 498 | –10.8 (1.0) | 253 | 2.3 (1.4) | –13.0 (–16.3 to –9.8) | <.001 |
| 24 | 485 | –10.2 (1.2) | 247 | 2.4 (1.6) | –12.6 (–16.6 to –8.7) | <.001 |
| 52 | 467 | –10.3 (1.2) | 237 | –0.4 (1.6) | –9.9 (–13.8 to –6.0) | <.001 |
| Total cholesterol | ||||||
| 12 | 499 | –9.9 (0.7) | 253 | 1.3 (1.0) | –11.2 (–13.6 to –8.8) | <.001 |
| 24 | 486 | –9.3 (0.9) | 247 | 1.5 (1.2) | –10.8 (–13.7 to –7.8) | <.001 |
| 52 | 467 | –10.3 (0.8) | 237 | –1.9 (1.2) | –8.4 (–11.2 to –5.5) | <.001 |
| Apolipoprotein B | ||||||
| 12 | 479 | –9.3 (0.9) | 245 | 3.7 (1.3) | –13.0 (–16.1 to –9.9) | <.001 |
| 24 | 294 | –8.6 (1.3) | 144 | 4.4 (2.1) | –13.0 (–17.8 to –8.2) | <.001 |
| 52 | 464 | –6.6 (1.0) | 237 | 3.0 (1.5) | –9.6 (–13.1 to –6.0) | <.001 |
| hsCRP | ||||||
| 12 | 467 | –18.7 (–46.1 to 23.9) | 240 | –9.4 (–36.3 to 35.2) | –8.7 (–17.2 to –0.4) | .04 |
| 24 | 271 | –24.1 (–51.5 to 14.0) | 127 | 1.6 (–32.2 to 47.5) | –21.3 (–32.3 to –10.0) | <.001 |
| 52 | 465 | –16.7 (–50.9 to 31.4) | 237 | –6.3 (–39.3 to 41.8) | –7.6 (–17.0 to 1.7) | .10 |
Abbreviations: hsCRP, high-sensitivity C-reactive protein; LS, least-squares; non–HDL-C, non–high-density lipoprotein cholesterol.
Data for hsCRP are presented as median (25th-75th percentile).
Percent changes from baseline in non–HDL-C, total cholesterol, and apolipoprotein B were analyzed using analysis of covariance with treatment group and randomization stratification parameters as factors and baseline value as a covariate. For secondary end points (week 12), missing data were imputed using a pattern-mixture model. For hsCRP, nonparametric analyses (Wilcoxon rank-sum test) with Hodges-Lehmann estimates of location shift and 95% asymptotic confidence limits were performed without imputation for missing values.
Prespecified sensitivity analyses confirmed the LDL-C lowering observed with bempedoic acid. Among patients receiving assigned study drug at week 12 (on-treatment analysis), bempedoic acid lowered LDL-C levels by 16.0% compared with an increase of 2.4% in the placebo group (difference, –18.4% [95% CI, –21.9% to –14.9%]; P < .001). Results from on-treatment analyses were also comparable with primary analyses for HDL-C, total cholesterol, apoB, and hsCRP. In all prespecified patient subgroups, LDL-C lowering at week 12 was significantly greater with bempedoic acid compared with placebo (P < .05) (eFigure in Supplement 3). The magnitude of LDL-C lowering was generally consistent among subgroups.
Post Hoc Analyses
Additional post hoc analyses found similar LDL-C lowering in patients receiving a low-/moderate-intensity or high-intensity statin and placebo-corrected decreases from baseline of 22.0% (95% CI, –33.4% to –10.6%; P < .001) in patients receiving no statin and 26.8% (95% CI, –40.2% to –13.3%; P < .001) in patients receiving no lipid-lowering therapy (eTable 3 in Supplement 3). In an exploratory analysis, LDL-C lowering at week 12 in patients who maintained stable background lipid-lowering therapy (≈95% of patients) was consistent with that in the overall population. A post hoc analysis detected no effect of study site on the primary end point (P = .28); LDL-C lowering with bempedoic acid vs placebo was 17.5% (95% CI, –21.0 to –13.9%; P < .001) when study site was added as a random effect in a mixed-effects model (eTable 4 in Supplement 3).
Adverse Events
Treatment-emergent adverse events occurred in 70.1% of patients in the bempedoic acid group and 70.8% of patients in the placebo group (Table 3). The majority of adverse events (430/548 [78.5%]) were mild or moderate in intensity, and most (425/548 [77.6%]) were classified by the investigator as not related or unlikely related to study drug treatment. Seventy-nine patients (57/522 [10.9%] bempedoic acid, 22/257 [8.6%] placebo) had an adverse event that led to discontinuation of study treatment. The difference in frequency was not caused by an imbalance in any single adverse event or class of adverse events. Adverse events leading to discontinuation that occurred in more than 0.5% of patients in either treatment group were myalgia (1.0% bempedoic acid, 0.8% placebo), increased aspartate aminotransferase level (0.6% bempedoic acid, 0% placebo), arthralgia (0.6% bempedoic acid, 0% placebo), muscle spasms (0.6% bempedoic acid, 0% placebo), cardiac arrest (0.2% bempedoic acid, 0.8% placebo), and fatigue (0.2% bempedoic acid, 0.8% placebo). Serious adverse events occurred in 19.8% of patients (154/779). Three serious adverse events were considered to be at least possibly related to study treatment: ulcerative colitis and ischemic stroke in the bempedoic acid group and upper abdominal pain in the placebo group.
Table 3. Treatment-Emergent Adverse Events and Key Safety Laboratory Parameters.
| Parameter | Bempedoic Acid (n = 522) | Placebo (n = 257) |
|---|---|---|
| Overview of AEs, No. (%) | ||
| Any AE | 366 (70.1) | 182 (70.8) |
| Serious AE | 106 (20.3) | 48 (18.7) |
| Study drug–related AEs | 91 (17.4) | 32 (12.5) |
| Discontinuation because of AE | 57 (10.9) | 22 (8.6) |
| Fatal treatment-emergent AE | 6 (1.1) | 2 (0.8) |
| Most common AEs, No. (%)a | ||
| Nasopharyngitis | 27 (5.2) | 13 (5.1) |
| Urinary tract infection | 26 (5.0) | 5 (1.9) |
| Hyperuricemia | 22 (4.2) | 5 (1.9) |
| Upper respiratory tract infection | 19 (3.6) | 9 (3.5) |
| Arthralgia | 18 (3.4) | 8 (3.1) |
| Diarrhea | 16 (3.1) | 7 (2.7) |
| Angina pectoris | 16 (3.1) | 5 (1.9) |
| Osteoarthritis | 16 (3.1) | 5 (1.9) |
| Dizziness | 8 (1.5) | 9 (3.5) |
| Lower respiratory tract infection | 8 (1.5) | 8 (3.1) |
| Fatigue | 6 (1.1) | 9 (3.5) |
| AEs of special interest, No. (%) | ||
| Myalgia | 15 (2.9) | 8 (3.1) |
| Muscle spasms | 11 (2.1) | 3 (1.2) |
| Pain in extremity | 11 (2.1) | 1 (0.4) |
| Muscular weakness | 2 (0.4) | 1 (0.4) |
| New-onset or worsening diabetes | 36 (6.9) | 19 (7.4) |
| Blood uric acid increased | 14 (2.7) | 1 (0.4) |
| Gout | 11 (2.1) | 2 (0.8) |
| Blood creatinine increased | 4 (0.8) | 1 (0.4) |
| Glomerular filtration rate decreased | 4 (0.8) | 1 (0.4) |
| Neurocognitive disorders | 3 (0.6) | 1 (0.4) |
| Laboratory results | ||
| ALT or AST >3× ULN, No. (%)b | 6 (1.1) | 2 (0.8) |
| Creatine kinase >5× ULN, No. (%)b | 0 | 1 (0.4) |
| Mean (SD) change, baseline to wk 52 | ||
| Uric acid, mg/dLc | 0.6 (1.2) | 0.1 (1.1) |
| Creatinine, mg/dL | 0.05 (0.16) | 0.01 (0.12) |
| eGFR, mL/min/1.73 m2 | –3.8 (10.2) | –1.1 (10.8) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; ULN, upper limit of normal.
SI conversion factors: To convert uric acid values to μmol/L, multiply by 59.485; creatinine values to μmol/L, multiply by 88.4.
Occurring in 3% or more of patients in either treatment group, excluding AEs of special interest.
Percentage of patients with repeated and confirmed elevations in aminotransferase or creatine kinase levels.
Baseline mean uric acid concentrations were 5.95 (SD, 1.47) mg/dL (bempedoic acid) and 5.97 (SD, 1.44) mg/dL (placebo).
Eight fatal treatment-emergent adverse events were reported during the study, including 1 case each of cardiac arrest, coronary artery arteriosclerosis, acute poisoning with carbon dioxide, myocardial infarction, septic shock (related to a prescheduled abdominal surgical procedure), and unknown cause in the bempedoic acid group and acute coronary syndrome and coronary artery disease in the placebo group. All fatal adverse events were assessed by the investigator as unrelated to study drug. Four events (0.8%) in the bempedoic acid group (including the death from unknown cause) and 2 events (0.8%) in the placebo group were positively adjudicated as cardiovascular deaths (eTable 5 in Supplement 3). Adjudicated clinical events occurred in 8.2% of patients (43/522) in the bempedoic acid group and 10.1% (26/257) in the placebo group. Positively adjudicated 3-component major adverse cardiovascular event rates of 2.7% and 4.7% were observed in the bempedoic acid and placebo groups, respectively (eTable 5 in Supplement 3); the difference between groups was not statistically significant.
Myalgia was reported by approximately 3% of patients and muscle weakness by 0.4% of patients who were receiving either bempedoic acid or placebo (Table 3). New-onset or worsening diabetes occurred in approximately 7% of patients in both treatment groups. Gout and increased blood uric acid level were experienced, respectively, by 2.1% and 2.7% of patients in the bempedoic acid group and 0.8% and 0.4% of patients in the placebo group. Among the 11 patients in the bempedoic acid group who experienced gout, 5 had a history of gout and 3 had a history of hyperuricemia before study enrollment. Uric acid levels were above the upper limit of normal at baseline for 10 of the 11 patients in the bempedoic acid group who reported gout.
Laboratory and Other Safety Measures
Among patients with diabetes at baseline, mean hemoglobin A1c (HbA1c) levels decreased from baseline to week 12 by 0.08 percentage point in the bempedoic acid group and increased by 0.13 percentage point in the placebo group. To further explore effects of bempedoic acid on glycemia, fasting glucose and HbA1c levels were evaluated in patients with a history of or laboratory evidence indicating diabetes or impaired fasting glucose. Changes in HbA1c levels across populations with diabetes and with impaired fasting glucose showed improvement or less worsening of glycemic control at week 12 (eTable 6 in Supplement 3). Favorable glycemic control and less worsening of diabetes persisted over the 1-year treatment period.
Overall, mean uric acid levels increased by 0.6 (SD, 1.2) mg/dL at week 52 in the bempedoic acid group compared with 0.1 (SD, 1.1) mg/dL in the placebo group (Table 3). Rates of aminotransferase level elevations greater than 3 times the upper limit of normal were 1.1% in the bempedoic acid group and 0.8% in the placebo group. Mean creatinine concentration increased by 0.5 (SD, 0.16) mg/dL and estimated glomerular filtration rate (calculated based on creatinine concentration) decreased by 3.8 (SD, 10.2) mL/min/1.73 m2 from baseline to week 52 among patients who received bempedoic acid.
Discussion
In this population of patients at high cardiovascular risk, addition of oral bempedoic acid (180 mg) once daily to maximally tolerated lipid-lowering therapy significantly lowered LDL-C levels compared with placebo. The results of this study reinforce observations from the similarly designed CLEAR Harmony clinical trial in which bempedoic acid lowered LDL-C levels at week 12 by 18.1% (95% CI, –20.0% to –16.1%; P < .001) compared with placebo in patients receiving maximally tolerated statin therapy.13 In both studies, LDL-C lowering was maintained through week 52, significant improvements were observed in secondary efficacy measures, and treatment effects were consistent across patient subgroups. In addition, despite prevalent background statin use, rates of adverse events associated with statin therapy such as muscle-related adverse events and new-onset or worsening diabetes were generally low. Similar to this 52-week trial, a statistically nonsignificant difference in the incidence of major adverse cardiovascular events with bempedoic acid (4.6%) vs placebo (5.7%) (relative risk, 0.81 [95% CI, 0.56 to 1.17]) was observed in CLEAR Harmony.
The study described herein expands on the findings of CLEAR Harmony in several key areas. The current study required that patients be receiving maximally tolerated lipid-lowering therapy, which may have included no statin or no background lipid-lowering therapy, whereas patients in CLEAR Harmony were required to be receiving a maximally tolerated statin. Inclusion of these different groups allowed for analysis of the effects of background therapies on lipid changes and revealed considerable LDL-C lowering among patients in the bempedoic acid treatment group who were not receiving statin therapy or not receiving any background lipid-lowering therapy. The screening LDL-C threshold in the current study (≥100 mg/dL) was also higher than that in CLEAR Harmony (≥70 mg/dL), which resulted in greater baseline mean LDL-C values (120.4 and 103.2 mg/dL, respectively).13 The higher baseline mean LDL-C level reflects a population in greater need of LDL-C lowering compared with the CLEAR Harmony population. Neither the difference in statin usage nor the variance in baseline LDL-C levels affected overall LDL-C reduction with bempedoic acid. In CLEAR Harmony, new-onset or worsening diabetes occurred in 3.3% of patients who received bempedoic acid vs 5.4% of patients who received placebo.13 These observations formed the basis for additional exploration in the current study, which revealed improvement or less worsening of glycemic control among patients receiving bempedoic acid. In the current study, there were no deaths due to cancer, and the rate of fatal treatment-emergent adverse events was 1.1% in the bempedoic acid group and 0.8% in the placebo group.
The treatment strategy applied in the current study, wherein an additional agent is added to maximally tolerated lipid-lowering therapy in patients at high cardiovascular risk who have not achieved sufficient LDL-C lowering, is consistent with cholesterol management guideline recommendations.1 At screening, all of the enrolled patients had LDL-C levels 100 mg/dL or greater despite background therapy rates of 90% for statins, the first-line therapy for LDL-C lowering, and 8% for ezetimibe, the guideline-recommended adjunct to statin therapy in patients requiring further LDL-C lowering. Although use of a background proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was allowed in the current study, few patients were using this therapy, likely because of barriers to access common during the enrollment period of the study.16 The risk associated with persistent on-treatment hypercholesterolemia in patients at high cardiovascular risk warrants investigation of new pharmacologic therapies that can be used in conjunction with statins as well as other nonstatin agents.
Limitations
This study has several limitations. First, although adherence to study drug was monitored, adherence to background therapy was not. Given the known problems with inconsistency in long-term adherence to statin therapy,17,18 it is reasonable to expect that some patients did not maintain a stable treatment regimen over time, which would have the effect of dampening the observed lipid lowering. Second, the study was only 52 weeks in duration; further data on long-term efficacy and safety are needed. Third, the study was not powered to evaluate cardiovascular outcomes. Greater clarity regarding event risk reduction with bempedoic acid will come from the ongoing 12 600-patient cardiovascular outcomes trial (CLEAR Outcomes [NCT02993406]).
Conclusions
Among patients at high risk for cardiovascular disease receiving maximally tolerated statins, the addition of bempedoic acid compared with placebo resulted in a significant lowering of LDL-C level over 12 weeks. Further research is needed to assess the durability and clinical effect as well as long-term safety.
Study Protocol
Statistical Analysis Plan
eTable 1. Effect of Bempedoic Acid on Low-Density Lipoprotein Cholesterol (LDL-C) Level
eTable 2. Percent Change From Baseline in Triglycerides and HDL-C
eTable 3. Low-Density Lipoprotein Cholesterol (LDL-C) Percent Change From Baseline to Week 12 by Statin Intensity and Background Lipid-Lowering Therapy, Post Hoc Subgroup Analysis
eTable 4. Post Hoc Analysis of the Effect of Study Site on the Primary Endpoint, Percent Change From Baseline to Week 12 in Low-Density Lipoprotein Cholesterol (LDL-C)
eTable 5. Positively Adjudicated Clinical Events
eTable 6. Measures of On-Treatment Glucose Control by Baseline Glycemic Status
eFigure. Low-Density Lipoprotein Cholesterol (LDL-C) Percent Change From Baseline to Week 12 by Patient Subgroup
Data Sharing Statement
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485-494. doi: 10.1016/j.jacc.2014.02.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deGoma EM, Ahmad ZS, O’Brien EC, et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH registry. Circ Cardiovasc Genet. 2016;9(3):240-249. doi: 10.1161/CIRCGENETICS.116.001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitt AK, Lautsch D, Ferrieres J, et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis. 2016;255:200-209. doi: 10.1016/j.atherosclerosis.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Menzin J, Aggarwal J, Boatman B, et al. Ezetimibe use and LDL-C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm. 2017;23(12):1270-1276. doi: 10.18553/jmcp.2017.16414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez de Isla L, Alonso R, Watts GF, et al. ; SAFEHEART Investigators . Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol. 2016;67(11):1278-1285. doi: 10.1016/j.jacc.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 9.Pinkosky SL, Newton RS, Day EA, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;7:13457. doi: 10.1038/ncomms13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380(11):1033-1042. doi: 10.1056/NEJMoa1806747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catapano AL, Graham I, De Backer G, et al. ; ESC Scientific Document Group . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058. doi: 10.1093/eurheartj/ehw272 [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195-203. doi: 10.1016/j.atherosclerosis.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Ray KK, Bays HE, Catapano AL, et al. ; CLEAR Harmony Trial . Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022-1032. doi: 10.1056/NEJMoa1803917 [DOI] [PubMed] [Google Scholar]
- 14.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi: 10.1161/JAHA.118.011662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 16.Cohen JD, Cziraky MJ, Jacobson TA, Maki KC, Karalis DG. Barriers to PCSK9 inhibitor prescriptions for patients with high cardiovascular risk: results of a healthcare provider survey conducted by the National Lipid Association. J Clin Lipidol. 2017;11(4):891-900. doi: 10.1016/j.jacl.2017.04.120 [DOI] [PubMed] [Google Scholar]
- 17.Stroes ES, Thompson PD, Corsini A, et al. ; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi: 10.1093/eurheartj/ehv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colantonio LD, Rosenson RS, Deng L, et al. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8(1):e010376. doi: 10.1161/JAHA.118.010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eTable 1. Effect of Bempedoic Acid on Low-Density Lipoprotein Cholesterol (LDL-C) Level
eTable 2. Percent Change From Baseline in Triglycerides and HDL-C
eTable 3. Low-Density Lipoprotein Cholesterol (LDL-C) Percent Change From Baseline to Week 12 by Statin Intensity and Background Lipid-Lowering Therapy, Post Hoc Subgroup Analysis
eTable 4. Post Hoc Analysis of the Effect of Study Site on the Primary Endpoint, Percent Change From Baseline to Week 12 in Low-Density Lipoprotein Cholesterol (LDL-C)
eTable 5. Positively Adjudicated Clinical Events
eTable 6. Measures of On-Treatment Glucose Control by Baseline Glycemic Status
eFigure. Low-Density Lipoprotein Cholesterol (LDL-C) Percent Change From Baseline to Week 12 by Patient Subgroup
Data Sharing Statement