Key Points
Question
Is the glucocorticoid-induced tumor necrosis factor receptor–related protein agonist BMS-986156 treatment with or without nivolumab tolerable and clinically active in patients with advanced solid tumors?
Findings
In this open-label, phase 1/2a study of 292 treated patients with advanced solid tumors (69 completed initial treatment), BMS-986156 therapy had a tolerable safety profile; combination therapy had a similar safety profile to that of nivolumab. No responses were seen with monotherapy; however, in combination therapy, response rates were comparable to those historically observed with nivolumab (<15% across tumor types).
Meaning
This study represents the largest data set on glucocorticoid-induced tumor necrosis factor receptor–related protein agonism with or without nivolumab to our knowledge; a clear signal has not emerged demonstrating that glucocorticoid-induced tumor necrosis factor receptor–related protein agonism may be an effective therapeutic strategy in a broad patient population.
Abstract
Importance
Multiple immunostimulatory agonist antibodies have been clinically tested in solid tumors to evaluate the role of targeting glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein in anticancer treatments.
Objective
To evaluate the safety and activity of the fully human glucocorticoid-induced TNF receptor–related protein agonist IgG1 monoclonal antibody BMS-986156 with or without nivolumab in patients with advanced solid tumors.
Design, Setting, and Participants
This global, open-label, phase 1/2a study of BMS-986156 with or without nivolumab enrolled 292 patients 18 years or older with advanced solid tumors and an Eastern Cooperative Oncology Group performance status of 1 or less. Prior checkpoint inhibitor therapy was allowed. Monotherapy and combination dose-escalation cohorts ran concurrently to guide expansion doses beginning October 16, 2015; the study is ongoing.
Interventions
The protein agonist BMS-986156 was administered intravenously at a dose of 10, 30, 100, 240, or 800 mg every 2 weeks as monotherapy, and in the combination group 30, 100, 240, or 800 mg plus 240 mg of nivolumab every 2 weeks; same-dose cohorts were pooled for analysis. One cohort also received 480 mg of BMS-986156 plus 480 mg of nivolumab every 4 weeks.
Main Outcomes and Measures
The primary end points were safety, tolerability, and dose-limiting toxic effects. Additional end points included antitumor activity per Response Evaluation Criteria in Solid Tumors, version 1.1, and exploratory biomarker analyses.
Results
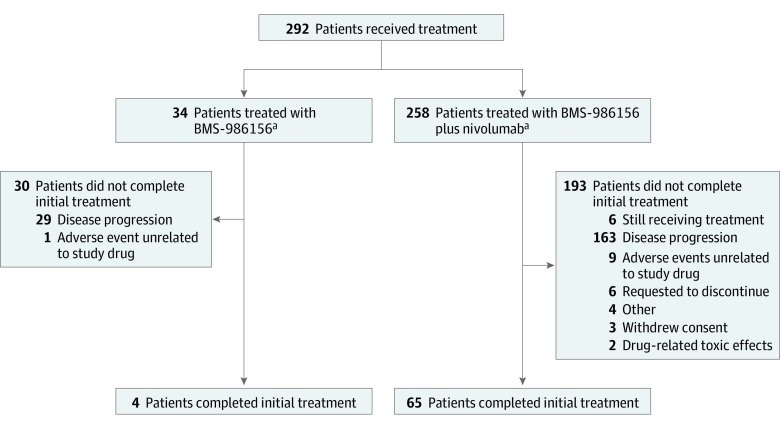
With a follow-up range of 1.4 to 101.7 weeks (follow-up ongoing), 34 patients (16 women and 18 men; median age, 56.6 years [range, 28-75 years]) received monotherapy (4 patients completed initial treatment), and 258 patients (140 women and 118 men; median age, 60 years [range, 21-87 years]) received combination therapy (65 patients completed initial treatment). No grade 3 to 5 treatment-related adverse events occurred with BMS-986156 monotherapy; grade 3 to 4 treatment-related adverse events occurred in 24 patients (9.3%) receiving BMS-986156 plus nivolumab, with no grade 5 treatment-related adverse events. One dose-limiting toxic effect (grade 4 elevated creatine phosphokinase levels) occurred in a patient receiving 800 mg of BMS-986156 plus 240 mg of nivolumab every 2 weeks; BMS-986156 with or without nivolumab exhibited linear pharmacokinetics with dose-related increase after a single dose. Peripheral T-cell and natural killer–cell proliferation increased after administration of BMS-986156 with or without nivolumab. No consistent and significant modulation of intratumoral CD8+ T cells and FoxP3+ regulatory T cells was observed. No responses were seen with BMS-986156 alone; objective response rates ranged from 0% to 11.1% (1 of 9) across combination therapy cohorts, with a few responses observed in patients previously treated with anti–programmed death receptor (ligand) 1 therapy.
Conclusions and Relevance
Based on this cohort, BMS-986156 appears to have had a manageable safety profile, and BMS-986156 plus nivolumab demonstrated safety and efficacy comparable to historical data reported for nivolumab monotherapy.
Trial Registration
ClinicalTrials.gov identifier: NCT02598960
This open-label, phase 1/2a study evaluates the safety and activity of fully human glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein agonist immunoglobulin G1 monoclonal antibody BMS-986156 with or without nivolumab in patients with advanced solid tumors.
Introduction
Immunotherapy treatment options are broad and include multiple approaches. One of the most successful strategies thus far has been the blockade of T-cell inhibitory checkpoints, such as cytotoxic T-lymphocyte–associated antigen 4 and programmed death receptor 1 (PD-1), and programmed death ligand 1 (PD-L1), to enhance the antitumor immune response.1 Immune checkpoint inhibitors lead to highly durable responses and significantly prolonged overall survival in many advanced tumor types.2,3,4 Dual checkpoint blockade, combining nivolumab with ipilimumab, has shown even more promising results in various tumor types.5,6,7,8,9,10 Although checkpoint inhibitors have provided advancements in anticancer treatment, a significant majority of patients, such as those with tumor types that are mismatch repair proficient or noninflamed, remain unresponsive to checkpoint inhibition.11,12,13,14 In addition, some patients with initial response to checkpoint inhibition eventually experience disease progression and face limited subsequent therapeutic options. Thus, new strategies for modulating the critical balance of T-cell activation and antigen tolerance that make an antitumor immune response possible are being investigated.15,16,17,18
A variety of T-cell costimulatory receptors exist whose activity and engagement may potentiate the T-cell response induced by checkpoint inhibitors. Promising therapeutic targets include the tumor necrosis factor receptor (TNFR) superfamily, such as the glucocorticoid-induced TNFR-related protein (GITR), TNFR superfamily member 4 (CD134/OX40), and TNFR superfamily member 9 (CD137/4-1BB). It was hypothesized that agonistic GITR antibodies may successfully activate costimulatory pathways to synergize with PD-1 inhibitors and PD-L1 inhibitors in the tumor microenvironment.1 To date, several anti-GITR antibodies and anti-GITR ligand antibodies have been used in clinical trials.19,20,21,22,23,24,25,26
The protein agonist BMS-986156 is an IgG1 agonist monoclonal antibody to GITR, a molecule that is constitutively expressed by intratumoral regulatory T cells at high levels and by effector T cells at low levels.27 In addition, GITR becomes upregulated on effector T cells on their activation. In preclinical studies, GITR engagement can deplete GITR-expressing cells or can induce T-cell proliferation depending on the system.27,28 BMS-986156 was engineered to increase T-cell activation and deplete intratumoral regulatory T cells in combination with anti–PD-1 therapy. Preclinical experiments with in vivo syngeneic mouse models show enhanced antitumor activity when a GITR agonist antibody is added to anti–PD-1 therapy.29 Thus, it was hypothesized that this combination may result in improved and prolonged antitumor responses in patients with advanced cancer.
In the present study, results are reported from a phase 1/2a dose-escalation and dose-expansion study investigating the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary clinical activity profiles of BMS-986156 as monotherapy treatment and in combination with nivolumab in patients with advanced solid tumors (NCT02598960).30
Methods
Patients
Twenty-seven sites across Australia, Belgium, Canada, France, Germany, Italy, the Netherlands, Spain, Switzerland, and the United States participated in this study, which began October 16, 2015, and is ongoing. Eligible patients were aged 18 years or older with confirmed, previously treated advanced solid tumors per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.131 and must have received and then progressed on or been intolerant to 1 or more standard treatment regimens in the advanced or metastatic setting, if such a therapy existed. Other key eligibility criteria included an Eastern Cooperative Oncology Group performance status of 1 or less. Prior anti–PD-1 therapy or anti–PD-L1 therapy was allowed. The study was conducted in compliance with the trial protocol (Supplement 1). The protocol, any amendments, and the patient informed consent form were reviewed and approved by an institutional review board or independent ethics committee (Australia: Linear Clinical Research Ltd, Liverpool Cancer Therapy Center, Princess Alexandra Hospital, Westmead Hospital; Belgium: Universitair Ziekenhuis Gent; Canada: Cross Cancer Institute, Princess Margaret Cancer Centre; France: Centre Claudius Regaud, Institut Curie, Institut Gustave Roussy; Germany: Klinikum Der Albrecht-Ludwigs-Universitat, Med. Klinik Und Poliklinik D. Uni Wuerzburg, Universitaetsklinikum Bonn; Italy: Istituto di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori Milano, Istituto Europeo Di Oncologia; Netherlands: NKI AVL; Spain: Fundacion Jimenez Diaz, Hosp Univer 12 De Octubre; Switzerland: Cantonal Hospital St Gallen, University Hospital Zurich; United States: Emory University, Providence Portland Medical Center, The Ohio State University, The West Clinic P.C., Thomas Jefferson University Hospital, University of California San Diego Moores Cancer Center, University of Alabama at Birmingham) prior to initiation of the study. Patients provided written informed consent.
Study Design and Treatment
This phase 1/2a, open-label study investigated BMS-986156 as monotherapy and in combination with nivolumab (study design, doses, and Consolidated Standards of Reporting Trials [CONSORT] diagram shown in Figure 1; trial protocol in Supplement 1; and eFigure 1 in Supplement 2).
Figure 1. CONSORT Diagram.
aThis was not a randomized trial and the monotherapy and combination therapy arms were not comparator arms. CONSORT indicates Consolidated Standards of Reporting Trials.
Study Outcomes
The primary objective of this study was to determine the safety, tolerability, dose-limiting toxic effects, and maximum tolerated dose of BMS-986156 either as monotherapy or in combination with nivolumab in patients with advanced solid tumors. Key secondary objectives included determining the antitumor activity and characterizing the pharmacokinetic profile and immunogenicity of BMS-986156 as monotherapy and in combination with nivolumab. Exploratory end points included nivolumab pharmacokinetics, overall survival, and the pharmacodynamics of BMS-986156 plus nivolumab via peripheral blood and intratumoral biomarker analysis.
Safety
Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.32 Adverse events were evaluated throughout the study while participants were receiving treatment and until 100 days after study completion.
Tumor Response
Assessment of tumor response was observed through computed tomography and/or magnetic resonance imaging at the start of the study and every 8 weeks until disease progression or treatment termination via RECIST, version 1.1.
Statistical Analysis
Detailed information on exclusion criteria, study treatment, and statistical, pharmacokinetic, and pharmacodynamic analyses are described in the eAppendix in Supplement 2.
Results
Patient Characteristics
Detailed patient demographics are presented in eTable 1 in Supplement 2. Baseline characteristics were similar between the monotherapy and combination therapy cohorts. Prior anti–PD-1 or anti–PD-L1 therapy was received by 11 of 34 patients (32.4%) in the monotherapy cohort and 51 of 258 patients (19.8%) in the combination cohort.
As of March 27, 2018, 34 patients received 10 to 800 mg of BMS-986156 monotherapy every 2 weeks and 258 patients received 30 to 800 mg of BMS-986156 plus 240 mg of nivolumab every 2 weeks or 480 mg of BMS-986156 plus 480 mg of nivolumab every 4 weeks. Duration of therapy ranged from 2.0 to 61.0 weeks, with follow-up time ranging from 1.4 to 101.7 weeks. Median treatment exposure (in weeks) is presented in eTables 2A and 2B in Supplement 2. All patients but 1 (n = 33) were able to receive 90% or more of the planned cumulative treatment dose of BMS-986156 in the dose-escalation phase (4 patients completed initial treatment), and more than 80% of patients in each dose cohort of the combination phase (n = 227) were able to receive 90% or more of the planned cumulative treatment dose of both drugs (65 patients completed initial treatment).
Safety
Overall, the safety profile of BMS-986156 was tolerable. Any treatment-related adverse events (TRAEs) by dose in the BMS-986156 monotherapy cohort and in 5% or more of patients in the BMS-986156 plus nivolumab combination cohorts, as well as any grade 3 or 4 TRAEs in either cohort, are shown in eTables 3A and 3B in Supplement 2. Grades 3 and 4 TRAEs occurred in 24 patients (9.3%) receiving BMS-986156 plus nivolumab. No grade 5 TRAEs were observed with either treatment. In addition, no TRAEs led to treatment discontinuation in the BMS-986156 monotherapy cohort. Three TRAEs led to treatment discontinuation of BMS-986156 plus nivolumab (grade 3 colitis, infusion-related reaction, and pancreatitis, all in the cohort receiving 240 mg of BMS-986156 plus 240 mg of nivolumab). Grade 1 to 2 pyrexia was seen in 6 patients (17.6%) treated with BMS-986156 monotherapy and 28 patients (10.9%) treated with combination therapy, usually within 24 hours of infusion without associated sequelae. Any grade and grades 3 and 4 serious TRAEs occurring in the overall monotherapy and combination cohorts are shown in eTable 4 in Supplement 2. Grade 2 pneumonitis was the only serious TRAE seen in patients treated with BMS-986156 monotherapy. With combination therapy, most serious TRAEs were observed in the 202 patients who received 240 mg of BMS-986156 plus 240 mg of nivolumab every 2 weeks. One dose-limiting toxic effect (grade 4 elevated creatine phosphokinase level) occurred in a patient receiving 800 mg of BMS-986156 plus 240 mg of nivolumab every 2 weeks. Causes of death in the trial are shown in eTable 5 in Supplement 2.
Pharmacokinetics and Immunogenicity
The pharmacokinetics for BMS-986156 monotherapy and BMS-986156 plus nivolumab combination after the first dose is shown in eFigure 2 in Supplement 2. Overall, the pharmacokinetics was linear and exhibited a dose-related increase in exposure that was not affected in combination with nivolumab.
Immunogenicity with BMS-986156 monotherapy was low; only 1 of 31 patients (at the dose of 10 mg every 2 weeks) was antidrug antibody (ADA) positive, and no patients had persistent ADA positivity (eTable 6A in Supplement 2). In the combination cohort, the frequency of anti-GITR antibodies at all tested doses of BMS-986156 remained low, with 223 of 229 patients (97.4%) remaining ADA negative, and no persistently ADA-positive patients. Immunogenicity with nivolumab was relatively higher, with 35 of 223 ADA-positive patients (15.7%), and 1 of 223 patients (0.4%) persistently positive, which is consistent with nivolumab monotherapy (eTable 6B in Supplement 2).
Pharmacodynamics
In analyzed patients, there was a trend toward an increase in CD8+ T-cell and natural killer–cell proliferation after administration of anti-GITR monotherapy tested up to a dose of 240 mg every 2 weeks, although patient numbers were small (eFigure 3 in Supplement 2). Enhanced CD8+ T-cell and natural killer–cell proliferation was observed in the combination cohort.
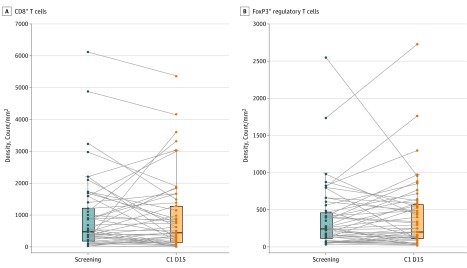
In the subset of patients with data available at baseline and all analyzed time points, results of flow cytometry revealed no clear depletion of regulatory T cells in the peripheral blood in response to BMS-986156 plus nivolumab (eFigure 4 in Supplement 2). In addition, CD8+ T cells and FoxP3+ regulatory T cells as assessed by immunohistochemistry revealed interpatient variability from before treatment to the treatment period with BMS-986156 plus nivolumab (Figure 2).
Figure 2. Intratumoral T-Cell and Regulatory T-Cell Modulation After BMS-986156 Plus Nivolumab Therapy.
Tumor-infiltrating CD8+ T cells (P = .72) (A) and FoxP3+ regulatory T cells (P = .44) (B) by immunohistochemistry biopsies before treatment and while receiving treatment for 53 matched pairs of patients receiving BMS-986156 plus nivolumab combination therapy (all patients included in this analysis received 240 mg of nivolumab every 2 weeks). The Wilcoxon signed rank test was performed for all the comparisons. The horizontal line in each box plot denotes the median level. C1 D15 indicates cycle 1, day 15.
Preliminary Clinical Activity
Response results for the BMS-986156 monotherapy cohort and the BMS-986156 plus nivolumab combination cohort are summarized in Table 1 and Table 2, and response results by tumor type for the combination treatment cohort are summarized in eTable 7 in Supplement 2. No complete or partial responses were observed with BMS-986156 alone. Objective response rates ranged from 0% to 11.1% (1 of 9) across combination therapy cohorts; an objective response rate of 9.0% (18 of 200) was observed in the patient cohort evaluable for response who received 240 mg of BMS-986156 plus 240 mg of nivolumab.
Table 1. Efficacy in the BMS-986156 Monotherapy Cohort.
| Outcome | 10 mg of BMS-986156 (n = 4) | 30 mg of BMS-986156 (n = 6) | 100 mg of BMS-986156g (n = 4) | 240 mg of BMS-986156 (n = 9) | 800 mg of BMS-986156 (n = 11) |
|---|---|---|---|---|---|
| BOR, No. (%) | |||||
| CR | 0 | 0 | 0 | 0 | 0 |
| PR | 0 | 0 | 0 | 0 | 0 |
| SD | 2 (50.0) | 1 (16.7) | 2 (50.0) | 2 (22.2) | 4 (36.4) |
| PD | 1 (25.0) | 3 (50.0) | 2 (50.0) | 6 (66.7) | 6 (54.5) |
| NE | 1 (25.0) | 2 (33.3) | 0 | 1 (11.1) | 1 (9.1) |
| Confirmed ORR, % (95% CI) | 0 (0.0-60.2) | 0 (0.0-45.9) | 0 (0.0-60.2) | 0 (0.0-33.6) | 0 (0.0-28.5) |
| Confirmed DCR, No. (%) [95% CI] | 2 (50.0) [6.8-93.2] | 1 (16.7) [0.4-64.1] | 2 (50.0) [6.8-93.2] | 2 (22.2) [2.8-60.0] | 4 (36.4) [10.9-69.2] |
Abbreviations: BOR, best overall response; CR, complete response; DCR, disease control rate; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Table 2. Efficacy in the BMS-986156 Plus Nivolumab Combination Cohort.
| Outcome | 30 mg of BMS-986156 + 240 mg of Nivolumab (n = 3) | 100 mg of BMS-986156 + 240 mg of Nivolumab (n = 9) | 240 mg of BMS-986156 + 240 mg of Nivolumab (n = 200) | 800 mg of BMS-986156 + 240 mg of Nivolumab (n = 11) | 480 mg of BMS-986156 + 480 mg of Nivolumab (n = 29) |
|---|---|---|---|---|---|
| BOR, No. (%) | |||||
| CR | 0 | 0 | 2 (1.0) | 0 | 0 |
| PR | 0 | 1 (11.1)a | 16 (8.0)b,c | 1 (9.1) | 1 (3.4) |
| SD | 1 (33.3) | 2 (22.2) | 65 (32.5) | 5 (45.5) | 11 (37.9) |
| PD | 2 (66.7) | 4 (44.4) | 95 (47.5) | 4 (36.4) | 12 (41.4) |
| NE | 0 | 2 (22.2) | 14 (7.0) | 0 | 2 (6.9) |
| Confirmed ORR, No. (%) [95% CI] | 0 [0.0-70.8] | 1 (11.1) [0.3-48.2] | 18 (9.0) [5.4-13.9] | 1 (9.1) [0.2-41.3] | 1 (3.4) [0.1-17.8] |
| Confirmed DCR, No. (%) [95% CI] | 1 (33.3) [0.8-90.6] | 3 (33.3) [7.5-70.1] | 83 (41.5) [34.6-48.7] | 6 (54.5) [23.4-83.3] | 12 (41.4) [23.5-61.1] |
Abbreviations: BOR, best overall response; CR, complete response; DCR, disease control rate; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
This patient had prior treatment with pembrolizumab, with a BOR of PD, ending 111 weeks before initiation of study treatment.
One of these patients had prior treatment with nivolumab, with a BOR of PD, ending 4 weeks before initiation of study treatment.
One of these patients had prior treatment with pembrolizumab, with a BOR of PR, ending 83 weeks before initiation of study treatment.
Discussion
Here, results are presented from a phase 1/2a dose-escalation and dose-expansion study of the GITR agonistic antibody BMS-986156 with or without nivolumab in 292 patients with advanced solid tumors. Overall, the safety profile of BMS-986156 monotherapy appeared to be manageable, with no unanticipated safety signals, no dose-limiting toxic effects, and no treatment discontinuations owing to TRAEs. The findings suggest that the safety profile of BMS-986156 plus nivolumab at all doses was manageable and tolerable and was similar to that of nivolumab alone.2,33,34 The rate of infusion-related reactions with combination therapy was the only TRAE that potentially appeared more frequently than observed with nivolumab alone.2,33,34
Limitations
Some limitations of this trial include the absence of comparator groups and the enrollment of an unselected population. For example, although combination therapy with BMS-986156 plus nivolumab yielded pharmacodynamic changes and clinical response rates similar to those historically observed with nivolumab monotherapy in patients with advanced solid tumors (range, 13%-20%),2,33,34,35 this trial was not designed to be a head-to-head comparison of nivolumab monotherapy vs nivolumab plus BMS-986156. Thus, without an appropriate comparator group (eg, nivolumab monotherapy), dissecting the effects of BMS-986156 and nivolumab is difficult, and the contribution of the GITR agonist to the combinatorial clinical activity remains unclear. In addition, no tumor type appeared to respond more favorably to combination therapy vs others. Most observed responses occurred in patients without prior anti–PD-1 or anti–PD-L1 therapy, although a few responses were observed in patients with prior anti–PD-1 or anti–PD-L1 therapy. Overall, however, the data appear to indicate the absence of substantial clinical activity of BMS-986156 in an unselected, broad population of patients with advanced solid tumors.
Similar observations of a tolerable safety profile and some immunomodulatory action, but limited clinical activity, have been made with other GITR agonist antibodies in clinical trials; across 4 other studies, more than 100 patients have been treated with GITR agonist monotherapy, demonstrating a manageable safety profile and limited clinical activity (the best overall response observed was stable disease).19,21,22,36 Based on current available data, to date, no synergistic activity has been observed between GITR agonists and pembrolizumab when administered as a combination treatment, except potentially in checkpoint inhibitor–naive patients with melanoma, based on a small cohort of 13 patients.21,37
Although checkpoint inhibitors have made strides in revolutionizing cancer therapy, to our knowledge, there has not been a clear path identified to date for costimulatory therapy combinations. Other members of the TNFR superfamily and classes of costimulatory molecules that are mechanistically different from TNFR are also under investigation, including inducible costimulator, a member of the CD28 family, which promotes T-cell proliferation and cytokine production after T-cell activation.15 The potential role of combination checkpoint inhibition plus agonistic induction of costimulatory T-cell pathways in certain patient subsets with immunotherapy-resistant or refractory tumors remains to be determined.
Conclusions
To our knowledge, this represents the largest clinical data set investigating GITR agonist therapy (BMS-986156) with or without nivolumab. We believe that BMS-986156 has a manageable safety profile, and its combination with nivolumab seems to show a similar safety signal to that of nivolumab monotherapy. Clinical activity of anti-GITR therapy plus anti–PD-1 therapy was similar to historically observed activity with anti–PD-1 therapy alone. Thus, no evidence of monotherapeutic clinical activity for GITR agonism was observed in this broad population. In addition, no clear signal has emerged to date demonstrating that GITR agonism may be an effective therapeutic strategy in a broad patient population.
Trial Protocol
eAppendix. Methods
eFigure 1. Study Design Schematic of NCT02598960
eFigure 2. Pharmacokinetics of BMS-986156 and BMS-986156 Plus Nivolumab
eFigure 3. Peripheral CD8 T-Cell and Natural Killer (NK)-Cell Proliferation in Response to BMS-986156 Monotherapy and BMS-986156, Alone and In Combination With Nivolumab Therapy
eFigure 4. Peripheral Regulatory T-cell (Treg) Depletion After BMS-986156 Plus Nivolumab Therapy
eTable 1. Patient Demographics and Baseline Characteristics in the Monotherapy and Combination Cohorts
eTable 2A. Treatment Exposure: BMS-986156 Monotherapy
eTable 2B. Treatment Exposure: BMS-986156 Plus Nivolumab Combination Therapy
eTable 3A. TRAEs in the BMS-986156 Monotherapy Dose Cohorts
eTable 3B. TRAEs in the BMS-986156 Plus Nivolumab Dose Cohorts
eTable 4. Serious TRAEs in the BMS-986156 Monotherapy and BMS-986156 Plus Nivolumab Cohorts
eTable 5. Causes of Death
eTable 6A. Immunogenicity in the BMS-986156 Monotherapy Cohort by Dose
eTable 6B. Immunogenicity in the BMS-986156 Plus Nivolumab Cohorts by Dose
eTable 7. Efficacy — ORR and DCR Per Investigator in the BMS-986156 Plus Nivolumab Combination Cohort by Tumor Type
Data Sharing Statement
References
- 1.Schaer DA, Hirschhorn-Cymerman D, Wolchok JD. Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer. 2014;2:7. doi: 10.1186/2051-1426-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G Jr, Fayette J, et al. . Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim DW, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schadendorf D, Hodi FS, Robert C, et al. . Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889-1894. doi: 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris SJ, Brown J, Lopez J, Yap TA. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med. 2016;13(2):171-193. doi: 10.20892/j.issn.2095-3941.2016.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. doi: 10.1186/s40425-017-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overman MJ, Lonardi S, Wong KYM, et al. . Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26(2):e15-e21. [DOI] [PubMed] [Google Scholar]
- 12.Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KM, Desai J. Nivolumab for the treatment of colorectal cancer. Expert Rev Anticancer Ther. 2018;18(7):611-618. doi: 10.1080/14737140.2018.1480942 [DOI] [PubMed] [Google Scholar]
- 14.Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166-173. doi: 10.1016/j.canlet.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227-242. doi: 10.1038/nri3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Bernatchez C, Sharma P, Radvanyi LG, Hwu P. Advances in the development of cancer immunotherapies. Trends Immunol. 2013;34(2):90-98. doi: 10.1016/j.it.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515-548. doi: 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- 18.O’Callaghan DS, Rexhepaj E, Gately K, et al. . Tumour islet Foxp3+ T-cell infiltration predicts poor outcome in nonsmall cell lung cancer. Eur Respir J. 2015;46(6):1762-1772. doi: 10.1183/13993003.00176-2014 [DOI] [PubMed] [Google Scholar]
- 19.Denlinger CS, Infante JR, Aljumaily R, et al. . A phase I study of MEDI1873, a novel GITR agonist, in advanced solid tumors [abstract 1154P]. Ann Oncol. 2018;29(suppl 8):viii400-viii441. doi: 10.1093/annonc/mdy288.027 [DOI] [Google Scholar]
- 20.Arnett SO, Teillaud JL, Wurch T, Reichert JM, Dunlop C, Huber M. IBC’s 21st Annual Antibody Engineering and 8th Annual Antibody Therapeutics International Conferences and 2010 Annual Meeting of the Antibody Society. December 5-9, 2010, San Diego, CA USA. MAbs. 2011;3(2):133-152. doi: 10.4161/mabs.3.2.14939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geva R, Voskoboynik M, Beebe A, et al. . First-in-human phase 1 study of MK-1248, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody, as monotherapy or in combination with pembrolizumab in patients with advanced solid tumors [abstract 3029]. J Clin Oncol. 2018;36(15_suppl):3029. doi: 10.1200/JCO.2018.36.15_suppl.3029 [DOI] [Google Scholar]
- 22.Tran B, Carvajal RD, Marabelle A, et al. . Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J Immunother Cancer. 2018;6(1):93. doi: 10.1186/s40425-018-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidney Kimmel Comprehensive Cancer Center Johns Hopkins, Bristol-Myers Squibb Biomarker-driven therapy using immune activators with nivolumab in patients with first recurrence of glioblastoma. ClinicalTrials.gov identifier: NCT03707457. https://clinicaltrials.gov/ct2/show/NCT03707457. Accessed October 3, 2019.
- 24.Novartis Pharmaceuticals Phase I/Ib study of GWN323 alone and in combination with PDR001 in patients with advanced malignancies and lymphomas. ClinicalTrials.gov identifier: NCT02740270. https://clinicaltrials.gov/ct2/show/NCT02740270. Accessed October 3, 2019.
- 25.OncoMed Pharmaceuticals, Inc. A study of OMP-336B11 in subjects with locally advanced or metastatic tumors. ClinicalTrials.gov identifier: NCT03295942. https://clinicaltrials.gov/ct2/show/NCT03295942. Accessed October 3, 2019.
- 26.Incyte Biosciences International Sàrl An open-label, dose-escalation, safety study of INCAGN01876 in subjects with advanced or metastatic solid tumors. ClinicalTrials.gov identifier: NCT02697591.https://clinicaltrials.gov/ct2/show/NCT02697591. Accessed October 3, 2019.
- 27.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1-10. doi: 10.1016/j.ejca.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 28.Cohen AD, Schaer DA, Liu C, et al. . Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5(5):e10436. doi: 10.1371/journal.pone.0010436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siu LL, Steeghs N, Meniawy T, et al. . Preliminary results of a phase I/IIa study of BMS-986156 (glucocorticoid-induced tumor necrosis factor receptor–related gene [GITR] agonist), alone and in combination with nivolumab in pts with advanced solid tumors [abstract 104]. J Clin Oncol. 2017;35(15_suppl):104. doi: 10.1200/JCO.2017.35.15_suppl.104 [DOI] [Google Scholar]
- 30.Bristol-Myers Squibb An investigational immuno-therapy study of experimental medication BMS-986156, given by itself or in combination with nivolumab in patients with solid cancers or cancers that have spread. ClinicalTrials.gov identifier: NCT02598960. https://clinicaltrials.gov/ct2/show/NCT02598960. Accessed October 3, 2019.
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services Common terminology criteria for adverse events (CTCAE): version 4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Published June 14, 2010. Accessed October 4, 2019.
- 33.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer JR, Drake CG, Wollner I, et al. . Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167-3175. doi: 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koon H, Shepard D, Merghoub T, Schaer D, Sirard C, Wolchok J. First-in-human phase 1 single-dose study of TRX-518, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody in adults with advanced solid tumors [abstract 3017]. J Clin Oncol. 2016;34(15_suppl):3017. doi: 10.1200/JCO.2016.34.15_suppl.3017 [DOI] [Google Scholar]
- 37.Papadopoulos KP, Autio KA, Golan T, et al. . Phase 1 study of MK-4166, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) antibody, as monotherapy or with pembrolizumab (pembro) in patients (pts) with advanced solid tumors [abstract 9509]. J Clin Oncol. 2019;37(15_suppl):9509. doi: 10.1200/JCO.2019.37.15_suppl.9509 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Methods
eFigure 1. Study Design Schematic of NCT02598960
eFigure 2. Pharmacokinetics of BMS-986156 and BMS-986156 Plus Nivolumab
eFigure 3. Peripheral CD8 T-Cell and Natural Killer (NK)-Cell Proliferation in Response to BMS-986156 Monotherapy and BMS-986156, Alone and In Combination With Nivolumab Therapy
eFigure 4. Peripheral Regulatory T-cell (Treg) Depletion After BMS-986156 Plus Nivolumab Therapy
eTable 1. Patient Demographics and Baseline Characteristics in the Monotherapy and Combination Cohorts
eTable 2A. Treatment Exposure: BMS-986156 Monotherapy
eTable 2B. Treatment Exposure: BMS-986156 Plus Nivolumab Combination Therapy
eTable 3A. TRAEs in the BMS-986156 Monotherapy Dose Cohorts
eTable 3B. TRAEs in the BMS-986156 Plus Nivolumab Dose Cohorts
eTable 4. Serious TRAEs in the BMS-986156 Monotherapy and BMS-986156 Plus Nivolumab Cohorts
eTable 5. Causes of Death
eTable 6A. Immunogenicity in the BMS-986156 Monotherapy Cohort by Dose
eTable 6B. Immunogenicity in the BMS-986156 Plus Nivolumab Cohorts by Dose
eTable 7. Efficacy — ORR and DCR Per Investigator in the BMS-986156 Plus Nivolumab Combination Cohort by Tumor Type
Data Sharing Statement