Key Points
Question
Is the incidence of melanoma in the United States changing between age groups?
Findings
In this population-based study including 988 103 cases of invasive melanoma reported between 2001 and 2015, the melanoma incidence increased overall but decreased among individuals aged 10 to 29 years at diagnosis in the United States from 2006 to 2015. These findings were based on data from the US Cancer Statistics National Program of Cancer Registries.
Meaning
The apparent decline in the incidence of melanoma in adolescents and young adults in the United States contrasts with increased incidence in melanoma in older ages and is possibly associated with sun protective interventions, providing support for ongoing prevention efforts.
Abstract
Importance
Melanoma is epidemiologically linked to UV exposure, particularly childhood sunburn. Public health campaigns are increasing sun-protective behavior in the United States, but the effect on melanoma incidence is unknown.
Objective
To examine the incidence of melanoma in the United States and whether any age-specific differences are present.
Design, Setting, and Participants
Observational, population-based registry data were extracted on July 3, 2018, from the combined National Program of Cancer Registries–Surveillance Epidemiology and End Results United States Cancer Statistics database for 2001-2015. Deidentified data for 988 103 cases of invasive melanoma, with International Classification of Diseases for Oncology histologic categorization codes 8720 to 8790, were used for analysis. Data analysis was performed from July 1, 2018, to March 1, 2019.
Main Outcomes and Measures
The annual rates of melanoma in pediatric, adolescent, young adult, and adult age groups were determined. Analyses were stratified by sex, and incidence rates were age-adjusted to the 2000 US standard population. Annual percentage change (APC) in incidence rate was calculated over the most recent decade for which data were available (2006-2015) using the weighted least squares method.
Results
In 2015, 83 362 cases of invasive melanoma were reported in the United States, including 67 in children younger than 10 years, 251 in adolescents (10-19 years), and 1973 in young adults (20-29 years). Between 2006 and 2015, the overall incidence rate increased from 200.1 to 229.1 cases per million person-years. In adults aged 40 years or older, melanoma rates increased by an APC of 1.8% in both men (95% CI, 1.4%-2.1%) and women (95% CI, 1.4%-2.2%). In contrast, clinically and statistically significant decreases were seen in melanoma incidence for adolescents and young adults. Specifically, incidence rates decreased by an APC of −4.4% for male adolescents (95% CI, −1.7% to −7.0%), −5.4% for female adolescents (95% CI, −3.3% to −7.4%), −3.7% for male young adults (95% CI, −2.5% to −4.8%), and −3.6% for female young adults (95% CI, −2.8% to −4.5%). Data on skin pigmentation and sun protection history were unavailable; similar trends were observed with data limited to non-Hispanic whites. Young adult women appeared to have twice the risk of melanoma as young adult men.
Conclusions and Relevance
The incidence of invasive melanoma in the United States appeared to decrease in adolescents and young adults from 2006 to 2015, and this finding contrasted with increases in older populations. These incidence trends suggest that public health efforts may be favorably influencing melanoma incidence in the United States.
This population-based study examines the incidence of melanoma in the United States overall as well as in different age cohorts.
Introduction
Melanoma is the fifth most common cause of cancer overall in the United States1 and is the most prominent factor in skin cancer death, with more than 9000 deaths per year.2 Although progress has been made in the treatment of regionally advanced3,4 and metastatic5,6,7,8,9,10,11,12 melanoma, there remains an important role for primary prevention.
The highest modifiable risk factor for melanoma is well accepted to be UV light exposure, either by sunlight13 or tanning beds.14 The risk is further increased when melanoma occurs early in life or is associated with sunburn.15,16 A randomized trial conducted in Australia in the 1990s concluded that sunscreen use may decrease melanoma risk, and this conclusion was supported by observational studies with similar findings.17,18 A number of public health measures were undertaken in the United States in the late 1990s and 2000s to increase sun-protective behavior, and data support modest improvements over this time period with increased sunscreen use, including in adolescents and young adults.19 However, the public health effects of these interventions are unknown, and the overall incidence of melanoma has reportedly increased to more than 80 000 cases per year in the United States despite these changes.20 Adolescent and young adult populations would be most likely to benefit from sun-protective behavior by altering early sun exposure. Thus, decreases in melanoma incidence in this population might suggest emerging benefit of sun protective campaigns or other favorable public health or ecologic changes.
The National Program of Cancer Registries (NPCR)–Surveillance Epidemiology and End Results (SEER) combined database, maintained by the US Cancer Statistics Public Use Database, encompasses more than 99% of incident cancer cases in the United States diagnosed between 2001 and 2015.20,21 Using this resource, we examined whether melanoma incidence rates are rising, stable, or falling among US adolescents and young adults and how any changes in these rates compare with the adult population. To determine the burden of disease, we also describe the absolute number of incident cases in adolescent and young adult populations in the United States for these years.
Methods
Nonidentifiable national registry data were extracted from the US Cancer Statistics Public Use Database, November 2017 submission,21 on July 3, 2018, for 2001-2015. The US Cancer Statistics data represent the official federal cancer statistics and combine data from the Centers for Disease Control and Prevention NPCR-SEER program.20,21 Use of the deidentified public registry data was determined to be exempt from review by the University of Washington Institutional Review Board because humans were not used.
All histologically confirmed cases of invasive melanoma (International Classification of Diseases for Oncology codes 8720-8790/3) diagnosed at any age were included in the primary analyses. Anatomic site was classified as head and neck, trunk, upper extremity, lower extremity, cutaneous unknown, ocular, and other sites. Histologic characteristics were classified as superficial spreading melanoma, nodular melanoma, spindle-cell melanoma, melanoma not otherwise specified, and other. Stage was categorized as local (localized), regional (regional direct extension, lymph node involvement, or both direct and lymph node), distant, and unknown. A total of 988 103 invasive melanomas were identified.
The primary population for this study was patients with invasive melanoma because these are the most consistently reported registries. However, similar data extraction and analyses were performed for in situ melanomas to determine whether the lower rates of invasive melanoma over time were associated with more precursor lesions being removed (in which one would expect a higher rate of in situ melanoma over time) or with development of fewer lesions at risk (in which a lower rate of in situ melanoma mirroring invasive melanoma would be expected). Data for in situ melanomas (all cases with histologic codes 8720-8790/2) were extracted from 2006 to 2015 (n = 382 533 in situ melanomas).
We sought to evaluate the potential reasons for observed decreases in reported melanoma incidence in adolescents and young adults that represent an explanation other than a true decrease in invasive melanomas. To evaluate for miscategorization of atypical spitzoid neoplasms as melanoma, the fraction of cases that were spitzoid were compared over time. To evaluate for overdiagnosis of early lesions, the percentage of patients diagnosed with localized disease was compared longitudinally for stability.
Statistical Analysis
Calculations and analyses were performed with standard functions of the SEER*Stat software.22 Given the different incidence patterns, analyses were performed separately for males and females. Two-tailed P value for significance was set a priori at .05. Incidence rates were age-adjusted to the 2000 US standard population (19 age groups; census P25-1130)20,21 to minimize potential confounding by differences of age distribution over time. In brief, the crude rates (number of new cases in the US population per year for each age and sex group) were used to generate a weighted average, in which the weights are proportional to the corresponding age group from the standard population. The methods of Tiwari et al23 were used to calculate 95% CIs.
Trends in incidence rate for the most recent decade of data available (2006-2015) were calculated using 2-year averages with annual percentage change calculated by the weighted least squares method,24,25 age-adjusted to the 2000 US standard population, and performed separately for males and females as described above. These trends were calculated within SEER*Stat software22; detailed formulas used in this calculation are available.26 Data analysis was performed from July 1, 2018, to March 1, 2019.
Results
Patient Characteristics
Of 988 103 invasive melanoma cases reported to SEER-NPCR between 2001 and 2015, 40 425 cases (4.1%) were diagnosed in persons younger than 30 years. Children (age, 0-9 years) differed substantially from adolescents (age, 10-19 years) and young adults (age, 20-29 years) in terms of demographics (Table). Compared with younger children, adolescents and young adults were more likely to be female (children, 474 [53.3%]; adolescents, 3251 [59.4%]; and young adults, 23 397 [68.7%]), non-Hispanic white (children, 675 [78.3%]; adolescents, 4824 [92.5%]; and young adults [30 484 [94.8%]), present with a primary melanoma on the trunk (children, 162 [18.2%]; adolescents, 1980 [36.2%]; and young adults, 13 719 [40.3%]), and/or have a superficial spreading melanoma (children, 82 [9.2%]; adolescents, 1764 [32.2%]; and young adults, 12 981 [38.1%]). Adolescents and young adults were less likely than younger children to present with metastatic disease (age 0-9 years: 46 [5.2%], 10-19 years: 114 [2.1%], and 20-29 years: 840 [2.5%]) or with a lesion on the head and neck (children, 259 [29.1%]; adolescents, 1087 [19.9%]; and young adults, 4420 [13.0%]).
Table. Age-Specific Demographic and Tumor Behavior Characteristics of Invasive Melanoma From NPCR-SEER Registry Data, 2001 to 2015a.
| Characteristic | Age, No. (%), y | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | ≥80 | |
| Sex | |||||||||
| Male | 416 (46.7) | 2222 (40.6) | 10 665 (31.3) | 27 549 (39.0) | 60 096 (46.6) | 109 665 (56.7) | 138 465 (64.1) | 129 782 (66.5) | 88 674 (61.9) |
| Female | 474 (53.3) | 3251 (59.4) | 23 397 (68.7) | 43 054 (61.0) | 68 926 (53.4) | 83 804 (43.3) | 77 674 (35.9) | 65 505 (33.5) | 54 484 (38.1) |
| Race/ethnicity | |||||||||
| White non-Hispanic | 675 (78.3) | 4824 (92.5) | 30 484 (94.8) | 63 267 (94.6) | 117 680 (95.6) | 179 261 (96.5 | 202 658 (96.9) | 185 103 (97.1) | 136 541 (97.2) |
| White Hispanic | 103 (11.9) | 247 (4.7) | 1089 (3.4) | 2428 (3.6) | 3626 (2.9) | 3965 (2.1) | 3825 (1.8) | 3213 (1.7) | 2170 (1.5) |
| Black | 47 (5.5) | 62 (1.2) | 179 (0.6) | 400 (0.6) | 652 (0.5) | 1116 (0.6) | 1244 (0.6) | 1218 (0.6) | 956 (0.7) |
| Other | 37 (4.3) | 80 (1.5) | 404 (1.3) | 811 (1.2) | 1093 (0.9) | 1469 (0.8) | 1463 (0.7) | 1151 (0.6) | 793 (0.6) |
| Primary site | |||||||||
| Skin, head and neck | 259 (29.1) | 1087 (19.9) | 4420 (13.0) | 7936 (11.2) | 14 296 (11.1) | 27 328 (14.1) | 42 833 (19.8) | 51 240 (26.2) | 48 174 (33.7) |
| Skin, trunk | 162 (18.2) | 1980 (36.2) | 13 719 (40.3) | 26 840 (38.0) | 47 017 (36.4) | 67 187 (34.7) | 68 348 (31.6) | 51 742 (26.5) | 28 456 (19.9) |
| Skin, upper extremity | 185 (20.8) | 961 (17.6) | 6148 (18.0) | 14 471 (20.5) | 28 877 (22.4) | 46 825 (24.2) | 54 143 (25.1) | 49 183 (25.2) | 34 619 (24.2) |
| Skin, lower extremity | 218 (24.5) | 1129 (20.6) | 8126 (23.9) | 17 414 (24.7) | 29 488 (22.9) | 34 918 (18.0) | 30 354 (14.0) | 23 614 (12.1) | 17 267 (12.1) |
| Skin, other or unknown | 29 (3.3) | 110 (2.0) | 902 (2.6) | 2205 (3.1) | 4856 (3.8) | 8519 (4.4) | 9954 (4.6) | 9685 (5.0) | 7603 (5.3) |
| Ocular | 19 (2.1) | 150 (2.7) | 554 (1.6) | 1412 (2.0) | 3528 (2.7) | 6754 (3.5) | 7794 (3.6) | 6542 (3.4) | 3762 (2.6) |
| Other nonskin | 18 (2.0) | 53 (1.0) | 184 (0.5) | 297 (0.4) | 881 (0.7) | 1800 (0.9) | 2573 (1.2) | 3119 (1.6) | 3152 (2.2) |
| Unknown | <16 (<1) | <16 (<1) | <16 (<1) | 27 (0.0) | 75 (0.1) | 123 (0.1) | 132 (0.1) | 155 (0.1) | 119 (0.1) |
| Histologic categorization | |||||||||
| Melanoma, not otherwise specified | 581 (65.3) | 2986 (54.6) | 17 834 (52.4) | 36 765 (52.1) | 68 508 (53.1) | 103 361 (53.4) | 116 036 (53.7) | 105 613 (54.1) | 76 632 (53.5) |
| Nodular | 83 (9.3) | 331 (6.0) | 1719 (5) | 3424 (4.8) | 7216 (5.6) | 11 989 (6.2) | 13 951 (6.5) | 14 471 (7.4) | 14 771 (10.3) |
| Superficial spreading | 82 (9.2) | 1764 (32.2) | 12 981 (38.1) | 27 091 (38.4) | 45 291 (35.1) | 61 109 (31.6) | 59 259 (27.4) | 44 229 (22.6) | 26 192 (18.3) |
| Spindle cell | <16 (<1) | 67 (1.2) | 231 (0.7) | 497 (0.7) | 1124 (0.9) | 2116 (1.1) | 2990 (1.4) | 3260 (1.7) | 2962 (2.1) |
| Other | 132 (14.8) | 325 (5.9) | 1297 (3.8) | 2826 (4.0) | 6883 (5.3) | 14 894 (7.7) | 23 903 (11.1) | 27 714 (14.2) | 22 601 (15.8) |
| Extent of disease at diagnosis | |||||||||
| Localized only | 483 (54.3) | 4040 (73.8) | 27 341 (80.3) | 56 787 (80.4) | 102 226 (79.2) | 150 904 (78.0) | 167 648 (77.6) | 148 795 (76.2) | 104 500 (73.0) |
| Regional, direct extension only | 42 (4.7) | 87 (1.6) | 298 (0.9) | 785 (1.1) | 1906 (1.5) | 3675 (1.9) | 5384 (2.5) | 6665 (3.4) | 8226 (5.7) |
| Regional, regional lymph nodes only | 148 (16.6) | 605 (11.1) | 2362 (6.9) | 4407 (6.2) | 7632 (5.9) | 10 863 (5.6) | 10 391 (4.8) | 7917 (4.1) | 4710 (3.3) |
| Regional, extension and lymph nodes | 41 (4.6) | 59 (1.1) | 252 (0.7) | 451 (0.6) | 1010 (0.8) | 1643 (0.8) | 1739 (0.8) | 1649 (0.8) | 1256 (0.9) |
| Regional, not otherwise specified | <16 (<1) | 33 (0.6) | 136 (0.4) | 312 (0.4) | 673 (0.5) | 1151 (0.6) | 1375 (0.6) | 1384 (0.7) | 1288 (0.9) |
| Distant sites/nodes involved | 46 (5.2) | 114 (2.1) | 840 (2.5) | 2039 (2.9) | 4783 (3.7) | 9001 (4.7) | 10 796 (5.0) | 10 563 (5.4) | 7945 (5.6) |
| Unknown, unstaged, unspecified | 116 (13.2) | 534 (9.8) | 2829 (8.3) | 5817 (8.2) | 10 789 (8.4) | 16 224 (8.4) | 18 799 (8.7) | 18 303 (9.4) | 15 227 (10.6) |
Abbreviation: NPCR-SEER, National Program of Cancer Registries–Surveillance Epidemiology and End Results.
Information was available for more than 95% of cases for race/ethnicity and more than 99.9% of included cases for all other fields. Counts of fewer than 16 were suppressed to maintain deidentified information. Melanomas totaled 988 103.
Melanoma Incidence
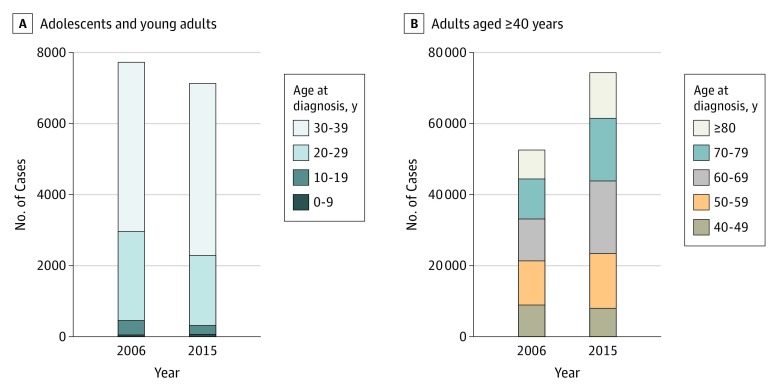
The total number of reported US incident cases of invasive melanoma for all ages increased steadily from 50 272 in 2001 to 61 551 in 2006 to 71 922 in 2011 to 83 362 in 2015, with 67 cases in children, 251 in adolescents, 1973 in young adults, and 81 071 in adults reported in 2015. Between 2006 and 2015, the overall incidence rate increased from 200.1 to 229.1 cases per million person-years. Over the past decade for which data are available (2006-2015), this change represents an absolute increase of 21 811 reported cases (Figure 1A). Furthermore, from 2006 to 2015, the population-adjusted incidence rate increased significantly in both men (from 251 to 288 cases per 1 000 000 person-years) and women (from 164 to 186 cases per 1 000 000 person-years). This increase corresponded to an annual percentage change (APC) per year of 1.5% (95% CI, 1.2%-1.8%) for men and 1.1% (95% CI, 0.7%-1.6%) for women. Increased melanoma incidence was largely associated with adults aged 40 years or older. In this group of persons, melanoma incidence rates rose for both men (APC, 1.8%; 95% CI, 1.4%- 2.1%) and women (APC, 1.8%; 95% CI, 1.4%-2.2%). The significant increase in adjusted incidence rates suggests that the increase in observed reported melanoma cases represented an elevated rate of melanoma occurrence and not simply a greater number of persons at risk. The annual percentage increase in melanoma in those aged 40 years or older was found not only in localized disease (APC, 1.9%; 95% CI, 1.4%-2.4%) but also in distant metastatic disease (APC, 4.8%; 95% CI, 3.9%-5.8%).
Figure 1. Changes in Melanoma Annual Incident Cases in the United States.
Reported incident cases of invasive melanoma in the United States in persons aged 0 to 39 years (A) and those 40 years or older (B).
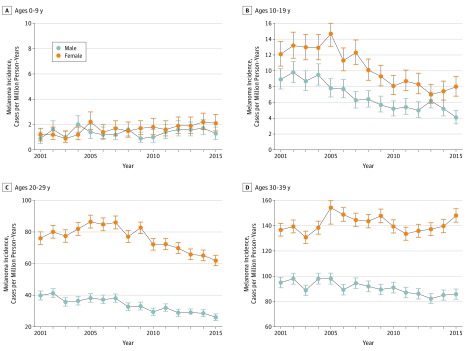
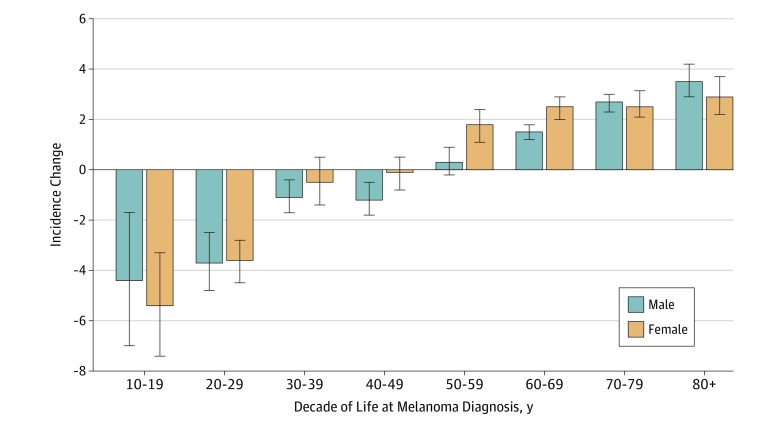
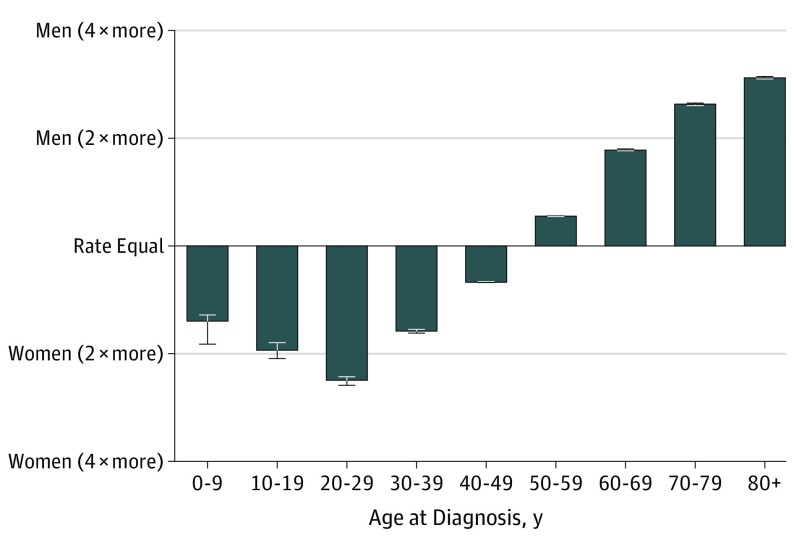
In contrast to the overall increased incidence, trends in melanoma incidence appeared to differ among children, adolescents, and young adults. Specifically, incident cases and incidence rates remained low and stable among children (age, 0-9 years). However, for both adolescents (age, 10-19 years) and young adults (age, 20-29 years), the incidence peaked at approximately 2004-2005 and then began to decrease (Figure 2; eFigure in the Supplement). This finding was true of both absolute number of cases (Figure 1) and annual incidence rate. Over the most recent 10-year period with data available from 2006 to 2015, a statistically significant (all P < .001) APC in incident rate of −4.4% was observed for adolescent boys (95% CI, −1.7% to −7.0%), −5.4% for adolescent girls (95% CI, −3.3% to −7.4%), −3.7% for young adult men (95% CI, −2.5% to −4.8%), and −3.6% for young adult women (95% CI, −2.8% to −4.5%) (Figure 2). An association with age was observed across the age spectrum. Between 2006 and 2015, incidence rates were decreasing for adolescents and young adults, approximately stable for middle-aged adults, and markedly increased for older adults (Figure 3). Although declining incidence was observed across both young men and young women, melanoma appeared to remain more common in females than males in younger individuals and more common in males than females in older individuals (Figure 4).
Figure 2. Melanoma Incidence Rate in the United States, 2001-2015.
Total number of melanoma incident cases are shown, with age at diagnosis indicated above graphs. For adolescents (age, 10-19 years) and young adults (age, 20-29 years) melanoma incidence peaked at approximately 2005 and then began to decrease. Bars represent 95% confidence intervals. Melanoma incidence peaked at approximately 2005 and then began to decrease.
Figure 3. Annual Percentage Change in Melanoma Incidence Rate in the United States, 2006-2015.
Annual percentage change in melanoma incidence rate in the United States between 2006 and 2015 (most recent decade with data available), with data shown by decade of life. Insufficient data were available for ages 0-9 years to determine annual percentage change (<100 cases per year in the US in this age group).
Figure 4. Melanoma Incidence Rate Ratio by Sex and Age, 2015.
Ratio of incidence rates in men and women are shown by age. In 2015, melanoma was more common in women than men before the age of 50 years. Error bars indicate 95% CIs.
Over the study period, the fraction of cases with a histologic category of other (including spitzoid neoplasms) was low and essentially stable between 2001-2005 and 2011-2015 for children (14.7% vs 14.9%), adolescents (5.8% vs 6.2%), and young adults (4.3% vs 3.5%) and insufficient to account for the changes in incidence observed. This finding argues against recategorization of spitzoid melanoma as explaining decreased incidence. Similarly, the percentage of adolescents presenting with localized disease was only slightly decreased (76.0% vs 71.8%), and the percentage of young adults presenting with localized disease was stable (79.4% vs 79.7%) in the past decade. This finding suggests that overdiagnosis was not a major factor in observed peak incidence rates.
Trends in in situ melanoma incidence in the US Cancer Statistics database were similar to the trends in invasive melanoma reported herein. Specifically, between 2006 and 2015, in situ melanoma significantly decreased in adolescent boys (APC, −4.6%; range, −7.9% to −1.3%), young adult men (APC, −3.6%; range, −5.5% to −1.5%), adolescent girls (APC, −6.0%; range, −8.5% to −3.5%), and young adult women (APC, −1.5%; range, −2.6% to −0.4%) while increasing significantly in adults aged 40 years or older (men: APC, 7.0%; range, 6.5%-7.6%; women: APC, 6.5%; range, 5.9%-7.1%).
As the best available surrogate for skin pigmentation, we evaluated whether trends in non-Hispanic white individuals mirrored those of the larger population; if these rates were instead stable, they would suggest that demographic changes are the major factor. However, the APC decrease in melanoma incidence rate between 2006 and 2015 in young non-Hispanic white individuals was similar in magnitude to the larger population. The APC was statistically significant for adolescent boys (−3.2%; 95% CI, −0.8% to −5.6%), adolescent girls (−5.0%; 95% CI, −3.1% to −6.9%), young adult men (−3.5%; 95% CI, −2.3% to −4.8%), and young adult women (−3.4%; 95% CI, −2.6% to −4.3%), supporting a possible decreased melanoma incidence in even the highest-risk populations.
Discussion
Despite changes in melanoma therapy associated with improved survival,27 melanoma remains the deadliest skin cancer in the United States.2 Public health efforts have been undertaken in the United States to recognize skin cancer as a major public health problem and improve sun-protective behavior with hopes of reducing melanoma incidence and mortality. The recent Surgeon General’s Call to Action supports efforts to improve sun-protective behavior, and potential improved health outcomes remain an active research interest.28,29 The model public health response was undertaken in Australia, where multiple public health efforts in Queensland, including the SunSmart Slip! Slop! Slap! campaign started in 1981, were successful in increasing sun-protective behaviors. These efforts were associated with specific reductions in melanomas diagnosed starting around 1998, and similar to our observations, results were particularly pronounced in younger populations.30
In the United States, reported melanoma incidence was rapidly increasing in all ages, including pediatric, adolescent, and young adult populations, through 2005.31 Between 2006 and 2009, data from the SEER registry suggested that the incidence of melanoma in adolescent populations may have finally peaked and started to downtrend.32,33 However, it was unclear whether this decrease represented a durable trend, and data from young adult populations were not reported. In this study, we used the large NPCR-SEER data set and found what appears to be a sustained, statistically and clinically significant downtrend in melanoma incidence in adolescent and young adult populations from 2006 to 2015, with the total number of US adolescent and young adult reported cases decreasing by 23.4%
In contrast to the reported observations in young adult populations, melanoma incidence markedly increased in persons 40 years or older across the same time period, with increases particularly pronounced in the oldest cohorts. Although detailed evaluation of trends in older adults is beyond the scope of this study, these increases did not appear to be simply associated with overdiagnosis of clinically insignificant localized lesions because the annual percentage increase in melanoma in those aged 40 years or older was found not only in localized disease (APC, 1.9%; 95% CI, 1.4%-2.4%) but also in distant metastatic disease (APC, 4.8%; 95% CI, 3.9%-5.8%). These disparate trends between adolescents and young adults vs older adults further suggest that observed differences reflect real clinical differences and not simply changes in database ascertainment. One potential explanation is that sun protection during younger years is especially beneficial and thus should be one key focus of public health efforts; however, we continue to advocate for lifetime UV light exposure protection. It will be interesting to determine the outcomes of the present adolescent and young adult cohorts over time and whether they maintain their lower incidence of melanoma.
Limitations
Our data have limitations that need to be considered when interpreting the study findings. The national registry data do not include information about skin pigmentation, UV light exposure, sunburn history, sun-protective behavior (eg, sunscreen), protective clothing, sun avoidance, or tanning bed use. Because of this lack of information, we cannot estimate the association between increased sun-protective behavior and reductions in melanoma incidence. However, this change in behavior remains a plausible explanation for decreased melanoma rates in adolescent and young adult populations. We further cannot isolate the association of any one intervention with reduced invasive melanoma incidence (eg, sunscreen; clothing; education campaigns, such as ABCDE [asymmetry, border, color, diameter, and evolution]; increased dermatologic care; and reduced access to tanning beds), and, to our knowledge, changes in any one of these factors cannot explain the specific year of peak invasive melanoma in approximately 2004-2005 in adolescent and young adult populations. The absence of observed association in pediatric (age, 0-9 years) age groups may reflect different causes in this population, with a greater genetic component and lower contribution of UV exposure.34,35 Similarly, results of sun-protective behavior in adults aged 40 years or older may still be present but not yet be evident owing to these cohorts adopting sun-protective behaviors later in life and accumulating a history of childhood/adolescent sunburns, tanning bed use, and cumulative sun exposure over their lifetime.36,37,38 Studies of melanoma genomics report a similar frequency of UV-associated mutations in adolescent and adult melanoma,39 and epidemiologic studies report UV exposure as a major risk factor for adolescent and adult melanoma.40 Together, these data support the contention that reduced UV exposure may be a factor in the reduced melanoma incidence observed in adolescent and young adult populations.
Conclusions
Melanoma has been increasing in incidence over the past several decades and is now the fifth most common invasive cancer in men and women.41 Despite this increasing incidence and overall association with health, 2 positive areas in melanoma care are suggested in treatment and prevention. The first is the apparent improvement in systemic therapies over the past 5 years, with new targeting and immunotherapies that are reporting improved survival outcomes for patients with metastatic disease.27 The second, reported herein, is an apparently marked ongoing downtrend in the incidence of melanoma in adolescent and young adult populations. The reported data are observational and thus cannot conclusively determine the cause of this statistically and clinically significant decrease. However, a likely explanation for the reduced melanoma incidence in adolescents and young adults is success at increased UV exposure protection. These data provide an impetus to further improve multimodal efforts aimed at reducing the burden of melanoma and encourage ongoing UV exposure protection efforts throughout the lifetime of individuals.
eFigure. Melanoma Incidence Rate in the USA From 2001-2015
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review (CSR) 1975-2015. https://seer.cancer.gov/csr/1975_2015/. Updated September 10, 2018. Accessed March 1, 2019.
- 3.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813-1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 4.Weber J, Mandala M, Del Vecchio M, et al. ; CheckMate 238 Collaborators . Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824-1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 5.D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226-235. doi: 10.1200/JCO.2016.67.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. doi: 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109-1117. doi: 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 Investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Rollin L, Larkin J. Nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(25):2503-2504. doi: 10.1056/NEJMc1714339 [DOI] [PubMed] [Google Scholar]
- 10.Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9. [PMC free article] [PubMed] [Google Scholar]
- 11.Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603-615. doi: 10.1016/S1470-2045(18)30142-6 [DOI] [PubMed] [Google Scholar]
- 12.Menzies AM, Long GV. Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res. 2014;20(8):2035-2043. doi: 10.1158/1078-0432.CCR-13-2054 [DOI] [PubMed] [Google Scholar]
- 13.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341-1348. doi: 10.1056/NEJM199904293401707 [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci LM, Vogel RI, Cartmel B, Lazovich D, Mayne ST. Indoor tanning in businesses and homes and risk of melanoma and nonmelanoma skin cancer in 2 US case-control studies. J Am Acad Dermatol. 2014;71(5):882-887. doi: 10.1016/j.jaad.2014.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: does age matter? a comprehensive meta-analysis. Ann Epidemiol. 2008;18(8):614-627. doi: 10.1016/j.annepidem.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi AA, Zhang M, Han J. Heterogeneity in host risk factors for incident melanoma and non-melanoma skin cancer in a cohort of US women. J Epidemiol. 2011;21(3):197-203. doi: 10.2188/jea.JE20100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257-263. doi: 10.1200/JCO.2010.28.7078 [DOI] [PubMed] [Google Scholar]
- 18.Watts CG, Drummond M, Goumas C, et al. Sunscreen use and melanoma risk among young Australian adults. JAMA Dermatol. 2018;154(9):1001-1009. doi: 10.1001/jamadermatol.2018.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cokkinides V, Weinstock M, Glanz K, Albano J, Ward E, Thun M. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among US adolescents, 1998-2004. Pediatrics. 2006;118(3):853-864. doi: 10.1542/peds.2005-3109 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Melanoma Incidence and Mortality, United States—2012–2016. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. [Google Scholar]
- 21.Centers for Disease Control and Prevention. 2001–2015 database: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence—US Cancer Statistics Public Use Research Database, November 2017 submission (2001–2015), United States Dept of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Created on 4/27/2018. https://www.cdc.gov/cancer/uscs/public-use. Published April 27, 2018. Accessed March 1, 2019.
- 22.Surveillance Research Program, National Cancer Institute. SEER*Stat software. https://seer.cancer.gov/seerstat. Accessed March 1, 2019.
- 23.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. doi: 10.1177/0962280206070621 [DOI] [PubMed] [Google Scholar]
- 24.Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models 2nd ed. New York, NY: Mc-Graw-Hill Higher Education; 1985. [Google Scholar]
- 25.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 26.Introduction to SEER*Stat. https://seer.cancer.gov/seerstat/WebHelp/seerstat.htm. Accessed August 1, 2019.
- 27.Albertini MR. The age of enlightenment in melanoma immunotherapy. J Immunother Cancer. 2018;6(1):80. doi: 10.1186/s40425-018-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holman DM, Ding H, Guy GP Jr, Watson M, Hartman AM, Perna FM. Prevalence of sun protection use and sunburn and association of demographic and behaviorial characteristics with sunburn among US adults. JAMA Dermatol. 2018;154(5):561-568. doi: 10.1001/jamadermatol.2018.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gefeller O. The garment protection factor: further advances in labelling sun-protective clothing. Br J Dermatol. 2018;178(4):835-836. doi: 10.1111/bjd.16344 [DOI] [PubMed] [Google Scholar]
- 30.Iannacone MR, Youlden DR, Baade PD, Aitken JF, Green AC. Melanoma incidence trends and survival in adolescents and young adults in Queensland, Australia. Int J Cancer. 2015;136(3):603-609. doi: 10.1002/ijc.28956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the Surveillance, Epidemiology and End Results database. J Clin Oncol. 2005;23(21):4735-4741. doi: 10.1200/JCO.2005.02.899 [DOI] [PubMed] [Google Scholar]
- 32.Wong JR, Harris JK, Rodriguez-Galindo C, Johnson KJ. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics. 2013;131(5):846-854. doi: 10.1542/peds.2012-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J, Bordeaux JS. Melanoma incidence in children and adolescents: decreasing trends in the United States. J Pediatr. 2015;166(6):1505-1513. doi: 10.1016/j.jpeds.2015.02.050 [DOI] [PubMed] [Google Scholar]
- 34.Aoude LG, Wadt KA, Pritchard AL, Hayward NK. Genetics of familial melanoma: 20 years after CDKN2A. Pigment Cell Melanoma Res. 2015;28(2):148-160. doi: 10.1111/pcmr.12333 [DOI] [PubMed] [Google Scholar]
- 35.Cordoro KM, Gupta D, Frieden IJ, McCalmont T, Kashani-Sabet M. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol. 2013;68(6):913-925. doi: 10.1016/j.jaad.2012.12.953 [DOI] [PubMed] [Google Scholar]
- 36.Gershenwald JE, Guy GP Jr. Stemming the rising incidence of melanoma: calling prevention to action. J Natl Cancer Inst. 2015;108(1):pii:djv381. doi: 10.1093/jnci/djv381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazovich D, Isaksson Vogel R, Weinstock MA, Nelson HH, Ahmed RL, Berwick M. Association between indoor tanning and melanoma in younger men and women. JAMA Dermatol. 2016;152(3):268-275. doi: 10.1001/jamadermatol.2015.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1080-1089. doi: 10.1158/1055-9965.EPI-13-0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C, Zhang J, Nagahawatte P, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol. 2015;135(3):816-823. doi: 10.1038/jid.2014.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojcik KY, Escobedo LA, Wysong A, et al. High birth weight, early UV exposure, and melanoma risk in children, adolescents, and young adults. Epidemiology. 2019;30(2):278-284. doi: 10.1097/EDE.0000000000000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Melanoma Incidence Rate in the USA From 2001-2015