This randomized clinical trial evaluates the effect of providing HIV self-tests on frequency of testing, diagnoses of HIV infection, and sexual risk behaviors among men who have sex with men.
Key Points
Question
Does provision of HIV self-tests to internet-recruited men who have sex with men increase HIV testing and diagnosis of infection over a 12-month period?
Findings
In this randomized clinical trial of 2665 US participants randomized to 2 groups, more participants in the arm that was mailed HIV self-tests reported testing for HIV 3 or more times during the trial compared with control participants, with 12 infections identified in the self-testing arm in the first 3 months compared with 2 infections in the control arm. Participants who shared the study self-tests reported 34 infections among their social network members.
Meaning
Providing free HIV self-tests helped increase awareness of infection among participants and their social network members.
Abstract
Importance
Undiagnosed HIV infection results in delayed access to treatment and increased transmission. Self-tests for HIV may increase awareness of infection among men who have sex with men (MSM).
Objective
To evaluate the effect of providing HIV self-tests on frequency of testing, diagnoses of HIV infection, and sexual risk behaviors.
Design, Setting, and Participants
This 12-month longitudinal, 2-group randomized clinical trial recruited MSM through online banner advertisements from March through August 2015. Those recruited were at least 18 years of age, reported engaging in anal sex with men in the past year, never tested positive for HIV, and were US residents with mailing addresses. Participants completed quarterly online surveys. Telephone call notes and laboratory test results were included in the analysis, which was completed from August 2017 through December 2018.
Interventions
All participants had access to online web-based HIV testing resources and telephone counseling on request. Participants were randomized in a 1:1 ratio to the control group or a self-testing (ST) group, which received 4 HIV self-tests after completing the baseline survey with the option to replenish self-tests after completing quarterly surveys. At study completion, all participants were offered 2 self-tests and 1 dried blood spot collection kit.
Main Outcomes and Measures
Primary outcomes were HIV testing frequency (tested ≥3 times during the trial) and number of newly identified HIV infections among participants in both groups and social network members who used the study HIV self-tests. Secondary outcomes included sex behaviors (eg, anal sex, serosorting).
Results
Of 2665 participants, the mean (SD) age was 30 (9.6) years, 1540 (57.8%) were white, and 443 (16.6%) had never tested for HIV before enrollment. Retention rates at each time point were more than 54%, and 1991 (74.7%) participants initiated 1 or more follow-up surveys. More ST participants reported testing 3 or more times during the trial than control participants (777 of 1014 [76.6%] vs 215 of 977 [22.0%]; P < .01). The cumulative number of newly identified infections during the trial was twice as high in the ST participants as the control participants (25 of 1325 [1.9%] vs 11 of 1340 [0.8%]; P = .02), with the largest difference in HIV infections identified in the first 3 months (12 of 1325 [0.9%] vs 2 of 1340 [0.1%]; P < .01). The ST participants reported 34 newly identified infections among social network members who used the self-tests.
Conclusions and Relevance
Distribution of HIV self-tests provides a worthwhile mechanism to increase awareness of HIV infection and prevent transmission among MSM.
Trial Registration
ClinicalTrials.gov identifier: NCT02067039
Introduction
In the United States, gay, bisexual, and other men who have sex with men (MSM) account for more than two-thirds of people with newly diagnosed HIV infections,1 among whom 38% were black or African American and 29% were Hispanic or Latino.2 Furthermore, 1 in 6 MSM is unaware of his infection. Diagnosing HIV infection as early as possible is one of the strategies for ending the HIV epidemic in the United States.3
Studies among high-risk populations, including formative work among MSM for this study, have shown that HIV self-testing is acceptable4,5,6,7,8 and feasible,5,6,7,9 and that providing HIV self-tests directly to participants can increase uptake or frequency of HIV screening.10,11,12,13,14 Despite its benefits, few US MSM reported self-testing in the past 12 months, with cost being frequently reported as a barrier.15,16,17 Concerns exist about the lack of in-person contact following a positive self-test result that could negatively affect linkage to care.18 Also, HIV self-testing prior to engaging in sexual intercourse (point-of-sex testing) has the potential for increased risk of transmission if a false-negative result is obtained when engaging in serosorting.19
To evaluate the effects of HIV self-testing, the Centers for Disease Control and Prevention (CDC) sponsored a randomized clinical trial, called Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP), on the effectiveness of mailing HIV self-tests to MSM in the United States. In this study, we examined the effect of providing HIV self-tests on frequency of testing, diagnoses of HIV infection, sexual risk behaviors, and the use of self-test by social-network members.
Methods
Before implementing the eSTAMP trial, we conducted 3 formative activities to obtain input from the study population on study design and materials,20 assess participants’ ability to follow test instructions and interpret results,9 and evaluate use of tests and study recruitment procedures.8,16 The protocol for this 12-month longitudinal cohort randomized clinical trial was approved by the Emory University Institutional Review Board (see protocol in Supplement 1). Participants were internet-recruited and provided online informed consent for eligibility screening, enrollment, and follow-up. Recruitment occurred from March 25, 2015, through August 4, 2015, and the trial follow-up and data collection was completed by November 22, 2016. This study followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
During the recruitment period, advertisements were placed on social network, music, and dating websites frequented by MSM (see eFigure in Supplement 2). Persons who clicked the advertisements were directed to the study website to complete eligibility screening. Eligible participants were born male, currently identified as male, were at least 18 years old, resided in the United States, reported engaging in anal sex with at least 1 man in the past 12 months, reported being HIV negative or unaware of HIV status, had never been diagnosed with a bleeding disorder, had never participated in an HIV vaccine trial, were not taking antiretroviral medications to prevent HIV, and did not participate in eSTAMP formative activities. Enrollment required participants to set up a password-protected online account by providing contact information (ie, an email address, a cell phone number to receive text messages, a shipping address, and a nickname or preferred name), and complete the baseline survey. After enrollment, follow-up online surveys were scheduled quarterly for 12 months. Participants could receive up to $90 for completing study activities: baseline survey ($20), follow-up surveys at 3, 6, 9, 12 months ($10 each), and return dried blood spot (DBS) card and report self-test results after the 12-month survey ($30).
Interventions
All participants were provided a link to AIDSvu.org, which has HIV prevention information and resources to locate local HIV testing services. Through the study telephone number, an HIV counselor was available 9 am through 5 pm EST, Monday through Friday, and mental health counseling was available after hours and on weekends. Participants could report and discuss their HIV test results during these calls.
Participants were randomly assigned to the self-testing (ST) arm or control arm using a computer-generated stochastic random 1:1 allocation. After completing the baseline survey, ST participants were mailed 2 oral fluid HIV self-tests (OraQuick In-Home HIV Test [OraSure Technologies Inc]), and 2 finger-stick whole blood HIV self-tests (SURE CHECK HIV 1/2 Assay [Chembio Diagnostics System Inc], used under an investigational device exemption from the US Food and Drug Administration [FDA]). After completing interim quarterly surveys at 3, 6, and 9 months, ST participants could order HIV self-tests to replace tests used or given away. Although we provided self-tests to participants in the ST arm, we did not provide advice or recommend how often participants should use the tests nor did we encourage participants to give the self-tests to friends, family members, or other people in their social network (ie, social network members). Online videos were provided on how to use all HIV-testing materials. The ST participants could report their self-test results through the study website in a results reporting survey after conducting the self-test, during the quarterly surveys, or during participant-initiated counseling telephone calls (see eMethods in Supplement 2).
After completing the 12-month survey at the end of the trial or after reporting a positive HIV result during the trial, ST and control participants were offered 1 DBS kit and 1 of each HIV self-test. Participants were instructed to mail the collected DBS specimens to Emory University, and the cards were later transported to the CDC for laboratory analyses (see eMethods in Supplement 2).
Outcomes
The primary study outcomes of these analyses were frequency of HIV testing (mean number of times tested [testing ≥3 times during the trial]) and number of newly identified HIV infections among participants and among the ST arm’s social network. To assess frequency of HIV testing, we summed the total number of HIV testing instances reported during the trial—both provider-based testing and self-testing. For this analysis, we categorized participants and their social network members as having a newly identified infection based on participant-reported information and laboratory test results when available (see eMethods in Supplement 2).
Secondary outcomes included any HIV testing during the study, HIV testing reported on at least 3 follow-up surveys, provider-based testing, testing among those who had never been tested at enrollment, and linkage to care (scheduled or attended appointment). We also assessed participants’ sexual behaviors during the trial. These included number of male anal sex partners, male anal sex partners without using condoms, total number of sex partners during the trial, and serosorting (engaging in sex with a person of the same serostatus).
Sample Size and Statistical Analyses
To calculate the sample size for the primary binary outcome of testing for HIV at least 3 times during the trial, we sought to determine whether a difference existed in the underlying success rates (testing ≥3 times during the trial) between the ST and control arms (10% and 5%, respectively). Using the 2-tailed χ2 test at the 5% significance level, we would have 90% power with a sample size of 582 per arm to detect a difference in frequency (testing ≥3 times during the trial) between these arms. Applying an adjustment suggested by Lachin,21 we inflated the sample size by 1/(1 − 0.4)2 to allow for a maximum 40% attrition rate, giving a target sample size of 1617 per arm, for a total target sample size of 3234.
Analyses for frequency of testing, newly identified infections, and sexual behaviors were intent to treat. The denominator for newly identified infections includes all study participants because data were included from multiple sources; otherwise, denominators reflect data available in surveys initiated by participants. Follow-up surveys asked about testing events since the last survey, which allowed us to sum the number of tests over time. We computed summary statistics (eg, means, standard deviations) and frequencies for the study outcomes. For comparing frequencies, we computed P values using the Fisher exact test. For comparing sets of frequencies, we computed P values using permutation tests. For comparing means, we performed t tests using the Satterthwaite approximation for unequal variances. In addition to frequency of testing, we calculated how dispersed HIV testing events were throughout the study by examining testing reported during each survey interval. All statistical computations were performed using SAS, version 9.4 (SAS Institute). Statistical significance was set at the 5% threshold.
Results
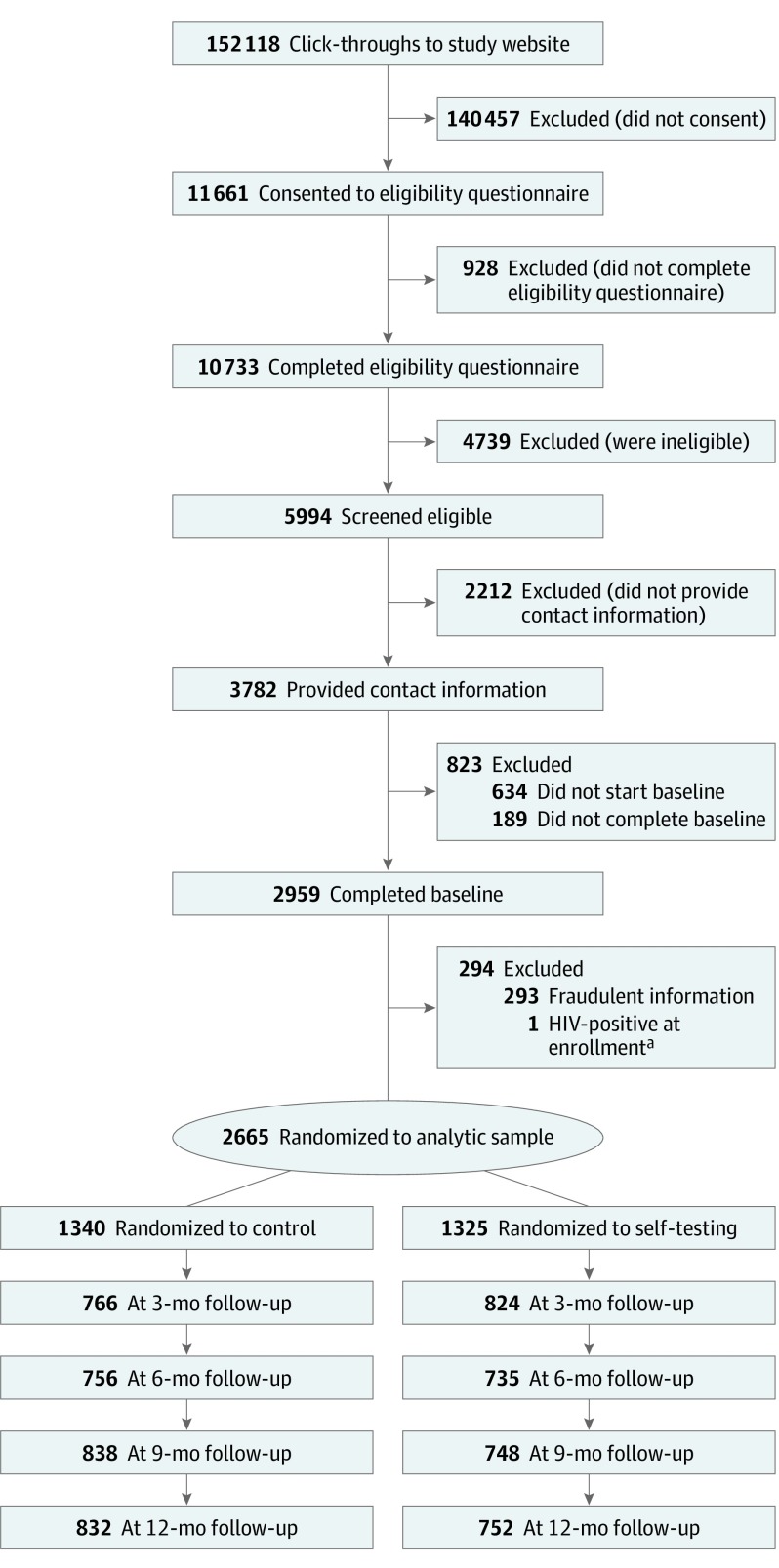
There were 152 118 click-throughs to the study website, 11 661 persons who initiated the eligibility screening, and 10 733 persons who completed the screening (Figure). A total of 5994 men were eligible, 3782 provided contact information and registered, and 2959 completed the baseline survey. Of the 4739 persons who were ineligible, while not mutually exclusive, the 2 most common reasons were (1) not having sex with a male partner in the past year (n = 1937) and (2) prior HIV-positive test results (n = 1078).
Figure. Recruitment, Eligibility, Assignment, and Retention of Participants in the Evaluation of Rapid HIV Self-testing Among MSM.
MSM indicates men who have sex with men.
aOne control group participant disclosed during his 3-month follow-up survey that his first HIV positive result was in 2001.
We excluded 293 participants who provided fraudulent or duplicate registration information and 1 person who reported during his 3-month survey that his first positive HIV test result was in 2001. This yielded an analytic sample of 2665 participants randomized into the ST (n = 1325) or control (n = 1340) arms. On average, 1562 of 2665 (58.6%) participants initiated each follow-up survey (Figure), and over the course of the study, 1014 ST (77%) and 977 control (73%) participants initiated at least 1 follow-up survey.
Most participants were recruited from dating (1517 of 2665 [56.9%]) or music (1063 of 2665 [39.9%]) websites; were younger than 30 years (1527 of 2665 [57.3%]); were non-Hispanic white (1540 of 2665 [57.8%]); had a high school education or higher (2222 of 2658 [83.6%]); identified as being homosexual, gay, or same gender–loving (2352 of 2653 [88.7%]); were employed (2236 of 2630 [85.0%); had health insurance (2155 of 2629 [82.0%]); and were recruited from the South US Census Region (1116 of 2665 [41.9%]) (Table 1). During the 3 months before study enrollment, approximately 4.0% (106 of 2665) of participants reported having female sex partners, and 7.9% (210 of 2665) had no sex partners. At enrollment, 16.6% (443 of 2665) of participants had never tested for HIV, 60.3% (1608 of 2665) had tested for HIV in the past year, and 17.3% (461 of 2665) had tested at least 3 times in the past year. Study retention rates were lower for black or African American and Hispanic MSM than white MSM (see eTable in Supplement 2).
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Self-testing (n = 1325) | Control (n = 1340) | ||
| Recruitment site | |||
| Music | 510 (38.5) | 553 (41.3) | <.001 |
| Dating | 754 (56.9) | 763 (56.9) | |
| Social network | 61 (4.6) | 24 (1.8) | |
| Age group, y | |||
| 18-29 | 752 (56.8) | 775 (57.8) | .58 |
| ≥30 | 573 (43.2) | 565 (42.2) | |
| Race/ethnicity | |||
| Non-Hispanic white | 773 (58.3) | 767 (57.2) | .73 |
| Non-Hispanic black | 133 (10.0) | 128 (9.6) | |
| Hispanic | 296 (22.3) | 324 (24.2) | |
| Other/mixed | 123 (9.3) | 121 (9.0) | |
| Educationa | |||
| ≤High school/GED | 209 (15.8) | 227 (17.0) | .43 |
| >High school | 1113 (84.2) | 1109 (83.0) | |
| Sexual identitya | |||
| Homosexual/gay | 1172 (88.9) | 1180 (88.5) | .11 |
| Bisexual | 134 (10.2) | 149 (11.2) | |
| Heterosexual/other | 13 (1.0) | 5 (0.4) | |
| US Census Region of residence | |||
| Northeast | 206 (15.6) | 219 (16.3) | .40 |
| Midwest | 226 (17.1) | 234 (17.5) | |
| South | 576 (43.5) | 540 (40.3) | |
| West | 317 (23.9) | 347 (25.9) | |
| Employmenta | |||
| Employed | 1101 (84.2) | 1135 (85.9) | .23 |
| Not employed | 207 (15.8) | 187 (14.1) | |
| Health insurancea | |||
| Yes | 1067 (81.6) | 1088 (82.4) | .61 |
| No | 241 (18.4) | 233 (17.6) | |
| Sex of partners in past 3 mo | |||
| Men only | 1180 (89.1) | 1169 (87.2) | .27 |
| Men and women | 5 (0.4) | 9 (0.7) | |
| Women only | 47 (3.5) | 45 (3.4) | |
| No partners | 93 (7.0) | 117 (8.7) | |
| HIV testing experience at enrollment | |||
| Never | 230 (17.4) | 213 (15.9) | .09 |
| In past 12 mo | 813 (61.4) | 795 (59.3) | |
| More than 12 mo ago | 282 (21.3) | 332 (24.8) | |
| At least 3 HIV tests in past year | |||
| Yes | 243 (18.3) | 218 (16.3) | .17 |
| No | 1082 (81.7) | 112 (83.7) | |
| HIV testing in past 12 mo, mean (SD)a | 1.35 (1.68) | 1.22 (1.41) | .03 |
Abbreviation: GED, General Equivalency Diploma.
Missing data were excluded.
During the trial, we mailed 8654 self-tests (4879 [56.4%] OraQuick In-Home HIV Tests and 3775 [43.6%] SURE CHECK HIV 1/2 Assays) to ST participants. Participants reported using 4501 (52.0%) self-tests and distributing 2864 (33.1%) self-tests to their social network members.
Primary Outcomes
The ST participants reported testing more frequently than control participants (mean number of tests over 12 months, 5.3 vs 1.5; P < .001), and most ST participants (777 of 1014 [76.6%]) reported testing 3 times or more before their 12-month survey compared with 22% (215 of 977; P < .001) of control participants (Table 2).
Table 2. HIV Testing Activity Among Study Participants Who Initiated at Least 1 Follow-up Surveya.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| Self-testing (n = 1014) | Control (n = 977) | ||
| HIV testing reported during study by testing type, mean (SD) | |||
| Any type of testing | 5.29 (3.59) | 1.50 (1.76) | <.001 |
| Health care provider–based testingb | 0.85 (1.49) | 1.50 (1.76) | <.001 |
| Participants by the total of HIV tests reported during the trial | |||
| 0 | 45 (4.4) | 358 (36.6) | <.001 |
| 1 | 76 (7.5) | 245 (25.1) | |
| 2 | 116 (11.4) | 159 (16.3) | |
| 3 | 127 (12.5) | 91 (9.3) | |
| 4 | 131 (12.9) | 62 (6.3) | |
| 5 | 114 (11.2) | 27 (2.8) | |
| ≥6 | 405 (39.9) | 35 (3.6) | |
| Participants who tested ≥3 times during the trial | 777 (76.6) | 215 (22.0) | <.001 |
| Participants who tested on at least 3 follow-up surveys (survey initiated) | 516 (50.9) | 168 (17.2) | <.001 |
| Participants who had at least 1 HIV test since last survey (survey initiated)c | |||
| 3-mo Follow-up | 755/811 (93.1) | 284/759 (37.4) | <.001 |
| 6-mo Follow-up | 617/704 (87.7) | 307/734 (41.8) | <.001 |
| 9-mo Follow-up | 566/738 (76.7) | 296/834 (35.5) | <.001 |
| 12-mo Follow-up | 564/748 (75.4) | 315/828 (38.0) | <.001 |
| Participants who had any health care provider–based HIV testing since last survey (survey initiated)b,c | |||
| 3-mo Follow-up | 132/811 (16.3) | 283/758 (37.3) | <.001 |
| 6 mo Follow-up | 181/704 (25.7) | 306/733 (41.8) | <.001 |
| 9-mo Follow-up | 164/736 (22.3) | 296/834 (35.5) | <.001 |
| 12-mo Follow-up | 163/747 (21.8) | 312/825 (37.8) | <.001 |
| During study | 403/1014 (40.1) | 617/977 (63.5) | <.001 |
| Among 443 participants who had never tested for HIV at enrollment (survey initiated) | |||
| Tested at least once during the trial | 159/166 (95.8) | 63/137 (46.0) | <.001 |
| Tested ≥3 times during the trial | 113/166 (68.1) | 10/137 (7.3) | <.001 |
During the trial, all participants were asked to complete follow-up surveys at 3, 6, 9, and 12 months.
Includes clinic, venue-based, and other testing. Excludes study rapid HIV self-tests.
Missing data were excluded.
During the trial there were 36 newly identified HIV infections among all study participants: 34 reported in surveys and the reporting system, and 2 during telephone calls (Table 3). More HIV infections were identified in the ST arm than in the control arm (12 of 1325 [0.9%] vs 2 of 1340 [0.1%]; P < .007) during the first 3 months of follow-up. During the whole 12-month follow-up period, more than twice as many HIV infections (25 of 1325 [1.9%] vs 11 of 1340 [0.8%]; P = .02) were identified. Nearly half of these infections (17 of 36 [47.2%]) were among participants who had not been tested in the preceding year, which included 9 participants who had never been tested before enrollment. After completion of the trial, the DBS kit and self-tests identified an additional 5 infections in the ST arm and 3 in the control arm, yielding a total of 44 newly diagnosed infections. We did not receive DBS cards from all study participants who reported a positive self-test result; therefore, we were only able to confirm 40.9% (18 of 44) of them by laboratory testing.
Table 3. Newly Identified HIV Infection Among Participants and Social Network Members Who Used Study Self-testsa.
| Reporting Interval | No. (%) | P Valueb | Survey | Social Network Members, No. (%) | |
|---|---|---|---|---|---|
| Self-testing | Control | ||||
| Day 1 to 3 mo | 12/1325 (0.91) | 2/1340 (0.15) | .007 | 3 mo | 11/710 (1.55) |
| >3-6 mo | 3/1313 (0.23) | 1/1338 (0.07) | .37 | 6 mo | 8/541 (1.48) |
| >6-9 mo | 5/1310 (0.38) | 5/1337 (0.37) | .99 | 9 mo | 8/512 (1.56) |
| >9 mo to end of trial | 5/1305 (0.38) | 3/1332 (0.23) | .50 | 12 mo | 7/559 (1.25) |
| Day 1 to end of trial | 25/1325 (1.88) | 11/1340 (0.82) | .02 | All surveys | 34/2152 (1.58) |
Includes all reported infections: participant-reported infections and Centers for Disease Control and Prevention laboratory-diagnosed infections.
Fisher exact test between self-testing and control groups.
Thirty-eight ST participants reported that 52 of their social network members obtained a positive test result. Of the 52 social network members with a positive test, 29 were unaware of their infection before obtaining a positive self-test result, and 5 had not made the ST participant aware of their infection prior to the test. Therefore, we classified 34 social network members as becoming aware of their HIV infection from using a study self-test, 11 (32%) of whom were reported in the first 3 months (Table 3).
Secondary Outcomes
Overall there was a 55.7% increase in annual HIV testing among ST participants: 61.4% (813 of 1325) before enrollment to 95.6% (969 of 1014) at end of the trial. We observed a 6.9% increase in annual HIV testing among control participants, from 59.3% (795 of 1340) before enrollment to 63.4% (619 of 977) at the end of the trial. Nearly all ST participants reported testing at least once during the study compared with fewer than two-thirds of control participants (Table 2). Although HIV testing was more frequent among ST participants, a smaller percentage of them received health care provider–based HIV testing than control participants. About half of ST participants (516 of 1014 [50.9%]) reported at least 1 HIV test on at least 3 separate follow-up surveys compared with about 1 in every 6 (168 of 977 [17.2%]) control participants. Among those who had never tested, a greater proportion of ST participants compared with control participants had a lower retention rate than those who had ever tested (159 of 166 [95.8%] vs 63 of 137 [46.0%]; P < .001) and tested 3 or more times (113 of 166 [68.1%] vs 10 of 137 [7.3%]; P < .001) during the trial.
During the trial, we observed differences in newly identified infections based on testing history of participants before study enrollment. The proportion of participants with newly identified infections was 2.0% (9 of 440) among those who had never tested, 1.3% (8 of 611) among those who tested more than 12 months ago, and lowest (19 of 1604 [1.2%]) among those who tested in the 12 months before study enrollment.
Of the 36 participants with newly identified infection during the trial, 26 (72%) reported that they had initiated linkage to care. There was no statistically significant difference in linkage rates between ST and control participants during the trial (16 of 25 [64%] vs 10 of 11 [91%]; P = .13).
There were no statistically significant differences between participants in the 2 study arms with respect to most of the secondary sexual behavior outcomes (Table 4). However, on each survey, the percentage who reported serosorting was lower among control participants throughout the study (Table 4) and reached statistical significance at 6 and 12 months.
Table 4. Sex Partners and Serosorting Reported by MSM After Enrollment.
| Variable | Self-testing (n = 1325) | Control (n = 1340) | P Value | ||
|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | ||
| No. of male sex partners in past 3 mo since last interview | |||||
| Baseline | 1323 | 3.53 (5.46) | 1340 | 3.69 (5.57) | .47 |
| 3 mo | 792 | 2.96 (5.81) | 754 | 2.86 (4.73) | .72 |
| 6 mo | 715 | 3.01 (5.03) | 745 | 2.68 (4.35) | .18 |
| 9 mo | 724 | 2.68 (5.02) | 829 | 2.49 (5.81) | .51 |
| 12 mo | 732 | 2.70 (4.81) | 827 | 2.29 (4.52) | .09 |
| No. of male sex partners without condoms in past 3 mo since last interview | |||||
| Baseline | 1322 | 1.96 (3.26) | 1340 | 1.96 (3.46) | .96 |
| 3 mo | 791 | 1.58 (4.29) | 754 | 1.62 (3.46) | .81 |
| 6 mo | 715 | 1.70 (2.94) | 745 | 1.65 (3.22) | .80 |
| 9 mo | 724 | 1.62 (3.95) | 829 | 1.46 (3.91) | .41 |
| 12 mo | 732 | 1.63 (3.45) | 826 | 1.41 (2.51) | .17 |
| No. of sex partners since enrollment reported on final survey | |||||
| Men and women | 724 | 9.01 (16.93) | 821 | 9.72 (19.75) | .45 |
| Man with positive HIV status | 724 | 0.42 (2.22) | 821 | 0.44 (2.80) | .88 |
| Man with unknown HIV status | 724 | 2.24 (7.47) | 821 | 2.56 (7.01) | .39 |
| Man with negative HIV status | 724 | 6.08 (12.06) | 821 | 6.59 (17.48) | .50 |
| HIV-negative participants reporting serosorting since last survey (surveys initiated)a | |||||
| 3-mo Follow-up | 160/704 (22.7) | 55/266 (20.7) | .54 | ||
| 6-mo Follow-up | 128/558 (22.9) | 39/295 (13.2) | <.001 | ||
| 9-mo Follow-up | 106/488 (21.7) | 47/289 (16.3) | .08 | ||
| 12-mo Follow-up | 108/474 (22.8) | 50/303 (16.5) | .04 | ||
Abbreviation: MSM, men who have sex with men.
Serosorting refers to men who reported a negative HIV test result since the last survey and have decided only to have sex with persons of the same serostatus.
Discussion
The distribution of HIV self-tests to MSM recruited via the internet significantly increased the frequency of testing among ST participants compared with control participants and facilitated the distribution and use of HIV self-tests to social network members. The intervention identified more previously undiagnosed HIV infections than in the control arm and identified infections among ST participants’ social network members. Approximately half of the newly identified infections among ST participants, and one-third of the infections among their social network members, were identified during the 3 months following the initial distribution of HIV self-tests. The initial excess of new infections reported early in the study was because of the identification of prevalent infections among ST participants as a result of using the study self-tests. Distribution of HIV self-test kits had a substantial prevention benefit among social network members: during the trial, more new HIV infections were reported among social network members who used the study self-tests than among the ST participants themselves.
Prior studies evaluating the use of HIV self-tests typically required interaction with health care providers or research staff. To our knowledge, this study is the first nationwide, large, internet-based, randomized clinical trial of HIV self-testing in the United States, and participants were not required to interact with health care providers, counselors, or peers. By mailing HIV self-tests, this design aimed to increase distribution efficiency and reduce some of the barriers associated with clinical and peer-based HIV testing programs, such as patient and physician time, testing costs, stigma, and face-to-face interactions.20 The recruitment strategy may also have resulted in reaching a higher risk population based on the nature of sites from which participants were recruited.
Consistent with other national studies,22,23 these findings indicate that the CDC recommendation for annual HIV screening for MSM was not being achieved in the year before their study participation. Only 4% of MSM in the ST arm did not achieve the CDC recommendation of annual HIV testing for sexually active MSM24 during the trial. These results also reinforce the importance of the screening recommendations: participants who had not tested in the past year accounted for nearly half of the newly identified infections in the study. Hence, this approach may be a useful strategy to reach MSM, especially those who are not testing at least annually, and those who have never accessed existing HIV testing services.
The full benefits of HIV testing are achieved once persons with HIV receive treatment. More than 70% of study participants who became aware of their HIV infection initiated linkage to HIV care, and there was not a significant difference in the percentage who initiated linkage to care between arms. However, some participants did not report initiating linkage to care, and additional efforts will be needed to reach the national goal of 85% for linkage to care for persons using HIV self-tests.25
Results of a modeling analysis showed that replacing all clinic-based HIV testing with the FDA-approved HIV self-test might increase HIV transmissions in the community owing to the lower sensitivity of the FDA-approved HIV self-test.26 In this study, we provide data on the substitution of health care provider–based tests by some ST participants. However, with documented greater HIV testing coverage and more frequent testing, we also report that more infections were identified in the ST arm.
The findings concerning sexual risk behavior between the 2 study arms support previous evidence that HIV self-testing is not associated with an increase in sexual risk behaviors.11,13,27 However, though not statistically significant at all intervals, a greater proportion of ST participants than control participants reported serosorting throughout the study. A previous study reported that HIV-negative MSM did not have intercourse with a partner who used an HIV self-test and obtained a positive result.28 The potential for HIV transmission exists if a false-negative result is obtained when the HIV self-test is used during acute HIV infection when viremia is high, or if the test is performed incorrectly.29 Therefore, people who use HIV self-tests should be discouraged from using the self-test as a point-of-sex test.
The intensive follow-up efforts in this study allowed us to obtain a higher retention rate than achieved in a web-based HIV self-test distribution to MSM in Los Angeles (17%) and a pilot HIV self-testing program in New York City (48%).30,31 Health departments and community-based organizations planning to implement an HIV self-testing program may not have the resources to obtain high follow-up rates for longitudinal program evaluation. Therefore, HIV self-testing program evaluators may need to consider other measures of program success, such as the number of tests distributed to high-risk persons, rather than rely on client-reported test results or linkage to care.
Limitations
Although recruiting MSM online is practical and we recruited a convenience sample of MSM from all regions of the United States, the sample is not representative of all MSM in the United States. However, we provide data on internet-using MSM, and these findings might portray HIV self-testing among this group. The recruitment materials and websites were only provided in English, and though we provided testing instructions in Spanish, the sample may underrepresent those whose first language is not English. We were not able to verify all positive results from study participants, and we were unable to verify the self-test results from the social network members. Additionally, possibly because of concerns of privacy or stigma, some study participants may have been unwilling to report a positive test result. Although our participation rate was nearly 60% at each survey, approximately 25% of the participants discontinued participation after the baseline survey, and follow-up retention rates were lower among black or African American and Hispanic MSM compared with white MSM (eTable in Supplement 2). Given that the retention rates were lower among MSM most affected by HIV in the United States, we likely underreport diagnoses of HIV infection and initiation of linkage. However, the large difference observed for the primary outcome suggests that the findings would remain significant with a greater study retention rate.
Conclusions
Internet recruitment of MSM for HIV self-test distribution by mail increased testing and awareness of HIV infection among study participants and their social network members, potentially contributing to the prevention of HIV transmission. This approach resulted in a high proportion of infections identified in the first 3 months and a consistently high proportion of infections identified among social network members during the 12-month study. Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations and providing high-risk MSM additional kits to promote distribution to social network members. Providing existing clients with additional tests is beneficial; however, programs should anticipate that the majority of newly identified infections will originate from the ongoing recruitment of new clients. Additional implementation research on HIV self-testing could provide information about the most cost-effective methods for online recruitment, optimal frequency of self-test provision, linkage of HIV-positive persons to care, and HIV behavioral messaging to improve operational expansion of HIV self-testing.
Trial Protocol
eMethods. Data collection methods and definition of newly identified infection in eSTAMP
eFigure. Example of banner add used for study recruitment
eTable. Retention rates by selected baseline characteristics among participants who initiated any follow-up survey and the 12-month survey
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention HIV Surveillance Report, 2017; volume 29: Diagnoses of HIV infection in the United States and dependent areas, 2017. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. Published November 2018. Accessed October 15, 2019.
- 2.Centers for Disease Control and Prevention HIV and gay and bisexual men. https://www.cdc.gov/hiv/pdf/group/msm/cdc-hiv-msm.pdf. Published September 2019. Accessed October 15, 2019.
- 3.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844-845. doi: 10.1001/jama.2019.1343 [DOI] [PubMed] [Google Scholar]
- 4.Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10(4):e1001414. doi: 10.1371/journal.pmed.1001414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health. 2013;13:735. doi: 10.1186/1471-2458-13-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav. 2015;19(11):1949-1965. doi: 10.1007/s10461-015-1097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngure K, Heffron R, Mugo N, et al. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc. 2017;20(1):21234. doi: 10.7448/IAS.20.1.21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Chavez PR, MacGowan RJ, et al. Willingness to distribute free rapid home HIV test kits and to test with social or sexual network associates among men who have sex with men in the United States. AIDS Care. 2017;29(12):1499-1503. doi: 10.1080/09540121.2017.1313386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGowan RJ, Chavez PR, Gravens L, et al. ; eSTAMP Study Group . Pilot evaluation of the ability of men who have sex with men to self-administer rapid HIV tests, prepare dried blood spot cards, and interpret test results, Atlanta, Georgia, 2013. AIDS Behav. 2018;22(1):117-126. doi: 10.1007/s10461-017-1932-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamil MS, Prestage G, Fairley CK, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): a waiting-list randomised controlled trial. Lancet HIV. 2017;4(6):e241-e250. doi: 10.1016/S2352-3018(17)30023-1 [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Lau JTF, Ip M, et al. A randomized controlled trial evaluating efficacy of promoting a home-based HIV self-testing with online counseling on increasing HIV testing among men who have sex with men. AIDS Behav. 2018;22(1):190-201. doi: 10.1007/s10461-017-1887-2 [DOI] [PubMed] [Google Scholar]
- 12.Ortblad K, Kibuuka Musoke D, Ngabirano T, et al. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial. PLoS Med. 2017;14(11):e1002458. doi: 10.1371/journal.pmed.1002458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz DA, Golden MR, Hughes JP, Farquhar C, Stekler JD. HIV self-testing increases HIV testing frequency in high-risk men who have sex with men: a randomized controlled trial. J Acquir Immune Defic Syndr. 2018;78(5):505-512. doi: 10.1097/QAI.0000000000001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacPherson P, Lalloo DG, Webb EL, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312(4):372-379. doi: 10.1001/jama.2014.6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Sullivan PS, Khosropour CM. Willingness to take a free home HIV test and associated factors among internet-using men who have sex with men. J Int Assoc Physicians AIDS Care (Chic). 2011;10(6):357-364. doi: 10.1177/1545109711404946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavez PR, Wesolowski LG, Owen SM, Gravens L, Sullivan P, MacGowan RJ Perceptions and performance of self-administered rapid HIV tests conducted by untrained users in real-world settings. Abstract presented at: 2016 HIV Diagnostics Conference; March 21-24, 2016; Atlanta, GA. http://hivtestingconference.org/wp-content/uploads/2016/03/2016-HIV-DX-Program-Book_WEB_FINAL.pdf. Accessed October 15, 2019.
- 17.Chavez PR, MacGowan RJ, Borkowf CB, et al. Characteristics associated with HIV self-testing reported by internet-recruited MSM in the United States, eSTAMP baseline data, 2015. AIDS Soc. 2017. http://programme.ias2017.org/Abstract/Abstract/2149. Accessed October 21, 2019. [Google Scholar]
- 18.Walensky RP, Paltiel AD. Rapid HIV testing at home: does it solve a problem or create one? Ann Intern Med. 2006;145(6):459-462. doi: 10.7326/0003-4819-145-6-200609190-00010 [DOI] [PubMed] [Google Scholar]
- 19.Wood BR, Ballenger C, Stekler JD. Arguments for and against HIV self-testing. HIV AIDS (Auckl). 2014;6:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman AE, Sullivan P, Higa D, et al. ; eSTAMP Study Group . Perceptions of HIV self-testing among men who have sex with men in the United States: a qualitative analysis. AIDS Educ Prev. 2018;30(1):47-62. doi: 10.1521/aeap.2018.30.1.47 [DOI] [PubMed] [Google Scholar]
- 21.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93-113. doi: 10.1016/0197-2456(81)90001-5 [DOI] [PubMed] [Google Scholar]
- 22.Pitasi MA, Delaney KP, Oraka E, et al. Interval since last HIV test for men and women with recent risk for HIV infection—United States, 2006–2016. MMWR Morb Mortal Wkly Rep. 2018;67(24):677-681. doi: 10.15585/mmwr.mm6724a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooley LA, Oster AM, Rose CE, Wejnert C, Le BC, Paz-Bailey G; NHBS Study Group . Increases in HIV testing among men who have sex with men—National HIV Behavioral Surveillance System, 20 U.S. Metropolitan Statistical Areas, 2008 and 2011. PLoS One. 2014;9(9):e104162. doi: 10.1371/journal.pone.0104162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiNenno EA, Prejean J, Irwin K, et al. Recommendations for HIV screening of gay, bisexual, and other men who have sex with men—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(31):830-832. doi: 10.15585/mmwr.mm6631a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Office of National AIDS Policy National HIV/AIDS strategy for the United States: updated to 2020. https://www.hiv.gov/federal-response/national-hiv-aids-strategy/nhas-update. Updated January 31, 2017. Accessed October 16, 2019.
- 26.Katz DA, Cassels SL, Stekler JD. Replacing clinic-based tests with home-use tests may increase HIV prevalence among Seattle men who have sex with men: evidence from a mathematical model. Sex Transm Dis. 2014;41(1):2-9. doi: 10.1097/OLQ.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson CC, Kennedy C, Fonner V, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20(1):21594. doi: 10.7448/IAS.20.1.21594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carballo-Diéguez A, Frasca T, Balan I, Ibitoye M, Dolezal C. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS Behav. 2012;16(7):1753-1760. doi: 10.1007/s10461-012-0274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration Summary of safety and effectiveness. OraQuick® In-Home HIV test. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM312534.pdf. Published 2012. Accessed April 4, 2017.
- 30.Rosengren AL, Huang E, Daniels J, Young SD, Marlin RW, Klausner JD. Feasibility of using GrindrTM to distribute HIV self-test kits to men who have sex with men in Los Angeles, California. Sex Health. 2016;13(4):389-392. doi: 10.1071/SH15236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelstein ZR, Salcuni PM, Tsoi B, et al. Feasibility and reach of a home-test giveaway in New York City, 2015–2016. Poster presented at: Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, Washington. http://www.croiconference.org/sessions/feasibility-and-reach-home-test-giveaway-new-york-city-2015%E2%80%932016. Accessed October 16, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Data collection methods and definition of newly identified infection in eSTAMP
eFigure. Example of banner add used for study recruitment
eTable. Retention rates by selected baseline characteristics among participants who initiated any follow-up survey and the 12-month survey
Data Sharing Statement