Key Points
Question
What are the patterns of change of albuminuria over the course of young adulthood, and is trajectory of albuminuria over a 20-year span associated with adverse changes to cardiac structure and function in middle age?
Findings
In this cohort study of 2647 black and white young adults, 5 distinct trajectory groups of urine albumin-to-creatinine ratio (UACR) were identified, which could not be identified by baseline UACR levels alone. Urine albumin-to-creatinine ratio trajectory group was independently associated with long-term adverse alterations to cardiac structure, left ventricular systolic function, and left ventricular diastolic function.
Meaning
There are distinct patterns of change in albuminuria among young adults over a 20-year span, and dynamic changes in UACR are independently associated with cardiac structural and functional remodeling.
This cohort study describes the trajectory of albuminuria as measured by urine albumin-to-creatinine ratio across a 20-year span and evaluates the association of albuminuria trajectory with echocardiographic indices of structure and function in middle age.
Abstract
Importance
Albuminuria, as measured by single urine albumin-to-creatinine ratio (UACR) levels, is associated with cardiac remodeling and adverse clinical outcomes. The longitudinal patterns of change in UACR through young adulthood and their associations with myocardial structure and function later in life remain unclear.
Objective
To describe the trajectory of albuminuria as measured by UACR across a 20-year span and evaluate the association of albuminuria trajectory with echocardiographic indices of structure and function in middle age.
Design, Setting, and Participants
In the Coronary Artery Risk Development in Young Adults (CARDIA) study, a prospective cohort of black and white participants aged 18 to 30 years at baseline (March 1985 to June 1986) were evaluated over 30 years. Participants underwent evaluations at 4 urban US sites. Data were collected from March 1985 to May 2016, and data were analyzed from September 2018 to April 2019.
Exposures
Trajectories of UACR from the year 10 examination to the year 30 examination as determined by latent class modeling.
Main Outcomes and Measures
Echocardiographic indices of myocardial structure, systolic function, and diastolic function at the year 30 examination.
Results
Of the 2647 included participants, 1441 (54.4%) were white, 1206 (45.6%) were black, and the mean (SD) age was 35.2 (3.6) years. A total of 5 trajectory groups of UACR were identified, including 1718 participants (64.9%) in the low-stable group, 682 (25.8%) in the moderate-stable group, 116 (4.4%) in the high-stable group, 88 (3.3%) in the moderate-increasing group, and 43 (1.6%) in the high-increasing group. Apart from the high-increasing cohort, the remaining 4 groups had median baseline UACR levels less than 30 mg/g. Male sex, current smoking, diabetes, and elevated blood pressure were more common in the moderate-increasing and high-increasing UACR groups. After adjustment for clinical variables and baseline UACR levels, there were significant differences in left ventricular (LV) mass by trajectory group (mean [SE] LV mass: high-increasing, 98.4 [3.4] g/m2; moderate-increasing, 91.7 [2.2] g/m2; high-stable, 86.0 [2.1] g/m2; moderate-stable, 82.3 [0.8] g/m2; low-stable, 78.6 [0.5] g/m2; P < .001). Significant differences by trajectory group were also noted in LV longitudinal strain, e′ tissue velocities, and estimated LV filling pressures, even after adjustment for clinical variables and baseline UACR level. The association of trajectory group with indices of myocardial structure and function remained significant after adjustment for clinical variables and cumulative UACR from the year 10 to year 25 examinations.
Conclusions and Relevance
There are distinct patterns of change in albuminuria among young adults over a 20-year span, and these trajectory groups cannot be identified by baseline UACR level alone. Dynamic changes in albuminuria are independently associated with adverse alterations to cardiac structure, LV systolic function, and LV diastolic function.
Introduction
Albuminuria is a biomarker of endothelial dysfunction and has been associated with higher cardiovascular mortality among individuals with and without diabetes.1,2 Indeed, significant associations exist between albuminuria and incident heart failure (HF), stroke, myocardial infarction, and cardiovascular death among those without preexisting cardiovascular disease (CVD).3,4,5 Albuminuria may precede the onset of traditional CVD risk factors, as 70% of young adults with prevalent albuminuria have normotension and normoglycemia.6
The presence of albuminuria, even at subclinical levels, has been associated with alterations to myocardial structure and function.7,8,9,10 To our knowledge, studies of albuminuria to date have relied on single measurements of urine albumin-to-creatinine ratio (UACR) level, and the trajectories and correlates of UACR across young adulthood have not been elucidated. Furthermore, the association of the trajectory of albuminuria from early adulthood onwards with myocardial structure and function in later life have not been studied. As such, we aimed to (1) describe the longitudinal changes in albuminuria levels over a 20-year span among individuals in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study and (2) evaluate the association of trajectory of albuminuria with several echocardiographic indices of myocardial structure and function in later life.
Methods
Study Sample
The CARDIA study is a prospective cohort study that was designed to understand risk factors for the development of subclinical and overt CVD. Black and white men and women aged 18 to 30 years were recruited from March 1985 to June 1986 across 4 urban US sites: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. The original cohort included 5115 participants. Participants have been observed for more than 30 years, with evaluation of demographic, clinical, laboratory, and imaging data, including height, weight, heart rate, blood pressure, lipid levels, glucose levels, smoking status, and education level. Complete information regarding the CARDIA study design, recruitment, and protocol for examinations has been described previously.11 Participants in the CARDIA study have undergone in-person examinations at baseline (year 0) and at years 2, 5, 7, 10, 15, 20, 25, and 30. Retention rates among surviving participants at each in-person examination were 90.5% (4622 of 5107), 85.7% (4351 of 5077), 80.6% (4085 of 5068), 78.5% (3948 of 5029), 73.6% (3671 of 4988), 71.9% (3548 of 4935), 72.1% (3497 of 4850), and 71.0% (3358 of 4730), respectively. Contact is maintained with participants via telephone, mail, or email every 6 months, with annual interim medical history ascertainment. Over the last 5 years, more than 90% of the surviving cohort members have been directly contacted, and follow-up for vital status is virtually complete through related contacts and intermittent National Death Index searches.
For the present analysis, participants with (1) echocardiography obtained at the year 30 examination and (2) UACR levels recorded at 3 or more examinations (including year 10, when urinary albumin and creatinine levels were measured for the first time, and year 30) were included in this analysis. Among 5115 CARDIA study participants, 2125 participants were excluded; 1756 did not attend the year 30 examination, 358 did not attend the year 10 examination, 1 withdrew consent, and 10 were missing more than 3 UACR measurements. There were 2990 participants included for trajectory group modeling. Of this group, 144 participants did not have echocardiography obtained at year 30, and 199 were excluded for missing covariate data at the year 10 examination. The final analytic cohort was composed of 2647 participants. The study was approved by the institutional review boards at each site, and all participants provided written informed consent.
Measurement of UACR Level
Urine samples were collected and analyzed for urine albumin and urine creatinine levels at 5 consecutive examinations (years 10, 15, 20, 25, and 30). A single untimed urine sample was collected during the clinic visits. Urine albumin was assessed using a nephelometric procedure with a specific antialbumin antibody, and urine creatinine was assessed using the Jaffe method.6 Previous studies have demonstrated reliability of these measurement techniques when tested for quality assurance among the CARDIA cohort (correlation coefficients greater than 0.96 among repeated sample measurement).6 The urine albumin-to-creatinine ratio was defined as the urine albumin level divided by the urine creatinine level and was expressed with standard units (milligrams per gram).
Echocardiography
Comprehensive, 2-dimensional, M-mode, and Doppler echocardiography were obtained at the year 30 examination. Standard machines were used for image acquisition at each site (Apilo Artida scanner; Toshiba Medical Systems). Sonographers from the 4 field sites underwent centralized training, and quality control procedures assessed intrasonographer and intersonographer reliability throughout the examination.12 Studies were digitized, and sonographers made measurements offline using standard software (Digisonics). Studies were sent electronically to the echocardiography core reading laboratory at Johns Hopkins University, Baltimore, Maryland. Left ventricular (LV) ejection fraction and left atrial (LA) volume were measured from the apical 2-chamber and 4-chamber views based on American Society of Echocardiography guidelines.13 Left ventricular mass was derived using the Devereux formula.14,15 Left ventricular diastolic dimension was measured from the parasternal long-axis view. Left ventricular mass, LA volume, and LV diastolic dimension were indexed to body surface area. Pulse-wave Doppler of mitral inflow during early diastole measured the peak velocity at early (E wave) and late (A wave) diastole. Tissue doppler imaging was used to assess early peak diastolic mitral annular velocity (e′) at the septal and lateral walls. E/e′ ratio, a surrogate for LV filling pressures, was calculated as the E wave divided by the average of the septal and lateral e′ velocities. All echocardiograms were assigned apical and short-axis image quality scores (1-4) based on degree of visualization of the myocardium and cardiac structures.
Speckle-tracking imaging was also performed offline after image acquisition at the year 30 examination using dedicated semiautomated software (Toshiba Medical Systems). Three cardiac cycles were used to generate strain curves. The apical 4-chamber view was used for generation of LV longitudinal strain curves, and the mid-LV cavity short-axis view was used for generation of LV circumferential strain curves. Peak systolic strain values were derived from 6 segments of the LV in either the apical or short-axis views. Left ventricular longitudinal and LV circumferential strain values were then calculated as the average of the peak systolic strain of the 6 segments in the apical 4-chamber and short-axis views, respectfully.
Statistical Analysis
Urine albumin-to-creatinine ratio values were log-transformed prior to trajectory modeling. Trajectory of albuminuria was modeled among participants in the CARDIA study who attended both the year 10 and year 30 examinations and were missing no more than 3 UACR measurements from year 10 through year 30. We used latent class models to identify subgroups within the CARDIA study that shared similar trajectories of UACR. Models were fit using the Traj procedure in SAS version 9.4 (SAS Institute).16,17,18 The Traj procedure is a group-based modeling strategy that uses a discrete mixture model to identify clusters of longitudinal data series. We tested models with groups ranging from 2 to 5 with cubic polynomial function parameters and examined bayesian information criteria to assess the optimal number of trajectories. Trajectory groups were data derived and subsequently labeled based on the groups found by latent class analysis. In addition to classifying UACR levels by trajectory groups, we also calculated cumulative UACR levels from year 10 through year 25 among CARDIA study participants who had UACR levels measured at the year 10 examination, year 25 examination, and at least 1 interim examination. Cumulative UACR levels from year 10 through year 25 were calculated by summing the product of UACR levels (milligrams per gram) at each examination by years of exposure. Years of exposure was defined as the number of years until subsequent UACR measurement.
Clinical characteristics at the year 10 examination (the baseline examination for this analysis) by trajectory group were compared using χ2 tests for categorical variables and univariate linear models for continuous variables. Multivariable general linear models were used to evaluate the association of trajectory group with the following echocardiographic assessments of myocardial performance: myocardial structure (LV mass index, LV diastolic dimension, and LA volume index), LV systolic function (LV ejection fraction, LV longitudinal strain, and LV circumferential strain), and LV diastolic function (E/A ratio, average e′ velocity, and average E/e′ ratio). The first model adjusted for the following clinical variables obtained at the year 10 examination: age, race, sex, average systolic blood pressure at the year 10 examination, use of antihypertensive medication, total cholesterol level, diabetes, number of cigarettes per day, body mass index, creatinine level, CARDIA field center, and apical and short-axis image quality scores. We also further adjusted for cumulative systolic blood pressure or cumulative number of years with diabetes from years 10 to 25. Cumulative systolic blood pressure was calculated by summing the product of systolic blood pressure at each examination by years of exposure. Years of exposure was defined as the number of years until subsequent systolic blood pressure measurement. Two further models were prespecified, each additionally adjusted for values of UACR. The first model further adjusted for log-transformed year 10 UACR levels, the first UACR measurement in the CARDIA cohort. The second model further adjusted for cumulative UACR levels. In sensitivity analyses, to account for participants who did not attend the year 30 examination, we used multivariable logistic regression models to determine the inverse probability of inclusion in our analysis. The inverse probability of inclusion was used to perform weighted regression analyses of the associations of trajectory groups with all echocardiographic outcomes. We evaluated the associations of cumulative albuminuria levels with echocardiographic outcomes in general linear models. Two-tailed P values less than .05 were considered statistically significant.
Results
Participant Characteristics and Albuminuria Trajectories
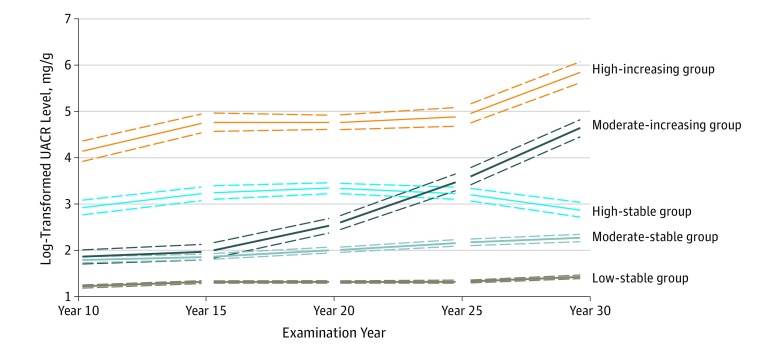
There were 5 trajectories of UACR among the 2990 participants included for the trajectory group modeling and among the subset of 2647 participants who comprised the final analytic cohort (Figure 1): 1718 participants (64.9%) had low UACR levels that remained low throughout follow-up (low-stable group), 682 (25.8%) had moderate UACR levels that remained stable (moderate-stable group), 116 (4.4%) had higher levels of UACR that were relatively stable throughout (high-stable group), 88 (3.3%) had moderate UACR levels that rose rapidly starting at the year 20 examination (moderate-increasing group), and 43 (1.6%) had high UACR that increased consistently during follow-up (high-increasing group). The mean posterior probability of group membership was high for each trajectory group (low-stable group, 0.914; moderate-stable group, 0.818; high-stable group, 0.877; moderate-increasing group, 0.888; high-increasing group, 0.954). Four of 5 groups had median UACR levels less than the clinical threshold of microalbuminuria (30 mg/g) at the year 10 examination (eTable 1 in the Supplement). The median (interquartile range) changes in UACR level from the year 10 to year 30 examinations by trajectory group were 0.6 (−0.7 to 2.1) mg/g in the low-stable group, 3.5 (−0.5 to 10.3) mg/g in the moderate-stable group, 4.7 (−14.7 to 14.5) mg/g in the high-stable group, 85.0 (53.4-171.0) mg/g in the moderate-increasing group, and 265.7 (116.5-539.6) mg/g in the high-increasing group (eTable 1 in the Supplement).
Figure 1. Trajectories of Albuminuria.
Of the 2990 participants included for trajectory group modeling, there were 1923 in the low-stable group, 785 in the moderate-stable group, 136 in the high-stable group, 99 in the moderate-increasing group, and 47 in the high-increasing group. Dotted lines indicate 95% CIs. UACR indicates urine albumin-to-creatinine ratio.
The baseline characteristics of participants by trajectory group are shown in Table 1. Compared with the low-stable group, the moderate-increasing and high-increasing groups had higher rates of participants who were male, black, and current smokers. Additionally, rates of diabetes and antihypertensive medication use were higher among the moderate-increasing and high-increasing groups. Average systolic and diastolic blood pressures, body mass index, and education level varied significantly by trajectory groups. While low-density lipoprotein cholesterol levels were relatively similar, high-density lipoprotein cholesterol levels were lower and creatinine levels were higher among the moderate-increasing and high-increasing groups. Compared with the analytic cohort, excluded participants were more likely to be younger, black, have lower education levels, and have higher smoking rates (eTable 2 in the Supplement).
Table 1. Baseline Characteristics at the Year 10 Examination Stratified by Albuminuria Trajectory Group.
| Characteristic | Trajectory Group, Mean (SD) | P Value | ||||
|---|---|---|---|---|---|---|
| Low-Stable (n = 1718) | Moderate-Stable (n = 682) | High-Stable (n = 116) | Moderate-Increasing (n = 88) | High-Increasing (n = 43) | ||
| Age, y | 35.1 (3.6) | 35.3 (3.6) | 35.2 (3.6) | 34.9 (3.5) | 36.0 (3.8) | .33 |
| Female, No. (%) | 892 (51.9) | 484 (71.0) | 61 (52.6) | 33 (38) | 19 (44) | <.001 |
| Black, No. (%) | 698 (40.6) | 344 (50.4) | 78 (67.2) | 51 (58) | 35 (81) | <.001 |
| BMIa | 26.5 (5.4) | 28.0 (7.0) | 30.4 (7.5) | 30.2 (7.1) | 31.6 (7.0) | <.001 |
| Blood pressure, mm Hg | ||||||
| Systolic | 108.0 (11.0) | 110.1 (12.5) | 113.7 (12.2) | 114.5 (12.8) | 120.6 (15.1) | <.001 |
| Diastolic | 71.1 (9.0) | 72.7 (10.3) | 74.5 (10.2) | 76.1 (10.7) | 81.6 (11.6) | <.001 |
| Antihypertensive medication use, No. (%) | 26 (1.5) | 17 (2.5) | 10 (8.6) | 5 (6) | 8 (19) | <.001 |
| Diabetes, No. (%) | 33 (1.9) | 23 (3.4) | 10 (8.6) | 11 (13) | 7 (16) | <.001 |
| Smoking status, No. (%) | ||||||
| Current | 310 (18.0) | 131 (19.2) | 25 (21.6) | 30 (34) | 16 (37) | .002 |
| Former | 318 (18.5) | 119 (17.5) | 18 (15.5) | 15 (17) | 4 (9) | |
| Never | 1090 (63.5) | 432 (63.3) | 73 (62.9) | 43 (49) | 23 (545) | |
| Years of education, y | 15.1 (2.5) | 14.7 (2.5) | 13.9 (2.7) | 14.2 (2.4) | 13.5 (2.4) | <.001 |
| Total cholesterol, mg/dL | 176.4 (33.2) | 178.7 (33.2) | 182.4 (37.4) | 179.8 (34.0) | 185.9 (36.0) | .08 |
| LDL-C | 108.9 (30.6) | 108.7 (31.4) | 112.6 (34.3) | 109.4 (30.2) | 115.1 (34.0) | .53 |
| HDL-C | 50.5 (13.3) | 51.6 (14.6) | 47.6 (13.9) | 45.6 (13.3) | 43.4 (13.6) | <.001 |
| Creatinine level, mg/dL | 0.86 (0.16) | 0.79 (0.16) | 0.85 (0.18) | 0.88 (0.21) | 0.91 (0.21) | <.001 |
| UACR, median (IQR), mg/g | 3.3 (2.4-4.4) | 5.8 (4.0-8.9) | 14.0 (8.6-35.0) | 6.2 (4.3-9.7) | 57.3 (22.5-206.2) | <.001 |
Abbreviations: BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; UACR, urine albumin-to-creatinine ratio.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Calculated as weight in kilograms divided by height in meters squared.
Trajectory of Albuminuria and Myocardial Structure and Function
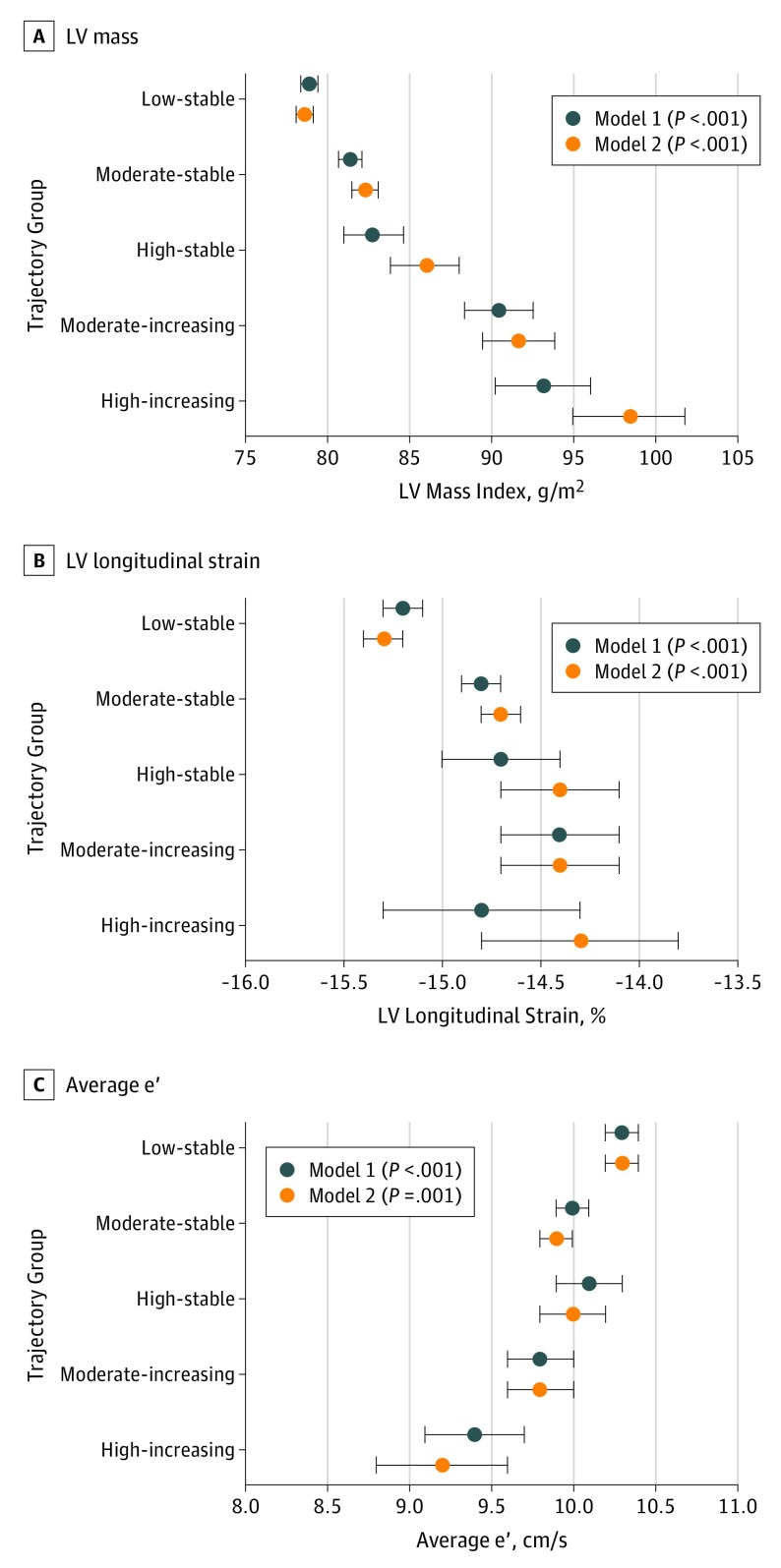
Values of myocardial structure and function, after adjustment for demographic information and clinical covariates, are shown in Table 2 and Figure 2, stratified by UACR trajectory group. The moderate-increasing and high-increasing groups were associated with higher indexed LV mass and indexed LA volumes. There was no association of trajectory of albuminuria with LV end diastolic dimension. While there was no significant association of trajectory group with LV ejection fraction, moderate-increasing and high-increasing trajectories were associated with reduced LV longitudinal strain values (Table 2) (Figure 2). Average e′ velocities were lower and E/e′ measurements were significantly higher among the moderate-increasing and high-increasing UACR groups. There were no significant associations of albuminuria trajectory groups with E/A ratio or circumferential strain. After further adjustment for cumulative systolic blood pressure or cumulative years of diabetes, the association of trajectory group with indices of cardiac structure and function were similar. The association of trajectory group with LV mass, LA volume, e′ velocity, E/e′ ratio, and LV longitudinal strain remained significant on further adjustment for log-transformed year 10 UACR level in addition to demographic and clinical variables (Table 2) (Figure 2). In sensitivity analyses, the association of trajectory group with all echocardiographic outcomes were generally consistent (eTable 3 in the Supplement).
Table 2. Association of Albuminuria Trajectory With Indices of Myocardial Structure and Function Adjusting for Clinical Variables and Baseline Urine Albumin-to-Creatinine Ratio.
| Echocardiographic Variable | Trajectory Group, Adjusted Least Squares Mean (SE) | P Value | ||||
|---|---|---|---|---|---|---|
| Low-Stable (n = 1718) | Moderate-Stable (n = 682) | High-Stable (n = 116) | Moderate-Increasing (n = 88) | High-Increasing (n = 43) | ||
| Myocardial Structure | ||||||
| LV mass indexed, g/m2 | ||||||
| Model 1a | 78.9 (0.5) | 81.4 (0.7) | 82.8 (1.8) | 90.5 (2.1) | 93.2 (2.9) | <.001 |
| Model 2b | 78.6 (0.5) | 82.3 (0.8) | 86.0 (2.1) | 91.7 (2.2) | 98.4 (3.4) | <.001 |
| LV diastolic dimension indexed, cm/m2 | ||||||
| Model 1a | 2.49 (0.01) | 2.50 (0.01) | 2.55 (0.03) | 2.47 (0.03) | 2.58 (0.05) | .05 |
| Model 2b | 2.48 (0.01) | 2.50 (0.01) | 2.54 (0.03) | 2.47 (0.03) | 2.59 (0.05) | .18 |
| LA volume indexed, mL/m2 | ||||||
| Model 1a | 25.3 (0.2) | 25.5 (0.3) | 26.2 (0.7) | 28.3 (0.8) | 29.0 (1.1) | <.001 |
| Model 2b | 25.4 (0.2) | 25.6 (0.3) | 26.4 (0.8) | 28.5 (0.8) | 29.3 (1.3) | <.001 |
| Myocardial Systolic Function | ||||||
| LVEF, % | ||||||
| Model 1a | 59.9 (0.1) | 59.4 (0.2) | 59.1 (0.5) | 59.8 (0.6) | 59.0 (0.9) | .30 |
| Model 2b | 59.8 (0.2) | 59.4 (0.2) | 58.7 (0.6) | 59.8 (0.6) | 58.9 (1.0) | .42 |
| LV, % | ||||||
| Longitudinal strain | ||||||
| Model 1a | −15.2 (0.1) | −14.8 (0.1) | −14.7 (0.3) | −14.4 (0.3) | −14.8 (0.5) | <.001 |
| Model 2b | −15.3 (0.1) | −14.7 (0.1) | −14.4 (0.3) | −14.4 (0.3) | −14.3 (0.5) | <.001 |
| Circumferential strain | ||||||
| Model 1a | −14.5 (0.1) | −14.3 (0.1) | −13.8 (0.4) | −14.4 (0.4) | −14.3 (0.6) | .31 |
| Model 2b | −14.6 (0.1) | −14.3 (0.2) | −13.6 (0.4) | −14.4 (0.4) | −14.2 (0.7) | .29 |
| Myocardial Diastolic Function | ||||||
| E/A | ||||||
| Model 1a | 1.20 (0.01) | 1.16 (0.01) | 1.15 (0.03) | 1.18 (0.04) | 1.22 (0.05) | .14 |
| Model 2b | 1.19 (0.01) | 1.17 (0.01) | 1.14 (0.04) | 1.19 (0.04) | 1.23 (0.06) | .32 |
| Average e′, cm/s | ||||||
| Model 1a | 10.3 (0.1) | 10.0 (0.1) | 10.1 (0.2) | 9.8 (0.2) | 9.4 (0.3) | <.001 |
| Model 2b | 10.3 (0.1) | 9.9 (0.1) | 10.0 (0.2) | 9.8 (0.2) | 9.2 (0.4) | .001 |
| E/e′ | ||||||
| Model 1a | 7.8 (0.1) | 8.2 (0.1) | 8.0 (0.2) | 8.9 (0.2) | 9.6 (0.3) | <.001 |
| Model 2b | 7.7 (0.1) | 8.3 (0.1) | 8.3 (0.2) | 9.1 (0.3) | 10.3 (0.4) | <.001 |
Abbreviations: LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction.
Adjusted for age, race, sex, average systolic blood pressure, antihypertensive medication use, total cholesterol level, diabetes, number of cigarettes per day, body mass index, creatinine level, field center, and image quality score.
Adjusted for model 1 variables and log-transformed year 10 urine albumin-to-creatinine ratio.
Figure 2. Association of Albuminuria Trajectory Groups With Indices of Myocardial Structure and Function.
A, Association of trajectory group with left ventricular (LV) mass, a representative metric for LV structure. B, Association of trajectory group with LV longitudinal strain, a representative metric for LV systolic function. C, Association of trajectory group with average e′, a representative metric for LV diastolic function. Model 1 adjusted for age, race, sex, average systolic blood pressure, antihypertensive medication use, total cholesterol level, diabetes, number of cigarettes per day, body mass index, creatinine level, field center, and image quality score. Model 2 adjusted for the model 1 variables as well as log-transformed year 10 urine albumin-to-creatinine ratio levels. Data points indicate adjusted least square means, and error bars indicate SEs.
Values of myocardial structure and function by trajectory group after adjustment for demographic characteristics, clinical covariates, and cumulative albuminuria levels are shown in Table 3. Association of trajectory group with indices of myocardial structure, LV systolic function, and LV diastolic function remained significant after adjustment for cumulative albuminuria level. Additionally, cumulative albuminuria level was significantly associated with worse LV mass, LV longitudinal strain, average e′ velocity, and E/e′.
Table 3. Association of Albuminuria Trajectory With Indices of Myocardial Structure and Function Adjusting for Cumulative Urine Albumin-to-Creatinine Ratio.
| Echocardiographic Variable | Trajectory Group, Adjusted Least Squares Mean (SE) | P Valuea | ||||
|---|---|---|---|---|---|---|
| Low-Stable (n = 1718) | Moderate-Stable (n = 682) | High-Stable (n = 116) | Moderate-Increasing (n = 88) | High-Increasing (n = 43) | ||
| Myocardial structure | ||||||
| LV mass indexed, g/m2 | 78.8 (0.5) | 81.7 (0.8) | 82.8 (2.1) | 91.2 (2.3) | 91.9 (4.3) | <.001 |
| LV diastolic dimension indexed, cm/m2 | 2.48 (0.01) | 2.51 (0.01) | 2.53 (0.03) | 2.46 (0.04) | 2.53 (0.07) | .37 |
| LA volume indexed, mL/m2 | 25.3 (0.2) | 25.6 (0.3) | 26.4 (0.8) | 28.6 (0.9) | 27.9 (1.6) | .005 |
| Myocardial systolic function | ||||||
| LVEF, % | 59.8 (0.2) | 59.3 (0.2) | 58.8 (0.6) | 60.7 (0.7) | 59.8 (1.3) | .11 |
| LV, % | ||||||
| Longitudinal strain | −15.2 (0.1) | −14.8 (0.1) | −14.6 (0.3) | −14.3 (0.3) | −14.5 (0.7) | .009 |
| Circumferential strain | −14.6 (0.1) | −14.3 (0.2) | −13.5 (0.4) | −14.6 (0.5) | −13.8 (1.0) | .15 |
| Myocardial diastolic function | ||||||
| E/A | 1.19 (0.01) | 1.17 (0.01) | 1.19 (0.04) | 1.16 (0.04) | 1.32 (0.08) | .20 |
| Average e′, cm/s | 10.3 (0.1) | 10.0 (0.1) | 10.2 (0.2) | 9.9 (0.2) | 9.2 (0.5) | .005 |
| E/e′ | 7.8 (0.1) | 8.2 (0.1) | 8.0 (0.2) | 8.9 (0.3) | 9.7 (0.5) | <.001 |
Abbreviations: LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction.
Adjusted for age, race, sex, average systolic blood pressure, antihypertensive medication use, total cholesterol level, diabetes, number of cigarettes per day, body mass index, creatinine level, field center, image quality score, and cumulative albuminuria.
Discussion
In this longitudinal analysis of a comprehensively phenotyped cohort, we describe the heterogeneous patterns of change in albuminuria over a 20-year span through young adulthood for the first time, to our knowledge. We identified 5 distinct trajectories of UACR that were independently associated with adverse myocardial remodeling with regard to cardiac structure, LV systolic function, and LV diastolic function. Specifically, the group of participants in the CARDIA study with dynamic, rapid increase in albuminuria, about 4% to 5% of the cohort, demonstrated the highest degree of maladaptive myocardial remodeling by year 30 echocardiography compared with those with stable levels of UACR. Notably, trajectory groups were not readily identifiable at the time of baseline UACR measurement: 4 of 5 groups had baseline UACR levels less than the criteria for microalbuminuria. Trajectory of albuminuria was associated with adverse remodeling independent of baseline UACR level.
Albuminuria, a marker of systemic endothelial dysfunction,19 is most commonly measured through spot UACR level. While 24-hour urine collection is considered the criterion standard for albuminuria quantification, spot metrics are strongly correlated with 24-hour urine measurements20 and are associated with CVD. To date, descriptions of the relationship between albuminuria and clinical and subclinical CVD have relied on single values of UACR. Through serial UACR measurements, we identified 5 distinct trajectory groups that could not be readily identified based on a single, baseline UACR value, and each group carried varying degrees of risk for adverse myocardial remodeling.
Albuminuria has been linked to adverse myocardial remodeling in cross-sectional analyses. Among a cohort of hypertensive and normotensive participants, UACR levels were associated with higher LV mass and lower LV longitudinal strain.7,9 The cross-sectional nature of prior investigations limits the ability to understand the temporal association of albuminuria with myocardial remodeling. Our findings demonstrate that albuminuria in early life and, importantly, its pattern of change over time are associated with global cardiac remodeling. Both dynamic increases in previously low levels of UACR and longstanding and continued elevations in UACR levels were associated with significant LV remodeling.
The association of trajectory of albuminuria with cardiac remodeling were independent of baseline UACR level, providing further evidence that cardiovascular risk cannot be readily identified at the time of initial UACR level measurement and that such risk groups differentiate during follow-up. The association of albuminuria trajectory with indices of cardiac remodeling were independent of cumulative exposure of blood pressure or diabetes, indicating that UACR level may not only reflect risk factor burden. The association of albuminuria trajectory group with worse echocardiographic outcomes remained significant after adjustment for cumulative albuminuria, suggesting that the pattern of change in UACR is independently associated with myocardial remodeling. In aggregate, our findings suggest a role for longitudinal measurement of UACR level among individuals at high risk for CVD, as identification of elevated UACR levels and attendant changes in myocardial structure and function at a later time point, rather than during antecedent years, may ultimately be too late to prevent adverse outcomes or improve myocardial factors that put one at risk for CVD events. Indeed, participants with controlled blood pressure after medical therapy had higher LV mass than those with ideal blood pressure, suggesting that LV remodeling may be difficult to reverse once present.21
Longitudinal measurement of UACR level to identify trajectories of change is relatively straightforward to implement and may carry important clinical implications. The electronic medical record allows for rapid integration of data across multiple time points. As such, it is feasible for a clinician to graph trends in UACR level to identify high-risk individuals who behave similarly to the moderate-increasing and high-increasing groups that we observed. Such patients may benefit from (1) earlier and more frequent screening for diabetes and (2) aggressive risk factor control (eg, blood pressure management, smoking cessation, weight loss). The clinical implications of UACR trajectory groups may be particularly important for CVD prevention in the setting of diabetes. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, has demonstrated reduction in clinically important cardiac and renal events among patients with diabetes and albuminuric kidney disease (UACR >300 mg/g).22 Recently, among 8000 patients with diabetes, UACR level was identified as 1 of only 5 variables predictive of HF hospitalizations.23 Indeed, UACR level, even in the microalbuminuric range, was incorporated in a novel risk score for incident HF hospitalizations.23 Notably, patients with higher risk scores were more likely to derive cardiovascular benefit from the sodium-glucose cotransporter 2 inhibitor dapagliflozin.23 Indeed, even among those with prevalent HF but without diabetes, dapagliflozin has demonstrated cardiovascular benefit.24 These findings highlight the clinical importance of measuring UACR levels longitudinally across the life course of diabetes, as increases in UACR, even into the microalbuminuric range, identify patients at higher risk for CVD who may benefit from initiation of specific therapies.
The mechanisms by which albuminuria may result in cardiac remodeling are currently unclear. It is possible that endothelial dysfunction, for which UACR level is a marker, has effects on the myocardium. If so, the subendocardial layer would be most vulnerable to endothelial dysfunction given its reliance on the microvasculature. Longitudinal strain, a sensitive metric of systolic function of the endocardium,25 was significantly worse in those with dynamic albuminuria trajectories, although differences between groups were relatively small. This finding suggests the potential negative effect of endothelial dysfunction, leading to deterioration of subendocardial cardiac function. Our findings may be particularly relevant to the development of HF with preserved ejection fraction, a disease state highlighted by endothelial dysfunction. Urine albumin-to-creatinine ratio has been associated with coronary microvascular dysfunction in HF with preserved ejection fraction.26 Further investigation is required to evaluate if UACR levels and their patterns of change are associated with microvascular dysfunction and incident HF with preserved ejection fraction.
Strengths and Limitations
Our study has strengths and limitations. The CARDIA study represents a biracial cohort that has been phenotyped over 9 examinations, allowing for the examination of patterns of albuminuria. While 24-hour urine assessment is the criterion standard for albuminuria, it was quantified by single UACR levels in the CARDIA study, which exhibits strong correlation when measured repeatedly and is representative of 24-hour urine levels.20 Echocardiographic imaging was performed across the 4 field centers and is subject to variability in image quality, which may confound the association of albuminuria with echocardiographic indices. Such variability would likely have attenuated these associations, and we adjusted for image quality and field center. Given the age of CARDIA study participants, the number of incident HF events was low, limiting the ability to evaluate the association of albuminuria trajectory with clinical outcomes. While excluded CARDIA study participants were more likely black, of lower education level, and had a higher comorbidity burden, our participant sample still represented a diverse cohort of a wide spectrum of cardiovascular risk factors, socioeconomic status, and race. The exclusion of these high-risk participants would likely bias toward the null, suggesting the association of UACR trajectory with myocardial structure and function may be underestimated. Finally, sensitivity analyses were consistent with the overall results.
Conclusions
In this analysis of a diverse cohort of young adults, we identified 5 distinct trajectories of albuminuria. Participants could not be readily categorized into their trajectory groups on the basis of their baseline UACR level, and 4 of 5 groups had UACR levels less than the clinical threshold of microalbuminuria. We identified 2 high-risk groups composed of select participants that demonstrated a dynamic rise in albuminuria levels over time. The trajectory of albuminuria from young adulthood to middle age was associated with adverse remodeling with respect to myocardial structure, LV systolic function, and LV diastolic function, independent of baseline UACR level or cumulative degree of albuminuria. Trajectory of albuminuria may provide additional insight into risk of subclinical CVD and offer early identification of high-risk cohorts with dynamic rises in albuminuria who may benefit from aggressive risk factor modification.
eTable 1. Urine albumin-to-creatinine ratio values at examination visits by albuminuria trajectory group.
eTable 2. Characteristics of CARDIA participants at year 0 examination stratified by inclusion in UACR trajectory analysis.
eTable 3. Sensitivity analyses accounting for participants with missing year 30 data: association of UACR trajectory with cardiac structure and function.
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073-2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstein HC, Mann JF, Yi Q, et al. ; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421-426. doi: 10.1001/jama.286.4.421 [DOI] [PubMed] [Google Scholar]
- 3.Ingelsson E, Sundström J, Lind L, et al. Low-grade albuminuria and the incidence of heart failure in a community-based cohort of elderly men. Eur Heart J. 2007;28(14):1739-1745. doi: 10.1093/eurheartj/ehm130 [DOI] [PubMed] [Google Scholar]
- 4.Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969-975. doi: 10.1161/CIRCULATIONAHA.105.538132 [DOI] [PubMed] [Google Scholar]
- 5.Hillege HL, Fidler V, Diercks GF, et al. ; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group . Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777-1782. doi: 10.1161/01.CIR.0000031732.78052.81 [DOI] [PubMed] [Google Scholar]
- 6.Murtaugh MA, Jacobs DR Jr, Yu X, Gross MD, Steffes M; Coronary Artery Risk Development in Young Adults Study . Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2003;158(7):676-686. doi: 10.1093/aje/kwg208 [DOI] [PubMed] [Google Scholar]
- 7.Djoussé L, Kochar J, Hunt SC, et al. Relation of albuminuria to left ventricular mass (from the HyperGEN Study). Am J Cardiol. 2008;101(2):212-216. doi: 10.1016/j.amjcard.2007.07.065 [DOI] [PubMed] [Google Scholar]
- 8.Shah AM, Lam CS, Cheng S, et al. The relationship between renal impairment and left ventricular structure, function, and ventricular-arterial interaction in hypertension. J Hypertens. 2011;29(9):1829-1836. doi: 10.1097/HJH.0b013e32834a4d38 [DOI] [PubMed] [Google Scholar]
- 9.Katz DH, Selvaraj S, Aguilar FG, et al. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the Hypertension Genetic Epidemiology Network (HyperGEN) Study. Circulation. 2014;129(1):42-50. doi: 10.1161/CIRCULATIONAHA.113.003429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer H, Jacobs DR Jr, Bild D, et al. ; The Multi-Ethnic Study of Atherosclerosis . Urine albumin excretion and subclinical cardiovascular disease. Hypertension. 2005;46(1):38-43. doi: 10.1161/01.HYP.0000171189.48911.18 [DOI] [PubMed] [Google Scholar]
- 11.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AC, Ricketts EP, Cox C, et al. Quality control and reproducibility in M-mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography. 2015;32(8):1233-1240. doi: 10.1111/echo.12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450-458. doi: 10.1016/0002-9149(86)90771-X [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109-138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 17.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015-2023. doi: 10.1177/0962280216673085 [DOI] [PubMed] [Google Scholar]
- 18.Nagin DS, Odgers CL. Group-based trajectory modeling (nearly) two decades later. J Quant Criminol. 2010;26(4):445-453. doi: 10.1007/s10940-010-9113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340(8815):319-323. doi: 10.1016/0140-6736(92)91401-S [DOI] [PubMed] [Google Scholar]
- 20.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10(4):414-418. doi: 10.2337/diacare.10.4.414 [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Colangelo LA, Daviglus ML, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2015;4(9):e002275. doi: 10.1161/JAHA.115.002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 23.Berg DD, Wiviott SD, Scirica BM, et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus [published online August 31, 2019]. Circulation. doi: 10.1161/CIRCULATIONAHA.119.042685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction [published online September 19, 2019]. N Engl J Med. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 25.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351-369. doi: 10.1016/j.echo.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 26.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39(37):3439-3450. doi: 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Urine albumin-to-creatinine ratio values at examination visits by albuminuria trajectory group.
eTable 2. Characteristics of CARDIA participants at year 0 examination stratified by inclusion in UACR trajectory analysis.
eTable 3. Sensitivity analyses accounting for participants with missing year 30 data: association of UACR trajectory with cardiac structure and function.