This cohort study examines data from the Swedish family-cancer data sets for family history, relative’s age at diagnosis, and other factors to identify the appropriate age for breast cancer screening.
Key Points
Question
At what age should women with differing family histories of breast cancer start screening?
Findings
In this nationwide cohort study of 5 099 172 women in Sweden, risk-adapted starting age of breast cancer screening varied by the number of affected first- or second-degree relatives and by age at diagnosis of first-degree relatives. For example, when mass screening was recommended at age 50 years, women with multiple affected first-degree relatives reached the screening risk level at age 27 to 36 years, depending on the youngest age at diagnosis in relatives.
Meaning
This study identifies risk-based starting ages for breast cancer screening that may provide guidance to clinicians and relatives of patients with breast cancer on when to start risk-adapted screening.
Abstract
Importance
Breast cancer screening guidelines acknowledge the need for earlier screening for women at increased risk but provide limited guidance for women with a family history of breast cancer. A risk-adapted starting age of screening for relatives of patients with breast cancer may help supplement current screening guidelines.
Objective
To identify the risk-adapted starting age of breast cancer screening on the basis of a woman’s detailed family history.
Design, Setting, and Participants
This nationwide cohort study analyzed data recorded in the Swedish family-cancer data sets. All women born from 1932 onward and with at least 1 known first-degree relative (FDR) were included (N = 5 099 172). Data from January 1, 1958, to December 31, 2015, were collected. Data were analyzed from October 1, 2017, to March 31, 2019.
Exposures
Family history of breast cancer in FDRs and second-degree relatives (SDRs).
Main Outcomes and Measures
Primary invasive breast cancer diagnosis and the age at which women with different constellations of family history attained the risk level at which breast screening is usually recommended.
Results
Of the 5 099 172 women included in the study, 118 953 (2.3%) received a diagnosis of primary invasive breast cancer. A total of 102 751 women (86.4%; mean [SD] age at diagnosis, 55.9 [11.1] years) did not have family history of breast cancer in FDRs and SDRs at the time of their diagnosis. Risk-adapted starting age of breast cancer screening varied by number of FDRs and SDRs with breast cancer diagnosis and the age at diagnosis of the FDRs. For example, for screening recommendation at age 50 years for the general population (2.2% 10-year cumulative risk), women with multiple affected FDRs, with the youngest affected relative receiving a diagnosis before age 50 years, reached the benchmark risk level at age 27 years. When the youngest relative received a diagnosis after age 50 years, however, this risk level was attained at age 36 years.
Conclusions and Relevance
This study identifies possible risk-based starting ages for breast cancer screening based on population-based registers. These results may serve as high-quality evidence to supplement current screening guidelines for relatives of patients with breast cancer.
Introduction
Breast cancer is a major cause of morbidity and mortality in women worldwide,1,2 accounting for one-quarter of all new cancer cases and 15% of all cancer deaths in women.3 Family history is a nonmodifiable risk factor for the disease.4 Breast cancer risk associated with family history varies with age of the individual, nature and number of affected relatives, and age at which the relative received a diagnosis.5,6,7 Family history of breast cancer among the extended family, such as second-degree relatives (SDRs), has also been reported as a major factor in breast cancer risk and should be considered in risk assessment.8
Early detection of breast tumors through screening can reduce breast cancer mortality.9,10,11 Reductions in breast cancer mortality in Europe over the past 2 decades have been associated at least in part with the implementation of screening programs.12,13 Available evidence suggests that implementation of a screening program can decrease breast cancer mortality by up to 20%.10 Screening enables the detection of tumors at an early stage, when more treatment options are feasible and most effective. However, screening is associated with substantial risks, such as overdiagnosis, false-positive results, and physical and psychological harms,9,11,14,15 particularly when large numbers of women with low risk are frequently screened.
Many national screening programs have adopted a one-size-fits-all approach, advising women aged 50 to 69 years to obtain a biennial mammogram.16,17,18 Similarly, most breast cancer screening guidelines recommend a general starting age for screening for all women, even those at increased risk, such as those with family history of breast cancer. Existing guidelines for women at increased risk, such as the American Cancer Society guidelines that recommend initiation of breast cancer screening at age 40 years or 10 years earlier than the youngest relative with a diagnosis, are largely based on expert opinion rather than empirical evidence. Because age-oriented screening suggestions pay limited attention to individual risks based on known risk factors, the approach is considered suboptimal, and personalized screening has been advocated.9,19,20,21 Although many risk assessment models and tools have been developed,22 none of them provide the evidence-based risk-adapted starting age of screening for women with different constellations of family history.
This cohort study used, to our knowledge, the world’s largest family-cancer data set to assess the absolute familial risks associated with the age at diagnosis and number of first-degree relatives (FDRs) and SDRs. The goal was to establish a risk-adapted starting age of breast cancer screening.
Methods
This study received approval from the Lund Regional Ethics Committee. Informed consent was waived because of the use of deidentified (pseudonymized) data. Data from January 1, 1958, to December 31, 2015, were collected. Data were analyzed from October 1, 2017, to March 31, 2019.
We used the Swedish family-cancer data sets, which link all records from the Swedish Multi-generation Register, Swedish Cancer Registry (incident cancers since 1958), national censuses (socioeconomic data), and Swedish Cause of Death Register.23 The combined data sets include all Swedish residents born after 1931 (offspring generation) linked to their biological parents (parental generation). The latest (2017) data set contained the records of 7.9 million women, including 6.3 million with at least 1 known FDR.23 To minimize cohort and period effects on the results, we restricted the current analysis to only the offspring generation. In this study, we included 5 099 172 women with at least 1 known FDR and born from 1932 onward. The study data set included cancers diagnosed through December 31, 2015. Cancer data were classified according to codes from the International Classification of Disease, Seventh Revision (or later revisions in recent decades). We used the 3-digit code 170 to identify all patients with invasive breast cancer.
For all individuals in the study, we identified family history of breast cancer (diagnosis among FDRs and SDRs), age at diagnosis of the relatives, and type of relationship. The number of affected relatives was included as a dynamic variable to establish the family history of every participant at entry into the study and the changing family history every time a new family member received a diagnosis. Similarly, for women with multiple affected FDRs, the dynamic nature of the age of the youngest relative with a diagnosis was taken into account, allowing for variation throughout the follow-up period whenever a subsequent affected FDR received a diagnosis at a younger age.
Only primary invasive breast cancers were considered in the current study. The start of follow-up was defined as the year of birth, year of the Swedish Cancer Registry initiation (1958), or year of immigration, whichever occurred latest. The end of follow-up was at the year of breast cancer diagnosis, emigration, death, or December 31, 2015, whichever occurred first.
Risk-adapted starting age of screening was defined as the age at which women with a particular family history of breast cancer attained a similar level of 10-year cumulative risk to the threshold level at which women in the general population are usually advised to initiate screening. The 10-year cumulative risk for the different constellations of breast cancer family history and earliest age at diagnosis of the affected relatives was calculated as follows: Annual incidence rate at age X = Number of cases at age X / Person-years at age X. The 10-year cumulative incidence rate was calculated as a sum of 10 consecutive age-specific annual incidence rates and was converted into a 10-year cumulative risk using the following formula: 10-year cumulative risk = 1-e−(10-year cumulative rate). Ten-year cumulative risk curves were used to determine the 10-year cumulative risk for women in the general population at benchmark ages of 40, 45, and 50 years. Corresponding ages at which women with different family history attained the same level of risk were identified as the ages at which the cumulative risk curves crossed the established 10-year cumulative risk for the general population. A 2-fold cross-validation method for internal validation of the cumulative incidence rates for different constellations of family history is presented in eMethods 1 in the Supplement.
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc). Curves were plotted using Excel 2010 (Microsoft Corp).
Results
A total of 118 953 women received a diagnosis of invasive breast cancer as the first primary tumor during the study period. Of this total, 102 751 women (86.4%; mean [SD] age at diagnosis, 55.9 [11.1] years) did not have family history of breast cancer in both FDRs and SDRs. The risk of developing breast cancer in the next 10 years for women in the general population was 1.1% at age 40 years, 1.8% at age 45 years, and 2.2% at age 50 years. The lifetime (0-79 years) risk for 16 202 women (13.6%) who had a family history of breast cancer ranged from 11% to 23%. The lifetime risk was 9.4% for 102 571 women (86.4%) without family history.
Number of Affected FDRs and SDRs
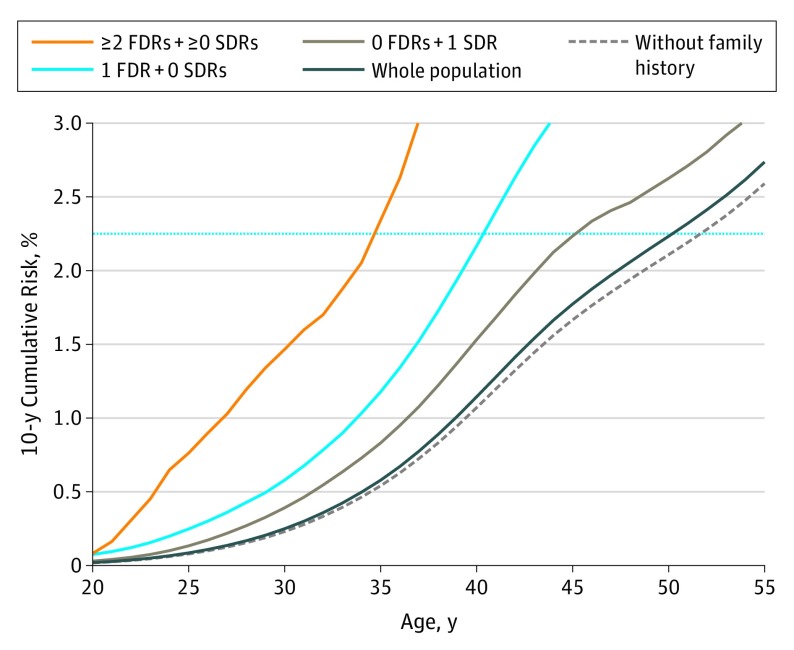
The age at which women attained risk equal to that of women with average risk at ages 40, 45, and 50 years in the general population varied according to the number of affected FDRs and SDRs (Table 1). For example, when screening is recommended at age 50 years for the general population (10-year cumulative risk of 2.2%), women with only 1 affected SDR and no affected FDR attained a similar risk level at age 45 years (Figure 1 and Table 1). For these women, there was no clinically relevant difference in the risk-adapted starting age of screening whether the affected SDR was from the maternal or the paternal side (eFigure 2 in the Supplement). Women with multiple affected SDRs and no affected FDRs reached similar risk level at age 41 years, 9 years earlier than the general recommendation (Table 1). Similarly, women with 1 affected FDR and no family history in SDRs reached a 10-year cumulative risk of 2.2% at age 40 years, whereas those who had 1 affected FDR and additional SDRs with breast cancer reached the same level of risk at age 38 years, and women with multiple affected FDRs either alone or with additional affected SDRs attained 2.2% 10-year cumulative risk at age 35 years.
Table 1. Risk-Adapted Starting Age of Screening by Family History of Breast Cancer and Age at Diagnosis of Youngest Affected First-Degree Relative.
| Family History | Youngest Age at Diagnosis in FDRs, y | No. of Patients | Risk-Adapted Starting Age of Screening, y | ||
|---|---|---|---|---|---|
| Whole population | NA | 118 953 | 40a | 45a | 50a |
| No family history | NA | 102 751 | 41 | 46 | 52 |
| 0 FDR + 1 SDR | NA | 2512 | 37 | 42 | 45 |
| 0 FDR + ≥2 SDRs | NA | 276 | 36 | 39 | 41 |
| 1 FDR + 0 SDR | All ages | 12 075 | 35 | 38 | 40 |
| <40 | 691 | 30 | 34b | 36 | |
| 40-44 | 916 | 32 | 36 | 38 | |
| 45-49 | 1426 | 34 | 37 | 39 | |
| ≥50 | 9042 | 36 | 39 | 41 | |
| 1 FDR + ≥1 SDRs | All ages | 544 | 32 | 36 | 38 |
| <40 | 55 | 23 | 25 | 26 | |
| 40-49 | 123 | 32 | 35 | 37 | |
| ≥50 | 366 | 34 | 37 | 38 | |
| ≥2 FDRs + ≥0 SDRs | All ages | 795 | 28 | 32 | 35 |
| <50 | 449 | 23 | 26 | 27 | |
| ≥50 | 346 | 33 | 35 | 36 | |
| 10-y Cumulative risk in the general population, % | NA | NA | 1.1 | 1.8b | 2.2 |
Abbreviations: FDR, first-degree relative; NA, not applicable; SDR, second-degree relative.
Ages 40, 45, and 50 years are recommended (benchmark) by the guidelines as the starting age of mass screening. Other ages are evidence-based risk-adapted starting age of screening recommended by the present study.
Example: When screening for women with average risk is recommended at age 45 years (10-year cumulative risk of 1.8%), women with 1 FDR who received a diagnosis before age 40 years reached the same level of risk at age 34 years, 11 years earlier than her peers in the general population.
Figure 1. Ten-Year Cumulative Breast Cancer Risk Curves by Number of Affected First-Degree Relatives (FDRs) and Second-Degree Relatives (SDRs) for Women.
The blue horizontal line represents population-level 10-year cumulative risk of 2.2%, when starting mass breast cancer screening is recommended at age 50 years.
Age at Diagnosis of Affected FDR
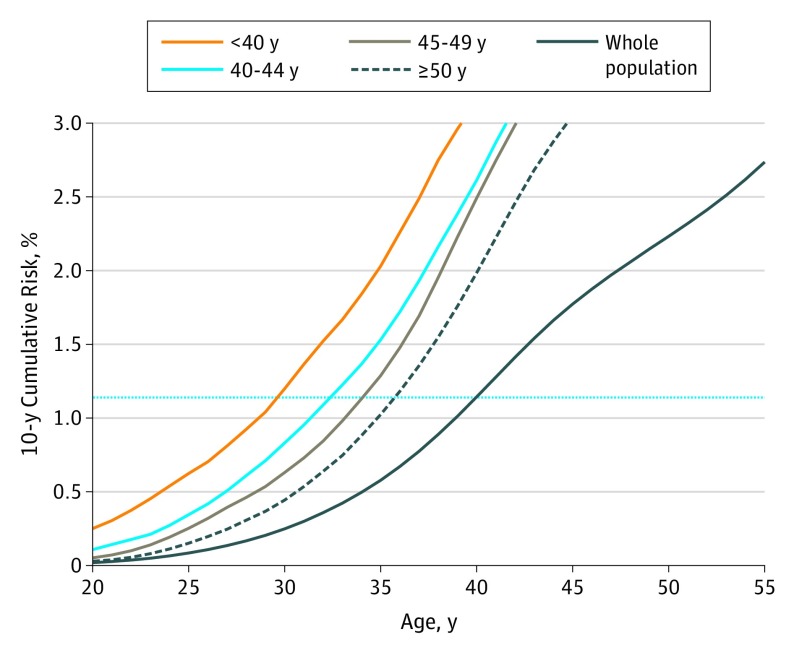
The age at diagnosis of the affected FDR also modified the age at which women with a family history of breast cancer reached a risk level equivalent to the age at which screening would be suggested (Table 1). For example, when screening for women with average risk is recommended at age 40 years (10-year cumulative risk of 1.1%), women with 1 FDR who received a diagnosis before age 40 years reached the same level of risk at age 30 years, but it was at age 32 years for women whose relative received a diagnosis at ages 40 to 44 years and at age 36 years for women with an FDR who received a diagnosis after age 50 years (Figure 2 and Table 1). The association with age at diagnosis of the youngest FDRs with a diagnosis also existed for women with family history of breast cancer in multiple FDRs. For such women, when the youngest affected relative received a diagnosis before age 50 years, they reached a 10-year cumulative risk of 1.1% (screening recommended at age 40 years for those with average risk) at age 23 years, but it was at age 33 years when the youngest relative received a diagnosis after age 50 years. The age at diagnosis of the SDR was not meaningfully associated with the age at which women reached the screening-level risk.
Figure 2. Ten-Year Cumulative Breast Cancer Risk Curves by Age at Diagnosis of First-Degree Relative (FDR) for Women With 1 Affected FDR.
The blue horizontal line is drawn at a 10-year cumulative risk of 1.1% and represents the population-level risk of starting screening at the recommended age of 40 years.
Internal Validation and Sensitivity Analyses
In a 2-fold cross-validation analysis and sensitivity analyses by calendar period, comparable results were found. The 10-year cumulative risk curves for women with 1 FDR or SDR from both the development and validation data sets did not show statistically significant differences, overlapped, and were close to each other across age (eFigure 1 in the Supplement). Similarly, the expected/observed ratios from the comparison of the development and validation sets showed that incidence rates were similar for premenopausal and lifetime breast cancer (all expected/observed ratios were close to 1.00 and their 95% CIs included unity [eTable in the Supplement]). These results are presented in the eTable, eFigure 1, and eMethods 1 and 2 in the Supplement.
Comparison With Current Guidelines
The International Agency for Research on Cancer and the US Preventive Services Task Force recommend that breast cancer screening should start at age 50 years and do not provide detailed guidance for women with different constellations of family history. Thus, under these guidelines, for example, a woman with 1 FDR who received breast cancer diagnosis at age 47 years should start breast cancer screening at age 50 years, which is 11 years later than the evidenced-based starting age of 39 years estimated in this study (Table 2). Compared with the American Cancer Society recommendation to start mass breast cancer screening at age 45 years, the estimated risk-adapted starting age was different by 8 years. In contrast, the difference was small between the risk-adapted starting age and the starting age suggested by the American College of Radiology, which is age 30 years or 10 years earlier than the youngest relative with a diagnosis. The American College of Radiology recommendation is only 4 years earlier than the risk-adapted starting age. The example ages in Table 2 are arbitrary. By selection of other ages at diagnosis in the relatives, the differences could be even larger, ranging from –6 to +24 years, than what was observed.
Table 2. Difference Between Starting Age of Breast Cancer Screening Recommended by Guidelines and Risk-Adapted Starting Age of Screening.
| Family History | Youngest Age at Diagnosis in FDRs, y | Example Age, ya | Patients, No. | Recommended Starting Age, y | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 45 | 50 | ||||||||||
| ACRb | Evidencec | Diffd | ACSb | Evidence | Diffd | USPSTF, IARCb | Evidence | Diffd | ||||
| Whole population | NA | NA | 118 953 | 40 | 40 | 0 | 45 | 45 | 0 | 50 | 50 | 0 |
| No family history | NA | NA | 102 751 | 40 | 41 | −1 | 45 | 46 | −1 | 50 | 52 | −2 |
| 0 FDR + 1 SDR | NA | NA | 2512 | 40 | 37 | 3 | 45 | 42 | 3 | 50 | 45 | 5 |
| 0 FDR + ≥2 SDRs | NA | NA | 276 | 40 | 36 | 4 | 45 | 39 | 6 | 50 | 41 | 9 |
| 1 FDR + 0 SDR | All ages | 50 | 12 075 | 30 | 35 | −5 | 45 | 38 | 7 | 50 | 40 | 10 |
| <40 | 35 | 691 | 30 | 30 | 0 | 45 | 34 | 11 | 50 | 36 | 14 | |
| 40-44 | 43 | 916 | 30 | 32 | −2 | 45 | 36 | 9 | 50 | 38 | 12 | |
| 45-49 | 47 | 1426 | 30 | 34 | −4 | 45 | 37 | 8 | 50 | 39 | 11 | |
| ≥50 | 55 | 9042 | 30 | 36 | −6 | 45 | 39 | 6 | 50 | 41 | 9 | |
| 1 FDR + ≥1 SDRs | All ages | 50 | 544 | 30 | 32 | −2 | 45 | 36 | 9 | 50 | 38 | 12 |
| <40 | 35 | 55 | 25 | 23 | 2 | 45 | 25 | 20 | 50 | 26 | 24 | |
| 40-49 | 45 | 123 | 30 | 32 | −2 | 45 | 35 | 10 | 50 | 37 | 13 | |
| ≥50 | 55 | 366 | 30 | 34 | −4 | 45 | 37 | 8 | 50 | 38 | 12 | |
| ≥2 FDRs + ≥0 SDRs | All ages | 50 | 795 | 30 | 28 | 2 | 45 | 32 | 13 | 50 | 35 | 15 |
| <50 | 45 | 449 | 30 | 23 | 7 | 45 | 26 | 19 | 50 | 27 | 23 | |
| ≥50 | 55 | 346 | 30 | 33 | −3 | 45 | 35 | 10 | 50 | 36 | 14 | |
Abbreviations: ACR, American College of Radiology; ACS, American Cancer Society; Diff, difference; FDR, first-degree relative; IARC, International Agency for Research on Cancer; NA, not applicable; SDR, second-degree relative; USPSTF, US Preventive Services Task Force.
Example of a possible youngest age at diagnosis of affected FDR.
Starting age of screening per screening guideline.
Evidence-based risk-adapted starting age of screening per the present study.
Diff: Age recommended by guidelines minus risk-adapted starting age per the present study. The difference indicates how many years earlier (denoted without minus sign) or later (denoted with minus sign) the screening should be done compared with the general recommended starting age of screening.
Discussion
The principle behind tailoring screening to individuals is to ensure screening begins for each woman at the age at which breast cancer risk is equal to the average risk at which screening is suggested. In this way, women with higher than average risk can begin screening at an earlier age.19,24,25 Using the world’s largest nationwide family-cancer data sets from Sweden, we found that the population-level 10-year cumulative risks at ages at which most breast cancer guidelines advise women to start screening were 1.1% at 40 years, 1.8% at 45 years, and 2.2% at 50 years. Using these constant population-level risks as benchmarks, we identified the evidence-based ages at which women with defined family history of breast cancer (also taking into account family history in SDRs and relative’s age at diagnosis) attained risk levels similar to those at which screening is suggested. For example, we found that when screening is recommended at age 50 years, women with 1 FDR who received a diagnosis before age 40 years attained population-level risk at age 36 years and could be counseled to start screening 14 years earlier than the general starting age recommendation.
Breast cancer screening guidelines and professional bodies recommend different starting ages of mammography screening for women with average risk. The recommended starting age is 40 years from the Society of Breast Imaging and the American College of Radiology,26 45 years from the American Cancer Society,27 and 50 years from the US Preventive Services Task Force and most European countries.17,28 Many guidelines also recognize the need for earlier initiation of screening for women with higher risk owing to family history, albeit with limited detail. We have identified the equivalent ages at which women with different constellations of family history of breast cancer attained 10-year cumulative risk similar to that of women with average risk at the age at which initiating screening is suggested, hence providing evidence-based risk-adapted starting ages of screening for women with family history. We believe this information is clinically useful for counseling women with particular constellations of family history, including family history in extended relatives or for whom current guidelines advise individualized decision making.
These risk-adapted starting ages of screening are not intended as an independent risk assessment model but rather as a supplement to available tools and a practical guide for women and clinicians in communicating risk and making decisions about starting screening. The suggested starting age of screening ranged from 27 to 45 years when the general population recommendation was at 50 years and from 23 to 37 years when the recommendation was at 40 years.
Screening young women at increased risks presents both opportunities and challenges. Tumors in young women tend to be biologically more aggressive and are associated with poor survival,29 and thus early detection through screening has the potential to be associated with improved outcomes. However, because young women generally have dense breasts, screening mammography does not perform well.30 Moreover, young women are more likely to be harmed by screening compared with older women.31 For these reasons, digital breast tomosynthesis and ultrasonography have been suggested for routine screening in young women with increased risk beginning at age 30 years and breast magnetic resonance imaging for women with increased risk aged 25 to 30 years.25,30 The suggested risk-adapted starting ages are independent of the screening modalities, although the implementation of suggested risk-adapted starting ages of screening in clinical practice should aim to maximize benefits while minimizing harms. This concern is expected to ease as more efficient screening modalities for young women become available.
Another relevant observation from this study was that women with no family history of breast cancer in both FDRs and SDRs attained screening-level risk at age 52 years and could thus be counseled to postpone the routine screening by 2 years. Most women (86.4% in this study) have no family history, and postponing screening for this group might represent substantial reductions in otherwise unnecessary mammograms and cost savings required for earlier screening initiation in high-risk women. A simulation analysis by the Independent UK Panel on Breast Cancer Screening suggests that such a delayed screening among low-risk women would still maintain reduction in mortality.32 More studies are needed to investigate the effectiveness of less screening than currently recommended for women with low risk of breast cancer and the cost-effectiveness of earlier initiation of screening for women with high risk owing to family history of breast cancer.
The 10-year cumulative risk thresholds used to identify the risk-adapted starting ages of screening were based on breast cancer incidence rates in Sweden. We used a 2-independent-sample cross-validation method using 50% of the study population. We demonstrated that the distribution of cases and the cumulative risk were comparable in the development and validation data sets across constellations of family history and that the 10-year cumulative risk curves as well as the expected/observed ratios were comparable, and thus the incidence rates were internally validated. Although the incidence rate of breast cancer varies by geography and race/ethnicity, the familial risks of cancers are generalizable across populations with approximately similar cancer incidence and pattern. Thus, the risk advancement time (the difference between the age at which the whole population and the age at which women with a particular family history constellation attain screening-level risk) is likely to be similarly generalizable. Nevertheless, it would be optimal if risk-adapted starting ages of screening for women with a family history were externally validated before implementation in clinical practice, although the current experts’ opinion-based recommendations for screening relatives of patients with breast cancer are already in practice without validation.
Overall, the results suggest that current screening guidelines do not adequately provide evidence-based recommendation on starting age of screening for women with a family history of breast cancer. The American College of Radiology, which offers more detailed guidance for women with increased risk, recommends starting ages of screening that are closest to the risk-adapted starting ages of screening calculated in this study, although differences still existed. Screening women with a family history under the existing guidelines represents missed opportunities for early detection of early-onset breast cancer in women at increased risk owing to family history. We believe this study thus provides high-quality evidence that can supplement the current guidelines by including detailed guidance for women with various family histories of breast cancer.
Strengths and Limitations
This study has several strengths. Many studies have established an association between a woman’s breast cancer risk and relatives with breast cancer and their age at diagnosis, but these studies provide relative risks, such as risk ratios and hazard ratios, that are not easily applied to clinical decision-making. The current study provides the age at which women attained risk at which screening is recommended, a finding that is clinically usable. This study used the Swedish family-cancer data sets, enabling the detailed analysis of family history and precise estimation of absolute risks. These data provided a complete and accurate recording of relationships and cancers, which eliminates selective ascertainment and information biases that are common in analyses of family pedigrees and cancer reports. The time-dependent nature of family history was also taken into account in the analyses to allow for more accurate estimation of familial risk. The internal validation that used a 2-fold cross-validation and presentation of examples within the context of current screening guidelines are other strengths of this study.
This study also has several limitations. A common underlying assumption in risk prediction analyses is that, other than the risk factor of interest (eg, family history of breast cancer), the risk factor profile of a woman with a family history is similar to that of an average woman in the general population. We did not have data on other breast cancer risk factors, such as predisposing genes (eg, BRCA1, BRCA2, and PALB2), mammographic breast density, and the estrogen receptor status of breast cancers. In general, women with other known risk factors, such as cancer predisposing genes, are advised to start screening as recommended by guidelines for women with high risk if the guidelines offer an earlier starting age of screening than the familial risk-adapted starting age of screening estimated in this study. More strengths and limitations of the study are discussed in eMethods 3 in the Supplement.
Integrating the Results Into Other Risk Assessment Models
The landscape of risk-based breast cancer screening is evolving, and many risk assessment models have been developed. To benefit from both the risk-adapted starting ages based on detailed family history from the study (which is lacking in other risk assessment models so far) and consideration of other breast cancer risk factors in other risk assessment tools (which are lacking in the study), we propose an incorporation strategy.
In risk prediction tools that consider age and family history alongside other risk factors, the family history status could be set to no family history, and the difference of years between the estimate for risk-adapted starting age of screening and the benchmark age of mass screening in the population could be added to the actual age. An equivalent familial risk–adapted age from the breast cancer point of view, instead of the actual calendar age, could be used in other models. For example, in a country with mass screening starting at age 50 years, a 35-year-old woman with a sister with breast cancer who received a diagnosis at age 43 years can reach the risk level of 50-year-old women in the general population at 38 years of age (ie, 12 years earlier; Table 1). With regard to breast cancer risk, this woman is akin to a 47-year-old woman in the general population (ie, her breast cancer–wise age is 12 years older than her actual age). In other risk prediction models that consider fewer details on family history but more details on other risk factors, this woman could be considered as a 47-year-old woman (35 + 12 = 47) without family history. Validation of such integration is warranted.
Conclusions
This cohort study appears to provide tangible and clinically useful guidance on how many years earlier women with a family history of breast cancer should start screening, which may be an important addition to personalized breast cancer screening information. This approach has the potential to improve the cost-effectiveness of breast cancer screening. The study presents what we believe to be high-quality evidence to supplement the recommendations in current guidelines on age at initial screening for the relatives of patients with breast cancer.
eMethods 1. Internal Validation
eMethods 2. Sensitivity Analysis
eMethods 3. Further Strengths and Limitations
eFigure 1. Ten-Year Cumulative Risk Curves for Women With Affected First-Degree Relatives (FDRs) or Second-Degree Relatives (SDRs) in the Development and Validation Data Sets
eFigure 2. Ten-Year Cumulative Risk Curves for Women With Affected Second-Degree Relatives (SDRs) by Maternal and Paternal Lineage
eTable. Comparison of Premenopausal and Lifetime Breast Cancer Risk Between Development and Validation Data Sets by Family History
References
- 1.Ferlay JSI, Ervik M, Dikshit R, et al. Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27. doi: 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Frank C, Fallah M, Sundquist J, Hemminki A, Hemminki K. Population landscape of familial cancer. Sci Rep. 2015;5:12891. doi: 10.1038/srep12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800-809. doi: [DOI] [PubMed] [Google Scholar]
- 6.Braithwaite D, Miglioretti DL, Zhu W, et al. ; Breast Cancer Surveillance Consortium . Family history and breast cancer risk among older women in the breast cancer surveillance consortium cohort. JAMA Intern Med. 2018;178(4):494-501. doi: 10.1001/jamainternmed.2017.8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389-1399. doi: 10.1016/S0140-6736(01)06524-2 [DOI] [PubMed] [Google Scholar]
- 8.Ahern TP, Sprague BL, Bissell MCS, et al. Family history of breast cancer, breast density, and breast cancer risk in a U.S. breast cancer screening population. Cancer Epidemiol Biomarkers Prev. 2017;26(6):938-944. doi: 10.1158/1055-9965.EPI-16-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327-1335. doi: 10.1001/jama.2014.1398 [DOI] [PubMed] [Google Scholar]
- 10.Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;4(6):CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447. doi: 10.1056/NEJMoa1600249 [DOI] [PubMed] [Google Scholar]
- 12.Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89-95. doi: 10.1016/j.breast.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Amaro J, Severo M, Vilela S, et al. Patterns of breast cancer mortality trends in Europe. Breast. 2013;22(3):244-253. doi: 10.1016/j.breast.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen KJ. Mammography screening. Benefits, harms, and informed choice. Dan Med J. 2013;60(4):B4614. [PubMed] [Google Scholar]
- 15.Brennan M, Houssami N. Discussing the benefits and harms of screening mammography. Maturitas. 2016;92:150-153. doi: 10.1016/j.maturitas.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Altobelli E, Rapacchietta L, Angeletti PM, Barbante L, Profeta FV, Fagnano R. Breast cancer screening programmes across the WHO European region: differences among countries based on national income level. Int J Environ Res Public Health. 2017;14(4):E452. doi: 10.3390/ijerph14040452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726, W-236. doi: 10.7326/0003-4819-151-10-200911170-00008 [DOI] [PubMed] [Google Scholar]
- 18.Lauby-Secretan B, Scoccianti C, Loomis D, et al. ; International Agency for Research on Cancer Handbook Working Group . Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353-2358. doi: 10.1056/NEJMsr1504363 [DOI] [PubMed] [Google Scholar]
- 19.Depypere H, Desreux J, Pérez-López FR, et al. EMAS position statement: individualized breast cancer screening versus population-based mammography screening programmes. Maturitas. 2014;79(4):481-486. doi: 10.1016/j.maturitas.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 20.Esserman LJ; WISDOM Study and Athena Investigators . The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer. 2017;3:34. doi: 10.1038/s41523-017-0035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120(19):2955-2964. doi: 10.1002/cncr.28771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680-691. doi: 10.1093/jnci/djq088 [DOI] [PubMed] [Google Scholar]
- 23.Hemminki K, Granström C, Chen B. The Swedish family-cancer database: update, application to colorectal cancer and clinical relevance. Hered Cancer Clin Pract. 2005;3(1):7-18. doi: 10.1186/1897-4287-3-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desreux J, Bleret V, Lifrange E. Should we individualize breast cancer screening? Maturitas. 2012;73(3):202-205. doi: 10.1016/j.maturitas.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 25.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408-414. doi: 10.1016/j.jacr.2017.11.034 [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18-27. doi: 10.1016/j.jacr.2009.09.022 [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297-316. doi: 10.3322/caac.21446 [DOI] [PubMed] [Google Scholar]
- 28.Armaroli P, Villain P, Suonio E, et al. European Code against cancer, 4th Edition: cancer screening. Cancer Epidemiol. 2015;39(suppl 1)(suppl 1):S139-S152. doi: 10.1016/j.canep.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Yang J, Cai H, Ye Y. Young age is an independent adverse prognostic factor in early stage breast cancer: a population-based study. Cancer Manag Res. 2018;10:4005-4018. doi: 10.2147/CMAR.S167363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desreux JAC. Breast cancer screening in young women. Eur J Obstet Gynecol Reprod Biol. 2018;230:208-211. doi: 10.1016/j.ejogrb.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 31.Siu AL; U.S. Preventive Services Task Force . Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279-296. doi: 10.7326/M15-2886 [DOI] [PubMed] [Google Scholar]
- 32.Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504-1510. doi: 10.1001/jamaoncol.2018.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Internal Validation
eMethods 2. Sensitivity Analysis
eMethods 3. Further Strengths and Limitations
eFigure 1. Ten-Year Cumulative Risk Curves for Women With Affected First-Degree Relatives (FDRs) or Second-Degree Relatives (SDRs) in the Development and Validation Data Sets
eFigure 2. Ten-Year Cumulative Risk Curves for Women With Affected Second-Degree Relatives (SDRs) by Maternal and Paternal Lineage
eTable. Comparison of Premenopausal and Lifetime Breast Cancer Risk Between Development and Validation Data Sets by Family History