This registry-based cohort study analyzes the association between varying combinations of neurohormonal blockade therapies and survival and quality of life in adult patients with left ventricular assist devices.
Key Points
Question
Does the association between the use of neurohormonal blockade (NHB) and improved clinical outcomes among patients with heart failure with reduced ejection fraction extend to patients with left ventricular assist devices (LVADs)?
Findings
In this cohort study of 12 144 patients with LVADs in the Interagency Registry for Mechanically Assisted Circulatory Support between January 2008 and June 2016, use of NHB was associated with significantly lower risk of death and higher quality of life compared with patients not receiving NHB. Survival at 4 years was greatest among patients receiving combination therapy with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, β-blocker, and mineralocorticoid antagonist.
Meaning
Use of NHB is associated with improved survival and quality of life among patients with LVADs, suggesting the potential for synergy between intensive NHB and mechanical unloading for patients with advanced heart failure.
Abstract
Importance
Left ventricular assist devices (LVADs) improve outcomes in patients with advanced heart failure, but little is known about the role of neurohormonal blockade (NHB) in treating these patients.
Objective
To analyze the association between NHB blockade and outcomes in patients with LVADs.
Design, Setting, and Participants
This retrospective cohort analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) included patients from more than 170 centers across the United States and Canada with continuous flow LVADs from 2008 to 2016 who were alive with the device in place at 6 months after implant. The data were analyzed between February and November 2019.
Exposures
Patients were stratified based on exposure to NHB and represented all permutations of the following drug classes: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, β-blockers, and mineralocorticoid antagonists.
Main Outcomes and Measures
The outcomes of interest were survival at 4 years and quality of life at 2 years based on Kansas City Cardiomyopathy Questionnaire scores and a 6-minute walk test.
Results
A total of 12 144 patients in INTERMACS met inclusion criteria, of whom 2526 (20.8% ) were women, 8088 (66.6%) were white, 3024 (24.9%) were African American, and 753 (6.2%) were Hispanic; the mean (SD) age was 56.8 (12.9) years. Of these, 10 419 (85.8%) were receiving NHB. Those receiving any NHB medication at 6 months had a better survival rate at 4 years compared with patients not receiving NHB (56.0%; 95% CI, 54.5%-57.5% vs 43.9%; 95% CI, 40.5%-47.7%). After sensitivity analyses with an adjusted model, this trend persisted with patients receiving triple therapy with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, β-blocker, and mineralocorticoid antagonist having the lowest hazard of death compared with patients in the other groups (hazard ratio, 0.34; 95% CI, 0.28-0.41). Compared with patients not receiving NHB, use of NHB was associated with a higher Kansas City Cardiomyopathy Questionnaire score (66.6; bootstrapped 95% CI, 65.8-67.3 vs 63.0; bootstrapped 95% CI, 60.1-65.8; P = .02) and a 6-minute walk test (1103 ft; bootstrapped 95% CI, 1084-1123 ft vs 987 ft; bootstrapped 95% CI, 913-1060 ft; P < .001).
Conclusions and Relevance
Among patients with LVADs who tolerated NHB therapy, continued treatment was associated with improved survival and quality of life. The optimal heart failure regimen for patients after LVAD implant may be the initiation and continuation of guideline-directed medical therapy.
Introduction
Patients with stage D heart failure (HF) have limited life-prolonging therapeutic options other than left ventricular assist devices (LVADs) and cardiac transplant.1 As few patients can undergo transplant and outcomes with mechanical circulatory support have greatly improved, the population of patients with durable LVADs is increasing.2 However, data on the use of pharmacotherapy beyond the use of anticoagulation to prevent thrombotic events are limited. Specifically, little is known about the association of neurohormonal blockade (NHB) with clinical outcomes in patients with LVADs.
Among patients with HF with reduced ejection fraction (HFrEF), using NHB leads to substantial improvements in long-term and short-term clinical outcomes.3 In patients with HF with LVAD support, studies have shown that the underlying HF phenotype remains.4,5,6 A subset of these patients can achieve myocardial remission that permits LVAD removal when treated aggressively with NHB.7 Therefore, it is highly plausible that using these therapies in the general population of patients with LVADs might lead to improvements in clinical outcomes. In this study, we sought to determine the role of guideline-directed medical therapy (GDMT) on survival and quality of life (QoL) among patients with LVADs who were included in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).
Methods
Data Source
We conducted a retrospective cohort analysis of the INTERMACS registry, a prospective database supported by the National Heart, Lung, and Blood Institute, the US Food and Drug Administration, the Centers for Medicare & Medicaid Services, medical industry, and participating centers. INTERMACS was established in 2005 to improve the quality of care and outcomes in patients with mechanical circulatory support devices.8 The registry includes patients with a US Food and Drug Administration–approved device throughout the United States and Canada. The data, analytical methods, and study materials are available via the Biological Specimen and Data Repository Information Coordinating Center.9 The institutional review board at Yale University School of Medicine approved the study and written informed consent was obtained by INTERMACS participating centers.
Study Population
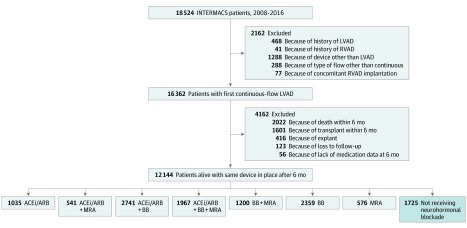
A Consolidated Standards of Reporting Trials diagram of patients included in our study is shown in Figure 1. Based on prior studies suggesting that the recovery of left ventricular function, medication use, and clinical status stabilizes by 6 months after LVAD implant, we included all adult patients with continuous-flow LVADs who underwent implant from January 2008 to June 2016 who were alive and still using the device at 6 months.10,11,12,13 Because of the small sample size and their unique pathophysiology, we excluded patients who had total artificial hearts, biventricular devices, right ventricular assist devices only, or pulsatile devices.
Figure 1. CONSORT Diagram of Study Population.
ACEi indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; MRA, mineralocorticoid antagonists; RVAD, right ventricular assist device.
Definitions
We considered NHB to include the HF medications listed as class I level of evidence A recommendations by the American College of Cardiology and American Heart Association guidelines.3 The medication groups consisted of all permutations of the following major medication classes: (1) angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ACEis/ARBs), (2) β-blockers (BBs), and (3) mineralocorticoid receptor antagonists (MRAs). A separate group comprised patients taking other medication classes that did not fit these criteria: digoxin, amiodarone, calcium channel blockers, hydralazine, and phosphodiesterase inhibitors.
Outcome Measures
Our primary outcome of interest was survival from baseline (6 months after LVAD implant) to 4 years. Our secondary outcome of interest was QoL as defined by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and a 6-minute walk test from baseline to 2 years. After 2 years, QoL measures become scarce in the data set.
Statistical Analysis
Continuous variables are summarized as means (SD) if normally distributed or median and interquartile range (IQR) if not normally distributed. Parametric variables were compared using the t test or analysis of variance and nonparametric variables were compared using the Wilcoxon rank sum and Kruskal-Wallis tests. Categorical variables are presented as frequencies with percentages and were compared using the χ2 test. We assessed associations between medication group and survival using a Kaplan-Meier survival analysis from 6 months to 4 years after implant. We censored patients who underwent explant because of recovery or transplant.
Given that the combination of medical therapies that patients receive changes over time, we treated medication group as a time-dependent variable in a Cox proportional hazards regression.14 Data for patients at each follow-up time were transformed into counting-process form using the survival package in R (R Foundation). Our multivariate sensitivity analysis adjusted for early hazards of mortality identified by the eighth annual INTERMACS report (age, sex, body mass index [calculated as weight in kilograms divided by height in meters squared], implantable cardioveter defibrillator (ICD), INTERMACS profile 1 or 2, albumin, dialysis, blood urea nitrogen, total bilirubin, history of cardiac surgery, concomitant cardiac surgery, and illness too severe to complete EQ-5D).15 In addition, we adjusted for modified Charlson Comorbidity Index (eMethods in the Supplement), institutional LVAD implant volume (averaged between 2014-2016), year of implant, and device strategy (bridge to transplant [BTT] or destination therapy [DT]).15,16 To ensure that the fully adjusted, time-varying Cox proportional hazards regression model did not violate the proportional hazards assumption, we applied time-transforms to coefficients for age, whether patients were too sick to complete the EQ-5D questionnaire, and dialysis prior to implant. In addition, we fit natural cubic splines to the modified Charlson Comorbidity Index score. To minimize skewness, blood urea nitrogen and total bilirubin levels were log-base-2–transformed. Using the Schoenfeld test for proportional hazards, all terms in the fully adjusted, time-varying Cox model upheld the proportional hazards assumption.17
To account for potential residual confounding in the multivariate regression model, we conducted a propensity-matched cohort analysis and, as a negative control, we assessed whether NHB is associated with events recorded by INTERMACS but without known association with the use of NHB (drive-line infection, psychiatric episode, device malfunction, and pump thrombosis). For the propensity-matched analysis, we used the early hazards for mortality identified by the eighth annual INTERMACS Report as covariates (age, sex, body mass index, ICD, INTERMACS profile 1 or 2, albumin, dialysis, blood urea nitrogen, total bilirubin, history of cardiac surgery, concomitant cardiac surgery, and illness too severe to complete EQ-5D) and used a genetic-matching algorithm to determine covariate weights (implemented with the MatchIt and Matching packages in R, respectively).15,18,19
We also conducted a Kaplan-Meier survival analysis using a new user cohort. After excluding patients receiving NHB before LVAD implant, we then performed a Kaplan-Meier survival analysis for 2 groups: those with incident NHB use and those without NHB use after 6 months of device implant. We calculated an adjusted hazard ratio (HR) based on the covariates used in our aforementioned multivariate regression model.
Lastly, to further understand association of NHB therapy with outcomes after LVAD implant, we performed a competing risks analysis by calculating the cumulative incidence of death, transplant, and explant due to recovery using the Aalen-Johansen estimate.20 For the competing risks analysis, we stratified patients according to whether their preimplant treatment strategy was BTT or DT and compared patients either receiving NHB, not receiving NHB, or receiving triple NHB therapy. We used R, version 3.5.3, and Stata, version 14.2 (StataCorp) for all the analyses. Two-tailed P values of <.05 were considered statistically significant.
Results
Characteristics of Study Population
We included 12 144 patients with continuous-flow LVADs between 2008 to 2016 who were alive and using the device 6 months after implant (Figure 1 and Table). There were 9618 men (79.2%) and 8088 (66.6%) were white. The indication for LVAD was BTT in 6800 (56.0%), DT in 5270 (43.4%), and bridge to recovery in 49 (0.4%). There were 2259 patients (18.6%) with chronic renal disease; 959 (7.9%) had severe diabetes, 2344 (19.3%) had pulmonary hypertension, and 1785 (14.7%) had atrial arrhythmias. Race/ethnicity, comorbidities, INTERMACS profile, and indication for LVAD implant were similar in the NHB and not receiving NHB groups (Table). The use of amiodarone, calcium channel blockers, and phosphodiesterase inhibitors were similar between those receiving NHB and those not receiving NHB, but there were significant differences in the use of hydralazine, warfarin, and digoxin between the 2 groups. Over the course of the study period, 17 patients (0.99%) not receiving NHB underwent explant because of recovery compared with 169 (1.62%) of those receiving NHB (P = .04).
Table. Characteristics of Study Populationa.
| Baseline Characteristic | Total Cohort (N = 12 144) | Not Receiving NHB (n = 1725) | Receiving NHB (n = 10 419) | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 56.8 (12.9) | 59.0 (12.5) | 56.5 (13.0) | <.001 |
| Female, No, (%) | 2526 (20.8) | 355 (20.6) | 2167 (20.8) | .90 |
| White, No. (%)% | 8088 (66.6) | 1170 (67.8) | 6918 (66.4) | .27 |
| African American, No. (%) | 3024 (24.9) | 393 (22.8) | 2636 (25.3) | .03 |
| Hispanic, No. (%) | 753 (6.2) | 116 (6.7) | 636 (6.1) | .38 |
| Body mass index, mean (SD)b | 28.6 (6.7) | 28.2(6.8) | 28.7(6.7) | .001 |
| Body surface area, mean (SD)c | 2.1 (0.3) | 2.04 (0.29) | 2.06 (0.29) | .006 |
| Diabetes, No. (%) | 959 (7.9) | 154 (8.9) | 802 (7.7) | .09 |
| Peripheral vascular disease, No. (%) | 486 (4.0) | 66 (3.8) | 417 (4.0) | .73 |
| Atrial arrhythmia, No. (%) | 1785 (14.7) | 248 (14.4) | 1542 (14.8) | .65 |
| Ischemic heart disease, No. (%) | 729 (6.0) | 116 (6.7) | 615 (5.9) | .19 |
| Previous cardiac surgery, No. (%) | 3923 (32.3) | 616 (35.7) | 3303 (31.7) | .001 |
| Lung disease, No. (%) | 923 (7.6) | 128 (7.4) | 792 (7.6) | .78 |
| Pulmonary HTN, No. (%) | 2344 (19.3) | 321 (18.6) | 2021 (19.4) | .42 |
| Active smoker, No. (%) | 668 (5.5) | 105 (6.1) | 563 (5.4) | .20 |
| Chronic kidney disease, No. (%) | 2259 (18.6) | 364 (21.1) | 1896 (18.2) | .004 |
| INTERMACS profile, No. (%) | ||||
| 1 | 1603 (13.2) | 266 (15.4) | 1334 (12.8) | <.001 |
| 2 | 4323 (35.6) | 650 (37.7) | 3667 (35.2) | |
| 3 | 4032 (33.2) | 516 (29.9) | 3522 (33.8) | |
| 4-7 | 2186 (18.0) | 293 (17.0) | 1886 (18.1) | |
| Modified Charlson Comorbidity Index | 3 | 3 | 4 | <.001 |
| Bridge to recovery, No. (%) | 49 (0.4) | 5 (0.3) | 42 (0.4) | .69 |
| Bridge to transplant, No. (%) | 5671 (46.7) | 735 (42.6) | 4939 (47.4) | <.001 |
| Destination therapy, No. (%) | 5270 (43.4) | 847 (49.1) | 4418 (42.4) | <.001 |
| NYHA class, No. (%) | ||||
| I-II | 85 (0.7) | 10 (0.6) | 83 (0.8) | .004 |
| III | 2174 (17.90 | 274 (15.9) | 1907 (18.3) | |
| IV | 9132 (75.2) | 1304 (75.6) | 7825 (75.1) | |
| Unknown | 753 (6.2) | 136 (7.9) | 615 (5.9) | |
| Amiodarone | 4760 (39.2) | 656 (38.0) | 4105 (39.4) | .27 |
| Calcium channel blocker, No. (%) | 1154 (9.5) | 140 (8.1) | 1011 (9.7) | .04 |
| Hydralazine, No. (%) | 1882 (15.5) | 212 (12.3) | 1667 (16.0) | <.001 |
| Loop diuretic, No. (%) | 7906 (65.1) | 899 (52.1) | 7012 (67.3) | <.001 |
| Digoxin, No. (%) | 1870 (15.4) | 154 (8.9) | 1719 (16.5) | <.001 |
| Phosphodiesterase inhibitor, No. (%) | 1955 (16.1) | 240 (13.9) | 1709 (16.4) | .008 |
| Warfarin, No. (%) | 11 160 (91.9) | 1258 (72.9) | 9898 (95.0) | <.001 |
Abbreviations: HTN, hypertension; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association.
Data are presented as mean (SD) for continuous measures and number (percentage) for categorical variables.
Calculated as weight in kilograms divided by height in meters squared.
Calculated as square root ([hgt_cm × wgt_kg]/3600).
Trends in Use of HF Therapies After LVAD Implant
The proportion of patients treated with NHB increased from 8865 (73.0%) before LVAD implant to 10 419 (85.8%) at 6 months after LVAD implant (data not shown). At the 6-month point, 1967 patients (16.2%) were receiving triple therapy with all 3 medication classes; 2742 (22.6%) were receiving a BB and ACEi/ARB, 1200 (9.8%) were receiving a BB and MRA, 541 (4.5%) were receiving an ACEi/ARB and MRA, 2359 (19.4%) were receiving a BB, 1035 (8.5%) were receiving an ACEi/ARB, 576 (4.7%) were receiving MRA, and 1725 (14.2%) were not receiving NHB (Figure 1). In eTable 1 in the Supplement, we present demographic data, comorbidities, INTERMACS profile, New York Heart Association class, and laboratory data among the 8 varying medication groups included in the analyses. For most variables, there were statistically significant differences among medication groups. Patients receiving triple therapy with an ACEi/ARB, BB, and MRA had the lowest N-terminal pro–B-type natriuretic peptide values with a mean (SD) of 1252 (1442) ng/L compared with those without NHB with a mean (SD) of 3276 (4712) ng/L. The average creatinine level was lowest in those receiving triple with a mean (SD) of 1.18 (0.43) mg/dL (to convert to micromoles per liter, multiply by 88.4). The degree of anticoagulation based on the international normalized ratio was similar in all groups (range, 2.14-2.27; P < .001).
HF Medication Use and Risk of Death
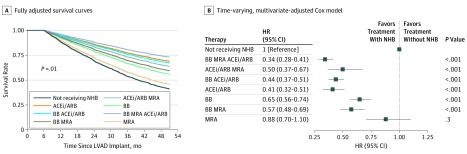
Unadjusted survival estimate curves by medication group are shown in eFigure 1 in the Supplement. The unadjusted 4-year survival estimate for patients receiving NHB was 56.0% (95% CI, 54.5%-57.5%) compared with 43.9% (95% CI, 40.5%-47.7%) for patients not receiving NHB (P < .001). This unadjusted model revealed that patients receiving triple therapy with an ACEi/ARB, BB, and MRA at 6 months had the longest survival estimate at 4 years (66.4%; 95% CI, 63.1%-70.0%).
Because the Kaplan-Meier analysis does not account for confounders or medication group switching over the study period, we performed a sensitivity analysis using a multivariate Cox proportional hazards regression that treated medication group as a time-dependent covariate. This analysis revealed that use of triple therapy with all NHB classes was associated with significantly improved survival compared with the other medication groups (HR, 0.34; 95% CI, 0.28-0.41; P < .001) (Figure 2). Every other medication group was associated with reduced hazard with the exception of MRA alone (adjusted HR, 0.88; 95% CI, 0.7-1.1). For all adjusted models of survival, there was improved survival among patients taking an ACEi/ARB alone or in combination with the other HF therapies compared with medication groups without an ACEi/ARB.
Figure 2. Sensitivity Analysis of Patients Receiving Varying Heart Failure Therapies.
A, Fully adjusted survival curves accounting for time variation in patients’ medications throughout the follow-up period. B, Forest plot for fully adjusted Cox proportional hazards regression model. ACEi indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; BB, β-blockers; HR, hazard ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; MRA, mineralocorticoid antagonists; NHB, neurohormonal blockade.
To account for residual confounding, we performed further sensitivity analyses using a propensity score–matched cohort. The results of the propensity-matched analysis indicated that use of any NHB was associated with significantly improved survival compared with medical regimens without NHB 4 years postimplant (59.3%; 95% CI, 57.8%-60.9% vs 50.4%; 95% CI, 46.6%-54.5%). When examining the survival estimate for patients specifically receiving triple therapy with an ACEi/ARB, BB, and MRA vs those not receiving NHB, the difference in survival rate was even greater (68.5%; 95% CI, 65.2%-72.0% vs 53.9%; 95% CI ,49.1%-59.4%) (eFigure 2 and eTable 2 in the Supplement).
Further sensitivity analyses were performed using drive-line infection, psychiatric episode, device malfunction, and pump thrombosis as negative controls (eTable 3 in the Supplement). We stratified survival based on exposure to either any NHB or triple therapy; no NHB was used as a reference. The cumulative incidence between 6 months and 4 years was 10.8% (n = 1307) for drive-line infection, 3.1% (n = 373) for psychiatric episode, 24.3% (n = 2965) for device malfunction, and 12.8% (n = 1557) for pump thrombosis. There were no statistically significant differences in the rates of these events across groups.
In an analysis restricted to patients with incident use of NHB 6 months after implant (ie, new users), the association between NHB and outcomes persisted. Of the 3381 preimplant NHB-naive patients, use of NHB was associated with a significant improvement in survival compared with nonuse of NHB (adjusted HR, 0.66; 95% CI, 0.56-0.78) (eFigure 3 in the Supplement).
To further describe the study cohort, we performed competing risk analyses by calculating the cumulative incidence of death, transplant, and explant due to recovery for those with a BTT or DT device strategy (eFigure 4 in the Supplement). Patients undergoing BTT receiving NHB were estimated to have a lower risk of death, a higher chance of undergoing explant due to recovery, and a higher chance of undergoing transplant (20.5% [95% CI, 19.3%-21.7%] vs 33.4% [95% CI, 30.0%-37.2%]; 3.6% [95% CI, 3.1%-4.1%] vs 1.8% [95% CI, 1.0%-3.2%]; and 51.7% [95% CI, 50.3%-53.1%] vs 43.3% [95% CI, 39.9%-47.1%], respectively; all P < .004). The patients undergoing BTT receiving triple therapy had even better estimated outcomes 4 years postimplant, with less than half of the probability of death, 3-fold probability of undergoing explant due to recovery, and greater than 10% probability of undergoing transplant compared with patients undergoing BTT not receiving NHB (15.1% [95% CI, 12.9%-17.7%] vs 33.4% [95% CI, 30.0%-37.2%]; 6.2% [95% CI, 4.8%-7.9%] vs 1.8% [95% CI, 1.0%-3.2%]; and 53.3% [95% CI, 50.2%-56.6%] vs 43.3% [95% CI, 39.9%-47.1%], respectively; all P < .001). The association between NHB therapy and survival also held for patients receiving LVADs for DT. Regardless of whether patients undergoing DT were receiving any NHB or triple therapy at 6 months postimplant, they had a lower estimated probability of death at 4 years and a higher chance of undergoing transplant compared with patients undergoing DT not receiving any NHB (P < .005). For those who underwent implant as DT, exposure to triple therapy was associated with a nearly 2-fold higher chance of undergoing explant due to recovery compared with patients undergoing DT not receiving NHB (P = .02).
Association of HF Therapies With QoL
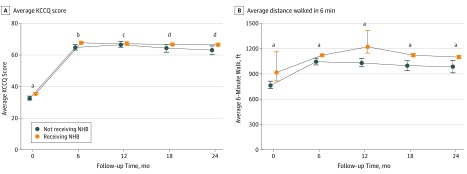
To assess whether various combinations of HF therapies were associated with QoL outcomes for patients with LVADs, we measured the KCCQ score and a 6-minute walk test for patients at each follow-up time from 6 months postimplantation to 2 years. The median KCCQ score at 2 years for patients receiving NHB was 68.8 (IQR, 52.6-82.3) vs 64.9 (IQR, 48.1-82.8) for patients not receiving any therapies (P = .02) (Figure 3A). The average KCCQ score at 2 years for patients receiving NHB was 66.6 (bootstrapped 95% CI, 65.8-67.3) vs 63.0 (bootstrapped 95% CI, 60.1-65.8) for patients not receiving NHB (P = .02) (Figure 3A). The average 6-minute walk test at 2 years was 1103 ft (bootstrapped 95% CI, 1084-1123 ft) compared with 987 ft (bootstrapped 95% CI, 913-1060 ft; P < .001) (Figure 3B).
Figure 3. Association of Neurohormonal Blockade (NHB) With Quality-of-Life Outcomes.
Quality of life for patients receiving NHB therapy. A, Average Kansas City Cardiomyopathy Questionnaire (KCCQ) scores based on therapy from time of implant to 24 months. Error bars show bootstrapped 95% CIs. B, Average distance walked in 6 minutes for patients receiving different heart failure therapy. Error bars show bootstrapped 95% CI. The difference between medication groups at each interval was compared using a 1-tailed Mann-Whitney U test.
aP ≤ .001.
bP ≤ .01.
cNot significant.
dP ≤ .05.
Discussion
To our knowledge, this study is the first analysis of varying combinations of NHB therapies and patient-centered outcomes in patients with LVADs; it has 4 key findings. First, there is a significant association between NHB therapy and improved survival compared with LVAD implant without NHB. Second, our unadjusted and adjusted models showed the lowest hazard of death for patients with LVADs receiving triple therapy with an ACEi/ARB, BB, and MRA, similar to GDMT for HFrEF. Third, patients receiving NHB enjoyed a better QoL as defined by KCCQ scores and 6-minute walk test. Fourth, there is substantial variation in the use of HF therapies among patients with LVADs.
These results support the findings of other studies that sought to augment LVAD function with medical therapies. Several small trials have shown that the use of combination therapy with an ACEi, ARB, BB, MRA, and a β-2 agonist can lead to LVAD weaning and explant for recovery in a limited subset of patients with primarily nonischemic cardiomyopathy.7,10,21,22,23,24 In this study, very few patients underwent explant for recovery, but there were significantly more in the group receiving NHB compared with those not receiving NHB. The associations we found between HF medication therapy and improved outcomes are also supported by a recent prospective study comparing the association of NHB with mechanical unloading with mechanical unloading alone.4 The histopathological parameters of reverse remodeling were greater in those receiving NHB compared with controls, but the study was not powered to detect differences in mortality between the groups. Both our unadjusted and adjusted models showed an association between survival and use of NHB, with patients receiving triple therapy with an ACEi/ARB, BB, and MRA performing best. The survival benefit seen in these groups was not surprising based on evidence that the combination of these therapies reduces mortality in patients with HFrEF without an LVAD.
We were also concerned about confounding associated with illness severity. Recently published data from the CHAMP-HF registry consisting of patients with HFrEF without mechanical circulatory support revealed that factors, such as low systolic blood pressure and poor QoL, were associated with GDMT discontinuation, whereas stable or improving renal function were associated with medication initiation or dose increase.25 Neither the CHAMP-HF registry nor the INTERMACS registry can indicate a causal relationship between medication use and health status or vice versa. To address whether inherently healthier participants are able to tolerate a more intensive NHB regimen, we adjusted our analysis for factors that could contribute to health status. Our multivariate-adjusted model, negative control analyses, propensity-matched cohort analyses, and competing risk analyses showed similar trends in survival with the group receiving all 3 NHB therapies performing the best when compared with those not receiving any NHB. Although we used different approaches to reduce bias and address confounders in our analyses, our results indicate an association between NHB and improved outcomes after LVAD and cannot conclude causality. Surprisingly, there was a significant difference in the use of warfarin therapy in the cohort receiving NHB compared with the cohort not receiving NHB. This difference could not be accounted for with proportionately greater use of other forms of anticoagulation. Given the nearly identical values of international normalized ratio among all the medication groups, including those not receiving NHB, it is possible that relative lack of warfarin use was secondary to coagulopathy.
When comparing all the groups against each other, groups containing ACEi/ARB therapy had the longest survival. The association of ACEi/ARB use and survival may be multifactorial. Renin angiotensin-aldosterone system antagonism with lisinopril was also associated with decreased rates of gastrointestinal bleeding among patients with continuous-flow LVADs according to a recent retrospective analysis, but there was no association with survival during the 2-year period of interest.26 The difference in survival benefit between the former study and ours may be explained by the large difference in sample size. If use of an ACEi/ARB is associated with improved survival and a decreased risk of gastrointestinal bleeding, then ACEi/ARBs may be preferred if patients are only able to tolerate 1 agent that targets neurohormonal dysfunction.
Similar to the survival analyses, patients receiving NHB enjoyed a higher QoL compared with those not receiving NHB. We found a 3.6-point absolute difference in mean KCCQ score between the groups after 2 years. This statistically significant result is also clinically significant given that the difference of only a few points can reflect patients’ ability to independently bathe or participate in hobbies.27 The improvement in KCCQ score is substantiated by the improvement in the 6-minute walk test between the 2 groups. Both of these QoL measures suggest that medical regimens that include NHB can be additive to the improvement in QoL by LVADs alone based on the original clinical trials.28,29
Unlike for patients with HFrEF without mechanical circulatory support, there are no GDMTs for HF after LVAD implant. The International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support only recommend ACEi/ARBs, BBs, and MRAs for patients with another indication for the drug class, such as hypertension, coronary artery disease, or diabetes with vascular complications. While 85.8% of patients in INTERMACS are receiving either an ACEi/ARB, BB, or MRA, there is substantial variation in the combined use of these medications. Together with the results of small studies that use intensive NHB in patients with LVADs, our results indicated that there is potential synergy between the traditional GDMT for HF and mechanical unloading for patients with advanced HF.
Limitations
Our sensitivity analyses were limited by a lack of site-level data, which prevented us from adjusting for differences in prescribing patterns and outcomes unique to the various LVAD centers. Furthermore, because change in medication exposure over time was likely not a random event, but rather associated with a patient’s clinical status, our time-varying covariate analysis is limited. INTERMACS does not contain data concerning medication dosage, medication compliance, nor the reason for medication initiation or discontinuation. Given these limitations, it was not clear whether patients receiving intensive NHB regimens were inherently healthier owing to their ability to tolerate these therapies. Conversely, it was not possible to conclude that patients not receiving NHB or less intensive NHB regimens had higher illness severity and were intolerant of such therapies. The interpretation of these results requires appreciation for the limitations of observational studies.
Conclusions
This large, nationwide analysis of patients undergoing LVAD implant demonstrates an association between varying combinations of HF therapies and patient-centered outcomes. Specifically, medical regimens with NHB similar to GDMT for HFrEF were associated with improved survival and QoL. These data suggest that there may be an optimal medical regimen for HF after LVAD implant that is similar to GDMT for HFrEF.
eMethods. Description of modified Charlson Comorbidities Index used for sensitivity analysis
eTable 1. Characteristics of Study Population by Cohort
eTable 2. Balance between Covariate Distributions for Propensity-Matched Patients
eFigure 1. Propensity Matched Cohort Analysis
eFigure 2. New User Analysis
eFigure 3. Competing Risk Analyses
References
- 1.Ahmad T, Patel CB, Milano CA, Rogers JG. When the heart runs out of heartbeats: treatment options for refractory end-stage heart failure. Circulation. 2012;125(23):2948-2955. doi: 10.1161/CIRCULATIONAHA.112.097337 [DOI] [PubMed] [Google Scholar]
- 2.Miller LW, Rogers JG. Evolution of left ventricular assist device therapy for advanced heart failure: a review. JAMA Cardiol. 2018;3(7):650-658. doi: 10.1001/jamacardio.2018.0522 [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 4.Catino AB, Ferrin P, Wever-Pinzon J, et al. . Clinical and histopathological effects of heart failure drug therapy in advanced heart failure patients on chronic mechanical circulatory support. Eur J Heart Fail. 2018;20(1):164-174. doi: 10.1002/ejhf.1018 [DOI] [PubMed] [Google Scholar]
- 5.Ahmad T, Kelly JP, McGarrah RW, et al. . Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol. 2016;67(3):291-299. doi: 10.1016/j.jacc.2015.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad T, Fiuzat M, Neely B, et al. . Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail. 2014;2(3):260-268. doi: 10.1016/j.jchf.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birks EJ, Tansley PD, Hardy J, et al. . Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355(18):1873-1884. doi: 10.1056/NEJMoa053063 [DOI] [PubMed] [Google Scholar]
- 8.Kormos RL, Cowger J, Pagani FD, et al. . The Society of Thoracic Surgeons INTERMACS database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114-126. doi: 10.1016/j.healun.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 9.Coady SA, Mensah GA, Wagner EL, Goldfarb ME, Hitchcock DM, Giffen CA. Use of the National Heart, Lung, and Blood Institute data repository. N Engl J Med. 2017;376(19):1849-1858. doi: 10.1056/NEJMsa1603542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drakos SG, Wever-Pinzon O, Selzman CH, et al. ; UCAR (Utah Cardiac Recovery Program) Investigators . Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61(19):1985-1994. doi: 10.1016/j.jacc.2013.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khazanie P, Hammill BG, Patel CB, et al. . Use of heart failure medical therapies among patients with left ventricular assist devices: insights from INTERMACS. J Card Fail. 2016;22(9):672-679. doi: 10.1016/j.cardfail.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakos SG, Mehra MR. Clinical myocardial recovery during long-term mechanical support in advanced heart failure: Insights into moving the field forward. J Heart Lung Transplant. 2016;35(4):413-420. doi: 10.1016/j.healun.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Ahmad T, Wang T, O’Brien EC, et al. . Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail. 2015;3(1):30-39. doi: 10.1016/j.jchf.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. doi: 10.21037/atm.2018.02.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirklin JK, Pagani FD, Kormos RL, et al. . Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080-1086. doi: 10.1016/j.healun.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 17.Granbsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 18.Ho D. MatchIT: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1. doi: 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 19.Diamond A. Genetic matching for estimating causal effects: a general multivariate matching method for achieving balance in observational studies. Rev Econ Stat. 2013;95(3):932-945. doi: 10.1162/REST_a_00318 [DOI] [Google Scholar]
- 20.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Berlin, Germany: Springer Science & Business Media; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 21.Yacoub MH. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J. 2001;22(7):534-540. doi: 10.1053/euhj.2001.2613 [DOI] [PubMed] [Google Scholar]
- 22.Aaronson KD, Pagani FD, Maybaum SW, et al. . 3 Combination therapy with pulsatile left ventricular assist device, heart failure medication and clenbuterol in chronic heart failure: results from HARPS. J Heart Lung Transplant. 2011;30(4):S8-S9. doi: 10.1016/j.healun.2011.01.010 21977502 [DOI] [Google Scholar]
- 23.Diakos NA, Selzman CH, Sachse FB, et al. . Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for cardiac assist device-induced myocardial recovery. J Am Coll Cardiol. 2014;64(15):1602-1612. doi: 10.1016/j.jacc.2014.05.073 [DOI] [PubMed] [Google Scholar]
- 24.Lamarche Y, Kearns M, Josan K, et al. . Successful weaning and explantation of the Heartmate II left ventricular assist device. Can J Cardiol. 2011;27(3):358-362. doi: 10.1016/j.cjca.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 25.Greene SJ, Fonarow GC, DeVore AD, et al. . Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Converse MP, Sobhanian M, Taber DJ, Houston BA, Meadows HB, Uber WE. Effect of angiotensin II inhibitors on gastrointestinal bleeding in patients with left ventricular assist devices. J Am Coll Cardiol. 2019;73(14):1769-1778. doi: 10.1016/j.jacc.2019.01.051 [DOI] [PubMed] [Google Scholar]
- 27.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469-476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani FD, Miller LW, Russell SD, et al. ; HeartMate II Investigators . Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312-321. doi: 10.1016/j.jacc.2009.03.055 [DOI] [PubMed] [Google Scholar]
- 29.Rogers JG, Pagani FD, Tatooles AJ, et al. . Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Description of modified Charlson Comorbidities Index used for sensitivity analysis
eTable 1. Characteristics of Study Population by Cohort
eTable 2. Balance between Covariate Distributions for Propensity-Matched Patients
eFigure 1. Propensity Matched Cohort Analysis
eFigure 2. New User Analysis
eFigure 3. Competing Risk Analyses