ABSTRACT
Cancer cell invasion is influenced by various biomechanical forces found within the microenvironment. We have previously found that invasion is enhanced in fibrosarcoma cells when transient mechanical stimulation is applied within an in vitro mechano-invasion assay. This enhancement of invasion is dependent on cofilin (CFL1), a known regulator of invadopodia maturation. Invadopodia are actin-rich structures present in invasive cancer cells that are enzymatically active and degrade the surrounding extracellular matrix to facilitate invasion. In this study, we examine changes in gene expression in response to tugging on matrix fibers. Interestingly, we find that integrin β3 expression is downregulated and leads to an increase in cofilin activity, as evidenced by a reduction in its Ser3 phosphorylation levels. As a result, invadopodia lengthen and have increased enzymatic activity, indicating that transient mechanical stimulation promotes the maturation of invadopodia leading to increased levels of cell invasion. Our results are unique in defining an invasive mechanism specific to the invasive process of cancer cells that is triggered by tugging forces in the microenvironment, as opposed to rigidity, compression or stretch forces.
KEY WORDS: Invadopodia, Cell mechanics, Cell invasion, Cancer, Cofilin
Summary: Tugging on ECM fibers promotes the maturation of invadopodia and cell invasion in highly invasive cancer cells. This specific form of mechanical signaling downregulates integrin β3 resulting in increased cofilin activity.
INTRODUCTION
Invasion is an acquired cellular process that drives the progression of cancer into the metastatic cascade. Without the ability to invade, the majority of solid tumor cancers would be treatable by surgical removal of the initial tumor, and would minimize the need for chemotherapeutics to kill the unseen invasion-competent cells. Unfortunately, at this time, our full understanding of how invasive ability is acquired and how invasion is executed is woefully lacking. However, with increasing knowledge about metastatic progression, cancer biologists have begun to appreciate the importance of multiple mechanical factors in initiating the multi-step metastatic cascade (Kumar and Weaver, 2009). These mechanical factors include changes in the structure and mechanics of the whole tissue, as well as local biophysical changes in the geometry and topology of the extracellular matrix (ECM). Much of the early focus has been on the obvious changes in stiffness, since the stroma surrounding most tumors often becomes more rigid and dense due to an enrichment of collagen type I and fibronectin (Miles and Sikes, 2014; Pickup et al., 2014). This increase in rigidity leads to an increase in tumor cell proliferation, migration and invasion (Alexander et al., 2008; Charras and Sahai, 2014; Jerrell and Parekh, 2014; Kostic et al., 2009; Parekh and Weaver, 2009; Tilghman et al., 2010; Ulrich et al., 2009; Umesh et al., 2014).
In addition to the rigidity of the matrix, stroma-associated cells, including the highly contractile myofibroblasts, produce forces in the ECM as they remodel and migrate through the matrix (Goffin et al., 2006; Shieh, 2011; Tripathi et al., 2012). During the remodeling process, a tugging force is generated on the surrounding collagen fibers as they are bundled and arranged (Castella et al., 2010; Goffin et al., 2006; Murrell et al., 2015; Oudin et al., 2016). The presence of the myofibroblasts and the forces they generate have been linked to increased cancer cell invasion and motility (De Wever et al., 2008; Elkabets et al., 2011; Fuyuhiro et al., 2012). A previous study in our laboratory found that transient tugging on fibers within a collagen–fibronectin matrix, to mimic the magnitude and organization of the forces that would be produced by both normal fibroblasts and fibrosarcoma cells within the ECM, can enhance the extent of cell invasion in highly invasive human fibrosarcoma (Menon and Beningo, 2011). This suggests that random transient tugging forces provide mechanical cues utilized by metastatic cancer cells to augment their invasion. These specific mechanical cues and others that are present within the ECM are detected by mechanoreceptors located on the surface of the cell (Gasparski and Beningo, 2015). In particular, the integrin family of receptors have been implicated in mechanoreception, and their significance in mechanotransduction has been widely studied (Roca-Cusachs et al., 2012; Ross et al., 2013). More generally, cellular structures including filopodia, lamellipodia and invadosomes (invadopodia and podosomes) are known to respond to mechanical cues (Mrkonjic et al., 2016; Schwarz and Gardel, 2012).
Invadopodia are thin, actin-rich cellular protrusions that are unique to invasive cells and contribute to the proteolysis of the ECM. They recruit various matrix metalloproteinases (MMPs) that allow invasive cancer cells to erode through the basement membrane to begin their metastatic journey (Frittoli et al., 2011; Jacob and Prekeris, 2015; Poincloux et al., 2009). These structures are enriched in actin-associated proteins, such as cortactin (CTTN) and cofilin (CFL1), which contribute to their dynamic behavior (Artym et al., 2006; Clark et al., 2007; Yamaguchi et al., 2005). Invadopodia undergo three distinct phases in their lifetime: initiation, assembly and maturation. The formation of a core structure containing N-WASp (also known as WASL), Tks5 (also known as SH3PXD2A), cofilin and cortactin defines the initiation step (Artym et al., 2006; Blouw et al., 2015; Oser et al., 2009). The assembly phase requires the stabilization of these precursor proteins to continue the actin polymerization needed for maturation. The maturation phase may be regulated by NHE-1 (also known as SLC9A1), a Na+/H+ exchanger, which induces the pH-dependent release of cofilin from its inhibitory interaction with cortactin (Magalhaes et al., 2011). It has been demonstrated that cofilin expression is necessary in cancer cells in order for them to retain their invasiveness (Menon and Beningo, 2011; Nagai et al., 2011; Walter et al., 2009). Cofilin activity is also regulated by its phosphorylation at the Ser3 position by LIM kinase 1 (LIMK1). When it is unphosphorylated, cofilin binds to F-actin filaments to promote actin polymerization, while phosphorylated cofilin cannot interact with these filaments. As a result, only active, unphosphorylated cofilin can facilitate actin polymerization via generation of free barbed ends (Blanchoin et al., 2000).
During the maturation phase, invadopodia become proteolytically active, which is a process that is characterized by the localization and/or secretion of functional MMP enzymes. There are three members of the MMP family that are associated with invadopodia: MMP-2, MMP-9 and MT1-MMP (also known as MMP-14) (Jacob and Prekeris, 2015). Of particular interest to this study is MMP-2, whose fibronectin type II repeats bind to its collagen substrate (Polette et al., 2004). MMP-2 localizes to invadopodia, where it is secreted into the extracellular environment to degrade the ECM (Clark and Weaver, 2008). Interestingly, the overexpression of cofilin in several invasive cancer cell lines increases cell invasiveness and MMP-2 enzymatic activity (Dang et al., 2006; Yap et al., 2005), while a decrease in cofilin expression reduces invadopodia maturation and MMP-2 enzymatic activity (Tahtamouni et al., 2013; Wang et al., 2007).
A variety of mechanical forces are present in the tumor environment; however, much effort has focused solely on the increased rigidity of the stroma (Kostic et al., 2009; Levental et al., 2009; Paszek et al., 2005). In this study, we utilize an in vitro mechano-invasion assay (Menon and Beningo, 2011) to test the impact of a different form of mechanical stimuli on the ability of the cell to invade (Fig. 1). Mechanical stimulation is provided in the form of transient tugging forces generated by magnetic beads randomly attached to anisotropic collagen and fibronectin fibers. These forces are not significant enough to induce whole substrate stretch, nor is the transient strain aligned in any particular axis of the substrate. We have previously described a significant increase in the invasion efficiency of cells when this type of mechanical cue is present (Menon and Beningo, 2011). In our previous study, we determined that enhanced invasion in response to this stimulus required that the cells already be invasive, as non-invasive cells could not be stimulated to become invasive. Hence, this mechanical cue is specifically exploited by metastatic cancer cells. We also found that both cofilin and fibronectin were necessary to respond to the mechanical cue. However, the mechanistic details regarding this type of mechanosensing have not been identified. Our objective is to uncover candidate genes that define a mechanosensing signaling pathway that ultimately leads to enhanced invasion and dissemination. Additionally, we examine the role of invadopodia in this process, as both cofilin and invadopodia are required for cancer cell invasion (Menon and Beningo, 2011). Based on our preliminary data, we speculated that in response to tugging forces in the stroma, cancer cells would show an altered expression of genes involved in mechanosensing. We used real-time quantitative PCR (qPCR) analysis to identify differentially expressed genes in mechanically stimulated invasive cancer cells. We identified the integrin β3-encoding gene as being differentially expressed and confirmed its functional importance to sensing this specific form of mechanical stimulation. Furthermore, we discovered that the downregulation of integrin β3 expression increases invadopodia maturation in response to stimulation and that knockdown of cofilin expression produces invadopodia that do not respond to stimulation. As a result of promoting the maturation of invadopodia, there is an accompanying increase in invadopodia-associated MMP activity. To our knowledge, this is the first study to implicate tugging forces as specifically promoting the invasion of metastatic cells and that they further provide a mechanical signal for the basis of mechanically induced maturation of invadopodia.
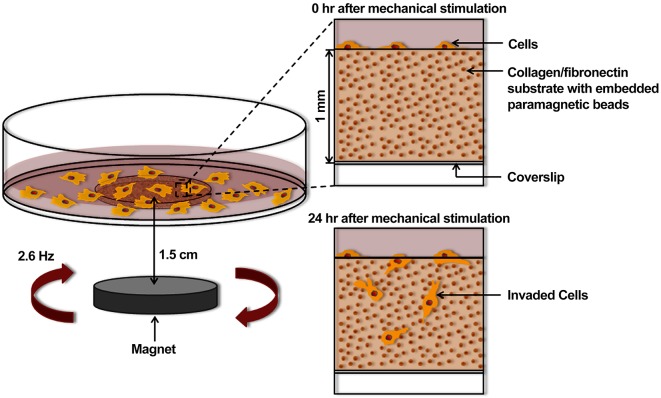
Fig. 1.
Design of an in vitro mechano-invasion assay. A 1 mm deep well is created in a 60 mm cell culture dish by drilling a hole in the bottom of the dish and attaching an activated glass coverslip with vacuum grease. The resulting well is filled with a collagen type I and fibronectin matrix containing 1 µm carboxylated paramagnetic beads, which covalently attach to the fibers upon polymerization. HT1080 fibrosarcoma cells are seeded onto the surface of the matrix and either cultured 1.5 cm above a rotating magnet or outside of the magnetic field (unstimulated). After 24 h, cells invade into the matrix and are counted to determine the percentage invasion.
RESULTS
Identification of differentially expressed genes by PCR array analysis
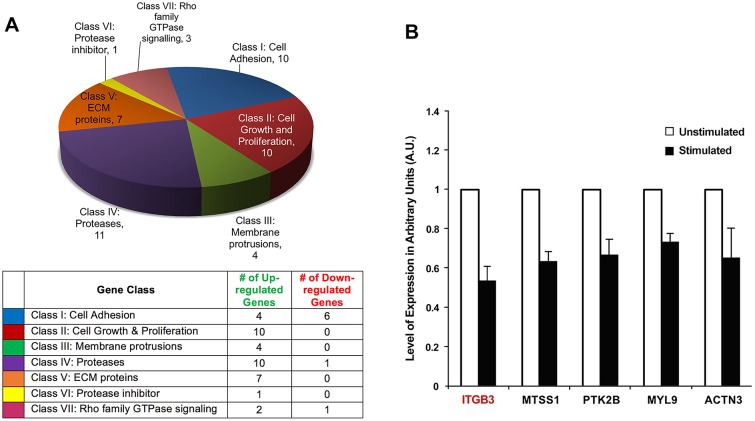
For identification of differentially expressed genes under mechanically stimulated and unstimulated conditions, three PCR arrays were used. The RNA was obtained from mechanically stimulated and unstimulated cells and was then used to prepare cDNA for PCR array analysis. The differentially expressed genes identified by PCR array analysis were classified into seven groups based on their function, as defined by the manufacturer of the gene array. Out of the 252 genes examined, 46 genes were differentially expressed upon mechanical stimulation using a cut-off value of 1.35-fold change in expression: 38 genes were upregulated and eight genes were downregulated (Fig. 2A).
Fig. 2.
Differentially expressed genes resulting from mechanical stimulation. (A) qPCR array analysis of genes found to have a ±1.35-fold differential expression upon mechanical stimulation. The genes were classified into seven groups. The pie chart illustrates the number of differentially expressed genes within each class and the table contains the number of up- and down-regulated genes within each class. (B) qPCR confirmation of select genes with downregulated expression upon stimulation. These differentially expressed genes were selected for further confirmation by qPCR. Three biological replicates were used for qPCR of ITGB3, MTSS1, MYL9 and ACTN3; four biological replicates were used for qPCR of PTK2B. For every biological replicate, two technical replicates were performed; values represent mean±s.e.m. All results were P<0.05 (two-tailed t-test).
From the differentially expressed genes, several were selected based upon their known association with cancer cell invasion and/or mechanosensing (Calvo et al., 2013; Ciobanasu et al., 2013; Prager-Khoutorsky et al., 2011; Shams et al., 2012; Xie et al., 2011) and confirmed by qPCR. The following downregulated genes were chosen: ITGB3 (encoding integrin β3), MTSS1 (encoding metastatic suppressor 1), PTK2B (encoding protein tyrosine kinase 2B), MYL9 (encoding myosin light chain 9) and ACTN3 (encoding actinin 3). Similar to the PCR array analysis, qPCR was performed with RNA extracted from cells incubated under stimulated and unstimulated conditions for 48 h. For every gene, at least two biological replicates were used, and for every biological replicate two technical replicates were performed. The housekeeping gene GAPDH was used for normalization of gene expression. qPCR analysis confirmed the downregulated expression of ITGB3, MTSS1, PTK2B, MYL9 and ACTN3 in response to mechanical stimulation (Fig. 2B).
Downregulation of integrin β3 expression upon mechanical stimulation
From the confirmed differentially expressed genes, ITGB3 was chosen for further analysis. ITGB3 codes for integrin β3, one of the β subunits within the integrin family, and is considered to be a mechanosensor (Rathinam and Alahari, 2010). More importantly, integrin β3 pairs with integrin αv or integrin αIIb to form heterodimeric integrin molecules. These integrin β3-containing heterodimers are known to bind arginine-glycine-aspartic acid (RGD) domains of fibronectin (Xiong et al., 2002). As fibronectin is required for the enhanced cellular response in our mechano-invasion assay (Menon and Beningo, 2011), the downregulation of integrin β3 upon mechanical stimulation was intriguing. Additionally, it is known that the expression levels of integrin β3 can vary in different types of cancer and that this change in expression can affect cell invasion (Jin and Varner, 2004; Seguin et al., 2015; Sheldrake and Patterson, 2009).
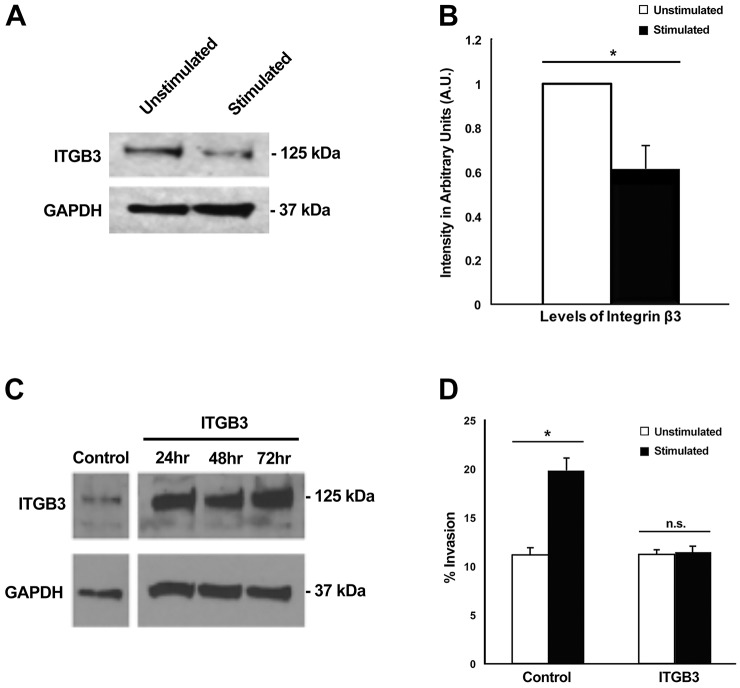
We first confirmed the downregulation of integrin β3 at the translational level. Western blot analysis was performed on cell lysates from cultures that were mechanically stimulated or unstimulated for 48 h in the mechano-invasion assay (Fig. 3A). Lysates from mechanically stimulated cells had ∼0.6 times integrin β3 compared to that from unstimulated cells. This value was comparable to the change seen on gene expression levels, where stimulated samples contained 0.5 times the ITGB3 mRNA compared to unstimulated samples (Fig. 2B), thereby validating that mechanical stimulation also reduced the amount of integrin β3 protein expressed by the cell.
Fig. 3.
Downregulation of integrin β3 expression upon mechanical stimulation enhances cell invasion. (A) Western blot of lysates, examining ITGB3 expression with and without stimulation. (B) Quantification of ITGB3 protein levels with and without stimulation. Results are mean±s.e.m. (n=4). *P=0.0059 (Student's t-test). (C) Integrin β3 protein levels when overexpressed (ITGB3) via nucleofection compared to mock nucleofected cells (control). (D) After 48 h with and without stimulation, the percentage of cell invasion was examined in mock nucleofected cells (control) and cells with integrin β3 overexpressed (ITGB3). Percentage invasion was determined by dividing the number of invaded cells (cells below the matrix surface) by the total number of cells. Results are mean±s.e.m. (n=4). *P<0.05; n.s., not significant (two-factor ANOVA).
It was entirely possible that downregulation of integrin β3 was just an outcome of enhanced invasion and not required for mechanosensing. To test the functional significance of the downregulation of integrin β3, we overexpressed the gene in HT1080 fibrosarcoma cells and tested the invasive response of these cells in the mechano-invasion assay. If downregulation of integrin β3 were required for cells to sense the mechanical stimulus and produce enhanced invasion, overexpression of integrin β3 would be expected to inhibit the enhancement of invasion.
Integrin β3 was overexpressed in HT1080 cells and a western blot confirmed that the protein was stably overexpressed by 2-fold for up to 72 h (Fig. 3C). Overexpressing cells were seeded on collagen–fibronectin matrices 24 h after transfection and incubated with or without mechanical stimulation for 48 h. It was observed that cells overexpressing integrin β3 failed to respond to mechanical stimulation, as they did not display enhanced invasion compared to control (Fig. 3D), thus confirming that downregulation of integrin β3 is required for the enhanced invasion as a result of stimulation.
Downregulation of integrin β3 is not accompanied by increased activity of integrin β1
Numerous instances of integrin crosstalk have been reported, where binding of one integrin to its ligand causes a perturbation in the expression or activity of other integrins. Such crosstalk is known to occur between integrin β3 and another fibronectin-binding family member, integrin β1 (Gonzalez et al., 2010). To address the potential of crosstalk between these two integrins, we tested the possibility that downregulation of integrin β3 is accompanied by an increase in the activation of integrin β1. A re-evaluation of the PCR array analysis indicated there was no difference in the expression levels of integrin β1 upon mechanical stimulation (Fig. S1A). However, as a result of crosstalk between integrin β1 and β3, the regulation may not occur at the level of expression but in the degree of receptor activation of integrin β1. We used an antibody specific to the active conformation of integrin β1 to test this idea. After 48 h with and without stimulation, we determined that the levels of activated integrin β1 upon mechanical stimulation were not significantly different from controls (Fig. S1B,C). These results suggest that no crosstalk occurs between integrin β1 and β3 subunits in the invasive response observed upon mechanical stimulation, therefore eliminating an obvious divergent pathway.
Downregulation of integrin β3 results in higher levels of the active form of cofilin upon mechanical stimulation
To identify potential pathways that are downstream of integrin β3, we focused on the relationship between integrins and cofilin. Cofilin is known to sever actin filaments and is necessary for invadopodia maturation (Yamaguchi et al., 2005). Moreover, in our in vitro mechano-invasion assay, cells treated with siRNA against cofilin failed to sense the mechanical stimulation and did not show enhanced invasion, yet retained a basal level of invasion (Menon and Beningo, 2011). As cofilin activity is controlled by its phosphorylation at Ser3 (Ser3 phospho-cofilin) (Pollard and Borisy, 2003), we speculated that mechanically stimulated cells would have lower levels of Ser3 phospho-cofilin (inactive state) compared to unstimulated cells. Additionally, if cofilin activity is dependent on integrin β3 expression, overexpression of integrin β3 should increase the levels of inactive phospho-cofilin upon mechanical stimulation.
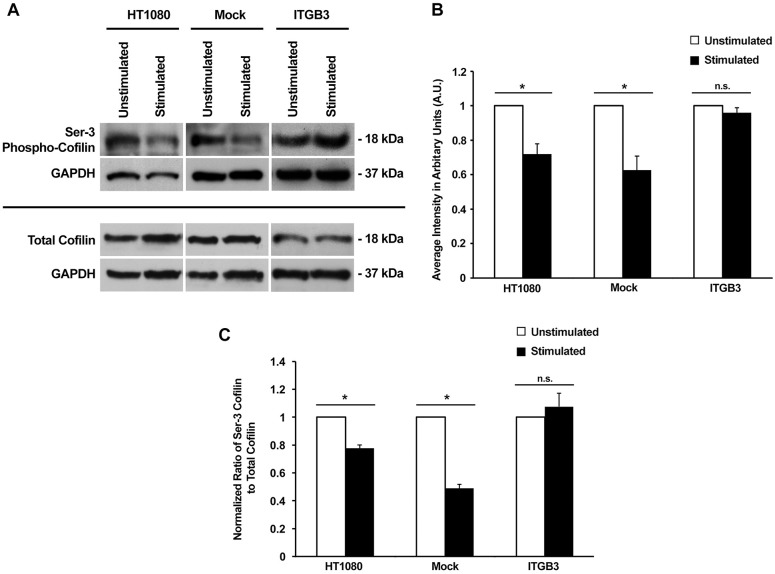
To test our prediction, cell lysates were collected from cells cultured on collagen–fibronectin matrices and incubated with or without mechanical stimulation for 48 h. It was determined that levels of Ser3 phospho-cofilin were indeed decreased upon mechanical stimulation, indicating that more cofilin was likely to be in its active state in those conditions (Fig. 4A). Lysates collected from mock nucleofected cells also showed a similar decrease in Ser3 phospho-cofilin upon mechanical stimulation. Quantitation of band intensities revealed that Ser3 phospho-cofilin levels upon stimulation were ∼0.65 times lower when compared to the unstimulated samples in both wild-type cells and mock nucleofected cells (Fig. 4B). However, when integrin β3 was overexpressed, the effect of mechanical stimulation on Ser3 phospho-cofilin level was reversed, such that similar levels of Ser3 phospho-cofilin were observed in integrin β3-overexpressing cells with or without mechanical stimulation. In addition, the amount of total cofilin was unchanged in wild-type cells, integrin β3-overexpressing cells and mock nucleofected cells between stimulated and unstimulated conditions (Fig. 4B). When the relative amount of Ser3 phospho-cofilin was compared to total cofilin levels, it was apparent that mechanical stimulation significantly reduced the fraction of cofilin that was phosphorylated in wild-type and mock nucleofected cells. When integrin β3 is overexpressed, this effect was abolished (Fig. 4C). Our data strongly suggest that the amount of active non-phosphorylated cofilin increases in response to mechanical stimulation, and this increase in active cofilin is brought about by downregulated expression of the integrin β3 mechanoreceptor.
Fig. 4.
The decrease in levels of Ser3 phospho-cofilin upon mechanical stimulation is dependent on the downregulation of integrin β3 expression. (A) Lysates from wild-type (HT1080), mock nucleofected (Mock) and overexpressing integrin β3 (ITGB3) cells cultured with or without stimulation were used for analysis of Ser3 phospho-cofilin and total cofilin levels. GAPDH served as a loading control. (B) Quantification of Ser3 phospho-cofilin levels with and without stimulation. Results are mean±s.e.m. from three biological replicates. *P<0.05; n.s., not significant (two-tailed t-test). (C) The normalized ratio of Ser3 phospho-cofilin to total cofilin based on data obtained in A. Results are mean±s.e.m. (n=3). *P<0.05; n.s., not significant (two-tailed t-test). In B and C, the data was normalized within each condition.
In vitro mechanical stimulation results in the lengthening of invadopodia
Previous studies have shown that invadopodia become more enzymatically active in response to changes in ECM rigidity (Jerrell and Parekh, 2016; Parekh and Weaver, 2016) but the maturation of invadopodia in response to contractile tugging forces present within the ECM has not been examined. In our assay, invasion occurred at a basal level without mechanical stimulation, but only in cells known to be invasive, suggesting that existing invasive machinery, such as invadopodia, were functioning without mechanical stimulation. Coupled with the observation that greater levels of active cofilin were available upon mechanical stimulation, we reasoned that transient mechanical stimulation was enriching the activity of the existing invadopodia. Specifically, we predicted that our form of mechanical stimulation was enhancing the invasive abilities of the cancer cells by promoting the maturation of invadopodia.
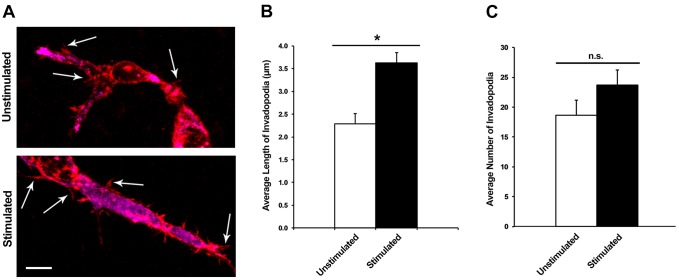
To test this prediction, we used confocal microscopy to observe changes in the number and length of invadopodia upon transient mechanical stimulation. Briefly, cells were seeded onto collagen–fibronectin matrices with and without mechanical stimulation. The matrices were then chemically fixed and probed with an antibody to cortactin, an identifying marker of invadopodia. Fluorescent secondary antibodies and phalloidin were used to label individual invadopodia for confocal microscopy. z-stack images were taken of randomly selected cells, and punctate areas of cortactin and actin colocalization served as a marker for individual invadopodia (Fig. 5A). These areas of colocalization were measured in the z-plane and an average length was calculated.
Fig. 5.
Mechanical stimulation produces an increase in the length of invadopodia without affecting the number of invadopodia. (A) Representative confocal fluorescent images of HT1080 cells fixed within the collagen–fibronectin matrix with and without mechanical stimulation. Red indicates actin and magenta indicates cortactin. Arrows indicate invadopodia, based on cortactin and actin colocalization. Scale bar: 10 µm. (B) The average length of invadopodia per cell after 48 h with and without stimulation. Results are mean±s.e.m. (n=16). *P<0.05 (two-tailed t-test). (C) The average number of invadopodia per cell with and without stimulation. Results are mean±s.e.m. (n=16). n.s., not significant (two-tailed t-test).
After 48 h of mechanical stimulation, we found that the invadopodia in stimulated cells had an average increase in length of 1.4 µm compared to unstimulated cells (Fig. 5B). This data suggested that invadopodia in stimulated cells were indeed increasing in length and likely to be more mature. Additionally, this maturation would likely be accompanied by an increase in MMP activity.
We also examined the change in the number of invadopodia per cell upon mechanical stimulation, as this may also account for the enhanced invasion observed in stimulated cells. Under the same conditions used to measure invadopodia length, we determined that no significant change (P>0.05) in number of invadopodia occurred between stimulated and unstimulated cells (Fig. 5C).
Integrin β3 overexpression inhibits invadopodia maturation upon stimulation
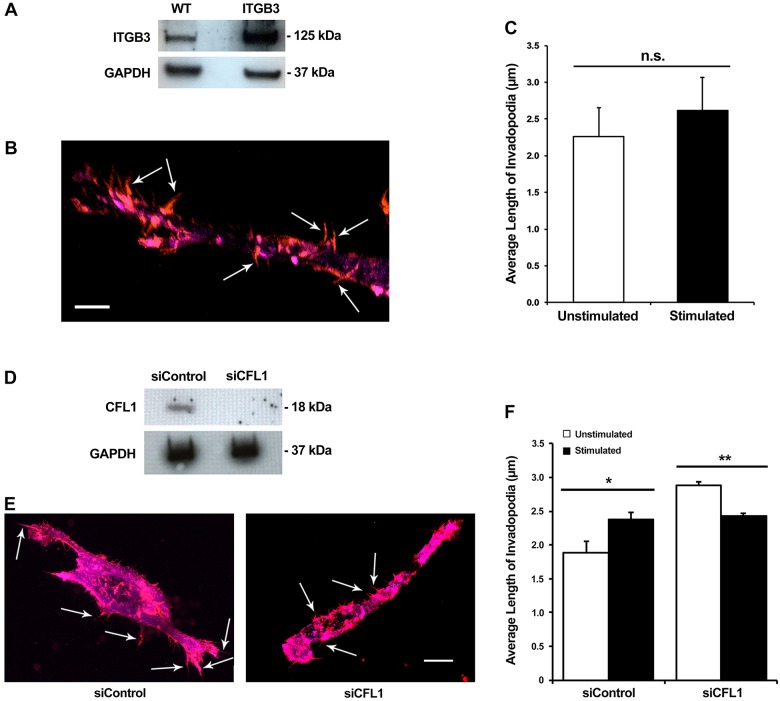
Integrin β3 is required for sensing the rigidity of matrices and is known to have an increased affinity for the ECM under mechanical strain (Jiang et al., 2006; Katsumi et al., 2005). However, unlike these previous studies, with our specific form of mechanical stimulation we find the opposite – integrin β3 expression is downregulated in response to tugging forces. To examine the effect of integrin β3 expression on invadopodia maturation, confocal microscopy was used to observe invadopodia in cells with overexpression of integrin β3 (Fig. 6A,B). As 24 h was the most effective time point for overexpression, cells were seeded onto invasion assay plates at that time point and allowed to invade into the matrix for 48 h with and without stimulation. After fixation and immunolabeling, z-stack confocal microscopy indicated that the length of invadopodia does not change significantly with transient mechanical stimulation when integrin β3 is overexpressed (Fig. 6C). This suggests that integrin β3 is regulating some aspect of a signaling pathway involved in the maturation of invadopodia, as overexpression of integrin β3 decreases invasion to basal levels when mechanical stimulation is applied.
Fig. 6.
Overexpression of integrin β3 and downregulation of cofilin expression both negatively affect the lengthening of invadopodia upon stimulation. (A) Western blot confirming overexpression of integrin β3 in protein lysates from control vector (WT) and integrin β3 (ITGB3)-overexpressing cells after 48 h. (B) A representative confocal fluorescent image of a fixed cell with integrin β3 overexpression residing within the collagen–fibronectin matrix. Red indicates actin and magenta indicates cortactin. Invadopodia are indicated by the arrows. Scale bar: 5 µm. (C) Measurement of invadopodia length with and without stimulation in cells that overexpress integrin β3. Results are mean±s.e.m. from four biological replicates. n.s., not significant (two-tailed t-test). (D) Western blot of cofilin knockdown by cofilin-specific siRNA (siCFL1). siControl, control siRNA. (E) Representative confocal fluorescent images of control siRNA and siCFL1 nucleofected cells chemically fixed within the matrix. Red indicates actin and magenta indicates cortactin. Arrows indicate individual invadopodia. Scale bar: 10 µm. (F) Invadopodia length with and without stimulation in cells nucleofected with control siRNA and siCFL1. Results are mean±s.e.m. from four biological replicates. *P<0.05, **P<0.01 (two-tailed t-test).
The elongation of invadopodia that occurs during mechanical stimulation is attenuated when cofilin is knocked down
It has been demonstrated that cofilin expression is necessary in cancer cells in order for them to be invasive (Menon and Beningo, 2011; Nagai et al., 2011). Furthermore, cofilin activity is required for the actin polymerization needed for the maturation of invadopodia; hence, knockdown of cofilin should reduce invadopodia elongation when cells are mechanically stimulated. siRNA was used to reduce cofilin expression in cells, with off-target siRNA serving as a negative control (Fig. 6D). Cells were mechanically stimulated after siRNA transfection, then fixed and immunolabeled to visualize invadopodia through confocal microscopy (Fig. 6E). When cofilin expression is reduced, cells produced significantly shorter invadopodia in stimulated cultures than do equivalent control siRNA cells (Fig. 6F). This result confirms that cofilin is a key factor in inducing the maturation of invadopodia in response to the mechanical stimulation provided by our assay.
Mechanical stimulation increases the expression and activity of MMP-2
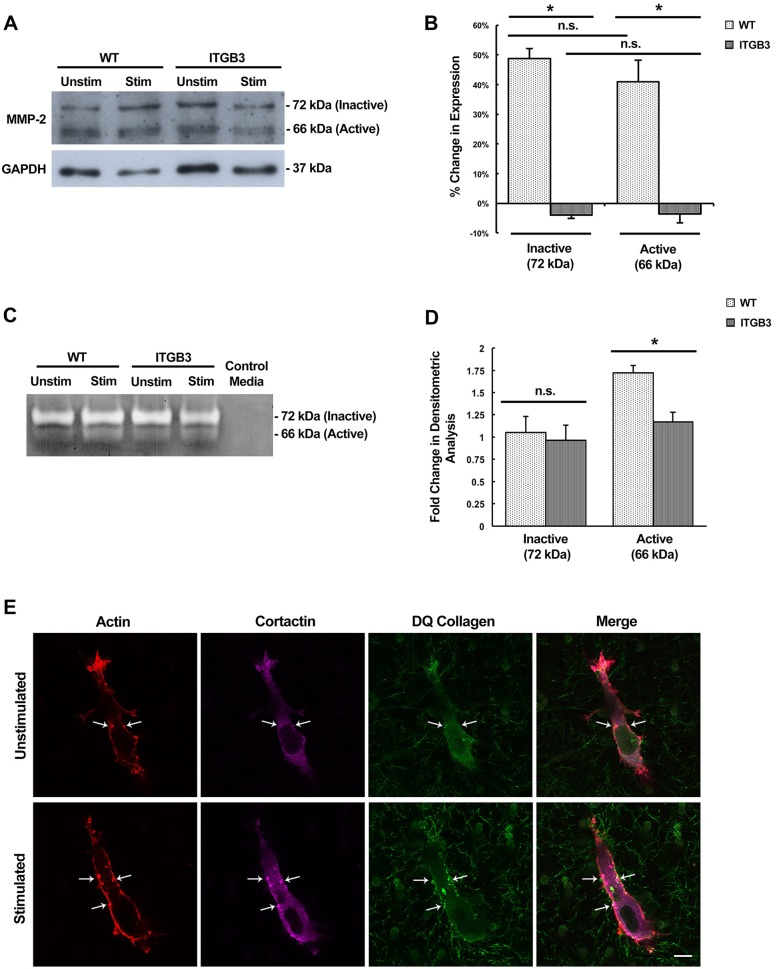
The maturation of invadopodia is associated with the targeted expression and secretion of MMP enzymes that degrade the surrounding ECM. One of these MMP enzymes associated with invadopodia is MMP-2, which specifically recognizes both collagen and fibronectin as its substrates. Two isoforms of MMP-2 are detectable by western blotting: the inactive pro-form (72 kDa) and the secreted proteolytically active form (66 kDa). We examined both the expression and secretion of MMP-2 with and without stimulation. Prior to cell lysis for total protein collection, conditioned medium was collected from cells containing the control vector and integrin β3 overexpression plasmid.
We determined that mechanical stimulation increases MMP-2 expression, supporting the idea that our mechanical stimulus is inducing the maturation of invadopodia. Upon stimulation, control cells have an average increase of ∼50% and ∼30% MMP-2 expression upon stimulation for the inactive and active isoforms, respectively (Fig. 7A,B). Furthermore, overexpression of the integrin β3 receptor negates this effect, as there was no detectable increase in expression of either MMP-2 isoform (Fig. 7A,B). This supports our observation that invadopodia maturation is reduced upon stimulation as a result of integrin β3 overexpression, as MMP-2 expression is a hallmark of invadopodia maturation.
Fig. 7.
MMP-2 protein expression and enzymatic activity is enhanced upon mechanical stimulation and inhibited by integrin β3 overexpression. (A) Western blot analysis of protein lysates from control vector (WT) and integrin β3 (ITGB3)-overexpressing cells after 48 h. Two isoforms of MMP-2 are detected: the inactive form (72 kDa) and the active form (66 kDa). GAPDH served as the loading control. (B) The change in expression of MMP-2 inactive and active isoforms upon stimulation in WT and ITGB3 cells. Results are mean±s.e.m. from three biological replicates. (C) Zymography of conditioned medium collected from WT and ITGB3 cells with and without mechanical stimulation. Control medium served as a negative control. Unstained bands (white) indicate enzymatic proteolysis. (D) Quantification of zymogram band intensities in WT and ITGB3 cells. Results are mean±s.e.m. from three biological replicates. (E) Representative confocal fluorescent images of DQ collagen-containing matrices from stimulated and unstimulated cells. Punctate structures with actin, cortactin and DQ collagen colocalization are invadopodia. Select invadopodia are indicated by the arrows. *P<0.01; n.s., not significant (two-tailed t-test). Scale bar: 10 µm.
When secretion of MMP-2 was examined by gelatin zymography of the conditioned medium, a similar observation was made. Cells containing the control plasmid showed an increase in MMP-2 proteolytic activity upon mechanical stimulation while integrin β3-overexpressing cells showed no change in secreted MMP-2 activity (Fig. 7C,D). This suggests that invadopodia in HT1080 cells are indeed increasingly mature upon stimulation, as both MMP-2 expression and secretion are upregulated. Overexpressing the integrin β3 receptor inhibits invadopodia maturation, which is evident by the lack of upregulated MMP-2 secretion and activity.
To visually examine the extent of collagen degradation activity in vitro, DQ collagen (type I) was mixed with the collagen–fibronectin matrices just prior to polymerization. This collagen allows for the microscopic analysis of proteolysis because it has an excessive amount of fluorescent dyes conjugated to it which causes a quenching effect. After it has been enzymatically degraded, the quenching is reduced and the local area becomes fluorescent (Jedeszko et al., 2008). Therefore, areas of DQ collagen fluorescence indicate collagenase proteolytic activity, which would be mediated by many of the invadopodia-associated MMP enzymes, including MMP-2. In our assay, areas of fluorescence were observed near areas of invadopodia that were deemed to be mature. Mechanically stimulated cells also appear to contain more numerous punctate degradation areas and at a higher fluorescence intensity (Fig. 7E). This suggests that ECM-degrading enzymatic activity is increased in mechanically stimulated cells.
DISCUSSION
Metastasis is a multistep process that is influenced by various biochemical and mechanical factors within the microenvironment of the tumor cell. In our previously described in vitro mechano-invasion assay, we mimicked the tugging forces produced by cellular movements within the ECM and discovered that they augment cell invasion (Menon and Beningo, 2011). We have now used this assay to identify the potential mechanism responsible for enhanced invasion in response to random tugging on the ECM fibers.
We identified several genes that are differentially expressed between mechanically stimulated and unstimulated conditions in the HT1080 human fibrosarcoma cell line. Many of the genes with differential expression are involved in cellular processes that are consistent upon mechanical stimulation enhancing cell invasion. For example, several genes involved in membrane protrusions were upregulated. As these genes are required for regulation of invadopodia activity in tumor cells, their upregulation suggested that more-active invadopodia were formed upon mechanical stimulation (Albiges-Rizo et al., 2009; Alexander et al., 2008). In addition, consistent with the idea that lower adhesion leads to an increase in the invasive capacity of a cell, we found that a greater number of cell adhesion genes were downregulated rather than upregulated. Among these genes discovered, we found that the expression of ITGB3, the integrin β3 receptor subunit, was downregulated upon stimulation. In agreement with our expression analysis, overexpression of integrin β3 inhibited the enhancement of invasion that typically occurs upon stimulation, confirming that integrin β3 must be downregulated upon mechanical stimulation in order for the cells to respond to the stimulus.
The mechanism of how the mechanical stimulation causes the downregulation of integrin β3 is currently unknown. Since fibronectin is required for sensing this mechanical stimulus (Menon and Beningo, 2011), it is entirely possible that downregulation of integrin β3 is caused by a feedback mechanism that is initiated by mechanosensing through integrin β3 itself. Alternatively, another integrin, such as integrin β1 could be responsible for the downregulation via a mechanism involving crosstalk. Nonetheless it was surprising that a known mechanoreceptor would be downregulated in response to mechanical stimulation. However, other studies have shown that expression of integrin β3 is variable in response to other forms of mechanical stimuli in various types of cancer (Felding-Habermann et al., 2001; Page et al., 2015). Additionally, a recent study found that HT1080 cells exhibit an MMP-independent ‘nuclear piston’ mechanism of invasion that requires integrin β3 activity (Petrie et al., 2017). It is an intriguing possibility that a cell uses the expression of integrin β3 to select between modes of invasion, such that downregulation promotes MMP-dependent invasion while normal levels of integrin β3 expression favors an MMP-independent nuclear piston mode of invasion.
Both integrin β1 and β3 are RGD-binding integrins (Branch et al., 2012) and are important in the regulation of invadopodia maturation (Beaty and Condeelis, 2014; Beaty et al., 2013; Knowles et al., 2013). As integrin β3 is downregulated upon mechanical stimulation, we examined the possibility that this was accompanied by an increase in activity of integrin β1, an integrin subunit known to localize to invadopodia (Mueller et al., 1999). Although we could not observe any significant change in the activation levels of integrin β1 by western blotting upon mechanical stimulation, it is possible that downregulation of integrin β3 might alter the localization of integrin β1 within the cell. For example, downregulation of integrin β3 may enrich integrin α5β1 at invadopodia, and thus increase their interaction with fibronectin resulting in enhanced mechanosensing. This has been observed previously where integrin β1 localization to invadopodia has led to increased invadopodia maturation via promotion of MMP secretion, interaction with Arg kinase (also known as ABL2) and signal complex formation with ezrin at lipid rafts (Antelmi et al., 2013; Beaty et al., 2013).
To confirm that maturation of invadopodia was stimulated by our mechanical signal, it was important to examine enzymatic activity, a hallmark of mature invadopodia (Jacob and Prekeris, 2015). Several MMPs have been associated with mature invadopodia, including MMP-2, which we found to have upregulated expression upon mechanical stimulation. Overexpression of integrin β3 abolished these effects, which further suggests that invadopodia maturation is increased upon downregulated integrin β3 expression. Microscopy of matrices containing DQ collagen showed increased areas of collagen degradation at and around individual invadopodia upon stimulation. Collectively, these results confirm that invadopodia maturation is upregulated upon mechanical stimulation, as increased enzymatic activity coincides with a measurable increase in invadopodia length. To what extent maturation is promoted is currently unknown. Mechanical stimulation may accelerate the rate of the maturation process and/or cause more of the nascent invadopodia to reach maturation compared to unstimulated conditions. However, the average number of invadopodia within a cell was relatively unaffected by mechanical stimulation, so it is likely that upregulated maturation is the dominant effect responsible for enhancing cell invasion instead of a disruption in invadopodia turnover.
The downstream signaling pathway that is affected by reduced expression of integrin β3 and the maturation of invadopodia is likely regulating cofilin activity. We have shown that cofilin is required for sensing the mechanical stimulation in our mechano-invasion assay (Menon and Beningo, 2011). The actin filament-severing activity of cofilin is regulated via phosphorylation by LIMK1 (Arber et al., 1998; Pollard and Borisy, 2003; Yamaguchi et al., 2005). The levels of Ser3-phosphorylated cofilin are low in highly metastatic cell lines derived from T-lymphoma and carcinoma (Nebl et al., 1996), and the invasiveness of cancer cells is correlated with overall output of the cofilin pathway (Wang et al., 2006; Zebda et al., 2000). The Ser3 phosphorylation of cofilin is linked to the ligand-binding ability of integrin αvβ3 as well as expression of MMP-2 (Dang et al., 2006). Our results suggest that cofilin is in its active state (low Ser3 phosphorylation) upon mechanical stimulation, and that this activation can be inhibited by overexpression of integrin β3. This suggests that an already established signaling pathway between integrin β3 and cofilin is likely affected by mechanical signaling leading to the maturation of invadopodia.
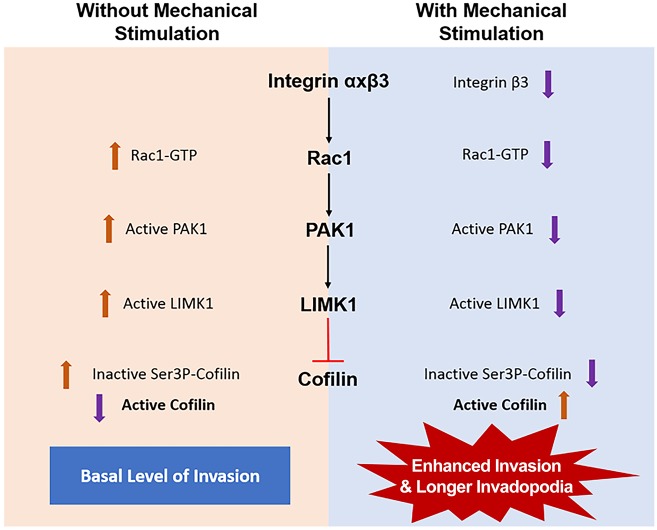
Based on our observations and current literature, we have developed a model signaling pathway connecting integrin β3 and cofilin activity and, consequently, the maturation of invadopodia (Fig. 8). We propose that integrin engagement and activation leads to activation of Rac1, a Rho GTPase known to be regulated by integrin β3 in focal complexes (Morgan et al., 2009). We hypothesize that upon mechanical stimulation, integrin β3 expression is reduced, resulting in poor activation of Rac1. The inactive GTP-bound Rac1 fails to phosphorylate and activate its down-stream effector p21-activated kinase-1 (PAK1) (del Pozo et al., 2000). The loss of PAK1 activity prevents the activation of LIMK1, which in turn fails to phosphorylate cofilin at the Ser3 position, rendering the cellular pool of cofilin active. Therefore, during unstimulated conditions, the amount of active cofilin is lower than inactive cofilin, yet it is enough to produce basal levels of both invasion and invadopodia maturation, such as those observed in our assay. During mechanical stimulation, the increase in active cofilin leads to a collective increase in invadopodia maturation that is responsible for enhancing invasion.
Fig. 8.
Potential pathway for enhanced invasion upon mechanical stimulation. Signaling downstream of integrin β3, through sequential activation of Rac1, PAK1 and LIMK1, results in inhibition of cofilin activity by phosphorylation at the Ser3 position. Upon mechanical stimulation, integrin β3 is downregulated thereby decreasing the levels of downstream kinases. A decrease in the activation of LIMK1 also decreases phosphorylation of cofilin. This leads to an increase in levels of active cofilin producing longer and more-mature invadopodia that enhance cell invasion.
As an aside, our study supports the idea that different types of force utilize different signaling pathways, with the force essentially acting as different ligands. In our previous study, we found that increasing the stiffness of the invasion assay matrix (4.5 mg/ml) did not affect the enhancement of invasion from tugging forces compared to that exerted by a less stiff matrix (2.5 mg/ml). In addition, an increase in stiffness alone, without the tugging force, did not increase the extent of invasion (Menon and Beningo, 2011). Importantly, it is worth noting that the matrix that we are using in this assay has a comparable stiffness to the stroma surrounding a tumor (Paszek et al., 2005), yet only with the transient tugging do invadopodia mature and enhance invasion. This implies that it is the tugging force that is responsible for the maturation of invadopodia and not the stiffness of the matrix. Furthermore, other studies performed in 2D cultures have shown that increasing collagen matrix stiffness results in an increase in the number of invadopodia (Alexander et al., 2008; Artym et al., 2015; Das et al., 2013). While our study has found that the number of invadopodia is unaffected by tugging forces, the length is, however, clearly affected. Taken together, these studies would suggest that highly invasive cells are capable of discerning a tugging force from a change in matrix stiffness and utilizing the message differently. Nonetheless, further investigation is needed to flush out this idea of selective cellular mechanical communication.
In conclusion, this study has uncovered a mechanosensitive signaling pathway that leads to the maturation of invadopodia and enhanced invasion of metastatic cancer cells in response to tugging forces. These findings suggest that transient tugging forces provide a unique biomechanical cue for the promotion of invasion, and extend our knowledge beyond those biomechanical cues currently recognized to promote tumor formation and cancer progression. This type of mechanical signal could be present in any extracellular microenvironment and is clearly exploited by the highly metastatic cancer cell to promote invasiveness, while non-invasive cells do not seem to have the capacity to use this specific form of mechanical signal. Untangling the multitude of biomechanical cues encountered by a cancer cell will take us closer to understanding how mechanical cues are used by the cancer cell for the progression of the disease.
MATERIALS AND METHODS
Cell culture
Experiments in this study were performed with HT1080 human fibrosarcoma cells (ATCC). Cells were maintained in Eagle's minimum essential medium (EMEM; ATCC) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin solution (100 U/ml penicillin and 100 µg/ml streptomycin; Life Technologies) at 37°C and 5% CO2. Cells were passaged via trypsinization with 0.25% trypsin (Sigma) and maintained up until the eighth consecutive passage. Cells were authenticated and tested negative for mycoplasma by the Biobanking and Correlative Services Core at the Karmanos Cancer Institute in October 2016.
In vitro mechano-invasion assay
The invasion matrices and the assay were setup and performed as previously described (Menon and Beningo, 2011). Briefly, 1.5×104 cells were seeded onto the sterilized collagen–fibronectin–paramagnetic bead matrix, and mechanical stimulation was provided by rotating a rare earth magnet of 12,100 Gauss (25 mm×5.5 mm) 1.5 cm beneath the culture on an orbital shaker (Barnstead Thermolyne) at 160 rpm (2.6 Hz). Unstimulated matrices were incubated outside of the magnetic field. After 24 h, cells within ten random microscopic fields of matrix were counted under a 10× phase objective on an Olympus IX81 microscope. Cells within the field were counted, beginning on the surface of the matrix and at eight increments of 100 µm/step within the z-plane of the matrix. The percentage invasion was determined by calculating the number of invaded cells (cells below the surface of the matrix) divided by the total number of cells. Numerous controls for this assay have been previously performed, including verification that cells did not phagocytose the magnetic beads, that paracrine secretions were not a factor and that the matrix was not being remodeled or stretched in a manner that could cause pores in the matrix; in addition, multiple cell lines have been tested (Menon and Beningo, 2011).
Collagen degradation
A 2 mg/ml solution of collagenase type IV (Worthington Biochemical) in Hank's balanced salt solution (Life Technologies) was warmed to 37°C, and 2 ml was added to the matrix (matrix was physically removed from the culture well by using a spatula and added to a sterile tube). The matrix was incubated in a 37°C water bath with intermittent shaking for 10 min to degrade the collagen. Cells were separated by centrifugation at ∼500 g for 5 min at 37°C. The resulting pellet contained both whole cells and the paramagnetic beads from the matrix. The pellet was washed with sterile 1× PBS at 37°C.
RNA extraction
The RNA was extracted from invaded cells following matrix degradation. The matrices for RNA extraction were seeded with 7×104 HT1080 cells on matrices scaled-up in larger wells of 1 mm×3 cm. For each independent experiment, unstimulated and stimulated matrices were made in duplicates. A Qiagen RNeasy mini kit was used for RNA extraction from pelleted cells. To prevent column clogging, paramagnetic beads from the pellet were pulled down with a magnet before loading the lysate onto the column. Genomic DNA contamination was removed by Qiagen on-column DNaseI digestion. RNA was eluted in 20 µl of DNase- and RNase-free water. The quality of the acquired RNA was assessed by using a NanoDrop spectrophotometer (ThermoScientific). Only RNA samples having 260 nm/280 nm absorption values≥2.0, 260 nm/230 nm absorption values≥1.7 and concentration ≥40 µg/ml were used.
PCR array and qPCR analysis
RNA obtained from both unstimulated and stimulated cells from the invasion assay were used to make cDNA and used for the PCR array as well as qPCR analysis. For each experiment, 1 µg of RNA from each unstimulated and stimulated sample was converted into cDNA using RT² First Strand Kit (SA Biosciences, for PCR array analysis) or GoScript™ Reverse Transcriptase (Promega, for qPCR). To identify differentially expressed genes between stimulated and unstimulated conditions from the invasion assay, the following PCR arrays were purchased from SA Biosciences; Cell Motility PCR Array, Tumor Metastasis PCR Array and ECM and Adhesion Molecules PCR Array. Each PCR array contained primers against 84 candidate genes related to cell motility, tumor metastasis and ECM and adhesion molecules. PCR array analysis was performed by using a RT² qPCR SYBR Green/ROX MasterMix-12 (SA Biosciences) on a Stratagene Mx3000P instrument. The raw data was analyzed using the web-based RT2 Profiler PCR Array Data Analysis software (SA Biosciences). Gene expression was normalized using five housekeeping genes: actin (ACTB), β-2-microglobulin (B2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxanthine phosphoribosyltransferase 1 (HPRT1) and large ribosomal protein (RPL13A). Data from the PCR array have been deposited in the Gene Expression Omnibus (GEO) database under accession ID GSE98917 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE98917). For confirming the differential expression, qPCR primers were designed for the genes showing more than 1.35-fold differential expression in the PCR array analysis. To design the necessary qPCR primers, online PrimerQuest software (Integrated DNA Technologies) was used. The following primers were synthesized: GAPDH (GAPDH-F, 5′-TTCGACAGTCAGCCGCATCTTCTT-3′; GAPDH-R, 5′-ACCAAATCCGTTGACTCCGACCTT-3′), ACTN3 (ACTN3-F, 5′-CAATGGCCTCAAACTCATGCTGCT-3′; ACTN3-R, 5′-TCTCTTCAGCACCAATGGACACCA-3′), CTTN (CTTN-F, 5′-TCCAAAGGATTCGGCGGGAAGTAT-3′; CTTN-R, 5′-ACCTGGGTGACATCCTCAAAGGTT-3′), MYL9 (MYL9-F, 5′-GGCCACATCCAATGTCTTCGCAAT-3′; MYL9-R, 5′-AGCCATCACGGTTCTGGTCAATCA-3′), PTK2B (PTK2B-F, 5′-AGAAGTTCATGAGCGAGGCAGTGA-3′; PTK2B-R, 5′-ATTCCATGATGATCCAGGTGGGCT-3′), ITGB3 (ITGB3-F, 5′-TGGACAAGCCTGTGTCACCATACA-3′; ITGB3-R, 5′-TTGTAGCCAAACATGGGCAAGCAG-3′), and MTSS1 (MTSS1-F, 5′-ATCAAGATGGGCTTTGCCGTTTCC-3′; MTSS1-R, 5′-AGCCAAACCGCTCTGTAGGGTATT-3′). The GAPDH gene was used for normalization during the analysis. The qPCR analysis of individual genes was performed using the RT² qPCR SYBR Green/ROX MasterMix-12 (SA Biosciences) on a Stratagene Mx3000P instrument. For every gene, at least two biological replicates were performed and for every biological replicate, two technical replicates were performed. The raw data obtained was analyzed using Stratagene Mx-Pro Mx3000P software. Student's t-test was performed to determine the significance of difference in gene expression.
Integrin β3 overexpression
A plasmid with the human ITGB3 gene, pcDNA3.1-beta-3, deposited by Timothy Springer (Addgene #27289; Takagi et al., 2002), was used for integrin β3 overexpression in HT1080 cells. Cells were grown to ∼85% confluency and nucleofected by using the Amaxa Nucleofector 2 device (Lonza) with the nucleofector kit T (Lonza). After nucleofection, cells were seeded onto 100 mm culture dishes for 24 h and incubated at 37°C with 5% CO2. In the case of invasion assays performed for protein extraction, nucleofected cells were directly seeded onto collagen–fibronectin matrices prior to incubation.
Protein extraction
Triple-detergent lysis buffer (TDLB; 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) mixed with Protease Inhibitor Cocktail (Sigma) and Halt™ Phosphatase Inhibitor Cocktail (ThermoScientific) was used for protein extraction for western blot analysis. Cells were isolated at 24, 48 and 72 h post-nucleofection, rinsed with 1× PBS and incubated with TDLB for 20 min under ice-cold conditions. Lysates were centrifuged at 4°C for 10 min at ∼5500 g to remove cell debris.
For protein extraction from invaded cells, duplicates of unstimulated and stimulated matrices were prepared. Matrices were degraded with collagenase, and cells were pelleted by centrifugation as described above. The cell pellet was incubated with 250 µl TDLB for 20 min under ice-cold conditions. Paramagnetic beads were separated from lysates with a magnet. Lysates were centrifuged at 4°C for 5 min at ∼7000 g to remove cell debris. This solution was then mixed with 50 µl of 6× Laemmli buffer (reducing or non-reducing, based on the antibody to be used; Boston BioProducts) and boiled for 10 min.
Conditioned medium
For the collection of secreted MMP-2, the first 24 h of the mechano-invasion assay was allowed to proceed as normal. The medium was then removed from the invasion assay cultures, and the cultures were rinsed twice with warmed 1× PBS. The medium was replaced with serum-free medium for an additional 24 h of stimulation. Medium was collected from the invasion assays immediately before the cells were lysed. The collected medium was concentrated using protein concentrators with a molecular mass cut-off of 10 kDa (ThermoFisher). Protein concentration was measured by using the DC protein assay (Bio-Rad).
Western blotting
Western blotting was performed with protein samples collected from cells following 48 h with and without mechanical stimulation. Proteins were then transferred onto a PVDF membrane (Bio-Rad) and blocked using 5% milk in 0.1% Tween-80 in PBS (PBS/T; for GAPDH and cofilin), 5% milk in 0.1% Tween-80 in TBS (TBS/T; for integrin β3, integrin β1 and MMP-2) or 5% BSA in 0.1% TBS/T (for phospho-cofilin). Primary antibody dilutions were made in the same solutions used for blocking (except for integrin β3 for which 1% milk in 0.1% TBS/T was used) and incubated at 4°C overnight (except for cofilin; 4 h at RT). The membrane was washed in 0.1% PBS/T (GAPDH and cofilin) or 0.1% TBS/T (phospho-cofilin, integrin β3, active integrin β1 and MMP-2). Secondary antibody dilutions were made in the same solution as that of primary and incubated at RT 1 h. The following antibodies were used: rabbit polyclonal against integrin β3 (1:300, cat# sc-14009, Santa Cruz Biotechnology), rat monoclonal against active integrin β1 (1:5000, cat# 553715, BD Pharmingen), mouse monoclonal against cofilin (1:300, cat# ab54532, Abcam), rabbit monoclonal against phospho-cofilin (Ser3) (1:1000, cat# 3313, Cell Signaling Technology), rabbit polyclonal against MMP-2 (1:500, cat# bs-4599R, Bioss), mouse monoclonal against GAPDH (1:15,000, cat# MAB374, Millipore), horseradish peroxidase (HRP)-tagged anti-mouse-IgG (Fisher), HRP-tagged anti-rat-IgG (Abcam), and HRP-tagged anti-rabbit-IgG (Amersham). Following secondary antibody incubation, membranes were washed with 0.1% PBS/T or 0.1% TBS/T and incubated with Amersham ECL Prime Western Blotting Detection Reagent. Band intensity readings were measured and normalized using ImageJ (NIH).
Immunofluorescence
Cells embedded within the invasion assay matrices were chemically fixed at 37°C in a solution containing 2.5% paraformaldehyde (Electron Microscopy Sciences) and 1.33× PBS for 10 min followed by a permeabilization solution of 2.5% paraformaldehyde, 1.33× PBS and Triton X-100 for 10 min. The samples were quenched in a 0.5 mg/ml solution of NaBH4 (Sigma) in 1× PBS for 5 min at room temperature. Matrices were blocked in a solution containing 5% BSA (Fisher) in 1× PBS overnight at 4°C. A mouse monoclonal anti-cortactin (1:500, cat# ab33333, Abcam) was prepared in blocking solution overnight at 4°C. A secondary anti-mouse-IgG conjugated to Alexa Fluor 647 (1:450, Life Technologies) and Alexa-Fluor-546–phalloidin (1:200, Life Technologies) prepared in blocking solution was incubated at room temperature for 1 h and followed by 3 washes in 1× PBS.
Imaging and measurements of invadopodia
For invasion assays requiring imaging of invadopodia, DQ collagen type I (ThermoFisher) was added to the matrix and mixed thoroughly, just prior to polymerization at a concentration of 25 µg/ml.
Fixed invasion assay matrices (except cofilin knockdown) were imaged by using a Zeiss LSM 550 META NLO confocal microscope with an Achroplan 63× water-immersion objective. The microscope is housed in the Microscopy, Imaging and Cytometry Resources Core located at Wayne State University (Detroit, MI). The cofilin-knockdown experiment was imaged by using a Zeiss LSM 880 microscope with a Plan-Apochromat 63× oil-immersion objective. Images were taken at 0.4 µm intervals along the z-plane and a minimum of five cells per condition in three individual trials were imaged for each experiment. Images were viewed and channels merged using the Zeiss Zen software (version 2012) and ImageJ (NIH).
Measurements of invadopodia were obtained after first identifying protrusions within each cell that displayed cortactin and actin colocalization. The lengths of each invadopodia were measured by identifying the first appearance of colocalization within the z-stack (‘starting’ point) and ending when the colocalization became undetectable within the z-stack (‘ending’ point). The difference in these two values was recorded as the length for each individual invadopodia within each cell. The average length for invadopodia was calculated and the Student's t-test was performed to determine statistical significance.
Zymography
Concentrated conditioned medium was mixed with 6× Laemmli non-reducing loading buffer (Boston BioProducts) and 30 µg loaded onto an 8% SDS-PAGE gel containing 1 mg/ml gelatin (Sigma). After running for 1.5 h at 75 V, the gel was incubated in renaturing solution (2.5% Triton X-100) for 30 min at room temperature. After rinsing the gel twice with water, it was incubated in developing buffer (50 mM Tris-HCl, pH 7.8, 0.2 M NaCl, 5 mM CaCl2 and 0.02% Triton X-100) for 1 h at room temperature. The buffer was then replaced with fresh developing buffer for 16 h at 37°C. The gel was rinsed and stained with 0.05% Coomassie blue and destained with methanol and acetic acid until bands were clearly visible. Medium that had not been used in culture served as a negative control. Band intensities were quantified with ImageJ (NIH).
Supplementary Material
Acknowledgements
The authors wish to thank Imjoo Jang for his careful reading of the manuscript and the Microscopy, Imaging and Cytometry Resources Core at Wayne State University for their technical support. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by a National Institutes of Health center grant (P30 CA022453) to the Karmanos Cancer Institute at Wayne State University, and the Perinatology Research Branch of the National Institutes of Child Health and Development at Wayne State University.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.N.G., S.O., K.A.B.; Methodology: A.N.G., S.O., K.A.B.; Formal analysis: K.A.B.; Investigation: A.N.G., S.O., K.A.B.; Resources: K.A.B.; Writing - original draft: S.O., K.A.B.; Writing - review & editing: A.N.G., K.A.B.; Supervision: K.A.B.; Project administration: K.A.B.; Funding acquisition: K.A.B. A.N.G. and S.O. contributed equally to the collection of all data, preparation of figures and writing of the manuscript.
Funding
This study was partially funded by a grant awarded to K.A.B. by the Karmanos Cancer Institute and McClaren Health Systems, funds from Wayne State University and graduate enhancement funds to S.O.
Data availability
Data from the PCR array have been deposited in the Gene Expression Omnibus (GEO) database under accession ID GSE98917 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE98917).
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.199760.supplemental
References
- Albiges-Rizo C., Destaing O., Fourcade B., Planus E. and Block M. R. (2009). Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 122, 3037-3049. 10.1242/jcs.052704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N. R., Branch K. M., Parekh A., Clark E. S., Iwueke I. C., Guelcher S. A. and Weaver A. M. (2008). Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 18, 1295-1299. 10.1016/j.cub.2008.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S., Barbayannis F. A., Hanser H., Schneider C., Stanyon C. A., Bernard O. and Caroni P. (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805-809. 10.1038/31729 [DOI] [PubMed] [Google Scholar]
- Antelmi E., Cardone R. A., Greco M. R., Rubino R., Di Sole F., Martino N. A., Casavola V., Carcangiu M. L., Moro L. and Reshkin S. J. (2013). β1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS ONE 8, e75113 10.1371/journal.pone.0075113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M. and Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034-3043. 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- Artym V. V., Swatkoski S., Matsumoto K., Campbell C. B., Petrie R. J., Dimitriadis E. K., Li X., Mueller S. C., Bugge T. H., Gucek M. et al. (2015). Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol. 208, 331-350. 10.1083/jcb.201405099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty B. T. and Condeelis J. (2014). Digging a little deeper: the stages of invadopodium formation and maturation. Eur. J. Cell Biol. 93, 438-444. 10.1016/j.ejcb.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty B. T., Sharma V. P., Bravo-Cordero J. J., Simpson M. A., Eddy R. J., Koleske A. J. and Condeelis J. (2013). beta1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol. Biol. Cell 24, 1661-1675. 10.1091/mbc.E12-12-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L., Pollard T. D. and Mullins R. D. (2000). Interactions of ADF/cofilin, Arp2/3 complex, capping protein and profilin in remodeling of branched actin filament networks. Curr. Biol. 10, 1273-1282. 10.1016/S0960-9822(00)00749-1 [DOI] [PubMed] [Google Scholar]
- Blouw B., Patel M., Iizuka S., Abdullah C., You W. K., Huang X., Li J.-L., Diaz B., Stallcup W. B. and Courtneidge S. A. (2015). The invadopodia scaffold protein Tks5 is required for the growth of human breast cancer cells in vitro and in vivo. PLoS ONE 10, e0121003 10.1371/journal.pone.0121003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch K. M., Hoshino D. and Weaver A. M. (2012). Adhesion rings surround invadopodia and promote maturation. Biol. Open 1, 711-722. 10.1242/bio.20121867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R. P., Chaudhry S. I., Harrington K., Williamson P., Moeendarbary E., Charras G. et al. (2013). Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637-646. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella L. F., Buscemi L., Godbout C., Meister J.-J. and Hinz B. (2010). A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J. Cell Sci. 123, 1751-1760. 10.1242/jcs.066795 [DOI] [PubMed] [Google Scholar]
- Charras G. and Sahai E. (2014). Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15, 813-824. 10.1038/nrm3897 [DOI] [PubMed] [Google Scholar]
- Ciobanasu C., Faivre B. and Le Clainche C. (2013). Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur. J. Cell Biol. 92, 339-348. 10.1016/j.ejcb.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Clark E. S. and Weaver A. M. (2008). A new role for cortactin in invadopodia: regulation of protease secretion. Eur. J. Cell Biol. 87, 581-590. 10.1016/j.ejcb.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. S., Whigham A. S., Yarbrough W. G. and Weaver A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227-4235. 10.1158/0008-5472.CAN-06-3928 [DOI] [PubMed] [Google Scholar]
- Dang D., Bamburg J. R. and Ramos D. M. (2006). Alphavbeta3 integrin and cofilin modulate K1735 melanoma cell invasion. Exp. Cell Res. 312, 468-477. 10.1016/j.yexcr.2005.11.011 [DOI] [PubMed] [Google Scholar]
- Das A., Kapoor A., Mehta G. D., Ghosh S. K. and Sen S. (2013). Extracellular matrix density regulates extracellular proteolysis via modulation of cellular contractility. Carcinogen. Mutagene. S13, 003 10.4172/2157-2518.s13-003 [DOI] [Google Scholar]
- De Wever O., Demetter P., Mareel M. and Bracke M. (2008). Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer 123, 2229-2238. 10.1002/ijc.23925 [DOI] [PubMed] [Google Scholar]
- del Pozo M. A., Price L. S., Alderson N. B., Ren X. D. and Schwartz M. A. (2000). Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008-2014. 10.1093/emboj/19.9.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabets M., Gifford A. M., Scheel C., Nilsson B., Reinhardt F., Bray M.-A., Carpenter A. E., Jirström K., Magnusson K., Ebert B. L. et al. (2011). Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J. Clin. Invest. 121, 784-799. 10.1172/JCI43757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B., O'Toole T. E., Smith J. W., Fransvea E., Ruggeri Z. M., Ginsberg M. H., Hughes P. E., Pampori N., Shattil S. J., Saven A. et al. (2001). Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA 98, 1853-1858. 10.1073/pnas.98.4.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frittoli E., Palamidessi A., Disanza A. and Scita G. (2011). Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur. J. Cell Biol. 90, 108-114. 10.1016/j.ejcb.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Fuyuhiro Y., Yashiro M., Noda S., Matsuoka J., Hasegawa T., Kato Y., Sawada T. and Hirakawa K. (2012). Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells. Cancer Sci. 103, 797-805. 10.1111/j.1349-7006.2012.02209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparski A. N. and Beningo K. A. (2015). Mechanoreception at the cell membrane: More than the integrins. Arch. Biochem. Biophys. 586, 20-26. 10.1016/j.abb.2015.07.017 [DOI] [PubMed] [Google Scholar]
- Goffin J. M., Pittet P., Csucs G., Lussi J. W., Meister J.-J. and Hinz B. (2006). Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 172, 259-268. 10.1083/jcb.200506179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. M., Bhattacharya R., deHart G. W. and Jones J. C. R. (2010). Transdominant regulation of integrin function: mechanisms of crosstalk. Cell. Signal. 22, 578-583. 10.1016/j.cellsig.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. and Prekeris R. (2015). The regulation of MMP targeting to invadopodia during cancer metastasis. Front. Cell Dev. Biol. 3, 4 10.3389/fcell.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedeszko C., Sameni M., Olive M. B., Moin K. and Sloane B. F. (2008). Visualizing protease activity in living cells: from two dimensions to four dimensions. Curr. Protoc. Cell Biol. Chapter 4, Unit 4.20 10.1002/0471143030.cb0420s39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrell R. J. and Parekh A. (2014). Cellular traction stresses mediate extracellular matrix degradation by invadopodia. Acta Biomater. 10, 1886-1896. 10.1016/j.actbio.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrell R. J. and Parekh A. (2016). Matrix rigidity differentially regulates invadopodia activity through ROCK1 and ROCK2. Biomaterials 84, 119-129. 10.1016/j.biomaterials.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Huang A. H., Cai Y., Tanase M. and Sheetz M. P. (2006). Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys. J. 90, 1804-1809. 10.1529/biophysj.105.072462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H. and Varner J. (2004). Integrins: roles in cancer development and as treatment targets. Br. J. Cancer 90, 561-565. 10.1038/sj.bjc.6601576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A., Naoe T., Matsushita T., Kaibuchi K. and Schwartz M. A. (2005). Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 280, 16546-16549. 10.1074/jbc.C400455200 [DOI] [PubMed] [Google Scholar]
- Knowles L. M., Gurski L. A., Engel C., Gnarra J. R., Maranchie J. K. and Pilch J. (2013). Integrin alphavbeta3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 73, 6175-6184. 10.1158/0008-5472.CAN-13-0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A., Lynch C. D. and Sheetz M. P. (2009). Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS ONE 4, e6361 10.1371/journal.pone.0006361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. and Weaver V. M. (2009). Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113-127. 10.1007/s10555-008-9173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F. T., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891-906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes M. A. O., Larson D. R., Mader C. C., Bravo-Cordero J. J., Gil-Henn H., Oser M., Chen X., Koleske A. J. and Condeelis J. (2011). Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J. Cell Biol. 195, 903-920. 10.1083/jcb.201103045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S. and Beningo K. A. (2011). Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS ONE 6, e17277 10.1371/journal.pone.0017277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles F. L. and Sikes R. A. (2014). Insidious changes in stromal matrix fuel cancer progression. Mol. Cancer Res. 12, 297-312. 10.1158/1541-7786.MCR-13-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. R., Byron A., Humphries M. J. and Bass M. D. (2009). Giving off mixed signals--distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life 61, 731-738. 10.1002/iub.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkonjic S., Destaing O. and Albiges-Rizo C. (2016). Mechanotransduction pulls the strings of matrix degradation at invadosome. Matrix Biol. 57-58,190-203. 10.1016/j.matbio.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Ghersi G., Akiyama S. K., Sang Q.-X. A., Howard L., Pineiro-Sanchez M., Nakahara H., Yeh Y. and Chen W.-T. (1999). A novel protease-docking function of integrin at invadopodia. J. Biol. Chem. 274, 24947-24952. 10.1074/jbc.274.35.24947 [DOI] [PubMed] [Google Scholar]
- Murrell M., Oakes P. W., Lenz M. and Gardel M. L. (2015). Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486-498. 10.1038/nrm4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Moreno O., Smith C. A., Ivanchuk S., Romagnuolo R., Golbourn B., Weeks A., Seol H. J. and Rutka J. T. (2011). Role of the cofilin activity cycle in astrocytoma migration and invasion. Genes Cancer 2, 859-869. 10.1177/1947601911431839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl G., Meuer S. C. and Samstag Y. (1996). Dephosphorylation of serine 3 regulates nuclear translocation of cofilin. J. Biol. Chem. 271, 26276-26280. 10.1074/jbc.271.42.26276 [DOI] [PubMed] [Google Scholar]
- Oser M., Yamaguchi H., Mader C. C., Bravo-Cordero J. J., Arias M., Chen X., Desmarais V., van Rheenen J., Koleske A. J. and Condeelis J. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571-587. 10.1083/jcb.200812176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin M. J., Jonas O., Kosciuk T., Broye L. C., Guido B. C., Wyckoff J., Riquelme D., Lamar J. M., Asokan S. B., Whittaker C. et al. (2016). Tumor cell-driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 6, 516-531. 10.1158/2159-8290.CD-15-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page J. M., Merkel A. R., Ruppender N. S., Guo R., Dadwal U. C., Cannonier S. A., Basu S., Guelcher S. A. and Sterling J. A. (2015). Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin beta3 and TGF-beta receptor type II. Biomaterials 64, 33-44. 10.1016/j.biomaterials.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. and Weaver A. M. (2009). Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adh. Migr. 3, 288-292. 10.4161/cam.3.3.8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. and Weaver A. M. (2016). Regulation of invadopodia by mechanical signaling. Exp. Cell Res. 343, 89-95. 10.1016/j.yexcr.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D. et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241-254. 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Petrie R. J., Harlin H. M., Korsak L. I. T. and Yamada K. M. (2017). Activating the nuclear piston mechanism of 3D migration in tumor cells. J. Cell Biol. 216, 93-100. 10.1083/jcb.201605097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M. W., Mouw J. K. and Weaver V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243-1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincloux R., Lizarraga F. and Chavrier P. (2009). Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015-3024. 10.1242/jcs.034561 [DOI] [PubMed] [Google Scholar]
- Polette M., Nawrocki-Raby B., Gilles C., Clavel C. and Birembaut P. (2004). Tumour invasion and matrix metalloproteinases. Crit. Rev. Oncol. Hematol. 49, 179-186. 10.1016/j.critrevonc.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. and Borisy G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Prager-Khoutorsky M., Lichtenstein A., Krishnan R., Rajendran K., Mayo A., Kam Z., Geiger B. and Bershadsky A. D. (2011). Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 13, 1457-1465. 10.1038/ncb2370 [DOI] [PubMed] [Google Scholar]
- Rathinam R. and Alahari S. K. (2010). Important role of integrins in the cancer biology. Cancer Metastasis Rev. 29, 223-237. 10.1007/s10555-010-9211-x [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P., Iskratsch T. and Sheetz M. P. (2012). Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 125, 3025-3038. 10.1242/jcs.095794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. D., Coon B. G., Yun S., Baeyens N., Tanaka K., Ouyang M. and Schwartz M. A. (2013). Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25, 613-618. 10.1016/j.ceb.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U. S. and Gardel M. L. (2012). United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J. Cell Sci. 125, 3051-3060. 10.1242/jcs.093716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L., Desgrosellier J. S., Weis S. M. and Cheresh D. A. (2015). Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 25, 234-240. 10.1016/j.tcb.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams H., Golji J. and Mofrad M. R. K. (2012). A molecular trajectory of alpha-actinin activation. Biophys. J. 103, 2050-2059. 10.1016/j.bpj.2012.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake H. M. and Patterson L. H. (2009). Function and antagonism of beta3 integrins in the development of cancer therapy. Curr. Cancer Drug Targets 9, 519-540. 10.2174/156800909788486713 [DOI] [PubMed] [Google Scholar]
- Shieh A. C. (2011). Biomechanical forces shape the tumor microenvironment. Ann. Biomed. Eng. 39, 1379-1389. 10.1007/s10439-011-0252-2 [DOI] [PubMed] [Google Scholar]
- Takagi J., Petre B.M., Walz T. and Springer T.A. (2002). Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 110, 599-611. 10.1016/S0092-8674(02)00935-2 [DOI] [PubMed] [Google Scholar]
- Tahtamouni L. H., Shaw A. E., Hasan M. H., Yasin S. R. and Bamburg J. R. (2013). Non-overlapping activities of ADF and cofilin-1 during the migration of metastatic breast tumor cells. BMC Cell Biol. 14, 45 10.1186/1471-2121-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman R. W., Cowan C. R., Mih J. D., Koryakina Y., Gioeli D., Slack-Davis J. K., Blackman B. R., Tschumperlin D. J. and Parsons J. T. (2010). Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS ONE 5, e12905 10.1371/journal.pone.0012905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi M., Billet S. and Bhowmick N. A. (2012). Understanding the role of stromal fibroblasts in cancer progression. Cell Adh. Migr. 6, 231-235. 10.4161/cam.20419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich T. A., de Juan Pardo E. M. and Kumar S. (2009). The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167-4174. 10.1158/0008-5472.CAN-08-4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh V., Rape A. D., Ulrich T. A. and Kumar S. (2014). Microenvironmental stiffness enhances glioma cell proliferation by stimulating epidermal growth factor receptor signaling. PLoS ONE 9, e101771 10.1371/journal.pone.0101771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Liang S., Ghosh S., Hornsby P. J. and Li R. (2009). Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 28, 2745-2755. 10.1038/onc.2009.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Eddy R. and Condeelis J. (2007). The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 7, 429-40. 10.1038/nrc2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Mouneimne G., Sidani M., Wyckoff J., Chen X., Makris A., Goswami S., Bresnick A. R. and Condeelis J. S. (2006). The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J. Cell Biol. 173, 395-404. 10.1083/jcb.200510115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., Ye L., Ta M., Zhang L. and Jiang W. G. (2011). MTSS1: a multifunctional protein and its role in cancer invasion and metastasis. Front. Biosci. 3, 621-631. 10.2741/s175 [DOI] [PubMed] [Google Scholar]
- Xiong J.-P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L. and Arnaout M. A. (2002). Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296, 151-155. 10.1126/science.1069040 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T. et al. (2005). Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 168, 441-452. 10.1083/jcb.200407076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap C. T., Simpson T. I., Pratt T., Price D. J. and Maciver S. K. (2005). The motility of glioblastoma tumour cells is modulated by intracellular cofilin expression in a concentration-dependent manner. Cell Motil Cytoskeleton 60, 153-165. 10.1002/cm.20053 [DOI] [PubMed] [Google Scholar]
- Zebda N., Bernard O., Bailly M., Welti S., Lawrence D. S. and Condeelis J. S. (2000). Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J. Cell Biol. 151, 1119-1128. 10.1083/jcb.151.5.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.