Abstract
This is a case report of necrosis of more than two-third of the dorsal tongue in a 74-year-old male following prolonged oral intubation for vascular surgery. This necrosis progressed from the left tongue to involve much of the dorsal tongue bilaterally. A diagnosis of rhabdomyolysis was made evident by compartment syndrome of the legs with an elevated creatine kinase level of 89 789 u/l (units per litre). The literature also reveals that vasopressin has been linked with reported cases of tongue necrosis. Other possible aetiological factors were discussed in this finding.
Keywords: Tongue, Necrosis, Creatine Kinase, vasopressin, intubation
INTRODUCTION
The tongue is a well-vascularized organ, with a blood supply from the lingual artery, a branch of the external carotid artery. It has a limited secondary contralateral supply from the submucosal plexus. Necrosis of the tongue is not a common finding [1].
Tongue necrosis has most frequently been associated with giant cell arthritis [1–5], as seen in temporal arthritis, often diagnosed by a high erythrocyte sedimentation rate [1, 2, 6, 7]. It may also occur with cardiogenic shock [1], leading to end-organ hypoperfusion. Vascular compromise may occur with use of vasoconstrictors [2, 3] such as vasopressin, especially when a high dose or continuous infusion is required to maintain blood pressure [2, 5].
Tongue necrosis has also been associated with vascular diseases such as, Kawasaki disease [7, 8], Wegener granulomatosis [1, 3, 6], hypercoagulable condition such as disseminated intravascular coagulation and rheumatoid hyperviscosity syndrome [6].
CASE REPORT
A 74-year-old male patient was admitted in 2018 by the Vascular Team for a fenestrated cuff repair (type 1a) of an aortic endovascular leak. The patient had been admitted in 2009 for a 7.1 cm abdominal aortic aneurysm of the infra-renal aorta, and an endovascular aneurysm repair had been performed with an endurant bifurcated device. At the latest admission, a computer angiogram of the aorta revealed a type 1a endovascular leak of 6.4 cm.
Regular medications included warfarin, bisoprolol, glicalzide, linagliptin, prednisolone, ramipril and salbutamol. There were significant medical comorbidities including chronic obstructive pulmonary disease (COPD), high body mass index, atrial fibrillation, diabetes mellitus type 2, hypertension, smoking and obstructive sleep apnoea.
The patient was admitted to the adult intensive care unit (AICU) at our institute. The vascular surgery was prolonged with over 10 hours with oral intubation.
On Day 2, a diagnosis of rhabdomyolysis was made with clinical and imaging evidence of compartment syndrome of the legs with an elevated creatine kinase of 90 000 u/l. Bilateral fasciotomies was performed of both lower limbs due to a sudden onset of bilateral compartment syndrome. The patient had developed acute renal failure, and continuous renal replacement therapy was commenced for metabolic acidosis.
On Day 3, a black tongue was noted, and referral was made to the maxillofacial team (Fig. 1). Blackness of the left tongue dorsum was obvious. The patient was very unwell. Chlorhexidine mouth was prescribed.
Figure 1.
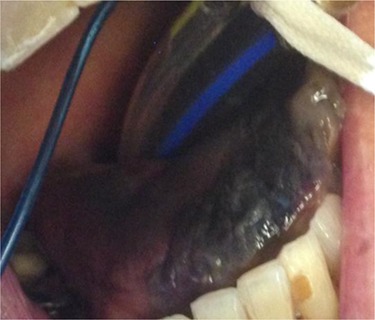
Necrosis of the tip of tongue.
At day 15, he was discharged to the ward but deteriorated the following day with respiratory failure secondary to hospital-acquired pneumonia and mucus plugging of the left lung.
He was reintubated and readmitted to AICU. Inotropic support was required for hypotension and bradycardia. Episodes of melana were noted and had blood transfused. Percutaneous tracheostomy was performed.
At Day 34, the patient was taken back to theatre by the maxillofacial team. Much of the anterior and part of the posterior third of the dorsum of the tongue was necrotic (Fig. 2). The necrotic area was removed, and the tongue debrided to healthy tissue.
Figure 2.
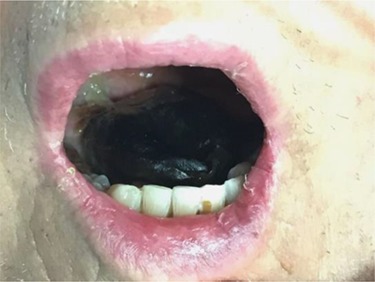
Bilateral dorsal tongue involvements.
At 12 months review, the dorsum of the tongue had healed with considerable scarring (Fig. 3). Despite the significant restriction in tongue movement and speech deficit, the patient however declined further surgical intervention.
Figure 3.
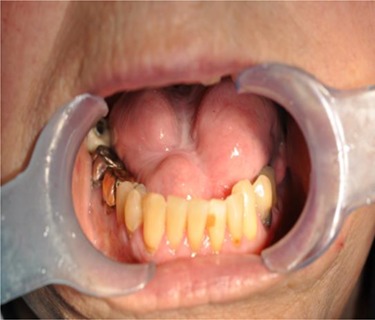
Extensive scarring of the tongue.
DISCUSSION
Ischaemic necrosis of the tongue has been reported in cases of giant cell arteritis (GCA), circulatory shock, Kawasaki disease, Wegener’s granulomatosis, disseminated intravascular coagulation and prolonged use of vasopressin. It is probably most commonly associated with GCA. There was no evidence of these alternative causes except for prolonged use of vasopressin.
Necrosis of the tongue in this case is unique because it was probably due to the prolonged oral intubation with compression by an anaesthetic tube. The necrosis of the dorsum of the tongue was progressive from the left to the right side to involve the side not compressed by the tube.
Using a flexible rather than a rigid tube would probably have minimized the risk of compression. In retrospect, a soft material (e.g. wet or greased sponge) could have been used as a barrier between the tongue and the oral tube.
It is speculated that type 2 respiratory failure, exacerbated by the pre-existing COPD and mucus plugging, together with the compartment syndrome may have resulted in reduced tissue perfusion and rhabdomyolysis. The pre-existing multiple comorbidities probably contributed to the development of multiple organ failure. The very high creatine kinase is a feature of this case not previously reported elsewhere.
ACKNOWLEDGEMENT
We are grateful to patient for allowing this report.
FUNDING
This study was funded by Leicester Royal Infirmary.
References
- 1. Benjamin RR, Immerman SB, Morris L. Ischemic necrosis of the tongue in patients with cardiogenic shock. Laryngoscope 2010;120:1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jinbeom C, Kiyoung S, Dosang L. Ischemic necrosis of the tongue in surgical patients with septic shock: a case report. BMC Surgery 2016;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter LM, Brizman E. Lingual infarction in Wegener’s granulomatosis: a case report and review of the literature. Head Face Med 2008;4:19. [PubMed: 18718013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sofferman RA. Lingual infarction in cranial arteritis. JAMA 1980;243:2422–3. [PubMed: 7373821]. [PubMed] [Google Scholar]
- 5. Zimmermann AT, Brown M.. Tongue infarction in giant cell (temporal) arteritis. Intern Med J 2008:38:376. [PubMed: 18402568]. [DOI] [PubMed] [Google Scholar]
- 6. Brodmann M, Dorr A, Hafner F, Gary T, Pilger E. Tongue necrosis as first symptom of giant cell arteritis (GCA). Clin Rheumatol 2009;28:S47–9. [PubMed: 19277817]. [DOI] [PubMed] [Google Scholar]
- 7. Paul MB, Alex DA, Wichita K. Tongue necrosis as an unusual presentation of carotid artery stenosis. J Vasc Surg 2011;54:837–9. [DOI] [PubMed] [Google Scholar]
- 8. Scardina GA, Fuca G, Carini F. Oral necrotizing microvasculitis in a patient affected by Kawasaki disease. Med Oral Patol Oral Cir Buccal 2007:12:E560–4. [PubMed: 18059239]. [PubMed] [Google Scholar]


