Abstract
Freezing of gait (FOG) in Parkinson's disease (PD) is frequently triggered upon passing through narrow spaces such as doorways. However, despite being common the neural mechanisms underlying this phenomenon are poorly understood. In our study, 19 patients who routinely experience FOG performed a previously validated virtual reality (VR) gait paradigm where they used foot‐pedals to navigate a series of doorways. Patients underwent testing randomised between both their “ON” and “OFF” medication states. Task performance in conjunction with blood oxygenation level dependent (BOLD) signal changes between “ON” and “OFF” states were compared within each patient. Specifically, as they passed through a doorway in the VR environment patients demonstrated significantly longer “footstep” latencies in the OFF state compared to the ON state. As seen clinically in FOG this locomotive delay was primarily triggered by narrow doorways rather than wide doorways. Functional magnetic resonance imaging revealed that footstep prolongation on passing through doorways was associated with selective hypoactivation in the presupplementary motor area (pSMA) bilaterally. Task‐based functional connectivity analyses revealed that increased latency in response to doorways was inversely correlated with the degree of functional connectivity between the pSMA and the subthalamic nucleus (STN) across both hemispheres. Furthermore, increased frequency of prolonged footstep latency was associated with increased connectivity between the bilateral STN. These findings suggest that the effect of environmental cues on triggering FOG reflects a degree of impaired processing within the pSMA and disrupted signalling between the pSMA and STN, thus implicating the “hyperdirect” pathway in the generation of this phenomenon.
Keywords: freezing of gait, functional magnetic resonance imaging, hyperdirect pathway, Parkinson's disease, presupplementary motor area, subthalamic nucleus
1. INTRODUCTION
Freezing of gait (FOG) is a distressing form of gait disturbance that affects over half of patients with advanced Parkinson's disease (PD; Giladi et al., 2001) and can lead to falls, nursing home placement and a reduced quality of life (Aarsland, Larsen, Tandberg, & Laake, 2000; Gray & Hildebrand, 2000; Walton et al., 2015). The freezing phenomenon is characterised by a paroxysmal involuntary cessation of stepping that can frequently last for several seconds during which patients experience a subjective sensation of being “glued to the floor” (Nutt et al., 2011). Freezing episodes can present either at the initiation of gait or during locomotion commonly when turning, dual tasking, reaching a destination, and when negotiating narrow passages such as doorways (Almeida & Lebold, 2010; Cohen, Chao, Nutt, & Horak, 2011; Schaafsma et al., 2003). Furthermore, it is well known that freezing occurs with increased severity and frequency in patients when they are in their clinical OFF state, suggesting a dopaminergic role in the manifestation of this phenomenon (Giladi, 2008). Indeed, dopaminergic treatment remains the mainstay of treatment for freezing especially in its earlier stages (Nonnekes et al., 2015).
Alterations in specific motor behaviours have been identified in patients with FOG even in the absence of specific freezing episodes. Examples of this include an increased variability in step amplitude and timing (Gilat et al., 2013; Hausdorff et al., 2003) and abnormal postural adjustments prior to footstep initiation (Almeida, Frank, Roy, Patla, & Jog, 2007). In addition, similar phenomena have been observed in nongait activities such as altered timing in bimanual upper limb tasks (Vercruysse et al., 2012) and increased step variability when performing a bipedal stepping task (Gilat et al., 2013; Nantel, de Solages, & Bronte‐Stewart, 2011). These findings suggest that the mechanism underlying freezing may reflect a more generalised impairment in networks sub‐serving motor output.
Until recently, defining the neural correlates of FOG had been challenging due to the inherent limitations accompanying the functional imaging of gait (Bakker, Verstappen, Bloem, & Toni, 2007), however neuroimaging studies employing novel paradigms have shed significant light on the pathophysiology underlying freezing. Such protocols have used motor imagery tasks (Snijders et al., 2011) and assessments of upper limb motor blocks (Vercruysse et al., 2014) to successfully define differences in patients with FOG. Meanwhile experiments from our own group have demonstrated the utility of a virtual reality (VR) paradigm in which subjects perform a bipedal lower limb motor task to move through a realistic three dimensional environment (Shine, Matar, Ward, Bolitho, et al., 2013; Shine, Mattar, Ward, Frank, et al., 2013). These studies have demonstrated that freezing behaviour provoked within the VR environment can actually be correlated with true FOG events during Timed Up‐and‐Go (TUG) tasks in the clinic (Shine et al., 2013).
Combining this VR approach with functional MRI, we have recently shown that these behavioural freezing episodes are associated with a significant increase in the blood oxygen level dependent (BOLD) response within the bilateral dorsolateral prefrontal cortex and the posterior parietal cortex and a concomitant decrease in activity within subcortical structures including the bilateral caudate nucleus, the globus pallidus internus (GPi), the subthalamic nucleus (STN), and the mesencephalic locomotor region (MLR; Shine, Matar, Ward, Bolitho, et al., 2013). In a separate cohort, functional connectivity analyses revealed that these paroxysmal motor arrests are associated with functional decoupling between the cognitive control network and basal ganglia in each hemisphere within patients who freeze (Shine et al., 2013).
These findings support an emerging conceptual framework for freezing that implicates frontoparietal dysfunction in the pathogenesis of FOG (Lewis & Barker, 2009; Shine, Moustafa, Matar, Frank, & Lewis, 2013). This is supported by the fact that FOG is associated with deficits in executive functions including attentional set‐shifting (Amboni, Cozzolino, Longo, Picillo, & Barone, 2008; Naismith, Shine, & Lewis, 2010), dual tasking (Spildooren et al., 2010), and conflict resolution (Matar, Shine, Naismith, & Lewis, 2013; Vandenbossche et al., 2011). According to this framework, internal and external stimuli activate a range of cortical networks across cognitive, limbic, and/or motor domains, which compete for a limited processing capacity within the striatum and other cortical structures responsible for conflict resolution (Lewis & Shine, 2016). Interestingly, a number of these processes have also been shown to be mediated by the “hyperdirect” pathway consisting of the presupplementary motor area (pSMA) and the STN (Aron, Behrens, Smith, Frank, & Poldrack, 2007; Cavanagh & Frank, 2013; Isoda & Hikosaka, 2008). Such a model of freezing incorporating the corticostriatal and hyperdirect pathways has the potential to account for a number of different clinical observations such as the fact that freezing may be triggered by cognitive, affective, and sensorimotor cues, and ameliorated in circumstances that externally orient attentional resources to walking such as through the use of auditory pacing and targets for stepping (Rahman, Griffin, Quinn, & Jahanshahi, 2008).
It has been established that freezing can often be triggered on passing through narrow spaces such as doorways (Almeida & Lebold, 2010; Cohen et al., 2011; Cowie, Limousin, Peters, Hariz, & Day, 2012). Furthermore, narrower doorways are more likely to trigger freezing especially in the relative absence of dopaminergic medication (Cowie et al., 2012; Schaafsma et al., 2003). Previously, we have explored whether similar freezing behaviour could be elicited by doorways presented in the VR gait paradigm (Matar et al., 2013; Matar, Shine, Naismith, & Lewis, 2014). Within these studies patients with FOG demonstrated intermittently increased foot‐stepping latency in response to doorways that when averaged across all trials were significantly greater compared to PD patients without freezing and controls. Furthermore, delayed footstep latency associated with doorways within the VR paradigm was significantly improved with dopaminergic medication exclusively in patients with FOG (Matar et al., 2014).
Following on with this in the present study, we combined the same VR gait paradigm with functional magnetic resonance imaging (fMRI) in an effort to investigate the neural basis underlying doorway provoked freezing behaviour and modulated dopaminergic input by assessing patients in both their ON and OFF conditions. We hypothesised that comparing the pattern of fMRI signal change between ON and OFF states during prolonged footstep latencies associated with doorways in the VR environment would highlight those regions underlying the freezing behaviour associated with environmental cues. In particular, we predicted that disturbances in activation of the pSMA and its connectivity with the STN, as part of the hyperdirect pathway, would correlate with the motor delay observed in response to doorways.
2. MATERIALS AND METHODS
2.1. Participants
Nineteen patients with idiopathic PD who were known to experience FOG were recruited from the Brain and Mind Centre, Parkinson's disease Research Clinic. All patients satisfied the United Kingdom Parkinson's Disease Society Brain Bank criteria (Gibb & Lees, 1988). The study was approved by the Human Ethics Research Committee of the University of Sydney and written informed consent was obtained from all patients. Demographic details are presented in Table 1. All patients were studied on two separate occasions separated by no less than 2 weeks (average time between scans = 55 days). Visit order was randomised and patients attended in their practically defined ON state (at least 1 hr following dopaminergic medications) and on a separate occasion in their OFF state (following overnight withdrawal of dopaminergic medication; average time off medication = 600 min; refer to Table 1 for mean Dopamine Dose Equivalence). The patients underwent neurological examination using section III of the Unified Parkinson's Disease Rating Scale (UPDRS; (Goetz et al., 2007) in their ON and OFF states just prior to entering the scanner. All patients demonstrated an absolute improvement in UPDRS‐III score in the ON state compared to their OFF state (Table 1). Characterisation of the predominant motor phenotype according to the UPDRS using Postural Instability and Gait Difficulty (PIGD) scores as well as Tremor Scores are also shown in Table 1 (see Stebbins et al. [2013] for calculation). As expected PIGD scores were greater than tremor scores for patients in our cohort.
Table 1.
Patient demographics
| Demographics | Range | Mean | SD |
|---|---|---|---|
| N = 19 | |||
| Age (years) | 50–76 | 65.32 | 6.3 |
| UPDRS III, on | 10–53 | 30.4 | 14.8 |
| UPDRS III, off | 13–71 | 35.1* | 15.7 |
| UPDRS III (FOG item), on | 0–4 | 1.4 | 1.2 |
| UPDRS III (FOG item), off | 0–4 | 2.1** | 1.2 |
| Hoehn & Yahr | 1–4 | 2.16 | 0.7 |
| PIGD score | 3–12 | 5.8 | 2.7 |
| Tremor score | 2–7 | 2.3 | 2.1 |
| BDI‐II | 0–42 | 12.33 | 10.58 |
| FOG | 2–19 | 10.16 | 4.4 |
| FOGQ3 | 1–4 | 2.37 | 0.9 |
| MMSE | 23–30 | 27.89 | 1.7 |
| DDE | 150–2,400 | 959.68 | 653.5 |
Demographic data shown for the 19 patients who participated in this study. UPDRS, Unified Parkinson's Disease Rating Scale; PIGD, Postural Instability and Gait Difficulty; FOGQ, Freezing of Gait Questionnaire; FOGQ3, FOGQ item three; BDI‐II, Beck Depression Inventory II; MMSE, Mini‐Mental State Examination; DDE, Dopamine Dose Equivalence.
p < 0.05.
p < 0.005 paired sign‐test (one‐tailed) between on‐ and off‐ testing conditions.
Patients were selected based on self‐reported FOG symptoms. This was assessed through completion of the FOG questionnaire (FOG‐Q) and by direct observation by the investigators (Giladi et al., 2000). All patients were classified as experiencing FOG (FOG+ patients) as per item three (FOG‐Q3: “Do you feel that your feet get glued to the floor while walking, making a turn or when trying to initiate walking (freezing)?”). A positive response to FOG‐Q3 (score 1–4) has been proven to be reliable for identifying patients with FOG (Giladi et al., 2009). Furthermore, 15 of the 19 patients (79%) also displayed overt freezing episodes during their clinical assessment (in the OFF state) just prior to testing.
All patients underwent cognitive testing with the Mini‐Mental state examination (MMSE; Folstein, Folstein, & McHugh, 1975) and were deemed not to have dementia based on MDS guidelines (Emre et al., 2007) or major depression according to DSM‐IV criteria (American Psychiatric Association, 2000) by consensus rating of a Neurologist (SJGL) and a Neuropsychologist (SLN). All Patients taking anti‐depressants, anti‐psychotics, and benzodiazepines were excluded from the study. Performance data is provided in Table 1.
2.2. VR paradigm
The VR paradigm employed the Virtual Gait Laboratory (VGL), which displays a three‐dimensional corridor presented in the first person. This environment was constructed using a software development kit made available by ID Software (Mesquite, TX). In this paradigm, the participant is supine with left and right feet positioned over corresponding foot pedals. The virtual environment is displayed on a small screen mounted on the head coils. Gait initiation (“WALK” presented in green) and stopping cues (“STOP” presented in red) were displayed on screen at predefined intervals as described previously (Matar et al., 2013). To navigate the virtual environment, the patient is instructed to step on the pedals, alternating between left and right with a speed akin to their natural walking rhythm. This action is associated with stepping movements on the display screen. While all pedal responses were recorded, the system was configured such that forward progression on screen is halted during “out of sequence” steps (i.e., left–left or right–right) thus enforcing that on‐screen movement was only associated with alternating left–right sequences. The time point corresponding to every step (pedal depression) made by the patient was recorded thus allowing for the calculation of time taken between any two successfully completed steps. On the screen, patients were required to navigate a virtual corridor along which “narrow” and “wide” doorways (wide doorways being twice the width of narrow) are encountered at irregular intervals. Each subject undertook a single 10‐min trial while in the scanner, which consisted of 10 interleaved blocks with 4–5 s of “rest” between blocks. The time points coinciding with the passing through a narrow or wide doorway were recorded to allow synchronisation with fMRI. All subjects underwent a practice run with the VR task outside of the MRI scanner to familiarize subjects with the format of the paradigm and minimize training effects. Participants were required to demonstrate adequate understanding and performance on the task prior to testing.
2.3. Behavioural analysis
The main output measure of the paradigm was “footstep latency”, defined as the time taken between two alternate (left–right or right–left) steps resulting in forward movement on screen. A previous study utilising this paradigm has shown the longest spontaneous “step” latency to correlate with self‐reported and actual FOG (Matar et al., 2013; Shine, Matar, Bolitho, Dilda, et al., 2013).
In order to characterize the effects of environmental stimuli on motor behaviour, the outcome of interest chosen in this study was the maximum footstep latency occurring within three steps of a virtual doorway (see Matar et al., 2013). This doorway‐associated maximum latency was then scaled (divided) by each subject's own modal footstep latency (henceforth referred to as simply “MFSL”). The modal latency was obtained by taking the most frequent footstep latency observed for each subject throughout the entire VGL as grouped into bins of 0.1 s. The modal footstep latency is considered to be a more robust measure of the cadence of natural stepping than the average footstep latency, which can be skewed by exceptionally long latencies that reflect episodes of freezing behaviour. This measure was chosen to increase the sensitivity of the paradigm to any stimulus‐provoked behavioural disturbances and has been previously shown to reliably separate groups of FOG+ PD patients from those without FOG (FOG−) and controls using a cognitive task within the paradigm (Matar et al., 2013, 2014; Shine, Matar, Ward, Bolitho, et al., 2013; Shine, Matar, Ward, Frank, et al., 2013).
The time points corresponding to the onset of the MFSL in response to each doorway were extracted and used for the event‐related imaging analysis (below). For the behavioural comparisons, we examined the scaled MFSLs in response to all doorways, with additional subanalysis of narrow doorways versus wide doorways. The MFSL in response to every doorway encountered during the paradigm was averaged across the entire run for each patient (mean MFSL). The mean MFSL was then used for statistical comparison between the ON and OFF performances (see Matar et al., 2013). As expected, the data was not normally distributed, thus paired statistical comparison were performed using the two‐tailed nonparametric sign test. Data analysis was performed using Statistical Package for the Social Sciences Version 17 (SPSS; Chicago, IL). Considering the sample size, the conventional alpha level of 0.05 was used to minimize Type II errors. Results are expressed as mean ± standard error unless otherwise specified.
2.4. Image acquisition
Imaging was conducted on a General Electric 3 Tesla MRI (General Electric, Milwaukee, WI). T2*‐weighted echo planar functional images were acquired in sequential order with repetition time (TR) = 3 s, echo time (TE) = 32 ms, flip angle = 90°, 32 axial slices covering the whole brain, field of view (FOV) = 220 mm, interslice gap = 0.4 mm, and raw voxel size = 1.72 mm by 1.95 mm by 4 mm thick. High‐resolution 3D T1‐weighted, anatomical images (voxel size 0.4 × 0.4 × 0.9 mm) were obtained for co‐registration with functional data.
2.5. Image preprocessing
Statistical parametric mapping software (SPM12, Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/) was used for image processing and analysis. Image processing and analysis was also conducted in SPM8 and the comparative results are shown in Supporting Information Figure S2. Functional images were slice‐time corrected to the median (17th) slice in each TR and were realigned to create a mean realigned image. The images were then normalised to the Echo Planar Image (EPI) template and smoothed using an 8‐mm full‐width half‐maximum isotropic Gaussian kernel. Due to the increased risk of head movements in this clinical population, each trial was subsequently analysed using ArtRepair (Mazaika, Whifield‐Gabrieli, Reiss, & Glover, 2007) and trials with a large amount of global drift or scan‐to‐scan head movements greater than 1 mm were corrected using interpolation. Head movements were minimised with the application of soft padding and positioners. Trials with scan‐to‐scan head‐movements of greater than 3 mm or 3° of movement were removed from the analysis.
2.6. Neuroimaging analysis
Statistical parametric maps were calculated for each subject using a general linear model analysis within an event‐related design in which the slowest step through a virtual doorway in the ON state was then contrasted with the corresponding slowest step in the OFF state for each patient (no other events were modelled). Contrast images from the first‐level analyses were then entered into a second‐level random‐effects design in order to determine the group‐level effects of the condition of interest. These images were analysed using an alpha level of 0.005 and a cluster threshold of 10, in order to strike a balance between Type I and Type II errors (Lieberman & Cunningham, 2009). The result was visualised using xjView toolbox (http://www.alivelearn.net/xjview).
In order to determine the relative contribution of the ON and OFF state to any relative differences between conditions in a given region, a 4 mm spherical region of interest (ROI) was drawn around the peak of any clusters that passed the statistical threshold applied to the second level analysis. The contrast value for this ROI was then extracted using the MarsBar toolbox (http://marsbar.sourceforge.net), allowing for the calculation of significant within and between condition effects. ROI coordinates in this study are reported in Montreal Neurological Institute (MNI) space.
2.7. Task based functional connectivity
In a recent study, we showed that freezing in the VR task is related to functional decoupling between the cortex and basal ganglia (Shine, Matar, Ward, Frank, et al., 2013). A direct prediction of these results is that footstep delay during the navigation of virtual doorways should similarly show a pattern of reduced connectivity between cortical and subcortical structures. To assess this prediction in the current study, we extracted BOLD data from a 4 mm spherical ROI (as defined above), along with a 4 mm spherical ROI in both the left and right STN (MNI: ±11 –14 −3; (Shine, Matar, Ward, Bolitho, et al., 2013) and a 4 mm spherical ROI in the bilateral auditory cortex (MNI: ±50 –14 4), which was used as a control analysis. After removing noise associated with the global brain signal, rigid head movements, cerebrospinal fluid and white matter signals, we then performed a correlation between the time courses of each ROI pair in all 19 participants in the study. This correlation coefficient was then converted to a Z‐score using a Pearson's r‐to‐Z transformation. These values were then compared between the ON and OFF state, and also correlated against the number of prolonged footstep latencies when navigating doorways in the relevant medication state.
To obtain a summary statistic reflecting pSMA‐STN connectivity over the course of the experiment, the correlation between the peak ROI of the pSMA and STN was averaged across the two hemispheres, leaving a single value that represented the normalised functional connectivity between the pSMA and the STN for the entire experimental run in both the ON and OFF state. To assess the relationship between this functional connectivity value and footstep latency through virtual doorways, we subtracted the functional connectivity score from the OFF state by the score from the ON state, leading to a summary score that represented the extent to which connectivity between the pSMA and the STN was higher in the ON state. This value was then correlated with the relative extent to which an individual patient experienced a prolonged footstep latency when navigating doorways in the OFF state.
3. RESULTS
3.1. Behavioural results
No statistically significant difference in modal latency was observed between the FOG+ patients in their OFF state (0.49 ± 0.04 s) compared to their ON state (0.50 ± 0.03 s; p = 0.75). Scaled mean MFSLs following all doorways and scaled mean MFSL following stratification into narrow and wide doorways are shown in Figure 1. When comparing all doorways together, scaled mean MFSL in their OFF state (1.87 ± 0.31) was significantly greater than the corresponding MFSL for each patient in their ON state (1.34 ± 0.09; p = 0.019). When narrow and wide doorways were analysed separately, this trend was observed in both conditions but was statistically significant only for narrow doorways (p = 0.019), with increased latencies in the OFF state (1.78 ± 0.22) versus those seen in the ON state (1.39 ± 0.13). No significant difference was seen for wide doorways (p = 0.167), which were also associated with greater across‐subject variability.
Figure 1.
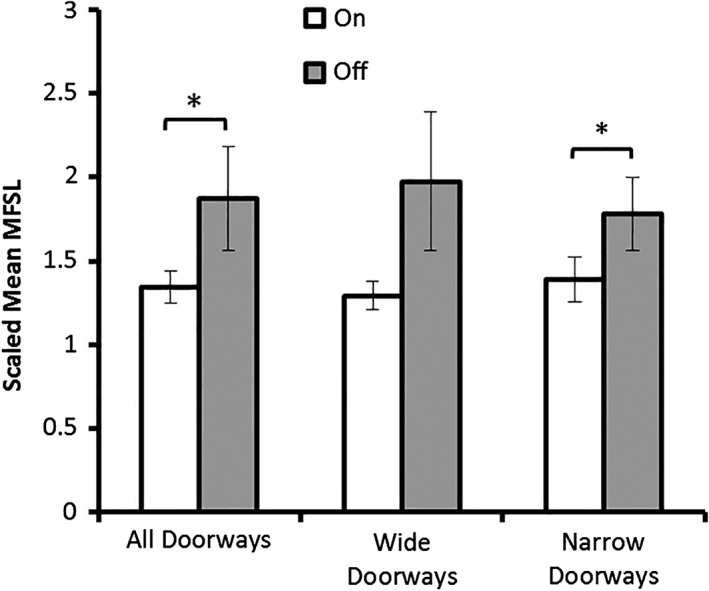
Scaled mean maximum footstep latency (MFSL) for a cohort of 19 patients with FOG triggered in response to all doorways, wide doorways and narrow doorways. A statistically significant increase in footstep latency was observed in participants off their medications (OFF) compared to when they were on their medications (ON) in response to all doorways and this was also seen when narrow doorways were subsequently analysed. Wide doorways alone did not elicit a significant difference between the on and off state. Error bars represent SEM
3.2. Imaging results
The second level analysis revealed a single cluster of decreased BOLD signal in the pSMA bilaterally distributed across the two hemispheres of the brain (MNI: left hemisphere peak voxel: −1 × 29 × 49; t = 3.71; right hemisphere peak voxel: 6 × 41 × 43; t = 3.58), which was driven by a significant decrease in this ROI in the OFF state (average = −0.67; t = 1.67 and p < 0.05) and a nonsignificant increase in the ON state (average = 0.15; t = 0.17 and p > 0.1). The results of these analyses are displayed graphically in Figure 2.
Figure 2.
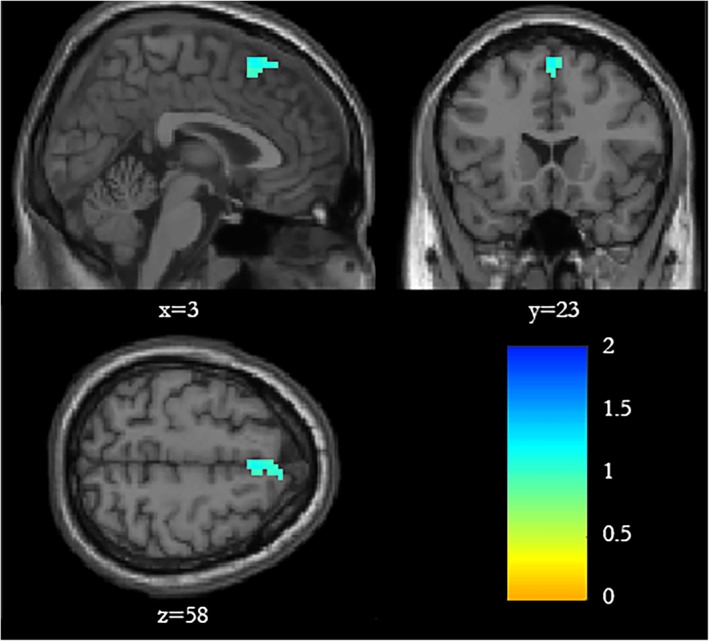
Spatial map showing that increased footstep latency while passing through a doorway in the OFF state compared to the ON state (contrast ON > OFF) was associated with reduced BOLD signal within the presupplementary motor area in both the right (peak voxel: 6 × 41 × 43) and left hemisphere (peak voxel: −1 × 29 × 49). Statistical parametric maps shown are analysed with p < 0.005, and a cluster threshold of 20 voxels. Maps with less stringent alpha values (p < 0.01 and p < 0.05) are provided in Supporting Information Figure S3 [Color figure can be viewed at https://wileyonlinelibrary.com]
To supplement the connectivity analyses below, we also performed a small volume correction on the bilateral STN. We found significantly reduced BOLD signal within the left STN in the OFF state relative to the ON state (beta = −0.32, t = 1.96; p = 0.033). A nonsignificant decrease in BOLD response was seen in the right STN (beta = −0.29, t = 1.16, p = 0.131) resulting on average in reduced activation of bilateral STN with a weak overall trend toward significance (beta = −0.31, t = 1.45; p = 0.083).
To exclude differences in head motion between the two conditions, the mean framewise displacement during scans was calculated and compared for both the ON (0.23 ± 0.19 mm) and OFF (0.21 ± 0.16 mm) medication states. Statistical analysis (paired t test) revealed no significant difference between the two conditions (t: −0.37; p = 0.72).
3.3. Task‐based functional connectivity
The pSMA connects with the STN as part of the “hyperdirect pathway” (Aron et al., 2007; Haynes & Haber, 2013; Nachev, Kennard, & Husain, 2008). Having identified hypoactivation in the pSMA during motor delays associated with doorways, we set out to explore the question of whether altered communication between the pSMA and the STN may account for the preferentially increased motor slowing seen in the paradigm in the OFF state. First, we calculated group differences in pSMA and STN task‐based functional connectivity (i.e., a Pearson's correlation between the time series) within and between hemispheres across the entire paradigm. The average r‐to‐Z scores for each ROI pair in both the ON and OFF states are displayed in Table 2. This analysis revealed significantly reduced STN to pSMA connectivity both within and between hemispheres in the OFF state (p < 0.05). There was no significant difference in STN–STN or pSMA–pSMA connectivity between hemispheres, and no significant difference between bilateral auditory cortex as a function of medication state.
Table 2.
Connectivity (r‐to‐Z scores) between bilateral STN and pSMA in the ON vs OFF states
| L STN | R STN | L pSMA | R pSMA | ||
| L STN | 1.17a , *** | 0.39b , * | 0.34b , * | ||
| R STN | 1.17a , *** | 0.40b , * | 0.34b , * | ||
| ON state | L pSMA | 0.39b , * | 0.40b , * | 1.31a , *** | |
| R pSMA | 0.34b , * | 0.34b , * | 1.31a , *** | ||
| L STN | 1.45a | 0.00c | −0.37d , * | ||
| OFF state | R STN | 1.45a , *** | 0.07c | −0.25d , * | |
| L pSMA | 0.00c | 0.07c | 1.04a , *** | ||
| R pSMA | −0.37d , * | −0.25d , * | 1.04a , *** |
Comparison of connectivity between the ON and OFF states for connections between the STN and pSMA across both hemispheres. pSMA, presupplementary motor area; STN, subthalamic nucleus; L, Left; R, Right. * p < .05; *** p < .001 (paired t test).
Z > 1.
Z > 0.3.
Z < 0.3.
Z > −0.3.
Next, we looked specifically to see whether differences in connectivity between the average pSMA and STN correlated with performance in the task. This analysis showed that the degree of impaired functional connectivity between the pSMA and STN in the OFF state (relative to the ON state) was positively correlated (r = 0.441, p < 0.05) with the degree of footstep latency when passing through doorways (see Figure 3).
Figure 3.
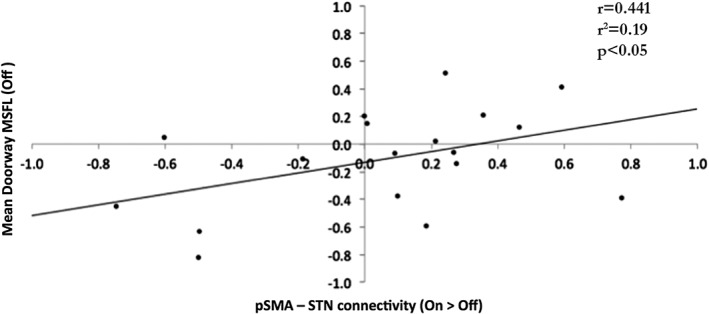
There was a positive correlation (r = 0.441, p < 0.05) between the degree of impaired functional connectivity between the pSMA and STN in the OFF state (relative to the ON state) and the degree of footstep delay (mean doorway MFSL) while navigating doorways in the OFF state
Finally, drawing on work relating increased aberrant oscillatory activity between STN nuclei to FOG (see Section 4) we wanted to explore whether the frequency of especially prolonged footsteps occurring while passing through a doorway may be associated with differences in connectivity between the left and right STN. We observed a strong positive correlation between the number of “prolonged footstep latencies” demonstrated by the patients in the OFF state and increased connectivity between the left and right STN (r = 0.575, p = 0.010; see Figure 4).
Figure 4.
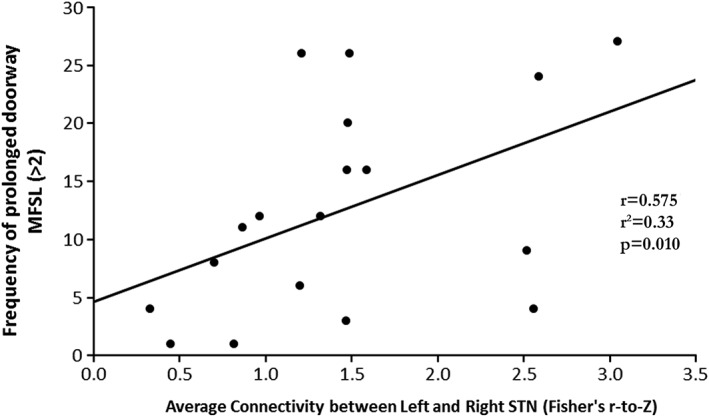
A strong positive correlation (r = 0.575, p = 0.01) was observed between the frequency of prolonged MFSL (defined as greater than 2) observed in patients in the OFF‐state and the functional connectivity between the left and right STN (Fisher's r‐to‐Z) of the same subjects averaged over the total duration of the VR paradigm
4. DISCUSSION
This is the first study to identify the neural correlates underlying freezing‐related motor behaviour in response to a recognised environmental trigger of FOG. Specifically, we found that the dopaminergic dependency of the freezing behaviour associated with doorways was mediated by hypoactivation in the pSMA and impaired functional connectivity between the pSMA and the STN. We were also able to demonstrate that the frequency of prolonged footstep latencies triggered by doorways was associated with increased connectivity between STN bilaterally.
4.1. Increased latency in response to virtual doorways
Analogous to the observation that doorway freezing is more common in the OFF state, we found a significant increase in footstep latency as participants passed through a virtual doorway in their OFF state compared to their clinical ON state. Importantly, the footstep latency observed over the entire paradigm, as judged by the modal latency, was not significantly different between the ON and OFF states for each subject suggesting that the increased latency seen was not due to a generalised slowing of motor output within the task but rather a specific response to the environmental stimulus. Interestingly, this effect was mainly associated with narrow rather than wide doorways, which also correlates well with the clinical behaviour of FOG (Cowie et al., 2012). Further evidence for the relation of this measure to freezing behaviour comes from our previous studies showing that footstep latency associated with doorways within the VR paradigm was increased in FOG+ relative to FOG− patients and controls (Matar et al., 2013), and additionally, that the effect was ameliorated by dopamine only in FOG+ patients (Matar et al., 2014). The implication of these results is that the phenomenon investigated in the present study pertains specifically to freezing and underscores the relevance of the imaging results to freezing behaviour more generally.
A significant degree of variability was observed in the group data with respect to MFSL. As expected, this was more evident in the OFF state relative to the ON state. Although not specifically tested here, this may reflect a spectrum of FOG severity represented within the cohort. This is suggested in the range of UPDRS‐III scores of the subjects (Table 1). Future studies will be required to investigate the possibility of a positive correlation between the MFSL and objective measures of FOG severity such as that obtained from direct video recording of gait (Morris et al., 2012; Shine et al., 2012).
As acknowledged in previous studies, a limitation of the use of this motor task to investigate gait is that it does not access other important variables of gait including posture, balance, and step length. Nonetheless, we have shown that footstep latency within the VR paradigm is a robust measure that is able to separate FOG+ from FOG− patients and allows tractable exploration of freezing behaviour (Matar et al., 2013, 2014; Shine, Matar, Ward, Frank, et al., 2013). In this experiment, the maximum latencies through all doorway cues was included in the imaging analysis to increase sensitivity of the study, however this technique cannot distinguish between subtle motor delays and true freezing. Although we argue below that the two may be mediated by shared underlying mechanisms, future studies incorporating accelerometry data may be able to capture individual freezing episodes and hence, increase the specificity of the findings.
4.2. Reduced activity in pSMA
In this study, increased footstep delay upon passing a doorway in the OFF state relative to the ON state was associated with hypoactivation in the pSMA. The pSMA is an area that has been associated with roles such as voluntary (internally cued) movement, updating and sequencing of motor plans, response inhibition, task switching and minimising interference from distracting cues (for a still relevant review of pSMA functions, see Nachev et al., 2008). Additionally, the pSMA is heavily interconnected with basal ganglia structures with direct projections to the striatum and STN, regions which combine to form the “hyperdirect” pathway of the basal ganglia (Aron & Poldrack, 2006; Nambu, Tokuno, & Takada, 2002). Reduced activity in the pSMA (together with the SMA) that can be ameliorated by the administration of dopaminergic agents has been previously reported in patients with PD (Haslinger et al., 2001). This reduced activity has been linked with a number of motor disturbances in PD such as hypokinesia and reduced stepping amplitude, as well as impairments in executive functions such as cognitive flexibility and task switching (Nachev et al., 2008; Sabatini et al., 2000). Furthermore, alterations in activity within the pSMA and SMA have recently been reported in PD patients with FOG (Shine, Matar, Ward, Bolitho, et al., 2013; Snijders et al., 2011). Reduced SMA activation was observed in our FOG+ relative to FOG− patients performing a motor imagery gait task (Snijders et al., 2011) and another experiment using the same VR task as described here showed reduced activity in the pSMA associated with cognitive and motor dual‐tasking (Shine, Matar, Ward, Frank, et al., 2013).
The findings in our study suggest that the dopaminergic improvement of doorway‐triggered footstep delay in patients with FOG may be modulated by activity within the pSMA. This observation could not be accounted for by more general changes in footstep amplitude or hypokinesia given that the modal footstep latency was comparable between the OFF and ON states. Instead, the effect appears to have been specifically provoked by a perceptual stimulus in the VR paradigm. We speculate that the perception of a doorway may trigger alternative motor plans associated with postural and gait adjustments, which results in additional demand on the pSMA to selectively activate or inhibit movement relating to the interfering stimulus. Therefore, reduced pSMA activation in the OFF state relative to the ON state may translate into the motor‐sequencing interference observed either as a result of the disinhibition of a “stop” cue implicit in the doorway stimulus (Matar et al., 2013; Vandenbossche et al., 2011) or simply inefficient/delayed neural processing of the stimulus and any competing motor plan (Nachev et al., 2008).
As discussed further below, the pSMA shares direct connections with the STN as part of the “hyper‐direct” pathway. In addition to the alterations in BOLD activity seen in the pSMA, we were able to show significant modulation of the BOLD response between ON and OFF states within the right STN with a trend toward significance of the bilateral STN. The lateralisation could reflect asymmetry of the pathology which is often clinically manifest in patients with PD. Alternatively, this result could also reflect reduced power owing to variation in the magnitude of dopamine responsiveness between individuals.
4.3. Altered functional connectivity between bilateral STN and pSMA
The mechanism by which the hypoactivation in the pSMA observed above may translate to paroxysmal inhibition of motor output has been proposed to be critically dependent on its connections with the STN as part of the “hyperdirect” pathway (Lewis & Shine, 2016; Shine, Moustafa, Matar, Frank, & Lewis, 2013). The STN is being increasingly recognised as an important structure in the pathophysiology of FOG. It is known deep brain stimulation targeting the STN results in partial alleviation of freezing symptoms (Ferraye, Debu, & Pollak, 2008; Moreau et al., 2008; Schlenstedt et al., 2017; Xie, Kang, & Warnke, 2012). Although traditionally the STN acts to inhibit motor output as part of the “indirect” pathway, the recent realisation of the “hyperdirect” pathway allows a means for the premotor regions to bypass the striatum and directly influence motor gating through its projections to the STN (Nambu et al., 2002). Data from neurophysiological and computational experiments suggest this pathway mediates the processes of conflict resolution and response inhibition (Cavanagh & Frank, 2013).
In this study, we were able to show that reduced functional connectivity between the pSMA with the STN correlated with doorway evoked motor delay in FOG+ patients in their OFF state. We were also able to demonstrate reduced connectivity between the pSMA and STN structures between and within both hemispheres in the OFF state. These results emphasize a potential role for the hyperdirect pathway and possibly impaired conflict processing in the mechanisms underlying freezing behaviour. This is in alignment with recent behavioural data showing impaired conflict resolution in FOG+ patients (Matar et al., 2013; Vandenbossche et al., 2011). Additionally, a recent electroencephalography (EEG) study in patients with FOG was able to associate transitions from walking to freezing with increases in the theta frequency band (Handojoseno et al., 2015; Shine et al., 2014). Increased power within this frequency band has been linked behaviourally with a delayed reaction time in high conflict scenarios and corresponds to activation with the STN (Cavanagh & Frank, 2013). Further to this, a more recent EEG study examining recordings from implanted STN electrodes for chronic stimulation found that FOG is associated with increased power in the high‐beta frequency in the OFF state which cohered maximally with midline cortical structures such as the SMA (and likely also the pSMA; Toledo et al., 2014).
An updated model of freezing incorporating the pSMA and STN has been proposed focusing on frontostriatal impairment in the pathogenesis of FOG (Shine, Moustafa, Matar, Frank, & Lewis, 2013). It is argued that pathology affecting various cortical and subcortical structures including the brainstem, the pedunculopontine nucleus, and the pSMA reduces efficient processing of conflict signals and gating of motor output thus triggering a state of aberrant oscillatory activity between the STN and the GPi. This state of oscillatory activity would lead to an overall decrease in energy demand and reduction in BOLD signal during freezing as has been confirmed in previous fMRI results (Shine, Matar, Ward, Bolitho, et al., 2013). In the context of the present study, approaching a doorway may lead to increased conflict via the activation of several competing motor programs that encode adjustments in posture, timing and stepping rhythm. Dysfunction within the pSMA and STN connectivity either extrinsically or due to focal pathology in the pSMA may then lead to impairments in the processing of the conflict signal manifesting at one end of the spectrum as a subtle motor deficit such as a foot stepping delay ranging through to motor arrest with freezing at the other. The freezing itself would represent a paroxysmal failure of the hyperdirect pathway to select a single motor response (by inhibition of alternate responses) resulting in an unrestrained increase in excitatory input into a dopamine depleted striatum. The altered signal timing may be the trigger for the aberrant oscillatory activity hypothesised to occur within basal ganglia nuclei during freezing.
Further in favour of this hypothesis, we were able to demonstrate increased STN‐STN connectivity in association with an increased frequency of prolonged footstep latencies (MFSL>2)—a measure which has been shown to correlate with severity of freezing in a timed “up and go” task (Shine et al., 2012). This novel result supports, albeit indirectly, the role of synchronous activity between the subcortical structures in freezing as proposed by the model above. Subcortical recordings during episodic freezing from deep‐brain stimulation electrodes implanted in bilateral STN might be able to confirm this result in the future.
5. CONCLUSION
In this study, we were able to directly probe the neural correlate of doorway evoked motor delays using event‐related functional imaging. We specifically examined the influence of environmental cues on footstep latency during the performance of a VR gait task and investigated the effect of dopaminergic depletion. We found that compared to the ON state, there was increased footstep latency upon passing through a doorway in the OFF state, which was associated with a specific reduced activation within the pSMA. Furthermore, functional decoupling of the pSMA and STN was observed corresponding to patients with freezing as they passed through doorways in their OFF state. Finally, we also detected a positive correlation between frequency of prolonged footsteps and connectivity between bilateral STN. This is the first study to directly explore the neural regions underlying the established phenomenon of doorway provoked FOG adding to growing evidence for the pSMA and the hyperdirect pathway as a potential therapeutic target in FOG.
AUTHOR CONTRIBUTIONS
E.M., J.M.S and S.J.G.L were involved in the study design and conception. E.M. was responsible for writing the first draft. E.M, J.M.S. and K.E. were responsible for data collection and analysis. S.L.N. assisted with statistical analysis and critical review of the manuscript. P.B.W., M.J.F. A.A.M., M.G. and S.J.G.L. were involved in the critical appraisal of the study design and final manuscript.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
Supporting information
Figure S1: Characterisation of motor fluctuations in the cohort according to MDS‐UPDRS Items 4.4 (Functional impact of motor fluctuations) and 4.5 (Complexity of motor fluctuations). A score of 0 denotes no impact, with 4 implying high level of functional impact of fluctuations with less than one quarter of OFF periods being predictable. The majority of patients reported low impact of fluctuations and high predictability.
Figure S2: Comparison of original analysis in initial submission using SPM8 (left) and revised re‐analysis of original images using SPM12 (right). Statistical parametric maps shown are analysed with p < 0.005, and a cluster threshold of 20 voxels.
Figure S3: Spatial maps of reduced BOLD signal shown in patients in the OFF state compared to their ON state corresponding to an increased MFSL as subjects passed through a doorway (ON > OFF). Statistical parametric maps shown are analysed with p < 0.005, p < 0.01 and p < 0.05 as indicated by the colour. A cluster threshold of 20 voxels was used. Fourteen clusters identified in total at p < 0.05. Peak coordinates and corresponding region shown in Supporting Information Table S1.
Table S1: Coordinates of clusters of reduced BOLD activation (ON > OFF) in FOG+ patients as they passed through a doorway (p < 0.05, cluster size>20 voxels).
ACKNOWLEDGMENTS
This work was supported by grants from the Michael J. Fox Foundation and the Brain Foundation of Australia. EM is supported by a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship and the Australian and New Zealand Association of Neurologists Gwen James Fellowship Award. SJGL is supported by an NHMRC‐Australian Research Council Dementia Fellowship (#1110414). JMS is supported by a NHMRC CJ Martin Fellowship.
Matar E, Shine JM, Gilat M, et al. Identifying the neural correlates of doorway freezing in Parkinson's disease. Hum Brain Mapp. 2019;40:2055–2064. 10.1002/hbm.24506
Funding information Michael J. Fox Foundation for Parkinson's Research; National Health and Medical Research Council, Grant/Award Number: #1110414; NHMRC CJ Martin Fellowship; Australian Research Council Dementia Fellowship, Grant/Award Number: 1110414; Australian and New Zealand Association of Neurologists Gwen James Fellowship Award; National Health and Medical Research Council (NHMRC) Postgraduate Scholarship; Brain Foundation of Australia; Michael J. Fox Foundation
REFERENCES
- Aarsland, D. , Larsen, J. P. , Tandberg, E. , & Laake, K. (2000). Predictors of nursing home placement in Parkinson's disease: A population‐based, prospective study. Journal of the American Geriatrics Society, 48, 938–942. [DOI] [PubMed] [Google Scholar]
- Almeida, Q. J. , Frank, J. S. , Roy, E. A. , Patla, A. E. , & Jog, M. S. (2007). Dopaminergic modulation of timing control and variability in the gait of Parkinson's disease. Movement Disorders, 22, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Almeida, Q. J. , & Lebold, C. A. (2010). Freezing of gait in Parkinson's disease: A perceptual cause for a motor impairment? Journal of Neurology, Neurosurgery, and Psychiatry, 81, 513–518. [DOI] [PubMed] [Google Scholar]
- Amboni, M. , Cozzolino, A. , Longo, K. , Picillo, M. , & Barone, P. (2008). Freezing of gait and executive functions in patients with Parkinson's disease. Movement Disorders, 23, 395–400. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM‐IV‐TR. Washington, DC: Author. [Google Scholar]
- Aron, A. R. , Behrens, T. E. , Smith, S. , Frank, M. J. , & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience, 27, 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. , & Poldrack, R. A. (2006). Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. The Journal of Neuroscience, 26, 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, M. , Verstappen, C. C. , Bloem, B. R. , & Toni, I. (2007). Recent advances in functional neuroimaging of gait. Journal of Neural Transmission (Vienna), 114, 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh, J. F. , & Frank, M. J. (2013). Stop! Stay tuned for more information. Experimental Neurology, 247, 289–291. [DOI] [PubMed] [Google Scholar]
- Cohen, R. G. , Chao, A. , Nutt, J. G. , & Horak, F. B. (2011). Freezing of gait is associated with a mismatch between motor imagery and motor execution in narrow doorways, not with failure to judge doorway passability. Neuropsychologia, 49, 3981–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie, D. , Limousin, P. , Peters, A. , Hariz, M. , & Day, B. L. (2012). Doorway‐provoked freezing of gait in Parkinson's disease. Movement Disorders, 27, 492–499. [DOI] [PubMed] [Google Scholar]
- Emre, M. , Aarsland, D. , Brown, R. , Burn, D.J. , Duyckaerts, C. , Mizuno, Y. , Broe, G.A. , Cummings, J. , Dickson, D.W. , Gauthier, S. , Goldman, J. , Goetz, C. , Korczyn, A. , Lees, A. , Levy, R. , Litvan, I. , McKeith, I. , Olanow, W. , Poewe, W. , Quinn, N. , Sampaio, C. , Tolosa, E. , Dubois, B. (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders, 22:1689–707. [DOI] [PubMed] [Google Scholar]
- Ferraye, M. U. , Debu, B. , & Pollak, P. (2008). Deep brain stimulation effect on freezing of gait. Movement Disorders, 23(Suppl 2), S489–S494. [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). "mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gibb, W. R. , & Lees, A. J. (1988). A comparison of clinical and pathological features of young‐ and old‐onset Parkinson's disease. Neurology, 38, 1402–1406. [DOI] [PubMed] [Google Scholar]
- Giladi, N. (2008). Medical treatment of freezing of gait. Movement Disorders, 23(Suppl 2), S482–S488. [DOI] [PubMed] [Google Scholar]
- Giladi, N. , Shabtai, H. , Simon, E. S. , Biran, S. , Tal, J. , & Korczyn, A. D. (2000). Construction of freezing of gait questionnaire for patients with parkinsonism. Parkinsonism & Related Disorders, 6, 165–170. [DOI] [PubMed] [Google Scholar]
- Giladi, N. , Tal, J. , Azulay, T. , Rascol, O. , Brooks, D. J. , Melamed, E. , … Tolosa, E. (2009). Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Movement Disorders, 24, 655–661. [DOI] [PubMed] [Google Scholar]
- Giladi, N. , Treves, T. A. , Simon, E. S. , Shabtai, H. , Orlov, Y. , Kandinov, B. , … Korczyn, A. D. (2001). Freezing of gait in patients with advanced Parkinson's disease. Journal of Neural Transmission (Vienna), 108, 53–61. [DOI] [PubMed] [Google Scholar]
- Gilat, M. , Shine, J. M. , Bolitho, S. J. , Matar, E. , Kamsma, Y. P. , Naismith, S. L. , & Lewis, S. J. (2013). Variability of stepping during a virtual reality paradigm in Parkinson's disease patients with and without freezing of gait. PLoS One, 8, e66718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, C. G. , Fahn, S. , Martinez‐Martin, P. , Poewe, W. , Sampaio, C. , Stebbins, G. T. , … Lapelle, N. (2007). Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): Process, format, and clinimetric testing plan. Movement Disorders, 22, 41–47. [DOI] [PubMed] [Google Scholar]
- Gray, P. , & Hildebrand, K. (2000). Fall risk factors in Parkinson's disease. The Journal of Neuroscience Nursing, 32, 222–228. [DOI] [PubMed] [Google Scholar]
- Handojoseno, A. M. , Gilat, M. , Ly, Q. T. , Chamtie, H. , Shine, J. M. , Nguyen, T. N. , … Nguyen, H. T. (2015). An EEG study of turning freeze in Parkinson's disease patients: The alteration of brain dynamic on the motor and visual cortex. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2015, 6618–6621. [DOI] [PubMed] [Google Scholar]
- Haslinger, B. , Erhard, P. , Kampfe, N. , Boecker, H. , Rummeny, E. , Schwaiger, M. , … Ceballos‐Baumann, A. O. (2001). Event‐related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain, 124, 558–570. [DOI] [PubMed] [Google Scholar]
- Hausdorff, J. M. , Schaafsma, J. D. , Balash, Y. , Bartels, A. L. , Gurevich, T. , & Giladi, N. (2003). Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Experimental Brain Research, 149, 187–194. [DOI] [PubMed] [Google Scholar]
- Haynes, W. I. , & Haber, S. N. (2013). The organization of prefrontal‐subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. The Journal of Neuroscience, 33, 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda, M. , & Hikosaka, O. (2008). Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. The Journal of Neuroscience, 28, 7209–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. J. , & Barker, R. A. (2009). A pathophysiological model of freezing of gait in Parkinson's disease. Parkinsonism & Related Disorders, 15, 333–338. [DOI] [PubMed] [Google Scholar]
- Lewis, S. J. , & Shine, J. M. (2016). The next step: A common neural mechanism for freezing of gait. The Neuroscientist, 22, 72–82. [DOI] [PubMed] [Google Scholar]
- Lieberman, M. D. , & Cunningham, W. A. (2009). Type I and type II error concerns in fMRI research: Re‐balancing the scale. Social Cognitive and Affective Neuroscience, 4, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar, E. , Shine, J. M. , Naismith, S. L. , & Lewis, S. J. (2013). Using virtual reality to explore the role of conflict resolution and environmental salience in freezing of gait in Parkinson's disease. Parkinsonism & Related Disorders, 19, 937–942. [DOI] [PubMed] [Google Scholar]
- Matar, E. , Shine, J. M. , Naismith, S. L. , & Lewis, S. J. (2014). Virtual reality walking and dopamine: Opening new doorways to understanding freezing of gait in Parkinson's disease. Journal of the Neurological Sciences, 344, 182–185. [DOI] [PubMed] [Google Scholar]
- Mazaika, P. K. , Whifield‐Gabrieli, S. , Reiss, A. , & Glover, G. (2007). Artifact repair of fMRI data from high motion clinical subjects [abstract]. Neuroimage, 36, S142. [Google Scholar]
- Moreau, C. , Defebvre, L. , Destee, A. , Bleuse, S. , Clement, F. , Blatt, J. L. , … Devos, D. (2008). STN‐DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology, 71, 80–84. [DOI] [PubMed] [Google Scholar]
- Morris, T. R. , Cho, C. , Dilda, V. , Shine, J. M. , Naismith, S. L. , Lewis, S. J. , & Moore, S. T. (2012). A comparison of clinical and objective measures of freezing of gait in Parkinson's disease. Parkinsonism & Related Disorders, 18, 572–577. [DOI] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews. Neuroscience, 9, 856–869. [DOI] [PubMed] [Google Scholar]
- Naismith, S. L. , Shine, J. M. , & Lewis, S. J. (2010). The specific contributions of set‐shifting to freezing of gait in Parkinson's disease. Movement Disorders, 25, 1000–1004. [DOI] [PubMed] [Google Scholar]
- Nambu, A. , Tokuno, H. , & Takada, M. (2002). Functional significance of the cortico‐subthalamo‐pallidal 'hyperdirect' pathway. Neuroscience Research, 43, 111–117. [DOI] [PubMed] [Google Scholar]
- Nantel, J. , de Solages, C. , & Bronte‐Stewart, H. (2011). Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson's disease. Gait & Posture, 34, 329–333. [DOI] [PubMed] [Google Scholar]
- Nonnekes, J. , Snijders, A. H. , Nutt, J. G. , Deuschl, G. , Giladi, N. , & Bloem, B. R. (2015). Freezing of gait: A practical approach to management. Lancet Neurology, 14, 768–778. [DOI] [PubMed] [Google Scholar]
- Nutt, J. G. , Bloem, B. R. , Giladi, N. , Hallett, M. , Horak, F. B. , & Nieuwboer, A. (2011). Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurology, 10, 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, S. , Griffin, H. J. , Quinn, N. P. , & Jahanshahi, M. (2008). The factors that induce or overcome freezing of gait in Parkinson's disease. Behavioural Neurology, 19, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini, U. , Boulanouar, K. , Fabre, N. , Martin, F. , Carel, C. , Colonnese, C. , … Rascol, O. (2000). Cortical motor reorganization in akinetic patients with Parkinson's disease: A functional MRI study. Brain, 123(Pt 2), 394–403. [DOI] [PubMed] [Google Scholar]
- Schaafsma, J. D. , Balash, Y. , Gurevich, T. , Bartels, A. L. , Hausdorff, J. M. , & Giladi, N. (2003). Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. European Journal of Neurology, 10, 391–398. [DOI] [PubMed] [Google Scholar]
- Schlenstedt, C. , Shalash, A. , Muthuraman, M. , Falk, D. , Witt, K. , & Deuschl, G. (2017). Effect of high‐frequency subthalamic neurostimulation on gait and freezing of gait in Parkinson's disease: A systematic review and meta‐analysis. European Journal of Neurology, 24, 18–26. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Handojoseno, A. M. , Nguyen, T. N. , Tran, Y. , Naismith, S. L. , Nguyen, H. , & Lewis, S. J. (2014). Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson's disease. Clinical Neurophysiology, 125, 569–576. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Matar, E. , Bolitho, S. J. , Dilda, V. , Morris, T. R. , Naismith, S. L. , … Lewis, S. J. (2013). Modeling freezing of gait in Parkinson's disease with a virtual reality paradigm. Gait & Posture, 38, 104–108. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Matar, E. , Ward, P. B. , Bolitho, S. J. , Gilat, M. , Pearson, M. , … Lewis, S. J. (2013). Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain, 136, 1204–1215. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Matar, E. , Ward, P. B. , Bolitho, S. J. , Pearson, M. , Naismith, S. L. , & Lewis, S. J. (2013). Differential neural activation patterns in patients with Parkinson's disease and freezing of gait in response to concurrent cognitive and motor load. PLoS One, 8, e52602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine, J. M. , Matar, E. , Ward, P. B. , Frank, M. J. , Moustafa, A. A. , Pearson, M. , … Lewis, S. J. (2013). Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain, 136, 3671–3681. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Moore, S. T. , Bolitho, S. J. , Morris, T. R. , Dilda, V. , Naismith, S. L. , & Lewis, S. J. (2012). Assessing the utility of freezing of gait questionnaires in Parkinson's disease. Parkinsonism & Related Disorders, 18, 25–29. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Moustafa, A. A. , Matar, E. , Frank, M. J. , & Lewis, S. J. (2013). The role of frontostriatal impairment in freezing of gait in Parkinson's disease. Frontiers in Systems Neuroscience, 7, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, A. H. , Leunissen, I. , Bakker, M. , Overeem, S. , Helmich, R. C. , Bloem, B. R. , & Toni, I. (2011). Gait‐related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain, 134, 59–72. [DOI] [PubMed] [Google Scholar]
- Spildooren, J. , Vercruysse, S. , Desloovere, K. , Vandenberghe, W. , Kerckhofs, E. , & Nieuwboer, A. (2010). Freezing of gait in Parkinson's disease: The impact of dual‐tasking and turning. Movement Disorders, 25, 2563–2570. [DOI] [PubMed] [Google Scholar]
- Stebbins, G. T. , Goetz, C. G. , Burn, D. J. , Jankovic, J. , Khoo, T. K. , & Tilley, B. C. (2013). How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: Comparison with the unified Parkinson's disease rating scale. Movement Disorders, 28, 668–670. [DOI] [PubMed] [Google Scholar]
- Toledo, J. B. , Lopez‐Azcarate, J. , Garcia‐Garcia, D. , Guridi, J. , Valencia, M. , Artieda, J. , … Rodriguez‐Oroz, M. (2014). High beta activity in the subthalamic nucleus and freezing of gait in Parkinson's disease. Neurobiology of Disease, 64, 60–65. [DOI] [PubMed] [Google Scholar]
- Vandenbossche, J. , Deroost, N. , Soetens, E. , Spildooren, J. , Vercruysse, S. , Nieuwboer, A. , & Kerckhofs, E. (2011). Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabilitation and Neural Repair, 25, 765–773. [DOI] [PubMed] [Google Scholar]
- Vercruysse, S. , Spildooren, J. , Heremans, E. , Vandenbossche, J. , Wenderoth, N. , Swinnen, S. P. , … Nieuwboer, A. (2012). Abnormalities and cue dependence of rhythmical upper‐limb movements in Parkinson patients with freezing of gait. Neurorehabilitation and Neural Repair, 26, 636–645. [DOI] [PubMed] [Google Scholar]
- Vercruysse, S. , Spildooren, J. , Heremans, E. , Wenderoth, N. , Swinnen, S. P. , Vandenberghe, W. , & Nieuwboer, A. (2014). The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cerebral Cortex, 24, 3154–3166. [DOI] [PubMed] [Google Scholar]
- Walton, C. C. , Shine, J. M. , Hall, J. M. , O'Callaghan, C. , Mowszowski, L. , Gilat, M. , … Lewis, S. J. (2015). The major impact of freezing of gait on quality of life in Parkinson's disease. Journal of Neurology, 262, 108–115. [DOI] [PubMed] [Google Scholar]
- Xie, T. , Kang, U. J. , & Warnke, P. (2012). Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 83, 1015–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Characterisation of motor fluctuations in the cohort according to MDS‐UPDRS Items 4.4 (Functional impact of motor fluctuations) and 4.5 (Complexity of motor fluctuations). A score of 0 denotes no impact, with 4 implying high level of functional impact of fluctuations with less than one quarter of OFF periods being predictable. The majority of patients reported low impact of fluctuations and high predictability.
Figure S2: Comparison of original analysis in initial submission using SPM8 (left) and revised re‐analysis of original images using SPM12 (right). Statistical parametric maps shown are analysed with p < 0.005, and a cluster threshold of 20 voxels.
Figure S3: Spatial maps of reduced BOLD signal shown in patients in the OFF state compared to their ON state corresponding to an increased MFSL as subjects passed through a doorway (ON > OFF). Statistical parametric maps shown are analysed with p < 0.005, p < 0.01 and p < 0.05 as indicated by the colour. A cluster threshold of 20 voxels was used. Fourteen clusters identified in total at p < 0.05. Peak coordinates and corresponding region shown in Supporting Information Table S1.
Table S1: Coordinates of clusters of reduced BOLD activation (ON > OFF) in FOG+ patients as they passed through a doorway (p < 0.05, cluster size>20 voxels).


