Abstract
Neurite orientation dispersion and density imaging (NODDI) uses a three‐compartment model to probe brain tissue microstructure, whereas free‐water (FW) imaging models two‐compartments. It is unknown if NODDI detects more disease‐specific effects related to neurodegeneration in Parkinson's disease (PD) and atypical Parkinsonism. We acquired multi‐ and single‐shell diffusion imaging at 3 Tesla across two sites. NODDI (using multi‐shell; isotropic volume [Viso]; intracellular volume [Vic]; orientation dispersion [ODI]) and FW imaging (using single‐shell; FW; free‐water corrected fractional anisotropy [FAt]) were compared with 44 PD, 21 multiple system atrophy Parkinsonian variant (MSAp), 26 progressive supranuclear palsy (PSP), and 24 healthy control subjects in the basal ganglia, midbrain/thalamus, cerebellum, and corpus callosum. There was elevated Viso in posterior substantia nigra across Parkinsonisms, and Viso, Vic, and ODI were altered in MSAp and PSP in the striatum, globus pallidus, midbrain, thalamus, cerebellum, and corpus callosum relative to controls. The mean effect size across regions for Viso was 0.163, ODI 0.131, Vic 0.122, FW 0.359, and FAt 0.125, with extracellular compartments having the greatest effect size. A key question addressed was if these techniques discriminate PD and atypical Parkinsonism. Both NODDI (AUC: 0.945) and FW imaging (AUC: 0.969) had high accuracy, with no significant difference between models. This study provides new evidence that NODDI and FW imaging offer similar discriminability between PD and atypical Parkinsonism, and FW had higher effect sizes for detecting Parkinsonism within regions across the basal ganglia and cerebellum.
Keywords: diffusion MRI, free‐water, isotropic volume, neurite density, orientation dispersion, Parkinsonism
1. INTRODUCTION
There is growing interest in novel multicompartmental diffusion imaging models that are able to more accurately describe the underlying biological microstructure in the human brain. Neurite orientation dispersion and density imaging (NODDI) is one such diffusion MRI technique that probes the microstructure of neurites (i.e., axons and dendrites) by modeling three‐compartments of the brain tissue. Three metrics are produced that quantify the fractional volume of extracellular fluid (isotropic volume fraction [Viso]), as well as describe the intracellular neurite dispersion (orientation dispersion index [ODI]) and neurite density (volume fraction [Vic]), after the effect of the extracellular fluid has been removed (Zhang, Hubbard, Parker, & Alexander, 2011; Zhang, Schneider, Wheeler‐Kingshott, & Alexander, 2012). In comparison, conventional single‐tensor diffusion imaging outcomes, such as fractional anisotropy, model the combined effects of multiple microstructures within a voxel. Prior work has compared a single‐tensor diffusion approach to the two‐compartment free‐water (FW) analysis model, and the two‐compartment model had increased effect sizes for the substantia nigra (Ofori et al., 2017). The NODDI model has not yet been implemented to compare between different subtypes of Parkinsonism. Furthermore, no studies have compared the NODDI model to the FW model, which has been widely used (Pasternak, Sochen, Gur, Intrator, & Assaf, 2009).
PD, progressive supranuclear palsy (PSP), and the Parkinsonian variant of multiple system atrophy (MSAp) are all Parkinsonian disorders with unique underlying pathology that are difficult to differentiate clinically in early stages of the disease. All three present with similar motor symptoms in early stages and have nigrostriatal degeneration (Dickson, 2012). However, MSAp and PSP have more widespread pathological changes characterized by distinct gray and white matter neurodegeneration throughout the basal ganglia, midbrain, thalamus, cerebellum, and corpus callosum (Ahmed, Josephs, Gonzalez, DelleDonne, & Dickson, 2008; Cykowski et al., 2015; Halliday, Macdonald, & Henderson, 2005; Ofori et al., 2017; Planetta et al., 2016; Salvesen et al., 2015; Tsuboi et al., 2003; Wenning, Tison, Ben Shlomo, Daniel, & Quinn, 1997). Moreover, differential diagnosis of these syndromes has important implications for good prognosis and selection for inclusion in clinical trials. Thus, it is critical to develop in vivo neuroimaging markers to differentiate these three Parkinsonian syndromes.
NODDI is a promising imaging technique to understand disease‐specific neurodegeneration that occurs in Parkinsonism. Viso is expected to be increased in cases of neurodegeneration where there should be an increase in extracellular fluid (Zhang et al., 2012). Increases in ODI in white matter correspond to increased dispersion of neurites, which could indicate axonal disorganization in white matter tracts, and decreases of ODI in gray matter could be indicative of dendritic thinning (Zhang et al., 2012). Similarly, Vic is lower in gray matter and higher in white matter and detects changes in density of neurites (Zhang et al., 2012). Indeed, this parcellation of the intracellular tissue into neurite dispersion and density, is unique to NODDI. An alternative model is the two‐compartment model that examines the extracellular compartment (i.e., free‐water), and the tissue compartment from which diffusion tensor imaging scalar metrics are computed, corrected for the free‐water compartment (i.e., free‐water corrected fractional anisotropy [FAt]) (Pasternak et al., 2009). The goal of the current study was to assess three‐compartment NODDI and two‐compartment FW imaging measures in a cohort of PD, MSAp, and PSP diagnosed by movement disorder specialists. A multi‐shell acquisition was used to conduct the NODDI model and the single‐shell was extracted from this acquisition to conduct the FW imaging model (FWms and FAtms). A more clinically feasible single‐shell acquisition was also acquired to compute FW imaging measures (FWss and FAtss) as has been done in prior studies (Burciu et al., 2017; Pasternak et al., 2009; Planetta et al., 2016).
We test the hypothesis that Viso, Vic, and ODI differs across forms of Parkinsonism within the basal ganglia, midbrain, thalamus, cerebellum, and corpus callosum. Viso is expected to be increased in the nigrostriatal regions in PD, MSAp, and PSP (Ofori et al., 2015; Planetta et al., 2016). ODI is expected to be decreased in the putamen and caudate for MSAp, and increased in the superior cerebellar peduncle in PSP, and we expect Vic will be decreased in all of these regions (Planetta et al., 2016).
Further, since prior work in a different cohort of subjects has shown that the FW model detects specific changes in PD compared with MSAp and PSP (Planetta et al., 2016), we compare NODDI and the two FW models in this new cohort, and directly compare NODDI and FW models for distinguishing PD from atypical Parkinsonism. We compute the effect size for disease comparisons to determine which of the models are sensitive to disease‐related changes across the basal ganglia, midbrain, thalamus, and cerebellum. We examine correlations with clinical scales to determine which imaging models relate to clinical status. Finally, we examine the three‐compartment NODDI and two‐compartment FW model at two sites, using two 3 T systems with the same pulse sequences, and a similar clinical protocol. As such, a final goal was to determine if the measurements were different across sites when the data collection is harmonized as part of the study design.
2. MATERIAL AND METHODS
2.1. Participants
The study included 115 participants: 44 with PD, 26 with PSP, 21 with MSAp, and 24 healthy control subjects (Table 1). Participants were referred and diagnosed by movement disorder specialists at the University of Florida, Northwestern University, Rush University, and University of Chicago. All imaging and data collection took place at University of Florida and Northwestern University between October 2017 and March 2019. All procedures were approved by the Institutional Review Board at all sites, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Table 1.
Subject demographics
| CON | PD | MSAp | PSP | |
|---|---|---|---|---|
| N | 24 | 44 | 21 | 26 |
| Site 01/Site 02 | 11/13 | 20/24 | 11/10 | 12/14 |
| Age, year | 67.9 (4.7) | 66.1 (7.8) | 65.9 (7.5) | 70.1 (5.0) |
| Sex, male/female | 10/14 | 26/18 | 15/6 | 11/15 |
| Disease duration, y | 4.3 (2.8) | 2.6 (2.6) | 3.0 (2.5) | |
| Hoehn and Yahr stage (I/II/III/IV/V) | — | 9/34/1/0/0 | 0/5/3/6/7 | 0/6/5/8/6 |
| MDS‐UPDRS III, total | 4.3 (2.5) | 30.7 (13.7) | 62.5 (19.2) | 49.9 (16.8) |
| MDS‐UPDRS III, posture and gait | 0.9 (1.3) | 2.2 (1.6) | 14.0 (8.5) | 10.5 (4.7) |
| UMSARS, parts I and II | 2.3 (1.8) | 17.8 (6.8) | 50.4 (15.5) | 46.6 (14.7) |
| UPSPRS, total | 2.6 (1.8) | 12.5 (5.3) | 36.5 (13.1) | 40.5 (14.4) |
| MoCA | 27.4 (2.0) | 27.3 (2.5) | 24.0 (4.9) | 23.0 (4.0) |
Note: All variables are presented as mean (SD) except sex and Hoehn and Yahr stage which are presented as proportions.
Abbreviation: MDS‐UPDRS III, movement disorders society unified Parkinson's disease rating scale part III; UMSARS, parts I and II, unified multiple systems atrophy rating scale parts I and II;, PSPRS, progressive supranuclear palsy rating scale, MoCA, Montreal cognitive assessment.
All laboratory assessments took place after overnight withdrawal from all dopaminergic medication. Motor impairment was assessed using Part III of the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS‐III) (Goetz et al., 2007). A sub‐score for posture and gait was derived from the summed score of items 9 to 13 of the MDS‐UPDRS‐III. MSAp and PSP specific tests for disease severity were also administered, the Unified Multiple Systems Atrophy Rating Scale (UMSARS) (Wenning et al., 2004) and the Progressive Supranuclear Palsy Rating Scale (PSPRS) (Golbe & Ohman‐Strickland, 2007). The Montreal Cognitive Assessment (MoCA) was used to assess cognitive function (Nasreddine et al., 2005). Disease duration was defined as the time since the current diagnosis. Age and sex‐matched control subjects were free from neurological disease and family history of PD.
2.2. Imaging and diffusion data acquisition
Data were collected at the University of Florida McKnight Brain Institute and Northwestern University Center for Translational Imaging using a 3 T Siemens Prisma (Florida) and 3 T Siemens Prisma Fit (Northwestern) with a 64‐channel head and neck coil. The same scanner software version (VE11C) and the same pulse sequences were implemented at each site. Quality assurance using the same phantom at each site provides quantitative evidence that the signal integrity was stable across sites throughout the study (Supplementary Material). T1‐weighted images were acquired with a three‐dimensional (3D) magnetization‐prepared 180° radio‐frequency pulses and rapid gradient‐echo (MP‐RAGE) sequence (repetition time: 2000 ms, echo time: 2.99 ms, flip angle: 8°, TI = 1,010 ms, GRAPPA factor = 2, 0.8 mm isotropic voxels, 208 contiguous sagittal slices, bandwidth: 240 Hz/pixel) were acquired. Multi‐shell diffusion imaging was acquired to implement the NODDI and FWms models (repetition time: 3200 ms, echo time: 70 ms, flip angle: 90°, field of view: 256 × 256 mm, resolution: 2 mm isotropic, 64 diffusion gradient directions, b‐values: 5 × 0, 64 × 1000, 64 × 2000, and 64 × 3,000 s/mm2, 69 axial slices, bandwidth: 2442 Hz/pixel, total acquisition time: 10 min 52 s) using a simultaneous multi‐slice acquisition (acceleration factor = 3). A single‐shell diffusion image was also acquired to implement the FWss two‐compartment model (repetition time: 6400 ms, echo time: 58 ms, flip angle: 90°, field of view: 256 × 256 mm, resolution: 2 mm isotropic, 64 diffusion gradient directions, b‐values: 5 × 0, and 64 × 1,000 s/mm2, 69 axial slices, bandwidth: 2442 Hz/pixel, total acquisition time: 7 min 41 s). The single‐shell and multi‐shell scans were acquired consecutively.
2.3. Diffusion data processing
All diffusion data were corrected for eddy‐currents and head motion using the FMRIB software library (FSL) eddy_correct tool, and gradient directions were rotated based on eddy current corrections (Smith et al., 2004). Nonbrain tissue was removed prior to data processing using the FSL brain extraction tool. NODDI maps (Viso, Vic, and ODI) were computed from the pre‐processed motion and eddy‐current corrected volumes using the NODDI toolbox in MATLAB (Figure 1) (Zhang et al., 2012). FW and FAt maps were computed on a single‐shell (b‐values: 5 × 0, and 64 × 1000s/mm2) extracted from the multi‐shell diffusion scan (FWms and FAtms) using custom written MATLAB (R2013a, The Mathworks, Natick, MA) code, as described in previous work (Archer et al., 2018; Burciu et al., 2017; Ofori et al., 2015; Pasternak et al., 2009). This was done to derive FW and FAt from the same acquisition that we quantified the NODDI measures. In addition, FW and FAt were computed on the single‐shell acquisition (FWss and FAtss) that has a reduced echo time, as well as a reduced acquisition time making it more clinically feasible. Nonlinear transformations to in‐house templates (Archer et al., 2018; Yang et al., 2019) were performed in order to warp the Viso, Vic, ODI, FW, and FAt maps into standard MNI space using the advanced normalization tools (ANTs) package (Avants, Epstein, Grossman, & Gee, 2008) (Figure 1).
Figure 1.
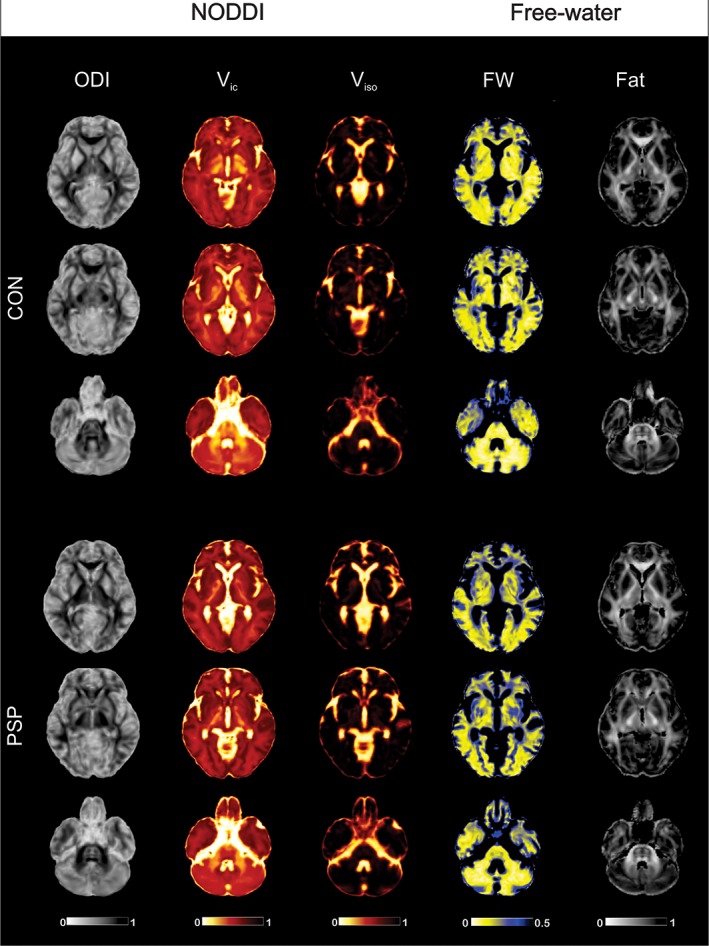
Free‐water imaging and NODDI maps. Free‐water imaging (left two columns) and NODDI maps (right three columns) in a single healthy control subject (CON; top three rows) and individual with progressive supranuclear palsy (PSP; bottom three rows). ODI, orientation dispersion index; Viso, isotropic volume fraction; Vic, intracellular volume fraction [Color figure can be viewed at http://wileyonlinelibrary.com]
A validated in‐house template of 17 regions of interest known to be implicated in Parkinsonism (basal ganglia [anterior substantia nigra, posterior substantia nigra, putamen, caudate nucleus, globus pallidus, and subthalamic nucleus], midbrain/thalamus [red nucleus, thalamus, and pedunculopontine nucleus], cerebellum [dentate nucleus, middle cerebellar peduncle, superior cerebellar peduncle, cerebellar lobule V, cerebellar lobule VI, and cerebellar vermis], and the corpus callosum [CC1, CC2]) was used to obtain regional mean diffusion measures across left and right hemispheres (Planetta et al., 2016).
The data that support the findings of this study are openly available in the Parkinson's disease biomarker program.
2.4. Statistical analyses
Statistical analyses were performed in IBM SPSS version 21.0 and R version 3.2.1. Participant demographics were compared between groups with an analysis of variance (ANOVA) or Welch's ANOVA in the case of nonparametric variables as determined by Levene's test. Distribution of sex was compared using a χ2 test. Diffusion measures were analyzed using a multivariate analysis of variance (MANCOVA) using sex, age, and site as covariates across all regions of interest. Significant group effects were adjusted for multiple comparisons using a 5% false discovery rate (FDR), followed by FDR‐corrected pairwise comparisons (Benjamini & Hochberg, 1995). All reported p‐values have been FDR corrected. The effect size for each outcome was determined using Partial Eta Squared, where 0.01 denotes a small effect size, 0.06 is a medium effect size, and above 0.14 is a large effect size (Lakens, 2013). The relationship between MoCA, MDS‐UPDRS III, and the MDS‐UPDRS III posture and gait sub‐score with diffusion outcomes was assessed across disease groups in regions of interest selected using Pearson's correlation (p < .05, FDR corrected). In order to determine if each imaging method differentiates PD from atypical Parkinsonism (MSAp/PSP), a sensitivity and specificity analysis was conducted. Leave‐one‐out cross‐validation variable selection was used to determine regions that were significant predictors of atypical Parkinsonism in each imaging model (Kuhn, 2008). The subset of regions which minimized the root‐mean‐square error was selected and entered into binary logistic regression and receiver operating characteristic (ROC) analyses. The area under the curve (AUC) was compared between models using Delong's test (DeLong, DeLong, & Clarke‐Pearson, 1988).
3. RESULTS
3.1. Participant characteristics
Group subject demographics are shown in Table 1. Sex distribution (χ2 3,112 = 5.93, p = .115) did not differ across groups, whereas age (Welch's F3,112 = 2.87, p = .054) and disease duration (F2,89 = 3.52, p = .051) approached significance. MDS‐UPDRS‐III scores were different between disease groups (F2,89 = 31.07, p < .001); MSAp and PSP had greater scores than PD (p < .001), and MSAp had greater scores than PSP (p < .012). The posture and gait sub‐score also differed across disease groups (Welch's F2,89 = 53.78, p < .001). Post hoc tests shows that both MSAp and PSP had greater posture and gait scores than PD (p < .001), and MSAp had greater posture and gait scores than PSP (p < .017). The groups also differed in cognitive impairment assessed by the MoCA (Welch's F3,112 = 11.48, p < .001), where both PSP and MSAp were more impaired compared to controls and PD (p ≤ .016).
3.2. Diffusion outcomes
The covariate for site was not significant for any of the diffusion measures across all regions of interest (p > .05, FDR corrected) except the Viso, Vic, and FW in the superior cerebellar peduncle (p ≤ .034, FDR corrected). Each diffusion outcome region‐based mean at each site is reported in the Table S1. Table 2 summarizes the group effects for all diffusion imaging measures derived from the multi‐shell acquisition across sites.
Table 2.
Group effects for each diffusion measure derived from the multi‐shell scan
| Viso | Vic | ODI | FWms | FAtms | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | p valuea | Partial Eta squared | p valuea | Partial Eta squared | p valuea | Partial Eta squared | p valuea | Partial Eta squared | p valuea | Partial Eta squared |
| aSN | <.001 | .173 b – d | .113 | .060 | .012 | .105 e | <.001 | .190 b , d | .189 | .052 |
| pSN | <.001 | .204 b – d , f | .084 | .068 | <.001 | .206 b , d , e | <.001 | .335 b – e | .153 | .059 |
| PUT | <.001 | .293 c , e , g | <.001 | .154 c , e , g | <.001 | .179 c , g | <.001 | .159 c , g | <.001 | .342 c , e , g |
| CN | <.001 | .175 b – d , g | .012 | .113 b , c | .086 | .064 | <.001 | .254 b – e | .998 | <.001 |
| GP | .009 | .106 b , c | <.001 | .162 c , e , g | .016 | .099 d | <.001 | .227 b – e | .007 | .131 c , g |
| STN | .401 | .026 | .013 | .108 b , d , e | <.001 | .157 b , d , e | <.001 | .392 b – e , g | .030 | .091 e |
| RN | .012 | .099 b | .003 | .148 b , d , e | .045 | .078 d | <.001 | .451 b – e | .727 | .017 |
| THA | .056 | .066 | <.001 | .206 b , d , e | <.001 | .203 b , d , e | <.001 | .210 b – d , g | .028 | .095 b |
| PPN | .009 | .106 b , d , e | <.001 | .457 b , d , e | <.001 | .404 b , d , e | <.001 | .428 b , d , e | <.001 | .380 b , d , e |
| DN | .003 | .123 c , g | .119 | .058 | .509 | .021 | <.001 | .239 b – e , g | .783 | .012 |
| MCP | .056 | .068 | <.001 | .307 c – e , g | .095 | .061 | <.001 | .319 c , e , g | .011 | .116 d , e |
| SCP | <.001 | .485 b – e , g | .015 | .103 c , g | <.001 | .206 b , d , e | <.001 | .533 b – e , g | <.001 | .340 b , d , e |
| VER | .056 | .068 | .932 | .006 | .009 | .112 d | .005 | .114 c , g | <.001 | .175 b , d |
| LB V | <.001 | .214 b – d , g | .687 | .017 | .009 | .115 b | <.001 | .260 b – d , g | .024 | .100 b |
| LB VI | <.001 | .207 b – d , g | .549 | .024 | .217 | .041 | <.001 | .251 b – d , g | .727 | .016 |
| CC1 | <.001 | .169 b – e , g | .039 | .084 b | .009 | .117 b | <.001 | .218 b – d | .303 | .041 |
| CC2 | <.001 | .185 b , d | .932 | .004 | .143 | .051 | <.001 | .289 b – e | .505 | .028 |
Note: Significant p‐values designated in bold.
Abbreviations: Viso, isotropic volume fraction; Vic, intracellular volume fraction; ODI, orientation dispersion index; FWms, free‐water derived from multi‐shell scan; FAtms, free‐water corrected fractional anisotropy derived from the multi‐shell scan; aSN, anterior substantia nigra; pSN, posterior substantia nigra; PUT, putamen; CN, caudate nucleus; GP, globus pallidus; STN, subthalamic nucleus; RN, red nucleus; THA, thalamus; PPN, pedunculopontine nucleus; DN, dentate nucleus; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; VER, the cerebellar vermis; CC1, prefrontal of corpus callosum; and CC2, premotor of the corpus callosum.
p‐value FDR‐corrected for multiple comparisons.
PSP versus CON.
MSAp versus CON.
PSP versus PD.
MSAp versus PSP.
PD versus CON.
MSAp versus PD.
3.2.1. NODDI using multi‐shell scan
Figure 2 shows significant group differences in NODDI measures. Viso differed between all groups in most regions of interest (p ≤ .012, Table 2), except the globus pallidus, thalamus, middle cerebellar peduncle, and vermis (p ≥ .056). Compared to controls, PD had greater Viso in the posterior substantia nigra; MSAp had greater Viso than controls in the anterior and posterior substantia nigra, putamen, caudate, globus pallidus, dentate nucleus, superior cerebellar peduncle, lobules V and VI, and the corpus callosum area CC1; and PSP had increased Viso in all regions except the putamen, thalamus, dentate nucleus, middle cerebellar peduncle, and vermis (Figure 2). ODI and Vic were different between all groups in the basal ganglia, midbrain, cerebellum, and corpus callosum (p ≤ .45). Compared to controls, MSAp had decreased ODI in putamen and PSP had increased ODI in the superior cerebellar peduncle, lobule V, corpus callosum area CC1, and decreased ODI in the posterior substantia nigra, subthalamic nucleus, thalamus, and pedunculopontine nucleus. Compared to controls, MSAp had greater Vic in the putamen, caudate, globus pallidus, and superior cerebellar peduncle, and lower Vic in the middle cerebellar peduncle. PSP had decreased Vic in the subthalamic nucleus, red nucleus, thalamus, pedunculopontine nucleus, and corpus callosum area CC1, and increased Vic in the caudate nucleus compared to controls. In summary, Viso differed significantly between groups in 76% (13/17) of the regions tested, Vic in 59%, (10/17) and ODI in 71% (12/17) (Table 2) The mean effect sizes across all regions for Viso was 0.163, Vic 0.122, and ODI 0.131 (Table 2).
Figure 2.
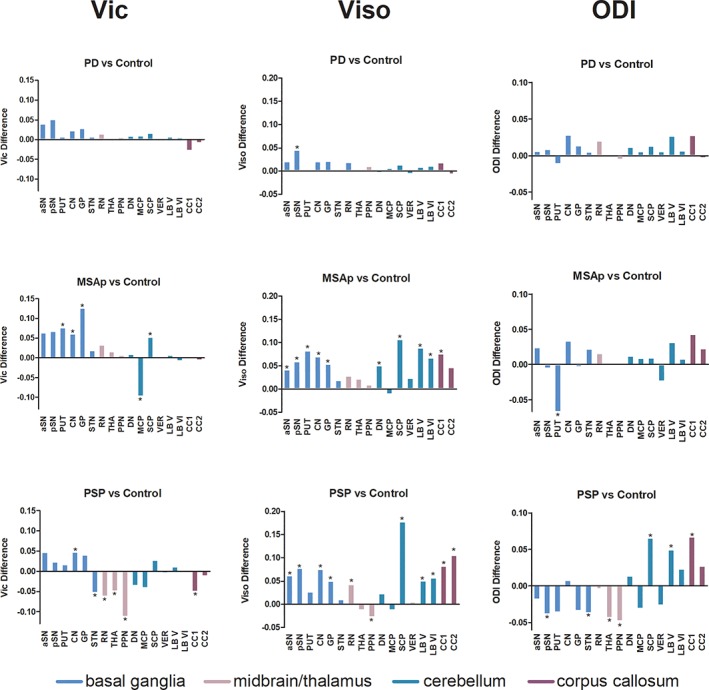
NODDI imaging in Parkinsonism. Between group differences between PD, MSAp, and PSP versus Controls at all regions of interest for each NODDI metric. ODI, orientation dispersion index; Viso, isotropic volume fraction; Vic, intracellular volume fraction; aSN, anterior substantia nigra; pSN, posterior substantia nigra; PUT, putamen; CN, caudate nucleus; GP, globus pallidus; STN, subthalamic nucleus; RN, red nucleus; THA, thalamus; PPN, pedunculopontine nucleus; DN, dentate nucleus; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; VER, the cerebellar vermis; CC1, prefrontal of corpus callosum; and CC2, premotor of the corpus callosum. *p < .05, FDR‐corrected [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.2. Free‐water from multi‐shell scan (FWms and FAtms)
FWms had a significant group effect in all regions of interest (p ≤ .005, Table 2), whereas FAtms differed significantly between groups in a subset of regions across the basal ganglia, midbrain, and cerebellum (p ≤ .030). FWms significantly differed between groups in 100% of all 17 regions tested and FAtms differed in 53% (9/17) of regions (Table 2). Furthermore, the mean effect size across all regions of interest for FWms was 0.286, FAtms 0.117 (Table 2).
3.2.3. Free‐water from single‐shell scan
Figure 3 shows significant group differences in free‐water from single‐shell scan (FWss and FAtss) derived from the single‐shell acquisition. Furthermore, FWss significantly differed between groups in 100% of all 17 regions tested and FAtss differed in 53% (9/17) of regions (Table 3). The mean effect size across all regions of interest for FWss was 0.359 and for FAtss 0.125 (Table 3).
Figure 3.
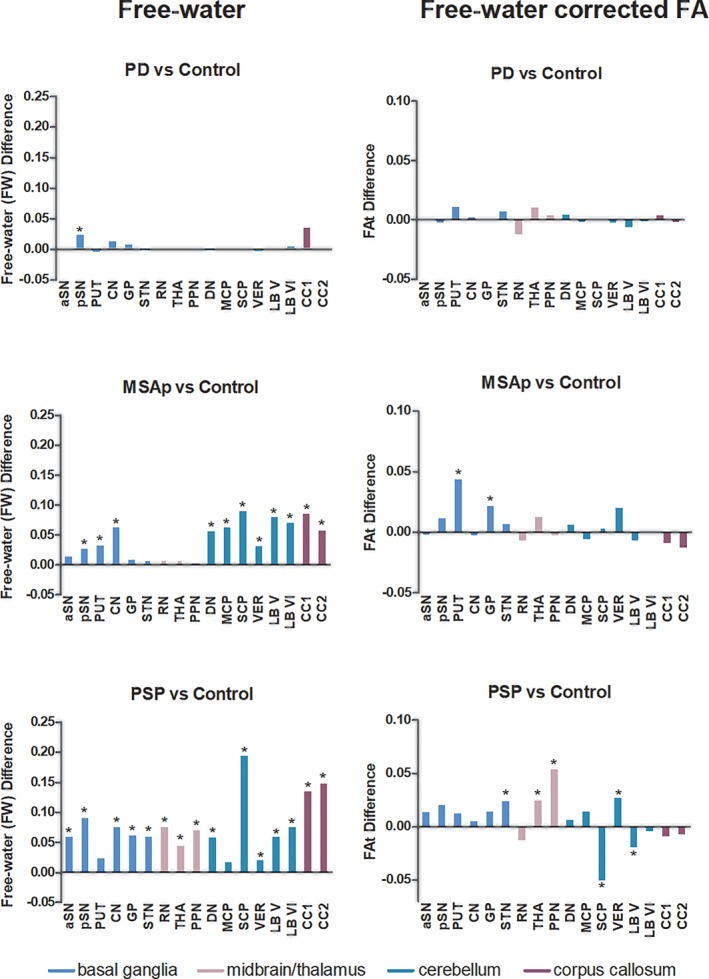
Free‐water imaging in Parkinsonism. Between group differences between PD, MSAp, and PSP versus Controls at all regions of interest for each single‐shell FW metric. aSN, anterior substantia nigra; pSN, posterior substantia nigra; PUT, putamen; CN, caudate nucleus; GP, globus pallidus; STN, subthalamic nucleus; RN, red nucleus; THA, thalamus; PPN, pedunculopontine nucleus; DN, dentate nucleus; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; VER, the cerebellar vermis; CC1, prefrontal of corpus callosum; and CC2, premotor of the corpus callosum. *p < .05, FDR‐corrected [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Free‐water imaging derived from the single‐shell scan
| FWss | FAtss | |||
|---|---|---|---|---|
| ROI | p valuea | Partial Eta squared | p valuea | Partial Eta squared |
| aSN | <.001 | .354 b – d | .190 | .052 |
| pSN | <.001 | .432 b , c , d , e , f | .011 | .108 c |
| PUT | .001 | .137 c , f , g | <.001 | .333 f , g , d |
| CN | <.001 | .280 b , c , f , g | .532 | .026 |
| GP | <.001 | .320 b – d | <.001 | .158 c , f , g |
| STN | <.001 | .596 b – d | <.001 | .154 b – d |
| RN | <.001 | .586 b – d | .532 | .025 |
| THA | <.001 | .392 b – d | .002 | .142 b |
| PPN | <.001 | .495 b – d | <.001 | .370 b – d |
| DN | <.001 | .271 b , c , f , g | .896 | .008 |
| MCP | <.001 | .336 f , g , d | .122 | .063 |
| SCP | <.001 | .565 b – d , f , g | <.001 | .336 b – d |
| VER | <.001 | .177 b , c , f , g | <.001 | .165 b , c , g |
| LB V | <.001 | .294 b , c , f , g | .009 | .116 b |
| LB VI | <.001 | .323 b , c , f , g | .907 | .005 |
| CC1 | <.001 | .269 b , c , f , g | .248 | .045 |
| CC2 | <.001 | .273 b – d , f , g | .579 | .021 |
Note: Significant p‐values designated in bold.
Abbreviations: FWss, free‐water derived from single‐shell scan; FAtss, free‐water corrected fractional anisotropy derived from the single‐shell scan. aSN, anterior substantia nigra; pSN, posterior substantia nigra; PUT, putamen; CN, caudate nucleus; GP, globus pallidus; STN, subthalamic nucleus; RN, red nucleus; THA, thalamus; PPN, pedunculopontine nucleus; DN, dentate nucleus; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; VER, the cerebellar vermis; CC1, prefrontal of corpus callosum; and CC2, premotor of the corpus callosum.
p‐value FDR‐corrected for multiple comparisons.
PSP versus CON.
PSP versus PD.
MSAp versus PSP.
PD versus CON.
MSAp versus CON.
MSAp versus PD.
3.3. Association of diffusion and clinical measures
Overall, FWms significantly correlated with global motor impairment (MDS‐UPDRS‐III total score) in 16 of the regions tested. FWss correlated with motor impairment in 13 regions, Viso and Vic correlated in 10 and 3 regions, respectively, whereas ODI and FAtms correlated in one region, and FAtss was not correlated with motor impairment (Table 4). FWms and FWss significantly correlated with posture and gait impairment (MDS‐UPDRS‐III posture and gait sub‐score) 17 and 16 regions, respectively, whereas Viso correlated with posture and gait impairment in seven regions, Vic and FAtss correlated in four regions, and ODI and FAtms correlated with posture and gait impairment in three regions. FWss correlated with cognitive impairment (MoCA score) in 14 regions, whereas FWms correlated in 10 regions, Viso in 5 regions, FAtms in 1 regions, and ODI, Vic, and FAtss did not correlate with cognitive impairment in any of the regions.
Table 4.
Association of diffusion outcomes and impairment
| aSN | pSN | PUT | CN | GP | STN | RN | THA | PPN | DN | MCP | SCP | VER | LV | LVI | CC1 | CC2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viso | |||||||||||||||||
| UPDRS III total | .35 | .06 | .44 | .39 | .25 | .00 | .11 | .09 | −.05 | .15 | −.18 | .28 | .36 | .31 | .31 | .26 | .25 |
| UPDRS III posture & gait | .48 | .21 | .67 | .31 | .32 | .21 | .19 | .25 | −.06 | .14 | −.13 | .43 | .16 | .25 | .22 | .31 | .31 |
| MoCA | −.27 | −.26 | −.07 | −.33 | −.11 | .07 | −.15 | .08 | .06 | −.06 | .11 | −.26 | −.04 | −.12 | −.15 | −.41 | −.35 |
| Vic | |||||||||||||||||
| UPDRS III total | .12 | .02 | .31 | .32 | .23 | −.03 | −.05 | −.09 | −.13 | −.09 | −.48 | .11 | .01 | .14 | −.02 | −.07 | −.13 |
| UPDRS III posture & gait | .18 | .03 | .46 | .25 | .37 | .03 | −.03 | .00 | −.22 | −.02 | −.52 | .17 | −.01 | .06 | −.04 | .14 | .03 |
| MoCA | .05 | .16 | .01 | −.15 | .07 | .19 | .23 | .25 | .26 | .17 | .22 | −.01 | .12 | −.12 | −.04 | .15 | .01 |
| ODI | |||||||||||||||||
| UPDRS III total | .17 | −.11 | −.32 | .02 | −.04 | .09 | −.07 | −.05 | −.03 | −.08 | −.07 | .13 | −.28 | .16 | .15 | .22 | .24 |
| UPDRS III posture & gait | .29 | −.15 | −.30 | .07 | .00 | .20 | .03 | .06 | −.13 | .04 | −.11 | .22 | −.23 | .28 | .20 | .15 | .31 |
| MoCA | .10 | .23 | .16 | .23 | .13 | .10 | .07 | .24 | .18 | .06 | .27 | −.17 | .27 | −.04 | −.11 | −.29 | −.18 |
| FW ms | |||||||||||||||||
| UPDRS III total | .25 | .24 | .32 | .35 | .25 | .27 | .31 | .31 | .17 | .31 | .37 | .32 | .23 | .32 | .26 | .19 | .20 |
| UPDRS III posture & gait | .36 | .26 | .54 | .35 | .29 | .36 | .35 | .40 | .23 | .35 | .44 | .42 | .27 | .40 | .34 | .23 | .28 |
| MoCA | −.42 | −.46 | −.24 | −.18 | −.38 | −.37 | −.40 | −.39 | −.45 | −.30 | −.28 | −.33 | −.04 | −.15 | −.19 | −.08 | −.20 |
| FAt ms | |||||||||||||||||
| UPDRS III total | −.21 | −.07 | .39 | .00 | .23 | −.03 | −.14 | .00 | −.10 | −.19 | −.05 | −.24 | .17 | .01 | .04 | −.17 | −.13 |
| UPDRS III posture & gait | −.11 | .06 | .54 | −.06 | .35 | .04 | .01 | .02 | .02 | −.16 | .00 | −.32 | .17 | −.06 | .00 | −.08 | −.09 |
| MoCA | .24 | .14 | −.04 | −.14 | −.06 | .06 | .14 | −.07 | −.05 | .12 | .03 | .35 | −.34 | .15 | .06 | .03 | −.08 |
| FW ss | |||||||||||||||||
| UPDRS III total | .32 | .12 | .36 | .27 | .20 | .21 | .22 | .26 | .21 | .35 | .45 | .33 | .41 | .37 | .40 | .29 | .32 |
| UPDRS III posture & gait | .41 | .21 | .53 | .31 | .26 | .36 | .32 | .34 | .30 | .33 | .51 | .48 | .30 | .37 | .39 | .27 | .32 |
| MoCA | −.43 | −.39 | −.17 | −.45 | −.33 | −.36 | −.33 | −.41 | −.38 | −.28 | −.21 | −.33 | −.20 | −.22 | −.25 | −.51 | −.46 |
| FAt ss | |||||||||||||||||
| UPDRS III total | .04 | .17 | .28 | −.11 | .14 | .01 | .00 | .02 | .04 | −.06 | .00 | −.17 | .30 | −.10 | −.09 | −.12 | −.07 |
| UPDRS III posture & gait | .02 | .23 | .37 | −.09 | .26 | .02 | .09 | −.03 | .15 | −.12 | .04 | −.35 | .28 | −.29 | −.20 | −.16 | −.11 |
| MoCA | .03 | −.05 | −.08 | −.06 | −.05 | −.06 | .23 | −.18 | −.19 | −.11 | −.21 | .24 | −.25 | .07 | .07 | .03 | −.06 |
Note: Correlations between motor and cognitive assessments across disease groups. Pearson correlation coefficients are presented, with significance (p < .05, FDR corrected) indicated in bold‐type.
Abbreviations: Viso, isotropic volume fraction; Vic, intracellular volume fraction; ODI, orientation dispersion index; FWms, free‐water derived from multi‐shell scan; FAtms, free‐water corrected fractional anisotropy derived from the multi‐shell scan; FWss, free‐water derived from single‐shell scan; FAtss, free‐water corrected fractional anisotropy derived from the single‐shell scan; aSN, anterior substantia nigra; pSN, posterior substantia nigra; PUT, putamen; CN, caudate nucleus; GP, globus pallidus; STN, subthalamic nucleus; RN, red nucleus; THA, thalamus; PPN, pedunculopontine nucleus; DN, dentate nucleus; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; VER, the cerebellar vermis; CC1, prefrontal of corpus callosum; and CC2, premotor of the corpus callosum.
3.4. Differentiation of Parkinsonism
Results from the classification analysis for detecting atypical Parkinsonism (PD vs. atypical Parkinsonsim [MSAp and PSP]) are illustrated in Figure 4. The AUC in the NODDI model was 0.945 (sensitivity 92%, specificity 93%) with predictors identified using leave‐one‐out cross‐validation being Viso in the superior cerebellar peduncle, lobule V, and the putamen, ODI in the globus pallidus, and Vic in the thalamus and middle cerebellar peduncle. For the FWms model the AUC was 0.969 (sensitivity 85%, specificity 100%) with predictors being FWms in the caudate, subthalamic nucleus, middle and superior cerebellar peduncles, and corpus callosum. For the FWss model the AUC was 0.977 (sensitivity 92%, specificity 100%) with predictors being FWss in the caudate, subthalamic nucleus, middle and superior cerebellar peduncles, lobules V and VI, and dentate nucleus, and FAtss in the globus pallidus. Delong's test determined that there was no difference in performance between any of the three models (p's ≥ .072).
Figure 4.
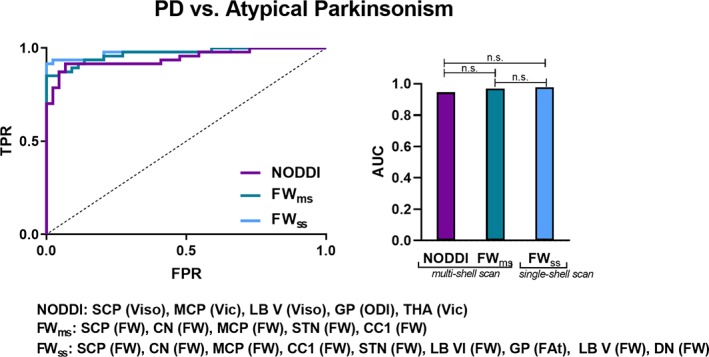
Differentiating atypical Parkinsonism. ROC analyses and corresponding AUC for each diffusion imaging model for PD versus atypical Parkinsonism (MSAp and PSP). The variables selected in each model are listed below. Delong's test was conducted to determine between‐model differences. ODI, orientation dispersion index; Viso, isotropic volume fraction; Vic, intracellular volume fraction; FWms, free‐water derived from the multi‐shell scan; FAtms, free‐water corrected fractional anisotropy derived from the multi‐shell scan. FWss, free‐water derived from the single‐shell scan; FAtss, free‐water corrected fractional anisotropy derived from the single‐shell scan. n.s.; not significant; TPR, true positive rate; FPR, false positive rate; SCP, superior cerebellar peduncle; MCP, middle cerebellar peduncle; LB V, cerebellar lobule V; LB VI, cerebellar lobule VI; GP, globus pallidus; THA, thalamus; STN, subthalamic nucleus; CC1, corpus callosum prefrontal area; CN, caudate nucleus; DN, dentate nucleus [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Both NODDI and FW imaging metrics were assessed in order to determine whether these techniques detect different microstructural effects in PD and atypical Parkinsonism, and whether these outcomes were consistent between two acquisition sites. The first novel finding was that extracellular metrics are more sensitive to disease group than microstructural measures across the two imaging models. Furthermore, our results demonstrate that FW imaging in this cohort detects more disease‐specific effects of neurodegeneration and correlates with clinical status in Parkinsonian syndromes across a greater number of individual brain regions. When combining a subset of regions, both NODDI and FW imaging offer high AUCs, and with no difference in their discriminability between PD and atypical Parkinsonism. When compared to a three‐compartment model using a multi‐shell scan, our findings suggest that data from a single‐shell scan that used a two‐compartment model had greater effect sizes, and no difference in the capacity for differentiation between PD and atypical Parkinsonism. Since the single‐shell scan requires shorter acquisition times, this approach may be more feasible than a multi‐shell scan for application in clinical settings.
Pathology in PD is characterized by degeneration of dopaminergic neurons in the substantia nigra and the aggregation of Lewy bodies (Braak et al., 2003; Fearnley & Lees, 1991). This study is the first to show elevated Viso in the posterior substantia nigra in PD, and both the anterior and posterior substantia nigra in MSAp and PSP compared to healthy controls. FWss imaging results derived from the single‐shell scan confirm previous work that FW in the posterior substantia nigra is elevated in PD, MSAp, and PSP compared to controls (Burciu et al., 2017; Guttuso Jr. et al., 2018; Ofori et al., 2015; Ofori et al., 2017; Planetta et al., 2016). Increased FW and Viso are likely indicative of neurodegeneration wherein the ratio of intracellular to extracellular space is reduced leading to an increase in the measured fractional volume of extracellular fluid (Pasternak et al., 2009). Together, these findings build on previous evidence and support the hypothesis that extracellular fluid in the posterior substantia nigra is increased in PD and atypical forms of Parkinsonism and is a robust marker of nigrostriatal degeneration.
In the present study, Viso and FW in the posterior substantia nigra are the only diffusion measures that differed in PD relative to healthy control subjects, indicating that substantia nigra differences are consistent and highly replicable across studies implementing multicompartmental models (Burciu et al., 2017; Guttuso Jr. et al., 2018; Ofori et al., 2017; Ofori et al., 2015; Planetta et al., 2016; Yang et al., 2019). Studies implementing the NODDI model in PD have shown reduced Vic in the substantia nigra (Kamagata et al., 2016), and reduced Vic along the pathway in which seeds are placed in the substantia nigra and globus pallidus (Andica et al., 2018). In a study using automated analysis of many regions (not including substantia nigra though), the authors found increases in Viso in the left and right caudate, and left putamen (Kamagata et al., 2017). Other studies have reported significant differences between PD and healthy control subjects in other brain regions using conventional diffusion tensor imaging measures (e.g., FA, mean diffusivity, and axial diffusivity); however, these results have also been inconsistent. These regions include the basal ganglia, thalamus (Peran et al., 2010; Zhan et al., 2012), corpus callosum (Gattellaro et al., 2009; Karagulle Kendi, Lehericy, Luciana, Ugurbil, & Tuite, 2008), cerebral cortex (Karagulle Kendi et al., 2008; Nicoletti et al., 2006; Zhan et al., 2012; Zhang et al., 2011), and the cerebellar hemispheres (Mormina et al., 2015; Zhang, Yu, et al., 2011). These disparities could be related to partial volume effects and differences in cohorts.
The pathology of MSA is characterized by alpha‐synuclein glial cytoplasmic inclusions throughout the nigrostriatal and olivopontocerebellar systems (Wenning et al., 1997), along with widespread neurodegeneration in the substantia nigra, striatum, olivopontocerebellar system, frontal and anterior cingulate cortices, and hypothalamus (Cykowski et al., 2015). In line with this pathology, MSAp had increased Viso and Vic in the substantia nigra, putamen, caudate, and globus pallidus compared to controls. These regions align with those in which we find elevated FW compared to controls (i.e., putamen and caudate, red nucleus, and cerebellar regions). It was also found that MSAp had increased FAt (effect size FAtms: .342; effect size FAtss: .333) and decreased ODI (effect size: .179) in the putamen compared to controls. These differences occurred in gray matter regions and thus may indicate thinning of dendrites, which is related to a reduction in the isotropy of diffusion (Nazeri et al., 2015; Zhang et al., 2012). Furthermore, decreased ODI and increased FAt in the putamen was associated with increased motor impairment. Many studies examining diffusion tensor metrics in MSA patients have also reported altered values in the putamen (Barbagallo et al., 2016; Baudrexel et al., 2014; Ito et al., 2007; Kanazawa et al., 2004; Kollensperger et al., 2007; Nicoletti et al., 2006; Planetta et al., 2016; Schocke et al., 2004; Seppi et al., 2006; Tsukamoto et al., 2012), with fewer reporting altered diffusion in the caudate (Barbagallo et al., 2016; Planetta et al., 2016). We also found decreased Vic in the middle cerebellar peduncle and increased Viso in the superior cerebellar peduncle and lobules V and VI compared to controls. Decreases in Vic in white matter regions are indicative of thinning axons and neurodegeneration (Zhang et al., 2012). In line with previous work, we report increased FW in the middle and superior cerebellar peduncles, dentate nucleus, and lobules V and VI compared to controls (Planetta et al., 2016). Previous studies examining conventional diffusion tensor metrics have also reported altered diffusion in the cerebellum, especially the middle cerebellar peduncle, in MSAc (cerebellar variant) (Kanazawa et al., 2004; Pellecchia et al., 2009), MSAp (Nicoletti et al., 2006), and combined MSA cohorts (Planetta et al., 2016). Furthermore, increased cerebellar Viso and FW in the present study was associated with increased motor impairment. Notably, this is the first study to look at multicompartmental diffusion in MSAp, excluding nonparkinsonian variants of MSA (i.e., MSAc). It is likely that early deficits in nigrostriatal and olivopontocerebellar pathways lead to the clinical phenotypes of MSAp and MSAc, respectively. However, postmortem investigations have shown that both olivopontocerebellar and nigrostriatal systems become compromised in both MSAp and MSAc (Cykowski et al., 2015). Recent work found increased FW compared to controls in the thalamus, red nucleus, and pedunculopontine nucleus in a cohort that included both MSAp and MSAc (Planetta et al., 2016). Here, we included only MSAp patients and found increased FW in the red nucleus but not the thalamus or the pedunculopontine nucleus compared to controls, and no changes in NODDI measures in the red nucleus or thalamus. These results suggest that increased thalamic degeneration could be more closely associated with cerebellar pathology. Moreover, both NODDI and FW imaging detects pathologically relevant changes in MSAp involving the nigrostriatal and olivopontocerebellar pathways.
PSP is a neurodegenerative tauopathy where neurofibrillary tangles and neuropil threads accumulate within neurons, astrocytes, and oligodendroglia and there is diffuse neuronal loss with structures affected including the substantia nigra, globus pallidus, subthalamic nucleus, pons, and cerebellum (Agosta et al., 2012; Ahmed et al., 2008; Dickson, 2012; Feany, Mattiace, & Dickson, 1996; Halliday et al., 2005; Hardman, Halliday, McRitchie, Cartwright, & Morris, 1997; Kanazawa et al., 2009; Tsuboi et al., 2003). Both NODDI and FW imaging metrics reflect this pathology. We found increased Viso in the substantia nigra, caudate, globus pallidus, superior cerebellar peduncle, lobule V, and decreased Viso in the pedunculopontine nucleus compared to controls. We found increased ODI in the superior cerebellar peduncle and lobule V, as well as decreased ODI in the posterior substantia nigra and pedunculopontine nucleus compared to controls. Previous FW imaging results (Planetta et al., 2016) and the current FW imaging results demonstrate increased FW compared to controls in these same regions of the basal ganglia and pons. Moreover, we found increased ODI in white matter regions and decreased ODI in gray matter regions. Increases in ODI in white matter regions is indicative of axonal disorganization, degeneration, and dispersion (Zhang, Hubbard, et al., 2011). Decreases in ODI in gray matter regions is indicative of dendritic thinning (Zhang, Hubbard, et al., 2011). There was also decreased ODI in the thalamus and decreased Vic in the subthalamic nucleus, red nucleus, thalamus, pedunculopontine nucleus, and middle cerebellar peduncle compared to controls. Our results show that FW and NODDI imaging detected changes in pathologically relevant regions across the basal ganglia and cerebellum in PSP. FW imaging detected involvement of the dentate nucleus, whereas NODDI detected involvement of the middle cerebellar peduncle. In addition, there was increased Viso and FW in the corpus callosum in MSAp and PSP compared to controls, and these measures were associated with increased cognitive impairment. Furthermore, increased FAt in the superior cerebellar peduncle was associated with increased motor and cognitive impairment. Moreover, the outcomes distinguishing PSP from MSAp and PD were marked reduction in FAt and increased ODI in the superior cerebellar peduncle, and the converse in the pedunculopontine nucleus.
As the clinical phenotype of these three Parkinsonian disorders overlaps in the early stages, and treatment plans may differ between them, there is a critical need to develop neuroimaging markers to differentiate atypical Parkinsonism from PD. This is the first study to compare NODDI and FW imaging in this regard. Both techniques identify similar regions that are affected in pathology in PD, MSAp, and PSP, with some differences between the diffusion metrics for MSAp and PSP, as discussed above. The present study demonstrates consistency across acquisition sites in both of these diffusion techniques in almost all regions of interest. This degree of experimental consistency has not been previously carried out for either NODDI or FW imaging studies in clinical populations. There was no effect of site in all regions except the superior cerebellar peduncle, suggesting that either of these modalities can be used at different neuroimaging sites when the same hardware and pulse sequences are used. Future studies should test whether these diffusion imaging protocols remain consistent across sites when using unmatched scanners, hardware, and pulse sequences. Notably, FWms had a greater number of significant group effects with larger effect size compared to Viso, Vic, ODI, and FAtms. In addition, FWms was correlated with both cognitive and motor impairment across a greater number of regions compared to Viso, Vic, ODI, and FAtms. The FW analysis conducted on the single‐shell acquisition had even greater effect sizes for FWss and a greater number of correlations compared to all other diffusion metrics. Moreover, the current outcomes suggest that FW imaging may better detect disease‐specific neurodegeneration in PD, MSAp, and PSP on a region basis. However, when combining a subset of disease‐relevant regions, there was no difference in performance between NODDI (Viso, Vic, and ODI) and FW imaging (FWms, FAtms or FWss, and FAtss) in separating PD from atypical Parkinsonism. It is important to note that the multi‐shell scan used here was different to the scanning parameters used in the article by Zhang and colleagues (Zhang et al., 2012), and thus the findings for the NODDI model could differ depending on the multi‐shell scan used. In addition, the MD was lower in the multi‐shell scan compared to the single‐shell scan (Figure S2), which could be related to noise from the increased gradient strength in the multi‐shell scan and thus have influenced the effect size estimates (Landman et al., 2007).
Increased water in the extracellular space is indexed by Viso in the NODDI model and FW in the FW model. Both of these measures were increased in the substantia nigra across Parkinsonism, however, we found that in some regions these measures were not consistent with each other. In PSP, Viso in the subthalamic nucleus, thalamus, dentate nucleus, and cerebellar vermis were not different from controls, whereas FW was increased across all of these regions. In MSAp, there was increased FW but not Viso in the middle cerebellar peduncle and cerebellar vermis compared to controls, whereas there was increased Viso but not FW in the anterior substantia nigra and globus pallidus. These discrepancies could be due to differences in how these cerebral spinal fluid constants are computed between the two imaging models (Pasternak et al., 2009; Zhang et al., 2012), and related to the additional noise in the multi‐shell scan (Figure S2). Further, Table 2 and Table 3 indicate that the FW and FAt effect sizes from the single‐shell and multi‐shell scans differ slightly, and we suspect this is related to differences in the scanning parameters of the two sequences.
In this study, NODDI and FW imaging were conducted on the same cohort of PD, MSAp, PSP, and healthy control subjects acquired at two sites. Moreover, FW differed between groups and correlated with impairment scores across a greater number of regions compared to each of the NODDI metrics and FAt. It is noteworthy that ODI and Vic combined had a greater number of significant group effects compared to FAt alone. When combining diffusion metrics, NODDI and FW performed equally well in differentiating PD and atypical Parkinsonism. Thus, this study provides new evidence that NODDI and FW imaging both offer high discriminability between PD and atypical Parkinsonism. Furthermore, as there is no difference in the capacity for differentiation between PD and atypical Parkinsonism and the single‐shell scan requires shorter acquisition times, this approach may be more feasible than a multi‐shell scan for application in clinical settings.
CONFLICT OF INTEREST
Dr. Derek B. Archer reports grant support from the Parkinson's Foundation. Dr. Nikolaus R. McFarland reports grants from the NIH and the Michael J. Fox Foundation, and has received personal honoraria from the NIH and the American Academy of Neurology. Dr. Michael S. Okun serves as consultant for the National Parkinson's Foundation, and has received research grants from the National Institutes of Health, National Parkinson's Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann‐Strauss Foundation, Tourette Syndrome Association, and UF Foundation. Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Tanya Simuni reports grants from NINDS, Michael J. Fox Foundation, Parkinson's Foundation, Biogen, Roche, Neuroderm, Sanofi, and Sun Pharma for research and clinical trials, and served as a consultant for Michael J. Fox Foundation, Parkinson's Foundation, Acadia, Abbvie, Adamas, Anavex, Allergan, Accorda, Denali, Neuroderm, Neurocrine, Revance, Sanofi, Sunovion, TEVA, Takeda, Voyager, and US World Meds. Dr. Simuni has received honorarium from Acadia, Adamas, and TEVA. Dr. Cynthia Comella recieves research support from the NIH, Parkinson's Foundation, Dystonia Medical Research Foundation, Merz Pharmaceutical, Revance Therapeutic, Retrophin and Acorda Therapeutic, and has served as a consultant or an advisory committee member for Acorda Therapeutics, Allergan Inc, Lundbeck Ltd., Medtronic Inc., Merz Pharmaceuticals, Acadia Pharmaceuticals, Jazz Pharmaceuticals, Neurocrine Biosciences Inc., Revance Therapeutic, Sunovion. Dr. Comella serves on the editorial board of Clinical Neuropharmacology and Sleep Medicine, and receives royalties from Cambridge, Wolters Kluwer. Dr. Tao Xie has been funded by the Parkinson's Foundation, NIH, Michael J Fox Foundation for Parkinson's Research, Abbvie, Bristol‐Myers Squibb, Biogen and the University of Chicago for research and clinical trials, and also served as consultant for Parkinson's Foundation, Abbvie, and CVS/Caremark. Dr. Daniel M. Corcos reports grants from NIH. Dr. David E. Vaillancourt reports grants from NIH, NSF, and Tyler's Hope Foundation during the conduct of the study, and personal honoraria from NIH and Parkinson's Foundation unrelated to the submitted work. All other authors report no disclosures.
Supporting information
The supplemental info document has red font as per the revisions made. All font should be black in the published version. Supplemental Figure 1 Quality Assurance over time on the 5B0 scan at each site
S01: Site 1, University of Florida, S02: Site 2, Northwestern University,
Supplemental Figure 2. Mean Diffusivity
Mean diffusivity (MD) in the single‐shell scan (b = 1,000), multi‐shell scan (b = 1,000, b = 2000, b = 3,000), multi‐shell scan (b = 1,000 only). aSN: anterior substantia nigra, pSN: posterior substantia nigra, PUT: putamen, CN: caudate nucleus, GP: globus pallidus, STN: subthalamic nucleus, RN: red nucleus, THA: thalamus, PPN: pedunculopontine nucleus, DN: dentate nucleus, MCP: middle cerebellar peduncle, SCP: superior cerebellar peduncle, LB V: cerebellar lobule V, LB VI: cerebellar lobule VI, VER: the cerebellar vermis, CC1: prefrontal of corpus callosum, and CC2: premotor of the corpus callosum.
Supplemental Table 1. Distribution across site
ACKNOWLEDGMENTS
We would like to thank all the participants for their time and commitment to this research. This work was supported by the National Institutes of Health [U01 NS102038]. This work was supported in part by an NIH award, S10 OD021726, for High End Instrumentation. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory's AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR‐1644779 and the State of Florida.
Mitchell T, Archer DB, Chu WT, et al. Neurite orientation dispersion and density imaging (NODDI) and free‐water imaging in Parkinsonism. Hum Brain Mapp. 2019;40:5094–5107. 10.1002/hbm.24760
Funding information National Institute of Neurological Disorders and Stroke, Grant/Award Number: U01 NS102038; National Institutes of Health
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Parkinson's disease biomarker program.
REFERENCES
- Agosta, F. , Pievani, M. , Svetel, M. , Jecmenica Lukic, M. , Copetti, M. , Tomic, A. , … Filippi, M. (2012). Diffusion tensor MRI contributes to differentiate Richardson's syndrome from PSP‐parkinsonism. Neurobiology of Aging, 33, 2817–2826. [DOI] [PubMed] [Google Scholar]
- Ahmed, Z. , Josephs, K. A. , Gonzalez, J. , DelleDonne, A. , & Dickson, D. W. (2008). Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido‐nigro‐luysial degeneration and axonal dystrophy. Brain, 131, 460–472. [DOI] [PubMed] [Google Scholar]
- Andica, C. , Kamagata, K. , Hatano, T. , Okuzumi, A. , Saito, A. , Nakazawa, M. , … Aoki, S. (2018). Neurite orientation dispersion and density imaging of the nigrostriatal pathway in Parkinson's disease: Retrograde degeneration observed by tract‐profile analysis. Parkinsonism & Related Disorders, 51, 55–60. [DOI] [PubMed] [Google Scholar]
- Archer, D. B. , Coombes, S. A. , Chu, W. T. , Chung, J. W. , Burciu, R. G. , Okun, M. S. , … Vaillancourt, D. E. (2018). A widespread visually‐sensitive functional network relates to symptoms in essential tremor. Brain, 141, 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Epstein, C. L. , Grossman, M. , & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo, G. , Sierra‐Pena, M. , Nemmi, F. , Traon, A. P. , Meissner, W. G. , Rascol, O. , & Peran, P. (2016). Multimodal MRI assessment of nigro‐striatal pathway in multiple system atrophy and Parkinson disease. Movement Disorders, 31, 325–334. [DOI] [PubMed] [Google Scholar]
- Baudrexel, S. , Seifried, C. , Penndorf, B. , Klein, J. C. , Middendorp, M. , Steinmetz, H. , … Hilker, R. (2014). The value of putaminal diffusion imaging versus 18‐fluorodeoxyglucose positron emission tomography for the differential diagnosis of the Parkinson variant of multiple system atrophy. Movement Disorders, 29, 380–387. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Braak, H. , Del Tredici, K. , Rub, U. , de Vos, R. A. , Jansen Steur, E. N. , & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging, 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Burciu, R. G. , Ofori, E. , Archer, D. B. , Wu, S. S. , Pasternak, O. , McFarland, N. R. , … Vaillancourt, D. E. (2017). Progression marker of Parkinson's disease: A 4‐year multi‐site imaging study. Brain, 140, 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski, M. D. , Coon, E. A. , Powell, S. Z. , Jenkins, S. M. , Benarroch, E. E. , Low, P. A. , … Parisi, J. E. (2015). Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain, 138, 2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, E. R. , DeLong, D. M. , & Clarke‐Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44, 837–845. [PubMed] [Google Scholar]
- Dickson, D. W. (2012). Parkinson's disease and parkinsonism: Neuropathology. Cold Spring Harbor Perspectives in Medicine, 2, a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany, M. B. , Mattiace, L. A. , & Dickson, D. W. (1996). Neuropathologic overlap of progressive supranuclear palsy, Pick's disease and corticobasal degeneration. Journal of Neuropathology and Experimental Neurology, 55, 53–67. [DOI] [PubMed] [Google Scholar]
- Fearnley, J. M. , & Lees, A. J. (1991). Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain, 114(Pt 5), 2283–2301. [DOI] [PubMed] [Google Scholar]
- Gattellaro, G. , Minati, L. , Grisoli, M. , Mariani, C. , Carella, F. , Osio, M. , … Bruzzone, M. G. (2009). White matter involvement in idiopathic Parkinson disease: A diffusion tensor imaging study. American Journal of Neuroradiology, 30, 1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, C. G. , Fahn, S. , Martinez‐Martin, P. , Poewe, W. , Sampaio, C. , Stebbins, G. T. , … LaPelle, N. (2007). Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): Process, format, and clinimetric testing plan. Movement Disorders, 22, 41–47. [DOI] [PubMed] [Google Scholar]
- Golbe, L. I. , & Ohman‐Strickland, P. A. (2007). A clinical rating scale for progressive supranuclear palsy. Brain, 130, 1552–1565. [DOI] [PubMed] [Google Scholar]
- Guttuso, T., Jr. , Bergsland, N. , Hagemeier, J. , Lichter, D. G. , Pasternak, O. , & Zivadinov, R. (2018). Substantia nigra free water increases longitudinally in Parkinson disease. American Journal of Neuroradiology, 39, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday, G. M. , Macdonald, V. , & Henderson, J. M. (2005). A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson's disease. Brain, 128, 2272–2280. [DOI] [PubMed] [Google Scholar]
- Hardman, C. D. , Halliday, G. M. , McRitchie, D. A. , Cartwright, H. R. , & Morris, J. G. (1997). Progressive supranuclear palsy affects both the substantia nigra pars compacta and reticulata. Experimental Neurology, 144, 183–192. [DOI] [PubMed] [Google Scholar]
- Ito, M. , Watanabe, H. , Kawai, Y. , Atsuta, N. , Tanaka, F. , Naganawa, S. , … Sobue, G. (2007). Usefulness of combined fractional anisotropy and apparent diffusion coefficient values for detection of involvement in multiple system atrophy. Journal of Neurology, Neurosurgery, and Psychiatry, 78, 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata, K. , Hatano, T. , Okuzumi, A. , Motoi, Y. , Abe, O. , Shimoji, K. , … Aoki, S. (2016). Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. European Radiology, 26, 2567–2577. [DOI] [PubMed] [Google Scholar]
- Kamagata, K. , Zalesky, A. , Hatano, T. , Ueda, R. , Di Biase, M. A. , Okuzumi, A. , … Aoki, S. (2017). Gray matter abnormalities in idiopathic Parkinson's disease: Evaluation by diffusional kurtosis imaging and Neurite orientation dispersion and density imaging. Human Brain Mapping, 38, 3704–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa, M. , Shimohata, T. , Terajima, K. , Onodera, O. , Tanaka, K. , Tsuji, S. , … Nishizawa, M. (2004). Quantitative evaluation of brainstem involvement in multiple system atrophy by diffusion‐weighted MR imaging. Journal of Neurology, 251, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Kanazawa, M. , Shimohata, T. , Toyoshima, Y. , Tada, M. , Kakita, A. , Morita, T. , … Nishizawa, M. (2009). Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Movement Disorders, 24, 1312–1318. [DOI] [PubMed] [Google Scholar]
- Karagulle Kendi, A. T. , Lehericy, S. , Luciana, M. , Ugurbil, K. , & Tuite, P. (2008). Altered diffusion in the frontal lobe in Parkinson disease. American Journal of Neuroradiology, 29, 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollensperger, M. , Seppi, K. , Liener, C. , Boesch, S. , Heute, D. , Mair, K. J. , … Poewe, W. (2007). Diffusion weighted imaging best discriminates PD from MSA‐P: A comparison with tilt table testing and heart MIBG scintigraphy. Movement Disorders, 22, 1771–1776. [DOI] [PubMed] [Google Scholar]
- Kuhn, M. (2008). Building predictive models in R using the caret package. Journal of Statistical Software, 28, 1–26.27774042 [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman, B. A. , Farrell, J. A. , Jones, C. K. , Smith, S. A. , Prince, J. L. , & Mori, S. (2007). Effects of diffusion weighting schemes on the reproducibility of DTI‐derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. NeuroImage, 36, 1123–1138. [DOI] [PubMed] [Google Scholar]
- Mormina, E. , Arrigo, A. , Calamuneri, A. , Granata, F. , Quartarone, A. , Ghilardi, M. F. , … Gaeta, M. (2015). Diffusion tensor imaging parameters' changes of cerebellar hemispheres in Parkinson's disease. Neuroradiology, 57, 327–334. [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Nazeri, A. , Chakravarty, M. M. , Rotenberg, D. J. , Rajji, T. K. , Rathi, Y. , Michailovich, O. V. , & Voineskos, A. N. (2015). Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. The Journal of Neuroscience, 35, 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti, G. , Lodi, R. , Condino, F. , Tonon, C. , Fera, F. , Malucelli, E. , … Quattrone, A. (2006). Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson's disease and progressive supranuclear palsy. Brain, 129, 2679–2687. [DOI] [PubMed] [Google Scholar]
- Ofori, E. , Krismer, F. , Burciu, R. G. , Pasternak, O. , McCracken, J. L. , Lewis, M. M. , … Vaillancourt, D. E. (2017). Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Movement Disorders, 32, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori, E. , Pasternak, O. , Planetta, P. J. , Burciu, R. , Snyder, A. , Febo, M. , … Vaillancourt, D. E. (2015). Increased free water in the substantia nigra of Parkinson's disease: A single‐site and multi‐site study. Neurobiology of Aging, 36, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak, O. , Sochen, N. , Gur, Y. , Intrator, N. , & Assaf, Y. (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine, 62, 717–730. [DOI] [PubMed] [Google Scholar]
- Pellecchia, M. T. , Barone, P. , Mollica, C. , Salvatore, E. , Ianniciello, M. , Longo, K. , … Pappata, S. (2009). Diffusion‐weighted imaging in multiple system atrophy: A comparison between clinical subtypes. Movement Disorders, 24, 689–696. [DOI] [PubMed] [Google Scholar]
- Peran, P. , Cherubini, A. , Assogna, F. , Piras, F. , Quattrocchi, C. , Peppe, A. , … Sabatini, U. (2010). Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain, 133, 3423–3433. [DOI] [PubMed] [Google Scholar]
- Planetta, P. J. , Ofori, E. , Pasternak, O. , Burciu, R. G. , Shukla, P. , DeSimone, J. C. , … Vaillancourt, D. E. (2016). Free‐water imaging in Parkinson's disease and atypical parkinsonism. Brain, 139, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen, L. , Ullerup, B. H. , Sunay, F. B. , Brudek, T. , Lokkegaard, A. , Agander, T. K. , … Pakkenberg, B. (2015). Changes in total cell numbers of the basal ganglia in patients with multiple system atrophy ‐ a stereological study. Neurobiology of Disease, 74, 104–113. [DOI] [PubMed] [Google Scholar]
- Schocke, M. F. , Seppi, K. , Esterhammer, R. , Kremser, C. , Mair, K. J. , Czermak, B. V. , … Wenning, G. K. (2004). Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson's disease. NeuroImage, 21, 1443–1451. [DOI] [PubMed] [Google Scholar]
- Seppi, K. , Schocke, M. F. , Mair, K. J. , Esterhammer, R. , Scherfler, C. , Geser, F. , … Wenning, G. K. (2006). Progression of putaminal degeneration in multiple system atrophy: A serial diffusion MR study. NeuroImage, 31, 240–245. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Tsuboi, Y. , Slowinski, J. , Josephs, K. A. , Honer, W. G. , Wszolek, Z. K. , & Dickson, D. W. (2003). Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology, 60, 1766–1769. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, K. , Matsusue, E. , Kanasaki, Y. , Kakite, S. , Fujii, S. , Kaminou, T. , & Ogawa, T. (2012). Significance of apparent diffusion coefficient measurement for the differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and Parkinson's disease: Evaluation by 3.0‐T MR imaging. Neuroradiology, 54, 947–955. [DOI] [PubMed] [Google Scholar]
- Wenning, G. K. , Tison, F. , Ben Shlomo, Y. , Daniel, S. E. , & Quinn, N. P. (1997). Multiple system atrophy: A review of 203 pathologically proven cases. Movement Disorders, 12, 133–147. [DOI] [PubMed] [Google Scholar]
- Wenning, G. K. , Tison, F. , Seppi, K. , Sampaio, C. , Diem, A. , Yekhlef, F. , … Multiple System Atrophy Study, G . (2004). Development and validation of the unified multiple system atrophy rating scale (UMSARS). Movement Disorders, 19, 1391–1402. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Archer, D. B. , Burciu, R. G. , Muller, M. , Roy, A. , Ofori, E. , … Vaillancourt, D. E. (2019). Multimodal dopaminergic and free‐water imaging in Parkinson's disease. Parkinsonism & Related Disorders, 62, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, W. , Kang, G. A. , Glass, G. A. , Zhang, Y. , Shirley, C. , Millin, R. , … Schuff, N. (2012). Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Movement Disorders, 27, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Hubbard, P. L. , Parker, G. J. , & Alexander, D. C. (2011). Axon diameter mapping in the presence of orientation dispersion with diffusion MRI. NeuroImage, 56, 1301–1315. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Schneider, T. , Wheeler‐Kingshott, C. A. , & Alexander, D. C. (2012). NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61, 1000–1016. [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Yu, C. , Zhang, Y. , Wu, X. , Zhu, C. , Chan, P. , & Li, K. (2011). Voxel‐based analysis of diffusion tensor indices in the brain in patients with Parkinson's disease. European Journal of Radiology, 77, 269–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplemental info document has red font as per the revisions made. All font should be black in the published version. Supplemental Figure 1 Quality Assurance over time on the 5B0 scan at each site
S01: Site 1, University of Florida, S02: Site 2, Northwestern University,
Supplemental Figure 2. Mean Diffusivity
Mean diffusivity (MD) in the single‐shell scan (b = 1,000), multi‐shell scan (b = 1,000, b = 2000, b = 3,000), multi‐shell scan (b = 1,000 only). aSN: anterior substantia nigra, pSN: posterior substantia nigra, PUT: putamen, CN: caudate nucleus, GP: globus pallidus, STN: subthalamic nucleus, RN: red nucleus, THA: thalamus, PPN: pedunculopontine nucleus, DN: dentate nucleus, MCP: middle cerebellar peduncle, SCP: superior cerebellar peduncle, LB V: cerebellar lobule V, LB VI: cerebellar lobule VI, VER: the cerebellar vermis, CC1: prefrontal of corpus callosum, and CC2: premotor of the corpus callosum.
Supplemental Table 1. Distribution across site
Data Availability Statement
The data that support the findings of this study are openly available in the Parkinson's disease biomarker program.


