Abstract
To investigate whether interindividual variability of white matter (WM) tract microstructure of the medial prefrontal cortex (mPFC)‐amygdala circuit could predict 8‐week placebo treatment outcomes in patients with migraine without aura (MO) using diffusion tensor imaging (DTI) with a tractography atlas‐based analysis algorithm and a linear support vector machine algorithm. This study received institutional review board approval, and all subjects gave informed consent. One hundred and twenty‐four MO had an 8‐week sham acupuncture treatment. Patients were subdivided into recovering (MOr, >50% improvement in migraine attack frequency after treatment) and persisting (MOp, <50% reduction in number of migraine days). Neuroimaging was collected via magnetic resonance imaging (MRI) in all subjects. Patients were imaged during the interictal phase of migraine (at least 72 hr after, and not within 24 hr of a migraine) before the treatment. WM microstructures were quantified along the selected fiber pathway and were used to evaluate the discrimination performance for classifying MOr and MOp. The combined features of diffusion measures from vertices along the pathways of the mPFC‐amygdala accurately discriminated MOr from MOp migraineurs with an accuracy of 84.0% (p < .005, permutation test). The most discriminative WM features that contributed to the classification were located in the external capsule and ACC/mPFC. Our findings suggested that the variability of placebo treatment outcomes in migraineurs could be predicted from priori diffusion measures along the fiber pathways of the mPFC‐amygdala, which may demonstrate a potential of WM neuroimaging features as imaging markers for identifying placebo responders in migraine patients.
Keywords: migraine, placebo, prediction, white matter tract microstructure
1. INTRODUCTION
Migraine, as characterized by episodic or chronic headache of a debilitating condition with a number of physiological and emotional stressors (Manack, Buse, & Lipton, 2011; Sprenger & Borsook, 2012), has been an important healthcare and social problem (Borsook, Maleki, Becerra, & Mcewen, 2012; Liu et al., 2015). Systematic review pointed out that placebo treatment in clinical trials of migraine prophylaxis results in responder rates ranging from 0% to 58%, which indicated the overall effect of clinical responses to migraine treatment might be partially due to placebo effects (Meissner et al., 2013; Tfelthansen & Hougaard, 2014). Previous studies pointed out that substantial interindividual variability existed in the ability for endogenous pain control in placebo paradigms (Petrovic, Kalso, Petersson, & Ingvar, 2002; Wager et al., 2004; Zubieta et al., 2005). However, only recent human neuroimaging studies have begun to unravel the interindividual differences in psychologically mediated placebo response in pain disorders (Hashmi et al., 2012; Hashmi et al., 2014; Tétreault et al., 2016).
Placebo effects are a powerful illustration of a learning phenomenon based on expectation and experience, and they could be observed ubiquitously in pain treatment trials (Amanzio, Benedetti, Porro, Palermo, & Cauda, 2013). Accumulating evidence indicates that the placebo response was believed to be driven by the central nervous system and cognitive/emotional processes play a central role in individual subject responses (Büchel, Geuter, Sprenger, & Eippert, 2014). So far, studies have focused on the brain neuroimaging markers for the placebo response to acute pain relief in healthy subjects (Amanzio et al., 2013), in which highly variable responses were found in different subjects (Büchel et al., 2014). Insights from neuroimaging studies have suggested that certain brain measures could predict acute pain elicited by noxious heat (Wager et al., 2013), development of chronic back pain (Baliki et al., 2012; Vachon‐Presseau et al., 2016), and responses to future behavioral treatments (Gabrieli, Ghosh, & Whitfieldgabrieli, 2015). It is quite possible that the placebo response to treatment could be predicted from baseline brain structure and function (Hashmi et al., 2014; Tétreault et al., 2016).
Recently, our group found that baseline gray matter volume of the mPFC and its functional connectivity could predict a future placebo response in an 8‐week sham acupuncture treatment for migraine (Liu et al., 2017b; Liu et al., 2017c). Additionally, the abnormal baseline gray matter volume of the amygdala may have susceptibility to persistent headache and diminished response to placebo treatment (Liu et al., 2017c). Hence, as white matter properties may be linked to gray matter changes, there is a possibility that the anatomical characteristics of the mPFC‐amygdala fiber pathway may also have differences between placebo responders and nonresponders in migraine.
Diffusion tensor imaging (DTI), as an important neuroscience technology, can noninvasively probe the abnormal brain white matter microstructure in vivo in migraine patients (Yu et al., 2013). Tractography atlas‐based analysis (TABS) combines spatial normalization of tensor images with a voxel‐wise statistical framework for tract‐oriented statistics for a single hypothesis test per tract (Liu et al., 2017a), which is a novel method used to investigate abnormal microstructural properties of cerebral white matter. In this study, by using the DTI and TABS, we investigated the diffusional characteristics of the fiber pathway between mPFC and amygdala migraine patients without aura (MO), and we hypothesized that interindividual variability of white matter tract microstructure of the mPFC‐amygdala circuit in the migraine brain could predict the placebo response before starting clinical treatment.
2. MATERIALS AND METHODS
All research procedures were approved by the Institutional Review Board of the First Affiliated Hospital of the Medical College in Xi'an Jiaotong University and were conducted in accordance with the Declaration of Helsinki. All subjects gave written, informed consent after the experimental procedures had been fully explained.
2.1. Participants
Inclusion criteria for the migraine patient group were according to the International Classification of Headache Disorders third edition (beta version) (2013): (1) migraine attacks lasted 4–72 hr (untreated or unsuccessfully treated); (2) featured at least two of the following characteristics: unilateral location, pulsating quality, moderate to severe pain intensity, and aggravation by causing avoidance of routine physical activity; and (3) there was nausea and/or vomiting, or photophobia and phonophobia during migraine. Exclusion criteria were (1) existence of acupuncture treatment; (2) any physical illness such as a brain tumors, hepatitis, or epilepsy as assessed according to clinical evaluations and medical records; (3) existence of other comorbid chronic pain conditions (e.g., tension type headache, fibromyalgia, etc.); (4) existence of a neurological disease or psychiatric disorder; (5) pregnancy or menstrual period; (6) use of prescription medications within the last month; (7) alcohol, nicotine or drug abuse; and (8) claustrophobia.
2.2. Acupuncture treatment
The duration of the study per patient was 16 weeks (Figure 1). Specifically, (1) during the 4 weeks before acupuncture treatment, patients would carefully rate the migraine diary, which included the average pain intensity of the attacks, migraine days, migraine attack duration per time, migraine‐specific quality‐of‐life questionnaire (MSQ), self‐rating anxiety scale (SAS), and self‐rating depression scale (SDS). (2) All patients received the magnetic resonance imaging (MRI) scan during the interictal phase of migraine (at least 72 hr after and not within 24 hr of a migraine), and then they participated in an 8‐week double‐blinded, randomized, placebo‐controlled acupuncture treatment lasting 24 sessions at 30 min duration per session (3 sessions per week) following a similar experimental model in Linde et al.’s (2005) study. The details of the acupuncture treatment were shown in our previous study (Liu et al., 2017c). Sham acupuncture was performed by using Streitberger needles, which retracted into their sheaths when pressed against the skin surface at nonacupoints. After 8 weeks of treatment, the success of blinding was tested using a credibility questionnaire: “When you volunteered for the trial, you were informed that you had an equal chance of receiving traditional acupuncture or sham acupuncture. Which acupuncture do you think you received?” (3) During the 4 weeks after acupuncture treatment, all patients rerated their migraine diary. The drugs used for the prophylaxis of migraine were stopped 4 weeks before the experiment; however, they were allowed to take pirprofen when their migraine was too difficult to endure during the experiment. Detailed information about the patients' drug intake was recorded.
Figure 1.
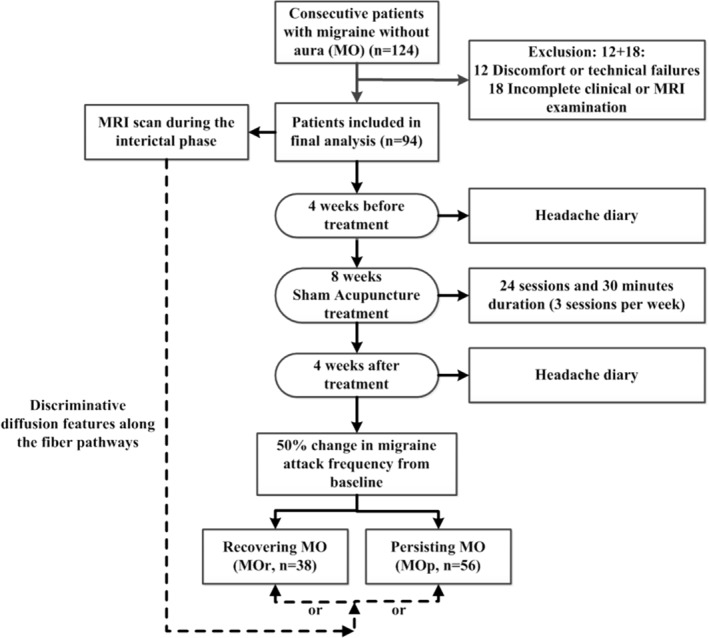
Flow chart of patient inclusion and experimental procedure
In this study, an 8‐week double‐blinded, randomized, placebo‐controlled acupuncture treatment was applied. One hundred and twenty‐four episodic migraineurs were randomly assigned to sham acupuncture treatment. It should be noted that 98 of the 124 patients have been previously reported (Liu et al., 2017b). This prior article dealt with the interindividual variability of the topological organization of the whole‐brain white matter network in migraine brain, whereas in this study, we reported the microstructure along a selected fiber bundle.
2.3. Response to sham acupuncture treatment
According to the guidelines for controlled trials of drugs in migraine (Tfelthansen et al., 2012), responses in preventive migraine randomized controlled trials (RCTs) are usually defined by a relatively strict threshold that has a >50% decrease in attack frequency or migraine days from baseline. In this study, we followed the same principles and defined placebo responders as patients with a reduction in migraine days of at least 50%. Hence, after the sham acupuncture treatment, migraineurs were subdivided into recovering (MOr, >50% improvement in migraine attack frequency after treatment) and persisting (MOp, <50% reduction in number of migraine days) patients.
2.4. Imaging acquisition
This experiment was carried out in a 3.0 Tesla Signa GE scanner with an 8‐channel phase array head coil. For each subject, a high‐resolution structural image was acquired by using a three‐dimensional MRI sequence with a voxel size of 1 mm × 1 mm × 1 mm using an axial Fast Spoiled Gradient Recalled sequence (FSGR) with the following parameters: repetition time (TR) = 1,900 ms; echo time (TE) = 2.26 ms; data matrix = 256 × 256; field of view (FOV) = 256 mm × 256 mm.
DTI was obtained with a single‐shot echo‐planar imaging sequence. Diffusion sensitizing gradients were applied along 30 noncollinear directions (b = 1,000 s/mm2) with five acquisitions without diffusion weighing (b = 0 s/mm2). The imaging parameters were 75 continuous axial slices with a slice thickness of 2 mm and no gap; field of view (FOV) = 256; TR = 9,400 ms; TE = 84 ms; and matrix size = 128 × 128, resulting in 2 mm isotropic voxels.
2.5. Data preprocessing and measurements for diffusion properties
Data preprocessing procedures for diffusion weighted imaging included qualitatively examining the data quality, correcting the motion and rotating the b‐matrix, and correcting for current distortions and echo planar imaging distortion (Liu et al., 2017a). Then, the diffusion tensor and diffusion properties—including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD)—were calculated. The changes of these measurements could be attributed to anisotropic diffusion, the overall degree of diffusivity, the diffusivity along the direction of the reconstructed streamlines, and the diffusivity perpendicular to the direction of the reconstructed streamlines, respectively (Thomason & Thompson, 2011). Hence, the FA, MD, RD, and AD could help us understand how the diffusion tensor is changing and present specific biomarkers of microstructural architecture in white matter (Thomason & Thompson, 2011).
2.6. TABS analysis along selected fiber tracts
Automated TABS is a global‐brain, fully automated, statistical analysis method for observation of white matter microstructure and measurement of microstructural properties along fiber bundles in diffusion MRI data (Liu et al., 2017a). The main contents of TABS contain three parts (Supplementary Information, Figure S1): construction of a population atlas, modeling of fiber tract properties, and collection of the diffusion measures in native space along the fiber tract of interest (Liu et al., 2017a). This method could quantify diffusion properties of white matter at specific locations on a reconstructed streamline, which facilitates an easy interpretation of the findings by directly reporting the resulting statistics on the fiber tracts. The detailed procedure of TABS was shown in our previous study (Liu et al., 2017a).
2.7. Tract selection in population atlas space
Based on our previous findings, baseline brain gray matter volume of the mPFC and amygdala was associated with future placebo responses in migraineurs (Liu et al., 2017c). In this study, these two regions were considered as regions of interest (ROI) to create a fiber bundle of interest in the population atlas space for the TABS analysis. The ROIs of the mPFC (Talairach: ±14 +58 +1) and amygdala (Talairach: ±24 −1 −15) were derived from the center of the mass coordinates by creating 10‐mm‐diameter spheres (Liu et al., 2017c). The ROIs were verified based on the Harvard‐Oxford Atlas. Based on the whole‐brain fiber tracking results, fiber bundles connecting the mPFC and amygdala were created.
In this study, using the TABS method, each diffusion measure (i.e., FA, MD, AD, and RD) from each vertex along the reconstructed streamline was concatenated to yield a single raw feature vector for each subject. The combined features were also concatenated (i.e., FA + MD, FA + RD, FA + AD, MD + RD, MD + AD, RD + AD, FA + MD + RD, FA + MD + AD, FA + RD + AD, MD + RD + AD, and FA + MD + RD + AD). Each feature vector was applied to evaluate the discrimination performance in the following multivariate machine learning analysis.
2.8. The LSVM‐based classification
Neuroimaging data were analyzed using a training, validation, and testing approach with a nested cross‐validation and feature selection routine to classify placebo responders and nonresponders of migraine in our study. While taking into account the complexity of the model, the LSVM was used and generated a classifier with a decision boundary optimizing the separation between the two groups by minimizing the empirical classification error in training data and achieving a higher accuracy on unseen data (Cui, Xia, Su, Shu, & Gong, 2016).
Since a combination of multi‐type features with different diffusion measurements was used, a nested‐leave‐one‐out cross validation (LOOCV) was adopted to select the discriminative features and validate the classification model. Specifically, the classification model was constructed as follows (Supporting Information, Figure S2): (i) in inner LOOCVs (N‐2 subjects), two sample t test was used to yield a p value for each feature, and the best p threshold with a higher classification accuracy was determined by traversing the values in a p threshold range from 0.01 to 1 with a 0.01 interval; (ii) the best p threshold was used to select the features for final classifier training; and (iii) this trained model was applied to the left‐out subject for the given fold. A similar method description can be found in Cui et al. (2016).The LIBSVM toolbox for MATLAB was applied to implement the LSVM classification (http://www.csie.ntu.edu.tw/~cjlin/libsvm/). Regularization parameter, C, was set at a default value of 1.
To quantify our classification performance, the accuracy (percentage of subjects correctly classified), sensitivity (percentage of placebo responders correctly classified), specificity (percentage of placebo nonresponders correctly classified), positive predictive value (PPV, percentage of correct placebo responder predictions), and negative predictive value (NPV, percentage of correct placebo nonresponder predictions) were computed.
Due to a slightly different sample subset in each circulation loop, this may result in a different feature set of selected features across LOOCV folds. The overlapped features across all circulation loops were chosen as the discriminative feature and their absolute value of discriminative weight was averaged. A higher averaged value of the feature indicated a greater contribution to the classification between placebo responders and nonresponders in migraine. The corresponding features to the classification were finally visualized in fiber bundles of a three‐dimensional brain.
2.9. Statistical analysis
To test the group differences in the subjects' basic information (age, years of education, height, weight, temperature, blood pressure, and heart rate), we analyzed the data with one‐way ANOVAs. Post hoc pairwise comparisons were then performed using t tests. The gender data were analyzed using a χ 2 test. The between‐group differences in the patients' headache activity were tested by using two sample t tests.
The between‐group differences in the subjects' diffusion properties along the interested fibers were tested using the permutation‐based nonparametric inferences (5,000 random permutations) in the TABS analysis. The threshold for the statistical significance was set as p < .01, using threshold‐free cluster enhancement (TFCE) with a family‐wise error (FWE) correction for multiple comparison corrections (5,000 permutations).
The permutation test was applied to evaluate the classification performance in the LSVM analysis. The label vector (i.e., +1 for treatment effective group and −1 for treatment ineffective group) for all subjects was randomly permuted 5,000 times, and the same cross‐validation procedure described above was conducted after each permutation. After 5,000 permutations, a null distribution for each model performance metric was obtained. The statistical significance value, p, was then achieved as the proportion of values that was greater than or equal to the value derived from the actual (i.e., nonpermuted) labels. Thus, the smaller p value stands for the more likely significant prediction for the group label, and p < .05 is acceptable.
3. RESULTS
3.1. Clinical and demographic characteristics
In this study, a total of 124 episodic migraineurs were randomly assigned to the sham group and received placebo treatment. Within these patients, 12 patients were excluded for discomfort and technical failures and 18 patients dropped out due to incomplete clinical data of the headache diary or MRI examination, and thus 94 patients completed the whole experiment. Thirteen patients in the sham group and 10 patients in the traditional acupuncture group believed that they had received sham acupuncture, and there were no significant between‐group differences in proportions of unblinding (p > .05, χ 2 test). Within the remaining 94 patients in the sham acupuncture group, no patients took drugs based on their records and reported no acupuncture adverse events during the whole experiment.
In our results, 38 migraineurs had 50% changes in migraine days from baseline after the sham acupuncture treatment (40.4% of the total patients). As can be seen from Table 1, there were no significant between‐group differences between different subject groups (Table 1).
Table 1.
Demographic characteristics of subjects
| Information | MOr (n = 38) | MOp (n = 56) | p value |
|---|---|---|---|
| Age (years) | 22.1 ± 0.45 | 21.5 ± 0.43 | 0.40 |
| Height (cm) | 161.4 ± 1.9 | 159.5 ± 1.81 | 0.45 |
| Weight (kg) | 53.3 ± 1.7 | 53.8 ± 1.64 | 0.85 |
| Temperature | 36.8 ± 0.08 | 36.9 ± 0.07 | 0.21 |
| Blood pressure | 109.1 ± 1.3/72.4 ± 1.4 | 106.6 ± 1.28/71.3 ± 1.33 | 0.17/0.52 |
| Heart rate | 78.1 ± 0.96 | 77.7 ± 0.91 | 0.81 |
| Disease duration (mh) | 51.1 ± 4.76 | 61.9 ± 5.58 | 0.09 |
| Headache days | 8.2 ± 1.8 | 6.1 ± 1.4 | 0.09 |
| VAS | 5.3 ± 0.25 | 5.7 ± 0.24 | 0.39 |
| Average duration of a migraine attack (h) | 8.2 ± 1.49 | 7.4 ± 1.42 | 0.66 |
| SAS | 45.1 ± 2.04 | 43.8 ± 1.94 | 0.59 |
| SDS | 43.5 ± 2.70 | 44.6 ± 2.57 | 0.72 |
| MSQ‐emotion | 63.3 ± 4.68 | 63.0 ± 4.45 | 0.96 |
| MSQ‐function limitation | 61.7 ± 3.90 | 57.9 ± 3.72 | 0.49 |
| MSQ‐function disorder | 69.4 ± 5.05 | 61.7 ± 4.81 | 0.29 |
MOr = migraine without aura, recovering; MOp = migraine without aura, persisting; MSQ = migraine‐specific questionnaire; SAS = self‐rating anxiety scale; SDS = self‐rating depression scale; VAS = visual analogue scale.
Note. Values are mean ± SEM. Data showed the headache activity and mood characteristics before sham acupuncture treatment, for MOr and MOp, respectively. MOp and MOr had similar headache activity at baseline, including migraine duration, attack frequency, pain intensity, and migraine attack duration. Patients also showed similar anxiety and depression characteristics at baseline.
The comparisons of the basic information were performed between MOp and MOr group using two‐sample t test.
3.2. Classification
The classifier could not discriminate MOr from MOp migraineurs using the combined demographics and clinical features (Table 2). The classification became improved using the combined mean values of the FA, MD, AD, and RD features of the fiber pathways of the mPFC‐amygdala, and the classification performance was significantly better than chance (Table 2).
Table 2.
Classification results of the LSVM classifier between MOr and MOp
| Discriminative weight | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Features from demographics and clinical profiles | ||||||
| Age | 0.16 | |||||
| Weight of body | 0.06 | |||||
| Duration | 0.73 | |||||
| VAS | 0.31 | |||||
| SAS | 0.32 | |||||
| SDS | 0.14 | 57.14 | 45.45 | 70.00 | 62.50 | 53.9 |
| MSQ‐emotion | 0.25 | |||||
| MSQ‐function limitation | 0.08 | |||||
| MSQ‐function disorder | 0.35 | |||||
| Features from diffusivity measures | ||||||
| FA | 0.83 | |||||
| MD | 0.21 | |||||
| AD | 0.24 | 57.5** | 68.2** | 48.00** | 53.6** | 63.2** |
| RD | 0.44 | |||||
*p < .01, *p < .005, p < .001 with 5,000 permutation tests.
MOp = migraine without aura, persisting; MOr = migraine without aura, recovering; MSQ = migraine‐specific questionnaire; NPV = negative predictive value; PPV = positive predictive value; SAS = self‐rating anxiety scale; SDS = self‐rating depression scale; VAS = visual analogue scale.
For the combined features of diffusion measures (FA, MD, and RD) from vertices along the fiber pathways of the mPFC‐amygdala, the LSVM classifier accurately discriminated MOr from MOp migraineurs. Specifically, the accuracy, sensitivity, specificity, PPV, and NPV were 84.0%, 90.2%, 76.7%, 82.1%, and 86.8%, respectively (Table 3). The permutation tests revealed that our classification performance of the above results was significantly higher than values expected by chance (p < .001), indicating excellent discriminative power for combined features of the diffusion measures. Additionally, the classification became worse using the single‐type metric of the diffusion measures (Table 3).
Table 3.
Classification results of the LSVM classifier between MOr and MOp
| Features from diffusion measures | Number of overlapped features | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| FA | 1,325 | 69.2 | 76.5 | 60.5 | 69.6 | 68.4 |
| MD | 663 | 62.8 | 70.6* | 53.5 | 64.3 | 60.5 |
| AD | 0 | 30.9 | 38.5 | 25.5 | 26.8 | 36.8 |
| RD | 1,325 | 64.9* | 74.5* | 55.3 | 62.5 | 68.4* |
| FA&MD | 1839 | 78.7** | 87.5* | 69.6* | 75.0* | 84.2** |
| FA&RD | 2,286 | 74.5** | 83.3* | 65.2 | 71.4* | 78.9* |
| MD&RD | 1,606 | 84.0*** | 90.2** | 76.7** | 82.1** | 86.8** |
| FA&MD&RD | 2,758 | 84.0*** | 90.2*** | 76.7*** | 82.1*** | 86.8*** |
| FA&MD&AD&RD | 2,758 | 84.0*** | 90.2*** | 76.7*** | 82.1*** | 86.8*** |
p < .01.
p < .005.
p < .001, with 5,000 permutation tests
AD = axial diffusivity; FA = fractional anisotropy; MD = mean diffusivity; MOr = migraine without aura, recovering; MOp = migraine without aura, persisting; NPV = negative predictive value; PPV = positive predictive value; RD = radial diffusivity.
3.3. Discriminative white matter features along the fiber pathways of the mPFC‐amygdala
As compared with the HC group, several areas exhibited abnormal white matter microstructure along the fiber pathways of the mPFC‐amygdala in patients with migraine, with increased FA, decreased MD, decreased AD, and decreased RD (Figure 2, p < .01, FWE corrected, permutation test). For the classification analysis by using the combined features of diffusion measures (FA, MD, and RD), Figure 3 shows the regions that significantly contributed to the classification performance to discriminate between MOr and MOp, and where areas located at the external capsule (cluster 1) and anterior cingulate cortex (ACC)/mPFC (cluster 2 and 3) were found (Figure 3).
Figure 2.
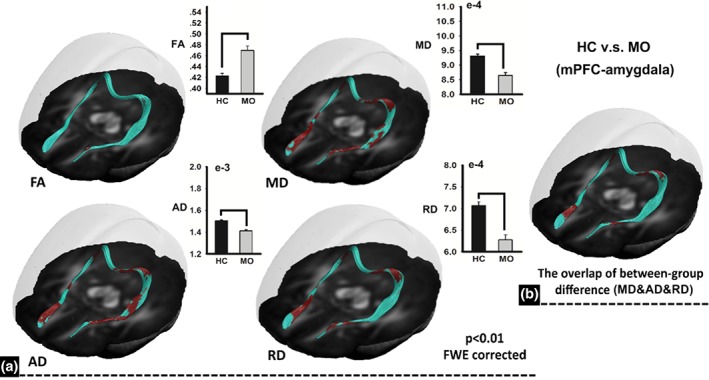
Between‐group differences of diffusion properties along the fiber bundle of the mPFC‐amygdala. As compared with controls, patients with migraine exhibited abnormal white matter microstructure with increased fractional anisotropy, decreased mean diffusivity, decreased axial diffusivity, and decreased radial diffusivity. FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; AD = axial diffusivity [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
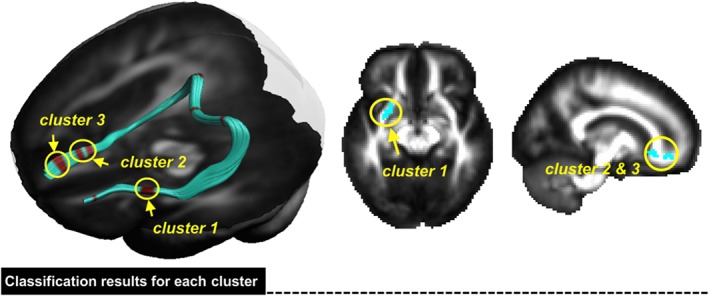
The corresponding features to the classification were finally visualized in fiber bundles of a three‐dimensional brain. In our results, areas located at the external capsule (cluster 1) and anterior cingulate cortex (ACC)/medial prefrontal cortex (mPFC) (cluster 2 and 3) significantly contributed to the classification performance to discriminate between MOr and MOp (MOr: migraine without aura, recovering; MOp: migraine without aura, persisting) [Color figure can be viewed at http://wileyonlinelibrary.com]
In our findings, the discriminative white matter features of the external capsule (cluster 1 in Figure 3) performed better than other clusters in classifying MOr from MOp migraineurs, with an accuracy of 74.5%, sensitivity of 83.3%, specificity of 65.2%, PPV of 71.4%, and NPV of 78.9% (Table 4).
Table 4.
Classification results of the LSVM classifier between MOr and MOp
| Features from combined diffusion measures (FA&MD&RD) | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Cluster 1 | 74.5*** | 83.3* | 65.2* | 71.4** | 78.9** |
| Cluster 2 | 67.0* | 76.6* | 57.4 | 64.3 | 71.1 |
| Cluster 3 | 64.9* | 74.5* | 55.3 | 62.5* | 68.4 |
p < .01.
p < .005.
p < .001, with 5,000 permutation tests
ADr = axial diffusivity; FAr = fractional anisotropy; MDr = mean diffusivity; MOr = migraine without aura, recovering; MOpr = migraine without aura, persisting; NPVr = negative predictive value; PPVr = positive predictive value; RDr = radial diffusivity.
3.4. Correlation between white matter microstructure and migraine days
In our findings, we did not find any significant correlation between the attack frequency or migraine days and diffusion properties (including the FA, MD, RD, and AD) along the fiber pathways of the mPFC‐amygdala in all patients with migraine (p > .05, FWE corrected).
4. DISCUSSION
This study indicated that the interindividual variability of the white matter tract microstructure of the mPFC‐amygdala may be a predisposition for subsequent responses to placebo treatment. Notably, the white matter located in the external capsule and ACC/mPFC within this fiber pathway could predict future placebo responses in migraineurs. Our study provided an alternative way for identifying placebo responders and nonresponders in migraine to sham acupuncture treatment, which offered valuable implication in a clinical setting.
Placebo effects interact with internal psychological and brain processes to context information that promotes health and well‐being, which are mediated by the patient's learning, expectations, and experience (Wager & Atlas, 2015). Studies of placebo effects by using modern neuroimaging techniques have provided an emerging picture of brain systems that are associated with the maintenance of context information and the generation of placebo effects in pain (Wager & Atlas, 2015). Functional neuroimaging studies with positron emission tomography showed reproducible findings of endogenous opioid activity in the PFC, nucleus accumbens‐ventral striatum, periaqueductal gray area, and the amygdala during placebo‐induced analgesia and expected pain intensity (Wager & Atlas, 2015), suggesting that placebo treatment could activate opioid and dopamine neurotransmission in the prefrontal‐subcortical system, which is associated with reward and descending pain inhibitory pathways.
However, studies regarding predicting interindividual variability in pain relief with a placebo treatment in chronic pain patient populations are sparse. By using a double‐blind brain imaging clinical trial, Hashmi et al. (2012) found that the mPFC‐related baseline functional connectivity predicted future placebo responses in chronic back pain patients (Hashmi et al., 2012). Additionally, the same group further reported that network efficiency in baseline brain functional networks could predict analgesic responses to placebo cues (Hashmi et al., 2014), and they observed that a stronger mPFC connection with limbic and memory circuitry was related to placebo analgesia in chronic knee osteoarthritis pain patients (Hashmi et al., 2014). In a randomized clinical trial without manipulating expectations, Tétreault et al. also pointed out that the extent of mPFC‐related functional connectivity at baseline could predict the placebo response in chronic knee osteoarthritis pain patients (Tétreault et al., 2016).
In line with the above findings, as exhibited in the 8‐week placebo treatment for migraine prophylaxis, earlier analyses of subsamples of these data showed that individual differences in brain gray matter volume of the mPFC and amygdala may act as a moderating variable of placebo effects and are associated with variability of treatment outcomes in migraineurs (Liu et al., 2017c). In this study, using DTI, individual variance of treatment success was associated with individual differences of white matter tract microstructure of the mPFC‐amygdala circuit in the migraine brain. Our results indicated that white matter in the brain connects the mPFC and amygdala, so that the information context surrounding placebo treatment could be transported for further processing and integrated differently in different migraine patients. The currently observed discriminability for white matter tracts between the mPFC and amygdala further highlights their essential role in a psychologically induced placebo response.
In our LSVM analysis, only the white matter microstructure located in the external capsule and ACC/mPFC significantly contributed to our classification of placebo responders. The laterocapsular division of the central nucleus (CeLC) receives nociceptive‐specific information from the spinal cord and brainstem and responds exclusively or preferentially to noxious stimuli (Ren & Neugebauer, 2010). It receives strong excitatory input from the mPFC via the external capsule which is known to provide cortical input to the amygdala (Amir, Amano, & Pare, 2011). Researchers showed that amygdalar output can be inhibited by increased infralimbic activity of the mPFC, resulting in eliminating negative emotions (Chang & Maren, 2010; Kim, Jo, Kim, Kim, & Choi, 2010). This was associated in cognitive control deficits in behavioral extinction models (Kim et al., 2010; Sierramercado, Padillacoreano, & Quirk, 2011) and behavioral disinhibition (Dalley, Everitt, & Robbins, 2011). For human studies, Kim et al. used diffusion tensor imaging and found a stronger structural connection linking the amygdala and mPFC which could predict the level of individual anxiety (Kim & Whalen, 2009). Additionally, prolonged stress could cause both a hyperactive amygdala‐mediated fear response to threats and a diminished capacity of the mPFC to regulate the activation of the amygdala following fear acquisition (Feng, Feng, Chen, & Lei, 2014). For migraine patients, frequent headache attacks associated with a number of physiological and emotional stressors (Borsook et al., 2012) may result in a new steady state of neural activity within limbic structures involved in learning and memory that may be maladaptive. As the elimination of stressors may diminish the chronification of migraine, our results suggested that the variability in pain relief with placebo treatment across the MOp and MOr groups, in part, may be due to different engagement of the prefrontal‐amygdala circuit involved in pain‐related emotion.
Previously, by examining brain white matter properties at an earlier time after onset of acute back pain, Mansour et al. reported that white matter structural differences could predict patients whose pain would resolve and those whose pain would persist 1 year later (Mansour et al., 2013). They pointed out that brain white matter abnormalities of some limbic regions impart a predisposition for developing chronic pain conditions (Mansour et al., 2013). Additionally, the same group further reported that higher incidence of white matter abnormalities within the mPFC‐amygdala‐accumbens circuit was associated with emotional overtones of pain representing personality traits and predisposing subjects to both negative emotions and pain chronification (Vachon‐Presseau et al., 2016). They argued that chronic pain might be a maladaptive response, contingent on this vulnerable brain circuit that renders the brain addicted to pain (Baliki et al., 2012; Vachon‐Presseau et al., 2016). Accordingly, an alternative explanation for their findings is that some patients have a diminished hypoalgesia response to placebo treatment which may be due to aberrations in mPFC‐amygdala circuitry, and be an overall contribution to the development and maintenance of persistent pain states (Tracey, 2016). Hence, when the placebo treatment was given, we inferred that some patients may have been locked in a persistent pain state and have diminished hypoalgesia responses (Tracey, 2016). There was no clear empirical verification of our hypothesis; hence, future studies will focus on a longitudinal experimental design for the placebo treatment in a group of patients who are in their early clinical stage of migraine.
There are several issues that should be addressed. First, we did not assess the time elapsed as the last and next migraine attack from the MRI. Second, the lack of a control group who underwent sham acupuncture treatment was also a limitation in our study. Future studies need to consider more extensive longitudinal experiments with healthy control groups.
5. CONCLUSIONS
Placebo responses are substantial across diverse clinical disorders and, in some cases, are related to objective pathology and survival (Wager & Atlas, 2015). Based on common clinical observations of migraineurs with similar headache activity, only some of them exhibited improvement in a clinical condition after placebo treatment (Meissner et al., 2013). Using DTI, we found that the white matter microstructure along the fiber pathway of the mPFC‐amygdala could be used to distinguish placebo responders with migraine in an 8‐week sham acupuncture treatment. This study investigated interindividual variability of the placebo responses from a brain systems‐oriented view which may help capitalize placebo effects in clinical outcomes and develop more efficient clinical trials in migraine patients.
CONFLICT OF INTEREST
None declared.
Supporting information
Figure S1 The pipeline of the tractography atlas‐based analysis (TABS). To provide spatial normalization for the analysis of diffusion values at corresponding locations, the tensor properties in the subjects' native space (a) were integrated to create a population‐specific tensor template (b). Based on the whole‐brain tractography maps in the population space (c), the tract of interest was selected (d). For the modeling of fiber tract properties (2), the prototype fiber was chosen and continuous function of the arc length was used to model the data within the prototype fiber by using a point match method (e). Fibers in the population space were warped back into the individual's native space (f) to collect the diffusion measure
Figure S2. Schematic overview of the nested leave‐one‐out cross‐validation (LOOCV) classification framework. A similar method description can be found in Cui et al. (2016). FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China under Grant Nos. 81471737, 81571640, 81473603, and 81501547; the Fundamental Research Funds for the Central Universities (JB171201); and the Science and Technology Planning Program of Henan Province under Grant Nos. 172106000074 and 162102210218.
Liu J, Mu J, Chen T, Zhang M, Tian J. White matter tract microstructure of the mPFC‐amygdala predicts interindividual differences in placebo response related to treatment in migraine patients. Hum Brain Mapp. 2019;40:284–292. 10.1002/hbm.24372
Funding information Science and Technology Planning Program of Henan Province, Grant/Award Numbers: 162102210218, 172106000074; Fundamental Research Funds for the Central Universities, Grant/Award Number: JB171201; National Natural Science Foundation of China, Grant/Award Numbers: 81501547, 81473603, 81571640, 81471737
Contributor Information
Ming Zhang, Email: zmmri@163.com.
Jie Tian, Email: tian@ieee.org.
REFERENCES
- Amanzio, M. , Benedetti, F. , Porro, C. A. , Palermo, S. , & Cauda, F. (2013). Activation likelihood estimation meta‐analysis of brain correlates of placebo analgesia in human experimental pain. Human Brain Mapping, 34, 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir, A. , Amano, T. , & Pare, D. (2011). Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. Journal of Neurophysiology, 105, 3054–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki, M. , Petre, B. , Torbey, S. , Herrmann, K. , Huang, L. , Schnitzer, T. , … Apkarian, A. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nature Neuroscience, 15, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook, D. , Maleki, N. , Becerra, L. , & Mcewen, B. (2012). Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron, 73, 219–234. [DOI] [PubMed] [Google Scholar]
- Büchel, C. , Geuter, S. , Sprenger, C. , & Eippert, F. (2014). Placebo analgesia: A predictive coding perspective. Neuron, 81, 1223–1239. [DOI] [PubMed] [Google Scholar]
- Chang, C. H. , & Maren, S. (2010). Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behavioral Neuroscience, 124, 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Z. , Xia, Z. , Su, M. , Shu, H. , & Gong, G. (2016). Disrupted white matter connectivity underlying developmental dyslexia: A machine learning approach. Human Brain Mapping, 37, 1443–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J. W. , Everitt, B. J. , & Robbins, T. W. (2011). Impulsivity, compulsivity, and top‐down cognitive control. Neuron, 69, 680–694. [DOI] [PubMed] [Google Scholar]
- Feng, P. , Feng, T. , Chen, Z. , & Lei, X. (2014). Memory consolidation of fear conditioning: Bi‐stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Social Cognitive and Affective Neuroscience, 9, 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli, J. D. , Ghosh, S. S. , & Whitfieldgabrieli, S. (2015). Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron, 85, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi, J. A. , Baria, A. T. , Baliki, M. N. , Huang, L. , Schnitzer, T. J. , & Apkarian, A. V. (2012). Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain, 153, 2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi, J. A. , Kong, J. , Spaeth, R. , Khan, S. , Kaptchuk, T. J. , & Gollub, R. L. (2014). Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. The Journal of Neuroscience, 34, 3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. J. , & Whalen, P. J. (2009). The structural integrity of an amygdala‐prefrontal pathway predicts trait anxiety. The Journal of Neuroscience, 29, 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. C. , Jo, Y. S. , Kim, I. H. , Kim, H. , & Choi, J. S. (2010). Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. The Journal of Neuroscience, 30, 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde, K. , Streng, A. , Jürgens, S. , Hoppe, A. , Brinkhaus, B. , Witt, C. , … Weidenhammer, W. (2005). Acupuncture for patients with migraine: A randomized controlled trial. JAMA, 293, 2118–2125. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Lan, L. , Mu, J. , Zhao, L. , Yuan, K. , Zhang, Y. , … Tian, J. (2015). Genetic contribution of catechol‐O‐methyltransferase in hippocampal structural and functional changes of female migraine sufferers. Human Brain Mapping, 36, 1782–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Liu, H. , Mu, J. , Xu, Q. , Chen, T. , Dun, W. , … Zhang, M. (2017a). Altered white matter microarchitecture in the cingulum bundle in women with primary dysmenorrhea: A tract‐based analysis study. Human Brain Mapping, 38, 4430–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Ma, S. , Mu, J. , Chen, T. , Xu, Q. , Dun, W. , … Zhang, M. (2017b). Integration of white matter network is associated with interindividual differences in psychologically mediated placebo response in migraine patients. Human Brain Mapping, 38, 5250–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Mu, J. , Liu, Q. , Dun, W. , Zhang, M. , & Tian, J. (2017c). Brain structural properties predict psychologically mediated hypoalgesia in an 8‐week sham acupuncture treatment for migraine. Human Brain Mapping, 38, 4386–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manack, A. N. , Buse, D. C. , & Lipton, R. B. (2011). Chronic migraine: Epidemiology and disease burden. Current Pain and Headache Reports, 15, 70–78. [DOI] [PubMed] [Google Scholar]
- Mansour, A. R. , Baliki, M. N. , Huang, L. , Torbey, S. , Herrmann, K. M. , Schnitzer, T. J. , & Apkarian, A. V. (2013). Brain white matter structural properties predict transition to chronic pain. Pain, 154, 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner, K. , Fässler, M. , Rücker, G. , Kleijnen, J. , Hróbjartsson, A. , Schneider, A. , … Linde, K. (2013). Differential effectiveness of placebo treatments: A systematic review of migraine prophylaxis. JAMA Internal Medicine, 173, 1941–1951. [DOI] [PubMed] [Google Scholar]
- Petrovic, P. , Kalso, E. , Petersson, K. M. , & Ingvar, M. (2002). Placebo and opioid analgesia: Imaging a shared neuronal network. Science, 295, 1737–1740. [DOI] [PubMed] [Google Scholar]
- Ren, W. , & Neugebauer, V. (2010). Pain‐related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Molecular Pain, 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierramercado, D. , Padillacoreano, N. , & Quirk, G. J. (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger, T. , & Borsook, D. (2012). Migraine changes the brain: Neuroimaging makes its mark. Current Opinion in Neurology, 25, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault, P. , Mansour, A. , Vachonpresseau, E. , Schnitzer, T. J. , Apkarian, A. V. , & Baliki, M. N. (2016). Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biology, 14, e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelthansen, P. , Pascual, J. , Ramadan, N. , Dahlöf, C. , D'Amico, D. , Diener, H. C. , … Mccrory, D. (2012). Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia, 32, 6–38. [DOI] [PubMed] [Google Scholar]
- Tfelthansen, P. C. , & Hougaard, A. (2014). Migraine: Differential effects of placebos in migraine clinical trials. Nature Reviews. Neurology, 10, 10–11. [DOI] [PubMed] [Google Scholar]
- Thomason, M. E. , & Thompson, P. M. (2011). Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology, 7, 63–85. [DOI] [PubMed] [Google Scholar]
- Tracey, I. (2016). A vulnerability to chronic pain and its interrelationship with resistance to analgesia. Brain, 139, 1869–1872. [DOI] [PubMed] [Google Scholar]
- Vachon‐Presseau, E. , Tétreault, P. , Petre, B. , Huang, L. , Berger, S. E. , Torbey, S. , … Griffith, J. W. (2016). Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain, 139, 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. , Atlas, L. , Lindquist, M. , Roy, M. , Woo, C. , & Kross, E. (2013). An fMRI‐based neurologic signature of physical pain. The New England Journal of Medicine, 368, 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , & Atlas, L. Y. (2015). The neuroscience of placebo effects: Connecting context, learning and health. Nature Reviews. Neurology, 16, 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Rilling, J. K. , Smith, E. E. , Sokolik, A. , Casey, K. L. , Davidson, R. J. , … Cohen, J. D. (2004). Placebo‐induced changes in FMRI in the anticipation and experience of pain. Science, 303, 1162–1167. [DOI] [PubMed] [Google Scholar]
- Yu, D. , Yuan, K. , Qin, W. , Zhao, L. , Dong, M. , Liu, P. , … Zhou, G. (2013). Axonal loss of white matter in migraine without aura: A tract‐based spatial statistics study. Cephalalgia, 33, 34–42. [DOI] [PubMed] [Google Scholar]
- Zubieta, J. K. , Bueller, J. A. , Jackson, L. R. , Scott, D. J. , Xu, Y. , Koeppe, R. A. , … Stohler, C. S. (2005). Placebo effects mediated by endogenous opioid activity on μ‐opioid receptors. The Journal of Neuroscience, 25, 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The pipeline of the tractography atlas‐based analysis (TABS). To provide spatial normalization for the analysis of diffusion values at corresponding locations, the tensor properties in the subjects' native space (a) were integrated to create a population‐specific tensor template (b). Based on the whole‐brain tractography maps in the population space (c), the tract of interest was selected (d). For the modeling of fiber tract properties (2), the prototype fiber was chosen and continuous function of the arc length was used to model the data within the prototype fiber by using a point match method (e). Fibers in the population space were warped back into the individual's native space (f) to collect the diffusion measure
Figure S2. Schematic overview of the nested leave‐one‐out cross‐validation (LOOCV) classification framework. A similar method description can be found in Cui et al. (2016). FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity


