Abstract
Spatial representations are processed in the service of several different cognitive functions. The present study capitalizes on the Activation Likelihood Estimation (ALE) method of meta‐analysis to identify: (a) the shared neural activations among spatial functions to reveal the “core” network of spatial processing; (b) the specific neural activations associated with each of these functions. Following PRISMA guidelines, a total of 133 fMRI and PET studies were included in the meta‐analysis. The overall analysis showed that the core network of spatial processing comprises regions that are symmetrically distributed on both hemispheres and that include dorsal frontoparietal regions, presupplementary motor area, anterior insula, and frontal operculum. The specific analyses revealed the brain regions that are selectively recruited for each spatial function, such as the right temporoparietal junction for shift of spatial attention, the right parahippocampal gyrus, and the retrosplenial cortex for navigation and spatial long‐term memory. The findings are integrated within a systematic review of the neuroimaging literature and a new neurocognitive model of spatial cognition is proposed.
Keywords: activation likelihood estimation, frontoparietal, long‐term memory, mental rotation, meta‐analysis, navigation, neuroimaging, spatial attention, spatial working memory, spatial cognition
“D'où venons‐nous? Que sommes‐nous? Où allons‐nous?. ”
Paul Gauguin.
1. INTRODUCTION
“Where Do We Come From? What Are We? Where Are We Going?” is one of the most famous paintings by French artist Paul Gauguin. “Where?” became indeed the core question that Gauguin asked in his art. We decided to begin the present work citing this painting to highlight how the “where?” is an intrinsic question of the human being, and permeates our lives in its most mysterious and transcendent aspects as well as in its everyday, practical scopes, such as “Where have I parked my car?”. Spatial processing is indeed an essential ability of humans that can be defined as sensing and integrating all spatial aspects of our environment, which comprises both the external multisensory information and the mentally constructed inner mental representations.
When speaking about the brain functional architecture of spatial cognition, one of the first, most influential, finding concerns the distinction between location‐based “where” information and object‐based “what” information that has been identified in posterior cortical regions (Ungerleider & Mishkin, 1982). More specifically, according to Mishkin, Ungerleider, and Macko (1983), the primate visual system is divided into two separate pathways: the ventral, or “what” stream, projecting from the occipital to temporal regions, and the dorsal, or “where” stream, projecting from the occipital to the parietal regions. Pioneering research by Goldman‐Rakic and colleagues pointed out that such a dorsal–ventral distinction between “where” and “what” information is carried through into the frontal regions (for a review, see Levy & Goldman‐Rakic, 2000).
Important for the aims of the present study, spatial representations are encoded and processed in the service of several different cognitive functions, including visuospatial attention, spatial working memory, mental rotation and spatial imagery, long‐term memory, and navigation. These functions encompass distinct cognitive operations and neural substrates, but they may also share common neural mechanisms since they all operate on spatial material.
1.1. Spatial attention
Spatial attention traditionally defines the capacity to select a location for focal visual or auditory processing and to filter out other inputs. Orienting of attention in space is controlled either endogenously, allocating top‐down and voluntary attentional resources; or exogenously, guided by external stimulation (bottom‐up, involuntary stimulus‐driven attention; Jonides, 1981). The most used paradigm to explore endogenous and exogenous spatial orienting is Posner's cueing paradigm (Posner, 1980), in which participants have to respond to a peripheral target that can be preceded by a peripheral or central cue.
A large body of neuroimaging studies has shown that spatial orienting is implemented in a bilateral circuit with core regions in parietal and frontal regions (Corbetta, Miezin, Shulman, & Petersen, 1993; Nobre et al., 1997; see Chica, Bartolomeo, & Lupiáñez, 2013 , for a review). More specifically, frontoparietal regions are segregated into two functional and anatomical networks: the dorsal and the ventral frontoparietal networks (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002). The dorsal frontoparietal network mediates the endogenous, top‐down allocation of attention, and allows the selection of sensory stimuli on the basis of internal goals or expectations. Core regions of this network include the dorsal parietal cortex, in particular, the intraparietal sulcus (IPS) and superior parietal lobule, and the dorsal frontal cortex, near or in the frontal eye fields (FEF). The ventral frontoparietal network supports the bottom‐up, exogenous allocation of attention, and acts to detect salient and task‐relevant stimuli in the environment. Core regions of this network are the temporoparietal junction (TPJ), the inferior frontal gyrus, the anterior insula, and the frontal operculum. Although the majority of the studies of spatial attention used visual stimuli rather than auditory stimuli, a recent study highlighted shared neural substrates supporting visual and auditory modalities spatial attention (Smith et al., 2010).
1.2. Spatial working memory
Spatial working memory is the ability to encode, maintain, and manipulate spatial information for perception and action. A prominent cognitive model of working memory was proposed by Baddeley (1986) that included a distinction between working memory for visuospatial versus verbal material. Subsequent studies have yielded a further fractionation for visuospatial material, proposing separate stores for spatial and visual working memory. This separation within the architecture for working memory systems were suggested to mirror the dorsal–ventral dissociation of the frontoparietal systems mentioned above (Levy & Goldman‐Rakic, 2000).
Particularly controversial have been, however, the functional contribution of the regions of frontal cortex. According to the “material‐specific” view and in line with the Baddeley's models (Baddeley & Logie, 1999), some early neuroimaging studies implicated DLPFC as a crucial site for the domain‐specific maintenance of spatial information (Courtney, Ungerleider, Keil, & Haxby, 1996; McCarthy et al., 1996; Ventre‐Dominey et al., 2005), whereas ventral frontal and prefrontal regions were found activated preferentially in verbal or object working memory (Sala, Rama, & Courtney, 2003).
In contrast, according to the more recent “process‐specific” view, the frontal subregions do not differ in their contribution depending on the material held in WM, but in the type of process they mediate (Nystrom et al., 2000; Owen, Iddon, Hodges, Summers, & Robbins, 1997; Petrides, 2005). Working memory is indeed the result of multiple sub‐processes, including the selective attention, monitoring, and manipulation within working memory, response selection, implementation of strategies to enhance memory, clustering, and organization of material, and control over the representations. All these functions were found to be associated with the activation of distinct regions of prefrontal regions (see Owen, McMillan, Laird, & Bullmore, 2005, for a review). More specifically, the ventrolateral PFC (BA 45/47) would support retrieval of recently encoded information, whereas the dorsolateral PFC (BA 9/46) would subserve monitoring and active processing (Curtis & D'Esposito, 2003).
Importantly, many studies found an hemispheric asymmetry in activation of frontoparietal networks, greater on the right for spatial working memory and on the left for verbal or object working memory (Belger et al., 1998; Jonides et al., 1993; Manoach et al., 2004; Thomason et al., 2009; Ventre‐Dominey et al., 2005), even if several studies failed to find any hemispheric specialization, showing no dissociation between right and left hemispheric activation with spatial versus nonspatial material (Nystrom et al., 2000).
1.3. Navigation and spatial long‐term memory
“I Have Often Walked Down This Street Before” is how the title of a seminal study by Rosenbaum, Ziegler, Winocur, Grady, and Moscovitch (2004) begins and is a representative paper highlighting that the processes of mental navigation and spatial memory are tightly linked and, hence, often explored together. This is the reason why also our meta‐analysis dealt with and investigated the neural substrates of these two abilities together.
Neurophysiological studies in animals have provided snapshots of neuronal responses to simplified stimuli, identifying several populations of cells that encode spatial properties useful for navigation: place cells in the hippocampus (O'Keefe & Nadel, 1978), grid cells in entorhinal cortex (Hafting, Fyhn, Molden, Moser, & Moser, 2005), and head direction cells in Papez circuit structures (Taube, 1998). Nevertheless, the neural systems supporting human spatial navigation in complex environments are less well understood. Neuroimaging studies of human navigation commonly revealed activations of a network of areas comprising a temporoparietal pathway running between the precuneus and parahippocampal cortices via retrosplenial cortex and the parieto‐occipital sulcus, hippocampal regions, precuneus and bilateral posterior parietal cortex, prefrontal and anterior cingulate cortex, and cerebellum (Burgess, Becker, King, & O'Keefe, 2001; Rochefort et al., 2011).
It has been proposed that parahippocampal cortex implements the recognition of specific views whereas the retrosplenial cortex converts the allocentric (environment‐based) representations in hippocampal–entorhinal regions to egocentric representations in posterior parietal cortex (Epstein, 2008; Spiers & Maguire, 2007). Retrosplenial cortex would use head directional information derived from the landmarks (recognized by the parahippocampus gyrus) to allow a representation within a survey (i.e., allocentric) framework. In contrast, posterior parietal cortex is more involved in route‐based (i.e., egocentric) representation of landmarks, processed in relation to the navigator. Recent studies also showed that the retrosplenial cortex and parahippocampal place area (PPA) have complementary roles in spatial navigation, with PPA being involved in representing local visual scenes, whereas retrosplenial cortex being more involved in situating the visual scene within a broader spatial environment (Epstein, 2008).
A central matter of debate is the differential contribution of the hippocampus versus parahippocampal gyrus to spatial memory and mental navigation. Early evidence from single‐cell recording and lesion studies in animals with the discovery of the “place cells” suggested that the hippocampus is necessary for the formation and storage of allocentric spatial representations (Morris, Garrud, Rawlins, & O'Keefe, 1982; O'Keefe & Nadel, 1978). On the other hand, in humans, both the studies with patients and neuroimaging studies indicated parahippocampal gyrus as the candidate area for spatial memory and navigation (Aguirre, Detre, Alsop, & D'Esposito, 1996; Maguire, Burgess, Donnett, O'Keefe, & Frith, 1998; Rosenbaum et al., 2004; Shelton & Gabrieli, 2002, see Spiers & Barry, 2015 for a review).
1.4. Spatial imagery and mental rotation
Mental imagery is a multifaceted cognitive construct and underlies the ability to generate, experience, and manipulate mental images without existing external input (Lückmann, Jacobs, & Sack, 2014). The most typical tasks used to assess spatial mental imagery are mental rotation tasks, which require subjects to judge whether two object stimuli presented at different orientations are identical or mirror images of each other. These tasks thus require subjects to generate a mental image of a geometric shape (or other stimuli, as hands, body parts) and to perform mental operations on this object (e.g., rotate and compare with another mental representation).
Several brain imaging studies have suggested that the bilateral frontoparietal networks, and in particular, the dorsal attention network (DAN), recruited during perceptual visuospatial tasks mediate also the spatial operations of mentally imagined objects (Kosslyn, DiGirolamo, Thompson, & Alpert, 1998; Cohen et al., 1996; Lamm, Windischberger, Moser, & Bauer, 2007; Sack et al., 2008). Studies employing transcranial magnetic stimulation (TMS) during mental rotation performance have been able to corroborate the assumption that successful mental imagery relies on efficiency in regions within the DAN as well as visual areas (Bestmann, Thilo, Sauner, Siebner, & Rothwell, 2002; Cona, Marino, & Semenza, 2017; Cona, Panozzo, & Semenza, 2017; Harris & Miniussi, 2003; van de Ven & Sack, 2013 for a review), whereas the role of primary motor area remains still unclear (Sauner, Bestmann, Siebner, & Rothwell, 2006).
The networks activated during mental imagery are mostly bilateral, although a functional specialization has been suggested: imagery tasks based on categorical spatial processing would be supported by the left posterior parietal cortex, whereas mental spatial transformations based on coordinate spatial processing would be supported by the right posterior parietal cortex (Bien & Sack, 2014; Kosslyn, Maljkovic, Hamilton, Horwitz, & Thompson, 1995; Palermo, Bureca, Matano, & Guariglia, 2008; Trojano et al., 2002). Nevertheless, despite these studies, the actual involvement of both hemispheric counterparts in the spatial mental imagery still remains speculative (Bien & Sack, 2014).
Furthermore, a prominent role is played by premotor cortices and pre‐SMA (Cona, Marino, & Semenza, 2017; Cona, Panozzo, & Semenza, 2017; Jordan, Wustenberg, Heinze, Peters, & Jancke, 2002; Lamm et al., 2007; Leek, Yuen, & Johnston, 2016; Kosslyn et al., 1998; see Zacks, 2008 for a review). Dorsal premotor cortices mediate visuospatial transformations by subserving the processing of spatial codes required to traduce the mental image of the to‐be‐rotated stimulus within a spatial coordinate system (Cona, Panozzo, & Semenza, 2017; Lamm et al., 2007; Milivojevic, Hamm, & Corballis, 2009; Richter et al., 2000). Pre‐SMA instead contributes to sequence processing routines, which would involve spatial mapping between the coordinates of the to‐be‐rotated stimulus, through the computation of vector transformations (Cona, Marino, & Semenza, 2017; Cona & Semenza, 2017, for a review; Leek et al., 2016).
1.5. The current study
The neuroanatomical basis of spatial processing has been a central matter of investigation since the early 1900s. Functional magnetic resonance imaging (MRI) has been widely used with this purpose, providing useful data on functional correlates associated with the processing of the spatial information. The research in the last decades has been very prolific, but no attempt has been made so far to identify consistent results across the available literature.
The present meta‐analysis was designed to reveal both the specific neural activations associated with each cognitive function that involves spatial material and the shared activations among these functions, which would represent the “core” neural substrate deputed to process spatial information. Indeed, since all the functions mentioned above work on the same type of material, that is, spatial material, it is possible to conceive that there is a degree of overlap among their neural substrates. Our starting‐point assumption is supported by the “multiple demand pattern model” (Duncan, 2010), according to which there is a set of regions, centered mainly on frontoparietal network, that is flexibly recruited to represent information—in this case, spatial information—in a variety of different tasks. Furthermore, a recent study that combined diffusion tractography with a meta‐analytic approach found shared activations in several areas within frontoparietal networks among 14 different functions (Parlatini et al., 2017). Other than dealing with the same material, these functions show some common cognitive features and require similar cognitive operations. For example, it has been suggested that attention acts in the service of working memory in three main ways: by directing attention to a memory (spatial) stimulus, by contributing to the attentional‐based rehearsal process, and by regulating the access of the information into the working memory, like a gating mechanism (Awh & Jonides, 2001; Zimmer, 2008). Likewise, all of the functions mentioned above inherently involve the allocation of either internal or external attention toward spatial stimuli/representations, as the individuals need to encode the spatial locations of the to‐be‐retrieved, rotated, or updated stimuli.
At the same time, it is conceivable that the operations that are uniquely recruited for a given function (e.g., retrieving past spatial information in the case of spatial long‐term memory) are subserved by a set of regions that are specifically and uniquely activated in that function (e.g., the parahippocampal gyrus and retrosplenial cortex for long‐term memory).
On these grounds, the present work carefully sought to address three main aims in order to uncover both common and distinct activations underlying spatial functions:
Aim 1. Is there a “core” neural substrate reflecting the processing of spatial properties in healthy individuals? To answer this question, a meta‐analysis including all the available studies investigating the spatial processing in healthy subjects was planned.
Aim 2. Could the results of the aim 1 be biased by the earlier studies on space processing, where the results have not been corrected for multiple comparisons? Indeed, it is well known that results should always be corrected for multiple comparisons, in order to reduce the likelihood of false positives (Bennett, Baird, Miller, & Wolford, 2009; Bennett, Wolford, & Miller, 2009). As the neuroscience literature has been recently under threat by the results replicability problem (Fanelli, 2018), in the current study we are interested in providing consistent and valid data. To answer this question, we performed an additional meta‐analysis including only the original studies where the correction for multiple comparison was applied in order to understand whether or not the inclusion of papers reporting uncorrected findings altered the results (for completeness, we also run a meta‐analysis including only the original studies that did not apply any correction for multiple comparisons. Finally, these two meta‐analyses were contrasted to identify if any statistically significant difference between the two maps exists).
Aim 3. Which are the brain regions recruited in each spatial function (aim 3a)? Which are the brain regions univocally and selectively recruited in each spatial function (aim 3b)? To answer these questions, a meta‐analysis on convergent results for each specific function described in the introduction was planned (aim 3a). In addition, we run discriminability meta‐analyses to identify the brain activations elicited in each specific spatial function against brain activations elicited in all the other functions pooled together (aim 3b).
2. MATERIALS AND METHODS
2.1. Studies selection
An in‐depth search was conducted up to March 2018. Two hundred and ninety possible eligible papers were identified through database search and additional 167 studies were found by means of the “related articles” function of the PubMed database and by tracing the references from review articles and the identified papers. This yielded to an initial identification of 457 papers.
To be included in the systematic review and meta‐analysis, studies have to meet the following inclusion criteria:
to use functional magnetic resonance imaging (fMRI) or positron emission tomography (PET);
to analyze the data using univariate approach that revealed localized increased activation (i.e., studies using machine learning and multivoxel pattern analysis were excluded; studies analyzing the data using functional connectivity or related techniques have been discharged);
to have performed a whole‐brain analysis (i.e., articles that performed only region of interest (ROI) or small volume correction (SVM) analysis have been excluded);
to be peer‐reviewed articles reporting novel data on the spatial processing in healthy individuals;
to use a task clearly linked to spatial processing (e.g., studies using mixed task, for example involving both space and time, as for example [Formisano et al., 2002], were excluded);
to report a clear higher activation during spatial processing compared with a control condition;
sample size >5 participants;
to report results in a standardized coordinate space (e.g. Talairach & Tournoux, 1988 or Montreal Neurologic Institute [MNI]).
2.2. Systematic review
The literature screening and final selection have been performed according to the PRISMA guidelines (Liberati et al., 2009; Moher, Liberati, Tetzlaff, & Altman, 2009). This procedure is summarized in the PRISMA flow diagram that is available within the file “Supporting Information A”. Applying the PRISMA procedure, a total of 133 original articles were found eligible to be included in the systematic review (see file “Supporting Information B” for the list of the studies included).
One author (CS) and a student (in the acknowledgments) extracted and checked the data independently. A second author (CG) double‐checked the data in case of discordance between the first two extractions. A database was created with the following features of each paper: the number of subjects, the cognitive function involved, the specific task used, the contrast performed, the coordinate system, the coordinate localization (brain regions), the p value criteria (corrected, uncorrected), and the associated statistic (t value, z score). In order to avoid to create dependency across experiment maps that might negatively impact on the validity of the meta‐analysis results, for each included paper only the contrast that most strongly reflected the process that the current meta‐analysis aimed to investigate has been selected, in line with the recent meta‐analysis guidelines (Muller et al., 2018). Four out of 133 included papers (Jordan et al., 2002; Kosslyn et al., 1998; Leek et al., 2016; Seurinck, Vingerhoets, de Lange, & Achten, 2004) performed the same experiment using two independent samples. As a consequence, two independent contrasts were selected from these papers without the need to adjust for multiple contrasts. Furthermore, another study (Mellet et al., 2000) performed the same experiment in two independent samples. However, one of the two groups included less than five participants and was thus excluded from the meta‐analysis, according to the inclusion criteria. These procedures led to the inclusion in the meta‐analysis of 133 studies, resulting in 137 experiments, with “study” referring to a paper, and “experiment” referring to an individual contrast reported in each paper.
2.3. The meta‐analysis
The meta‐analysis guidelines (Muller et al., 2018) have been used in the current paper. Talairach coordinates were reported into MNI space before performing the meta‐analysis using a linear transformation (Laird et al., 2010; Lancaster et al., 2007). For a quantitative assessment of inter‐study concordance the Activation Likelihood Estimation (ALE) method (Eickhoff et al., 2009; Laird et al., 2005; Turkeltaub, Eden, Jones, & Zeffiro, 2002) has been applied. The peaks of enhanced activation during spatial processing compared to the control condition were used to generate an ALE map, using the revised ALE algorithm (Turkeltaub et al., 2012) running under Ginger ALE software (http://brainmap.org/ale/) version 2.3.6. This approach aims to identify areas with a convergence of reported coordinates across experiments that are higher than expected from a random distribution of foci. Briefly, this algorithm treats activated foci of brain regions as three‐dimensional Gaussian probability distributions centered at the given coordinates (Eickhoff et al., 2009; Laird et al., 2005). The algorithm incorporates the size of the probability distributions by considering the sample size of each study. Moreover, the algorithm utilizes the random‐effect rather than the fixed‐effect inference. It does so by testing the above‐chance clustering between contrasts rather than the above‐chance clustering between foci. Inference is then sought regarding regions where the likelihood of activation being reported in a particular set of experiments is higher than expected by chance, that is, where there is a nonrandom convergence. For further details on the ALE method please refer to the original publications (Eickhoff et al., 2009; Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Turkeltaub et al., 2012). The checklist for neuroimaging meta‐analysis is available within the file “Supporting Information C”.
According to the experimental hypotheses, different meta‐analyses were run. First, a meta‐analysis was performed including all the studies investigating spatial processing, regardless of the specific cognitive function (aim 1). Second, a meta‐analysis was performed only on those studies where the results were corrected for multiple comparisons (aim 2). For completeness, a meta‐analysis on original studies not corrected for multiple comparisons was also performed, and the two ALE maps were directly contrasted. Third, different meta‐analyses were performed separately for each cognitive function, dividing the studies according to the cognitive operation recruited for the spatial material (aim 3a). Finally, each function specific meta‐analysis was contrasted against the meta‐analysis from the remaining cognitive functions pooled together (e.g., the meta‐analysis on attention was contrasted against the meta‐analysis including working memory, mental rotation, and spatial navigation/long‐term memory) (aim 3b), using the method described in Laird et al. (2005).
Twenty‐five experiments were classified as spatial working memory; 44 as spatial attention (both with visual and auditory modality); 24 as spatial long‐term memory and/or spatial navigation; and 35 as mental rotation and spatial imagery. Eight experiments were not included in the specific analyses since they used tasks that do not clearly involve any specific cognitive function took into account in our meta‐analysis, making the classification of these experiments within one or the other sub‐meta‐analysis artificial.
Concerning the aims 1 and 2 (i.e., investigating the presence of a “core” neural network for space processing and investigating whether or not the results of this first analysis might be biased by the presence of articles presenting uncorrected results), statistical ALE maps were thresholded using voxel level family‐wise error (FWE) correction at p < 0.05 (5,000 permutations) and an extent threshold of 200 voxels, as we wanted to be very conservative. Regarding aim 3a (i.e., to investigate the specific neural functional correlates of space underlying each cognitive spatial function), statistical ALE maps were thresholded using cluster‐level FWE correction at p < 0.05 (cluster‐forming threshold at voxel level p < 0.001) (Eickhoff et al., 2016) according with the recent guidelines for coordinate‐based meta‐analysis (Muller et al., 2018). Finally, regarding aim 3b (i.e., to investigate the brain regions recruited specifically in each cognitive function), statistical ALE maps were thresholded using False Discovery Rate (FDR) correction at p < 0.05 (Laird et al., 2005).
3. RESULTS
3.1. Spatial processing: the core network
The meta‐analysis of all studies involving spatial processing (aim 1) included 1,696 foci from 137 experiments for a total of 1882 participants. The results are reported in Table 1 and graphically represented in Figure 1.
Table 1.
Brain areas commonly activated by all studies of spatial cognition
| Cluster size | Brain regions | Broadman areas | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| All the studies investigating space processing | |||||
| 5,648 | Precuneus | 7 | 12 | −64 | 56 |
| Superior parietal lobule | 7 | 36 | −76 | 51 | |
| Superior parietal lobule | 7 | 30 | −68 | 50 | |
| 4,688 | Middle frontal Gyrus | 6 | 28 | −2 | 56 |
| 4,072 | Precuneus | 7 | −12 | −66 | 54 |
| Superior parietal lobule | 7 | −24 | −58 | 58 | |
| 4,064 | Presupplementary motor area | 6 | 2 | 14 | 50 |
| 3,200 | Middle frontal Gyrus | 6 | −28 | 2 | 54 |
| 1800 | Inferior parietal lobule | 7 | 34 | −48 | 52 |
| Inferior parietal lobule | 40 | 40 | −42 | 44 | |
| 1,768 | Inferior parietal lobule | 40 | −38 | −44 | 44 |
| 1,376 | Insula | 13 | −34 | 22 | −4 |
| 1,320 | Inferior frontal Gyrus, pars opercularis | 9 | 52 | 10 | 24 |
| 808 | Inferior frontal Gyrus, pars opercularis | 9 | −50 | 12 | 28 |
| 504 | Insula | 13 | 34 | 22 | 2 |
| 488 | Fusiform Gyrus | 37 | −35 | −64 | −8 |
| Only studies with results corrected for multiple comparisons | |||||
| 3,720 | Middle frontal Gyrus | 6 | 28 | −2 | 56 |
| Middle frontal Gyrus | 6 | 30 | 8 | 54 | |
| 3,392 | Precuneus | 7 | 12 | −64 | 56 |
| 3,088 | Presupplementary motor area | 32 | 0 | 14 | 48 |
| 2,680 | Superior parietal lobule | 7 | −18 | −68 | 54 |
| Superior parietal lobule | 7 | −22 | −58 | 60 | |
| 2,360 | Middle frontal Gyrus | 6 | −28 | 2 | 56 |
| 1,432 | Inferior parietal lobule | 40 | −38 | −44 | 44 |
| 1,088 | Insula | 13 | −32 | 24 | −4 |
| 800 | Fusiform Gyrus | 37 | −35 | −65 | −8 |
| 792 | Inferior frontal Gyrus, pars opercularis | 9 | 52 | 10 | 24 |
| 696 | Inferior parietal lobule | 7 | 36 | −46 | 52 |
| Inferior parietal lobule | 40 | 40 | −42 | 46 | |
| 608 | Superior parietal lobule | 7 | 30 | −66 | 50 |
| Superior parietal lobule | 19 | 34 | −74 | 54 | |
| 400 | Insula | 13 | 34 | 22 | 2 |
| 328 | Inferior frontal Gyrus, pars opercularis | 9 | −50 | 12 | 28 |
| Precentral Gyrus | 6 | −46 | 2 | 32 | |
Figure 1.
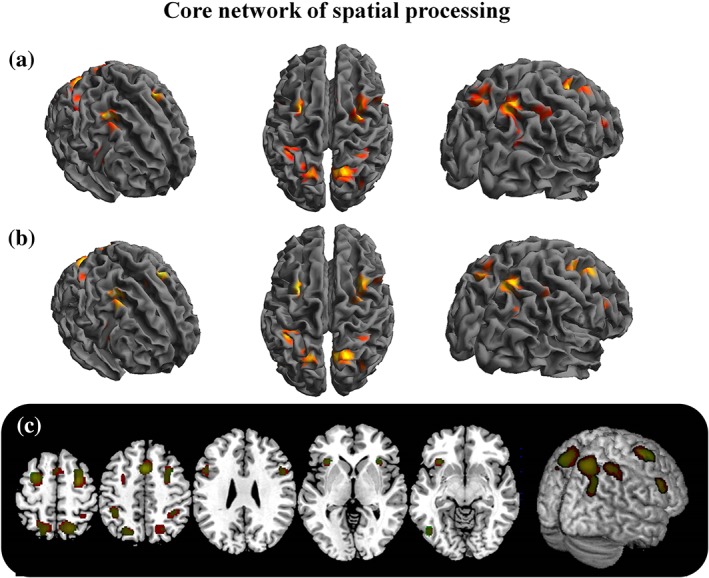
Core network for spatial processing. The figure represents the results of the meta‐analysis including all the eligible studies (a) and the results of the meta‐analysis including only the studies that applied the correction for multiple comparisons to their results (b); in the third panel (c), the overlap between the two meta‐analyses is graphically represented
This analysis showed a symmetrical pattern of activation between the hemispheres. Activations were located bilaterally in dorsal regions of frontal and parietal lobes (BA 6 and BA 7, bilaterally), and medially, in the pre‐SMA. More specifically, concerning the dorsal frontal regions, activations were found mainly over right and left middle frontal gyrus, in regions corresponding to the FEF and dorsal premotor cortex, BA 6); whereas over dorsal parietal regions, the activations involved precuneus, bilateral superior parietal lobules, and intraparietal sulci.
Although with a lesser extent, a symmetrical pattern of activation was also found in insular cortices and in ventral frontoparietal regions, which include the right and left frontal opercula (BA 9), and the inferior parietal lobules (BA 40). Significant convergence was also found in the left fusiform gyrus (BA 37).
The meta‐analysis comprising only the sub‐group of studies where the results have been corrected for multiple comparisons (aim 2) included 1,110 foci from 82 experiments for a total of 1,215 participants. The results are reported in Table 1 and graphically represented in Figure 1. Notably, the same cortical regions of the first meta‐analysis were identified by this meta‐analysis too, although the size of the clusters was reasonably slightly different from that observed in the previous analysis. This difference in cluster size is likely to be due to the smaller number of included experiments (137 vs. 82) and, consequently, foci of activation (1,697 vs. 1,110), rather than functional differences between the two meta‐analyses. For completeness, the meta‐analysis comprising only the studies where the results have not been corrected for multiple comparisons included 586 foci from 55 experiments for a total of 667 participants. Convergent results are revealed in the dorsal region of the frontal lobe (BA 6; 30, −6, 58) and in the inferior parietal lobule (BA 40; 40, −42, 44). The direct comparison between the two meta‐analyses (i.e., that one including only studies corrected and that one including only studies uncorrected for multiple comparisons) revealed no statistically significant differences.
In the following paragraphs, the results of each cognitive function involving space are presented (aims 3a and 3b). Eight experiments (132 foci and 107 participants) have not been included in the following analyses as the task used could not be categorized univocally in any of the following cognitive functions.
3.2. Spatial attention
We then considered separately the 516 foci reported in 44 experiments of spatial attention for a total of 602 participants. The results are reported in Table 2 and graphically represented in Figure 2.
Table 2.
Brain areas activated in spatial attention
| Cluster size | Brain region | Broadman area | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 9,424 | Superior parietal lobule | 7 | 16 | −62 | 56 |
| Precuneus | 7 | 8 | −60 | 54 | |
| Middle occipital Gyrus | 19 | 36 | −78 | 32 | |
| Inferior parietal lobule | 40 | 40 | −46 | 46 | |
| Superior occipital gyrus | 19 | 32 | −68 | 40 | |
| 5,920 | Presupplementary motor area | 6 | 0 | 12 | 50 |
| 5,672 | Middle frontal Gyrus | 6 | −26 | −4 | 58 |
| Middle frontal Gyrus | 6 | −28 | 4 | 54 | |
| Precentral Gyrus | 6 | −42 | −2 | 52 | |
| Precentral Gyrus | 6 | −26 | −8 | 46 | |
| Precentral Gyrus | 4 | −40 | −14 | 56 | |
| 4,112 | Superior parietal lobule | 7 | −16 | −60 | 60 |
| Superior parietal lobule | 7 | −20 | −70 | 56 | |
| Superior parietal lobule | 7 | −24 | −62 | 44 | |
| Superior parietal lobule | 7 | −26 | −60 | 54 | |
| 3,912 | Middle frontal Gyrus | 6 | 30 | −2 | 56 |
| Middle frontal Gyrus | 6 | 28 | 16 | 46 | |
| 3,016 | Inferior frontal Gyrus, pars opercularis | 9 | 48 | 14 | 28 |
| Middle frontal Gyrus | 6 | 44 | 10 | 42 | |
| 2,088 | Insula | 13 | −32 | 24 | −2 |
| 1,832 | Insula | 13 | 36 | 22 | 0 |
| 1,664 | Inferior parietal lobule | 40 | −36 | −46 | 44 |
| 1,216 | Precentral Gyrus | 6 | −44 | 2 | 34 |
| Precentral Gyrus | 9 | −48 | 6 | 22 | |
| 1,114 | Fusiform Gyrus | 37 | −34 | −64 | −8 |
| 976 | Superior temporal Gyrus | 13 | 58 | −42 | 18 |
| Attention: Specific activations | |||||
| 104 | Superior temporal Gyrus | 22 | 58 | −42 | 17 |
| 80 | Inferior frontal Gyrus, pars opercularis | 9 | 45 | 12 | 34 |
Figure 2.
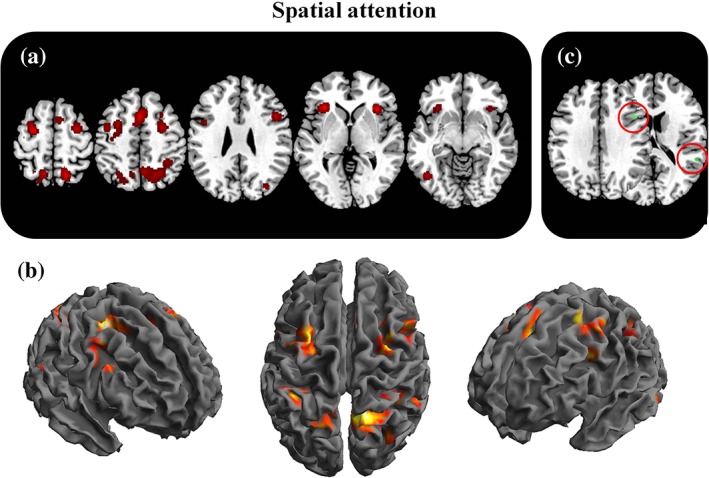
Spatial attention related activations. The figure represents the 2D (a) and 3D (b) brain activations during attention tasks. In the C panel, the selective activations for attention (attention—Working memory+navigation+mental rotation) are represented [Color figure can be viewed at http://wileyonlinelibrary.com]
The meta‐analysis on the studies involving spatial attention (aim 3a) revealed the following clusters of activation: bilateral superior parietal lobule and precuneus (BA 7) extending to the inferior parietal lobe (BA 40); and to right superior occipital gyrus (BA 19). In frontal regions, we found concordant bilateral activation over FEF, dorsal premotor cortices (BA 6) and frontal opercula (BA 9). SMA and pre‐SMA; pre‐central gyrus (BA 4; BA 6). Insular cortices (BA 13), the left fusiform gyrus (BA 37) and the right superior temporal gyrus (BA 13) were also active.
Notably, in the meta‐analysis of studies specifically involved in spatial attention (aim 3b: attention vs. working memory+navigation+mental rotation) we found that two brain regions were activated selectively in spatial attention: the right superior temporal gyrus, close to the TPJ (BA 22) and a region close to the right inferior frontal sulcus (BA 9).
3.3. Spatial working memory
The meta‐analysis of studies on spatial working memory (aim 3a) included 337 foci from 25 experiments for a total of 360 participants. The results are reported in Table 3 and graphically represented in Figure 3. The largest clusters of activation were found over dorsal frontal regions (BA 6) of both the hemispheres, over the precuneus and bilateral superior parietal lobules (BA 7) and over the pre‐SMA.
Table 3.
Brain areas activated in spatial working memory
| Cluster size | Brain region | Broadman area | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 4,488 | Middle frontal Gyrus | 6 | 34 | 12 | 52 |
| Middle frontal Gyrus | 6 | 28 | 4 | 52 | |
| Middle frontal Gyrus | 6 | 30 | 0 | 58 | |
| 4,000 | Middle frontal Gyrus | 6 | −26 | 4 | 52 |
| Precentral gyrus | 8 | −44 | 8 | 46 | |
| 3,496 | Superior parietal lobule | 7 | −22 | −56 | 60 |
| Superior parietal lobule | 7 | −14 | −64 | 54 | |
| 3,408 | Presupplementary motor area | 6 | −2 | 14 | 50 |
| Presupplementary motor area | 6 | −4 | 8 | 56 | |
| Presupplementary motor area | 6 | 12 | 6 | 56 | |
| 2,304 | Superior parietal lobule | 7 | 20 | −62 | 60 |
| Precuneus | 7 | 8 | −66 | 56 | |
| 1,760 | Inferior frontal Gyrus | 47 | −36 | 22 | −8 |
| Insula | 13 | −34 | 18 | 4 | |
| 1,680 | Inferior parietal lobule | 40 | −36 | −40 | 44 |
| 1,024 | Inferior frontal Gyrus, pars opercularis | 9 | 52 | 12 | 26 |
| 928 | Middle occipital Gyrus | 19 | −28 | −82 | 24 |
| 896 | Inferior frontal Gyrus, pars triangularis | 9 | −48 | 14 | 30 |
| Working memory: Specific activations | |||||
| 240 | Middle frontal Gyrus | 6 | −28 | 6 | 47 |
| 184 | Inferior frontal Gyrus, pars opercularis | 47 | −32 | 22 | −12 |
Figure 3.
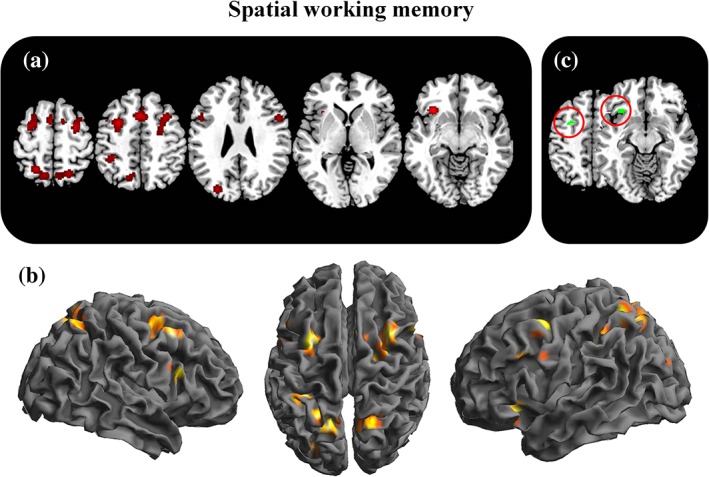
Spatial working memory related activations. The figure represents the 2D (a) and 3D (b) brain activations during working memory tasks. In the (c) panel, the selective activations for working memory (working memory—Attention+navigation+mental rotation) are represented [Color figure can be viewed at http://wileyonlinelibrary.com]
Convergent loci of activation were also found in the left ventrolateral prefrontal region (BA 47) extending to insular cortex (BA 13) and in left inferior parietal lobule (BA 40) and occipital gyrus. Dorsolateral prefrontal cortex (BA 9) was shown consistently activated over both the left and right hemisphere.
As compared with the other spatial functions (aim 3b: working memory vs. attention+navigation+mental rotation), spatial working memory was associated with specific activation of a region located over the left middle frontal gyrus (BA 6). Also, the left ventrolateral prefrontal cortex (BA 47) was found to be engaged uniquely by this function.
3.4. Long‐term spatial memory and spatial navigation
The meta‐analysis of studies that investigated long‐term spatial memory and spatial navigation (aim 3a) included 363 foci from 24 experiments for a total of 338 participants. The results are reported in Table 4 and graphically represented in Figure 4. The analysis showed consistent activations mainly located in the right hemisphere and in particular in right parahippocampal gyrus (BA 36), in posterior cingulate cortex and specifically in the retrosplenial cortex (BA 30, BA 29), in the precuneus (BA 7, 19, and 31), in the right middle occipital gyrus (BA 39) and in the right inferior parietal lobule (BA 40).
Table 4.
Brain areas activated in spatial long‐term memory and navigation
| Cluster size | Brain region | Broadman area | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 2,984 | Parahippocampus | 36 | 24 | −40 | −10 |
| 2,408 | Posterior cingulate (Retrosplenial cortex) | 30 | −12 | −50 | 18 |
| Posterior cingulate (Retrosplenial cortex) | 29 | −6 | −45 | 8 | |
| 1,720 | Posterior cingulate(Retrosplenial cortex) | 30 | 14 | −45 | 18 |
| Precuneus | 31 | 12 | −60 | 28 | |
| 1,216 | Middle occipital Gyrus | 19 | 34 | −74 | 36 |
| Middle occipital Gyrus | 30 | 46 | −70 | 28 | |
| 992 | Precuneus | 7 | 10 | −62 | 54 |
| 808 | Inferior parietal lobule | 40 | 40 | −44 | 54 |
| Long‐term memory and navigation: Specific activations | |||||
| 936 | Parahippocampus | 36 | 25 | −39 | −10 |
| 200 |
Posterior cingulate (Retrosplenial cortex) |
29 | −13 | −51 | 19 |
| 56 | Posterior cingulate (Retrosplenial cortex) | 30 | 10 | −45 | 17 |
Figure 4.
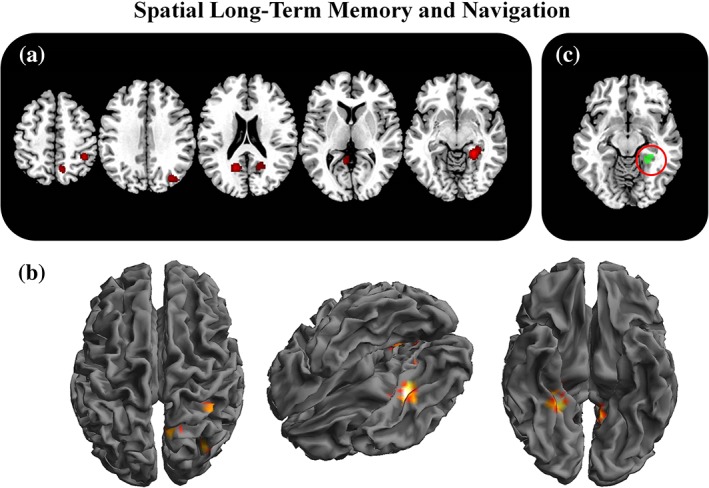
Spatial long‐term memory and navigation related activations. The figure represents the 2D (a) and 3D (b) brain activations during long‐term memory and navigation tasks. In the (c) panel, the selective activations for long‐term memory and navigation (LTM + navigation—Mental rotation+navigation+working memory) are represented [Color figure can be viewed at http://wileyonlinelibrary.com]
Importantly, in the meta‐analysis of studies specifically involved in navigation and long‐term memory (aim 3b: navigation vs. working memory+attention+mental rotation), with respect to the other functions, the right parahippocampal gyrus and two bilateral regions over the retrosplenial cortex (BA 30; BA 29) were elicited selectively in long‐term spatial memory and navigation tasks.
3.5. Mental rotation and spatial imagery
The meta‐analysis of mental rotation studies (aim 3a) included 348 foci from 35 experiments for a total of 475 participants. The results are reported in Table 5 and graphically represented in Figure 5. Consistent clusters of activation were found over the right and left superior parietal lobules (BA 7) extending to precuneus, the bilateral middle frontal regions involving the FEF and dorsal premotor regions (BA 6), and the pre‐SMA. Consistent clusters of activation were found also bilaterally in ventral frontoparietal regions: over inferior parietal lobule (BA 40) and the inferior frontal gyrus (BA 9). Bilateral temporal and occipital regions were found consistently activated and included fusiform gyrus (BA 19, BA 37), and middle occipital gyri (BA 18, BA 19).
Table 5.
Brain areas activated in mental rotation and spatial imagery
| Cluster size | Brain region | Broadman area | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 4,576 | Superior parietal lobule | 7 | 16 | −66 | 54 |
| Superior parietal lobule | 7 | 26 | −76 | 52 | |
| 3,816 | Middle frontal Gyrus | 6 | 28 | −4 | 56 |
| 3,744 | Superior parietal lobule | 7 | 32 | −48 | 56 |
| Inferior parietal lobule | 40 | 40 | −40 | 44 | |
| Superior parietal lobule | 7 | 26 | −50 | 66 | |
| 3,400 | Presupplementary motor area | 6 | 2 | 14 | 50 |
| 3,328 | Inferior parietal lobule | 40 | −38 | −44 | 46 |
| Postcentral Gyrus | 40 | −40 | −32 | 44 | |
| 2,992 | Middle frontal Gyrus | 6 | −26 | −6 | 52 |
| Superior frontal Gyrus | 6 | −22 | 2 | 58 | |
| Superior frontal Gyrus | 6 | −28 | −2 | 64 | |
| 2056 | Superior parietal lobule | 7 | −26 | −70 | 46 |
| Precuneus | 7 | −13 | −66 | 54 | |
| 1,760 | Fusiform Gyrus | 19 | −35 | −65 | −8 |
| 1,584 | Inferior frontal Gyrus, pars opercularis | 9 | −50 | 12 | 28 |
| 1,520 | Middle occipital Gyrus | 19 | 40 | −78 | 8 |
| Middle occipital Gyrus | 18 | 38 | −86 | 2 | |
| Middle occipital Gyrus | 19 | 34 | −84 | 16 | |
| Inferior occipital Gyrus | 19 | 48 | −82 | 2 | |
| 1,248 | Fusiform Gyrus | 37 | 26 | −74 | −8 |
| 1,232 | Inferior frontal Gyrus, pars opercularis | 9 | 54 | 8 | 24 |
| Mental rotation: Specific activations | |||||
| 96 | Post central Gyrus | 7 | 30 | −49 | 62 |
| 72 | Inferior occipital Gyrus | 19 | −46 | −73 | −5 |
Figure 5.
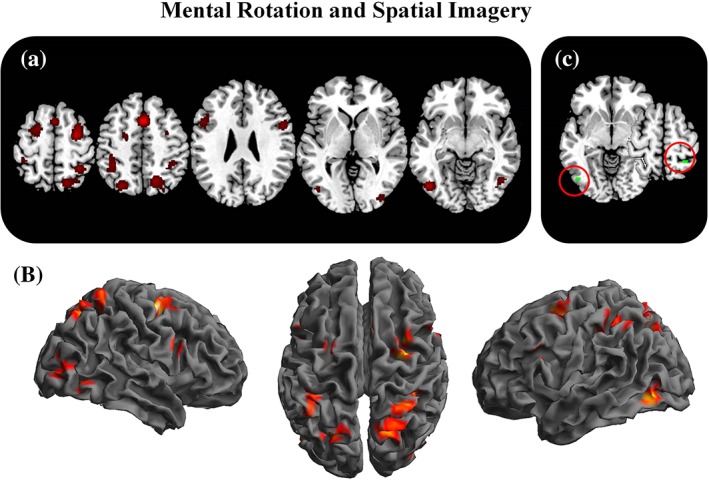
Mental rotation and spatial imagery related activations. The figure represents the 2D (a) and 3D (b) brain activations during mental rotation and spatial imagery tasks. In the (c) panel, the selective activations for these processes (mental rotation+spatial imagery—Attention+navigation+working memory) are represented [Color figure can be viewed at http://wileyonlinelibrary.com]
In the meta‐analysis of studies specifically involved in mental rotation (aim 3b: mental rotation and spatial imagery vs. working memory+attention+navigation), we found that, as compared with the other spatial functions, spatial imagery specifically elicited a region in the right postcentral sulcus, between the somatosensory cortex and the superior parietal lobule (BA 7); and a region over the left inferior occipital gyrus (BA 19).
4. DISCUSSION
We are constantly dealing with space in our everyday life. Spatial material represents indeed the target information to process in the service of multiple distinct cognitive functions that range from attention, working memory, to imagery, navigation, and long‐term memory.
Our understanding of the heterogeneous findings regarding brain function connoting cognitive operations may be enriched by a cohesive view of the structures that are consistently implicated. Through a systematic review and coordinate‐based meta‐analysis, this study aimed at determining the brain regions that are commonly recruited by all the cognitive functions operating on spatial information and thus to identify the core network underlying spatial processing. In addition, the current study aimed at identifying the distinct regions that are specifically involved in each spatial function. As far as we know, this is the first systematic review and meta‐analysis of spatial cognition. Critically, we also empirically demonstrated that the results of the current meta‐analyses are not affected by the original studies that did not correct their results for multiple comparisons since the results of the “core” network meta‐analysis did not change including or excluding these studies. This is of outstanding importance as it allows us to safely affirm that the results of the meta‐analysis are not driven by the earlier studies, where the results were presented without any correction for multiple comparisons.
Importantly, these results do not in any way indicate that results not corrected for multiple comparisons are as reliable as the results corrected for multiple comparisons. Rather, these results suggest that the consistent brain activation for spatial processing did not significantly differ between the two sets of studies.
4.1. Spatial processing: the core network
In relation to the first aim, we found that spatial information is processed in a network of brain regions symmetrically distributed on both hemispheres and that comprises dorsal frontal and parietal regions, pre‐SMA, anterior insula and, to less extent, frontal operculum, and inferior parietal lobule.
The symmetrical distribution evidenced in the omnibus meta‐analysis rules against the idea of a right hemisphere specialization for spatial cognition proposed by past studies (Fink et al., 2000; Halligan, Fink, Marshall, & Vallar, 2003; Marshall & Fink, 2001), at least if we consider the general spatial processing, regardless of the function investigated.
A bilateral dorsal frontoparietal network that includes dorsal premotor regions and frontal eye fields in frontal cortex, and precuneus, superior parietal lobule and intraparietal sulcus in parietal cortex was found commonly activated in all spatial tasks. Although to lesser extent, bilateral activations in ventral frontoparietal network were also found in the inferior frontal gyrus and in inferior parietal lobule.
This pattern of results suggests that the frontoparietal networks support a shared mechanism that is involved in the execution of all spatial functions, rather than being implicated to several task‐specific processes. The challenge is to identify such putative mechanism underlying the spatial functions.
The most recent studies have converged on a conceptual framework positing that dorsal frontoparietal regions contain topographic maps and their activity presumably constitutes a rank‐ordered or “prioritized” representations of relevant locations within the visual field (Bisley & Goldberg, 2010; Itti & Koch, 2001; Serences & Yantis, 2007; Szczepanski, Konen, & Kastner, 2010).
Topographic maps have been indeed discovered also outside the visual cortex, in associative regions of frontal and parietal network that closely mirror those found in the present meta‐analysis (Hagler Jr & Sereno, 2006; Konen & Kastner, 2008; Saygin & Sereno, 2008; Schluppeck, Glimcher, & Heeger, 2005; Sereno, Pitzalis, & Martinez, 2001; Silver & Kastner, 2009; Silver, Ress, & Heeger, 2005). In particular, Hagler Jr and Sereno (2006) discovered spatial maps in both superior precentral sulcus, in a location corresponding to the FEF and in the inferior frontal sulcus, anterior to the precentral sulcus. They also found maps in the superior parietal regions, close to the intraparietal sulcus. Likewise, Jerde and Curtis (2013) identified reliable topographic maps in the same brain regions.
A recent study using novel procedures characterized the topographic organization of visual field maps in human frontoparietal regions and found that these maps are organized by polar angle and eccentricity, containing a representation of all the gradients of polar angles of the contralateral visual field (Mackey, Winawer, & Curtis, 2017).
The topographic maps in frontoparietal regions were shown to be the best candidate priority maps of space (Jerde, 2012; Jerde & Curtis, 2013). Such maps would dynamically code for the priority of locations in the environment on the basis of the behavioral context, tagging the locations that are salient and behaviorally relevant (Serences & Yantis, 2007; Thompson & Bichot, 2005). The behavioral priority changes indeed continuously over time and is shaped by both cognitive inputs (goals, prior information, and so forth) and visual information. It is still, however, unclear which is the specific mechanism used to prioritize the relevant spatial representations. A possible mechanism might be the allocation of the attention to the internal representations in a similar manner as evidenced for external attentional allocation (Lückmann et al., 2014).
The activation of dorsal frontoparietal regions such as the FEF, superior parietal lobule, and IPS has been quite consensually classified the dorsal attention network (DAN; see Corbetta et al., 2008; Power et al., 2011). The finding of DAN activation across different tasks requiring the internal maintenance of task‐related representations has given rise to a set of neurocognitive models that considered attentional orienting as a crucial mechanism in the field of working memory (internal attention; Nobre et al., 2004), episodic retrieval (attention to memory; Cabeza, Ciaramelli, Olson, & Moscovitch, 2008), prospective memory (attention to delayed intention; Cona, Scarpazza, Sartori, Moscovitch, & Bisiacchi, 2015; Cona, Bisiacchi, Sartori, & Scarpazza, 2016) and mental imagery (top‐down control; e.g., Kosslyn, 2005; Sack & Schuhmann, 2012). The sustained DAN activation during the delay “maintenance” period in all these kinds of tasks is likely to reflect the attentional orienting toward the internal representation to keep it active (Lückmann et al., 2014).
Other regions seem to support such prioritizing mechanism and were found commonly activated in all the spatial tasks of our meta‐analysis. The anterior insula and frontal operculum, which comprise network labeled cingulo‐opercular control network (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008) or salience network (Seeley et al., 2007) were found consistently activated in the present meta‐analysis. These regions are thought to serve the detection of the most relevant (internal or external) stimuli in order to influence and guide thoughts and behaviors (Seeley et al., 2007). Notably, a recent neurocognitive model (Myers, Stokes, & Nobre, 2017) built up in the working memory context, conceptualized the prioritizing mechanism as a multi‐step process: after an encoding phase, a cue indicating that one item in the working memory is behaviorally relevant leads to orientation of attention toward the corresponding representation, and, in turn, to the selection of such representation. This operation would be mediated by the dorsal frontal–parietal network (DAN). In a second step, the identified representation needs to be prioritized by recruiting the anterior insula, frontal operculum and ventrolateral prefrontal cortex, and possibly pre‐SMA or ACC. In the third step, the selected representation is reformatted into an action‐oriented format. This model fits in nicely with the findings and interpretations presented in the present paper, although a clear understanding of the specific contribution of some regions, such as the pre‐SMA, is still lacking.
In this regard, the finding of persistent and convergent activation of pre‐SMA across different spatial tasks is consistent with the “unified account” that pre‐SMA supports domain‐general sequence operations in visuospatial tasks (Cona, Marino, & Semenza, 2017; Leek et al., 2016) as well as in other cognitive tasks (see Cona & Semenza, 2017, for a review). Sequential operations are necessary to create a representation of space (Leek et al., 2016), as well as of time, or other magnitude dimensions, such as numbers (Dehaene et al., 1996). According to this account, SMA regions (and pre‐SMA in particular) play a central role in the integration of sequential items into higher‐order structural representations regardless of the nature of such items (spatial, motor, temporal, numerical, linguistic, and so forth), providing also evidence from TMS and lesion studies of the causal contribution of pre‐SMA in this process (Cona & Semenza, 2017). In accordance with this idea, another study specifically focused on spatial processing showed that pre‐SMA supports hierarchical sequence processing in visuospatial tasks (Bahlmann, Schubotz, Mueller, Koester, & Friederici, 2009). Interestingly, we found that pre‐SMA only was deputed to spatial processes, whereas SMA was not consistently activated among the tasks. This result is consistent with the notion that the pre‐SMA has “higher”, cognitive functions, in contrast to the SMA, which is associated with “lower” motor functions (Grezes & Decety, 2001).
In this first part, we started with a discussion of the task‐general brain activations in spatial cognition. Now we outline and discuss the findings of task‐ and function‐specific activations in brain regions and finally, we will provide a framework that allows for the integration of all these findings.
4.2. Spatial attention
The current meta‐analysis highlighted that spatial attention processes elicit concordant activity of both dorsal and ventral frontoparietal networks. Furthermore, pre‐SMA regions and bilateral insular cortices (BA 13) were associated with spatial attention as well. This pattern of activity seems to mirror that one observed in the omnibus analysis (although it comprises larger clusters of activations). This would support the idea that attention is an overarching mechanism involved in multiple functions.
According to the models developed by Corbetta and collaborators (Corbetta et al., 2008; Corbetta & Shulman, 2002), the dorsal frontoparietal network—whose core regions comprise dorsal premotor cortex and FEF in frontal cortex, and the SPL and IPS in parietal cortex—enables the selection of external sensory stimuli based on goals (top‐down, goal‐driven attention) and links them to appropriate behaviorally responses. As discussed above in the previous paragraph, our results not only support this account, but extend it revealing that this network, labeled as DAN, subserves the sustained top‐down allocation of attention not only toward the external sensory stimuli but also toward the internal spatial representations.
On the other hand, according to Corbetta's models, the ventral frontoparietal network, including the temporoparietal junction (TPJ), parts of the middle and inferior frontal gyrus, the frontal operculum, and the anterior insula, allows the detection of salient and behaviorally relevant external stimuli, especially when these are unattended (stimulus‐driven or bottom‐up attention).
Several studies supported this dorsal‐ventral segregation in frontoparietal networks (Chica, Bartolomeo, & Valero‐Cabre, 2011; Hahn, Ross, & Stein, 2006; Indovina & Macaluso, 2007; Liu, Pestilli, & Carrasco, 2005; Yantis et al., 2002), others instead found no modulation on some regions of the ventral system associated with exogenous attentional orienting, favoring the idea of partly overlapping network for endogenous and exogenous orienting (Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005). The results from the present meta‐analysis are clear‐cut in this respect, demonstrating a functional segregation of top‐down and bottom‐up systems: The bilateral dorsal frontoparietal network is shared by all the spatial functions and it is likely to mediate an overarching mechanism such as the endogenous, top‐down control of attention. Conversely, two regions in the ventral frontoparietal network of the right hemisphere are instead selectively implied in spatial attention, thus would be engaged in the exogenous shift of attention toward the part of the space in which salient (and probably unexpected) stimuli occur. Notably, the right lateralization of TPJ and inferior frontal activation as specific in visuospatial attention is consistent with the classical findings of spatial attention deficits shown in the neglect syndrome after lesions of TPJ or of its connections to the ipsilateral frontal regions (Bartolomeo, Decaix, & Siéroff, 2007; Bourgeois, Chica, Migliaccio, Thiebaut de Schotten, & Bartolomeo, 2012, for recent studies). Anatomical lesion data in patients with neglect pointed often to the TPJ as the best candidate for spatial attention shift (Vallar & Perani, 1986).
While the functional contribution of frontoparietal networks to attention has been extensively studied in the past, the role of fusiform gyrus in relation to visuospatial attention is still less understood. We found a consistent activation of the left fusiform gyrus across spatial attention tasks. The left fusiform gyrus is part of the ventral processing stream and has been typically linked to encoding object properties such as shape, color, and texture (Zeki & Marini, 1998) and object categorization. Notably, more recent studies are concordant with our findings in suggesting a specific role of this region in exogenous, stimulus‐driven attention (Hahn et al., 2006; Kincade et al., 2005).
4.3. Spatial working memory
The spatial working memory analysis revealed the most prominent activation in the dorsal frontoparietal network that mirrors the DAN, corroborating the idea that this network contributes to an overarching mechanism, which is likely to be the internal attention toward priority maps of space.
This analysis showed that several sets of activations were common to both spatial attention and working memory, including bilateral FEF and dorsal premotor cortices, pre‐SMA, inferior frontal regions, bilateral superior parietal lobules, and insula (although for the spatial working memory the activation was restricted to the left insula). These findings are consistent with previous views that spatial attention and working memory share common cognitive operations and neural mechanisms (McCarthy, Nobre, Bentin, & Spencer, 1995; Awh & Jonides, 2001; Corbetta, Kincade, & Shulman, 2002; La Bar, Gitelman, Parrish, & Mesulam, 1999).
Notably, the present analysis revealed that spatial working memory did not activate only dorsal frontal and dorsolateral prefrontal regions but also ventral frontal regions including the bilateral inferior frontal regions and the left ventrolateral prefrontal cortex (BA 47), which appears to be recruited uniquely by this function.
While the contribution of the frontal lobe to spatial working memory is beyond dispute (Owen et al., 2005; Rottschy et al., 2012; Wager & Smith, 2003), the stimulus‐specific segregation of prefrontal cortical areas is still controversial (Hautzel et al., 2002; Zimmer, 2008).
Two major dissociations in functional contribution of prefrontal cortex have been discussed and still represent a matter of debate: (a) the left/right dissociation of verbal (left hemisphere) versus spatial working memory (right hemisphere); and (b) the ventral (object, shape working memory) versus dorsal (spatial working memory] dissociation.
Concerning the first point, past studies proposed a stimulus‐specific hemispheric lateralization in frontal regions, with a left hemisphere dominance for verbal working memory (Wager & Smith, 2003); and right hemisphere dominance for visuospatial working memory (van der Ham, Raemaekers, van Wezel, Oleksiak, & Postma, 2009; Walter et al., 2003). However, this dominance of the right hemisphere for spatial working memory was not substantiated in many studies (Nystrom et al., 2000; Wager & Smith, 2003). In most experiments on visuospatial working memory, indeed, the activation was bilateral (Zimmer, 2008). Likewise, in our analysis, we did not find a right hemisphere dominance for spatial working memory.
Concerning the second point, two different views have been pointed out. Some authors have suggested that the distinction between “what” and “where” shown in the posterior cortex has a correspondence in the frontal lobe. According to this “material‐specific” view, the dorsolateral PFC regions maintain spatial information, whereas the ventrolateral PFC regions maintain object information (Sala et al., 2003; Ungerleider, Courtney, & Haxby, 1998; Ventre‐Dominey et al., 2005).
Other authors proposed instead that the frontal subregions do not differ in their contribution depending on the material held in WM, but in the type of process, they mediate (Nystrom et al., 2000; Owen et al., 1997; Petrides, 2005). More specifically, according to this “process‐specific” view, the ventrolateral PFC (BA 45/47) would support retrieval of recently encoded information, whereas the dorsolateral PFC (BA 9/46) would subserve monitoring and active processing (Curtis & D'Esposito, 2003).
The pattern of our results is in line with the process‐specific view, as we found that spatial working memory activates both dorsal and ventral PFC regions. Notably, while the activations in dorsal frontal regions underlie multiple, distinct spatial functions, the left ventrolateral PFC seems to be preferentially recruited by working memory, thus it might contribute to a specific working memory process, namely the retrieval of information as previously proposed.
4.4. Navigation and spatial long‐term memory
Navigation and spatial long‐term memory tasks elicit a set of activations over the posterior regions mainly lateralized in the right hemisphere. In contrast to the left, the right medial temporal and posterior regions have been indeed strongly implicated in spatial navigation from both neuroimaging studies (Aguirre et al., 1996; Ghaem et al., 1997) and neuropsychological cases (Luzzi, Pucci, Di, & Piccirilli, 2000).
These activations were located in the parahippocampal gyrus, in posterior cingulate cortex—particularly in the retrosplenial cortex—in posterior parietal cortex (i.e., precuneus and inferior parietal lobule) and in middle occipital gyrus (BA 39).
Notably, we found that the right parahippocampal gyrus and the retrosplenial cortex were involved selectively in these tasks, providing supporting evidence for their crucial and preferential role in the navigation and memory system (Epstein, 2008; Spiers & Maguire, 2007). Neuroimaging studies of spatial navigation most commonly found activations in the posterior parahippocampal and retrosplenial regions (Epstein, 2008, for a review) and damage to these regions is often associated with wayfinding deficits (Aguirre & D'Esposito, 1999).
The present finding can also disentangle the relative contribution of the hippocampus versus the parahippocampal gyrus to spatial memory and mental navigation, highlighting that, at least in humans, parahippocampal gyrus represents the best candidate for the memory of spatial relations (Rosenbaum et al., 2004). Moreover, in line with previous studies, a critical role seems to be also played by the right precuneus, likely because of its well‐established involvement in imagery and episodic memory (Cabeza & Nyberg, 2000).
It has been proposed that parahippocampal gyrus, posterior parietal cortex, and retrosplenial cortex subserve different functions in navigation and spatial memory. The parahippocampal gyrus is deputed to form memories of places and to recognize specific landmarks and views, thus encoding a representation of the local scene that enables it to be subsequently recognized (Epstein, 2008).
The posterior parietal cortex (and the precuneus in particular in our study) is involved in route‐based (i.e., egocentric) representation of landmarks that allows reaching objects, moving with respect to those landmarks in the environment (Farrell, 1996; Milner & Goodale, 1995) and it is implicated in imagining places from an egocentric perspective (Bisiach, Brouchon, Poncet, & Rusconi, 1993; Burgess et al., 2001).
The retrosplenial cortex provides instead an allocentric mapping of the environment (Epstein, 2008; Spiers & Barry, 2015; Spiers & Maguire, 2007). There is not, however, a clear consensus about the specific contribution of this region. According to some authors, the retrosplenial cortex would act as a device for converting the representation from an allocentric (environment‐based) framework, stored in hippocampal–entorhinal regions, to an egocentric perspective in posterior parietal cortex (Burgess et al., 2001; Byrne, Becker, & Burgess, 2007; Epstein, 2008; Spiers & Maguire, 2007). According to other authors, instead, retrosplenial cortex is not a mere relay structure but it encodes and stores its own allocentric representation of the spatial world (Moscovitch et al., 2005). Retrosplenial and posterior parietal cortex have thus complementary functions in the spatial navigation system, providing allocentric and egocentric representation, respectively (Rosenbaum et al., 2004). Furthermore, the evidence that rodent retrosplenial cells code head direction (Chen, Lin, Green, Barnes, & McNaughton, 1994) led to the idea that this structure is responsible for coding allocentric heading direction (Takahashi, Kawamura, Shiota, Kasahata, & Hirayama, 1997).
Finally, some studies found a prefrontal involvement, which is considered to reflect route planning and switching among different navigation strategies (Poucet et al., 2004; Spiers, 2008). In contrast, we did not find a consistent and concordant activation of prefrontal cortex. This might suggest that prefrontal cortex has a corollary role in mental navigation and memory, and its involvement is likely modulated by the control demands rather than being related to specific spatial operations required in these tasks.
4.5. Spatial imagery and mental rotation
The robust involvement of dorsal frontoparietal regions in spatial imagery tasks provides a further strong support to the idea that DAN network is engaged not only in externally directed cognitive tasks but also in internally directed processes. As proposed before, these processes are likely to be the allocation of attention and implementation of the spatial maps, as also pointed out by a meta‐analysis of the neuroimaging studies on mental rotation tasks (Zacks, 2008). Interestingly, the study by Zacks showed that activation in those areas was modulated by the amount of mental rotation performed, indicating an important role for this network in visuospatial representation transformations.
Together with dorsal frontoparietal regions, the pre‐SMA was shown consistently activated. Previous studies found a modulation in pre‐SMA activity as a function of mental rotation: the higher the angle of rotation, the greater the pre‐SMA activation (Milivojevic et al., 2009). Furthermore, TMS over pre‐SMA influenced mental rotation, but only in trials with higher angular displacement between stimuli with larger angular disparity (Cona, Marino, & Semenza, 2017). These results suggest a role of pre‐SMA in transforming the spatial representations, which is likely to be the sequential integration of elements into higher‐order representation (Cona & Semenza, 2017).
Bilateral inferior frontal regions were found also active, although their functional meaning is less clear. As noted before, Hagler Jr and Sereno (2006) discovered spatial maps in the inferior frontal sulcus, in a region very similar to that one evidenced in our study, just anterior to the precentral sulcus. Likewise, Jerde and Curtis (2013) identified reliable topographic maps in both the superior and inferior precentral sulcus and considered those regions as good candidate priority map (Kastner et al., 2007).
In view of the tight link between imagining and executing actions, it has been suggested that the primary motor cortex (M1) is engaged in the mental rotation to support motor imagery. Nevertheless, this is still an ongoing matter of debate, with mixing results (Cona, Marino, & Semenza, 2017; Cona, Panozzo, & Semenza, 2017; Ganis, Keenan, Kosslyn, & Pascual‐Leone, 2000; Pelgrims, Michaux, Olivier, & Andres, 2011; Sauner et al., 2006; Tomasino, Borroni, Isaja, & Rumiati, 2005). The present meta‐analysis did not observe a consistent activation in M1 across studies, suggesting that this region is not consistently activated in spatial imagery tasks. The M1 involvement observed in previous studies might be thus related to the specific strategy adopted in that task, and/or it might be associated with motor planning and execution rather than spatial imagery processes per se.
Interestingly, as compared with the other spatial functions, spatial imagery was shown to rely more upon a region over the left inferior occipital gyrus (BA 19) and a region in the right postcentral sulcus, between the somatosensory cortex and the superior parietal lobule (BA 7). A possible interpretation is that the process of mentally simulation/imagery is likely to rely upon the integration of information that comes from different modalities, such as somatosensory and visual, thus it activates the brain regions deputed to code such information.
4.6. Strengths and limitations
This systematic review and meta‐analysis has a number of important strengths as well as limitations. The strengths are mainly three. First, this study tested the eventual impact that studies presenting the results without a correction for multiple comparisons might have had on the whole meta‐analysis findings. Indeed, during the systematic review of the literature, we noticed that the majority of the earlier study did not provide corrected results. In this way, we have been able to demonstrate that these studies, although anachronistically can be defined not technically perfect, did not negatively impact on the results. To the best of our knowledge, this is the first study providing this evidence. Second, unlike the previous meta‐analysis on spatial cognition which investigates the neural correlates of mental rotation only (Zacks, 2008), the current meta‐analysis took into considerations the different cognitive functions that involve processing of spatial information. This allowed us to identify the functional brain mechanisms involved in the processing of the spatial information for each cognitive function. Third, our approach to compare the brain activation of each cognitive function against the brain activation of all the other cognitive functions pooled together allowed us to highlight the brain regions that contributed selectively to a specific spatial cognitive function. To the best of our knowledge, this is the first study using this methodology.
Despite the undoubted strengths, this work is not free from limitations. The biggest limitation is that related to the potential publication bias, which might lead the results to be partial. The ALE method, indeed, does not take into account results that are not statistically significant therefore we had to exclude from the meta‐analysis those [published] studies reporting results that were not statistically significant. Nevertheless, through the systematic review, we identified only one study reporting a negative result for the contrast of interest, therefore, we can be reassured that the current results are not significantly affected by that. In addition, the ALE method used in the current study considers only the reported coordinates and the number of subjects in each study, that is, ALE does not consider the strength of the statistics associated with each result.
4.7. Schematic model of spatial processing and open questions
Based on the findings of both common and distinct networks involved in various spatial functions, we have come up with a schematic framework to describe the brain mechanisms underlying spatial processing (Figure 6).
Figure 6.
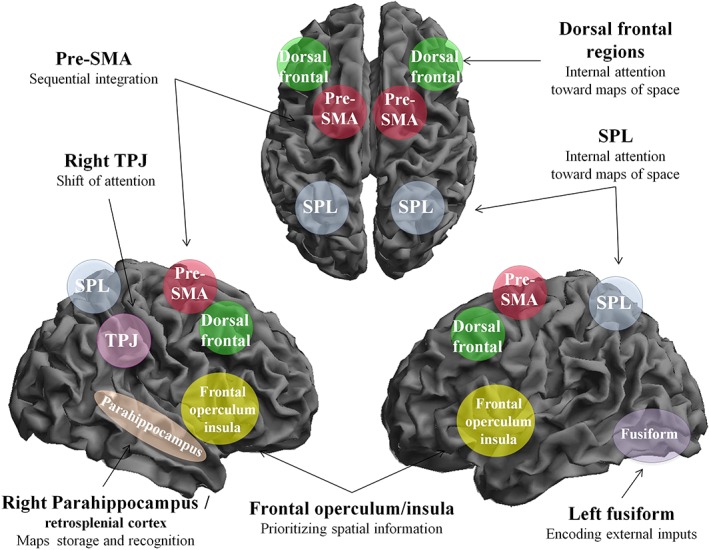
Schematic model of spatial processing. This figure represents a schematic illustration of the proposed model for spatial processing. Spatial inputs processed in visual regions lead to a shift of attention, mediated by the right ventral frontoparietal network, comprising TPJ and inferior frontal gyrus. Dorsal frontal and superior parietal lobe regions, forming the dorsal attention network (DAN), are then activated and serve to allocate internal attention toward the spatial representations to keep them active. The pre‐SMA acts in cooperation with the DAN and is responsible to integrate sequential spatial information into a coherent map of space. Such maps of space are then prioritized by frontal operculum and the insular cortex based on both the individuals' goals and the external stimuli. Posterior parahippocampal and retrosplenial regions are instead recruited selectively in long‐term memory processes, such as spatial maps storage and recognition of landmarks. Pre‐SMA, presupplementary motor area; SPL, superior parietal lobe; TPJ, temporoparietal junction [Color figure can be viewed at http://wileyonlinelibrary.com]
We sought to group different brain regions on the basis of their roles in different spatial functions, although we must acknowledge that each region is likely to mediate multiple functions and may interact with other brain regions in a far more complex manner. In the assumptions developed in section 1, we hypothesized that spatial functions share some areas of activations and that such activations would reflect spatial information processing. This assumption was also based on previous works (Duncan, 2010; Ikkai & Curtis, 2011; Parlatini et al., 2017), which highlighted the presence of a set of regions of overlap in the maps of activations across different functions and tasks.
We found shared areas of activation in the dorsal frontoparietal network (DAN). This result closely parallels the pattern of findings derived from another recent meta‐analysis (Parlatini et al., 2017), which showed overlapping activations in dorsal frontoparietal regions among a variety of tasks that, although different, require all the manipulation of spatial information. Interestingly, this study demonstrated a segregation into dorsal spatial versus ventral nonspatial frontoparietal networks, revealing that such dissociation overlaps with the projections of the dorsal branch versus ventral branch of the superior longitudinal fasciculus, respectively. We proposed that the DAN serves to implement spatial representation (i.e., priority maps of space) and to allocate top‐down internal attention toward such representations. The first open question is, however, whether the dorsal frontoparietal network has mainly a representational function, an attentional function, or both (Jerde & Curtis, 2013; Lückmann et al., 2014).
The pre‐SMA is also commonly activated in all spatial functions, which is in line with the flexible role attributed to this area by previous studies (Duncan and Owen, 2000; Parlatini et al., 2017; Cona & Semenza, 2017). Based on previous findings, the pre‐SMA would cooperate with the DAN by integrating the sequential space‐related elements in order to obtain higher‐order structural representations (Bahlmann et al., 2009; Cona & Semenza, 2017).
Other regions commonly activated in all spatial functions are the frontal operculum and the insular cortex of both hemispheres, which represent the cingulo‐opercular control network (Dosenbach et al., 2008) or salience network (Seeley et al., 2007). These regions would contribute to dynamically prioritize the maps of space formed in the DAN on the basis of both the saliency (i.e., bottom‐up inputs from early visual neurons about the physical features of environmental stimuli) and the top‐down internal information, which includes individual's goals, previous knowledge and all the other information provided by higher association cortices (Serences & Yantis, 2007). Previous studies discovered topographical maps also in a region close to the frontal operculum (Hagler Jr & Sereno, 2006). A possibility is that such prioritizing process acts on the representations held in the frontal operculum. The second open question is therefore whether superior and inferior frontal regions represent distinct maps of space (or distinct fields of the map), or whether they subserve different operational processes on the same map of space.
Interestingly, the fusiform gyrus, selectively over the left hemisphere, was found consistently involved in the spatial tasks, as evidenced by the omnibus analysis. The present finding suggests that fusiform region, typically implicated in object encoding and recognition (Zeki & Marini, 1998), might have a more “overarching” role, being responsible for the encoding the external inputs, regardless of its nature.
Together with common activations, we observed brain activations that were specific for the spatial function explored. Concerning this point, we found that two regions in the ventral frontoparietal network of the right hemisphere (i.e., TPJ and inferior frontal gyrus) were selectively implied in the spatial attention. Based on previous reports, we propose that they play a pivotal role in exogenous shift of attention (Corbetta et al., 2008). In line with our interpretations, lesions studies showed that damage to the ventral part of the right PPC is usually associated with spatial neglect, a syndrome that involves deficits in directing attention to the part of the space contralateral to the side of the lesion (Critchley, 1953; Robertson & Marshall, 1993). Likewise, noninvasive brain stimulation of ventral PPC regions was consistently shown able to disrupt performance in visuospatial attentional tasks that require visual detection and reorienting (Chambers, Payne, Stokes, & Mattingley, 2004; Ellison, Schindler, Pattison, & Milner, 2004; Meister et al., 2006).
The right parahippocampal gyrus and two bilateral regions over the retrosplenial cortex are specifically involved in long‐term spatial memory and navigation. The parahippocampal cortex implements the recognition of specific landmarks and views whereas the retrosplenial cortex is responsible for coding allocentric heading direction and based on this would provide an allocentric mapping of the environment (Aguirre & D'Esposito, 1999; Epstein, 2008; Spiers & Maguire, 2007). The third open question is whether the retrosplenial cortex functions as device for translating between hippocampal (allocentric) and precuneus (egocentric) spatial frameworks or it encodes its own representations of the world (Epstein, 2008).
Surprisingly, the left ventrolateral PFC was shown to be preferentially recruited by spatial working memory, thus it might contribute to a specific working memory process, which is likely to be the retrieval of information.
Spatial imagery recruits additional regions in occipital and posterior parietal cortex, probably to enable the integration of information coming from somatosensory and visual modalities into a coherent representation in the absence of external stimuli. A TMS study by Pasalar, Ro, and Beauchamp (2010) supports our view, as it revealed a causal contribution of PPC to visual–tactile multisensory integration, suggesting that the PPC contains maps of space that receive inputs from both the somatosensory and visual modalities.
Considering together the functional contribution of each region, it is possible to develop a neurocognitive model for spatial processing where spatial inputs from visual regions lead to a shift of attention toward stimuli, mediated by the right ventral frontoparietal network (i.e., TPJ and inferior frontal gyrus). In order to encode and maintain the spatial features of these stimuli, the dorsal frontoparietal network is then activated and allocates internal attention toward the corresponding spatial representations, in cooperation with the pre‐SMA that is responsible for the integration of sequential spatial information into a coherent representation. Then, the derived maps of space are prioritized by frontal operculum and the insular cortex of both hemispheres based on both the individuals' goals and the external stimuli. The maps of space are updated in line with the changes in the environment and/or in individuals' goals, and this process is mediated by frontal and prefrontal regions of both the hemispheres. Posterior parahippocampal and retrosplenial regions are involved instead only when long‐term memory processes are needed, as they subserve spatial maps storage and recognition of landmarks.
Supporting information
Supporting Information A PRISMA 2009 Flow Diagram (last update March 2018)
Supporting Information B List of studies included
Supporting Information C Checklist for neuroimaging meta‐analysis (Muller et al., 2017)
ACKNOWLEDGMENTS
The present work was carried out within the scope of the research program “Dipartimenti di Eccellenza”, which is supported by a grant from MIUR to the Department of General Psychology, University of Padua. We thank Anastasia Chatzilia for her help in extracting the data.
Cona G, Scarpazza C. Where is the “where” in the brain? A meta‐analysis of neuroimaging studies on spatial cognition. Hum Brain Mapp. 2019;40:1867–1886. 10.1002/hbm.24496
REFERENCES
- Aguirre, G. K. , & D'Esposito, M. (1999). Topographical disorientation: A synthesis and taxonomy. Brain, 122, 1613–1628. [DOI] [PubMed] [Google Scholar]
- Aguirre, G. K. , Detre, J. A. , Alsop, D. C. , & D'Esposito, M. (1996). The parahippocampus subserves topographical learning in man. Cerebral Cortex, 6, 823–829. [DOI] [PubMed] [Google Scholar]
- Awh, E. , & Jonides, J. (2001). Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences, 5(3), 119–126. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. , & Logie, R. (1999). Working memory: The multiple‐component model In Miyake A. & Shah P. (Eds.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 28–61). New York: Cambridge University Press. [Google Scholar]
- Baddeley, A. D. (1986). Working memory. New York: Oxford University Press. [Google Scholar]
- Bahlmann, J. , Schubotz, R. I. , Mueller, J. L. , Koester, D. , & Friederici, A. D. (2009). Neural circuits of hierarchical visuo‐spatial sequence processing. Brain Research, 1298, 161–170. [DOI] [PubMed] [Google Scholar]
- Bartolomeo, P. , Decaix, C. , & Siéroff, E. (2007). The phenomenology of endogenous orienting. Consciousness and Cognition, 16, 144–161. [DOI] [PubMed] [Google Scholar]
- Belger, A. , Puce, A. , Krystal, J. H. , Gore, J. C. , Goldman‐Rakic, P. , & McCarthy, G. (1998). Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Human Brain Mapping, 6, 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, C. M. , Baird, A. A. , Miller, M. B. , & Wolford, G. L. (2009). Neural correlates of interspecies perspective taking in the post mortem Atlantic Samon: An argument for multiple comparison correction. NeuroImage, 47, S125. [Google Scholar]
- Bennett, C. M. , Wolford, G. L. , & Miller, M. B. (2009). The principled control of false positives in neuroimaging. Social Cognitive & Affective Neuroscience, 4(4), 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann, S. , Thilo, K. V. , Sauner, D. , Siebner, H. R. , & Rothwell, J. C. (2002). Parietal magnetic stimulation delays visuomotor mental rotation at increased processing demands. NeuroImage, 17(3), 1512–1520. [DOI] [PubMed] [Google Scholar]
- Bien, N. , & Sack, A. T. (2014). Dissecting hemisphere‐specific contributions to visual spatial imagery using parametric brain mapping. NeuroImage, 94, 231–238. [DOI] [PubMed] [Google Scholar]
- Bisiach, E. , Brouchon, M. , Poncet, M. , & Rusconi, M. L. (1993). Unilateral neglect in route description. Neuropsychologia, 31, 1255–1262. [DOI] [PubMed] [Google Scholar]
- Bisley, J. W. , & Goldberg, M. E. (2010). Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience, 33(1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois, A. , Chica, A. B. , Migliaccio, R. , Thiebaut de Schotten, M. , & Bartolomeo, P. (2012). Cortical control of inhibition of return: Evidence from patients with inferior parietal damage and visual neglect. Neuropsychologia, 50, 800–809. [DOI] [PubMed] [Google Scholar]
- Burgess, N. , Becker, S. , King, J. A. , & O'Keefe, J. (2001). Memory for events and their spatial context: Models and experiments. Philosophical Transactions of the Royal Society B, Biological Sciences, 356(1413), 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, P. , Becker, S. , & Burgess, N. (2007). Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychological Review, 114(2), 340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. , Ciaramelli, E. , Olson, I. R. , & Moscovitch, M. (2008). The parietal cortex and episodic memory: An attentional account. Nature Review Neuroscience, 9, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. , & Nyberg, L. (2000). Neural bases of learning and memory: Functional neuroimaging evidence. Current Opinion in Neurology, 13, 415–421. [DOI] [PubMed] [Google Scholar]
- Chambers, C. D. , Payne, J. M. , Stokes, M. G. , & Mattingley, J. B. (2004). Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience, 7(3), 217–218. [DOI] [PubMed] [Google Scholar]
- Chen, L. L. , Lin, L. H. , Green, E. J. , Barnes, C. A. , & McNaughton, B. L. (1994). Head‐direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Experimental Brain Research, 101, 8–23. [DOI] [PubMed] [Google Scholar]
- Chica, A. B. , Bartolomeo, P. , & Lupiáñez, J. (2013). Two cognitive and neural systems for endogenous and exogenous spatial attention. Behavioural Brain Research, 237, 107–123. [DOI] [PubMed] [Google Scholar]
- Chica, A. B. , Bartolomeo, P. , & Valero‐Cabre, A. (2011). Dorsal and ventral parietal contributions to spatial orienting in the human brain. Journal of Neuroscience, 31, 8143–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. S. , Kosslyn, S. M. , Breiter, H. C. , DiGirolamo, G. J. , Thompson, W. L. , Anderson, A. K. , … Belliveau, J. W. (1996). Changes in cortical activity during mental rotation a mapping study using functional MRI. Brain, 119(1), 89–100. [DOI] [PubMed] [Google Scholar]
- Cona, G. , Bisiacchi, P. S. , Sartori, G. , & Scarpazza, C. (2016). Effects of cue focality on the neural mechanisms of prospective memory: A meta‐analysis of neuroimaging studies. Scientific Reports, 6, 25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona, G. , Marino, G. , & Semenza, C. (2017). TMS of supplementary motor area (SMA) facilitates mental rotation performance: Evidence for sequence processing in SMA. NeuroImage, 146, 770–777. [DOI] [PubMed] [Google Scholar]
- Cona, G. , Panozzo, G. , & Semenza, C. (2017). The role of dorsal premotor cortex in mental rotation: A transcranial magnetic stimulation study. Brain and Cognition, 116, 71–78. [DOI] [PubMed] [Google Scholar]
- Cona, G. , Scarpazza, C. , Sartori, G. , Moscovitch, M. , & Bisiacchi, P. S. (2015). Neural bases of prospective memory: A meta‐analysis and the “attention to delayed intention” (AtoDI) model. Neuroscience and Biobehavioral Reviews, 52, 21–37. [DOI] [PubMed] [Google Scholar]
- Cona, G. , & Semenza, C. (2017). Supplementary motor area as key structure for domain‐general sequence processing: A unified account. Neuroscience and Biobehavioral Reviews, 72, 28–42. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , & Shulman, G. L. (2002). Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience, 14(3), 508–523. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Miezin, F. M. , Shulman, G. L. , & Petersen, S. E. (1993). A PET study of visuospatial attention. Journal of Neuroscience, 13(3), 1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Review Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Courtney, S. M. , Ungerleider, L. G. , Keil, K. , & Haxby, J. V. (1996). Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex, 6, 39–49. [DOI] [PubMed] [Google Scholar]
- Critchley, M. (1953). The parietal lobes. London: Edward Arnold. [Google Scholar]
- Curtis, C. E. , & D'Esposito, M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423. [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Tzourio, N. , Frak, V. , Raynaud, L. , Cohen, L. , Mehler, J. , & Mazoyer, B. (1996). Cerebral activations during number multiplication and comparison: A PET study. Neuropsychologia, 34, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, J. (2010). The multiple‐demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. [DOI] [PubMed] [Google Scholar]
- Duncan, J. , & Owen, A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience, 23, 475–483. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood meta‐analysis revisited. NeuroImage, 59, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate based activation likelihood estimation meta‐analysis of neuroimaging data: A random effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , … Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison, A. , Schindler, I. , Pattison, L. L. , & Milner, A. D. (2004). An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain, 127, 2307–2315. [DOI] [PubMed] [Google Scholar]
- Epstein, R. A. (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences, 12(10), 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli, D. (2018). Opinion: Is science really facing a reproducibility crisis, and do we need it to? Proceedings of the National Academy of Sciences of the United States of America, 115(11), 2628–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, M. J. (1996). Topographical disorientation. Neurocase, 2, 509–520. [Google Scholar]
- Fink, G. R. , Marshall, J. C. , Shah, N. J. , Weiss, P. H. , Halligan, P. W. , Grosse‐Ruyken, M. , … Freund, H. J. (2000). Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology, 54(6), 1324–1331. [DOI] [PubMed] [Google Scholar]
- Formisano, E. , Linden, D.E. , Di Salle, F. , Trojano, L. , Esposito, F. , Sack, A.T. , Grossi D., Zanella FE, Goebel R. (2002). Tracking the mind's image in the brain I: Time‐resolved fMRI during visuospatial mental imagery. Neuron, 35(1), 195–94. [DOI] [PubMed] [Google Scholar]
- Ganis, G. , Keenan, J. P. , Kosslyn, S. M. , & Pascual‐Leone, A. (2000). Transcranial magnetic stimulation of primary motor cortex affects mental rotation. Cerebral Cortex, 10, 175–180. [DOI] [PubMed] [Google Scholar]
- Ghaem, O. , Mellet, E. , Crivello, F. , Tzourio, N. , Mazoyer, B. , Berthoz, A. , & Denis, M. (1997). Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport, 8, 739–744. [DOI] [PubMed] [Google Scholar]
- Grezes, J. , & Decety, J. (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Human Brain Mapping, 12(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting, T. , Fyhn, M. , Molden, S. , Moser, M. B. , & Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature, 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hagler, D. J., Jr. , & Sereno, M. I. (2006). Spatial maps in frontal and prefrontal cortex. NeuroImage, 29(2), 567–577. [DOI] [PubMed] [Google Scholar]
- Hahn, B. , Ross, T. J. , & Stein, E. A. (2006). Neuroanatomical dissociation between bottom‐up and top‐down processes of visuospatial selective attention. NeuroImage, 32(2), 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan, P. W. , Fink, G. R. , Marshall, J. C. , & Vallar, G. (2003). Spatial cognition: Evidence from visual neglect. Trends in Cognitive Sciences, 7, 125–133. [DOI] [PubMed] [Google Scholar]
- Harris, I. M. , & Miniussi, C. (2003). Parietal lobe contribution to mental rotation demonstrated with rTMS. Journal of Cognitive Neuroscience, 15(3), 315–323. [DOI] [PubMed] [Google Scholar]
- Hautzel, H. , Mottaghy, F. M. , Schmidt, D. , Zemb, M. , Shah, N. J. , Müller‐Gärtner, H. W. , & Krause, B. J. (2002). Topographic segregation and convergence of verbal, object, shape and spatial working memory in humans. Neuroscience Letters, 323(2), 156–160. [DOI] [PubMed] [Google Scholar]
- Ikkai, A. , & Curtis, C. E. (2011). Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia, 49, 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina, I. , & Macaluso, E. (2007). Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cerebral Cortex, 17, 1701–1711. [DOI] [PubMed] [Google Scholar]
- Itti, L. , & Koch, C. (2001). Computational modelling of visual attention. Nature Review Neuroscience, 2(3), 194–203. [DOI] [PubMed] [Google Scholar]
- Jerde, T. A. (2012). Prioritized maps of space in human frontoparietal cortex. Journal of Neuroscience, 107(6), 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde, T. A. , & Curtis, C. E. (2013). Maps of space in human frontoparietal cortex. Journal of Physiology, 107(6), 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides, J. (1981). Voluntary versus automatic control over the mind's eye's movement In Long J. B. & Baddeley A. D. (Eds.), Attention and performance IX (pp. 187–203). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Jonides, J. , Smith, E. E. , Koeppe, R. A. , Awh, E. , Minoshima, S. , & Mintun, M. A. (1993). Spatial working memory in humans as revealed by PET. Nature, 363(6430), 623–625. [DOI] [PubMed] [Google Scholar]
- Jordan, K. , Wustenberg, T. , Heinze, H. J. , Peters, M. , & Jancke, L. (2002). Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia, 40, 2397–2408. [DOI] [PubMed] [Google Scholar]
- Kastner, S. , DeSimone, K. , Konen, C. S.m. , Szczepanski, S. M. , Weiner, K. S. , & Schneider, K. A. (2007). Topographic maps in human frontal cortex revealed in memory‐guided saccade and spatial working‐memory tasks. Journal of Neurophysiology, 97, 3494–3507. [DOI] [PubMed] [Google Scholar]
- Kincade, J. M. , Abrams, R. A. , Astafiev, S. V. , Shulman, G. L. , & Corbetta, M. (2005). An event‐related functional magnetic resonance imaging study of voluntary and stimulus‐driven orienting of attention. Journal of Neuroscience, 25, 4593–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen, C. S. , & Kastner, S. (2008). Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. Journal of Neuroscience, 28, 8361–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn, S. M. (2005). Mental images and the brain. Cognitive Neuropsychology, 22(3–4), 333–347. [DOI] [PubMed] [Google Scholar]
- Kosslyn, S. M. , DiGirolamo, G. J. , Thompson, W. L. , & Alpert, N. M. (1998). Mental rotation of objects versus hands: Neural mechanisms revealed by positron emission tomography. Psychophysiology, 35(2), 151–161. [PubMed] [Google Scholar]
- Kosslyn, S. M. , Maljkovic, V. , Hamilton, S. E. , Horwitz, G. , & Thompson, W. L. (1995). Two types of image generation: Evidence for left and right hemisphere processes. Neuropsychologia, 33(11), 1485–1510. [DOI] [PubMed] [Google Scholar]
- La Bar, K. S. , Gitelman, D. R. , Parrish, T. B. , & Mesulam, M. M. (1999). Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. NeuroImage, 10, 695–704. [DOI] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Price, C. J. , Glahn, D. C. , Uecker, A. M. , Lancaster, J. L. , … Fox, P. T. (2005). ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Robinson, J. L. , McMillan, K. M. , Tordesillas‐Gutierrez, D. , Moran, S. T. , Gonzales, S. M. , … Lancaster, J. L. (2010). Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage, 51, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm, C. , Windischberger, C. , Moser, E. , & Bauer, H. (2007). The functional role of dorsolateral premotor cortex during mental rotation. An event‐related fMRI study separating cognitive processing steps using a novel task paradigm. NeuroImage, 36, 1374–1386. [DOI] [PubMed] [Google Scholar]
- Lancaster, J. L. , Tordesillas‐Gutierrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28, 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek, E. C. , Yuen, K. S. , & Johnston, S. J. (2016). Domain general sequence operations contribute to pre‐SMA involvement in visuo‐spatial processing. Frontiers in Human Neuroscience, 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, R. , & Goldman‐Rakic, P. S. (2000). Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research, 133(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gotzsche, P. C. , Ioannidis, J. P. , … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: Explanation and elaboration. British Medical Journal, 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Pestilli, F. , & Carrasco, M. (2005). Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron, 45(3), 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückmann, H. C. , Jacobs, H. I. L. , & Sack, A. T. (2014). The cross‐functional role of frontoparietal regions in cognition: Internal attention as the overarching mechanism. Progress in Neurobiology, 116, 66–86. [DOI] [PubMed] [Google Scholar]
- Luzzi, S. , Pucci, E. , Di, P. B. , & Piccirilli, M. (2000). Topographical disorientation consequent to amnesia of spatial location in a patient with right parahippocampal damage. Cortex, 36(3), 427–434. [DOI] [PubMed] [Google Scholar]
- Mackey, W. E. , Winawer, J. , & Curtis, C. E. (2017). Visual field map clusters in human frontoparietal cortex. eLife, 6, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A. , Burgess, N. , Donnett, J. G. , O'Keefe, J. , & Frith, C. D. (1998). Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large‐scale space. Journal of Cognitive Neuroscience, 10, 61–76. [DOI] [PubMed] [Google Scholar]
- Manoach, D. S. , White, N. S. , Lindgren, K. A. , Heckers, S. , Coleman, M. J. , Dubal, S. , & Holzman, P. S. (2004). Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory. NeuroImage, 21, 894–903. [DOI] [PubMed] [Google Scholar]
- Marshall, J. C. , & Fink, G. R. (2001). Spatial cognition: Where we were and where we are. NeuroImage, 14, S2–S7. [DOI] [PubMed] [Google Scholar]
- McCarthy, G. , Nobre, A. C. , Bentin, S. , & Spencer, D. D. (1995). Language‐related field potentials in the anterior‐medial temporal lobe. I. Intracranial distribution and neural generators. Journal of Neuroscience, 15, 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, G. , Puce, A. , Constable, R. T. , Krystal, J. H. , Gore, J. C. , & Goldman‐Rakic, P. (1996). Activation of human‐prefrontal cortex during spatial and nonspatial working‐memory tasks measured by functional MRI. Cerebral Cortex, 6, 600–611. [DOI] [PubMed] [Google Scholar]
- Meister, I. G. , Wienemann, M. , Buelte, D. , Grunewald, C. , Sparing, R. , Dam‐ beck, N. , & Boroojerdi, B. (2006). Hemiextinction induced by transcranial magnetic stimulation over the right temporo‐parietal junction. Neuroscience, 142, 119–123. [DOI] [PubMed] [Google Scholar]
- Mellet, E. , Briscogne, S. , Tzourio‐Mazoyer, N. , Ghaem, O. , Petit, L. , Zago, L. , … Denis, M. (2000). Neural correlates of topographical mental exploration: The impact of route versus survey perspective learning. NeuroImage, 12(5), 588–600. [DOI] [PubMed] [Google Scholar]
- Milivojevic, B. , Hamm, J. P. , & Corballis, M. C. (2009). Functional neuroanatomy of mental rotation. Journal of Cognitive Neuroscience, 21, 945–959. [DOI] [PubMed] [Google Scholar]
- Milner, A. D. , & Goodale, M. A. (1995). The visual brain in action. Oxford, UK: Oxford University Press. [Google Scholar]
- Mishkin, M. , Ungerleider, L. G. , & Macko, K. A. (1983). Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences, 6, 414–417. [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. British Medical Journal, 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R. G. M. , Garrud, P. , Rawlins, J. N. , & O'Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Moscovitch, M. , Rosenbaum, R. S. , Gilboa, A. , Addis, D. R. , Westmacott, R. , Grady, C. , … Nadel, L. (2005). Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. Journal of Anatomy, 207, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, V. I. , Cieslik, E. C. , Laird, A. R. , Fox, P. T. , Radua, J. , Mataix‐Cols, D. , … Eickhoff, S. B. (2018). Ten simple rules for neuroimaging meta‐analysis. Neuroscience and Biobehavioral Reviews, 84, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N. E. , Stokes, M. G. , & Nobre, A. C. (2017). Prioritizing information during working memory: Beyond sustained internal attention. Trends in Cognitive Sciences, 21(6), 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre, A. C. , Coull, J. T. , Maquet, P. , Frith, C. D. , Vandenberghe, R. , & Mesulam, M. M. (2004). Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience, 16(3), 363–373. [DOI] [PubMed] [Google Scholar]
- Nobre, A. C. , Sebestyen, G. N. , Gitelman, D. R. , Mesulam, M. M. , Frackowiak, R. S. , & Frith, C. D. (1997). Functional localization of the system for visuospatial attention using positron emission tomography. Brain, 120(3), 515–533. [DOI] [PubMed] [Google Scholar]
- Nystrom, L. E. , Braver, T. S. , Sabb, F. W. , Delgado, M. R. , Noll, D. C. , & Cohen, J. D. (2000). Working memory for letters, shapes and locations: fMRI evidence against stimulus‐based regional organization of human prefrontal cortex. NeuroImage, 11, 424–446. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. , & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon. [Google Scholar]
- Owen, A. M. , Iddon, J. L. , Hodges, J. R. , Summers, B. A. , & Robbins, T. W. (1997). Spatial and non‐spatial working memory at different stages of Parkinson's disease. Neuropsychologia, 35(4), 519–532. [DOI] [PubMed] [Google Scholar]
- Owen, A. M. , McMillan, K. M. , Laird, A. R. , & Bullmore, E. (2005). N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo, L. , Bureca, I. , Matano, A. , & Guariglia, C. (2008). Hemispheric contribution to categorical and coordinate representational processes: A study on brain‐damaged patients. Neuropsychologia, 46(11), 2802–2807. [DOI] [PubMed] [Google Scholar]
- Parlatini, V. , Radua, J. , Dell'Acqua, F. , Leslie, A. , Simmons, A. , Murphy, D. G. , … Thiebaut de Schotten, M. (2017). Functional segregation and integration within fronto‐parietal networks. NeuroImage, 146, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalar, S. , Ro, T. , & Beauchamp, M. S. (2010). TMS of posterior parietal cortex disrupts visual tactile multisensory integration. European Journal of Neuroscience, 31(10), 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims, B. , Michaux, N. , Olivier, E. , & Andres, M. (2011). Contribution of the primary motor cortex to motor imagery: A subthreshold TMS study. Human Brain Mapping, 32, 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides, M. (2005). Lateral prefrontal cortex: Architectonic and functional organization. Philosophical Transactions of the Royal Society B, Biological Sciences, 360(1456), 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology (Hove), 32(1), 3–25. [DOI] [PubMed] [Google Scholar]
- Poucet, B. , Lenck‐Santini, P. P. , Hok, V. , Save, E. , Banquet, J. P. , Gaussier, P. , & Muller, R. U. (2004). Spatial navigation and hippocampal place cell firing: The problem of goal encoding. Reviews in the Neurosciences, 15, 89–107. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , & Church, J. A. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, W. , Somorjai, R. , Summers, R. , Jarmasz, M. , Menon, R. S. , Gati, J. S. , … Kim, S. G. (2000). Motor area activity during mental rotation studied by time‐resolved single‐trial fMRI. Journal of Cognitive Neuroscience, 12(2), 310–320. [DOI] [PubMed] [Google Scholar]
- Robertson, I. , & Marshall, J. (1993). Unilateral neglect clinical and experimental studies (pp. 3–25). Hove, UK: Lawrence Erlbaum Associates. [Google Scholar]
- Rochefort, C. , Arabo, A. , André, M. , Poucet, B. , Save, E. , & Rondi‐Reig, L. (2011). Cerebellum shapes hippocampal spatial code. Science, 334(6054), 385–389. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, R. S. , Ziegler, M. , Winocur, G. , Grady, C. L. , & Moscovitch, M. (2004). “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus, 14(7), 826–835. [DOI] [PubMed] [Google Scholar]
- Rottschy, C. , Langner, R. , Dogan, I. , Reetz, K. , Laird, A. R. , Schulz, J. B. , … Eickhoff, S. B. (2012). Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. NeuroImage, 60, 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, A. T. , Jacobs, C. , De Martino, F. , Staeren, N. , Goebel, R. , & Formisano, E. (2008). Dynamic premotor‐to‐parietal interactions during spatial imagery. Journal of Neuroscience, 28(34), 8417–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, A. T. , & Schuhmann, T. (2012). Hemispheric differences within the fronto‐parietal network dynamics underlying spatial imagery. Frontiers in Psychology, 3, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala, J. , Rama, P. , & Courtney, S. M. (2003). Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia, 41, 341–356. [DOI] [PubMed] [Google Scholar]
- Sauner, D. , Bestmann, S. , Siebner, H. R. , & Rothwell, J. C. (2006). No evidence for a substantial involvement of primary motor hand area in handedness judgements: A transcranial magnetic stimulation study. European Journal of Neuroscience, 23, 2215–2224. [DOI] [PubMed] [Google Scholar]
- Saygin, A. P. , & Sereno, M. I. (2008). Retinotopy and attention in human occipital, temporal, parietal, and frontal cortex. Cerebral Cortex, 18(9), 2158–2168. [DOI] [PubMed] [Google Scholar]
- Schluppeck, D. , Glimcher, P. , & Heeger, D. J. (2005). Topographic organization for delayed saccades in human posterior parietal cortex. Journal of Neurophysiology, 94(2), 1372–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences, J. T. , & Yantis, S. (2007). Spatially selective representations of voluntary and stimulus‐driven attentional priority in human occipital, parietal, and frontal cortex. Cerebral Cortex, 17, 284–293. [DOI] [PubMed] [Google Scholar]
- Sereno, M. I. , Pitzalis, S. , & Martinez, A. (2001). Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science, 294(5545), 1350–1354. [DOI] [PubMed] [Google Scholar]
- Seurinck, R. , Vingerhoets, G. , de Lange, F. P. , & Achten, E. (2004). Does egocentric mental rotation elicit sex differences? NeuroImage, 23(4), 1440–1449. [DOI] [PubMed] [Google Scholar]
- Shelton, A. L. , & Gabrieli, J. D. (2002). Neural correlates of encoding space from route and survey perspectives. Journal of Neuroscience, 22, 2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M. , & Kastner, S. (2009). Topographic maps in human frontal and parietal cortex. Trends in Cognitive Sciences, 13, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M. A. , Ress, D. , & Heeger, D. J. (2005). Topographic maps of visual spatial attention in human parietal cortex. Journal of Neurophysiology, 94, 1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. V. , Davis, B. , Niu, K. , Healy, E. W. , Bonilha, L. , Fridriksson, J. , … Rorden, C. (2010). Spatial attention evokes similar activation patterns for visual and auditory stimuli. Journal of Cognitive Neuroscience, 22(2), 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers, H. J. (2008). Keeping the goal in mind: Prefrontal contributions to spatial navigation. Neuropsychologia, 46(7), 2106–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers, H. J. , & Barry, C. (2015). Neural systems supporting navigation. Current Opinion in Behavioral Sciences, 1, 47–55. [Google Scholar]
- Spiers, H. J. , & Maguire, E. A. (2007). The neuroscience of remote spatial memory: A tale of two cities. Neuroscience, 149(1), 7–27. [DOI] [PubMed] [Google Scholar]
- Szczepanski, S. M. , Konen, C. S. , & Kastner, S. (2010). Mechanisms of spatial attention control in frontal and parietal cortex. Journal of Neuroscience, 30(1), 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, N. , Kawamura, M. , Shiota, J. , Kasahata, N. , & Hirayama, K. (1997). Pure topographic disorientation due to right retrosplenial lesion. Neurology, 49, 464–469. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Taube, J. (1998). Head direction cells and the neurophysiological basis for a sense of direction. Progress in Neurobiology, 55(3), 225–256. [DOI] [PubMed] [Google Scholar]
- Thomason, M. E. , Race, E. , Burrows, B. , Whitfield‐Gabrieli, S. , Glover, G. H. , & Gabrieli, J. D. (2009). Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience, 21(2), 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, K. G. , & Bichot, N. P. (2005). A visual salience map in the primate frontal eye field. Progress in Brain Research, 147, 251–262. [DOI] [PubMed] [Google Scholar]
- Tomasino, B. , Borroni, P. , Isaja, A. , & Rumiati, R. I. (2005). The role of the primary motor cortex in mental rotation: A TMS study. Cognitive Neuropsychology, 22, 348–363. [DOI] [PubMed] [Google Scholar]
- Trojano, L. , Grossi, D. , Linden, D. E. , Formisano, E. , Goebel, R. , Cirillo, S. , … Di Salle, F. (2002). Coordinate and categorical judgements in spatial imagery. An fMRI study. Neuropsychologia, 40(10), 1666–1674. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single–word reading: Methods and validation. NeuroImage, 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in Activation Likelihood Estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider, L. , & Mishkin, M. (1982). Two cortical visual systems In Ingle D., Goodale M. A., & Mansfield R. J. W. (Eds.), Analysis of visual behaviour (pp. 549–586). Boston: The MIT Press. [Google Scholar]
- Ungerleider, L. G. , Courtney, S. M. , & Haxby, J. V. (1998). A neural system for human visual working memory. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar, G. , & Perani, D. (1986). The anatomy of unilateral neglect after right‐hemisphere stroke lesions. A clinical/ CT‐scan correlation study in man. Neuropsychologia, 24, 609–622. [DOI] [PubMed] [Google Scholar]
- van de Ven, V. , & Sack, A. T. (2013). Transcranial magnetic stimulation of visual cortex in memory: Cortical state interference and reactivation of visual content in memory. Behavioural Brain Research, 236, 67–77. [DOI] [PubMed] [Google Scholar]
- van der Ham, I. J. , Raemaekers, M. , van Wezel, R. J. , Oleksiak, A. , & Postma, A. (2009). Categorical and coordinate spatial relations in working memory: An fMRI study. Brain Research, 1297, 70–79. [DOI] [PubMed] [Google Scholar]
- Ventre‐Dominey, J. , Bailly, A. , Lavenne, F. , Lebars, D. , Mollion, H. , Costes, N. , & Dominey, P. F. (2005). Double dissociation in neural correlates of visual working memory: A PET study. Brain Research. Cognitive Brain Research, 25(3), 747–759. [DOI] [PubMed] [Google Scholar]
- Wager, T. , & Smith, E. E. (2003). Neuroimaging studies of working memory: A meta‐analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(4), 255–274. [DOI] [PubMed] [Google Scholar]
- Walter, H. , Bretschneider, V. , Grön, G. , Zurowski, B. , Wunderlich, A. P. , Tomczak, R. , & Spitzer, M. (2003). Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex, 39(4), 897–911. [DOI] [PubMed] [Google Scholar]
- Yantis, S. , Schwarzbach, J. , Serences, J. T. , Carlson, R. L. , Steinmetz, M. A. , Pekar, J. J. , & Courtney, S. M. (2002). Transient neural activity in human parietal cortex during spatial attention shifts. Nature Neuroscience, 5, 995–1002. [DOI] [PubMed] [Google Scholar]
- Zacks, J. M. (2008). Neuroimaging studies of mental rotation: A meta‐analysis and review. Journal of Cognitive Neuroscience, 20, 1–19. [DOI] [PubMed] [Google Scholar]
- Zeki, S. M. , & Marini, L. (1998). Three cortical stages of color processing in the human brain. Brain, 121, 1669–1685. [DOI] [PubMed] [Google Scholar]
- Zimmer, H. D. (2008). Visual and spatial working memory: From boxes to networks. Neuroscience & Biobehavioral Reviews, 32, 1373–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information A PRISMA 2009 Flow Diagram (last update March 2018)
Supporting Information B List of studies included
Supporting Information C Checklist for neuroimaging meta‐analysis (Muller et al., 2017)


