Abstract
It remains unclear whether and to what extent working memory (WM) temporal subprocesses (i.e., encoding, maintenance, and retrieval) involve shared or distinct intrinsic networks. To address this issue, I constructed a model of intrinsic network contributions to different WM phases and then evaluated the validity of the model by performing a quantitative meta‐analysis of relevant functional neuroimaging data. The model suggests that the transition from the encoding to maintenance and to retrieval stages involves progressively decreasing involvement of the dorsal attention network (DAN), but progressively increasing involvement of the frontoparietal control network (FPCN). Separate meta‐analysis of each phase effect and direct comparisons between them yielded results that were largely consistent with the model. This evidence included between‐phase double dissociations that were consistent with the model, such as encoding > maintenance contrast showing some DAN, but no FPCN, regions, and maintenance > encoding contrast showing the reverse, that is, some FPCN, but no DAN, regions. Two closely juxtaposed regions that are members of the DAN and FPCN, such as inferior frontal junction versus caudal prefrontal cortex and superior versus inferior intraparietal sulcus, showed a high degree of functional differentiation. Although all regions identified in the present study were already identified in previous WM studies, this study uniquely enhances our understating of their roles by clarifying their network membership and specific associations with different WM phases.
Keywords: encoding, fMRI, maintenance, meta‐analysis, retrieval, working memory
1. INTRODUCTION
1.1. The aim of the study
Working memory (WM), an ability to hold and manipulate information “in the mind,” is a fundamental component of many everyday cognitive activities, such as reading comprehension, learning, problem solving, and planning. An important goal of WM cognitive neuroscience is to identify the neural mechanisms underlying its encoding, maintenance, and retrieval processes. To this end, many functional neuroimaging studies employ various “delayed‐response” paradigms that can dissociate the WM stages in time, such as the Sternberg Item Recognition Task, which consists of an encoding phase, during which subjects memorize a set of items; a maintenance phase, during which the information is held “online”; and a retrieval phase, during which subjects respond to a probe by indicating whether or not it was a member of the memorized set. While these studies expanded our understanding of the neural bases of WM temporal subprocesses, they also produced many between‐study discrepancies and even direct contradictions. For example, while many studies indicated that maintenance involved the prefrontal cortex (PFC), specific prefrontal sites showing this effect varied widely across studies (Cairo, Liddle, Woodward, & Ngan, 2004; Habeck et al., 2005; Manoach, Greve, Lindgren, & Dale, 2003; Passaro et al., 2013; Rypma & D'Esposito, 2000) and some even found no specific evidence of PFC involvement (Beatty et al., 2015; Majerus, Salmon, & Attout, 2013; Murty et al., 2011). While these diverse results per se are not necessarily surprising, given the many different stimuli types, procedures, and analytic methods employed, they make it difficult to draw general conclusions as to which brain regions reliably contribute to different WM phases.
Several studies performed quantitative meta‐analyses of WM‐neuroimaging data to identify significant concordance across individual studies. These studies typically focused exclusively on “n‐back” tasks (Owen, McMillan, Laird, & Bullmore, 2005; Wang et al., 2019; Yaple & Arsalidou, 2018), which do not permit the separation of the different temporal phases, or collapsed analyses across data regarding n‐back and other delayed‐response tasks (Hill, Laird, & Robinson, 2014; Rottschy et al., 2011; Wager & Smith, 2003). Although a recent study (Daniel, Katz, & Robinson, 2016) focused exclusively on a delayed‐response paradigm, namely, delayed match to sample, it did not perform any specific analysis relevant to the separation of different phase effects. In this regard, the present study aimed to provide the first meta‐analysis identifying which specific brain regions are recruited during different WM phases. To formulate hypotheses for the meta‐analysis and interpret the results, the present study also constructed a neurocognitive model of WM temporal subprocesses, as described in more detail below. Thus, this study strives to go beyond a generic purpose of integrating results across studies and potentially also contribute to neurocognitive theorizing of WM.
1.2. An overview of the meta‐analysis
A most fundamental aspect of all meta‐analysis designs is determining which studies are included in and excluded from the analysis. Candidate studies for meta‐analysis necessarily used WM tasks that separated three phase‐epochs in time, but only a minority actually reported the activation effects of all three phases, whereas a majority reported only one‐ or two‐phase effects (e.g., Bledowski et al., 2006; Todd, Han, Harrison, & Marois, 2011; Wendelken, Bunge, & Carter, 2008). Although the inclusion of the latter group of studies increases the statistical power, it is otherwise problematic because ensuing encoding‐, maintenance‐, and retrieval‐phase datasets differ in several ways that have nothing to do with phase per se, such as stimuli type, task requirement, and reference condition. Therefore, despite some loss in statistical power, I chose to include only studies that reported the activation effects of all three phases, thereby rendering different task‐phase data well matched in terms of potential confounding factors. Fortunately, the collected dataset was relatively large even with this restriction, and provided reasonably high statistical power.
In the present study, and likely in some of the previous studies, the ultimate purpose of separating encoding‐, maintenance‐, and retrieval‐phase effects was to evaluate whether and to what extent different phase effects involve common or distinct neural regions. Although this purpose naturally demands direct comparisons between different phase effects, the vast majority of previous studies that separated three‐phase effects did not make such comparisons (e.g., Cairo et al., 2004; Manoach et al., 2003; Passaro et al., 2013; Pessoa, Gutierrez, Bandettini, & Ungerleider, 2002). While this practice may merely follow from the goal of these studies not including any comparisons, it necessarily precludes a clear appreciation of which regions are commonly or distinctly involved in different WM phases. Obviously, “visual comparisons” cannot substitute formal inferences based on statistical testing. In this regard, the present study emphasized not only separate meta‐analysis of encoding‐, maintenance‐ and retrieval‐phase data but also direct comparisons between them.
A large body of evidence now indicates that the brain is organized into multiple large‐scale intrinsic networks, each comprising a set of discontinuous but intimately interacting regions (Eickhoff, Yeo, & Genon, 2018; Gordon et al., 2017; Yeo et al., 2011). This evidence resonates with the theoretical stance that WM does not involve a dedicated system, but rather emerges from the interactions among the systems that support more basic functions, such as attention, perception, and action (D'Esposito & Postle, 2015; Eriksson, Vogel, Lansner, Bergström, & Nyberg, 2015; Postle, 2006). Thus, activations during WM tasks can be seen more suitably as components of large‐scale networks that act in concert rather than regions responding in isolation. In this regard, an increasing number of recent WM studies have adopted a specific network‐based approach (Dagenbach, 2019; Santangelo & Bordier, 2019; Wallis, Stokes, Cousijn, Woolrich, & Nobre, 2015). Based on the global and broad match between intrinsic‐network topography and concentrations of WM activations across studies, WM is most closely associated with two intrinsic networks: dorsal attention network (DAN) and frontoparietal control network (FPCN; Fox, Corbetta, Snyder, Vincent, & Raichle, 2006; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008; Yeo et al., 2011). Thus, the present study emphasized these two networks in formulating hypotheses for the meta‐analysis and interpreting the results, as described in more detail below.
1.3. A network‐based model of WM stages
Figure 1a shows the major DAN and FPCN regions, along with the terms used to refer to them in this study. The activity of the DAN increases during externally directed cognition, such as stimulus processing, visual orienting, and target detection, suggesting that it embodies a mechanism for orienting attention to the external environment (Corbetta & Shulman, 2002; Fox et al., 2005; Sestieri, Shulman, & Corbetta, 2012). The FPCN shows high activity during executive function tasks, such as conflict resolution, task switching, and decision making, providing evidence that it mediates cognitive control processes (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Niendam et al., 2012; Vincent et al., 2008). A Neurosynth‐based decoding of DAN and FPCN regions performed herein supports the notion that they play a strong role in external attention and cognitive control processes, respectively. As Figure 1b illustrates, top semantic associates of the DAN include terms such as “spatial,” “attention,” “visuospatial,” and “eye movements” and those of the FPCN terms such as “working memory,” “cognitive control,” “executive,” and “demands” (for methodological details, see Figure 1). The DAN and FPCN include many (but not all) of the cortical sites where WM‐related activations have been most consistently observed, including the mid‐lateral PFC, inferior frontal junction (IFJ), and intraparietal sulcus (IPS). The DAN and FPCN subregions are closely juxtaposed in both frontal and posterior cortices, such as IFJ versus caudal PFC and superior versus inferior IPS, indicating that two very adjacent WM‐activations can subserve highly differential functions.
Figure 1.
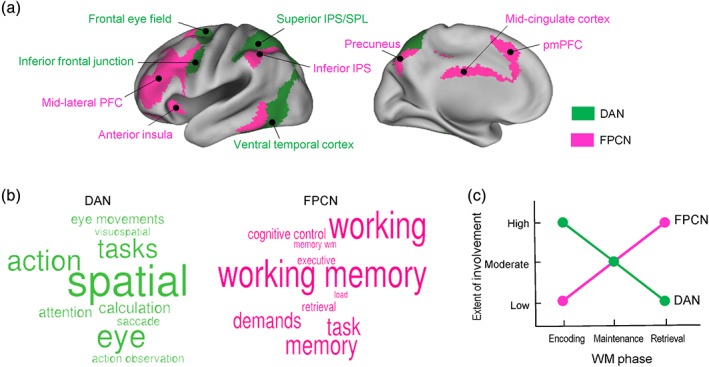
(a) Estimates of the dorsal attention network (DAN; shown in green) and the frontoparietal cognitive control network (FPCN; pink), produced using data from Yeo et al. (2011). (b) A Neurosynth function called “decoding” can generate a rank‐ordered list of associated terms given an activation image, based on the similarity between the image and a large number of meta‐analytic maps (http://neurosynth.org; Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). This function was used to extract the top 10 semantic associates of the DAN and FPCN regions shown in (a), which are presented here in word clouds. The size of a term in each cloud is proportional to the strength of similarity. (c) A graphic illustration of the proposed model of intrinsic network involvement in working memory (WM) encoding, maintenance, and retrieval. IPS, intraparietal sulcus; pmPFC, posteromedial prefrontal cortex; SPL, superior parietal lobe [Color figure can be viewed at http://wileyonlinelibrary.com]
The model of WM stages proposed herein involves the following three core hypotheses:
Hypothesis 1
Encoding is extensively associated with the DAN, but much less with the FPCN.
Hypothesis 2
Maintenance is associated with each of the DAN and FPCN to a moderate extent.
Hypothesis 3
Retrieval is extensively associated with the FPCN, but much less with the DAN.
Figure 1c graphically illustrates these hypotheses. Below, I first discuss the rationale for these hypotheses and then describe additional hypotheses derived from them.
With regard to Hypothesis 1 (encoding), both anecdotal and scientific evidence indicates that attention “gates” or determines what will be encoded into WM (Gazzaley, 2011; Rutman, Clapp, Chadick, & Gazzaley, 2010; Schmidt, Vogel, Woodman, & Luck, 2002), providing a basis to expect relatively extensive recruitment of the DAN during an encoding phase. In contrast, the FPCN is likely involved more conditionally because its activity scales with the amount and complexity of the memoranda. Given that WM studies typically employ very simple and repetitive stimuli as memoranda, such as letters, digits, and dots (Cairo et al., 2004; Chang, Crottaz‐Herbette, & Menon, 2007; Habeck et al., 2005; Manoach et al., 2003; Rowe & Passingham, 2001), an encoding phase may in most cases recruit the FPCN to a relatively weak extent. In sum, an encoding phase may typically recruit the DAN to a greater extent, but the FPCN to a lesser extent.
Hypothesis 2 (maintenance) reflects multiple lines of evidence. First, as regards the involvement of the DAN, previous studies established that orienting attention in, or selecting information from, visual space versus WM involves extensively overlapping regions that are largely within the DAN (Ikkai & Curtis, 2011; Nee & Jonides, 2009; Nobre et al., 2004; Tamber‐Rosenau, Esterman, Chiu, & Yantis, 2011; for a meta‐analysis, see Wallis et al., 2015). Thus, the DAN, perhaps more commonly characterized as “external attention network,” also plays a role in WM‐based attentional operations, providing a basis to expect the participation of the DAN in WM maintenance. However, studies also indicated that despite this commonality, some DAN regions were associated more strongly with attentional operations in visual space than in WM (Corbetta, Kincade, & Shulman, 2002; Emrich, Riggall, LaRocque, & Postle, 2013; Nee & Jonides, 2009; Nobre et al., 2004), suggesting that if other things are held constant, the network tends to be more strongly recruited in the presence than in the absence of stimulus. In this regard, the DAN may be recruited with moderate, rather than high, strength during a maintenance stage. Although the term “moderate” is relatively ambiguous, the strength can be more precisely specified relative to that in other stages, that is, intermediate between the encoding and retrieval stages, as illustrated in Figure 1c. Next, as regards the involvement of the FPCN, keeping information “online” taxes some control‐demanding processes such as rehearsal, sustained attention, and distractor resistance (Awh et al., 1999; Chun, 2011; Sakai, Rowe, & Passingham, 2002a). However, given that such processes tend to involve relatively monotonous operations rather than very complex manipulation of information (e.g., decision making, conflict resolution), the FPCN may also be recruited with moderate, rather than high, strength during a maintenance stage. In sum, a maintenance phase may typically recruit each of the DAN and FPCN to a moderate extent.
With regard to Hypothesis 3 (retrieval), a retrieval phase strongly demands executive control functions, such as memory scanning, matching operation involving the sample and probe stimuli, decision making, and response selection (for reviews, see Eriksson et al., 2015; Jonides et al., 2008), which supports the expectation for relatively extensive recruitment of the FPCN during the phase. A retrieval phase also demands external attention because it requires processing of a probe. However, given that externally and internally directed cognition are largely antagonistic to each other (Boly et al., 2007; Dixon, Fox, & Christoff, 2014; Huijbers, Pennartz, Cabeza, & Daselaar, 2009; Kim, 2018), persistent attention to the external environment would interfere with memory scanning, decision making, and other controlled processes that are based on internal representations. Thus, subjects' initial attention to a probe may soon be replaced by more persistent attention to internal representations (of a probe and sample stimuli), which supports the expectation for relatively weak recruitment of the DAN during the phase. Moreover, in trials when a probe is a repeat of a stimulus presented at encoding, neural priming effects, known as “repetition suppression” (for reviews, see Grill‐Spector, Henson, & Martin, 2006; Schacter, Wig, & Stevens, 2007) would further constrain activation of the DAN. In sum, a retrieval phase may typically recruit the FPCN to a greater extent, but the DAN to a lesser extent.
The three core hypotheses were used to derive the following six hypotheses:
Hypothesis 4
Regions more strongly associated with encoding than maintenance involve some DAN, but few FPCN, regions, whereas the reverse, that is, the involvement of some FPCN, but few DAN, regions, is true for regions more strongly associated with maintenance than encoding.
Hypothesis 5
Regions more strongly associated with maintenance than retrieval involve some DAN, but few FPCN, regions, whereas the reverse is true for regions more strongly associated with retrieval than maintenance.
Hypothesis 6
Regions more strongly associated with encoding than retrieval involve some DAN, but few FPCN, regions, whereas the reverse is true for regions more strongly associated with retrieval than encoding.
Hypothesis 7
Regions common to encoding and maintenance involve the DAN to a greater extent, but the FPCN to a lesser extent.
Hypothesis 8
Regions common to maintenance and retrieval involve the FPCN to a greater extent, but the DAN to a lesser extent.
Hypothesis 9
Regions common to encoding and retrieval involve both the DAN and FPCN to a limited extent.
Hypotheses 4 through 6 address the distinct substrates of different WM phases and Hypotheses 7 through 9 address the common substrates. I will not further dwell on these hypotheses because they can be seen largely as logical extensions of the three core hypotheses.
To summarize, the proposed model comprises nine hypotheses regarding the recruitment of the DAN and FPCN during three WM stages and their shared and distinct effects. In essence, it suggests that the transition from the encoding to maintenance and to retrieval stages involves progressively decreasing involvement of the DAN, but progressively increasing involvement of the FPCN. Although the model is broadly specified in terms of whole networks rather than their constituent components, this limitation does not prevent the model from providing a useful framework for further research efforts on related topics.
2. MATERIALS AND METHODS
2.1. Data collection
Candidate studies employed functional magnetic resonance imaging (fMRI) as the imaging modality and various delayed‐response tasks as the behavioral paradigm. Positron emission tomography studies were not considered for inclusion, because specific separation of different task phases requires an event‐related design. To identify candidate studies, the PubMed database was searched on July 7, 2018, with the search strings: “fMRI AND ‘working memory’ AND (encoding OR maintenance OR retrieval OR Sternberg OR ‘delayed match to sample’ OR ‘delayed simple matching’)”. In addition, the reference sections from relevant meta‐analysis studies (e.g., Rottschy et al., 2011; Wager & Smith, 2003; Wang et al., 2019) were screened to identify studies not identified by the online database search. Search results were filtered to include only studies that met the following inclusion/exclusion criteria:
Only studies that tested healthy subjects with no sign of mental or neurological illness were included.
Only studies that involved the presentation of stimuli through a visual modality were included.
Only studies that reported whole‐brain data and peak activation foci in standard stereotaxic coordinates (i.e., Talairach or Montreal Neurological Institute [MNI]) were included.
Only studies that reported activation effects of all three phases were included (see section 1 for the rationale for this criterion).
A few studies employed a design that involved the presentation of external stimuli during a maintenance phase, such as distractors. These studies were excluded to guard against the potential confounding of maintenance‐related effects with perceptually driven ones.
A few studies reported multiple activation contrasts of similar types, such as load 5 > load 3 and load 3 > load 1. In each of these studies, only one contrast was included to keep that study from overly affecting the results. The activation contrast that involved the highest number of peak activation foci was selected, based on the rationale that it would maximize the sensitivity of the meta‐analysis.
Deactivation data (e.g., baseline > WM task) were excluded because such data have been rarely reported and are not directly related to the present hypotheses.
2.2. Sample characteristics
Forty‐seven studies met the inclusion/exclusion criteria and were included in the meta‐analysis. These studies are listed in Table 1 by authors and year of publication, along with information regarding the number of subjects, composition of activation contrast, stimuli type, retrieval task, and the number of foci. The mean number of subjects per study was 17.7 (SD = 10.1). Behavioral performance was in the good to excellent range in most included studies. Of 32 studies that numerically reported the proportion of correct responses, it was in 0.90 s in 14, 0.80 s in 12, 0.70 s in 3, and <0.70 s in 3. Thus, this study may involve possible biasing effects linked to poor behavioral performance to a minimal degree. The composition of activation contrast was task > baseline in 20 experiments, load‐related increase in 11, task > sensorimotor control in 8, difficult condition > easy condition (e.g., manipulation > no‐manipulation) in 6, and correct‐response trials > incorrect‐response trials in 2. The stimuli type was verbal (letters, words, numbers) in 25 experiments, abstract shapes (e.g., dots, polygons) in 13, concrete objects (e.g., faces, scenes) in 7, and a combination of verbal and shape stimuli in 2. The retrieval task was identity verification in 28 experiments, location verification or recall in 6, both identity and location verification in 6, order verification in 2, and other operations (e.g., verbal recall, semantic verification) in 5. The delay‐period interval was fixed (e.g., 5 s) in 32 experiments and variable (e.g., 4, 8, 12 s) in 15. The mean number of foci was 11.2 (SD = 7.2) for encoding, 9.5 (SD = 6.6) for maintenance, and 11.6 (SD = 8.8) for retrieval conditions.
Table 1.
Overview of studies included in the present meta‐analysis
| Foci | |||||||
|---|---|---|---|---|---|---|---|
| Study | Subjects | Contrast | Stimulus | Task | E | M | R |
| Beatty et al., 2015 | 43 | Task > baseline | Shapes | Identity verification | 4 | 3 | 3 |
| Bedwell et al., 2005 | 14 | Task > control | Letters | Identity verification | 19 | 24 | 10 |
| Bennett, Rivera, & Rypma, 2013 | 42 | Load‐related increase | Letters | Identity verification | 5 | 10 | 2 |
| Bergmann, Daselaar, Fernandez, & Kessels, 2016 | 24 | Correct > incorrect | Objects/scenes | Location verification | 0 | 7 | 15 |
| Bokde et al., 2010 | 8 | Task > baseline | Letters | Identity verification | 18 | 8 | 17 |
| Cairo et al., 2004 | 18 | Task > baseline | Letters | Identity verification | 19 | 12 | 18 |
| Chang et al., 2007 | 14 | Load‐related increase | Numbers | Identity verification | 11 | 31 | 9 |
| Chein & Fiez, 2001 | 12 | Task > baseline | Words | Verbal recall | 13 | 10 | 15 |
| Chen & Desmond, 2005 | 15 | Load‐related increase | Letters | Identity verification | 37 | 14 | 12 |
| Curtis, Rao, & D'Esposito, 2004 | 15 | Difficult > easy | Shapes | Oculomotor‐based location recall | 2 | 7 | 7 |
| de Leeuw, Kahn, Zandbelt, Widschwendter, & Vink, 2013 | 24 | Task > control | Letters | Identity verification | 16 | 7 | 14 |
| de Leeuw et al., 2013 | 24 | Task > control | Letters | Identity verification | 13 | 9 | 10 |
| Fiebach, Friederici, Smith, & Swinney, 2007 | 12 | Difficult > easy | Words | Semantic verification | 0 | 1 | 3 |
| Fiehler et al., 2011 | 21 | Task > baseline | Shapes | Grasping‐based shape recall | 7 | 3 | 7 |
| Gibbs & D'Esposito, 2005a | 10 | Task > baseline | Shapes | Identity/location verification | 8 | 8 | 15 |
| Gibbs & D'Esposito, 2005b | 13 | Task > baseline | Letters | Identity verification | 14 | 5 | 17 |
| Gibbs & D'Esposito, 2006 | 9 | Task > baseline | Shapes | Identity/location verification | 6 | 3 | 15 |
| Grot et al., 2017 | 23 | Difficult > easy | Words/shapes | Location verification | 9 | 5 | 0 |
| Habeck et al., 2005 | 40 | Load‐related increase | Letters | Identity verification | 8 | 22 | 10 |
| Jenness et al., 2018 | 54 | Task > baseline | Faces/scenes | Identity verification | 5 | 9 | 2 |
| Karlsgodt, Shirinyan, van Erp, Cohen, & Cannon, 2005 | 13 | Task > baseline | Words | Identity verification | 14 | 8 | 16 |
| Klaassen et al., 2013 | 21 | Load‐related increase | Letters | Identity verification | 14 | 15 | 8 |
| Kochan et al., 2011 | 18 | Load‐related increase | Shapes | Identity/location verification | 6 | 7 | 10 |
| Kondo, Nomura, & Kashino, 2015 | 28 | Task > baseline | Shapes | Location verification | 11 | 9 | 5 |
| Lagopoulos, Ivanovski, & Malhi, 2007 | 10 | Load‐related increase | Words | Identity verification | 7 | 17 | 11 |
| Landau, Lal, O'Neil, Baker, & Jagust, 2009 | 23 | Load‐related increase | Letters | Identity verification | 24 | 12 | 1 |
| Landau, Schumacher, Garavan, Druzgal, & D'Esposito, 2004 | 10 | Task > baseline | Faces | Identity verification | 6 | 2 | 8 |
| Luck et al., 2010 | 17 | Task > baseline | Letters/shapes | Identity/location verification | 12 | 4 | 14 |
| Majerus et al., 2013 | 21 | Task > control | Words | Identity verification | 14 | 0 | 27 |
| Manoach et al., 2003 | 12 | Task > baseline | Numbers | Identity verification | 20 | 11 | 49 |
| Marvel & Desmond, 2010 | 16 | Difficult > easy | Letters | Identity verification | 21 | 21 | 5 |
| Mohr, Goebel, & Linden, 2006 | 13 | Difficult > easy | Shapes | Color/angle verification | 20 | 20 | 11 |
| Narayanan et al., 2005 | 12 | Task > baseline | Letters | Identity verification | 12 | 8 | 16 |
| Panwar et al., 2014 | 18 | Task > baseline | Words | Identity verification | 7 | 5 | 5 |
| Passaro et al., 2013 | 10 | Task > baseline | Shapes | Identity/location verification | 8 | 16 | 13 |
| Pessoa et al., 2002 | 9 | Correct > incorrect | Shapes | Identity verification | 9 | 9 | 9 |
| Rämä & Courtney, 2005 | 12 | Task > control | Faces | Identity verification | 11 | 12 | 17 |
| Ravizza, Hazeltine, Ruiz, & Zhu, 2011 | 17 | Load‐related increase | Letters | Order verification | 3 | 4 | 1 |
| Röder, Mohr, & Linden, 2011 | 22 | Load‐related increase | Faces | Identity/emotion verification | 11 | 5 | 3 |
| Ross, LoPresti, Schon, & Stern, 2013 | 16 | Difficult > easy | Faces | Identity verification | 1 | 4 | 36 |
| Rowe & Passingham, 2001 | 6 | Task > baseline | Shapes | Joystick‐based location recall | 12 | 5 | 14 |
| Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000 | 6 | Task > control | Shapes | Joystick‐based location recall | 4 | 5 | 7 |
| Rypma & D'Esposito, 2000 | 25 | Task > baseline | Letters | Identity verification | 18 | 9 | 19 |
| Sakai, Rowe, & Passingham, 2002b | 12 | Task > control | Letters | Order verification | 4 | 11 | 6 |
| Sayala, Sala, & Courtney, 2006 | 10 | Task > control | Faces | Identity/location verification | 22 | 14 | 12 |
| Sheridan, Hinshaw, & D'Esposito, 2007 | 10 | Task > baseline | Letters | Identity verification | 8 | 0 | 12 |
| Taylor et al., 2004 | 10 | Load‐related increase | Shapes | Identity verification | 12 | 16 | 7 |
Abbreviations: E, encoding; M, maintenance; R, retrieval.
2.3. Meta‐analysis
Three sets of meta‐analyses were performed. First was a series of single‐study meta‐analyses that separately evaluated each phase effect, namely, encoding, maintenance, and retrieval. The second was a series of pairwise subtraction analyses between different phase effects to evaluate the extent to which they involved distinct regions. The third was a series of pairwise conjunction analyses between different phase effects to evaluate the extent to which they involved similar regions. All analyses were conducted using the activation likelihood estimation (ALE) algorithm (Eickhoff et al., 2009; Turkeltaub et al., 2012) implemented in the software program GingerALE 3.0.2 (http://www.brainmap.org). ALE is a peak‐coordinate‐based meta‐analysis approach that can be used to estimate above‐chance activation convergence across independent studies. The ALE algorithm was applied to analyze the present data in the following steps (I have previously described similar methodological steps in Kim (2019) and other similar studies).
All activation coordinates reported in the Talairach space were converted to the MNI space using the GingerALE transformation algorithm. The reported foci for each study were modeled as the centers of 3‐dimensional (3D) Gaussian probability distributions, thereby accounting for the spatial uncertainty associated with each focus. The size of the full‐width at half maximum (FWHM) of the Gaussian kernel was not manually specified, but determined by an extended ALE algorithm which weighted, among other things, the individual sample size, to accommodate the fact that larger sample sizes provide more certain estimates of the true spatial locations. As an example, the meta‐analysis of the encoding‐phase data involved minimum, median, and maximum FWHM values of 8.73, 9.50, and 10.94 mm, respectively. The 3D Gaussian probability values were combined across all of the reported foci in a given study and then across all of the studies included in the meta‐analysis, thereby estimating the likelihood of activation at every voxel. These ALE values were tested against the null hypothesis of a uniform distribution of foci, using a cluster‐level, familywise error (FWE)‐corrected threshold of p < .05 with a cluster‐forming, voxel‐level threshold of p < .005.
A conjunction analysis between two ALE maps was performed based on the intersection between the two thresholded maps. Thus, a voxel determined to be significant in a conjunction analysis survived a cluster‐level, FWE‐corrected threshold of p < .05 with a cluster‐forming, voxel‐level threshold of p < .005 in both ALE analyses. A spatial extent threshold exceeding 500 mm3 was imposed to further constrain the possibility of false‐positive findings. To conduct a subtraction analysis between two ALE maps, all studies contributing to either map were combined and randomly split into two groups of the same size as the original two groups. Voxel‐wise ALE values for these two randomly formed groups were computed and subtracted from each other. Repeating this process 10,000 times created an expected distribution of ALE‐value differences under the null hypothesis. The observed differences in the ALE scores were tested against this null distribution, using a voxel‐wise threshold of p < .05. A correction for multiple comparisons was not applied to a subtraction analysis because (a) the present approach is largely hypothesis‐driven, (b) between‐phase differences were expected to be relatively subtle in the magnitude given that they referred to within‐trial effects, and (c) the possibility of false‐positive findings was further constrained by a spatial extent threshold exceeding 500 mm3.
To visualize the meta‐analysis results, the thresholded ALE maps were projected onto the inflated surface of a population‐average, and landmark‐ and surface‐based (PALS) atlas by using the Caret software suite (Van Essen, 2005). To visualize the subcortical structures not visible on the PALS atlas, the thresholded ALE maps were also imported into the Mango software program (http://ric.uthscsa.edu/mango) as an overlay on an International Consortium for Brain Mapping template. Yeo et al. (2011) parcellated the cerebral cortex region into seven intrinsic networks based on functional connectivity analyses of resting‐state data. Estimated boundaries of the DAN and FPCN in this study (Figure 1a) were also projected onto the PALS to evaluate whether convergence clusters were located inside or outside these networks. These boundaries were used as flexible guidelines rather than fixed templates, with the understanding that intrinsic network boundaries are dynamically organized rather than strictly fixed, as discussed in more detail below.
2.4. Conjunction analysis between WM‐phase maps and Yeo 7 networks
To further evaluate the present model, a conjunction analysis was performed between WM‐phase maps and Yeo et al.'s (2011) 7 networks (data available from https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011), using the Mango software program. For each WM‐phase map, this analysis indicated what percentage of it overlaps with the DAN, FPCN, and other networks. The advantages of Yeo 7‐network model, relative to other similar models of brain parcellation, are that it is built on a large dataset (n = 1,000), it involves an extensive range of validation efforts, including a split‐half replication, its topography corresponds closely to that of activation maps observed in subtraction imaging studies, and its usefulness as a frame of reference in interpreting task‐based activations has been repeatedly demonstrated (e.g., Benoit & Schacter, 2015; Fox, Spreng, Ellamil, Andrews‐Hanna, & Christoff, 2015; Kim, 2015, 2018; Rosen, Stern, Michalka, Devaney, & Somers, 2016). However, any resting state‐based connectivity models are at best an approximate framework for interpreting task‐based fMRI activations, because functional coupling across dispersed brain regions differs between rest and task, although overall network configuration remains preserved (Buckner, Krienen, & Yeo, 2013; Gonzalez‐Castillo & Bandettini, 2018; Krienen, Yeo, & Buckner, 2014). Thus, the breakdown of WM‐phase maps as a function of Yeo 7 networks was meant to aid in interpreting meta‐analysis results rather than to test the model in a rigorously quantitative way.
2.5. Neurosynth‐based decoding of WM‐phase maps
Neurosynth is a meta‐analytic platform comprising over 14,000 published fMRI studies and over 1,300 topic terms (http://neurosynth.org). A Neurosynth function called “decoding” can generate a rank‐ordered list of associated terms given an activation image in standard space, based on similarity coefficients (Pearson r) between the input image and a large number of meta‐analytic maps (Yarkoni et al., 2011). This function was used herein to decode WM‐phase maps, with the general expectation that some terms would be common to all phase maps, reflecting anatomical overlaps between them, whereas others would be more specific to each map, reflecting anatomical differentiation between them. This analysis was performed with the following restrictions. First, anatomical terms were excluded from a list to focus on image‐to‐function, as opposed to image‐to‐anatomy, decoding. Second, when a list had multiple terms having the same base, only the one with the highest‐ranking was included. For each decoding, “task‐tasks” was the only instance to which this rule was actually applied. Third, to strike a balance between sensitivity and specificity of decoding, only the top 10 strongest associates were examined in detail for each decoding. Finally, decoding of unthresholded and thresholded maps yielded highly comparable results, but similarity coefficients were generally greater for the former. Thus, only the results based on unthresholded maps are reported below.
3. RESULTS
3.1. Encoding‐, maintenance‐, and retrieval‐phase effects
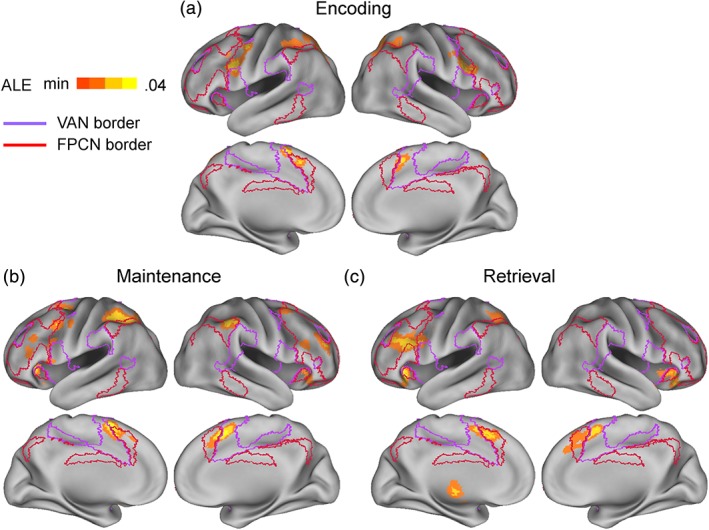
Table 2 shows the results of the separate ALE meta‐analyses of different phase effects. Figures 2 and 3 illustrate above‐threshold cortical and subcortical regions, respectively. Consistent with Hypothesis 1, the encoding phase involved many DAN regions, that is, bilateral IFJ and bilateral superior IPS/superior parietal lobe, but only one FPCN region, that is, left posteromedial PFC (pmPFC; see Figure 2a). Outside the DAN and FPCN, it mainly involved the bilateral supplementary motor area (SMA) and left striatum regions.
Table 2.
Results of separate ALE meta‐analyses of encoding‐, maintenance‐, and retrieval‐phase effects
| MNI | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | y | z | ALE | Region |
| Encoding | |||||
| 8,496 | −24 | −68 | 40 | 0.029 | Left superior IPS, SPL |
| 6,512 | −48 | −6 | 50 | 0.033 | Left IFJ, PCG |
| 6,176 | −4 | 2 | 62 | 0.039 | Left pmPFC, bilateral SMA |
| 5,808 | 16 | −68 | 56 | 0.024 | Right superior IPS, SPL |
| 4,864 | 48 | 6 | 28 | 0.030 | Right IFJ, PCG |
| 3,128 | −18 | 10 | 2 | 0.031 | Left striatum |
| Maintenance | |||||
| 15,560 | −4 | 4 | 58 | 0.055 | Bilateral pmPFC, SMA |
| −54 | −4 | 44 | 0.026 | Left frontal eye field, PCG | |
| 8,944 | −28 | −64 | 42 | 0.030 | Left superior/inferior IPS |
| 7,128 | −30 | 24 | 4 | 0.029 | Left anterior insula, FO |
| −20 | 10 | −6 | 0.028 | Left striatum | |
| 4,768 | 38 | 42 | 26 | 0.028 | Right mid‐lateral PFC |
| 3,600 | −54 | 8 | 28 | 0.021 | Left mid‐lateral PFC, IFJ |
| 3,208 | 46 | −44 | 46 | 0.032 | Right superior/inferior IPS |
| 2,920 | 36 | 26 | −6 | 0.027 | Right anterior insula, FO |
| 2,088 | 26 | 6 | 56 | 0.022 | Right dorsal caudal PFC |
| Retrieval | |||||
| 11,384 | −4 | 8 | 50 | 0.037 | Bilateral pmPFC, SMA |
| 8,256 | −48 | 12 | 28 | 0.033 | Left mid‐lateral PFC, IFJ |
| 6,976 | 34 | 24 | −2 | 0.047 | Right anterior insula, FO |
| 6,296 | −32 | 24 | −4 | 0.048 | Left anterior insula, FO |
| 2,528 | −30 | −56 | 38 | 0.018 | Left superior/inferior IPS |
| 2,024 | −12 | −16 | 4 | 0.028 | Left thalamus |
Abbreviations: FO, frontal operculum; IFJ, inferior frontal junction; IPS, intraparietal sulcus; PCG, precentral gyrus; pmPFC, posteromedial PFC; SMA, supplementary motor area; SPL, superior parietal lobe.
Figure 2.
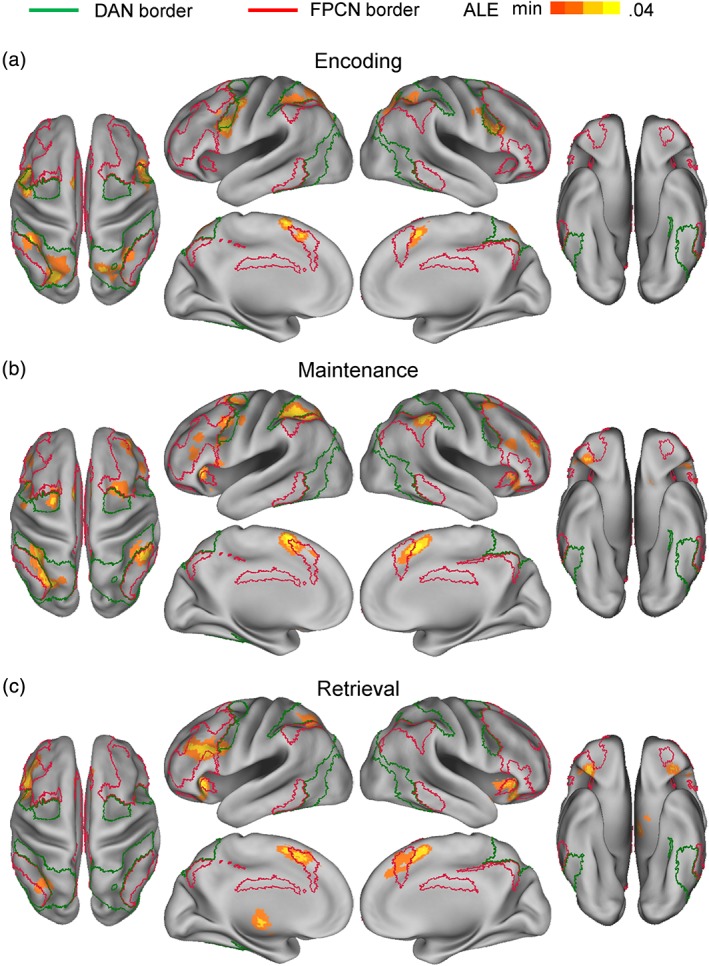
Above‐threshold regions in separate ALE meta‐analyses of (a) encoding‐, (b) maintenance‐, and (c) retrieval‐phase data. Green and crimson lines indicate estimated boundaries of the dorsal attention network (DAN) and the frontoparietal control network (FPCN) in Yeo et al.'s (2011) 7‐network model, respectively. NIFTI files available at https://identifiers.org/neurovault.collection:5506 [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
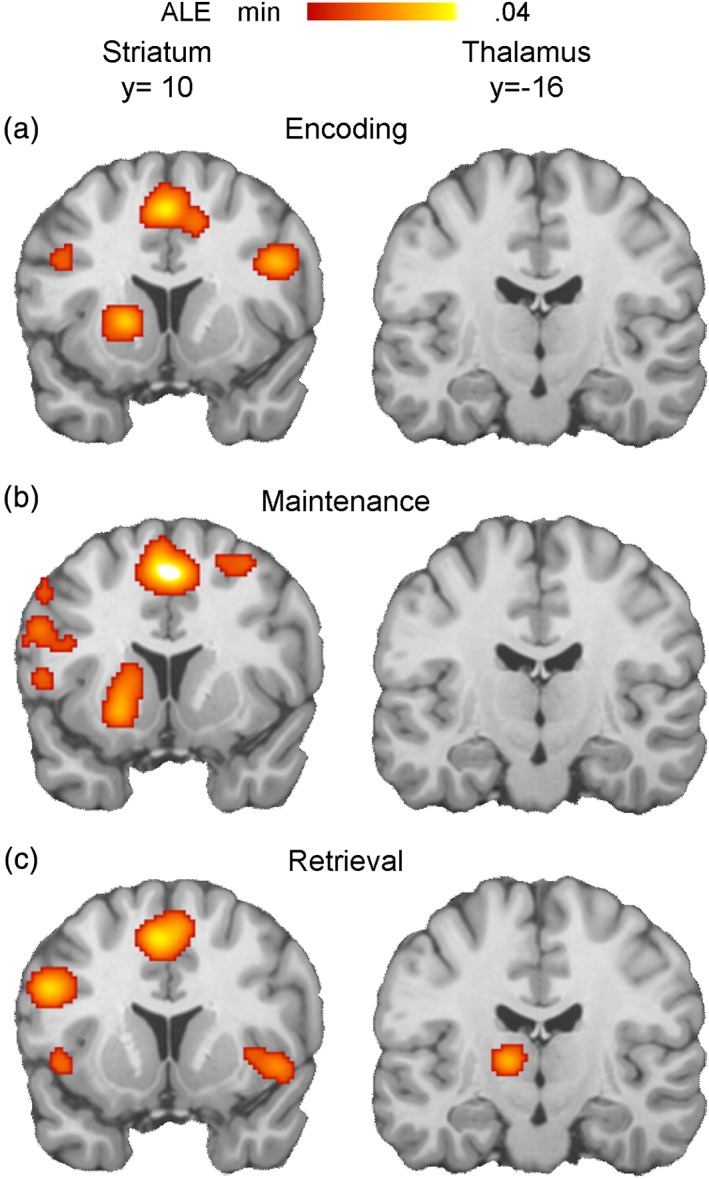
Above‐threshold subcortical regions in separate ALE meta‐analyses of (a) encoding‐, (b) maintenance‐, and (c) retrieval‐phase data [Color figure can be viewed at http://wileyonlinelibrary.com]
Consistent with Hypothesis 2, the maintenance phase involved many DAN regions, that is, left IFJ, left frontal eye field, and bilateral superior IPS, and also many FPCN regions, that is, bilateral mid‐lateral PFC, anterior insula, pmPFC, and inferior IPS (Figure 2b). Outside the DAN and FPCN, it mainly involved the bilateral SMA, bilateral frontal operculum, and left striatum regions.
Consistent with Hypothesis 3, the retrieval phase involved many FPCN regions, that is, left mid‐lateral PFC, bilateral anterior insula, bilateral pmPFC, and left inferior IPS, but only two DAN regions, that is, left IFJ and superior IPS (Figure 2c). Outside the DAN and FPCN, it mainly involved the bilateral SMA, bilateral frontal operculum, and left thalamus regions.
3.2. Pairwise subtraction analyses
Table 3 and Figure 4 show the results of pairwise subtraction analyses between different phase effects. A subtraction analysis between encoding and maintenance effects indicated a double dissociation consistent with Hypothesis 4. Specifically, preferential encoding effects indicated two DAN regions, that is, right IFJ and left superior IPS, but no FPCN region. In contrast, preferential maintenance effects indicated many FPCN regions, that is, right mid‐lateral PFC, left pmPFC, bilateral anterior insula, and bilateral inferior IPS, but no DAN region (Figure 4a). Outside the DAN and FPCN, preferential encoding effects involved no above‐threshold region, and preferential maintenance effects mainly involved the right frontal operculum area.
Table 3.
Results of pairwise subtraction analyses among encoding‐, maintenance‐, and retrieval‐phase effects
| MNI | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | y | z | Z | Region |
| Encoding > maintenance | |||||
| 1,800 | −24 | −76 | 34 | 3.72 | Left superior IPS |
| 1,752 | 45 | 5 | 24 | 3.35 | Right IFJ |
| Maintenance > encoding | |||||
| 1,288 | −28 | 24 | 8 | 2.77 | Left anterior insula, FO |
| 1,016 | −32 | −60 | 42 | 2.37 | Left inferior IPS |
| 976 | 32 | 28 | −8 | 2.40 | Right anterior insula, FO |
| 784 | 46 | 24 | 20 | 2.89 | Right mid‐lateral PFC |
| 632 | 52 | −44 | 48 | 2.42 | Right inferior IPS |
| 592 | −14 | 6 | 54 | 2.35 | Left pmPFC |
| Maintenance > retrieval | |||||
| 3,064 | −12 | 4 | 62 | 3.35 | Left SMA |
| −24 | −2 | 46 | 2.86 | Left frontal eye field | |
| 2,296 | −35 | −47 | 44 | 2.77 | Left superior IPS |
| 904 | −18 | 12 | −8 | 2.19 | Left striatum |
| 712 | −52 | −10 | 50 | 2.74 | Left PCG |
| 632 | 24 | 6 | 52 | 2.52 | Right dorsal caudal PFC |
| Retrieval > Maintenance | |||||
| 2,952 | 37 | 12 | −8 | 3.89 | Right anterior insula, FO |
| 2,040 | −30 | 23 | −8 | 3.89 | Left anterior insula, FO |
| 888 | −46 | 10 | 32 | 2.29 | Left mid‐lateral PFC |
| 768 | 2 | 28 | 28 | 2.95 | Right pmPFC |
| 704 | −8 | −30 | 0 | 3.29 | Left thalamus |
| 672 | 2 | 28 | 58 | 2.62 | Bilateral pmPFC |
| Encoding > retrieval | |||||
| 3,352 | −22 | −76 | 42 | 3.54 | Left superior IPS, SPL |
| 2,616 | 20 | −68 | 60 | 3.29 | Right superior IPS, SPL |
| 2,336 | 56 | 3 | 46 | 3.54 | Right IFJ, PCG |
| 2,032 | −51 | −8 | 52 | 3.89 | Left PCG |
| 856 | −18 | 6 | 8 | 2.26 | Left striatum |
| 856 | −8 | 2 | 66 | 2.49 | Left SMA |
| Retrieval > encoding | |||||
| 4,536 | 34 | 23 | −14 | 3.89 | Right anterior insula, FO |
| 3,840 | −37 | 21 | −10 | 2.99 | Left anterior insula, FO |
| 3,440 | −49 | 19 | 23 | 3.89 | Left mid‐lateral PFC |
| 1,488 | 0 | 32 | 26 | 3.72 | Bilateral pmPFC |
| 1,096 | 2 | 26 | 54 | 2.55 | Bilateral pmPFC |
| 672 | −14 | −10 | 4 | 2.54 | Left thalamus |
For abbreviations, see Table 2.
Figure 4.
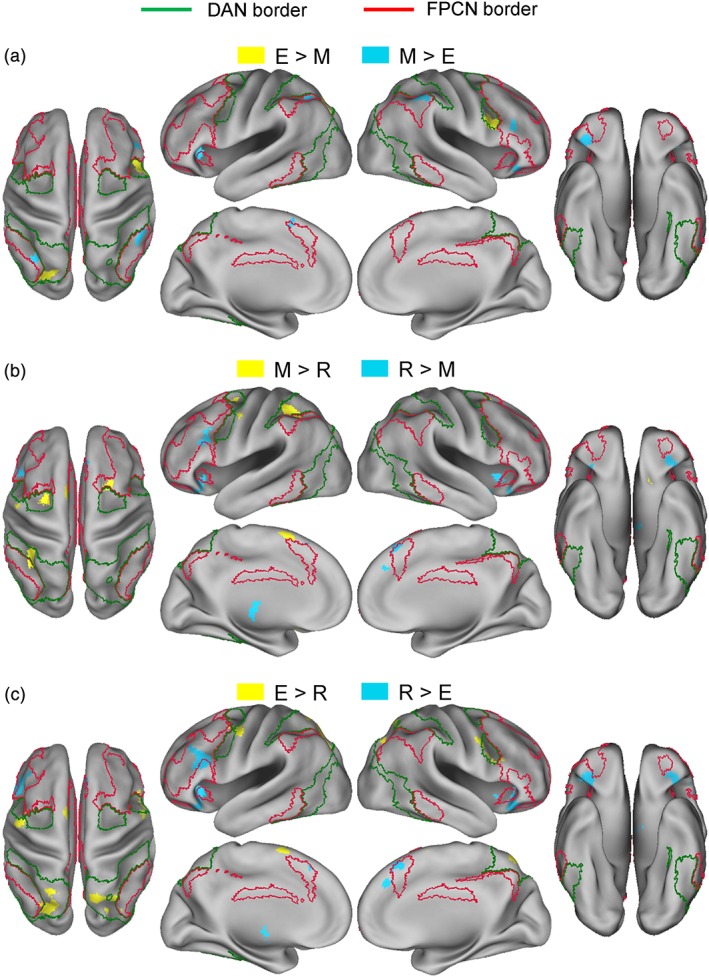
Above‐threshold regions in a pairwise subtraction analysis between encoding (E), maintenance (M), and retrieval (R) ALE results [Color figure can be viewed at http://wileyonlinelibrary.com]
A subtraction analysis between maintenance and retrieval effects indicated a double dissociation consistent with Hypothesis 5, with only one minor area of exception. Specifically, preferential maintenance effects involved two DAN regions, that is, left frontal eye field and superior IPS, and one minor FPCN region, that is, right dorsal caudal PFC. In contrast, preferential retrieval effects involved many FPCN regions, that is, left mid‐lateral PFC, bilateral pmPFC, and bilateral anterior insula (Figure 4b). Outside the DAN and FPCN, preferential maintenance effects mainly involved the left SMA and striatum regions, and preferential retrieval effects mainly involved the bilateral frontal operculum and left thalamic regions.
A subtraction analysis between encoding and retrieval effects indicated a double dissociation consistent with Hypothesis 6. Specifically, preferential encoding effects involved many DAN regions, that is, right IFJ and bilateral superior IPS/superior parietal lobe, but no FPCN region. In contrast, preferential retrieval effects involved many FPCN regions, that is, left mid‐lateral PFC, bilateral pmPFC, and bilateral anterior insula, but no DAN region (Figure 4c). Outside the DAN and FPCN, preferential encoding effects mainly involved the left SMA and striatum regions, and preferential retrieval effects mainly involved the bilateral frontal operculum and left thalamic regions.
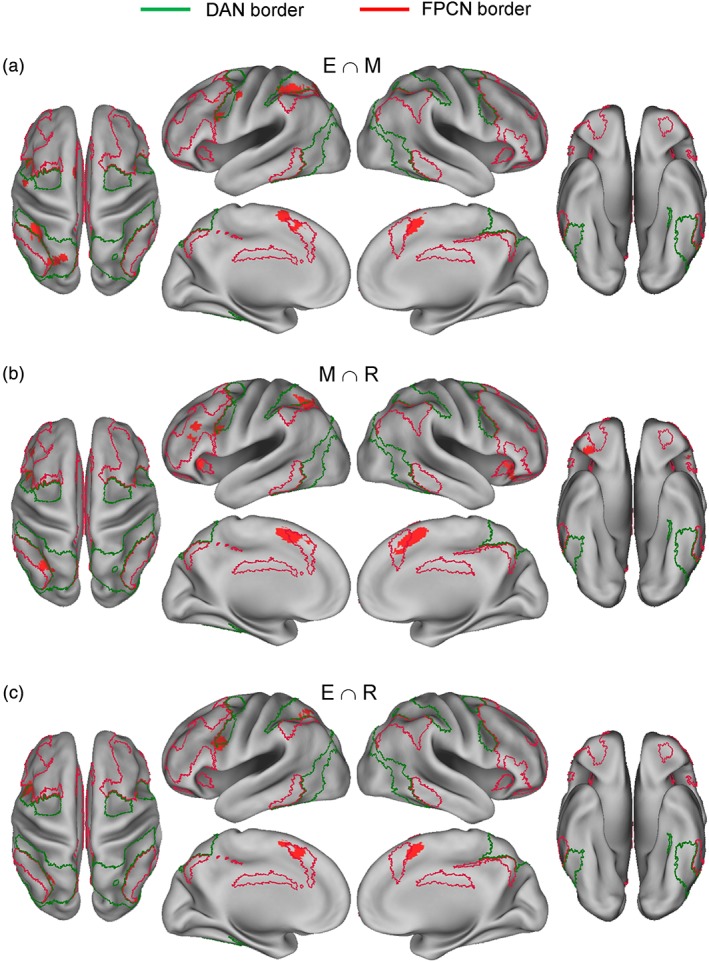
3.3. Pairwise conjunction analyses
Table 4 and Figure 5 show the results of pairwise conjunction analyses between different phase effects. Consistent with Hypothesis 7, encoding and maintenance effects commonly involved many DAN regions, that is, left IFJ and left superior IPS/superior parietal lobe, but only one FPCN region, that is, left pmPFC (Figure 5a). Outside the DAN and FPCN, they commonly involved the bilateral SMA and left striatum regions.
Table 4.
Results of pairwise conjunction analyses among encoding‐, maintenance‐, and retrieval‐phase effects
| MNI | |||||
|---|---|---|---|---|---|
| Volume (mm3) | x | y | z | ALE | Region |
| Encoding ∩ maintenance | |||||
| 4,880 | −4 | 2 | 62 | 0.039 | Left pmPFC, bilateral SMA |
| 3,000 | −26 | −66 | 42 | 0.023 | Left superior IPS |
| 1,816 | −18 | 12 | 0 | 0.024 | Left striatum |
| 1,616 | −54 | −2 | 46 | 0.025 | Left PCG |
| 616 | −52 | 6 | 26 | 0.016 | Left IFJ |
| Maintenance ∩ retrieval | |||||
| 6,704 | −4 | 8 | 50 | 0.037 | Bilateral pmPFC, SMA |
| 2,304 | −54 | 8 | 28 | 0.021 | Left mid‐lateral PFC, IFJ |
| 2,280 | 36 | 26 | −6 | 0.027 | Right anterior insula, FO |
| 1,704 | −30 | −56 | 38 | 0.018 | Left superior/inferior IPS |
| 1,536 | −30 | 24 | 4 | 0.029 | Left anterior insula, FO |
| 840 | −46 | 16 | 0 | 0.020 | Left anterior insula, FO |
| Encoding ∩ retrieval | |||||
| 4,000 | −2 | 10 | 50 | 0.036 | Left pmPFC, bilateral SMA |
| 1,800 | −48 | 6 | 28 | 0.026 | Left IFJ |
| 728 | −28 | −56 | 48 | 0.018 | Left superior IPS |
For abbreviations, see Table 2.
Figure 5.
Above‐threshold regions in a pairwise conjunction analysis between encoding (E), maintenance (M), and retrieval (R) ALE results [Color figure can be viewed at http://wileyonlinelibrary.com]
Consistent with Hypothesis 8, maintenance and retrieval effects commonly involved many FPCN regions, that is, left mid‐lateral PFC, bilateral anterior insula, bilateral pmPFC, and left inferior IPS, but only two DAN regions, that is, left IFJ and superior IPS (Figure 5b). Outside the DAN and FPCN, they commonly involved the bilateral SMA and right frontal operculum regions.
Consistent with Hypothesis 9, encoding and retrieval effects commonly involved only two DAN regions, that is, left IFJ and superior IPS, and only one FPCN region, that is, left pmPFC (Figure 5c). Outside the DAN and FPCN, they commonly involved the bilateral SMA region.
3.4. Association of WM‐phase maps with Yeo 7 networks
Table 5 shows a breakdown of WM‐phase maps as a function of Yeo 7 networks. Network‐composition of each map was generally consistent with the present model. First, consistent with Hypothesis 1, the encoding map overlapped more extensively with the DAN than the FPCN (52.7 vs. 13.7%). Second, consistent with Hypothesis 2, the maintenance map overlapped more equally with the DAN and FPCN (25.6 vs. 41.1%). Third, consistent with Hypothesis 3, the retrieval map overlapped more extensively with the FPCN than the DAN (40.6 vs. 8.6%). Fourth, consistent with the model, overlaps of the phase maps with the DAN progressively decreased from the encoding (52.7%) to maintenance (25.6%) and to retrieval (8.6%) stages. Fifth, also consistent with the model, overlaps with the FPCN increased from the encoding (13.7%) to maintenance (41.1%) stages. However, they did not increase from the maintenance (41.1%) to retrieval (40.6%) stages, providing a potentially important exception to the model. While retrieval involved more regions within the frontal components of the FPCN than maintenance, the reverse was true for the inferior IPS component of the FPCN (compare Figure 2b,c), producing relatively equal overlaps of the two maps with the whole FPCN.
Table 5.
Proportion (%) of encoding‐, maintenance‐, and retrieval‐phase above‐threshold regions as a function of Yeo 7 networks
| Network | Encoding | Maintenance | Retrieval |
|---|---|---|---|
| Dorsal attention | 52.7 | 25.6 | 8.6 |
| Frontoparietal control | 13.7 | 41.1 | 40.6 |
| Ventral attention | 20.0 | 23.3 | 33.7 |
| Default mode | 0.6 | 4.6 | 12.3 |
| Visual | 2.5 | 0.0 | 0.0 |
| Somatomotor | 10.4 | 5.4 | 3.9 |
| Limbic | 0.0 | 0.0 | 0.9 |
Aside from the DAN and FPCN, the ventral attention network (VAN) was the only network that showed substantial overlaps with each WM‐phase map. As Figure 6 illustrates, these within‐VAN effects were mostly in the SMA and frontal operculum regions and co‐extensive with adjacent FPCN effects involving the pmPFC and anterior insula. While these regions may act in concert with the VAN during a resting state, they may interact more closely with the FPCN than the VAN during the WM task state (and also likely during other cognitively intensive task states), as discussed in more detail below.
Figure 6.
Above‐threshold regions in separate ALE meta‐analyses of (a) encoding‐, (b) maintenance‐, and (c) retrieval‐phase data (the same data shown in Figure 2) are shown with estimated boundaries of the ventral attention network (VAN) and the frontoparietal control network (FPCN) in Yeo et al.'s (2011) 7‐network model [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. Image‐to‐text decoding of WM‐phase maps
Table 6 shows the results of the Neurosynth‐based decoding of WM‐phase maps. Three findings were notable. First, all phase maps were commonly associated with three very general, task‐related terms, that is, “tasks,” “goal,” and “demands,” consistent with the hypothesis that WM‐related regions serve multiple cognitive, not just WM, demands. Second, all phase maps were also commonly associated with three WM‐related terms, that is, “working memory,” “working,” and “load,” indicating the presence of a degree of similarity between Neurosynth‐based automatic versus current, annotated meta‐analysis of WM‐related studies. Finally, most of the other terms were specific to each stage. Specific terms for the encoding map included vision‐ and attention‐related terms such as “visual,” “attentional,” and “eye,” consistent with the hypothesis that this phase greatly demands external attention. Specific terms for the maintenance map included WM‐related terms such as “memory wm” and “maintenance” and also the term “phonological,” suggesting that this phase may typically recruit verbal rehearsal operation. Specific terms for the retrieval map included control demand‐related terms such as “difficulty,” “task difficulty,” and “verbal,” consistent with the hypothesis that this phase taxes cognitive control processes to a strong extent.
Table 6.
Neurosynth‐based image‐to‐text decoding of encoding‐, maintenance‐, and retrieval‐phase maps
| Encoding | Maintenance | Retrieval | |||
|---|---|---|---|---|---|
| Text | r | Text | r | Text | r |
| Tasks | .463 | Task | .559 | Goal | .527 |
| Goal | .445 | Working memory | .534 | Task | .504 |
| Working memory | .391 | Working | .530 | Demands | .400 |
| Working | .388 | Goal | .524 | Working memory | .392 |
| Visual | .326 | Load | .414 | Working | .388 |
| Load | .314 | Demands | .388 | Difficulty | .301 |
| Calculation | .288 | Memory wm | .362 | Load | .297 |
| Attentional | .278 | Maintenance | .327 | Fear | .258 |
| Demands | .273 | Calculation | .319 | Task difficulty | .256 |
| Eye | .267 | Phonological | .311 | Verbal | .240 |
3.6. Supplementary analysis
A criticism of a meta‐analysis is that it integrates studies with largely different experimental parameters, thereby producing no easily interpretable results. To address this potential limitation, I additionally performed a subgroup meta‐analysis, including only studies that required identity verification of verbal memoranda (n = 20) and using a relatively lenient statistical threshold (voxel‐level, p < .005, uncorrected; spatial extent, <1,000 mm3). Essential results of this analysis were very similar to those of the main analysis. As illustrated in Figure S1 available online, the encoding phase was more extensively associated with the DAN than the FPCN; the maintenance phase was more equally associated with the two networks; and the retrieval phase involved the FPCN to a greater extent than the DAN. Subcortical effects were limited largely to the striatum at encoding and maintenance, but to the thalamus at retrieval. Thus, essential results of the main analysis are unlikely to strongly reflect possible biasing effects linked to heterogeneity of included studies.
4. DISCUSSION
4.1. Contribution of the DAN and FPCN to the encoding phase
The encoding phase was associated extensively with the DAN, but only slightly with the FPCN. Specifically, within the DAN, it involved the bilateral IFJ and superior IPS/superior parietal lobe regions to a strong extent, but within the FPCN, only a small portion of the left pmPFC region. Because an encoding phase requires attention to sample stimuli, the involvement of the DAN in the phase adds to existing evidence that the network plays a strong role in external attention (Corbetta & Shulman, 2002; Fox et al., 2005; Sestieri et al., 2012). Despite its close association with the DAN, the encoding phase did not specifically involve its ventral temporal component. Given that this region is part of the “what” pathway, the absence of its involvement may partly reflect the fact that WM studies typically employed very simple and repetitive stimuli. Consistent with this hypothesis, previous studies that presented perceptually more complex stimuli, such as faces and scenes, indicated activation of the region (Jenness et al., 2018; Landau et al., 2004; Rämä & Courtney, 2005; Röder et al., 2011; Sayala et al., 2006). On a related note, studies on long‐term memory encoding consistently indicated involvement of the ventral temporal cortex, along with other DAN regions (for a meta‐analysis, see Kim, 2011). Thus, an important agenda for future studies is to determine whether activation of this visual region during long‐term, but not WM, encoding is due to intrinsic differences between memory domains or some extrinsic, procedural factors that have nothing to do with the memory domain per se.
The limited association of encoding with the FPCN suggests that transforming perceptual inputs into WM representations does not rely heavily on executive control functions. In this regard, the pattern is not necessarily consistent with the view that the central executive system controls the flow of information to its slave, storage systems (Baddeley, 2012), but fits more comfortably with the view that memory formation is a “by‐product” or direct consequence of attention focused on relevant information (Chun & Johnson, 2011). However, studies that used a parametric variation of memory load indicated corresponding increases in PFC activity at encoding and other phases (Bennett et al., 2013; Kochan et al., 2011; Landau et al., 2009; Ravizza et al., 2011; Röder et al., 2011), consistent with the hypothesis that the strength of the FPCN involvement scales with the amount and complexity of the memoranda. In this regard, the limited association of encoding with the FPCN is attributable partly to the use of perceptually impoverished stimuli in the majority of previous WM studies.
4.2. Contribution of the DAN and FPCN to the maintenance phase
The maintenance phase involved the DAN and FPCN to a more equal extent. Specifically, within the DAN, it involved the left IFJ, left frontal eye field, and bilateral superior IPS regions and, within the FPCN, the bilateral mid‐lateral PFC, anterior insula, pmPFC, and inferior IPS regions. The involvement of the DAN in the maintenance phase indicates that the network goes beyond subserving attention to external information but also plays a role in maintaining information from external sources when the source is no longer present. Previous studies indicated that orienting attention in, or selecting information from, WM involves many regions within the DAN (Ikkai & Curtis, 2011; Nee & Jonides, 2009; Nobre et al., 2004; Tamber‐Rosenau et al., 2011; for a meta‐analysis, see Wallis et al., 2015), which indirectly suggests a role of the network in WM maintenance. Like the encoding phase, the maintenance phase did not specifically involve the ventral temporal component of the DAN. Studies using multi‐voxel pattern analysis showed decoding of the maintained content in visual areas, even in V1, thereby providing evidence for a sensory recruitment hypothesis of WM (Ester, Serences, & Awh, 2009; Nelissen, Stokes, Nobre, & Rushworth, 2013; Riggall & Postle, 2012; for a review, see Lee & Baker, 2016). Thus, despite the lack of specific supporting evidence in subtraction‐based studies, the ventral temporal region may play some role in representing information in WM.
The involvement of the FPCN in the maintenance phase indicates that maintaining information in WM cannot be supported by the DAN alone, but typically requires an interplay between the DAN and the FPCN. In this regard, the previous literature provided evidence that the PFC plays a strong role in sustained attention to WM and the related function of protecting the information against external interference or task‐unrelated thoughts (Feredoes, Heinen, Weiskopf, Ruff, & Driver, 2011; Nee et al., 2012; Nichols, Kao, Verfaellie, & Gabrieli, 2006; Sakai et al., 2002a). Studies also indicated that the FPCN is anti‐correlated with the default mode network during the maintenance, but not encoding or retrieval, phase (Piccoli et al., 2015; Santangelo & Bordier, 2019), likely indicating its role in suppressing intrusive thoughts during the delay period (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Dixon et al., 2014). The FPCN may also be required because the DAN alone can only support the maintenance of a very limited amount of information. In this regard, Linden et al. (2003) showed that activity in the lateral PFC and medial frontal regions increased monotonically in response to memory load, even up to the maximum load condition in the study. In contrast, activity in the frontal eye field and IPS regions started to decline as memory load approached the behavioral capacity limit. Other studies (Todd & Marois, 2004; Xu & Chun, 2005) reported comparable dissociations between inferior IPS (FPCN) versus superior IPS (DAN) regions.
4.3. Contribution of the DAN and FPCN to the retrieval phase
The retrieval phase was associated extensively with the FPCN, but only slightly with the DAN. Specifically, within the FPCN, it involved the left mid‐lateral PFC, bilateral anterior insula, bilateral pmPFC, and left inferior IPS regions, but within the DAN, a small portion of left IFJ and superior IPS regions only. Because a retrieval phase almost certainly demands control functions to a great extent, such as memory scanning, matching operation involving the sample and probe stimuli, decision making, and response selection, the strong involvement of the FPCN in the phase is consistent with the hypothesis that the network embodies executive function mechanisms (Dosenbach et al., 2008; Niendam et al., 2012; Vincent et al., 2008). Despite its close association with the FPCN, the retrieval phase did not specifically involve its precuneus and mid‐cingulate cortex components. On a related note, previous studies showed that long‐term memory retrieval recruits these posteromedial regions to a great extent, along with other FPCN regions (for a meta‐analysis, see Kim, 2013). Thus, future studies are needed to determine whether recruitment of these regions during long‐term, but not WM, retrieval is due to intrinsic differences between memory domains, that is, retrieval of “offline” versus “online” information, or some extrinsic, procedural factors (for related discussion, see Gilmore, Nelson, & McDermott, 2015; Kim, 2018).
The limited involvement of the DAN in the retrieval phase likely reflects multiple factors. First, attention to a probe may be quickly replaced by more persistent attention to internal representations (of the probe and sample stimuli), although such transition likely occurs to some degree in parallel rather than in a strictly serial manner (Bledowski et al., 2006). As discussed earlier, such re‐focusing is necessary for efficient memory scanning, decision making, and other controlled processes that are based on internal representations. Second, when a probe is a repeat of a stimulus presented at encoding, repetition suppression effects constrain re‐activation of the DAN regions. Indeed, previous studies indicated that many regions within the DAN show repetition suppression to a great extent (for a meta‐analysis, see Kim, 2017). Finally, while the memoranda typically consist of multiple items, a probe is in most cases a single item, providing a basis to expect lesser demand on external attention during a retrieval than encoding phase.
4.4. Comparisons between different phase effects
The current model suggests that the transition from the encoding to maintenance and to retrieval stages involves progressively decreasing recruitment of the DAN, but progressively increasing recruitment of the FPCN. Distributions of regions specific to different phase effects were largely consistent with the model. A subtraction analysis of encoding and maintenance effects indicated that preferential encoding effects involved two DAN regions, that is, right IFJ and left superior IPS, but no FPCN region, whereas preferential maintenance effects involved many FPCN regions, that is, right mid‐lateral PFC, left pmPFC, bilateral anterior insula, and bilateral inferior IPS, but no DAN region. With some minor areas of exception, subtraction analysis between maintenance and retrieval effects and encoding and retrieval effects also indicated similar double dissociations involving the DAN and FPCN that are consistent with the model, although specific within‐network sites of dissociations were variable across analyses.
Distributions of regions common to different phase effects were also largely consistent with the model. A conjunction analysis indicates that encoding and maintenance effects commonly involved many DAN regions, that is, left IFJ and left superior IPS/superior parietal lobe, but only one FPCN region, that is, left pmPFC. In contrast, maintenance and retrieval effects commonly involved many FPCN regions, that is, left mid‐lateral PFC, bilateral anterior insula, bilateral pmPFC, and left inferior IPS, but only two DAN regions, that is, left IFJ and superior IPS. Only two DAN regions, that is, left IFJ and superior IPS, and one FPCN region, that is, left pmPFC, contributed to both encoding and retrieval phases and to all three phases for that matter. Previous studies showed that the left IFJ contributes to a wide variety of cognitive control tasks, including go/no‐go, stop signal, task‐switching, Stroop, Posner‐cueing, and oddball detection (for meta‐analyses, see Derrfuss, Brass, Neumann, & Von Cramon, 2005; Kim, 2014; Levy & Wagner, 2011; Owen et al., 2005), suggesting a more general, cross‐functional role than other DAN components. Noting that “the IFJ is located at the junction of three functional neuroanatomical domains, namely the premotor domain, the language domain, and the working memory domain” (p. 316), Brass, Derrfuss, Forstmann, and Cramon (2005) proposed that the region may play an important role in “task representations,” such as representation of stimulus–response mapping rules. Based on this hypothesis, the recruitment of this region in each WM phase may reflect the necessity of maintaining task rules “online” during the whole trial period.
A conjunction analysis between WM‐phase maps and Yeo 7 networks also produced results that were largely consistent with the model. Overlap of the phase maps with the DAN progressively decreased from the encoding to maintenance and to retrieval phase, whereas overlaps with the FPCN increased from the encoding to maintenance phase. However, in an apparent contradiction to the model, the maintenance‐ and retrieval‐phase maps did not appreciably differ in overlaps with the FPCN. While more regions within the frontal components of the FPCN were associated with retrieval than maintenance, the reverse was true for the inferior IPS component of the FPCN (Figure 2b,c), driving relatively equal overlaps with the whole FPCN. A direct, subtraction analysis between maintenance and retrieval effects indicated prominent differences only in the frontal, but not inferior IPS, components of the FPCN (Figure 4b), indicating that any difference involving the inferior IPS component is rather subtle in the magnitude. Taken together, these findings indicate that a greater association of the FPCN with retrieval than maintenance holds for the frontal, but not inferior IPS, components of the FPCN, adding to existing evidence that anterior versus posterior components of the FPCN support differential functions (Brass, Ullsperger, Knoesche, Cramon, & Phillips, 2005; Kim, 2018; Rosen et al., 2016).
A Neurosynth‐based decoding showed that WM‐phase maps had some commonalities as well as differences in top semantic associates. Common associates included very general task‐related terms such as “tasks,” “goal,” and “demands,” and also WM‐related terms such as “working memory,” “working,” and “load,” collectively suggesting that WM‐related regions serve multiple cognitive, not just WM, demands (Duncan, 2010; Postle, 2006). Specific associates for the encoding map included terms such as “visual,” “attentional,” and “eye” consistent with the hypothesis that WM encoding relies critically on external attention. Those for the maintenance map included terms such as “memory wm,” “maintenance,” and “phonological” suggesting that WM maintenance is typically associated with verbal rehearsal operation (Baddeley, 2012). Those for the retrieval map included terms such as “difficulty,” “task difficulty,” and “verbal” consistent with the hypothesis that WM retrieval demands cognitive control to an extensive extent. Taken together, these results provide valuable information regarding the similarity (and differences thereof) between WM and other cognitive‐domain activity maps.
4.5. Convergence clusters outside the DAN and FPCN
Two cortical clusters, one involving the SMA and the other the frontal operculum, were observed outside the DAN and FPCN. These two clusters were largely within the VAN, contributing to the relatively prominent overlaps of each WM‐phase map with the VAN. Two findings indicate that despite their association with the VAN during a resting state, these regions may interact more closely with the FPCN than the VAN during the WM task state. First, most of these effects were co‐extensive with adjacent FPCN effects involving the pmPFC and anterior insula. Second, practically no other VAN component, even the posterior core of the VAN known as the temporoparietal junction (Corbetta & Shulman, 2002; Fox et al., 2006; Kim, 2014), specifically contributed to any WM phase. According to Yeo et al. (2011, p. 1138), “the violet ventral attention network is likely an aggregate of (or closely adjacent to) multiple networks in the literature variably referred to as the salience (Seeley et al., 2007) and cingulo‐opercular networks (Dosenbach et al., 2007).” Based on this aggregation and relevant task‐based activation and functional connectivity findings (Dosenbach et al., 2007; Gonzalez‐Castillo & Bandettini, 2018; Krienen et al., 2014), the anterior insula and pmPFC components of the FPCN likely extend to adjacent frontal operculum and SMA regions during the WM task and also likely during other similar cognitively intensive states.
Two subcortical clusters, one involving the left striatum and the other involving the left thalamus, were observed. First, the left striatum region was associated with the encoding and maintenance phases, but little with the retrieval phase and subtraction analysis indicated a greater association with the encoding or maintenance than retrieval phase. A prominent hypothesis in the WM literature suggests that the striatum subserves gating access to WM or filtering irrelevant information from WM (Geiger et al., 2018; McNab & Klingberg, 2007). The present pattern of results is broadly consistent with this hypothesis in that demand on such gating or filtering function is likely greater during the encoding or maintenance than retrieval phase. Second, the left thalamic region was associated with the retrieval, but not encoding or maintenance, phase. A subtraction analysis indicated a greater association of this region with the retrieval than encoding or maintenance phase, suggesting that it may play an important role in retrieval‐related control operations, likely in association with the FPCN. Consistent with this hypothesis, previous functional connectivity studies provided evidence that part of the thalamus is a subcortical module closely associated with the FPCN, perhaps contributing to rapid coordination of control‐related signals across the cortex (Beckmann, DeLuca, Devlin, & Smith, 2005; Dosenbach et al., 2007; Halassa & Kastner, 2017).
4.6. Limitations and conclusions
This study has several limitations, including relatively simplified modeling and hypotheses and the drawbacks of coordinate‐based meta‐analysis. A more specific limitation is that while the networks provided by Yeo et al.'s (2011) study refer to the “resting state,” the current dataset was obtained during the WM task state. Given that functional coupling across dispersed brain regions differs to some degree between task and rest, despite overall correspondence in network configuration (Buckner et al., 2013; Krienen et al., 2014), Yeo et al.'s 7‐network model can only provide an approximate, but not exact, framework for interpreting the current results. Another limitation is that some results may be related more to the specifics of widely employed procedures, such as the use of perceptually simple stimuli and the requirement of decision making during a retrieval, but not other, phase, than to the intrinsic properties of WM.
Evidence from electroencephalography (EEG) and magnetoencephalography (MEG) studies show that the nature of the information representation in a brain region can dynamically change in the order of milliseconds, posing an inferential challenge for studies using low temporal resolution measures such as fMRI (Ghuman & Martin, 2019). Using a Sternberg‐type WM task and complex source reconstruction algorithm, an MEG study (Heinrichs‐Graham & Wilson, 2015) indicated that occipital areas showed alpha/beta desynchronizations beginning immediately after the onset of encoding stimuli, which became gradually weaker as a function of time during encoding and eventually evolved into a strong synchronization during the late maintenance period. In contrast, similar desynchronizations in the left dorsolateral PFC and superior temporal areas became progressively stronger as a function of time during encoding and were sustained during most of the maintenance period until being sharply dispelled just before retrieval. This and other related EEG/MEG findings (e.g., Brookes et al., 2011; Palva, Kulashekhar, Hämäläinen, & Palva, 2011; Pinal, Zurrón, & Díaz, 2014) indicate that WM‐related activity observed in fMRI studies is mix of dynamic changes on the level of milliseconds, warranting its cautious interpretation. A multi‐modal approach that combines fMRI and EEG/MEG has great potential to enhance our understanding of within‐phase activity changes during WM tasks.
Within the limitations described above, the present study reveals the dynamic unfolding of intrinsic network participation in WM temporal subprocesses. In particular, the proposed model essentially suggests that the transition from the encoding to maintenance and to retrieval stages involves progressively decreasing involvement of the DAN, but progressively increasing involvement of the FPCN. Separate meta‐analysis of each phase effect and direct comparisons between them yielded results that were largely consistent with the model. Pairwise contrasts between different phase effects indicated double dissociations involving the DAN and FPCN, thereby providing the strongest supporting evidence for the model. Two closely juxtaposed regions that are members of the DAN and FPCN, such as IFJ versus caudal PFC and superior versus inferior IPS, showed a high degree of functional differentiation. In conclusion, although all regions identified in the present study were already identified in previous WM studies, this study uniquely enhances our understating of their roles by clarifying their network membership and specific associations with different WM phases.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
Supporting information
Figure S1 Above‐threshold regions in separate ALE meta‐analyses of (a) encoding‐, (b) maintenance‐, and (c) retrieval‐phase data, including only studies that used identity verification of vernal memoranda and using a voxel‐level threshold of p < .005 (uncorrected) and spatial extent threshold exceeding 1,000 mm3. Green and crimson lines indicate estimated boundaries of the dorsal attention network (DAN) and the frontoparietal control network (FPCN) in Yeo et al.'s (2011) 7‐network model, respectively.
ACKNOWLEDGMENTS
This research was supported by a Daegu University Research Grant, 2018.
Kim H. Neural activity during working memory encoding, maintenance, and retrieval: A network‐based model and meta‐analysis. Hum Brain Mapp. 2019;40:4912–4933. 10.1002/hbm.24747
Funding information Daegu University Research Grant, 2018
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in NeuroVault at https://identifiers.org/neurovault.collection:5506.
REFERENCES
- Awh, E. , Jonides, J. , Smith, E. E. , Buxton, R. B. , Frank, L. R. , Love, T. , … Gmeindl, L. (1999). Rehearsal in spatial working memory: Evidence from neuroimaging. Psychological Science, 10(5), 433–437. 10.1111/1467-9280.00182 [DOI] [Google Scholar]
- Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63(1), 1–29. 10.1146/annurev-psych-120710-100422 [DOI] [PubMed] [Google Scholar]
- Beatty, E. L. , Jobidon, M. E. , Bouak, F. , Nakashima, A. , Smith, I. , Lam, Q. , … Vartanian, O. (2015). Transfer of training from one working memory task to another: Behavioural and neural evidence. Frontiers in Systems Neuroscience, 9, 86 10.3389/fnsys.2015.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, C. , DeLuca, M. , Devlin, J. , & Smith, S. (2005). Investigations into resting‐state connectivity using independent component analysis. Philosophical Transactions of the Royal Society, B: Biological Sciences, 360(1457), 1001–1013. 10.1098/rstb.2005.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell, J. S. , Horner, M. D. , Yamanaka, K. , Li, X. , Myrick, H. , Nahas, Z. , & George, M. S. (2005). Functional neuroanatomy of subcomponent cognitive processes involved in verbal working memory. International Journal of Neuroscience, 115(7), 1017–1032. 10.1080/00207450590901530 [DOI] [PubMed] [Google Scholar]
- Bennett, I. J. , Rivera, H. G. , & Rypma, B. (2013). Isolating age‐group differences in working memory load‐related neural activity: Assessing the contribution of working memory capacity using a partial‐trial fMRI method. NeuroImage, 72, 20–32. 10.1016/j.neuroimage.2013.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit, R. G. , & Schacter, D. L. (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. 10.1016/j.neuropsychologia.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, H. C. , Daselaar, S. M. , Fernandez, G. , & Kessels, R. P. (2016). Neural substrates of successful working memory and long‐term memory formation in a relational spatial memory task. Cognitive Processing, 17(4), 377–387. 10.1007/s10339-016-0772-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski, C. , Cohen Kadosh, K. , Wibral, M. , Rahm, B. , Bittner, R. A. , Hoechstetter, K. , … Linden, D. E. (2006). Mental chronometry of working memory retrieval: A combined functional magnetic resonance imaging and event‐related potentials approach. The Journal of Neuroscience, 26(3), 821–829. 10.1523/JNEUROSCI.3542-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde, A. L. , Karmann, M. , Born, C. , Teipel, S. J. , Omerovic, M. , Ewers, M. , … Hampel, H. (2010). Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. Journal of Alzheimer's Disease, 21(1), 103–118. 10.3233/jad-2010-091054 [DOI] [PubMed] [Google Scholar]
- Boly, M. , Balteau, E. , Schnakers, C. , Degueldre, C. , Moonen, G. , Luxen, A. , … Laureys, S. (2007). Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(29), 12187–12192. 10.1073/pnas.0611404104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass, M. , Derrfuss, J. , Forstmann, B. , & Cramon, D. (2005). The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences, 9(7), 314–316. 10.1016/j.tics.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Brass, M. , Ullsperger, M. , Knoesche, T. , Cramon, D. , & Phillips, N. (2005). Who comes first? The role of the prefrontal and parietal cortex in cognitive control. Journal of Cognitive Neuroscience, 17(9), 1367–1375. 10.1162/0898929054985400 [DOI] [PubMed] [Google Scholar]
- Brookes, M. J. , Wood, J. R. , Stevenson, C. M. , Zumer, J. M. , White, T. P. , Liddle, P. F. , & Morris, P. G. (2011). Changes in brain network activity during working memory tasks: A magnetoencephalography study. NeuroImage, 55(4), 1804–1815. 10.1016/j.neuroimage.2010.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , & Yeo, B. T. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience, 16, 832–837. [DOI] [PubMed] [Google Scholar]
- Cairo, T. A. , Liddle, P. F. , Woodward, T. S. , & Ngan, E. T. (2004). The influence of working memory load on phase specific patterns of cortical activity. Cognitive Brain Research, 21(3), 377–387. 10.1016/j.cogbrainres.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Chang, C. , Crottaz‐Herbette, S. , & Menon, V. (2007). Temporal dynamics of basal ganglia response and connectivity during verbal working memory. NeuroImage, 34(3), 1253–1269. 10.1016/j.neuroimage.2006.08.056 [DOI] [PubMed] [Google Scholar]
- Chein, J. M. , & Fiez, J. A. (2001). Dissociation of verbal working memory system components using a delayed serial recall task. Cerebral Cortex, 11(11), 1003–1014. 10.1093/cercor/11.11.1003 [DOI] [PubMed] [Google Scholar]
- Chen, S. H. , & Desmond, J. E. (2005). Temporal dynamics of cerebro‐cerebellar network recruitment during a cognitive task. Neuropsychologia, 43(9), 1227–1237. 10.1016/j.neuropsychologia.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. , Smallwood, J. , Smith, R. , & Schooler, J. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, M. M. (2011). Visual working memory as visual attention sustained internally over time. Neuropsychologia, 49(6), 1407–1409. 10.1016/j.neuropsychologia.2011.01.029 [DOI] [PubMed] [Google Scholar]
- Chun, M. M. , & Johnson, M. K. (2011). Memory: Enduring traces of perceptual and reflective attention. Neuron, 72(4), 520–535. 10.1016/j.neuron.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , Kincade, J. M. , & Shulman, G. L. (2002). Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience, 14(3), 508–523. 10.1162/089892902317362029 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Curtis, C. E. , Rao, V. Y. , & D'Esposito, M. (2004). Maintenance of spatial and motor codes during oculomotor delayed response tasks. The Journal of Neuroscience, 24(16), 3944–3952. 10.1523/JNEUROSCI.5640-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenbach, D. (2019). Insights into cognition from network science analyses of human brain functional connectivity: Working memory as a test case In Munsell B. C., Wu G., Bonilha L., & Laurienti P. J. (Eds.), Connectomics: Applications to neuroimaging (pp. 27–41). Cambridge, MA: Acadmic Press; 10.1016/B978-0-12-813838-0.00002-9 [DOI] [Google Scholar]
- Daniel, T. A. , Katz, J. S. , & Robinson, J. L. (2016). Delayed match‐to‐sample in working memory: A BrainMap meta‐analysis. Biological Psychology, 120, 10–20. 10.1016/j.biopsycho.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw, M. , Kahn, R. S. , Zandbelt, B. B. , Widschwendter, C. G. , & Vink, M. (2013). Working memory and default mode network abnormalities in unaffected siblings of schizophrenia patients. Schizophrenia Research, 150(2–3), 555–562. 10.1016/j.schres.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Derrfuss, J. , Brass, M. , Neumann, J. , & Von Cramon, D. (2005). Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Human Brain Mapping, 25(1), 22–34. 10.1002/hbm.20127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito, M. , & Postle, B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66(1), 115–142. 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , Fox, K. C. , & Christoff, K. (2014). A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia, 62, 321–330. 10.1016/j.neuropsychologia.2014.05.024 [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. , Fair, D. , Cohen, A. , Schlaggar, B. , & Petersen, S. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. , Fair, D. , Miezin, F. , Cohen, A. , Wenger, K. , Dosenbach, R. , … Raichle, M. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, J. (2010). The multiple‐demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Yeo, B. T. T. , & Genon, S. (2018). Imaging‐based parcellations of the human brain. Nature Reviews Neuroscience, 19(11), 672–686. 10.1038/s41583-018-0071-7 [DOI] [PubMed] [Google Scholar]
- Emrich, S. M. , Riggall, A. C. , LaRocque, J. J. , & Postle, B. R. (2013). Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short‐term memory. The Journal of Neuroscience, 33(15), 6516–6523. 10.1523/JNEUROSCI.5732-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, J. , Vogel, E. K. , Lansner, A. , Bergström, F. , & Nyberg, L. (2015). Neurocognitive architecture of working memory. Neuron, 88(1), 33–46. 10.1016/j.neuron.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester, E. F. , Serences, J. T. , & Awh, E. (2009). Spatially global representations in human primary visual cortex during working memory maintenance. The Journal of Neuroscience, 29(48), 15258–15265. 10.1523/JNEUROSCI.4388-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes, E. , Heinen, K. , Weiskopf, N. , Ruff, C. , & Driver, J. (2011). Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proceedings of the National Academy of Sciences of the United States of America, 108(42), 17510–17515. 10.1073/pnas.1106439108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach, C. J. , Friederici, A. D. , Smith, E. E. , & Swinney, D. (2007). Lateral inferotemporal cortex maintains conceptual‐semantic representations in verbal working memory. Journal of Cognitive Neuroscience, 19(12), 2035–2049. 10.1162/jocn.2007.19.12.2035 [DOI] [PubMed] [Google Scholar]
- Fiehler, K. , Bannert, M. M. , Bischoff, M. , Blecker, C. , Stark, R. , Vaitl, D. , … Rosler, F. (2011). Working memory maintenance of grasp‐target information in the human posterior parietal cortex. NeuroImage, 54(3), 2401–2411. 10.1016/j.neuroimage.2010.09.080 [DOI] [PubMed] [Google Scholar]
- Fox, K. C. R. , Spreng, R. N. , Ellamil, M. , Andrews‐Hanna, J. R. , & Christoff, K. (2015). The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Fox, M. , Corbetta, M. , Snyder, A. , Vincent, J. , & Raichle, M. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–10051. 10.1073/pnas.0604187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. , Snyder, A. , Vincent, J. , Corbetta, M. , Van Essen, D. , & Raichle, M. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley, A. (2011). Influence of early attentional modulation on working memory. Neuropsychologia, 49(6), 1410–1424. 10.1016/j.neuropsychologia.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, L. S. , Moessnang, C. , Schäfer, A. , Zang, Z. , Zangl, M. , Cao, H. , … Tost, H. (2018). Novelty modulates human striatal activation and prefrontal–striatal effective connectivity during working memory encoding. Brain Structure and Function, 223(7), 3121–3132. 10.1007/s00429-018-1679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuman, A. S. , & Martin, A. (2019). Dynamic neural representations: An inferential challenge for fMRI. Trends in Cognitive Sciences, 23(7), 534–536. 10.1016/j.tics.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, S. E. , & D'Esposito, M. (2005a). A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology, 180(4), 644–653. 10.1007/s00213-005-0077-5 [DOI] [PubMed] [Google Scholar]
- Gibbs, S. E. , & D'Esposito, M. (2005b). Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cognitive, Affective, & Behavioral Neuroscience, 5(2), 212–221. 10.3758/CABN.5.2.212 [DOI] [PubMed] [Google Scholar]
- Gibbs, S. E. , & D'Esposito, M. (2006). A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience, 139(1), 359–371. 10.1016/j.neuroscience.2005.11.055 [DOI] [PubMed] [Google Scholar]
- Gilmore, A. W. , Nelson, S. M. , & McDermott, K. B. (2015). A parietal memory network revealed by multiple MRI methods. Trends in Cognitive Sciences, 19(9), 534–543. 10.1016/j.tics.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Castillo, J. , & Bandettini, P. A. (2018). Task‐based dynamic functional connectivity: Recent findings and open questions. NeuroImage, 180, 526–533. 10.1016/j.neuroimage.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, E. M. , Laumann, T. O. , Gilmore, A. W. , Newbold, D. J. , Greene, D. J. , Berg, J. J. , … Dosenbach, N. U. F. (2017). Precision functional mapping of individual human brains. Neuron, 95(4), 791–807. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector, K. , Henson, R. , & Martin, A. (2006). Repetition and the brain: Neural models of stimulus‐specific effects. Trends in Cognitive Sciences, 10(1), 14–23. 10.1016/j.tics.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Grot, S. , Legare, V. P. , Lipp, O. , Soulieres, I. , Dolcos, F. , & Luck, D. (2017). Abnormal prefrontal and parietal activity linked to deficient active binding in working memory in schizophrenia. Schizophrenia Research, 188, 68–74. 10.1016/j.schres.2017.01.021 [DOI] [PubMed] [Google Scholar]
- Habeck, C. , Rakitin, B. C. , Moeller, J. , Scarmeas, N. , Zarahn, E. , Brown, T. , & Stern, Y. (2005). An event‐related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed‐match‐to‐sample task. Cognitive Brain Research, 23(2–3), 207–220. 10.1016/j.cogbrainres.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Halassa, M. , & Kastner, S. (2017). Thalamic functions in distributed cognitive control. Nature Neuroscience, 20(12), 1669–1679. 10.1038/s41593-017-0020-1 [DOI] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2015). Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex, 69, 121–130. 10.1016/j.cortex.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A. C. , Laird, A. R. , & Robinson, J. L. (2014). Gender differences in working memory networks: A BrainMap meta‐analysis. Biological Psychology, 102, 18–29. 10.1016/j.biopsycho.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers, W. , Pennartz, C. M. , Cabeza, R. , & Daselaar, S. M. (2009). When learning and remembering compete: A functional MRI study. PLoS Biology, 7(1), e1000011 10.1371/journal.pbio.1000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikkai, A. , & Curtis, C. E. (2011). Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia, 49(6), 1428–1434. 10.1016/j.neuropsychologia.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness, J. L. , Rosen, M. L. , Sambrook, K. A. , Dennison, M. J. , Lambert, H. K. , Sheridan, M. A. , & McLaughlin, K. A. (2018). Violence exposure and neural systems underlying working memory for emotional stimuli in youth. Development and Psychopathology, 30(4), 1517–1528. 10.1017/S0954579417001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides, J. , Lewis, R. L. , Nee, D. E. , Lustig, C. A. , Berman, M. G. , & Moore, K. S. (2008). The mind and brain of short‐term memory. Annual Review of Psychology, 59(1), 193–224. 10.1146/annurev.psych.59.103006.093615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt, K. H. , Shirinyan, D. , van Erp, T. G. , Cohen, M. S. , & Cannon, T. D. (2005). Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. NeuroImage, 25(4), 1224–1231. 10.1016/j.neuroimage.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Kim, H. (2011). Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. NeuroImage, 54(3), 2446–2461. 10.1016/j.neuroimage.2010.09.045 [DOI] [PubMed] [Google Scholar]
- Kim, H. (2013). Differential neural activity in the recognition of old versus new events: An activation likelihood estimation meta‐analysis. Human Brain Mapping, 34(4), 814–836. 10.1002/hbm.21474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. (2014). Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta‐analysis. Human Brain Mapping, 35(5), 2265–2284. 10.1002/hbm.22326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. (2015). Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: The HERNET model. Hippocampus, 25(4), 500–510. 10.1002/hipo.22387 [DOI] [PubMed] [Google Scholar]
- Kim, H. (2017). Brain regions that show repetition suppression and enhancement: A meta‐analysis of 137 neuroimaging experiments. Human Brain Mapping, 38(4), 1894–1913. 10.1002/hbm.23492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. (2018). Parietal control network activation during memory tasks may be associated with the co‐occurrence of externally and internally directed cognition: A cross‐function meta‐analysis. Brain Research, 1683, 55–66. 10.1016/j.brainres.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Kim, H. (2019). Neural correlates of explicit and implicit memory at encoding and retrieval: A unified framework and meta‐analysis of functional neuroimaging studies. Biological Psychology, 145, 96–111. 10.1016/j.biopsycho.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Klaassen, E. B. , de Groot, R. H. , Evers, E. A. , Snel, J. , Veerman, E. C. , Ligtenberg, A. J. , … Veltman, D. J. (2013). The effect of caffeine on working memory load‐related brain activation in middle‐aged males. Neuropharmacology, 64, 160–167. 10.1016/j.neuropharm.2012.06.026 [DOI] [PubMed] [Google Scholar]
- Kochan, N. A. , Valenzuela, M. , Slavin, M. J. , McCraw, S. , Sachdev, P. S. , & Breakspear, M. (2011). Impact of load‐related neural processes on feature binding in visuospatial working memory. PLoS One, 6(8), e23960 10.1371/journal.pone.0023960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, H. M. , Nomura, M. , & Kashino, M. (2015). Different roles of COMT and HTR2A genotypes in working memory subprocesses. PLoS One, 10(5), e0126511 10.1371/journal.pone.0126511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen, F. M. , Yeo, B. T. , & Buckner, R. L. (2014). Reconfigurable task‐dependent functional coupling modes cluster around a core functional architecture. Philosophical Transactions of the Royal Society, B: Biological Sciences, 369(1653), 20130526 10.1098/rstb.2013.0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos, J. , Ivanovski, B. , & Malhi, G. S. (2007). An event‐related functional MRI study of working memory in euthymic bipolar disorder. Journal of Psychiatry and Neuroscience, 32(3), 174–184. [PMC free article] [PubMed] [Google Scholar]
- Landau, S. M. , Lal, R. , O'Neil, J. P. , Baker, S. , & Jagust, W. J. (2009). Striatal dopamine and working memory. Cerebral Cortex, 19(2), 445–454. 10.1093/cercor/bhn095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau, S. M. , Schumacher, E. H. , Garavan, H. , Druzgal, T. J. , & D'Esposito, M. (2004). A functional MRI study of the influence of practice on component processes of working memory. NeuroImage, 22(1), 211–221. 10.1016/j.neuroimage.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , & Baker, C. I. (2016). Multi‐voxel decoding and the topography of maintained information during visual working memory. Frontiers in Systems Neuroscience, 10, 2 10.3389/fnsys.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, B. J. , & Wagner, A. D. (2011). Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224(1), 40–62. 10.1111/j.1749-6632.2011.05958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, D. E. J. , Bittner, R. A. , Muckli, L. , Waltz, J. A. , Kriegeskorte, N. , Goebel, R. , … Munk, M. H. J. (2003). Cortical capacity constraints for visual working memory: Dissociation of fMRI load effects in a fronto‐parietal network. NeuroImage, 20(3), 1518–1530. 10.1016/j.neuroimage.2003.07.021 [DOI] [PubMed] [Google Scholar]
- Luck, D. , Danion, J. M. , Marrer, C. , Pham, B. T. , Gounot, D. , & Foucher, J. (2010). The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain and Cognition, 72(2), 255–263. 10.1016/j.bandc.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Salmon, E. , & Attout, L. (2013). The importance of encoding‐related neural dynamics in the prediction of inter‐individual differences in verbal working memory performance. PLoS One, 8(7), e69278 10.1371/journal.pone.0069278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach, D. S. , Greve, D. N. , Lindgren, K. A. , & Dale, A. M. (2003). Identifying regional activity associated with temporally separated components of working memory using event‐related functional MRI. NeuroImage, 20(3), 1670–1684. 10.1016/j.neuroimage.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Marvel, C. L. , & Desmond, J. E. (2010). The contributions of cerebro‐cerebellar circuitry to executive verbal working memory. Cortex, 46(7), 880–895. 10.1016/j.cortex.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab, F. , & Klingberg, T. (2007). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103–107. 10.1038/nn2024 [DOI] [PubMed] [Google Scholar]
- Mohr, H. M. , Goebel, R. , & Linden, D. E. (2006). Content‐ and task‐specific dissociations of frontal activity during maintenance and manipulation in visual working memory. The Journal of Neuroscience, 26(17), 4465–4471. 10.1523/JNEUROSCI.5232-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty, V. P. , Sambataro, F. , Radulescu, E. , Altamura, M. , Iudicello, J. , Zoltick, B. , … Mattay, V. S. (2011). Selective updating of working memory content modulates meso‐cortico‐striatal activity. NeuroImage, 57(3), 1264–1272. 10.1016/j.neuroimage.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, N. S. , Prabhakaran, V. , Bunge, S. A. , Christoff, K. , Fine, E. M. , & Gabrieli, J. D. (2005). The role of the prefrontal cortex in the maintenance of verbal working memory: An event‐related FMRI analysis. Neuropsychology, 19(2), 223–232. 10.1037/0894-4105.19.2.223 [DOI] [PubMed] [Google Scholar]
- Nee, D. E. , Brown, J. W. , Askren, M. K. , Berman, M. G. , Demiralp, E. , Krawitz, A. , & Jonides, J. (2012). A meta‐analysis of executive components of working memory. Cerebral Cortex, 23(2), 264–282. 10.1093/cercor/bhs007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee, D. E. , & Jonides, J. (2009). Common and distinct neural correlates of perceptual and memorial selection. NeuroImage, 45(3), 963–975. 10.1016/j.neuroimage.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen, N. , Stokes, M. , Nobre, A. C. , & Rushworth, M. F. S. (2013). Frontal and parietal cortical interactions with distributed visual representations during selective attention and action selection. The Journal of Neuroscience, 33(42), 16443–16458. 10.1523/JNEUROSCI.2625-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, E. A. , Kao, Y. C. , Verfaellie, M. , & Gabrieli, J. D. (2006). Working memory and long‐term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus, 16(7), 604–616. 10.1002/hipo.20190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam, T. A. , Laird, A. R. , Ray, K. L. , Dean, Y. M. , Glahn, D. C. , & Carter, C. S. (2012). Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre, A. C. , Coull, J. T. , Maquet, P. , Frith, C. D. , Vandenberghe, R. , & Mesulam, M. M. (2004). Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience, 16(3), 363–373. 10.1162/089892904322926700 [DOI] [PubMed] [Google Scholar]
- Owen, A. , McMillan, K. , Laird, A. , & Bullmore, E. (2005). N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva, S. , Kulashekhar, S. , Hämäläinen, M. , & Palva, J. M. (2011). Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. The Journal of Neuroscience, 31(13), 5013–5025. 10.1523/JNEUROSCI.5592-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar, K. , Rutherford, H. J. , Mencl, W. E. , Lacadie, C. M. , Potenza, M. N. , & Mayes, L. C. (2014). Differential associations between impulsivity and risk‐taking and brain activations underlying working memory in adolescents. Addictive Behaviors, 39(11), 1606–1621. 10.1016/j.addbeh.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro, A. D. , Elmore, L. C. , Ellmore, T. M. , Leising, K. J. , Papanicolaou, A. C. , & Wright, A. A. (2013). Explorations of object and location memory using fMRI. Frontiers in Behavioral Neuroscience, 7, 105 10.3389/fnbeh.2013.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. , Gutierrez, E. , Bandettini, P. , & Ungerleider, L. (2002). Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron, 35(5), 975–987. 10.1016/S0896-6273(02)00817-6 [DOI] [PubMed] [Google Scholar]
- Piccoli, T. , Valente, G. , Linden, D. E. J. , Re, M. , Esposito, F. , Sack, A. T. , & Salle, F. D. (2015). The default mode network and the working memory network are not anti‐correlated during all phases of a working memory task. PLoS One, 10(4), e0123354 10.1371/journal.pone.0123354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal, D. , Zurrón, M. , & Díaz, F. (2014). Effects of load and maintenance duration on the time course of information encoding and retrieval in working memory: From perceptual analysis to post‐categorization processes. Frontiers in Human Neuroscience, 8, 165 10.3389/fnhum.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle, B. R. (2006). Working memory as an emergent property of the mind and brain. Neuroscience, 139(1), 23–38. 10.1016/j.neuroscience.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rämä, P. , & Courtney, S. M. (2005). Functional topography of working memory for face or voice identity. NeuroImage, 24(1), 224–234. 10.1016/j.neuroimage.2004.08.024 [DOI] [PubMed] [Google Scholar]
- Ravizza, S. M. , Hazeltine, E. , Ruiz, S. , & Zhu, D. C. (2011). Left TPJ activity in verbal working memory: Implications for storage‐ and sensory‐specific models of short term memory. NeuroImage, 55(4), 1836–1846. 10.1016/j.neuroimage.2010.12.021 [DOI] [PubMed] [Google Scholar]
- Riggall, A. C. , & Postle, B. R. (2012). The relationship between working memory storage and elevated activity as measured with functional magnetic resonance imaging. The Journal of Neuroscience, 32(38), 12990–12998. 10.1523/JNEUROSCI.1892-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder, C. H. , Mohr, H. , & Linden, D. E. J. (2011). Retention of identity versus expression of emotional faces differs in the recruitment of limbic areas. Neuropsychologia, 49(3), 444–453. 10.1016/j.neuropsychologia.2010.11.040 [DOI] [PubMed] [Google Scholar]
- Rosen, M. L. , Stern, C. E. , Michalka, S. W. , Devaney, K. J. , & Somers, D. C. (2016). Cognitive control network contributions to memory‐guided visual attention. Cerebral Cortex, 26(5), 2059–2073. 10.1093/cercor/bhv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, R. S. , LoPresti, M. L. , Schon, K. , & Stern, C. E. (2013). Role of the hippocampus and orbitofrontal cortex during the disambiguation of social cues in working memory. Cognitive, Affective, & Behavioral Neuroscience, 13(4), 900–915. 10.3758/s13415-013-0170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy, C. , Langner, R. , Dogan, I. , Reetz, K. , Laird, A. , Schulz, J. , … Eickhoff, S. (2011). Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. NeuroImage, 60(1), 830–846. 10.1016/j.neuroimage.2011.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, J. B. , & Passingham, R. E. (2001). Working memory for location and time: Activity in prefrontal area 46 relates to selection rather than maintenance in memory. NeuroImage, 14(1), 77–86. 10.1006/nimg.2001.0784 [DOI] [PubMed] [Google Scholar]
- Rowe, J. B. , Toni, I. , Josephs, O. , Frackowiak, R. S. , & Passingham, R. E. (2000). The prefrontal cortex: Response selection or maintenance within working memory? Science, 288(5471), 1656–1660. 10.1126/science.288.5471.1656 [DOI] [PubMed] [Google Scholar]
- Rutman, A. M. , Clapp, W. C. , Chadick, J. Z. , & Gazzaley, A. (2010). Early top–down control of visual processing predicts working memory performance. Journal of Cognitive Neuroscience, 22(6), 1224–1234. 10.1162/jocn.2009.21257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma, B. , & D'Esposito, M. (2000). Isolating the neural mechanisms of age‐related changes in human working memory. Nature Neuroscience, 3(5), 509–515. 10.1038/74889 [DOI] [PubMed] [Google Scholar]
- Sakai, K. , Rowe, J. B. , & Passingham, R. E. (2002a). Active maintenance in prefrontal area 46 creates distractor‐resistant memory. Nature Neuroscience, 5(5), 479–484. 10.1038/nn846 [DOI] [PubMed] [Google Scholar]
- Sakai, K. , Rowe, J. B. , & Passingham, R. E. (2002b). Parahippocampal reactivation signal at retrieval after interruption of rehearsal. The Journal of Neuroscience, 22(15), 6315–6320. 10.1523/JNEUROSCI.22-15-06315.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo, V. , & Bordier, C. (2019). Large‐scale brain networks underlying successful and unsuccessful encoding, maintenance, and retrieval of everyday scenes in visuospatial working memory. Frontiers in Psychology, 10, 233 10.3389/fpsyg.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayala, S. , Sala, J. B. , & Courtney, S. M. (2006). Increased neural efficiency with repeated performance of a working memory task is information‐type dependent. Cerebral Cortex, 16(5), 609–617. 10.1093/cercor/bhj007 [DOI] [PubMed] [Google Scholar]
- Schacter, D. L. , Wig, G. S. , & Stevens, W. D. (2007). Reductions in cortical activity during priming. Current Opinion in Neurobiology, 17(2), 171–176. 10.1016/j.conb.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Schmidt, B. K. , Vogel, E. K. , Woodman, G. F. , & Luck, S. J. (2002). Voluntary and automatic attentional control of visual working memory. Perception & Psychophysics, 64(5), 754–763. 10.3758/BF03194742 [DOI] [PubMed] [Google Scholar]
- Seeley, W., Menon,V., Schatzberg, A., Keller, J., Glover, G., Kenna, H., … Greicius, M. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri, C. , Shulman, G. L. , & Corbetta, M. (2012). Orienting to the environment: Separate contributions of dorsal and ventral frontoparietal attention networks In Mangun G. R. (Ed.), The neuroscience of attention: Attentional control and selection (pp. 100–130). New York, NY: Oxford University Press. [Google Scholar]
- Sheridan, M. A. , Hinshaw, S. , & D'Esposito, M. (2007). Efficiency of the prefrontal cortex during working memory in attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 46(10), 1357–1366. 10.1097/chi.0b013e31812eecf7 [DOI] [PubMed] [Google Scholar]
- Tamber‐Rosenau, B. J. , Esterman, M. , Chiu, Y.‐C. , & Yantis, S. (2011). Cortical mechanisms of cognitive control for shifting attention in vision and working memory. Journal of Cognitive Neuroscience, 23(10), 2905–2919. 10.1162/jocn.2011.21608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. F. , Welsh, R. C. , Wager, T. D. , Phan, K. L. , Fitzgerald, K. D. , & Gehring, W. J. (2004). A functional neuroimaging study of motivation and executive function. NeuroImage, 21(3), 1045–1054. 10.1016/j.neuroimage.2003.10.032 [DOI] [PubMed] [Google Scholar]
- Todd, J. J. , Han, S. W. , Harrison, S. , & Marois, R. (2011). The neural correlates of visual working memory encoding: A time‐resolved fMRI study. Neuropsychologia, 49(6), 1527–1536. 10.1016/j.neuropsychologia.2011.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, J. J. , & Marois, R. (2004). Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature, 428(6984), 751–754. 10.1038/nature02466 [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within experiment and within group effects in activation likelihood estimation meta analyses. Human Brain Mapping, 33(1), 1–13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. (2005). A population‐average, landmark‐ and surface‐based (PALS) atlas of human cerebral cortex. NeuroImage, 28(3), 635–662. 10.1016/j.neuroimage.2005.06.058 [DOI] [PubMed] [Google Scholar]
- Vincent, J. , Kahn, I. , Snyder, A. , Raichle, M. , & Buckner, R. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. , & Smith, E. (2003). Neuroimaging studies of working memory: A meta‐analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(4), 255–274. 10.3758/CABN.3.4.255 [DOI] [PubMed] [Google Scholar]
- Wallis, G. , Stokes, M. , Cousijn, H. , Woolrich, M. , & Nobre, A. C. (2015). Frontoparietal and cingulo‐opercular networks play dissociable roles in control of working memory. Journal of Cognitive Neuroscience, 27(10), 2019–2034. 10.1162/jocn_a_00838 [DOI] [PubMed] [Google Scholar]
- Wang, H. , He, W. , Wu, J. , Zhang, J. , Jin, Z. , & Li, L. (2019). A coordinate‐based meta‐analysis of the n‐back working memory paradigm using activation likelihood estimation. Brain and Cognition, 132, 1–12. 10.1016/j.bandc.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Wendelken, C. , Bunge, S. A. , & Carter, C. S. (2008). Maintaining structured information: An investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia, 46(2), 665–678. 10.1016/j.neuropsychologia.2007.09.015 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , & Chun, M. M. (2005). Dissociable neural mechanisms supporting visual short‐term memory for objects. Nature, 440, 91–95. 10.1038/nature04262 [DOI] [PubMed] [Google Scholar]
- Yaple, Z. , & Arsalidou, M. (2018). N‐back working memory task: Meta‐analysis of normative fMRI studies with children. Child Development, 89(6), 2010–2022. 10.1111/cdev.13080 [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, B. T. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Polimeni, J. R. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Above‐threshold regions in separate ALE meta‐analyses of (a) encoding‐, (b) maintenance‐, and (c) retrieval‐phase data, including only studies that used identity verification of vernal memoranda and using a voxel‐level threshold of p < .005 (uncorrected) and spatial extent threshold exceeding 1,000 mm3. Green and crimson lines indicate estimated boundaries of the dorsal attention network (DAN) and the frontoparietal control network (FPCN) in Yeo et al.'s (2011) 7‐network model, respectively.
Data Availability Statement
The data that support the findings of this study are openly available in NeuroVault at https://identifiers.org/neurovault.collection:5506.


