Abstract
The prefrontal‐limbic network in the human brain plays a major role in social cognition, especially cognitive control of emotion. The medial frontopolar cortex (mFP; Brodmann Area 10) and the amygdala are part of this network and display correlated neuronal activity in time, as measured by functional magnetic resonance imaging (fMRI). This functional connectivity is dynamic, sensitive to training, and affected in mental disorders. However, the effects of neurostimulation on functional connectivity within this network have not yet been systematically investigated. Here, we investigate the effects of both low‐ and high‐frequency repetitive transcranial magnetic stimulation (rTMS) to the right mFP on functional connectivity between mFP and amygdala, as measured with resting state fMRI (rsfMRI). Three groups of healthy participants received either low‐frequency rTMS (1 Hz; N = 18), sham TMS (1 Hz, subthreshold; N = 18) or high‐frequency rTMS (20 Hz; N = 19). rsfMRI was acquired before and after (separate days). We hypothesized a modulation of functional connectivity in opposite directions compared to sham TMS through adjustment of the stimulation frequency. Groups differed in functional connectivity between mFP and amygdala after stimulation compared to before stimulation (low‐frequency: decrease, high‐frequency: increase). Motion or induced changes in neuronal activity were excluded as confounders. Results show that rTMS is effective for increasing and decreasing functional coherence between prefrontal and limbic regions. This finding is relevant for social and affective neuroscience as well as novel treatment approaches in psychiatry.
Keywords: amygdala, emotion, frontopolar cortex, functional connectivity, resting state MRI, social cognition, transcranial magnetic stimulation
1. INTRODUCTION
The medial frontopolar cortex (mFP; Brodmann Area 10) and the amygdala are part of a prefrontal‐limbic network, that plays a major role for social cognition, especially cognitive control of emotion. Neuronal activity within the mFP and the amygdala, as measured by functional magnetic resonance imaging (fMRI), is correlated in time, that is, these regions exhibit functional connectivity (Delli Pizzi et al., 2017; Eickhoff, Laird, Fox, Bzdok, & Hensel, 2016; Folloni et al., 2019; Gold, Morey, & McCarthy, 2015; Liu et al., 2013; Sallet et al., 2013). A direct anatomical connection between the mFP and the amygdala is less well supported by existing knowledge. Nevertheless, white matter tracts between amygdala and prefrontal cortex extending to the ventral mFP were reported by some individual studies (Folloni et al., 2019; Thiebaut de Schotten, Dell'Acqua, Valabregue, & Catani, 2012). Consistent with these reports, Liu et al. (2013) found that the anatomical connection of the FP with the amygdala is strongest in its orbital region. Interestingly, the functional connectivity with the amygdala is relatively constant throughout the three FP regions (incl. mFP; Liu et al., 2013). This can be explained in part by indirect anatomical pathways (Honey et al., 2009), for example, via the ventromedial prefrontal cortex (vmPFC), the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), and the subgenual cingulate area (Beckmann, Johansen‐Berg, & Rushworth, 2009; Goetschius et al., 2019; Liu et al., 2013; Moayedi, Salomons, Dunlop, Downar, & Davis, 2015; Thiebaut de Schotten et al., 2012; Von Der Heide, Skipper, Klobusicky, & Olson, 2013). For the purpose of this study, functional connectivity between mFP and amygdala is a proxy for the coherence in the prefrontal‐limbic network that underlies various socio‐emotional processes (Abraham et al., 2014; Forbes & Grafman, 2010; Raine, 2018). This functional connectivity between mFP and amygdala is dynamic: it increases through amygdala real‐time fMRI neurofeedback (Young et al., 2018; Zotev, Phillips, Young, Drevets, & Bodurka, 2013) and it is decreased during negative emotion processing in major depressive disorder (Kong et al., 2013; Young, Siegle, Bodurka, & Drevets, 2016). And previous studies showed a reduction in negative emotions by frontopolar repetitive transcranial magnetic stimulation (rTMS; Guhn et al., 2014; Herrmann et al., 2017).
Despite the importance of this prefrontal‐limbic network, the influence of neurostimulation on its functional connectivity has not yet been systematically investigated. In this study, we investigate the effects of both low‐ and high‐frequency rTMS on resting state functional connectivity between mFP and amygdala in healthy participants. TMS has proven to be an effective tool to interfere with neuronal activity and functional connectivity (Cirillo et al., 2017; Eldaief, Halko, Buckner, & Pascual‐Leone, 2011; Fitzgerald, Fountain, & Daskalakis, 2006; Fox, Halko, Eldaief, & Pascual‐Leone, 2012; Pascual‐Leone et al., 1998; Pascual‐Leone, Walsh, & Rothwell, 2000; Paus, Castro‐Alamancos, & Petrides, 2001; van der Werf, Sanz‐Arigita, Menning, & van den Heuvel, 2010; Watanabe et al., 2014; Ziemann, 2017).
With regard to the efficacy of low‐ and high‐frequency rTMS to modulate PFC function, past studies in healthy participants showed that TMS changes neuronal activity in the PFC as measured indirectly with positron emission tomography (PET; Speer et al., 2000), single‐photon emission computed tomography (SPECT; George et al., 1999; Loo et al., 2003), arterial spin labeling (ASL; Gratton, Lee, Nomura, & D'Esposito, 2014), electroencephalography (EEG; Graf et al., 2001; Griskova, Ruksenas, Dapsys, Herpertz, & Hoppner, 2007; Grossheinrich et al., 2009; Okamura, Jing, & Takigawa, 2001; Pripfl, Tomova, Riecansky, & Lamm, 2014; Wozniak‐Kwasniewska, Szekely, Aussedat, Bougerol, & David, 2014), near‐infrared spectroscopy (NIRS; Tupak et al., 2013) and fMRI (Hanlon et al., 2013; Nahas et al., 2001). Although challenged by some authors (de Jesus et al., 2014) and not universally valid, the well accepted convention is that high‐frequency rTMS (≥5 Hz) increases neuronal excitability and low‐frequency rTMS (≤1 Hz) decreases neuronal excitability (Fitzgerald et al., 2006; Hoogendam, Ramakers, & Di Lazzaro, 2010; Kobayashi & Pascual‐Leone, 2003), that is, they have opposing effects on neuronal excitability (George et al., 1999; Graf et al., 2001; Gratton et al., 2014; Griskova et al., 2007; Grossheinrich et al., 2009; Hanlon et al., 2013; Loo et al., 2003; Nahas et al., 2001; Okamura et al., 2001; Pripfl et al., 2014; Speer et al., 2000; Tupak et al., 2013; Wozniak‐Kwasniewska et al., 2014).
With regard to the efficacy of low‐ and high‐frequency rTMS to additionally modulate functional connectivity of the PFC, previous studies showed encouraging but heterogeneous results. In healthy participants, van der Werf et al. showed a reduced activity within the default mode network (DMN) after low‐frequency rTMS to the dlPFC (2010). Eldaief et al. (2011) and Watanabe et al. (2014) used both inhibitory and excitatory protocols to regions other than PFC. Watanabe et al. (2014) reported a decrease in interhemispheric functional connectivity induced by excitatory rTMS over the M1 (quadripulse TMS [QPS] with interstimulus intervals of 5 ms), whereas inhibitory rTMS (QPS with interstimulus intervals of 50 ms) induced an increase in functional connectivity. Eldaief et al. (2011) reported a decrease in functional connectivity between the left posterior inferior parietal lobule (lpIPL) and the mPFC induced by excitatory rTMS (20 Hz) over the lpIPL, whereas inhibitory rTMS (1 Hz) induced an increase in functional connectivity between lpIPL and the hippocampal formation. However, in the above studies, inhibitory and excitatory rTMS had no differential effects on functional connectivity between two particular regions.
With respect to our main objective, there are only a few studies indicating how rTMS to the mFP might alter activity in prefrontal‐limbic circuits. Hanlon et al. (2013), for example, reported an increased neuronal activity in subcortical brain regions (i.e., amygdala, hippocampus, caudate, putamen, thalamus) after single pulse TMS applied to the FP. Recently, Downar (2017) reported preliminary results of an increase in functional connectivity within the salience network after 20 Hz rTMS to bilateral dorsomedial PFC in patients with medication‐resistant depression. To our knowledge, there are no studies so far in healthy participants using combined rTMS and resting state fMRI (rsfMRI) to examine functional connectivity in the prefrontal‐limbic neurocircuitry. Additionally, there are no studies which assessed the effects of both high‐ and low‐frequency rTMS on FP or PFC activity or functional connectivity.
In the current study, three groups of participants either received low‐frequency rTMS (1 Hz), sham TMS (1 Hz, subthreshold) or high‐frequency rTMS (20 Hz) to the right mFP (MNI coordinate: x = 10, y = 63, z = 25) outside the MRI scanner. Functional connectivity was measured twice via rsfMRI: first, on a separate day before rTMS (Pre‐TMS, 10 min duration) and second, directly after rTMS (Post‐TMS, 20 min duration; Figure 1). The rsfMRI session after TMS (Post‐TMS), which had a total duration of 20 min, was split for statistical analysis in two sub‐sessions of 10 min duration each (Post‐1‐TMS, Post‐2‐TMS; Figure 1) to assess potential return‐to‐baseline effects.
Figure 1.
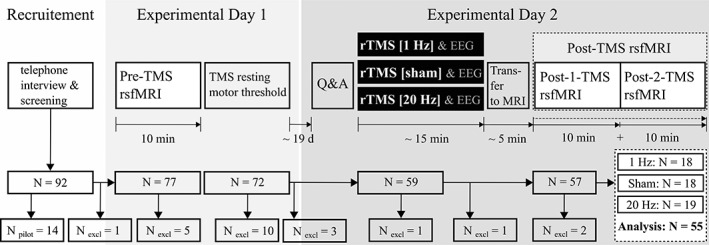
Study design in chronological order. On the first experimental day, participants underwent the first rsfMRI session (Pre‐TMS). Subsequently, participants' individual resting motor thresholds (RMT) were acquired. On the second experimental day and outside the MRI scanner (“offline”), the three groups of participants received one of the following stimulations to the right mFP: Low‐frequency rTMS (1 Hz, 100% RMT; N = 18), sham TMS (1 Hz, subthreshold; N = 18), high‐frequency rTMS (20 Hz, 100% RMT; N = 19). During rTMS simultaneous EEG data were acquired (see Section 2). After rTMS, participants underwent the second rsfMRI session (Post‐TMS). This session was split in half to assess an expected return‐to‐baseline effect. Furthermore, participants completed a set of mood and anxiety questionnaires (Q&A). Fifty‐five participants were included in the statistical analysis
Although there is strong anatomical and functional connectivity between the vmPFC and OFC, on the one hand, and the amygdala, on the other (see above), and this connectivity is highly relevant for psychiatric disorders (Downar & Daskalakis, 2013; Kamphausen et al., 2013; Likhtik & Paz, 2015; Mukherjee et al., 2016; Myers‐Schulz & Koenigs, 2012; Park et al., 2018; A. K. Roy et al., 2013), the mFP was selected for TMS instead. The mFP is located both proximal to the scalp, which ensures effective stimulation with a conventional TMS coil (Downar & Daskalakis, 2013), and atop a medial brain circuit that includes pathways between vmPFC, OFC and amygdala (Forbes & Grafman, 2010). Bearing in mind future clinical applications, the right mFP was chosen over the left mFP, because, in depression, EEG alpha power is increased over the right frontal lobe relative to the left side (Allen & Reznik, 2015; Perera et al., 2016). Consequently, disruptive 1 Hz rTMS is usually applied on the right side to balance this frontal asymmetry. We followed this approach given that we were more confident to observe a disruptive effect (rather than an amplifying effect via 20 Hz rTMS to the left mFP). A recent study also showed no difference in anti‐depressive treatment effects when comparing high‐frequency left PFC rTMS to low‐frequency right PFC rTMS (Donse, Padberg, Sack, Rush, & Arns, 2018).
Furthermore, we previously found a concurrent increase in fMRI neuronal activity in the mFP, the vmPFC and the amygdala when contrasting pictures with negative valence to pictures with neutral valence during an emotional attention capture task (Figure S1). This is in line with a well‐known interaction between amygdala and medial prefrontal regions to process negative emotions (Buhle et al., 2014; Bzdok et al., 2013; Courtin, Bienvenu, Einarsson, & Herry, 2013; Delli Pizzi et al., 2017; Eickhoff et al., 2016; Etkin, Egner, & Kalisch, 2011; Kober et al., 2008; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015; Phan, Wager, Taylor, & Liberzon, 2002; Phelps, Delgado, Nearing, & LeDoux, 2004; Phelps & LeDoux, 2005; Phillips, Drevets, Rauch, & Lane, 2003; Shin & Liberzon, 2010). The mFP coordinates used for TMS here were the most inferior, superficial access point to stimulate this network.
We hypothesized that the three stimulation‐groups (1 Hz rTMS, sham TMS, 20 Hz rTMS) would differ in functional connectivity between mFP and amygdala at the Post‐1‐TMS session compared to the Pre‐TMS session. We also hypothesized that low‐ and high‐frequency rTMS would have opposing effects on this functional connectivity—Without being able to directly predict the specific directionality of these changes due to scarce and heterogeneous previous results. Additionally, we expected a return‐to‐baseline effect, that is, that functional connectivity at Post‐2‐TMS is between functional connectivity at Post‐1‐TMS and functional connectivity at Pre‐TMS.
Even though, rTMS is an FDA‐approved treatment option for depression (Perera et al., 2016), it is still unclear how rTMS affects fronto‐limbic socio‐emotional brain circuits. If the above hypotheses were confirmed, this would provide evidence that functional connectivity within this network can be modulated by rTMS per se and that bidirectional modulation can be achieved through adjustment of the stimulation frequency. Such a finding would be highly relevant for studying brain processes related to, for example, threat, anxiety or fear processing. It would also provide the foundation for further research into the optimal application of rTMS for treating conditions such as anxiety‐, mood‐, personality‐ and psychotic disorders (Bengtsson, Olsson, Wass, & Bodén, 2015; Burt, Lisanby, & Sackeim, 2002; Camchong, MacDonald 3rd, Bell, Mueller, & Lim, 2011; Downar & Daskalakis, 2013; Hasan, Strube, Palm, & Wobrock, 2016; Kamphausen et al., 2013; Mukherjee et al., 2016; Shin & Liberzon, 2010).
2. MATERIAL AND METHODS
The study was approved by the Ethics Committee of the Technische Universität Dresden, Germany. The study was carried out at the Neuroimaging Center of the Technische Universität Dresden, Germany.
2.1. Participants
Ninety‐two participants were initially recruited for the study (18–35 years of age, M = 23.8, SD = 3.0, 50 female). Fourteen participants were recruited for piloting only. Twenty‐three participants were excluded for several reasons: lack of a resting motor threshold (RMT; N = 9), very high RMT (N = 1), technical problems (N = 3), side effects of TMS or MRI (N = 3), no appearance on the second day of the experiment (N = 3), contraindications for MRI (N = 1), neuroanatomical irregularity (N = 1), and participant falling asleep during MRI (N = 2).
Fifty‐five participants were included in the statistical analysis (18–30 years of age, M = 23.5, SD = 2.8, 31 female). They had no history of mental disorder as assessed with a customized, structured interview based on Diagnostic and Statistical Manual of Mental Disorders (DSM) IV criteria. They were free of any medication and reported no history of neurologic disease and no pregnancy. Participants were screened for exclusion criteria for MRI and TMS (Rossi, Hallett, Rossini, & Pascual‐Leone, 2011). All participants were right‐handed (as assessed with the Edinburgh Handedness Inventory [Oldfield, 1971]). Participants were encouraged to appear well‐rested to the experiment, to avoid alcohol 24 hr before the experiment, and not to drink coffee on the day of the experiment. Written informed consent was obtained from each participant. All were unaware of the hypotheses of the study and received a financial compensation for their participation.
2.2. Experimental design
The study was conducted on two separate days. The entire study design in chronological order is presented in Figure 1. On the first experimental day and before TMS, participants underwent the first rsfMRI session (Pre‐TMS; 10 min). Subsequently, participants' individual RMTs were acquired. Participants eligible for the second experimental day were randomly assigned, initially, to the 1 Hz rTMS or sham TMS group. Upon securing the required resources for an additional experimental group, participants were also randomized to the 20 Hz rTMS group. To finish all groups at the same time, a bias for an assignment to the 20 Hz rTMS group was introduced. After an average of 19 days (SD = 36), participants returned to the second experimental session. The mean days between the first and second experimental session did not differ between stimulation‐groups (two‐way univariate ANOVA: F[2, 52] = 0.746, p = .479). The mean‐centered number of days between sessions was used as a covariate only for Supporting Information (Supporting Information Methods, Tables S1–S3). In the second session, three groups of participants either received low‐frequency rTMS (1 Hz), sham TMS (1 Hz, subthreshold) or high‐frequency rTMS (20 Hz) to the right mFP outside the MRI scanner (“offline”). The stimulation‐groups did not differ significantly in age or gender (two‐way multivariate analysis of variance [MANOVA] for age: F[1, 52] = 1.264/p = .291, for gender: F[1, 52] = .760/p = .473). During TMS, simultaneous EEG data were acquired. The EEG data are part of a different research question and will be reported elsewhere. After rTMS, participants underwent the second rsfMRI session (Post‐TMS; 20 min). For the statistical analysis, this session was split in half (Post‐1‐TMS, Post‐2‐TMS; 10 min each) to assess potential return‐to‐baseline effects after rTMS. The average time between the end of the stimulation and the beginning of the rsfMRI scan was 318 s (SD = 92 s). The stimulation‐groups differed in this transfer time (M 1 Hz = 343 s, M sham = 349 s, M 20 Hz = 273 s; F(2, 52) = 3.386/p = .041). This was due to outliers with higher transfer times in the 1 Hz and sham group and an outlier with a lower transfer time in the 20 Hz group. The mean‐centered transfer‐time was used as a covariate only for Supporting Information (Supporting Information Methods). Furthermore, participants completed a set of mood and anxiety questionnaires (STAI, PSS, BDI‐II) on the second experimental day via TestMaker, a software for web‐based assessments (Milbradt, Zimmerhofer, & Hornke, 2007), to test for group differences.
2.3. Acquisition of neuroimaging data
Images were acquired on a 3‐Tesla Siemens Tim Trio scanner using the Siemens 32‐channel head coil (Siemens, Erlangen, Germany). T1‐weighted images were acquired with a 3D magnetization‐prepared rapid gradient echo (MP‐RAGE) sequence (repetition time [TR] = 1.9 s, echo time [TE] = 2.26 ms, field of view [FOV] = 256 × 224 × 176 mm3, voxel size = 1 × 1 × 1 mm3, inversion time = 0.9 s, flip angle [FA] = 9°, phase partial Fourier 7/8, bandwidth [BW] = 200 Hz/Px). rsfMRI data were acquired using a T2*‐weighted multiband accelerated EPI (Moeller et al., 2010; Setsompop et al., 2012; Xu et al., 2013; TR = 376 ms, TE = 25 ms, flip angle = 42°, voxel size = 2.5 × 2.5 × 3 mm3, slice thickness = 3 mm, matrix size = 78 × 78, number of slices = 48, multiband acceleration factor = 8, phase partial Fourier factor = 6/8, bandwidth = 2,466 Hz/Px, excite pulse duration = 4,620 μs). During scans the participants' head movements were reduced by cushions placed between the ears and the head coil. Participants were instructed to keep their eyes open and look at a black fixation cross centered on a white background, which was presented during rsfMRI.
2.4. Neurostimulation
2.4.1. Setting
Noninvasive neurostimulation was performed in a TMS research laboratory in a nonclinical setting. The laboratory was equipped with a MagProX100 stimulator and a MCF‐B65 Butterfly Coil (MagVenture, Hueckelhoven, Germany; CE‐certificate 0543). Two operators, trained and experienced with TMS were present to perform the stimulation and assure data quality of EEG recordings.
2.4.2. Resting motor threshold
On the first day of the study, each participant's RMT was determined subsequent to MRI. Electromyographic recording of the first dorsal interosseus muscle of the right hand was performed to assess muscle contraction in response to single TMS pulses; hence the TMS coil was placed over the left primary motor cortex. Electrodes were placed in a standard belly‐tendon‐montage. The RMT was defined by the minimum single pulse intensity required to evoke a motor evoked potential with a peak‐to‐peak amplitude of at least 50 μV on 5 of 10 consecutive trials. RMTs were not acquired again on the second experimental day. The participants' RMT acquired on the first experimental day was used to adjust stimulation intensity.
2.4.3. Neuronavigation
Neuronavigation was performed on the second experimental day. The individual structural MRI scan acquired on the first day was transformed to MNI standard space. A series of anatomical landmarks (nasion, left, and right tragus) was marked on this 3D‐scan. The registration points were subsequently sampled using a Polaris position sensor (Northern Digital, Inc., Waterloo, Ontario, CA), a tracker attached to the participant's head, and a pointer tool to define the participant's position in space. The target MNI coordinate (MNI: x = 10, y = 63, z = 25) was displayed on the individual participant's 3D cortical surface model. Target sites were tracked using image guided frameless stereotaxy (TMS Neuronavigator [version 1.1.0.186] as part of Turbo‐BrainVoyager® [Brain Innovation, Maastricht, NL]) and marked on the scalp.
2.4.4. rTMS
After neuronavigation, we employed rTMS outside the MRI scanner (i.e., “offline”). For right mFP stimulation, the figure‐of‐eight coil was positioned slightly to the right of the midline as shown in Figure 2. The goal of the TMS protocol was to (a) apply a standard 15 min rTMS session (Fitzgerald et al., 2006) and (b) simultaneously integrate cortical excitability measurements using TMS and EEG (to be published separately). Therefore, each TMS protocol consisted of four blocks: (a) 25 single TMS pulses to record a baseline TEP (TMS‐evoked potential), (b) 10 min of rTMS followed by a 30 s break, (c) 25 single pulses followed by a 30 s break, and (d) 5 min of rTMS. As a tradeoff, the EEG measurement (Block 3) was included already after 10 min of rTMS (Block 2) and not at the end of the 15 min rTMS session in order to minimize the time between the end of rTMS and the start of MRI, that is, to maximize rTMS effects on functional connectivity (further referred to as FC in 2, 3). The three different rTMS protocols (1 Hz rTMS, sham TMS, 20 Hz rTMS) were defined as follows: 1 Hz rTMS (100% RMT, 10‐min‐train + 5‐min‐train, 900 pulses in total), sham TMS (1 Hz, subthreshold stimulation intensity of 60% RMT, 10‐min‐train + 5‐min‐train, 900 pulses in total), 20 Hz rTMS (100% RMT, inter‐train‐interval [ITI] = 18 s, train duration = 2 s, 15 trains in 10 min followed by eight trains in 5 min, 920 pulses in total).
Figure 2.
Photographs of a staged TMS setup. The right mFP was stimulated using a figure‐of‐eight coil. The stimulation coil was positioned slightly to the right of the midline [Color figure can be viewed at http://wileyonlinelibrary.com]
2.5. Analysis of rsfMRI data
2.5.1. Preprocessing
Standard fMRI preprocessing was performed on the rsfMRI data (Weissenbacher et al., 2009) using statistical parametric mapping (SPM8, Wellcome Trust Centre for Neuroimaging, University College London, London, UK, http://www.fil.ion.ucl.ac.uk/spm) and Matlab 2017a (The MathWorks, Inc., Natick, MA). Preprocessing included: (a) spatial realignment (motion correction), (b) T1‐based normalization, (c) float‐conversion, and (d) smoothing with an isotropic, 8 mm Gaussian kernel (FWHM; Penny, Friston, Ashburner, Kiebel, & Nichols, 2011), to improve signal‐to‐noise ratio and level residual anatomical variations. Slice timing correction was not performed. After basic preprocessing, the 20 min Post‐TMS rsfMRI scan was split into two 10 min sub‐sessions. This resulted in three rsfMRI sessions in total (Pre‐TMS, Post‐1‐TMS, Post‐2‐TMS; Figure 1). Subsequently, we performed nuisance regression using the following regressors: (a) average signals of cerebrospinal fluid (CSF) and white matter (WM) masks to correct for physiological artifacts including respiration and cardiac effects (Weissenbacher et al., 2009; Windischberger et al., 2002); (b) six motion parameters derived from spatial realignment. Next, we employed a censoring‐based artifact removal strategy (scrubbing) to tag volumes in our RS time series that were potentially affected by motion (Carp, 2013; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Power et al., 2014; Power, Schlaggar, & Petersen, 2015; see Supporting Information for details). Finally, we performed temporal filtering via a band‐pass filter (0.009–0.08 Hz). After temporal filtering, the tagged volumes were censored, that is eliminated before computing correlations.
2.5.2. Correlation analysis
A seed‐based approach was used. The seed within the right mFP was the voxel closest to the target coordinate (see Section 2.4.3; MNI: x = 10, y = 63, z = 25). Because smoothing was performed during preprocessing, the seed represents a weighted average of the signal within a sphere (8 mm FWHM kernel). The time course of the seed voxel was extracted from the preprocessed images for each participant and each 10 min rsfMRI session (Pre‐TMS, Post‐1‐TMS, Post‐2‐TMS). Seed‐to‐voxel whole brain functional connectivity analysis was performed for each session by calculating Pearson's correlation coefficients (r). A Fisher's r‐to‐z was done before further statistical analyses. Subsequently, the mean z‐values of all voxels within the right and left amygdala (WFU Pickatlas, version 3.0.5b; Lancaster et al., 2000; Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003) were extracted.
2.5.3. Statistical analysis
Subsequent statistical analyses were performed in SPSS Statistics 23 (IBM SPSS Statistics, Armonk, NY).
Initially (see Section 3.1), we used t‐tests, (a) to test whether mFP and amygdala neuronal activity show a positive correlation (i.e., positive FC), (b) to test whether right mFP (site of rTMS) and right (ipsilateral) amygdala show a stronger FC than right mFP and left (contralateral) amygdala, and (c) to exclude differences in FC between mFP and amygdala (mFP‐amygdala‐FC) between stimulation‐groups at Pre‐TMS (baseline). MANOVA was used to assess group differences in mood and anxiety scores at Pre‐TMS (baseline; independent variable: stimulation‐group [1 Hz rTMS, sham TMS, 20 Hz rTMS]; dependent variables: scores of STAI‐State, STAI‐Trait, PSS, BDI‐II).
Subsequent analyses focused on right‐mFP‐right‐amygdala‐FC. Note that we did not expect that rTMS changes FC between mFP and amygdala specifically within one hemisphere only. However, we assumed that the induced change in FC could be more pronounced within one hemisphere than across hemispheres. In fact, we also had to assume that the effects of rTMS are not reproduced at all across hemispheres, as such effects can only be mediated by additional commissural fibers. This is consistent with our assumption that FC between mFP and amygdala is mediated by either direct or indirect anatomical connections. Hence, we performed separate analyses for right‐mFP‐right‐amygdala FC (see Section 3.2) and right‐mFP‐left‐amygdala FC (see Section 3.3). However, since we did not have any specific hypothesis regarding the difference between intra‐ and interhemispheric effects of rTMS, we did not statistically test for such.
First, we tested for any effect of stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) or/and session (Pre‐TMS, Post‐1‐TMS, Post‐2‐TMS) on mFP‐amygdala‐FC using a 3 × 3 factorial mixed design ANOVA (MD‐ANOVA; see also Donse et al., 2018). Second, we tested our main hypothesis, that is, that the three stimulation‐groups (1 Hz rTMS, sham TMS, 20 Hz rTMS) would differ in mFP‐amygdala‐FC at the Post‐1‐TMS session compared to the Pre‐TMS session using a 3 × 2 factorial MD‐ANOVA. We expected a significant stimulation‐group * session interaction. Additionally, differences in mean z‐values and their standard error of the mean (SEM) were calculated to show the direction of rTMS induced FC changes and to estimate effect sizes. Fourth, we assessed return‐to‐baseline effects by testing for a main effect of stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) on the slope of the linear function defining mFP‐amygdala‐FC from Pre‐TMS over Post‐2‐TMS to Post‐1‐TMS (linear session effect) in a 3 × 3 factorial MD‐ANOVA. We expected opposite slopes for 1 Hz and 20 Hz rTMS and a slope of about 0 in sham TMS.
Because the mFP is implicated in a variety of neuronal processes and shows FC with several brain regions, we also conducted an explorative whole brain analysis using a 3 × 2 factorial MD‐ANOVA in SPM8 testing for the interaction between stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and session (Pre‐TMS, Post‐1‐TMS; see Section 3.4).
In rsfMRI, BOLD signal correlations between brain regions (FC) are susceptible to motion, that is, correlations could be introduced or masked by head‐motion (Power et al., 2012). To exclude that the observed effect of TMS on mFP‐amygdala‐FC (see Section 3.2) was a spurious result due to head‐motion, we further performed two 3 × 2 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐1‐TMS & Post‐1‐TMS, Post‐2‐TMS) on the number of censored volumes (NCV; see Supporting Information Methods and Results 3.5).
At last, we investigated whether we could identify stimulation effects on the BOLD‐signal at the seed region (see Supporting Information Methods and Results 3.6) by conducting a 3 × 2 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐1‐TMS) on the dependent variables temporal “mean BOLD‐mFP(R)/mean BOLD‐mFP(L) ratio” and the “mFP(R)‐BOLD coefficient of variation” (coefficient of variation [CV] = SD/mean) at the seed/target location.
3. RESULTS
3.1. Baseline mFP‐amygdala‐FC and baseline mood and anxiety scores
The left panel in Figure 3 depicts a network positively (Fisher's z‐value > 0.1; orange) and a network negatively correlated (Fisher's z‐value < −0.1; blue) with the right mFP (mFP(R); seed ≙ one voxel at MNI: x = 10, y = 63, z = 25, which is depicted via a crosshair) before rTMS (Pre‐TMS; baseline) across the three stimulation‐groups. The right panel in Figure 3 shows an overlay of the amygdala masks used for later analysis and a map of voxels showing a positive FC with the mFP(R) seed. There was a stronger FC of the mFP(R) with the right amygdala (amygdala[R]) compared to the left amygdala (amygdala[L]; t[54] = 3.37, p = .001). Both amygdala(R) (t[54] = 7.361, p < .001) and amygdala(L) (t[54] = 4.618, p < .001) showed mean z‐values significantly greater than 0. The stimulation‐groups did not significantly differ in mFP‐amygdala‐FC before rTMS (amygdala[R]: F[2, 52] = 2.623, p = .082; amygdala[L]: F[2, 52] = 0.718, p = .492).
Figure 3.
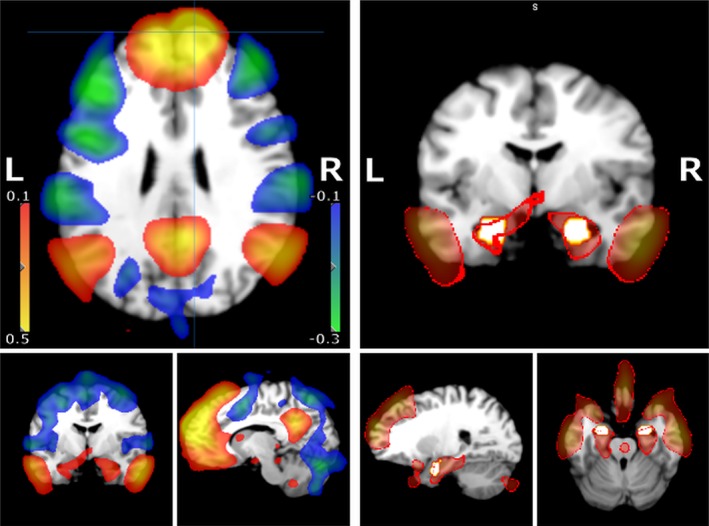
Heat map of whole brain functional connectivity of the right mFP (z‐values). Left panel: Depicted are thresholded mean Fisher's z‐values derived from the correlation analysis (see Section 2) overlayed on a generic T1 MRI scan. z‐Values between 0.1 to 0.5 (orange) and −0.1 to −0.3 (blue) are shown. The seed region (mFP[R]) is marked by crosshairs (sagittal and transversal plane). Additionally, an amygdala slice is shown (coronal plane). Right panel: The position of the utilized right and left amygdala masks (in white with an orange rim) are depicted together with the z‐map from the left panel with a threshold for z‐values >0.1. The edges of the z‐map are highlighted in red (0.15 > z‐values > 0.1). The amygdala masks are largely overlapping within the thresholded z‐map [Color figure can be viewed at http://wileyonlinelibrary.com]
The mean results of the State‐Trait Anxiety Inventory (STAI; Spielberger, 2010), Perceived Stress Scale (PSS; Sheldon Cohen, Kamarck, & Mermelstein, 1994), Beck Depression Inventory (BDI‐II; Beck, Steer, & Brown, 1996) were within the range of one SD of the normative data of representative healthy populations (Sheldon Cohen, 1988; S. Cohen & Janicki‐Deverts, 2012; Knight, Waalmanning, & Spears, 1983; Whisman & Richardson, 2015). Stimulation‐groups did not differ in the STAI‐State (MANOVA; main effect of stimulation‐group: F[2, 53] = 1.189, p = .313), STAI‐Trait (F[2, 53] = 0.681, p = .511), PSS (F[2, 53] = 0.515, p = .601), or BDI‐II scores (F[2, 53] = 1.216, p = .305).
3.2. Effects of rTMS to right mFP on right‐mFP‐right‐amygdala‐FC
Mean‐z‐values within the right amygdala (mFP[R]‐amygdala[R]‐FC) across stimulation‐groups and sessions are depicted in Figure 4 (upper panel). In addition, participants' individual z‐values are depicted in Figure 5. First, a 3 × 3 factorial mixed design ANOVA (MD‐ANOVA) with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐2‐TMS, Post‐1‐TMS) revealed a significant stimulation‐group * session interaction (F[4, 104] = 2.9, p = .024, partial η 2 = 0.1), a significant main effect of session (F[2, 51] = 3.7, p = .031, partial η 2 = 0.13), and no significant main effect of stimulation‐group (F[2, 52] = 0.2, p = .796, partial η 2 = 0.01). Second, we performed a 3 × 2 factorial MD‐ANOVA to test our main hypothesis, that is, that stimulation‐groups (1 Hz rTMS, sham TMS, 20 Hz rTMS) will differ in mFP‐amygdala‐FC at the Post‐1‐TMS session compared to the Pre‐TMS session. In line with this hypothesis, analysis revealed a significant stimulation‐group * session interaction (F[2, 52] = 4.893, p = .012, partial η 2 = 0.16), no significant main effect of session (F[1, 52] = 0.681, p = .413, partial η 2 = 0.013), and no significant main effect of stimulation‐group (F[2, 51] = 1.025, p = .366, partial η 2 = 0.038; Figure 4). Third, mean difference of mFP(R)‐amygdala(R)‐FC at Post‐1‐TMS and Pre‐TMS were calculated with respect to stimulation‐group (ΔM in z‐values, with SEM): ΔM 1Hz = −0.07 (SEM = 0.03), ΔM sham = −0.03 (SEM = 0.03), and ΔM 20Hz = 0.06 (SEM = 0.03). Fourth, we assessed our hypothesis that mFP(R)‐amygdala(R)‐FC at Post‐2‐TMS is between FC at Post‐1‐TMS and FC at Pre‐TMS for the 1 Hz and 20 Hz rTMS group (return‐to‐baseline effects of rTMS). A 3 × 3 factorial MD‐ANOVA (see above) revealed a significant main effect of stimulation‐group on the slope (linear session effect) when ordering the sessions Pre‐TMS – Post‐2‐TMS – Post‐1‐TMS (stimulation‐group * linear effect of session interaction: F[2, 52] = 4.8, p = .012, partial η 2 = 0.16). However, a return‐to‐baseline effect was only observed in the 20 Hz rTMS group while the 1 Hz rTMS group did not behave as expected (please refer to Figure 4). Therefore, return‐to‐baseline effects were not consistently observed and, therefore, not further pursued statistically.
Figure 4.
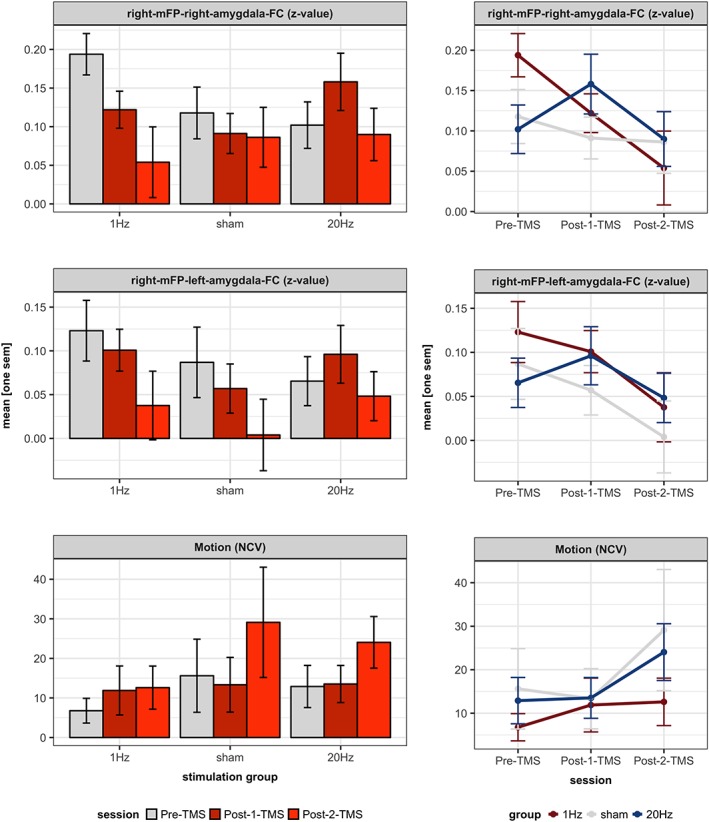
Effects of rTMS to right mFP on its functional connectivity with right and left amygdala. Upper and middle panel: Depicted are the mean‐z‐values (and ±1 standard error of the mean [SEM]) within the right and left amygdala across the three stimulation‐groups and sessions. The mean differences (ΔM ± 1 SD) of mFP(R)‐amygdala(R)‐FC at Post‐1‐TMS and Pre‐TMS for the three stimulation groups were: ΔM1 Hz = −0.07 (SEM = 0.03), ΔM sham = −0.03 (SEM = 0.03), ΔM20 Hz = 0.06 (SEM = 0.03). The statistical analyses revealed a significant interaction of stimulation‐group and session for mFP(R)‐amygdala(R)‐FC (see Section 3 for more details). Interactions between stimulation‐groups and sessions are depicted on the right. Lower panel: Depicted are the number of censored volumes (NCV; and ±1 SEM) across the stimulation‐groups and sessions. The ΔM (SD) of NCV at Post‐2‐TMS and Post‐1‐TMS was 9 (26). Interactions between stimulation‐groups and sessions are depicted on the right. For statistical analyses please see Section 3 [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
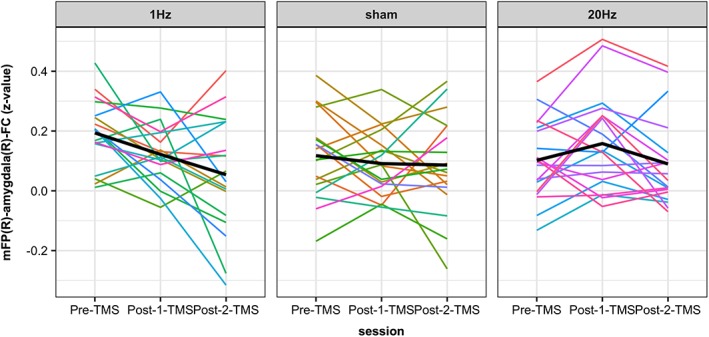
Individual effects of rTMS to right mFP on its functional connectivity with the right amygdala. Depicted are the participants' individual z‐values within the right amygdala (representing mFP[R]‐amygdala[R]‐FC) across the three stimulation‐groups and sessions [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Effects of rTMS to right mFP on right‐mFP‐left‐amygdala‐FC
Mean‐z‐values within the left amygdala (mFP[R]‐amygdala[L]‐FC) are depicted in Figure 4 (middle panel). A 3 × 3 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐2‐TMS, Post‐1‐TMS) revealed no significant stimulation‐group * session interaction (F[4, 104] = 0.6, p = .683, partial η 2 = 0.02), a significant main effect of session (F[2, 51] = 4.8, p = .012, partial η 2 = 0.16) and no significant main effect of stimulation‐group (F[2, 52] = 0.5, p = .58, partial η 2 = 0.02). There was neither a significant difference between stimulation‐groups in the slope (linear session effect; order: Pre‐TMS – Post‐2‐TMS – Post‐1‐TMS; stimulation‐group * session interaction: F[2, 52] = 0.8, p = .462, partial η 2 = 0.03) nor a significant linear main effect of session across groups (F[1, 52] = 0.1, p = .742, partial η 2 = 0).
3.4. Effects of rTMS to right mFP on right‐mFP‐whole‐brain‐FC
Because the mFP shows FC with several brain regions (Hiser & Koenigs, 2018; M. Roy, Shohamy, & Wager, 2012), we additionally performed seed‐based (mFP[R], i.e., MNI: x = 10, y = 63, z = 25) explorative whole brain FC analyses in SPM8 for graphical illustration (p < .005 uncorrected). Only the 3 × 2 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐1‐TMS; see Section 3.2) was conducted. In addition to the amygdala, we found three additional clusters in line with our main hypothesis namely within the right insula, the right fusiform face area (FFA) and the right ACC (Figures S2 and S3). There were no significant results at voxel‐ or cluster‐level at a threshold of pFWE <0.05.
3.5. Motion artifacts
The NCVs (out of 1,595 volumes per session) across stimulation‐groups and session are depicted in Figure 4 (lower panel). In rsfMRI, BOLD signal correlations between brain regions (FC) are susceptible to motion, that is, correlations could be introduced or masked by head‐motion (Power et al., 2012). To exclude that the observed effect of TMS on mFP(R)‐amygdala(R)‐FC (Section 3.2) was a spurious result due to head‐motion, we performed an equivalent 3 × 2 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group (1 Hz rTMS, sham TMS, 20 Hz rTMS) and the within‐subject‐factor session (Pre‐TMS, Post‐1‐TMS), which showed no significant results (all p > .4). An additional 3 × 2 factorial MD‐ANOVA with the between‐subject‐factor stimulation‐group and the within‐subject‐factor session (Post‐1‐TMS, Post‐2‐TMS) yielded a significant main effect of session (F[1, 52] = 6.9, p = .012, partial η 2 = 0.12). Increased participants' movement over time in the MRI scanner was expected. As also expected, there was again no interaction with stimulation‐group (F[1, 52] = 1.6, p = .209, partial η 2 = 0.06). The total group mean NCVs were N = 12 (range: 0–166) for Pre‐TMS, N = 13 (0–122) for Post‐1‐TMS, and N = 22 (0–254) for Post‐2‐TMS.
3.6. Effects of rTMS on BOLD signal at right mFP
There were no significant stimulation‐group * session interactions on BOLD‐measures (also see Section 2.5.3) at the right mFP (p > .1). However, there was a main effect of stimulation‐group on the BOLD‐mFP(R)/BOLD‐mFP(L)‐ratio (F[1, 52] = 3.797, p = .029). There also was a trend toward a main effect of session on the mFP(R)‐BOLD coefficient of variation (F[1, 52] = 3.230, p = .078). Please, refer to Figure S4 for further results.
4. DISCUSSION
In line with our hypotheses, the results of this study demonstrate that rTMS to the mFP modulates functional connectivity within a prefrontal‐limbic network of brain regions that is important for socio‐emotional processes. More precisely, low‐ and high‐frequency rTMS to the right mFP had opposing effects on functional connectivity between the mFP and the amygdala in the right hemisphere. Low‐frequency rTMS (1 Hz) resulted in a decrease of functional connectivity and high‐frequency rTMS (20 Hz) resulted in an increase (Figures 4 and 5). Note that our test for any interaction between stimulation‐group and Pre–Post functional connectivity change is conservative compared to testing the directional hypothesis that 1 Hz rTMS would lead to a decrease in functional connectivity while 20 Hz rTMS would lead to an increase in functional connectivity.
Alterations of neuronal activity by rTMS at the site of stimulation have been widely reported and are thought to be the result of neuron assemblies being depolarized by TMS‐induced electric currents in brain tissue (Valero‐Cabre, Amengual, Stengel, Pascual‐Leone, & Coubard, 2017). This way, pyramidal cells within a brain region can be activated directly or indirectly (trans‐synaptic; Kobayashi & Pascual‐Leone, 2003). A rTMS‐induced modulation of functional connectivity between brain regions (as observed in this study) can be explained by altered trans‐synaptic connections between regions (Eldaief et al., 2011; Hanlon et al., 2013; Ilmoniemi et al., 1997; Lenz et al., 2016) or by an induction of neurotransmitter release that in consequence affects the coupling with remote regions (Lamusuo et al., 2017; Ohnishi et al., 2004; Strafella, Paus, Barrett, & Dagher, 2001). However, both of these processes require direct or at least indirect anatomical pathways (i.e., white matter tracts and synapses) between regions. Such structural connections exist between the mFP and the amygdala (see Section 1). Therefore, an effect of rTMS on functional connectivity is principally plausible, but the directionality of functional connectivity alterations is not easily predicted. As summarized in the introduction, the findings of inhibitory and excitatory TMS effects on functional connectivity are heterogeneous. We, therefore, only hypothesized differential effects of the two stimulation conditions. This was partially based on the differential effects of the stimulation protocols on excitability as measured by EEG and our hope that protocol‐specific effects would increase the utility of rTMS as an interventional tool. It was however, within the realm of possibilities that both stimulation conditions would, for example, decrease functional connectivity by disrupting natural rhythms. This seems exceedingly unlikely, now, given our results.
We observed an effect of stimulation on functional connectivity with the right/ipsilateral amygdala but not with the left. This is not surprising in light of the following considerations. Functional connectivity between mFP and amygdala in part reflects direct and indirect anatomical pathways (see Section 1). Each anatomical pathway between the right mFP and the left amygdala requires commissural fibers across the hemispheres and association fibers within a hemisphere. However, association fibers alone are sufficient for connectivity between the right mFP and the right amygdala. Therefore, the effects of rTMS across hemispheres are more difficult to reproduce as they have to affect a larger network. This may explain why effects in the left amygdala can be detected less or not at all. An additional explanation is that even though the left and right amygdalae have a high degree of concordance in their functional connectivity, there are still subtle differences in functional connectivity and function (A. K. Roy et al., 2009).
When considering effects on conventional resting state networks, there is an overlap of the seed‐based network at the Pre‐TMS session (Figure 3, left panel), with the default mode network and the salience network (Buckner, Andrews‐Hanna, & Schacter, 2008; Uddin, 2015). Whole brain analysis suggests an effect of rTMS on the salience network. But this finding needs to be regarded with caution as it did not survive whole brain correction (Figures S2 and S3). However, this could be one route for future studies. Regarding the DMN, we did not find any changes in functional connectivity between mFP and any other brain region of the DMN in the direction of our hypothesis. However, it would not be unexpected that such modulations by rTMS could be detected with an ICA‐analysis of the rsfMRI data at hand. This would be part of a secondary analysis of the data, but was not the focus of this study.
Group differences in motion are known to be able to contaminate rsfMRI data (Power et al., 2012; Power et al., 2014; Power et al., 2015). However, we did not find such group differences. In addition, we censored rsfMRI datasets in line with common procedures to reduce the likelihood of such a type I error. Additionally, we found no evidence that changes in functional connectivity arose from changes in BOLD signal in the seed region. Although we found an effect of session on the BOLD coefficient of variation at the right mFP, there was no interaction with group. Therefore, neither observed changes in BOLD variance can be explained by active TMS (1 Hz, 20 Hz), nor can the effects of active TMS on functional connectivity be explained by changes in BOLD variance.
Some potential limitations of our study should be considered: rsfMRI scanning on two separate days, the between‐subject design, and potential TMS‐related side effects. Unlike Eldaief et al. (2011) and after initial piloting, we decided to conduct rsfMRI scans before rTMS and after rTMS not on the same date to facilitate the scheduling and preparation of MRI, EEG and TMS sessions and not to cause undue subject burden (e.g., rush, anxiety). Beyond the logistical advantage, it is easily possible that the amount of variance added to the rsfMRI data due to subject burden would be larger than the effect of separate scanning days, especially given that Honey et al. (2009) showed that reliability within one rsfMRI scan was almost identical to reliability across two separate scans. We showed that the three stimulation‐groups did not differ in number of days between the two rsfMRI scans.
Our choice for a between‐subject design was based on the considerations that (a) we were planning a subsequent combination of rTMS with intensive neurofeedback training, which would have required a between‐subject design, (b) we expected recruitment difficulties for a within‐subject study and increased drop‐out rates, that would interact with TMS protocol order due to the variable discomfort of the stimulation protocols, and (c) the between‐subject design avoided “unblinding” of participants due to differences in stimulation‐related sensations and potential order effects that could have been counter‐balanced but may have increased residual variance. However, we acknowledge that a within‐subject design would have had a power advantage, especially given individual differences in structural and functional anatomy in the mPFC (Braga & Buckner, 2017), which may have led to a stimulation of different networks in different individuals. The latter could in part explain the variance in individual responses to rTMS within a stimulation‐group (Figure 5). However, since the group effect in the high‐frequency rTMS group was significantly opposite to the low‐frequency rTMS group (as expected), we assume that we stimulated the same target region in each individual participant relatively consistently.
A typical limitation of TMS studies is that we cannot entirely discard the possibility that high‐ and low‐frequency rTMS affected participants differently due to differences in sounds or sensory perception (esp. given that the frontalis muscle was directly stimulated), a principle confounder in TMS studies. Trained personal frequently and repeatedly screened each individual during TMS to monitor participants' comfort. In these encounters, primarily minor levels of discomfort, such as eye twitching, were reported, qualitatively. Only three participants had to be excluded because of stronger side effects such as headaches. It is plausible that participants did not have much discomfort or side effects, because (a) the stimulation protocol was split into four short blocks and (b) we used a stimulation intensity that was adjusted to 100% of the individual RMT instead of 120% as in other studies. However, we did not enquire discomfort quantitatively.
A further limitation of our design is that we cannot exclude that accidental group differences are responsible for the group effects. At least with respect to the STAI and BDI questionnaires, we did not see such differences. While functional connectivity between mFP and amygdala before TMS (baseline) did not statistically differ between stimulation‐groups, the p‐value is on the level of a trend. Therefore, we cannot exclude that regression‐to‐the‐mean effects may have contributed to our observations. However, this observation could be related to the also observed group difference in the mean BOLD‐mFP(R)/BOLD‐mFP(L)‐ratio, which indicates a stable group difference over all sessions. This makes regression‐to‐the‐mean effects less likely. Furthermore, we did not observe any return‐to‐baseline‐effect. This is not surprising due to power limitations as the effect size of any return‐to‐baseline‐effect has to be smaller than the Pre–Post stimulation effect. Nevertheless, future studies should address this effect and attempt to quantify it as the time scale of the return‐to‐baseline is crucial for future clinical or scientific applications.
Taken together, the results of this study are highly relevant for different fields of research. First, they complement the scarce literature on differential effects of low‐ and high‐frequency rTMS on functional connectivity. Low‐frequency rTMS (1 Hz) resulted in a decrease of functional connectivity and high‐frequency rTMS (20 Hz) resulted in an increase. Second, results suggest rTMS as a promising tool to investigate the neural basis of emotions or/and their cognitive control. Third, results could guide intervention strategies using noninvasive neurostimulation in mental disorders with respect to the stimulation target and stimulation protocol (Bengtsson et al., 2015; Burt et al., 2002; Camchong et al., 2011; Chen et al., 2013; Downar & Daskalakis, 2013; Hasan et al., 2016; Kamphausen et al., 2013; Mukherjee et al., 2016; Shin & Liberzon, 2010). For example: (a) because of the differential effects of low‐ and high‐frequency rTMS, it would be feasible to adjust the stimulation frequency in nonresponders of prefrontal TMS treatment (Fitzgerald et al., 2018), (b) because of the significant effects of mFP stimulation in this study, the stimulation site in nonresponders of prefrontal TMS treatment to the dlPFC could be adjusted (Downar & Daskalakis, 2013), and (c) because of the specific effect of mFP stimulation on fronto‐limbic functional connectivity, it would be feasible to consider this protocol for treatment of symptoms that accompany clinical depression and are related to alterations in this functional connectivity (Kong et al., 2013; Phillips et al., 2015; Young et al., 2016). In addition to all the foregoing, the present study addresses an important short‐coming emphasized, for example, by Post and Keck: “compared to the growing number of clinical studies on its putative therapeutic properties, the studies on the basic mechanisms of rTMS are surprisingly scarce.” (Post & Keck, 2001).
5. CONCLUSION
Despite certain limitations, the results of this study clearly provide initial evidence for opposing effects of low‐ and high‐frequency rTMS on prefrontal‐limbic functional connectivity. This finding has crucial implications in both social neuroscience and clinical research. However, replication of our study in a larger sample would be desirable, especially before drawing final conclusions for medical applications.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
Data S1 Supporting Information.
ACKNOWLEDGMENTS
This research was supported by the Deutsche Forschungsgemeinschaft (DFG) grants SFB 940/1 and SFB 940/2. We thank the staff of the Neuroimaging Center (NIC) of the TU Dresden for help with the data acquisition and the developers of the employed multiband EPI sequence that we received from the Center for Magnetic Resonance Research at the University of Minnesota.
Riedel P, Heil M, Bender S, et al. Modulating functional connectivity between medial frontopolar cortex and amygdala by inhibitory and excitatory transcranial magnetic stimulation. Hum Brain Mapp. 2019;40:4301–4315. 10.1002/hbm.24703
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Numbers: SFB 940/2, SFB 940/1
DATA ACCESSIBILITY
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abraham, E. , Hendler, T. , Shapira‐Lichter, I. , Kanat‐Maymon, Y. , Zagoory‐Sharon, O. , & Feldman, R. (2014). Father's brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences of the United States of America, 111(27), 9792–9797. 10.1073/pnas.1402569111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J. J. B. , & Reznik, S. J. (2015). Frontal EEG asymmetry as a promising marker of depression vulnerability: Summary and methodological considerations. Current Opinion in Psychology, 4, 93–97. 10.1016/j.copsyc.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck Depression Inventory‐II (BDI‐II). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beckmann, M. , Johansen‐Berg, H. , & Rushworth, M. F. (2009). Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience, 29(4), 1175–1190. 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson, J. , Olsson, E. , Wass, C. , & Bodén, R. (2015). Theta burst transcranial magnetic stimulation of the dorsomedial prefrontal cortex in schizophrenia and depression. Brain Stimulation, 8(2), 373. [Google Scholar]
- Braga, R. M. , & Buckner, R. L. (2017). Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron, 95(2), 457 10.1016/j.neuron.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, T. , Lisanby, S. H. , & Sackeim, H. A. (2002). Neuropsychiatric applications of transcranial magnetic stimulation: A meta analysis. International Journal of Neuropsychopharmacology, 5(1), 73–103. 10.1017/S1461145702002791 [DOI] [PubMed] [Google Scholar]
- Bzdok, D. , Langner, R. , Schilbach, L. , Engemann, D. A. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2013). Segregation of the human medial prefrontal cortex in social cognition. Frontiers in Human Neuroscience, 7, 232 10.3389/fnhum.2013.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong, J. , MacDonald, A. W., 3rd , Bell, C. , Mueller, B. A. , & Lim, K. O. (2011). Altered functional and anatomical connectivity in schizophrenia. Schizophrenia Bulletin, 37(3), 640–650. 10.1093/schbul/sbp131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp, J. (2013). Optimizing the order of operations for movement scrubbing: Comment on Power et al. NeuroImage, 76, 436–438. 10.1016/j.neuroimage.2011.12.061 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Zhou, C. , Wu, B. , Wang, Y. , Li, Q. , Wei, Y. , … Xie, P. (2013). Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta‐analysis of randomised controlled trials. Psychiatry Research, 210(3), 1260–1264. 10.1016/j.psychres.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Cirillo, G. , Di Pino, G. , Capone, F. , Ranieri, F. , Florio, L. , Todisco, V. , … Di Lazzaro, V. (2017). Neurobiological after‐effects of non‐invasive brain stimulation. Brain Stimulation, 10(1), 1–18. 10.1016/j.brs.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Cohen, S. (1988). Perceived stress in a probability sample of the United States. In S. Spacapan & S. Oskamp (Eds.), The Claremont Symposium on Applied Social Psychology. The social psychology of health (pp. 31–67). Thousand Oaks, CA, US: Sage Publications, Inc.
- Cohen, S. , & Janicki‐Deverts, D. (2012). Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. Journal of Applied Social Psychology, 42(6), 1320–1334. 10.1111/j.1559-1816.2012.00900.x [DOI] [Google Scholar]
- Cohen, S. , Kamarck, T. , & Mermelstein, R. (1994). Perceived stress scale In S. Cohen, R. C. Kessler, & L. U. Gordon, (Eds.), Measuring stress: A guide for health and social scientists (pp. 235–283). New York, NY, US: Oxford University Press.
- Courtin, J. , Bienvenu, T. C. M. , Einarsson, E. O. , & Herry, C. (2013). Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience, 240, 219–242. 10.1016/j.neuroscience.2013.03.001 [DOI] [PubMed] [Google Scholar]
- de Jesus, D. R. , Favalli, G. P. D. , Hoppenbrouwers, S. S. , Barr, M. S. , Chen, R. , Fitzgerald, P. B. , & Daskalakis, Z. J. (2014). Determining optimal rTMS parameters through changes in cortical inhibition. Clinical Neurophysiology, 125(4), 755–762. 10.1016/j.clinph.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Delli Pizzi, S. , Chiacchiaretta, P. , Mantini, D. , Bubbico, G. , Ferretti, A. , Edden, R. A. , … Bonanni, L. (2017). Functional and neurochemical interactions within the amygdala‐medial prefrontal cortex circuit and their relevance to emotional processing. Brain Structure & Function, 222(3), 1267–1279. 10.1007/s00429-016-1276-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donse, L. , Padberg, F. , Sack, A. T. , Rush, A. J. , & Arns, M. (2018). Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimulation, 11(2), 337–345. 10.1016/j.brs.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Downar, J. (2017). Predictors and correlates of rTMS response on resting‐state functional MRI. Biological Psychiatry, 81(10), S12–S12. [Google Scholar]
- Downar, J. , & Daskalakis, Z. J. (2013). New targets for rTMS in depression: A review of convergent evidence. Brain Stimulation, 6(3), 231–240. 10.1016/j.brs.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. T. , Bzdok, D. , & Hensel, L. (2016). Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex, 26(1), 304–321. 10.1093/cercor/bhu250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief, M. C. , Halko, M. A. , Buckner, R. L. , & Pascual‐Leone, A. (2011). Transcranial magnetic stimulation modulates the brain's intrinsic activity in a frequency‐dependent manner. Proceedings of the National Academy of Sciences of the United States of America, 108(52), 21229–21234. 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Fountain, S. , & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596. 10.1016/j.clinph.2006.06.712 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Hoy, K. E. , Elliot, D. , McQueen, S. , Wambeek, L. E. , & Daskalakis, Z. J. (2018). Exploring alternative rTMS strategies in non‐responders to standard high frequency left‐sided treatment: A switching study. Journal of Affective Disorders, 232, 79–82. 10.1016/j.jad.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Folloni, D. , Sallet, J. , Khrapitchev, A. A. , Sibson, N. R. , Verhagen, L. , & Mars, R. B. (2019). Two fiber pathways connecting amygdala and prefrontal cortex in humans and monkeys. bioRxiv, 561811 10.1101/561811 [DOI] [Google Scholar]
- Forbes, C. E. , & Grafman, J. (2010). The role of the human prefrontal cortex in social cognition and moral judgment. Annual Review of Neuroscience, 33, 299–324. 10.1146/annurev-neuro-060909-153230 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Halko, M. A. , Eldaief, M. C. , & Pascual‐Leone, A. (2012). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). NeuroImage, 62(4), 2232–2243. 10.1016/j.neuroimage.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M. S. , Stallings, L. E. , Speer, A. M. , Nahas, Z. , Spicer, K. M. , Vincent, D. J. , … Risch, S. C. (1999). Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Human Psychopharmacology—Clinical and Experimental, 14(3), 161 [DOI] [Google Scholar]
- Goetschius, L. G. , Hein, T. C. , Mattson, W. I. , Lopez‐Duran, N. , Dotterer, H. L. , Welsh, R. C. , … Monk, C. S. (2019). Amygdala‐prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. NeuroImage, 191, 278–291. 10.1016/j.neuroimage.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, A. L. , Morey, R. A. , & McCarthy, G. (2015). Amygdala‐prefrontal cortex functional connectivity during threat‐induced anxiety and goal distraction. Biological Psychiatry, 77(4), 394–403. 10.1016/j.biopsych.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, T. , Engeler, J. , Achermann, P. , Mosimann, U. P. , Noss, R. , Fisch, H. U. , & Schlaepfer, T. E. (2001). High frequency repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral cortex: EEG topography during waking and subsequent sleep. Psychiatry Research, 107(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Gratton, C. , Lee, T. G. , Nomura, E. M. , & D'Esposito, M. (2014). Perfusion MRI indexes variability in the functional brain effects of theta‐burst transcranial magnetic stimulation. PLoS One, 9(7), e101430 10.1371/journal.pone.0101430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griskova, I. , Ruksenas, O. , Dapsys, K. , Herpertz, S. , & Hoppner, J. (2007). The effects of 10 Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neuroscience Letters, 419(2), 162–167. 10.1016/j.neulet.2007.04.030 [DOI] [PubMed] [Google Scholar]
- Grossheinrich, N. , Rau, A. , Pogarell, O. , Hennig‐Fast, K. , Reinl, M. , Karch, S. , … Padberg, F. (2009). Theta burst stimulation of the prefrontal cortex: Safety and impact on cognition, mood, and resting electroencephalogram. Biological Psychiatry, 65(9), 778–784. 10.1016/j.biopsych.2008.10.029 [DOI] [PubMed] [Google Scholar]
- Guhn, A. , Dresler, T. , Andreatta, M. , Muller, L. D. , Hahn, T. , Tupak, S. V. , … Herrmann, M. J. (2014). Medial prefrontal cortex stimulation modulates the processing of conditioned fear. Frontiers in Behavioral Neuroscience, 8, 44 10.3389/fnbeh.2014.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon, C. A. , Canterberry, M. , Taylor, J. J. , DeVries, W. , Li, X. B. , Brown, T. R. , & George, M. S. (2013). Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: A pilot study. PLoS One, 8(7), e67917 10.1371/journal.pone.0067917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, A. , Strube, W. , Palm, U. , & Wobrock, T. (2016). Repetitive noninvasive brain stimulation to modulate cognitive functions in schizophrenia: A systematic review of primary and secondary outcomes. Schizophrenia Bulletin, 42(Suppl. 1), S95–S109. 10.1093/schbul/sbv158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, M. J. , Katzorke, A. , Busch, Y. , Gromer, D. , Polak, T. , Pauli, P. , & Deckert, J. (2017). Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimulation, 10(2), 291–297. 10.1016/j.brs.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Hiser, J. , & Koenigs, M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J. , Sporns, O. , Cammoun, L. , Gigandet, X. , Thiran, J. P. , Meuli, R. , & Hagmann, P. (2009). Predicting human resting‐state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. 10.1073/pnas.0811168106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam, J. M. , Ramakers, G. M. , & Di Lazzaro, V. (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimulation, 3(2), 95–118. 10.1016/j.brs.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Ilmoniemi, R. J. , Virtanen, J. , Ruohonen, J. , Karhu, J. , Aronen, H. J. , Naatanen, R. , & Katila, T. (1997). Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport, 8(16), 3537–3540. 10.1097/00001756-199711100-00024 [DOI] [PubMed] [Google Scholar]
- Kamphausen, S. , Schroder, P. , Maier, S. , Bader, K. , Feige, B. , Kaller, C. P. , … Tuscher, O. (2013). Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. World Journal of Biological Psychiatry, 14(4), 307–318, S301–304. 10.3109/15622975.2012.665174 [DOI] [PubMed] [Google Scholar]
- Knight, R. G. , Waalmanning, H. J. , & Spears, G. F. (1983). Some norms and reliability data for the state trait anxiety inventory and the Zung self‐rating depression scale. British Journal of Clinical Psychology, 22(November), 245–249. [DOI] [PubMed] [Google Scholar]
- Kobayashi, M. , & Pascual‐Leone, A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurology, 2(3), 145–156. [DOI] [PubMed] [Google Scholar]
- Kober, H. , Barrett, L. F. , Joseph, J. , Bliss‐Moreau, E. , Lindquist, K. , & Wager, T. D. (2008). Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. NeuroImage, 42(2), 998–1031. 10.1016/j.neuroimage.2008.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L. T. , Chen, K. Y. , Tang, Y. Q. , Wu, F. , Driesen, N. , Womer, F. , … Wang, F. (2013). Functional connectivity between the amygdala and prefrontal cortex in medication‐naive individuals with major depressive disorder. Journal of Psychiatry and Neuroscience, 38(6), 417–422. 10.1503/jpn.120117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamusuo, S. , Hirvonen, J. , Lindholm, P. , Martikainen, I. K. , Hagelberg, N. , Parkkola, R. , … Jaaskelainen, S. K. (2017). Neurotransmitters behind pain relief with transcranial magnetic stimulation—Positron emission tomography evidence for release of endogenous opioids. European Journal of Pain (London, England), 21(9), 1505–1515. 10.1002/ejp.1052 [DOI] [PubMed] [Google Scholar]
- Lancaster, J. L. , Woldorff, M. G. , Parsons, L. M. , Liotti, M. , Freitas, C. S. , Rainey, L. , … Fox, P. T. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz, M. , Galanis, C. , Muller‐Dahlhaus, F. , Opitz, A. , Wierenga, C. J. , Szabo, G. , … Vlachos, A. (2016). Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nature Communications, 7, 10020 10.1038/ncomms10020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik, E. , & Paz, R. (2015). Amygdala‐prefrontal interactions in (mal)adaptive learning. Trends in Neurosciences, 38(3), 158–166. 10.1016/j.tins.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Qin, W. , Li, W. , Fan, L. , Wang, J. , Jiang, T. , & Yu, C. (2013). Connectivity‐based parcellation of the human frontal pole with diffusion tensor imaging. Journal of Neuroscience, 33(16), 6782–6790. 10.1523/JNEUROSCI.4882-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo, C. K. , Sachdev, P. S. , Haindl, W. , Wen, W. , Mitchell, P. B. , Croker, V. M. , & Malhi, G. S. (2003). High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychological Medicine, 33(6), 997–1006. 10.1017/S0033291703007955 [DOI] [PubMed] [Google Scholar]
- Maldjian, J. A. , Laurienti, P. J. , & Burdette, J. H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian, J. A. , Laurienti, P. J. , Kraft, R. A. , & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Milbradt, A. , Zimmerhofer, A. , & Hornke, L. (2007. –2018). testMaker—A computer software for web‐based assessments. Department of Industrial and Organizational Psychology, Aachen, Germany: RWTH Aachen University. [Google Scholar]
- Moayedi, M. , Salomons, T. V. , Dunlop, K. A. , Downar, J. , & Davis, K. D. (2015). Connectivity‐based parcellation of the human frontal polar cortex. Brain Structure & Function, 220(5), 2603–2616. 10.1007/s00429-014-0809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, S. , Yacoub, E. , Olman, C. A. , Auerbach, E. , Strupp, J. , Harel, N. , & Ugurbil, K. (2010). Multiband multislice GE‐EPI at 7 tesla, with 16‐fold acceleration using partial parallel imaging with application to high spatial and temporal whole‐brain FMRI. Magnetic Resonance in Medicine, 63(5), 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin, J. C. , Philippi, C. L. , Wolf, R. C. , Baskaya, M. K. , & Koenigs, M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–284. 10.1016/j.biopsych.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, P. , Sabharwal, A. , Kotov, R. , Szekely, A. , Parsey, R. , Barch, D. M. , & Mohanty, A. (2016). Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophrenia Bulletin, 42(4), 1056–1067. 10.1093/schbul/sbw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers‐Schulz, B. , & Koenigs, M. (2012). Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Molecular Psychiatry, 17(2), 132–141. 10.1038/mp.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas, Z. , Lomarev, M. , Roberts, D. R. , Shastri, A. , Lorberbaum, J. P. , Teneback, C. , … Bohning, D. E. (2001). Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity‐dependent bilateral effects as measured by interleaved BOLD fMRI. Biological Psychiatry, 50(9), 712–720. 10.1016/S0006-3223(01)01199-4 [DOI] [PubMed] [Google Scholar]
- Ohnishi, T. , Hayashi, T. , Okabe, S. , Nonaka, I. , Matsuda, H. , Iida, H. , … Ugawa, Y. (2004). Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: An [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biological Psychiatry, 55(5), 484–489. 10.1016/j.biopsych.2003.09.016 [DOI] [PubMed] [Google Scholar]
- Okamura, H. , Jing, H. , & Takigawa, M. (2001). EEG modification induced by repetitive transcranial magnetic stimulation. Journal of Clinical Neurophysiology, 18(4), 318–325. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Park, A. T. , Leonard, J. A. , Saxler, P. K. , Cyr, A. B. , Gabrieli, J. D. E. , & Mackey, A. P. (2018). Amygdala‐medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Social Cognitive and Affective Neuroscience, 13(4), 430–439. 10.1093/scan/nsy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Tormos, J. M. , Keenan, J. , Tarazona, F. , Canete, C. , & Catala, M. D. (1998). Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology, 15(4), 333–343. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Walsh, V. , & Rothwell, J. (2000). Transcranial magnetic stimulation in cognitive neuroscience—Virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology, 10(2), 232–237. [DOI] [PubMed] [Google Scholar]
- Paus, T. , Castro‐Alamancos, M. A. , & Petrides, M. (2001). Cortico‐cortical connectivity of the human mid‐dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. European Journal of Neuroscience, 14(8), 1405–1411. [DOI] [PubMed] [Google Scholar]
- Penny, W. D. , Friston, K. J. , Ashburner, J. T. , Kiebel, S. J. , & Nichols, T. E. (2011). Statistical parametric mapping: The analysis of functional brain images: The analysis of functional brain images. Cambridge, MA: Academic Press. [Google Scholar]
- Perera, T. , George, M. S. , Grammer, G. , Janicak, P. G. , Pascual‐Leone, A. , & Wirecki, T. S. (2016). The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L. , Wager, T. , Taylor, S. F. , & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331–348. 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , Delgado, M. R. , Nearing, K. I. , & LeDoux, J. E. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43(6), 897–905. 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. , & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48(2), 175–187. 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Chase, H. W. , Sheline, Y. I. , Etkin, A. , Almeida, J. R. , Deckersbach, T. , & Trivedi, M. H. (2015). Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: Neuroimaging approaches. American Journal of Psychiatry, 172(2), 124–138. 10.1176/appi.ajp.2014.14010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514. [DOI] [PubMed] [Google Scholar]
- Post, A. , & Keck, M. E. (2001). Transcranial magnetic stimulation as a therapeutic tool in psychiatry: What do we know about the neurobiological mechanisms? Journal of Psychiatric Research, 35(4), 193–215. 10.1016/S0022-3956(01)00023-1 [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripfl, J. , Tomova, L. , Riecansky, I. , & Lamm, C. (2014). Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue‐induced nicotine craving and EEG delta power. Brain Stimulation, 7(2), 226–233. 10.1016/j.brs.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Raine, A. (2018). The neuromoral theory of antisocial, violent, and psychopathic behavior. Psychiatry Research, 277, 64–69. 10.1016/j.psychres.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Rossi, S. , Hallett, M. , Rossini, P. M. , & Pascual‐Leone, A. (2011). Screening questionnaire before TMS: An update. Clinical Neurophysiology, 122(8), 1686 10.1016/j.clinph.2010.12.037 [DOI] [PubMed] [Google Scholar]
- Roy, A. K. , Fudge, J. L. , Kelly, C. , Perry, J. S. , Daniele, T. , Carlisi, C. , … Ernst, M. (2013). Intrinsic functional connectivity of amygdala‐based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 52(3), 290–299 e292. 10.1016/j.jaac.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, A. K. , Shehzad, Z. , Margulies, D. S. , Kelly, A. M. C. , Uddin, L. Q. , Gotimer, K. , … Milham, M. P. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. 10.1016/j.neuroimage.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, M. , Shohamy, D. , & Wager, T. D. (2012). Ventromedial prefrontal‐subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet, J. , Mars, R. B. , Noonan, M. P. , Neubert, F. X. , Jbabdi, S. , O'Reilly, J. X. , … Rushworth, M. F. (2013). The organization of dorsal frontal cortex in humans and macaques. Journal of Neuroscience, 33(30), 12255–12274. 10.1523/Jneurosci.5108-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop, K. , Gagoski, B. A. , Polimeni, J. R. , Witzel, T. , Wedeen, V. J. , & Wald, L. L. (2012). Blipped‐controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g‐factor penalty. Magnetic Resonance in Medicine, 67(5), 1210–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, L. M. , & Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer, A. M. , Kimbrell, T. A. , Wassermann, E. M. , Repella, J. D. , Willis, M. W. , Herscovitch, P. , & Post, R. M. (2000). Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biological Psychiatry, 48(12), 1133–1141. 10.1016/S0006-3223(00)01065-9 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. (2010). State‐Trait anxiety inventory. In I. B. Weiner, & W. E. Craighead (Eds.), The Corsini encyclopedia of psychology (Vol. 4. pp. 1–1). Hoboken, NJ, US: John Wiley & Sons, Inc. [Google Scholar]
- Strafella, A. P. , Paus, T. , Barrett, J. , & Dagher, A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience, 21(15), RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten, M. , Dell'Acqua, F. , Valabregue, R. , & Catani, M. (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex, 48(1), 82–96. 10.1016/j.cortex.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Tupak, S. V. , Dresler, T. , Badewien, M. , Hahn, T. , Ernst, L. H. , Herrmann, M. J. , … Fallgatter, A. J. (2013). Inhibitory transcranial magnetic theta burst stimulation attenuates prefrontal cortex oxygenation. Human Brain Mapping, 34(1), 150–157. 10.1002/hbm.21421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews: Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Valero‐Cabre, A. , Amengual, J. L. , Stengel, C. , Pascual‐Leone, A. , & Coubard, O. A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience and Biobehavioral Reviews, 83, 381–404. 10.1016/j.neubiorev.2017.10.006 [DOI] [PubMed] [Google Scholar]
- van der Werf, Y. D. , Sanz‐Arigita, E. J. , Menning, S. , & van den Heuvel, O. A. (2010). Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neuroscience, 11, 145. 10.1186/1471-2202-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide, R. J. , Skipper, L. M. , Klobusicky, E. , & Olson, I. R. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136(Pt 6), 1692–1707. 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T. , Hanajima, R. , Shirota, Y. , Ohminami, S. , Tsutsumi, R. , Terao, Y. , … Ohtomo, K. (2014). Bidirectional effects on interhemispheric resting‐state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Human Brain Mapping, 35(5), 1896–1905. 10.1002/hbm.22300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher, A. , Kasess, C. , Gerstl, F. , Lanzenberger, R. , Moser, E. , & Windischberger, C. (2009). Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage, 47(4), 1408–1416. 10.1016/j.neuroimage.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Whisman, M. A. , & Richardson, E. D. (2015). Normative data on the Beck Depression Inventory—Second edition (BDI‐II) in college students. Journal of Clinical Psychology, 71, 898–907. [DOI] [PubMed] [Google Scholar]
- Windischberger, C. , Langenberger, H. , Sycha, T. , Tschernko, E. M. , Fuchsjager‐Mayerl, G. , Schmetterer, L. , & Moser, E. (2002). On the origin of respiratory artifacts in BOLD‐EPI of the human brain. Magnetic Resonance Imaging, 20(8), 575–582. [DOI] [PubMed] [Google Scholar]
- Wozniak‐Kwasniewska, A. , Szekely, D. , Aussedat, P. , Bougerol, T. , & David, O. (2014). Changes of oscillatory brain activity induced by repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in healthy subjects. NeuroImage, 88, 91–99. 10.1016/j.neuroimage.2013.11.029 [DOI] [PubMed] [Google Scholar]
- Xu, J. Q. , Moeller, S. , Auerbach, E. J. , Strupp, J. , Smith, S. M. , Feinberg, D. A. , … Ugurbil, K. (2013). Evaluation of slice accelerations using multiband echo planar imaging at 3 T. NeuroImage, 83, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Bodurka, J. , & Drevets, W. C. (2016). Amygdala activity during autobiographical memory recall in depressed and vulnerable individuals: Association with symptom severity and autobiographical overgenerality. American Journal of Psychiatry, 173(1), 78–89. 10.1176/appi.ajp.2015.15010119 [DOI] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Misaki, M. , Zotev, V. , Phillips, R. , Drevets, W. C. , & Bodurka, J. (2018). Altered task‐based and resting‐state amygdala functional connectivity following real‐time fMRI amygdala neurofeedback training in major depressive disorder. NeuroImage: Clinical, 17, 691–703. 10.1016/j.nicl.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann, U. (2017). Thirty years of transcranial magnetic stimulation: Where do we stand? Experimental Brain Research, 235(4), 973–984. 10.1007/s00221-016-4865-4 [DOI] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Young, K. D. , Drevets, W. C. , & Bodurka, J. (2013). Prefrontal control of the amygdala during real‐time fMRI neurofeedback training of emotion regulation. PLoS One, 8(11), e79184 10.1371/journal.pone.0079184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


