Abstract
Reach movements are characterized by multiple kinematic variables that can change with age or due to medical conditions such as movement disorders. While the neural control of reach direction is well investigated, the elements of the neural network regulating speed (the nondirectional component of velocity) remain uncertain. Here, we used a custom made magnetic resonance (MR)‐compatible arm movement tracking system to capture the real kinematics of the arm movements while measuring brain activation with functional magnetic resonance imaging to reveal areas in the human brain in which BOLD‐activation covaries with the speed of arm movements. We found significant activation in multiple cortical and subcortical brain regions positively correlated with endpoint (wrist) speed (speed‐related activation), including contralateral premotor cortex (PMC), supplementary motor area (SMA), thalamus (putative VL/VA nuclei), and bilateral putamen. The hand and arm regions of primary sensorimotor cortex (SMC) and a posterior region of thalamus were significantly activated by reach movements but showed a more binary response characteristics (movement present or absent) than with continuously varying speed. Moreover, a subregion of contralateral SMA also showed binary movement activation but no speed‐related BOLD‐activation. Effect size analysis revealed bilateral putamen as the most speed‐specific region among the speed‐related clusters whereas primary SMC showed the strongest specificity for movement versus non‐movement discrimination, independent of speed variations. The results reveal a network of multiple cortical and subcortical brain regions that are involved in speed regulation among which putamen, anterior thalamus, and PMC show highest specificity to speed, suggesting a basal‐ganglia‐thalamo‐cortical loop for speed regulation.
Keywords: arm movement, arm movement tracking, basal‐ganglia‐thalamo‐cortical loop, functional magnetic resonance imaging, kinematics, neural control of speed
1. INTRODUCTION
Reach movement velocity comprises two kinematic variables: direction and speed. While neural correlates of movement direction have been relatively well characterized (Eisenberg, Shmuelof, Vaadia, & Zohary, 2010; Fabbri, Caramazza, & Lingnau, 2010; Georgopoulos, Kalaska, Caminiti, & Massey, 1982), less is known about the neural control of movement speed, in particular at the level of the whole human brain. Understanding brain control of movement speed is important as bradykinesia, the inability to perform fast movements, is a cardinal problem in movement disorders like Parkinson disease (Berardelli, Rothwell, Thompson, & Hallett, 2001). Moreover, a major problem of controlling prosthesis kinematics with motor brain‐machine interfaces is based in the difficulty to decode reliable speed signals from motor cortex (Golub, Yu, Schwartz, & Chase, 2014).
Electrophysiological studies have investigated movement speed representations at the single neuron and population response level with mixed results. Schwartz (1992) recorded single neuron activity from monkey motor cortex during an arm tracking task and reported strong orientation tuning but only very little correlation between firing rates and tracking speed. Employing a center‐out‐task, Moran and Schwartz (1999) found that single‐cell firing rates in monkey primary motor cortex (M1) coded the speed of wrist movement only along the neurons' preferred direction. Using a similar task, Churchland, Santhanam, and Shenoy (2006b) trained monkeys to perform center‐out movements at two different speeds and found that the direction tuning of many neurons in M1 and dorsal premotor cortex (PMd) was altered by instructed speed in the preparatory phase of the movement. Interestingly, trial‐wise variations of single neuron activations in M1 and PMd in the preparatory phase predicted some of the speed variability in the executed reach movement (Churchland, Afshar, & Shenoy, 2006a). Using information based analyses, Golub et al. (2014) found that the single trial activation of small populations of motor cortical neurons conveyed much more information about endpoint movement direction than speed. In contrast to the monkey single‐cell studies, Hammer et al. (2016) recorded iEEG in and around human hand/arm motor cortex from large neuronal populations and found that single iEEG‐electrode time series' correlated stronger with the undirected speed of movement than directed velocity. This difference between studies may be due to the different population sizes that were used for speed prediction (Hammer et al., 2016) in the different studies or due to sampling from different motor cortical areas with different functional properties. Although single‐cell electrophysiological studies can provide information about speed coding at great spatial and functional detail, they usually record from few cortical brain areas and rarely record activations in deeper brain structures.
While lacking the spatial specificity of single‐cell recordings, neuroimaging studies can provide a spatially more complete picture of movement speed coding in an individual's whole brain. A positron emission tomography (PET) study conducted by Turner, Grafton, Votaw, Delong, and Hoffman (1998) investigated regional cerebral blood flow (rCBF) changes in the human brain correlated with repetitive arm movements performed at different rates and reported correlations in primary sensorimotor cortices (SMC), globus pallidus, and cerebellum. In another PET study, Turner, Desmurget, Grethe, Crutcher, and Grafton (2003) investigated the correlation of arm movement extent and speed with rCBF changes. Here, the authors observed that activation in ipsilateral cerebellum and bilateral basal ganglia (putamen and globus pallidus) correlated with the extent and speed of movement. However, rCBF changes were hardly detectable in SMC where single‐cell electrophysiological studies reported varying degrees of correlation between movement speed and neuronal activation. More recently, functional magnetic resonance imaging (fMRI) correlates of foot movement speed were studied in power and endurance athletes (Wenzel, Taubert, Ragert, Krug, & Villringer, 2014). In this study, fast plantar flexions caused increased blood‐oxygenation‐level‐dependent (BOLD) activation in the cerebellar anterior lobe, but no speed‐specific functional difference was observed in M1 and supplementary motor area (SMA) which showed activation during repetitive dorsi‐ and plantar‐flexions. These neuroimaging studies provide valuable information about potential neural substrates of movement speed control. However, the experimental paradigms, utilized in these studies, confounded the speed of movement with a second variable, that is, movement extent (Turner et al., 2003) or rate (repetitions per time unit, Turner et al., 1998). Hence, the reported results might not accurately reflect the speed‐specific activation pattern in the brain. Moreover, these studies employed rather simple single‐joint movements which may result in different activation pattern compared to multi‐joint movements (Moran & Schwartz, 1999). One reason for reducing the complexity of the motor task is that the kinematic parameters of multi‐joint movements are hard to measure in the magnetic resonance (MR) environment due to the multiple degrees of freedom involved.
To the best of our knowledge, the current study is the first to investigate the fMRI correlates of in situ measured endpoint (wrist) speed of naturalistic multi‐joint arm movements in the human brain independently of variations in rate and distance. Unlike single‐cell recordings, which are restricted to a small brain region/neuronal population, fMRI allows us to noninvasively probe reach movement speed effects in both cortical and subcortical regions of the human brain. In our study we defined movement speed as the amplitude of the movement velocity vector. We measured the actual endpoint speed of reach movements with a custom build MR‐compatible arm movement tracking system (Shirinbayan & Rieger, 2017) during fMRI‐scanning and used these measurements of actual (wrist) movement speed to reveal speed‐related brain activity. We compare the patterns of brain activation correlating with varying endpoint speed to patterns of brain areas exhibiting a categorical response, independent of speed variation, to the presence or absence of movement (denoted “movement per se” from here on) and report effect sizes to characterize the selectivity of brain areas for speed and movement per se.
2. MATERIALS AND METHODS
2.1. Subjects
Fourteen volunteers (six females; mean age: 24.5 years; range: 21–35) participated in the experiment. All subjects were right‐handed, as assessed with the Edinburg handedness inventory, and had no motor impairment. They provided their written informed consent prior to the experiment. The experimental procedure was approved by the ethics committee of the Carl von Ossietzky University of Oldenburg.
2.2. Experimental paradigm
Subjects took part in four sessions each lasting 12 min. A half‐circle wooden plate was placed on the bed of the scanner above the torso (Figure 1). It comprised two paths differing with 90° and connecting a start point to two targets indicated by “1” and “2” (distance between the targets and the start point: 22 cm). Subjects were instructed to move with their extended index finger from the start point toward the targets at slow or normal speed and immediately return without stopping at the targets. In order to minimize potential movement trajectory differences between the slow/normal speed conditions, we also instructed the subjects to perform the slow and normal reaches consistently along the lines drawn between the start point and the targets. There was a training session prior to the experiment where the subjects practiced the slow and normal movements until the movement speeds were confirmed by the experimenter. Each session included 32 randomly ordered movements in an event‐related design, eight movements for each permutation of target position and speed: Target 1, slow speed; Target 1, normal speed; Target 2, slow speed; Target 2, normal speed. The intertrial intervals were randomly varied between 14 and 16 s. Auditory cues (spoken commands through MR‐compatible headphones) indicated the start of each trial as well as the intended target and movement speed (slow or normal). Baseline periods (35 s duration) without movement were included at the beginning and end of each session. We mounted a double‐mirror on the head coil to provide the subjects with vision to the start and target points and cushions were placed under their arm for comfort at the start position.
Figure 1.

Experimental setup. Targets were shown by “1” and “2” on the wooden plate at the end of the instructed movement paths differing with 90°. G1–5 illustrate the movement tracking gyroscopes mounted on the arm for capturing the kinematics of arm movements
2.3. Arm movement tracking
We used an MR‐compatible gyroscope‐based arm movement tracking system to capture the movement kinematics while the subjects performed the task inside the scanner (G1–5 in Figure 1). For individual arm joints, the movement tracker converts the measured angular velocity (provided by MEMS gyroscopes) to angular displacement and combines the resulting rotations with the movement simulation of a realistic arm skeleton model in the OpenSim software (Delp et al., 2007; Seth, Sherman, Eastman, & Delp, 2010) to provide different kinematic variables such as displacement, velocity, and trajectory of points of interest that can be placed at any position on the arm (Shirinbayan & Rieger, 2017). For the fMRI‐analyses, we use the average speed of individual movements (as opposed to maximum speed) measured at the wrist, because the slow BOLD‐response cannot follow the rapid changes in the movement speed profile and wrist speed is the independent variable that was varied in many studies on neuronal speed coding (e.g., Churchland, Afshar, et al., 2006a; Churchland, Santhanam, et al. 2006b; Golub et al., 2014; Moran & Schwartz, 1999; Schwartz, 1992). The onset and end of the movement intervals were manually extracted by visual inspection of the movement sensor signals (gyroscopes) for each trial. The movement intervals included both the forward and backward movements and the unsigned wrist speed signal was then averaged over this interval (see Figure 2a).
Figure 2.
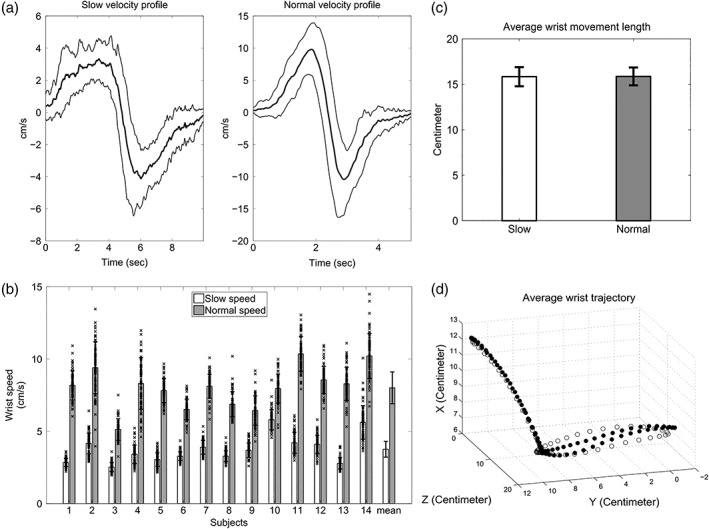
(a) Example average velocity profile (S03). Each average velocity profile (thick curves) was obtained by averaging over the individual slow or normal velocities (64 trials per velocity type). Thin curves depict the corresponding standard deviations. (b) Average wrist speed. The first 14 grouped bars show the average wrist speed for individual subjects and for slow (white bars) and normal (gray bars) movements separately. Error bars depict the corresponding standard deviations. The last grouped bars show the average wrist speeds calculated for each movement type over all subjects and sessions. The crosses depict the average wrist speeds of individual trials and show naturally occurring trial‐by‐trial variations. (c) Average wrist movement length. The bars illustrate the mean length of wrist movements averaged over all subjects for slow and normal speeds separately. Standard deviations are shown by error bars. (d) Wrist movement trajectory. The white and black circles depict the average wrist trajectories (over all trials/subjects) for normal and slow speeds, respectively
2.4. FMRI data acquisition
We acquired brain images using a 3T Siemens Verio MR scanner (Erlangen, Germany) equipped with a 20‐channel head coil. The functional images were acquired through 35 axial slices with a gradient‐recalled echo planer imaging sequence (repetition time = 2000 ms; voxel resolution = 3 × 3 × 3 mm; field of view = 190 mm; echo time = 30 ms; flip angle = 80°). A T1 structural image was acquired using an MPRAGE‐sequence with a voxel size of 1 × 1 × 1 mm in a 256 mm field of view and 1900 ms repetition time.
2.5. FMRI data analysis
The statistical parametric mapping (SPM 12) toolbox (2014, Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) was used to analyze the functional images acquired during the individual sessions. For preprocessing, the images were first realigned with respect to the mean image and then slice‐time corrected. Co‐registration was performed afterwards to adjust the structural image with the mean image yielded from realignment. The resulting functional images were spatially normalized to the standard Montreal Neurological Institute (MNI) template (Collins et al., 1998), smoothed with full‐width half‐maximum Gaussian kernel (of 6 mm), and the voxel time series were high‐pass filtered with a cut‐off period of 128 s to attenuate low‐frequency artifacts. During the preprocessing steps, we used the highest order of interpolation (seventh Degree B‐Spline) to get more accurate results (Thévenaz, Blu, & Unser, 2000).
Following preprocessing, we employed two general linear models (GLM) and performed a first‐level analysis on individual subjects' data in order to separately characterize the effects of movement speed and movement per se on BOLD‐activation. We designed the first GLM to reveal speed‐related effects independent of movement per se‐related effects. Therefore, we included one binary movement per se (movement on/off) regressor with the appropriate movement durations and one parametrically modulated speed regressor with the average wrist speed and movement duration of individual trials. Then, we orthogonalized the speed regressor with respect to the movement per se regressor in order to obtain an independent estimate of the speed‐related effect. In this way, the speed regressor captures the speed‐related effects independent of the movement per se effects, while the parameter estimates associated with the movement per se effects are no longer interpretable (Mumford, Poline, & Poldrack, 2015). In addition, we included two regressors for movement direction and one regressor accounting for the baseline intervals (no movements) at the start and end of each session. Moreover, the head motion parameters were included as covariates of no interest. We then employed a second GLM in order to identify the brain regions where the BOLD‐activation positively correlated with movement per se. The second model included all regressors of the first model, except the speed regressor.
Based on the parameter estimates of the individual first‐level analyses, we performed two second‐level random effects analyses to reveal brain regions that show statistically significant BOLD‐activation across subjects associated with movement speed and movement per se. The reported results indicate voxels with positive correlations between BOLD‐responses and the GLM‐regressors that exceed a voxel‐level FWE‐corrected threshold at p < .05 (correction without cluster size constraints [Eklund, Nichols, & Knutsson, 2016] as implemented in SPM12) and form clusters with an extent of at least five voxels.
For functional labeling of statistically significant activation clusters in the cortex, we used the automated anatomical labeling atlas (Tzourio‐Mazoyer et al., 2002) and for subcortical clusters the Chakravarty atlas (Chakravarty, Bertrand, Hodge, Sadikot, & Collins, 2006).
3. RESULTS
In this section, we demonstrate the behavioral results as well as the BOLD‐activations yielded from the fMRI data analyses.
3.1. Behavioral results
The motion tracking results confirmed that all subjects were able to execute distinguishable slow and normal speed movements. Figure 2a illustrates exemplary average velocity profiles of one subject (S03). Each velocity profile (thick curves) was obtained by averaging over the individual slow or normal velocities (64 trials per velocity type). The thin curves depict the corresponding standard deviations. The grouped bars in Figure 2b show the average wrist speed for each subject and for slow and normal speeds separately (white bars: slow speed; gray bars: normal speed). As expected, instructed normal movements were performed faster (range: 5.1–10.3 cm/s) compared to the slow movements (range: 2.5–5.8 cm/s). The corresponding standard deviations, illustrated by the error bars, were also larger for the normal movements (range: 0.7–1.8 cm/s) compared to the slow ones (range: 0.3–1.2 cm/s). Over all subjects, the wrist speed for the instructed normal movements was approximately twice the speed of instructed slow movements (last grouped bars; mean of normal speeds: 8.1 cm/s, SD: 1.1 cm/s; mean of slow speeds: 3.8 cm/s, SD: 0.5 cm/s). The crosses in Figure 2b depict the average wrist speeds for individual trials and show a considerable natural variation over trials. In six subjects (2, 3, 4, 9, 10, and 14), the average trial speeds even overlapped between the instructed slow and normal conditions. We took these trial‐by‐trial speed variations into account in our analysis of speed effects by including the average speed of each single trial into the GLM.
The average wrist movement lengths are shown in Figure 2c for slow and normal speeds separately. The lengths of the movement paths (mean 15.8 cm) did not differ significantly between slow and normal speeds in a paired‐sample t‐test across subjects (df = 13, t = 0.23, p = .82). Moreover, the curvatures of the slow and normal movement trajectories were virtually identical for leftward movements and differed only slightly for rightward movements (Figure 2d). As our analysis of the speed effect did not distinguish between the directions (see Section 2.5), we consider it highly unlikely that curvature had an effect on the speed results.
3.2. FMRI results
3.2.1. Speed‐related activation
The MRI‐compatible movement tracker allowed us to measure the continuous arm movement kinematics concurrently with the fMRI data acquisition. In the following analysis we used the average wrist speed of individual trials to identify the brain regions where the BOLD‐activation positively correlated with the varying levels of speed. This approach models naturally occurring in addition to instructed movement speed variations in the GLM. Performing second‐level analysis with a FWE‐corrected threshold (p < .05; minimum cluster size: five voxels), we found multiple cortical and subcortical brain regions. These regions are shown by encircled areas in Figure 3.
Figure 3.
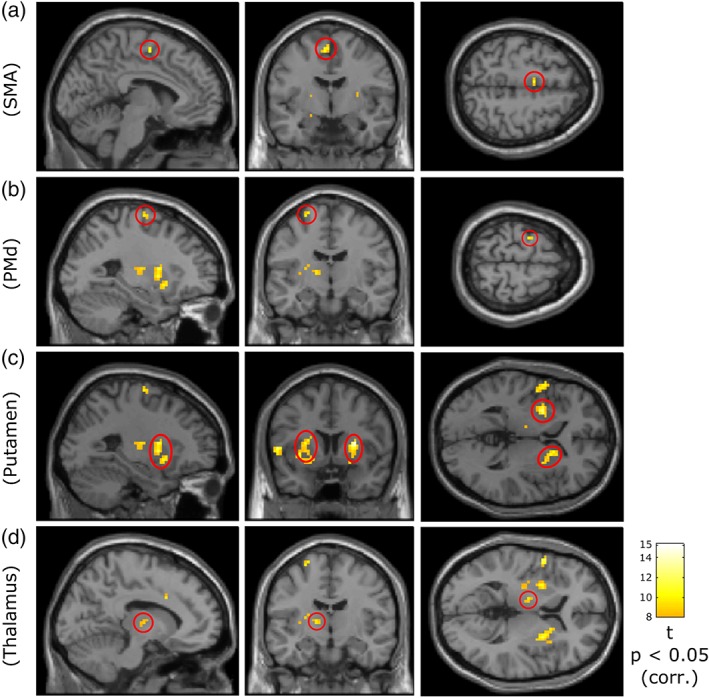
Cortical and subcortical brain areas in which BOLD‐activation significantly correlates with movement speed. Panels from left to right show the sagittal, coronal, and axial sections. The encircled clusters in different rows illustrate the speed‐related BOLD‐activations in the cortical (rows a and b) and subcortical (rows c and d) regions. The corresponding names are shown on the left. Row a: contralateral SMA proper (MNI coordinates: X = −5.2, Y = −5.1, Z = 56). Row b: contralateral PMd (MNI coordinates: X = −26, Y = −11, Z = 68). Row c: bilateral putamen (MNI coordinates: X = −26, Y = 6.8, Z = 2). Row d: contralateral thalamus (VL/VA; MNI coordinates: X = −11.1, Y = −11, Z = 5). The results were derived from second‐level analysis based on a contrast identifying the BOLD‐activation positively correlated with the wrist speed. Statistical threshold: p < .05 FWE‐corrected; minimum cluster size: five voxels. Colors indicate effect size (t values) for the voxels exceeding the significance‐threshold [Color figure can be viewed at http://wileyonlinelibrary.com]
In the cerebral cortex, we found significant wrist speed‐related activation in the posterior part of contralateral SMA, so called SMA proper (Figure 3, row a, MNI coordinates: X = −5.2, Y = −5.1, Z = 56), and in contralateral PMd (Figure 3, row b, MNI coordinates: X = −26, Y = −11, Z = 68). Both of these regions have been shown to be involved in higher levels of motor control: SMA is thought to play a key role in preparation and execution of voluntary movements (Ball et al., 1999; Lee, Chang, & Roh, 1999; Nachev, Kennard, & Husain, 2008), and from monkey studies it is known that PMd is involved in movement initiation and control of reaching movements (Cisek & Kalaska, 2005; Johnson, Coltz, & Ebner, 1999). The current results indicate that SMA and PMd also contribute to speed regulation.
In subcortical regions, we found bilateral significant positive correlations between the BOLD‐activation and the wrist speed in putamen (Figure 3, row c, MNI coordinates: X = −26, Y = 6.8, Z = 2) which are thought to serve motor function and receive input from cortical motor areas (Alexander & Crutcher, 1990; Choi, Yeo, & Buckner, 2012). More specifically, the speed‐related activation predominantly occupied the postcommissural (posterior) portions of bilateral putamen that have been shown to have connections with bilateral SMA and premotor cortices (PMC) in humans (Draganski et al., 2008; Lehéricy et al., 2004; Marchand et al., 2008). In addition to putamen, we observed speed‐related BOLD‐activation in contralateral thalamus (Figure 3, row d, MNI coordinates: X = −11.1, Y = −11, Z = 5). The comparison with Chakravarty atlas (Chakravarty et al., 2006) suggests that this thalamic activation was localized in the ventrolateral (VL) and ventroanterior (VA) nuclei of thalamus both of which receive input from the basal ganglia and support motor function by projection to motor cortical regions (Alexander & Crutcher, 1990; Herrero, Barcia, & Navarro, 2002; Sommer, 2003). The speed‐related activations we found in SMA, PMd, postcommissural putamen, and putative VL/VA in thalamus correspond to nodes of the motor cortico‐basal ganglia loop described by Alexander and Crutcher (1990).
3.2.2. Movement per se‐related activation
For the movement per se effect, the second‐level analysis (FWE‐corrected at p < .05; minimum cluster size: five voxels) revealed multiple cortical and subcortical brain regions which are shown by encircled areas in Figure 4 (from left to right: sagittal, coronal, and axial sections). In the cortical regions, we observed significant movement per se‐related activation in contralateral primary SMC as well as in SMA proper (Figure 4, axial section, Z = 59). The primary SMC activation surrounded the left central sulcus predominantly in the arm and hand regions of M1 and primary somatosensory cortex (S1). Additionally, bilateral motor cingulate gyri showed activations positively correlated with movement per se (Figure 4, coronal section, Y = 3.8). These activations are in accordance with the activation patterns reported by other studies focusing on arm/hand movements (Filimon, 2010; Filimon, Nelson, Hagler, & Sereno, 2007).
Figure 4.
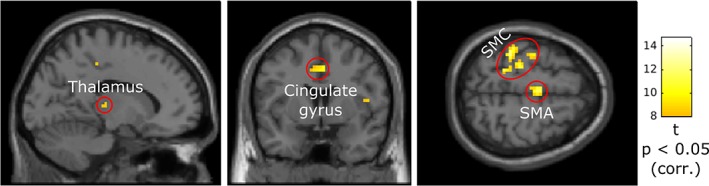
BOLD‐activation correlated with movement per se. Panels illustrate sagittal, coronal and axial views from left to right. MNI coordinates of the sections: X = −14.1, Y = 3.8, Z = 59. The activation pattern (encircled clusters) was obtained from second‐level analysis with corrected p value threshold (p < .05, FWE‐corrected). Significant cortical activation: contralateral primary SMC, SMA proper (axial section) and bilateral motor cingulate gyri (coronal section). Significant subcortical activation: contralateral thalamus (sagittal section). The color bar shows the effect size (t values) for the voxels with activation exceeding the significance‐threshold. Minimum cluster size: five voxels [Color figure can be viewed at http://wileyonlinelibrary.com]
In subcortical regions, movement per se revealed significant activation in posterior contralateral thalamus (Figure 4, sagittal section, X = −14.1). Comparison with the Chakravarty atlas suggests that this activation mainly fell in anterior pulvinar (MNI coordinates of local maximum: X = −14.1, Y = −22.9, Z = 8), which is involved in somatosensory processing (Grieve, Acuña, & Cudeiro, 2000).
3.2.3. Brain areas showing speed‐ and movement per se‐related activations
The analyses with the movement per se and the orthogonalized speed regressor revealed multiple brain areas in two separate GLMs. Figure 5 merges the information from Figures 3 and 4 by overlaying the statistically significant speed‐ and movement per se‐related activations of the two analyses on the same MNI brain in order to put the spatial characteristics of the two brain networks side by side. The speed‐related activations are shown in green, the movement per se‐related activations in red and the shared activations in yellow. Note that Figure 5 shows an overlay of significant activations revealed by two orthogonal regressors rather than maps with significant activation differences between two conditions.
Figure 5.
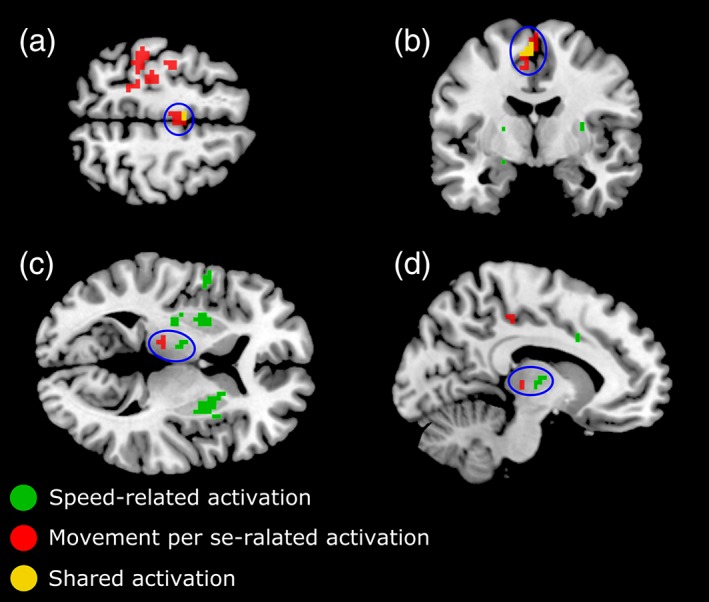
Overlay of speed‐ and movement per se‐related BOLD‐activations in MNI space. Speed‐related activations are depicted in green and movement per se‐related activation in red. Overlapping activation regions are depicted in yellow. Encircled areas in (a) and (b) show the activations in contralateral SMA proper (MNI coordinates of the sections: (a) Z = 58, (b) Y = −5), and in (c) and (d) illustrate the activations in contralateral thalamus (MNI coordinates of the sections: (c) Z = 6, (d) X = −11). Voxels passing FWE‐corrected significance‐threshold p < .05 are shown [Color figure can be viewed at http://wileyonlinelibrary.com]
It can be seen that the movement per se activated a broad region covering the arm and hand areas of the primary SMC, whereas no significant speed‐related activity was observed in these areas (Figure 5a, Z = 58). Similarly, wrist speed variation activated bilateral putamen which showed no significant movement per se activation (Figure 5c, Z = 6). In the contralateral thalamus, we found BOLD‐activation related to the speed and movement per se but in different nuclei. The more posterior BOLD‐activation, related to movement per se, was in putative anterior pulvinar (regional MNI coordinates: X = −14.1, Y = −22.9, Z = 8) that is connected with somatosensory regions (Grieve et al., 2000), whereas the anterior speed‐related activations fell in the putative VL and VA nuclei of thalamus (regional MNI coordinates: X = −11, Y = −11, Z = 5) which are motor thalamic nuclei and assumed to receive inputs from basal ganglia and project to premotor areas including SMA (Herrero et al., 2002; Sommer, 2003).
We found overlapping speed‐ and movement per se‐related activations only in the contralateral SMA proper, but the speed‐related activation was limited to a smaller region (regional MNI coordinates: X = −5, Y = −5, Z = 56) compared to the movement per se activation. This is shown by the encircled areas in Figure 5a,b. Voxels that showed speed‐related activation in SMA were a subset of the voxels showing movement per se‐related activation, as indicated by their yellow color. The shared activation suggests that SMA proper which is involved in control of movement initiation may also play a role in movement speed regulation.
In order to explore the strength of the speed and movement per se effects, we extracted the effect sizes (t values) of the second‐level analysis for regions of interest (ROIs) independently defined in the two GLM analyses. SPM MarsBaR toolbox was used to define the ROIs of statistically significant effects. In this analysis we do not intend to make additional inferences about statistical differences between movement per se‐ and speed‐related effects. It should be noted that defining ROIs from two independent analyses does not bias the magnitude of effect size differences between speed and movement per se within a cluster as in principle the same ROI could be found in each analysis. Therefore, we only compare effect size differences among clusters revealed by the same type of GLM analysis. We defined ROIs based on significant clusters revealed by the speed analysis (left PMd, left SMA, left and right putamen, and left VL/VA) and ROIs based on the significant clusters revealed by the movement per se analysis (left primary SMC, left pulvinar as well as left SMA and cingulate combined). In Figure 6 light and dark gray bars show the voxel averaged effect sizes for the movement per se and the speed regressors, respectively for each activation cluster. The bars indicate how much the effect sizes differ within each cluster between the speed and movement per se. The largest effect size difference among the speed‐related clusters was found in the right and left putamen where the average effects of speed variation were much higher than the effects of movement per se (R‐Put: Δt = 7.1, L‐Put: Δt = 6.9). Although higher speed effect sizes in right and left putamen are expected (as they are given by the speed‐related GLM), this large difference suggests that the activations in bilateral putamen are mainly modulated by varying levels of speed. In other words, the bilateral putamen appear to be the most speed‐specific regions among the speed‐related clusters. The effect differences among the speed‐related left PMd (Δt: 2.5) and VL/VA (Δt: 3.3) clusters were comparable. Among the movement per se‐related clusters, the largest average effect difference was found in the left primary SMC (Δt: 4.4) indicating that M1 and S1 are mainly involved in movement per se control but not in speed regulation. This makes the primary SMC the most movement per se‐specific cluster among the other clusters of the same type. In the overlap region of the left SMA cluster (see Figure 5a,b) the speed and movement per se regressors were associated with comparable effects sizes (Δt: 0.9).
Figure 6.
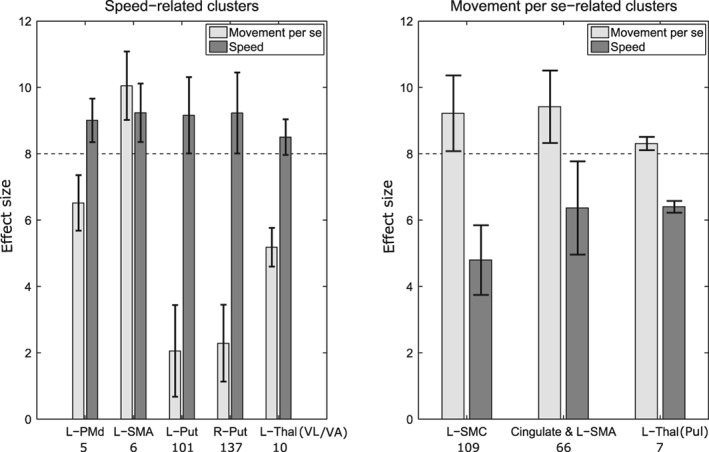
Effect sizes (from second‐level analysis) within the significant activation clusters associated with speed and movement per se (see Figures 3 and 4). The light and dark gray bars indicate the average effect sizes related to the movement per se and speed effects, respectively. The error bars show the corresponding standard deviations. The numbers of voxels included are provided under the cluster labels. The horizontal dashed lines indicate the significance‐threshold. L: left, Put: putamen, Thal: thalamus, VL/VA: ventrolateral/ventroanterior nucleus, Pul: pulvinar
We emphasize that we do not report any brain regions differentially activated by speed and movement per se, as we cannot contrast these effects directly on one GLM (Mumford et al., 2015). However, the separate GLM analyses enable us to report regional differences of activation patterns associated separately with speed and movement per se. In the GLM used for modeling the speed effect, the orthogonalization guarantees that the corresponding activations are due to the movement speed only, excluding effects of movement per se. In contrast, the activations associated with the movement per se regressor may represent any aspect of the movement orthogonal to the speed regressor. Note that this does not preclude overlapping activation for both regressors in a brain area, as indicated in the SMA.
3.2.4. Direction‐related activation
In order to explore direction tuning for arm movements we contrasted the direction regressors. However, the second‐level analysis with FWE‐corrected p value threshold (p < .05; minimum cluster size: five voxels) revealed no significant direction‐related activation differences. This was not consistent with our expectations. We discuss the potential reasons in the next section.
4. DISCUSSION
We investigated the neural control of naturalistic arm movement speed in the human brain by combining fMRI‐BOLD imaging with concurrent measurements of actual wrist speed using a custom build movement tracking system (Shirinbayan & Rieger, 2017). In our approach, we took care of separating speed‐specific from speed‐unspecific BOLD‐activation by orthogonalizing the continuous speed predictor with respect to the binary movement per se predictor in the GLM. We found that BOLD‐activation significantly correlated with wrist speed in contralateral PMd, SMA, in putative thalamic VL and VA nuclei, as well as bilaterally in the putamen. These brain areas are nodes in the basal‐ganglia‐thalamo‐cortical motor loop (Alexander & Crutcher, 1990). We found the strongest speed‐related effects in the putamen of the basal ganglia. Additionally, we found movement‐related but speed‐unspecific BOLD‐modulation in primary SMC, cingulate gyrus, in the putative anterior pulvinar of thalamus, and in SMA. The analysis of effect sizes showed that SMC was most specific to movement per se. SMA was the only region which exhibited partial overlap between speed‐ and movement per se‐specific voxels and consequently showed the smallest difference of effect sizes. To our knowledge, this is the first human neuroimaging study which considers the magnitude of movement speed as an independent variable without confounding it with other kinematic parameters like rate or distance of movement. The results support for the notion that voluntary control of movement speed is mediated by a basal‐ganglia‐thalamo‐cortical loop which includes premotor cortices, but not the primary SMC.
4.1. Brain regions with speed‐related activation
In the bilateral postcommissural putamen, contralateral PMd, contralateral VL/VA of the thalamus, and a small region within SMA BOLD‐activation was specifically correlated with the endpoint speed of arm movements in our study. Several previous studies have shown that these brain areas are involved in motor control but did not specifically test for movement speed (putamen: Gerardin et al. (2003), Marchand et al. (2008); PMd: Cisek and Kalaska (2005), Johnson et al. (1999); and VL/VA thalamus: Herrero et al. (2002), Sommer (2003)). Studies focusing on control of movement kinematics found overlapping sets of brain regions but confounded movement speed with other kinematic parameters such as rate (repetition frequency) or extent of movement (Riecker, Wildgruber, Mathiak, Grodd, & Ackermann, 2003; Taniwaki et al., 2003; Turner et al., 1998, 2003) preventing an unequivocal interpretation of the functional significance of the observed brain activations. By keeping rate and distance of the movements constant and by separating speed‐related from movement per se‐related effects in our GLM‐based analysis, we were able to reveal four speed‐specific brain regions including the contralateral SMA proper, contralateral PMd, bilateral postcommissural putamen, and contralateral VL/VA of thalamus.
In principle, movement duration could confound our speed‐related results as it took longer to perform slower than fast movements. To address this issue, we included the movement duration in the speed and movement per se regressors to model duration difference of the BOLD‐responses. Furthermore, we orthogonalized the speed regressor with respect to the movement regressor which removes duration‐related variance from the speed regressor (Mumford et al., 2015). Finally, if movement duration had an effect on the measured BOLD‐responses other than what was considered in the GLM design, we should have observed similar activation patterns for the movement per se and the speed analysis, which is not the case. Thus, we conclude that movement duration has little or no influence on the speed‐related activations we report.
The set of speed‐specific brain areas we found closely matches the components of the basal‐ganglia‐thalamo‐motor cortical loop suggested by Alexander and Crutcher (1990) in which cortical SMA and PMC send input to the dorsal putamen that modulates the thalamic motor nuclei VA/VL which then close the loop by projecting to SMA and PMC. In humans, this connectivity pattern has been confirmed with diffusion tensor imaging based fiber tracking (Draganski et al., 2008; Lehéricy et al., 2004). In an fMRI study Taniwaki et al. (2003) demonstrated modulation of this network and selective dynamical interactions between the elements which were approximately in concordance with the topology of the motor control loop suggested by Alexander and Crutcher (1990). The experimental design of our study was not optimized for connectivity analysis restricting us to activation analyses. Moreover, the speed‐specific network in our results is in accordance with the model of reach movements suggested by Shadmehr and Krakauer (2008) in the optimal feedback control framework (Todorov & Jordan, 2002). The suggested model presents a basal‐ganglia‐thalamo‐cortical loop comprising motor thalamus, SMA, PMC, and putamen as a module that computes the utility of a movement. Moreover, the model assumes that the motor cortices implement the movement control commands. Shadmehr and Krakauer (2008) suggested that PMC computes motor commands based on visual information whereas the primary motor cortices compute motor commands based on proprioceptive signals. Our results are in correspondence with these predictions as PMd activation correlated with the speed of the visually guided movements our subjects performed. Furthermore, the somatosensory and primary motor cortices exhibited similar response characteristics in representing the movement per se.
Among the cortical and subcortical regions, where the BOLD‐activation positively correlated with the speed of movement, the putamen was the only region bilaterally activated by the unilaterally performed arm movements. The bilateral speed‐related activation in putamen is in line with previous studies focusing on anatomical and functional striatal connections. The striatum of the basal ganglia has been shown to be connected to the cortical areas of both hemispheres in nonhuman primates (Flaherty & Graybiel, 1993; Künzle, 1975) and therefore might activate bilaterally more often than cortical regions when movement is performed unilaterally. Moreover, human neuroimaging studies reported bilateral activations in the putamen correlated with different types of unilateral movements (Gerardin et al., 2003; Marchand et al., 2008; Turner et al., 2003). Turner et al. (2003) observed rCBF changes in bilateral putamen when extent and speed of unilateral arm movement simultaneously increased which is consistent with our results.
It should be noted that activation in cerebellum has also been reported to covary with movement kinematics (Lewis et al., 2003; Roitman, Pasalar, Johnson, & Ebner, 2005; Turner et al., 1998, 2003). However, due to limitations in the number of slices we could not consistently measure cerebellar activation.
Our gyroscope‐based arm tracking system (Shirinbayan & Rieger, 2017) allowed us to measure naturalistic multi‐joint arm movements and to compare the wrist kinematics to the shoulder and elbow kinematics of the reach movements. Particularly, the speed profiles (averaged over all subjects) were highly correlated (wrist‐elbow: 0.92, wrist‐shoulder: 0.87, elbow‐shoulder: 0.96). Thus, the three variables are expected to produce very similar results in a GLM analysis. However, our study was not designed to disentangle between the BOLD‐responses associated with wrist, elbow and shoulder speed.
4.2. Brain regions with movement‐related activation irrespective of speed
We found movement per se‐related activation in primary SMC around the hand knob, in extended regions of SMA, and in the anterior pulvinar. We modeled movement per se‐related activations in the GLM as effects that are related to movement but do not vary with speed. We expected such a regressor to capture activations related to movement initiation and the sensory consequences of movements which we did not expect to vary with speed.
Primary somatosensory cortex (S1) receives the proprioceptive and somatosensory inputs as a consequence of the performed movement. The localization of the activation in S1 in and around the hand knob (Yousry et al., 1997), the anatomically identifiable cortical region of the hand/arm representation in somatosensory cortex, is consistent with the somatotopic organization of SMC. In the monkey, somatosensory cortex is connected with the anterior pulvinar which is involved in somatosensory functions (Cusick & Gould, 1990; Pons & Kaas, 1985).
SMA is known to be involved in the planning and initiation of movements (Ball et al., 1999; Deecke, 1990; Eccles, 1982). Activation in SMA is thus expected to be revealed by the movement per se regressor. However, in addition, we found speed‐related activation in a small rostral area of SMA proper that fully overlapped with movement per se activation (see Figure 5a,b). Our finding that activation in a subsection of SMA correlates with arm movement speed in addition to movement initiation is in concordance with results from a lesion study (Bell, Traylor, Anderson, Berger, & Ojemann, 1994) which found that humans have difficulties in scaling arm movement speed after excision of SMA. Further, single‐cell recordings in humans indicated that movement speed and direction are coded in the response of a subpopulation of SMA neurons (Tankus, Yeshurun, Flash, & Fried, 2009). Thus, it seems likely that a subsection of SMA is also involved in regulating movement speed but a more fine‐grained functional subdivision of SMA proper itself requires further investigations.
The involvement of M1 in the control of movement speed is a matter of debate. In concordance with our finding that M1 activation is modulated by the execution of a movement but not by the speed, Schwartz (1992) found in single‐cell recordings of monkey M1 that orientation and distance of arm movement explained the largest part of the neural response variance whereas speed explained the smallest amount of variance. Most importantly, speed information was only available in neurons with preferred direction tuning in the movement direction which was later elaborated by Moran and Schwartz (1999). Similarly, Golub et al. (2014) found little information about movement speed when they decoded kinematic parameters from M1 neural population spike data. These studies suggest that speed‐related response modulation is negligible in the population response that integrates over neurons with different orientation tunings in M1. This population response drives the BOLD‐signal which is picked up by fMRI (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001) and therefore explains the insignificant speed‐related M1 activation in our results. Moreover, human studies provide a mixed picture. Similar to our results, Turner et al. (2003) did not find significant activation in M1 in a PET study that varied movement extent/speed. Likewise, Wenzel et al. (2014) found no speed‐specific BOLD‐activation in M1 for fast versus slow plantar flexion of the ankle. However, in an earlier study Turner et al. (1998) found significant activation in contralateral primary SMC when they studied the influence of increasing movement rates on the rCBF changes. Similarly, Taniwaki et al. (2003) found significant activation increases in the primary SMC with increasing movement rates. The results of these studies together with our result, that SMC activation is better modeled by movement onsets than movement speed modulations, suggest that activation in primary SMC is driven by the rate of movement onsets rather than the speed movements.
We also observed weak activations in the posterior intraparietal areas of both hemispheres, presumably including parietal reach region (not shown). Contralateral to the reach movements these activations extended into the superior parieto‐occipital sulcus. However, these activations were only observed at a less strict uncorrected significance‐threshold of p < .001. The movement per se‐related activations in posterior parietal areas are in accordance with their functional role in visual coordination of reach movements (Filimon, Nelson, Huang, & Sereno, 2009; Konen, Mruczek, Montoya, & Kastner, 2013).
4.3. Directional tuning
Single‐cell studies in monkey motor cortex repeatedly showed directional tuning of neuronal responses (Georgopoulos et al., 1982; Schwartz, 1992). However, the two arm movement directions our subjects performed yielded no significant modulation of activation in the motor areas. We believe that these directions might not be sensitive enough to assess directional tuning with fMRI, as neurons with different preferred directions might fall within the same voxel and cancel each other out. According to Hammer et al. (2016), directional tuning can be better characterized on the micro level, but its specificity degrades when the brain activity is recorded from larger neuronal populations (i.e., above 10,000 neurons) which is the case in the standard fMRI studies where each voxel represents the activation of approximately 500,000 neurons. Accordingly, two fMRI adaptation studies (Eisenberg et al., 2010; Fabbri et al., 2010) found orientation tuning in motor cortices when subjects repeatedly performed movements in the same direction. This manipulation is thought to adapt the responses of neuronal populations with a certain orientation selectivity and, as a consequence, increases the selectivity of voxels' BOLD‐responses. Moreover, the pattern of direction tunings in the study of Eisenberg et al. (2010) showed a complex spatial organization of direction tuning preferences among M1 voxels which was likely lost in our second‐level analysis.
5. CONCLUSION
We investigated the fMRI correlates of arm movement speed in the human brain. Our results suggest a basal‐ganglia‐thalamo‐cortical loop for speed regulation including contralateral PMd, a subregion of contralateral SMA (SMA proper), bilateral putamen and contralateral VL/VA thalamus with bilateral putamen exhibiting the highest speed specificity. Moreover, activation in primary SMC appears to be more related to other kinematic parameters than speed. The observation that activation in a large part of SMA was modulated by movements per se but not by speed suggests functional subdivisions of SMA.
Supporting information
Figure S1 Movement_Unc_Tmap
Figure S2 Speed_Unc_Tmap
ACKNOWLEDGMENTS
This work was supported by the “Signals and Cognition” graduate program of the University of Oldenburg, the “Forschungspool” of the School of Medicine and Health Sciences, University of Oldenburg (grant 2017‐006), and the Deutsche Forschungsgemeinschaft (Cluster of Excellence 1077 “Hearing4all”).
The full statistical maps can be made available upon request.
Shirinbayan SI, Dreyer AM, Rieger JW. Cortical and subcortical areas involved in the regulation of reach movement speed in the human brain: An fMRI study. Hum Brain Mapp. 2019;40:151–162. 10.1002/hbm.24361
Funding information “Forschungspool” of the School of Medicine and Health Sciences, University of Oldenburg, Grant/Award Number: 2017‐006; “Signals and Cognition; graduate program funded by the State of Lower Saxony; Deutsche Forschungsgemeinschaft, Grant/Award Number: Cluster of Excellence 1077 “Hearing4all”
REFERENCES
- Alexander, G. E. , & Crutcher, M. D. (1990). Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neurosciences, 13, 266–271. 10.1016/0166-2236(90)90107-L [DOI] [PubMed] [Google Scholar]
- Ball, T. , Schreiber, A. , Feige, B. , Wagner, M. , Lücking, C. H. , & Kristeva‐Feige, R. (1999). The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. NeuroImage, 10, 682–694. 10.1006/nimg.1999.0507 [DOI] [PubMed] [Google Scholar]
- Bell, K. R. , Traylor, G. H. , Anderson, M. E. , Berger, M. S. , & Ojemann, G. A. (1994). Features of targeted arm movement after unilateral excisions that included the supplementary motor area in humans. Brain Research, 655, 202–212. 10.1016/0006-8993(94)91615-2 [DOI] [PubMed] [Google Scholar]
- Berardelli, A. , Rothwell, J. C. , Thompson, P. D. , & Hallett, M. (2001). Pathophysiology of bradykinesia in Parkinson's disease. Brain, 124, 2131–2146. 10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- Chakravarty, M. M. , Bertrand, G. , Hodge, C. P. , Sadikot, A. F. , & Collins, D. L. (2006). The creation of a brain atlas for image guided neurosurgery using serial histological data. NeuroImage, 30, 359–376. 10.1016/j.neuroimage.2005.09.041 [DOI] [PubMed] [Google Scholar]
- Choi, E. Y. , Yeo, B. T. T. , & Buckner, R. L. (2012). The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108, 2242–2263. 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland, M. M. , Afshar, A. , & Shenoy, K. V. (2006a). A central source of movement variability. Neuron, 52, 1085–1096. 10.1016/j.neuron.2006.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland, M. M. , Santhanam, G. , & Shenoy, K. V. (2006b). Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. Journal of Neurophysiology, 96, 3130–3146. 10.1152/jn.00307.2006 [DOI] [PubMed] [Google Scholar]
- Cisek, P. , & Kalaska, J. F. (2005). Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron, 45, 801–814. 10.1016/j.neuron.2005.01.027 [DOI] [PubMed] [Google Scholar]
- Collins, D. L. , Zijdenbos, A. P. , Kollokian, V. , Sled, J. G. , Kabani, N. J. , Holmes, C. J. , & Evans, A. C. (1998). Design and construction of a realistic digital brain phantom. IEEE Transactions on Medical Imaging, 17, 463–468. 10.1109/42.712135 [DOI] [PubMed] [Google Scholar]
- Cusick, C. G. , & Gould, H. J. (1990). Connections between area 3b of the somatosensory cortex and subdivisions of the ventroposterior nuclear complex and the anterior pulvinar nucleus in squirrel monkeys. The Journal of Comparative Neurology, 292, 83–102. 10.1002/cne.902920106 [DOI] [PubMed] [Google Scholar]
- Deecke, L. (1990). Electrophysiological correlates of movement initiation. Revue Neurologique (Paris), 146, 612–619. [PubMed] [Google Scholar]
- Delp, S. L. , Anderson, F. C. , Arnold, A. S. , Loan, P. , Habib, A. , John, C. T. , … Thelen, D. G. (2007). OpenSim: Open‐source software to create and analyze dynamic simulations of movement. IEEE Transactions on Biomedical Engineering, 54, 1940–1950. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- Draganski, B. , Kherif, F. , Klöppel, S. , Cook, P. A. , Alexander, D. C. , Parker, G. J. M. , … Frackowiak, R. S. J. (2008). Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28, 7143–7152. 10.1523/JNEUROSCI.1486-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, J. C. (1982). The initiation of voluntary movements by the supplementary motor area. Archiv für Psychiatrie und Nervenkrankheiten, 231, 423–441. 10.1007/BF00342722 [DOI] [PubMed] [Google Scholar]
- Eisenberg, M. , Shmuelof, L. , Vaadia, E. , & Zohary, E. (2010). Functional organization of human motor cortex: Directional selectivity for movement. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 8897–8905. 10.1523/JNEUROSCI.0007-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113, 201602413 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri, S. , Caramazza, A. , & Lingnau, A. (2010). Tuning curves for movement direction in the human visuomotor system. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 13488–13498. 10.1523/JNEUROSCI.2571-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon, F. (2010). Human cortical control of hand movements: Parietofrontal networks for reaching, grasping, and pointing. The Neuroscientist, 16, 388–407. 10.1177/1073858410375468 [DOI] [PubMed] [Google Scholar]
- Filimon, F. , Nelson, J. D. , Hagler, D. J. , & Sereno, M. I. (2007). Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. NeuroImage, 37, 1315–1328. 10.1016/j.neuroimage.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon, F. , Nelson, J. D. , Huang, R.‐S. , & Sereno, M. I. (2009). Multiple parietal reach regions in humans: Cortical representations for visual and proprioceptive feedback during on‐line reaching. The Journal of Neuroscience, 29, 2961–2971. 10.1523/JNEUROSCI.3211-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty, A. W. , & Graybiel, A. M. (1993). Two input systems for body representations in the primate striatal matrix: Experimental evidence in the squirrel monkey. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13, 1120–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos, A. P. , Kalaska, J. F. , Caminiti, R. , & Massey, J. T. (1982). On the relations between the direction of two‐dimensional arm movements and cell discharge in primate motor cortex. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 2, 1527–1537 https://doi.org/citeulike-article-id:444841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin, E. , Lehéricy, S. , Pochon, J.‐B. , Tézenas du Montcel, S. , Mangin, J.‐F. , Poupon, F. , … Marsault, C. (2003). Foot, hand, face and eye representation in the human striatum. Cerebral Cortex (New York, N.Y. 1991), 13, 162–169. 10.1093/cercor/13.2.162 [DOI] [PubMed] [Google Scholar]
- Golub, M. D. , Yu, B. M. , Schwartz, A. B. , & Chase, S. M. (2014). Motor cortical control of movement speed with implications for brain‐machine interface control. Journal of Neurophysiology, 112, 411–429. 10.1152/jn.00391.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve, K. L. , Acuña, C. , & Cudeiro, J. (2000). The primate pulvinar nuclei: Vision and action. Trends in Neurosciences, 23, 35–39. 10.1016/S0166-2236(99)01482-4 [DOI] [PubMed] [Google Scholar]
- Hammer, J. , Pistohl, T. , Fischer, J. , Kršek, P. , Tomášek, M. , Marusič, P. , … Ball, T. (2016). Predominance of movement speed over direction in neuronal population signals of motor cortex: Intracranial EEG data and a simple explanatory model. Cerebral Cortex (New York, N.Y. 1991), 26, 2863–2881. 10.1093/cercor/bhw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero, M.‐T. , Barcia, C. , & Navarro, J. M. (2002). Functional anatomy of thalamus and basal ganglia. Child's Nervous System, 18, 386–404. 10.1007/s00381-002-0604-1 [DOI] [PubMed] [Google Scholar]
- Johnson, M. T. , Coltz, J. D. , & Ebner, T. J. (1999). Encoding of target direction and speed during visual instruction and arm tracking in dorsal premotor and primary motor cortical neurons. The European Journal of Neuroscience, 11, 4433–4445. 10.1046/j.1460-9568.1999.00846.x [DOI] [PubMed] [Google Scholar]
- Konen, C. S. , Mruczek, R. E. B. , Montoya, J. L. , & Kastner, S. (2013). Functional organization of human posterior parietal cortex: Grasping‐ and reaching‐related activations relative to topographically organized cortex. Journal of Neurophysiology, 109, 2897–2908. 10.1152/jn.00657.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle, H. (1975). Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis . Brain Research, 88, 195–209. 10.1016/0006-8993(75)90384-4 [DOI] [PubMed] [Google Scholar]
- Lee, K. M. , Chang, K. H. , & Roh, J. K. (1999). Subregions within the supplementary motor area activated at different stages of movement preparation and execution. NeuroImage, 9, 117–123. 10.1006/nimg.1998.0393 [DOI] [PubMed] [Google Scholar]
- Lehéricy, S. , Ducros, M. , Van de Moortele, P.‐F. , Francois, C. , Thivard, L. , Poupon, C. , … Kim, D.‐S. (2004). Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology, 55, 522–529. 10.1002/ana.20030 [DOI] [PubMed] [Google Scholar]
- Lewis, S. M. , Jerde, T. A. , Tzagarakis, C. , Georgopoulos, M.‐A. , Tsekos, N. , Amirikian, B. , … Georgopoulos, A. P. (2003). Cerebellar activation during copying geometrical shapes. Journal of Neurophysiology, 90, 3874–3887. 10.1152/jn.00009.2003 [DOI] [PubMed] [Google Scholar]
- Logothetis, N. K. , Pauls, J. , Augath, M. , Trinath, T. , & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412, 150–157. 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Marchand, W. R. , Lee, J. N. , Thatcher, J. W. , Hsu, E. W. , Rashkin, E. , Suchy, Y. , … Barbera, S. S. (2008). Putamen coactivation during motor task execution. Neuroreport, 19, 957–960. 10.1097/WNR.0b013e328302c873 [DOI] [PubMed] [Google Scholar]
- Moran, D. W. , & Schwartz, A. B. (1999). Motor cortical representation of speed and direction during reaching. Journal of Neurophysiology, 82, 2676–2692 https://doi.org/citeulike-article-id:560567 [DOI] [PubMed] [Google Scholar]
- Mumford, J. A. , Poline, J.‐B. , & Poldrack, R. A. (2015). Orthogonalization of regressors in FMRI models. PLoS One, 10, e0126255 10.1371/journal.pone.0126255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews. Neuroscience, 9, 856–869. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- Pons, T. P. , & Kaas, J. H. (1985). Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. The Journal of Comparative Neurology, 240, 16–36. 10.1002/cne.902400103 [DOI] [PubMed] [Google Scholar]
- Riecker, A. , Wildgruber, D. , Mathiak, K. , Grodd, W. , & Ackermann, H. (2003). Parametric analysis of rate‐dependent hemodynamic response functions of cortical and subcortical brain structures during auditorily cued finger tapping: A fMRI study. NeuroImage, 18, 731–739. 10.1016/S1053-8119(03)00003-X [DOI] [PubMed] [Google Scholar]
- Roitman, A. V. , Pasalar, S. , Johnson, M. T. V. , & Ebner, T. J. (2005). Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25, 9244–9257. 10.1523/JNEUROSCI.1886-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, A. B. (1992). Motor cortical activity during drawing movements: Single‐unit activity during sinusoid tracing. Journal of Neurophysiology, 68, 528–541. [DOI] [PubMed] [Google Scholar]
- Seth, A. , Sherman, M. , Eastman, P. , & Delp, S. (2010). Minimal formulation of joint motion for biomechanisms. Nonlinear Dynamics, 62, 291–303. 10.1007/s11071-010-9717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr, R. , & Krakauer, J. W. (2008). A computational neuroanatomy for motor control. Experimental Brain Research, 185, 359–381. 10.1007/s00221-008-1280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirinbayan, S. I. , & Rieger, J. W. (2017). An MR‐compatible gyroscope‐based arm movement tracking system. Journal of Neuroscience Methods, 280, 16–26. 10.1016/j.jneumeth.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Sommer, M. A. (2003). The role of the thalamus in motor control. Current Opinion in Neurobiology, 13, 663–670. 10.1016/j.conb.2003.10.014 [DOI] [PubMed] [Google Scholar]
- Taniwaki, T. , Okayama, A. , Yoshiura, T. , Nakamura, Y. , Goto, Y. , Kira, J. , & Tobimatsu, S. (2003). Reappraisal of the motor role of basal ganglia: A functional magnetic resonance image study. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 3432–3438. https://doi.org/23/8/3432 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankus, A. , Yeshurun, Y. , Flash, T. , & Fried, I. (2009). Encoding of speed and direction of movement in the human supplementary motor area. Journal of Neurosurgery, 110, 1304–1316. 10.3171/2008.10.JNS08466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz, P. , Blu, T. , & Unser, M. (2000). Interpolation revisited. IEEE Transanctions on Medical Imaging, 19, 739–758. 10.1109/42.875199 [DOI] [PubMed] [Google Scholar]
- Todorov, E. , & Jordan, M. I. (2002). Optimal feedback control as a theory of motor coordination. Nature Neuroscience, 5, 1226–1235. 10.1038/nn963 [DOI] [PubMed] [Google Scholar]
- Turner, R. S. , Desmurget, M. , Grethe, J. , Crutcher, M. D. , & Grafton, S. T. (2003). Motor subcircuits mediating the control of movement extent and speed. Journal of Neurophysiology, 90, 3958–3966. 10.1152/jn.00323.2003 [DOI] [PubMed] [Google Scholar]
- Turner, R. S. , Grafton, S. T. , Votaw, J. R. , Delong, M. R. , & Hoffman, J. M. (1998). Motor subcircuits mediating the control of movement velocity: A PET study. Journal of Neurophysiology, 80, 2162–2176. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wenzel, U. , Taubert, M. , Ragert, P. , Krug, J. , & Villringer, A. (2014). Functional and structural correlates of motor speed in the cerebellar anterior lobe. PLoS One, 9, 1–8. 10.1371/journal.pone.0096871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Buettner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120(Pt 1), 141–157. 10.1093/brain/120.1.141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Movement_Unc_Tmap
Figure S2 Speed_Unc_Tmap


