Abstract
Aberrant structural (diffusion tensor imaging [DTI]) and resting‐state functional magnetic resonance imagining connectivity are core features of bipolar disorder. However, few studies have explored the integrity agreement between structural and functional connectivity (SC–FC) in bipolar disorder. We examine SC connectivity coupling index whether could potentially provide additional clinical predictive value for bipolar disorder spectrum disorders besides the intramodality network measures. By examining the structural (DTI) and resting‐state functional network properties, as well as their coupling index, among 57 euthymic bipolar disorder patients (age 13–28 years, 18 females) and 42 age‐ and gender‐matched healthy controls (age 13–28 years, 16 females), we found that compared to controls, bipolar disorder patients showed increased structural rich‐club connectivity as well as decreased functional modularity. Importantly, the coupling strength between structural and functional connectome was decreased in patients compared to controls, which emerged as the most powerful feature discriminating the two groups. Our findings suggest that structural–functional coupling strength could serve as a valuable biological trait‐like feature for bipolar disorder over and above the intramodality network measures. Such measure can have important clinical implications for early identification of bipolar disorder individuals, and inform strategies for prevention of bipolar disorder onset and relapse.
Keywords: bipolar disorder, modularity, predictive analysis, rich club, structural–functional coupling
1. INTRODUCTION
Bipolar disorder (BD) is characterized by mood dysregulation and recurrent episodes of depression and mania with variable interepisode remission periods (Saunders & Goodwin, 2010). As a common disorder with 2.4% prevalence, BD is a disabling psychiatric disorder that severely impacts the daily life of patients and their families (Merikangas et al., 2011). Thus, advanced understanding of objective neural markers of BD etiology can critically inform early stage diagnosis, illness intervention, and prevention (Frías, Palma, & Farriols, 2015; Mcintyre, Konarski, Misener, & Kennedy, 2005).
Recent neuroscientific investigations have identified abnormalities in both structural and functional connectivity (SC–FC) patterns in BD. For example, Roberts et al. (2018) reported BD‐related white matter microstructural abnormalities in connections between prefrontal and anterior limbic structures. Employing a face affect task, Wang et al. (2009) demonstrated abnormal FC between perigenual anterior cingulate cortex and amygdala during processing fearful and happy faces in BD. Moreover, aberrant FC of the inferior frontal gyrus that is implicated in emotional regulation may represent a trait abnormality for BD (Roberts et al., 2018). Together, evidence suggests that impaired neural connectivity play critical roles in BD‐related deficits in emotion processing and regulation (Strakowski et al., 2012). However, few studies have examined the interaction between structural and FC profiles in BD (Wang et al., 2009).
Structural connectivity (SC) is highly predictive of and place anatomical constraints on FC across the human brain network over various spatial scales (Honey et al., 2009). Conversely, functional connections exert effects on structural connections through mechanisms of plasticity (Guerra‐Carrillo, Mackey, & Bunge, 2014; Hagmann et al., 2010). The use of correlation estimates between subject‐level resting‐state functional magnetic resonance imagining (rsfMRI) and diffusion tensor imaging (DTI) connectivity matrices was proposed and allows for quantification of the agreement between structural and functional networks (Honey, Thivierge, & Sporns, 2010). This measure, known as SC–FC coupling, has demonstrated its potential for characterizing changes in structural–functional network relationships with age during development (Hagmann et al., 2010), and in disease states (Chiang, Stern, Engel Jr, & Haneef, 2015; Zhang et al., 2011). Of particular relevance, limited existing evidence indicates that the SC–FC coupling strength of the limbic system might be altered in BD patients relative to matched controls (Versace et al., 2010). Further, changes in SC–FC coupling for long‐distance connections were also detected in nonpsychotic offspring of BD patients (Collin, Scholtens, Kahn, Hillegers, & van den Heuvel, 2017). Moreover, recent studies suggested that the SC–FC coupling strength may be intrinsically a reflection of mental states (Batista‐García‐Ramó & Fernández‐Verdecia, 2018; Huang & Ding, 2016). However, it is still unclear whether and how the global SC–FC coupling strength is changed in young BD patients, and whether global SC–FC coupling may provide significant additional value for prediction of BD status over and above that offered by single‐modality network indices. To fill in these knowledge gaps and further clarify the role of SC–FC coupling in the neuropathology of BD, the current study systematically explored the global SC–FC coupling profile as well as SC and FC measures alone in BD patients.
Since the structural rich club (RC) lays the anatomical foundation for linking and integrating functional resting state networks in the human cortex (Van den Heuvel & Sporns, 2013), the anatomical infrastructure of the brain's RC is critically important for facilitating neuronal signaling and thus global network information communication between functionally segregated domains of the human brain (functional modularity). While recent studies have begun to reveal aberrant brain structural connections between hubs and nonhub nodes, as well as among hubs themselves (i.e., RC connections) in relate to BD (Roberts et al., 2018), it remains unclear whether abnormal RC connectivity profiles impact the global functional communication capacity in BD.
In this study, we examined the interaction between whole brain structural and functional network in BD using SC–FC coupling. Since existing literature suggests that factors such as age, BD clinical states, and illness course may exert confounding effects on structural and functional network measures (Collin et al., 2016; O'Donoghue et al., 2017; Roberts et al., 2018), we recruited a homogenous sample of euthymic BD patients within a restricted young age range (13–28 years) encompassing the peak age of BD onset (Faraone, Lasky‐Su, Glatt, Eerdewegh, & Tsuang, 2006). Given SC–FC coupling could be impacted through changes to SC, FC, or both, our network analysis consisted of three sequential steps: first, we tested whether SC of the brain's hubs showed alterations in euthymic BD patients compared with controls using DTI; second, we tested whether the global network functional communication capacity (i.e., functional modularity) showed aberrant patterns in euthymic BD patients using rsfMRI; and third, we compared the global SC–FC coupling index between the BD patients and matched healthy controls (HCs). We hypothesized that in BD patients, the SC–FC coupling would be aberrant in networks that exhibited BD‐related alterations in SC among the RC systems, or in functional modularity. Furthermore, logistic regression analysis was conducted to formally test the additional value of SC–FC coupling in differentiating BD from controls over and above that of the SC (i.e., RC) and FC (i.e., functional modularity) measures. Collectively, the current findings would advance our knowledge on BD trait and state neural network measures that could potentially emerge as complementary illness markers, and offer important insights on early phase identification of BD individuals and inform strategies for prevention of BD onset and relapse.
2. METHODS
2.1. Participants
The current study forms part of the ongoing Recognition and Early Intervention on Prodromal BDs project, initiated by the Global Mood and Brain Science Initiative (Lin et al., 2015; Lin et al., 2017; Mansur et al., 2018; McIntyre et al., 2017). This study was approved by the Institutional Review Board of the Guangzhou Brain Hospital.
Sixty‐two BD patients aged 13–28 years were recruited from the Guangzhou Brain Hospital. All patients were diagnosed by research psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria. These BD patients were in euthymic state, defined as <7 scores on the Hamilton Depression Rating Scale (HAMD) and <6 scores on the Young Mania Rating Scale (YMRS) lasting for at least 1 month. Prescribed medications for the patients are listed in Table 1. To match the BD patients, 42 HCs who had no personal or family history of any psychiatric disorders were recruited. HCs underwent the same systematic psychiatric assessments as did BD patients. The HAMD, YMRS, and the Brief Psychiatric Rating Scale (BPRS) were used to assess the severity of depressive, manic, and psychotic symptoms, respectively. Written informed consents were obtained from all participants (if ≥18 years) and their guardians (if <18 years). Details about participant characteristics are included in Table 1.
Table 1.
Demographic and clinical characteristics of the BD and HC groups
| BD group (n = 57) | HC (n = 42) | t/X 2, p | |
|---|---|---|---|
| Age | 20.29 (3.56) | 19.14 (3.45) | t = 1.68, p = .11 |
| Gender | 39M/18F | 26M/16F | X 2 = 0.46, p = .50 |
| Handedness | 56 right/1 left | 42 right/0 left | X 2 = 0.74, p = .38 |
| IQ (TONI) | 29.5 (9.63) | 35 (6.15) | t = −1.81, p = .07 |
| HAMD | 3.13 (3.88) | 0.29 (0.66) | t = 3.84, p = .002 |
| YMRS | 0.40 (1.00) | 0.07 (0.26) | t = 1.67, p = .100 |
| BPRS | 20.17 (3.30) | 18.14 (0.45) | t = 3.22, p = .002 |
| Illness onset (years) | 16.55 (3.95) | n.a | |
| Illness duration (month) | 27.98 (36.63) | n.a | |
| Medication | Mood stabilizer (n = 34) | n.a | |
| Selective Serotonin Reuptake Inhibitor (n = 17) | |||
| Serotonin and Norepinephrine Reuptake Inhibitors (n = 4) | |||
| Antipsychotic drugs (n = 30) |
Note. Both mean and SD are shown. IQ is measured using the Test of Nonverbal Intelligence, Third Edition.
Bold values indicate p < 0.05.
Abbreviations: BD, bipolar disorder; BPRS, the Brief Psychiatric Rating Scale; HC, healthy controls; HAMD, the Hamilton Depression Rating Scale; YMRS, the Young Mania Rating Scale.
2.2. Data acquisition and preprocessing
All neuroimaging data were acquired on a 3.0 Tesla MR imaging system (Achieva X‐series; Philips Medical Systems, Best, Netherlands) with an eight‐channel SENSE head coil, in the Department of Radiology, Guangzhou Brain Hospital. Tight but comfortable foam padding was used to reduce head motion, and earplugs were used to muffle scanner noise. DTI images were collected with the following parameters: 32 diffusion‐weighted (b = 1,000 s/mm2) and one nondiffusion‐weighted scans; repetition time (TR) = 10,100 ms; echo time (TE) = 90 ms, field of view (FOV) = 256 × 256 mm2, and voxel size = 2 × 2 × 2 mm3. rsfMRI images were acquired using a gradient‐echo echo‐planar imaging sequence with TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix = 64 × 64, FOV = 220 × 220 mm2, slice thickness = 4 mm with interslice gap = 0.6 mm, 33 interleaved axial slices, and 240 time points. T1 images were acquired with an interleaved sequence (188 sagittal slices, TR/TE/flip angle =8.2 ms/3.7 ms/7°, matrix = 256 × 256 mm2, FOV = 256 × 256 × 188 mm, voxel size = 1 × 1 × 1 mm3).
2.3. Structural connectome construction and analysis
2.3.1. DTI preprocessing
The effects of head motion and image distortion caused by eddy current were corrected by applying an affine alignment to register all other diffusion volumes to the original b0 volume using the FSL/FDT toolbox (FSL 5.1: http://www.fmrib.ox.ac.uk/fsl). Rotation corrections were applied to the corresponding diffusion‐sensitive gradient directions (Leemans & Jones, 2009). Before proceeding to analysis, each participant's data were manually assessed for quality. This process involved checking for precise image alignment, minimal head motion, accurate brain extraction, good tensor fitting, and minimal voxels containing the theoretically impossible value of factional anisotropy (FA) greater than 1. No participant had to be excluded due to bad data quality. The corrected DTI data were then processed using DSI studio (http://dsi-studio.labsolver.org/) to map whole‐brain white matter wiring based on the fiber assignment by continuous tracking algorithm. Fiber tracking was stopped when FA <0.15 or the angle between the eigenvectors of two consecutive voxels was large than 60°.
2.3.2. Structural connectome construction
Node and edge are two basic elements of a network. We defined the nodes of the structural connectome following our previous studies (Zhang et al., 2015; Zhang et al., 2016). Briefly, we first coregistered T1‐weighted image to the original b0 volume resulting in the coregistered T1 image for each individual, using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The coregistered T1 images were then nonlinearly transformed to the ICBM‐152 T1‐weighted template in the standard Montreal Neurological Institute (MNI) space. The inverse transformations were used to warp the automated anatomical labeling template with 90 regions (AAL90, Table S1) from the standard MNI space to the individual native DTI space using a nearest‐neighbor interpolation approach.
We used an abstract model of brain network to represent the brain systems at the macroscale level, with each node corresponding to a brain region and each edge to an internodal connection. Any two regions of the AAL90 were considered structurally connected if there were at least three streamline counts (counts ≥3) located between them. This way, we obtained a symmetric 90 × 90 connectivity matrix representing the brain structural connectome for each subject. The processing flowchart is depicted in Figure 1.
Figure 1.

Flowchart of data analysis. (a) The AAL atlas based on which the structural and functional connectomes were constructed; (b) white‐matter fiber tracking; (c) computation of structural connectome matrix; (d) structural connectome analysis; (e) construction of the functional connectome by extracting the time series of each region in the AAL atlas; (f) estimating the FC matrix through Pearson correlation; and (g) structural–functional coupling analysis. AAL, automated anatomical labeling; FC, functional connectivity
2.3.3. RC organization
High‐degree hubs connect to other highly connected hubs more often than to peripheral nodes of low degree simply by virtue of their high degree (Van Den Heuvel & Sporns, 2011). An RC is considered to exist when the connections among high‐degree hubs are enriched above what would be predicted by their degree alone. This organization can be summarized by the RC coefficient (ϕ). The presence of RC organization was verified in the current sample (see Figure S1, Supporting Information). We defined hubs for each individual such that if the degree of a node was greater than 1.5 times of the average nodal degree of that individual, then that node was defined as a hub. Accordingly, all connections were classified into one of three classes (Van Den Heuvel & Sporns, 2011): highly interconnected hubs as RC, connections between hubs and nonhubs as feeder, and connections between nonhubs as local. For each individual data set, RC, feeder, and local connectivity were computed as the sum of the weights of each edge class.
2.3.4. SC deficits in BD
To test whether group difference in RC connectivity was contributed by connectivity among the network components that were also different between groups, we first identified subnetworks in which the interregional structural connections were significantly changed in BD patients compared to HCs, using a network‐based statistic (NBS) (Zalesky, Fornito, & Bullmore, 2010) approach (Appendix S1, Supporting Information). The NBS is a permutation‐based method that allows correcting for the family wise error due to the large number of structural edges tested, and has been applied widely to test for between‐group differences in structural network connectivity (Bassett & Sporns, 2017). Second, we estimated the Pearson correlation coefficients between the RC connectivity strength and connectivity strengths of the altered component(s) derived from NBS.
2.4. Functional connectome construction and analysis
2.4.1. rsfMRI preprocessing and functional connectome construction
The rsfMRI data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and DPABI (Yan, Wang, Zuo, & Zang, 2016). For each subject, the first 10 volumes of the rsfMRI data set were discarded to allow for MR signal equilibrium, leaving 230 volumes for further analysis. The remaining images were then corrected for slice timing, realigned to the first volume to correct for head motions, and spatially normalized to a standard MNI template and resampled to a voxel size of 3 × 3 × 3 mm3. Then, normalized images were smoothed with 6‐mm kernel. Finally, band‐pass filtering with 0.01–0.08 Hz was applied to each voxel to reduce low‐frequency drift and high‐frequency physiological noises. Five participants whose translational or rotational head motion exceeded 2 mm or 2° in any direction were excluded. Motion effect was further reduced by applying scrubbing procedures for time points with a power frame displacement (FD) greater than 0.5. t‐Test on the FD revealed there was no significant difference between the two groups (p > .05). Finally, a total of 57 BD patients and 42 age‐ and gender‐matched HCs were entered into data analysis (Table 1).
As in the structural connectome analysis, we deployed the AAL atlas to delineate the functional connectome nodes, thus allowing for direct comparisons of the structural and functional analysis results. For each subject, after regressing out white matter signal, cerebral spinal fluid signal, and motion parameters, Pearson's correlation coefficient was calculated on the residual time series between each pair of nodes as index for the between‐node FC. As a result, we obtained a 90 × 90 symmetric correlation matrix representing the functional connectome of each individual.
2.4.2. Functional modularity detection
Following the construction of functional connectomes, nonparametric method of locally adaptive network sparsification (Foti, Hughes, & Rockmore, 2011) was applied to extract the backbone of weighed networks, and then the weighted functional connectome was entered into functional modularity analysis using the Louvain modularity detection algorithm (1,000 runs per subject, selecting the highest functional modularity [Q fc] index). The Louvain algorithm (Blondel, Guillaume, Lambiotte, & Lefebvre, 2011) was used because it is suitable for networks with both positively and negatively weighted connections, as is the case in rsMRI functional networks. The level of whole‐brain functional modularity informs global network functional communication capacity (Colombo, 2013). Furthermore, the sum of connectivity values between functional modules was calculated as a measure of intermodular functional integration.
2.5. SC–FC coupling analysis
SC–FC coupling analysis has been described in previous studies (Honey et al., 2009; Zhang et al., 2011). Here, we briefly summarized the processing pipeline. The coupling analysis proceeded in two ways.
First, for each network, a correlation analysis was performed between the strength of the structural connections and their functional counterparts. All nonzero entries of the SC matrix were selected, rescaled to a Gaussian distribution, and correlated with their functional counterparts selected from the FC matrix. This resulted in a single SC–FC coupling metric for each subject, indicating the SC–FC coherence across all brain subregions. Rescaling of the structural weights to a Gaussian distribution was used to normalize the distribution of SC values. To further test whether group differences in SC–FC coupling were contributed by connections between (interhemispheric) or within (intrahemispheric) the hemispheres, we conducted the coupling analysis separately between and within hemispheres. Furthermore, we also assessed the potential impact of fiber length on structural–function coupling, through classifying the reconstructed fibers into three layers: short (<50 mm), intermediate (50–100 mm), and long distance (≥100 mm).
Second, it has been suggested that RC acts as an anatomical infrastructure for the integration of information among functional modules (Senden, Deco, de Reus, Goebel, & van den Heuvel, 2014). To investigate whether structural RC connectivity impacts modular‐level functional integration, we examined: (a) the association between structural RC connectivity and whole‐brain functional modularity (Q fc), and (b) the association between structural RC connectivity and intermodular FC strengths, in BD patients and controls using linear regression analysis correcting for the effects of age and gender.
2.6. Statistical analysis
A nonparametric permutation test was used to assess the statistical significance of between‐group differences. This randomization procedure was repeated 5,000 times for a given network parameter and the corresponding distribution of t values was obtained. We set the critical value as 95% of the distribution for each of the metrics to test the null hypothesis, with a nominal statistical threshold of p < .05. Age and gender were entered as confounders before running the permutation tests.
For metrics showing significant between‐group differences, we performed multiple linear regression analyses to explore the relationship between each parameter and each of the BD‐related clinical variables. The clinical variables included the HAMD, YMRS, and BPRS scores, the age of illness onset, illness duration, and IQ (i.e., TONI).
We further conducted logistic regression analyses to examine whether SC–FC coupling measure provided additional predictive value for differentiating the BD patient and HC groups over and above that of the intramodality measures. To achieve such aim, we employed a stepwise logistic regression model on BD diagnosis (patients vs. controls), in which the SC (structural RC) and FC (functional modularity) measures were entered first into the model, followed by the SC–FC coupling measure. The data analysis was performed on SPSS v.20.
2.7. Control analysis
2.7.1. Edge definition on structural connectome
Studies have suggested that different edge definitions could affect the spatial pattern of white matter connections (Zhong, He, & Gong, 2015). To test whether the specific edge definition adopted by us contributed to our results, we applied alterative edge definition (edges weighted by the FA value along the streamlines, or FA × Fiber Number (FN)) and reestimated the RC, feeder, and local connectivity profile in all subjects.
2.7.2. Hub identification scheme
In the main analysis, RC regions were selected for each participant individually as those with nodal degrees great than 1.5 times of the average nodal degree of that individual, thus the number of hub nodes in each individual was different. In this regard, we further explored the pattern under a wide range of hub thresholds, and when all participants had the same “hub” organization. Two hub definitions were considered: the rank of degree distribution or the RC coefficient profile. For each threshold, group differences in RC connectivity were examined using the nonparametric permutation test (10,000 times).
2.7.3. Medication effects
The BD patients were under various pharmacological treatment regimens at the time of study (see Table 1), which could impact the brain functional and SC (Schmidt et al., 2013). To test whether different medication protocols may have confounded our main findings, we conducted further multiple regression analyses which controlled for the effects of medication types (see Appendix S1, Supporting Information for the detailed method).
2.7.4. Split‐half reliability analysis
A split‐half analysis was performed testing the consistency of the results. The analyses were repeated in each of two BD and HC subgroups, formed by splitting each of the BD and HC groups into two equal‐sized halves, which were matched for age and gender (HC1: 13M/8F, mean age = 19.47 years; HC2: 13M/8F, mean age = 18.81 years; BD1: 19 M/9F, mean age = 19.78 years; BD2: 20 M/9F, mean age = 20.79 years) (see Supporting Information).
3. RESULTS
3.1. Demographic and clinical variables
BD and HC groups were age and gender matched (ps > .05). Among patients, the mean age of BD onset was 16.55 years of age, with mean illness duration of 2.33 years. BD scored significantly higher on the HAMD and BPRS than did the controls (ps < .05) (Table 1).
3.2. Structural connectome analysis
There was no significant between‐group difference in total network strength, defined as the sum of the weights of all structural connections in the network (p = .32, Cohen's d = 0.21, power = 0.16) (Figure 2a). However, when characterizing the shape of edge weights distribution, we found that edge weights displayed a shift towards smaller values in the BD group (Figure 2b), a phenomenon referred to as “relative paucity of white matter resources” (Karolis et al., 2016).To further localize the origins of the “relative paucity of white matter resources” in BD, we first identified hubs including: the bilateral superior frontal gyri (dorsal lateral and medial part), middle frontal gyri, precuneus, middle temporal gyri, and left inferior temporal gyrus (Figure 2c). Then, we classified the connectivity into three layers: RC, feeder, and local. Group analysis revealed increased RC (p = .01, Cohen's d = 0.48, power = 0.65) and a trend of decreased local connectivity (p = .05, Cohen's d = 0.40, power = 0.47) in the BD group compared to the HCs (Figure 2d–f).
Figure 2.
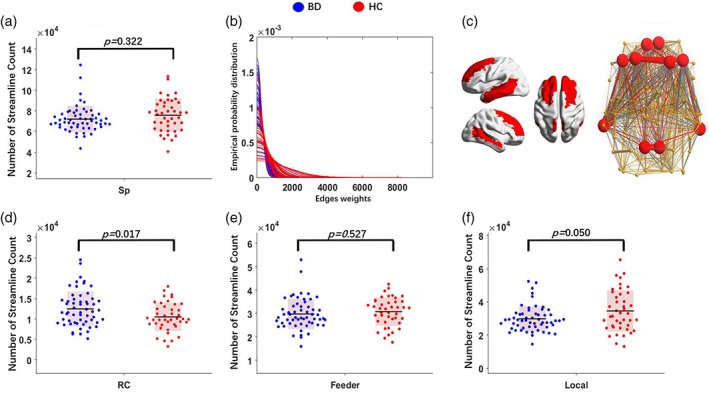
Structural RC organization profile. (a) Network strength (S p): compared to the HCs, the BD group showed no significant difference; (b) edge weights distribution in BD and HCs: compared to the HCs, BD showed left shift trends, which may indicate the relative paucity of white matter resources in the BD; (c) defined hubs coded with red; connectivity profile were divided into three distinct layers: (d) RC connections linking hubs, (e) feeder connections linking hubs to nonhubs, and (f) local connections connecting nonhub nodes. Compared with controls, BD showed significantly increased RC connectivity density (d) and a trend of reduction in local connectivity density (f). BD, bipolar disorder; HC, healthy control; RC, rich club
NBS analysis revealed that BD patients showed altered SC in two large‐scale brain networks compared to the HCs. First, decreased SC in the BD group was found in a component comprised of 35 nodes with 45 edges (p < .001, corrected, Figure 3a), which was mainly located in the frontal, parietal, and temporal areas. Conversely, increased connectivity strengths in BD patients were found in a network consisting of 33 nodes with 40 edges (p < .001, corrected, Figure 3b), mainly in the frontal cortex and subcortical areas (Table S2, Supporting Information). Interestingly, edges of the increased component in the BD group were correlated with hub nodes: further correlation analysis revealed significant positive correlations between the connectivity strength derived from the increased component and RC only in the BD group (r = .28, p = .03), but not in HCs (Figure 3d).
Figure 3.
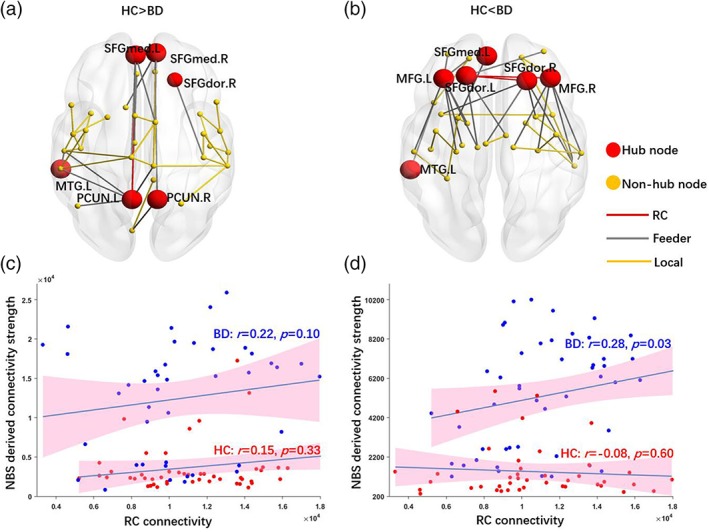
NBS on structural connectome. (a) Component showing decreased SC in BD compared with HCs; (b) component showing increased structural connectivity in BD compared with HCs; (c) connectivity density derived from the component showing decreased connectivity in BD correlated with RC connectivity density; and (d) connectivity density derived from the component showing increased connectivity in BD correlated with RC connectivity density. The BD, but not HC, group showed positive relationships between RC connectivity and NBS‐derived connectivity strengths for both increased and decreased components. AAL, automated anatomical labeling; BD, bipolar disorder; FC, functional connectivity; HC, healthy control; NBS, network‐based statistic; RC, rich club
3.3. Functional connectome analysis
Functional modularity (Q fc) in BD patients decreased when compared with HCs (p = .04, Cohen's d = 0.41, power = 0.51, Figure 4b). Further analysis showed that BD patients exhibited decreased intramodule connectivity (p < .001, Cohen's d = 1.21, power = 0.99, Figure 4d) and marginally increased intermodule connectivity (p = .05, Cohen's d = 0.45, power = 0.55) compared with the HCs.
Figure 4.
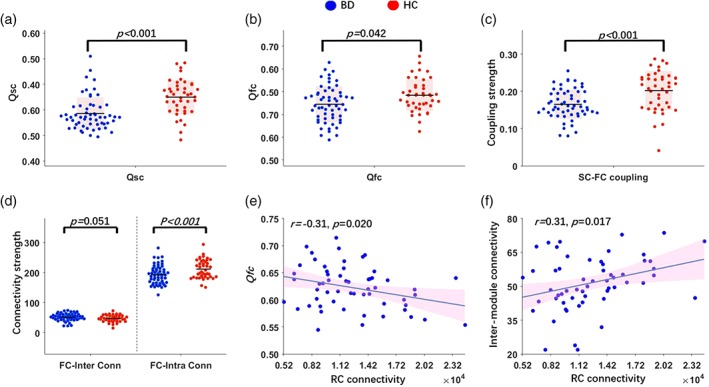
Relationship between functional modularity and RC connectivity density. (a) Decreased structural connectome modularity (Qsc) in BD compared with HCs; (b) decreased functional connectome modularity (Qfc) in BD compared with HCs; (c) decreased structural–functional coupling strength in BD compared with HCs; (d) two layers of FC profile: Intermodule (FC‐inter conn) and intramodule (FC‐intra conn). Compared with HCs, BD showed less intramodule (but not intermodule) connectivity; (e) RC connectivity showed negative relationship with functional modularity; and (f) RC showed positive relationship with intermodule FC. BD, bipolar disorder; FC, functional connectivity; HC, healthy control; RC, rich club
3.4. SC–FC coupling analysis
Compared with HCs (0.20 ± 0.05), the BD group (0.16 ± 0.04) showed a significant decrease in the SC–FC coupling strength (p < .001, Cohen's d = 0.70, power = 0.93, Figure 4c). Moreover, total intrahemisphere coupling strength was significantly decreased in the BD group compared with HCs (p = .008, Cohen's d = 0.55, power = 0.77). No significant group difference was observed for total interhemisphere connectivity strength. Furthermore, significant group differences (p < .05) were observed across different fiber lengths (≦50 mm, 50–100 mm, and ≥100 mm).
A negative correlation between functional modularity (Q fc) and structural RC connectivity was additionally found in the BD group (r = −.31, p = .02, Figure 4e), but not in HCs. Consistent with this finding, structural RC showed a trend of positive correlation with intermodular functional integration in BD (r = .26, p = .05, Figure 4f).
3.5. Clinical predictive analysis
When the logistic predictive model contained only the SC and FC measures, the predictive strength of the model was significant (X 2 = 10.47, p = .005, accuracy = 61.6%). The predictive strength of functional modularity was significant (Wald = 4.17, p = .041), whereas that of RC was marginal (Wald = 3.75, p = .053). When the SC–FC coupling measure was additionally and subsequently entered into the model, the predictive strength of the total model quantitatively increased (X 2 = 13.66, p < .001, accuracy = 72.7%). Importantly, while the predictive strength for the SC–FC coupling measure was clearly significant (Wald = 11.5, p = .001), those for the SC and FC measures both reduced to being marginally significant (Wald ≥ 3.63, p ≥ .057). Detailed model and variable parameters are included in Table S3, Supporting Information.
3.6. Auxiliary analyses
3.6.1. Clinical variables
Partial correlation analyses reveal no significant association (ps > .05) between measures that showed group difference and the clinical variables including age of illness onset, illness duration, clinical assessment (HAMD, YMRS, and BPRS), or IQ.
3.6.2. Control analysis
First, using alternative edge definition (FA × FN), the structural–functional coupling strength in the BD patients was significantly decreased compared with the HCs, which was in line with our main analysis (Figure S2, Supporting Information). Second, no matter which hub definition protocols adopted, BD patients showed enhanced RC SC (Figures S3 and S4, Supporting Information). Third, correcting for medication protocols in the models confirmed the main results (RC connectivity: F (1,91) = 4.25, p = .042; functional modularity: F (1,91) = 3.96, p = .049, and SC–FC coupling: F (1,91) = 4.42, p = .038). Last but not least, we tested the split‐half reliability of our main results (Figure S5, Supporting Information). High cross‐subgroup correlations on both SC and FC connectivity patterns were observed for both BD patients (r = .95 and r = .96 for structural and functional connectome, respectively) and HCs (r = .96 and r = .95 for structural and functional connectome, respectively), and direct comparisons of the split‐half subgroups revealed no significant difference for BD or HC participants (ps > .05) in all connectivity measures including structural RC, functional modularity, and SC–FC coupling strength. In contrast, when the subgroups of BD patients were compared against those of the HCs, we found that Q fc (HC1 vs. BD1, HC2 vs. BD2, HC1 vs. BD2, HC2 vs. BD1), structural RC (HC2 vs. BD1), and SC–FC coupling (HC1 vs. BD1, HC2 vs. BD1) showed significant group differences (ps < .05). Association analysis demonstrated that structural RC connectivity was significantly and negatively associated with functional modularity in one subgroup of BD patients (r = −.45, p = .01), and the relation was insignificantly negative in the other subgroup (r = −.14, p = .45). These results collectively suggested that our main findings have high level of reproductivity.
4. DISCUSSION
This is the first study exploring the BD neuropathology from the perspective of functional–structural connectome coalescence, employing combined DTI and rsfMRI methods. The main findings were that euthymic young BD patients exhibited increased structural RC connectivity density and decreased functional modularity, as well as decreased coupling of SC–FC, compared with HCs. Given RCs act as core structures in the brain's network topology, and connections among hubs play essential roles in information integration, our results suggest that the changes of structural RC organization might potentially result in altered global functional communication capacity and altered functional brain dynamics in euthymic young BD patients (Bressler & Menon, 2010; Hagmann et al., 2010; Honey, Kötter, Breakspear, & Sporns, 2007; Supekar et al., 2010). Conversely, changes in functional network characteristics might potentially, with time, also lead to corresponding transformations of the underlying structural network features. On the other hand, the reduced SC–FC coupling strength in BD patients may serve as a trait‐like feature and showed higher predictive power in distinguishing euthymic BD patients from HCs than the intramodality network measures.
RCs were hypothesized to act as a central backbone for signal traffic, allowing for integration and dissemination of information conveyance (van den Heuvel, Kahn, Goñi, & Sporns, 2012). In healthy state, RC shapes information flow and funnels traffic towards some nodes and away from others, which processes may become disordered with negative impact on resource allocation and information transfer (Mišić, Sporns, & McIntosh, 2014). Notably, our study involved BD patients and HCs in adolescence, a critical period for brain network development. Existing evidence consistently suggests that white matter structural network connectivity indices show changes during adolescence, manifesting either decreases or increases that may depend on the location of the network (e.g., frontal lobe vs. temporal lobe) (Dennis et al., 2013). Recent studies tend to suggest reduction of white matter RC connectivity particularly in the frontal cortex in BD patients (Forde et al., 2015; O'Donoghue, Holleran, Cannon, & McDonald, 2017; Wang et al., 2019), although one study failed to observe such difference between BD patients and controls (Collin et al., 2016). The discrepant findings could be due to the latter study involving only BD Type‐I patients with a mixture of mood status. On the other hand, transitioning from pathological affective states (mania or depressed) to mood stabilization has been associated with reduction of cytokine levels and inflammatory responses, with limited evidence that these reductions might be in turn linked to normalized gray matter structures (Berk, 2009), although the associations between cytokine levels, white matter, and functional network characteristics are still unclear. Also, medications were shown to reorganize neural profiles by preventing biochemical processes from causing tissue damage (Bai, Zhang, & Li, 2004), although it remains to be seen whether such neuroprotective property of psychotropic medication can be measured at a brain network level using MRI in humans. All BD patients included in the current study were in euthymic state and on mood stabilizers at the time of study. Thus, increased RC connectivity and a trend of decrease in local connectivity as observed in euthymic BD patients may indicate reorganization of RC connections that are part of the processes blocking the neuroprogression of BD.
Consistent with the view that reorganization of RC connectivity conveys neuroprotective effects in euthymic BD, our findings further showed increased connectivity strength in euthymic BD which were mostly observed among hub regions. Specifically, the frontal cortex and subcortical areas including bilateral putamen and bilateral pallidum were the major regions showing increased SC in euthymic BD. Wessa, Kanske, and Linke (2014) found altered SC between the prefrontal and limbic cortex in BD, which they proposed underlies emotional and motivational dysregulation in BD and might represent relevant vulnerability and disease neural maker. Moreover, heightened FC between the amygdala and the lateral regions of the ventral prefrontal cortex during rest or emotional processing were found in BD (Chase & Phillips, 2016). Furthermore, structural studies suggested that fractional anisotropy was significantly increased in euthymic bipolar patients relative to HCs in the medial frontal, precentral, inferior parietal, and occipital white matters (Versace et al., 2008; Michèle Wessa et al., 2009). Finally, during brain development in adolescence, white matter network in the frontal cortex was found to show major decreases in connectivity density, which is possibly related to pruning (Dennis et al., 2013). Thus, increased connectivity density between these RC structures may indicate that the structural network is reorganized in shaping information flow and may partially reflect white matter plasticity in increasing fiber streamline counts, possibly underlying the stronger regulatory control of the dorsal neocortical brain network over ventral limbic activity in euthymic BD.
Compared with controls, euthymic BD patients showed decreased functional modularity, further characterized by decreased intramodule connectivity and somewhat increased intermodule connectivity. Decrease in intramodule connectivity was also found for the default mode, limbic, and cognitive control networks among BD Type‐II patients (Chase & Phillips, 2016; Wang et al., 2016; Wang et al., 2017), suggesting disrupted self‐referential affective processing and top‐down regulation. Modularity, a network metrics for functional segregation, quantifies the ease with which the whole‐brain network can be divided into distinct subnetworks, or “modules” (Sporns, 2013). Brain regions that belong to a particular module tend to interconnect with each other and sparsely connect with areas outside the module. There is evidence that during brain maturation in late childhood and adolescence, functional modularity transforms from being organized largely on anatomical proximity to being congregated functionally, highlighting its critical role in functional specialization and coordination (Fair et al., 2009; Power, Fair, Schlaggar, & Petersen, 2010). Decreased modularity may indicate that euthymic BD patients showed sparse intramodular connectivity, coupled with increased intermodular connectivity. Moreover, connectivity density of RC was positively correlated with intermodular connectivity in BD patients, confirming the notion that structural RC connectivity provides the anatomical infrastructure for the integration of neural information between segregated functional modules in the brain (Van Den Heuvel & Sporns, 2011). Recent model hypothesized that a disruption in the integration of neural information among functional brain circuits may underlie psychotic symptoms and mood dysregulation in BD (Chase & Phillips, 2016; Strakowski et al., 2012). Conversely, decreased modularity coupled with increased functional integration, and its relationship with structural RC in euthymic BD may suggest that functional integration plays an essential role in mood normalization during the remission process of BD.
Preliminary evidence suggests that SC and FC interact with one another and the SC–FC coupling strength may be intrinsically a reflection of mental states (Batista‐García‐Ramó & Fernández‐Verdecia, 2018; Huang & Ding, 2016), yet its applications are to be researched. Limited existing evidence reveals significant coupling of structural and functional networks in the limbic system in BD patients and in HCs (Versace et al., 2010). Also, one study showed increased long‐distance (but not short or intermediate range) SC–FC coupling in young bipolar offspring (Collin et al., 2017). However, it is still unknown whether global SC–FC coupling strength in BD patients is changed. In the current study, we show that euthymic BD patients exhibited decreased intrahemispheric SC–FC coupling strength. It is considered that the SC provides scaffolding for FC and that FC shapes SC by mechanisms of neuroplasticity (Honey et al., 2010). Convergent functional and SC patterns have been found at different scales from single cortical slice to resting‐state networks (Greicius, Supekar, Menon, & Dougherty, 2009) and even for large‐scale whole‐brain network (Honey et al., 2009). Furthermore, pilot studies demonstrated that SC–FC coupling can be configured under normal physiological (Honey et al., 2009) and pathological states (e.g., van den Heuvel et al., 2013). Interestingly, preliminary evidence suggests that the relationship between SC–FC was not observed in children but became noticeable in young adults within the default mode network (Supekar et al., 2010), accompanying the development of self‐referential affective regulatory function throughout adolescence. Similarly, Hagmann et al. (2010) showed in a group of children and adolescents that the correlation between SC–FC increased with age. Decrease in SC–FC coupling may suggest a loss of coherence of functional and structural connectomes. We found such a neural pattern existed in BD patients of euthymic state, a state in which BD patients were clinically recovered, suggestive of a neural trait‐like feature for BD. Compared with SC and FC measures, the SC–FC coupling measure seemingly was a more robust predictor in distinguishing euthymic BD patients from HCs. This finding resonates with that of a previous study which reported that the level of intermodality integration is an additional measure for detecting subtle brain alterations that may be undetected by examining intramodality network indices (Zhang et al., 2011). However, our current findings cannot address the directionality of the BD‐related changes in SC–FC coupling and in SC and FC measures, which need to be explored in future longitudinal research.
4.1. Limitations
A few limitations need to be noted. First, all the euthymic patients were unavoidably medicated. Medication might have an impact on the white matter integrity and FC (Diler et al., 2013; Machado‐Vieira, Manji, & Zarate Jr, 2009). Given BD is a highly heritable and chronic, recurrent illness, future research could search for trait markers (or endophenotypes) in genetically at‐risk subjects who are medication‐naïve. Second, the current study did not compare the euthymic BD patients to a sample of acute‐phase patients, which limits the interpretation of the findings. Future studies including BD patients at different illness phases as well as HCs could provide more definitive evidence on BD trait‐ and state‐related brain network characteristics. Third, the diagnosis of BD in pediatric populations can be controversial, although considerable progress has been made in improving the diagnostic validity (Youngstrom, Birmaher, & Findling, 2008). Our on‐going project has been following up the pediatric patients at regular intervals, which will provide us with further confirmation on the clinical status of those individuals in the near future.
5. CONCLUSION
By characterizing the structural and functional connectomes in euthymic BD, we found increased structural RC, decreased functional modularization, and decreased structural–functional coupling strength in BD. The increased RC connectivity in BD patients of a euthymic state might be a consequence of mood normalization from acute states (i.e., depressive and hypo/manic states), while decreased structural–functional coupling strength could be suggestive of a neural trait‐like feature of BD, assisting in distinguishing BD from HCs with promising capacity. Future longitudinal studies incorporating BD patients in both acute and euthymic phases would be particularly valuable for elucidating the importance of structural–functional coupling in BD etiology, transition, and recovery.
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflict of interest.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This study was funded by the National Natural Science Foundation of China (NSFC: 81671347), Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders (201805010009), and Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory (201805010009), which had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Zhang R, Shao R, Xu G, et al. Aberrant brain structural–functional connectivity coupling in euthymic bipolar disorder. Hum Brain Mapp. 2019;40:3452–3463. 10.1002/hbm.24608
Ruibin Zhang and Robin Shao contributed equally to this study.
Funding information Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory, Grant/Award Number: 201805010009; Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders; National Natural Science Foundation of China, Grant/Award Number: 81671347
Contributor Information
Guiyun Xu, Email: xuguiyun2908@hotmail.com.
Kangguang Lin, Email: linkangguang@163.com.
DATA ACCESSIBILITY
Data will be made available on request.
REFERENCES
- Bai, O. , Zhang, H. , & Li, X.‐M. (2004). Antipsychotic drugs clozapine and olanzapine upregulate bcl‐2 mRNA and protein in rat frontal cortex and hippocampus. Brain Research, 1010(1–2), 81–86. [DOI] [PubMed] [Google Scholar]
- Bassett, D. S. , & Sporns, O. (2017). Network neuroscience. Nature Neuroscience, 20(3), 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista‐García‐Ramó, K. , & Fernández‐Verdecia, C. I. (2018). What we know about the brain structure–function relationship. Behavioral Sciences, 8(4), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk, M. (2009). Neuroprogression: Pathways to progressive brain changes in bipolar disorder. International Journal of Neuropsychopharmacology, 12(4), 441–445. [DOI] [PubMed] [Google Scholar]
- Blondel, V. D. , Guillaume, J.‐L. , Lambiotte, R. , & Lefebvre, É. (2011). The Louvain method for community detection in large networks. Journal of Statistical Mechanics: Theory and Experiment, 10, P10008. [Google Scholar]
- Bressler, S. L. , & Menon, V. (2010). Large‐scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. [DOI] [PubMed] [Google Scholar]
- Chase, H. W. , & Phillips, M. L. (2016). Elucidating neural network functional connectivity abnormalities in bipolar disorder: Toward a harmonized methodological approach. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, S. , Stern, J. M. , Engel, J., Jr. , & Haneef, Z. (2015). Structural–functional coupling changes in temporal lobe epilepsy. Brain Research, 1616, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, G. , Scholtens, L. H. , Kahn, R. S. , Hillegers, M. H. , & van den Heuvel, M. P. (2017). Affected anatomical rich club and structural–functional coupling in young offspring of schizophrenia and bipolar disorder patients. Biological Psychiatry, 82(10), 746–755. [DOI] [PubMed] [Google Scholar]
- Collin, G. , van den Heuvel, M. P. , Abramovic, L. , Vreeker, A. , de Reus, M. A. , van Haren, N. E. , … Kahn, R. S. (2016). Brain network analysis reveals affected connectome structure in bipolar I disorder. Human Brain Mapping, 37(1), 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, M. (2013). Moving forward (and beyond) the modularity debate: A network perspective. Philosophy of Science, 80(3), 356–377. [Google Scholar]
- Dennis, E. L. , Jahanshad, N. , McMahon, K. L. , de Zubicaray, G. I. , Martin, N. G. , Hickie, I. B. , … Thompson, P. M. (2013). Development of brain structural connectivity between ages 12 and 30: A 4‐Tesla diffusion imaging study in 439 adolescents and adults. NeuroImage, 64, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler, R. S. , Ladouceur, C. D. , Segreti, A. , Almeida, J. R. , Birmaher, B. , Axelson, D. A. , … Pan, L. A. (2013). Neural correlates of treatment response in depressed bipolar adolescents during emotion processing. Brain Imaging and Behavior, 7(2), 227–235. [DOI] [PubMed] [Google Scholar]
- Fair, D. A. , Cohen, A. L. , Power, J. D. , Dosenbach, N. U. , Church, J. A. , Miezin, F. M. , … Petersen, S. E. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5(5), e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone, S. V. , Lasky‐Su, J. , Glatt, S. J. , Eerdewegh, P. V. , & Tsuang, M. T. (2006). Early onset bipolar disorder: Possible linkage to chromosome 9q34. Bipolar Disorders, 8(2), 144–151. [DOI] [PubMed] [Google Scholar]
- Forde, N. J. , O'Donoghue, S. , Scanlon, C. , Emsell, L. , Chaddock, C. , Leemans, A. , … Murray, R. M. (2015). Structural brain network analysis in families multiply affected with bipolar I disorder. Psychiatry Research: Neuroimaging, 234(1), 44–51. [DOI] [PubMed] [Google Scholar]
- Foti, N. J. , Hughes, J. M. , & Rockmore, D. N. (2011). Nonparametric sparsification of complex multiscale networks. PLoS One, 6(2), e16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías, Á. , Palma, C. , & Farriols, N. (2015). Comorbidity in pediatric bipolar disorder: Prevalence, clinical impact, etiology and treatment. Journal of Affective Disorders, 174, 378–389. [DOI] [PubMed] [Google Scholar]
- Greicius, M. D. , Supekar, K. , Menon, V. , & Dougherty, R. F. (2009). Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex, 19(1), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra‐Carrillo, B. , Mackey, A. P. , & Bunge, S. A. (2014). Resting‐state fMRI: A window into human brain plasticity. The Neuroscientist, 20(5), 522–533. [DOI] [PubMed] [Google Scholar]
- Hagmann, P. , Sporns, O. , Madan, N. , Cammoun, L. , Pienaar, R. , Wedeen, V. J. , … Grant, P. (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proceedings of the National Academy of Sciences of the United States of America, 107(44), 19067–19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. , Sporns, O. , Cammoun, L. , Gigandet, X. , Thiran, J.‐P. , Meuli, R. , & Hagmann, P. (2009). Predicting human resting‐state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J. , Kötter, R. , Breakspear, M. , & Sporns, O. (2007). Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proceedings of the National Academy of Sciences of the United States of America, 104(24), 10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J. , Thivierge, J.‐P. , & Sporns, O. (2010). Can structure predict function in the human brain? NeuroImage, 52(3), 766–776. [DOI] [PubMed] [Google Scholar]
- Huang, H. , & Ding, M. (2016). Linking functional connectivity and structural connectivity quantitatively: A comparison of methods. Brain Connectivity, 6(2), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolis, V. R. , Froudist‐Walsh, S. , Brittain, P. J. , Kroll, J. , Ball, G. , Edwards, A. D. , … Nosarti, C. (2016). Reinforcement of the brain's rich‐club architecture following early neurodevelopmental disruption caused by very preterm birth. Cerebral Cortex, 26(3), 1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans, A. , & Jones, D. K. (2009). The B‐matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. [DOI] [PubMed] [Google Scholar]
- Lin, K. , Lu, R. , Chen, K. , Li, T. , Lu, W. , Kong, J. , & Xu, G. (2017). Differences in cognitive deficits in individuals with subthreshold syndromes with and without family history of bipolar disorder. Journal of Psychiatric Research, 91, 177–183. [DOI] [PubMed] [Google Scholar]
- Lin, K. , Xu, G. , Wong, N. M. , Wu, H. , Li, T. , Lu, W. , … Zhong, L. (2015). A multi‐dimensional and integrative approach to examining the high‐risk and ultra‐high‐risk stages of bipolar disorder. EBioMedicine, 2(8), 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado‐Vieira, R. , Manji, H. K. , & Zarate, C. A., Jr. (2009). The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disorders, 11(s2), 92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur, R. B. , McIntyre, R. S. , Cao, B. , Lee, Y. , Japiassú, L. , Chen, K. , … Li, T. (2018). Obesity and frontal‐striatal brain structures in offspring of individuals with bipolar disorder: Results from the global mood and brain science initiative. Bipolar Disorders, 20(1), 42–48. [DOI] [PubMed] [Google Scholar]
- Mcintyre, R. S. , Konarski, J. Z. , Misener, V. L. , & Kennedy, S. H. (2005). Bipolar disorder and diabetes mellitus: Epidemiology, etiology, and treatment implications. Annals of Clinical Psychiatry, 17(2), 83–93. [DOI] [PubMed] [Google Scholar]
- McIntyre, R. S. , Mansur, R. B. , Lee, Y. , Japiassú, L. , Chen, K. , Lu, R. , … Xu, G. (2017). Adverse effects of obesity on cognitive functions in individuals at ultra high risk for bipolar disorder: Results from the global mood and brain science initiative. Bipolar Disorders, 19(2), 128–134. [DOI] [PubMed] [Google Scholar]
- Merikangas, K. R. , Jin, R. , He, J.‐P. , Kessler, R. C. , Lee, S. , Sampson, N. A. , … Karam, E. G. (2011). Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry, 68(3), 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić, B. , Sporns, O. , & McIntosh, A. R. (2014). Communication efficiency and congestion of signal traffic in large‐scale brain networks. PLoS Computational Biology, 10(1), e1003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue, S. , Holleran, L. , Cannon, D. M. , & McDonald, C. (2017). Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: A selective review of structural network analyses using diffusion MRI. Journal of Affective Disorders, 209, 217–228. [DOI] [PubMed] [Google Scholar]
- O'Donoghue, S. , Kilmartin, L. , O'Hora, D. , Emsell, L. , Langan, C. , McInerney, S. , … Barker, G. J. (2017). Anatomical integration and rich‐club connectivity in euthymic bipolar disorder. Psychological Medicine, 47(9), 1609–1623. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Fair, D. A. , Schlaggar, B. L. , & Petersen, S. E. (2010). The development of human functional brain networks. Neuron, 67(5), 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, G. , Perry, A. , Lord, A. , Frankland, A. , Leung, V. , Holmes‐Preston, E. , … Breakspear, M. (2018). Structural dysconnectivity of key cognitive and emotional hubs in young people at high genetic risk for bipolar disorder. Molecular Psychiatry, 23(2), 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, K. E. , & Goodwin, G. M. (2010). The course of bipolar disorder. Advances in Psychiatric Treatment, 16(5), 318–328. [Google Scholar]
- Schmidt, A. , Smieskova, R. , Aston, J. , Simon, A. , Allen, P. , Fusar‐Poli, P. , … Borgwardt, S. (2013). Brain connectivity abnormalities predating the onset of psychosis: Correlation with the effect of medication. JAMA Psychiatry, 70(9), 903–912. [DOI] [PubMed] [Google Scholar]
- Senden, M. , Deco, G. , de Reus, M. A. , Goebel, R. , & van den Heuvel, M. P. (2014). Rich club organization supports a diverse set of functional network configurations. NeuroImage, 96, 174–182. [DOI] [PubMed] [Google Scholar]
- Sporns, O. (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23(2), 162–171. [DOI] [PubMed] [Google Scholar]
- Strakowski, S. M. , Adler, C. M. , Almeida, J. , Altshuler, L. L. , Blumberg, H. P. , Chang, K. D. , … Phillips, M. L. (2012). The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disorders, 14(4), 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar, K. , Uddin, L. Q. , Prater, K. , Amin, H. , Greicius, M. D. , & Menon, V. (2010). Development of functional and structural connectivity within the default mode network in young children. NeuroImage, 52(1), 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Kahn, R. S. , Goñi, J. , & Sporns, O. (2012). High‐cost, high‐capacity backbone for global brain communication. Proceedings of the National Academy of Sciences of the United States of America, 109(28), 11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel, M. P. , & Sporns, O. (2011). Rich‐club organization of the human connectome. Journal of Neuroscience, 31(44), 15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel, M. P. , & Sporns, O. (2013). An anatomical substrate for integration among functional networks in human cortex. Journal of Neuroscience, 33(36), 14489–14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Sporns, O. , Collin, G. , Scheewe, T. , Mandl, R. C. , Cahn, W. , … Kahn, R. S. (2013). Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry, 70(8), 783–792. [DOI] [PubMed] [Google Scholar]
- Versace, A. , Almeida, J. R. , Hassel, S. , Walsh, N. D. , Novelli, M. , Klein, C. R. , … Phillips, M. L. (2008). Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract‐based spatial statistics. Archives of General Psychiatry, 65(9), 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace, A. , Thompson, W. K. , Zhou, D. , Almeida, J. R. , Hassel, S. , Klein, C. R. , … Phillips, M. L. (2010). Abnormal left and right amygdala‐orbitofrontal cortical functional connectivity to emotional faces: State versus trait vulnerability markers of depression in bipolar disorder. Biological Psychiatry, 67(5), 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Kalmar, J. H. , He, Y. , Jackowski, M. , Chepenik, L. G. , Edmiston, E. E. , … Jones, M. (2009). Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological Psychiatry, 66(5), 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Deng, F. , Jia, Y. , Wang, J. , Zhong, S. , Huang, H. , … Huang, L. (2019). Disrupted rich club organization and structural brain connectome in unmedicated bipolar disorder. Psychological Medicine, 49(3), 510–518. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Wang, J. , Jia, Y. , Zhong, S. , Zhong, M. , Sun, Y. , … Huang, L. (2017). Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Translational Psychiatry, 7(7), e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhong, S. , Jia, Y. , Sun, Y. , Wang, B. , Liu, T. , … Huang, L. (2016). Disrupted resting‐state functional connectivity in nonmedicated bipolar disorder. Radiology, 280(2), 529–536. [DOI] [PubMed] [Google Scholar]
- Wessa, M. , Houenou, J. , Leboyer, M. , Chanraud, S. , Poupon, C. , Martinot, J. L. , & Paillère‐Martinot, M. L. (2009). Microstructural white matter changes in euthymic bipolar patients: A whole‐brain diffusion tensor imaging study. Bipolar Disorders, 11(5), 504–514. [DOI] [PubMed] [Google Scholar]
- Wessa, M. , Kanske, P. , & Linke, J. (2014). Bipolar disorder: A neural network perspective on a disorder of emotion and motivation. Restorative Neurology and Neuroscience, 32(1), 51–62. [DOI] [PubMed] [Google Scholar]
- Yan, C.‐G. , Wang, X.‐D. , Zuo, X.‐N. , & Zang, Y.‐F. (2016). DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Youngstrom, E. A. , Birmaher, B. , & Findling, R. L. (2008). Pediatric bipolar disorder: Validity, phenomenology, and recommendations for diagnosis. Bipolar Disorders, 10(1p2), 194–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Jiang, G. , Tian, J. , Qiu, Y. , Wen, X. , Zalesky, A. , … Huang, R. (2016). Abnormal white matter structural networks characterize heroin‐dependent individuals: A network analysis. Addiction Biology, 21(3), 667–678. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Wei, Q. , Kang, Z. , Zalesky, A. , Li, M. , Xu, Y. , … Huang, R. (2015). Disrupted brain anatomical connectivity in medication‐naïve patients with first‐episode schizophrenia. Brain Structure and Function, 220(2), 1145–1159. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Liao, W. , Chen, H. , Mantini, D. , Ding, J.‐R. , Xu, Q. , … Jiao, Q. (2011). Altered functional–structural coupling of large‐scale brain networks in idiopathic generalized epilepsy. Brain, 134(10), 2912–2928. [DOI] [PubMed] [Google Scholar]
- Zhong, S. , He, Y. , & Gong, G. (2015). Convergence and divergence across construction methods for human brain white matter networks: An assessment based on individual differences. Human Brain Mapping, 36(5), 1995–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data will be made available on request.


