Abstract
Mind blanking (MB) is the state where our minds are seemingly “nowhere,” and attention calls no perceptual input into conscious awareness. It is little investigated, perhaps partly because it is difficult to detect the mysterious periods of blanking. In this study, we found that our participants could intentionally produce a state of MB whose neural correlates were deactivation of Broca's area and parts of the default mode network (namely, the hippocampus) which would be active during mind wandering (MW), in addition to activity in another region in the default mode network (namely, anterior cingulate cortex). Because the behavioral finding replicates a previous report of ours, we suggest that the simple instructions that we used to induce MB should be effective. From the neuroimaging data, we conclude that we cannot define the content of our thoughts during MB because our inner speech system does not work at that time. Another possibility is that we actually think of nothing in the MB state. Although more sophisticated studies would be needed to uncover the mechanism of such a phenomenon, the present study provides a methodology and clues for understanding MB and related concepts such as MW, awareness, and metacognitive ability.
Keywords: Broca's area, consciousness, experience sampling, metacognition, mind wandering
1. INTRODUCTION
Our mind often generates activity without the guidance of sensory input from the external world. This shift of attention away from a primary task at hand toward internal thought is referred to as mind wandering (MW; Smallwood & Schooler, 2006). Our mind wanders anytime and anywhere, often unintended and unaware, about 30–50% of our waking hours (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Killingsworth & Gilbert, 2010; Smallwood, McSpadden, & Schooler, 2007). This psychological state has been considered to be related to the activity of the default mode network (DMN), which is a set of regions consisting of the medial prefrontal cortex (PFC) including anterior cingulate cortex (ACC), posterior cingulate cortex, precuneus, and inferior parietal lobule including angular gyrus (AG) that are consistently deactivated across a range of externally oriented experimental tasks (Christoff, Irving, Fox, Spreng, & Andrews‐Hanna, 2016; Fox, Spreng, Ellamil, Andrews‐Hanna, & Christoff, 2015; Raichle et al., 2001). Although this psychological state occupies about half of our waking mind, interestingly, at other times, our mind may go to “nowhere.” That is, there may be times when our minds are blank (Ward & Wegner, 2013).
During this state of mind blanking (MB), the individual is not focally aware of internal or external stimuli. Several previous works indicate that people often spontaneously describe their minds as being “blank” in a free‐response format (McCormick, Rosenthal, Miller, & Maguire, 2018; Schooler, Reichle, & Halpern, 2004; Watts & Sharrock, 1985). Although several experiments have indicated that MB is an independent psychological state, it remains difficult to detect. The blank mind may be obscured by internal self‐examination, in that any attempts to assess one's own mental states will necessarily find conscious thoughts and thus fail to find a blank mind (Ward & Wegner, 2013). Nonetheless, we recently reported that people could deliberately make their mind blank for a certain time, during which functional network connectivity (i.e., connectivity between resting‐state brain network, such as visual, frontal, and DMN) decreased relative to the MW state (Kawagoe, Onoda, & Yamaguchi, 2018). In our task, participants were instructed to think of nothing as best they could, and the results showed that they reported experience of MB for 58% of total time during the scan. Because the previous study set two conditions (MW and MB) as long‐range (10 min) functional magnetic resonance imaging (fMRI) tasks to specify resting‐state brain connectivity, however, we could not define brain activity specific to MB. In the present study, we thus conducted fMRI investigation to delineate MB‐related brain activity. To address this, we utilized temporal analysis of brain activation (Hasenkamp, Wilson‐Mendenhall, Duncan, & Barsalou, 2012). This analytical method provides an admirable opportunity to specify activity related to particular events given by a participant's cue, such as a button press (see Section 2).
To our knowledge, this is the first study to investigate the neural correlates of MB. Several brain regions or networks might be related to this psychological state—for example, DMN and executive network (Christoff et al., 2009; Fox et al., 2015), as in MW, because MB and MW are seemingly similar constructs. Based on the psychological finding that these two are independent (Ward & Wegner, 2013), however, it is highly plausible that neural correlates of MB will be detected that are distinct from those of MW.
2. MATERIALS AND METHODS
2.1. Participants
Twenty‐nine subjects (12 females and 17 males; mean age: 23.6 years, SD: 3.5; mean years of education: 16.5, SD: 1.9) were enrolled after providing written informed consent. This sample size was set based on a previous report suggesting that more than 20 subjects are necessary to achieve reliable results for fMRI group analysis (Thirion et al., 2007). The subjects had no history of neurological or psychiatric illness and had normal vision or corrected‐to‐normal vision with MRI‐compatible glasses.
2.2. fMRI task
After each individual's consent to participate was obtained, they went through the fMRI task. Before the scan, they were instructed to “Keep awake, fixate on the cross, and think of nothing as best you can. When you realize that you are thinking about something, disengage your attention and again try to think of nothing during the scans.” This simple instruction was intended to decrease MW during the scan and suppress intrinsic large‐scale brain connectivity during resting state, which might be due to MW (Kawagoe et al., 2018). During this long‐range fMRI task, we applied an experience sampling method, which enables online assessment of momentary changes in the content of consciousness by collecting self‐reports during the task at hand, which would capture even MW without awareness (Christoff et al., 2009; Smallwood et al., 2007). Therefore, participants were also instructed as follows: “You may be suddenly asked what you are thinking about during the scan through the presentation of a probe with a question and choices on it. Please answer the question by pressing one of the four buttons at hand.” The individuals were interrupted during fixation and were asked whether they were in a MW state, as Q1: “When was your mind at immediately before this probe?” with choices of “[index] past,” “[middle] present,” “[third] future,” and “[little] nowhere.” The words in square brackets indicated which finger should be used to press the corresponding button to give that answer. The second probe (Q2) differed depending on participants' response to the previous question. We primarily focused on MB in this study; thus, if an individual answered that their mind had just blanked out by pressing the button corresponding to the little finger in Q1, the Q2 probe was “You mean…” and choices were “[index] that you were trying to think of nothing (as instructed)” and “[middle] that your mind genuinely blanked out.” The latter was taken to indicate that the participant was in a state of MB. For the other Q1 answers, the Q2 probe was “The content was…,” with choices as follows: for the “past” MW trial, “[index] positive” and “[middle] negative”; for the “present” MW trial, “[index] the information originated from an internal sensation” and “[middle] the information originated from an external stimulation”; and for the “future” MW trial, “[index] goal‐directed planning” and “[middle] not goal‐directed.” These Q2s for past, present, and future MW were fillers, so that motor behavior was not different among the choices during the scan. In total, 15 pairs of probes (Q1 and Q2) were presented at random times during a single scan; the inter‐probe intervals were between 20 and 60 s. To prevent social desirability bias as much as possible, we emphasized that “Following the instruction to think of nothing would be difficult for us [humans], so be honest and tell me the truth about what you thought [about].”
The above experience sampling procedure produced evidence of several types of psychological state. We named them as follows: MB1 (answer “nothing” to Q1 and “try to think of nothing” to Q2), MB2 (answer “nothing” to Q1 and “genuinely blanked” to Q2), past1 (answer “past” to Q1 and “positive” to Q2), past2 (answer “past” to Q1 and “negative” to Q2), present1 (answer “present” to Q1 and “from internal sensation” to Q2), present2 (answer “present” to Q1 and “external stimulation” to Q2), future1 (answer “future” to Q1 and “goal‐directed planning” to Q2), and future2 (answer “future” to Q1 and “not goal‐directed” to Q2). MB2 was the primary condition of interest, and we considered past1, past2, future1, and future2 to indicate a state of MW.
Finally, participants' motivation and sleepiness during the scan were assessed after consecutive scans via 10‐cm visual analogue scale, because motivation for the task affects occurrence rate of MW during the task (Seli, Cheyne, Xu, Purdon, & Smilek, 2015), while sleepiness during the scan impacts resting‐state brain activity (Stoffers et al., 2015).
2.3. fMRI data acquisition and preprocessing
We conducted a 10‐min scan three times using a 3T MRI scanner (Phillips Ingenia 3.0T CX) at the Shimane Institute of Health Science (Izumo, Shimane, Japan). Subjects were instructed to keep as still as possible throughout the scanning procedure. Functional imaging data were acquired using the MRI scanner. Forty slices parallel to the plane connecting the anterior and posterior commissures were acquired using a T2*‐weighted gradient‐echo spiral pulse sequence with the following settings: repetition time = 2,500 ms, echo time = 30 ms, flip angle = 80°, ascending order, matrix size = 64 × 64, field of view = 212 × 212 mm2, isotropic spatial resolution = 3.3 mm, 3.2‐mm slice thickness, and 0.8‐mm slice gaps. The four initial scans were dummies in every run. After the three runs of functional scans, T1‐weighted images of the entire brain were obtained (170 slices, repetition time = 6.8 ms, echo time = 3.1 ms, flip angle = 9°, matrix size = 256 × 256, field of view = 256 × 256 mm2, and isotropic spatial resolution = 1 mm).
We used Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm) for temporal and spatial preprocessing. Images were spatially realigned to the first functional image to remove any artifacts due to head movements (X, Y, Z, pitch, yaw, roll, and their derivatives) and corrected for differences in image acquisition time between slices. Movement for all of the subjects was found to be within 3 mm in each of the functional runs and was fully corrected. The realigned images were normalized to Montreal Neurological Institute template standard space using DARTEL (Ashburner, 2007) and resliced with a 3 × 3 × 3‐mm voxel size. Spatial smoothing was then applied with an 8‐mm full width at half maximum. A high‐pass filter of 1/128 Hz was used to remove low‐frequency noise, and an AR (1) model was used to correct temporal autocorrelation.
2.4. fMRI analyses
For each subject, a beta value was obtained at each voxel for each condition of interest by fitting a GLM to the subject's BOLD signal data. This GLM included regressors for each condition of interest (MB1 and 2, past1 and 2, present1 and 2, and future1 and 2), modeled by convolving box car functions with a canonical hemodynamic response function based on the participants' responses to Q1 and Q2. The duration of interest was 5 s (2TR) immediately before the presentation of Q1 (Figure 1). Used as confound regressors were subjects' motion parameters produced at preprocessing and block regressors representing each run due to initial concatenation. Motivation and sleepiness were set as covariates for confounding adjustment due to their effect on thinking‐ and on resting‐state brain activity (Seli et al., 2015; Stoffers et al., 2015). The given beta values for each participant were warped into MNI space in preparation for group analyses, by applying a one‐sample t‐test to the MNI‐warped maps for each condition of interest (i.e., MB and MW). Although the spatial extent threshold was basically set at p < .05 with FDR correction, some items were more liberally thresholded at p < .001 without correction (k > 100). All of the above processing steps and statistical calculations were run on the MATLAB software (version R2017a; The Mathworks, Natick, MA).
Figure 1.
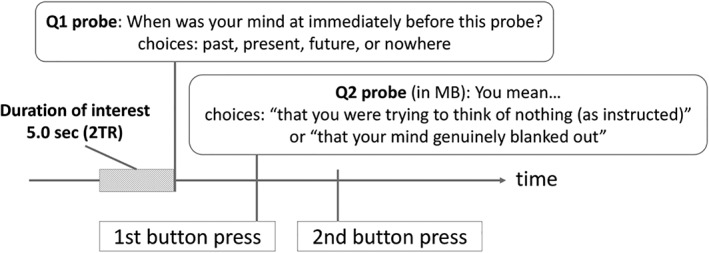
The task during the functional magnetic resonance imaging (fMRI) scan. The duration of interest was 2.5 ms before the first probe. The category of GLM regressors for psychological state of interest was defined based on the participants' responses to the first and second probes
3. RESULTS
3.1. Behavioral data
Participants reported that their minds went somewhere or nowhere during the scan (Figure 2) and reported a negligible amount of MW, accounting for 11.89% of past and 4.3% of future thinking. They also indicated that they were thinking of the present moment 26.5% of the time, with close amounts for internal sensation and external stimulation. MB (1 + 2) was found to be 57.3%, which is very consistent with our previous published value (58%) via the same task (Kawagoe et al., 2018). Of this MB, the MB2, which we focused on here, was 32.0%. This indicates that participants' minds were blanked out in at least almost one‐third of the samplings during the current scan. This result provides a clue to their neural activity during this intriguing psychological state. In addition, the means of the motivation and sleepiness scales were 6.32 (SD: 1.84) and 5.84 (SD: 2.39), respectively, on the 10‐cm visual analogue scale.
Figure 2.
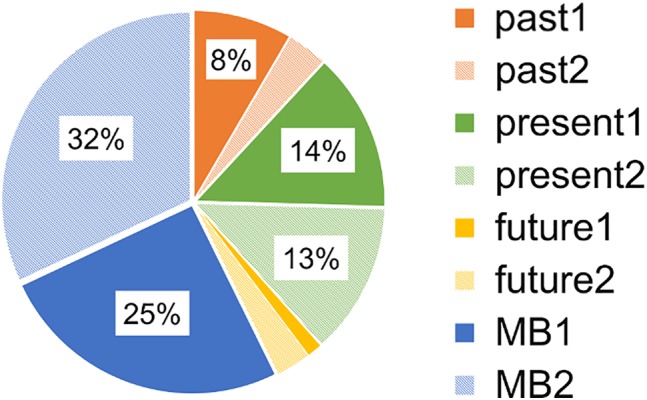
Behavioral data for experience sampling. Participants were, in the case of past1: thinking about positive things in the past; past2: thinking about negative things in the past; present1: thinking about their internal sensations; present2: thinking about external stimulation; future1: planning for the future; future2: thinking about the future; MB1: trying to think of nothing; and MB2: genuinely blanked
Based on our hypothesis and the behavioral results, we set the conditions as “pure MB,” modeled by MB2, and “MW,” modeled by a mixture of past1 and 2 and future1 and 2. We excluded present1 and 2 from the MW condition because their proportions were different from those of future and past (χ 2 (3) = 65.5, ps < .001) and because the “present” participants reported afterward that they were thinking about the meaning of the task (e.g., “What is nothing?” or “How can I think of nothing?”), the surroundings (e.g., sounds in the scanner or the fixation point), or sensations of their body (e.g., an itch), which might be not the case in MW. The numbers for each individual's responses are described in Table S1.
3.2. fMRI data
As a main purpose of this study, we investigated brain activities related to “pure” MB. Three participants were excluded from this analysis because they did not report MB at all. Table 1 and Figure 3 display significant clusters during pure MB (MB vs. baseline). A main effect of MB was found in the ACC/medial PFC as a positive effect. Meanwhile, a negative effect of MB was found in the frontal regions, including the inferior frontal gyrus (IFG) and Broca's area, superior frontal gyrus/supplementary motor cortex (SMC), and hippocampus.
Table 1.
Activations during “Pure Mind Blanking” condition (MB2 state; FDR‐corrected)
| Conditional effect/structure | BA | Cluster | L/R | T‐value | MNI coordinate | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| MB (positive) | |||||||
| ACC/medial PFC | 11 | 457 | Bilateral | 5.07 | 2 | 27 | −6 |
| 11 | 4.91 | 3 | 39 | −5 | |||
| MB (negative) | |||||||
| SFG/SMC | 8 | 678 | Bilateral | 6.04 | −18 | 17 | 62 |
| 6 | 4.87 | 3 | 17 | 57 | |||
| 8 | 4.34 | 17 | 15 | 60 | |||
| Hippocampus | 37 | 449 | Left | 6.03 | −27 | −33 | −3 |
| Broca's area | 45 | 562 | Left | 5.61 | −47 | 26 | 20 |
| 45 | 4.16 | −42 | 26 | 9 | |||
| IFG, Broca's area | 38 | 444 | Left | 4.72 | −50 | 24 | −9 |
| 45 | 3.99 | −53 | 35 | −3 | |||
| 45 | 3.75 | −48 | 39 | 5 | |||
Abbreviations: ACC, anterior cingulate cortex; BA, Brodmann area; IFG, inferior frontal gyrus; PFC, prefrontal cortex; SFG, superior frontal gyrus; SMC, supplementary motor cortex.
Figure 3.
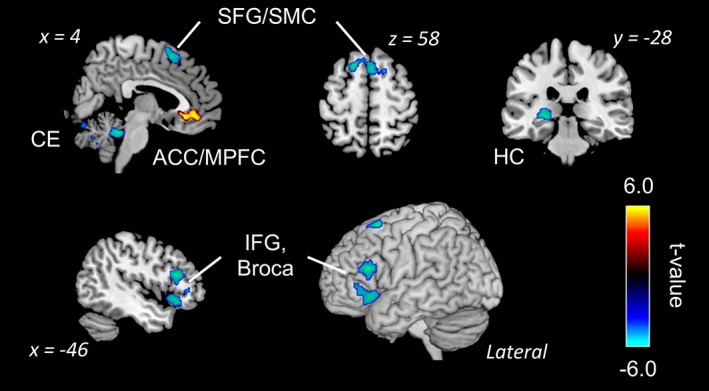
Activation and deactivation during mind blanking condition (FDR‐corrected, p < .05). ACC, anterior cingulate cortex; Broca, Broca's area; CE, cerebellum; HC, hippocampus; IFG, inferior frontal gyrus; MPFC, medial prefrontal cortex; SFG, superior frontal gyrus; SMC, supplementary motor cortex
We also assessed functional activation during MW (MW vs. baseline). The result showed that the SFG and medial PFC were in positive contrast. Although no activation was found in the negative contrast at the conservative threshold (FDR‐corrected), the AG/supramarginal gyrus (SMG) and lateral PFC does decrease during MW state at a liberal threshold (uncorrected, p < .001, k > 100; Table 2 and Figure 4).
Table 2.
Activations during mind wandering (past1 and 2 and future1 and 2)
| Conditional effect/structure | BA | Cluster | L/R | T‐value | MNI coordinate | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| MW (positive, FDR‐corrected) | |||||||
| Medial SFG | 10 | 553 | Bilateral | 5.85 | 8 | 56 | 23 |
| 11 | 4.21 | −3 | 54 | 15 | |||
| ACC/medial PFC | 11 | 850 | Bilateral | 5.32 | 3 | 35 | −6 |
| 10 | 5.04 | 5 | 45 | −9 | |||
| 11 | 3.97 | −8 | 39 | −8 | |||
| MW (negative, uncorrected, k > 100) | |||||||
| SMG/AG | 40 | 429 | Right | 5.72 | 51 | −41 | 42 |
| 40 | 4.00 | 50 | −44 | 56 | |||
| Lateral PFC | 46 | 165 | Right | 4.38 | 45 | 50 | 2 |
| 45 | 4.27 | 47 | 42 | −2 | |||
| 46 | 3.99 | 41 | 53 | 8 | |||
Abbreviations: AG, angular gyrus; BA, Brodmann area; PFC, prefrontal cortex; SFG, superior frontal gyrus; SMG, supramarginal gyrus.
Figure 4.
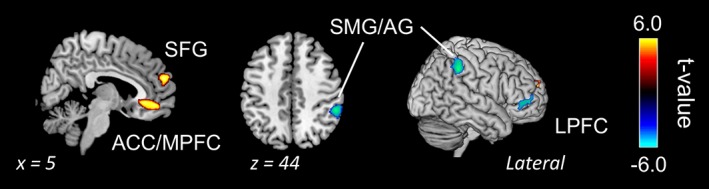
Activation and deactivation during mind wandering condition. The data were thresholded at p < .001 without correction (k > 100). Abbreviations: ACC, anterior cingulate cortex; AG, angular gyrus; LPFC, lateral prefrontal cortex; MPFC, medial prefrontal cortex; SFG, superior frontal gyrus; SMG, supramarginal gyrus
In addition, we compared activation between MB and MW. With a liberal threshold (uncorrected, p < .001, k > 100), some decreased activations were found in MB: left anterior insula (220 voxels at [−24 23 −6]) and Broca's area (80 voxels at [−53 17 11]; Figure 5).
Figure 5.
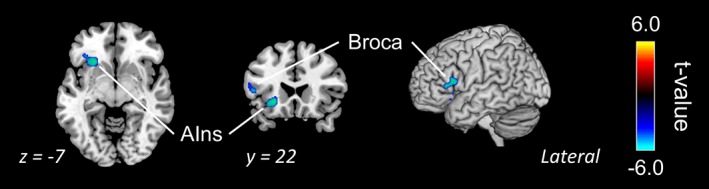
Comparison between the states of mind wandering and mind blanking. The blue‐cyan areas show higher activation during mind blanking than mind wandering. The data were thresholded at p < .001 without correction (k > 100). AIns, anterior insula; Broca, Broca's area
4. DISCUSSION
We investigated the neural correlates of MB, during which people's minds simply “disappear.” This state is independent from other mental states such as focusing and MW (Ward & Wegner, 2013), although its occurrence is not very frequent, ranging in previous studies from a few instances to 18% of total mental state (McCormick et al., 2018; Schooler et al., 2004; Ward & Wegner, 2013). However, the behavioral data from the current study suggest that the state of MB can be produced intentionally (Figure 2). Like our previous report (Kawagoe et al., 2018), the current result showed that participants answered they were in a state of MB for 57% of probes. Because such responses might include the state in which they were just trying to blank their minds to follow our instructions, we further asked them whether their mind was literally blank or they were just trying to make it blank, and found that 32% of the total were “pure” MB. Considering the similar amounts of MB reports in Kawagoe et al. (2018) and the initial question in the current study, we suggest that our instructions, in which the participants were told not to think of anything, could have the capability of inducing the rare psychological state of MB. Although we should have had a control condition to clearly indicate this, the result could still provide some clues on how to investigate MB in an experimental setting. In addition, the proportions of MW with different temporal aspects (i.e., past, present, and future) were not consistent with those of previous studies in which participants went through a monotonous cognitive task (Baird, Smallwood, & Schooler, 2011; Smallwood et al., 2011). The discrepancy of temporal focus might come from the mix‐up of on‐ and off‐task thought in addition to the difference of the tasks.
The fMRI results added important information about MB. The most notable finding was that the left‐lateralized inferior frontal region including Broca's area was deactivated during MB (Table 1 and Figure 3). Given the importance of Broca's area for semantic articulation, it is plausible that suppression of it caused the state of MB. Such an explanation has been frequently given for patients with traumatic brain injury, in which deactivation of Broca's area has been repeatedly shown (Hull, 2002). It is suggested that because of the deactivation, persons with trauma exhibit their emotions physically but not orally, due to the difficulty of verbalizing their feelings (Van der Kolk, McFarlane, & Weisæth, 1996). A recent neuropsychological study indicated that patients with poststroke Broca's aphasia had deficits in “inner speech,” and suggested that this impairment could affect metacognitive function (Langland‐Hassan, Gauker, Richardson, Dietz, & Faries, 2017), an assertion endorsed by recent a meta‐analysis (Vaccaro & Fleming, 2018). Inner speech has also been reported to play an important role in bringing thought to consciousness (Feinberg, Gonzalez Rothi, & Heilman, 1986; Morin, 2009). Another study indicated that the amount of intrusive inner speech, which negatively affects the task at hand, was associated with the activation of Broca's area (Kühn et al., 2013). Based on these previous studies, deactivation of Broca's area in the current results seems to constitute the neural representation of MB. When our inner speech system is “silent,” we cannot identify our psychological experience during that period—our minds are blanked out.
Our other finding on the MB state, demonstrated positive activation of medial PFC. Because this region is included in the core hubs of the DMN, which is related to MW (Christoff et al., 2016; Fox et al., 2015; Raichle et al., 2001), it is suggested that subjects' minds may have wandered anywhere during the periods in which they reported that their mind had been blank. However, we can also interpret the result as showing that there is literally no active, or at least active phonological, thinking in these periods. This interpretation was supported by the deactivation of the hippocampus during MB, which is a part of the DMN (Christoff et al., 2016). Because it has been reported that the hippocampus is the region that spontaneous thoughts emerge from (Ellamil et al., 2016) and hippocampal damage decreases future and past MW (McCormick et al., 2018), participants might actually think of nothing in MB periods; in this view, the deactivation of Broca's area would prove the absence of active thought. Altogether, in other words, we can consider the mechanism of MB in two ways: MB is caused by metacognitive deficits and/or by literally no thinking happening. The current experiment did not focus on this aspect, however, so these two mechanisms might be mixed up in the results. Future study should try to separate the two different backgrounds, although they may cause the same psychological phenomenon, MB.
As shown in Table 2 and Figure 4, the fMRI data for the MW state would endorse the validity of the methods in this study, because they clearly exhibit the activation of the core DMN region (Christoff et al., 2016; Fox et al., 2015; Raichle et al., 2001) and the deactivation of task‐positive networks such as the fronto‐parietal control network, which is involved in goal‐oriented thought (Petersen & Posner, 2012). An anticorrelation between these is usually observed (Christoff et al., 2016; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008), indicating that the current results are consistent with previous findings with regard to brain activation in MW. Moreover, the comparison of brain activation between MW and MB indicates that, in addition to Broca's area, the left anterior insula shows less activation in MB than MW. Because that the insula would be involved in awareness of MW (Hasenkamp et al., 2012), the explanation in which transient metacognitive deficit can be a trigger of MB, mentioned above, seems to identify a plausible mechanism for MB. Meanwhile, this cluster may include the claustrum, a mysterious region in the brain. There is an idea that the claustrum is responsible for consciousness because it anatomically receives input from almost all regions of the cortex and projects it back to them (Crick & Koch, 2005). A case report has shown this possibility (Koubeissi, Bartolomei, Beltagy, & Picard, 2014), which might make the “no thinking” hypothesis possible for the MB.
In sum, the current results indicate that we can deliberately make our mind blank, as also reported for MW (Seli, Risko, & Smilek, 2016) and that the deactivation of the Broca's area is a characteristic neural correlate of MB, as is the deactivation of the hippocampus. Future research should focus on determining a causal relationship between the deactivation of Broca's area and the MB state.
CONFLICT OF INTEREST
The authors declare no conflicts of interest with respect to the authorship or the publication of this article.
5.
DATA AVAILABILITY STATEMENT
Owing to ethical concerns, supporting data cannot be made openly available. However, further information about the data can be provided by the corresponding author, upon request.
AUTHOR CONTRIBUTIONS
T.K.: study concept and design; collecting, analyzing, and interpreting the data; and manuscript preparation. K.O.: supporting study design; collecting, analyzing, and interpreting the data; and critical review of the manuscript. S.Y.: critical review of the manuscript.
Supporting information
Table S1 The number of response for the probes of each participant.
ACKNOWLEDGMENTS
The study was conducted in accordance with the Declaration of Helsinki (1975, as revised in 2008) and the regulations of the Japanese Ministry of Health, Labour and Welfare. Shimane University medical ethics committee approved this study. Written informed consent was obtained from all participants. This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant Number 19K14481, 18K07558 and Rikkyo University Special Fund for Research (SFR) grant.
Kawagoe T, Onoda K, Yamaguchi S. The neural correlates of “mind blanking”: When the mind goes away. Hum Brain Mapp. 2019;40:4934–4940. 10.1002/hbm.24748
Funding information Japan Society for the Promotion of Science, Grant/Award Numbers: 19K14481, 18K07558; Rikkyo University Special Fund for Research
REFERENCES
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Baird, B. , Smallwood, J. , & Schooler, J. W. (2011). Back to the future: Autobiographical planning and the functionality of mind‐wandering. Consciousness and Cognition, 20(4), 1604–1611. 10.1016/j.concog.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Irving, Z. C. , Fox, K. C. R. , Spreng, R. N. , & Andrews‐Hanna, J. R. (2016). Mind‐wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Crick, F. C. , & Koch, C. (2005). What is the function of the claustrum? Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1458), 1271–1279. 10.1098/rstb.2005.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil, M. , Fox, K. C. R. , Dixon, M. L. , Pritchard, S. , Todd, R. M. , Thompson, E. , & Christoff, K. (2016). Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. NeuroImage, 136, 186–196. 10.1016/j.neuroimage.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Feinberg, T. E. , Gonzalez Rothi, L. J. , & Heilman, K. M. (1986). “Inner speech” in conduction aphasia. Archives of Neurology, 43(6), 591–593 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3718287 [DOI] [PubMed] [Google Scholar]
- Fox, K. C. R. , Spreng, R. N. , Ellamil, M. , Andrews‐Hanna, J. R. , & Christoff, K. (2015). The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Hasenkamp, W. , Wilson‐Mendenhall, C. D. , Duncan, E. , & Barsalou, L. W. (2012). Mind wandering and attention during focused meditation: A fine‐grained temporal analysis of fluctuating cognitive states. NeuroImage, 59(1), 750–760. 10.1016/j.neuroimage.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hull, A. M. (2002). Neuroimaging findings in post‐traumatic stress disorder. Systematic review. The British Journal of Psychiatry: The Journal of Mental Science, 181, 102–110 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12151279 [PubMed] [Google Scholar]
- Kawagoe, T. , Onoda, K. , & Yamaguchi, S. (2018). Different pre‐scanning instructions induce distinct psychological and resting brain states during functional magnetic resonance imaging. European Journal of Neuroscience, 47(1), 77–82. 10.1111/ejn.13787 [DOI] [PubMed] [Google Scholar]
- Kelly, A. M. C. , Uddin, L. Q. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2008). Competition between functional brain networks mediates behavioral variability. NeuroImage, 39(1), 527–537. 10.1016/J.NEUROIMAGE.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Killingsworth, M. A. , & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science (New York), 330(6006), 932 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Koubeissi, M. Z. , Bartolomei, F. , Beltagy, A. , & Picard, F. (2014). Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy & Behavior, 37, 32–35. 10.1016/j.yebeh.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Kühn, S. , Schmiedek, F. , Brose, A. , Schott, B. H. , Lindenberger, U. , & Lövden, M. (2013). The neural representation of intrusive thoughts. Social Cognitive and Affective Neuroscience, 8(6), 688–693. 10.1093/scan/nss047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland‐Hassan, P. , Gauker, C. , Richardson, M. J. , Dietz, A. , & Faries, F. R. (2017). Metacognitive deficits in categorization tasks in a population with impaired inner speech. Acta Psychologica, 181, 62–74. 10.1016/j.actpsy.2017.10.004 [DOI] [PubMed] [Google Scholar]
- McCormick, C. , Rosenthal, C. R. , Miller, T. D. , & Maguire, E. A. (2018). Mind‐wandering in people with hippocampal damage. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(11), 2745–2754. 10.1523/JNEUROSCI.1812-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, A. (2009). Self‐awareness deficits following loss of inner speech: Dr. Jill Bolte Taylor's case study. Consciousness and Cognition, 18(2), 524–529. 10.1016/j.concog.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Petersen, S. E. , & Posner, M. I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35(1), 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler, J. W. , Reichle, E. D. , & Halpern, D. V. (2004). Zoning out while reading: Evidence for dissociations between experience and metaconsciousness In Thinking and seeing: Visual metacognition in adults and children (pp. 203–226). Cambridge, MA: MIT Press. [Google Scholar]
- Seli, P. , Cheyne, J. A. , Xu, M. , Purdon, C. , & Smilek, D. (2015). Motivation, intentionality, and mind wandering: Implications for assessments of task‐unrelated thought. Journal of Experimental Psychology: Learning Memory and Cognition, 41(5), 1417–1425. 10.1037/xlm0000116 [DOI] [PubMed] [Google Scholar]
- Seli, P. , Risko, E. F. , & Smilek, D. (2016). On the necessity of distinguishing between unintentional and intentional mind wandering. Psychological Science, 27(5), 685–691. 10.1177/0956797616634068 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , McSpadden, M. , & Schooler, J. W. (2007). The lights are on but no one's home: Meta‐awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin and Review, 14(3), 527–533. 10.3758/BF03194102 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , & Schooler, J. W. (2006). The restless mind. Psychological Bulletin, 132(6), 946–958. 10.1037/0033-2909.132.6.946 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , Schooler, J. W. , Turk, D. J. , Cunningham, S. J. , Burns, P. , & Macrae, C. N. (2011). Self‐reflection and the temporal focus of the wandering mind. Consciousness and Cognition, 20(4), 1120–1126. 10.1016/j.concog.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Stoffers, D. , Diaz, B. A. , Chen, G. , Den Braber, A. , Van't Ent, D. , Boomsma, D. I. , … Linkenkaer‐Hansen, K. (2015). Resting‐state fMRI functional connectivity is associated with sleepiness, imagery, and discontinuity of mind. PLoS One, 10(11), e0142014 10.1371/journal.pone.0142014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion, B. , Pinel, P. , Mériaux, S. , Roche, A. , Dehaene, S. , & Poline, J.‐B. (2007). Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage, 35(1), 105–120. 10.1016/j.neuroimage.2006.11.054 [DOI] [PubMed] [Google Scholar]
- Vaccaro, A. G. , & Fleming, S. M. (2018). Thinking about thinking: A coordinate‐based meta‐analysis of neuroimaging studies of metacognitive judgements. Brain and Neuroscience Advances, 2, 239821281881059 10.1177/2398212818810591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kolk, B. A. , McFarlane, A. C. , & Weisæth, L. (1996). Traumatic stress : The effects of overwhelming experience on mind, body, and society. New York: Guilford Press. [Google Scholar]
- Ward, A. F. , & Wegner, D. M. (2013). Mind‐blanking: When the mind goes away. Frontiers in Psychology, 4(Sep), 1–15. 10.3389/fpsyg.2013.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, F. N. , & Sharrock, R. (1985). Description and measurement of concentration problems on depressed patients. Psychological Medicine, 15(2), 317–326. 10.1017/S003329170002359X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The number of response for the probes of each participant.
Data Availability Statement
Owing to ethical concerns, supporting data cannot be made openly available. However, further information about the data can be provided by the corresponding author, upon request.


