Abstract
Hand motor function is often severely affected in stroke patients. Non‐satisfying recovery limits reintegration into normal daily life. Understanding stroke‐related network changes and identifying common principles that might underlie recovered motor function is a prerequisite for the development of interventional therapies to support recovery. Here, we combine the evaluation of functional activity (multichannel electroencephalography) and structural integrity (diffusion tensor imaging) in order to explain the degree of residual motor function in chronic stroke patients. By recording neural activity during a reaching and grasping task that mimics activities of daily living, the study focuses on deficit‐related neural activation patterns. The study showed that the functional role of movement‐related beta desynchronization in the supplementary motor area (SMA) for residual hand motor function in stroke patients depends on the microstructural integrity of the corticospinal tract (CST). In particular, in patients with damaged CST, stronger task‐related activity in the SMA was associated with worse residual motor function. Neither CST damage nor functional brain activity alone sufficiently explained residual hand motor function. The findings suggest a central role of the SMA in the motor network during reaching and grasping in stroke patients, the degree of functional relevance of the SMA is depending on CST integrity.
Keywords: DTI, EEG, functional and structural neuroimaging, motor function, SMA, stroke recovery
1. INTRODUCTION
Stroke is the leading cause for long‐term disability. Despite the fact that acute treatment has substantially improved in recent years, the number of patients with severe neurological deficits, requiring intensive rehabilitation is still rising (Feigin, Forouzanfar, & Krishnamurthi, 2014). Hand motor function is often severely affected in stroke patients and substantially limits activity of daily living, quality of life and re‐integration in professional activity (Ones, Yilmaz, Cetinkaya, & Caglar, 2005). Recovery of hand function is one primary goal in stroke rehabilitation treatment programs (Bernhardt et al., 2016). The course of recovery and response to rehabilitative treatment, however, is heterogeneous across patients and subject to large inter‐patient variability (Veerbeek, Kwakkel, van Wegen, Ket, & Heymans, 2011). Hence, a mechanistic understanding of adaptive and maladaptive neuroplastic changes occurring during recovery is mandatory for the development of patient‐tailored therapeutic protocols including individualized rehabilitative training and targeted neurostimulation protocols (Di Pino et al., 2014; Koch & Hummel, 2017; Raffin & Hummel, 2018).
Previous studies have extracted clinical, electrophysiological and neuroimaging markers indicative of a favorable outcome, which not only increased our understanding of network adaptation to lesion, but might also offer prognostic markers in clinical care (for a review see [Raffin & Hummel, 2018; Stinear, 2017]). It is even more important to identify biomarkers for expected treatment responses toward precision medicine‐based interventions. The degree of impairment is influenced by several factors, not only initial impairment, lesion location, but also by structural and functional changes within the motor network. As for the structural changes, the lesion volume has little explanatory value of motor function after stroke (Puig et al., 2011; Schiemanck, Kwakkel, Post, Kappelle, & Prevo, 2006), whereas the lesion location (Crafton, 2003; Shelton & Reding, 2001) and the microstructural white matter integrity has been shown to strongly determine motor function (Koch & Hummel, 2017). In particular, better integrity of the lesioned corticospinal tract (CST) is positivity correlated with better motor function (Lindenberg et al., 2010; Schulz et al., 2012; Schulz et al., 2015). Disconnection from descending pathways results in neuroplastic changes toward alternative motor network activation patterns. Especially in the subacute phase, patients show stronger activation in the contralesional primary motor cortex (M1), bilateral ventral premotor cortex (PMv) and supplementary motor area (SMA) relative to healthy subjects while using the paretic arm. Furthermore, ipsilesional M1, pre‐SMA, contralesional premotor cortex, and cerebellar activity correlated with better residual motor performance (Bönstrup et al., 2015; Lee et al., 2017; Rehme, Eickhoff, Wang, Fink, & Grefkes, 2011).
These unimodal findings give rise to the assumption that best prediction of residual motor function might be obtained when integrating information from multiple modalities. Stinear proposed the PREP algorithm, which integrated information from diffusion tensor imaging (DTI) measurements, transcranial magnetic stimulation (TMS) and initial motor function to classify motor outcome 12 weeks post stroke in different groups of recovery (Stinear, 2010). Moreover, a recent study by Volz et al. demonstrated that individual motor impairment is the best explained by changes in cortical excitability obtained from TMS, functional connectivity in‐between ipsi‐ and contralesional primary motor cortex, as well as structural damage to the CST (Volz et al., 2015). Similarly, functional improvement after rehabilitative therapy was best explained by CST integrity and functional connectivity of the primary motor cortices (Burke Quinlan et al., 2015). Even though neural oscillatory activation patterns have been shown to be altered after stroke depending on the degree of motor impairment (Bönstrup et al., 2015; Nicolo et al., 2015; Rossiter, Boudrias, & Ward, 2014), integrating oscillatory and structural information has only rarely been investigated. Novel results suggest that the combination of motor network connectivity measured via electroencephalography (EEG) and CST integrity holds further information on the motor impairment status (Wu et al., 2015) and motor recovery (Guggisberg, Nicolo, Cohen, Schnider, & Buch, 2017). Importantly, these biomarkers were extracted from the task‐free brain state. Task‐free neuronal activations can demonstrate general, not functionally specific neuroplastic changes occurring after a lesion. Task‐free activations are also less informative regarding task‐specific network adaptations and their functional role for residual motor function. The relationship between aberrant cortical oscillatory activation patterns during everyday tasks, like grasping and reaching, CST integrity and motor function has not been studied in detail so far.
Here, we link information of oscillatory changes measured with multichannel EEG during specific grasping movements and the structural integrity of the CST, in order to explain hand motor outcome in chronic stroke patients. Importantly, we combined functional and structural information into one multivariate model and analyzed the interaction between both factors and its influence on residual motor function. Notably, patients with mild to moderate hand motor deficits performed two different grasping movements during EEG measurement, allowing us to study neural activation patterns during fine skilled movements. Grasping movements are central for the usage of tools and are impaired regularly, even in otherwise well recovered patients, and significantly limit their activities of daily living. Here, a task was implemented feasible even for the patient subgroup with stronger deficits, but still challenging for the better‐recovered patients. We hypothesized that the cortical representation of residual motor function during a skilled grasping task, depends on the structural integrity of the CST.
2. MATERIAL AND METHODS
2.1. Participants and motor outcome
Twenty individuals (mean age 63.9 years ±8.3 SD, range 51–79, 7 females) with mild to moderate unilateral hand motor impairment due to a first‐ever ischemic stroke were recruited. Of the 20 participants, 3 participants had to be excluded due to extensive muscle artifacts during EEG recordings. All participants were in the chronic stage after stroke (> 6 month). Lesion locations included cortical and subcortical areas (5 cortical, 12 subcortical) (see Figure 1 for lesion location). Ten patients had lesions within the dominant hemisphere (see Table 1 for clinical data). DTI data of a subgroup of these patients (n = 9) has been already analyzed in a previous study with focus on parietofrontal structural connectivity (Schulz et al., 2015). Motor outcome was evaluated by means of the Fugl–Meyer score for the upper extremity (UEFM, (Fugl‐Meyer, Jääskö, Leyman, Olsson, & Steglind, 1975)), as well as grip‐ and pinch force (three measurements, Strength JAMAR hand evaluation kit, Elite healthcare, Wigan, UK). For grip‐ and pinch force, the ratio affected/unaffected hand was computed. Subsequently, we calculated one composite motor score explaining 72% of the variance in all three measurements by extracting the first eigenvariate of a principal component analysis (Schulz et al., 2015). Thus, this value was used for further analysis, with higher values indicating better motor outcome (Table 1). Participants gave written informed consent according to the Declaration of Helsinki. The study was approved by the local ethics committee of the Medical Association of Hamburg (PV3777).
Figure 1.
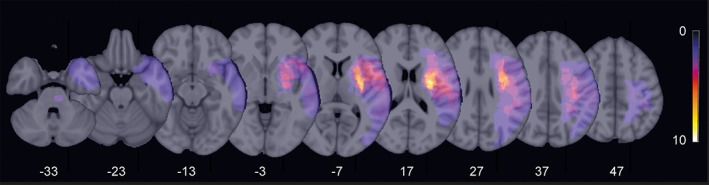
Stroke lesion overlay. Stroke lesions were projected to the left hemisphere for each patient and overlaid onto a T1 template in MNI standard space. Color indicates the number of patients with stroke lesions in the corresponding voxel. Z values mark the MNI coordinates of the transverse section
Table 1.
Clinical data
| Patient ID | Gender | Age | Dom | TAS | Lesion location | UE‐FM | Pinch‐ratio (A/U) | Grip‐ratio (A/U) | Motor score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 59 | 1 | 42 | PLIC | 60 | 0.8 | 0.75 | −0.69 |
| 2 | f | 60 | 0 | 44 | PONS | 64 | 1.15 | 0.89 | 1.46 |
| 3 | m | 55 | 0 | 22 | CR | 66 | 0.89 | 0.79 | 0.33 |
| 4 | m | 53 | 1 | 32 | CR, BG | 66 | 1.2 | 0.89 | 1.84 |
| 5 | m | 70 | 1 | 113 | MED | 49 | 0.82 | 0.77 | −1.52 |
| 6 | m | 65 | 1 | 35 | CR, CI | 66 | 0.84 | 0.79 | 0.16 |
| 7 | m | 52 | 0 | 26 | MED, IC | 64 | 0.95 | 0.89 | 0.76 |
| 8 | f | 70 | 0 | 31 | PONS | 66 | 1.03 | 0.8 | 0.88 |
| 9 | f | 66 | 1 | 27 | TC, PLIC | 55 | 0.8 | 0.63 | −1.59 |
| 10 | f | 51 | 0 | 97 | MED | 54 | 0.86 | 0.7 | −1.19 |
| 11 | m | 73 | 0 | 54 | CR | 49 | 0.82 | 0.66 | −1.95 |
| 12 | f | 79 | 1 | 11 | TC, PLIC | 66 | 1.28 | 1.1 | 2.89 |
| 13 | f | 75 | 1 | 197 | IC, BG, CR | 61 | 1.1 | 1.04 | 1.60 |
| 14 | m | 65 | 0 | 22 | CR | 60 | 0.82 | 0.42 | −1.87 |
| 15 | f | 65 | 0 | 81 | MED | 60 | 0.68 | 0.72 | −1.23 |
| 16 | m | 60 | 1 | 51 | CR, BG, IC | 59 | 0.77 | 0.82 | −0.58 |
| 17 | m | 71 | 1 | 39 | BG, CR | 66 | 0.88 | 0.89 | 0.71 |
Note. Age (in years), gender (m, male; f, female), Dom, dominant hemisphere affected (0 = no; 1 = yes); TAS, time after stroke in month, stroke location (MED, media infarct; PLIC, posterior limb of the internal capsule; CR, corona radiate; CI, capsula interna; TC, thalamocapsular; BG, basal ganglia; PONS, pons; IC, insular cortex), UEFM, upper extremity Fugl‐Meyer, pinch and grip force ratio A/U (A, affected hand; U, unaffected hand). UEFM, pinch and grip force ration have been merged into one composite motor score.
2.2. Motor tasks
Participants performed repetitive reaching and grasping movements with their affected upper extremity in an event‐related design. They were seated in front of a monitor with their arms placed on a custom‐made platform. The affected hand was placed on a socket installed on the platform with the elbow 90° flexed. Instructions were visually presented on a screen, consisting of the word “pinch grip” or “whole hand grip,” followed by a “Go” cue 2–3 s later (Figure 2). After the “Go” cue patients had to reach for a 200 g weight positioned 35 cm in front of them. During the “pinch grip” condition, participants had to lift the weight 10–20 cm off the table using the affected thumb and index finger. During the whole hand grip condition, the weight was lifted with all fingers. The weight was reset immediately after. Subsequently, the hand was placed on the socket again. Condition “pinch” and condition “whole” were presented in a random, counterbalanced order. Each trial started 8–10 s after the replacement to the socket. Each participant performed 80 pinch and 80 whole hand grips during EEG recording.
Figure 2.
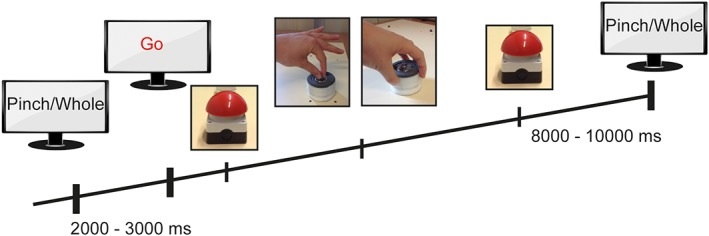
Motor task. The affected hand was rested on the red socket. Instructions were visually presented on a screen (pinch grip/whole hand grip), followed by a “Go” cue 2–3 s later. After the “Go” cue, participants had to lift a weight off the table using a pinch grip or whole hand grip of the affected hand. The weight was reset immediately after and the hand was returned to the socket. Eight to 10 s later the next visual cue was presented
2.3. Recording and preprocessing
2.3.1. Electroencephalography
Data were sampled at 1000 Hz using a 63‐channel EEG system positioned according to the 10–10 System of the American Electroencephalographic Society (using actiCAP®, Brain Products GmbH, Gilching, Germany; Electro‐Cap International, Inc., Eaton, OH, USA) and referenced to the Cz electrode. The impedance of the EEG electrodes was kept below 25 kΩ. Data were segmented from −7 to 5 s with respect to the “Go” cue for further analysis. Data were filtered from 2 to 60 Hz with a bandpass‐filter of fourth order and a bandstop filter at 49–51 Hz and resampled to 125 Hz. Eye‐movement artifacts were removed employing an independent component analysis (Makeig, Bell, & Jung, 1996). Epochs containing electrode artifacts, muscle artifacts, head movements or incompletely rejected blink artifacts were marked manually by visual inspection. Subsequently, data were re‐referenced to a common average reference. Artifact rejection resulted in an overall number of μ = 55.6/66.8, SD = 11.2/8.4 trials (pinch grip /whole hand grip). The Fieldtrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011) as well as custom written software using MATLAB Version 8.2.0 (R2013b, Mathworks Inc., Massachusetts) were used for EEG data analysis.
2.3.2. Brain imaging
Diffusion‐weighted imaging and high‐resolution T1‐weighted anatomical images data were acquired using a 3 T Siemens Skyra MRI scanner (Siemens, Erlangen, Germany). For the former, 75 axial slices were obtained covering the whole brain with gradients (b = 1,500 s/mm2) applied along 64 noncollinear directions with the following sequence parameters: Repetition time = 10,000 ms, echo time = 82 ms, field of view = 256 × 204, slice thickness = 2 mm, in‐plane resolution = 2 × 2 mm. For the anatomical imaging, a 3D magnetization‐prepared, rapid acquisition gradient‐echo sequence (MPRAGE) was used with the following parameters: repetition time = 2,500 ms, echo time = 2.12 ms, field of view = 256 × 208 mm, 256 axial slices, slice thickness = 0.94 mm, in‐plane resolution = 0.83 × 0.83 mm (Schulz et al., 2015).
2.4. Data analysis
2.4.1. EEG data analysis
Source reconstruction
To resolve time‐frequency dynamics in source space, we reconstructed activity at pre‐defined coordinates of interest (COI) using spatial filtering. Coordinates for M1, PMv, and SMA were derived from a previous functional Magnetic Resonance Imaging (fMRI) experiment involving a hand grip task in healthy elderly participants (Schulz et al., 2016). All lesions were flipped to the left side in order to allow a comparison of contralateral and ipsilateral activations across patients. As a forward model, we computed a Boundary Element Method volume conduction model (Fuchs, Kastner, Wagner, Hawes, & Ebersole, 2002) using the source space modeling functions of the Statistical Parametric Mapping software (SPM12b, Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm). These forward models were based on individual T1‐weighted structural MRIs and individual electrode positions registered with an ultrasound localization system (CMS20, Zebris, Isny, Germany). Leadfields for the dipolar sources at the COIs were computed and in junction with the channel covariance matrix used to calculate a linearly constrained minimum variance (LCMV) beamformer (Van Veen, van Drongelen, Yuchtman, & Suzuki, 1997) for each location. Channel time series were then projected to source space with the filter oriented along the maximal signal variance.
Frequency analysis
The time resolved spectral analysis was calculated from 1 to 25 Hz in steps of 1 Hz applying a fast Fourier transformation using one Hanning taper. The window length was frequency dependent (5 cycles per time window) and the center of the moving window was shifted in steps of 50 ms. Subsequently, we calculated the task‐related power change during movement relative to baseline (−7,000 ms to −4,000 ms with respect to the “Go” cue) and averaged all trials within one participant.
2.4.2. DTI data analysis
The FSL software package 5.1 (http://www.fmrib.ox.ac.uk/fsl) was used to analyze the imaging data of the stroke patients. In brief, after correcting for eddy currents and head motion, brains were skull stripped and fractional anisotropy (FA) maps were calculated for each participant fitting the diffusion tensor model at each voxel (Behrens et al., 2003). The FA maps were then registered non‐linearly to the Montreal Neurological Institute (MNI) standard space applying FSL's flirt and fnirt commands. Subsequently, stroke lesions were masked out and were not considered during the registration process. The characterization of the microstructural state of the CST in the stroke patients was conducted using structural and diffusion‐weighted imaging data of 26 healthy controls taken from a previous study (Schulz et al., 2017). For the CST, normalized and binarized group‐average masks of varying thresholds of the left and right average CST at the level from the mesencephalon to the cerebral peduncle (MNI coordinates z = −25 to z = −20) were already available. These masks were used to calculate the mean FA for the affected and unaffected tract in the stroke patients. We report the integrity of the CST as proportional FA values (affected/unaffected tract). Please refer to the Supporting Information Methods for further details.
2.5. Multimodal model
We used the R statistical package 3.2.3 (CDT, 2008) to perform a linear regression analysis of the relationship between the motor score and functional activation parameters combined with measures of structural integrity. As fixed effects, we entered the CST‐Ratio and EEG relative power (with interaction term) into the model. Age (Quandt et al., 2016; Veerbeek et al., 2011) and information on the dominance/non‐dominance of the lesioned hemisphere were included as additional fixed effects. This model was iteratively tested for the six COIs (left/right SMA, PMv, M1), three frequency bands (4–7 Hz, 8–13 Hz, 14–25 Hz) as well as during movement (0.2 to 0.6 s with respect to the “Go” cue) and baseline (−7 to −4 s). Frequency bands and time intervals were chosen based on the detected time‐frequency patterns (see results section 3.1). Moreover, the time interval was chosen to avoid muscle artifacts in the later phase of the movement. p‐values of the F‐statistic were adapted for multiple comparisons using a Bonferroni correction (α = 0.05/36). Estimates are reported with 95% confidence intervals. When comparing the full model against the reduced model p‐values were obtained using likelihood ratio tests.
3. RESULTS
3.1. Source spectral power dynamics
We found task‐related amplitude changes in all brain regions across the theta, alpha, and beta frequency range. As illustrated in Figure 3, task‐related power decrease in the alpha and beta frequency range (8–25 Hz) started about 200 ms after the “Go” cue, whereas beta oscillations (14–25 Hz) occurred even before in the period between the instruction, which grip to execute, and the “Go” cue. Task‐related power decreases (i.e., activation) were larger in the contralateral (i.e., ipsilesional) hemisphere to movement and stronger in M1 and SMA compared to PMv. In all regions, we detected induced theta oscillations right after the “Go” cue that was strongest in the ipsilateral PMv. In summary, the distribution of these time‐frequency responses were in line with previous findings determined during hand movements in healthy subjects (Crone et al., 1998; Pfurtscheller & Lopes da Silva, 1999; Quandt et al., 2016; van Wijk, Beek, & Daffertshofer, 2012). Comparing the spectral power dynamics between pinch‐ and whole hand grip revealed no significant clusters within the time‐frequency maps of all regions (paired t‐test, p > .05, Bonferroni corrected).
Figure 3.
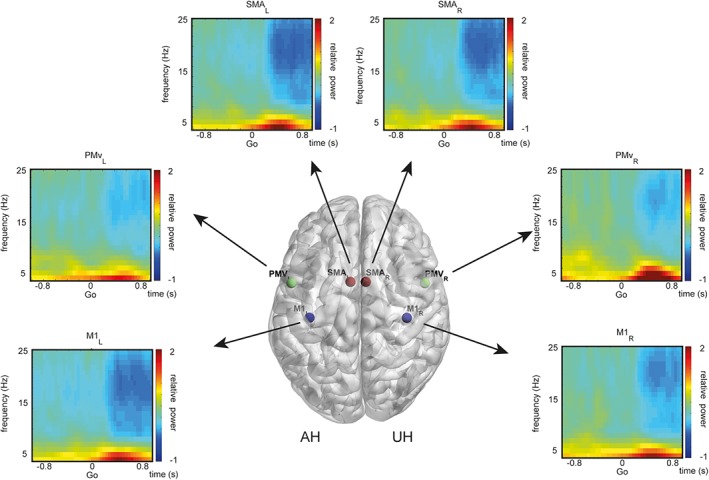
Time‐frequency maps of task‐related spectral power dynamics. Group average of time‐frequency responses during pinch grip at the coordinates of interest. Location of coordinates of interests are rendered on a template brain for visualization. Stroke lesions were projected to the left hemisphere (AH) for each patient and overlaid onto a T1 template in MNI standard space. Blue indicates a relative power decrease, red displays a power increase relative to baseline. Affected/unaffected hemisphere (AH/UH)
3.2. White matter integrity of the CST
Figure 4 summarizes the tract‐related absolute FA values for the affected and unaffected hemispheres of the stroke patients, as well as for the ratio affected/unaffected.
Figure 4.
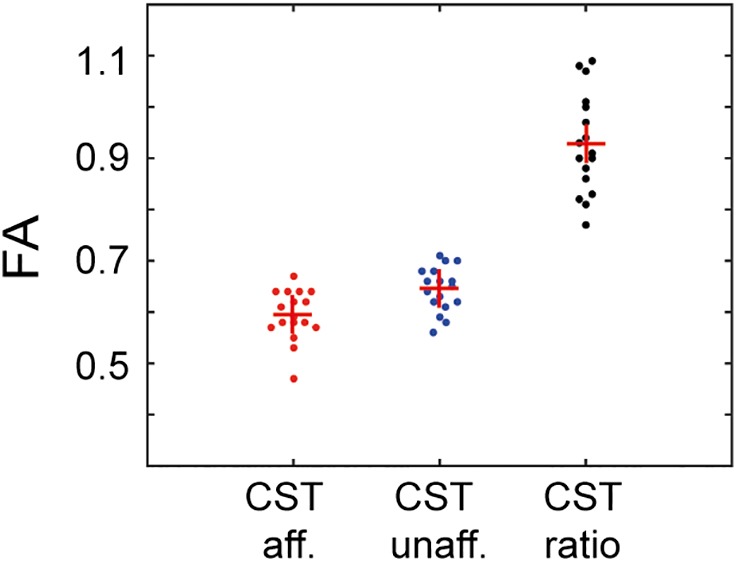
Corticospinal tract (CST) integrity. FA values of the CST for the affected and unaffected side, as well as for the ratio affected/unaffected for each individual. Red crosses mark the group mean
3.3. Multimodal model explaining motor outcome
In an exploratory approach, we performed multiple linear regression analyses in order to explain the hand motor score in chronic stroke patients by functional activation of the motor network combined with structural integrity of the CST. We iteratively tested the model with the fixed effects varying over frequency bands (theta, alpha, and beta), time intervals (baseline, movement), and regions (six coordinates of interest; 36 models). EEG activations were taken from the pinch grip condition. Movement‐related beta desynchronization (MRBD) in the ipsilesional SMA in combination with structural integrity of the CST best explained residual motor function of the paretic upper extremity of the patients. All other models were not significant after correction for multiple comparisons (for all model results, please refer to Table S1). The SMA model was checked for validity, visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. However, since we detected one influential point (patient id # 1, based on Cook's distance and high advantage), we re‐ran the model analysis (as reported in Table 2). Model results kept stable after exclusion of the influential point (F[5,10] = 11.58, p = .0007, R2 = 0.85).
Table 2.
Linear regression modeling of hand motor outcome and supplementary motor area (SMA) power and CST integrity
| Outcome | Predictor | Estimate | 95% CI | p‐value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Motor score | Intercept | 15.11 | −2.72 | 32.95 | .088 |
| AGE | 0.07 | 0.007 | 0.14 | .034* | |
| DOM | 0.30 | −0.54 | 1.15 | .443 | |
| EEG | 57.00 | 24.04 | 89.97 | .003** | |
| CST‐R | −19.61 | −39.15 | −0.07 | .049* | |
| EEG × CST‐R | −56.58 | −93.53 | −19.63 | .006** | |
Note. Linear regression results on composite motor score, with age (AGE), lesion side in relationship to the dominant hemisphere (DOM), relative beta power desynchronization during movement in the ipsilesional SMA (EEG), the FA values of the CST affected/CST unaffected ratio (CST‐R), as well as the interaction of EEG and CST‐R. Model results are reported after exclusion of the influential point. Asterisks mark significance level with *p < .05, **p < .01.
Hence, 85% of residual hand motor function in these chronic stroke patients could be explained by means of beta desynchronization during movement in the ipsilesional SMA in combination with the structural integrity of the CST, as well as age and the lesion side in relation to the dominant hemisphere. Importantly, apart from the EEG, CST‐R, and age predictor, the interaction of structural (CST‐R) and functional parameters during movement (EEG) was highly significant. The effect plot for the crossmodal interaction between structural integrity and movement‐related beta desynchronization revealed that beta power mostly contributes to the residual motor outcome in patients with a greater damage to the CST (Figure 5). In patients with a less affected CST, beta power did not play a pivotal role in explaining hand motor outcome. Moreover, the multimodal model outperformed unimodal models with either solely structural information (χ2 [2] = 23.72, p < .001) or solely functional activation information (χ2 [2] = 23.25, p < .001). Unimodal models only explained 35% (CST‐R) and 37% (EEG) of the hand motor outcome, thus the additionally explained variance by combining EEG and CST‐R is ∼ 50%.
Figure 5.
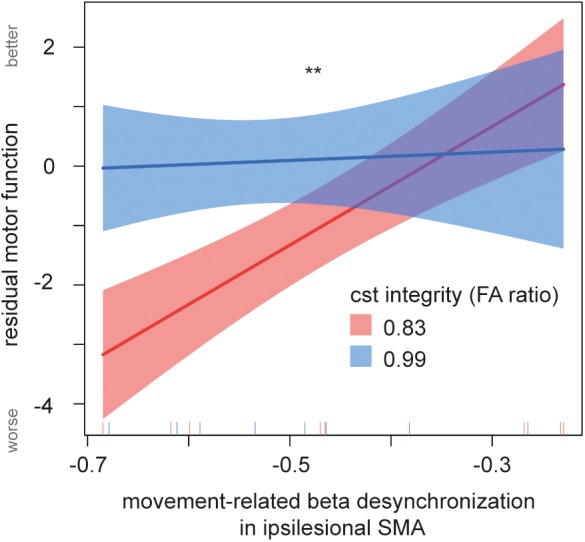
Effect of multimodal interaction of movement‐related beta desynchronization and corticospinal tract (CST) integrity. The multimodal interaction between MRBD and CST integrity significantly contributed to the residual motor outcome in chronic stroke patients. With greater damage to the CST, a higher desynchronization of beta power in ipsilesional supplementary motor area (SMA) was found in patients with a worse motor outcome. With less damage to the CST, beta power does not vary with motor outcome. The CST is split by the 50th quantile (blue, red). Lines display the prediction lines, shaded areas the 95% confidence interval. Asterisk marks significance level **p < .01
4. DISCUSSION
The present data reveal a central functional role of SMA for precision grip in chronic stroke patients with a mild to moderate deficit that are capable of dexterous grasping. Specifically, in patients with more damage to the CST, movement‐related beta power in the SMA is negatively correlated with residual motor function. Neither structural CST damage nor functional activations in the SMA alone sufficiently explained the degree of residual hand motor function.
Previous structural brain imaging studies have demonstrated that the integrity of the CST relates to residual motor outcome in chronic stroke patients (Lindenberg et al., 2010; Schulz et al., 2012; Schulz et al., 2015). CST integrity alone, however, is not the only predictive parameter and cannot sufficiently explain motor outcome. Even patients with a severely damaged CST may show a variable motor outcome, suggesting that other factors, such as alterations of association‐, commissural‐, and corticofugal fibers might influence recovery (Koch & Hummel, 2017; Schulz et al., 2017b; Schulz et al., 2017c; Schulz, Frey, et al., 2017).
On a cortical level, stroke patients show additional recruitment of ipsilateral and premotor areas (Fridman et al., 2004; Johansen‐Berg et al., 2002; Lee et al., 2017). In healthy controls, cortical control of movements emerges from activity in cortical motor areas via direct corticospinal projections from primary motor cortex as well as to a varying degree, from secondary motor areas (Dum & Strick, 1991). The involvement of the latter is depending on particular task demands and stages like visuomotor integration (Kravitz, Saleem, Baker, & Mishkin, 2011), degree of complexity (Hummel, Kirsammer, & Gerloff, 2003), hand shaping for reaching/grasping (Fluet, Baumann, & Scherberger, 2010) or sequential movements (Hummel et al., 2004; Nachev, Kennard, & Husain, 2008). Early after stroke, secondary motor areas may contribute to recovery via taking over of functionality from lesion‐damaged or decoupled areas or via stronger integration of specific areas in the network. Corresponding reconfiguration of the movement‐related activation patterns (Rehme, Eickhoff, Rottschy, Fink, & Grefkes, 2012) as well as white matter associations (Koch & Hummel, 2017; Koch, Schulz, & Hummel, 2016; Schulz, Park, et al., 2017c) and functional connections are detectable in chronic stroke patients.
Former multimodal studies found a positive correlation between structural integrity of the CST and cortical activation patterns (Hannanu et al., 2017; Schaechter & Perdue, 2008), as well as functional connectivity between motor areas (Carter et al., 2012; Guggisberg et al., 2017; Liu, Qin, Zhang, Zhang, & Yu, 2015; Pichiorri et al., 2018). Accordingly, complementary information from brain structure (CST integrity) and metabolic activations has been shown to provide a better understanding of motor outcome (Volz et al., 2015), as well as motor recovery after stroke (Burke Quinlan et al., 2015). Correspondingly, we found that hand motor outcome after stroke is best explained by integrating information from functional activation in terms of movement‐related beta power decrease in SMA with the degree of structural integrity of the CST. The combined measures account for 85% of the variance, whereas unimodal models explain only 35% (CST‐R) and 37% (EEG power) of the variance. We included age as a predictive factor in the model because younger age has been shown to be favorable for outcome (Nakayama, Jørgensen, Raaschou, & Olsen, 1994; Stinear, 2017; Veerbeek et al., 2011). In the present model, however, age was positively associated to motor function, which might well be an unspecific result given the small sample size. Pointing to a similar direction, a recent study by Wu et al. demonstrated that coupling parameters derived from task‐free EEG in combination with the structural integrity of the CST contribute synergistic information on motor impairment after stroke (Wu et al., 2015).
Motor control is paralleled by dynamics in several sensorimotor rhythms (Babiloni et al., 2016; Crone et al., 1998). Some of which show pathophysiologic patterns after stroke (Chen, Lee, Wang, Lin, & Su, 2017; Nicolo et al., 2015; Rossiter et al., 2014). We explored the predictive value of oscillations in the theta, alpha, and beta band. In the present study, especially oscillations in the beta band during movement preparation contained sufficient information to explain motor outcome after stroke. This was, however, only the case for movement‐related spectral perturbations, whereas the baseline period before movement did not contain predictive information in any frequency band, as previously reported by Rossiter et al. (Rossiter et al., 2014). When comparing the two conditions “pinch grip” and “whole hand grip,” we did not find any evident differences of oscillatory activity between the two conditions (please see Figure S2). As stroke patients show more widespread, less distinct activation patterns during different movements (Ward, 2003), we suspect that after suffering a stroke the representational oscillatory EEG activity is not anywhere distinguishable between grip forms. Moreover, higher frequencies like gamma might be more sensitive toward movement types (Cheyne & Ferrari, 2013). EEG, however, lacks the temporal resolution to detect reliably high gamma with the amount of movement repetitions done in this study.
The beta rhythm strongly varies with different aspects of motor control. A decrease of beta power is related to higher excitability in the primary motor cortex as revealed in TMS studies (Chen, Yaseen, Cohen, & Hallett, 1998). Desynchronization of ongoing beta oscillatory activity occurs during active and passive movements, as well as motor imagery and movement anticipation (Brinkman, Stolk, Dijkerman, de Lange, & Toni, 2014; Pfurtscheller & Lopes da Silva, 1999). During weak to moderate muscle contraction, corticomuscular coherence is prominent specifically in the beta band (Mima & Hallett, 1999) and has been related to specific movement parameters such as precision and force constraints (Chen, Entakli, Bonnard, Berton, & De Graaf, 2013; Kilner, Baker, Salenius, Hari, & Lemon, 2000), which are distinctive features of the present task.
How can the region‐specific effect in SMA and the dependency on CST integrity be explained in terms of network adaptation? We propose three scenarios: First, SMA might directly control movements through descending projections. Second, activity in SMA might influence connected motor areas through cortico‐cortical interactions. Third, higher SMA activity could be a correlate of increased task demands in impaired patients to perform the task as well as possible.
The first scenario would require a structural path for downstream commands from SMA. Direct projections with distinct arm representation from the SMA to cervical segments through the cerebral peduncle have been described in monkeys (He, Dum, & Strick, 1995) and in humans (Newton, 2006). How functionally relevant are these connections for dexterous movements in humans and for plastic reorganization after stroke? Although monosynaptic pathways via corticomotoneuronal connections are most likely unique to M1 cells (Rathelot & Strick, 2006), the SMA contains pyramidal tract neurons, which could exert more general cortical influences on motor neurons via oligosynaptic pathways along the corticospinal projections (Lemon, 2008). Moreover, direct projections from SMA have been shown to undergo functionally relevant post‐stroke plasticity (McNeal et al., 2009). If SMA contributes to descending motor commands in stroke patients, its motor commands would likely require an increase of spiking activity in pyramidal tract (PT) neurons. Assessing this is beyond the scope of non‐invasive recording techniques. Oscillations picked up at the scalp level, however, are a reflection of local field potentials (LFP) (da Silva, 2013). Especially for beta oscillations, a relationship with the firing rate of PT neurons has been advocated (Miller et al., 2012; van Wijk et al., 2012). Invasive recordings have revealed that spiking behavior of PT neurons is phase locked to LFP beta oscillations (Murthy & Fetz, 1992). Concomitant with movement onset, the firing rate of PT neurons increases and decouples from the LFP beta oscillations. This is paralleled by a drop in amplitude of the latter, corresponding to a desynchronization of the scalp potentials measured with EEG. In this context, the present finding of MRBD in SMA being closely linked to motor impairment could be interpreted as direct descending motor control from pyramidal neurons.
In the second scenario, the increased MRBD in the SMA for patients with higher motor impairment could be interpreted in the context of enhanced cortico‐cortical connectivity within in the motor system to adapt for the motor deficits. SMA is highly connected to M1, PMv and PMd, and a major source of input to digit representation in these areas (Dum & Strick, 2005; He et al., 1995). Intracranial recordings in humans have revealed strong increases in SMA‐M1 coherence prior to movement onset (Ohara et al., 2001). Moreover, a neuroimaging study in stroke patients investigating motor network effective coupling point to a facilitatory role of SMA‐M1 coupling in motor recovery (Grefkes et al., 2008). Although the effective coupling from ipsilesional SMA‐M1 was reduced in stroke patients compared to healthy controls, its magnitude positively correlates with motor outcome in the acute stage (Rehme et al., 2011). Furthermore, the facilitatory coupling increases with time after stroke, this increase again correlates with motor recovery (Grefkes et al., 2008). Assuming that a strong SMA to M1 link is necessary for proper motor output, our finding of a stronger SMA‐MRBD for patients with damaged CST and residual deficits could be a signature of increased SMA‐M1 connectivity. This represents an interesting hypothesis to be followed up on in future research.
Third, another intuitive interpretation would be the increase of task demand and complexity during a pinch grip in stroke patients. SMA activity is linked to the complexity of a task (Hummel et al., 2003; Hummel, Andres, Altenmüller, Dichgans, & Gerloff, 2002; Manganotti et al., 1998). The precision constraints during the object driven pinch grip particularly engage SMA activity, as it has been shown in previous work (Haller, Chapuis, Gassert, Burdet, & Klarhöfer, 2009). If complexity would be the primary factor, however, one would expect a linear relationship of MRBD in SMA with motor outcome that is not, as in our case, related to the structural integrity of the CST. In a previous study in mild stroke patients, we demonstrated that stronger motor area activation in stroke patients is not related to the enhanced effort but rather reflects neural processes involved in reorganization (Bönstrup et al., 2015).
An EEG study by Amengual et al. (Amengual et al., 2014) showed an overactivation of SMA in chronic stroke patients however, did not investigate the relationship to motor recovery. To our knowledge, this is the first EEG study, relating oscillatory activity in SMA to motor recovery after stroke. So far, our interpretation is in line with a compensatory role of SMA activity. The increase of activity in SMA, however, could represent two functionally different mechanisms. On the one hand, the enhanced activity in SMA could be interpreted to play a maladaptive role (Di Pino et al., 2014; Jang, 2013), being the cause for the poor motor outcome. On the other hand, the high activity in SMA could serve as a compensatory mechanism to support limited motor outcome (Raffin & Hummel, 2018; Ward, 2003).
Hence, in the former case, inhibiting neural activity in the SMA should lead to an increase of motor performance, whereas in the latter case, it should decrease motor performance of the impaired hand. Even though we might be able to speculate on the role of SMA activity in patients with lesioned CST, functional activations have an associative character and cannot provide strong causal evidence with regard to functional significance. Here, TMS offers the opportunity to study causal, functional relevance by interfering with SMA activity and testing the behavioral consequences. In order to address this point, we conducted a preliminary, hypothesis‐driven, pilot experiment, in which we aimed to temporally inhibit SMA with repetitive TMS in patients with good (n = 3) and limited (n = 3) motor outcome combined with an impaired CST (red group in Figure 5). We hypothesized that patients with impaired CST integrity might show a decrease of motor performance upon inhibiting SMA, with a more pronounced decrease in patients with worse motor outcome. Stimulation was carried out using a crossover design with one real stimulation (cTBS, inhibitory) and one sham stimulation session (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005; Zenon, Sidibe, & Olivier, 2015), motor performance was measured before and after stimulation using a simple reaction time task (for details please refer to the Supporting Information). A decrease of performance would hint toward a compensatory role, whereas an increase of performance would point toward a maladaptive role of SMA activity. We found no change in performance in the sham condition in any patient (n = 6). Whereas four patients did not show changes in performance during the stimulation condition, two patients, however, (one good recovery, one bad recovery) exhibited a significant worsening of motor performance after SMA stimulation (for results please refer to Figure S3). The decrease in performance supports the notion that the increased SMA activity in the patients has not a maladaptive role, but rather favors a beneficial role. The results, however, have to be interpreted with caution due to the small sample size. Larger activation of secondary motor areas may serve as a compensatory mechanism. Correlative evidence for the involvement of secondary motor areas in the process of poststroke plasticity and functional reorganization has been provided in recent longitudinal and cross‐sectional structural and functional neuroimaging studies (for review please see [Grefkes & Fink, 2014; Koch & Hummel, 2017]). Within this framework especially the ventral and dorsal premotor cortex, the SMA and the parietal areas might play a relevant role (Grefkes et al., 2008; Schulz, Park, et al., 2017c). Parietal areas demonstrate stronger connectivity with the motor cortex during hand movements in chronic stroke patients (Pool et al., 2018; Schulz et al., 2016; Bönstrup et al. 2018). Causal evidence for this assumption comes from TMS deactivation studies, where inhibition of PMd (contralesional and ipsilesional), as well as the parietal lobes and contralesional M1 led to decreased motor performance (e.g., Bestmann et al., 2010; Fridman et al., 2004; Johansen‐Berg et al., 2002). The compensatory nature of this functional re‐mapping, nevertheless, needs to be fully confirmed.
Over the last years, it became clear that treatment outcomes are quite heterogeneous across patients including responders and nonresponders (Stinear, 2017). Likely, there might not be one generalizable interventional treatment protocol for all patients. Thus, it is the ultimate goal to head toward patient‐tailored treatment strategies, that is, noninvasive brain stimulation sites (Koch & Hummel, 2017; Raffin & Hummel, 2018), to enhance motor recovery. Increasing knowledge on possible individual predictors (biomarkers) of treatment response such as structural integrity of the CST or local activation patterns will be of great value for the development of personalized neuro‐rehabilitative, neurotechnology‐based treatment strategies toward precision medicine.
4.1. Limitations
The present data revealed a strong association between movement‐related beta desynchronization in the SMA combined with CST‐integrity and motor impairment after stroke. As mentioned above, correlations do not provide proof of causal relationships, which hampers interpretation of the predictive nature of SMA beta oscillations. We designed a task that mimics frequent motoric action in daily living. Feasibility constraints limited the cohort to patients with a mild to moderate deficit. Severely affected patients typically show more widespread and stronger activity during motor actions (Grefkes & Fink, 2014) and also larger lesion load of the CST. Thus, a similar structure–function relationship may hold true in severely affected patients. However, this hypothesis has to be addressed in detail in upcoming multimodal studies including more severely impaired patients. Another general limitation is the signal reconstruction of deep sources due to the limited spatial resolution of EEG (Nunez, Srinivasan, & Fields, 2015). The mean coordinates for left and right SMA has a distance of about 4 cm. Thus, a reliable dissociation between left and right SMA is beyond the scope of the methodology of this study. In fact, the ERD signal from the right SMA was very similar to the left, although more variable across the group. Likewise, we excluded the dorsal premotor cortex from this analysis, due to low selectivity from M1. Neuroimaging of brain oscillatory activity with a higher spatiotemporal resolution like magnetoencephalography (Baillet, 2017) could help localize the relevant beta activity with higher accuracy and could extend the detectable frequency range, providing higher sensitivity for fine movements. Taken together, our interpretations are within the well‐known limits of sample size, low spatial resolution of the EEG and the correlative aspect of the study design. To validate the MRBD in SMA as a biomarker for motor recovery and to further characterize it as a target for noninvasive brain stimulation intervention, our findings have to be confirmed in larger cohorts with additional methodology for spatial localization. A longitudinally designed study and a neuromodulatory approach could elucidate the qualitative nature of the signal (Morishita & Hummel, 2017).
In conclusion, the present findings extend current concepts of functional reorganization toward a potential biomarker by combining MRBD magnitude in the SMA and the integrity of the CST in stroke patients. The combination of CST‐integrity and MRBD in the SMA accounted for 85% of the variance in this group of chronic stroke patients' residual motor outcome, whereas unimodal models explain only 35% (CST‐R) and 37% (EEG power) of the variance. This study further emphasizes the use of combinatory multimodal approaches for predictive models toward translation to clinical application and the value of task‐related measurements to detect functional biomarkers for individualized predictive models and patient‐tailored treatment strategies. Additionally, these findings shed light on the reorganization within motor areas after stroke, with SMA potentially taking over or at least supporting M1 functionality, in the case that the downstream pathways of the latter (CST) are damaged significantly by stroke.
CONFLICT OF INTEREST
Authors do not state conflicts of interest.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
6 DTI data sets were obtained within SFB936 C1/C2.
Quandt F, Bönstrup M, Schulz R, et al. The functional role of beta‐oscillations in the supplementary motor area during reaching and grasping after stroke: A question of structural damage to the corticospinal tract. Hum Brain Mapp. 2019;40:3091–3101. 10.1002/hbm.24582
Funding information Defitech Foundation; Deutsche Forschungsgemeinschaft, Grant/Award Number: SFB936‐C4 (to F.C.H); German National Academy of Sciences Leopoldina, Grant/Award Number: LPDS 2016‐01, to M.B.; Wyss Foundation, Grant/Award Number: WCP024A to F.C.H.
REFERENCES
- Amengual, J. L. , Münte, T. F. , Marco‐Pallarés, J. , Rojo, N. , Grau‐Sánchez, J. , Rubio, F. , … Rodríguez‐Fornells, A. (2014). Overactivation of the supplementary motor area in chronic stroke patients. Journal of Neurophysiology, 112, 2251–2263. [DOI] [PubMed] [Google Scholar]
- Babiloni, C. , Del Percio, C. , Vecchio, F. , Sebastiano, F. , Di Gennaro, G. , Quarato, P. P. , … Mirabella, G. (2016). Clinical neurophysiology. Clinical Neurophysiology, 127, 641–654. [DOI] [PubMed] [Google Scholar]
- Baillet, S. (2017). Magnetoencephalography for brain electrophysiology and imaging. Nature Neuroscience, 20, 327–339. [DOI] [PubMed] [Google Scholar]
- Behrens, T. E. J. , Woolrich, M. W. , Jenkinson, M. , Johansen‐Berg, H. , Nunes, R. G. , Clare, S. , … Smith, S. M. (2003). Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine, 50, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Bernhardt, J. , Borschmann, K. , Boyd, L. , Thomas Carmichael, S. , Corbett, D. , Cramer, S. C. , … Walker, M. (2016). Moving rehabilitation research forward: Developing consensus statements for rehabilitation and recovery research. International journal of stroke, 11(4), 454–458. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , Swayne, O. , Blankenburg, F. , Ruff, C. C. , Teo, J. , Weiskopf, N. , … Ward, N. S. (2010). The role of Contralesional dorsal premotor cortex after stroke as studied with concurrent TMS‐fMRI. The Journal of Neuroscience, 30(36), 11926–11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönstrup, M. , Schulz, R. , Cheng, B. , Feldheim, J. , Zimerman, M. , Thomalla, G. , … Gerloff, C. (2015). Evolution of brain activation after stroke in a constant‐effort versus constant‐output motor task. Restorative Neurology and Neuroscience, 33, 845–864. [DOI] [PubMed] [Google Scholar]
- Bönstrup, M. , Schulz, R. , Schön, G. , Cheng, B. , Feldheim, J. , Thomalla, G. , & Gerloff, C. (2018). Parietofrontal network upregulation after motor stroke. NeuroImage: Clinical, 18, 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman, L. , Stolk, A. , Dijkerman, H. C. , de Lange, F. P. , & Toni, I. (2014). Distinct roles for alpha‐ and Beta‐band oscillations during mental simulation of goal‐directed actions. Journal of Neuroscience, 34, 14783–14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke Quinlan, E. , Dodakian, L. , See, J. , McKenzie, A. , Le, V. , Wojnowicz, M. , … Cramer, S. C. (2015). Neural function, injury, and stroke subtype predict treatment gains after stroke. Annals of Neurology, 77, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, A. R. , Patel, K. R. , Astafiev, S. V. , Snyder, A. Z. , Rengachary, J. , Strube, M. J. , … Corbetta, M. (2012). Upstream dysfunction of Somatomotor functional connectivity after Corticospinal damage in stroke. Neurorehabilitation and Neural Repair, 26, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDT R . (2008). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Chen, C.‐C. , Lee, S.‐H. , Wang, W.‐J. , Lin, Y.‐C. , & Su, M.‐C. (2017). EEG‐based motor network biomarkers for identifying target patients with stroke for upper limb rehabilitation and its construct validity. PLoS ONE, 12, e0178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Yaseen, Z. , Cohen, L. G. , & Hallett, M. (1998). Time course of corticospinal excitability in reaction time and self‐paced movements. Annals of Neurology, 44(3), 317–325. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Entakli, J. , Bonnard, M. , Berton, E. , & De Graaf, J. B. (2013). Functional Corticospinal projections from human supplementary motor area revealed by Corticomuscular coherence during precise grip force control. PLoS ONE, 8, e60291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne, D. , & Ferrari, P. (2013). MEG studies of motor cortex gamma oscillations: Evidence for a gamma “fingerprint” in the brain? Frontiers in Human Neuroscience, 7, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafton, K. R. (2003). Improved understanding of cortical injury by incorporating measures of functional anatomy. Brain, 126, 1650–1659. [DOI] [PubMed] [Google Scholar]
- Crone, N. E. , Miglioretti, D. L. , Gordon, B. , Sieracki, J. M. , Wilson, M. T. , Uematsu, S. , & Lesser, R. P. (1998). Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event‐related desynchronization. Brain, 121(Pt 12), 2271–2299. [DOI] [PubMed] [Google Scholar]
- da Silva, F. L. (2013). EEG and MEG: Relevance to neuroscience. Neuron, 80(5), 1112–1128. [DOI] [PubMed] [Google Scholar]
- Di Pino, G. , Pellegrino, G. , Assenza, G. , Capone, F. , Ferreri, F. , Formica, D. , … Di Lazzaro, V. (2014). Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nature Reviews Neurology, 10, 597–608. [DOI] [PubMed] [Google Scholar]
- Dum, R. P. , & Strick, P. L. (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. The Journal of Neuroscience, 11, 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum, R. P. , & Strick, P. L. (2005). Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. The Journal of Neuroscience, 25, 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin, V. L. , Forouzanfar, M. H. , & Krishnamurthi, R. (2014). Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. The Lancet, 383(9924), 1205–1206. [DOI] [PubMed] [Google Scholar]
- Fluet, M.‐C. , Baumann, M. A. , & Scherberger, H. (2010). Context‐specific grasp movement representation in macaque ventral premotor cortex. Journal of Neuroscience, 30, 15175–15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman, E. A. , Hanakawa, T. , Chung, M. , Hummel, F. , Leiguarda, R. C. , & Cohen, L. G. (2004). Reorganization of the human ipsilesional premotor cortex after stroke. Brain, 127, 747–758. [DOI] [PubMed] [Google Scholar]
- Fuchs, M. , Kastner, J. , Wagner, M. , Hawes, S. , & Ebersole, J. S. (2002). A standardized boundary element method volume conductor model. Clinical Neurophysiology, 113, 702–712. [DOI] [PubMed] [Google Scholar]
- Fugl‐Meyer, A. R. , Jääskö, L. , Leyman, I. , Olsson, S. , & Steglind, S. (1975). The post‐stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine, 7, 13–31. [PubMed] [Google Scholar]
- Grefkes, C. , & Fink, G. R. (2014). Connectivity‐based approaches in stroke and recovery of function. Lancet Neurology, 13, 206–216. [DOI] [PubMed] [Google Scholar]
- Grefkes, C. , Nowak, D. A. , Eickhoff, S. B. , Dafotakis, M. , Küst, J. , Karbe, H. , & Fink, G. R. (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Annals of Neurology, 63, 236–246. [DOI] [PubMed] [Google Scholar]
- Guggisberg, A. G. , Nicolo, P. , Cohen, L. G. , Schnider, A. , & Buch, E. R. (2017). Longitudinal structural and functional differences between proportional and poor motor recovery after stroke. Neurorehabilitation and Neural Repair, 31, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, S. , Chapuis, D. , Gassert, R. , Burdet, E. , & Klarhöfer, M. (2009). Supplementary motor area and anterior intraparietal area integrate fine‐graded timing and force control during precision grip. The European Journal of Neuroscience, 30, 2401–2406. [DOI] [PubMed] [Google Scholar]
- Hannanu, F. F. , Zeffiro, T. A. , Lamalle, L. , Heck, O. , Renard, F. , Thuriot, A. , … ISIS‐HERMES Study Group . (2017). Parietal operculum and motor cortex activities predict motor recovery in moderate to severe stroke. NeuroImage: Clinical, 14, 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S. Q. , Dum, R. P. , & Strick, P. L. (1995). Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere. The Journal of Neuroscience, 15(5 Pt 1), 3284–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.‐Z. , Edwards, M. J. , Rounis, E. , Bhatia, K. P. , & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45, 201–206. [DOI] [PubMed] [Google Scholar]
- Hummel, F. , Andres, F. , Altenmüller, E. , Dichgans, J. , & Gerloff, C. (2002). Inhibitory control of acquired motor programmes in the human brain. Brain, 125, 404–420. [DOI] [PubMed] [Google Scholar]
- Hummel, F. , Kirsammer, R. , & Gerloff, C. (2003). Ipsilateral cortical activation during finger sequences of increasing complexity: Representation of movement difficulty or memory load? Clinical Neurophysiology, 114(4), 605–613. [DOI] [PubMed] [Google Scholar]
- Hummel, F. , Saur, R. , Lasogga, S. , Plewnia, C. , Erb, M. , Wildgruber, D. , … Gerloff, C. (2004). To act or not to act. Neural correlates of executive control of learned motor behavior. NeuroImage, 23, 1391–1401. [DOI] [PubMed] [Google Scholar]
- Jang, S. H. (2013). Motor function‐related maladaptive plasticity in stroke: A review. NeuroRehabilitation, 32, 311–316. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg, H. , Dawes, H. , Guy, C. , Smith, S. M. , Wade, D. T. , & Matthews, P. M. (2002). Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain, 125, 2731–2742. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg, H. , Rushworth, M. F. S. , Bogdanovic, M. D. , Kischka, U. , Wimalaratna, S. , & Matthews, P. M. (2002). The role of ipsilateral premotor cortex in hand movement after stroke. Proceedings of the National Academy of Sciences of the United States of America, 99, 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner, J. M. , Baker, S. N. , Salenius, S. , Hari, R. , & Lemon, R. N. (2000). Human cortical muscle coherence is directly related to specific motor parameters. Journal of Neuroscience, 20, 8838–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, P. , Schulz, R. , & Hummel, F. C. (2016). Structural connectivity analyses in motor recovery research after stroke. Annals of Clinical Translational Neurology, 3, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, P. J. , & Hummel, F. C. (2017). Toward precision medicine. Current Opinion in Neurology, 30, 388–397. [DOI] [PubMed] [Google Scholar]
- Kravitz, D. J. , Saleem, K. S. , Baker, C. I. , & Mishkin, M. (2011). A new neural framework for visuospatial processing. Nature Reviews Neuroscience, 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Park, E. , Lee, A. , Chang, W. H. , Kim, D.‐S. , & Kim, Y.‐H. (2017). Recovery‐related indicators of motor network plasticity according to impairment severity after stroke. European Journal of Neurology, 24, 1290–1299. [DOI] [PubMed] [Google Scholar]
- Lemon, R. N. (2008). Descending pathways in motor control. Annual Review of Neuroscience, 31, 195–218. [DOI] [PubMed] [Google Scholar]
- Lindenberg, R. , Renga, V. , Zhu, L. L. , Betzler, F. , Alsop, D. , & Schlaug, G. (2010). Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology, 74, 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Qin, W. , Zhang, J. , Zhang, X. , & Yu, C. (2015). Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke, 46(4), 1045–1051. [DOI] [PubMed] [Google Scholar]
- Makeig, S. , Bell, A. , & Jung, T. (1996). Independent component analysis of electroencephalographic data. “Advances in neural information processing systems 8”. Cambridge MA: MIT Press. [Google Scholar]
- Manganotti, P. , Gerloff, C. , Toro, C. , Katsuta, H. , Sadato, N. , Zhuang, P. , … Hallett, M. (1998). Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroencephalography and Clinical Neurophysiology, 109, 50–62. [DOI] [PubMed] [Google Scholar]
- McNeal, D. W. , Darling, W. G. , Ge, J. , Stilwell‐Morecraft, K. S. , Solon, K. M. , Hynes, S. M. , … Morecraft, R. J. (2009). Selective long‐term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. The Journal of Comparative Neurology, 518, 586–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. J. , Hermes, D. , Honey, C. J. , Hebb, A. O. , Ramsey, N. F. , Knight, R. T. , … Fetz, E. E. (2012). Human motor cortical activity is selectively phase‐entrained on underlying rhythms. PLoS Computational Biology, 8, e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima, T. , & Hallett, M. (1999). Corticomuscular coherence: A review. Journal of Clinical Neurophysiology, 16, 501–511. [DOI] [PubMed] [Google Scholar]
- Morishita, T. , & Hummel, F. C. (2017). Non‐invasive brain stimulation (NIBS) in motor recovery after stroke: Concepts to increase efficacy. Current Behavioral Neuroscience Reports, 4, 280–289. [Google Scholar]
- Murthy, V. N. , & Fetz, E. E. (1992). Coherent 25‐ to 35‐Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proceedings of the National Academy of Sciences of the United States of America, 89, 5670–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews. Neuroscience, 9, 856–869. [DOI] [PubMed] [Google Scholar]
- Nakayama, H. , Jørgensen, H. S. , Raaschou, H. O. , & Olsen, T. S. (1994). The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke, 25, 808–813. [DOI] [PubMed] [Google Scholar]
- Newton, J. M. (2006). Non‐invasive mapping of corticofugal fibres from multiple motor areas‐‐relevance to stroke recovery. Brain, 129, 1844–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo, P. , Rizk, S. , Magnin, C. , Pietro, M. D. , Schnider, A. , & Guggisberg, A. G. (2015). Coherent neural oscillations predict future motor and language improvement after stroke. Brain, 138, 3048–3060. [DOI] [PubMed] [Google Scholar]
- Nunez, P. L. , Srinivasan, R. , & Fields, R. D. (2015). EEG functional connectivity, axon delays and white matter disease. Clinical Neurophysiology, 126, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara, S. , Mima, T. , Baba, K. , Ikeda, A. , Kunieda, T. , Matsumoto, R. , … Shibasaki, H. (2001). Increased synchronization of cortical oscillatory activities between human supplementary motor and primary sensorimotor areas during voluntary movements. Journal of Neuroscience, 21, 9377–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ones, K. , Yilmaz, E. , Cetinkaya, B. , & Caglar, N. (2005). Quality of life for patients poststroke and the factors affecting it. Journal of Stroke and Cerebrovascular Diseases, 14, 261–266. [DOI] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J.‐M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. (1999). Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pichiorri, F. , Petti, M. , Caschera, S. , Astolfi, L. , Cincotti, F. , & Mattia, D. (2018). An EEG index of sensorimotor interhemispheric coupling after unilateral stroke: Clinical and neurophysiological study. The European Journal of Neuroscience, 47, 158–163. [DOI] [PubMed] [Google Scholar]
- Pool, E. M. , Leimbach, M. , Binder, E. , Nettekoven, C. , Eickhoff, S. B. , Fink, G. R. , & Grefkes, C. (2018). Network dynamics engaged in the modulation of motor behavior in stroke patients. Human brain mapping, 39(3), 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, J. , Pedraza, S. , Blasco, G. , Daunis‐i‐Estadella, J. , Prados, F. , Remollo, S. , … Serena, J. (2011). Acute damage to the posterior limb of the internal capsule on diffusion tensor Tractography as an early imaging predictor of motor outcome after stroke. American Journal of Neuroradiology, 32, 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt, F. , Bönstrup, M. , Schulz, R. , Timmermann, J. , Zimerman, M. , Nolte, G. , & Hummel, F. (2016). Spectral variability in the aged brain during fine motor control. Frontiers in Aging Neuroscience, 8, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin, E. , & Hummel, F. C. (2018). Restoring motor functions after stroke: Multiple approaches and opportunities. The Neuroscientist, 24(4), 400–416. [DOI] [PubMed] [Google Scholar]
- Rathelot, J.‐A. , & Strick, P. L. (2006). Muscle representation in the macaque motor cortex: An anatomical perspective. Proceedings of the National Academy of Sciences of the United States of America, 103, 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme, A. K. , Eickhoff, S. B. , Rottschy, C. , Fink, G. R. , & Grefkes, C. (2012). Activation likelihood estimation meta‐analysis of motor‐related neural activity after stroke. NeuroImage, 59, 2771–2782. [DOI] [PubMed] [Google Scholar]
- Rehme, A. K. , Eickhoff, S. B. , Wang, L. E. , Fink, G. R. , & Grefkes, C. (2011). Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage, 55, 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter, H. E. , Boudrias, M.‐H. E. L. E. N. , & Ward, N. S. (2014). Do movement‐related beta oscillations change after stroke? Journal of Neurophysiology, 112, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter, J. D. , & Perdue, K. L. (2008). Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cerebral Cortex, 18, 638–647. [DOI] [PubMed] [Google Scholar]
- Schiemanck, S. K. , Kwakkel, G. , Post, M. W. M. , Kappelle, L. J. , & Prevo, A. J. H. (2006). Predicting long‐term independency in activities of daily living after middle cerebral artery stroke: Does information from MRI have added predictive value compared with clinical information? Stroke, 37, 1050–1054. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , Buchholz, A. , Frey, B. M. , Bönstrup, M. , Cheng, B. , Thomalla, G. , … Gerloff, C. (2016). Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well‐recovered stroke patients. Stroke, 47(2), 482–489. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , Frey, B. M. , Koch, P. , Zimerman, M. , Bönstrup, M. , Feldheim, J. , … Hummel, F. C. (2017). Cortico‐cerebellar structural connectivity is related to residual motor output in chronic stroke. Cerebral Cortex, 27, 635–645. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , Koch, P. , Zimerman, M. , Wessel, M. , Bönstrup, M. , Thomalla, G. , … Hummel, F. C. (2015). Parietofrontal motor pathways and their association with motor function after stroke. Brain, 138, 1949–1960. [DOI] [PubMed] [Google Scholar]
- Schulz, R. , Park, C.‐H. , Boudrias, M.‐H. , Gerloff, C. , Hummel, F. C. , & Ward, N. S. (2012). Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke, 43, 2248–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, R. , Park, E. , Lee, J. , Chang, W. H. , Lee, A. , Kim, Y.‐H. , & Hummel, F. C. (2017b). Synergistic but independent: The role of corticospinal and alternate motor fibers for residual motor output after stroke. NeuroImage: Clinical, 15, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, R. , Park, E. , Lee, J. , Chang, W. H. , Lee, A. , Kim, Y.‐H. , & Hummel, F. C. (2017c). Interactions between the corticospinal tract and premotor‐motor pathways for residual motor output after stroke. Stroke, 48, 2805–2811. [DOI] [PubMed] [Google Scholar]
- Shelton, F. N. , & Reding, M. J. (2001). Effect of lesion location on upper limb motor recovery after stroke. Stroke, 32, 107–112. [DOI] [PubMed] [Google Scholar]
- Stinear, C. (2010). Prediction of recovery of motor function after stroke. Lancet Neurology, 9, 1228–1232. [DOI] [PubMed] [Google Scholar]
- Stinear, D. C. M. (2017). Prediction of motor recovery after stroke: Advances in biomarkers. The Lancet Neurology, 16, 826–836. [DOI] [PubMed] [Google Scholar]
- Van Veen, B. D. , van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering, 44, 867–880. [DOI] [PubMed] [Google Scholar]
- van Wijk, B. C. M. , Beek, P. J. , & Daffertshofer, A. (2012). Neural synchrony within the motor system: What have we learned so far? Frontiers in Human Neuroscience, 6, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerbeek, J. M. , Kwakkel, G. , van Wegen, E. E. H. , Ket, J. C. F. , & Heymans, M. W. (2011). Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke, 42, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Volz, L. J. , Sarfeld, A.‐S. , Diekhoff, S. , Rehme, A. K. , Pool, E.‐M. , Eickhoff, S. B. , … Grefkes, C. (2015). Motor cortex excitability and connectivity in chronic stroke: A multimodal model of functional reorganization. Brain Structure & Function, 220, 1093–1107. [DOI] [PubMed] [Google Scholar]
- Ward, N. S. (2003). Neural correlates of outcome after stroke: A cross‐sectional fMRI study. Brain, 126, 1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Quinlan, E. B. , Dodakian, L. , McKenzie, A. , Kathuria, N. , Zhou, R. J. , … Cramer, S. C. (2015). Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain, 138, 2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon, A. , Sidibe, M. , & Olivier, E. (2015). Disrupting the supplementary motor area makes physical effort appear less effortful. Journal of Neuroscience, 35, 8737–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


