Abstract
Cognitive flexibility is a major facet of executive functions and often refers to sequential task control; that is, it is very likely that one may re‐encounter a task that has previously been abandoned to carry out a different task. In the context of sequential cognitive flexibility, the “backward inhibition (BI) effect” has been studied quite extensively. Here we ask whether there are age‐related differences between adolescents and adults to overcome BI and what system‐neurophysiological mechanisms underlie these modulations. This was examined using a system neurophysiological study procedure combining event‐related potentials data with source localization and EEG signal decomposition methods. We show that sequential cognitive flexibility, and the ability overcome backward inhibition, is inferior in adolescents compared with adults. Accounting for intra‐individual variability in the neurophysiological data, this data suggest that two partly inter‐related processes underlie the differences between adolescents than adults to overcome backward inhibition: One process refers to the suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial during perceptual categorization of incoming information that is associated with right inferior frontal regions. The other process refers to immature response selection and conflict monitoring mechanisms associated with regions in the medial frontal cortex.
Keywords: adolescents, adults, backward inhibition, cognitive flexibility, EEG, sequential cognitive flexibility, source localization
1. INTRODUCTION
Cognitive flexibility is a major facet of executive functions and enables us to quickly adapt thinking and acting in consideration of changing environmental conditions (Diamond, 2002). However, it is very likely that one may re‐encounter a task that has previously been abandoned in favor of a different task. This resembles a situation where one switches from operating a desktop computer to a cellular phone and then back to the desktop computer. Therefore, cognitive flexibility often refers to sequential task control.
Processes involved in sequential cognitive flexibility are reflected by the “backward inhibition (BI) effect” (Allport, Styles, & Hsieh, 1994; Allport & Wylie, 1999). It relates to the assumption that efficient cognitive flexibility requires the activation of a new task set (or rule), which warrants the deactivation of the no longer needed, competing task through inhibitory processes (Dajani & Uddin, 2015; Klimesch, 2011; Mayr & Keele, 2000). Formally, the BI effect concerns the interplay of the n‐2 and n‐1 trials and its effect on the nth trial (Zhang, Stock, & Beste, 2016a; Zhang, Stock, Fischer, & Beste, 2016b). Hence, when switching back to a recently suppressed task set (e.g., task A) after one intermediate trial (e.g., aba triplet/ BI condition), the performance costs are higher compared with trials without n‐2 task repetition in sequence (e.g., DBA task triplet/BASE condition). This effect is termed “backward inhibition effect” and refers to the time cost of overcoming the inhibition of the lately abandoned task set that becomes relevant again (Mayr & Keele, 2000). Therefore, a strong BI effect suggests that an individual has large costs to overcome the inhibitory effect of the n‐1 trial on the n‐2 trial. This shows that a strong BI effect is disadvantageous and leads to difficulties in task performance when a previously inhibited task becomes relevant again (Allport et al., 1994; Allport & Wylie, 1999).
Since cognitive flexibility mechanisms are generally under‐developed in adolescents in comparison to adults (Davidson, Amso, Anderson, & Diamond, 2006; Diamond, 2002; Doebel & Zelazo, 2015; Lehto, Juujarvi, Kooistra, & Pulkkinen, 2003), a natural hypothesis is that also sequential cognitive flexibility is dysfunctional: the difficulty to overcome the BI effect should be larger in adolescents than adults. Performance in the BASE condition may not differ, because it does not require overcoming the inhibitory effect of the n‐1 trial on the n‐2 trial (i.e., sequential cognitive flexibility). The difference in reaction times RTs between the BI and the BASE condition should be larger in adolescents than adults and especially RTs in the BI condition should be prolonged. This hypothesis has never been tested. Considering this hypothesis, it is, however, important to examine cognitive–neurophysiological subprocesses and associated functional neuroanatomical structures in detail. The reason is that cognitive flexibility depends on multiple cognitive systems (Gruner & Pittenger, 2017) and the concept of the “Interactive Specialization” (Johnson, 2011) states that emerging cognitive functions (i.e., developmental/age‐related changes) are due to the maturation and interregional interactions of specific brain regions and processes associated with these regions. In combination with source localization techniques, the quantification of event‐related potentials (ERPs) allows to identify and dissociate both cognitive sub‐processes and brain regions that are differentially modulated by age groups during sequential cognitive flexibility. There is ample evidence that especially two partly interrelated cognitive‐neurophysiological processes play an important role during backward inhibition:
Previous studies have shown that cognitive neurophysiological subprocesses underlying backward inhibition are reflected by the N2 ERP‐component, likely reflecting response selection mechanisms (Beste, Baune, Falkenstein, & Konrad, 2010; Gajewski, Kleinsorge, & Falkenstein, 2010; Gehring, Bryck, Jonides, Albin, & Badre, 2003) in the context of backward inhibition (Zhang, Stock, & Beste, 2016a; Zhang, Stock, Fischer, et al., 2016b). Although the N2 component has been related to conflict monitoring (Deng, Wang, Ding, & Tang, 2015; Donkers & van Boxtel, 2004; Larson, Clayson, & Clawson, 2014), it has been shown that in the context of backward inhibition modulations of the N2 are not in line with the conflict‐monitoring approach (Zhang, Stock, & Beste, 2016a), because the N2 was not enhanced in the BI condition. A conflict‐like N2 enhancement is often associated with the orientation to an infrequent stimulus/task rule, which is not necessarily the case during backward inhibition (Sinai, Goffaux, & Phillips, 2007; Zhang, Stock, & Beste, 2016a). Response selection mechanisms during processes to overcome backward inhibition have been shown to be modulated by the dopaminergic system (Zhang, Stock, & Beste, 2016a) and are associated with anterior cingulate cortex activity (Zhang, Stock, & Beste, 2016a). This is in line with fMRI results suggesting that the BI effect relates to functions of the basal ganglia, the supplementary motor area and premotor area (BA6) (Whitmer & Banich, 2012). Both, the dopaminergic system and medial frontal brain structures are still underdeveloped in adolescence (Giorgio et al., 2010; Gogtay et al., 2004; Hämmerer, Müller, & Li, 2014; Sowell et al., 2003) and also the N2 has been shown undergo considerable developmental effects (Chmielewski, Mückschel, Roessner, & Beste, 2014; Espinet, Anderson, & Zelazo, 2012; Lamm, Zelazo, & Lewis, 2006; Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006; Waxer & Morton, 2011). Considering all this, we hypothesize that deficits to overcome backward inhibition in adolescence are due to deficits in response selection mechanisms reflected by processes in the N2 time interval, which are associated with activation differences in medial frontal areas. Following the consideration, that adolescents show a bigger BI effect, we hypothesize that this will be paralleled by larger difference in the N2 between the two conditions and in particular show a smaller N2 in BI condition compared with adults.
However, another process that may be associated with differences in backward inhibition between adolescents and adults is ability to update mental representations. This updating requires the suppression of the no longer needed, competing task (Dajani & Uddin, 2015; Klimesch, 2011; Mayr & Keele, 2000). It can be seen as the suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial. Notably, these processes have recently been shown to be important during backward inhibition (Wolff, Buse, Tost, Roessner, & Beste, 2017; Wolff, Giller, Buse, Roessner, & Beste, 2018). The P1 ERP‐component is assumed to represent mechanisms related to the suppression of information in task irrelevant networks during early categorization thereby controlling access to information stored in a knowledge system (Klimesch, 2011). The knowledge system is thought to be a storage system, which includes procedural and implicit‐perceptual knowledge (Klimesch, 2011; Petruo, Stock, Münchau, & Beste, 2016) important during cognitive flexibility (Wolff et al., 2017). Recent work suggesting that the suppression of the no longer needed, competing task is relevant during backward inhibition accounted for modulations in the P1 ERP component (Wolff et al., 2017, 2018) reflecting inhibitory gating mechanisms which control the access to a previous task sets/representations (Klimesch, 2011). We expect that adolescents will show a greater difference in the P1 between BI and BASE condition driven by a larger P1 in the BI condition, paralleling effects of persistent inhibitory processes and deficits in overcoming these. According to the assumption, that the P1 is reflecting inhibitory gating mechanisms which control the access to a previous task set (Klimesch, 2011) and corresponding to EEG and fMRI studies that the right inferior frontal gyrus (rIFG) is associated with inhibitory processes (Aron, Robbins, & Poldrack, 2014; Whitmer & Banich, 2012; Wolff et al., 2017, 2018), we expect that the rIFG will be associated with modulations in the P1 between adolescents and adults.
Considering the electrophysiological measures used, it is important that ERPs can only provide reliable insights into neurophysiological mechanisms when there is little intra‐individual variability in the EEG data (Ouyang, Herzmann, Zhou, & Sommer, 2011; Ouyang, Sommer, & Zhou, 2015a, b). Yet, intra‐individual variability is strongly affected by developmental processes (Bielak, Cherbuin, Bunce, & Anstey, 2014; Garrett, Macdonald, & Craik, 2012; Mella, Fagot, Lecerf, & de Ribaupierre, 2015; Störmer, Eppinger, & Li, 2014; Tamnes, Fjell, Westlye, Østby, & Walhovd, 2012) and impedes reliable comparisons between adolescents and adults when it comes to neurophysiological correlates of cognitive flexibility (Bodmer, Mückschel, Roessner, & Beste, 2018). It is, therefore, very likely, that there are no reliable effects in electrophysiological correlates when analyzing above‐mentioned standard ERP‐components without accounting for intra‐individual variability. This can be done using residue iteration decomposition (RIDE), which reduces intra‐individual variability in the data (Ouyang et al., 2011, 2015b; Verleger, Metzner, Ouyang, Śmigasiewicz, & Zhou, 2014). RIDE is a temporal EEG signal decomposition method that overcomes limitations of other methods (Ouyang et al., 2011, 2015b; Verleger et al., 2014): Principal component analysis (PCA), for example, only assumes that the amplitude but not the latency varies across trials (Ouyang et al., 2011). Other deconvolution methods do not work for latency‐variable ERP components that are not locked to a response. Yet, such processes are typical in tasks measuring complex cognitive processes (Ouyang et al., 2011). RIDE has been shown to overcome these limitations (Ouyang et al., 2011, 2015b; Verleger et al., 2014). RIDE decomposes event‐related potential (ERP) data into several component clusters with different functional relevance while accounting for intra‐individual variability in the data: the S component cluster is related to stimulus‐related processes (e.g., perception and attention), the R component cluster pertains to response‐related processes (e.g., motor preparation/execution), and the C component cluster refers to intermediate processes between S and R (e.g., response selection) (Ouyang et al., 2011). Utilizing RIDE analysis, recent examinations show that N2‐related processes are captured by the S‐cluster and the C‐cluster in response selection and inhibition paradigms (Mückschel, Chmielewski, Ziemssen, & Beste, 2017). Previous findings have shown that the N2 reflects intermingled perceptual and response‐related (selection) processes (Folstein & Van Petten, 2008). Using RIDE it was recently shown that these intermingled coding levels in the N2 are reflected by the S‐cluster and the C‐cluster (Chmielewski, Mückschel, & Beste, 2018; Mückschel et al., 2017). If the modulation of response selection processes is the most important factor underlying processes to overcome backward inhibition in adolescents compared with adults, it is likely that the above hypothesized effects for the N2 will be evident in the C‐cluster data in the N2 time window. Similarly, the above hypothesized effects for the P1, will be evident in the S‐cluster data in the P1 time window.
To summarize, we hypothesize that adolescents will show increased difficulties in overcoming backward inhibition, which will be reflected by a larger BI effect compared with adults. On a neurophysiological level, we hypothesize that this will be paralleled by a larger P1 amplitude in the BI condition compared with adults, which is related to persistent inhibitory processes and deficits in overcoming these. Moreover, we hypothesize that response selection mechanisms, as reflected by the N2 component, are associated with the above‐mentioned behavioral impairments. Thus, we suppose that adolescents will show a smaller N2‐amplitude in the BI condition compared with adults. Lastly, we hypothesize that these effects will not be observable until controlling for intra‐individual variability. Only after applying RIDE procedure, effects for the P1 component will be evident in the S‐cluster and for the N2 component in the C‐cluster.
2. MATERIALS AND METHODS
2.1. Participants
For an a‐priori power calculation we conservatively assumed a small effect size for the hypothesized interaction “condition (BI vs. BASE) × group (adolescents vs. adults)” around ηp 2 = .05, which should be detectable with a power of at least 85%. The power calculation using G*Power revealed a total sample size of N = 46; that is, N = 23 subjects per group. However, as outlined below, a total sample for the data analysis included N = 23 adolescents and N = 24 adults. In fact, the actually obtained effect sizes (refer results section) were twice as large (ηp 2 = .115 for the behavioral data; ηp 2 = .115 for the S‐cluster data; ηp 2 = .096 for the C‐cluster data). Based on this, the post‐hoc power analysis actually revealed a power greater than 95%. Overall, N = 54 healthy subjects were recruited. N = 2 adolescents had to be excluded because of ADHD symptoms that were identified during the inclusion process involving a clinical screening for ADHD symptoms. Moreover, N = 4 participants from the adolescent group and N = 1 from the adult group were excluded because of low EEG‐data quality. The remaining N = 23 adolescents (mean age 14.7 ± 1.9, 10 males, 13 females) and N = 24 adults (23.8 ± 3.3, 9 males, 15 females) were included in the data analysis. None of them had any neurological or psychiatric disorders. All participants had normal or corrected‐to‐normal vision. After written informed consent was obtained from the participants and their legal guardians the experiment started. All participants were treated according to the declaration of Helsinki. The study was approved by the ethics committee of the TU Dresden.
2.2. Experimental setting and task
The experiment used in the present study was a modified version of a BI paradigm proposed by Koch, Gade, and Philipp (2004). Aiming to examine BI processes and the neural substrates associated with developmental differences in task performance. The same paradigm was used in previous EEG studies (Zhang, Stock, & Beste, 2016a; Zhang, Stock, Fischer, et al., 2016b). Figure 1 shows the outline of the task:
Figure 1.
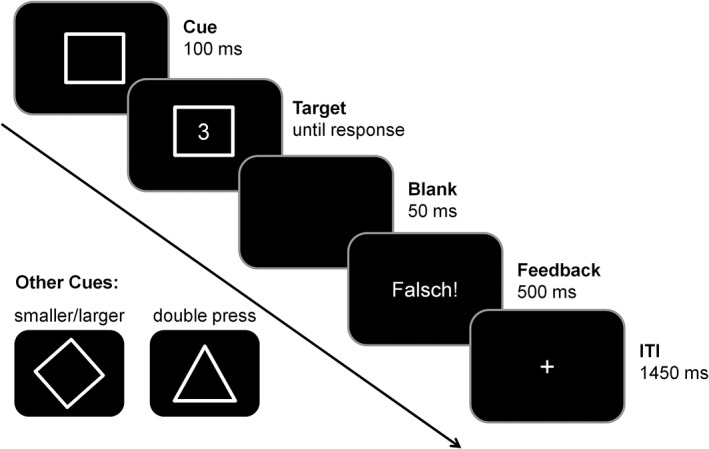
Trials started with the presentation of one of three cues, indicating the odd or even rule (square cue, task A), the smaller or larger rule (diamond cue, task B) or the double‐press rule (triangle cue, task D). The target (digit from 1 to 9, except 5) was presented centrally inside the cue stimulus 100 ms after cue onset. The target was shown until a key‐press was executed, except in the double‐press task. The inter‐trial interval ranged over 2000 ms. In incorrect trials a feedback was shown for 500 ms. In case of correct responses, a fixation cross appeared in the middle of the screen
Subjects were placed in front of a CRT computer monitor. Cues and targets were presented in white color on a black background at the screen center. For responding, participants were instructed to press one, respectively two buttons (left and right Ctrl‐buttons) on the keyboard using the respective index finger. The presentation of the stimuli, response recordings (RT and errors) and EEG‐triggers were implemented using “Presentation” software (Neurobehavioral Systems, Inc.). Altogether, the experiment consisted of 768 trials, which were divided into eight blocks with an equal number of trials per block. Three different figures served as cues for the task rules. A square indicated task A (odd/ even), a diamond indicated task B (smaller/ larger), and a triangle served as a cue for task D (double‐press). The trials began with the presentation of one of the above‐mentioned cues. After 100 ms (=stimulus onset asynchrony, SOA), a digit from 1 to 9 (except 5) was shown inside of the cue. This digit served as the target stimulus. When a square (task A, odd/ even) was presented, participants were instructed to answer whether the target was odd (left index finger) or even (right index finger). The display of the diamond indicated task B (smaller/larger). Here, subjects were supposed to assess whether the target was smaller (left index finger) or larger (right index finger) than 5. In task A and B, the participants needed to make a selection between two response options. In these two conditions, the cue and the target disappeared not until a response was given. In contrast, when a triangle was shown (task D, double press) participants were invited to press both buttons at the same time (left and right Ctrl‐button). In this condition, it was necessary to respond within 1,000 ms after the stimulus appeared. If they failed to press the buttons within 1,000 ms, a speed‐up sign (German word “Schneller!,” translated: “Faster!”) was shown right over the cue frame. To ensure the comparability between the conditions, the speed‐up sign was shown in each of the three tasks. Incorrect key‐presses, too slow responses and non‐simultaneous key‐presses in task D (i.e., in case of an asynchrony of more than 50 ms) were counted as errors. In those cases, an error feedback (German word “Falsch!,” translated: “Wrong!”) was presented for 500 ms. At this point, it needs to be stressed that the cueing of task D (double‐press rule) does not require a response selection like in task A or B. In this case, the response does not depend on the stimulus identity (digit between 1 and 9). The presentation of the cue already implies the response and thus can be interpreted as an unconditional response which has to be given as soon as the cue appears. Hence, the double‐press rule can be seen as a rather simple task compared with task A and B (Koch et al., 2004). After a response was executed, the cue stimulus of the next trial was presented after an offset of 1,500 ms. Within this time interval (=response stimulus interval, RSI), a fixation cross was shown centrally on the screen. The frequency of A‐tasks, B‐tasks, and D‐tasks was the same in each block.
After each of the eight blocks, a feedback about the mean reaction time (RT) during the last block was presented in the center of the screen. Each trial (except for the first two trials) built a triplet with the last two preceding trials. Thus, there were 12 possible triplet‐combinations (ABA; ADA; BAB; BDB; DAD; DBD; DBA; BDA; DAB; ADB; BAD; ABD). The frequency of their presentation did not differ across the eight blocks. Triplets where the last trial had the same cue as the n‐2 trial represented backward inhibition (BI) triplets. Triplets without n‐2 cue repetitions represented baseline (BASE) triplets. Participants received both written and verbal instructions. They were instructed to respond as fast and accurately they can. To guarantee the understanding of the instructions and that participants kept the task rules in mind, a practice was run before the main experiment started.
2.3. EEG‐recordings and data processing
For the recording of the EEG and event‐related potentials, an equidistant 60 Ag‐AgCl‐EEG setup was used. The sampling rate was 500 Hz (BrainAmp, Brain Products Inc.). Impedances were kept below 5 kΩ. Data processing was executed utilizing the BrainVision Analyzer 2 software package (Brain Products Inc.). During off‐line data processing, the recorded data were down‐sampled to 256 Hz and a band‐pass filter from 0.5 to 20 Hz with a slope of 48 dB/oct was applied. Afterward, a raw data inspection was conducted to remove technical artifacts. This was followed by an independent component analysis (ICA, infomax algorithm), which was used to correct blinks, pulse artifacts, and vertical eye‐movements. The number of ICs which were removed and corrected were 4 (±1.8). For trials comprising correct responses, cue‐locked segments were formed for all conditions separately. Segments started −200 ms prior cue onset and ended 1,200 ms thereafter. Subsequently, an automated artifact rejection procedure was performed. Amplitude differences above 200 μV in a 200 ms time span as well as an activity below 0.5 μV in a 100 ms period were used as rejection criteria. Afterward, current source density (CSD) transformation was performed to yield a reference‐free evaluation of the electrophysiological data, resulting in values stated in μV/m2 (Nunez & Pilgreen, 1991). It improves spatial representation of the location, course and intensity of the signal source. By means of this transformation, it is possible to identify electrodes which best reflect ERP‐component reflecting relevant cognitive neurophysiological subprocesses (Bodmer et al., 2018; Bodmer & Beste, 2017; Kamarajan, Pandey, Chorlian, & Porjesz, 2015; Kayser & Tenke, 2015a). It needs to be stressed that the CSD transformation was used in other studies using the same task and therefore fosters comparability across studies and examined populations (Wolff et al., 2018; Zhang, Stock, & Beste, 2016a; Zhang, Stock, Fischer, et al., 2016b). Moreover, CSD data transformed data are well‐known to be interpretable in the same way as conventional ERP data (Kayser & Tenke, 2015a, b). A baseline correction from −200 to 0 ms before cue onset was conducted and followed by the averaging of the segments for each condition and the individual subject level.
The choice of electrodes and search intervals for data quantification was based on the inspection of scalp topographies with a subsequent statistical validation of this choice. This validation has been introduced before (Mückschel, Stock, & Beste, 2014) and is as follows: An extraction of the mean amplitude was conducted for all 60 electrodes in each of the defined search intervals. To compare each electrode against the average of all other electrodes, Bonferroni‐correction was applied for multiple comparisons (critical threshold p = .0007). Solely electrodes exhibiting significantly larger mean amplitudes (i.e., negative for N‐potentials and positive for the P‐potentials) compared with the remaining electrodes were chosen. It is important to note that procedure revealed the same electrodes as identified in the visual inspection of the data. Details about the quantified and validated time windows/electrode sites can be found in Table 1.
Table 1.
Summary of the quantified ERP components and RIDE clusters including information on the quantified time windows (mean amplitudes are quantified) and electrode sites
| Component/parameter | Electrodes | Time windows |
|---|---|---|
| P1 (cue) | P7, P8 | 90–100 ms (both groups, all conditions) |
| P1 (target) | P7, P8 |
220–240 ms (adults, all conditions) 250–280 ms (adolescents, all conditions) |
| P1 (target) | CP6 | 240–260 ms (both groups, all conditions) |
| N1 (cue) | P7, P8 | 170–185 ms (both groups, all conditions) |
| N1 (target) | P7, P8 |
350–370 ms (adults, all conditions) 320–340 ms (adolescents, all conditions) |
| N2 | Cz, Pz | 395–410 ms (both groups, all conditions) |
| P3 | Cz, Pz | 560–590 ms (both groups, all conditions) |
| S‐cluster: | ||
| P1 (cue) | P7, P8 | 90–100 ms (both groups, all conditions) |
| P1 (target) | P7, P8 |
220–235 ms (adults, all conditions) 250–270 ms (adolescents, all conditions) |
| P1 (target) | CP6 | 240–255 ms (both groups, all conditions) |
| N1 (cue) | P7, P8 | 170–185 ms (both groups, all conditions) |
| N1 (target) | P7, P8 |
350–370 ms (adults, all conditions) 310–340 ms (adolescents, all conditions) |
| N2 | Cz | 395–425 ms (both groups, all conditions) |
| C‐cluster: | ||
| N2 | Cz |
360–370 ms (both groups, BI condition) 380–390 ms (both groups, BASE condition) |
| P3 | Pz | 540–580 ms (both groups, all conditions) |
| R‐cluster: | ||
| Motor processes | C3, C4 |
805–905 ms (adults, BI condition) 750–850 ms (adults; BASE condition) 925–1,025 ms (adolescents, BI condition) 835–935 ms (adolescents, BASE condition) |
For the RIDE R‐cluster the time windows correspond the mean reaction times (as outlined in the text).
It may be argued that due to the short cue‐stimulus interval of 100 ms (see description of the experiment), this causes some overlap ERPs reflecting of early attentional visual processing. Importantly, and as previously been shown (Zhang, Stock, & Beste, 2016a), this does not cause any problem in the interpretation of the N2/P2 time windows. To quote from that study: “The reason for this is that when analyzing the BI effect in the last trial of a triplet, one does not directly compare different conditions or trials as there are no differences in the conditions of the last trial the triplets used to compare the BASE and BI conditions. The BI condition comprises the two triplets ABA and BAB, so that ERPs obtained from both A and B trials will be averaged to form the BI condition. The BASE condition comprises the two triplets DBA and DAB, so that again, EEG data obtained from both A and B trials will again be averaged to form the BASE condition. Therefore, comparing the BI and BASE conditions means comparing an average of A&B trials to an average of A&B trials. When investigating the BI effect, we are interested in the interplay of the n‐2 and n‐1 trials and its effect on the nth trial. Due to the sufficiently long RSI of 1,500 ms, there can be no overlap between the ERPs evoked by the n‐1 or n‐2 trial and the trial we quantified for analyses. Aside from this, the experimental paradigms used in both groups were absolutely identical. Hence, it is entirely impossible that any of the reported effects caused by either group or BI condition are caused by an overlap of ERP components.” (Zhang, Stock, & Beste, 2016a).
2.4. Residue iteration decomposition
The RIDE analysis was applied following established procedures and methods (Ouyang et al., 2011; Verleger et al., 2014) using the toolbox package and manual of RIDE available on http://cns.hkbu.edu.hk/RIDE.htm. ERPs are conventionally obtained by averaging single trials, which are assumed to be locked to the stimulus onset. Previous approaches suppose that residues between the single trial data and the average are just noise. This view is questionable because it hardly reflects the variability in the latency of brain activities. It needs to be considered that latencies as well as amplitudes vary between single trials. The averaging of them result in an overlap and smearing of the components, changing their shape and amplitude (Ouyang et al., 2015b). Accordingly, the theoretical approach of RIDE postulates that an ERP consists of diverse components associated with different stages of cognitive processing and with variable inter‐component delays. The aim is to separate these components with and without time markers and to reconstruct the ERP reliably. Therefore, RIDE uses an iteration procedure of the residues of the averaged ERPs and decomposes ERP components using an L1‐norm minimization (i.e., obtaining median waveforms). The decomposition is applied individually for every single electrode (Ouyang et al., 2015b). Thus, it reduces residual error due to noise in the data and attenuates intra‐individual variability (Ouyang et al., 2015a; b). ERPs are decomposed locked to the stimulus onsets and reaction times, denoted as S‐ and R‐cluster (components). As opposed to this, it is assumed that there is a central component, referred to as C‐component, having variable latency over single trials and being neither locked to the stimulus onset nor to reaction times (Ouyang et al., 2015b; Verleger et al., 2014). Considering this, time markers for deriving the C‐cluster are estimated and iteratively improved.
Based on this estimation and by using the time markers of stimulus onsets as well as reaction times, self‐optimized iteration scheme is used for latency estimation, which amends the latency estimation of the C component cluster. For the computation of the RIDE components (S‐, R‐, C‐cluster), a time window function is used, which is supposed to cover the range in within each component is maximal. More details of the RIDE decomposition procedure can be found in Ouyang et al. (2015b). The search interval for the S‐cluster was defined from −200 to 600 ms, from 0 to 900 ms for the C‐cluster, and 300 ms around the response trigger (−300 to 300 ms) for the R‐cluster (Ouyang et al., 2015a). Each cluster was quantified on the single subject level. The quantifications of the mean amplitudes in the relevant RIDE cluster were conducted using the same procedure as described for the quantification in the original ERP data. The choice of electrode sites and time windows quantified in each RIDE cluster was validated using the same method as used for the ERP data (see ERP section). Since the R‐cluster is thought to reflect processes of motor response execution, the R‐cluster was quantified at electrodes overlying the motor cortex (i.e., C3 and C4). Details about the quantified and validates time windows/electrode sites can also be found in Table 1.
2.5. Source localization analysis (sLORETA)
The estimated RIDE clusters served as the basis for the source localization analysis. This was done because only the RIDE decomposed data yielded significant interactions “BI/base × group”. For source localization sLORETA (standardized low resolution brain electromagnetic tomography; (Pascual‐Marqui, 2002) was applied. This algorithm provides a single linear solution for the inverse problem without localization bias (Marco‐Pallarés, Grau, & Ruffini, 2005; Sekihara, Sahani, & Nagarajan, 2005). The validity of sources estimated via sLORETA analysis has been corroborated by evidence from fMRI and EEG/ TMS‐studies (Dippel & Beste, 2015; Sekihara et al., 2005). The computation of the standardized current density at each voxel was executed using the MNI152 template (Fuchs, Kastner, Wagner, Hawes, & Ebersole, 2002). The sLORETA images (partitioned into 6,239 voxels at 5 mm spatial resolution) of the intracerebral volume in adolescents were contrasted with the images from adults. This comparison was based on statistical nonparametric mapping utilizing the sLORETA—built‐in voxel—wise randomization test with 2,000 permutations (p < .01, corrected for multiple comparisons). Significant differences between voxels in contrasted conditions were located in the MNI brain (http://www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm).
2.6. Statistics
For the behavioral and neurophysiological data analysis, mixed effects ANOVAs including a within‐subject factors condition (backward inhibition/BI vs. baseline/BASE) and electrode (wherever applicable) were conducted. The factor “Group” (adolescents vs. adults) served as between‐subject factor. Separate ANOVAs were calculated for each behavioral and neurophysiological measure. Greenhouse–Geisser correction was applied whenever necessary. Post‐hoc tests were Bonferroni‐corrected whenever necessary. All included variables were normally distributed as tested with Kolmogorov–Smirnov tests (all z < .9; p > .3).
Before the analysis of the behavioral and neurophysiological data, the first two trials of each block were excluded. Similarly, all trials with an error and the following two trials were eliminated and not considered for data analysis. Trials with higher RTs than 2,500 ms or lower than 100 ms were discarded. The latter affected 0.55% (±1.44) of all trials. Focus of this study is the magnitude differences in the BI effect between healthy adolescents and adults on a system‐neurophysiological level. Therefore, for each baseline triplet the respective back‐switching triplet was chosen which only differed in cue presented in n‐2.
The data was analyzed as done in previous studies by our research group (Zhang, Stock, Fischer, et al., 2016b): the two back‐switching triplets as well as the two baseline triplets were averaged individually to achieve a measure for the BI condition and the baseline (BASE) condition, respectively. Subsequently, we calculated the “BI effect” as the RT difference between BI and BASE conditions [BI effect = mean (ABA, BAB) – mean (DBA, DAB)]. Given that the present study focuses on the examination of the basic BI effect, for the statistical analysis, solely the following triplets were included: ABA, BAB, DBA, and DAB. Other triplet combinations were not considered since they were stated by Koch et al. (2004) to examine not only the basic BI effect but additionally response‐related factors and especially the role of response modes in backward inhibition (Koch et al., 2004).
Since we intended to investigate age‐group effects to overcome the BI effect on a system neurophysiological level it is important to have strong BI effects, because this is critical considering the signal‐to‐noise ratio in the neurophysiological data. This is all the more the case because intra‐individual variability of behavioral and EEG data is an issue in developmental studies (refer introduction section). Therefore, and to keep the data comparable to other published studies using that paradigm (Wolff et al., 2018; Zhang, Stock, & Beste, 2016a; Zhang, Stock, Fischer, et al., 2016b), those triplets were not included in the data analysis.
3. RESULTS
3.1. Behavioral data
The behavioral data are shown Figure 2.
Figure 2.
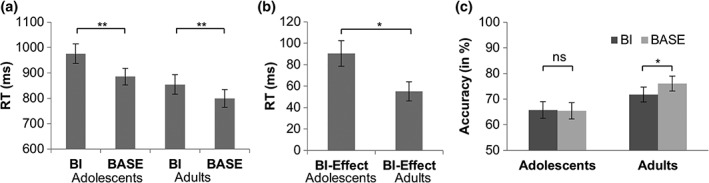
(a) Reaction time data for the BI and the BASE condition in adolescents (= Adol) and adults. (b) Data showing the BI‐effect (i.e., BI minus BASE) in each group. (c) Percentage of hits (accuracy) for the BI and the BASE condition in adolescents and adults is shown on the right. Means and SEMs are given. Stars represent significant differences between conditions or groups
For the RTs, the mixed effects ANOVA revealed a main effect of “condition” (F[1,44] = 97.46, p < .001, ηp 2 = .689), showing that RTs were longer (slower) in the BI condition (915 ms ± 27), than in the BASE condition (842 ms ± 24). There was a main effect of “group” (F[1,44] = 4.19, p = .047, ηp 2 = .087), showing longer RTs in adolescents (931 ms ± 37) than in adults (827 ms ± 35). Moreover, the analysis revealed a significant interaction of “condition × group” (F[1,44] = 5.71, p = .021, ηp 2 = .115) (Figure 2a). This interaction indicates a bigger difference between BI versus BASE condition in adolescents (976 ms ± 39 vs. 885 ms ± 35; difference: 90 ms ± 12) than in adults (854 ms ± 37 vs. 799 ms ± 33; difference: 55 ms ± 9). Post‐hoc tests showed that the group difference in the BI effect was driven by the BI condition, since adolescents differed from adults in the BI condition (t[44] = 2.24, p = .030) but not in the BASE condition (t[44] = 1.79, p = .162). A Post‐hoc test revealed that this difference (i.e., the BI effect) was larger in adolescents than in adults (t[44] = 2.39, p = .011) (Figure 2b).
The mixed effects ANOVA on performance accuracy (percentage of hits, Figure 2c) revealed an interaction effect of “condition × group” (F[1,44] = 4.32, p = .044, ηp 2 = .089). This interaction is shown in Figure 2 and indicates decreased percentage of hits during BI versus BASE condition in adults (72% ± 3 vs. 76% ± 3), but not in adolescents (65% ± 3 vs. 65% ± 3). Post‐hoc tests showed that these differences between BI and BASE condition were significant in adults (t[23] = −3.46, p = .002) and not in adolescents (t[21] = 0.036, p = .486). There was a main effect of “group” (F[1,44] = 4.32, p = .044, ηp 2 = .089), showing overall decreased accuracy in adolescents (65% ± 3) than in adults (74% ± 3). Moreover the analysis revealed a significant main effect of “condition” (F[1,44] = 4.08, p = .050, ηp 2 = .085), showing decreased accuracy in BI (68% ± 2) compared with BASE condition (70% ± 2).
3.2. Standard event‐related potentials (ERPs)
The standard ERP‐component are shown in Figure 3.
Figure 3.
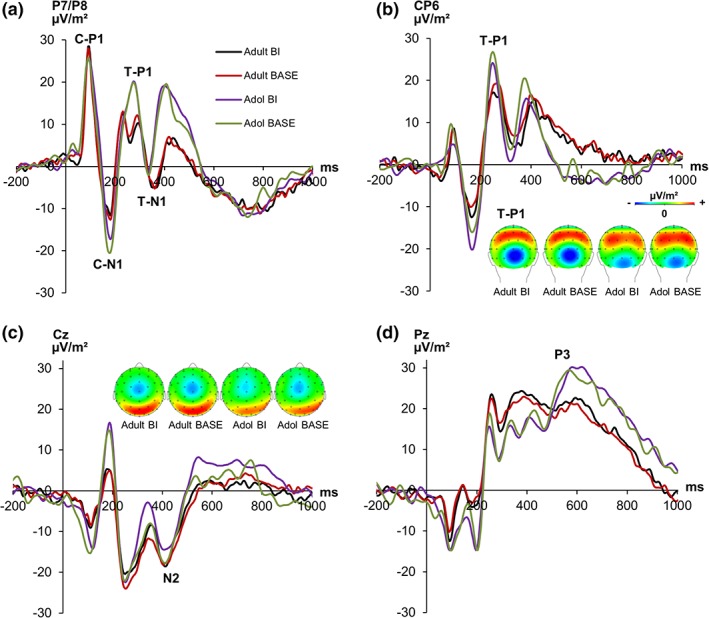
Standard event‐related potential (ERP) components. Time point zero represents the cue onset. The target appeared 100 ms later. (a) P1 and N1 ERP components on the cue and target pooled across electrodes P7/ P8. P1 and N1 elicited by the cue (C‐P1, C‐N1) are shown in the first two peaks. The target P1 and N1 (T‐P1, T‐N1) are represented by the following two peaks. (b) The target‐P1 at electrode CP6 including scalp topography maps (at 250 ms) in each group and condition. Red colors denote positivity, blue colors negativity. (c) The N2 ERP was quantified at electrode Cz with corresponding scalp topography (at 410 ms) for the time point of the N2‐peak. (d) The P3 ERP‐component at electrode Pz [Color figure can be viewed at http://wileyonlinelibrary.com]
The P1 and N1 following the cue can be seen in Figure 3a. For the P1 component following the cue stimulus, the mixed effects ANOVA showed a main effect of “electrode” (F[1,43] = 5.92, p = .019, ηp 2 = .121), showing that the P1 was smaller at electrode P7 (19.71 μV/m2 ± 1.86) than at electrode P8 (26.66 μV/m2 ± 2.64). Other main or interaction effects were not significant (all F < 0.47, p > .458). The mixed effects ANOVA for the N1 evoked by the cue stimulus revealed no significant main or interaction effects (all F < 3.77, p > .059). The P1 and N1‐ERP component following the target stimulus are also shown in Figure 3. For the P1 component on the target stimulus at electrodes P7/P8, the mixed effects ANOVA showed no further significant effects (all F < 1.72, p > .196). However, as can be seen in Figure 3b, the difference between the BI and the BASE condition was prominent at cento‐parietal electrode sites. The same electrode site showing a P1 effect in backward inhibition has recently been used (Wolff et al., 2018). Analyzing the P1 component at electrode CP6, significant main and interaction effects remained absent (all F < 3.26, p > .078). The mixed effects ANOVA for the target N1 revealed a significant main effect of “electrode” (F[1,43] = 7.07, p = .011, ηp 2 = .147), showing a more negative activation at electrode P7 (−5.65 μV/m2 ± 2.98) than at electrode P8 (3.93 μV/m2 ± 3.50). No further main or interaction effects were obtained (all F < 1.81, p > .186).
For the target N2 (Figure 3c), the mixed effects ANOVA revealed no significant main or interaction effects (all F < 1.56, p > .219). The mixed effects ANOVA for the target P3 (Figure 3d) showed a significant main effect of “group” (F[1,43] = 5.01, p = .031, ηp 2 = .107), indicating a larger P3 in adolescents (27.53 μV/m2 ± 3.16) than in adults (17.54 μV/m2 ± 3.16). Further significant effects could not be obtained (all F < 2.32; p > .136).
To summarize, the analysis of standard ERP components did not reveal interactive effects between BI/BASE condition and group. Underlining this, also a sLORETA analysis did not reveal reliable differences. The sLORETA analysis was performed because the choice of time and electrodes to quantify the ERP data may have failed to capture the difference. However, this is an expected finding since it is likely that intra‐individual variability in neurophysiological data may preclude the detection of reliable differences between adolescents and adults (Bodmer et al., 2018). To account for this, the RIDE analysis was performed.
3.3. Residue iteration decomposition
3.3.1. S‐cluster
The S‐cluster data is shown in Figure 4.
Figure 4.
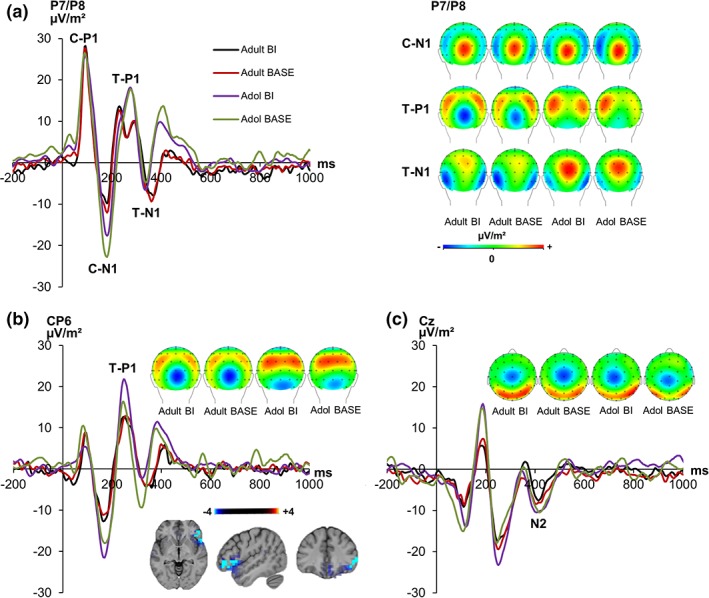
The S‐cluster at electrodes P7/P8, CP6, and Cz is shown for adolescents (= Adol) and adults in the BI and BASE condition. Time point zero represents the beginning of the cue presentation. The presentation of the target stimulus started 100 ms later (a) depiction of the P1 and N1 ERPs as reflected in the S‐cluster elicited by the cue and target stimuli pooled across electrode P7 and P8. P1 and N1 elicited by the cue (C‐P1, C‐N1) are shown in the first two peaks. The target P1 and N1 (T‐P1, T‐N1) are represented by the following two peaks. The scalp topography maps given for the C‐N1 (at 184 ms), T‐P1 (at 270 ms), and T‐N1 (at 367 ms) pooled across electrodes P7/P8 for each group and condition are shown at the right. Red colors denote positivity, blue colors negativity. (b) the P1 ERP as reflected in the S‐cluster at electrode CP6 along with the corresponding scalp topography maps (at 242 ms). The sLORETA plots show a source of difference in the right inferior frontal gyrus (BA47) (corrected for multiple comparisons). (c) The N2 ERP at electrode Cz including scalp topography (at 414 ms) [Color figure can be viewed at http://wileyonlinelibrary.com]
The mixed effects ANOVA in the P1 time window following the cue (Figure 4a) showed no significant interaction effects (all F < 5.57, p > .455). A significant main effect of “electrode” was obtained (F[1,43] = 6.41, p = .015, ηp 2 = .130), which showed a more positive activation on the P8 electrode (P7: 19.46 μV/m2 ± 1.86; P8 26.76 μV/m2 ± 2.66). There were no other significant effects (all F < .39; p > .536). A significant main effect of “group” was obtained for the cue N1 (F[1,43] = 4.37, p = .043, ηp 2 = .092), indicating a more negative activation in adolescents (−19.16 μV/m2 ± 3.92) than in adults (−7.44 μV/m2 ± 4.01). No other significant effects were observed (all F < 3.88; p > .055).
Examining the P1 on the target stimulus at electrodes P7/ P8 (Figure 4a), the amplitudes revealed no main or interaction effects (all F < 2.30, p > .137). However, as with the ERP data, the difference between the BI and the BASE condition was prominent at cento‐parietal electrode sites, and electrode CP6 in particular (Figure 4b). The same electrode site showing a P1 effect in backward inhibition has recently been used [Wolff et al., 2018]. The mixed effects ANOVA in the P1 after target stimulus presentation at electrode CP6 showed a significant main effect of “condition” (F[1,43] = 4.54, p = .039, ηp 2 = .097), indicating a larger P1 amplitude in the BI compared with the BASE condition (BI: 15.16 μV/m2 ± 2.16; BASE: 12.60 μV/m2 ± 1.76). A significant interaction “condition × group” was obtained (F[1,43] = 5.48, p = .024, ηp 2 = .115). Post‐hoc tests showed that there was a significant difference between the BI (17.95 μV/m2 ± 3.80) and the BASE (12.56 μV/m2 ± 3.16) condition in adolescents (t[21] = 2.53, p = .010), but not in adults (BI: 12.37 μV/m2 ± 2.11; BASE: 12.63 μV/m2 ± 1.71; t[22] = −0.21, p = .839). There were no further significant main or interaction effects (all F < .55; p > .461). It is shown that the BI effect (i.e., BI minus BASE) was larger in adolescents (3.93 μV/m2 ± 1.63) than adults (−0.25 μV/m2 ± 1.24) (t[43] = 2.07, p = .022). The sLORETA analysis revealed that difference in the strength of the BI‐effect between adolescents and adults in the target‐locked S‐cluster P1 was due to activation differences in the right inferior frontal gyrus (BA47) (refer Figure 4b).
The mixed effects ANOVA on the target N1 amplitudes at electrodes P7 and P8 following the target stimulus showed an interaction effect of “condition × electrodes” (F[1,43] = 4.51, p = .040, ηp 2 = .097) which indicates a stronger BI effect at electrode P7 (BI: −9.11 μV/m2 ± 2.52; BASE: −4.79 μV/m2 ± 2.47) than at P8 (BI: −2.04 μV/m2 ± 3.03; BASE: −3.35 μV/m2 ± 3.12). Post‐hoc tests revealed significant differences between BI and BASE condition on P7 (t[43] = −2.11, p = .021) but not on P8 electrode (t[43] = .82, p = .207). No other main or interaction effects were found (all F < 2.12; p > .153).
The mixed effects ANOVA for the N2 peaks (Figure 4c) in the S‐cluster revealed no significant main or interaction effects (all F < 0.59; p > .448).
3.4. C‐cluster
The C‐cluster data is shown in Figure 5.
Figure 5.
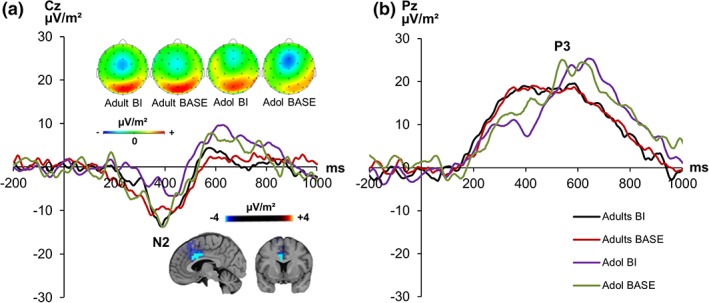
The C‐cluster at electrodes Cz and Pz is shown for adolescents (= Adol) and adults in the BI and BASE condition. Time point zero represents the onset of the cue presentation. It was followed by the target stimulus 100 ms later. (a) The N2 as reflected in the C‐cluster at electrode Cz as well as the corresponding scalp topography (at 387 ms) maps is shown. Activation differences in the anterior cingulate cortex (ACC, BA24, BA32) can be seen in corresponding sLORETA plots (corrected for multiple comparisons); (b) the P3 as reflected in the C‐cluster at electrode Pz [Color figure can be viewed at http://wileyonlinelibrary.com]
The mixed effects ANOVA in the N2 time window (Figure 5a) at electrode Cz revealed a significant interaction effect “condition × group” (F[1,43] = 4.48, p = .040, ηp 2 = .096), which indicates a bigger difference between BI and BASE condition in adolescents (BI: −1.14 μV/m2 ± 3.57; BASE: −9.33 μV/m2 ± 3.16) compared with adults (BI: −10.48 μV/m2 ± 3.57; BASE: −7.88 μV/m2 ± 3.16). Post‐hoc tests showed that this difference was significant in adolescents (t[21] = 1.73, p = .049) but not in adults (t[22] = −1.36, p = .094). Thus, the BI effect (BI minus BASE) was larger in adolescents (8.19 μV/m2 ± 4.72) than adults (−2.60 μV/m2 ± 1.91) (t[22.68] = −2.12, p = .022). Furthermore, post‐hoc tests revealed that the group difference in the BI effect was driven by the BI condition, since adolescents differed from adults in the BI condition (t[43] = 2.18, p = .034) but not in the BASE condition (t[28.35] = −0.35, p = .728). The sLORETA analysis showed that C‐cluster amplitude differences in the N2 time window were due to activation differences in the anterior cingulate cortex (ACC, BA24, BA32). The mixed effects ANOVA of the effects in the P3 time window (Figure 5b) at electrode Pz revealed no significant main or interaction effects (all F < 1.25; p > .278).
3.5. R‐cluster
The R‐Cluster is shown in Figure 6.
Figure 6.
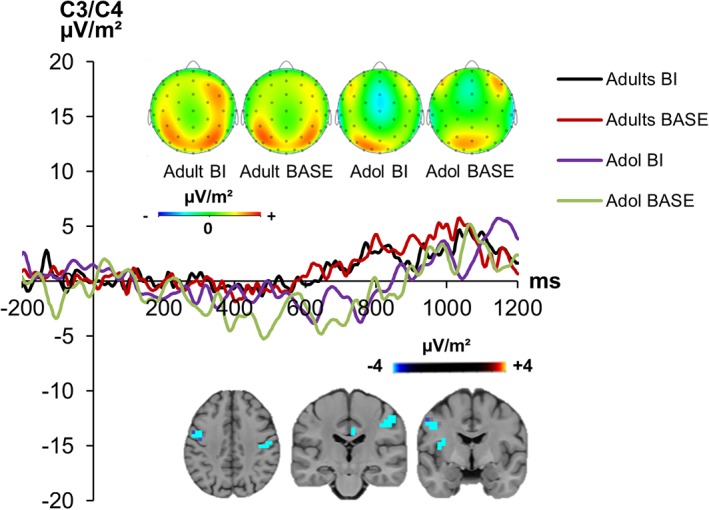
The R‐cluster at electrodes C3 and C4 (pooled across the electrodes) is shown for adolescents (= Adol) and adults in the BI and BASE condition including scalp topography maps (at 836 ms). The scalp topography maps are shown of the time point where the response is executed (this varies depending on group and condition, cf. behavioral data). Time point zero represents the onset of the cue. It was followed by the target 100 ms later [Color figure can be viewed at http://wileyonlinelibrary.com]
The mixed effects ANOVA at electrodes C3/ C4 showed a significant main effect of “condition” (F[1,43] = 6.40, p = .015, ηp 2 = .138), showing larger amplitudes in the BI condition (2.78 μV/m2 ± 0.75), compared with the BASE condition (0.96 μV/m2 ± 0.99). Moreover, the interaction between “condition × group” was significant (F[1,43] = 11.90, p = .001, ηp 2 = .229), indicating a bigger difference between BI and BASE condition in adolescents (BI: 3.35 μV/m2 ± 1.10; BASE: −0.95 μV/m2 ± 1.47) than in adults (BI: 2.22 μV/m2 ± 1.00; BASE: 2.88 μV/m2 ± 1.34). Thus, the BI effect (BI minus BASE) was larger in adolescents (4.03 μV/m2 ± 1.40) than adults (−0.29 μV/m2 ± 0.52) (t[22.96] = 3.08, p = .005). However, post‐hoc tests showed that the group difference in the BI effect was driven by the BASE condition, since adolescents differed from adults in the BASE condition (t[43] = −2.47, p = .017) but not in the BI condition (t[43] = 0.76, p = .452). Yet, the sLORETA analysis showed that modulations in the R‐cluster are associated with activation differences in the pre‐central gyrus (BA4).
4. DISCUSSION
The present study examines the system‐neurophysiological basis of developmental changes in sequential cognitive flexibility between adolescents and adults. The study focuses on mechanisms to overcome backward inhibition. We hypothesized that these deficits in processes to overcome backward inhibition in adolescence are either due to deficits in response selection processes associated with medial frontal regions (i.e., in the N2 time window) and/or modulations in processes likely reflecting inhibitory control of incoming information to a knowledge system associated with inferior frontal brain regions (i.e., processes in the P1 time window).
The behavioral data shows that adolescents have more difficulties to overcome the BI effect compared with adults; that is, reaction times in the BI condition were longer in adolescents than adults, no difference between groups was evident in the BASE condition. Adolescents thus encounter difficulties to overcome the inhibition of the lately suppressed task set that becomes relevant again (Allport et al., 1994; Allport & Wylie, 1999; Mayr & Keele, 2000). Currently, there is no data on processes used to overcome the BI effect between adolescents and adults and also across the entire life span such data is sparse (Schuch, 2016). Existing data in older ages using comparable experiments mostly report no effects (Lawo, Philipp, Schuch, & Koch, 2012; Mayr, 2001). It has been suggested that this is due to not examined subtle differences in the distribution of the behavioral data and intra‐individual variability (Schuch, 2016). While the intra‐individual variability does not seem to cause problems for the analysis of the behavioral data in the current study, the EEG data clearly shows that this is important to consider:
As hypothesized, using a standard ERP analysis, no processes could be detected at the neurophysiological level that reflected the differential modulation between adolescents and adults to overcome backward inhibition, that is, there was no reliable interaction “condition × group.”. Yet, reliable interactions “condition × group” were obtained after accounting for intra‐individual variability in the data using RIDE (Ouyang et al., 2011). Already previous results suggested that differences in intra‐individual variability bias comparisons between adolescents and adults when it comes to neurophysiological correlates of cognitive control (Bodmer et al., 2018). It is shown that the interaction “condition × group” was obtained for the S‐cluster in the time window of the target‐P1 and the C‐cluster in the search interval of the target‐N2.
In adolescents, the P1 was larger in the BI than the BASE condition. As outlined in the introduction, modulations of the P1 have been suggested to reflect processes related to the suppression of information in task irrelevant networks during early stimulus categorization (Klimesch, 2011). One important mechanism during cognitive flexibility is the ability to update mental representations (Dajani & Uddin, 2015), which requires the inhibition of the no longer relevant, competing task (Dajani & Uddin, 2015; Klimesch, 2011; Mayr & Keele, 2000). During backward inhibition, it is the suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial that fosters cognitive flexibility and reduces the degree of backward inhibition. The larger P1 in the nth trial of the BI compared with the BASE condition therefore suggests that the suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial is stronger in the BI condition. Underlining this interpretation, the sLORETA shows that regions in the right inferior frontal gyrus (rIFG) were associated with this modulation. This region is well‐known to play a central role in inhibitory control processes (e.g., Aron, Monsell, Sahakian, & Robbins, 2004; Bodmer & Beste, 2017; Chmielewski, Mückschel, Ziemssen, & Beste, 2017; Dippel & Beste, 2015; Stock, Popescu, Neuhaus, & Beste, 2016) and has previously been shown to be associated with inhibitory gating processes reflected by the P1 during backward inhibition (Wolff et al., 2018). Importantly, the rIFG is involved in stimulus detection (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010), which further underlines the interpretation of an inhibitory stimulus‐related gating mechanism. As mentioned, the BI effect thus concerns the interplay of the n‐2 and n‐1 trials and its effect on the nth trial. If the inhibitory effect of the n‐1 trial on the n‐2 trial is strong, costs to overcome this inhibition are high (= high BI effect). The larger P1 in the nth trial likely reflects the inhibition n‐1 task sets in the knowledge system. The resulting effect is that these task sets can no longer guide information processing and the top‐down inhibitory impact of the n‐1 trial on the n‐2 trial is weakened. In principal, this makes it easier to re‐use the previously abandoned n‐2 trial task set in the nth trial. However, the behavioral data shows that this was not the case. This suggests that adolescents do not benefit from the stronger inhibitory gating mechanisms, compared with adults. To explain this, the effects observed for the C‐cluster in the N2 time window are important to consider.
For the C‐cluster N2 it is shown that the amplitude was smaller in the BI than the BASE condition in adolescents. This process occurred approximately 100 ms later than processes reflected by the P1 in the S‐cluster. The C‐cluster has previously been found to be associated with response selection and control processes in the N2 time window (Chmielewski et al., 2018; Mückschel et al., 2017), which is in line with theoretical considerations suggesting that the N2 reflects intermingled perceptual and response‐related (selection) processes (Folstein & Van Petten, 2008). The effect observed for the C‐cluster was associated with the anterior cingulate cortex (BA24, BA32). No difference between BI and BASE conditions was evident in adults (adults also showed a smaller BI effect). In the BI condition, the C‐cluster N2 amplitudes were larger in adults than adolescents. No group differences were evident in the BASE condition. These results suggest that response selection processes were attenuated in the BI condition in adolescents and not sufficient to re‐use the recently abandoned task set. Previous results have shown that cognitive effort is required to re‐use the recently abandoned task set, which is accompanied by activation alterations in anterior cingulate structure (Zhang, Stock, & Beste, 2016a). When effort is increased to overcome the inhibition of a recently abandoned task set, amplitudes in the target N2 time window also increase (Zhang, Stock, & Beste, 2016a). This interpretation is in line with other studies suggesting that increased N2 amplitudes and an activation of medial frontal cortical structures is associated with an increased effort (Larson et al., 2014; Stock, Wolff, & Beste, 2017). It is therefore possible that adolescents do not (or are not able) to invest enough effort to re‐use the recently abandoned task set. Yet, a partly related interpretation refers to conflict monitoring processes (Folstein & Van Petten, 2008). In the BI condition, conflict may arise from the carry‐over of inhibition from n‐2 trials and the reactivation of this currently abandoned task. This requires that more cognitive control needs to be exerted to overcome BI (Costa & Friedrich, 2012; Grange & Houghton, 2010). The smaller C‐cluster amplitude in the N2 time window in the BI condition may, therefore, also reflect insufficient conflict monitoring processes in adolescents when they try to overcome the backward inhibition. This is also in line with the literature showing that conflict monitoring functions are under‐developed in adolescence (Adleman et al., 2002; Checa, Castellanos, Abundis‐Gutiérrez, & Rosario Rueda, 2014; Chmielewski et al., 2014; Fitzgerald et al., 2010; Hämmerer et al., 2014; Ladouceur, Dahl, & Carter, 2007; Perkins, Welsh, Stern, Taylor, & Fitzgerald, 2013; Rubia, Smith, Taylor, & Brammer, 2007; Schroeter, Zysset, Wahl, & von Cramon, 2004).
Interestingly, also the R‐cluster showed differential effects between BI/BASE condition and group (i.e., an interaction “condition × group”). However, this interactive effect was driven by the BASE condition and does therefore not explain the behavioral modulation in backward inhibition between adolescents and adults. The sLORETA analysis shows that modulations in the R‐cluster are associated with modulation of motor cortex activity. Since the R‐cluster reflects motor response related processes, this seems reasonable (Ouyang et al., 2015b). The R‐cluster data suggest that adolescents show an altered activation of the motor response system during cognitive flexibility. Yet, this peculiarity does not contribute to performance differences compared with adults.
Taken together, it seems that two, partly inter‐related processes underlie difficulties to overcome backward inhibition effects in adolescents than adults: One process refers to the suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial that is associated with right inferior frontal regions. The other process refers to response selection and conflict monitoring processes associated with medial frontal cortical regions. This suggests that developmental changes in sequential cognitive flexibility processes to overcome backward inhibition reflect the maturation of two, neurophysiological and functional dissociable processes that are further associated with distinct functional neuroanatomical structures in the frontal cortex. This is well in line with the concept of the “Interactive Specialization” (Johnson, 2011), which states that emerging cognitive, sensory as well as motor functions are due to the maturation of specific brain regions. Maturation refers to a developmental level at which adult functions are reached. Moreover, during development, the specific functionality of those regions are sharpened by activity‐dependent interactions between particular brain regions (Johnson, 2011). From the current data it seems that sequential cognitive flexibility relates to the maturation of processes related to inferior frontal and anterior cingulate structures. Motor processes do not seem to be important. Yet, the “Interactive Specialization” viewpoint stresses that functional brain development, at least within cerebral cortex, involves a process of organizing patterns of inter‐regional interactions. Future studies shall therefore investigate, using longitudinal data, how a possible interplay between inferior frontal and anterior cingulate structures develops and may shape the development of sequential cognitive flexibility. In that regard it will be important to consider that the current analysis using RIDE showed that there are neurophysiological component clusters with different functional relevance.
In summary, the study shows that sequential cognitive flexibility and the ability to overcome backward inhibition is subject to strong developmental (age‐related) changes between adolescence and adulthood. Sequential cognitive flexibility, as assessed using a backward inhibition experiment, is inferior in adolescents compared with adults. That is, the adolescents show more difficulties to overcome backward inhibition, which impedes sequential cognitive flexibility. The neurophysiological data suggest that two partly inter‐related processes underlie these effects in adolescents than adults: One process refers to an insufficient suppression of the inhibitory effect of the n‐1 trial on the n‐2 trial that is associated with right inferior frontal regions. The other process refers to immature response selection and conflict monitoring processes associated with medial frontal cortical regions.
ACKNOWLEDGMENTS
This work was partly supported by Grants from the Deutsche Forschungsgemeinschaft (DFG) SFB 940 project B8 and the BMBF 01GL1741C. We thank the two unknown reviewers for their constructive comments on an earlier version of the manuscript.
Giller F, Zhang R, Roessner V, Beste C. The neurophysiological basis of developmental changes during sequential cognitive flexibility between adolescents and adults. Hum Brain Mapp. 2019;40:552–565. 10.1002/hbm.24394
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Number: 01GL1741C; Deutsche Forschungsgemeinschaft, Grant/Award Number: SFB 940 project B8
REFERENCES
- Adleman, N. E. , Menon, V. , Blasey, C. M. , White, C. D. , Warsofsky, I. S. , Glover, G. H. , & Reiss, A. L. (2002). A developmental fMRI study of the Stroop color‐word task. NeuroImage, 16, 61–75. [DOI] [PubMed] [Google Scholar]
- Allport, A. , Styles, E. A. , & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of task In Umiltà C. & Moscovitch M. (Eds.), Attention and performance XV: Conscious and nonconscious information processing (pp. 421–452). Cambridge, MA: MIT Press. [Google Scholar]
- Allport, D. A. , & Wylie, G. (1999). Task‐switching: Positive and negative priming of task‐set In Humphreys G. W., Duncan J., & Treisman A. (Eds.), Attention, space, and action: Studies in cognitive neuroscience (pp. 273–296). New York, NY: Oxford University Press. [Google Scholar]
- Aron, A. R. , Monsell, S. , Sahakian, B. J. , & Robbins, T. W. (2004). A componential analysis of task‐switching deficits associated with lesions of left and right frontal cortex. Brain, 127, 1561–1573. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18, 177–185. [DOI] [PubMed] [Google Scholar]
- Beste, C. , Baune, B. T. , Falkenstein, M. , & Konrad, C. (2010). Variations in the TNF‐α gene (TNF‐α ‐308G→a) affect attention and action selection mechanisms in a dissociated fashion. Journal of Neurophysiology, 104, 2523–2531. [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M. , Cherbuin, N. , Bunce, D. , & Anstey, K. J. (2014). Intraindividual variability is a fundamental phenomenon of aging: Evidence from an 8‐year longitudinal study across young, middle, and older adulthood. Developmental Psychology, 50, 143–151. [DOI] [PubMed] [Google Scholar]
- Bodmer, B. , & Beste, C. (2017). On the dependence of response inhibition processes on sensory modality. Human Brain Mapping, 38, 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer, B. , Mückschel, M. , Roessner, V. , & Beste, C. (2018). Neurophysiological variability masks differences in functional neuroanatomical networks and their effectiveness to modulate response inhibition between children and adults. Brain Structure & Function, 223, 1797–1810. [DOI] [PubMed] [Google Scholar]
- Checa, P. , Castellanos, M. C. , Abundis‐Gutiérrez, A. , & Rosario Rueda, M. (2014). Development of neural mechanisms of conflict and error processing during childhood: Implications for self‐regulation. Frontiers in Psychology, 5, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski, W. X. , Mückschel, M. , & Beste, C. (2018). Response selection codes in neurophysiological data predict conjoint effects of controlled and automatic processes during response inhibition. Human Brain Mapping, 39, 1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski, W. X. , Mückschel, M. , Roessner, V. , & Beste, C. (2014). Expectancy effects during response selection modulate attentional selection and inhibitory control networks. Behavioural Brain Research, 274, 53–61. [DOI] [PubMed] [Google Scholar]
- Chmielewski, W. X. , Mückschel, M. , Ziemssen, T. , & Beste, C. (2017). The norepinephrine system affects specific neurophysiological subprocesses in the modulation of inhibitory control by working memory demands. Human Brain Mapping, 38, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, R. E. , & Friedrich, F. J. (2012). Inhibition, interference, and conflict in task switching. Psychonomic Bulletin & Review, 19, 1193–1201. [DOI] [PubMed] [Google Scholar]
- Dajani, D. R. , & Uddin, L. Q. (2015). Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in Neurosciences, 38, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, M. C. , Amso, D. , Anderson, L. C. , & Diamond, A. (2006). Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia, 44, 2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. , Wang, Y. , Ding, X. , & Tang, Y.‐Y. (2015). Conflict monitoring and adjustment in the task‐switching paradigm under different memory load conditions: An ERP/sLORETA analysis. Neuroreport, 26, 124–130. [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry In Stuss D. T. & Knight R. T. (Eds.), Principles of frontal lobe function (pp. 466–503). New York, NY: Oxford University Press. [Google Scholar]
- Dippel, G. , & Beste, C. (2015). A causal role of the right inferior frontal cortex in implementing strategies for multi‐component behaviour. Nature Communications, 6, 6587. [DOI] [PubMed] [Google Scholar]
- Doebel, S. , & Zelazo, P. D. (2015). A meta‐analysis of the dimensional change card Sort: Implications for developmental theories and the measurement of executive function in children. Developmental Review, 38, 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers, F. C. L. , & van Boxtel, G. J. M. (2004). The N2 in go/no‐go tasks reflects conflict monitoring not response inhibition: Neurocognitive mechanisms of performance monitoring and inhibitory control. Brain and Cognition, 56, 165–176. [DOI] [PubMed] [Google Scholar]
- Espinet, S. D. , Anderson, J. E. , & Zelazo, P. D. (2012). N2 amplitude as a neural marker of executive function in young children: An ERP study of children who switch versus perseverate on the dimensional change card Sort. Developmental Cognitive Neuroscience, 2, S49–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, K. D. , Perkins, S. C. , Angstadt, M. , Johnson, T. , Stern, E. R. , Welsh, R. C. , & Taylor, S. F. (2010). The development of performance‐monitoring function in the posterior medial frontal cortex. NeuroImage, 49, 3463–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, J. R. , & Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45, 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, M. , Kastner, J. , Wagner, M. , Hawes, S. , & Ebersole, J. S. (2002). A standardized boundary element method volume conductor model. Clinical Neurophysiology, 113, 702–712. [DOI] [PubMed] [Google Scholar]
- Gajewski, P. D. , Kleinsorge, T. , & Falkenstein, M. (2010). Electrophysiological correlates of residual switch costs. Cortex, 46, 1138–1148. [DOI] [PubMed] [Google Scholar]
- Garrett, D. D. , Macdonald, S. W. S. , & Craik, F. I. M. (2012). Intraindividual reaction time variability is malleable: Feedback‐ and education‐related reductions in variability with age. Frontiers in Human Neuroscience, 6, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, W. J. , Bryck, R. L. , Jonides, J. , Albin, R. L. , & Badre, D. (2003). The mind's eye, looking inward? In search of executive control in internal attention shifting. Psychophysiology, 40, 572–585. [DOI] [PubMed] [Google Scholar]
- Giorgio, A. , Santelli, L. , Tomassini, V. , Bosnell, R. , Smith, S. , De Stefano, N. , & Johansen‐Berg, H. (2010). Age‐related changes in grey and white matter structure throughout adulthood. NeuroImage, 51, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, A. C. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange, J. A. , & Houghton, G. (2010). Heightened conflict in cue‐target translation increases backward inhibition in set switching. Journal of Experimental Psychology. Learning, Memory, and Cognition, 36, 1003–1009. [DOI] [PubMed] [Google Scholar]
- Gruner, P. , & Pittenger, C. (2017). Cognitive inflexibility in obsessive‐compulsive disorder. Neuroscience, 345, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer, D. , Müller, V. , & Li, S.‐C. (2014). Performance monitoring across the lifespan: Still maturing post‐conflict regulation in children and declining task‐set monitoring in older adults. Neuroscience & Biobehavioral Reviews, 1, 105–123. [DOI] [PubMed] [Google Scholar]
- Hampshire, A. , Chamberlain, S. R. , Monti, M. M. , Duncan, J. , & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage, 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2011). Interactive specialization: A domain‐general framework for human functional brain development? Developmental Cognitive Neuroscience, 1, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan, C. , Pandey, A. K. , Chorlian, D. B. , & Porjesz, B. (2015). The use of current source density as electrophysiological correlates in neuropsychiatric disorders: A review of human studies. The International Journal of Psychophysiology, 97, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, J. , & Tenke, C. E. (2015a). Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. The International Journal of Psychophysiology, 97, 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, J. , & Tenke, C. E. (2015b). On the benefits of using surface Laplacian (current source density) methodology in electrophysiology. The International Journal of Psychophysiology, 97, 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. (2011). Evoked alpha and early access to the knowledge system: The P1 inhibition timing hypothesis. Brain Research, 1408, 52–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, I. , Gade, M. , & Philipp, A. M. (2004). Inhibition of response mode in task switching. Experimental Psychology, 51, 52–58. [DOI] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Dahl, R. E. , & Carter, C. S. (2007). Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science, 10, 874–891. [DOI] [PubMed] [Google Scholar]
- Lamm, C. , Zelazo, P. D. , & Lewis, M. D. (2006). Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia, 44, 2139–2148. [DOI] [PubMed] [Google Scholar]
- Larson, M. J. , Clayson, P. E. , & Clawson, A. (2014). Making sense of all the conflict: A theoretical review and critique of conflict‐related ERPs. The International Journal of Psychophysiology, 93, 283–297. [DOI] [PubMed] [Google Scholar]
- Lawo, V. , Philipp, A. M. , Schuch, S. , & Koch, I. (2012). The role of task preparation and task inhibition in age‐related task‐switching deficits. Psychology and Aging, 27, 1130–1137. [DOI] [PubMed] [Google Scholar]
- Lehto, J. E. , Juujarvi, P. , Kooistra, L. , & Pulkkinen, L. (2003). Dimensions of executive functioning: Evidence from children. The British Journal of Developmental Psychology, 21, 59–80. [Google Scholar]
- Lewis, M. D. , Lamm, C. , Segalowitz, S. J. , Stieben, J. , & Zelazo, P. D. (2006). Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience, 18, 430–443. [DOI] [PubMed] [Google Scholar]
- Marco‐Pallarés, J. , Grau, C. , & Ruffini, G. (2005). Combined ICA‐LORETA analysis of mismatch negativity. NeuroImage, 25, 471–477. [DOI] [PubMed] [Google Scholar]
- Mayr, U. (2001). Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response‐set overlap. Psychology and Aging, 16, 96–109. [DOI] [PubMed] [Google Scholar]
- Mayr, U. , & Keele, S. W. (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology. General, 129, 4–26. [DOI] [PubMed] [Google Scholar]
- Mella, N. , Fagot, D. , Lecerf, T. , & de Ribaupierre, A. (2015). Working memory and intraindividual variability in processing speed: A lifespan developmental and individual‐differences study. Memory & Cognition, 43, 340–356. [DOI] [PubMed] [Google Scholar]
- Mückschel, M. , Chmielewski, W. , Ziemssen, T. , & Beste, C. (2017). The norepinephrine system shows information‐content specific properties during cognitive control ‐ evidence from EEG and pupillary responses. NeuroImage, 149, 44–52. [DOI] [PubMed] [Google Scholar]
- Mückschel, M. , Stock, A.‐K. , & Beste, C. (2014). Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cerebral Cortex, 24, 2120–2129. [DOI] [PubMed] [Google Scholar]
- Nunez, P. L. , Pilgreen, K. L. (1991): The spline‐Laplacian in clinical neurophysiology: A method to improve EEG spatial resolution. [review]. Journal of Clinical Neurophysiology 8:397–413. [PubMed] [Google Scholar]
- Ouyang, G. , Herzmann, G. , Zhou, C. , & Sommer, W. (2011). Residue iteration decomposition (RIDE): A new method to separate ERP components on the basis of latency variability in single trials. Psychophysiology, 48, 1631–1647. [DOI] [PubMed] [Google Scholar]
- Ouyang, G. , Sommer, W. , & Zhou, C. (2015a). Updating and validating a new framework for restoring and analyzing latency‐variable ERP components from single trials with residue iteration decomposition (RIDE). Psychophysiology, 52, 839–856. [DOI] [PubMed] [Google Scholar]
- Ouyang, G. , Sommer, W. , & Zhou, C. (2015b). A toolbox for residue iteration decomposition (RIDE)‐‐a method for the decomposition, reconstruction, and single trial analysis of event related potentials. Journal of Neuroscience Methods, 250, 7–21. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui, R. D. (2002). Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl D), 5–12. [PubMed] [Google Scholar]
- Perkins, S. C. , Welsh, R. C. , Stern, E. R. , Taylor, S. F. , & Fitzgerald, K. D. (2013). Topographic analysis of the development of individual activation patterns during performance monitoring in medial frontal cortex. Developmental Cognitive Neuroscience, 6, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruo, V. A. , Stock, A.‐K. , Münchau, A. , & Beste, C. (2016). A systems neurophysiology approach to voluntary event coding. NeuroImage, 135, 324–332. [DOI] [PubMed] [Google Scholar]
- Rubia, K. , Smith, A. B. , Taylor, E. , & Brammer, M. (2007). Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Human Brain Mapping, 28, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter, M. L. , Zysset, S. , Wahl, M. , & von Cramon, D. Y. (2004). Prefrontal activation due to Stroop interference increases during development‐‐an event‐related fNIRS study. NeuroImage, 23, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Schuch, S. (2016). Task inhibition and response inhibition in older vs. younger adults: A diffusion model analysis. Frontiers in Psychology, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekihara, K. , Sahani, M. , & Nagarajan, S. S. (2005). Localization bias and spatial resolution of adaptive and non‐adaptive spatial filters for MEG source reconstruction. NeuroImage, 25, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai, M. , Goffaux, P. , & Phillips, N. A. (2007). Cue‐ versus response‐locked processes in backward inhibition: Evidence from ERPs. Psychophysiology, 44, 596–609. [DOI] [PubMed] [Google Scholar]
- Sowell, E. R. , Peterson, B. S. , Thompson, P. M. , Welcome, S. E. , Henkenius, A. L. , & Toga, A. W. (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Stock, A.‐K. , Popescu, F. , Neuhaus, A. H. , & Beste, C. (2016). Single‐subject prediction of response inhibition behavior by event‐related potentials. Journal of Neurophysiology, 115, 1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, A.‐K. , Wolff, N. , & Beste, C. (2017). Opposite effects of binge drinking on consciously vs. subliminally induced cognitive conflicts. NeuroImage, 162, 117–126. [DOI] [PubMed] [Google Scholar]
- Störmer, V. , Eppinger, B. , & Li, S.‐C. (2014). Reward speeds up and increases consistency of visual selective attention: A lifespan comparison. Cognitive, Affective, & Behavioral Neuroscience, 14, 659–671. [DOI] [PubMed] [Google Scholar]
- Tamnes, C. K. , Fjell, A. M. , Westlye, L. T. , Østby, Y. , & Walhovd, K. B. (2012). Becoming consistent: Developmental reductions in intraindividual variability in reaction time are related to white matter integrity. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32, 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger, R. , Metzner, M. F. , Ouyang, G. , Śmigasiewicz, K. , & Zhou, C. (2014). Testing the stimulus‐to‐response bridging function of the oddball‐P3 by delayed response signals and residue iteration decomposition (RIDE). NeuroImage, 100, 271–280. [DOI] [PubMed] [Google Scholar]
- Waxer, M. , & Morton, J. B. (2011). Multiple processes underlying dimensional change card sort performance: A developmental electrophysiological investigation. Journal of Cognitive Neuroscience, 23, 3267–3279. [DOI] [PubMed] [Google Scholar]
- Whitmer, A. J. , & Banich, M. T. (2012). Brain activity related to the ability to inhibit previous task sets: An fMRI study. Cognitive, Affective, & Behavioral Neuroscience, 12, 661–670. [DOI] [PubMed] [Google Scholar]
- Wolff, N. , Buse, J. , Tost, J. , Roessner, V. , & Beste, C. (2017). Modulations of cognitive flexibility in obsessive compulsive disorder reflect dysfunctions of perceptual categorization. Journal of Child Psychology and Psychiatry, 58, 939–949. [DOI] [PubMed] [Google Scholar]
- Wolff, N. , Giller, F. , Buse, J. , Roessner, V. , & Beste, C. (2018). When repetitive mental sets increase cognitive flexibility in adolescent obsessive‐compulsive disorder. Journal of Child Psychology and Psychiatry, 59, 1–9. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Stock, A.‐K. , & Beste, C. (2016a). The neurophysiological basis of reward effects on backward inhibition processes. NeuroImage, 142, 163–171. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Stock, A.‐K. , Fischer, R. , & Beste, C. (2016b). The system neurophysiological basis of backward inhibition. Brain Structure & Function, 221, 4575–4587. [DOI] [PubMed] [Google Scholar]


