Abstract
There is a high prevalence of neurodevelopmental impairments in individuals living with congenital heart disease (CHD) and the neural correlates of these impairments are not yet fully understood. Recent studies have shown that hippocampal volume and shape differences may provide unique biomarkers for neurodevelopmental disorders. The hippocampus is vulnerable to early life injury, especially in populations at risk for hypoxemia or hemodynamic instability such as in neonates with CHD. We compared hippocampal gray and white matter volume and morphometry between youth born with CHD (n = 50) aged 16–24 years and healthy peers (n = 48). We also explored whether hippocampal gray and white matter volume and morphometry are associated with executive function and self‐regulation deficits. To do so, participants underwent 3T brain magnetic resonance imaging and completed the self‐reported Behavior Rating Inventory of Executive Function—Adult version. We found that youth with CHD had smaller hippocampal volumes (all statistics corrected for false discovery rate; q < 0.05) as compared to controls. We also observed significant smaller surface area bilaterally and inward displacement on the left hippocampus predominantly on the ventral side (q < 0.10) in the CHD group that were not present in the controls. Left CA1 and CA2/3 were negatively associated with working memory (p < .05). Here, we report, for the first‐time, hippocampal morphometric alterations in youth born with CHD when compared to healthy peers, as well as, structure–function relationships between hippocampal volumes and executive function. These differences may reflect long lasting alterations in brain development specific to individual with CHD.
Keywords: congenital heart disease, executive function, hippocampus, magnetic resonance imaging, neurodevelopment, self‐regulation
Abbreviations
- BMI
body mass index
- BRIEF‐A
Behavior Rating Inventory of Executive Function—Adult scale
- CHD
congenital heart disease
- FDR
false discovery rate
- IQ
intelligence quotient
- MRI
magnetic resonance imaging
- SES
socioeconomic status
- TBV
total brain volume
1. INTRODUCTION
With an estimated incidence of eight per 1,000 live births, congenital heart disease (CHD) is the most common neonatal malformation (Bernier, Stefanescu, Samoukovic, & Tchervenkov, 2010). Neonates born with complex CHD require open heart surgery with cardiopulmonary bypass within the first year of life (Costello et al., 2014). With advances in surgical techniques and neonatal care, upwards of 90% of babies born with CHD reach adulthood (Marelli, Miller, Marino, Jefferson, & Newburger, 2016). There is a high prevalence of neurodevelopmental disorders (20–60%) in individuals born with CHD (Latal, 2016; Marino et al., 2012). While the exact nature of these neurodevelopmental disorders remains to be fully understood, it is acknowledged that the brains of individuals with CHD are vulnerable to multiple factors that can interfere with its development. The brains of individuals with CHD are at high risk for injury from the cumulative effect of hemodynamic stress and brain dysmaturation that occur in utero to the high risk for hypoxic‐ischemia during the pre‐, intra‐, and post‐operative periods (Andropoulos et al., 2010; Brossard‐Racine et al., 2014; Dimitropoulos et al., 2013; Miller et al., 2007). The downstream impact of CHD on neural maturational trajectories in the adolescent and early adulthood period requires better characterization (Marelli et al., 2016). We have yet to understand whether the multiple early life risk factors have a persisting impact on their brain morphology 15–20 years after their open heart surgery. A recent meta‐analysis concluded that youth with CHD were 15.6 times more likely to present with a brain abnormality (based on neuroimaging findings) compared to healthy controls (Bolduc, Lambert, Ganeshamoorthy, & Brossard‐Racine, 2018). Even in the absence of brain abnormalities seen on conventional magnetic resonance images (MRI), many youths with CHD presented with worse neurocognitive outcomes when compared to healthy peers (von Rhein et al., 2014). While conventional MRI can identify overt brain abnormalities, it may not be sensitive enough to identify brain variation associated with neurodevelopmental impairments.
Lack of brain oxygen due to systemic hypoxemia or hemodynamic instability is a common complication of CHD in early life (Andropoulos et al., 2010; Donofrio et al., 2003; Licht et al., 2004). The hippocampus is particularly vulnerable to neonatal hypoxia‐ischemia, and is therefore of particular interest in the CHD population (Cooper et al., 2015). Associations between hippocampal volumes, morphometry and persisting learning difficulties have been shown in adolescents born preterm, another population at risk for neonatal hypoxia (Aanes, Bjuland, Skranes, & Lohaugen, 2015; Brunnemann et al., 2013; Cole et al., 2015; Thompson et al., 2013; Thompson et al., 2014; Tseng et al., 2017). The hippocampus has important roles in higher order cognitive processes including memory, learning and behavior (Catani, Dell'acqua, & Thiebaut de Schotten, 2013; Rajmohan & Mohandas, 2007), as well as, emotional perception and regulation (Phillips, Drevets, Rauch, & Lane, 2003). Additionally, the importance of the hippocampal circuitry in executive function is increasingly recognized (Khan et al., 2015; O'Brien, Lloyd, McKeith, Gholkar, & Ferrier, 2004; Wall & Messier, 2001). Impairments in executive function are frequent in adolescents with CHD (Calderon, 2016; Calderon & Bellinger, 2015; Jackson, Gerardo, Monti, Schofield, & Vannatta, 2018), and can manifest by behavioral dysregulation, attentional problems, lower working memory, and difficulties with organization and planning (Makowski et al., 2017). The few studies which have explored the hippocampal integrity in CHD have specifically focused on volumetric measurement and have reported nonspecific volume reduction associated with total intelligence quotient (IQ), working memory, and verbal comprehension (Latal et al., 2016; Munoz‐Lopez et al., 2017; von Rhein et al., 2014). However, evaluation of the hippocampus performed to date has been restricted to whole volume measurement only. Shape analysis has the potential to further identify structural differences that cannot be captured by gross volume measurement and has been shown to be an index that is sensitive to neurodevelopmental abnormalities (Achterberg et al., 2014; M. M. Chakravarty et al., 2015; McDermott et al., 2019; Schroeder et al., 2017).
Considering that comprehensive evaluation of the hippocampal is lacking in the CHD population, we used an approach involving a segmentation of the hippocampus into its subfields and surrounding white matter structure to evaluate volumetric and morphometric differences. The primary objective of this study was to compare hippocampal volumes and morphometry between youth with CHD and age‐ and sex‐matched healthy peers. A secondary objective was to explore whether the differences in hippocampal measurements would be associated with executive function and self‐regulation.
2. MATERIALS AND METHODS
2.1. Participants
We enrolled term born (>36 weeks gestational age) youth (aged 16–24 years) who underwent open heart surgery utilizing cardiopulmonary bypass for complex CHD before the age of two, and without documented congenital infection, chromosomal abnormalities and/or multiorgan dysmorphic conditions. Participants were first recruited from a previously completed study on determinants of leisure in adolescents, the Determinants of Active Involvement in Leisure for Youth (DAILY) study (Majnemer et al., 2017). Additionally, to achieve targetted sample size, we recruited eligible participants from the pediatric and the adult cardiology units affiliated with the McGill University Health Center (MUHC) using the same inclusion/exclusion criteria as for the DAILY sample.
A control group of age‐ and sex‐matched healthy participants was subsequently recruited through the local university, community fliers, and word of mouth. Controls were considered healthy if they had no history of brain tumor or malformation, traumatic brain injury, developmental or neurologic conditions and had not received rehabilitation or special education services (i.e., occupational therapy, psychology, speech therapy) during childhood or adolescence.
Participants in both groups needed to be proficient in English or French and be able to safely complete a brain MRI (e.g., no pacemakers or ferromagnetic implant). Written informed consent was obtained from the participants or legal guardians when younger than 18 years of age. All participants came to the Montreal Children's Hospital (MCH) at the MUHC for a one‐time study visit and underwent brain MRI and filled out questionnaires. Only newly enrolled CHD participants were administered an additional IQ assessment, as IQ scores obtained in the last 7 years were available for the DAILY participants. IQ was evaluated in participants from the DAILY study with the Leiter Brief IQ (Roid & Miller, 2011). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (first edition) for all newly enrolled CHD participants (Stano, 1999). Both IQ assessment tools are well validated and were chosen because they are short reliable tests of intellectual ability (Axelrod, 2002; Hooper & Bell, 2006; Ryan et al., 2003; Saklofske, Caravan, & Schwartz, 2000; Tsatsanis et al., 2003).We did not re‐assess youth from the DAILY as part of the current study because IQ is expected to remain stable from adolescence to adulthood (Hooper & Bell, 2006; Ryan et al., 2003; Saklofske et al., 2000). Considering that IQ was not a primary outcome in this study, presence of delay was dichotomized similarly using IQ < 70 as the cutoff value and the potential confounding effect of such delay was explored in our analyses. The study was approved by the MUHC Pediatric Research Ethics Board.
2.2. Individual and clinical variables
At the time of the study visit, height and weight were measured for all participants to calculate the body mass index (BMI). Socioeconomic status (SES) was evaluated using the Hollingshead Four‐Factor Index questionnaire (Hollingshead, 1975). CHD participants' medical records were reviewed to extract relevant clinical data surrounding the peri‐operative period including time of cardiopulmonary bypass, presence of deep hypothermic circulatory arrest, age at the first open heart surgery, number of catheterizations, and whether a balloon atrial septostomy was performed prior to the first surgery.
2.3. Executive function and self‐regulation
To assess executive function and self‐regulation, all participants completed the Behavior Rating Inventory of Executive Function—Adult scale (BRIEF‐A; Rouel, Raman, Hay, & Smith, 2016), a norm‐referenced questionnaire. This self‐report executive function measure includes 75 items from nine scales: inhibit, shift, emotional control, self‐monitor, initiate, working memory, plan/organize, task monitor, and organization of materials. Scores were considered clinically abnormal on the BRIEF‐A scales if T scores were greater than or equal to 65 (Roth & Gioia, 2005).
3. MRI
3.1. Image acquisition
All participants underwent a brain MRI on a clinical 3.0 Tesla MRI system (Achieva X‐Series, Philips Healthcare, Best, The Netherlands) using a 32‐channel head coil at the MCH‐MUHC. Three‐dimensional 1 mm isotropic anatomical T1‐weighted images were acquired (TE = 3.7 ms, TR = 8.1 ms, TI = 1,010 ms, shot interval = 3,000 ms, bandwidth = 191.4 Hz/pixel, FOV = 240 × 240 × 180 mm, slice thickness 1 mm, flip angle = 8°, acquisition time = 6 min 20 s). All images were subsequently reviewed by an experienced neuroradiologist (C.S.M.) blinded to the details of the participant's medical history.
3.2. Image analysis
All acquired T1‐weighted images underwent visual quality assessment and were excluded if excessive motion or scanner artifacts were observed. All raw images were then preprocessed using the minc‐bpipe‐library (https://github.com/CobraLab/minc-bpipe-library; Sadedin, Pope, & Oshlack, 2012; Vincent et al., 2016). This pipeline uses a “clean_and_center” stage to uniformize the direction of cosines and the zero‐point of the scan to the center of the image, followed by a bias field correction for contrast inhomogeneity using the N4ITK algorithm (Tustison et al., 2010), and a brain extraction step to isolate the brain from nonbrain tissues based on nonlocal segmentation technique (BEaST; Eskildsen et al., 2012). Following the brain extraction, a final step to crop all images to remove the neck completes the preprocessing. Total brain volume (TBV) estimates were acquired from the BEaST mask (Eskildsen et al., 2012). All T1‐weighted images were also processed with CIVET 2.1.0 to extract total cerebral gray and white matter volumes (Zijdenbos, Forghani, & Evans, 2002).
To delineate the hippocampal circuitry, all preprocessed images were subsequently postprocessed using the standard sequence with a well validated automatic segmentation pipeline, the Multiple Automatically Generated Templates for different brains (MAGeT‐Brain; https://github.com/CobraLab/MAGeTbrain; M. Mallar Chakravarty et al., 2013; Pipitone et al., 2014; Winterburn et al., 2013). The MAGeT‐Brain uses a multiatlas voting algorithm based on templates defined from a subset of images to produce a segmentation for each image. MAGeT‐Brain segmentation has been validated across many samples and was found to be highly accurate presenting with a Dice Similarity Coefficient of 0.892 when compared to manual segmentation in people aged 14–37 years with a first episode of psychosis (Pipitone et al., 2014). This pipeline further delineates the hippocampus into four subfields: Cornu Ammonis (CA) 1, CA2/CA3, CA4/dentate gyrus (DG) and stratum, as well as the surrounding white matter structures: alveus, fornix and fimbria. Figure 1 represents an example of a good hippocampal segmentation. Images were thereafter submitted to the morphometric branch of MAGeT‐Brain to yield vertex‐wise surface area maps for each segmentation. All computations were performed on the Scinet University of Toronto cluster (Chris et al., 2010). Outputs from the MAGeT‐Brain pipeline included hippocampal volumes, surface area and displacement measurements. All raw images and outputs were visually inspected for quality and segmentation accuracy prior to each step. Vertex‐wise morphometry analyses were conducted through the RMINC library in R (version 3.4.4; Lerch, 2014; R Core Team, 2013).
Figure 1.
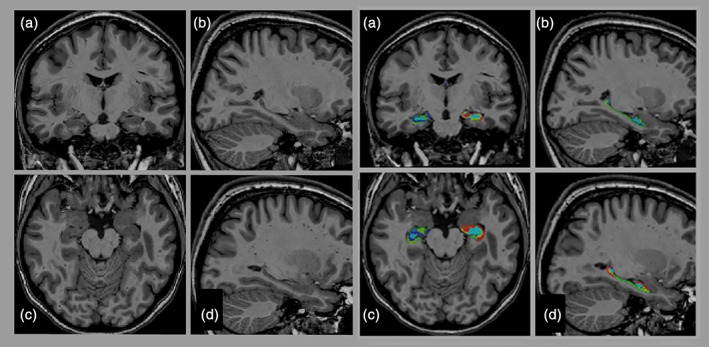
Segmentation of the hippocampus. T1‐weighted image of a female participant with CHD (21 years old) with delineation of the different hippocampal subfields: CA1 (red), subiculum (lime green), CA4/DG (miami turquoise), CA2/CA3 (light slate blue), stratum radiatum/stratum lacunosum/stratum moleculare (blue), in (a) coronal, (c) axial, (b, d) sagittal planes [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Statistical analysis
Descriptive statistics were first used to characterize the sample and outcomes for both groups. Shapiro–Wilk tests were then performed to assess if the variables were normally distributed. Subjects' characteristics were compared between groups using independent t‐tests or Mann–Whitney tests when appropriate for continuous variables and chi‐square tests for categorical variables. Variables significantly different between the two groups were considered as confounders and added to the subsequent models.
Hippocampal, total brain, total cerebral gray matter, and white matter volumetric differences between CHD and controls were assessed using general linear modeling, with group, TBV and SES as covariates. To illustrate the magnitude of the volumetric differences between the two groups, we also calculated the percentage of difference for each of the volumetric measurement using the following formula: Percentage of difference = (mean volume of controls − mean volume of CHD)/(mean volume of CHD) × 100. Morphometric differences were thereafter calculated also using general linear modeling also controlling for TBV and SES. We conducted post hoc significance testing with false discovery rate (FDR) for all general linear models to account for multiple comparisons. To additionally investigate associations between hippocampal volumes and morphometry with risk factors, such as CHD severity, IQ and other clinical variables, Spearman correlations were calculated in the CHD group. Analyses were thereafter rerun excluding the subjects with brain abnormalities to evaluate the potential effect of this variable. Thereafter, association between hippocampal volumes and the executive function scales that were identified to be significantly different between controls and CHD were explored conducting stepwise multiple linear regression analyses. The BRIEF‐A scale was entered as the dependent variable, while hippocampal volume was entered as the independent variable covarying for SES and TBV. To assess associations between hippocampal morphometry and executive function general linear modeling was used. Surface area and displacement models were run with group, TBV and SES in the model. These analyses were conducted in both groups (i.e., CHD and controls) independently. We conducted post hoc significance testing with FDR with a threshold of q < 0.10. Statistical analyses were performed using R (version 3.4.4; R Core Team, 2013).
4. RESULTS
4.1. Individual and clinical characteristics
We enrolled 23 participants with CHD from the DAILY study and 27 from the clinics. There were no significant differences with respect to age, sex, BMI and SES between the DAILY participants and the newly enrolled participants. Our complete sample consisted of 50 participants with CHD (21/50 male, mean age 20.0 years) and 48 controls (20/48 male, mean age 20.5 years). There were no significant differences with respect to age, sex and BMI between the CHD and controls. Only SES was significantly higher in controls compared to CHD. The participant characteristics are presented in Table 1.
Table 1.
Participants' characteristics
| Variables; mean (range), N (%) | CHD (n = 50) | Control (n = 48) | p value |
|---|---|---|---|
| Age at MRI, years | 20.02 (2.26) | 20.48 (1.99) | .289 |
| Sex | 1.00 | ||
| Male | 21 (42.0%) | 20 (41.67%) | |
| Female | 29 (58.0%) | 28 (58.33%) | |
| BMI | 23.45 (4.74) | 23.54 (3.87) | .906 |
| Socioeconomic statusa | 40.0 (12.85) | 49.91 (10.41) | <.001 |
| IQ < 70 | 2 (4.0%) | – | – |
| Type of CHD | |||
| Single ventricle | 12/50 (24.0%) | ||
| Tetralogy of Fallot | 11/50 (22.0%) | ||
| TGA | 18/50 (36.0%) | ||
| Other two ventricle | 9/50 (18.0%) | ||
| Number of open heart surgery per individual (n = 44) | 1.81 (1–4) | ||
| Age at first surgery, days (n = 42) | 92.89 (0–702) | ||
| Total bypass time, min (n = 36) | 138.15 (40–292) | ||
| Total aortic cross clamp time, min (n = 36) | 76.05 (0–162) | ||
| DHCA at first surgery (n = 35) | 23/39 (59.0%) | ||
| Catheterizations (n = 41) | 1.5 (0–5) | ||
| BAS before surgery (n = 41) | 15/42 (35.7%) |
Abbreviations: BMI, body mass index; DHCA, deep hypothermic circulatory arrest; IQ, intellectual quotient; TGA, transposition of the great arteries.
Score on the Hollingshead Four‐Factor Index of Social Status, with higher scores indicating higher social status.
Of the 50 youth with CHD, 38 (76.0%) presented with a biventricular cardiac physiology: d‐TGA (n = 18), Tetralogy of Fallot (n = 11), total anomalous pulmonary venous connection (n = 4), ventricular and atrial septal defects (n = 3) and truncus arteriosus type I (n = 2). Only 12/50 (24.0%) presented with a univentricular physiology: double outlet right ventricle (n = 4), pulmonary atresia (n = 4), double inlet left ventricle (n = 2), hypoplastic left heart syndrome (n = 1), and Ebstein's pulmonary atresia (n = 1). Youth with CHD had between one and four open heart surgeries (median 1.8) and between zero and five cardiac catheterizations (median 1.44) during their lifetime. Mean age at first surgery was 72.13 days old, with a range from zero to 353 days old.
4.2. Conventional MRI findings
No MRI image was rejected after quality assessment. Brain abnormalities detected by conventional MRI were more frequent in youth with CHD and were detected in 24.0% (12/50) of the CHD participants as compared to 10.4% (5/48) in the controls (χ 2 [1, n = 98] = 3.151, p = .065). Five participants had more than one abnormality: 8.0% (4/50) of the participants with CHD and 2.0% (1/48) of the controls. Overall, the brain abnormalities detected on conventional MRI were all considered to be mild and from a remote origin. Nevertheless, we further classified the observed abnormalities by type of origin between most likely developmental in origin (i.e., gray matter heterotopia, cortical and venous developmental anomalies, and Chiari I malformation) and most likely acquired in origin (i.e., space occupying lesion, periventricular white matter injuries, asymmetrical ventricles, enlarged perivascular spaces, and susceptibility artifacts). Acquired abnormalities (CHD; n = 15, controls; n = 3) were more frequent compared to developmental abnormalities (CHD; n = 7, controls; n = 3). The type of brain abnormalities detected in our sample is reported in Table 2. While we recognize that some of the minor abnormalities observed in the CHD group, such as venous abnormalities, asymmetrical ventricles and enlarged periventricular spaces, could be considered as normal developmental variants, the cross‐sectional nature of this study limits our ability to comment on their true origin. Considering their high prevalence in this clinical group at greater risk for brain abnormalities, we have considered the presence of these variants as potentially abnormal. There were no significant differences in sex, age, BMI or SES between subjects with and without a cerebral lesion, when comparing both within the control group and within the CHD group.
Table 2.
Conventional MRI results
| CHD (n = 50) n (%) | Control (n = 48) n (%) | |
|---|---|---|
| Normal brain MRI | 38 (76.0%) | 43 (89.6%) |
| Brain abnormalities most likely developmental in origin | ||
| Gray matter heterotopia | 3 (6.00%) | 1 (2.08%) |
| Cortical developmental anomaly | 1 (2.00%) | – |
| Developmental venous anomaly | 3 (6.00%) | 2 (4.17%) |
| Chiari 1 | 1 (2.00%) | – |
| Brain abnormalities most likely acquired in origin | ||
| Space occupying lesion | 1 (2.00%) | – |
| Periventricular white matter injury | 3 (6.00%) | – |
| Asymmetrical ventricles | 3 (6.00%) | 1 (2.08%) |
| Enlarged perivascular spaces | 4 (8.00%) | 1 (2.08%) |
| Focal rounded susceptibility artifact | 2 (4.00%) | – |
| Diffuse susceptibility artifacts | 2 (4.00%) | 1 (2.08%) |
| Encephalomalacia | 1 (2.00%) | – |
4.3. Hippocampal volumes
Both TBV and SES differed significantly between groups and correlated significantly with hippocampal volumes. These variables were therefore included in all the general linear models. Mean total cerebral gray matter and white matter volumes were smaller in youth with CHD, but the difference did not reach statistical significance when controlling for TBV and SES. Youth with CHD had significantly smaller total hippocampal volume (F[1, 94] = 15.36, q < 0.001), shown in Figure 2. The subfield segmentation further revealed that youth with CHD presented with significantly smaller volumes (q < 0.05) in all regions (CA1, subiculum, CA4/DG, stratum radiatum/stratum lacunosum/stratum moleculare and in the right CA2/CA3, as well as, in the surrounding white matter (left fimbria and bilateral fornix). Only the bilateral alveus and the right fimbria did not show a significant volumetric difference. Total and regional hippocampal volumes, as well as percentage of volumetric difference between groups, are reported in Table 3.
Figure 2.
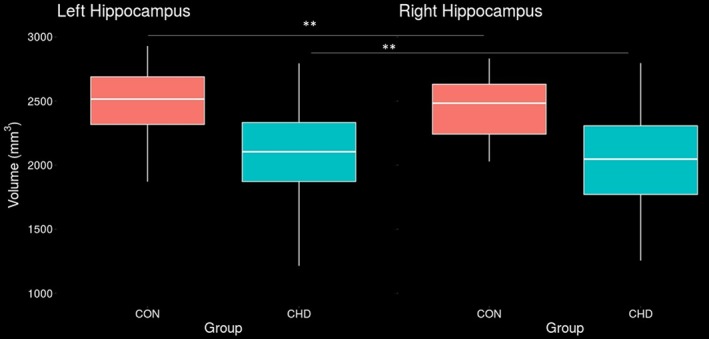
A general linear model controlling for total brain volume, sex and age indicated robust significant volumetric differences bilaterally between youth with CHD and healthy controls. Youth with CHD had significantly smaller volumes than controls in the left hippocampus (left panel), difference = 16.6%, p < .001, and right hippocampus (right panel), difference = 17.2%, p < .001 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Total and regional brain volumes differences between CHD and controls
| Variables volumes in mm3 controlling for TBV and SES (SD) | CHD (n = 50) | Control (n = 48) | % difference | q value |
|---|---|---|---|---|
| TBV (105) | 12.13 (1.46) | 13.30 (1.19) | 8.80 | <0.001 |
| Total white matter (104) | 38.68 (6.02) | 44.70 (5.13) | 13.46 | 0.022 |
| Total cerebral gray matter (104) | 52.16 (5.86) | 56.28 (4.90) | 7.32 | 0.385 |
| Right CA1 | 679.87 (128.95) | 819.18 (93.96) | 17.01 | 0.002 |
| Right subiculum | 312.36 (61.65) | 370.71 (47.50) | 15.74 | 0.01 |
| Right CA4/DG | 493.49 (99.99) | 599.75 (67.86) | 17.72 | 0.001 |
| Right CA2/CA3 | 127.60 (28.94) | 153.81 (28.84) | 17.04 | 0.012 |
| Right stratuma | 420.57 (86.43) | 514.06 (69.64) | 18.19 | 0.001 |
| Left CA1 | 675.96 (129.39) | 797.38 (94.52) | 15.23 | 0.012 |
| Left subiculum | 279.06 (53.83) | 325.34 (38.30) | 14.23 | 0.044 |
| Left CA4/DG | 500.74 (101.23) | 620.97 (66.57) | 19.36 | <0.001 |
| Left CA2/CA3 | 130.15 (26.78) | 158.55 (27.30) | 17.91 | <0.001 |
| Left stratuma | 499.24 (98.32) | 598.78 (69.85) | 16.62 | 0.002 |
| Right fimbria | 108.33 (21.50) | 124.58 (15.15) | 13.04 | 0.061 |
| Left fimbria | 103.34 (18.63) | 122.06 (16.84) | 15.34 | 0.004 |
| Right fornix | 501.57 (97.50) | 606.23 (53.67) | 17.26 | <0.001 |
| Left fornix | 453.11 (79.66) | 537.46 (50.92) | 15.69 | <0.001 |
| Right alveus | 270.20 (47.70) | 309.24 (38.98) | 12.62 | 0.061 |
| Left alveus | 360.77 (64.26) | 413.37 (56.38) | 12.72 | 0.068 |
Abbreviations: CA, cornu ammonis; DG, dentate gyrus; SES, socioeconomic status; TBV, total brain volume.
Includes stratum radiatum/stratum lacunosum/stratum moleculare.
In youth with CHD, use of deep hypothermic circulatory arrest at first open heart surgery was associated with smaller total hippocampal volumes independently of TBV, age and type of CHD (r[39] = −0.371, p = .020). This association was present bilaterally. When examining age at first open heart surgery as a dichotomous variable, participants who had surgery after 1 month of age, were associated with smaller hippocampal volumes than those who had surgery earlier (r[46] = −0.321, p = .029). Hippocampal volume was not associated with any other individual or clinical variables, including with brain abnormalities detected on conventional MRI.
4.4. Hippocampal morphometry
Significant vertex‐wise shape differences were found between the two groups when controlling for SES and TBV. Overall, youth with CHD had significantly smaller surface area (F[3, 94] ≥ 2.72, q < 0.05) bilaterally and inward displacement on the left hippocampus (F[3, 94] ≥ 3.44, q < 0.10) compared to controls. Deformations were concentrated bilaterally to the ventral side of the hippocampus. Significant differences in local surface area for left and right hippocampus are presented in Figure 3, while regions with significant displacement differences are presented in Figure 4. No clinical variables were found to significantly contribute to the shape analysis modeling.
Figure 3.
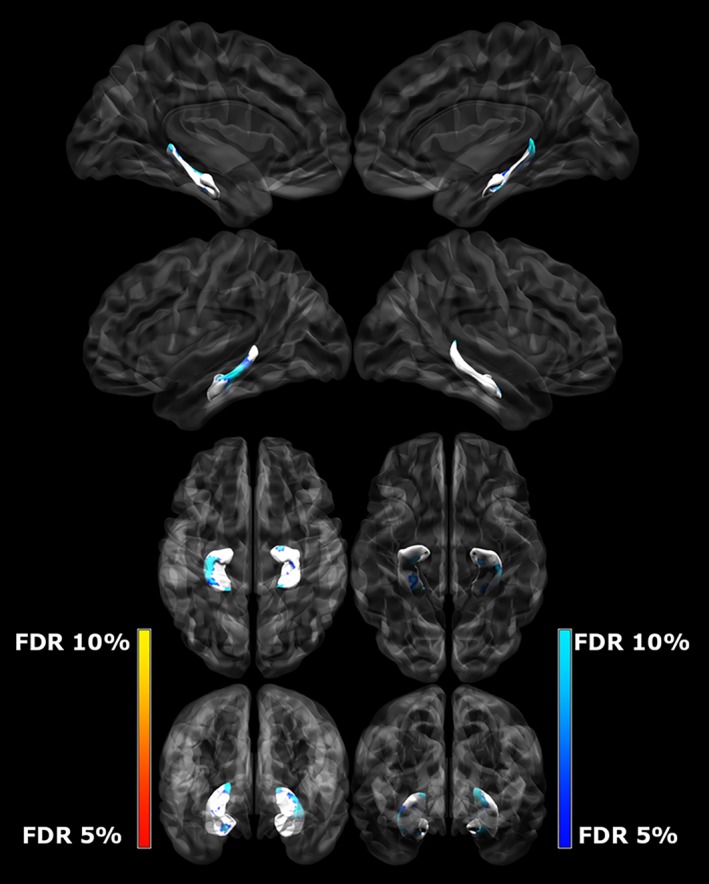
Differences between left and right hippocampal surface area (mm2) and group, controlling for TBV and SES. The darker blue colors indicate regions of significantly smaller surface area (more concave) in CHD subjects when compared to controls (q < 0.10) [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
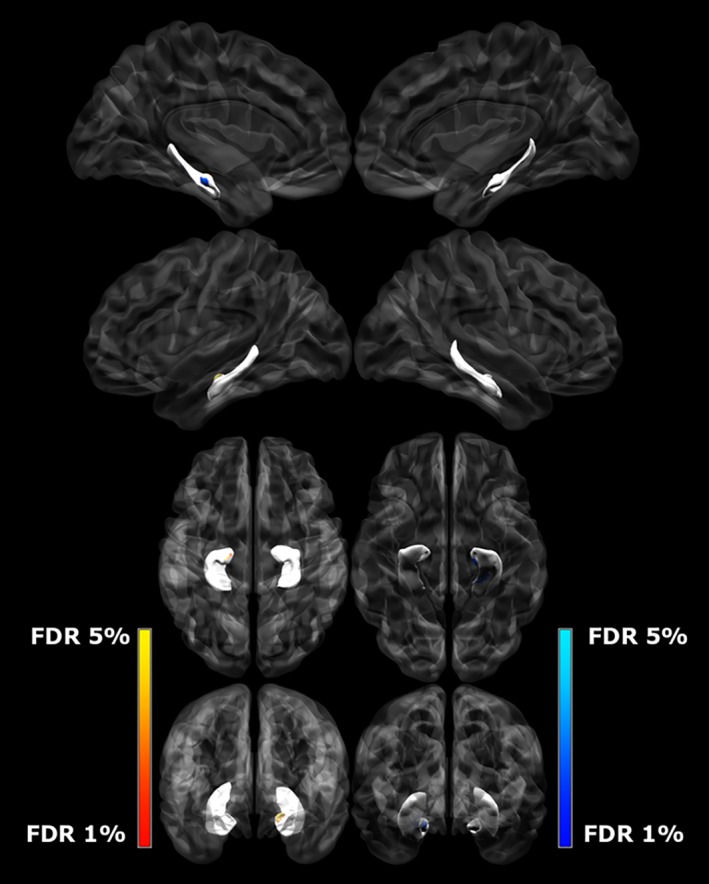
Differences between left hippocampal displacement (mm) and group, controlling for TBV and SES. The darker blue colors indicate regions significant inward displacement in CHD subjects when compared to controls (q < 0.05) [Color figure can be viewed at http://wileyonlinelibrary.com]
4.5. Executive function and self‐regulation
Two CHD participants and one control participant did not complete the entire BRIEF‐A questionnaire and were excluded from these analyses. Youth with CHD presented with significantly worse on six of the nine scales of the BRIEF‐A (U ≤ 843.5, p < .05) when compared to controls. There were no significant differences between groups on the Initiate, Task Monitor and Shift scales. The differences in mean scores obtained on the different scales are reported in Table 4. Overall a greater percentage of youths with CHD performed below clinical cutoff than control (10.4–33.3% in CHD vs. 0–10.8% in controls) reaching statistical difference for the inhibit, self‐monitor, working memory and organization of material scales (Table 5).
Table 4.
BRIEF‐A mean score differences between CHD and controls
| Mean (SD) | CHD (n = 48) | Control (n = 47) | p value |
|---|---|---|---|
| Inhibit | 54.85 (12.7) | 49.38 (8.7) | .018 |
| Shift | 53.15 (10.4) | 49.30 (8.5) | .052 |
| Emotional control | 56.00 (11.1) | 48.70 (10.7) | .001 |
| Self‐monitor | 54.44 (13.4) | 45.47 (8.5) | <.001 |
| Initiate | 54.85 (10.5) | 50.94 (10.0) | .072 |
| Working memory | 57.88 (11.7) | 52.64 (9.7) | .030 |
| Plan/organize | 54.54 (9.8) | 50.19 (8.1) | .026 |
| Task monitor | 56.31 (11.5) | 52.72 (10.8) | .089 |
| Organization of materials | 55.13 (12.4) | 46.15 (9.2) | <.001 |
Note. Higher scores on the BRIEF‐A indicate worse functioning.
Table 5.
Percentage of participants with a T score ≥65 (abnormal) on the BRIEF‐A scales for CHD and controls
| n (%) | CHD (n = 48) | Control (n = 47) | p value |
|---|---|---|---|
| Inhibit | 12 (25.0) | 0 (0) | <.001 |
| Shift | 5 (10.4) | 1 (2.1) | .204 |
| Emotional control | 11 (22.9) | 4 (8.5) | .089 |
| Self‐monitor | 11 (22.9) | 2 (4.3) | .014 |
| Initiate | 7 (14.6) | 5 (10.6) | .759 |
| Working memory | 16 (33.3) | 6 (12.8) | .027 |
| Plan/organize | 8 (16.7) | 4 (8.5) | .355 |
| Task monitor | 11 (22.9) | 6 (12.8) | .285 |
| Organization of materials | 12 (25.0) | 1 (2.1) | .002 |
In the CHD group, only smaller volumes in the left CA1, and left CA2/3 were significantly associated with worse working memory scores and are presented in Table 6. This association was not present in the control group. None of the individual or clinical characteristics were significantly associated with executive function. Furthermore, the addition of age sex, SES, TBV, and total hippocampal volume did not contribute to the models explored. There were no significant associations when running the general linear models for hippocampal surface area and displacement, and any of the executive function scales.
Table 6.
Stepwise multiple linear regression models of hippocampal volumes with executive function and self‐regulation in youth with CHD
| BRIEF‐A scale | Independent variables | Corrected R 2 | Beta | p value |
|---|---|---|---|---|
| Working memory | Left CA1 | .082 | −0.318 | .028 |
| Working memory | Left CA2/CA3 | .096 | −0.339 | .018 |
Note. Linear regression analyses (dependent variable: functional outcomes). Variables included: total hippocampal volume, total brain volume, SES, sex, and age.
5. DISCUSSION
This study used advanced structural MRI techniques to characterize hippocampal shape and volume in youth with CHD. We found that youth with CHD had smaller total and nonspecific regional hippocampal volumes and in the surrounding white matter, (right fimbria and the bilateral fornix) bilaterally, when compared to healthy peers of the same age. Consistent with our hypothesis, we found that the hippocampus is indeed vulnerable to volume loss in youth with CHD. Moreover, we found that hippocampal volume differences were more pronounced than differences in overt total cerebral gray matter and total white matter, suggesting a specific hippocampal vulnerability. It is important to recognize that the observed hippocampal alterations may be part of a more complex picture of brain abnormalities that have not been captured in this focused and hypothesis driven study. Future study combining hippocampal measurements with advanced quantitative evaluation of cortical and subcortical structural integrity will help to portray a comprehensive assessment of the at‐risk brain in this population.
Our results are congruent with the literature in children and younger adolescents with CHD which similarly reported smaller hippocampal volumes compared to healthy peers, and hippocampal difference of greater magnitude when compared to total gray matter volumes (Latal et al., 2016; Munoz‐Lopez et al., 2017; Watson, Stopp, Wypij, Newburger, & Rivkin, 2017). Our findings are also in line with animal models of hypoxia which suggest that many of the structural changes observed in the hippocampus could be the result of an early life injury (Rees, Harding, & Walker, 2008). Only Watson et al. (2016) did not find significant differences in hippocampal volumes in adolescents with d‐TGA evaluated at a mean age of 16 years, which might have been caused by their choice of hippocampal delineation arising from a whole brain region of interest cortical parcellation rather than a hypothesis driven approach (Watson et al., 2016). Taken together, ours and the findings of others suggest that the hippocampal volume loss observed in children with CHD persists into late adolescence and early adulthood. Nevertheless, without longitudinal data, the evolution of these findings remains speculative as only cross‐sectional group differences have been examined to date as opposed to differences in neurodevelopmental trajectories.
The hippocampal alterations reported in our study were not associated with the presence of brain abnormalities as detected by conventional MRI. Mild brain abnormalities were detected in 24% of the CHD group which is consistent with previous studies performed in similar cohorts (Bolduc et al., 2018). Per their nature, the observed brain abnormalities in our cohort are thought to be sequelae of early life injury in neonates with CHD. Brain injury in neonates has been extensively documented; however, the link between these injuries and later neurodevelopmental outcomes is less well understood (Peyvandi, Latal, Miller, & McQuillen, 2018). Thus, while conventional MRI provides insight into the nature and frequency of more severe brain abnormalities, it does not capture the subtle structural brain alterations that could underlie many of the neurodevelopment deficits observed in this population. Only one previous study has reported brain abnormalities to be associated with smaller hippocampal volumes (Latal et al., 2016). Our lack of agreement with this study likely resides in part in the difference in the type of brain abnormality evaluation and classification. We choose to use an inclusive classification that is capturing the minor abnormalities often considered as normal variants in the absence of other health condition because we considered that their high prevalence in a clinical group may be indicative of true abnormality. Nevertheless, this inclusive classification has also enabled us to report greater proportion of minor brain abnormalities in the control group than typically reported in the literature, which may have limited our ability to detect association with overt brain lesion.
The only clinical factor found to be associated with smaller hippocampal volume in our study was hypothermic circulatory arrest time at first surgery. Prolonged circulatory arrest has also been linked to increased adverse neurodevelopmental outcomes in infants and children with CHD (McQuillen & Miller, 2010). More importantly, hypothermic circulatory arrest time is a potentially modifiable risk factor and our findings support that surgical management should be optimized to minimize prolonged arrest time. Interestingly, when looking at age at first open heart surgery as a continuous variable, we did not find an association with hippocampal volumes. When exploring age as a dichotomous variable, we found that those operated after a 1‐month cutoff had smaller hippocampal volumes than those who had surgery earlier. Similar studies found that risk factors for reduced gray matter volumes included older age at first surgery in SV heart disease and d‐TGA adolescents (Watson et al., 2016; Watson et al., 2017). These findings suggest that operating earlier is beneficial for later hippocampal growth. A possible explanation for this is that infants who undergo surgery later after birth endure abnormal hemodynamics for a longer period and the restoration of circulation and oxygen delivery to the brain is later which may contribute to the prolonged brain maldevelopment. The number of missing clinical variables collected due to retrospective nature of the chart review may have limited our ability to identify other significant associations with other risk factors. However, recent research suggests that variables surrounding first cardiac surgery are less important at predicting neurodevelopmental outcomes in infants compared to innate patient factors and environmental factors (Gaynor et al., 2015; Hovels‐Gurich, 2016; McQuillen & Miller, 2010).
We report significant differences in hippocampal morphometry between CHD and controls marked by smaller surface area and inward displacement bilaterally predominantly on the ventral side of the hippocampus in the CHD group that were not present in controls. Our findings further suggest that volumetric measures alone may not be sufficient to detect changes in brain structures (M. M. Chakravarty et al., 2015). Shape analyses have the potential to explain structural differences such as bending, flattening or local differences which may provide biologically meaningful information about the development of the hippocampus (M. M. Chakravarty et al., 2015). To our knowledge, no study has explored hippocampal morphometry in youth with CHD. Nevertheless, we found similarities between our findings and the morphometric analyses performed in very preterm children and adolescents. It has been suggested before that the brain's maldevelopment and injury pattern, as well as, the neurodevelopmental outcomes profile in preterm children are comparable to children with CHD (Back & Miller, 2014; Easson et al., 2019; McQuillen & Miller, 2010; Miller et al., 2007; Miller & Ferriero, 2009; Miller & McQuillen, 2007). In very preterm children, the hippocampus has been described to be less inverted when compared to term children imaged at a similar corrected age (Thompson et al., 2013). This correspond to a less arched hippocampus along the inferior–superior axis in very preterm children which is comparable to the inward displacement and a smaller surface area we observed in youth with CHD in the current study. Another study exploring hippocampal morphometry in very preterm adolescents, found that at 19 years of age, there were significant surface contractions when compared to healthy peers (Cole et al., 2015). In the very preterm adolescents, the deformations were concentrated mainly in the tail, subiculum and CA1 of the hippocampus bilaterally (Cole et al., 2015). In our sample of CHD, we found greater areas of deformation predominantly in the tail of the hippocampus (i.e., the CA1 and the subiculum) which converges with the findings reported in the very preterm literature and is suggestive of similar delayed or disrupted hippocampal development (Thompson et al., 2013).
Youth with CHD in our study had significantly worse executive function and self‐regulation than healthy controls. We found that the percentage of participants with CHD with clinically significant scores was significantly higher than the controls for the inhibit, self‐monitor, working memory, and organization of materials scales. The BRIEF‐A self‐report questionnaire has been shown to be valid and reliable for measuring executive function. However, it is important to recognize that the self‐report scoring often underestimates difficulties compared to the informant report scoring, therefore, the difficulties reflected here may actually be underrepresented (Steward, Tan, Delgaty, Gonzales, & Bunner, 2017). Future work seeking to expand on this research might consider adding parent or teacher reports of the BRIEF‐A as well as objective performance‐based measures of executive function for a more complete evaluation. Our results are concurrent with previous reports which suggest that impairments in executive function are frequent in adolescents with CHD (Bellinger et al., 2011; Bellinger et al., 2015; Cassidy, White, DeMaso, Newburger, & Bellinger, 2015).
Previous studies in young adolescents with CHD, have found associations between whole hippocampal volumes and total IQ, verbal comprehension and working memory (Latal et al., 2016; Munoz‐Lopez et al., 2017; von Rhein et al., 2014). Although we did not find association between total hippocampal volume and neurocognition, we found that smaller left CA1 volume and left CA2/3 volume were independently associated with worse working memory ability in youth with CHD. Our ability to detect other structure–function relationships in our sample may have been limited by the self‐reported format of our executive function measure. As mentioned, although the BRIEF‐A is a well validated tool, it may have provided a narrower range of responses than formal executive function testing. Nevertheless, by delineating the hippocampal subfields, we reported structure–function relationships that were not captured by total hippocampal volume. This finding suggests that whole hippocampus segmentation may not be precise enough to detect subtle differences in structure–function relationships. In other clinical populations where executive function is thought to be impaired, MRI studies have revealed differential susceptibility of the hippocampus (Achterberg et al., 2014; Bartsch & Wulff, 2015; Haukvik et al., 2014; Makowski et al., 2017). For instance, studies have found that in youth with ADHD smaller hippocampal volumes correlated with indices of inattentiveness and impulsivity (Al‐Amin, Zinchenko, & Geyer, 2018) and that in mood disorders, subfield volumes were associated with illness progression (Cao et al., 2017).
Previous research suggests that the hippocampal subfields are highly interconnected and that their difference in structure and connectivity may contribute to distinctive stages of memory processing (Duvernoy, 2005). We found that the left hippocampal subfields CA1 and CA2/3 were associated with working memory in youth with CHD. The CA1 is a subfield which has been identified as a region of increased vulnerability in the case of prolonged and short‐term ischemia (Petito, Feldmann, Pulsinelli, & Plum, 1987; Schmidt‐Kastner & Freund, 1991). Subfield specialization research has proposed that the CA3 and DG are associated with early recall and CA1 with consolidation and retrieval of visual information (Mueller, Chao, Berman, & Weiner, 2011; Zammit et al., 2017). Further hypothesis‐driven studies are needed to better understand the subfield specialization as it pertains to working memory.
Finally, our approach was able to detect major morphometric changes; however, it may have been conservative in identifying associations between subtle variations and function. As this was an exploratory analysis, we examined differences between groups across the whole surface of the hippocampus on a vertex‐wise level, instead of using a region of interest approach. This remains the best technique for exploring morphometric group differences in vivo (Cole et al., 2015). Similarly, while we did find some associations between hippocampal volumes and executive function scales, the strength of our association was modest. Additional variance may be explained indirectly by the hippocampal functional connectivity and should be investigated by future multimodal imaging studies combining structural and functional MRI.
5.1. Limitations
Our sample is composed of a mixed cohort of CHD diagnoses and therefore, the finding cannot be generalized to a specific subtype. Nevertheless, the fact that our sample was not limited to the most complex forms of CHD and included a representative range of cardiac diagnoses is a strength as it accurately represents the clinical diversity in CHD. While we did our best to extract all relevant clinical variables in our sample, considering that many of our participants had surgery more than 15 years ago, and before the digitization of the medical data, some of the clinical information could not be retrieved. Additionally, in the absence of peri‐operative brain MRI, our interpretation of the origin of the observed brain abnormalities remains limited considering the cross‐sectional design of the study. Finally, a caveat with our comparison group is the higher SES, caused by the increased interest from healthy volunteers coming from the local universities; however, we took this limitation into account and included this variable in all our analyses.
6. CONCLUSION
This study provides the first evidence for morphometric differences in the hippocampus of CHD youth compared to controls. Our findings also suggest that adolescents and young adults with CHD present deficits in executive function which may be related to differences in hippocampal volumes. Specifically, we have highlighted the importance of delineating the hippocampus into its structural units, as we identified structure–function relationships between hippocampal subfields and working memory. Future studies should focus on using advanced structural MRI to better understand the relationship between structural brain differences and functional deficits in youth with CHD.
CONFLICT OF INTERESTS
Guillaume Gilbert is an employee of Philips Healthcare. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the youth and their families for their participation, and the clinicians, technologists and research assistants for their implication in this study.
At the time of the study, K.F. received studentship support from McGill University's Faculty of Medicine Internal Studentship Award, given as: Joseph Schubert Memorial Award & Jeannette and Abram Victor Fellowship Award and the Research Institute of the McGill University Health Centre—Desjardins Studentship in Child Health Research.
Computations were performed on the Niagara supercomputer at the SciNet HPC Consortium. SciNet is funded by: the Canada Foundation for Innovation under the auspices of Compute Canada; the Government of Ontario; Ontario Research Fund—Research Excellence; and the University of Toronto.
Fontes K, Rohlicek CV, Saint‐Martin C, et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum Brain Mapp. 2019;40:3548–3560. 10.1002/hbm.24615
Funding information Faculty of Medicine, McGill University; Research Institute of the McGill University Health Centre, Montreal Children's Hospital Foundation
REFERENCES
- Aanes, S. , Bjuland, K. J. , Skranes, J. , & Lohaugen, G. C. (2015). Memory function and hippocampal volumes in preterm born very‐low‐birth‐weight (VLBW) young adults. NeuroImage, 105, 76–83. 10.1016/j.neuroimage.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Achterberg, H. C. , van der Lijn, F. , den Heijer, T. , Vernooij, M. W. , Ikram, M. A. , Niessen, W. J. , & de Bruijne, M. (2014). Hippocampal shape is predictive for the development of dementia in a normal, elderly population. Human Brain Mapping, 35(5), 2359–2371. 10.1002/hbm.22333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Amin, M. , Zinchenko, A. , & Geyer, T. (2018). Hippocampal subfield volume changes in subtypes of attention deficit hyperactivity disorder. Brain Research, 1685, 1–8. 10.1016/j.brainres.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Andropoulos, D. B. , Hunter, J. V. , Nelson, D. P. , Stayer, S. A. , Stark, A. R. , McKenzie, E. D. , … Fraser, C. D., Jr. (2010). Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high‐flow bypass and cerebral oxygenation monitoring. The Journal of Thoracic and Cardiovascular Surgery, 139(3), 543–556. 10.1016/j.jtcvs.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, B. N. (2002). Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment, 9(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Back, S. A. , & Miller, S. P. (2014). Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Annals of Neurology, 75(4), 469–486. 10.1002/ana.24132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch, T. , & Wulff, P. (2015). The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience, 309, 1–16. 10.1016/j.neuroscience.2015.07.084 [DOI] [PubMed] [Google Scholar]
- Bellinger, D. C. , Rivkin, M. J. , DeMaso, D. , Robertson, R. L. , Stopp, C. , Dunbar‐Masterson, C. , … Newburger, J. W. (2015). Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiology in the Young, 25(2), 338–347. 10.1017/s1047951114000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger, D. C. , Wypij, D. , Rivkin, M. J. , DeMaso, D. R. , Robertson, R. L., Jr. , Dunbar‐Masterson, C. , … Newburger, J. W. (2011). Adolescents with d‐transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation, 124(12), 1361–1369. 10.1161/circulationaha.111.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier, P. L. , Stefanescu, A. , Samoukovic, G. , & Tchervenkov, C. I. (2010). The challenge of congenital heart disease worldwide: Epidemiologic and demographic facts. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual, 13(1), 26–34. 10.1053/j.pcsu.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Bolduc, M. E. , Lambert, H. , Ganeshamoorthy, S. , & Brossard‐Racine, M. (2018). Structural brain abnormalities in adolescents and young adults with congenital heart defect: A systematic review. Developmental Medicine and Child Neurology, 60(12), 1209–1224. 10.1111/dmcn.13975 [DOI] [PubMed] [Google Scholar]
- Brewster, R. C. , King, T. Z. , Burns, T. G. , Drossner, D. M. , & Mahle, W. T. (2015). White matter integrity dissociates verbal memory and auditory attention span in emerging adults with congenital heart disease. Journal of the International Neuropsychological Society, 21(1), 22–33. 10.1017/s135561771400109x [DOI] [PubMed] [Google Scholar]
- Brossard‐Racine, M. , du Plessis, A. J. , Vezina, G. , Robertson, R. , Bulas, D. , Evangelou, I. E. , … Limperopoulos, C. (2014). Prevalence and spectrum of in utero structural brain abnormalities in fetuses with complex congenital heart disease. AJNR. American Journal of Neuroradiology, 35(8), 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnemann, N. , Kipp, K. H. , Gortner, L. , Meng‐Hentschel, J. , Papanagiotou, P. , Reith, W. , & Shamdeen, M. G. (2013). Alterations in the relationship between hippocampal volume and episodic memory performance in preterm children. Developmental Neuropsychology, 38(4), 226–235. 10.1080/87565641.2013.773003 [DOI] [PubMed] [Google Scholar]
- Calderon, J. (2016). Executive function in patients with congenital heart disease: Only the tip of the iceberg? The Journal of Pediatrics, 173, 7–9. 10.1016/j.jpeds.2016.02.066 [DOI] [PubMed] [Google Scholar]
- Calderon, J. , & Bellinger, D. C. (2015). Executive function deficits in congenital heart disease: Why is intervention important? Cardiology in the Young, 25(7), 1238–1246. 10.1017/s1047951115001134 [DOI] [PubMed] [Google Scholar]
- Cao, B. , Passos, I. C. , Mwangi, B. , Amaral‐Silva, H. , Tannous, J. , Wu, M. J. , … Soares, J. C. (2017). Hippocampal subfield volumes in mood disorders. Molecular Psychiatry, 22(9), 1352–1358. 10.1038/mp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy, A. R. , White, M. T. , DeMaso, D. R. , Newburger, J. W. , & Bellinger, D. C. (2015). Executive function in children and adolescents with critical cyanotic congenital heart disease. Journal of the International Neuropsychological Society, 21(1), 34–49. 10.1017/s1355617714001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Dell'acqua, F. , & Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience and Biobehavioral Reviews, 37(8), 1724–1737. 10.1016/j.neubiorev.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Chakravarty, M. M. , Rapoport, J. L. , Giedd, J. N. , Raznahan, A. , Shaw, P. , Collins, D. L. , … Gogtay, N. (2015). Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: A longitudinal study. Human Brain Mapping, 36(4), 1458–1469. 10.1002/hbm.22715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty, M. M. , Steadman, P. , van Eede, M. C. , Calcott, R. D. , Gu, V. , Shaw, P. , … Lerch, J. P. (2013). Performing label‐fusion‐based segmentation using multiple automatically generated templates. Human Brain Mapping, 34(10), 2635–2654. 10.1002/hbm.22092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chris, L. , Daniel, G. , Leslie, G. , Richard, P. , Neil, B. , Michael, C. , … Ramses Van, Z. (2010). SciNet: Lessons learned from building a power‐efficient Top‐20 system and data centre. Journal of Physics: Conference Series, 256(1), 012026. [Google Scholar]
- Cole, J. H. , Filippetti, M. L. , Allin, M. P. , Walshe, M. , Nam, K. W. , Gutman, B. A. , … Nosarti, C. (2015). Subregional hippocampal morphology and psychiatric outcome in adolescents who were born very preterm and at term. PLoS One, 10(6), e0130094 10.1371/journal.pone.0130094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. M. , Gadian, D. G. , Jentschke, S. , Goldman, A. , Munoz, M. , Pitts, G. , … Vargha‐Khadem, F. (2015). Neonatal hypoxia, hippocampal atrophy, and memory impairment: Evidence of a causal sequence. Cerebral Cortex, 25(6), 1469–1476. 10.1093/cercor/bht332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello, J. M. , Pasquali, S. K. , Jacobs, J. P. , He, X. , Hill, K. D. , Cooper, D. S. , … Jacobs, M. L. (2014). Gestational age at birth and outcomes after neonatal cardiac surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation, 129(24), 2511–2517. 10.1161/circulationaha.113.005864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos, A. , McQuillen, P. S. , Sethi, V. , Moosa, A. , Chau, V. , Xu, D. , … Miller, S. P. (2013). Brain injury and development in newborns with critical congenital heart disease. Neurology, 81(3), 241–248. 10.1212/WNL.0b013e31829bfdcf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio, M. T. , Bremer, Y. A. , Schieken, R. M. , Gennings, C. , Morton, L. D. , Eidem, B. W. , … Kleinman, C. S. (2003). Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatric Cardiology, 24(5), 436–443. 10.1007/s00246-002-0404-0 [DOI] [PubMed] [Google Scholar]
- Duvernoy, H. M. (2005). The human hippocampus. Berlin/Heidelberg: Springer‐Verlag. [Google Scholar]
- Easson, K. , Dahan‐Oliel, N. , Rohlicek, C. , Sahakian, S. , Brossard‐Racine, M. , Mazer, B. , … Majnemer, A. (2019). Comparison of developmental outcomes of adolescent NICU survivors born with a congenital heart defect or born preterm. The Journal of Pediatrics, 207, 34–41.e2. [DOI] [PubMed] [Google Scholar]
- Eskildsen, S. F. , Coupé, P. , Fonov, V. , Manjón, J. V. , Leung, K. K. , Guizard, N. , … Collins, D. L. (2012). BEaST: Brain extraction based on nonlocal segmentation technique. NeuroImage, 59(3), 2362–2373. 10.1016/j.neuroimage.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Gaynor, J. W. , Stopp, C. , Wypij, D. , Andropoulos, D. B. , Atallah, J. , Atz, A. M. , … Newburger, J. W. (2015). Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics, 135(5), 816–825. 10.1542/peds.2014-3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik, U. K. , McNeil, T. , Lange, E. H. , Melle, I. , Dale, A. M. , Andreassen, O. A. , & Agartz, I. (2014). Pre‐ and perinatal hypoxia associated with hippocampus/amygdala volume in bipolar disorder. Psychological Medicine, 44(5), 975–985. 10.1017/s0033291713001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead, A. B. (1975). Four factor index of social status New Haven, CT: Yale University. [Google Scholar]
- Hooper, V. S. , & Bell, S. M. (2006). Concurrent validity of the universal nonverbal intelligence test and the Leiter International Performance scale—Revised. Psychology in the Schools, 43(2), 143–148. [Google Scholar]
- Hovels‐Gurich, H. H. (2016). Factors influencing neurodevelopment after cardiac surgery during infancy. Frontiers in Pediatrics, 4, 137 10.3389/fped.2016.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, J. L. , Gerardo, G. M. , Monti, J. D. , Schofield, K. A. , & Vannatta, K. (2018). Executive function and internalizing symptoms in adolescents and young adults with congenital heart disease: The role of coping. Journal of Pediatric Psychology, 43, 906–915. 10.1093/jpepsy/jsx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, S. A. , Ryali, V. , Bhat, P. S. , Prakash, J. , Srivastava, K. , & Khanam, S. (2015). The hippocampus and executive functions in depression. Industrial Psychiatry Journal, 24(1), 18–22. 10.4103/0972-6748.160920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latal, B. (2016). Neurodevelopmental outcomes of the child with congenital heart disease. Clinics in Perinatology, 43(1), 173–185. 10.1016/j.clp.2015.11.012 [DOI] [PubMed] [Google Scholar]
- Latal, B. , Patel, P. , Liamlahi, R. , Knirsch, W. , O'Gorman Tuura, R. , & von Rhein, M. (2016). Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatric Research, 80(4), 531–537. 10.1038/pr.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch, J. (2014). Voxel‐wise morphometry using RMINC (version 3.4.4).
- Licht, D. J. , Wang, J. , Silvestre, D. W. , Nicolson, S. C. , Montenegro, L. M. , Wernovsky, G. , … Detre, J. A. (2004). Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery, 128(6), 841–849. 10.1016/j.jtcvs.2004.07.022 [DOI] [PubMed] [Google Scholar]
- Majnemer, A. , Dahan‐Oliel, N. , Rohlicek, C. , Hatzigeorgiou, S. , Mazer, B. , Maltais, D. B. , & Schmitz, N. (2017). Educational and rehabilitation service utilization in adolescents born preterm or with a congenital heart defect and at high risk for disability. Developmental Medicine and Child Neurology, 59(10), 1056–1062. 10.1111/dmcn.13520 [DOI] [PubMed] [Google Scholar]
- Makowski, C. , Bodnar, M. , Shenker, J. J. , Malla, A. K. , Joober, R. , Chakravarty, M. M. , & Lepage, M. (2017). Linking persistent negative symptoms to amygdala‐hippocampus structure in first‐episode psychosis. Translational Psychiatry, 7(8), e1195 10.1038/tp.2017.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli, A. , Miller, S. P. , Marino, B. S. , Jefferson, A. L. , & Newburger, J. W. (2016). Brain in congenital heart disease across the lifespan: The cumulative burden of injury. Circulation, 133(20), 1951–1962. 10.1161/circulationaha.115.019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, B. S. , Lipkin, P. H. , Newburger, J. W. , Peacock, G. , Gerdes, M. , Gaynor, J. W. , … Mahle, W. T. (2012). Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation, 126(9), 1143–1172. 10.1161/CIR.0b013e318265ee8a [DOI] [PubMed] [Google Scholar]
- McDermott, C. , Seidlitz, J. , Nadig, A. , Liu, S. , Clasen, L. , Blumenthal, J. , … Raznahan, A. (2019). Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. The Journal of Neuroscience, 39(8), 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen, P. S. , & Miller, S. P. (2010). Congenital heart disease and brain development. Annals of the New York Academy of Sciences, 1184, 68–86. 10.1111/j.1749-6632.2009.05116.x [DOI] [PubMed] [Google Scholar]
- Miller, S. P. , & Ferriero, D. M. (2009). From selective vulnerability to connectivity: Insights from newborn brain imaging. Trends in Neurosciences, 32(9), 496–505. 10.1016/j.tins.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. P. , & McQuillen, P. S. (2007). Neurology of congenital heart disease: Insight from brain imaging. Archives of Disease in Childhood. Fetal and Neonatal Edition, 92(6), F435–F437. 10.1136/adc.2006.108845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. P. , McQuillen, P. S. , Hamrick, S. , Xu, D. , Glidden, D. V. , Charlton, N. , … Vigneron, D. B. (2007). Abnormal brain development in newborns with congenital heart disease. The New England Journal of Medicine, 357(19), 1928–1938. 10.1056/NEJMoa067393 [DOI] [PubMed] [Google Scholar]
- Mueller, S. G. , Chao, L. L. , Berman, B. , & Weiner, M. W. (2011). Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4T. NeuroImage, 56(3), 851–857. 10.1016/j.neuroimage.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz‐Lopez, M. , Hoskote, A. , Chadwick, M. J. , Dzieciol, A. M. , Gadian, D. G. , Chong, K. , … Vargha‐Khadem, F. (2017). Hippocampal damage and memory impairment in congenital cyanotic heart disease. Hippocampus, 27(4), 417–424. 10.1002/hipo.22700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, J. T. , Lloyd, A. , McKeith, I. , Gholkar, A. , & Ferrier, N. (2004). A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. The American Journal of Psychiatry, 161(11), 2081–2090. 10.1176/appi.ajp.161.11.2081 [DOI] [PubMed] [Google Scholar]
- Petito, C. K. , Feldmann, E. , Pulsinelli, W. A. , & Plum, F. (1987). Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology, 37(8), 1281–1286. [DOI] [PubMed] [Google Scholar]
- Peyvandi, S. , Latal, B. , Miller, S. P. , & McQuillen, P. S. (2018). The neonatal brain in critical congenital heart disease: Insights and future directions. NeuroImage, 185, 776–782. 10.1016/j.neuroimage.2018.05.045 [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54(5), 515–528. [DOI] [PubMed] [Google Scholar]
- Pipitone, J. , Park, M. T. , Winterburn, J. , Lett, T. A. , Lerch, J. P. , Pruessner, J. C. , … Chakravarty, M. M. (2014). Multi‐atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. NeuroImage, 101, 494–512. 10.1016/j.neuroimage.2014.04.054 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2013). R: A language and environment for statistical computing (version 3.4.4).
- Rajmohan, V. , & Mohandas, E. (2007). The limbic system. Indian Journal of Psychiatry, 49(2), 132–139. 10.4103/0019-5545.33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, S. , Harding, R. , & Walker, D. (2008). An adverse intrauterine environment: Implications for injury and altered development of the brain. International Journal of Developmental Neuroscience, 26(1), 3–11. 10.1016/j.ijdevneu.2007.08.020 [DOI] [PubMed] [Google Scholar]
- Roid, G. H. , & Miller, L. J. (2011). Leiter international performance scale‐revised (Leiter‐R). Madrid: Psymtec. [Google Scholar]
- Roth, R. M. , & Gioia, G. A. (2005). Behavior Rating Inventory of Executive Function—Adult version. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Rouel, M. , Raman, J. , Hay, P. , & Smith, E. (2016). Validation of the behaviour rating inventory of executive function—Adult version (BRIEF‐A) in the obese with and without binge eating disorder. Eating Behaviors, 23, 58–65. 10.1016/j.eatbeh.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Ryan, J. J. , Carruthers, C. A. , Miller, L. J. , Souheaver, G. T. , Gontkovsky, S. T. , & Zehr, M. D. (2003). Exploratory factor analysis of the Wechsler abbreviated scale of intelligence (WASI) in adult standardization and clinical samples. Applied Neuropsychology, 10(4), 252–256. [DOI] [PubMed] [Google Scholar]
- Sadedin, S. P. , Pope, B. , & Oshlack, A. (2012). Bpipe: A tool for running and managing bioinformatics pipelines. Bioinformatics, 28(11), 1525–1526. 10.1093/bioinformatics/bts167 [DOI] [PubMed] [Google Scholar]
- Saklofske, D. H. , Caravan, G. , & Schwartz, C. (2000). Concurrent validity of the Wechsler abbreviated scale of intelligence (WASI) with a sample of Canadian children. Canadian Journal of School Psychology, 16(1), 87–94. [Google Scholar]
- Schmidt‐Kastner, R. , & Freund, T. F. (1991). Selective vulnerability of the hippocampus in brain ischemia. Neuroscience, 40(3), 599–636. [DOI] [PubMed] [Google Scholar]
- Schroeder, C. , Park, M. T. M. , Germann, J. , Chakravarty, M. M. , Michels, L. , Kollias, S. , … Leh, S. E. (2017). Hippocampal shape alterations are associated with regional Abeta load in cognitively normal elderly individuals. The European Journal of Neuroscience, 45(10), 1241–1251. 10.1111/ejn.13408 [DOI] [PubMed] [Google Scholar]
- Stano, J. (1999). Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Steward, K. A. , Tan, A. , Delgaty, L. , Gonzales, M. M. , & Bunner, M. (2017). Self‐awareness of executive functioning deficits in adolescents with ADHD. Journal of Attention Disorders, 21(4), 316–322. 10.1177/1087054714530782 [DOI] [PubMed] [Google Scholar]
- Thompson, D. K. , Adamson, C. , Roberts, G. , Faggian, N. , Wood, S. J. , Warfield, S. K. , … Inder, T. E. (2013). Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: Perinatal predictors and functional significance at age 7. NeuroImage, 70, 278–287. 10.1016/j.neuroimage.2012.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, D. K. , Omizzolo, C. , Adamson, C. , Lee, K. J. , Stargatt, R. , Egan, G. F. , … Anderson, P. J. (2014). Longitudinal growth and morphology of the hippocampus through childhood: Impact of prematurity and implications for memory and learning. Human Brain Mapping, 35(8), 4129–4139. 10.1002/hbm.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis, K. D. , Dartnall, N. , Cicchetti, D. , Sparrow, S. S. , Klin, A. , & Volkmar, F. R. (2003). Concurrent validity and classification accuracy of the Leiter and Leiter‐R in low‐functioning children with autism. Journal of Autism and Developmental Disorders, 33(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Tseng, C. J. , Froudist‐Walsh, S. , Brittain, P. J. , Karolis, V. , Caldinelli, C. , Kroll, J. , … Nosarti, C. (2017). A multimodal imaging study of recognition memory in very preterm born adults. Human Brain Mapping, 38(2), 644–655. 10.1002/hbm.23405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison, N. J. , Avants, B. B. , Cook, P. A. , Zheng, Y. , Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/tmi.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, R. D. , Neelin, P. , Khalili‐Mahani, N. , Janke, A. L. , Fonov, V. S. , Robbins, S. M. , … Evans, A. C. (2016). MINC 2.0: A flexible format for multi‐modal images. Frontiers in Neuroinformatics, 10, 35 10.3389/fninf.2016.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rhein, M. , Buchmann, A. , Hagmann, C. , Huber, R. , Klaver, P. , Knirsch, W. , & Latal, B. (2014). Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain, 137(Pt. 1), 268–276. 10.1093/brain/awt322 [DOI] [PubMed] [Google Scholar]
- Wall, P. M. , & Messier, C. (2001). The hippocampal formation—Orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behavioural Brain Research, 127(1–2), 99–117. [DOI] [PubMed] [Google Scholar]
- Watson, C. G. , Asaro, L. A. , Wypij, D. , Robertson, R. L., Jr. , Newburger, J. W. , & Rivkin, M. J. (2016). Altered gray matter in adolescents with d‐transposition of the great arteries. The Journal of Pediatrics, 169, 36–43.e31. 10.1016/j.jpeds.2015.09.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, C. G. , Stopp, C. , Wypij, D. , Newburger, J. W. , & Rivkin, M. J. (2017). Reduced cortical volume and thickness and their relationship to medical and operative features in post‐Fontan children and adolescents. Pediatric Research, 81(6), 881–890. 10.1038/pr.2017.30 [DOI] [PubMed] [Google Scholar]
- Winterburn, J. L. , Pruessner, J. C. , Chavez, S. , Schira, M. M. , Lobaugh, N. J. , Voineskos, A. N. , & Chakravarty, M. M. (2013). A novel in vivo atlas of human hippocampal subfields using high‐resolution 3 T magnetic resonance imaging. NeuroImage, 74, 254–265. 10.1016/j.neuroimage.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Zammit, A. R. , Ezzati, A. , Zimmerman, M. E. , Lipton, R. B. , Lipton, M. L. , & Katz, M. J. (2017). Roles of hippocampal subfields in verbal and visual episodic memory. Behavioural Brain Research, 317, 157–162. 10.1016/j.bbr.2016.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos, A. P. , Forghani, R. , & Evans, A. C. (2002). Automatic “pipeline” analysis of 3‐D MRI data for clinical trials: Application to multiple sclerosis. IEEE Transactions on Medical Imaging, 21(10), 1280–1291. 10.1109/tmi.2002.806283 [DOI] [PubMed] [Google Scholar]


