Abstract
Behavioral and developmental studies have made a critical distinction between item and serial order processing components of verbal working memory (WM). This functional magnetic resonance imaging (fMRI) study determined the extent to which item and serial order WM components are characterized by specialized neural networks already in young children or whether this specialization emerges at a later developmental stage. Total of 59 children aged 7–12 years performed item and serial order short‐term probe recognition tasks in an fMRI experiment. While a left frontoparietal network was recruited in both item and serial order WM conditions, the right intraparietal sulcus was selectively involved in the serial order WM condition. This neural segregation was modulated by age, with both networks becoming increasingly separated in older children. Our results indicate a progressive specialization of networks involved in item and order WM processes during cognitive development.
Keywords: development, neuroimaging, serial order, short‐term memory, working memory
1. INTRODUCTION
The neural networks associated with the development of working memory (WM) have recently received considerable research interest. WM capacity matures quickly during childhood and is a critical determinant of other cognitive abilities such as learning of oral and written language, mathematical cognition, or problem solving (Gathercole, Pickering, Ambridge, & Wearing, 2004). At the neural level, the recruitment of a large frontoparietal network has been observed in several verbal and visuospatial WM tasks similarly to the network engaged in adults (Ciesielski, Lesnik, Savoy, Grant, & Ahlfors, 2006; Klingberg, Forssberg, & Westerberg, 2002; Scherf, Sweeney, & Luna, 2006; Siffredi et al., 2017). Numerous studies suggest that improvements in WM capacity are associated with a progressive maturation of white and gray matter in a frontoparietal network (see, e.g., Darki & Klingberg, 2014). At the functional level, increased recruitment of right dorsolateral prefrontal and bilateral parietal cortices is also observed from childhood to adolescence (Bunge & Wright, 2007; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006). At the same time, these activation differences appear to be at least partly related to task difficulty: when matching WM task difficulty for children and adults, developmental differences in frontoparietal cortex activation are strongly diminished (Kharitonova, Winter, & Sheridan, 2015).
The studies conducted so far, however, did not consider a crucial distinction between two aspects of short‐term storage: the processing of item information (i.e., the identity of the items with their phonological and semantic characteristics), on the one hand, and the processing of order information (i.e., the serial order in which the items have been presented), on the other hand. These aspects have been shown to rely on distinct neural networks in healthy adults and can be selectively impaired in patients with brain lesions (Kalm & Norris, 2014; Majerus et al., 2006; Majerus, Attout, Artielle, & Van der Kaa, 2015). The aim of this study is to achieve a more refined understanding of the developmental neural correlates of short‐term storage abilities by focusing specifically on item and serial order WM aspects and by determining their degree of neural specialization at different developmental stages. We will determine whether specialized item and serial order WM networks are already observed in young children or whether this distinction emerges at a later developmental stage, possibly as a result of metacognitive and strategic processes such as a scanning or a chunking strategy. It is important to note here that we used the term “WM” in a generic manner by considering that it applies to all short‐term storage tasks, whether they involve additional processing requirements or not (see Cowan, 2016, for a recent discussion of this issue). This study focused mainly on the maintenance aspect of WM tasks.
At the behavioral level, it has been shown that the distinction between item and order verbal WM processes is crucial for understanding the links between verbal WM abilities and cognitive development in various domains (Attout, Noël, & Majerus, 2014; Leclercq & Majerus, 2010; Martinez Perez, Majerus, & Poncelet, 2012; Ordonez Magro, Attout, Majerus, & Szmalec, 2018). Several previous behavioral and neuropsychological studies in adults already supported this dissociation between item and order verbal WM by showing for example selective influence of psycholinguistic variables on each process and specific deficits in brain damaged patients or patients with semantic dementia (Attout, Van der Kaa, George, & Majerus, 2012; Majerus, Norris, & Patterson, 2007). This distinction has important implications at the theoretical level by proposing a new verbal WM model, the Attentional‐Order‐Short‐term memory (A‐O‐STM) model where item WM is considered as a temporary activation of the phonological and lexico‐semantic language system and order WM as an independent component dedicated to process the serial order of phonemes/words (Majerus, 2009). In children, a distinction between item and serial order WM processes is supported by a series of developmental and neurodevelopmental studies. Specific alterations in serial order WM abilities, as opposed to item WM abilities, have been observed in genetic syndromes such as 22q11.2 microdeletion syndrome or Down syndrome (Brock & Jarrold, 2005; Majerus, Glaser, Van der Linden, & Eliez, 2006; Majerus, Van der Linden, Braissand, & Eliez, 2007) as well as in learning disorders such as dyslexia or dyscalculia (Attout & Majerus, 2014; De Visscher, Szmalec, Van Der Linden, & Noël, 2015; Martinez Perez, Majerus, Mahot, & Poncelet, 2012), and this for both verbal and visuospatial material. Developmental studies have also shown that estimates of serial order WM capacity (in the verbal modality) are particularly robust predictors of lexical, reading, spelling, and calculation abilities, as compared to estimates of item WM (Attout et al., 2014; Binamé & Poncelet, 2016; Leclercq & Majerus, 2010; Majerus & Boukebza, 2013; Majerus, Poncelet, Greffe, & Van der Linden, 2006; Martinez Perez, Majerus, & Poncelet, 2012; Ordonez Magro et al., 2018). These studies highlight the importance of distinguishing between item and serial order WM processes, both in order to understand the nature of WM impairment in specific neurodevelopmental populations as well as in order to understand the functional role of WM abilities in cognitive development.
As regards the neural substrates associated with item and serial order WM components, neuroimaging studies in adult participants have highlighted distinct as well as overlapping neural networks for item and serial order WM. Both item and serial order WM tasks, in the verbal and visuospatial modality, have been associated with a left hemisphere dominant frontoparietal network centered around the left intraparietal sulcus (IPS), while serial order WM has been associated more specifically with a right frontoparietal network centered on the right IPS (Henson, Burgess, & Frith, 2000; Majerus et al., 2010; Majerus, Poncelet, Van der Linden, et al., 2006; Marshuetz, Reuter‐Lorenz, Smith, Jonides, & Noll, 2006; Marshuetz, Smith, Jonides, DeGutis, & Chenevert, 2000). The left frontoparietal network has been proposed to support attentional control during WM tasks as it overlaps with the dorsal attention pathway. It is observed both in verbal and visuospatial WM tasks, and it is sensitive to WM load in both modalities (Majerus, Péters, Bouffier, Cowan, & Phillips, 2018; Todd & Marois, 2004). The right IPS involvement in serial order WM tasks has been associated with temporal and spatial serial order coding processes (Majerus et al., 2007; Majerus et al., 2010; van Dijck & Fias, 2011). For verbal item WM conditions, additional neural recruitment has been observed in areas associated with linguistic processing (see Friederici & Alter, 2004 for a review) such as the bilateral temporal gyrus (superior temporal and fusiform gyrus) and the left inferior parietal cortex (supramarginal cortex).
Currently, our knowledge about the development of the functional neural architecture of WM remains restricted to WM tasks that do not distinguish between item and serial order components of WM. Most neuroimaging studies in children observed a large frontoparietal network recruitment in several verbal and visuospatial WM tasks (Ciesielski et al., 2006; Klingberg et al., 2002; Scherf et al., 2006; Siffredi et al., 2017), and this in children as young as 5 years of age (Thomason et al., 2009). Furthermore, activity of the IPS and prefrontal cortex has been shown to increase with age (from 7 to 22 years old) in parallel to the increase of behavioral performance levels (Crone et al., 2006; Kwon, Reiss, & Menon, 2002). Most of these results stem from studies that used storage and processing tasks, making it difficult to distinguish neural substrates involved in core WM aspects such as temporary storage from those involved in task manipulation and further associated with executive control (Engle & Kane, 2004). The few studies focusing on storage processes were mostly in the visuospatial domain and showed age‐related differences, with an increase of activations in the parietal network and to some extent in the frontal areas (Kharitonova et al., 2015; Klingberg et al., 2002; Spencer‐Smith et al., 2013; Thomason et al., 2009). One study focusing on verbal maintenance and recognition via a modified Sternberg item recognition task (Van den Bosch et al., 2014) demonstrated similar results with an increase in the left motor area and right cerebellum, left prefrontal, and left parietal cortex activations when they considered age as a continuous variable. However, no study so far has specifically investigated the neural aspects that support the development of serial order versus item WM.
The aim of this study was to achieve a deeper understanding of the developmental neural substrates of WM storage abilities, by making the critical distinction between item and serial order storage abilities. In 59 children aged from 7‐to‐12 years, we used a functional magnetic resonance imaging (fMRI) study design to examine whether item and serial order WM components are characterized by specialized neural networks already in young children or whether this specialization emerges progressively with age. This last prediction may come from the implication of more general metacognitive processes. Children performed two probe recognition tasks focusing either on the storage of item or serial‐order information. The tasks were adaptations of paradigms already used in adult populations for exploring the neural substrates associated with item and serial order WM processes (Majerus, Bastin, et al., 2007; Majerus, Poncelet, Van der Linden, et al., 2006; Marshuetz et al., 2000).
2. METHODS
2.1. Participants
Total of 59 right‐handed children from second to sixth grade participated in the study. All parents declared that their children were native French speakers and had no history of neurological disorder, sensory impairment, or learning difficulties. Families received a 20 euros gift card for their participation. Data from two participants were excluded because of excessive movement in the scanner (i.e., see criteria below) and one participant was excluded due to floor‐level task performance (i.e., accuracy lower than .50 in each position of the item WM task, corresponding to chance level performance). The data from 56 participants (29 girls and 27 boys) were included in the analyses (mean age = 9.16 years old, range = 6.7–12.2 years old). Then, 13 participants were in second grade, 14 in third grade, 8 in fourth grade, 4 in fifth grade, and 13 in sixth grade.
Given the uneven distribution of age across participants, we also explored age effects by contrasting the children from 6 to 8 versus those from 9 to 12 years old, leading to two age groups with N = 20 for the younger group and N = 24 for the older group. The study has been approved by the ethics committee of the Faculty of Medicine at the University of Liège. In line with the Declaration of Helsinki, each parent and child gave their written informed consent prior to inclusion in the study.
2.2. Task description
The experiment assessed item and serial order WM capacities by using item and serial order probe recognition tasks. After the experimental WM tasks, children completed a second, independent task (Hebb learning task) reported elsewhere. In the serial order probe recognition condition, an auditory sequence of four words (one item per second) was presented and was followed, after a maintenance delay of variable duration, by the auditory presentation of two items of the memory list. The child had to decide whether the two words were presented in the same order as in the memory list. For positive and negative probe trials, items from adjacent serial positions were presented in order to probe memory for fine‐grained serial order representations. The item probe recognition condition had the same design as the serial order probe recognition task, except for the probe trials which consisted of the presentation of twice the same items (in order to match the amount of sensory information presented for the item and serial order probes). The probe items either matched one of the words in the list (positive probe) or differed by a single phoneme exchange (negative probe). The task setup was based on the tasks previously used to assess the neural substrates of item and serial order WM in adults (Henson, Hartley, Burgess, Hitch, & Flude, 2003; Majerus et al., 2008; Majerus et al., 2010; Majerus, Poncelet, Van der Linden, et al., 2006).
The WM tasks are illustrated in Figure 1. The memory list stimuli were presented at the speed of one item per second (corresponding to the encoding phase), followed by a maintenance phase during which a fixation cross was displayed for a variable duration (random Gaussian distribution with a mean duration of 3,000 ± 1,000 ms). A final screen with a question mark at the center marked the beginning of the probe recognition phase. Children had to respond within 7,000 ms whether or not the probe words were matching the target information in the memory list, by pressing the left button for “yes” responses or the right button for “no” responses. A scratch with a soft tissue was applied on the left button in order to avoid some confusion of the left/right dimension by the child and order stipulated that the response “yes” corresponded to this soft button. The different trials were separated by an intertrial interval of 3,500 ± 250 ms (random Gaussian distribution). A relatively short duration has been chosen in order to keep the child focused between trials and to shorten the total duration of the fMRI experiment). For each condition, baseline trials allowed to isolate neural substrates associated with auditory sensory processing and with general recognition and decision processes. The baseline trials consisted of the presentation of four identical words, a delay and a response phase in which the same word was represented twice in either standard or backward auditory format. The children had to decide whether the probe word was the same or not as in the list. The item and serial order conditions were presented using a blocked design in order to avoid difficulties in switching between item and serial order response modes. The blocks were presented in pseudorandom order. Both conditions were presented on a workstation running MATLAB 12 and the Cogent toolbox (UCL, http://www.vislab.ucl.ac.uk/cogent.php) for stimulus presentation. The auditory stimuli were presented via a high‐quality headset (Serene Sound System, Resonance Technology Inc., Northridge, CA).
Figure 1.
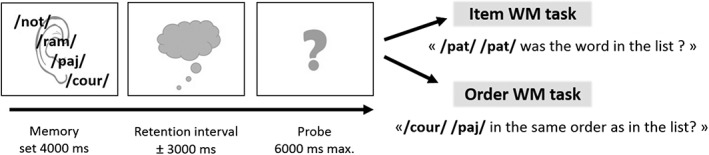
Experimental design and timing of the two WM tasks. For each condition, a negative probe trial is illustrated
The words had been sampled from a pool of 30 pairs of unisyllabic words that differed by the second consonant (e.g., “puce”‐“pull”‐“route”‐“rouge” translated in English as “flea”‐“sweater”‐“road”‐“red”). This enabled us to increase the difficulty of the item WM conditions, maximizing the retention of item information by constructing negative probes that differed only very minimally from the target word. Mean lexical frequency was matched within the minimal word pairs: for the first and second words of the pairs, mean lexical frequency was 70.38 (range 2.47–672) and 60.99 (range 0.77–453.13), respectively (Lexique3 database; New, Pallier, & Ferrand, 2005). For constructing the memory lists, the stimuli were pseudorandomly sampled from the stimulus set of 60 words, by ensuring that words from the same minimal pair did not co‐occur in the same memory list and that the different words appeared equally often in all serial positions. For the serial order probe recognition task, the probe trials always contained two adjacent words of the target stimulus list, but they were either from the first and second position (10 trials), from the second and third position (eight trials) or, from the third and the last position (10 trials) of the memory list. For the item probe recognition task, the target of the probe items were in the first (eight trials), second (six trials), third (six trials), or fourth (eight trials) position of the memory list. Therefore, eight baseline trials were included in each block. Each block included 36 trials (28 target and 8 baseline trials). There were an equal number of positive and negative probe trials, and all serial positions were probed equally often.
2.3. Procedure
A first practice session outside the scanner was organized 1 week before the fMRI experiment. During this practice session, children completed several tests assessing their reading, mathematical, and nonverbal intellectual abilities. These tests will be used elsewhere but allow us here to check and confirm that all children obtained scores in the normal range for their age or school level. The fMRI environment and procedure was also explained in details with pictures and with a book describing a space story. The children also practiced the WM tasks for the upcoming fMRI session. The tasks were further adapted by presenting each task as a game, the whole fMRI experiment being presented as journey on a space shuttle with the child playing the role of an astronaut. The fMRI session started with the administration of at least four practice trials outside the scanner. All participants demonstrated sufficient understanding of the WM tasks when being placed in the scanner. To minimize head motion, children were trained outside the scanner to not move their head and cushions were inserted around their head to fill the gap between it and the coil.
2.4. MRI acquisition
fMRI time series were acquired on a whole‐body 3T scanner (MAGNETOM Prisma; Siemens Medical Solutions, Erlangen, Germany) operated with a 20‐channel receiver head coil. Multislice T2*‐weighted functional images were acquired with the multiband gradient‐echo echo‐planar imaging sequence (CMRR, University of Minnesota) using axial slice orientation and covering the whole brain (32 slices, multiband factor = 2, FoV = 192 × 192 mm2, voxel size 3 × 3 × 3 mm3, 25% interslice gap, matrix size 64 × 64 × 32, TR = 978 ms, TE = 30 ms, FA = 90°). The five initial volumes were discarded to avoid T1 saturation effects. A gradient‐recalled sequence was applied to acquire two complex images with different echo times (TE = 10.00 and 12.46 ms, respectively) and generate field maps for distortion correction of the echo‐planar images (EPIs) (TR = 634 ms, FoV = 192 × 192 mm2, 64 × 64 matrix, 40 transverse slices [3 mm thickness, 25% interslice gap], flip angle = 90°, bandwidth = 260 Hz/pixel). For anatomical reference, a high‐resolution T1‐weighted image was acquired for each subject (T1‐weighted 3D magnetization‐prepared rapid gradient echo sequence, TR = 1900 ms, TE = 2.19 ms, inversion time (TI) = 900 ms, FoV = 256 × 240 mm2, matrix size = 256 × 240 × 224, voxel size = 1 × 1 × 1 mm3). For the item WM task, between 506 and 611 functional volumes were acquired (M = 554.78, SD = 19.29) while for order WM tasks, between 529 and 617 functional volumes were acquired (M = 574.88, SD = 21.62). The stimuli were displayed on a screen positioned at the rear of the scanner, which the participant could comfortably see via a head coil mounted mirror.
2.5. fMRI analyses
The functional images were preprocessed and analyzed using SPM12 software (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (MathWorks, Inc., Sherborn, MA). The MNI template was used for normalization; the use of this template has been shown not to significantly change the localization of neural activity foci in children (Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003). EPI time series were corrected for motion and distortion using the Realign and Unwarp with default settings functions together with the FieldMap toolbox (implemented in SPM12) (Andersson, Hutton, Ashburner, Turner, & Friston, 2001; Hutton et al., 2002). A mean realigned functional image was then calculated by averaging all the realigned and unwarped functional scans and the structural T1 image was coregistered to this mean functional image (using a rigid body transformation optimized to maximize the normalized mutual information between the two images). The mapping from subject to MNI space was estimated from the structural image with the “unified segmentation” approach (Ashburner & Friston, 2005). The warping parameters were then separately applied to the functional and structural images to produce normalized images of resolution 2 × 2 × 2 mm3 and 1 × 1 × 1 mm3, respectively. Finally, the warped functional images were spatially smoothed with a Gaussian kernel of 6 mm FWHM. ArtRepair was used to remove residual motion from the functional images prior to normalization (Mazaika, Hoeft, Glover, & Reiss, 2009). Volumes with rapid scan‐to‐scan movements greater than 1.5 mm were repaired by interpolation of the two nearest nonrepaired scans. Each trial with more than 15% of the total number of volumes replaced was removed from the analyses. The mean number of repaired scans was 2.45 ± 3.38%.
For each participant, brain responses were estimated at each voxel, using a general linear model with epoch regressors and event‐related regressors. For the WM tasks, regressors were defined to cover encoding, maintenance, and retrieval phases. Encoding and maintenance phases were modeled via a single regressor due to the short duration of the encoding phase leading to high autocorrelation between these two phases. Thus, the possible shared variance between the retrieval phase and the late encoding/maintenance phases was attributed to the retrieval regressor. The encoding/maintenance regressor ranged from the onset of each trial until 2000 ms after the last word presented of the encoding phase. For the WM retrieval stage, the regressor ranged from the onset of the probe display to the participant's response. On this basis, two linear contrasts were obtained for each task. The baseline trials were modeled implicitly. More precisely, at the first level, the model for each WM task refers to the following design: y = b1x1 + b2x2 + e with y = voxel response, b1 and x1 = beta and regressor for the encoding/maintenance phase, b2 and x2 = beta and regressor for the recognition phase, and e = the unmodeled variance including the baseline trials.
The resulting contrast images were then entered in second‐level analyses, corresponding to random effects models: y = b1x1 + b2x2 + b3x3 + b4x4 + e (order WM encoding/maintenance + order WM recognition + item WM encoding/maintenance + item WM recognition + error). For each model, the design matrix also included the realignment parameters to account for any residual movement‐related effect. A high‐pass filter was implemented using a cutoff period of 128 s in order to remove the low‐frequency drifts from the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model of order 1 (+white noise). Statistical inferences were performed at the cluster level at p < .05, with family‐wise error (FWE) corrections for multiple comparisons across the entire brain volume; a cluster‐forming threshold of p < .001 uncorrected was used in order to reduce false positives (Eklund, Nichols, & Knutsson, 2016). For the region of interest (ROI) analyses, the threshold was defined at p < .05, with small volume corrections based on Gaussian random field theory. We considered activations with a minimum extent threshold of 15 voxels.
2.6. ROI analysis
To further investigate potential task differences in brain activation and age‐brain correlations, we extracted ROIs using the anatomical WFU PickAtlas Toolbox (Wake Forrest University 312 PickAtlas, http://fmri.wfubmc.edu/cms/software). The selection of ROIs was guided by focusing on functional activity foci that had been reported earlier to be involved in order and item WM tasks in adults (Asplund, Todd, Snyder, & Marois, 2010; Gillebert et al., 2012; Majerus et al., 2010; Majerus, Bastin, et al., 2007; Majerus, Poncelet, Van der Linden, et al., 2006; Marshuetz et al., 2000; Marshuetz & Bates, 2004) as well as in more general WM tasks in children (Siffredi et al., 2017). We created two ROIs, a posterior ROI including parietal areas of interest and an anterior ROI including frontal areas of interest. The parietal ROI included the three parts of the IPS, the anterior [±43, −40, 43], the middle [±34, −49, 45], and the posterior [±26, −60, 41]. The frontal ROI included the bilateral superior fontal gyrus [24, 16, 56; −22, 5, 55, the bilateral middle frontal gyrus [46, 36, 22; −44, 24, 32], the bilateral inferior frontal gyrus [42, 12, 22; −54, 6, 18], and the SMA [−3, 8, 54]. We applied a sphere of 10 mm around the coordinates of interest and then regrouped the different ROIs in a single ROI mask in order to reduce the likelihood of false positives by increasing the number of voxels used for FWE correction.
3. RESULTS
3.1. Behavioral performance
Behavioral results are shown in Figure 2. For accuracy, a paired t test with task as repeated measure showed a significant difference between both tasks (t(55) = 4.57, n 2 = .28, p < .001). The item WM task led to slightly higher performance levels as compared to the order WM task (item: error rate = 22 ± 0.10%; order: error rate = 30 ± 0.14%). For response times (RT), a paired t test with task as repeated measure also showed a significant difference between both tasks (t(54) = 7.78, n 2 = .53, p < .001), the children being faster to judge item WM (3,317.88 ± 437.62 ms) than order WM (3,866.64 ± 471.79 ms) in accordance with previous studies. Note that RT data for one participant for the item WM task were missing due to a technical error during response registration.
Figure 2.
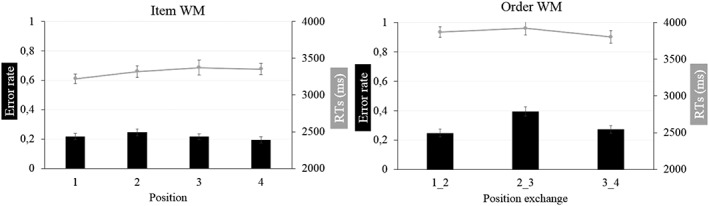
Error rate and RTs (mean and SE) for behavioral performance as a function of item and order WM conditions
We further explored response accuracy as a function of the serial position that was probed. For the item WM task, a repeated measures anova with accuracy showed a nonsignificant effect of position (F (3,162) = 1.18; n 2 = .02; p = .32); in Figure 2, a very mild recency effect can be observed. For the order WM task, the same repeated measures anova showed a significant effect of position (F (2,110) = 11.21; n 2 = .17; p < .001) with clear primacy and recency effects (more serial position exchanges involving the second and the third positions relative to the first and second positions as well as the third and fourth items, all Ps < .001). No significant serial position effects were observed for RTs on the item WM task (F (3,162) = 1.20; n 2 = .02; p = .31), nor for the order WM task (F (2,110) = 0.88; n 2 = .03; p = .28).
Given the uneven distribution of age across participants, we further explored age effects by contrasting subgroups of young and older children (subgroup 1: ≤8 years old, N = 20; subgroup 2: ≥9‐years old, N = 24; see Section 2). A 2 (group) × 2 (WM task) anova was conducted and showed a main effect of group (F (1,42) = 6.36; n 2 = .13; p < .05), a main effect of WM task (F (1,42) = 19.52; n 2 = .32; p < .001) but no interaction (F (1,42) = 0.85; n 2 = .02; p = .36). These results indicate that younger children performed less well than older children and this for both WM tasks while, as already underlined before, the item WM task was better performed than the order WM task (see Figure 3).
Figure 3.
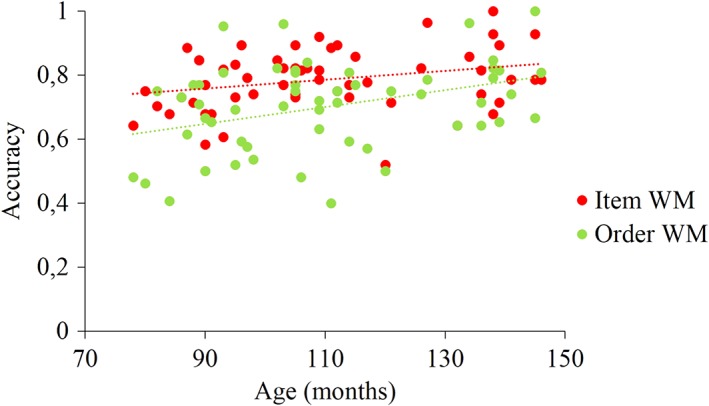
Scatterplot of performance in item and order WM tasks as a function of age in months [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. fMRI results
3.2.1. Main effects
The effects of WM condition on neural activity were explored using a 2 (item vs. order WM task) × 2 (encoding vs. retrieval WM phases) anova (see Table 1 and Figure 4). At the whole brain level, encoding of item information was associated with activity foci in the bilateral precuneus, the left SMA, the right supramarginal gyrus (SMG) the bilateral insula, the right IPS, the left middle temporal gyrus, the left caudate nucleus, and the cerebellum. Retrieval of item information was associated with activity foci in the SMA, the right middle frontal gyrus, the left precentral gyrus, the right postcentral gyrus, the left IPS, the bilateral insula, the cerebellum VI, and the bilateral lingual gyrus; additional activity foci, as revealed by the ROI analysis, were the left middle frontal gyrus and the bilateral inferior frontal gyrus. For encoding of the serial‐order information, we observed activity foci in the SMA, the left precentral gyrus, the right IPS, the right precuneus, the left insula, the left caudate and the cerebellum, and, at the ROI level, the left middle and inferior frontal gyrus. Retrieval of serial‐order information elicited activity foci in a large frontoparietal network, including, in the left hemisphere, the SMA, the middle and the inferior frontal gyrus, the insula, the thalamus, and the cerebellum; and in the right hemisphere, the middle frontal gyrus, the anterior IPS, the insula, and the cerebellum; additional activity foci as revealed by ROI analysis were the right superior frontal gyrus, the bilateral inferior frontal gyrus and the left IPS.
Table 1.
Activation peaks for the two WM tasks, as a function of encoding and retrieval. If not otherwise stated, all regions are significant at p < .05, corrected for whole brain volume
| Anatomical region | No. voxels | Left/right | x | y | z | BA area | SPM Z‐value |
|---|---|---|---|---|---|---|---|
| Item WM—encoding | |||||||
| SMA | 331 | L | −4 | 2 | 64 | 6 | >7.80 |
| SMG | 70 | R | 42 | −28 | 44 | 40 | 5.47 |
| Precentral | 82 | L | −54 | −4 | 52 | 6 | 7.10 |
| Intraparietal | 44 | R | 38 | −32 | 44 | 40 | 4.70* |
| Insula | 124 | B | −30 | 16 | 12 | 48 | 6.29 |
| 27 | 32 | 18 | 10 | 5.31 | |||
| Middle temporal gyrus | 26 | L | −62 | −32 | 6 | 21 | 5.32 |
| Precuneus | 234 | B | −24 | −44 | 12 | 37 | 6.89 |
| 275 | 26 | −44 | 10 | 6.68 | |||
| Caudate | 19 | L | −6 | 0 | 26 | 5.97 | |
| Cerebellum VI | 32 | R | 24 | −64 | −24 | 5.63 | |
| Item WM—retrieval | |||||||
| SMA | 550 | L | −6 | 0 | 52 | 6 | >7.80* |
| Middle frontal gyrus | 32 | B | −40 | 32 | 24 | 46 | 5.01 |
| 40 | 42 | 30 | 28 | 4.05* | |||
| Inferior frontal gyrus | 325 | B | −46 | 2 | 14 | 6 | 7.07* |
| 47 | 38 | 16 | 14 | 48 | 6.08* | ||
| Postcentral | 248 | R | 50 | −22 | 46 | 40 | 6.32 |
| Precentral | 6,977 | L | −34 | −24 | 54 | 40 | >7.80 |
| Intraparietal | 288 | L | −42 | −30 | 44 | 40 | >7.80* |
| Insula | 2,194 | B | −30 | 18 | 12 | 48 | >7.80 |
| 807 | 32 | 22 | 8 | 48 | >7.80 | ||
| Thalamus | 54 | L | −18 | −24 | 12 | 7.13 | |
| Lingual gyrus | 281 | B | −10 | −84 | 0 | 18 | 5.71 |
| Cerebellum VI | 1,498 | R | 22 | −52 | −24 | >7.80 | |
| Order WM—encoding | |||||||
| SMA | 453 | B | −4 | 4 | 64 | 6 | >7.80 |
| Middle frontal gyrus | 68 | L | −46 | 20 | 28 | 46 | 3.89* |
| Inferior frontal gyrus | 61 | L | −62 | 2 | 22 | 44 | 4.55* |
| Precentral gyrus | 163 | B | −54 | −4 | 52 | 40 | >7.80 |
| 96 | 44 | −24 | 68 | 5.64 | |||
| Intraparietal | 53 | R | 44 | −30 | 48 | 40 | 5.09 |
| Insula | 195 | B | −30 | 16 | 12 | 48 | 6.90 |
| 65 | 32 | 18 | 10 | 6.41 | |||
| Middle temporal gyrus | 162 | −64 | −32 | 6 | 21 | 6.04 | |
| Precuneus | 137 | B | −28 | −50 | 8 | 37 | 5.95 |
| 135 | 26 | −46 | 10 | 5.84 | |||
| Caudate | 18 | L | −14 | 24 | 6 | 4.95 | |
| Cerebellum VI | 47 | R | 30 | −62 | −24 | 7.27 | |
| Order WM—retrieval | |||||||
| SMA | 8,127 | L | −6 | 18 | 46 | 6 | >7.80 |
| Superior frontal gyrus | 95 | R | 28 | 8 | 56 | 8 | 4.92* |
| Middle frontal gyrus | 462 | B | −38 | 28 | 28 | 48 | 6.68* |
| 499 | 42 | 32 | 32 | 45 | 6.26 | ||
| Inferior frontal gyrus | 350 | L | −58 | 10 | 24 | 6 | 7.68* |
| 48 | 38 | 16 | 14 | 48 | 7.31* | ||
| Postcentral | 8,127 | L | −44 | −26 | 50 | 40 | >7.80 |
| Intraparietal (ant) | 797 | B | 48 | −24 | 46 | 40 | 6.91 |
| 44 | −36 | 46 | 6.72 | ||||
| 46 | −44 | 52 | 6.46 | ||||
| 941 | −42 | −30 | 44 | >7.80* | |||
| Insula | 3,569 | B | −30 | 18 | 10 | 48 | >7.80 |
| 930 | 32 | 22 | 6 | 47 | >7.80 | ||
| Thalamus (mammillary body) | 64 | L | −12 | −16 | 14 | 6.73 | |
| Cerebellum | 1,419 | R | 22 | −52 | −24 | IV | >7.80 |
| Cerebellum | 65 | L | −40 | −66 | −28 | Crus 1 | 6.45 |
WM = working memory.
p < .05, small volume corrections.
Figure 4.
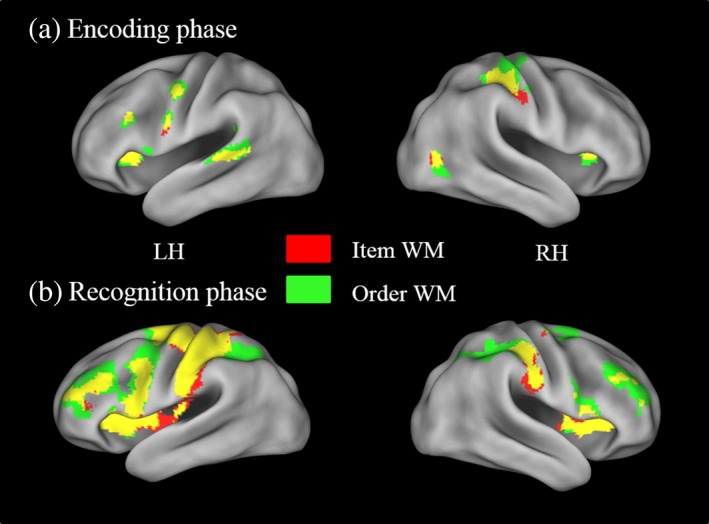
Activity foci for the (a) encoding and (b) recognition phase as a function of WM task (item vs. order). LH = left hemisphere; RH = right hemisphere. All activity foci displayed here are significant at p < .001 (uncorrected) and are mapped onto an inflated brain template using caret 5.64 with the PALS‐B12 atlas (Van Essen et al., 2001) [Color figure can be viewed at http://wileyonlinelibrary.com]
The common and specific activity foci for the item and serial order WM conditions were examined via conjunction null and contrast analyses (see Table 2). A conjunction null analysis over item and serial‐order information encoding conditions revealed activity foci in the SMA, the right IPS, the bilateral precuneus, and the right cerebellum. A conjunction null analysis over item and serial order retrieval conditions showed elevated activity in the SMA, the bilateral middle and inferior frontal gyrus, the bilateral inferior parietal gyrus, the left anterior IPS, the right cerebellum, the left thalamus, and the left lingual gyrus. A direct contrast between the two WM retrieval conditions showed increased activity in the bilateral superior frontal gyrus and in the right anterior IPS for the serial order condition relative to the item condition (see Table 2 and Figure 4).
Table 2.
Common and specific transient activation peaks (null conjunction and contrast) for the two WM tasks, as a function of encoding and retrieval. If not otherwise stated, all regions are significant at p < .05, corrected for whole brain volume
| Anatomical region | No. voxels | Left/right | x | y | z | BA area | SPM Z‐value |
|---|---|---|---|---|---|---|---|
| Item ∩ order WM—encoding | |||||||
| SMA | 324 | B | −4 | 2 | 64 | 6 | >7.80 |
| Inferior frontal gyrus | 117 | B | −30 | 16 | 12 | 48 | 6.29 |
| 27 | 32 | 18 | 10 | 5.31 | |||
| Middle frontal gyrus | 26 | L | −44 | 22 | 26 | 46 | 3.69* |
| Precentral gyrus | 82 | L | −54 | −4 | 52 | 40 | 7.10 |
| Intraparietal | 21 | R | 44 | −30 | 46 | 40 | 4.93 |
| Middle temporal gyrus | 26 | L | −62 | −32 | 6 | 21 | 5.32 |
| Precuneus | 120 | B | −26 | −48 | 8 | 37 | 5.85 |
| 132 | 26 | −46 | 10 | 5.84 | |||
| Cerebellum VI | 29 | R | 24 | −64 | −24 | 5.63 | |
| Item ∩ order WM—retrieval | |||||||
| SMA | 550 | B | −2 | 10 | 54 | 6 | >7.80* |
| Middle frontal gyrus | 32 | B | −40 | 32 | 24 | 45 | 5.01 |
| 40 | 42 | 30 | 28 | 4.05* | |||
| Inferior frontal gyrus | 1,743 | B | −30 | 18 | 12 | 48 | >7.80 |
| 642 | 32 | 22 | 8 | >7.80 | |||
| 316 | −46 | 2 | 16 | 6 | 6.42* | ||
| Inferior parietal gyrus | 6,222 | B | −36 | −24 | 56 | 40 | >7.80 |
| 108 | 50 | −22 | 46 | 6.32 | |||
| Intraparietal | 284 | L | −42 | −30 | 44 | 40 | >7.80* |
| Lingual gyrus | 18 | L | −12 | −84 | 0 | 18 | 4.83 |
| Thalamus | 40 | L | −16 | −24 | 14 | 6.56 | |
| Cerebellum | 1,057 | R | 22 | −52 | −24 | VI | >7.80 |
| Item WM > order WM | |||||||
| No voxel above threshold for any contrast, encoding or retrieval | |||||||
| Order WM > item WM encoding | |||||||
| No voxel above threshold | |||||||
| Order WM > item WM retrieval | |||||||
| Superior frontal gyrus | 119 | B | −30 | 8 | 58 | 8 | 4.06* |
| 60 | 28 | 10 | 54 | 5.20 | |||
| Intraparietal | 331 | R | 42 | −46 | 50 | 40 | 4.73* |
WM = working memory.
p < .05, small volume corrections.
Next, we assessed the impact of behavioral performance differences between the item and serial order WM on the neural activity differences, by introducing behavioral scores as covariates in addition to the item and serial order retrieval regressors in a paired t‐test analysis on functional images. When examining activity associated specifically with the behavioral covariates, no significant activity foci were observed. Critically, the serial order versus item WM contrast still showed elevated activity in the right superior frontal gyrus (28, 10, 64; k = 38; Z = 5.12; p < .05) and in the right IPS (40, −38, 44; k = 22; Z = 4.86; p < .05), despite the introduction of the behavioral scores associated with each condition. This means that the neural activity differences observed between the order and item WM conditions cannot be simply attributed to differences in task accuracy.
3.2.2. fMRI age‐related effects
First, we explored the effect of age by correlating activity for each condition and WM phase with age. For item encoding and retrieval, no significant correlations with age were observed. For the order WM condition, no significant correlation with age was observed for the encoding phase but age was positively correlated to right middle frontal gyrus and left IPS activity during the retrieval phase (see Table 3). Moreover, we extracted individual beta values from the parietal ROI for the retrieval phases of the order and item WM conditions. All measures had acceptable skewness (age = .36; order WM = .16; item WM = .30) and kurtosis (age = −1.04; order WM = −.56; item WM = −.05) values (values within the recommended two SE range with a cutoff of .64 for skewness and of 1.24 for kurtosis). A correlation analysis with these beta values confirmed the previous results by showing a significant correlation between age and activity levels in the parietal gyrus during the order WM task (r = .32, p < .05), while no significant correlation was observed for item WM (r = −.03, p = .81) (see Figure 5). We also checked for the presence and influence of atypical data points by calculating Cook's distances for the correlations between beta coefficients from parietal ROIs and age. No participant was found to exceed the usual cutoff value of 1. Furthermore, for subjects close to the more sensitive cutoff of 4/(n‐p‐1) for Cook's distance (no participants in item WM and four participants in order WM > .071), we reran the analysis with betas from order WM by removing these four participants. This did not change the outcome of results, as ρ = 0.35.
Table 3.
Correlations with age, as a function of encoding and retrieval. If not otherwise stated, all regions are significant at p < .05, corrected for whole brain volume
| Anatomical region | No. voxels | Left/right | x | y | z | BA area | SPM Z‐value |
|---|---|---|---|---|---|---|---|
| Item WM—encoding | |||||||
| No voxel above threshold | |||||||
| Item WM—Retrieval | |||||||
| No voxel above threshold | |||||||
| Order WM—Encoding | |||||||
| No voxel above threshold | |||||||
| Order WM—Retrieval positive | |||||||
| Middle frontal gyrus | 20 | R | 46 | 30 | 28 | 45 | 3.53* |
| Intraparietal sulcus | 42 | L | −28 | −48 | 44 | 40 | 4.12* |
WM = working memory.
p < .05, small volume corrections.
Figure 5.
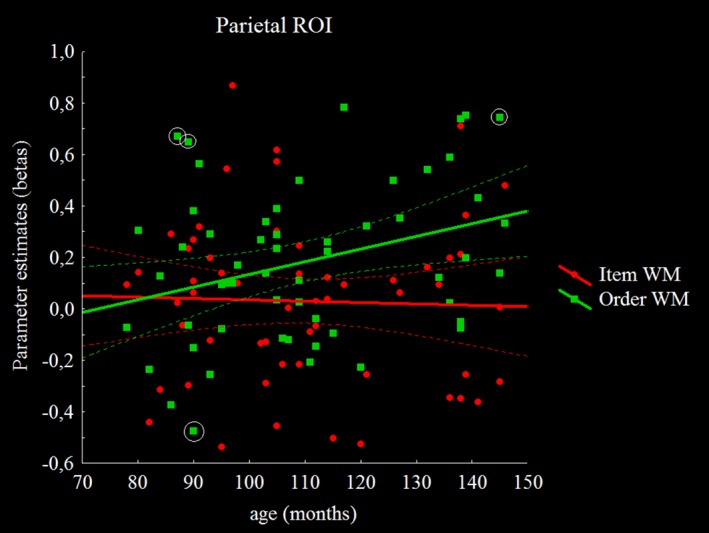
Correlation between age and parameter estimates (beta values) during the recognition phase for both WM tasks in the parietal ROI. Values identified as outside of the sensitive cutoff (<.071) by means of Cook's distance are circled [Color figure can be viewed at http://wileyonlinelibrary.com]
We further explored age effects by contrasting subgroups of young (≤8 years old) and older children (≥9 years old) (see methods and behavioral section). As shown in Figure 6, a 2 × 2 × 2 anova with group as between‐subject factor (young vs. old) and task (item WM vs. order WM) and phase (encoding vs. retrieval) as within‐subject factors showed that older children exhibited greater activity in the left SMA (z = 3.90, k = 19, p < .05) for both tasks. Critically, we observed an interaction between group and task in the right IPS (42, −38, 36; z = 3.78, k = 16, p < .05). Contrasts highlighted that children from the older subgroup showed increased activity in the right IPS during the order WM relatively to the item WM condition for the retrieval phase whereas there was no difference between both tasks in children from the younger subgroup. In order to further characterize this interaction, beta values were extracted from the parietal ROI for each participant. A 2 × 2 × 2 anova with subgroup as between‐subject factor and task and phase as within‐subject factor on the parameter estimates (beta values) was conducted. This analysis showed a marginally significant effect of the task (F (1,42) = 3.49; n 2 = .08; p = .08) and critically, a significant interaction between subgroup and task (F (1,42) = 6.75; η2 = .14; p < .05), the younger subgroup showing similar IPS activity levels for item and order WM tasks while the older subgroup exhibiting higher activity levels in the right IPS for the order WM as compared to the item WM task (p < .05) (see Figure 6). Moreover, there was a significant interaction between task and phase, the retrieval phase leading to higher right IPS activity in the order WM than in the item WM as compared to the encoding phase (see Figure 6).
Figure 6.
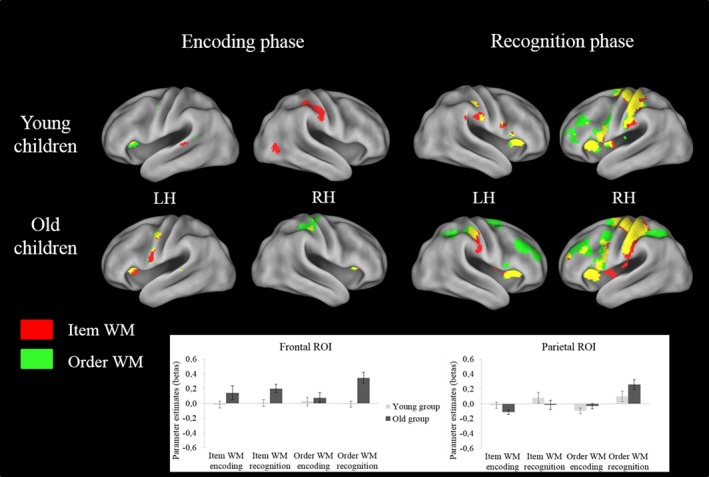
Brain regions activated in the (a) encoding and (b) recognition phase from the two WM tasks (item vs. order) × 2 WM phases (encoding vs. recognition) × 2 groups (young vs. old) factorial design. Below, parameter estimates (beta values) for each group, WM task and phase are showed at the parietal and frontal ROI level. LH = left hemisphere; RH = right hemisphere. All brain activations showed are significant at p < .001 (uncorrected) and are mapped onto an inflated brain template using Caret 5.64 with the PALSB12 atlas (Van Essen et al., 2001) [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The present study determined the extent to which the critical distinction between item and serial order WM storage neural networks observed in adults also characterizes WM storage networks in young children. In 7–12‐year old children, we observed that both item and serial order WM storage recruited a frontoparietal network. At the same time, order WM activated the bilateral superior frontal gyrus and the right anterior IPS to a larger extent relative to the item WM condition, replicating previous findings in adult populations. Critically, the right IPS was specifically associated with the temporary maintenance of serial‐order information only in older children.
First of all, our results replicate in a developmental population the results of previous studies that reported specific neural networks for the processing of serial order versus item information in adult WM (Majerus et al., 2010; Majerus, Bastin, et al., 2007; Majerus, Poncelet, Van der Linden, et al., 2006; Marshuetz et al., 2000). While the left IPS was involved in the retrieval of both item and serial‐order information, the right anterior IPS and the bilateral superior frontal gyrus were more strongly recruited in the serial order WM condition, in accordance with previous studies in adults. Furthermore, these differences in neural activity were independent of differences in the level of task difficulty.
Furthermore, selective activity in right IPS for the serial order WM condition was most strongly expressed in older children. However, at behavioral level, a global increase of performance was observed for both WM tasks in older children relatively to the younger subgroup. This finding is important in terms of the nature and development of WM components. The fact that serial order WM networks appear to specialize later at neural level, may reflect a more strategic and controlled nature of the processes involved in serial order WM, at least for the type of task used in this study. This interpretation is consistent with previous data showing more accurate coding of serial‐order information in 11‐year old compared to 7‐year‐old children (McCormack, Brown, Vousden, & Henson, 2000). However, even if neural data suggested that children may progressively use more specific strategies for maintaining serial‐order information in WM, our behavioral data indicated that item and order WM performance increase similarly with age. Therefore, the precise nature of these serial order coding strategies, just like the nature of serial order coding more generally, remains an open question and many different hypotheses having been proposed so far (see Majerus et al., 2018 for a discussion).
Our results also revealed activity foci in a large frontoparietal network including the left anterior IPS that were common to the order and item WM tasks. An interpretation advanced by a number of studies that mainly focused on WM cognitive tasks is that this frontoparietal network, also called the dorsal attention network, supports a more general role of attentional control (Cowan, 1999; Fougnie & Marois, 2007; Majerus et al., 2012; Ravizza, Delgado, Chein, Becker, & Fiez, 2004). Two meta‐analytic studies examining the neural substrates associated with attention shifting and executive processes in WM demonstrated a consistent involvement of the left anterior IPS (Nee & Jonides, 2013; Wager, Jonides, & Reading, 2004). The studies included in the meta‐analyses involved experiments that require either the retention of multiple items (as in the present study) or updating of information in WM. However, in this study, we focused on the storage component of WM since no manipulation of items was required and therefore no executive control processes. The present study suggests that the networks supporting attentional control processes during WM storage tasks could be already engaged in young children, also in line with the results of previous behavioral studies. Indeed, even if the use of attentional refreshing ability increases until 14 years to reach a similar level of use and efficiency as in adulthood, children as young as 6 years are already able to use attentional refreshing or other attentional control strategies in WM tasks (Oftinger & Camos, 2015; Tam, Jarrold, Baddeley, & Sabatos‐DeVito, 2010).
At a more general, developmental level, the age‐related differences in WM neural networks observed in this study, particularly for serial order WM, are in line with the hypothesis of a progressive neural specialization for WM that is not yet fully established in young children (Kharitonova et al., 2015; Klingberg et al., 2002; Spencer‐Smith et al., 2013; Thomason et al., 2009). While some neuroimaging studies observed a similar frontoparietal network involvement for WM tasks in children, adolescents, and adults (Thomason et al., 2009; Ciesielski et al., 2006; Siffredi et al., 2017; Klingberg et al., 2006; Scherf et al., 2006), other studies showed that activation levels of the parietal and prefrontal cortex increased with age (Crone et al., 2006; Kharitonova et al., 2015; Klingberg et al., 2002; Kwon et al., 2002; Spencer‐Smith et al., 2013; Thomason et al., 2009; Van den Bosch et al., 2014). In the present study, we observed similar levels of neural activity in young and older children, except for frontoparietal involvement specifically in the serial order WM condition, and which appeared to increase with age. It is also important to note that these specific developmental changes in the right superior frontal and IPS were not related to differences in task accuracy between the item and serial order WM conditions, performance in both tasks increasing similarly with age. However, our results concern mainly the storage aspects of information while most studies on the developmental neural correlates of WM focused on both storage and processing tasks. This suggests that there is also a specific maturation at the storage level and this mainly for serial order WM processing.
A further noteworthy finding of this study is the reduced level of overall brain activity observed during the encoding/maintenance stage as compared to the recognition phase. Studies in adults using very similar WM probe recognition paradigms as those in this study generally observe comparable levels of frontoparietal activity during encoding and recognition stages (see, e.g., Majerus et al., 2010). Although studies in children rarely distinguished specific WM stages, it should be noted that two studies in children populations observed activity foci in more posterior, perceptual networks during encoding and more extensive activity in fronto‐patietal areas only during the retrieval stage (van den Bosch et al., 2014; Siffredi et al., 2017). Our results are in agreement with these observations. Therefore, it could be interesting in further studies to investigate with an appropriate design if poorer activation in children during the encoding phase of a WM task reflects more passive encoding of information as compared to adults.
To conclude, this study shows that a distinction between item and order WM processing appears progressively in childhood from 7 to 12‐years old. While we observed common activity foci in a large frontoparietal network for both item and serial order WM tasks, supplementary and specific activity foci in the bilateral superior frontal areas and the right IPS appeared during the order WM task, and this only for older children. This study highlights progressive specialization of the neural correlates involved in serial order WM over the course of cognitive development.
CONFLICT OF INTEREST
There is no conflict of interest in connection with this work.
ACKNOWLEDGMENTS
This work was supported by grants F.R.S.‐FNRS (Fund for Scientific Research, FNRS, Belgium). The authors would like to thank all the children and their parents for their time and effort invested in this study. The authors would also like to thank some colleagues for their time spent to assist us and the children in fMRI, Laurens Van Calster, David Stawarczyk, and Benjamin Kowialiewski.
Attout L, Ordonez Magro L, Szmalec A, Majerus S. The developmental neural substrates of item and serial order components of verbal working memory. Hum Brain Mapp. 2019;40:1541–1553. 10.1002/hbm.24466
Funding information Fonds De La Recherche Scientifique ‐ FNRS, Grant/Award Number: T.1003.15
REFERENCES
- Andersson, J. L. , Hutton, C. , Ashburner, J. , Turner, R. , & Friston, K. (2001). Modeling geometric deformations in EPI time series. NeuroImage, 13(5), 903–919. 10.1006/nimg.2001.074 [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Asplund, C. L. , Todd, J. J. , Snyder, A. P. , & Marois, R. (2010). A central role for the lateral prefrontal cortex in goal‐directed and stimulus‐driven attention. Nature Neuroscience, 13(4), 507–512. 10.1038/nn.2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attout, L. , & Majerus, S. (2014). Working memory deficits in developmental dyscalculia: The importance of serial order. Child Neuropsychology, 21(4), 432–450. 10.1080/09297049.2014.922170 [DOI] [PubMed] [Google Scholar]
- Attout, L. , Noël, M. P. , & Majerus, S. (2014). The relationship between working memory for serial order and numerical development: A longitudinal study. Developmental Psychology, 50(6), 1667–1679. 10.1037/a0036496 [DOI] [PubMed] [Google Scholar]
- Attout, L. , Van der Kaa, M. A. , George, M. , & Majerus, S. (2012). Dissociating short‐term memory and language impairment: The importance of item and serial order information. Aphasiology, 26(3–4), 355–382. 10.1080/02687038.2011.604303 [DOI] [Google Scholar]
- Binamé, F. , & Poncelet, M. (2016). Order short‐term memory capacity predicts nonword reading and spelling in first and second grade. Reading and Writing, 29(1), 1–20. 10.1007/s11145-015-9577-9 [DOI] [Google Scholar]
- Brock, J. , & Jarrold, C. (2005). Serial order reconstruction in down syndrome: Evidence for a selective deficit in verbal short‐term memory. Journal of Child Psychology and Psychiatry, 46(3), 304–316. 10.1111/j.1469-7610.2004.00352.x [DOI] [PubMed] [Google Scholar]
- Bunge, S. A. , & Wright, S. B. (2007). Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology, 17(2), 243–250. 10.1016/j.conb.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Ciesielski, K. T. , Lesnik, P. G. , Savoy, R. L. , Grant, E. P. , & Ahlfors, S. P. (2006). Developmental neural networks in children performing a categorical N‐back task. NeuroImage, 33(3), 980–990. 10.1016/j.neuroimage.2006.07.028 [DOI] [PubMed] [Google Scholar]
- Cowan, N. (1999). Models of working memory: Mechanisms of active maintenance and executive control In Miyake A. & Shah P. (Eds.), An embedded‐processes model of working memory (pp. 62–101). New York, NY: Oxford University Press. [Google Scholar]
- Cowan, N. (2016). Working memory maturation: Can we get at the essence of cognitive growth? Perspectives on Psychological Science, 11(2), 239–264. 10.1177/1745691615621279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , Wendelken, C. , Donohue, S. , van Leijenhorst, L. , & Bunge, S. A. (2006). Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America, 103(24), 9315–9320. 10.1073/pnas.0510088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki, F. , & Klingberg, T. (2014). The role of fronto‐parietal and fronto‐striatal networks in the development of working memory: A longitudinal study. Cerebral Cortex, 25(6), 1587–1595. 10.1093/cercor/bht352 [DOI] [PubMed] [Google Scholar]
- De Visscher, A. , Szmalec, A. , Van Der Linden, L. , & Noël, M.‐P. (2015). Serial‐order learning impairment and hypersensitivity‐to‐interference in dyscalculia. Cognition, 144, 38–48. 10.1016/j.cognition.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, R. W. , & Kane, M. J. (2004). Executive attention, working memory capacity, and a two‐factor theory of cognitive control. Psychology of Learning and Motivation, 44, 145–200. [Google Scholar]
- Fougnie, D. , & Marois, R. (2007). Executive working memory load induces inattentional blindness. Psychonomic Bulletin & Review, 14(1), 142–147. [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. , & Alter, K. (2004). Lateralization of auditory language functions: A dynamic dual pathway model. Brain and Language, 89(2), 267–276. 10.1016/S0093-934X(03)00351-1 [DOI] [PubMed] [Google Scholar]
- Gathercole, S. E. , Pickering, S. J. , Ambridge, B. , & Wearing, H. (2004). The structure of working memory from 4 to 15 years of age. Developmental Psychology, 40(2), 177–190. 10.1037/0012-1649.40.2.177 [DOI] [PubMed] [Google Scholar]
- Gillebert, C. R. , Dyrholm, M. , Vangkilde, S. , Kyllingsbæk, S. , Peeters, R. , & Vandenberghe, R. (2012). Attentional priorities and access to short‐term memory: Parietal interactions. NeuroImage, 62(3), 1551–1562. 10.1016/j.neuroimage.2012.05.038 [DOI] [PubMed] [Google Scholar]
- Henson, R. N. A. , Burgess, N. , & Frith, C. D. (2000). Recoding, storage, rehearsal and grouping in verbal short‐term memory: An fMRI study. Neuropsychologia, 38(4), 426–440. 10.1016/S0028-3932(99)00098-6 [DOI] [PubMed] [Google Scholar]
- Henson, R. N. A. , Hartley, T. , Burgess, N. , Hitch, G. J. , & Flude, B. (2003). Selective interference with verbal short‐term memory for serial order information: A new paradigm and tests of a timing‐signal hypothesis. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology, 56(8), 1307–1334. 10.1080/02724980244000747 [DOI] [PubMed] [Google Scholar]
- Hutton, C. , Bork, A. , Josephs, O. , Deichmann, R. , Ashburner, J. , & Turner, R. (2002). Image distortion correction in fMRI: A quantitative evaluation. NeuroImage, 16(1), 217–240. 10.1006/nimg.2001.1054 [DOI] [PubMed] [Google Scholar]
- Kalm, K. , & Norris, D. (2014). The representation of order information in auditory‐verbal short‐term memory. The Journal of Neuroscience, 34(20), 6879–6886. 10.1523/JNEUROSCI.4104-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H. C. , Burgund, E. D. , Lugar, H. M. , Petersen, S. E. , & Schlaggar, B. L. (2003). Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage, 19(1), 16–28. 10.1016/S1053-8119(03)00038-7 [DOI] [PubMed] [Google Scholar]
- Kharitonova, M. , Winter, W. , & Sheridan, M. A. (2015). As working memory grows: A developmental account of neural bases of working memory capacity in 5‐to 8‐year old children and adults. Journal of Cognitive Neuroscience, 27(9), 1775–1788. 10.1162/jocn_a_00824 [DOI] [PubMed] [Google Scholar]
- Klingberg, T. (2006). Development of a superior frontal‐intraparietal network for visuo‐spatial working memory. Neuropsychologia, 44(11), 2171–2177. 10.1016/j.neuropsychologia.2005.11.019 [DOI] [PubMed] [Google Scholar]
- Klingberg, T. , Forssberg, H. , & Westerberg, H. (2002). Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience, 14(1), 1–10. 10.1162/089892902317205276 [DOI] [PubMed] [Google Scholar]
- Kwon, H. , Reiss, A. L. , & Menon, V. (2002). Neural basis of protracted developmental changes in visuo‐spatial working memory. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 13336–13341. 10.1073/pnas.162486399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq, A. L. , & Majerus, S. (2010). Serial‐order short‐term memory predicts vocabulary development: Evidence from a longitudinal study. Developmental Psychology, 46(2), 417–427. 10.1037/a0018540 [DOI] [PubMed] [Google Scholar]
- Majerus, S. (2009). Verbal short‐term memory and temporary activation of language representations: The importance of distinguishing item and order information In Thorn A. (Ed.), Interactions between short‐term and long‐term memory in the verbal domain (pp. 244–276). New York, NY: Psychology Press. [Google Scholar]
- Majerus, S. , Attout, L. , Artielle, M.‐A. , & Van der Kaa, M.‐A. (2015). The heterogeneity of verbal short‐term memory impairment in aphasia. Neuropsychologia, 77, 165–176. [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Attout, L. , D'Argembeau, A. , Degueldre, C. , Fias, W. , Maquet, P. , … Van der Linden, M. (2012). Attention supports verbal short‐term memory via competition between dorsal and ventral attention networks. Cerebral Cortex, 22(5), 1086–1097. 10.1093/cercor/bhr174 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Bastin, C. , Poncelet, M. , Van der Linden, M. , Salmon, E. , Collette, F. , & Maquet, P. (2007). Short‐term memory and the left intraparietal sulcus: Focus of attention? Further evidence from a face short‐term memory paradigm. NeuroImage, 35(1), 353–367. 10.1016/j.neuroimage.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Belayachi, S. , De Smedt, B. , Leclercq, A. L. , Martinez Perez, T. , Schmidt, C. , … Maquet, P. (2008). Neural networks for short‐term memory for order differentiate high and low proficiency bilinguals. NeuroImage, 42(4), 1698–1713. 10.1016/j.neuroimage.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , & Boukebza, C. (2013). Short‐term memory for serial order supports vocabulary development: New evidence from a novel word learning paradigm. Journal of Experimental Child Psychology, 116(4), 811–828. 10.1016/j.jecp.2013.07.014 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , D'Argembeau, A. , Martinez Perez, T. , Belayachi, S. , Van der Linden, M. , Collette, F. , … Maquet, P. (2010). The commonality of neural networks for verbal and visual short‐term memory. Journal of Cognitive Neuroscience, 22(11), 2570–2593. 10.1162/jocn.2009.21378 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Glaser, B. , Van der Linden, M. , & Eliez, S. (2006). A multiple case study of verbal short‐term memory in velo‐cardio‐facial syndrome. Journal of Intellectual Disability Research, 50(6), 457–469. 10.1111/j.1365-2788.2006.00791.x [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Norris, D. , & Patterson, K. (2007). What does a patient with semantic dementia remember in verbal short‐term memory? Order and sound but not words. Cognitive Neuropsychology, 24(2), 131–151. 10.1080/02643290600989376 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Péters, F. , Bouffier, M. , Cowan, N. , & Phillips, C. (2018). The dorsal attention network reflects both encoding load and top–down control during working memory. Journal of Cognitive Neuroscience, 30(2), 144–159. 10.1162/jocn_a_01195 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Poncelet, M. , Greffe, C. , & Van der Linden, M. (2006). Relations between vocabulary development and verbal short‐term memory: The relative importance of short‐term memory for serial order and item information. Journal of Experimental Child Psychology, 93(2), 95–119. 10.1016/j.jecp.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Poncelet, M. , Van der Linden, M. , Albouy, G. , Salmon, E. , Sterpenich, V. , … Maquet, P. (2006). The left intraparietal sulcus and verbal short‐term memory: Focus of attention or serial order? NeuroImage, 32(2), 880–891. 10.1016/j.neuroimage.2006.03.048 [DOI] [PubMed] [Google Scholar]
- Majerus, S. , Van der Linden, M. , Braissand, V. , & Eliez, S. (2007). Verbal short‐term memory in individuals with chromosome 22q11. 2 deletion: Specific deficit in serial order retention capacities? American Journal on Mental Retardation, 112(2), 79–93. 10.1352/0895-8017(2007)112 [DOI] [PubMed] [Google Scholar]
- Marshuetz, C. , & Bates, J. (2004). Functional neuroimaging and the prefrontal cortex: Organization by stimulus domain? In Satoru O. (Ed.), Prefrontal cortex: From synaptic plasticity to cognition (pp. 289–313). Boston, MA: Kluwer Academic. [Google Scholar]
- Marshuetz, C. , Reuter‐Lorenz, P. A. , Smith, E. E. , Jonides, J. , & Noll, D. C. (2006). Working memory for order and the parietal cortex: An event‐related functional magnetic resonance imaging study. Neuroscience, 139(1), 311–316. 10.1016/j.neuroscience.2005.04.071 [DOI] [PubMed] [Google Scholar]
- Marshuetz, C. , Smith, E. E. , Jonides, J. , DeGutis, J. , & Chenevert, T. L. (2000). Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. Journal of Cognitive Neuroscience, 12(Suppl. 2), 130–144. 10.1162/08989290051137459 [DOI] [PubMed] [Google Scholar]
- Martinez Perez, T. , Majerus, S. , Mahot, A. , & Poncelet, M. (2012). Evidence for a specific impairment of serial order short‐term memory in dyslexic children. Dyslexia, 18(2), 94–109. 10.1002/dys.1438 [DOI] [PubMed] [Google Scholar]
- Martinez Perez, T. , Majerus, S. , & Poncelet, M. (2012). The contribution of short‐term memory for serial order to early reading acquisition: Evidence from a longitudinal study. Journal of Experimental Child Psychology, 111, 708–723. 10.1016/j.jecp.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Mazaika, P. K. , Hoeft, F. , Glover, G. H. , & Reiss, A. L. (2009). Methods and software for fMRI analysis of clinical subjects. NeuroImage, 47, S58 10.1016/S1053-8119(09)70238-1 [DOI] [Google Scholar]
- McCormack, T. , Brown, G. D. A. , Vousden, J. I. , & Henson, R. N. A. (2000). Children's serial recall errors: Implications for theories of short‐term memory development. Journal of Experimental Child Psychology, 76(3), 222–252. 10.1006/jecp.1999.2550 [DOI] [PubMed] [Google Scholar]
- Nee, D. E. , & Jonides, J. (2013). Trisecting representational states in short‐term memory. Frontiers in Human Neuroscience, 7, 796 10.3389/fnhum.2013.00796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, B. , Pallier, C. , & Ferrand, L. (2005). Manuel de Lexique, 3, (3.03 ed.) Computer software manual. France. [Google Scholar]
- Oftinger, A.‐L. , & Camos, V. (2015). Maintenance mechanisms in children's verbal working memory. Journal of Educational and Developmental Psychology, 6(1), 16 10.5539/jedp.v6n1p16 [DOI] [Google Scholar]
- Ordonez Magro, L. , Attout, L. , Majerus, S. , & Szmalec, A. (2018). Short‐and long‐term memory determinants of novel word form learning. Cognitive Development, 47, 146–157. 10.1016/j.cogdev.2018.06.002 [DOI] [Google Scholar]
- Ravizza, S. M. , Delgado, M. R. , Chein, J. M. , Becker, J. T. , & Fiez, J. A. (2004). Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage, 22(2), 562–573. 10.1016/j.neuroimage.2004.01.039 [DOI] [PubMed] [Google Scholar]
- Scherf, K. S. , Sweeney, J. A. , & Luna, B. (2006). Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience, 18(7), 1045–1058. 10.1162/jocn.2006.18.7.1045 [DOI] [PubMed] [Google Scholar]
- Siffredi, V. , Barrouillet, P. , Spencer‐Smith, M. , Vaessen, M. , Anderson, V. , & Vuilleumier, P. (2017). Examining distinct working memory processes in children and adolescents using fMRI: Results and validation of a modified Brown‐Peterson paradigm. PLoS One, 12(7), e0179959 10.1371/journal.pone.0179959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer‐Smith, M. , Ritter, B. C. , Mürner‐Lavanchy, I. , El‐Koussy, M. , Steinlin, M. , & Everts, R. (2013). Age, sex, and performance influence the visuospatial working memory network in childhood. Developmental Neuropsychology, 38(4), 236–255. 10.1080/87565641.2013.784321 [DOI] [PubMed] [Google Scholar]
- Tam, H. , Jarrold, C. , Baddeley, A. D. , & Sabatos‐DeVito, M. (2010). The development of memory maintenance: Children's use of phonological rehearsal and attentional refreshment in working memory tasks. Journal of Experimental Child Psychology, 107(3), 306–324. 10.1016/j.jecp.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Thomason, M. E. , Race, E. , Burrows, B. , Whitfield‐Gabrieli, S. , Glover, G. H. , & Gabrieli, J. D. (2009). Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience, 21(2), 316–332. 10.1162/jocn.2008.21028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, J. J. , & Marois, R. (2004). Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature, 428(6984), 751–754. 10.1038/nature02466 [DOI] [PubMed] [Google Scholar]
- Van den Bosch, G. E. , Marroun, H. E. , Schmidt, M. N. , Tibboel, D. , Manoach, D. S. , Calhoun, V. D. , & White, T. J. (2014). Brain connectivity during verbal working memory in children and adolescents. Human Brain Mapping, 35(2), 698–711. 10.1002/hbm.22193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijck, J. P. , & Fias, W. (2011). A working memory account for spatial‐numerical associations. Cognition, 119(1), 114–119. 10.1016/j.cognition.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Van Essen, D. C. , Drury, H. A. , Dickson, J. , Harwell, J. , Hanlon, D. , & Anderson, C. H. (2001). An integrated software suite for surface‐based analyses of cerebral cortex. Journal of the American Medical Informatics Association, 8(5), 443–459. 10.1136/jamia.2001.0080443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Jonides, J. , & Reading, S. (2004). Neuroimaging studies of shifting attention: A meta‐analysis. NeuroImage, 22(4), 1679–1693. 10.1016/j.neuroimage.2004.03.052 [DOI] [PubMed] [Google Scholar]


