Abstract
Working memory (WM) refers to a set of cognitive processes that allows for the temporary storage and manipulation of information, crucial for everyday life skills. WM deficits are present in several neurological, psychiatric, and neurodevelopmental disorders, thus making the full understanding of its neural correlates a key aspect for the implementation of cognitive training interventions. Here, we present a quantitative meta‐analysis focusing on the underlying neural substrates upon which the n‐back, one of the most commonly used tasks for WM assessment, is believed to rely on, as highlighted by functional magnetic resonance imaging and positron emission tomography findings. Relevant published work was scrutinized through the activation likelihood estimate (ALE) statistical framework in order to generate a set of task‐specific activation maps, according to n‐back difficulty. Our results confirm the known involvement of frontoparietal areas across different types of n‐back tasks, as well as the recruitment of subcortical structures, cerebellum and precuneus. Specific activations maps for four stimuli types, six presentation modalities, three WM loads and their combination are provided and discussed. Moreover, functional overlap with resting‐state networks highlighted a strong similarity between n‐back nodes and the Dorsal Attention Network, with less overlap with other networks like Salience, Language, and Sensorimotor ones. Additionally, neural deactivations during n‐back tasks and their functional connectivity profile were examined. Clinical and functional implications are discussed in the context of potential noninvasive brain stimulation and cognitive enhancement/rehabilitation programs.
Keywords: executive function, fMRI, functional connectivity, n‐back, PET, quantitative meta‐analysis, working memory
1. INTRODUCTION
Working memory (WM) is generally defined as the capacity to temporary maintain and manipulate goal‐relevant information, as well as to concurrently remember and process information over brief periods of time (Baddeley, 1992). Compared to short‐term memory, WM allows to manipulate incoming information, thus not being limited to storage capacity (Baddeley, Della Sala, Robbins, & Baddeley, 1996). Due to its many plausible links with other high‐order cognitive functions—such as fluid intelligence (Friedman et al., 2006), inhibition (Miyake et al., 2000), switching (Miyake et al., 2000), and attention (Corbetta & Shulman, 2002)—a large number of studies has recently focused on understanding its mechanisms and neural correlates, not only in the context of cognitive neuroscience but also in clinical psychology, cognitive rehabilitation, and within the field of cognitive enhancement. Indeed, WM deficits are present in many psychiatric and neurodegenerative disorders, including depression (Rose & Ebmeier, 2006), schizophrenia (Lee & Park, 2005), Alzheimer's and Parkinson's diseases (Baddeley, Bressi, Della Sala, Logie, & Spinnler, 1991), as well as in some neurodevelopmental disorders (e.g., Attention Deficit Hyperactivity Disorder [ADHD], and Autistic Spectrum Disorder [ASD]; Martinussen, Hayden, Hogg‐Johnson, & Tannock, 2005; Williams, Goldstein, Carpenter, & Minshew, 2005).
Studies in both healthy humans and clinical populations have highlighted a set of brain regions playing a relevant role during WM‐related cognitive processing, with hundreds of studies being published so far. The complexity of WM has also led to a vast number of assessment tools being created, each one stressing a particular aspect of WM processing: for example, WM capacity versus manipulation, processing of visual stimuli with or without emotional valence, processing of auditory stimuli versus verbal ones, and so on. This has led to a consensus over which brain regions or lobe might play a role in WM, but with little to no specificity when it comes to the neural substrates underlying specific WM tasks that might be deployed in neurorehabilitation protocols or become a target for noninvasive brain stimulation (NIBS) techniques. The most recent activation likelihood estimate (ALE) meta‐analysis (Rottschy et al., 2012) examined a total of 189 WM experiments employing a variety of WM tasks. The authors reported a highly consistent activation of a core WM network across task variants, relying mostly on frontoparietal regions, with some differentiation depending on the type of stimuli and cognitive processes examined. However, due to the great variability in the WM tasks being examined, the relevant contribution of Rottschy et al. (2012) did not define task‐specific activation clusters or maps.
Among the most used WM tasks, examples include the Digit Span task, the Sternberg task, the n‐back task, and the delayed match‐to‐sample. The n‐back task—first described by Kirchner (1958)—is, however, the most popular measure of WM used in functional magnetic resonance imaging (fMRI) studies, relying on the presentation of “rapidly, continuously changing information” to measure very short‐term retention. In this task, participants are presented with a series of stimuli and are asked to indicate whether the current stimulus matches the stimulus presented n‐stimuli back in the series. The majority of fMRI studies using n‐back paradigms has so far focused on the effects of task load or type of material (e.g., verbal vs. spatial) in adults (Owen, McMillan, Laird, & Bullmore, 2005) and confirmed the well‐established frontoparietal network of activation mentioned above. Indeed, both differences in stimuli types (e.g., letters, numbers, faces, words, objects, and images) and presentation modalities (visual, auditory, and tactile) can be used to personalize a variety of features in n‐back paradigms, reason for which fMRI studies can be informative in revealing which brain areas are more active for a specific condition, or if instead, the activated WM network remains the same independently from changes in such features. Few studies have proven WM network activation to be material‐independent (Nystrom et al., 2000; Owen et al., 2005; Ragland et al., 2002; Schumacher et al., 1996), with the opposite finding holding true for other studies: while maintaining a bilateral frontoparietal activation, greater network activation has been reported in the left hemisphere for verbal inputs, and on the right hemisphere when subjects were presented with visuospatial material (Owen et al., 2005; Rottschy et al., 2012). The latest N‐back meta‐analysis dates back to 2005. Owen et al. (2005) examined 24 studies manipulating either the process required for task performance (i.e., location/spatial‐ vs. identity/nonspatial‐monitoring) or stimulus material (i.e., verbal or nonverbal). However, only some neural activations related to stimuli or presentation modalities were presented: specifically, three activation maps (“identity verbal”, “identity nonverbal” and “nonverbal location”) and two comparison maps (“verbal vs. non‐verbal” and “identity vs. location”).
A detailed knowledge of the pool of regions upon which a specific task or stimulus used in an n‐back paradigm relies on can be very useful in the field of cognitive rehabilitation: if a patient has a lesion on the left hemisphere and he/she presents a WM deficit, it would be important to implement a cognitive training capable of stressing that area or the efficiency of surrounding healthy tissue. In addition, several neuroimaging studies have shown an increase in activity at the neural level over specific regions, such as the bilateral prefrontal and parietal cortices, as a function of processing load (Jonides et al., 1997; Owen et al., 2005; Rottschy et al., 2012), suggesting the importance of mapping the neural substrates of WM load as well. The creation of well‐defined maps based on established task‐specific clusters of activity would also allow to selectively stimulate such areas, possibly through the implementation of Noninvasvie brain stimulation (NIBS) approaches.
Due to the aforementioned rationale, we hereby aim to present a quantitative meta‐analysis of the n‐back literature available to date, summarizing published experimental work involving task‐fMRI and positron emission tomography (PET) data. The ALE statistical framework (Eickhoff et al., 2009; Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012) was implemented in providing readers with a state‐of‐the‐art update on activation maps along with a “general to specific” gradient of WM n‐back paradigm characterization. In addition, a clear differentiation of the role played by the regions activated during WM tasks with respect to existing resting‐state networks (RSN; Biswal et al., 2010) would also be valuable, but it is still not available in the literature. To provide this information, we compared each n‐back map with those representing different RSNs (e.g., dorsal attention, executive control, language, sensorimotor, visual, default and auditory networks). Results offer an overview of the link between n‐back related brain activity and brain connectivity in humans.
2. METHODS
2.1. Literature search
Potentially relevant articles were retrieved by performing a search on PubMed and Google Scholar databases without temporal restrictions. The following terms “Working Memory”, “WM”, “N‐back task”, “Working Memory Task”, “Memory”, “Short‐term Memory”, were individually combined with “Functional Magnetic Resonance Imaging”, “Position Emission Tomography” and their acronyms. References from the retrieved material were examined for relevant publications too. Following abstract screening, a total of 152 studies were selected and analyzed (Supporting Information Figure S1). We intentionally excluded (a) studies involving patients with organic illnesses, pathological neurological exam, psychiatric conditions or history of drug abuse, (b) studies discussing magic ideation, (c) review papers, (d) studies not mentioning any of the keywords in their abstract unless they cite specific n‐back tasks, (e) studies not reporting fMRI/PET activations coordinates in MNI or Talairach space, (f) studies not reporting activation foci in table format or reporting statistical values without corresponding coordinates, (g) studies that used predefined Regions of Interest (ROIs), (h) studies not using classic n‐back tasks, defined as those where subjects must respond when the stimulus presented is the same as the stimulus presented n times before, (i) studies that used placebo or pharmacological interventions, (j) studies with only one subject, (k) studies reporting results obtained with small volume correction (SVC). The final selection comprised 85 studies reporting either fMRI or PET findings. For each study, the following information were retrieved: (a) number of participants, (b) mean age, (c) experimental design, (d) cognitive task specifics, and (e) main results. Data of the specific activation foci were collected and included in a quantitative ALE analysis for the identification of brain regions most commonly reported as involved in n‐back tasks.
2.2. N‐back maps
Different maps were created, carefully inspecting each manuscript and extracting activation foci from tables referring to the contrast of interest. A (1) global “WM” map was obtained including all the coordinates referring to n‐back tasks, regardless of presentation modality and stimulus type. We created (2) three maps of WM load containing all experiments that contrasted a high load n‐back condition with a low load n‐back condition (e.g., 3 back vs. 1 back tasks). Both (3) “visual” and (4) “auditory” n‐back maps were computed from studies using visual or auditory presentation modalities; words, letters, numbers, faces, objects, and images are examples of the most commonly displayed visual stimuli within former studies. Moreover, (5) “spatial” and (6) “nonspatial” n‐back maps were computed from studies using spatial designs (e.g., “judge whether if the current position of the box is the same or n position before the current position”) or nonspatial design (e.g., “judge whether if the current number is the same of n numbers before the current number”). In addition, (7) “verbal” and (8) “nonverbal” maps were also created, in which we included all studies with a nonspatial design that have used stimuli like letters, numbers or any other type of stimuli requiring a stimulus‐dependent semantic process (verbal WM); or nonverbal stimuli like faces, objects, images (nonverbal WM). For “verbal” and “nonverbal” maps, we produced a set of sub‐maps, one for each type of stimuli used: (9) “letters,” (10) “numbers,” (11) “faces,” and (12) “objects/images” maps. Finally, (12) a map reporting neural deactivations during the n‐back task (i.e., negative BOLD signal), as well as (13) a modality and stimuli‐unspecific map, showing the activation nodes common to each n‐back task were also created (for methods and results see the Supporting Information).
2.3. ALE maps computation
The quantitative evaluation of spatial PET and fMRI patterns was carried out using the ALE technique implemented using GingerALE software v2.3.2 (http://www.brainmap.org; Eickhoff et al., 2009; Eickhoff et al., 2012). The method yields a statistical map that indicates the set of significant voxels while considering the magnitude of the effect, the number of studies and the number of participants in each study.
First, lists of coordinates were carefully checked for duplication of data across publications, in order to avoid artifactual inflation of a given foci significance. Differences in coordinate spaces (MNI vs. Talairach space) between experiments were accounted for by transforming coordinates reported in Talairach space into MNI coordinates through the “tal2mni” algorithm implemented in GingerALE. The reported foci of activation for each study were modeled as Gaussian distributions and merged into a single 3D volume. Equally weighted coordinates were used to form estimates of the probability of activation for each voxel in the brain, using an estimation of the inter‐subject and inter‐study variability usually observed in neuroimaging experiments, rather than applying a priori full‐width half maximum (FWHM) kernel. Therefore, the number of participants in each study influenced the spatial extent of the Gaussian function used. We first modeled the probability of activation at each spatial point in the brain, returning localized “activation likelihood estimates” or ALE values. Values were then compared to a null distribution created from simulated datasets with randomly placed foci to identify significantly activated clusters (permutations test = 1,000 run). Corrections based on false‐discovery rate (FDR) at the cluster‐level and voxel‐level family‐wise error (FWE) estimation (Eickhoff et al., 2012) were applied. In details, cluster correction for multiple comparisons with a p < .001 threshold for cluster‐formation and a p < .05 for cluster‐level inference were set. Furthermore, in all tables, only clusters with a size exceeding the cluster size recommended by the authors of the ALE software were reported (range 500–1,000 mm3), except for Table 10 reporting deactivation clusters; given the small number of studies reporting deactivation patterns, we opted for a less stringent cluster size threshold in order to ease the interpretation of the resulting spatial topography. Specifically, we considered clusters with a volume <500 mm3, resulting in a deactivation pattern highly resembling the topography of a specific RSN, that is, the default mode network (DMN; Figure 12). All values were chosen based on their common use in similar meta‐analyses (Eickhoff et al., 2012; Turkeltaub et al., 2012).
Contrast images were created from the subtraction of each pair of ALE maps, together with a map showing their statistically significant overlap. Given that the resulting subtraction image has the major drawback of not considering the differences in the dataset sizes between the two original maps, GingerALE's simulated data of the pooled foci datasets—obtained by randomly dividing the pooled data into two new groupings of the same size as the original datasets—were considered. An ALE image is hereby created for each new dataset, then subtracted from the other and compared to the original data.
Finally, to further demonstrate the robustness and reliability of our results, we ran the analysis with a different version of GingerALE (3.0), focusing on maps based on a low (e.g., visual shape) and high (e.g., verbal letter) number of articles. We applied FWE correction both at cluster‐level and voxel‐level (p < .001 for cluster‐formation; p < .05 for cluster‐level inference). ALE maps were visualized using MRICronGL on an MNI standard brain.
2.4. Anatomic‐functional characterization of WM load maps
For the specific contrasts examining the impact of WM load on n‐back activation maps, an additional analysis was carried out to investigate the anatomo‐functional profile of activations characterizing the highest load conditions (i.e., 3‐back). Specifically, the anterior cingulate cortex (ACC) parcellation by Neubert, Mars, Sallet, and Rushworth (2015) and the cerebellar parcellation by Buckner, Krienen, Castellanos, Diaz, and Yeo (2011) were used. We imported each WM‐load map in MATLAB (The MathWorks, Release 2016b) and computed their quantitative overlap.
2.5. Overlap between n‐back and resting‐state fMRI networks
Shirer, Ryali, Rykhlevskaia, Menon, and Greicius (2012) defined 14 non‐overlapping maps representing distinct resting state networks: dorsal and ventral default mode (vDMN, dDMN), dorsal and ventral attention (AN), anterior and posterior salience (AS, PS), right and left executive control (RECN, LECN), language (LANG), basal ganglia (BG), high and primary visual (HVIS, PVIS), precuneus (PREC), somatosensory (SM), and auditory (AUD) networks (Shirer et al., 2012). This parcellation of RSNs was used to further estimate how much—in terms of percentage of voxels—each n‐back map correspond with functional networks.
3. RESULTS
3.1. Ale maps
A total of 152 studies were retrieved and examined. Only 85 of them were found to match our inclusion criteria and were ultimately entered in the analysis (a more detailed overview of the literature search is reported in Figure S1). A complete list of the included studies, reporting the type of n‐back tasks examined, the stimuli, their presentation modalities, the reference systems (MNI or Talairach), the number of foci and imaging techniques (e.g., fMRI, PET) are reported in Table S1. Moreover, information about sample size, gender, age, acquisition parameters (e.g., MRI scanner, TR, TE, FA) and the neuroimaging software used for fMRI/PET data analysis are shown for each paper in Table S2.
In the following section, tables and figures for each n‐back map are reported. A discussion about the role of each specific node is provided in the Discussion section of the article. Each map is available for download as a nifti.nii volumetric file at http://www.tmslab.org/santalab.php. Both full‐network and single‐node level maps are provided, along with the corresponding cluster size, MNI coordinates, and anatomical labeling.
3.2. Working memory network
The resulting map and coordinates of the comprehensive set of activation patterns during n‐back task execution are reported in Figure 1 and Table 1. The map includes 15 separate nodes highlighting a bilateral frontoparietal network of regions. Moreover, additional contribution from regions in the left cerebellum, fusiform gyrus, as well as from subcortical structures including the insula, claustrum, caudate, and lentiform nucleus, were found.
Figure 1.
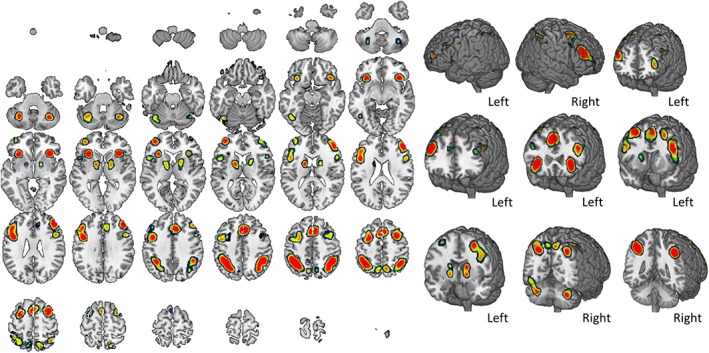
Brain activation during n‐back task. The result of cluster‐based statistics performed on the entire dataset of studies is shown. The map summarizes all 85 studies assessing n‐back tasks considered in the meta‐analysis, without distinction in material type or presentation modality. A complete set of coordinates for each cluster is available in Table 1 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
N‐back nodes information
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 20,608 | −41.2 | 11.6 | 34.6 | 0.089 | −42 | 4 | 30 | 6 | L | Frontal | Precentral gyrus |
| 0.079 | −28 | 2 | 54 | 6 | L | Frontal | Middle frontal gyrus | |||||
| 0.060 | −46 | 26 | 30 | 9 | L | Frontal | Middle frontal gyrus | |||||
| 0.036 | −52 | 14 | 4 | 44 | L | Frontal | Precentral gyrus | |||||
| 2 | 14,288 | −36.1 | −51.1 | 46.2 | 0.128 | −36 | −50 | 44 | 40 | L | Parietal | Inferior parietal lobule |
| 0.111 | −42 | −46 | 46 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 3 | 12,968 | 1.6 | 19.2 | 46.4 | 0.116 | 4 | 20 | 46 | 6 | R | Frontal | Medial frontal gyrus |
| 0.063 | −4 | 10 | 58 | 6 | L | Frontal | Medial frontal gyrus | |||||
| 4 | 11,112 | 39.6 | −52 | 45.8 | 0.138 | 44 | −46 | 44 | 40 | R | Parietal | Inferior parietal lobule |
| 0.072 | 34 | −64 | 44 | 19 | R | Parietal | Precuneus | |||||
| 5 | 9,024 | 36.3 | 10.2 | 46.7 | 0.101 | 30 | 8 | 56 | 6 | R | Frontal | Subgyral |
| 0.045 | 48 | 12 | 28 | 9 | R | Frontal | Inferior frontal gyrus | |||||
| 6 | 7,976 | 43.1 | 38.6 | 23 | 0.083 | 46 | 40 | 24 | 9 | R | Frontal | Superior frontal gyrus |
| 0.077 | 44 | 32 | 30 | 9 | R | Frontal | Middle frontal gyrus | |||||
| 0.028 | 38 | 58 | 2 | 10 | R | Frontal | Middle frontal gyrus | |||||
| 0.027 | 40 | 58 | −8 | 10 | R | Frontal | Middle frontal gyrus | |||||
| 7 | 5,456 | −35.9 | −62.5 | −26.8 | 0.064 | −30 | −58 | −34 | L | Cerebellum | Cerebellar tonsil | |
| 0.037 | −44 | −66 | −16 | L | Cerebellum | Declive | ||||||
| 0.037 | −46 | −62 | −14 | 37 | L | Temporal | Fusiform gyrus | |||||
| 8 | 5,408 | 35.3 | 24.1 | −4.2 | 0.134 | 34 | 24 | −2 | 13 | R | Subcortical | Insula |
| 9 | 4,920 | −32.2 | 23.1 | −3 | 0.109 | −32 | 22 | −2 | L | Subcortical | Claustrum | |
| 10 | 3,920 | −15.5 | −0.4 | 8.8 | 0.069 | −16 | 0 | 14 | L | Subcortical | Caudate | |
| 0.054 | −16 | 0 | 2 | L | Subcortical | Lentiform nucleus | ||||||
| 11 | 3,904 | −38.1 | 50.8 | 7.8 | 0.068 | −38 | 52 | 10 | 10 | L | Frontal | Middle frontal gyrus |
| 12 | 3,200 | 16.5 | 1.6 | 5.8 | 0.048 | 16 | 0 | 0 | R | Subcortical | Lentiform nucleus | |
| 0.037 | 16 | 0 | 16 | R | Subcortical | Caudate | ||||||
| 13 | 2,736 | 34.2 | −62 | −31.7 | 0.069 | 32 | −62 | −32 | R | Cerebellum | ||
| 0.027 | 36 | −68 | −18 | R | Cerebellum | Declive | ||||||
| 14 | 2,448 | 11.5 | −64.9 | 54.9 | 0.048 | 8 | −64 | 52 | 7 | R | Parietal | Precuneus |
| 0.045 | 16 | −66 | 60 | 7 | R | Parietal | Precuneus | |||||
| 15 | 1,664 | −9 | −65.8 | 51.9 | 0.040 | −8 | −68 | 48 | 7 | L | Parietal | Precuneus |
| 0.035 | −8 | −62 | 54 | 7 | L | Parietal | Precuneus | |||||
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for general n‐back.
3.3. N‐back load
Activation patterns resulting from the contrasts between different n‐back loads (high vs. low) are reported in Figure 2. Maps include different nodes of activation for different contrasts: 3 versus 1 back are reported in green, 3 versus 2 back in blue, 2 versus 1 back in red.
Figure 2.
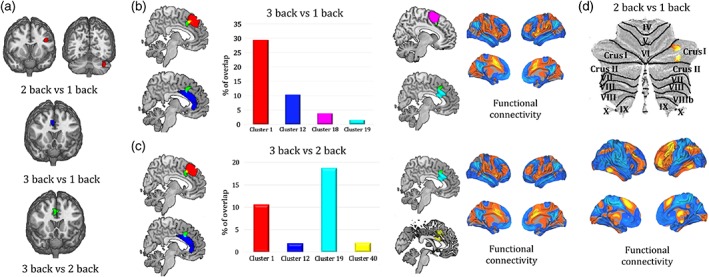
Anatomical and functional mapping of regions resulting from contrast maps. (a) Cerebral and cerebellar nodes act as a function of task load. (b, c) Mapping of ACC activation (3 vs. 1 back and 3 vs. 2 back; green) and respective overlap with clusters of ACC parcellation by Neubert et al. (2015). Functional connectivity maps for each contrast are shown to the right of each panel. (d) Functional mapping of cerebellar activation (2 back vs. 1 back) and corresponding functional connectivity map were computed according to parcellation by Buckner et al. (2011) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Anatomical and functional mapping
The difference between 3‐back and 2/1‐back maps highlighted clusters mostly located in the ACC and right cerebellum (Figure 2a). Significant nodes of interest in the 3 back versus 1 back map were found to overlap with four regions belonging to the aforementioned parcellation by Neubert et al. (Figure 2b):
area8m (red), representing an area in the medial portion of the human superior frontal gyrus extending down to the paracingulate sulcus;
right area 25 (blue), i.e., the subgenual area;
right anterior rostral cingulate zone (RCZa) (purple);
right posterior rostral cingulate zone (RCZp) (cyan).
Of those, rostral regions have already proven to be implicated in learning and in the update of choices' value (Walton, Devlin, & Rushworth, 2004), as well as in cognitive control tasks (Picard & Strick, 1996).
The 3 versus 2 back contrast showed overlap with the same areas of the 3 versus 1 back map (8 m, area 25, right RCZp) with the addition of left RCZp (shown in yellow; Figure 2c).
For what concerns the difference between 2 back versus 1 back maps, a significant node of interest in the contrast was found to correspond with the crus‐I region of the cerebellar parcellation by Buckner et al. (Figure 2d).
To characterize the spontaneous functional connectivity of ACC and crus‐I, a seed‐to‐voxel analysis was run on a database of 1,000 healthy participants from Yeo et al. (2011). Unthreshold functional connectivity maps are shown in Figure 2b–d.
3.5. Verbal n‐back
Figure 3 and Table 2 report neural activation patterns during verbal n‐back tasks, without distinguishing between type of stimuli (letters, numbers, or words). The map includes 21 clusters (i.e., nodes) of activation highlighting the involvement of bilateral frontal and parietal cortices, cerebellum as well as various other subcortical structures bilaterally. Results were successfully replicated using GingerALE 3.0 (see Supporting Information Figure S3).
Figure 3.
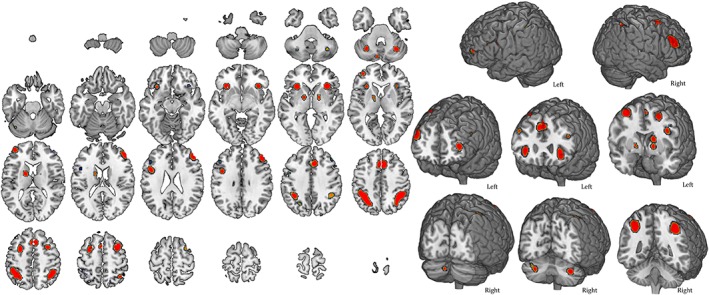
Areas of activation during verbal n‐back tasks. The map refers to 61 verbal n‐back studies in which stimuli (letters, numbers, or words) were presented visually. A complete set of coordinates for each cluster is available in Table 2 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Activity patterns in verbal n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 6,800 | −36 | −50 | 45.2 | 0.104 | −36 | −48 | 42 | 40 | L | Parietal | Inferior parietal lobule |
| 0.063 | −30 | −58 | 44 | 39 | L | Parietal | Angular gyrus | |||||
| 2 | 5,168 | 40.1 | −49.7 | 45.7 | 0.111 | 44 | −46 | 46 | 40 | R | Parietal | Inferior parietal lobule |
| 0.046 | 34 | −64 | 44 | 19 | R | Parietal | Precuneus | |||||
| 3 | 4,752 | 2.4 | 19.6 | 45.2 | 0.096 | 4 | 20 | 44 | 6 | R | Frontal | Medial frontal gyrus |
| 0.043 | −4 | 8 | 58 | 6 | L | Frontal | Medial frontal gyrus | |||||
| 4 | 3,568 | 45.1 | 37.9 | 25.7 | 0.064 | 46 | 40 | 24 | 9 | R | Frontal | Superior frontal gyrus |
| 0.059 | 46 | 34 | 30 | 9 | R | Frontal | Middle frontal gyrus | |||||
| 5 | 2,376 | 31.2 | 8.3 | 56 | 0.083 | 30 | 8 | 56 | 6 | R | Frontal | Subgyral |
| 6 | 2,216 | −32.2 | 23 | −3.6 | 0.082 | −32 | 22 | −2 | L | Subcortical | Claustrum | |
| 7 | 1880 | 34.2 | 23.8 | −2 | 0.090 | 34 | 24 | 0 | 13 | R | Subcortical | Insula |
| 8 | 1,632 | −27.7 | 4.4 | 53.5 | 0.062 | −28 | 2 | 54 | 6 | L | Frontal | Middle frontal gyrus |
| 9 | 1,480 | −44.8 | 8.5 | 27 | 0.052 | −42 | 6 | 28 | 6 | L | Frontal | Precentral gyrus |
| 10 | 1,360 | −16.1 | 0.2 | 8.7 | 0.060 | −16 | 0 | 14 | L | Subcortical | Caudate | |
| 0.052 | −16 | 0 | 0 | L | Subcortical | Lentiform nucleus | ||||||
| 11 | 944 | −39.1 | 51.6 | 9.5 | 0.059 | −40 | 52 | 10 | 46 | L | Frontal | Middle frontal gyrus |
| 12 | 792 | 33.2 | −62.4 | −33.5 | 0.057 | 34 | −64 | −34 | R | Cerebellum | Cerebellar tonsil | |
| 13 | 680 | −32.1 | −62 | −32.8 | 0.046 | −30 | −62 | −34 | L | Cerebellum | Cerebellar tonsil | |
| 14 | 328 | 16.3 | 0 | 0.6 | 0.041 | 16 | 0 | 0 | R | Subcortical | Lentiform nucleus | |
| 15 | 192 | −8.5 | −78 | −32 | 0.048 | −8 | −78 | −32 | L | Cerebellum | Pyramis | |
| 16 | 184 | −47.5 | 25.4 | 27.9 | 0.037 | −48 | 26 | 28 | 9 | L | Frontal | Middle frontal gyrus |
| 17 | 56 | −48.3 | 2.3 | 38.9 | 0.033 | −48 | 2 | 38 | 6 | L | Frontal | Precentral gyrus |
| 18 | 24 | 16.7 | 0 | 15.3 | 0.035 | 16 | 0 | 16 | R | Subcortical | Caudate | |
| 19 | 24 | 16 | −65.3 | 58.7 | 0.033 | 16 | −66 | 58 | 7 | R | Parietal | Precuneus |
| 20 | 8 | 0 | −48 | −22 | 0.033 | 0 | −48 | −22 | L | Cerebellum | Cerebellar lingual | |
| 21 | 8 | −12 | −66 | 54 | 0.033 | −12 | −66 | 54 | 7 | L | Parietal | Precuneus |
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for verbal n‐back.
Maps and coordinates of the activity patterns elicited during the performance of letters or numbers n‐back tasks are shown in Figures 4 and 5 and Tables 3 and 4. N‐back tasks using letters show a clear frontoparietal activation, left‐lateralized over frontal structures. Moreover, an involvement of the bilateral cerebellum and subcortical structures is also present (Figure 3, Table 2).
Figure 4.
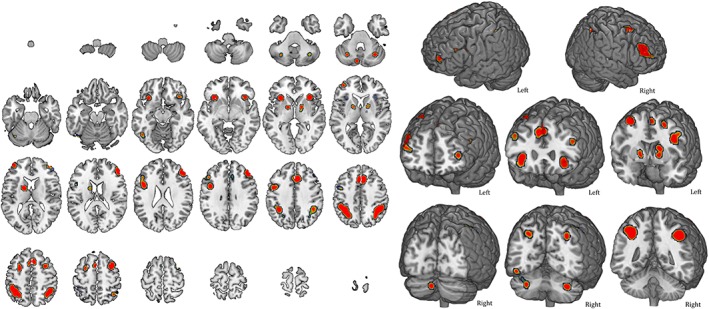
Average activity during letter n‐back tasks. The map refers to studies that only used letters as stimuli (49). A complete set of coordinates for each cluster is available in Table 3 [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
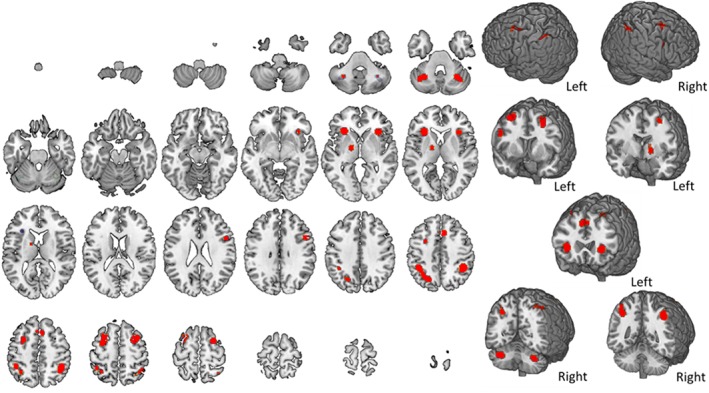
Brain activity during number n‐back tasks. The map refers to 12 number n‐back studies. A complete set of coordinates for each cluster is available in Table 4 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Activity patterns for letter n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 7,920 | −36.4 | −49.09 | 45.35 | 0.086 | −36 | −48 | 42 | 40 | L | Parietal | Inferior parietal lobule |
| 0.057 | −44 | −42 | 48 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 0.049 | −28 | −58 | 46 | 7 | L | Parietal | Superior parietal lobule | |||||
| 2 | 6,616 | 2.19 | 19.92 | 44.69 | 0.076 | 4 | 20 | 44 | 6 | R | Frontal | Medial frontal gyrus |
| 0.038 | −4 | 10 | 56 | 6 | L | Frontal | Medial frontal gyrus | |||||
| 3 | 6,344 | 40.12 | −50.39 | 45.26 | 0.077 | 44 | −46 | 46 | 40 | R | Parietal | Inferior parietal lobule |
| 0.052 | 36 | −54 | 48 | 7 | R | Parietal | Inferior parietal lobule | |||||
| 4 | 5,032 | 45.14 | 38.44 | 24.49 | 0.062 | 46 | 40 | 24 | 9 | R | Frontal | Superior frontal gyrus |
| 0.029 | 40 | 52 | 12 | 10 | R | Frontal | Middle frontal gyrus | |||||
| 5 | 4,080 | −45.07 | 8.71 | 30.67 | 0.050 | −42 | 6 | 28 | 6 | L | Frontal | Precentral gyrus |
| 0.033 | −48 | 2 | 38 | 6 | L | Frontal | Precentral gyrus | |||||
| 0.029 | −46 | 26 | 28 | 9 | L | Frontal | Middle frontal gyrus | |||||
| 6 | 2,472 | 31.93 | 9.21 | 55.85 | 0.062 | 30 | 8 | 54 | 6 | R | Frontal | Subgyral |
| 7 | 2,440 | −32.58 | 22.31 | −5.41 | 0.073 | −32 | 22 | −4 | L | Subcortical | Claustrum | |
| 8 | 2,224 | 34.68 | 23.81 | −3.85 | 0.066 | 34 | 24 | −2 | 13 | R | Subcortical | Insula |
| 9 | 1816 | −16.46 | 1.36 | 8.92 | 0.050 | −16 | 2 | 14 | L | Subcortical | Caudate | |
| 0.044 | −16 | 0 | 0 | L | Subcortical | Lentiform nucleus | ||||||
| 10 | 1,344 | −27.12 | 4.54 | 52.5 | 0.048 | −28 | 2 | 52 | 6 | L | Frontal | Middle frontal gyrus |
| 11 | 1,160 | −39.41 | 52.62 | 9.74 | 0.045 | −40 | 52 | 10 | 46 | L | Frontal | Middle frontal gyrus |
| 12 | 824 | 15.57 | 0.66 | 1.3 | 0.035 | 16 | 2 | 0 | R | Subcortical | Lentiform nucleus | |
| 0.025 | 14 | −6 | 0 | R | Subcortical | Thalamus | ||||||
| 13 | 720 | 32.83 | −63.53 | −33.84 | 0.045 | 32 | −64 | −34 | R | Cerebellum | Cerebellar tonsil | |
| 14 | 656 | −31.93 | −64.21 | −33.44 | 0.037 | −30 | −64 | −34 | L | Cerebellum | Cerebellar tonsil | |
| 0.024 | −38 | −66 | −26 | L | Cerebellum | Tuber | ||||||
| 15 | 568 | −46.06 | −63.51 | −15.36 | 0.031 | −46 | −64 | −16 | 37 | L | Temporal | Fusiform gyrus |
| 16 | 480 | −8.72 | −78.15 | −32.26 | 0.045 | −8 | −78 | −32 | L | Cerebellum | Pyramis | |
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for letter n‐back.
Table 4.
Activity patterns in number n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 3,576 | −34.8 | −53.4 | 45.7 | 0.024 | −40 | −46 | 44 | 40 | L | Parietal | Inferior parietal lobule |
| 0.023 | −24 | −66 | 42 | 7 | L | Parietal | Precuneus | |||||
| 0.016 | −34 | −54 | 54 | 7 | L | Parietal | Superior parietal lobule | |||||
| 0.015 | −42 | −48 | 58 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 2 | 2,792 | −29 | 5.6 | 55.3 | 0.023 | −30 | 0 | 58 | 6 | L | Frontal | Middle frontal gyrus |
| 3 | 2,664 | 41.3 | −47.2 | 47.7 | 0.043 | 42 | −44 | 46 | 40 | R | Parietal | Inferior parietal lobule |
| 0.013 | 34 | −58 | 60 | 7 | R | Parietal | Superior parietal lobule | |||||
| 4 | 1928 | 28.2 | 6 | 57.8 | 0.028 | 28 | 4 | 58 | 6 | R | Frontal | Subgyral |
| 5 | 1,632 | −30.5 | 24.6 | 3.1 | 0.027 | −30 | 26 | 2 | 13 | L | Subcortical | Insula |
| 6 | 1,328 | 33.9 | 23.7 | .5 | 0.031 | 34 | 24 | 2 | 13 | R | Subcortical | Insula |
| 7 | 1,264 | −34.1 | −59.4 | −32.6 | 0.021 | −32 | −58 | −32 | L | Cerebellum | Anterior lobe | |
| 8 | 1,152 | 3.9 | 19.4 | 46.8 | 0.023 | 6 | 20 | 46 | 6 | R | Frontal | Medial frontal gyrus |
| 0.013 | −4 | 22 | 48 | 8 | L | Frontal | Superior frontal gyrus | |||||
| 9 | 1,064 | 32.3 | −59.6 | −32.3 | 0.023 | 30 | −58 | −32 | R | Cerebellum | Anterior lobe | |
| 10 | 816 | 49 | 11 | 28.7 | 0.017 | 50 | 10 | 28 | 9 | R | Frontal | Inferior frontal gyrus |
| 11 | 736 | −16.6 | −5.4 | 2.2 | 0.020 | −18 | −6 | 0 | L | Subcortical | Lentiform nucleus | |
| 0.012 | −16 | 0 | 12 | L | Subcortical | Caudate | ||||||
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for number n‐back.
For what concerns n‐back tasks based on the visual presentation of numbers, 11 clusters of activity emerged (Table 4), involving mostly the parietal cortex bilaterally, the medial frontal cortex, the right and left insula, and the anterior lobe of the cerebellum in both hemispheres (Figure 5).
3.6. Visual–Nonverbal n‐back
Brain activity during nonverbal n‐back tasks and their corresponding set of coordinates are reported in Figure 6 and Table 5. Studies considered in this section refer to those in which nonverbal stimuli (e.g., images, faces, and objects) were visually presented during task execution. Figure 6 shows the results of 15 studies, without distinguishing for material type. The map includes nine nodes highlighting the involvement of the left frontal cortex, the inferior parietal lobule (IPL) bilaterally and various subcortical structures, including the left insula and the right claustrum, right limbic structures—in particular, the cingulate gyrus—and the left cerebellum. No active nodes were found over temporal lobe regions.
Figure 6.
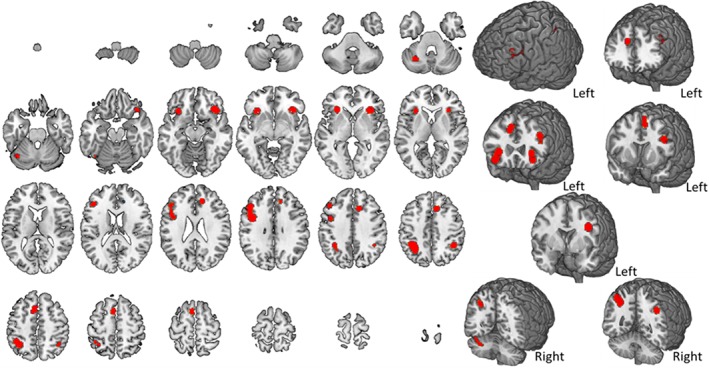
Average activity during visual–nonverbal n‐back tasks. The map refers to 15 studies relying on nonverbal n‐back tasks where stimuli (faces, objects, and images) were presented visually. A complete set of coordinates for each cluster is available in Table 5 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 5.
Activity patterns for nonverbal n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 4,216 | −44.2 | 15.2 | 29.9 | 0.029 | −42 | 4 | 32 | 6 | L | Frontal | Precentral gyrus |
| 0.023 | −46 | 24 | 32 | 9 | L | Frontal | Middle frontal gyrus | |||||
| 0.022 | −44 | 28 | 34 | 9 | L | Frontal | Precentral gyrus | |||||
| 0.017 | −44 | 30 | 20 | 46 | L | Frontal | Middle frontal gyrus | |||||
| 2 | 3,800 | −35.6 | −50.8 | 45.9 | 0.029 | −34 | −52 | 44 | 40 | L | Parietal | Inferior parietal lobule |
| 0.023 | −40 | −48 | 50 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 3 | 3,208 | 35 | 25.4 | −6.4 | 0.037 | 32 | 24 | 0 | R | Subcortical | Claustrum | |
| 0.022 | 40 | 24 | −14 | 47 | R | Frontal | Inferior frontal gyrus | |||||
| 4 | 2072 | −31.7 | 24.1 | −4.6 | 0.024 | −34 | 22 | −10 | 47 | L | Frontal | Inferior frontal gyrus |
| 0.021 | −30 | 26 | 2 | 13 | L | Subcortical | Insula | |||||
| 5 | 2008 | −6.3 | 12.6 | 54.7 | 0.023 | −6 | 16 | 50 | 6 | L | Frontal | Superior frontal gyrus |
| 0.019 | −6 | 10 | 62 | 6 | L | Frontal | Medial frontal gyrus | |||||
| 6 | 1,120 | 10.2 | 22.3 | 41 | 0.021 | 12 | 22 | 40 | 32 | R | Limbic | Cingulate gyrus |
| 7 | 1,032 | 41.2 | −48.8 | 44.3 | 0.022 | 42 | −48 | 44 | 40 | R | Parietal | Inferior parietal lobule |
| 8 | 728 | −35.9 | −61.8 | −28.3 | 0.014 | −40 | −62 | −24 | L | Cerebellum | Declive | |
| 0.014 | −36 | −62 | −30 | L | Cerebellum | |||||||
| 9 | 632 | 12.5 | 35.9 | 25.9 | 0.020 | 12 | 36 | 26 | 32 | R | Limbic | Cingulate gyrus |
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for nonverbal n‐back.
When the same studies were differentiated based on stimuli's characteristics, two different maps and tables of coordinates were obtained. Figure 7 and Table 6 refer to those studies in which faces were presented as stimuli. Six clusters (i.e., nodes) of activity over the left frontal and parietal cortices and subcortical structures (left insula and right claustrum mainly) are shown. Similarly to what observed for the general nonverbal map, no activity in the temporal lobe was found during face n‐back tasks.
Figure 7.
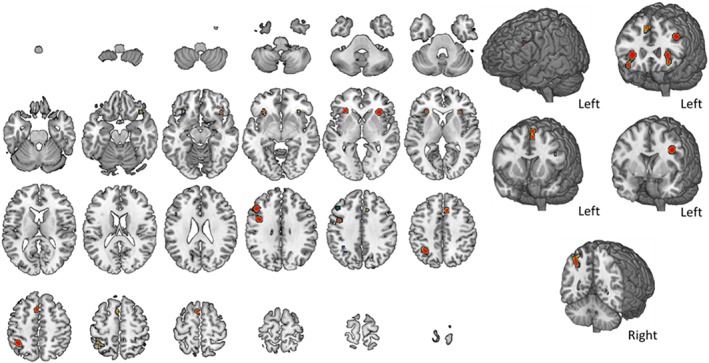
Brain activity during face n‐back tasks. The map summarizes findings from nine studies. A complete set of coordinates for each cluster is available in Table 6 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 6.
Activation patterns for face n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 1,768 | 0 | 16.8 | 51.3 | 0.015 | −4 | 16 | 52 | 6 | L | Frontal | Superior frontal gyrus |
| 0.013 | −6 | 12 | 64 | 6 | L | Frontal | Medial frontal gyrus | |||||
| 0.013 | 8 | 22 | 44 | 6 | R | Frontal | Medial frontal gyrus | |||||
| 2 | 1,752 | −36.3 | −51.9 | 48 | 0.018 | −40 | −48 | 52 | 40 | L | Parietal | Inferior parietal lobule |
| 0.016 | −32 | −58 | 42 | 39 | L | Parietal | Angular gyrus | |||||
| 0.010 | −36 | −48 | 38 | 40 | L | Parietal | Supramarginal gyrus | |||||
| 0.010 | −32 | −52 | 58 | 7 | L | Parietal | Superior parietal lobule | |||||
| 3 | 1,328 | 33.2 | 25.2 | −3.6 | 0.023 | 32 | 26 | 2 | R | Subcortical | Claustrum | |
| 0.013 | 38 | 24 | −16 | 47 | R | Frontal | Inferior frontal gyrus | |||||
| 4 | 1,064 | −31.4 | 26.8 | −2.3 | 0.017 | −30 | 28 | 2 | 13 | L | Subcortical | Insula |
| 0.012 | −32 | 26 | −10 | 47 | L | Frontal | Inferior frontal gyrus | |||||
| 5 | 928 | −41.8 | 3.6 | 32.7 | 0.017 | −42 | 2 | 34 | 6 | L | Frontal | Precentral gyrus |
| 6 | 832 | −45.8 | 24.3 | 31.8 | 0.018 | −46 | 26 | 32 | 9 | L | Frontal | Middle frontal gyrus |
| 0.010 | −46 | 14 | 26 | 9 | L | Frontal | Inferior frontal gyrus | |||||
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for face n‐back.
Figure 8 and Table 7 refer to studies in which object or images were used as stimuli. The resulting map shows six activation clusters, involving the frontal cortex bilaterally, the left parietal cortex—in particular, the IPL—various subcortical structures bilaterally and the right cingulate gyrus. Neither the cerebellum nor the temporal lobe showed an involvement within this type of stimuli. The same exact results were obtained using GingerALE 3.0 (Supporting Information Figure S4).
Figure 8.
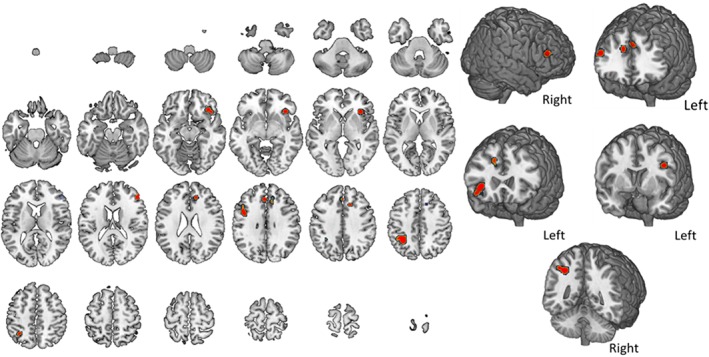
Average of the activity during objects/images n‐back. The map summarizes findings from six studies. A complete set of coordinates for each cluster is available in Table 7 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 7.
Activity patterns for objects/images n‐back tasks
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 1952 | −34.3 | −48.7 | 44.4 | 0.019 | −32 | −50 | 44 | 40 | L | Parietal | Inferior parietal lobule |
| 0.012 | −44 | −48 | 46 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 2 | 1,664 | 36.5 | 25.6 | −6.9 | 0.018 | 34 | 24 | −2 | 13 | R | Subcortical | Insula |
| 0.012 | 38 | 30 | −12 | 47 | R | Frontal | Inferior frontal gyrus | |||||
| 0.012 | 42 | 24 | −12 | 47 | R | Subcortical | Extra‐nuclear | |||||
| 3 | 904 | −44.5 | 8 | 30.6 | 0.014 | −44 | 6 | 30 | 9 | L | Frontal | Inferior frontal gyrus |
| 0.011 | −46 | 20 | 30 | 9 | L | Frontal | Inferior frontal gyrus | |||||
| 4 | 864 | 12.3 | 30.1 | 30.8 | 0.013 | 12 | 36 | 26 | 32 | R | Limbic | Cingulate gyrus |
| 0.012 | 14 | 22 | 38 | 32 | R | Limbic | Cingulate gyrus | |||||
| 5 | 792 | 48.1 | 37.7 | 18.1 | 0.014 | 48 | 36 | 18 | 46 | R | Frontal | Middle frontal gyrus |
| 6 | 688 | −2.6 | 33 | 33.2 | 0.015 | −4 | 34 | 32 | 6 | L | Frontal | Medial frontal gyrus |
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for objects/images n‐back.
3.7. Spatial n‐back
Studies characterized by the spatial presentation of stimuli were selected and their results displayed in Figure 9. Spatial n‐back requires to monitor the location of dots within a diamond shaped box on the screen at a given delay (0‐, 1‐, or 2‐back; Kumari et al., 2006). Coordinates of brain activity for this type of task are shown in Table 8. A great involvement of the parietal cortex bilaterally and of the bilateral frontal regions, right cingulate gyrus and right insula were found. Neither the cerebellum nor the temporal lobe showed significant activations.
Figure 9.
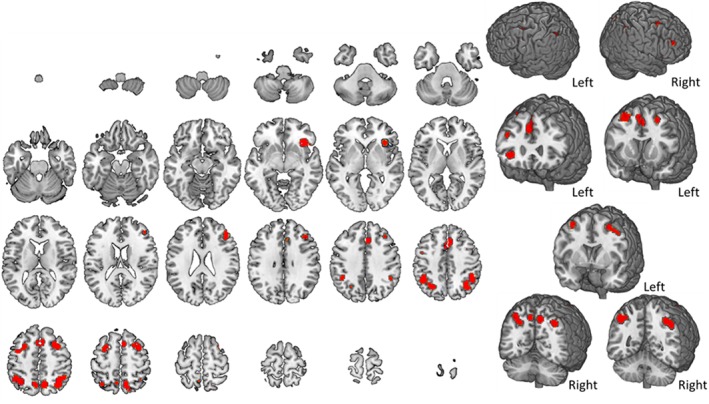
Brain activity during spatial n‐back tasks. The map summarizes findings from nine studies. A complete set of coordinates for each cluster is available in Table 8 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 8.
Spatial n‐back activation foci
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 3,784 | 39.2 | −54 | 46.8 | 0.028 | 36 | −54 | 50 | 7 | R | Parietal | Inferior parietal lobule |
| 0.022 | 34 | −64 | 48 | 19 | R | Parietal | Precuneus | |||||
| 0.020 | 46 | −48 | 44 | 40 | R | Parietal | Inferior parietal lobule | |||||
| 2 | 3,656 | −38.3 | −53.8 | 45.9 | 0.028 | −44 | −48 | 44 | 40 | L | Parietal | Inferior parietal lobule |
| 0.021 | −28 | −62 | 42 | 7 | L | Parietal | Precuneus | |||||
| 0.019 | −34 | −56 | 50 | 7 | L | Parietal | Inferior parietal lobule | |||||
| 3 | 3,272 | 2.5 | 18.4 | 46.2 | 0.024 | 0 | 16 | 48 | 32 | L | Frontal | Medial frontal gyrus |
| 0.024 | 4 | 14 | 54 | 6 | R | Frontal | Superior frontal gyrus | |||||
| 0.022 | 4 | 24 | 38 | 32 | R | Limbic | Cingulate gyrus | |||||
| 4 | 2,280 | 29.8 | 10 | 53.6 | 0.030 | 28 | 12 | 52 | 6 | R | Frontal | Subgyral |
| 5 | 1,760 | 36.8 | 23.6 | −5.1 | 0.029 | 36 | 24 | −6 | 13 | R | Subcortical | Insula |
| 0.014 | 46 | 20 | −6 | R | Subcortical | Insula | ||||||
| 6 | 1,632 | −31 | 5.3 | 52.9 | 0.024 | −28 | 6 | 54 | 6 | L | Frontal | Subgyral |
| 0.015 | −40 | 0 | 50 | 6 | L | Frontal | Middle frontal gyrus | |||||
| 7 | 1,544 | 9.8 | −63.8 | 52.9 | 0.027 | 10 | −62 | 52 | 7 | R | Parietal | Precuneus |
| 0.017 | 14 | −72 | 58 | 7 | R | Parietal | Precuneus | |||||
| 8 | 1,408 | 41.1 | 34.4 | 26.8 | 0.018 | 44 | 40 | 22 | 9 | R | Frontal | Middle frontal gyrus |
| 0.016 | 42 | 30 | 26 | 9 | R | Frontal | Middle frontal gyrus | |||||
| 9 | 752 | −7.9 | −62 | 51.6 | 0.016 | −8 | −60 | 54 | 7 | L | Parietal | Precuneus |
| 0.014 | −8 | −66 | 48 | 7 | L | Parietal | Precuneus | |||||
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for spatial n‐back.
3.8. Auditory n‐back
Map and coordinates of the activity patterns elicited during the performance of auditory n‐back tasks are shown in Figure 10 and Table 9. The distinction is hereby made based on presentation modality, rather than on stimuli's type, such as that only studies characterized by the auditory presentation of stimuli were considered. The map includes five separate nodes located in the left frontal and parietal lobe bilaterally, together with the activation of the right cingulate gyrus and left insula.
Figure 10.
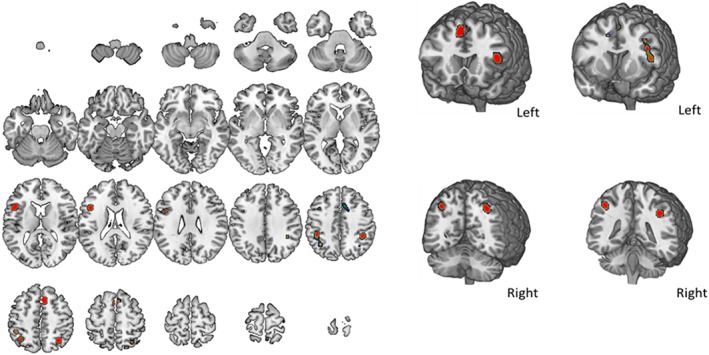
Areas of activity during auditory n‐back task. The map refers to seven auditory n‐back studies. A complete set of coordinates for each cluster is available in Table 9 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 9.
Auditory n‐back activation foci
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| 1 | 1936 | 4.2 | 17.7 | 50.8 | 0.017 | 6 | 20 | 50 | 6 | R | Frontal | Superior frontal gyrus |
| 0.016 | 2 | 20 | 50 | 6 | L | Frontal | Superior frontal gyrus | |||||
| 0.010 | −2 | 12 | 56 | 6 | L | Frontal | Superior frontal gyrus | |||||
| 0.008 | 14 | 8 | 44 | 24 | R | Limbic | Cingulate gyrus | |||||
| 2 | 1,736 | −46 | 14.1 | 18.1 | 0.015 | −46 | 16 | 16 | 13 | L | Subcortical | Insula |
| 0.010 | −44 | 8 | 28 | 9 | L | Frontal | Inferior frontal gyrus | |||||
| 0.008 | −40 | 10 | 40 | 9 | L | Frontal | Precentral gyrus | |||||
| 3 | 1,480 | −40.6 | −47.1 | 46.1 | 0.013 | −36 | −58 | 48 | 39 | L | Parietal | Inferior parietal lobule |
| 0.012 | −44 | −42 | 46 | 40 | L | Parietal | Inferior parietal lobule | |||||
| 4 | 872 | 31.5 | −60.5 | 51.2 | 0.013 | 32 | −60 | 50 | 7 | R | Parietal | Superior parietal lobule |
| 0.013 | 30 | −62 | 54 | 7 | R | Parietal | Superior parietal lobule | |||||
| 5 | 680 | 39.8 | −41.2 | 40.4 | 0.015 | 40 | −42 | 40 | 40 | R | Parietal | Inferior parietal lobule |
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere, and regional labels are reported for each cluster included in the ALE map for auditory n‐back.
3.9. N‐back and resting state networks
The overlap between RSNs and n‐back maps is presented in Figure 11.
Figure 11.
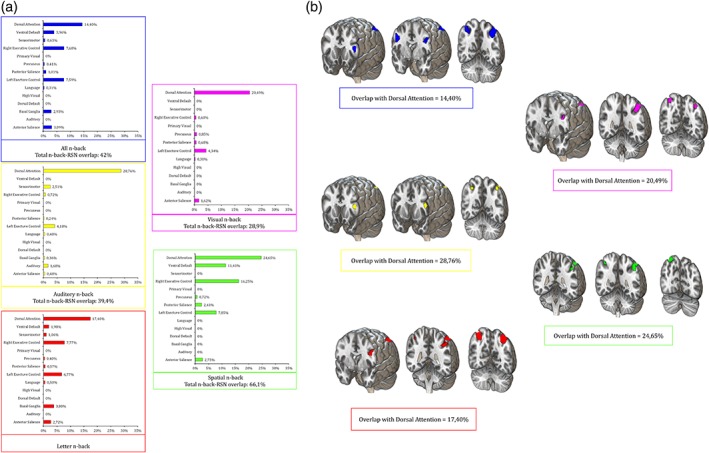
Overlaps between n‐back maps and RSNs. Panel a shows the total percentage of overlap between ALE maps for each n‐back task and the surface representation of RSNs according to Shirer et al. (2012). All resulting maps show greater correlation between n‐back tasks and the DAN, compared to other RSNs. Specific overlap percentages are reported in panel b [Color figure can be viewed at http://wileyonlinelibrary.com]
For what concerns stimuli‐dependent maps, verbal letter n‐back showed a 43% of overlap, compared to 28.9% reported for the nonverbal map. The greatest overlap is however observed for spatial maps, reaching a 66.1% of overlap. For what concerns auditory n‐back, a 39.4% of overlap is reported.
At the single RSN‐level, the greatest overlap involves the dorsal attention network (DAN) for all n‐back maps (Figure 11b). The overlap between n‐back regions and the DAN nodes appears especially over the parietal lobes and left frontal lobe. For the general n‐back task, overlap is observed with the Ventral Default (4%) and right (7.7%) and left (7.6%) Executive Control networks. The verbal letter n‐back task shows overlap with the right (7.8%) and left (6.8%) Executive Control network. On the other hand, both the auditory and visual maps show overlap only with the DAN.
3.10. Neural deactivations during the n‐back task
We collected 15 papers that used verbal (stimuli: letters/numbers) or face n‐back task and reported BOLD deactivations as part of the main results. From this database, activation and deactivation coordinates were extracted and corresponding ALE maps were obtained. The results are shown in Figure 12 and Table 10, without distinguishing between stimuli type. The map includes eight activation nodes corresponding to bilateral frontoparietal regions and five deactivation nodes, mostly located in the bilateral temporal lobe and the posterior cingulate cortex.
Figure 12.
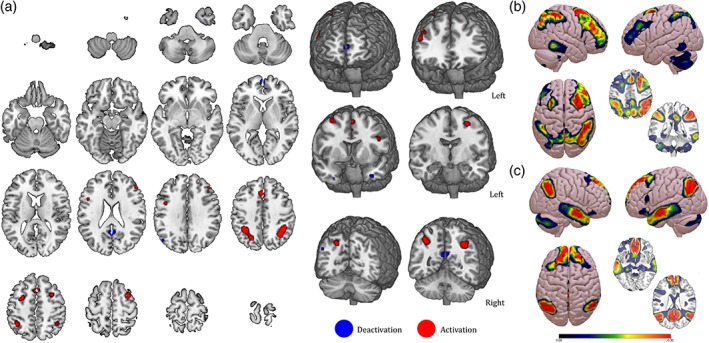
Increase and decrease during the n‐back task. (a) Activation (red) and deactivation (blue) nodes resulting from the analysis conducted on 15 articles. (b,c) Functional connectivity maps for the activation and deactivation nodes, respectively [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 10.
Volume, coordinates and corresponding Brodmann area, lobe, hemisphere and regional labels are reported for each cluster included in the ALE map for the increase and the decrease of neural activity during the n‐back task
| Weighted center | Extrema value coordinates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster number | Volume (mm3) | x | y | z | Extrema value | x | y | z | Brodmann area | Hemisphere | Lobe | Label |
| Deactivation | ||||||||||||
| 1 | 576 | −0.4 | −53.4 | 22.9 | 0.035 | −2 | −54 | 22 | 23 | L | Subcortical | Posterior cingulate |
| 2 | 304 | −1.5 | 59.6 | 5.7 | 0.029 | −2 | 60 | 6 | 10 | L | Frontal | Medial frontal gyrus |
| 3 | 64 | −34 | 5.2 | −36.8 | 0.025 | −34 | 6 | −36 | 38 | L | Temporal | Superior temporal gyrus |
| 4 | 64 | −49 | −69 | 31 | 0.026 | −50 | −68 | 32 | 39 | L | Temporal | Middle temporal gyrus |
| 5 | 16 | 30 | 6 | −41 | 0.025 | 30 | 6 | −40 | 38 | R | Temporal | Superior temporal gyrus |
| Activation | ||||||||||||
| 1 | 2,640 | 37.55 | −51.73 | 43.53 | 0.036 | 40 | −48 | 44 | 40 | R | Parietal | Inferior parietal lobule |
| 0.031 | 34 | −58 | 44 | 19 | R | Parietal | Precuneus | |||||
| 2 | 2,504 | −33.85 | −52.44 | 44.04 | 0.037 | −36 | −48 | 42 | 40 | L | Parietal | Inferior parietal lobule |
| 0.024 | −22 | −66 | 42 | 7 | L | Parietal | Precuneus | |||||
| 3 | 1,096 | 30.98 | 6.58 | 56.2 | 0.030 | 30 | 6 | 56 | 6 | R | Frontal | Subgyral |
| 4 | 720 | −2.97 | 21.94 | 42.66 | 0.024 | −4 | 24 | 44 | 6 | L | Frontal | Medial frontal gyrus |
| 5 | 600 | −44.5 | 6.3 | 29.98 | 0.019 | −46 | 2 | 36 | 6 | L | Frontal | Precentral gyrus |
| 0.018 | −42 | 6 | 30 | 6 | L | Frontal | Precentral gyrus | |||||
| 0.017 | −48 | 14 | 22 | 9 | L | Frontal | Inferior frontal gyrus | |||||
| 6 | 504 | −0.94 | 15.17 | 52.91 | 0.022 | −2 | 14 | 54 | 6 | L | Frontal | Superior frontal gyrus |
| 7 | 408 | 46.44 | 35.75 | 25.84 | 0.020 | 48 | 36 | 22 | 9 | R | Frontal | Middle frontal gyrus |
| 0.017 | 44 | 34 | 34 | 9 | R | Frontal | Middle frontal gyrus | |||||
| 8 | 408 | −28.53 | 0.25 | 51.65 | 0.020 | −28 | 0 | 52 | 6 | L | Frontal | Middle frontal gyrus |
Moreover, to characterize the functional connectivity profile of both activation and deactivation nodes, a seed‐to‐voxel analysis was run on a database of 1,000 healthy participants (Yeo et al., 2011). As expected, this analysis shows a strong positive connectivity profile between activation nodes and the DAN (Figure 12, panel b), and between deactivation nodes and the DMN (Figure 12, panel c).
4. DISCUSSION
We reviewed studies reporting fMRI or PET findings during n‐back task execution, aiming to create a set of activation maps specifically ideated to depict stimuli‐ and modality‐dependent activation patterns. In the following paragraphs we will discuss the functional role of the retrieved core regions for each n‐back task as well as of the observed overlap between n‐back related brain regions and RSNs related to executive control, salience, and attention. We will then discuss possible future functional and clinical applications, with a particular focus on neurostimulation and cognitive enhancement programs in both healthy subjects and neuropsychiatric patients showing dysfunctional WM performance.
4.1. Core regions in n‐back tasks
Congruent with the literature in the field, a frontoparietal network involvement underlying WM task execution ‐including n‐back performance‐ was confirmed in our ALE maps. Previous investigations (Owen et al., 2005; Rottschy et al., 2012) have reported the bilateral activation of a frontoparietal network and promoted the dorsolateral prefrontal cortex (DLPFC) as the area playing a key role in monitoring incoming information (Bagherzadeh, Khorrami, Zarrindast, Shariat, & Pantazis, 2016; Brunoni & Vanderhasselt, 2014; D'Esposito et al., 1998; Owen, 1997; Owen et al., 2005). DLPFC is known to be involved in the updating of goal representations based on contextual information and task‐related demands (Barch, Sheline, Csernansky, & Snyder, 2003; D'Esposito et al., 1995; D'Esposito, Postle, & Rypma, 2000), as well as in maintaining comprehensive representations by encoding task‐relevant rules and associated responses, stimulus features and conflictual information (Mansouri, Tanaka, & Buckley, 2009). However, differently from previous WM studies, we did not find a material‐dependent activation of DLPFC. Opposite to what we expected, a strong involvement of parietal cortices in verbal n‐back task was instead noticed, which has been previously described during short term storage of verbal material (Jonides et al., 1998; Miyauchi, Kitajo, & Kawasaki, 2016). Nevertheless, the IPL is known to underlie many higher‐order functions, including numerical judgments and arithmetic (Göbel & Rushworth, 2004; Hubbard, Piazza, Pinel, & Dehaene, 2005), reading (Turkeltaub, Eden, Jones, & Zeffiro, 2002), and semantic processing (Chou et al., 2006; Raposo, Moss, Stamatakis, & Tyler, 2006). Moreover, this area seems to be involved in the maintenance of goal‐directed attention (Corbetta & Shulman, 2002), action observation (Buccino et al., 2001), and visual presentation of graspable objects (Chao & Martin, 2000). IPL is further known to be split into two cytoarchitecturally distinct areas: Brodmann's area 40 corresponding to the supramarginal gyrus and Brodmann's area 39 representing the angular gyrus. Phonological processing, short‐term memory, and phonemes sequencing have been reported to engage the former (Gelfand & Bookheimer, 2003; Jacquemot, Pallier, LeBihan, Dehaene, & Dupoux, 2003; Paulesu, Frith, & Frackowiak, 1993), while the latter is involved in reading‐related tasks (e.g., understanding of the relationship among different characters; Inui et al., 1998). Despite few studies reporting an activation of IPL linked to spatial tasks (Cieslik, Zilles, Kurth, & Eickhoff, 2010), we failed to prove any stimuli‐driven specificity of this area, suggesting a more general involvement during n‐back task execution.
4.2. Activations outside the frontoparietal network
On top of a stronger involvement of fronto parietal regions, many other regions, including subcortical areas, the cerebellum, and the bilateral precuneus were found supporting n‐back processing (see Figure 1).
The bilateral activation of the anterior insula during WM tasks has already been reported in previous studies (Rottschy et al., 2012; Wager & Smith, 2003). A lateralized activation of the right insula emerged within all n‐back modalities in our analysis, perhaps in line with its proposed role in regulating the interaction between the ventral and dorsal attentional systems (environmentally driven) as well as selective attentional mechanisms (task driven) with the aim to ensure optimal performance execution (Eckert et al., 2009).
The role of the bilateral precuneus within a wide range of high‐order cognitive functions has been proposed by several studies (Cavanna & Trimble, 2006), e.g. its involvement in episodic and semantic retrieval tasks (Shallice et al., 1994). This could explain its bilateral activation in the general n‐back map, as expression of the crucial aspect of maintaining and retrieving information during n‐back task.
Finally, a focus of activation in the bilateral cerebellum was found. Embracing modern brain activity models, in which all brain areas must be considered functioning as an ensemble, the cerebellum might no longer be considered only in its role within the motor domain, but rather for its cognitive contribution too. Indeed, both neuroimaging studies and evidences from patients with cerebellar cognitive affective syndrome, report a cerebellar involvement during executive functions, including WM, planning and abstract reasoning, as well as in spatial cognition (Schmahmann & Sherman, 1998). Recent models by Marvel and Desmond (2010) suggest that WM is supported by the cerebellum through the engagement of inner speech mechanism, possibly supporting the creation of memory traces that facilitate the processing of new information (Ackermann, 2008; Ravizza, Delgado, Chein, Becker, & Fiez, 2004).
4.3. Neural activity and task load
When considering load effects, a similar activation in frontoparietal areas is found for all contrasts, with the added engagement of both ACC and right cerebellum as a function of task load. Both areas have already been mentioned as associated with increased difficulty in memory tasks (Haxby, Petit, Ungerleider, & Courtney, 2000). Moreover, the anatomical mapping of the cerebellar activation is observed to specifically overlap with the crus‐I cerebellar parcellation by Buckner, previously associated to cognitive control—including WM—and as part of the default mode network (Buckner et al., 2011; Stoodley & Schmahmann, 2009). Crus‐I projections to the prefrontal cortex and cingulate gyrus, and the concurrent positive association between ACC, frontal areas and the cerebellum (Buckner et al., 2011), might justify the involvement of such areas during memory performance.
4.4. Brain activity in verbal n‐back tasks
The resulting map from all studies using verbal material was not found to differ from the generic n‐back task map, as shown in Figure 3. The frontoparietal network remains the central core of activity, with the addition of the precuneus, as well as cerebellar and subcortical structures bilaterally. Differently from what we expected based on previous findings (D'Esposito et al., 1998; Nystrom et al., 2000), we did not observe a striking left lateralization in the prefrontal areas, particularly in Broca's area (Brodmann's areas 44/45), but a more distributed brain activation during verbal n‐back tasks. The dissociation between the neural areas involved in storage and rehearsal has been amply discussed in the literature. Indeed, Awh et al. (1996) have revealed through a PET study a different model of brain activation for these two components of verbal WM: when rehearsal‐based activation was detracted from the activation due to storage and rehearsal together, some of the anterior brain activations (including Broca's area) were subtracted, while the posterior parietal regions remained active. In line with neuropsychological evidence (Smith & Jonides, 1997), inferior parietal areas were implicated in storage, whereas inferior frontal areas were implicated in rehearsal, and Broca's area may be important for articulatory processes involved in recording visual material but not for maintenance of stimuli order per se (Henson, Burgess, & Frith, 2000).
Rather, greater activity is reported in the left parietal area, more specifically in the supramarginal and angular gyri of IPL (Brodmann's areas 39/40). In the field of visual word recognition, the supramarginal gyrus has been reported as particularly active when participants are focused on words' sound, whereas the angular gyrus is mostly related to words' meaning (Démonet, Price, Wise, & Frackowiak, 1994; Devlin, Jamison, Gonnerman, & Matthews, 2006; Mummery, Patterson, Hodges, & Price, 1998). Besides being involved in the convergence of visual, auditory, and somatosensory information, both regions appear involved in language comprehension, together with Wernicke's area (Kim, Karunanayaka, Privitera, Holland, & Szaflarski, 2011). The central role of parietal areas for WM storage capacity was moreover demonstrated by a recent transcranial alternating current stimulation (tACS) study that provided the first causal relation between theta activity and n‐back tasks (Jaušovec & Jaušovec, 2014).
Although in a recent meta‐analysis by Rottschy et al. (2012) greater recruitment of the left inferior frontal gyrus (IFG) was observed for verbal tasks, in our study we did not find an activation for this area on the left hemisphere, but rather on the right. The divergence in the results might be due to the fact that Rottschy et al. (2012) considered a greater variance of WM tasks, not limited to the n‐back paradigm.
4.5. Brain activity in visual n‐back tasks
Visual n‐back tasks refer to those paradigms in which nonverbal visual stimuli, such as objects, images, and faces, are presented. The map of brain activity for this type of task confirms once again the core role of the frontoparietal network.
For object n‐back, results revealed greater activity in the right limbic structures, in particular in the anterior cingulate gyrus. Several functional neuroimaging studies have associated greater ACC activity with the execution of various high‐order functional tasks, such as Go‐No‐Go tasks (Schulz, Bédard, Czarnecki, & Fan, 2011), theory of mind (Kobayashi, Glover, & Temple, 2008) and high‐load WM tasks (Haxby et al., 2000). Moreover, Smith and Jonides (1999) have described ACC as implicated in the temporary storage and manipulation of information, and in the resolution of cognitive conflicts. However, the most plausible explanation for the activity of this area in image n‐back tasks might be that of its role in the judgment of pleasantness/averseness level (Lindgren et al., 2012). Indeed, coactivation of ACC with orbitofrontal structures is typically found when emotional stimuli are presented (Miller & Cohen, 2001).
4.6. Overlap between n‐back task network and RSN
From the first fMRI study aimed at analyzing brain activation during WM task, the role of the frontoparietal network has been suggested and further confirmed by the present meta‐analysis. The frontoparietal network includes a set of regions that are implicated in a variety of executive functions, including inhibition, switching, mental rotation, and fluid intelligence. Recently, the research effort has shifted from single areas to the study of well‐interconnected networks. Our results suggest a higher degree of overlap between n‐back regions and the DAN, as opposed to other networks like the Salience, Language, and Sensorimotor networks. The synergy between WM and other functions such as attention has been postulated (Shipstead, Harrison, & Engle, 2015). A similar overlap has been recently identified for fluid intelligence (Santarnecchi, Emmendorfer, & Pascual‐Leone, 2017, Santarnecchi, Khanna, Musaeus, et al., 2017), a function highly correlated with WM (Friedman et al., 2006).
Human functional evidences recognize four main regions as underlying DAN activity: the intraparietal sulcus, the superior parietal lobule, the superior and inferior precentral sulci, and the middle temporal area (Fox et al., 2005; Yeo et al., 2011). The distinction between dorsal and ventral attention networks relates back to the work of Corbetta and Shulman (2002), who first proposed the former as involved in mediating top‐down guided voluntary allocation of attention, while the latter detects salient and behaviorally relevant stimuli. Moreover, several neuroimaging studies have proved the DAN to be modulated during search and detection processes (Corbetta, Shulman, Miezin, & Petersen, 1995; Shulman, Ollinger, Linenweber, Petersen, & Corbetta, 2001). This might explain the reported overlap between DAN and n‐back networks, where the manipulation and maintenance of incoming information represents a core aspect of WM.
4.7. Functional connectivity profile of neural activation and deactivation
Neural activation patterns during the n‐back task have been amply discussed. However, for a more in‐depth understanding of the neural substrate of this specific WM task, deactivation patterns are also highly relevant and should be considered. Even though previous studies have reported deactivation coordinates for single analysis, to the best of our knowledge here we originally provide an ALE map showing the neural deactivation during the n‐back task, revealing a significant decrease in BOLD response of posterior cingulate cortex as well as frontal and temporal regions. As expected, the functional connectivity profile of such ALE nodes highly resembles the topography of the DMN. This network includes the posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), middle frontal regions, lateral parietal, and medial temporal regions, and is believed to be involved in introspection and background processing (Andrews‐Hanna, Smallwood, & Spreng, 2014; Fox et al., 2005; Raichle et al., 2001; Sheline et al., 2009).
In line with the “deactivation of the DMN,” we identified a high resemblance between the connectivity profile of regions activated during n‐back processing and the DAN. Interestingly, the interplay between these two networks has been suggested as a major candidate biomarker for normal and pathological aging (Spreng & Schacter, 2012; Spreng, Stevens, Viviano, & Schacter, 2016) and has been correlated with cognition in healthy young participants (Santarnecchi, Emmendorfer, & Pascual‐Leone, 2017; Santarnecchi, Emmendorfer, Tadayon, et al., 2017). A clear overlap with DMN and DAN can suggest the modulation of their interplay as a candidate target for brain stimulation interventions based on transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES), able to modulate network‐level activity and elicit cognitive enhancement (Ruffini, Wendling, Sanchez‐Todo, & Santarnecchi, 2018; Santarnecchi et al., 2018).
4.8. Limitations of the study and future directions
Our ALE maps allow us to know which areas are more active for a specific n‐back task. Although we consider the results of our meta‐analysis as accurate as possible given the currently available literature, there are some publication bias that should be evaluated. For example, based on a recent simulation study (Eickhoff et al., 2016), it is known that the results of a meta‐analysis with a low number of papers could be driven by few experiments (as evidenced by our analysis, see Supporting Information Figure S5 ). Since a limited number of studies is available for specific N‐back maps (e.g., number, face, and object n‐back), corresponding results should be interpreted carefully.
WM deficits characterize many psychiatric, neurodegenerative, and neurodevelopmental disorders, including depression (Rose & Ebmeier, 2006), schizophrenia (Lee & Park, 2005), Alzheimer's and Parkinson's diseases (Baddeley et al., 1991), ADHD, and Autistic spectrum disorder (Martinussen et al., 2005; Williams et al., 2005). Future work should focus on characterizing the n‐back activation profile of specific patient populations, also looking at differences between conditions and their network‐level representations. This could suggest potential targets for TMS and tES neuromodulatory interventions (Bestmann, de Berker, & Bonaiuto, 2015; Santarnecchi et al., 2015; Tatti, Rossi, Innocenti, Rossi, & Santarnecchi, 2016), testing, e.g., approaches aimed at (a) enhancing the activity of nodes still active even in the presence of a pathological condition and memory deficit, versus (b) those focusing on reactivating nodes showing lack of activation as compared to healthy controls.
5. CONCLUSION
We identified stimuli‐, presentation modality‐ and contrast‐dependent brain activity maps for n‐back processing in humans. While providing insight on WM processing and potentially inform future neuroimaging investigations, the present work also intends to reduce the observed variability in the outcome of cognitive enhancement studies, unifying and guiding targets for future brain stimulation protocols.
CONFLICT OF INTEREST
All authors report no conflict of interest.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
Dr. Santarnecchi is partially supported by Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014‐13121700007. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the ODNI, IARPA, or the U.S. Government. Dr. Santarnecchi is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) Award 2017, and the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers.
Mencarelli L, Neri F, Momi D, et al. Stimuli, presentation modality, and load‐specific brain activity patterns during n‐back task. Hum Brain Mapp. 2019;40:3810–3831. 10.1002/hbm.24633
Funding information Defense Advanced Research Projects Agency (DARPA), Grant/Award Number: HR001117S0030; Beth Israel Deaconess Medical Center (BIDMC); Intelligence Advanced Research Projects Activity (IARPA), Grant/Award Number: 2014‐13121700007; Office of the Director of National Intelligence (ODNI)
REFERENCES
- Ackermann, H. (2008). Cerebellar contributions to speech production and speech perception: Psycholinguistic and neurobiological perspectives. Trends in Neurosciences, 31, 265–272. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Smallwood, J. , & Spreng, R. N. (2014). The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh, E. , Jonides, J. , Smith, E. E. , Schumacher, E. H. , Koeppe, R. A. , & Katz, S. (1996). Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science, 7, 25–31. [Google Scholar]
- Baddeley, A. (1992). Working memory. Science, 255, 556–559. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. , Della Sala, S. , Robbins, T. W. , & Baddeley, A. (1996). Working memory and executive control [and discussion]. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 351, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. D. , Bressi, S. , Della Sala, S. , Logie, R. , & Spinnler, H. (1991). The decline of working memory in Alzheimer's disease: A longitudinal study. Brain, 114, 2521–2542. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh, Y. , Khorrami, A. , Zarrindast, M. R. , Shariat, S. V. , & Pantazis, D. (2016). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex enhances working memory. Experimental Brain Research, 234, 1807–1818. [DOI] [PubMed] [Google Scholar]
- Barch, D. M. , Sheline, Y. I. , Csernansky, J. G. , & Snyder, A. Z. (2003). Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biological Psychiatry, 53, 376–384. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , de Berker, A. O. , & Bonaiuto, J. (2015). Understanding the behavioural consequences of noninvasive brain stimulation. Trends in Cognitive Sciences, 19, 13–20. [DOI] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes, M. , Zuo, X.‐N. , Gohel, S. , Kelly, C. , Smith, S. M. , … Milham, M. P. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences, 107, 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni, A. R. , & Vanderhasselt, M.‐A. (2014). Working memory improvement with non‐invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta‐analysis. Brain and Cognition, 86, 1–9. [DOI] [PubMed] [Google Scholar]
- Buccino, G. , Binkofski, F. , Fink, G. R. , Fadiga, L. , Fogassi, L. , Gallese, V. , … Freund, H. J. (2001). Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. The European Journal of Neuroscience, 13, 400–404. [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , Castellanos, A. , Diaz, J. C. , & Yeo, B. T. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology, 129, 564–583. [DOI] [PubMed] [Google Scholar]
- Chao, L. L. , & Martin, A. (2000). Representation of manipulable man‐made objects in the dorsal stream. NeuroImage, 12, 478–484. [DOI] [PubMed] [Google Scholar]
- Chou, T.‐L. , Booth, J. R. , Burman, D. D. , Bitan, T. , Bigio, J. D. , Lu, D. , & Cone, N. E. (2006). Developmental changes in the neural correlates of semantic processing. NeuroImage, 29, 1141–1149. [DOI] [PubMed] [Google Scholar]
- Cieslik, E. C. , Zilles, K. , Kurth, F. , & Eickhoff, S. B. (2010). Dissociating bottom‐up and top‐down processes in a manual stimulus‐response compatibility task. Journal of Neurophysiology, 104, 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Shulman, G. L. , Miezin, F. M. , & Petersen, S. E. (1995). Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science, 270, 802–805. [DOI] [PubMed] [Google Scholar]
- Démonet, J. F. , Price, C. , Wise, R. , & Frackowiak, R. S. (1994). Differential activation of right and left posterior Sylvian regions by semantic and phonological tasks: A positron‐emission tomography study in normal human subjects. Neuroscience Letters, 182, 25–28. [DOI] [PubMed] [Google Scholar]
- D'Esposito, M. , Aguirre, G. K. , Zarahn, E. , Ballard, D. , Shin, R. K. , & Lease, J. (1998). Functional MRI studies of spatial and nonspatial working memory. Brain Research. Cognitive Brain Research, 7, 1–13. [DOI] [PubMed] [Google Scholar]
- D'Esposito, M. , Detre, J. A. , Alsop, D. C. , Shin, R. K. , Atlas, S. , & Grossman, M. (1995). The neural basis of the central executive system of working memory. Nature, 378, 279–281. [DOI] [PubMed] [Google Scholar]
- D'Esposito, M. , Postle, B. R. , & Rypma, B. (2000). Prefrontal cortical contributions to working memory: Evidence from event‐related fMRI studies. Experimental Brain Research, 133, 3–11. [DOI] [PubMed] [Google Scholar]
- Devlin, J. T. , Jamison, H. L. , Gonnerman, L. M. , & Matthews, P. M. (2006). The role of the posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience, 18, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, M. , Vinod, M. , Adam, W. , Jayne, A. , Stewart, D. , Amy, H. , & DJ, R. (2009). At the heart of the ventral attention system: The right anterior insula. Human Brain Mapping, 30, 2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , … Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Essen, D. C. V. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, N. P. , Miyake, A. , Corley, R. P. , Young, S. E. , DeFries, J. C. , & Hewitt, J. K. (2006). Not all executive functions are related to intelligence. Psychological Science, 17, 172–179. [DOI] [PubMed] [Google Scholar]
- Gelfand, J. R. , & Bookheimer, S. Y. (2003). Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron, 38, 831–842. [DOI] [PubMed] [Google Scholar]
- Göbel, S. M. , & Rushworth, M. F. S. (2004). Cognitive neuroscience: Acting on numbers. Current Biology, 14, R517–R519. [DOI] [PubMed] [Google Scholar]
- Haxby, J. V. , Petit, L. , Ungerleider, L. G. , & Courtney, S. M. (2000). Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. NeuroImage, 11, 145–156. [DOI] [PubMed] [Google Scholar]
- Henson, R. N. A. , Burgess, N. , & Frith, C. D. (2000). Recoding, storage, rehearsal and grouping in verbal short‐term memory: An fMRI study. Neuropsychologia, 38, 426–440. [DOI] [PubMed] [Google Scholar]
- Hubbard, E. M. , Piazza, M. , Pinel, P. , & Dehaene, S. (2005). Interactions between number and space in parietal cortex. Nature Reviews. Neuroscience, 6, 435–448. [DOI] [PubMed] [Google Scholar]
- Inui, T. , Otsu, Y. , Tanaka, S. , Okada, T. , Nishizawa, S. , & Konishi, J. (1998). A functional MRI analysis of comprehension processes of Japanese sentences. Neuroreport, 9, 3325–3328. [DOI] [PubMed] [Google Scholar]
- Jacquemot, C. , Pallier, C. , LeBihan, D. , Dehaene, S. , & Dupoux, E. (2003). Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. Journal of Neuroscience, 23, 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaušovec, N. , & Jaušovec, K. (2014). Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Biological Psychology, 96, 42–47. [DOI] [PubMed] [Google Scholar]
- Jonides, J. , Schumacher, E. H. , Smith, E. E. , Koeppe, R. A. , Awh, E. , Reuter‐Lorenz, P. A. , … Willis, C. R. (1998). The role of parietal cortex in verbal working memory. Journal of Neuroscience, 18, 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides, J. , Schumacher, E. H. , Smith, E. E. , Lauber, E. J. , Awh, E. , Minoshima, S. , & Koeppe, R. A. (1997). Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience, 9, 462–475. [DOI] [PubMed] [Google Scholar]
- Kim, K. K. , Karunanayaka, P. , Privitera, M. D. , Holland, S. K. , & Szaflarski, J. P. (2011). Semantic association investigated with fMRI and independent component analysis. Epilepsy & Behavior, 20, 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner, W. K. (1958). Age differences in short‐term retention of rapidly changing information. Journal of Experimental Psychology, 55, 352–358. [DOI] [PubMed] [Google Scholar]
- Kobayashi, C. , Glover, G. H. , & Temple, E. (2008). Switching language switches mind: Linguistic effects on developmental neural bases of ‘theory of mind. Social Cognitive and Affective Neuroscience, 3, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, V. , Aasen, I. , Taylor, P. , Ffytche, D. H. , Das, M. , Barkataki, I. , … Sharma, T. (2006). Neural dysfunction and violence in schizophrenia: An fMRI investigation. Schizophrenia Research, 84, 144–164. [DOI] [PubMed] [Google Scholar]
- Lee, J. , & Park, S. (2005). Working memory impairments in schizophrenia: A meta‐analysis. Journal of Abnormal Psychology, 114, 599–611. [DOI] [PubMed] [Google Scholar]
- Lindgren, L. , Westling, G. , Brulin, C. , Lehtipalo, S. , Andersson, M. , & Nyberg, L. (2012). Pleasant human touch is represented in pregenual anterior cingulate cortex. NeuroImage, 59, 3427–3432. [DOI] [PubMed] [Google Scholar]
- Mansouri, F. A. , Tanaka, K. , & Buckley, M. J. (2009). Conflict‐induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nature Reviews. Neuroscience, 10, 141–152. [DOI] [PubMed] [Google Scholar]
- Martinussen, R. , Hayden, J. , Hogg‐Johnson, S. , & Tannock, R. (2005). A meta‐analysis of working memory impairments in children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 377–384. [DOI] [PubMed] [Google Scholar]
- Marvel, C. L. , & Desmond, J. E. (2010). Functional topography of the cerebellum in verbal working memory. Neuropsychology Review, 20, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake, A. , Friedman, N. P. , Emerson, M. J. , Witzki, A. H. , Howerter, A. , & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Miyauchi, E. , Kitajo, K. , & Kawasaki, M. (2016). TMS‐induced theta phase synchrony reveals a bottom‐up network in working memory. Neuroscience Letters, 622, 10–14. [DOI] [PubMed] [Google Scholar]
- Mummery, C. J. , Patterson, K. , Hodges, J. R. , & Price, C. J. (1998). Functional neuroanatomy of the semantic system: Divisible by what? Journal of Cognitive Neuroscience, 10, 766–777. [DOI] [PubMed] [Google Scholar]
- Neubert, F.‐X. , Mars, R. B. , Sallet, J. , & Rushworth, M. F. S. (2015). Connectivity reveals relationship of brain areas for reward‐guided learning and decision making in human and monkey frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 112, E2695–E2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom, L. E. , Braver, T. S. , Sabb, F. W. , Delgado, M. R. , Noll, D. C. , & Cohen, J. D. (2000). Working memory for letters, shapes, and locations: fMRI evidence against stimulus‐based regional organization in human prefrontal cortex. NeuroImage, 11, 424–446. [DOI] [PubMed] [Google Scholar]
- Owen, A. M. (1997). The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. The European Journal of Neuroscience, 9, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Owen, A. M. , McMillan, K. M. , Laird, A. R. , & Bullmore, E. (2005). N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu, E. , Frith, C. D. , & Frackowiak, R. S. (1993). The neural correlates of the verbal component of working memory. Nature, 362, 342–345. [DOI] [PubMed] [Google Scholar]
- Picard, N. , & Strick, P. L. (1996). Motor areas of the Medial Wall: A review of their location and functional activation. Cerebral Cortex, 6, 342–353. [DOI] [PubMed] [Google Scholar]
- Ragland, J. D. , Turetsky, B. I. , Gur, R. C. , Gunning‐Dixon, F. , Turner, T. , Schroeder, L. , … Gur, R. E. (2002). Working memory for complex figures: An fMRI comparison of letter and fractal n‐back tasks. Neuropsychology, 16, 370–379. [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo, A. , Moss, H. E. , Stamatakis, E. A. , & Tyler, L. K. (2006). Repetition suppression and semantic enhancement: An investigation of the neural correlates of priming. Neuropsychologia, 44, 2284–2295. [DOI] [PubMed] [Google Scholar]
- Ravizza, S. M. , Delgado, M. R. , Chein, J. M. , Becker, J. T. , & Fiez, J. A. (2004). Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage, 22, 562–573. [DOI] [PubMed] [Google Scholar]
- Rose, E. J. , & Ebmeier, K. P. (2006). Pattern of impaired working memory during major depression. Journal of Affective Disorders, 90, 149–161. [DOI] [PubMed] [Google Scholar]
- Rottschy, C. , Langner, R. , Dogan, I. , Reetz, K. , Laird, A. R. , Schulz, J. B. , … Eickhoff, S. B. (2012). Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. NeuroImage, 60, 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini, G. , Wendling, F. , Sanchez‐Todo, R. , & Santarnecchi, E. (2018). Targeting brain networks with multichannel transcranial current stimulation (tCS). Current Opinion in Biomedical Engineering, 8, 70–77. [Google Scholar]
- Santarnecchi, E. , Brem, A.‐K. , Levenbaum, E. , Thompson, T. , Kadosh, R. C. , & Pascual‐Leone, A. (2015). Enhancing cognition using transcranial electrical stimulation. Current Opinion in Behavioral Sciences, 4, 171–178. [Google Scholar]
- Santarnecchi, E. , Emmendorfer, A. , & Pascual‐Leone, A. (2017). Dissecting the parieto‐frontal correlates of fluid intelligence: A comprehensive ALE meta‐analysis study. Intelligence, 63, 9–28. [Google Scholar]
- Santarnecchi, E. , Emmendorfer, A. , Tadayon, S. , Rossi, S. , Rossi, A. , & Pascual‐Leone, A. (2017). Network connectivity correlates of variability in fluid intelligence performance. Intelligence, 65, 35–47. [Google Scholar]
- Santarnecchi, E. , Khanna, A. R. , Musaeus, C. S. , Benwell, C. S. Y. , Davila, P. , Farzan, F. , … Authors on behalf of HST . (2017). EEG microstate correlates of fluid intelligence and response to cognitive training. Brain Topography, 30, 502–520. [DOI] [PubMed] [Google Scholar]
- Santarnecchi, E. , Momi, D. , Sprugnoli, G. , Neri, F. , Pascual‐Leone, A. , Rossi, A. , & Rossi, S. (2018). Modulation of network‐to‐network connectivity via spike‐timing‐dependent noninvasive brain stimulation. Human Brain Mapping, 39, 4870–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. , & Sherman, J. C. (1998). The cerebellar cognitive affective syndrome. Brain: A Journal of Neurology, 121(Pt 4), 561–579. [DOI] [PubMed] [Google Scholar]
- Schulz, K. P. , Bédard, A.‐C. V. , Czarnecki, R. , & Fan, J. (2011). Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. NeuroImage, 57, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, E. H. , Lauber, E. , Awh, E. , Jonides, J. , Smith, E. E. , & Koeppe, R. A. (1996). PET evidence for an amodal verbal working memory system. NeuroImage, 3, 79–88. [DOI] [PubMed] [Google Scholar]
- Shallice, T. , Fletcher, P. , Frith, C. D. , Grasby, P. , Frackowiak, R. S. J. , & Dolan, R. J. (1994). Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature, 368, 633–635. [DOI] [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Price, J. L. , Rundle, M. M. , Vaishnavi, S. N. , Snyder, A. Z. , … Raichle, M. E. (2009). The default mode network and self‐referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106, 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead, Z. , Harrison, T. L. , & Engle, R. W. (2015). Working memory capacity and the scope and control of attention. Attention, Perception, & Psychophysics, 77, 1863–1880. [DOI] [PubMed] [Google Scholar]
- Shirer, W. R. , Ryali, S. , Rykhlevskaia, E. , Menon, V. , & Greicius, M. D. (2012). Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cerebral Cortex (New York, NY: 1991), 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, G. L. , Ollinger, J. M. , Linenweber, M. , Petersen, S. E. , & Corbetta, M. (2001). Multiple neural correlates of detection in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98, 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. E. , & Jonides, J. (1997). Working memory: A view from neuroimaging. Cognitive Psychology, 33, 5–42. [DOI] [PubMed] [Google Scholar]
- Smith, E. E. , & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- Spreng, R. N. , & Schacter, D. L. (2012). Default network modulation and large‐scale network interactivity in healthy Young and old adults. Cerebral cortex (New York, NY: 1991), 22, 2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Viviano, J. D. , & Schacter, D. L. (2016). Attenuated anticorrelation between the default and dorsal attention networks with aging: Evidence from task and rest. Neurobiology of Aging, 45, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage, 44, 489–501. [DOI] [PubMed] [Google Scholar]
- Tatti, E. , Rossi, S. , Innocenti, I. , Rossi, A. , & Santarnecchi, E. (2016). Non‐invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Research Reviews, 29, 66–89. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , & Smith, E. E. (2003). Neuroimaging studies of working memory: A meta‐analysis. Cognitive, Affective, & Behavioral Neuroscience, 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Walton, M. E. , Devlin, J. T. , & Rushworth, M. F. S. (2004). Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience, 7, 1259–1265. [DOI] [PubMed] [Google Scholar]
- Williams, D. L. , Goldstein, G. , Carpenter, P. A. , & Minshew, N. J. (2005). Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders, 35, 747–756. [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


