Abstract
Recent research found lasting increases in personality trait Openness in healthy individuals and patients after administration of the serotonin 2A receptor (5‐HT2AR) agonist psilocybin. However, no studies have investigated whether 5‐HT2AR availability as imaged using positron emission tomography (PET) is associated with this trait. In 159 healthy individuals (53 females), the association between 5‐HT2AR binding in neocortex imaged with [18F]altanserin or [11C]Cimbi‐36 PET and personality trait Openness was investigated using linear regression models. In these models the influence of sex on the association was also investigated. Trait Openness was assessed with the NEO Personality Inventory‐Revised. No significant associations between neocortical 5‐HT2AR binding and trait Openness were found for [18F]altanserin (p = 0.5) or [11C]Cimbi‐36 (p = 0.8). Pooling the data in a combined model did not substantially change our results (p = 0.4). No significant interactions with sex were found (p > 0.35). Our results indicate that differences in 5‐HT2AR availability are not related to variations in trait Openness in healthy individuals. Although stimulation of the 5‐HT2AR with compounds such as psilocybin may contribute to long‐term changes in trait Openness, there is no evidence in favor of an association between 5‐HT2AR and trait Openness.
Keywords: [11C]Cimbi‐36, [18F]altanserin, 5‐HT2A, biomarkers, NEO personality inventory, personality, PET scan, serotonin, trait Openness
1. INTRODUCTION
Personality trait Openness to experience (“trait Openness”) was formulated by Costa and McCrae as part of their 5‐factor model of personality (McCrae & Costa, 2006). It pertains to relatively stable individual differences in sensitivity to feeling and esthetic aspects of experience as well as openness to ideas and values both at the level of action and fantasy (McCrae & Costa, 2006; Schwaba & Bleidorn, 2017). Individuals with high scores on this trait tend to be creative (Kandler et al., 2016; Kaufman et al., 2016), keep an open mind as to consider novel opinions and revise ideas and values over the life span (Schwaba, Luhmann, Denissen, Chung, & Bleidorn, 2017). In recent years, trait Openness has attracted researcher's interest because of increases in this trait after intake of serotonergic psychedelics (Carhart‐Harris, Kaelen, et al., 2016; Erritzoe et al., 2018; MacLean, Johnson, & Griffiths, 2011), suggesting a link between brain serotonin (5‐HT) and trait Openness. A previous positron emission tomography (PET) study from our lab reported a negative correlation between trait Openness and midbrain serotonin transporter (5‐HTT) levels in healthy individuals (Kalbitzer et al., 2009), but this could not be replicated by another recent PET study of trait Openness and cerebral 5‐HTT levels in healthy individuals (Tuominen et al., 2017). While the 5‐HTT is a central feature of serotonergic neurotransmission and a main target for therapeutic drugs, such as selective serotonin reuptake inhibitors, the association between key postsynaptic 5‐HT receptors targeted by psychedelics and trait Openness remains to be evaluated.
Psilocybin is a naturally occurring prodrug of psilocin, a serotonergic psychedelic produced by psilocybin mushrooms which shows promise as a novel therapeutic drug (Bogenschutz et al., 2015; Carhart‐Harris, Bolstridge, et al., 2016; Griffiths et al., 2016; Grob et al., 2011; Johnson, Garcia‐Romeu, Cosimano, & Griffiths, 2014; Moreno, Wiegand, Taitano, & Delgado, 2006; Ross et al., 2016) and is known to induce highly meaningful and, occasionally, mystical‐type experiences (Baumeister, Barnes, Giaroli, & Tracy, 2014; Griffiths et al., 2011; Griffiths et al., 2016; Griffiths, Richards, McCann, & Jesse, 2006; Johnson et al., 2014; MacLean et al., 2011; Ross et al., 2016). These psychedelic effects of psilocybin are mediated primarily by its actions as an agonist at the serotonin 2A receptor (5‐HT2AR) (Vollenweider, Vollenweider‐Scherpenhuyzen, Babler, Vogel, & Hell, 1998). The 5‐HT2AR is an excitatory 5‐HT receptor in the human brain most highly expressed throughout the cerebral cortex (Beliveau & Ganz, 2017; Varnas, Halldin, & Hall, 2004), and dysfunction of 5‐HT2AR is implicated in neuropsychiatric disorders including depression and schizophrenia (Albert, Benkelfat, & Descarries, 2012; Bhagwagar et al., 2006; Lin, Jiang, Kan, & Chu, 2014; Naughton, Mulrooney, & Leonard, 2000; Nikolaus, Muller, & Hautzel, 2016). It is therefore possible that 5‐HT2AR‐mediated effects of psilocybin and other psychedelics contribute to enduring increases in trait Openness. An even stronger case for a direct coupling between the 5‐HT2AR and trait Openness would be to show that individual differences in 5‐HT2AR availability in psychedelic‐naïve healthy individuals are associated with variation within this trait. However, such an association remains to be empirically tested.
Here, we evaluate the association between 5‐HT2AR levels in neocortex and trait Openness in 159 healthy individuals in vivo using either the 5‐HT2AR antagonist PET radioligand [18F]altanserin or the 5‐HT2AR agonist PET radioligand [11C]Cimbi‐36 developed by our lab (Ettrup et al., 2014).
2. METHODS AND MATERIALS
2.1. Participants
Data were extracted from the Center for Integrated Molecular Brain Imaging (Cimbi) database for healthy individuals with either a [18F]altanserin or [11C]Cimbi‐36 PET scan and NEO Personality Inventory‐Revised (NEO PI‐R) data (Knudsen et al., 2016). Of the initial 200 healthy individuals identified, we excluded individuals with more than 365 days between their PET scan and NEO PI‐R assessment (n = 5), and who had a body mass index of >30, corresponding to obese (n = 36), in reference to an earlier study of personality differences between obese and nonobese individuals in our database (Haahr et al., 2015). Of the remaining 159 individuals (53 females), eight completed both a [18F]altanserin and [11C]Cimbi‐36 PET scan for a specific study (Ettrup et al., 2016). Unless otherwise stated, n = 139 [18F]altanserin and n = 28 [11C]Cimbi‐36 for the reported analyses.
All individuals were recruited by advertisement for different research protocols approved by the Ethics Committee of Copenhagen and Frederiksberg, Denmark (H‐4‐2012‐105, KF‐02‐058‐99, KF‐01‐156‐04, KF‐11‐061‐03, KF‐01‐001‐02, KF‐01‐124‐04, KF‐01‐2006‐20). Written informed consent was obtained from all individuals after a complete description of the respective study. Although inclusion criteria varied slightly across studies, all individuals included in the current study were healthy and without: (a) primary psychiatric disease, (b) substance or drug abuse, and (c) severe systemic or neurological disease based on self‐reported history and physical/neurological examination by a trained clinician. Of participants asked (n = 134), all self‐reported being naïve to psychedelic drugs. However, some [18F]altanserin data sets were acquired before this information was systematically collected and is not known (n = 33). A detailed description of the Cimbi database and the PET biomarkers that it contains can be found elsewhere (Beliveau & Ganz, 2017; Knudsen et al., 2016).
2.2. Measures
2.2.1. The NEO Personality Inventory‐Revised
The Danish version of the NEO PI‐R was used to assess personality; this version has previously been normed in a sample of 600 individuals (Skovdahl, Mortensen, & Schiøtz, 2011). The NEO PI‐R is a self‐report questionnaire comprising 240 items which measures five major traits of personality: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness, where each trait consists of six subfacets (McCrae & Costa, 2006). The participants rated each item on a 5‐point Likert scale from 0 (strongly disagree) to 4 (strongly agree). For the purpose of this study, we used trait Openness and its constituent subfacets: Fantasy, Esthetics, Feelings, Actions, Ideas, and Values. The scores of the items loading on trait Openness were summed to a total raw score, which was used in the analyses. Across all participants, we had two missing item responses for the trait Openness scale (one item response for Openness subscale 1 and one item response for Openness subscale 6), which were substituted with the most neutral response “2” on the 0–4 Likert scale. Internal consistency (measured with Cronbach's alpha, α) for trait Openness was high, α = 0.87.
2.2.2. Educational attainment
Educational scores were rated on a 5‐point Likert scale; 1 (no vocational degree), 2 (<2 years of vocational education), 3 (2–4 years of vocational secondary education), 4 (2–4 years of academic education including a prior high school degree), and 5 (>4 years of academic education including a prior high school degree).
2.2.3. Magnetic resonance imaging
Magnetic resonance imaging (MRI) scans were acquired for all participants on one of the following scanners: (a) a Siemens 1.5T Vision scanner (Erlangen, DE), (b) a Siemens 3T Magnetom Trio scanner (Erlangen, DE), or (c) a Siemens 3 T Magnetom Verio scanner (Erlangen, DE). A high‐resolution T1‐weighted structural brain scan (1.5T Vision: TE/TR/TI: 4.40/1140/100 ms, flip angle: 8°, in‐plane matrix: 256 × 256, slices: 158, voxel size: 1.2 × 1.2 × 1.1 mm; 3T Trio: TE/TR/TI: 3.04/1550/800 ms, flip angle: 9°, in‐plane matrix: 256 × 256, slices: 192, voxel size: 1 × 1 × 1 mm; 3T Verio: TE/TR/TI: 2.32/1900/900 ms, flip angle: 9°, in‐plane matrix: 256 × 256, slices: 224, voxel size: 0.9 × 0.9 × 0.9 mm) was acquired for each participant and used for segmentation into gray matter, white matter, and cerebrospinal fluid and delineation of regions of interest (ROIs).
2.2.4. PET imaging
[18F]altanserin (Lemaire, Cantineau, Guillaume, Plenevaux, & Christiaens, 1991) and [11C]Cimbi‐36 (Ettrup et al., 2014) were produced as described earlier and imaging acquisitions parameters have been described previously (Ettrup et al., 2014; Pinborg et al., 2003). Briefly, participants were scanned with either: (a) an High Resolution Research Tomograph (HRRT) scanner (CTI/Siemens, Knoxville, TN) with an approximate in‐plane resolution of 2 mm or 2) an 18‐ring GE‐Advance scanner (GE, Milwaukee, WI) operating in 3D‐acquisition mode with an approximate in‐plane resolution of 6 mm. [18F]altanserin was administered as a bolus injection followed by continuous infusion to obtain steady state of [18F]altanserin in blood and tissue with a bolus–infusion ratio of 1.75 hr (Pinborg et al., 2003). Following a 10‐min transmission scan, a 40‐min emission scan was acquired 2 hr after bolus administration and reconstructed into five frames (5 × 8 min). Following a 6‐min transmission scan, [11C]Cimbi‐36 was administered as a bolus and a dynamic 120‐min emission scan was acquired and reconstructed into 45 frames (6 × 10 s, 6 × 20 s, 6 × 60 s, 8 × 120 s, 19 × 300 s). Dynamic PET images acquired on the GE‐Advance scanner were reconstructed using filtered back projection and corrected for attenuation, dead time, and scatter using a 6 mm Hann filter. Dynamic PET images acquired on the HRRT scanner were reconstructed using an iterative OP‐OSEM3D method with resolution modeling (10 iterations and 16 subsets). Detailed venous and arterial blood sampling procedures for [18F]altanserin and [11C]Cimbi‐36 scans, respectively, for assessing plasma radioactivity concentrations and radiometabolites have been described elsewhere (Ettrup et al., 2014; Pinborg et al., 2003).
2.2.5. Brain image analysis and outcome parameters
To determine single‐subject within PET scan motion and realignment the automatic image registration algorithm was used (Woods, Cherry, & Mazziotta, 1992) with PET scans smoothed using a 12 mm (GE‐Advance) or a 10 mm (HRRT) within‐frame Gaussian filter before alignment. Nonfiltered PET images were resliced using these parameters. [18F]altanserin scans acquired on the GE‐Advance scanner were coregistered to high‐resolution MR images using a manual method described previously (Pinborg et al., 2003). Otherwise, Statistical Parametric Mapping (SPM) was used to coregister PET and high‐resolution MR images and segment high‐resolution MR images. Accurate coregistration and segmentation was confirmed visually for all data sets.
We used PVElab to automatically delineate ROIs on the participant's high‐resolution T1‐weighted MRI scan (Svarer et al., 2005). Mean time‐activity curves were extracted from the gray matter voxels within ROIs. We selected a large neocortex ROI because 5‐HT2AR shows low binding subcortically, is widely expressed throughout neocortex, and displays high interregional correlation across neocortical subregions (Erritzoe et al., 2010). Our neocortex region comprises occipital‐, orbitofrontal‐, and parietal cortex, pre/post central‐, middle/inferior frontal‐, middle/inferior temporal‐, superior frontal‐, and superior temporal gyrus as defined in PVElab.
The primary outcome parameter for [18F]altanserin was the binding potential relative to total plasma concentration (BPP), a reliable quantification method that effectively accounts for radiolabeled metabolites crossing the blood–brain barrier (Pinborg et al., 2003). BPP is defined as: BPP = (C T–C ND)/C P = f P(Bavail/K D), where C T and C ND are the steady‐state mean count densities in the ROI and the reference region (cerebellum), respectively; C P is the steady‐state activity of nonmetabolized tracer in plasma; fP is the free fraction of radiotracer in plasma; B avail is the number of receptor sites available for tracer binding; and KD is the dissociation constant reflecting affinity of the radiotracer for the receptor (Pinborg et al., 2003). The primary outcome parameter for [11C]Cimbi‐36 was the nondisplaceable binding potential (BPND), a validated and stable quantification for this tracer (Ettrup et al., 2014; Ettrup et al., 2016). BPND is defined as: BPND = (V T–V ND)/V ND = f ND(B avail/K D), where V T and V ND are the distribution volumes in the ROI and reference region (cerebellum), respectively, from two‐tissue compartment modeling with arterial input measurements as previously described (Ettrup et al., 2014). Cerebellum has been previously validated as an appropriate reference region for both [18F]altanserin and [11C]Cimbi‐36 (Ettrup et al., 2014; Pinborg et al., 2003).
2.3. Statistics
The associations between 5‐HT2AR availability in neocortex and trait Openness were analyzed in two separate ordinary least‐squares linear regression models: one for [18F]altanserin and one for [11C]Cimbi‐36. We considered scanner‐specific [18F]altanserin effects (HRRT vs. GE); however, separate analyses for the two scanners yielded similar effects. Pooled results including both HRRT and GE scans are reported.
Age and sex were included as covariates a priori in both models, based on previous reports from a Danish norm sample of an association with trait Openness (Skovdahl et al., 2011). Education has also been associated with trait Openness (Poropat, 2009), but this information was only available for 91 of the 139 [18F]altanserin scans, and its inclusion did not substantively affect the results, so we report the [18F]altanserin association with trait Openness excluding education as covariate. Body mass index was considered as an additional covariate but was not associated with trait Openness (p = 0.14) and therefore excluded from the final model. Subsequently, we pooled the [18F]altanserin and [11C]Cimbi‐36 data in a combined model adding radioligand as a covariate together with age and sex. This was done in an effort to increase statistical power with a larger sample size.
We compared the reported trait Openness in our study to the Danish norm sample (mean = 104.8, SD = 18.5) (Skovdahl et al., 2011), using a one‐sample t test. p‐Values <0.05 were considered statistically significant. For our main results, both the regression coefficient and the standardized regression coefficient are reported in Table 1. Model assumptions (e.g., normality of residuals, QQ‐plots) were considered and showed no evidence of model violation. Consistent with a previous study (Moses‐Kolko et al., 2011), 5‐HT2AR was negatively associated with age (p < 0.001). However, the variance inflation factor for age and neocortex were ~1.8, indicating that model parameter estimation is unlikely to be affected by collinearity. Statistical analyses were carried out in SPSS (v20.0) and R (v3.3.1) (https://cran.r-project.org/).
Table 1.
The associations between neocortex 5‐HT2AR binding and trait Openness
| Predictors | Beta | SE | p‐Value | 95% CI | Stand. Beta |
|---|---|---|---|---|---|
| Model 1 | [18F]altanserin, n = 139 | ||||
| Neocortex 2A binding | −2.54 | 3.75 | 0.50 | −9.95, 4.88 | −0.068 |
| Age | −0.43 | 0.11 | 0.000078 | −0.64, −0.22 | −0.40 |
| Sex | −4.32 | 3.14 | 0.17 | −10.53, 1.89 | −0.11 |
| Model 2 | [11C]‐Cimbi‐36, n = 28 | ||||
| Neocortex 2A binding | 9.03 | 29.72 | 0.76 | −52.31, 70.37 | 0.097 |
| Age | 1.02 | 0.26 | 0.31 | −0.99, 3.03 | 0.26 |
| Sex | −8.81 | 10.36 | 0.40 | −30.20, 12.57 | −0.22 |
| Model 3 | Combined [18F]altanserin and [11C]‐Cimbi‐36, n = 159 | ||||
| Neocortex 2A binding | −2.92 | 3.50 | 0.40 | −9.79, 3.94 | −0.07 |
| Age | −0.42 | 0.10 | 0.000020 | −0.62, −0.23 | −0.38 |
| Radioligand | −9.55 | 4.46 | 0.032 | −18.30, −0.81 | −0.19 |
| Sex | −5.24 | 3.06 | 0.087 | −11.24, 0.77 | −0.14 |
Note. Parameters from the models used to investigate associations between neocortex 5‐HT2AR binding and trait Openness, including the combined model with both radioligands, [18F]altanserin and [11C]‐Cimbi‐36, in Model 3. Combined analyses included 167 observations (eight individuals scanned two times). Sex parameter estimates reflect difference in trait Openness relative to female. SE = standard error, 95% CI = 95% confidence interval, Stand. Beta = standardized regression coefficient.
3. RESULTS
3.1. Descriptive data
Table 2 shows descriptive data for the [18F]altanserin and [11C]Cimbi‐36 groups. Average age in the [18F]altanserin group was significantly higher than in the [11C]Cimbi‐36 group (p < 0.001), due to differing age limits associated with specific studies. Reported trait Openness scores did not differ significantly between the [18F]altanserin and [11C]Cimbi‐36 groups (p = 0.62). Across both groups, reported trait Openness was significantly higher than reported in a Danish norm sample (our sample vs. Danish norm, mean: +10.3 trait Openness units, p < 0.001). The median days between 5‐HT2AR scans and personality assessments were 0 days (range: 0–358) in the [18F]altanserin group and 6 days (range: 0–60) in the [11C]Cimbi‐36 group.
Table 2.
Descriptive information
| [18F]altanserin, n = 139 | [11C]‐Cimbi‐36, n = 28 | |||||
|---|---|---|---|---|---|---|
| Measures | Mean ± SD or % | Median | Range, min–max | Mean ± SD or % | Median | Range, min–max |
| Age in years | 38.7 ± 17.4 | 33.4 | 18.5–81.7 | 23.5 ± 5.1 | 22.8 | 18.4–46.1 |
| BMI kg/m2 | 24.1 ± 2.5 | 24.1 | 18.4–29.7 | 23.7 ± 2.7 | 23.1 | 20.6–30.6 |
| Sex (female) | 38% | 48% | ||||
| Education | 3.9 ± 1.3 | 4 | 1–5 | 3.8 ± 1.7 | 5 | 1–5 |
| Trait Openness | 115.5 ± 18.7 | 114 | 68–170 | 113.7 ± 19.7 | 111 | 68–160 |
| Injected dose MBq | 265.2 ± 55.4 | 272 | 155–412 | 499.9 ± 117.3 | 563.2 | 213–603.9 |
| Injected mass | 2.7 ± 2.3 | 2.1 | 0.2–11 | 0.8 ± 0.5 | 0.6 | 0.1–1.5 |
| Specific activity MBq/μmol | 79.8 ± 74.2 | 58.4 | 10.3–441.7 | 378.5 ± 275.5 | 249.3 | 95.4–1,033 |
| Neocortex 2A BP or BND | 1.5 ± 0.5 | 1.5 | 0.4–3.7 | 1.3 ± 0.2 | 1.3 | 0.8–1.7 |
| PET scanner (HRRT) | 32% | 100% | ||||
| Days from PET to NEO | 11.09 ± 40.28 | 0 | 0–314 | 11.2 ± 14.2 | 6 | 0–60 |
Note. Descriptive information about the study participants. SD = standard deviation, BMI = body mass index, MBq = megabecquerel, μmol = micromole, B P = binding potential, B ND = nondisplaceable binding potential, PET = positron emission tomography, NEO PI‐R = NEO Personality Inventory.
3.2. 5‐HT2AR binding and trait Openness
Table 1 shows the results from our regression models for the [18F]altanserin and [11C]Cimbi‐36 group as well as the combined model with pooled data across both groups. Figure 1 shows scatterplots of neocortex 5‐HT2AR binding against trait Openness scores for the [18F]altanserin and [11C]Cimbi‐36 group. Neither [18F]altanserin neocortex 5‐HT2AR BPP (p = 0.5) nor [11C]Cimbi‐36 neocortex 5‐HT2AR BPND (p = 0.8) were significantly associated with trait Openness. Age was significantly negatively associated with trait Openness in the [18F]altanserin group (p < 0.001), whereas this was not the case in the [11C]Cimbi‐36 group (p = 0.3). Pooling the [18F]altanserin and [11C]Cimbi‐36 data in a combined model did not substantially change the observed associations between neocortex 5‐HT2AR and trait Openness (p = 0.4). Exploratory interaction effects with age and sex did not reveal any condition‐specific associations between neocortex and trait Openness (p > 0.35).
Figure 1.
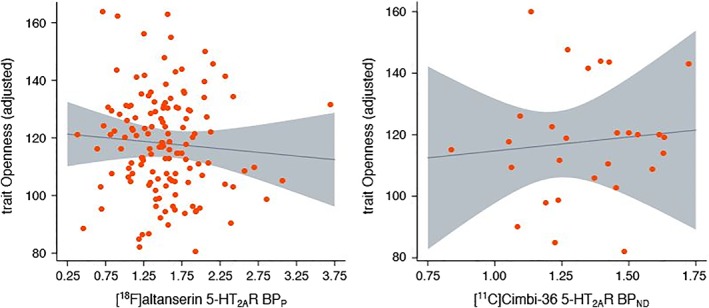
Two scatterplots of neocortex 5‐HT2AR binding imaged with [18F]altanserin (n = 139) and [11C]Cimbi‐36 (n = 28), respectively, against trait Openness scores is shown. Orange dots represent individual measurement points and lines and shading for each line represent slope estimates and 95% confidence intervals. Data shown are adjusted for age and sex [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
This is the first study to investigate the association between in vivo 5‐HT2AR PET binding and personality trait Openness, here in a large sample of 159 healthy individuals. We did not observe any significant associations between 5‐HT2AR and trait Openness with either of the two applied PET radioligands ([18F]altanserin or [11C]Cimbi‐36) separately or when combined, indicating that the 5‐HT2AR is not directly involved in this personality trait.
Although we were not able to detect a relation between trait Openness and 5‐HT2AR availability, other in vivo PET 5‐HT biomarker studies have been undertaken to examine similar relations. In line with the previously mentioned study from our own lab (Kalbitzer et al., 2009) in which a negative correlation between trait Openness and 5‐HTT binding was found, other studies reported negative correlations between a similar personality trait Self‐transcendence and 5‐HTT binding (Kim et al., 2015) and 5‐HT1AR levels (Borg, Andree, Soderstrom, & Farde, 2003), respectively. Self‐transcendence is a personality trait inherent in Cloninger's Temperament and Character Inventory (TCI) which has some overlap with trait Openness (Cloninger, Svrakic, & Przybeck, 1993; De Fruyt, Van De Wiele, & Van Heeringen, 2000). In line with the findings from the current study, another study found no significant association between 5‐HT1AR and trait Self‐transcendence (Karlsson, Hirvonen, Salminen, & Hietala, 2011), and two other studies reported nonsignificant associations between trait Openness and 5‐HT4R (Stenbaek et al., 2017) and 5‐HT1AR (Tauscher et al., 2001), respectively. Some studies reported significant associations between 5‐HT receptor or 5‐HTT binding and other personality traits from NEO PI‐R and TCI, but none in relation to trait Openness or Self‐transcendence (Tauscher et al., 2001; Tuominen et al., 2013; Tuominen et al., 2017). Conversely, other PET studies investigated associations between 5‐HT receptor or 5‐HTT binding and personality traits from NEO PI‐R and TCI, but did not report associations with trait Openness or Self‐transcendence (Frokjaer et al., 2008; Frokjaer et al., 2010; Gerretsen et al., 2010; Moresco et al., 2002; Reimold et al., 2008; Soloff, Chiappetta, Mason, Becker, & Price, 2014; Soloff, Price, Mason, Becker, & Meltzer, 2010; Takano et al., 2007), possibly reflecting null findings. A full review of the existing literature on PET biomarkers of the 5‐HT system and trait Openness and Self‐transcendence can be found in supplementary materials (Table S1, Supporting Information). In reference to these studies, our current study represents the largest to date and does not support an association between 5‐HT2AR availability and trait Openness.
Although studies of the 5‐HT system with PET and trait Openness reveal no obvious patterns across healthy individuals, classic serotonergic psychedelics do appear to provide novel information about possible change mechanisms in trait Openness and related traits. Observational studies have shown that, compared to controls, long time users of ayahuasca (containing the 5‐HT2AR agonist N,N‐dimethyltryptamine) exhibit higher trait Openness (Barbosa et al., 2016) and similar studies revealed higher trait Self‐transcendence in ayahuasca users than controls (Bouso et al., 2012; Bouso et al., 2015). Since observational and nonprospective studies are unable to measure changes in personality traits, it is uncertain whether these results reflect an effect of psychedelics or that individuals with specific trait profiles are more prone to seek out psychedelics (in either experimental or recreational settings). Recent experimental studies in healthy individuals have reported increases in trait Openness measured 2 weeks after administration of the serotonin psychedelic lysergic acid diethylamide (Carhart‐Harris, Kaelen, et al., 2016), and enduring increases in trait Openness 1 year and 3 months after administration of psilocybin in healthy individuals (MacLean et al., 2011) and patients with treatment‐resistant depression (Erritzoe et al., 2018), respectively. Intriguingly, it has also been reported that change in trait Openness was predicted by the occurrence of psilocybin‐induced mystical experiences, whereas baseline measures of Openness were unable to predict whether a mystical experience would occur (Griffiths et al., 2011). Considering that the occurrence of mystical experiences is positively related to psilocybin dose (Griffiths et al., 2011), our findings suggest a limited association between 5‐HT2AR and trait Openness at baseline, whereas the malleability of trait Openness may nevertheless be related to the capacity for 5‐HT2AR modulation by serotonergic psychedelics (e.g., 5‐HT2AR baseline levels). Future studies evaluating 5‐HT2AR‐mediated mechanisms of changes in personality after intake of psilocybin (or other serotonergic psychedelics) would shed light on this link.
4.1. Methodological considerations
A major strength of the current study is its sample size. It is the largest PET study to examine the association between an imaging marker of 5‐HT signaling and trait Openness. It is also the first to study the association between 5‐HT2AR and trait Openness. However, some limitations of the study should be considered. First, with our sample size of 159 participants, we have sufficient statistical power (β = 0.8) to detect effect sizes of r ≥ 0.2 (small/medium and greater). Although we are powered to detect in the higher end of small to medium effect sizes, we are underpowered to detect smaller effects. Our standardized regression coefficient (−0.07, 95% CI: −0.27, 0.13) is such that even a statistically significant association of this kind may be of limited practical relevance. Second, study exclusion criteria including family or personal history of psychiatric disorders may have caused us to miss a potential association between 5‐HT2AR and trait Openness in more vulnerable populations. Third, self‐report biases, for example, censorship, social desirability biases, or systematic manipulation of answers on items (Domino, 2006), are inherent problems with applied psychometric tools such as the NEO PI‐R and may have biased associations with 5‐HT2AR binding. However, studies of the correlation between self‐report scores and ratings by spouse on the NEO PI‐R supports the reliability of the self‐reported personality traits (McCrae & Costa, 2006).
In summary, we applied PET imaging to study 5‐HT2AR availability as a possible molecular marker associated with trait Openness in a large sample of 159 healthy individuals with no current or prior psychiatric history. No significant association was observed, suggesting that there is no evidence in favor of an association between 5‐HT2AR and trait Openness.
CONFLICT OF INTERESTS
All authors declare no conflict of interests.
Supporting information
Table S1 An overview of the existing literature on PET biomarkers of the 5‐HT system and trait Openness and Self‐transcendence. NEO PI‐R = NEO Personality Inventory; TCI = Temperament and Character Inventory; Blue indicates NEO‐PI‐R has been administrated. 5‐HT = Serotonin; 5‐HTT = Serotonin transporter; R = receptor; RN = Raphe nuclei; DLPC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; PC = parietal cortex and OC = occipital cortex; OFC = orbitofrontal cortex, Lt. MTC = left medial frontal cortex
ACKNOWLEDGMENTS
The authors would like to thank B. Dall, G. Thomsen, S. Larsen, A. Dyssegaard, and L. Freyr for their assistance with the data collection. The authors would also like to acknowledge and thank The John and Birthe Meyer Foundation for the donation of the Cyclotron and PET scanner.
Stenbæk DS, Kristiansen S, Burmester D, et al. Trait Openness and serotonin 2A receptors in healthy volunteers: A positron emission tomography study. Hum Brain Mapp. 2019;40:2117–2124. 10.1002/hbm.24511
REFERENCES
- Albert, P. R. , Benkelfat, C. , & Descarries, L. (2012). The neurobiology of depression‐‐revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 367(1601), 2378–2381. 10.1098/rstb.2012.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, P. C. , Strassman, R. J. , da Silveira, D. X. , Areco, K. , Hoy, R. , Pommy, J. , … Bogenschutz, M. (2016). Psychological and neuropsychological assessment of regular hoasca users. Comprehensive Psychiatry, 71, 95–105. 10.1016/j.comppsych.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Baumeister, D. , Barnes, G. , Giaroli, G. , & Tracy, D. (2014). Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Therapeutic Advances in Psychopharmacology, 4(4), 156–169. 10.1177/2045125314527985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau, V. , & Ganz, M. (2017). A high‐resolution in vivo atlas of the human Brain's serotonin system. Journal of Neuroscience, 37(1), 120–128. 10.1523/jneurosci.2830-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar, Z. , Hinz, R. , Taylor, M. , Fancy, S. , Cowen, P. , & Grasby, P. (2006). Increased 5‐HT(2A) receptor binding in euthymic, medication‐free patients recovered from depression: A positron emission study with [(11)C]MDL 100,907. The American Journal of Psychiatry, 163(9), 1580–1587. 10.1176/ajp.2006.163.9.1580 [DOI] [PubMed] [Google Scholar]
- Bogenschutz, M. P. , Forcehimes, A. A. , Pommy, J. A. , Wilcox, C. E. , Barbosa, P. C. , & Strassman, R. J. (2015). Psilocybin‐assisted treatment for alcohol dependence: A proof‐of‐concept study. Journal of Psychopharmacology, 29(3), 289–299. 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- Borg, J. , Andree, B. , Soderstrom, H. , & Farde, L. (2003). The serotonin system and spiritual experiences. The American Journal of Psychiatry, 160(11), 1965–1969. 10.1176/appi.ajp.160.11.1965 [DOI] [PubMed] [Google Scholar]
- Bouso, J. C. , Gonzalez, D. , Fondevila, S. , Cutchet, M. , Fernandez, X. , Ribeiro Barbosa, P. C. , … Riba, J. (2012). Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: A longitudinal study. PLoS One, 7(8), e42421 10.1371/journal.pone.0042421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouso, J. C. , Palhano‐Fontes, F. , Rodriguez‐Fornells, A. , Ribeiro, S. , Sanches, R. , Crippa, J. A. , … Riba, J. (2015). Long‐term use of psychedelic drugs is associated with differences in brain structure and personality in humans. European Neuropsychopharmacology, 25(4), 483–492. 10.1016/j.euroneuro.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Bolstridge, M. , Rucker, J. , Day, C. M. , Erritzoe, D. , Kaelen, M. , … Nutt, D. J. (2016). Psilocybin with psychological support for treatment‐resistant depression: An open‐label feasibility study. Lancet Psychiatry, 3(7), 619–627. 10.1016/s2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Kaelen, M. , Bolstridge, M. , Williams, T. M. , Williams, L. T. , Underwood, R. , … Nutt, D. J. (2016). The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychological Medicine, 46(7), 1379–1390. 10.1017/s0033291715002901 [DOI] [PubMed] [Google Scholar]
- Cloninger, C. R. , Svrakic, D. M. , & Przybeck, T. R. (1993). A psychobiological model of temperament and character. Archives of General Psychiatry, 50(12), 975–990. [DOI] [PubMed] [Google Scholar]
- De Fruyt, F. , Van De Wiele, L. , & Van Heeringen, C. (2000). Cloninger's psychobiological model of temperament and character and the five‐factor model of personality. Personality and Individual Differences, 29(3), 441–452. 10.1016/S0191-8869(99)00204-4 [DOI] [Google Scholar]
- Domino, G. (2006). Psychological testing, an introduction (2nd ed). Cambridge, NY: Cambridge University Press. [Google Scholar]
- Erritzoe, D. , Holst, K. , Frokjaer, V. G. , Licht, C. L. , Kalbitzer, J. , Nielsen, F. A. , … Knudsen, G. (2010). A nonlinear relationship between cerebral serotonin transporter and 5‐HT(2A) receptor binding: An in vivo molecular imaging study in humans. The Journal of Neuroscience, 30(9), 3391–3397. 10.1523/jneurosci.2852-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe, D. , Roseman, L. , Nour, M. M. , MacLean, K. , Kaelen, M. , Nutt, D. J. , & Carhart‐Harris, R. L. (2018). Effects of psilocybin therapy on personality structure. Acta Psychiatrica Scandinavica, 138, 368–378. 10.1111/acps.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup, A. , da Cunha‐Bang, S. , McMahon, B. , Lehel, S. , Dyssegaard, A. , Skibsted, A. W. , … Knudsen, G. M. (2014). Serotonin 2A receptor agonist binding in the human brain with [(11)C]Cimbi‐36. Journal of Cerebral Blood Flow & Metabolism, 34(7), 1188–1196. 10.1038/jcbfm.2014.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup, A. , Svarer, C. , McMahon, B. , da Cunha‐Bang, S. , Lehel, S. , Moller, K. , … Knudsen, G. M. (2016). Serotonin 2A receptor agonist binding in the human brain with [(11)C]Cimbi‐36: Test‐retest reproducibility and head‐to‐head comparison with the antagonist [(18)F]altanserin. Neuroimage, 130, 167–174. 10.1016/j.neuroimage.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Frokjaer, V. G. , Mortensen, E. L. , Nielsen, F. A. , Haugbol, S. , Pinborg, L. H. , Adams, K. H. , … Knudsen, G. M. (2008). Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry, 63(6), 569–576. 10.1016/j.biopsych.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Frokjaer, V. G. , Vinberg, M. , Erritzoe, D. , Baare, W. , Holst, K. K. , Mortensen, E. L. , … Knudsen, G. M. (2010). Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology, 35(5), 1129–1137. 10.1038/npp.2009.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen, P. , Graff‐Guerrero, A. , Menon, M. , Pollock, B. G. , Kapur, S. , Vasdev, N. , … Mamo, D. (2010). Is desire for social relationships mediated by the serotonergic system in the prefrontal cortex? An [(18)F]setoperone PET study. Social Neuroscience, 5(4), 375–383. 10.1080/17470911003589309 [DOI] [PubMed] [Google Scholar]
- Griffiths, R. R. , Johnson, M. W. , Carducci, M. A. , Umbricht, A. , Richards, W. A. , Richards, B. D. , … Klinedinst, M. A. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life‐threatening cancer: A randomized double‐blind trial. Journal of Psychopharmacology, 30(12), 1181–1197. 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, R. R. , Johnson, M. W. , Richards, W. A. , Richards, B. D. , McCann, U. , & Jesse, R. (2011). Psilocybin occasioned mystical‐type experiences: Immediate and persisting dose‐related effects. Psychopharmacology, 218(4), 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, R. R. , Richards, W. A. , McCann, U. , & Jesse, R. (2006). Psilocybin can occasion mystical‐type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology, 187(3), 268–283; discussion 284‐292. 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- Grob, C. S. , Danforth, A. L. , Chopra, G. S. , Hagerty, M. , McKay, C. R. , Halberstadt, A. L. , & Greer, G. R. (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced‐stage cancer. Archives of General Psychiatry, 68(1), 71–78. 10.1001/archgenpsychiatry.2010.116 [DOI] [PubMed] [Google Scholar]
- Haahr, M. E. , Hansen, D. L. , Fisher, P. M. , Svarer, C. , Stenbaek, D. S. , Madsen, K. , … Knudsen, G. M. (2015). Central 5‐HT neurotransmission modulates weight loss following gastric bypass surgery in obese individuals. The Journal of Neuroscience, 35(14), 5884–5889. 10.1523/jneurosci.3348-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. W. , Garcia‐Romeu, A. , Cosimano, M. P. , & Griffiths, R. R. (2014). Pilot study of the 5‐HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology, 28(11), 983–992. 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitzer, J. , Frokjaer, V. G. , Erritzoe, D. , Svarer, C. , Cumming, P. , Nielsen, F. A. , … Knudsen, G. M. (2009). The personality trait openness is related to cerebral 5‐HTT levels. Neuroimage, 45(2), 280–285. 10.1016/j.neuroimage.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Kandler, C. , Riemann, R. , Angleitner, A. , Spinath, F. M. , Borkenau, P. , & Penke, L. (2016). The nature of creativity: The roles of genetic factors, personality traits, cognitive abilities, and environmental sources. Journal of Personality and Social Psychology, 111(2), 230–249. 10.1037/pspp0000087 [DOI] [PubMed] [Google Scholar]
- Karlsson, H. , Hirvonen, J. , Salminen, J. K. , & Hietala, J. (2011). No association between serotonin 5‐HT 1A receptors and spirituality among patients with major depressive disorders or healthy volunteers. Molecular Psychiatry, 16(3), 282–285. 10.1038/mp.2009.126 [DOI] [PubMed] [Google Scholar]
- Kaufman, S. B. , Quilty, L. C. , Grazioplene, R. G. , Hirsh, J. B. , Gray, J. R. , Peterson, J. B. , & DeYoung, C. G. (2016). Openness to experience and intellect differentially predict creative achievement in the arts and sciences. Journal of Personality, 84(2), 248–258. 10.1111/jopy.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.‐H. , Son, Y.‐D. , Kim, J.‐H. , Choi, E.‐J. , Lee, S.‐Y. , Joo, Y.‐H. , … Cho, Z.‐H. (2015). Self‐transcendence trait and its relationship with in vivo serotonin transporter availability in brainstem raphe nuclei: An ultra‐high resolution PET‐MRI study. Brain Research, 1629, 63–71. 10.1016/j.brainres.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Knudsen, G. M. , Jensen, P. S. , Erritzoe, D. , Baare, W. F. , Ettrup, A. , Fisher, P. M. , … Frokjaer, V. G. (2016). The Center for Integrated Molecular Brain Imaging (Cimbi) database. Neuroimage, 124(Pt. B), 1213–1219. 10.1016/j.neuroimage.2015.04.025 [DOI] [PubMed] [Google Scholar]
- Lemaire, C. , Cantineau, R. , Guillaume, M. , Plenevaux, A. , & Christiaens, L. (1991). Fluorine‐18‐altanserin: A radioligand for the study of serotonin receptors with PET: Radiolabeling and in vivo biologic behavior in rats. Journal of Nuclear Medicine, 32(12), 2266–2272. [PubMed] [Google Scholar]
- Lin, J. Y. , Jiang, M. Y. , Kan, Z. M. , & Chu, Y. (2014). Influence of 5‐HTR2A genetic polymorphisms on the efficacy of antidepressants in the treatment of major depressive disorder: A meta‐analysis. Journal of Affective Disorders, 168, 430–438. 10.1016/j.jad.2014.06.012 [DOI] [PubMed] [Google Scholar]
- MacLean, K. A. , Johnson, M. W. , & Griffiths, R. R. (2011). Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology, 25(11), 1453–1461. 10.1177/0269881111420188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae, R. R. , & Costa, P. T. (2006). Personality in adulthood: A five‐factor theory perspective. New York, NY: The Guilford Press. [Google Scholar]
- Moreno, F. A. , Wiegand, C. B. , Taitano, E. K. , & Delgado, P. L. (2006). Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive‐compulsive disorder. The Journal of Clinical Psychiatry, 67(11), 1735–1740. [DOI] [PubMed] [Google Scholar]
- Moresco, F. M. , Dieci, M. , Vita, A. , Messa, C. , Gobbo, C. , Galli, L. , … Fazio, F. (2002). In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: A positron emission tomography study. Neuroimage, 17(3), 1470–1478. [DOI] [PubMed] [Google Scholar]
- Moses‐Kolko, E. L. , Price, J. C. , Shah, N. , Berga, S. , Sereika, S. M. , Fisher, P. M. , … Meltzer, C. C. (2011). Age, sex, and reproductive hormone effects on brain serotonin‐1A and serotonin‐2A receptor binding in a healthy population. Neuropsychopharmacology, 36(13), 2729–2740. 10.1038/npp.2011.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton, M. , Mulrooney, J. B. , & Leonard, B. E. (2000). A review of the role of serotonin receptors in psychiatric disorders. Human Psychopharmacology, 15(6), 397–415. [DOI] [PubMed] [Google Scholar]
- Nikolaus, S. , Muller, H. W. , & Hautzel, H. (2016). Different patterns of 5‐HT receptor and transporter dysfunction in neuropsychiatric disorders—A comparative analysis of in vivo imaging findings. Reviews in the Neurosciences, 27(1), 27–59. 10.1515/revneuro-2015-0014 [DOI] [PubMed] [Google Scholar]
- Pinborg, L. H. , Adams, K. H. , Svarer, C. , Holm, S. , Hasselbalch, S. G. , Haugbol, S. , … Knudsen, G. M. (2003). Quantification of 5‐HT2A receptors in the human brain using [18F]altanserin‐PET and the bolus/infusion approach. Journal of Cerebral Blood Flow and Metabolism, 23(8), 985–996. 10.1097/01.wcb.0000074092.59115.23 [DOI] [PubMed] [Google Scholar]
- Poropat, A. E. (2009). A meta‐analysis of the five‐factor model of personality and academic performance. Psychological Bulletin, 135(2), 322–338. 10.1037/a0014996 [DOI] [PubMed] [Google Scholar]
- Reimold, M. , Batra, A. , Knobel, A. , Smolka, M. N. , Zimmer, A. , Mann, K. , … Heinz, A. (2008). Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: A [11C]DASB PET study. Molecular Psychiatry, 13(6), 606–613, 557. 10.1038/sj.mp.4002149 [DOI] [PubMed] [Google Scholar]
- Ross, S. , Bossis, A. , Guss, J. , Agin‐Liebes, G. , Malone, T. , Cohen, B. , … Schmidt, B. L. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life‐threatening cancer: A randomized controlled trial. Journal of Psychopharmacology, 30(12), 1165–1180. 10.1177/0269881116675512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaba, T. , & Bleidorn, W. (2017). Individual differences in personality change across the adult life span. Journal of Personality, 86, 450–464. 10.1111/jopy.12327 [DOI] [PubMed] [Google Scholar]
- Schwaba, T. , Luhmann, M. , Denissen, J. J. A. , Chung, J. M. , & Bleidorn, W. (2017). Openness to experience and culture‐openness transactions across the lifespan. Journal of Personality and Social Psychology, 115, 118–136. 10.1037/pspp0000150 [DOI] [PubMed] [Google Scholar]
- Skovdahl, H. H. , Mortensen, E. L. , & Schiøtz, H. K. (2011). NEO PI‐R, manual – klinisk, (1. udgave, 5. oplag ed.). Copenhagen, Denmark: Hogrefe Psykologisk Forlag. [Google Scholar]
- Soloff, P. H. , Chiappetta, L. , Mason, N. S. , Becker, C. , & Price, J. C. (2014). Effects of serotonin‐2A receptor binding and gender on personality traits and suicidal behavior in borderline personality disorder. Psychiatry Research, 222(3), 140–148. 10.1016/j.pscychresns.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff, P. H. , Price, J. C. , Mason, N. S. , Becker, C. , & Meltzer, C. C. (2010). Gender, personality, and serotonin‐2A receptor binding in healthy subjects. Psychiatry Research, 181(1), 77–84. 10.1016/j.pscychresns.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbaek, D. S. , Dam, V. H. , Fisher, P. M. , Hansen, N. , Hjordt, L. V. , & Frokjaer, V. G. (2017). No evidence for a role of the serotonin 4 receptor in five‐factor personality traits: A positron emission tomography brain study. PLoS One, 12(9), e0184403 10.1371/journal.pone.0184403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarer, C. , Madsen, K. , Hasselbalch, S. G. , Pinborg, L. H. , Haugbol, S. , Frokjaer, V. G. , … Knudsen, G. M. (2005). MR‐based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage, 24(4), 969–979. 10.1016/j.neuroimage.2004.10.017 [DOI] [PubMed] [Google Scholar]
- Takano, A. , Arakawa, R. , Hayashi, M. , Takahashi, H. , Ito, H. , & Suhara, T. (2007). Relationship between neuroticism personality trait and serotonin transporter binding. Biological Psychiatry, 62(6), 588–592. 10.1016/j.biopsych.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Tauscher, J. , Bagby, R. M. , Javanmard, M. , Christensen, B. K. , Kasper, S. , & Kapur, S. (2001). Inverse relationship between serotonin 5‐HT(1A) receptor binding and anxiety: A [(11)C]WAY‐100635 PET investigation in healthy volunteers. The American Journal of Psychiatry, 158(8), 1326–1328. 10.1176/appi.ajp.158.8.1326 [DOI] [PubMed] [Google Scholar]
- Tuominen, L. , Miettunen, J. , Cannon, D. M. , Drevets, W. C. , Frokjaer, V. G. , Hirvonen, J. , … Hietala, J. (2017). Neuroticism associates with cerebral in vivo serotonin transporter binding differently in males and females. The International Journal of Neuropsychopharmacology, 20(12), 963–970. 10.1093/ijnp/pyx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen, L. , Salo, J. , Hirvonen, J. , Nagren, K. , Laine, P. , Melartin, T. , … Keltikangas‐Jarvinen, L. (2013). Temperament, character and serotonin activity in the human brain: A positron emission tomography study based on a general population cohort. Psychological Medicine, 43(4), 881–894. 10.1017/s003329171200164x [DOI] [PubMed] [Google Scholar]
- Varnas, K. , Halldin, C. , & Hall, H. (2004). Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping, 22(3), 246–260. 10.1002/hbm.20035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider, F. X. , Vollenweider‐Scherpenhuyzen, M. F. , Babler, A. , Vogel, H. , & Hell, D. (1998). Psilocybin induces schizophrenia‐like psychosis in humans via a serotonin‐2 agonist action. Neuroreport, 9(17), 3897–3902. [DOI] [PubMed] [Google Scholar]
- Woods, R. P. , Cherry, S. R. , & Mazziotta, J. C. (1992). Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography, 16(4), 620–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 An overview of the existing literature on PET biomarkers of the 5‐HT system and trait Openness and Self‐transcendence. NEO PI‐R = NEO Personality Inventory; TCI = Temperament and Character Inventory; Blue indicates NEO‐PI‐R has been administrated. 5‐HT = Serotonin; 5‐HTT = Serotonin transporter; R = receptor; RN = Raphe nuclei; DLPC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; PC = parietal cortex and OC = occipital cortex; OFC = orbitofrontal cortex, Lt. MTC = left medial frontal cortex


