Abstract
Vitamin D deficiency may exacerbate adverse neurocognitive outcomes in the progression of diseases such as Parkinson's, Alzheimer's, and other dementias. Mild cognitive impairment (MCI) is prodromal for these neurocognitive disorders and neuroimaging studies suggest that, in the elderly, this cognitive impairment is associated with a reduction in hippocampal volume and white matter structural integrity. To test whether vitamin D is associated with neuroanatomical correlates of MCI, we analyzed an existing structural and diffusion MRI dataset of elderly patients with MCI. Based on serum 25‐OHD levels, patients were categorized into serum 25‐OHD deficient (<12 ng/mL, n = 27) or not‐deficient (>12 ng/mL, n = 29). Freesurfer 6.0 was used to parcellate the whole brain into 164 structures and segment the hippocampal subfields. Whole‐brain structural connectomes were generated using probabilistic tractography with MRtrix. The network‐based statistic (NBS) was used to identify subnetworks of connections that significantly differed between the groups. We found a significant reduction in total hippocampal volume in the serum 25‐OHD deficient group especially in the CA1, molecular layer, dentate gyrus, and fimbria. We observed a connection deficit in 13 regions with the right hippocampus at the center of the disrupted network. Our results demonstrate that low vitamin D is associated with reduced volumes of hippocampal subfields and connection deficits in elderly people with MCI, which may exacerbate neurocognitive outcomes. Longitudinal studies are now required to determine if vitamin D can serve as a biomarker for Alzheimer's disease and if intervention can prevent the progression from MCI to major cognitive disorders.
Keywords: cognition, MRI, older adults, vitamin D
1. INTRODUCTION
Vitamin D deficiency is a global public health burden (van Schoor & Lips, 2011) affecting millions of people in all age groups (Palacios & Gonzalez, 2014). Low‐serum vitamin D has been described as a risk factor for many brain diseases, including schizophrenia (Chiang, Natarajan, & Fan, 2016), autism spectrum disorders (Fernell et al., 2015), depression (Brouwer‐Brolsma et al., 2015), multiple sclerosis (Eskandari et al., 2015), dementia (Afzal, Bojesen, & Nordestgaard, 2014), and Alzheimer's disease. Beyond being a risk factor, vitamin D insufficiency purportedly contributes to the progression of schizophrenia (Valipour, Saneei, & Esmaillzadeh, 2014), and autism (Mazahery et al., 2016), and impaired cognition (Balion et al., 2012; Hooshmand et al., 2014; van der Schaft et al., 2013; Yesil et al., 2015) and slow cognitive processing (Annweiler et al., 2013). Notably, vitamin D deficiency accelerates the decline of cognitive function in older adults (Miller et al., 2015), with lower cognitive performance in vitamin D‐deficient subjects reported in a number of cross‐sectional and longitudinal studies (Oudshoorn, Mattace‐Raso, van der Velde, Colin, & van der Cammen, 2008; Przybelski & Binkley, 2007; Vicente, Herr, Mahieux, & Ankri, 2015; Wilkins, Sheline, Roe, Birge, & Morris, 2006).
Various neuropsychological tests have been used to measure the impact of vitamin D deficiency on cognitive abilities. However, the mini‐mental state examination (MMSE) has been the most commonly administered scale to assess the global cognitive functioning (Goodwill & Szoeke, 2017). MMSE assesses various cognitive domains such as memory, attention, and executive function. Importantly, the score of MMSE subscale had shown an association with the alterations of specific brain structures (Khachiyants & Kim, 2012). For example, hippocampal changes are associated with the delayed recall impairment, and orientation to time and place (Khachiyants & Kim, 2012). The hippocampus also plays a vital role in spatial and temporal memory information and updates memory with the continuous changes of environment (Howard & Eichenbaum, 2015; Meck, Church, & Matell, 2013). In addition, the hippocampus is a key brain region involve in cognitive functioning (Ekstrom & Ranganath, 2017; O'keefe & Nadel, 1978). Vitamin D deficiency has been shown to be associated with impaired visual memory, and verbal memory (Vicente et al., 2015), but not verbal episodic memory (Lam et al., 2016). Vitamin D deficiency was also shown to impair executive function (Annweiler et al., 2013). Remarkably, vitamin D supplementation was found to rescue nonverbal memory performance in vitamin D insufficient elderly people (Pettersen, 2017). Collectively, these studies outline an important link between vitamin D and cognitive performance that is teasingly beyond our current understanding.
The vitamin D receptor is present in various hippocampal subfields (Eyles, Smith, Kinobe, Hewison, & McGrath, 2005; Langub, Herman, Malluche, & Koszewski, 2001), suggesting that vitamin D plays an important role in the hippocampus (Gezen‐Ak, Dursun, & Yilmazer, 2013), which in turn plays a vital role in memory formation (Cho et al., 2015; Virley et al., 1999), encoding, and retrieval (Greicius et al., 2003; Lepage, Habib, & Tulving, 1998). The hippocampus also facilitates the conversion of short‐term memory (STM) to long‐term memory (LTM). This integral role in memory function indicates that memory impairment results from hippocampal volume loss. Several studies have shown a relationship between hippocampal volume loss and cognitive impairment leading to dementia (Shi, Liu, Zhou, Yu, & Jiang, 2009). Emerging evidence from several lines of inquiry suggest that vitamin D may contribute to hippocampal volume loss. Evidence from a large community‐based study (n = 1,663) indicated that low‐serum vitamin D was associated with a lower hippocampal volume (Karakis et al., 2016) and reduction of hippocampal grey matter volume has been shown in schizophrenia patients with vitamin D deficiency. Importantly, a reduction of hippocampal subfield volume has been shown in the same brain diseases in which vitamin D deficiency is implicated, including major depressive disorder (Han, Won, Sim, & Tae, 2016; Ota et al., 2017; Samuels, Leonardo, & Hen, 2015), schizophrenia (Ota et al., 2017), Alzheimer's disease (de Flores, La Joie, & Chetelat, 2015; La Joie et al., 2013), and dementia (La Joie et al., 2013). Taken together, these studies suggest that vitamin D deficiency may contribute to reduced hippocampal subfield volume. However, to our knowledge, the structural changes of the hippocampal subfield in vitamin D deficiency have not been examined.
The hippocampus is a deep subcortical region consisting of anatomically distinct regions; cornu ammonis (CA; further categorized into CA1, CA2, CA3, CA4), dentate gyrus (DG), subiculum, pre‐ and para‐ subiculum, entorhinal cortex, hippocampal fissure, fimbria, and the hippocampal–amygdaloid transition area (HATA) (Andersen, 2007).
In this study, we test the hypothesis that vitamin D deficiency is associated with exacerbated neurocognitive outcomes in elderly individuals with mild cognitive impairment through reduced hippocampal volume and altered white matter connectivity. Individuals were separated into deficient and not‐deficient vitamin D groups, based on serum 25‐OHD levels. Psychometric measures and MRI data were analyzed to compare these two groups for cognitive impairment, mean hippocampal volumes, subfield volumes, and structural connectivity.
2. METHODS
2.1. Participants
Patients with a memory complaint visited the Memory Clinic of the Konkuk University Hospital (KUH) in South Korea during 2011–2013. Demographic information and any previous related history was collected during the first visit. Each participant was further examined using the Mini‐Mental Status Examination (MMSE); as well as the Clinical Dementia Rating Scale (CDR), Clinical Dementia Rating Scale Sum of Boxes (CDR‐SOB), Geriatric Depression Scale (GDepS), and the Global Deterioration Scale (GDS). Total physical activity was calculated by multiplying the metabolic equivalent of task (MET) values and frequency by the summed hours in 1 week. Physical activity scores of 0, 0.1–100, and >100 were classified as 0, 1, or 2, respectively. A routine clinical examination on known covariates was also conducted including incidence of diabetes or hypertension, and measures of serum creatinine and glomerular filtration [see more details in (Moon, Moon, Kwon, Lee, & Han, 2015)]. Patients were excluded if they had a previously diagnosed neurodegenerative disease, cancer, respiratory disorder, renal and hepatic disease, psychological, or substance abuse disorder (Langub et al., 2001). Patients with probable AD or amnesic mild cognitive impairment (aMCI) were diagnosed according to DSM‐IV (APA, 2000) and (Petersen et al., 1999), respectively. Demographic characteristics from these patients (N = 56), were provided to the Queensland Brain Institute (QBI) including sex, age, education years, together with vitamin D level, health metrics, and cognitive functions (MMSE, CDR, CDRSOB, GDepS, GDS). Brain magnetic resonance image (MRI) data acquired and deidentified by KUH were also provided for all but two patients (n = 54).
According to the Institute of Medicine (IOM), serum levels of <12 ng/mL (30 nmol/L) of 25‐OHD are associated with vitamin D deficiency. Levels of 12–20 ng/mL indicate risk of inadequacy, whereas higher than 20 ng/mL is considered to be sufficient (Ross et al., 2011). In our study, the mean serum level of 25‐OHD was 15.41 ng/mL (Figure 1). We followed the IOM categorization to select the cutoff value (12 ng/mL) to categories MCI patients into two groups: deficient 25‐OHD (n = 27, 18 females, M = 8, range 4.32–10.89 ng/mL) and not‐deficient 25‐OHD (n = 29, 20 females, M = 21.61, range 15–41.74 ng/mL).
Figure 1.
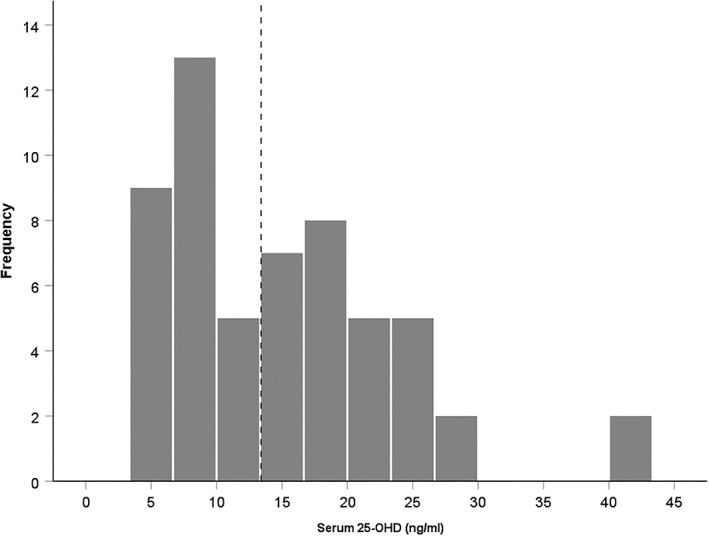
Histogram showing the frequency of serum 25‐OHD level of the participants (N = 56). Mean serum 25‐OHD level was 15.41 ng/mL. The participants were divided into two groups (dotted line): deficient 25‐OHD (n = 27) and not‐deficient 25‐OHD (n = 29) based on Institute of Medicine specifications (Ross et al., 2011)
The Institutional Review Board of KUH approved the study and the collection of samples and images (Moon et al., 2015). Hippocampal subfield segmentation was undertaken at the Queensland Brain Institute, The University of Queensland, with approval from the human ethics committee at the University of Queensland for the use of data and further analysis (Approval number 2017000165).
2.2. Image acquisition
Structural MRIs were acquired to diagnose and identify the possible neuroanatomical cause of dementia. Both structural T1‐weighted images and T2 flair images were acquired at the Konkuk University Medical Center on a Magnetom Skyra 3.0 Tesla unit (Siemens Germany). The following parameters were used during image acquisition: echo time (TE) = 9 ms, repetition time 2,000 ms, matrix size = 320 × 256, slice thickness = 5 mm, total number of slices = 240. The interpolated voxel size was 0.9 mm isotropic. Diffusion tensor images were acquired using the following parameters TR = 8,600 ms, TE = 67 ms, matrix 128 × 128; section thickness 3.5 mm. The T2‐flair images were acquired using parameters of TR = 9,000, TE = 95, TI = 2,500 ms; section thickness = 5 mm and matrix size = 320 × 188. Images were acquired with 21 directions with a b value of 1,000. MRI data are missing for two patients in the deficient group.
2.3. Hippocampal subfield segmentation
Hippocampal subfield segmentation was undertaken with Freesurfer, version 6.0 (http://surfer.nmr.mgh.harvard.edu). T1‐weighted structural images were motion corrected before using “recon‐all” script with settings kept as default for whole brain segmentation (Bohland, Saperstein, Pereira, Rapin, & Grady, 2012; Jovicich et al., 2006; Maclaren, Han, Vos, Fischbein, & Bammer, 2014). Segmentation errors were carefully checked by an investigator (MA) blinded to the identity of each sample and segmented images edited manually if parcellation was poor.
Hippocampal subfields were delineated with the automated Freesurfer 6.0 subfield segmentation script (Iglesias et al., 2015). This script uses a probabilistic atlas to create the internal boundaries between the hippocampal subfields. The atlas was built on ex vivo MRI data (~0.1 m isotropic). Previous studies have used manual tracing (Adler et al., 2014; Ballmaier et al., 2008) and automated labeling (Haukvik et al., 2015; Ota et al., 2017; Treadway et al., 2015) to determine the subfield volumes. We used the automated Freesurfer 6.0 script as this is the most widely used automated method for hippocampal subfield segmentation.
The recent development of automated, state‐of‐the‐art techniques has overcome previous challenges to identify the boundary of each hippocampal subfield. This method achieves reliability and accuracy that is comparable to manual tracing (Whelan et al., 2016), and consistent with histological segmentation (Iglesias et al., 2015). We quantified the volume of 24 hippocampal subregions (12/hemisphere). These included hippocampal tail, subiculum, CA1, hippocampal fissure, presubiculum, parasubiculum, molecular layer within the subiculum (ML), granular cell layer within the dentate gyrus (GC‐DG), CA3, CA4, fimbria, and the hippocampus–amygdaloid transition area (HATA) (Figure 2) (Iglesias et al., 2015). The labeling of each subfield was inspected manually by an investigator (MA) blind to the identity of each individual. Each segmented label file was visually cross‐checked by overlaying the respective 3D structural brain image to exclude any defect appearing in image registration and corresponding anatomical labelling of the hippocampal subfields. We did not exclude any sample due to poor registration or segmentation, although an individual was manually corrected to fit the labels with the corresponding 3D T1 structural image. We have not performed spatial smoothing for T1 image to extract the hippocampal subfield volumes, although spatial smoothing was used to produce Figure 2, for presentation only. We performed intracranial volume (ICV) analysis to correct any inter‐individual variances in head or whole brain size.
Figure 2.
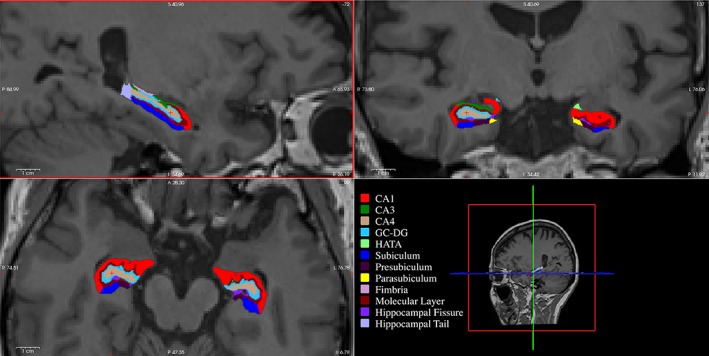
Hippocampal subfield segmentation using Freesurfer v 6.0 overlaid on a structural MRI scan to provide anatomical context. Three sections are shown: sagittal view of the left hippocampus (top left), coronal view of the both hippocampi (top right), and axial view of both hippocampi (bottom left). Each subfield is colored distinctly (bottom right). CA, Cornu Ammonis; GC‐DG, granular cell layer within the dentate gyrus; HATA, hippocampus–amygdaloid transition area [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. T1 image processing for node preparation
The FSL brain extraction tool (BET) (http://fsl.fmrib.ox.ac.uk) was used to remove nonbrain structures from the T1 weighted structural images (Smith, 2002). The five‐tissue‐type image comprising cortical grey matter, subcortical grey matter, white matter, cerebrospinal fluid (CSF), and pathological tissues were produced using 5ttgen from MRtrix. The parcellation of cortical ribbon into 148 distinct regions was done using the Destrieux atlas, which contains 74 cortical structures/hemisphere (Fischl et al., 2004). The parcellated image was converted using MRtrix to produce 164 nodes for the structural connectome analysis.
2.5. Diffusion‐weighted imaging (DWI) processing and tractography
Diffusion‐weighted imaging DWI datasets were processed using FSL denoising, eddy current, and bias correction as implemented in MRtrix 3 (Figure 3). Subsequently, the brain tissue was extracted, and a multi‐tissue response function was estimated (Dhollander, Raffelt, & Connelly, 2016) to calculate the fiber orientation distributions using the constrained spherical deconvolution algorithm (Tournier, Calamante, & Connelly, 2007).
Figure 3.
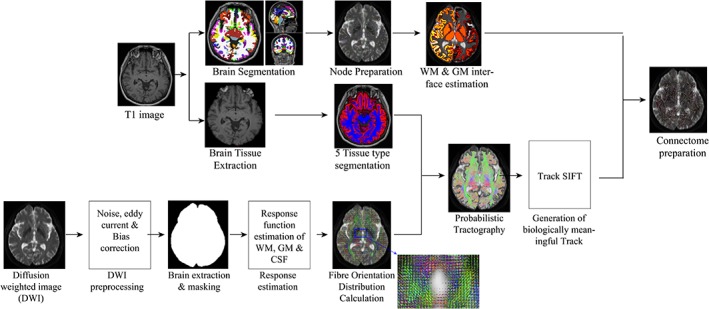
Processing pipeline of probabilistic tractography and connectome preparation. DWI, diffusion‐weighted image; GM, grey matter; SIFT, spherical‐deconvolution informed filtering of tractograms; WM, white matter [Color figure can be viewed at http://wileyonlinelibrary.com]
Five‐tissue‐type segmented T1 image and anatomically constrained tractography (Smith, Tournier, Calamante, & Connelly, 2012) were used to generate 10 million whole‐brain tractograms. In this method, tracts were allowed to be truncated if a poor structural termination was encountered, and the tracts were cropped at the grey matter white matter interface. Spherical‐deconvolution informed filtering of tractograms (SIFT) (Smith, Tournier, Calamante, & Connelly, 2013) was used to filter the tracts to 5 million. SIFT conversion can potentially improve the biological plausibility of some streamline counts. These tracks were mapped into the 164 nodes to produce a 164 × 164 connectivity matrix. Each matrix element stores the number of valid streamlines (streamline count) connecting a given pair of regions.
2.6. Connectivity analysis
The network based statistic (NBS) (Zalesky, Fornito, & Bullmore, 2010) was used to identify subnetworks (i.e., group of connections) for which the streamline count significantly differed between the deficient and not‐deficient 25‐OHD subgroups. The null hypothesis tested was equality in the mean streamline count between the two subgroups. The NBS is a nonparametric, permutation‐based approach for performing statistical inference on the connectome. With the NBS, the null hypothesis can be rejected at the level of subnetworks. This affords increased statistical power compared to testing each connection independently. We measured the size of a subnetwork as the number of connections it comprised (extent) and sum of the t statistic across these connections (intensity). A range of primary thresholds (2.5–3.5) were considered and 5,000 permutations were generated to estimate p values. Gender and age were included as a nuisance covariate. For each analysis, we used two different contrasts (deficient 25‐OHD > not‐deficient 25‐OHD; deficient 25‐OHD < not‐deficient 25‐OHD).
2.7. Data analysis
We conducted statistical analyses using Prism (Version 7, GraphPad Software, California) and SPSS (Version 24.0, IBM Analytics, Australia). Serum 25‐OHD was treated as an independent variable, with cognitive scores and hippocampal volumes as dependent variables, and patient demographics of age, BMI, years of education, levels of serum creatinine, diabetes, hypertension, and physical activity scores treated as covariates. The family wise error rate (FWER) was corrected using Bonferroni's multiple comparison test for the 12 hippocampal subfields. The p values <.05 were considered significant. Data are presented as mean ± SEM.
3. RESULTS
3.1. Low vitamin D serum levels are associated with degree of cognitive impairment
The mean serum 25‐OHD level for pooled participants was 15.41 ng/mL. As we used the IOM defined cutoff of <12 ng/mL for deficient levels, we performed student's t test to ensure the two groups were sufficiently different to compare. We found that serum 25‐OHD was significantly lower in the 25‐OHD deficient group (n = 27) (M = 8 ± 0.39) compared to the sufficient group (n = 29) (M = 21.61 ± 1.28) (where t[54] = 9.89, p < .0001) (Figure 4a).
Figure 4.
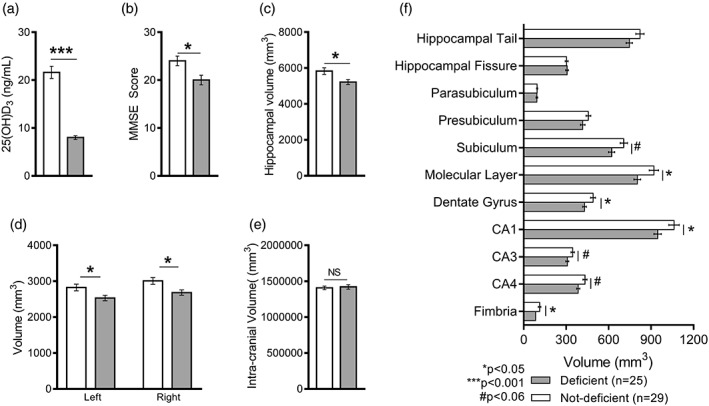
Serum vitamin D (25‐OHD) levels (N = 56) and hippocampal subfield volumes (N = 54) in deficient and not‐deficient serum 25‐OHD groups. Significant differences between deficient and not‐deficient groups were found for levels of 25‐OHD (a) MMSE score (b), and total hippocampal volume (c), with no effect of laterality (d) or brain/head size (e). Hippocampal subfield volume analysis revealed a significant difference in the CA1 region, the molecular layer, the dentate gyrus, and the fimbria (f). Smaller volumes in the subiculum, CA3 and CA4 did not reach significance. CA, Cornu Ammonis; GC‐DG, granular cell layer within the dentate gyrus; HATA, hippocampus–amygdaloid transition area. * p < .05; *** p < .001; # p < .06. Data present as mean ± SEM (standard error of mean). Family‐wise error rate (FWER) corrected. MRI data are missing for two patients in the deficient group
There were significant differences in overall cognitive impairment between the groups, with lower scores measured by all scales except the Geriatric Depression Scale (Figure 4b and Table 1). The groups did not differ significantly with respect to physical activity scores (Table 1). As education can influence MMSE scores, we confirmed that there were no significant differences in years of education between deficient (M = 8 ± 1.00) and not‐deficient (M = 11 ± 1.00) groups (p > .05).
Table 1.
Vitamin D‐deficient MCI patients have significantly lower scores on cognitive tests than do MCI patients who are not vitamin D deficient
| Deficient (n = 27) | Not‐deficient (n = 29) | ||||
|---|---|---|---|---|---|
| Mean | Std error | Mean | Std error | p value | |
| 25‐OHD | 8.00 | .39 | 21.61 | 1.28 | <.0001 |
| MMSE | 20.15 | 1.17 | 23.86 | .85 | .011a |
| Clinical Dementia Rating Scale | .80 | .10 | .52 | .05 | .015a |
| Clinical Dementia Rating Scale Sum of Boxes | 4.15 | .65 | 2.12 | .31 | .006a |
| Geriatric Depression Scaleb | 11.22 | 1.34 | 9.08 | 1.40 | .28 |
| Global Deterioration Scale | 4.15 | .19 | 3.41 | .14 | .003a |
| Physical Activity | n (%) | n (%) | |||
| 0 | 13 (48.1) | 11 (37.9) | |||
| 1 | 14 (51.9) | 14 (51.9) | |||
| 2 | 0 (0) | 4 (13.8) | |||
Indicates significance.
Data available for a subset of patients, deficient (n = 23) and not‐deficient (n = 26).
To gain greater insight into the neurocognitive tasks impacted by vitamin D deficiency, we examined MMSE subscales. The MMSE measures six domains comprising orientation, registration, attention, recall, language, and complex commands (in this case, drawing). Subscale data were missing for two patients in the not‐deficient group. We found significant differences in subscales between deficient and not‐deficient groups in the orientation subscale for both time (M = 2.85 ± 0.36 and M = 4.04 ± 0.26, respectively, where t[52] = 2.67, p = .01) and place (M = 3.63 ± 0.29 and M = 4.44 ± 0.13, respectively, where t[52] = 2.56, p = .013). No significant differences were found in subscales for registration, attention, recall, language, or drawing.
3.2. Low vitamin D serum levels are associated with smaller hippocampal volume
Analysis with ANOVA revealed a significant main effect of 25‐OHD on whole hippocampal volumes (F 1,52 = 6.60; p = .013), with the hippocampal volume of the deficient 25‐OHD group smaller than that of the not‐deficient 25‐OHD group (Figure 4c). Further analysis revealed that there was no significant main effect of laterality (F 1,104 = 3.62; p = .059) as the volume of both hippocampi were reduced compared to the not‐deficient group (Figure 4d). Intracranial volume (ICV) analysis showed that this effect was independent of brain or head size, as ICV did not differ significantly between deficient and not‐deficient groups (p > .05) (Figure 4e). A moderate correlation (r = .35) between levels of serum 25‐OHD and whole hippocampus volume was found to be significant (p = .01). Further correlation analysis showed a significant correlation between serum 25‐OHD and each hippocampal subfield volumes except hippocampal fissure, presubiculum, and parasubiculum (see Supporting Information, Table S1).
We also found a significant main effect of serum 25‐OHD in CA1 (F 1,52 = 4.41; p = .041), molecular layer of CA1 (F 1,52 = 5.35; p = .025), dentate gyrus (F 1,52 = 5.05; p = .029), and fimbria (F 1,52 = 4.38; p = .041) (Figure 4f). Volumes were smaller but did not reach statistical significance in the subiculum (F 1,52 = 3.98; p = .051), CA3 (F 1,52 = 3.77; p = .058), and CA4 (F 1,52 = 3.98; p = .051). There were no significant main effects of serum 25‐OHD in hippocampal fissure (F 1,52 = 0.07; p = .783), hippocampal tail (F 1,52 = 1.35; p = .25), presubiculum (F 1,52 = 1.96; p = .167), parasubiculum (F 1,52 = 0.001; p = .970), or HATA (F 1,52 = 1.24; p = .269).
Together these data suggest that low vitamin D status is related to smaller hippocampal volume which in turn impacts on hippocampal‐dependent tasks, such as orientation. Before examining neuroanatomical correlates in further detail, we first ruled out the effects of potential confounding variables collected at the time of presentation (Moon et al., 2015). We found no significant differences in terms of age (M = 76.56 ± 1.09 vs 73.59 ± 1.64), BMI (M = 22.70 ± 0.67 vs 21.74 ± 0.92), levels of serum creatinine (M = 0.88 ± 0.05 vs 0.87 ± 0.04), or glomerular filtration rate (M = 71.81 ± 3.12 vs 72.79 ± 2.48) between deficient and not‐deficient groups (p > .05) (data not shown). Diabetes and hypertension were recorded as incidence only, and therefore could not be included as covariates. No significant differences were found between the deficient and not‐deficient groups in incidence of diabetes (M = 0.30 ± 0.07 vs 0.14 ± 0.09) or hypertension (M = 0.61 ± 0.08 vs 0.35 ± 0.13).
3.3. Vitamin D deficiency is associated with structural connectivity deficits primarily in the right hemisphere
The NBS was used across a range of primary thresholds (t = 2.5–3.5) to identify structural connections that differed between the two groups. Widespread reductions in the number of connections between regions (streamline count) were found in the 25‐OHD‐deficient group compared to the not‐deficient group. As the primary threshold was increased, these reductions converged on a subnetwork centered on the hippocampus (Figure 5). No significant increases in connectivity in the deficient 25‐OHD group were found (p > .05). Using a threshold setting of 3.5 showed lower p values in terms of extent (p = .003) and intensity (p = .002) (Supporting Information, Figure S1). This threshold setting revealed the following networks to be disrupted in the brains of MCI patients with vitamin D deficiency (Figure 5).
Figure 5.
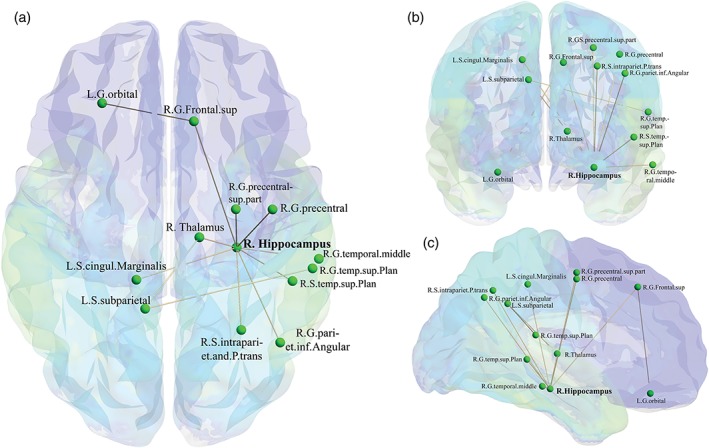
Disrupted intra‐ and interhemispheric connectivity in serum deficient 25‐OHD MCI patients (n = 25). This disrupted network was found at threshold t = 3.5. (a) Axial view. (b) Coronal view. (c) Sagittal view. The figure made using BrainNet Viewer (Xia, Wang, & He, 2013). In total, 13 nodes and 12 edges were disrupted in the brains of MCI patients with vitamin D deficiency. The 3D view of this disrupted network is available in Supporting Information, Video S2 [Color figure can be viewed at http://wileyonlinelibrary.com]
We found that 13 brain regions had a connection deficit in the 25‐OHD‐deficient group. The greatest disruption to connectivity was found in the right hemisphere with connection deficits in 10 regions, including eight cortical regions (right frontal superior gyrus, right precentral gyrus superior part, right precentral gyrus, right temporal middle gyrus, right temporal plane superior gyrus, right temporal plane superior sulcus, right parietal inferior‐angular gyrus, and right intraparietal sulcus and parietal transverse) and two subcortical regions (right thalamus and right hippocampus). There were three regions in the left cortex (left orbital gyrus, left cingulate marginalis sulcus, and left subparietal sulcus). Additionally, four interhemispheric connections were disrupted in the 25‐OHD‐deficient group compared to the not‐deficient group. The four inter‐hemispheric disrupted connections were observed connecting the (1) right frontal superior gyrus and left orbital gyrus; (2) right hippocampus and left cingulate marginalis sulcus; (3) right thalamus and left subparietal sulcus; and (4) right temporal superior plane sulcus and left subparietal sulcus. Interestingly, the right hippocampus was placed as the central hub of the disrupted network. Out of the 13 regions, nine regions were connected to the right hippocampus.
Further correlation analysis revealed that the serum 25‐OHD was associated with the total hippocampal connectivity (Supporting Information, Table S1). In addition, we observed that there was an association between serum 25‐OHD and right hippocampal connectivity but not left hippocampal connectivity.
3.4. Hippocampal CA1 volume and structural connectivity is associated with the MMSE scale
We observed a significant association between hippocampal volume and MMSE, CDR, and CDRSOB scores. There was also a significant association between hippocampal connectivity with MMSE, CDR, and CDRSOB scores (Supporting Information, Table S2).
We also analyzed the correlation between hippocampal CA1 volume and neuropsychological test scores. A moderate correlation (r = .513) between CA1 volume and global MMSE score was found to be significant (p < .001). In addition, a moderate correlation was observed between CA1 volume and MMSE subscales such as time, place, attention, and recall (Supporting Information, Table S2).
A mediation analysis among the variables serum 25‐OHD, right hippocampal volume, and right hippocampal structural connectivity was conducted. We showed that the connectome deficit was mediated by both serum vitamin D and right hippocampal volume (Supporting Information, Figure S2).
4. DISCUSSION
In elderly patients with mild cognitive impairment, we found that serum vitamin D levels were associated with hippocampal volume and structural brain connectivity. Specifically, we observed smaller whole hippocampal volume in those with deficient serum 25‐OHD levels, which on further analysis was found to be due to smaller volumes of the CA1, molecular layer, dentate gyrus, and fimbria subfields. We also found disrupted structural brain connectivity in 13 regions in patients with deficient levels of serum vitamin D. Network disruption was most evident in the right hemisphere, with the right hippocampus as the central hub. These patients had significantly more severe neurocognitive outcomes than the patients who were not vitamin D deficient, as reflected in scores on a cognitive battery including MMSE, CDR, CDR‐SOB, and GDS. The neuroanatomical correlates of this mild cognitive impairment were independent of age, years of education, or ICV.
4.1. Reduced hippocampal volumes are characteristic of both vitamin D deficiency and MCI
The smaller hippocampal volumes observed in the low vitamin D group are consistent with a recent large community study, which also suggested an association between low vitamin D and reduced hippocampal volume (Karakis et al., 2016). Similarly, low serum vitamin D has been associated with smaller hippocampal volumes in patients with schizophrenia and in patients with psychosis (Gurholt et al., 2018). Our finding of a smaller CA1 subfield is in alignment with Shivakumar et al.'s claim that volume loss is due to reduction of hippocampal grey matter (Shivakumar et al., 2015). The moderate positive correlation between serum 25‐OHD and hippocampal volume found in both our study and Shivakumar et al.'s suggests that the effect of low vitamin D on hippocampal volume loss may be dose dependent.
To further understand the structural changes of the hippocampus in low vitamin D conditions, we looked at the hippocampal subfields (Maruszak & Thuret, 2014). We observed CA1 hippocampal subfield measurements were differentially associated with vitamin D levels, as were volumes of the molecular layer, dentate gyrus, and fimbria regions. We also observed a trend of volume reduction in the subiculum, CA3, and CA4; however, multiple comparison tests found these differences fell short of significance. Our findings of smaller CA1 is not without precedent in neuroimaging studies with MCI patients. Reduced volume of the subiculum, right CA1, CA3, and dentate gyrus have been reported in MCI patients (Yassa et al., 2010), although vitamin D levels were not assessed. In addition, a reduction of dentate gyrus volume has been found to be associated with memory deficits in major depressive disorder (Travis et al., 2015).
4.2. Hippocampal volume loss is associated with disrupted networks which impact on cognitive processing
Besides hippocampal subfield volume loss, we also found a hippocampal‐centered connectivity deficit in the 25‐OHD‐deficient group. It is plausible that global hippocampal volume reduction impairs connectivity with both cortical and subcortical regions and that the reduction of subfield volume interrupts internal hippocampal connectivity. We observed that nine out of 12 disrupted edges (connections) were linked to the right hippocampus. Our hippocampal subfield volume analysis showed that the 25‐OHD‐deficient group had reduced whole hippocampal volume without effects of laterality. While hippocampal volume asymmetry has been observed in many studies of MCI patients (Shi et al., 2009), right hippocampal atrophy is associated with patients who progress from MCI to AD (Apostolova et al., 2006; Zhang et al., 2007).
Hippocampal volume loss could be linked with N‐acetyl aspartate (NAA) levels. We have not found any studies in which both hippocampal NAA (N‐acetyl aspartate) and hippocampal volume were measured in the same study on vitamin‐D‐deficient participants. However, there is evidence to show an association of lower NAA/Cr ratio with low serum 25‐OHD concentration (Annweiler, Beauchet, Bartha, Hachinski, & Montero‐Odasso, 2014). In addition, a reduced NAA level has been shown linked with the reduced hippocampal volume in many brain diseases (Abdallah et al., 2013; Schuff et al., 1997). Based on the existing evidence, we hypothesised that the reduction of NAA in the hippocampus could contribute to hippocampal volume loss.
Hippocampal subfields have a distinct role in memory processing (Bahar, Shirvalkar, & Shapiro, 2011; Coras et al., 2014; Lewis, 2016). Therefore, the reduction of the hippocampal subfield volumes would likely affect cognitive function. For example, the CA1 region is essential for retrieval of contextual memory (Ji & Maren, 2008), autobiographical memory (Bartsch, Dohring, Rohr, Jansen, & Deuschl, 2011), consolidation of long‐term memory (Remondes & Schuman, 2004; Stackman, Cohen, Lora, & Rios, 2016). This is consistent with the finding of significantly poorer scores in the orientation subscales of MMSE in patients with deficient levels of vitamin D. The CA1 region connects the cortical regions via the subiculum. Our results indicate a trend of reduction in the subiculum. Of all the subfields, the subiculum and presubiculum showed the greatest laterality with volumes in the right hemisphere reduced to a greater extent than those in the left. This finding potentially explains why the disrupted networks are lateralized to the right, despite similar volume loss in both hippocampi.
The subiculum, CA3, and CA4 regions are extensively connected with the CA1. The subiculum is the last relay center of the hippocampal formation prior to the cortex (Commins, Aggleton, & O'Mara, 2002; O'Mara, Commins, & Anderson, 2000), projecting to many cortical and subcortical targets (O'Mara, Commins, Anderson, & Gigg, 2001), with a broad range of information processing including spatial learning, stress, anxiety, and reward processing (O'Mara, Sanchez‐Vives, Brotons‐Mas, & O'Hare, 2009). The CA3 region has richer internal connectivity than any of the other hippocampal subfields (Cherubini & Miles, 2015), and has a vital role for rapid encoding of memory (Rebola, Carta, & Mulle, 2017), retrieving sequences of spatial memory (Hunsaker, Lee, & Kesner, 2008), and predicting memory sequence (Jensen & Lisman, 1996). CA3 is also involved in linking memory encoding and rebuilding memory representations while retrieval using pattern separation and pattern completion (Deuker et al., 2013). The CA4 region, also known as the hilus, receives inputs from the granule cells of the dentate gyrus (Amaral, 1978) and major excitatory input from the cortex. The reduction in volume of the CA4 region may induce a synaptic disruption between the cortex and hippocampus. The dentate gyrus plays a vital role in neurogenesis (Aimone, Deng, & Gage, 2014), and is linked with memory formation (Anacker & Hen, 2017). Damage to dentate gyrus neurons significantly affects spatial learning (Lee & Kesner, 2004a; Walsh, Schulz, Tilson, & Schmechel, 1986) by impaired encoding of spatial cues (Jerman, Kesner, & Hunsaker, 2006; Lee & Kesner, 2004b). Taken together, the reduction of CA1 would disrupt the internal hippocampal network and connections with many cortical and subcortical regions.
We performed a whole‐brain connectome analysis using NBS and DTI to identify altered connections and networks and volumetric analysis of the hippocampal subfields to determine changes associated with vitamin D deficiency. Importantly, DTI parameters are helpful to understand the pathological basis of MCI, with reports of a lower DTI index such as a lower median diffusivity in the hippocampus (Fellgiebel et al., 2004) and a lower fractional anisotropy of white‐matter tracts (Medina et al., 2006) in MCI patients. Moreover, a DTI study revealed a disruption of hippocampal structural connectivity within the higher cortical regions in MCI patients (Zhou et al., 2008).
In this study, we observed a hippocampal–thalamic–prefrontal connection disruption in patients with 25‐OHD deficiency. The thalamus is the primary relay hub of the brain due to widespread connectivity with cortical and subcortical regions and is associated with cognitive function. The alteration of thalamic connectivity may affect a range of cognitive processing, such as processes of attention, speed of information processing, and working memory (Fama & Sullivan, 2015).
4.3. Serum vitamin D as a potential biomarker
MMSE is a widely used parameter to assess cognitive dysfunction (Dinomais et al., 2016; Spering et al., 2012). In this study, there were significant differences in all cognitive scales (MMSE, CDR, CDR‐SOB, and GDS) except the GDepS between the vitamin D‐deficient and not‐deficient groups. Our result is consistent with a large volume of published studies that have found an association between MMSE score and serum vitamin D (Balion et al., 2012; Matchar et al., 2016; Vedak et al., 2015). A recent study purports the combination of MMSE score with serum 25‐OHD could have a predictive value of 98% in diagnosing MCI and AD (Ouma et al., 2018). Similarly, in elderly MCI patients, hippocampal atrophy is predictive of progression to AD (Jack Jr. et al., 1999). Further mediation analysis revealed a right hippocampal connectome deficit, which was mediated by both serum vitamin D levels and right hippocampal volumes. Together our results suggest that low levels of vitamin D are associated with the loss of hippocampal volume, which in turn impact on the connectivity that underpins hippocampal‐dependent tasks.
To ensure our results were valid, we ruled out as many potential confounds as the data allowed. We compared the intracranial volumes and observed that head/brain size did not differ between these groups. We compared demographic factors (age, gender, and education) and disease conditions (cardiovascular, diabetes, and kidney disease) that could have had an impact on our results and found these did not differ between the deficient and not‐deficient groups. Therefore, our data strongly suggest that the reduction of hippocampal subfield volumes is associated with a deficiency in serum vitamin D.
4.4. Potential mechanisms for vitamin D‐dependent hippocampal deficits
Low levels of vitamin D could contribute to hippocampal deficits through a number of mechanisms including increased proinflammatory cytokines, increased oxidative stress, reduced level of neurotrophic factors, lower synaptic protein, and increased excitotoxicity, all of which may result in reductions in hippocampal sub field volumes. Animal studies suggest that vitamin D deficiency alters immune function (Balden, Selvamani, & Sohrabji, 2012; Brett, Agellon, Vanstone, & Weiler, 2014), which may result in an increase in the production of proinflammatory cytokines (IL‐6), which damage the hippocampus (Samuelsson, Jennische, Hansson, & Holmang, 2006). Vitamin D deficiency reduces antioxidant properties (Garcion, Sindji, Leblondel, Brachet, & Darcy, 1999; Mutlu et al., 2016) by altered regulation of nuclear factor kappa‐B (NF‐kB)‐mediated elevation of iNOS enzyme (Keeney et al., 2013). Furthermore, vitamin D contributes to many neurotrophic factors (Feron et al., 2005; Naveilhan, Neveu, Wion, & Brachet, 1996), such as GDNF mRNA expression (Naveilhan et al., 1996), and increases nerve growth factor (NGF) concentration (Musiol & Feldman, 1997).
Vitamin D has been shown to enhance hippocampal synaptic function in rats (Latimer et al., 2014). Therefore, the disruptions of hippocampal structural connectivity and the reduction of hippocampal volume could be due to the loss of hippocampal synapses and a reduced level of synaptic protein. The lower level of synaptic protein might be a cause of hippocampal volume reduction which then leads to lower neuronal connectivity.
4.5. Limitations of the study
There were a number of limitations with this study, including the cross‐sectional design, small sample size, and lack of detailed data on potential covariates (hypertension and diabetes). The level of serum vitamin D was considered as a dichotomous variable, with a cutoff in line with the IOM classification of risk of deficiency. However, greater insight into the value of vitamin D as a predictive biomarker for progression from MCI to AD might be obtained from treating serum vitamin D as a continuous variable with a larger cohort of patients. No data were available on the potential limiters of level of serum vitamin D, such as reduced sunlight exposure, which could account for mild neurocognitive decline. Despite these limitations, this study has provided new insights into the impact of vitamin D deficiency on hippocampal subfield volume and structural brain connectivity. Animal models of vitamin D deficiency may be a feasible way to examine the impact of vitamin D on hippocampal volume. We suggest that longitudinal studies now be used to explore the causal relationship between cognitive impairment and volumetric changes in hippocampus subfields.
5. CONCLUSION
This study provides the first evidence that vitamin D deficiency leads to network disruption centered in the right hippocampus in elderly patients with MCI. Our results suggest that the deficits in hippocampal subfield volume and structural connectivity underlie accelerated neurocognitive decline particularly for hippocampal‐dependent tasks. Our findings have therapeutic and diagnostic implications for future studies in patients with MCI to determine if vitamin D can be used as a biomarker of neurodegeneration, circumventing the need for MRI in elderly patients. Furthermore, it remains to be seen if neurocognitive decline can be slowed or blocked using vitamin D supplementation, which is a safe, cheap, and publically acceptable treatment option. These findings bring us closer to an understanding of the acceleration of neurodegeneration in MCI and how vitamin D may play a role in predicting, prolonging, or one day even preventing the progression from MCI to AD.
FUNDING
This research was supported by the National Health and Medical Research Council grant APP1078159 to TB and a University of Queensland International PhD Scholarship to MA. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Supporting information
Supporting Information Figure S1
Supporting Information Figure S2
Figure Legends
Supporting Information, Table 1. Association of serum vitamin D concentrations with hippocampal subfield volumes and hippocampal connectomes
Supporting Information, Table 2. Correlation of hippocampal CA1 volume and hippocampal connectivity with neurocognitive outcomes
Al‐Amin M, Bradford DK, Sullivan RKP, et al. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum Brain Mapp. 2019;40:394–406. 10.1002/hbm.24380
Funding information National Health and Medical Research Council, Grant/Award Number: APP1078159
REFERENCES
- Abdallah, C. G. , Coplan, J. D. , Jackowski, A. , Sato, J. R. , Mao, X. , Shungu, D. C. , & Mathew, S. J. (2013). A pilot study of hippocampal volume and N‐acetylaspartate (NAA) as response biomarkers in riluzole‐treated patients with GAD. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 23(4), 276–284. 10.1016/j.euroneuro.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, D. H. , Pluta, J. , Kadivar, S. , Craige, C. , Gee, J. C. , Avants, B. B. , & Yushkevich, P. A. (2014). Histology‐derived volumetric annotation of the human hippocampal subfields in postmortem MRI. NeuroImage, 84, 505–523. 10.1016/j.neuroimage.2013.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, S. , Bojesen, S. E. , & Nordestgaard, B. G. (2014). Reduced 25‐hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement, 10(3), 296–302. 10.1016/j.jalz.2013.05.1765 [DOI] [PubMed] [Google Scholar]
- Aimone, J. B. , Deng, W. , & Gage, F. H. (2014). Adult neurogenesis in the dentate gyrus In D. Derdikman & J. J. Knierim (eds.). Space, time and memory in the hippocampal formation (pp. 409–429). Wien, Austria: Springer; –Verlag. [Google Scholar]
- Amaral, D. G. (1978). A Golgi study of cell types in the hilar region of the hippocampus in the rat. The Journal of Comparative Neurology, 182(4 Pt 2), 851–914. [DOI] [PubMed] [Google Scholar]
- Anacker, C. , & Hen, R. (2017). Adult hippocampal neurogenesis and cognitive flexibility [mdash] linking memory and mood. Nature Reviews Neuroscience, 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, P. , Morris, R., Amaral, D., Bliss, T. and O'Keefe, J. (eds.) (2007). The hippocampus book. New York: Oxford University Press. [Google Scholar]
- Annweiler, C. , Beauchet, O. , Bartha, R. , Hachinski, V. , & Montero‐Odasso, M. (2014). Vitamin D and caudal primary motor cortex: A magnetic resonance spectroscopy study. PLoS One, 9(1), e87314 10.1371/journal.pone.0087314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler, C. , Montero‐Odasso, M. , Llewellyn, D. J. , Richard‐Devantoy, S. , Duque, G. , & Beauchet, O. (2013). Meta‐analysis of memory and executive dysfunctions in relation to vitamin D. Journal of Alzheimer's Disease, 37(1), 147–171. 10.3233/jad-130452 [DOI] [PubMed] [Google Scholar]
- APA . (2000). Diagnostic and statistical manual of mental disorders: DSM‐IV‐TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- Apostolova, L. G. , Dutton, R. A. , Dinov, I. D. , Hayashi, K. M. , Toga, A. W. , Cummings, J. L. , & Thompson, P. M. (2006). Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Archives of Neurology, 63(5), 693–699. 10.1001/archneur.63.5.693 [DOI] [PubMed] [Google Scholar]
- Bahar, A. S. , Shirvalkar, P. R. , & Shapiro, M. L. (2011). Memory‐guided learning: CA1 and CA3 neuronal ensembles differentially encode the commonalities and differences between situations. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 31(34), 12270–12281. 10.1523/jneurosci.1671-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balden, R. , Selvamani, A. , & Sohrabji, F. (2012). Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia‐induced inflammation in adult rats. Endocrinology, 153(5), 2420–2435. 10.1210/en.2011-1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balion, C. , Griffith, L. E. , Strifler, L. , Henderson, M. , Patterson, C. , Heckman, G. , … Raina, P. (2012). Vitamin D, cognition, and dementia: A systematic review and meta‐analysis. Neurology, 79(13), 1397–1405. 10.1212/WNL.0b013e31826c197f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier, M. , Narr, K. L. , Toga, A. W. , Elderkin‐Thompson, V. , Thompson, P. M. , Hamilton, L. , … Kumar, A. (2008). Hippocampal morphology and distinguishing late‐onset from early‐onset elderly depression. The American Journal of Psychiatry, 165(2), 229–237. 10.1176/appi.ajp.2007.07030506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch, T. , Dohring, J. , Rohr, A. , Jansen, O. , & Deuschl, G. (2011). CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proceedings of the National Academy of Sciences of the United States of America, 108(42), 17562–17567. 10.1073/pnas.1110266108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland, J. W. , Saperstein, S. , Pereira, F. , Rapin, J. , & Grady, L. (2012). Network, anatomical, and non‐imaging measures for the prediction of ADHD diagnosis in individual subjects. Frontiers in Systems Neuroscience, 6, 78 10.3389/fnsys.2012.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett, N. , Agellon, S. , Vanstone, C. , & Weiler, H. (2014). Vitamin D status positively associates with interleukin‐6 and tumour necrosis factor alpha in healthy young children. The FASEB Journal, 28(1 Supplement), 40.8. [Google Scholar]
- Brouwer‐Brolsma, E. M. , Dhonukshe‐Rutten, R. A. , van Wijngaarden, J. P. , van der Zwaluw, N. L. , Sohl, E. , In't Veld, P. H. , … de Groot, L. C. (2015). Low vitamin D status is associated with more depressive symptoms in Dutch older adults. European Journal of Nutrition, 55, 1525–1534. 10.1007/s00394-015-0970-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini, E. , & Miles, R. (2015). The CA3 region of the hippocampus: How is it? What is it for? How does it do it? Frontiers in Cellular Neuroscience, 9, 19 10.3389/fncel.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M. , Natarajan, R. , & Fan, X. (2016). Vitamin D in schizophrenia: A clinical review. Evidence‐Based Mental Health, 19(1), 6–9. 10.1136/eb-2015-102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, J. , Yu, N. K. , Choi, J. H. , Sim, S. E. , Kang, S. J. , Kwak, C. , … Kaang, B. K. (2015). Multiple repressive mechanisms in the hippocampus during memory formation. Science (New York, N.Y.), 350(6256), 82–87. 10.1126/science.aac7368 [DOI] [PubMed] [Google Scholar]
- Commins, S. , Aggleton, J. P. , & O'Mara, S. M. (2002). Physiological evidence for a possible projection from dorsal subiculum to hippocampal area CA1. Experimental Brain Research, 146(2), 155–160. 10.1007/s00221-002-1158-x [DOI] [PubMed] [Google Scholar]
- Coras, R. , Pauli, E. , Li, J. , Schwarz, M. , Rossler, K. , Buchfelder, M. , … Blumcke, I. (2014). Differential influence of hippocampal subfields to memory formation: Insights from patients with temporal lobe epilepsy. Brain, 137(Pt 7), 1945–1957. 10.1093/brain/awu100 [DOI] [PubMed] [Google Scholar]
- de Flores, R. , La Joie, R. , & Chetelat, G. (2015). Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience, 309, 29–50. 10.1016/j.neuroscience.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Deuker, L. , Olligs, J. , Fell, J. , Kranz, T. A. , Mormann, F. , Montag, C. , … Axmacher, N. (2013). Memory consolidation by replay of stimulus‐specific neural activity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(49), 19373–19383. 10.1523/jneurosci.0414-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhollander T, Raffelt D, Connelly A Unsupervised 3‐tissue response function estimation from single‐shell or multi‐shell diffusion MR data without a co‐registered T1 image. In: Proc ISMRM workshop on breaking the barriers of diffusion MRI, 2016.
- Dinomais, M. , Celle, S. , Duval, G. T. , Roche, F. , Henni, S. , Bartha, R. , … Annweiler, C. (2016). Anatomic correlation of the mini‐mental state examination: A voxel‐based morphometric study in older adults. PLoS One, 11(10), e0162889 10.1371/journal.pone.0162889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, A. D. , & Ranganath, C. (2017). Space, time, and episodic memory: The hippocampus is all over the cognitive map. Hippocampus; 1–8. 10.1002/hipo.22750 [DOI] [PubMed] [Google Scholar]
- Eskandari, G. , Ghajarzadeh, M. , Yekaninejad, M. S. , Sahraian, M. A. , Gorji, R. , Rajaei, F. , … Azimi, A. (2015). Comparison of serum vitamin D level in multiple sclerosis patients, their siblings, and healthy controls. Iranian Journal of Neurology, 14(2), 81–85. [PMC free article] [PubMed] [Google Scholar]
- Eyles, D. W. , Smith, S. , Kinobe, R. , Hewison, M. , & McGrath, J. J. (2005). Distribution of the vitamin D receptor and 1 alpha‐hydroxylase in human brain. Journal of Chemical Neuroanatomy, 29(1), 21–30. 10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Fama, R. , & Sullivan, E. V. (2015). Thalamic structures and associated cognitive functions: Relations with age and aging. Neuroscience and Biobehavioral Reviews, 54, 29–37. 10.1016/j.neubiorev.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel, A. , Wille, P. , Muller, M. J. , Winterer, G. , Scheurich, A. , Vucurevic, G. , … Stoeter, P. (2004). Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Dementia and Geriatric Cognitive Disorders, 18(1), 101–108. 10.1159/000077817 [DOI] [PubMed] [Google Scholar]
- Fernell, E. , Bejerot, S. , Westerlund, J. , Miniscalco, C. , Simila, H. , Eyles, D. , … Humble, M. B. (2015). Autism spectrum disorder and low vitamin D at birth: A sibling control study. Molecular Autism, 6, 3 10.1186/2040-2392-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron, F. , Burne, T. H. , Brown, J. , Smith, E. , McGrath, J. J. , Mackay‐Sim, A. , & Eyles, D. W. (2005). Developmental vitamin D3 deficiency alters the adult rat brain. Brain Research Bulletin, 65(2), 141–148. 10.1016/j.brainresbull.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , van der Kouwe, A. , Destrieux, C. , Halgren, E. , Segonne, F. , Salat, D. H. , … Dale, A. M. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y. : 1991), 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Garcion, E. , Sindji, L. , Leblondel, G. , Brachet, P. , & Darcy, F. (1999). 1,25‐dihydroxyvitamin D3 regulates the synthesis of gamma‐glutamyl transpeptidase and glutathione levels in rat primary astrocytes. Journal of Neurochemistry, 73(2), 859–866. [DOI] [PubMed] [Google Scholar]
- Gezen‐Ak, D. , Dursun, E. , & Yilmazer, S. (2013). Vitamin D inquiry in hippocampal neurons: Consequences of vitamin D‐VDR pathway disruption on calcium channel and the vitamin D requirement. Neurological Sciences, 34(8), 1453–1458. 10.1007/s10072-012-1268-6 [DOI] [PubMed] [Google Scholar]
- Goodwill, A. M. , & Szoeke, C. (2017). A systematic review and meta‐analysis of the effect of low vitamin D on cognition. Journal of the American Geriatrics Society, 65(10), 2161–2168. 10.1111/jgs.15012 [DOI] [PubMed] [Google Scholar]
- Greicius, M. D. , Krasnow, B. , Boyett‐Anderson, J. M. , Eliez, S. , Schatzberg, A. F. , Reiss, A. L. , & Menon, V. (2003). Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus, 13(1), 164–174. 10.1002/hipo.10064 [DOI] [PubMed] [Google Scholar]
- Gurholt, T. P. , Nerhus, M. , Osnes, K. , Berg, A. O. , Andreassen, O. A. , Melle, I. , & Agartz, I. (2018). Hippocampus volume reduction in psychosis spectrum could be ameliorated by vitamin D. Schizophrenia Research. 10.1016/j.schres.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Han, K. M. , Won, E. , Sim, Y. , & Tae, W. S. (2016). Hippocampal subfield analysis in medication‐naive female patients with major depressive disorder. Journal of Affective Disorders, 194, 21–29. 10.1016/j.jad.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K. , Westlye, L. T. , Morch‐Johnsen, L. , Jorgensen, K. N. , Lange, E. H. , Dale, A. M. , … Agartz, I. (2015). In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biological Psychiatry, 77(6), 581–588. 10.1016/j.biopsych.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Hooshmand, B. , Lokk, J. , Solomon, A. , Mangialasche, F. , Miralbell, J. , Spulber, G. , … Kivipelto, M. (2014). Vitamin D in relation to cognitive impairment, cerebrospinal fluid biomarkers, and brain volumes. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 69(9), 1132–1138. 10.1093/gerona/glu022 [DOI] [PubMed] [Google Scholar]
- Howard, M. W. , & Eichenbaum, H. (2015). Time and space in the hippocampus. Brain Research, 1621, 345–354. 10.1016/j.brainres.2014.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker, M. R. , Lee, B. , & Kesner, R. P. (2008). Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behavioural Brain Research, 188(2), 310–315. 10.1016/j.bbr.2007.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J. E. , Augustinack, J. C. , Nguyen, K. , Player, C. M. , Player, A. , Wright, M. , … Van Leemput, K. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra‐high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage, 115, 117–137. 10.1016/j.neuroimage.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Petersen, R. C. , Xu, Y. C. , O'Brien, P. C. , Smith, G. E. , Ivnik, R. J. , … Kokmen, E. (1999). Prediction of AD with MRI‐based hippocampal volume in mild cognitive impairment. Neurology, 52(7), 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, O. , & Lisman, J. E. (1996). Hippocampal CA3 region predicts memory sequences: Accounting for the phase precession of place cells. Learning & Memory (Cold Spring Harbor, NY), 3(2–3), 279–287. [DOI] [PubMed] [Google Scholar]
- Jerman, T. , Kesner, R. P. , & Hunsaker, M. R. (2006). Disconnection analysis of CA3 and DG in mediating encoding but not retrieval in a spatial maze learning task. Learning & Memory (Cold Spring Harbor, NY), 13(4), 458–464. 10.1101/lm.246906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, J. , & Maren, S. (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory (Cold Spring Harbor, NY), 15(4), 244–251. 10.1101/lm.794808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich, J. , Czanner, S. , Greve, D. , Haley, E. , van der Kouwe, A. , Gollub, R. , … Dale, A. (2006). Reliability in multi‐site structural MRI studies: Effects of gradient non‐linearity correction on phantom and human data. NeuroImage, 30(2), 436–443. 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Karakis, I. , Pase, M. P. , Beiser, A. , Booth, S. L. , Jacques, P. F. , Rogers, G. , … Seshadri, S. (2016). Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham heart study. Journal of Alzheimer's Disease : JAD, 51(2), 451–461. 10.3233/jad-150991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, J. T. , Forster, S. , Sultana, R. , Brewer, L. D. , Latimer, C. S. , Cai, J. , … Butterfield, D. A. (2013). Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D‐dependent age‐related cognitive decline. Free Radical Biology & Medicine, 65, 324–334. 10.1016/j.freeradbiomed.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachiyants, N. , & Kim, K. (2012). Mini‐mental status examination mapping to the corresponding brain areas in dementia. Applied Innovations and Technologies, 7(2), 55–58. [Google Scholar]
- La Joie, R. , Perrotin, A. , de La Sayette, V. , Egret, S. , Doeuvre, L. , Belliard, S. , … Chetelat, G. (2013). Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. NeuroImage. Clinical, 3, 155–162. 10.1016/j.nicl.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, V. , Albrecht, M. A. , Takechi, R. , Prasopsang, P. , Lee, Y. P. , Foster, J. K. , & Mamo, J. C. (2016). Serum 25‐hydroxyvitamin D is associated with reduced verbal episodic memory in healthy, middle‐aged and older adults. European Journal of Nutrition, 55(4), 1503–1513. 10.1007/s00394-015-0968-0 [DOI] [PubMed] [Google Scholar]
- Langub, M. C. , Herman, J. P. , Malluche, H. H. , & Koszewski, N. J. (2001). Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience, 104(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Latimer, C. S. , Brewer, L. D. , Searcy, J. L. , Chen, K.‐C. , Popović, J. , Kraner, S. D. , … Porter, N. M. (2014). Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proceedings of the National Academy of Sciences of the United States of America, 111(41), E4359–E4366. 10.1073/pnas.1404477111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I. , & Kesner, R. P. (2004a). Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear‐conditioning. Hippocampus, 14(3), 301–310. 10.1002/hipo.10177 [DOI] [PubMed] [Google Scholar]
- Lee, I. , & Kesner, R. P. (2004b). Encoding versus retrieval of spatial memory: Double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus, 14(1), 66–76. 10.1002/hipo.10167 [DOI] [PubMed] [Google Scholar]
- Lepage, M. , Habib, R. , & Tulving, E. (1998). Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus, 8(4), 313–322. [DOI] [PubMed] [Google Scholar]
- Lewis, S. (2016). Spatial processing: Location, location, location. Nature Reviews Neuroscience, 17(9), 535 10.1038/nrn.2016.106 [DOI] [PubMed] [Google Scholar]
- Maclaren, J. , Han, Z. , Vos, S. B. , Fischbein, N. , & Bammer, R. (2014). Reliability of brain volume measurements: A test‐retest dataset. Scientific Data, 1, 140037 10.1038/sdata.2014.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruszak, A. , & Thuret, S. (2014). Why looking at the whole hippocampus is not enough‐a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer's disease diagnosis. Frontiers in Cellular Neuroscience, 8, 95 10.3389/fncel.2014.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchar, D. B. , Chei, C. L. , Yin, Z. X. , Koh, V. , Chakraborty, B. , Shi, X. M. , & Zeng, Y. (2016). Vitamin D levels and the risk of cognitive decline in Chinese elderly people: The Chinese longitudinal healthy longevity survey. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 71(10), 1363–1368. 10.1093/gerona/glw128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazahery, H. , Camargo, C. A. , Conlon, C. , Beck, K. L. , Kruger, M. C. , & von Hurst, P. R. (2016). Vitamin D and autism spectrum disorder: A literature review. Nutrients, 8(4) 236, 1–35. 10.3390/nu8040236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck, W. H. , Church, R. M. , & Matell, M. S. (2013). Hippocampus, time, and memory‐‐a retrospective analysis. Behavioral Neuroscience, 127(5), 642–654. 10.1037/a0034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, D. , DeToledo‐Morrell, L. , Urresta, F. , Gabrieli, J. D. , Moseley, M. , Fleischman, D. , … Stebbins, G. T. (2006). White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiology of Aging, 27(5), 663–672. 10.1016/j.neurobiolaging.2005.03.026 [DOI] [PubMed] [Google Scholar]
- Miller, J. W. , Harvey, D. J. , Beckett, L. A. , Green, R. , Farias, S. T. , Reed, B. R. , … DeCarli, C. (2015). Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurology, 72(11), 1295–1303. 10.1001/jamaneurol.2015.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, Y. , Moon, W. J. , Kwon, H. , Lee, J. M. , & Han, S. H. (2015). Vitamin D deficiency disrupts neuronal integrity in cognitively impaired patients. Journal of Alzheimer's Disease : JAD, 45(4), 1089–1096. 10.3233/jad-143063 [DOI] [PubMed] [Google Scholar]
- Musiol, I. M. , & Feldman, D. (1997). 1,25‐dihydroxyvitamin D3 induction of nerve growth factor in L929 mouse fibroblasts: Effect of vitamin D receptor regulation and potency of vitamin D3 analogs. Endocrinology, 138(1), 12–18. 10.1210/endo.138.1.4858 [DOI] [PubMed] [Google Scholar]
- Mutlu, M. , Sariaydin, M. , Aslan, Y. , Kader, S. , Dereci, S. , Kart, C. , … Kural, B. (2016). Status of vitamin D, antioxidant enzymes, and antioxidant substances in neonates with neonatal hypoxic‐ischemic encephalopathy. The Journal of Maternal‐Fetal & Neonatal Medicine, 29(14), 2259–2263. 10.3109/14767058.2015.1081889 [DOI] [PubMed] [Google Scholar]
- Naveilhan, P. , Neveu, I. , Wion, D. , & Brachet, P. (1996). 1,25‐Dihydroxyvitamin D3, an inducer of glial cell line‐derived neurotrophic factor. NeuroReport, 7(13), 2171–2175. [DOI] [PubMed] [Google Scholar]
- O'keefe, J. , & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon Press. [Google Scholar]
- O'Mara, S. M. , Commins, S. , & Anderson, M. (2000). Synaptic plasticity in the hippocampal area CA1‐subiculum projection: Implications for theories of memory. Hippocampus, 10(4), 447–456. [DOI] [PubMed] [Google Scholar]
- O'Mara, S. M. , Commins, S. , Anderson, M. , & Gigg, J. (2001). The subiculum: A review of form, physiology and function. Progress in Neurobiology, 64(2), 129–155. [DOI] [PubMed] [Google Scholar]
- O'Mara, S. M. , Sanchez‐Vives, M. V. , Brotons‐Mas, J. R. , & O'Hare, E. (2009). Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 33(5), 782–790. 10.1016/j.pnpbp.2009.03.040 [DOI] [PubMed] [Google Scholar]
- Ota, M. , Sato, N. , Hidese, S. , Teraishi, T. , Maikusa, N. , Matsuda, H. , … Kunugi, H. (2017). Structural differences in hippocampal subfields among schizophrenia patients, major depressive disorder patients, and healthy subjects. Psychiatry Research, 259, 54–59. 10.1016/j.pscychresns.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Oudshoorn, C. , Mattace‐Raso, F. U. , van der Velde, N. , Colin, E. M. , & van der Cammen, T. J. (2008). Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 25(6), 539–543. 10.1159/000134382 [DOI] [PubMed] [Google Scholar]
- Ouma, S. , Suenaga, M. , Bolukbasi Hatip, F. F. , Hatip‐Al‐Khatib, I. , Tsuboi, Y. , & Matsunaga, Y. (2018). Serum vitamin D in patients with mild cognitive impairment and Alzheimer's disease. Brain and Behavior: A Cognitive Neuroscience Perspective, 8(3), e00936 10.1002/brb3.936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, C. , & Gonzalez, L. (2014). Is vitamin D deficiency a major global public health problem? The Journal of Steroid Biochemistry and Molecular Biology, 144(Pt A), 138–145. 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, R. C. , Smith, G. E. , Waring, S. C. , Ivnik, R. J. , Tangalos, E. G. , & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308. [DOI] [PubMed] [Google Scholar]
- Pettersen, J. A. (2017). Does high dose vitamin D supplementation enhance cognition?: A randomized trial in healthy adults. Experimental Gerontology, 90, 90–97. 10.1016/j.exger.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Przybelski, R. J. , & Binkley, N. C. (2007). Is vitamin D important for preserving cognition? A positive correlation of serum 25‐hydroxyvitamin D concentration with cognitive function. Archives of Biochemistry and Biophysics, 460(2), 202–205. 10.1016/j.abb.2006.12.018 [DOI] [PubMed] [Google Scholar]
- Rebola, N. , Carta, M. , & Mulle, C. (2017). Operation and plasticity of hippocampal CA3 circuits: Implications for memory encoding. Nature Reviews Neuroscience, 18(4), 208–220. 10.1038/nrn.2017.10 [DOI] [PubMed] [Google Scholar]
- Remondes, M. , & Schuman, E. M. (2004). Role for a cortical input to hippocampal area CA1 in the consolidation of a long‐term memory. Nature, 431(7009), 699–703. 10.1038/nature02965 [DOI] [PubMed] [Google Scholar]
- Ross, A. C. , Manson, J. E. , Abrams, S. A. , Aloia, J. F. , Brannon, P. M. , Clinton, S. K. , … Shapses, S. A. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. The Journal of Clinical Endocrinology & Metabolism, 96(1), 53–58. 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels, B. A. , Leonardo, E. D. , & Hen, R. (2015). Hippocampal subfields and major depressive disorder. Biological Psychiatry, 77(3), 210–211. 10.1016/j.biopsych.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson, A. M. , Jennische, E. , Hansson, H. A. , & Holmang, A. (2006). Prenatal exposure to interleukin‐6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(a) dysregulation and impaired spatial learning. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 290(5), R1345–R1356. 10.1152/ajpregu.00268.2005 [DOI] [PubMed] [Google Scholar]
- Schuff, N. , Marmar, C. R. , Weiss, D. S. , Neylan, T. C. , Schoenfeld, F. , Fein, G. , & Weiner, M. W. (1997). Reduced hippocampal volume and n‐acetyl aspartate in posttraumatic stress disorder. Annals of the New York Academy of Sciences, 821, 516–520. [DOI] [PubMed] [Google Scholar]
- Shi, F. , Liu, B. , Zhou, Y. , Yu, C. , & Jiang, T. (2009). Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta‐analyses of MRI studies. Hippocampus, 19(11), 1055–1064. 10.1002/hipo.20573 [DOI] [PubMed] [Google Scholar]
- Shivakumar, V. , Kalmady, S. V. , Amaresha, A. C. , Jose, D. , Narayanaswamy, J. C. , Agarwal, S. M. , … Gangadhar, B. N. (2015). Serum vitamin D and hippocampal gray matter volume in schizophrenia. Psychiatry Research: Neuroimaging, 233(2), 175–179. 10.1016/j.pscychresns.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Smith, R. E. , Tournier, J.‐D. , Calamante, F. , & Connelly, A. (2012). Anatomically‐constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage, 62(3), 1924–1938. [DOI] [PubMed] [Google Scholar]
- Smith, R. E. , Tournier, J.‐D. , Calamante, F. , & Connelly, A. (2013). SIFT: Spherical‐deconvolution informed filtering of tractograms. NeuroImage, 67, 298–312. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering, C. C. , Hobson, V. , Lucas, J. A. , Menon, C. V. , Hall, J. R. , & O'Bryant, S. E. (2012). Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer's disease in ethnically diverse highly educated individuals: An analysis of the NACC database. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 67(8), 890–896. 10.1093/gerona/gls006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman, R. W. , Cohen, S. J. , Lora, J. C. , & Rios, L. M. (2016). Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long‐term object memory and spatial memory. Neurobiology of Learning and Memory, 133, 118–128. 10.1016/j.nlm.2016.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, J.‐D. , Calamante, F. , & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. NeuroImage, 35(4), 1459–1472. [DOI] [PubMed] [Google Scholar]
- Travis, S. , Coupland, N. J. , Silversone, P. H. , Huang, Y. , Fujiwara, E. , Carter, R. , … Malykhin, N. V. (2015). Dentate gyrus volume and memory performance in major depressive disorder. Journal of Affective Disorders, 172, 159–164. 10.1016/j.jad.2014.09.048 [DOI] [PubMed] [Google Scholar]
- Treadway, M. T. , Waskom, M. L. , Dillon, D. G. , Holmes, A. J. , Park, M. T. , Chakravarty, M. M. , … Pizzagalli, D. A. (2015). Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biological Psychiatry, 77(3), 285–294. 10.1016/j.biopsych.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valipour, G. , Saneei, P. , & Esmaillzadeh, A. (2014). Serum vitamin D levels in relation to schizophrenia: A systematic review and meta‐analysis of observational studies. The Journal of Clinical Endocrinology and Metabolism, 99(10), 3863–3872. 10.1210/jc.2014-1887 [DOI] [PubMed] [Google Scholar]
- van der Schaft, J. , Koek, H. L. , Dijkstra, E. , Verhaar, H. J. , van der Schouw, Y. T. , & Emmelot‐Vonk, M. H. (2013). The association between vitamin D and cognition: A systematic review. Ageing Research Reviews, 12(4), 1013–1023. 10.1016/j.arr.2013.05.004 [DOI] [PubMed] [Google Scholar]
- van Schoor, N. M. , & Lips, P. (2011). Worldwide vitamin D status. Best Practice & Research Clinical Endocrinology & Metabolism, 25(4), 671–680. 10.1016/j.beem.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Vedak, T. K. , Ganwir, V. , Shah, A. B. , Pinto, C. , Lele, V. R. , Subramanyam, A. , … Deo, S. S. (2015). Vitamin D as a marker of cognitive decline in elderly Indian population. Annals of Indian Academy of Neurology, 18(3), 314–319. 10.4103/0972-2327.160052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente, P. , Herr, M. , Mahieux, F. , & Ankri, J. (2015). Vitamin D and neuropsychological assessment of cognitive functions: A study of their relationships in a sample of 244 patients attending a memory clinic. Geriatrie et psychologie neuropsychiatrie du vieillissement, 13(4), 452–461. 10.1684/pnv.2015.0579 [DOI] [PubMed] [Google Scholar]
- Virley, D. , Ridley, R. M. , Sinden, J. D. , Kershaw, T. R. , Harland, S. , Rashid, T. , … Hodges, H. (1999). Primary CA1 and conditionally immortal MHP36 cell grafts restore conditional discrimination learning and recall in marmosets after excitotoxic lesions of the hippocampal CA1 field. Brain, 122(Pt 12), 2321–2335. [DOI] [PubMed] [Google Scholar]
- Walsh, T. J. , Schulz, D. W. , Tilson, H. A. , & Schmechel, D. E. (1986). Colchicine‐induced granule cell loss in rat hippocampus: Selective behavioral and histological alterations. Brain Research, 398(1), 23–36. [DOI] [PubMed] [Google Scholar]
- Whelan, C. D. , Hibar, D. P. , van Velzen, L. S. , Zannas, A. S. , Carrillo‐Roa, T. , McMahon, K. , … Thompson, P. M. (2016). Heritability and reliability of automatically segmented human hippocampal formation subregions. NeuroImage, 128, 125–137. 10.1016/j.neuroimage.2015.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, C. H. , Sheline, Y. I. , Roe, C. M. , Birge, S. J. , & Morris, J. C. (2006). Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 14(12), 1032–1040. 10.1097/01.JGP.0000240986.74642.7c [DOI] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8(7), e68910 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa, M. A. , Stark, S. M. , Bakker, A. , Albert, M. S. , Gallagher, M. , & Stark, C. E. (2010). High‐resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage, 51(3), 1242–1252. 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesil, Y. , Kuyumcu, M. E. , Kara, O. , Halacli, B. , Etgul, S. , Kizilarslanoglu, M. C. , … Ariogul, S. (2015). Vitamin D status and its association with gradual decline in cognitive function. Turkish Journal of Medical Sciences, 45(5), 1051–1057. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Schuff, N. , Jahng, G. H. , Bayne, W. , Mori, S. , Schad, L. , … Weiner, M. W. (2007). Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology, 68(1), 13–19. 10.1212/01.wnl.0000250326.77323.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Dougherty, J. H., Jr. , Hubner, K. F. , Bai, B. , Cannon, R. L. , & Hutson, R. K. (2008). Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement, 4(4), 265–270. 10.1016/j.jalz.2008.04.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1
Supporting Information Figure S2
Figure Legends
Supporting Information, Table 1. Association of serum vitamin D concentrations with hippocampal subfield volumes and hippocampal connectomes
Supporting Information, Table 2. Correlation of hippocampal CA1 volume and hippocampal connectivity with neurocognitive outcomes


