Abstract
Type 2 diabetes (T2D) is associated with an accelerated episodic memory decline, but the underlying pathophysiological mechanisms are not well understood. Hallmarks of T2D comprise impairment of insulin secretion and insulin sensitivity. Insulin signaling modulates cerebral neurotransmitter activity, including the excitatory glutamate and inhibitory gamma‐aminobutyric acid (GABA) systems. Here we tested the hypothesis that the glutamate and GABA systems are altered in T2D patients and this relates to memory decline and insulin resistance. Using 1H‐magnetic resonance spectroscopy (MRS), we examined glutamate and GABA concentrations in episodic memory relevant brain regions (medial prefrontal cortex and precuneus) of T2D patients and matched controls. Insulin sensitivity was measured by hyperinsulinemic‐euglycemic clamps and memory performance was assessed using a face‐profession associations test. T2D patients exhibited peripheral insulin resistance and had a decreased memory for face‐profession associations as well as elevated GABA concentration in the medial prefrontal cortex but not precuneus. In addition, medial prefrontal cortex GABA concentration was negatively associated with memory performance suggesting that abnormal GABA levels in the medial prefrontal cortex are linked to the episodic memory decline that occurs in T2D patients.
Keywords: diabetes, magnetic resonance spectroscopy, Memory
1. INTRODUCTION
Type 2 diabetes (T2D), the most prevalent diabetes type, is characterized by insulin resistance of target tissues and relative, rather than absolute, insulin deficiency. With the increasing life expectation of the Western population, the incidence of T2D will be further increasing in the next decades (Cowie et al., 2009; Gispen & Biessels, 2000; Zheng, Ley, & Hu, 2018).
Patients with T2D suffer from impairment in a variety of cognitive domains (Biessels, Strachan, Visseren, Kappelle, & Whitmer, 2014). T2D has been associated with decreased complex psychomotor functioning (Reaven, Thompson, Nahum, & Haskins, 1990), psychomotor speed (Gregg et al., 2000; Reaven et al., 1990), processing speed (Messier, 2005), verbal fluency (Kanaya, Barrett‐Connor, Gildengorin, & Yaffe, 2004; Reaven et al., 1990), executive function (Munshi et al., 2006; Perlmuter et al., 1984; Reaven et al., 1990), attention (Fontbonne, Berr, Ducimetiere, & Alperovitch, 2001), and several domains of memory (Grodstein, Chen, Wilson, & Manson, 2001; Messier, 2005; Mooradian, Perryman, Fitten, Kavonian, & Morley, 1988; Munshi et al., 2006; Perlmuter et al., 1984). Among these impairments, the most robust and frequently reported cognitive deficits in T2D are episodic memory dysfunction (Jones, Riby, Mitchell, & Smith, 2014). Not surprisingly, T2D associates with a greater prevalence of neurocognitive disorders and Alzheimer's disease in particular (Biessels, van der Heide, Kamal, Bleys, & Gispen, 2002; Leibson et al., 1997; Ott et al., 1996, 1999). Alzheimer's disease has also been associated with decreased insulin concentrations, abnormal insulin receptor function, and density in the brain (Frölich, Blum‐Degen, Riederer, & Hoyer, 1999; Rivera et al., 2005; Steen et al., 2005).
The pathophysiological mechanisms of T2D related cognitive dysfunction are not entirely understood. In recent years, magnetic resonance imaging (MRI) techniques have been increasingly utilized to investigate the cognitive decline in T2D patients (Geijselaers, Sep, Stehouwer, & Biessels, 2015). A recent meta‐analysis identified changes in functional MRI (fMRI) resting‐state brain activity (e.g., in the precuneus or frontal cortex) in T2D patients (Xia, Chen, & Ma, 2017). Moreover, Cui et al. (2014) showed that decreased resting‐state brain activity in T2D is associated with poorer memory performance and executive functioning. The amino‐acids glutamate and gamma‐aminobutyric acid (GABA) have been associated with both, human brain activation (Buzsaki, Kaila, & Raichle, 2007; Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001) and T2D‐related cognitive impairment (Lyoo et al., 2009; Van Bussel et al. (2016)). In rodents, it has been shown that T2D is associated with a malfunctioning homeostasis of GABA and glutamate in the brain (Sickmann, Waagepetersen, Schousboe, Benie, & Bouman, 2010). The homeostasis between GABA and glutamate is regulated by glia cells which metabolize these amino acids after neurotransmission into glutamine that, in turn, is transported to the neurons where it is synthesized back to glutamate and GABA (Bak, Schousboe, & Waagepetersen, 2006; Behar & Rothman, 2001; Patel et al., 2005; Sickmann, Waagepetersen, Schousboe, Benie, & Bouman, 2012). GABA and glutamate are the main inhibitory and excitatory neurotransmitters in the brain, respectively, and are essential for the formation of episodic memories (Day, Langston, & Morris, 2003; Miranda, 2007; Solomonia & McCabe, 2015; Thielen et al., 2018). Therefore, investigating the quantity of GABA, glutamate and glutamine in relevant brain regions may reveal important new insights into the pathogenesis of T2D related episodic memory impairments.
Magnet resonance spectroscopy (MRS) provides a unique opportunity to assess noninvasively the concentrations of GABA, glutamate and glutamine in vivo. By utilizing MRS at a 1.5 T scanner, Sinha et al. (2014) found an increased glutamate + glutamine (GLx) concentration in the right frontal region of T2D patients. Unfortunately, whether this effect was related to glutamate, glutamine or both could not be elucidated since it is difficult to separate glutamate from glutamine at lower magnetic field strengths (Moser, Stahlberg, Ladd, & Trattnig, 2012). In addition, Van Bussel et al. (2016) found an increased GABA concentration in the occipital lobe of T2D patients that was related to lower cognitive performance. However, the occipital lobe is thought to be a visual processing area that is not primary involved in the control of higher cognitive functions as episodic memory. Therefore, investigating GABA, glutamate and glutamine concentrations in specific, episodic memory related, brain regions would potentially offer deeper insight into the mechanisms underlying episodic memory impairment in T2D patients.
The present study utilized MRS at ultra‐high magnetic field strength (7 T) to investigate T2D related changes of amino acid concentrations in episodic memory specific regions. Since the medial prefrontal cortex (mPFC) and precuneus exhibit increased episodic memory related brain activation (fMRI) in healthy elderly (Thielen et al., 2016), we measured GABA, glutamate and glutamine concentrations in these areas and studied the relation of these concentrations to episodic memory performance and T2D. We hypothesized that abnormal amino acid concentration in T2D patients are associated with reduced episodic memory performance occurring in T2D.
2. METHODS
2.1. Participants
This study was conducted in 17 T2D patients and 14 healthy nondiabetic humans, serving as a control group (Table 1). Participants were consecutively recruited from the German Diabetes Center in Düsseldorf, which prospectively monitors patients with recent onset diabetes mellitus (Szendroedi et al., 2016) and from an interventional clinical study also at the German Diabetes Center in Düsseldorf (Reg. Nr. NCT02039934). Inclusion criteria for the present analysis were age of 30–65 years, BMI above 25 kg/m2. Main exclusion criteria were smoking, alcohol or drug abuse, psychiatric, autoimmune, hematologic, cardiovascular, pulmonary, pulmological, or thyroid diseases.
Table 1.
Participants' characteristics of Type 2 diabetes patients (T2D) and healthy humans serving as control (CON)
| T2D | CON | |
|---|---|---|
| Sex (male/female) | 14/3 | 13/1 |
| Age (years) | 55.1 ± 6 | 55.0 ± 8 |
| IQ (MWTB total score) | 31.9 ± 2.4 | 32.5 ± 2.0 |
| BMI (kg/cm2) | 31.8 ± 3.8 | 30.4 ± 4.6 |
| HbA1c (%) | 6.7 ± 0.7 | 5.3 ± 0.3 |
| M‐value (mL/min/kg) | 4.0 ± 1.9 | 6.5 ± 2.6 |
| Blood glucose (mg/dL) | 136.7 ± 28.0 | 87.6 ± 5.7 |
| Insulin (μU/mL) | 21.5 ± 16.7 | 9.7 ± 6.3 |
| Triglyceride (mg/dL) | 211.8 ± 131.4 | 144.4 ± 80.6 |
| Free fatty acids (μmol/L) | 577.2 ± 240.3 | 429.8 ± 121.6 |
2.2. Experimental procedures
All participants underwent intensive metabolic phenotyping in the morning (7:00 a.m.). After fasting blood sampling, a two‐step hyperinsulinaemic–euglycaemic clamp was performed for 4 hr using a primed‐continuous insulin infusion (40 mU [body weight in kg]−1 min−1 for 8 min, followed by 20 mU [body weight in kg]−1 min−1 for 2 hr and then 80 mU [body weight in kg]−1 min−1 for 8 min, followed by 40 mU [body weight in kg]−1 min−1 for another 2 hr; Insuman Rapid, sanofi‐aventis, Frankfurt am Main, Germany) to assess insulin sensitivity as shown previously (Phielix et al., 2013). A variable infusion of 20% glucose (wt/vol.; B. Braun, Melsungen, Germany) was administered to maintain blood glucose at 5 mmol/L. Blood glucose was measured at 5 min intervals.
Blood samples were immediately chilled and centrifuged, and supernatants stored at −20°C until analysis. Whole‐blood glucose concentration was measured at the bedside (EKF biosen C‐Line glucose analyzer, EKF Diagnostics, Barleben, Germany). Serum triacylglycerols, cholesterol, free fatty acids, HbA1c, C‐peptide, and insulin were measured as previously described (Nowotny et al., 2013). On a separate day (between 16:00 p.m. and 18:00 p.m.), the Mehrfachwahl‐Wortschatz‐Intelligenztest (MWTB; Lehrl, 2005) was conducted to estimate subject's general educational status as measurement for IQ. After that, 1H spectroscopy (MRS) was performed in the mPFC and the precuneus. Subsequently, volunteers had to memorize face‐occupation associations inside the scanner. Memory performance was subsequently tested outside the scanner.
2.3. Memory paradigm
We used the episodic memory paradigm and voxel placement described by Thielen et al. (2016) to ensure that the MRS voxels were placed in brain regions involved in the memory task. The face‐profession encoding task was performed within the scanner. Six blocks of an episodic memory condition (face‐profession encoding task) consisting of four stimuli as described below were interleaved with six blocks of a visuo‐motor condition consisting of six stimuli whereby each block lasted 22.8 s. During the episodic memory condition, a series of four novel faces uniquely associated with occupational titles were shown. Each face with its associated occupational title underneath was displayed at the center of the screen for 5.7 s. Participants were instructed to memorize face‐profession associations for a subsequent memory test and to judge (via button box) whether the face fitted well with the underlined profession or not. In the visuo‐motor condition each block (as in the episodic memory condition) started with the presentation of a brief instruction for 2.0 s, and followed by showing a series of six shadow‐masked face contours with the presentation time of 3.8 s each. Participants were required to judge (via button box) whether the ears of a shadow‐masked face contour were closer to the left or the right shoulder. Thereafter, the volunteers performed a recall test for the associated profession outside the scanner whereby they were presented with all the faces (printed on papers in A4 format) and had to write down the associated profession (Figure 1). Memory performance was determined by the amount of correct remembered face‐occupation associations. To probe T2D related changes in memory performance an ANOVA was conducted with the number of correct remembered face‐occupation associations as inputs.
Figure 1.
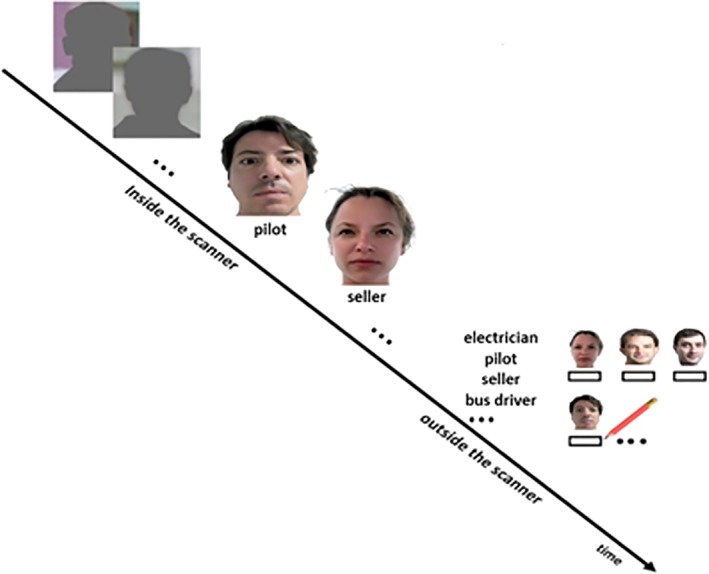
Design of the face profession association task. Inside the magnetic resonance scanner, persons performed the face‐profession association task that was interleaved with a visuo‐motor task. During the visuo‐motor task, they were required to judge whether the ears of a shadow‐masked face contour were closer to the left or the right shoulder. In the face profession association task participants had to memorize the face‐profession associations and to judge whether the face fitted well with the underlined profession or not. Thereafter, they performed a recall test outside the scanner. The participants were provided with a printed list of all the professions and faces seen in the scanner. They had to write down the associated professions below the faces. The figure is adapted with permission from Thielen, J. W., Hong, D., Rohani Rankouhi, S., Wiltfang, J., Fernández, G., Norris, D.G. et al (2018). The increase in medial prefrontal glutamate/glutamine concentration during memory encoding is associated with better memory performance and stronger functional connectivity in the human medial prefrontal‐thalamus‐hippocampus network. Human Brain Mapping, 39, 2381–2390. doi: 10.1002/hbm.24008 [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. MRS data acquisition
Scanning was performed on a whole‐body 7 T MR system (Magnetom 7 T, Siemens Healthcare, Germany) using a 32‐channel Rx/Tx head coil (Nova Medical, Wilmington, MA). Before MRS acquisition, a three‐dimensional T1‐weighted structural image (MP2RAGE; FOV = 240 × 240 mm, TR = 2,300 ms, TI = 1,100 ms, TE = 3.03 ms, 192 slices, spatial resolution = 1 × 1 × 1 mm3, flip angle = 8°) was acquired to guide MRS voxel placement (Figure 2). Afterward, single voxel edited 1H‐MR Spectra from the medial prefrontal cortex (voxel size: 20 × 20 × 20 mm) and precuneus (voxel size: 20 × 20 × 20 mm) were acquired when the shimming procedure resulted in FWHM lower than 30 Hz. MEGA‐sLASER (TR = 4,500 ms, TE = 80 ms, NEX = 64 [32 on, 32 off], scan time = 5:06 min) was used as an editing method (Andreychenko, Boer, Arteaga de Castro, Luijten, & Klomp, 2012).
Figure 2.
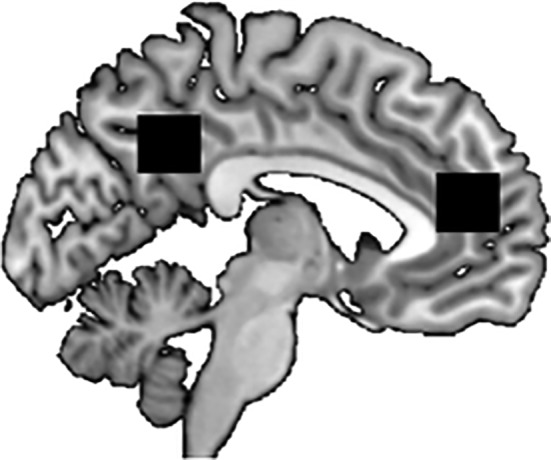
The positions of the MRS voxels (black squares) are depicted. The right (anterior) voxel is located in the medial prefrontal cortec (mPFC) whereas the left (posterior) voxel is located in the precuneus. Voxel size: 20 × 20 × 20 mm
2.5. MRS voxel placement
Since Thielen et al. (2016) showed that the memory task used in the present study activates brain regions in the mPFC and precuneus we placed the MRS voxels in this brain regions. The voxels were placed according to anatomical landmarks. The mPFC voxel was placed at the anterior edge of the bent of corpus callosum whereas the precuneus voxel was placed between the edge of the posterior bend of corpus callosum and the parieto‐occipital fissure (see Figure 2).
2.6. MRS data analysis
We utilized ratios in place of absolute metabolite quantification since absolute quantification requires an internal reference as water. However, water content may differ in disease states (Tognarelli et al., 2015) as diabetes. Additionally, gray and white matter and CSF have different water contents. As we placed the voxels along the midline of the brain, CSF contamination was inevitable. Since the ratio approach can account for CSF differences (Hoch, Kirov, & Tal, 2017), we preferred ratios over absolute values. Quantification and data processing of all spectra were performed with LCModel software (Provencher, 2001) using the edited basis set. This edited basis set was created by combining edited five spectra: N‐acetyl aspartate (NAA), N‐acetylaspartyl glutamate (NAAG), glutamate (Glu), glutamine (Gln), glutathione (GSH), and GABA, which were affected by the 1.9 ppm editing pulse. Edited spectral models were calculated by subtracting the MEGA off spectrum from the MEGA on spectrum. All spectral models were simulated using NMRSIM module included in the TOPSPIN package (version 3.6, Bruker BioSpin, Rheinstetten, Germany) with identical scan parameters to the in‐vivo spectroscopic scan. To probe T2D related changes in GABA, glutamate and glutamine concentrations in mPFC and precuneus, one way ANOVA's were conducted with GABA/NAA, glutamate/NAA and glutamine/NAA as inputs with Bonferroni correction for multiple testing.
2.7. Correlation analysis
Pearson's correlation analyses were performed by means of SPSS (IBM 21) software to assess the potential relationship between memory performance and GABA, glutamate and glutamine concentrations. To minimize the number of analyses we included only metabolites that were significantly different between T2D and controls.
3. RESULTS
3.1. Anthropometry
Sex, age, BMI, and IQ did not differ between both groups (Table 1). T2D patients exhibited excellent glycemic control, but were more insulin resistant compared to controls (Table 1). In addition and as expected, the T2D patients had higher concentrations of fasting triglycerides, free fatty acids, glucose, and insulin (Table 1).
We found a difference in performance of the paired associate memory task as measured by the number of correct remembered face‐profession associations between the groups (Figure 3). The controls remembered statistically significantly more items (F [1,30] = 4.376, p = .045), namely on average 6.14 (SD = 2.90) items, whereas the T2D patients remembered on average 3.94 (SD = 2.92) items.
Figure 3.
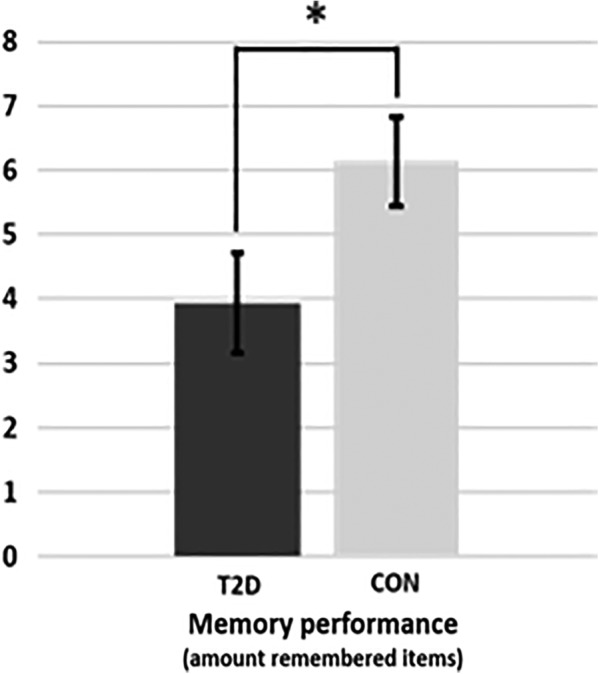
Memory performance of Type 2 diabetes patients (T2D) and healthy humans serving as control (CON). * p = .045
3.2. MRS analysis
The mean voxel position (center) in the mPFC was x = 1, y = 41, z = 12 in MNI space with a mean percentage of 63% (SD = 8.1) gray matter, 20% (SD = 9.1) white matter, and 17% (SD = 8.2) CSF. The mean voxel position (center) in the precuneus was x = 1, y = −48, z = 36 in MNI space with a mean percentage of 65% (SD = 10.4) gray matter, 21% (SD = 9.5) white matter, and 14% (SD = 6.2) CSF. There was no statistical significant difference between percentage of gray matter, white matter, and CSF between T2D and healthy control subjects neither in the mPFC voxel (GM: F [1,30] = .197, p = .661; WM: F [1,30] = .197, p = .661; CSF: F [1,30] = .122, p = .770) nor precuneus voxel (GM: F [1,30] = .504, p = .484; WM: F [1,30] = 1.30, p = .263; CSF: F [1,30] = .223. p = .640). Further, we found no difference in the NAA signal between T2D and healthy controls, neither in the mPFC (F [1,30] = .081, p = .778) nor in the precuneus (F [1,30] = 1.096, p = .304). Therefore, we used the ratio to NAA to indicate GABA, glutamate and glutamine concentrations. Regarding the GABA/NAA ratio, we found a significant increase in GABA in the mPFC of T2D patients compared to healthy controls (F [1,30] = 8.220, p = .008; figure 4) while there were no significant group differences in the precuneus (F [1,30] = .100, p = .754). We found no significant group differences in glutamate/NAA ratios in the mPFC (F [1,30] = .716, p = .406) or the precuneus (F [1,30] = .777, p = .386). The same was true of glutamine/NAA ratios: mPFC (F [1,30] = 1.186, p = .286), precuneus (F [1,30] = .309, p = .583).
Figure 4.
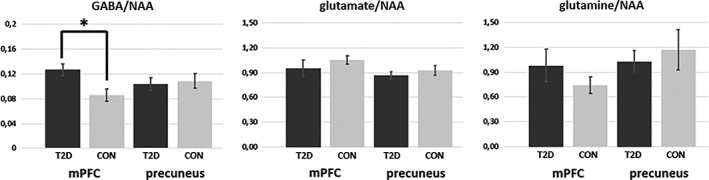
Results of the MRS analysis. The figure shows the mean concentrations of GABA (left), glutamate (middle), and glutamine (right) in the medial prefrontal cortex (mPFC) and precuneus of Type 2 diabetes (T2D) patients (dark gray) and healthy control subjects (bright gray). Only the GABA/NAA ratio in the mPFC was different between the groups. * p = .008
3.3. Correlation analysis
All variables included in the correlation analyses were normally distributed (Kolmogorov–Smirnov tests = p < .100). Because of the differences between groups in mPFC GABA concentrations and memory performance, we assessed the association between GABA and memory performance. Using Pearson's correlation, we found a negative correlation between mPFC GABA/NAA ratio and memory performance across all volunteers (r = −.432; p = .015; Figure 5). Hence, those individuals with the highest mPFC GABA concentration were those that performed poorest and vice versa. It is important to note, that Pearson's correlation analysis in the T2D group alone was not significant (r = −.297; p = .123), which may be due to the small sample size (N = 17). Regarding precuneus, there was no significant correlation (r = .225; p = .224) between GABA/NAA ratio and memory performance.
Figure 5.
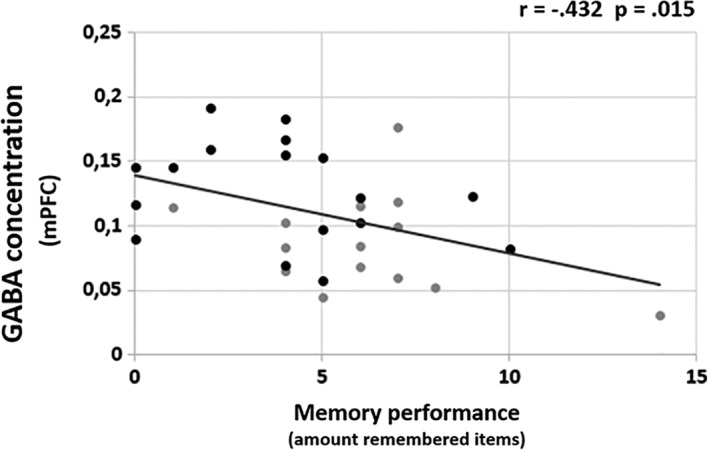
Correlation between mPFC GABA/NAA ratio and memory performance across all participants. Black dots represent Type 2 diabetes patients, gray dots represent healthy humans serving as control
To assess whether the metabolic measures (M‐value, fasting triglyceride, fasting free fatty acids, fasting glucose and insulin) are related to mPFC GABA concentration and memory performance we performed an additional Pearson's correlation analysis between these variables. This analysis revealed no correlation between any metabolic measure and mPFC GABA concentration. With respect to memory performance, there was a significant correlation with fasting triglyceride (r = −.399; p = .026).
4. DISCUSSION
The present study found a decreased memory for face‐occupation associations already in metabolically well‐controlled T2D patients with short known disease duration, which is related to increased GABA concentration in the mPFC. Interestingly, the increased GABA concentration appeared to be unrelated to metabolic measures. This is not in line with the findings of Van Bussel et al. (2016). These Authors reported an association between GABA concentration in occipital lobe and fasting blood glucose levels and HB1AC levels. A possible explanation may be either the small sample size of the present study or the brain region of interest assessed.
To date, only a limited number of studies examined neurotransmitter concentrations in T2D patients and their relation to cognitive performance. Van Bussel et al. (2016) showed that T2D patients exhibit higher GABA concentrations in the occipital lobe, which was associated with poorer cognitive performance as measured with the mini‐mental‐state‐examination (MMSE) test. However, the MMSE is a rather unspecific test, measuring different cognitive domains such as visual construction, attention, or calculation. In the present study, we focused on episodic memory and found indeed for the first time that T2D patients had a severely decreased memory performance for face‐occupation associations, which was correlated with increased GABA concentration in the mPFC but not precuneus. Though of course our single voxel based analyses limits a generalization of our results, our findings provide first evidence that abnormal GABA levels in T2D patients maybe region‐specific and not a general phenomenon. A possible explanation of our findings may be a T2D related change in the amino‐acid homeostasis. As previously mentioned, after synaptic transmission of GABA, surrounding glia cells metabolize GABA to glutamate and glutamate to glutamine, which is in turn transported to the neurons where it is synthesized to glutamate (Petroff, 2002). In GABAergic neurons, GABA is synthesized from glutamate via glutamate decarboxylase (GAD). Alterations within this GABA–glutamate–glutamine cycle may contribute to altered GABA concentrations in the brains of T2D patients. Indeed, a rat model of T2D has demonstrated that the GABA–glutamate–glutamine cycle is affected. In this regard, Sickmann et al. (2010) showed an increased glutamate‐GABA synthesis in memory related brain regions such as hippocampus. Notably, increased GABA synthesis was region specific and not evident in all brain regions examined. This supports our finding of a T2D related increased glutamate‐GABA synthesis in the mPFC, but not precuneus.
Regarding glutamate and glutamine, Sinha et al. (2014) reported an increased glutamate + glutamine (GLx) concentration in the brain of T2D patients but could not further dissociate this because of methodological shortcomings. In the present study, we assessed glutamate and glutamine separately. However, we did not find significant changes in glutamate or glutamine concentrations in T2D neither in the mPFC nor in precuneus. This is similar to the findings of Ajilore et al. (2007) who reported decreased Glx levels in T2D patients with depression but not in T2D patients without depression. A possible explanation for these differences may be the location of the brain regions examined. For instance, Sinha and colleagues measured GLx in right frontal, right parieto‐occipital, and right parieto‐temporal white matter and found increased GLx in the right frontal area only. Moreover, Sickmann et al. (2010) showed not only a region‐specific increase in glutamate‐GABA synthesis, but also a region‐specific decrease in glutamate‐glutamine metabolism in diabetic rats.
Our data demonstrates that T2D patients have a decreased memory for face‐profession associations and elevated GABA concentration in the mPFC but not in precuneus. Moreover, mPFC GABA concertation, across all subjects, was negatively associated with memory performance suggesting that abnormal GABA levels in the mPFC is linked to the episodic memory deficit occurring in T2D patients. Notably, our design allows the assessment of associative episodic memory. In this regard, previous work has mainly investigated T2D related decline in single item memory, for example, word list learning (Jones et al., 2014; Stewart & Liolitsa, 1999), which is a different process with distinct neural substrates as compared to associative memory (Ranganath, 2010; Wang, Li, Li, & Zhang, 2013). As associative memory, compared to single item memory, is more affected in Alzheimer's disease (Blackwell et al., 2004; Fowler, Saling, Conway, Semple, & Louis, 2002; O'Connell et al., 2004; Parra et al., 2009; Swainson et al., 2001) and T2D patients exhibit an increased risk in developing Alzheimer disease (Kuusisto et al., 1997; Leibson et al., 1997; Ott et al., 1999), our study of associative episodic memory in T2D provides a relevant contribution to the understanding of cognitive decline in T2D.
The absence of any effect regarding glutamate and glutamine may be due to the limited sample size. Second, we used the ratio of GABA to NAA, which has previously been associated with T2D (Sinha et al., 2014). However, we tested whether NAA concentrations were different in T2D patients and, in line with other studies (Ajilore et al., 2007; Sahin et al., 2008), we did not find any significant differences in NAA between T2D patients and healthy subjects. Therefore, it is unlikely that our findings are biased by NAA. Third, the present study focused just on GABA levels in the brain ignoring peripheral GABA. To get a more holistic view regarding the relationship between brain changes and other organs primarily involved in the pathophysiology of T2D, future studies should additionally assess GABA concentrations in peripheral organs such as the pancreas. In this regard, it has been shown that GABA is also produced by β‐cells of the islets where it functions as an intra‐islet transmitter regulating islet‐cell secretion and function (Wan, Wang, & Prud'homme, 2015). For instance, it has been shown that the islets' GABA signaling regulates insulin and glucagon secretion in both healthy subjects and T2D patients and that their GABA signaling system appears to be compromised in T2D individuals (Taneera et al., 2012). We can hence speculate that there may be a relationship between maladaptive GABA signaling in the islets and altered GABA concentrations in the CNS of T2D patients. Fourth, we did not control for psychological stress factors. Stress is a potential contributor to chronic hyperglycemia in diabetes. Stress stimulates the release of numerous hormones, which can result in higher blood glucose levels (Surwit, Schneider, & Feinglos, 1992). Although, this is of adaptive importance in healthy organisms, in T2D, as a result of relative lack of insulin, stress‐induced increases in glucose cannot be metabolized properly (Surwit et al., 1992). In this regard, Van Bussel et al. (2016) showed that GABA concentrations in the occipital lobe were increased in both, T2D patients and in subjects with higher fasting blood glucose levels. Therefore, it may possible that psychological stress, as for instance being in the scanner, serves as a confounding variable, which may affect GABA concentrations differentially in healthy controls and T2D patients. However, recent studies showed that stress decreases GABA concentrations in the prefrontal cortex (Strasser et al., 2019; Hasler, van der Veen, Grillon, Drevets, & Shen, 2010; but see also Houtepen et al., 2017). Therefore, it seems unlikely that our findings are due to processes related to stress.
The present study provides new evidence that GABA concentrations in T2D are higher only in specific regions and are negatively associated with associative episodic memory performance in T2D. The absence of T2D related abnormalities in glutamate and glutamine concentrations might be due to the small sample size or the localization of measurement. Nevertheless, our results provide important insight into the relation of amino acid neurotransmitter abnormalities in T2D and its relation to episodic memory deficits, occurring in T2D.
Species Studied
Human.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
This work was supported in part by the Ministry of Culture and Science of the State of North Rhine‐Westphalia (MKW NRW) and the German Federal Ministry of Health (BMG). This study was supported in part by grants of the Federal Ministry for Research (BMBF) to the German Center for Diabetes Research (DZD e.V.), the German Research Foundation (DFG, SFB1116, project B05), the ICEMED Hemlholtz Initiative with Universities and the Schmutzler‐Stiftung. J.W. Thielen was supported by the ICEMED grant to I. Tendolkar, D.G. Norris, and J. Wiltfang from the Helmholtz Alliance, Germany.
Thielen J‐W, Gancheva S, Hong D, et al. Higher GABA concentration in the medial prefrontal cortex of Type 2 diabetes patients is associated with episodic memory dysfunction. Hum Brain Mapp. 2019;40:4287–4295. 10.1002/hbm.24702
REFERENCES
- Ajilore, O. , Haroon, E. , Kumaran, S. , Darwin, C. , Binesh, N. , Mintz, J. , … Kumar, A. (2007). Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology, 32, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Andreychenko, A. , Boer, V. O. , Arteaga de Castro, C. S. , Luijten, P. R. , & Klomp, D. W. (2012). Efficient spectral editing at 7 T: GABA detection with MEGA‐sLASER. Magnetic Resonance in Medicine, 68, 1018–1025. [DOI] [PubMed] [Google Scholar]
- Bak, L. K. , Schousboe, A. , & Waagepetersen, H. S. (2006). The glutamate/GABA‐glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry, 98, 641–653. [DOI] [PubMed] [Google Scholar]
- Behar, K. L. , & Rothman, D. L. (2001). In vivo nuclear magnetic resonance studies of glutamate‐ gamma‐aminobutyric acid‐glutamine cycling in rodent and human cortex: The central role of glutamine. The Journal of Nutrition, 131, 2498–24504. [DOI] [PubMed] [Google Scholar]
- Biessels, G. J. , Strachan, M. W. , Visseren, F. L. , Kappelle, L. J. , & Whitmer, R. A. (2014). Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. The Lancet Diabetes and Endocrinology, 2, 246–255. [DOI] [PubMed] [Google Scholar]
- Biessels, G. J. , van der Heide, L. P. , Kamal, A. , Bleys, R. L. , & Gispen, W. H. (2002). Ageing and diabetes: Implications for brain function. European Journal of Pharmacology, 441, 1–14. [DOI] [PubMed] [Google Scholar]
- Blackwell, A. D. , Sahakian, B. J. , Vesey, R. , Semple, J. M. , Robbins, T. W. , & Hodges, J. R. (2004). Detecting dementia: Novel neuropsychological markers of preclinical Alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 17, 42–48. [DOI] [PubMed] [Google Scholar]
- Buzsaki, G. , Kaila, K. , & Raichle, M. (2007). Inhibition and brain work. Neuron, 56, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie, C. C. , Rust, K. F. , Ford, E. S. , Eberhardt, M. S. , Byrd‐Holt, D. D. , Li, C. , et al. (2009). Full accounting of diabetes and pre‐diabetes in the U. S. Population in 1988–1994 and 2005–2006. Diabetes Care, 32, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Jiao, Y. , Chen, Y. C. , Wang, K. , Gao, B. , & Wen, S. (2014). Altered spontaneous brain activity in type 2 diabetes: A resting state functional MRI study. Diabetes, 63, 749–760. [DOI] [PubMed] [Google Scholar]
- Day, M. , Langston, R. , & Morris, R. G. (2003). Glutamate‐receptor‐mediated encoding and retrieval of paired‐associate learning. Nature, 424, 205–209. [DOI] [PubMed] [Google Scholar]
- Fontbonne, A. , Berr, C. , Ducimetiere, P. , & Alperovitch, A. (2001). Changes in cognitive abilities over a 4‐year period are unfavorably affected in elderly diabetic subjects: Results of the epidemiology of vascular aging study. Diabetes Care, 24, 366–370. [DOI] [PubMed] [Google Scholar]
- Fowler, K. S. , Saling, M. M. , Conway, E. L. , Semple, J. M. , & Louis, W. J. (2002). Paired associate performance in the early detection of DAT. Journal of the International Neuropsychological Society, 8, 58–71. [PubMed] [Google Scholar]
- Frölich, L. , Blum‐Degen, D. , Riederer, P. , & Hoyer, S. (1999). A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Annals of the New York Academy of Sciences, 893, 290–293. [DOI] [PubMed] [Google Scholar]
- Geijselaers, S. L. C. , Sep, S. J. S. , Stehouwer, C. D. A. , & Biessels, G. J. (2015). Glucose regulation, cognition, and brain MRI in type 2 diabetes: A systematic review. The Lancet Diabetes and Endocrinology, 1, 75–89. [DOI] [PubMed] [Google Scholar]
- Gispen, W. H. , & Biessels, G. J. (2000). Cognition and synaptic plasticity in diabetes mellitus. Trends in Neurosciences, 23, 542–549. [DOI] [PubMed] [Google Scholar]
- Gregg, E. W. , Yaffe, K. , Cauley, J. A. , Rolka, D. B. , Blackwell, T. L. , Narayan, K. M. , & Cummings, S. R. (2000). Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of osteoporotic fractures research group. Archives of Internal Medicine, 160, 174–180. [DOI] [PubMed] [Google Scholar]
- Grodstein, F. , Chen, J. , Wilson, R. S. , & Manson, J. E. (2001). Type 2 diabetes and cognitive function in community‐dwelling elderly women. Diabetes Care, 24, 1060–1065. [DOI] [PubMed] [Google Scholar]
- Hasler, G. , van der Veen, J. W. , Grillon, C. , Drevets, W. C. , & Shen, J. (2010). Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. The American Journal of Psychiatry, 167, 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch, S. E. , Kirov, I. I. , & Tal, A. (2017). When are metabolic ratios superior to absolute quantification? A statistical analysis. NMR in Biomedicine, 30, e3710 10.1002/nbm.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtepen, L. C. , Schür, R. R. , Wijnen, J. P. , Boer, V. O. , Boks, M. P. , Kahn, R. S. , et al. (2017). Acute stress effects on GABA and glutamate levels in the prefrontal cortex: A 7T 1H magnetic resonance spectroscopy study. NeuroImage: Clinical, 14, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, N. , Riby, L. M. , Mitchell, R. L. , & Smith, M. A. (2014). Type 2 diabetes and memory: Using neuroimaging to understand the mechanisms. Current Diabetes Reviews, 10, 118–123. [DOI] [PubMed] [Google Scholar]
- Kanaya, A. M. , Barrett‐Connor, E. , Gildengorin, G. , & Yaffe, K. (2004). Change in cognitive function by glucose tolerance status in older adults: A 4‐year prospective study of the rancho Bernardo study cohort. Archives of Internal Medicine, 164, 1327–1333. [DOI] [PubMed] [Google Scholar]
- Kuusisto, J. , Koivisto, K. , Mykkanen, L. , Helkala, E.‐L. , Vanhanen, M. , Hanninen, T. , … Laakso, M. (1997). Association between features of the insulin resistance syndrome and Alzheimer's disease independently of apolipoprotein e4 phenotype: Cross sectional population based study. BMJ, 315, 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl, S. (2005). Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Spitta Verlag: Balingen. [Google Scholar]
- Leibson, C. L. , Rocca, W. A. , Hanson, V. A. , Cha, R. , Kokmen, E. , O'Brien, P. C. , & Palumbo, P. J. (1997). Risk of dementia among persons with diabetes mellitus: A population‐based cohort study. American Journal of Epidemiology, 145, 301–308. [DOI] [PubMed] [Google Scholar]
- Logothetis, N. K. , Pauls, J. , Augath, M. , Trinath, T. , & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412, 150–157. [DOI] [PubMed] [Google Scholar]
- Lyoo, I. K. , Yoon, S. J. , Musen, G. , Simonson, D. C. , Weinger, K. , Bolo, N. , … Jacobson, A. M. (2009). Altered prefrontal glutamate‐glutamine‐gamma‐aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Archives of General Psychiatry, 66, 878–887. [DOI] [PubMed] [Google Scholar]
- Messier, C. (2005). Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiology of Aging, 26, 26–30. [DOI] [PubMed] [Google Scholar]
- Miranda, M. I. (2007). Changes in neurotransmitter extracellular levels during memory formation In Bermúdez‐Rattoni F. (Ed.), Neural plasticity and memory: From genes to brain imaging. Boca Raton, FL: CRC Press/Taylor & Francis; Chapter 7. [PubMed] [Google Scholar]
- Mooradian, A. D. , Perryman, K. , Fitten, J. , Kavonian, G. D. , & Morley, J. E. (1988). Cortical function in elderly non‐insulin dependent diabetic patients: Behavioral and electrophysiologic studies. Archives of Internal Medicine, 148, 2369–2372. [PubMed] [Google Scholar]
- Moser, E. , Stahlberg, F. , Ladd, M. E. , & Trattnig, S. (2012). 7‐T MR‐‐from research to clinical applications? NMR in Biomedicine, 25, 695–716. [DOI] [PubMed] [Google Scholar]
- Munshi, M. , Grande, L. , Hayes, M. , Ayres, D. , Suhl, E. , Capelson, R. , … Weinger, K. (2006). Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care, 29, 1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny, B. , Zahiragic, L. , Krog, D. , Nowotny, P. J. , Herder, C. , Carstensen, M. , … Roden, M. (2013). Mechanisms underlying the onset of oral lipid‐induced skeletal muscle insulin resistance in humans. Diabetes, 62, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, H. , Coen, R. , Kidd, N. , Warsi, M. , Chin, A. V. , & Lawlor, B. A. (2004). Early detection of Alzheimer's disease (AD) using the CANTAB paired associates learning test. International Journal of Geriatric Psychiatry, 19, 1207–1208. [DOI] [PubMed] [Google Scholar]
- Ott, A. , Stolk, R. P. , Hofman, A. , van Harskamp, F. , Grobbee, D. E. , & Breteler, M. M. (1996). Association of diabetes mellitus and dementia: The Rotterdam study. Diabetologia, 39, 1392–1397. [DOI] [PubMed] [Google Scholar]
- Ott, A. , Stolk, R. P. , Van Harskamp, F. , Pols, H. A. , Hofman, A. , & Breteler, M. M. (1999). Diabetes mellitus and the risk of dementia: The Rotterdam study. Neurology, 53, 1937–1942. [DOI] [PubMed] [Google Scholar]
- Parra, M. A. , Abrahams, S. , Fabi, K. , Logie, R. , Luzzi, S. , & Della Sala, S. (2009). Short‐term memory binding deficits in Alzheimer's disease. Brain, 132, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Patel, A. B. , de Graaf, R. A. , Mason, G. F. , Rothman, D. L. , Shulman, R. G. , & Behar, K. L. (2005). The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proceedings of the National Academy of Sciences of the United States of America, 102, 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmuter, L. C. , Hakami, M. K. , Hodgson‐Harrington, C. , Ginsberg, J. , Katz, J. , Singer, D. E. , & Nathan, D. M. (1984). Decreased cognitive function in aging non‐insulin‐dependent diabetic patients. The American Journal of Medicine, 77, 1043–1048. [DOI] [PubMed] [Google Scholar]
- Petroff, O. A. (2002). GABA and glutamate in the human brain. The Neuroscientist, 8, 562–573. [DOI] [PubMed] [Google Scholar]
- Phielix, E. , Brehm, A. , Bernroider, E. , Krssak, M. , Anderwald, C. H. , Krebs, M. , … Roden, M. (2013). Effects of pioglitazone versus glimepiride exposure on hepatocellular fat content in type 2 diabetes. Diabetes, Obesity & Metabolism, 15, 915–922. [DOI] [PubMed] [Google Scholar]
- Provencher, S. W. (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 14, 260–264. [DOI] [PubMed] [Google Scholar]
- Ranganath, C. (2010). Binding items and contexts: The cognitive neuroscience of episodic memory. Current Directions in Psychological Science, 19, 131–137. [Google Scholar]
- Reaven, G. M. , Thompson, L. W. , Nahum, D. , & Haskins, E. (1990). Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care, 13, 16–21. [DOI] [PubMed] [Google Scholar]
- Rivera, E. J. , Goldin, A. , Fulmer, N. , Tavares, R. , Wands, J. R. , & de la Monte, S. M. (2005). Insulin and insulin‐like growth factor expression and function deteriorate with progression of Alzheimer's disease: Link to brain reductions in acetylcholine. Journal of Alzheimer's Disease, 8, 247–268. [DOI] [PubMed] [Google Scholar]
- Sahin, I. , Alkan, A. , Keskin, L. , Cikim, A. , Karakas, H. M. , Firat, A. K. , & Sigirci, A. (2008). Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. Journal of Diabetes and its Complications, 22, 254–260. [DOI] [PubMed] [Google Scholar]
- Sickmann, H. M. , Waagepetersen, H. S. , Schousboe, A. , Benie, A. J. , & Bouman, S. D. (2010). Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino‐acid homeostasis. Journal of Cerebral Blood Flow and Metabolism, 30, 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann, H. M. , Waagepetersen, H. S. , Schousboe, A. , Benie, A. J. , & Bouman, S. D. (2012). Brain glycogen and its role in supporting glutamate and GABA homeostasis in a type 2 diabetes rat model. Neurochemistry International, 60, 267–275. [DOI] [PubMed] [Google Scholar]
- Sinha, S. , Ekka, M. , Sharma, U. , P, R. , Pandey, R. M. , & Jagannathan, N. R. (2014). Assessment of changes in brain metabolites in Indian patients with type‐2 diabetes mellitus using proton magnetic resonance spectroscopy. BMC Research Notes, 7, 41 10.1186/1756-0500-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonia, R. O. , & McCabe, B. J. (2015). Molecular mechanisms of memory in imprinting. Neuroscience and Biobehavioral Reviews, 50, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen, E. , Terry, B. M. , Rivera, E. J. , Cannon, J. L. , Neely, T. R. , Tavares, R. , et al. (2005). Impaired insulin and insulin‐like growth factor expression and signaling mechanisms in Alzheimer's disease‐‐is this type 3 diabetes? Journal of Alzheimer's Disease, 7, 63–80. [DOI] [PubMed] [Google Scholar]
- Stewart, R. , & Liolitsa, D. (1999). Type 2 diabetes mellitus, cognitive impairment and dementia. Diabetic Medicine, 16, 93–112. [DOI] [PubMed] [Google Scholar]
- Strasser, A. , … Sandi, C. (2019). Nucleus accumbens neurochemistry in human anxiety: A 7 T 1H‐MRS study. European Neuropsychopharmacology, 29, 365–375. [DOI] [PubMed] [Google Scholar]
- Surwit, R. S. , Schneider, M. S. , & Feinglos, M. N. (1992). Stress and diabetes mellitus. Diabetes Care, 15, 1413–1422. [DOI] [PubMed] [Google Scholar]
- Swainson, R. , Hodges, J. R. , Galton, C. J. , Semple, J. , Michael, A. , Dunn, B. D. , … Sahakian, B. J. (2001). Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders, 12, 265–280. [DOI] [PubMed] [Google Scholar]
- Szendroedi, J. , Saxena, A. , Weber, K. S. , Strassburger, K. , Herder, C. , Burkart, V. , et al. (2016). Cohort profile: The German diabetes study (GDS). Cardiovascular Diabetology, 15, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneera, J. , Jin, Z. , Jin, Y. , Muhammed, S. J. , Zhang, E. , Lang, S. , … Birnir, B. (2012). γ‐Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia, 55, 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen, J. W. , Hong, D. , Rohani Rankouhi, S. , Wiltfang, J. , Fernández, G. , Norris, D. G. , & Tendolkar, I. (2018). The increase in medial prefrontal glutamate/glutamine concentration during memory encoding is associated with better memory performance and stronger functional connectivity in the human medial prefrontal‐thalamus‐hippocampus network. Human Brain Mapping, 39, 2381–2390. 10.1002/hbm.24008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen, J. W. , Kärgel, C. , Müller, B. W. , Rasche, I. , Genius, J. , Bus, B. , … Tendolkar, I. (2016). Aerobic activity in the healthy elderly is associated with larger plasticity in memory related brain structures and lower systemic inflammation. Frontiers in Aging Neuroscience, 8, 319 10.3389/fnagi.2016.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognarelli, J. M. , Dawood, M. , Shariff, M. I. , Grover, V. P. , Crossey, M. M. , Cox, I. J. , et al. (2015). Magnetic resonance spectroscopy: Principles and techniques: Lessons for clinicians. Journal of Clinical and Experimental Hepatology, 5, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bussel, F. C. , Backes, W. H. , Hofman, P. A. , Puts, N. A. , Edden, R. A. , van Boxtel, M. P. , et al. (2016). Increased GABA concentrations in type 2 diabetes mellitus are related to lower cognitive functioning. Medicine, 95, e4803 10.1097/MD.0000000000004803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Wang, Q. , & Prud'homme, G. J. (2015). GABAergic system in the endocrine pancreas: A new target for diabetes treatment. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 8, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Li, J. , Li, H. , & Zhang, S. (2013). Differences in learning rates for item and associative memories between amnestic mild cognitive impairment and healthy controls. Behavioral and Brain Functions, 9, 29 10.1186/1744-9081-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, W. , Chen, Y. C. , & Ma, J. (2017). Resting‐state brain anomalies in type 2 diabetes: A meta‐analysis. Front. Aging Neuroscience, 31, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , Ley, S. H. , & Hu, F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews. Endocrinology, 14, 88–98. [DOI] [PubMed] [Google Scholar]


