Abstract
Schizophrenia (SZ) is a highly heritable disease with neurodevelopmental origins and significant functional brain network dysfunction. Functional network is heavily influenced by neurodevelopment processes and can be characterized by the degree of segregation and integration. This study examines functional segregation and integration in SZ and their first‐degree relatives (high risk [HR]) to better understand the dynamic changes in vulnerability and resiliency, and disease markers. Resting‐state functional magnetic resonance imaging data acquired from 137 SZ, 89 HR, and 210 healthy controls (HCs). Small‐worldness σ was computed at voxel level to quantify balance between segregation and integration. Interregional functional associations were examined based on Euclidean distance between regions and reflect degree of segregation and integration. Distance strength maps were used to localize regions of altered distance‐based functional connectivity. σ was significantly decreased in SZ compared to HC, with no differences in high risk (HR). In three‐group comparison, significant differences were noted in short‐range connectivity (primarily in the primary sensory, motor and their association cortices, and the thalamus) and medium/long‐range connectivity (in the prefrontal cortices [PFCs]). Decreased short‐ and increased medium/long‐range connectivity was found in SZ. Decreased short‐range connectivity was seen in SZ and HR, while HR had decreased medium/long‐range connectivity. We observed disrupted balance between segregation and integration in SZ, whereas relatively preserved in HR. Similarities and differences between SZ and HR, specific changes of SZ were found. These might reflect dynamic changes of segregation in primary cortices and integration in PFCs in vulnerability and resilience, and disease markers in SZ.
Keywords: fMRI, functional network, genetic risk, resilience, schizophrenia, vulnerability
1. INTRODUCTION
Schizophrenia (SZ) is a highly heritable disease with significant dysfunction within the functional brain network (Fornito, Zalesky, & Breakspear, 2015; Insel, 2010). Human brain functional network has two major fundamental organizational principles: segregation (specialized processing within interconnected brain regions) and integration (of different brain areas in terms of functional and effective connectivity) (Friston, 2009; Rubinov et al., 2009; Tononi, Edelman, & Sporns, 1998). Optimal brain function occurs when there is balance between segregation and integration, which is vital for effective information processing and synthesis (Liu et al., 2008; Tononi et al., 1998). Previous research in SZ and other psychiatric disorders has demonstrated disrupted balance between segregation and integration within the functional brain network (Liu et al., 2008; Lynall et al., 2010; Rubinov et al., 2009; Wang et al., 2016).
The HR population provides an unique opportunity to identify vulnerability and resiliency factors when comparing them with affected individuals and healthy controls (HCs). Similarities between HR and disease samples may reflect vulnerability markers whereas differences may reflect resiliency markers. The lifetime prevalence of SZ in general populations varies in different populations but is approximately 1%. First‐degree relatives of individuals with SZ (HR individuals) have almost a 10‐fold increased risk of developing SZ and their lifetime prevalence is 13% (Tsuang, 2000). However, despite the increased genetic risk, most HR does not later develop SZ. Prior studies have found similarities between SZ and HR in alterations of functional brain network (Li, Xia, Bertisch, Branch, & Delisi, 2012). They have also shown functional connectivity alterations that potentially indicate protective factors in HR (Anticevic et al., 2014; Liu et al., 2016) and the presence of resiliency features in HR (Chang et al., 2016). At present, the relationship between illness onset, genetic susceptibility, and resilience and their underlying mechanisms remain unclear. While the balance between segregation and integration within the functional brain network is disrupted in SZ, it is unclear whether impaired balance is also observed in HR. Further studies are needed to further understand brain network alterations in SZ and HR.
Degrees of segregation and integration could be reflected in physical distance‐dependent connectivity within functional networks, with short‐range connections reflecting segregation and long‐range connections reflecting integration, and this could lead to the discovery of explicit alteration regions (Aerts, Fias, Caeyenberghs, & Marinazzo, 2016a; Yu, Sui, Kiehl, Pearlson, & Calhoun, 2013). Study of SZ and HR could identify dynamic changes in functional segregation and integration related to disease vulnerability and resilience in SZ. Brain network function is heavily influenced by neurodevelopment processes (Barthélemy, 2011; Ghisleni et al., 2015). These processes appeared to be dependent on the distance range of neural connections. As children develop, short‐range connections shift to more long‐range connections (Dosenbach et al., 2010; Fair et al., 2007; Fair et al., 2009). The observed shift likely reflects the balance between synaptic pruning and growth of length‐dependent neuronal connections during development (Biane, Scanziani, Tuszynski, & Conner, 2015; Toga, Thompson, & Sowell, 2006).
Recent studies have found alterations in distance‐dependent connectivity in SZ (Alexander‐Bloch et al., 2013; Lo et al., 2015) and HR (Guo et al., 2014). However, the findings in HR are inconsistent (Guo et al., 2014; Shi et al., 2012). This may relate to differences in parcellation approaches and brain parcellation atlases used (Wang et al., 2009). In addition, atlas‐based parcellation of functional brain networks would provide insight for connections between parcellated regions but not within them. Prior network studies have primarily focused on vulnerability but not resiliency markers (Lo et al., 2015).
In this study, we used voxel‐based graph analysis of resting‐state functional magnetic resonance imaging (R‐fMRI) to minimize parcellation‐dependent effects on brain networks and examine connections between and within regions. Specifically, we examined: (a) small worldness (a measure of the balance between segregation and integration), (b) whole‐brain functional connectivity based on connectivity distance, and (c) distance strength maps to understand alterations in functional brain network of SZ and HR. We hypothesized that SZ and HR have altered functional connectivity distance, which could suggest abnormal segregation and integration, and changes in HR would reveal the dynamic changes in vulnerability and resilience to SZ.
2. MATERIALS AND METHODS
2.1. Participants
A total of 481 participants (ages 13–45 years) were included in this study, including 159 SZ, 95 HR, and 227 HCs. SZ and HR participants were recruited from the inpatient and outpatient services at Shenyang Mental Health Center and the Department of Psychiatry, the First Affiliated Hospital of China Medical University, Shenyang, China. HC participants were recruited from Shenyang, China and surrounding cities by publically posted advertisement. The study was approved by the Institutional Review Board of China Medical University.
The presence or absence of Axis I diagnoses was determined by consensus between independent evaluations by two trained psychiatrists using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) Axis I Disorders, in participants 18 years and older and the Schedule for Affective Disorders and SZ for School‐Age Children‐present and Lifetime Version in those younger than 18 years. SZ participants met DSM‐IV diagnostic criteria for SZ and not any other Axis I disorders. HR participants were first‐degree relatives of individuals with SZ and did not meet criteria for any DSM‐IV Axis I disorder. HC participants did not have current or lifetime Axis I disorder or history of psychotic, mood, or other Axis I disorders in first‐degree relatives as determined by detailed family history. All individuals were excluded for: (a) lifetime substance/alcohol abuse or dependence, (b) concomitant major medical disorder, (c) any MRI contraindications, (d) history of head trauma with loss of consciousness ≥ 5 min or any neurological disorder, and (e) suboptimal imaging data quality (see below for details). Symptom severity was measured using the Brief Psychiatric Rating Scale (BPRS), and cognitive function was assessed using the Wisconsin Card Sorting Test (WCST). Detailed demographic and clinical information are presented in Table 1.
Table 1.
Demographics and clinical characteristics of HCs, SZ, and genetic HR
| HC | HR | SZ | |||
|---|---|---|---|---|---|
| (n = 210) | (n = 89) | (n = 137) | F/χ 2 values | p‐Values | |
| Demographic characteristic | |||||
| Age at scans (year) | 26.02 ± 7.36 | 24.83 ± 8.04 | 24.12 ± 8.88 | 2.458 | 0.087 |
| Gender (male/female) | 79/131 | 52/37 | 54/83 | 11.823 | 0.003 |
| Handedness (R/L/MIX) | 195/0/11 | 75/5/7 | 113/3/13 | 14.021 | 0.007 |
| Education (years) | 15.05 ± 3.08 | 12.13 ± 3.31 | 10.75 ± 3.07 | 1.703 | <0.001 |
| BMI (mean ± SD) | 23.84 ± 33.21 | 22.89 ± 4.12 | 22.88 ± 4.69 | 1.703 | 0.148 |
| Clinical characteristic | |||||
| Duration (months, mean ± SD) | N/A | N/A | 25.03 ± 39.00 | ||
| First episode (yes/no) | N/A | N/A | 91/42 | ||
| Medication (yes/no) | N/A | N/A | 92/43 | ||
| BPRS (mean ± SD) | n = 128 | n = 83 | n = 79 | ||
| 18.44 ± 1.07 | 18.81 ± 2.11 | 34.08 ± 10.86 | 194.68 | <0.001 | |
| Cognitive function | |||||
| WCST | n = 146 | n = 83 | n = 77 | ||
| Corrected responses | 31.46 ± 11.60 | 26.24 ± 11.28 | 19.40 ± 11.80 | 32.00 | <0.001 |
| Categories completed | 4.17 ± 2.10 | 3.21 ± 2.07 | 1.70 ± 1.90 | 41.43 | <0.001 |
| Total errors | 16.73 ± 11.73 | 21.76 ± 11.28 | 28.60 ± 11.80 | 30.84 | <0.001 |
| Perseverative errors | 6.15 ± 7.22 | 8.27 ± 8.03 | 12.08 ± 11.53 | 13.06 | <0.001 |
| Nonperseverative errors | 10.43 ± 6.41 | 13.36 ± 7.43 | 16.52 ± 8.71 | 20.90 | <0.001 |
HC = healthy control; HR = high risk; SZ = schizophrenia; BPRS = Brief Psychiatric Rating Scale; WCST = Wisconsin Card Sorting Test; handedness (R/L/MIX): right/left/mix handedness. Data are presented as mean ± SD.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Medical Science Research Ethics Committee of the First Affiliated Hospital of China Medical University (approval reference number [2012]25‐1). All participants provided written informed consent by themselves or by their parents/guardians if they were under 18 years old after a complete description of the study.
2.2. MRI acquisition
MRI data were acquired in a GE Sigma HD 3.0T scanner (General Electric, Milwaukee, WI) with a standard eight‐channel head coil at the First Affiliated Hospital of China Medical University. Functional images were collected with a gradient‐echo planar imaging (EPI) sequence. The parameters were as follows: TR = 2000 ms, TE = 30 ms, flip angle = 90°, field of view = 240 × 240 mm2, matrix = 64 × 64. Thirty‐five axial slices were collected with 3 mm thickness without gap. The scan lasted for 6 min and 40 s, resulting in 200 volumes. Participants were instructed to rest and relax with their eyes closed but remain awake during scanning.
2.3. Data preprocessing
Preprocessing of all R‐fMRI images was performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and DPARSF (Yan & Zang, 2010). The first 10 time points were discarded for magnetic field stabilization and allowing participants to adapt to the scanning environment. The subsequent preprocessing steps included slice time correction and head motion correction. During the head motion correction, 45 subjects (22 SZs, 6 HRs, and 17 HCs) were excluded from subsequent analysis due to excessive head motion larger than 3 mm or 3°. Next, the corrected functional images were normalized to Montreal Neurological Institute (MNI) space using the EPI template in SPM12, resampled to 3 mm isotropic voxels, and further smoothed via a Gaussian kernel with a 4 mm full‐width at half‐maximum. Linear detrending was performed and several confounding covariates, including the Friston‐24 head motion parameters (Friston, Williams, Howard, Frackowiak, & Turner, 1996), white matter, cerebrospinal fluid, and global signals, were regressed from the BOLD time series for all voxels. Finally, temporal band‐pass filtering (0.01–0.1 Hz) was applied to the regressed fMRI data.
2.4. Network construction
Individual functional network construction was constrained at a voxel level within a gray matter (GM) mask of 45,381 voxels, which was generated by extracting overlapping voxels in the automated anatomical labeling template (Tzourio‐Mazoyer et al., 2002) and the thresholded prior GM probability map (>0.2) provided by SPM12. We computed Pearson's correlations between all pairs of nodes (i.e., GM voxels), resulting in a 45,381 × 45,381 correlation matrix for each subject. These individual correlation matrices were further binarized with a density threshold of 1%, corresponding to remaining the 10,296,948 edges with top positive strength.
2.5. Global network measurement σ
We calculated global network measurement σ (small worldness) for each subject. σ is a measure of the balance segregation and integration (Aerts, Fias, Caeyenberghs, & Marinazzo, 2016b). The network construction and calculation of global network measurement were carried out using PAGANI toolkit (https://www.nitrc.org/projects/pagani_toolkit/) based on a hybrid CPU–GPU framework (Wang et al., 2013).
2.6. Distance‐dependent distribution of network connections
After network construction, we calculated the Euclidean distance, dij, as an approximate anatomical distance of functional connectivity between Voxels i and j. Then, we divided whole‐brain functional connectivity based on anatomical distance into 18 distance bins in increments of 10 mm Euclidean distance ranging from 0 to 180 mm (the longest distance between voxels in the GM mask). We performed subsequent analyses in Bins 1–14 (0–140 mm) as Bins 15–18 (140–180 mm) only accounted for less than 2% of the total connectivity. Finally, we counted the number of edges within each distance bin for each subject and compared them across groups.
2.7. Regional distance strength
For each distance bin, we calculated the distance strength for each node to further examine specific regions with altered distance‐dependent connectivity. The distance strength of a given node was calculated as the total length of the edges linking to this node within the connectivity distance range, capturing both the number of connections and approximate physical cost. Individual distance strength maps were generated for each distance bin and were compared across groups in a voxel‐wise fashion.
2.8. Statistical analyses
Group effects on clinical variables, connections in distance bins, and distance strength maps were examined using one‐way analysis of covariance (ancova) with age and gender as covariates. Significance for analyses of demographic and clinical characteristics, and global network measurement σ was set at p < 0.05. For the analyses involving multiple distance bins, false discovery rate (FDR) correction was applied for multiple comparisons, and significance was set at a corrected p < 0.05. Analyses of distance strength maps were performed in a voxel‐wise manner and significance was set at voxel‐level inference of p < 0.001 with Gaussian random field correction for cluster‐level inference of p < 0.05. Post hoc analyses were performed between SZ, HR, and HC using a general linear model for significant group effects in the ancova analyses. For each cluster with significant three‐group difference in Bins 1 and 8 (0–10 and 70–80 mm, respectively; analyses for other bins can be found in Appendix S1, Supporting Information), distance strength values were extracted and analyzed in pairwise two sample t tests, FDR correction was applied for multiple comparisons and significance was set at a corrected p < 0.05. To explore distance strength differences between the HR and HC, two‐sample t test was performed using the ancova results as masks on the individually distance strength maps with age and gender as covariates. Statistical significance was set at voxel‐level inference of p < 0.01 with Gaussian random field correction for cluster‐level inference of p < 0.05.
Analyses were also performed to examine the effects of age, education, clinical and cognitive variables measures on range‐dependent strength for the significant clusters of Bins 1 and 8 found in the three‐group analyses in Bins 1 and 8 (Appendix S1, Supporting Information). Due to a wide age range in the present sample (13–45 years), univariate analyses of variance were performed to assess potential diagnosis by age interactions in extracted distance strength values.
3. RESULTS
3.1. Demographic and clinical data
There were no significant differences in age among the SZ, HR, and HC groups. Significant differences were observed in sex (degrees of freedom [df] = 2, χ 2 = 11.823, p < 0.01, Table 1) and education (df = 2, χ 2 = 1.703, p < 0.001, Table 1). Significant differences were observed in BPRS and WCST scores among the three groups (Table 1). Post hoc analyses showed that BPRS scores were significantly increased in SZ, compared to the HR and HC groups (df = 1, p < 0.001) but not significantly different between HR and HC. WCST scores were lower in SZ than HR (df = 1, all p's < 0.005), and in HR than HC (df = 1, all p's < 0.01) except in perseverative errors (p = 0.059).
3.2. Global Network Measurement σ
Significant group effects were observed in global network measurement σ in the three group analysis (df = 2, p < 0.001) in descending order from HC to HR to SZ in mean values (Figure 1). Post hoc analysis revealed significantly decreased σ in the SZ groups, compared with HC. There was no statistical difference between HR and HC, or HR and SZ.
Figure 1.
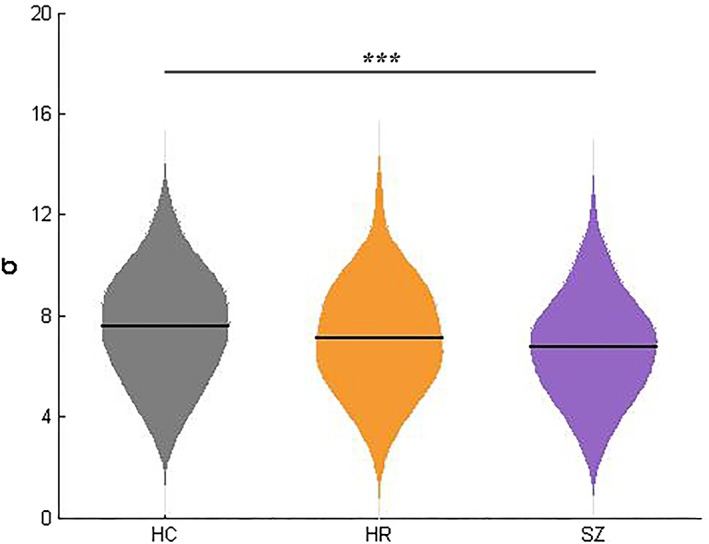
Differences in global network parameter σ among three groups. The violin plots represent the distribution of global network parameter σ in each group and the solid lines indicate the mean value in HC, HR, and SZ. Significance level was set as p < 0.05. ***, p < 0.001. HC = healthy control; HR = high risk; SZ = schizophrenia
3.3. Altered distance‐dependent functional connectivity
Significant group effects were observed in distance Bins 1 (0–10 mm), 2 (10–20 mm), 3 (20–30 mm), 5 (40–50 mm), 6 (50–60 mm), 7 (60–70 mm), and 8 (70–80 mm) (df = 2, all p < 0.01, FDR corrected, Figure 2). There were no significant differences among the three groups in other distance bins (distance Bins 4, and 9–14).
Figure 2.
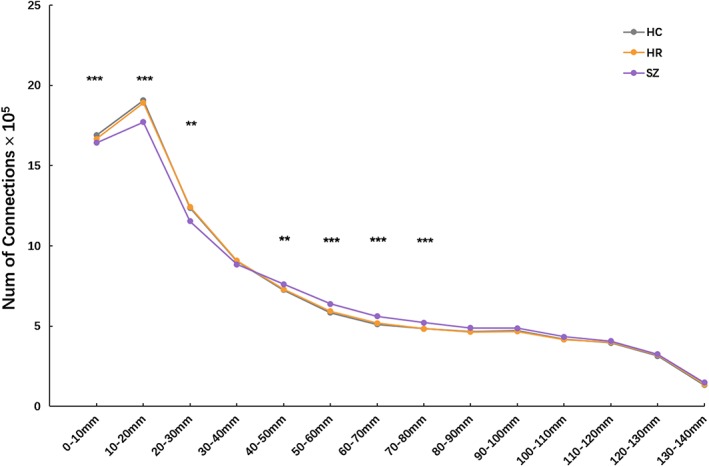
Distance‐dependent differences in amounts of network connections among three groups. Group effects on number of functional connections in each distance bin were detected by one‐way analysis of covariance. Significance level was set as p < 0.05 with false discovery rate correction for multiple comparisons. ***, p < 0.001; **, p < 0.01. HC = healthy control; HR = high risk; SZ = schizophrenia
3.4. Altered regional distance strength
3.4.1. Three‐group comparison
Significant group differences in distance strength of short‐range connections (distance Bins 1 [0–10 mm], 2 [10–20 mm], and 3 [20‐30 mm]) were mainly localized to the primary sensory, motor, and their association cortices, as well as the thalamus (Figure 3a, Figures S1 and S2, and Table 2). Significant group differences of medium/long‐range connections (distance Bins 5 [40–50 mm], 6 [50–60 mm], 7 [60–70 mm], and 8 [70–80 mm]) were primarily in the prefrontal cortex (PFC), as well as some regions in the primary cortex and parietal lobe (Figure 4a, Figures S3–S5, and Table 2).
Figure 3.
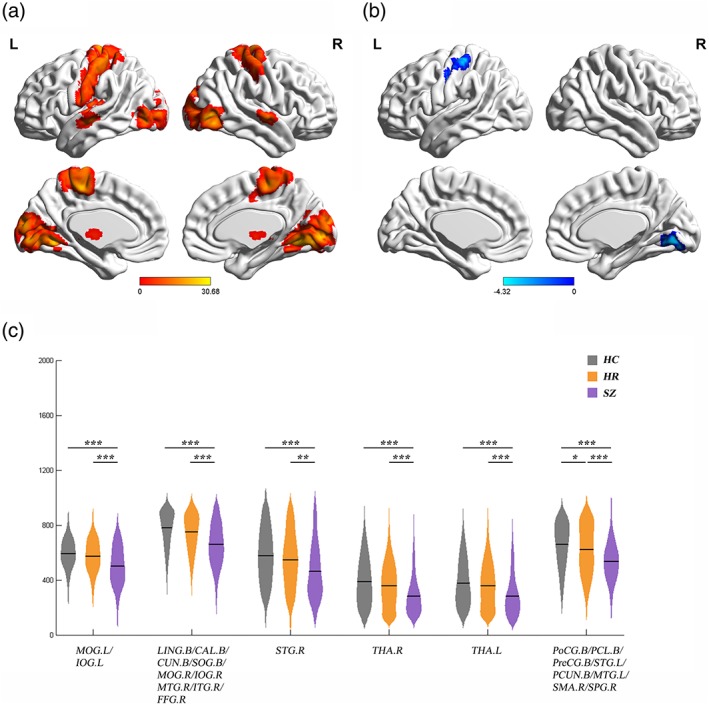
Significant difference of short‐range distance strength among SZ, HR, and HCs. (a) Regions with significant group effects on short‐range distance strength (Bin 1, 0–10 mm). Significance level was set as p < 0.001 at voxel level with Gaussian random field correction for multiple comparisons. (b, c) Post hoc analysis of Bin 1. (b) Regions showing significant difference of Bin 1 in the HR and HC. Significance level was set as p < 0.01 at voxel level with Gaussian random field correction for multiple comparisons. (c) Distance strength values of Bin 1 in regions showing significant differences among the participants with SZ, HR, and HCs. The violin plots represent the distribution of distance strength values in each group and the solid lines indicate the mean value. Significance level was set as p < 0.05 with false discovery rate correction for multiple comparisons. ***, p < 0.001; **, p < 0.01; *, p < 0.05. B = bilateral; CAL = calcarine cortex; CUN = cuneus; FFG = fusiform gyrus; HC = healthy control; HR = high risk; IOG = inferior occipital gyrus; ITG = inferior temporal gyrus; L = left; LING = lingual gyrus; MOG = middle occipital gyrus; MTG = middle temporal gyrus; PCL = paracentral lobule; PCUN = precuneus; PoCG = postcentral gyrus; PreCG = precentral gyrus; R = right; SMA = supplementary motor area; SOG = superior occipital gyrus; SPG = superior parietal gyrus; STG = superior temporal gyrus; SZ = schizophrenia; THA = thalamus. The surface visualization was conducted by using BrainNet Viewer (Xia, Wang, & Yong, 2013)
Table 2.
Regions showing abnormal distance strength in the three‐group analysis (HC, SZ, and HR)
| MNI coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Distance | Bins | Regions | BA | Cluster size | X | Y | Z | F values |
| Short‐range connection | 1 | Left middle occipital gyrus | 18/19 | 248 | −42 | −81 | −6 | 21.08 |
| Left inferior occipital gyrus | ||||||||
| Bilateral lingual gyrus | 17/18/19/30 | 2024 | 48 | −69 | −6 | 30.68 | ||
| Bilateral calcarine cortex | ||||||||
| Bilateral cuneus | ||||||||
| Bilateral superior occipital gyrus | ||||||||
| Right middle occipital gyrus | ||||||||
| Right inferior occipital gyrus | ||||||||
| Right middle temporal gyrus | ||||||||
| Right inferior temporal gyrus | ||||||||
| Right fusiform gyrus | ||||||||
| Right superior temporal gyrus | N/A | 84 | 63 | −9 | −3 | 17.05 | ||
| Right thalamus | N/A | 110 | 9 | −6 | 0 | 30.33 | ||
| Left thalamus | N/A | 122 | −6 | −9 | 3 | 23.65 | ||
| Bilateral postcentral gyrus | 1/2/3/4/5/6 | 2,133 | −33 | −42 | 69 | 25.31 | ||
| Bilateral paracentral lobule | ||||||||
| Bilateral precentral gyrus | ||||||||
| Left superior temporal gyrus | ||||||||
| Bilateral precuneus | ||||||||
| Left middle temporal gyrus | ||||||||
| Right supplementary motor area | ||||||||
| Right superior parietal gyrus | ||||||||
| 2 | Right middle temporal gyrus | N/A | 162 | 54 | −72 | −6 | 16.66 | |
| Right inferior temporal gyrus | ||||||||
| Right middle occipital gyrus | ||||||||
| Left superior temporal gyrus | N/A | 107 | −54 | −15 | 0 | 14.86 | ||
| Left middle temporal gyrus | ||||||||
| Bilateral cuneus | 17/18/19/30 | 1,533 | 21 | −66 | −3 | 26.48 | ||
| Bilateral lingual gyrus | ||||||||
| Bilateral calcarine cortex | ||||||||
| Right fusiform gyrus | ||||||||
| Right thalamus | N/A | 88 | 6 | −12 | 0 | 23.86 | ||
| Left thalamus | N/A | 89 | −6 | −15 | 3 | 21.95 | ||
| Right superior frontal gyrus | N/A | 37 | 27 | 51 | 6 | 12.58 | ||
| Right middle frontal gyrus | ||||||||
| Left middle frontal gyrus | N/A | 67 | −39 | 42 | 24 | 12.27 | ||
| Left superior frontal gyrus | N/A | 59 | −12 | 39 | 45 | 10.09 | ||
| Left inferior frontal gyrus, orbital part | N/A | 39 | −42 | 27 | −12 | 13.92 | ||
| Bilateral postcentral gyrus | 2/3/4/5/6/ | 1,758 | −3 | −27 | 57 | 24.05 | ||
| Bilateral precentral gyrus | ||||||||
| Bilateral paracentral lobule | ||||||||
| Bilateral precuneus | ||||||||
| Bilateral supplementary motor area | ||||||||
| Left median cingulate and paracingulate gyri | ||||||||
| Left supplementary motor area | N/A | 75 | −3 | 3 | 63 | 12.77 | ||
| 3 | Bilateral cuneus | 18/19/30 | 886 | 21 | −48 | −9 | 24.41 | |
| Bilateral lingual gyrus | ||||||||
| Bilateral calcarine cortex | ||||||||
| Right fusiform gyrus | ||||||||
| Right thalamus | N/A | 56 | 12 | −12 | 6 | 15.45 | ||
| Left thalamus | N/A | 59 | −12 | −18 | 3 | 18.38 | ||
| Right supramarginal gyrus | 40 | 108 | 63 | −39 | 27 | 16.75 | ||
| Left postcentral gyrus | N/A | 108 | −51 | −18 | 54 | 11.94 | ||
| Bilateral paracentral lobule | 3/4/5/6/7 | 996 | −12 | −33 | 75 | 22.76 | ||
| Bilateral precuneus | ||||||||
| Bilateral postcentral gyrus | ||||||||
| Right supplementary motor area | ||||||||
| Right precentral gyrus | ||||||||
| Left median cingulate and paracingulate gyri | ||||||||
| Medium/long‐range connection | 5 | Left lingual gyrus | 19 | 189 | −21 | −54 | −9 | 17.59 |
| Left calcarine cortex | ||||||||
| Right lingual gyrus | 19 | 185 | 21 | −48 | −9 | 23.43 | ||
| Right calcarine cortex | ||||||||
| Right fusiform gyrus | ||||||||
| Right supramarginal gyrus | N/A | 37 | 60 | −39 | 30 | 10.66 | ||
| Left middle frontal gyrus | N/A | 52 | −24 | 51 | 18 | 14.09 | ||
| Left cuneus | N/A | 67 | −6 | −87 | 18 | 10.69 | ||
| Left postcingulate gyrus | N/A | 25 | −3 | −45 | 24 | 10.04 | ||
| Left supplementary motor area | N/A | 73 | −3 | 18 | 69 | 12.90 | ||
| 6 | Right middle occipital gyrus | N/A | 36 | 30 | −90 | 0 | 12.83 | |
| Right inferior occipital gyrus | ||||||||
| Left lingual gyrus | N/A | 37 | −24 | −51 | −12 | 14.32 | ||
| Right lingual gyrus | N/A | 45 | 21 | −48 | −9 | 17.86 | ||
| Right fusiform gyrus | ||||||||
| Right middle temporal gyrus | N/A | 38 | 57 | −30 | −3 | 10.97 | ||
| Left inferior frontal gyrus, orbital part | N/A | 34 | −36 | 27 | −9 | 13.68 | ||
| Right Rolandic operculum | N/A | 26 | 60 | 3 | 6 | 10.75 | ||
| Left middle frontal gyrus | 10 | 77 | −24 | 51 | 18 | 11.70 | ||
| Left superior frontal gyrus | ||||||||
| Right middle frontal gyrus | N/A | 22 | 33 | 45 | 30 | 10.63 | ||
| Right cuneus | N/A | 36 | 12 | −93 | 27 | 11.81 | ||
| Left median cingulate and paracingulate gyri | N/A | 25 | −3 | −42 | 33 | 11.65 | ||
| Bilateral medial superior frontal gyrus | N/A | 33 | 0 | 42 | 33 | 11.77 | ||
| Right supplementary motor area | N/A | 36 | 3 | −3 | 75 | 12.91 | ||
| 7 | Right middle temporal gyrus | N/A | 48 | 54 | −30 | −3 | 11.01 | |
| Right inferior frontal gyrus, orbital part | N/A | 44 | 42 | 27 | −12 | 9.90 | ||
| Right inferior occipital gyrus | 18 | 79 | 33 | −90 | 3 | 16.41 | ||
| Right middle occipital gyrus | ||||||||
| Left middle temporal gyrus | N/A | 27 | −69 | −36 | −12 | 12.23 | ||
| Left inferior frontal gyrus, orbital part | N/A | 40 | −42 | 18 | −12 | 14.69 | ||
| Left middle occipital gyrus | N/A | 37 | −33 | −93 | 0 | 12.24 | ||
| Left middle frontal gyrus | N/A | 26 | −30 | 48 | 27 | 9.19 | ||
| 8 | Right inferior frontal gyrus, orbital part | N/A | 28 | 39 | 39 | −12 | 13.67 | |
| Left middle frontal gyrus, orbital part | N/A | 62 | −36 | 48 | −9 | 14.74 | ||
| Right inferior occipital gyrus | N/A | 29 | 39 | −90 | −9 | 13.42 | ||
| Left medial superior frontal gyrus | N/A | 21 | −6 | 57 | 18 | 10.89 | ||
| Left precuneus | N/A | 43 | 0 | −57 | 27 | 11.11 | ||
| Left inferior frontal gyrus, orbital part | N/A | 25 | −39 | 27 | −9 | 11.16 | ||
| Right middle frontal gyrus | N/A | 24 | 39 | 54 | −3 | 13.93 | ||
| Left superior frontal gyrus | N/A | 20 | −12 | 42 | 45 | 12.76 | ||
Significance level was set as p < 0.05 corrected by p < 0.001 at voxel level with Gaussian Random Field correction for multiple comparisons.
Figure 4.
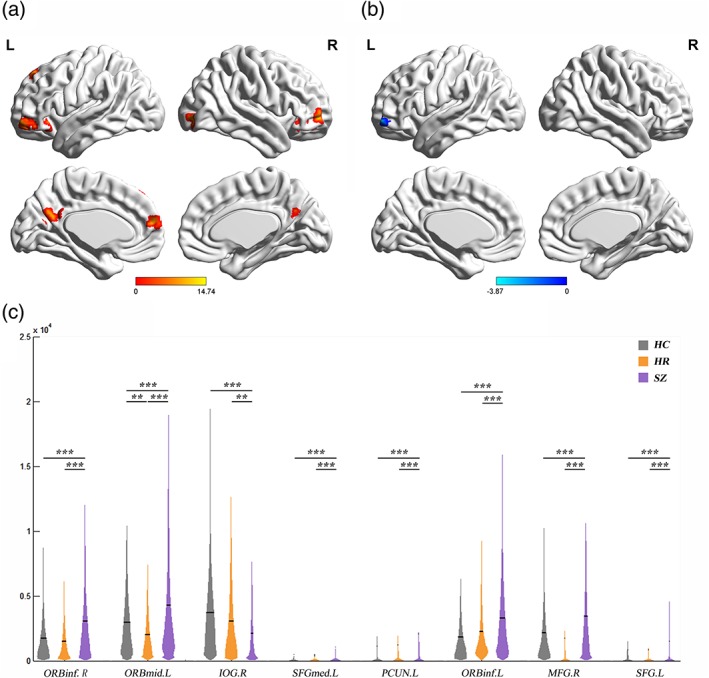
Significant difference of medium/long‐range distance strength among SZ, HR, and HCs. (a) Regions with significant group effects on medium/long‐range distance strength (Bin 8, 70–80 mm). Significance level was set as p < 0.001 at voxel level with Gaussian random field correction for multiple comparisons. (b, c) Post hoc analysis of Bin 8. (b) Regions showing significant difference of Bin 8 in the HR and HC. Significance level was set as p < 0.01 at voxel level with Gaussian random field correction for multiple comparisons. (c) Distance strength values of Bin 8 in regions showing significant differences among the participants with SZ, HR, and HCs. The violin plots represent the distribution of distance strength values in each group and the solid lines indicate the mean value. Significance level was set as p < 0.05 with false discovery rate correction for multiple comparisons. ***, p < 0.001; **, p < 0.01; *, p < 0.05. B = bilateral; HC = healthy control; HR = high risk; IOG = inferior occipital gyrus; L = left; MFG = middle frontal gyrus; ORBinf = inferior frontal gyrus, orbital part; ORBmid = middle frontal gyrus, orbital part; PCUN = precuneus; R = right; SFG = superior frontal gyrus; SFGmed = medial superior frontal gyrus; SZ = schizophrenia
3.4.2. SZ‐specific changes
SZ had specific significantly lower short‐range distance strength in the left middle and inferior occipital gyrus, left lingual gyrus, bilateral calcarine cortex, bilateral cuneus, bilateral superior occipital gyrus, right middle and inferior occipital gyrus, right middle and inferior temporal gyrus, right superior temporal gyrus, bilateral thalamus, and right postcentral gyrus, compared to HC (Figure 3c). The SZ group also had significantly increased distance strength of medium/long‐range connections in the PFC and the left precuneus, and significant decreased distance strength in the right inferior occipital gyrus, compared to the HC group (Figure 4c).
3.4.3. Similarities between SZ and HR
Post hoc analysis revealed that compared with HC, both SZ and HR groups had significantly lower short‐range distance strength in right lingual gyrus and left postcentral gyrus (Figure 3b,c). There were discrepant results between two‐sample t test (HR vs. HC) of distance strength maps and extracted values in right lingual gyrus. This may be due to the cluster including right lingual gyrus was too large in the ancova analysis.
3.4.4. Differences between SZ and HR
Compared with HC, SZ and HR had opposing changes in medium/long‐range distance strength in left orbital frontal cortex (OFC). The HR group had significantly lower medium/long‐range distance strength, compared to HC (Figure 4b,c).
3.4.5. HR‐specific changes
There was no HR‐specific change. There was no significant interaction between diagnosis and age on the distance strength.
The details of the effects of age, education, clinical, or cognitive variables on distance strength can be found in Appendix S1, Supporting Information.
4. DISCUSSION
To the best of our knowledge, this is the first study of the functional brain network in SZ and HR using a voxel‐wise approach. In this study, we observed altered balance between network segregation and integration in SZ with relatively preserved balance in HR. We found significant group differences in short‐range connections (0–30 mm) and long‐range connections (40–80 mm). We also found significant differences in short‐range distance strength primarily in the primary sensory, motor, and their association cortices, as well as the thalamus and in medium/long‐range distance strength primarily in the PFC in the three‐group comparison. Specific to SZ, we found decreased short‐range distance strength and increased medium/long‐range distance strength. Furthermore, we observed similarities and differences between SZ and HR. SZ and HR were similar in of short‐range distance strength in right lingual and left postcentral gyrus, and different in medium/long‐range distance strength in left OFC.
Decreased short‐range distance strength found herein suggests diminished segregation of neural processing in the primary sensory, motor, and their association cortices and thalamus in SZ, and increased medium/long‐range distance strength suggests over integration in connectivity involving the PFC in SZ. In addition, decreased short‐range distance strength in HR indicate subtly diminished network segregation, while decreased medium/long‐range distance strength may reflect decreased network integration. Potentially, the decreased medium/long‐range distance strength may attenuate the effect of decreased short‐range distance strength on the whole brain and lead to overall preserved balance between network segregation and integration.
The findings in this study are consistent with previous studies in SZ (Alexander‐Bloch et al., 2013; Rubinov et al., 2009), although there are discrepancies. Guo et al. (2014) found significantly increased strength of short‐range connections in SZ and their siblings. For strength of long‐range connections, they observed significant decreases in SZ and increases in siblings of SZ. Inconsistencies between this study and Guo et al. may relate to methodological differences that confound comparison between the two studies. First, negative connections, anticorrelations, were retained in Guo et al. but not in this study. Multiple modeling studies have shown that “artefactual” anticorrelations that were not originally present in the modeled data could be introduce after global signal regression (Anderson et al., 2011; Saad et al., 2011). Furthermore, there is less neurophysiological understanding for negative connections than positive ones (Rubinov & Sporns, 2010). In this study, we removed negative connections from the network to avoid interference (Murphy & Fox, 2016). Second, Guo et al. divided short‐ and long‐range connections into two categories based on median distance with a threshold of 75 mm. We divided connections into 18 distance bins ranging from 0–10 mm to 170–180 mm in 10 mm increments. We classified connections with distance of 0–30 mm as short and 40–80 mm as medium/long; in Guo et al, these would almost all be classified as short. Third, Guo et al. used a template‐driven method to parcellate the brain into 90 regions of interest, whereas a voxel‐wise approach was used in the present study to minimize parcellation‐dependent effects on network analyses (de Reus & van den Heuvel, 2013; van den Heuvel, Stam, Boersma, & Hulshoff Pol, 2008). Finally, Guo et al. computed mean strength of short‐ and long‐range connections within the whole brain or specific network, which reflects the overall changes in whole brain or specific networks. In this study, the distance strength was calculated for each node, which reflects more refined changes in the brain. In addition, Guo et al. localized single connections with group‐specific abnormalities, whereas this study localized specific regions with altered distance‐dependent network connectivity. There were also some clinical differenced. In the study of Guo et al., the participants were older, and had higher rates of medication, a less marked group difference in education as well.
Alterations specific to SZ observed in this study may indicate disease‐related functional network impairments. The thalamus is a critical hub for multiple brain pathways implicated in processing of sensorial, visual, and motor inputs (Pergola, Selvaggi, Trizio, Bertolino, & Blasi, 2015; Starke, Ball, Heinze, & Noesselt, 2017). The PFC is the key cortical region supporting human high‐order cognitive function (Amodio & Frith, 2006; Ramnani & Owen, 2004) and have function in the integration of sensory information from different modalities (Kolb et al., 2012). Diminished segregation in the primary sensory and their association cortices and thalamus may cause abnormal sensory input to the PFC, and overintegration in the PFC could lead to impaired information integration resulting in abnormal output. Furthermore, Kolb et al. (2012) implicate cortical output as components of feedback loops within the brain, suggesting that altered outputs of the motor cortex may also feedback onto sensory processing.
The similarities between SZ and HR found herein may implicate genetic susceptibility to SZ involving right lingual gyrus and left postcentral gyrus. Lin et al. (2017) identified the lingual gyrus as a genetic risk region in SZ. The postcentral gyrus consists of the primary somatosensory cortex, and previous studies have found functional changes in the postcentral gyrus in early onset SZ and HR (Jiang et al., 2015; Liu et al., 2017; Tang et al., 2015). We consider these changes in short‐range connections as vulnerability to SZ that are not sufficient for disease expression. Neurological soft signs, which typically precede disease onset, have been associated with structural and functional abnormalities of primary cortex (Dazzan et al., 2004; Zhao et al., 2014). We speculate that changes in the postcentral gyrus are associated with neurological soft signs in HR; however, we did not collect data to examine this relationship in the study.
The differences between SZ and HR involving the OFC could reflect resiliency to SZ. In contrast to SZ, HR had decreased medium/long‐range distance strength in the left OFC that were even lower than HC. Diminished integration of the PFC could reduce the effect of inaccurate input due to subtly impaired segregation. Diminished integration of the PFC in HR may represent an adaptive response to diminished segregation of the primary cortex to maintain relative balance of network segregation and integration.
While not specifically examined in this study, the present results may be causally linked to the reduction of glutamatergic neurotransmission (Goff & Coyle, 2001) and reduced GABA in the PFC (Gonzalez‐Burgos & Lewis, 2008; Lewis, Hashimoto, & Volk, 2005) reported in SZ. Dynamic changes of network characteristics (Barthélemy, 2011; Ghisleni et al., 2015) appeared to be dependent on neurotransmitter milieu. Neurotransmitters, which include inhibitory and excitatory ones, are mainly GABA or glutamate (Glu)‐ergic, respectively. It has been shown that Glu mediates the reduction of short‐range connections within subcortical regions including the thalamus, whereas GABA or GABA‐dopamine interaction may shape long‐range connections in the frontal lobe (Ghisleni et al., 2015). The resultant normative brain architecture is a balance between short‐ and long‐range connections, with predominant short‐range connections within the primary sensory and motor cortical areas, and subcortical regions such as thalamus, and predominant long‐range connections in heteromodal cortices, consistent with our findings (Sepulcre et al., 2010). These characteristics suggest that there is an optimal balance between network segregation and integration for information processing (Glausier & Lewis, 2013). Further studies are needed to confirm these postulations.
There were several limitations in this study. First, there was a relatively wide age range in the sample (13–45 years). Thus, developmental influences may confound our findings. However, we performed analysis to test for interactions between age and diagnosis and found no significant interaction between diagnosis and age on the distance strength. In addition, there were no significant differences in age among the SZ, HR, and HC groups. Second, there may be confounding effects of medications in the study (Ho, 2011). Future studies in medication naïve patients are needed to clarify these issues. Third, matching of the samples among groups was not very well. Sex, handedness, and education level showed significant differences across groups. However, we have performed analysis to test for interactions between sex and diagnosis, handedness and diagnosis, and education level and diagnosis. And we found there was no significant interaction between gender and diagnosis and between handedness and diagnosis on distance strength. There was also no significant interaction effect of diagnosis × education level on distance strength after FDR corrected. Furthermore, a moderate sample size might mitigate these issues and future studies with matched groups are needed to clarify these issues. Finally, the study was cross sectional. Longitudinal studies of HR individuals are needed to monitor brain alteration in relation to SZ onset to identify vulnerability versus resiliency markers in SZ development.
Specifically, education has important influence on brain function. The educational differences between groups may confound our findings. However, we found that regions of significantly alter distance strength did not show significant correlation with education in each group, and regions did not show significant interaction effects after FDR correction. Furthermore, means of distance strength values in HR and SZ groups stratified by low and high education level had similar trends as the combined groups when compared to HC. As we know, education do has the effect on brain function, and mainly improved cognitive function. In a study by Arenaza‐Urquijo et al. (2013), education mainly correlated to functional connectivity within the limbic system and heteromodal cortical areas (angular gyrus), which was itself associated with improved cognitive performance. However, we mainly found decreased short‐range connectivity in the primary and their association cortices, and increased medium/long‐range connectivity in the PFCs in SZ, suggesting these alterations largely relate to the disorder. Regions affected by education of brain function might be inconsistent with regions affected by disease, and we think that the effect of education was relatively small on our findings.
5. CONCLUSION
In summary, the present study found impaired balance between network segregation and integration in SZ with relative preservation in HR. We found significant differences of connectivity distance in multiple brain regions among the SZ, HR, and HC groups. We also found similarities and differences between SZ and HR as well as alterations specific to SZ. These findings may reflect to dynamic changes in network segregation within primary cortices as well as integration within the PFC and indicate disease, vulnerability, and resiliency markers in SZ. Identification of such markers could improve prediction of psychotic conversion in HR individuals and contribute to the development of more effective diagnosis and early intervention in SZ.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
Supporting information
Figure S1. Distance strength values in low (≤12 years, HC=59, HR=51, SZ=106) and high (>12 years, HC=151, HR=38, SZ=31) education groups.
ACKNOWLEDGMENTS
The authors were supported by research grants from the National Natural Science Foundation of China Funding support: National Science Fund for Distinguished Young Scholars (81725005 to F.W.), National Natural Science Foundation of China (81571311, 81071099, and 81271499 to Y.T., and 81571331 to F.W.), Liaoning Education Foundation (Pandeng Scholar, F.W.), National Key Research and Development Program (2016YFC0904300 to F.W.), National Key Research and Development Program (2016YFC1306900 to Y.T.), National High Tech Development Plan (863) (2015AA020513 to F.W.). The authors gratefully acknowledge the support of NVIDIA Corporation with the donation of the Tesla K40 GPU used for this research.
Duan J, Xia M, Womer FY, et al. Dynamic changes of functional segregation and integration in vulnerability and resilience to schizophrenia. Hum Brain Mapp. 2019;40:2200–2211. 10.1002/hbm.24518
Funding information Liaoning Education Foundation, Grant/Award Number: Pandeng Scholar; National High Tech Development Plan (863), Grant/Award Number: 2015AA020513; National Key Research and Development Program, Grant/Award Number: 2016YFC0904300, 2016YFC1306900; National Natural Science Foundation of China, Grant/Award Number: 81071099, 81271499, 81571311, 81571331; National Science Fund for Distinguished Young Scholars, Grant/Award Number: 81725005
Contributor Information
Yanqing Tang, Email: yanqingtang@163.com.
Fei Wang, Email: fei.wang@cmu.edu.cn.
REFERENCES
- Aerts, H. , Fias, W. , Caeyenberghs, K. , & Marinazzo, D. (2016a). Brain networks under attack: Robustness properties and the impact of lesions. Brain: A Journal of Neurology, 139, aww194. [DOI] [PubMed] [Google Scholar]
- Aerts, H. , Fias, W. , Caeyenberghs, K. , & Marinazzo, D. (2016b). Brain networks under attack: Robustness properties and the impact of lesions. Brain, 139, 3063–3083. 10.1093/brain/aww194 [DOI] [PubMed] [Google Scholar]
- Alexander‐Bloch, A. F. , Vertes, P. E. , Stidd, R. , Lalonde, F. , Clasen, L. , Rapoport, J. , … Gogtay, N. (2013). The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cerebral Cortex, 23, 127–138. 10.1093/cercor/bhr388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio, D. , & Frith, C. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–277. [DOI] [PubMed] [Google Scholar]
- Anderson, J. S. , Druzgal, T. J. , Lopezlarson, M. , Jeong, E. K. , Desai, K. , & Yurgeluntodd, D. (2011). Network anticorrelations, global regression, and phase‐shifted soft tissue correction. Human Brain Mapping, 32, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic, A. , Tang, Y. , Cho, Y. T. , Repovs, G. , Cole, M. W. , Savic, A. , … Xu, K. (2014). Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophrenia Bulletin, 40, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza‐Urquijo, E. M. , Landeau, B. , Joie, R. L. , Mevel, K. , Mézenge, F. , Perrotin, A. , … Chételat, G. (2013). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage, 83, 450–457. [DOI] [PubMed] [Google Scholar]
- Barthélemy, M. (2011). Spatial networks. Physics Reports, 499, 1–101. [Google Scholar]
- Biane, J. S. , Scanziani, M. , Tuszynski, M. H. , & Conner, J. M. (2015). Motor cortex maturation is associated with reductions in recurrent connectivity among functional subpopulations and increases in intrinsic excitability. Journal of Neuroscience, 35, 4719–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. , Womer, F. Y. , Bai, C. , Zhou, Q. , Wei, S. , Jiang, X. , … Wang, F. (2016). Voxel‐based morphometry in individuals at genetic high risk for schizophrenia and patients with schizophrenia during their first episode of psychosis. PLoS One, 11, e0163749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzan, P. , Morgan, K. D. , Orr, K. G. , Hutchinson, G. , Chitnis, X. , Suckling, J. , … Mallett, R. M. (2004). The structural brain correlates of neurological soft signs in AESOP first‐episode psychoses study. Brain: A Journal of Neurology, 127, 143–153. [DOI] [PubMed] [Google Scholar]
- de Reus, M. A. , & van den Heuvel, M. P. (2013). The parcellation‐based connectome: Limitations and extensions. Neuroimage, 80, 397–404. 10.1016/j.neuroimage.2013.03.053 [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Nardos, B. , Cohen, A. L. , Fair, D. A. , Power, J. D. , Church, J. A. , … Lessov‐Schlaggar, C. N. (2010). Prediction of individual brain maturity using fMRI. Science, 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Cohen, A. L. , Power, J. D. , Dosenbach, N. U. , Church, J. A. , Miezin, F. M. , … Petersen, S. E. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5, e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Dosenbach, N. U. , Church, J. A. , Cohen, A. L. , Brahmbhatt, S. , Miezin, F. M. , … Schlaggar, B. L. (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America, 104, 13507–13512.17679691 [Google Scholar]
- Fornito, A. , Zalesky, A. , & Breakspear, M. (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16, 159–172. 10.1038/nrn3901 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. (2009). Modalities, modes, and models in functional neuroimaging. Science, 326, 399–403. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Ghisleni, C. , Bollmann, S. , Poil, S. S. , Brandeis, D. , Martin, E. , Michels, L. , … Klaver, P. (2015). Subcortical glutamate mediates the reduction of short‐range functional connectivity with age in a developmental cohort. Journal of Neuroscience, 35, 8433–8441. 10.1523/jneurosci.4375-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier, J. R. , & Lewis, D. A. (2013). Dendritic spine pathology in schizophrenia. Neuroscience, 251, 90–107. 10.1016/j.neuroscience.2012.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, D. C. , & Coyle, J. T. (2001). The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry, 158, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Burgos, G. , & Lewis, D. A. (2008). GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin, 34, 944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S. , Palaniyappan, L. , Yang, B. , Liu, Z. , Xue, Z. , & Feng, J. (2014). Anatomical distance affects functional connectivity in patients with schizophrenia and their siblings. Schizophrenia Bulletin, 40, 449–459. 10.1093/schbul/sbt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, B. C. (2011). Long‐term antipsychotic treatment and brain volumes. Archives of General Psychiatry, 68, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, T. R. (2010). Rethinking schizophrenia. Nature, 468, 187–193. 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- Jiang, L. , Xu, Y. , Zhu, X. T. , Yang, Z. , Li, H. J. , & Zuo, X. N. (2015). Local‐to‐remote cortical connectivity in early‐ and adulthood‐onset schizophrenia. Translational Psychiatry, 5, e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, B. , Mychasiuk, R. , Muhammad, A. , Li, Y. , Frost, D. O. , & Gibb, R. (2012). Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 109, 17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, D. A. , Hashimoto, T. , & Volk, D. W. (2005). Cortical inhibitory neurons and schizophrenia. Nature Reviews. Neuroscience, 6, 312–324. [DOI] [PubMed] [Google Scholar]
- Li, X. , Xia, S. , Bertisch, H. C. , Branch, C. A. , & Delisi, L. E. (2012). Unique topology of language processing brain network: A systems‐level biomarker of schizophrenia. Schizophrenia Research, 141, 128–136. 10.1016/j.schres.2012.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, D. , Chen, J. , Ehrlich, S. , Bustillo, J. R. , Perrone‐Bizzozero, N. , Walton, E. , … Du, Y. (2017). Cross‐tissue exploration of genetic and epigenetic effects on brain gray matter in schizophrenia. Schizophrenia Bulletin 44(2), 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , Xue, Z. , Palaniyappan, L. , Zhou, L. , Liu, H. , Qi, C. , … Chen, X. (2016). Abnormally increased and incoherent resting‐state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophrenia Research, 171, 158–165. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Liang, M. , Zhou, Y. , He, Y. , Hao, Y. , Song, M. , … Jiang, T. (2008). Disrupted small‐world networks in schizophrenia. Brain, 131, 945–961. 10.1093/brain/awn018 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Zhang, Y. , Lv, L. , Wu, R. , Zhao, J. , & Guo, W. (2017). Abnormal neural activity as a potential biomarker for drug‐naive first‐episode adolescent‐onset schizophrenia with coherence regional homogeneity and support vector machine analyses. Schizophrenia Research 192, 408–415. [DOI] [PubMed] [Google Scholar]
- Lo, C. Y. , Su, T. W. , Huang, C. C. , Hung, C. C. , Chen, W. L. , Lan, T. H. , … Bullmore, E. T. (2015). Randomization and resilience of brain functional networks as systems‐level endophenotypes of schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 112, 9123–9128. 10.1073/pnas.1502052112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall, M. E. , Bassett, D. S. , Kerwin, R. , Mckenna, P. J. , Kitzbichler, M. , Muller, U. , & Bullmore, E. (2010). Functional connectivity and brain networks in schizophrenia. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K. , & Fox, M. D. (2016). Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage, 154, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola, G. , Selvaggi, P. , Trizio, S. , Bertolino, A. , & Blasi, G. (2015). The role of the thalamus in schizophrenia from a neuroimaging perspective. Neuroscience and Biobehavioral Reviews, 54, 57–75. [DOI] [PubMed] [Google Scholar]
- Ramnani, N. , & Owen, A. M. (2004). Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience, 5, 184–194. [DOI] [PubMed] [Google Scholar]
- Rubinov, M. , Knock, S. A. , Stam, C. J. , Micheloyannis, S. , Harris, A. W. , Williams, L. M. , & Breakspear, M. (2009). Small‐world properties of nonlinear brain activity in schizophrenia. Human Brain Mapping, 30, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Saad, Z. S. , Gotts, S. J. , Murphy, K. , Chen, G. , Jo, H. J. , Martin, A. , & Cox, R. W. (2011). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre, J. , Liu, H. , Talukdar, T. , Martincorena, I. , Yeo, B. T. , & Buckner, R. L. (2010). The organization of local and distant functional connectivity in the human brain. PLoS Computational Biology, 6, e1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, F. , Yap, P.‐T. , Gao, W. , Lin, W. , Gilmore, J. H. , & Shen, D. (2012). Altered structural connectivity in neonates at genetic risk for schizophrenia: A combined study using morphological and white matter networks. NeuroImage, 62, 1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke, J. , Ball, F. , Heinze, H. J. , & Noesselt, T. (2017). The spatio‐temporal profile of multisensory integration. European Journal of Neuroscience. Epub ahead of print. 10.1111/ejn.13753 [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Chen, K. , Zhou, Y. , Liu, J. , Wang, Y. , Driesen, N. , … Kong, L. (2015). Neural activity changes in unaffected children of patients with schizophrenia: A resting‐state fMRI study. Schizophrenia Research, 168, 360–365. [DOI] [PubMed] [Google Scholar]
- Toga, A. W. , Thompson, P. M. , & Sowell, E. R. (2006). Mapping brain maturation. Focus, 4, 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi, G. , Edelman, G. M. , & Sporns, O. (1998). Complexity and coherency: Integrating information in the brain. Trends in Cognitive Sciences, 2, 474–484. [DOI] [PubMed] [Google Scholar]
- Tsuang, M. (2000). Schizophrenia: genes and environment. Biological Psychiatry, 47, 210–220. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Stam, C. J. , Boersma, M. , & Hulshoff Pol, H. E. (2008). Small‐world and scale‐free organization of voxel‐based resting‐state functional connectivity in the human brain. NeuroImage, 43, 528–539. 10.1016/j.neuroimage.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, L. , Zang, Y. , Yang, H. , Tang, H. , Gong, Q. , … He, Y. (2009). Parcellation‐dependent small‐world brain functional networks: A resting‐state fMRI study. Human Brain Mapping, 30, 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Du, H. , Xia, M. , Ren, L. , Xu, M. , Xie, T. , … He, Y. (2013). A hybrid CPU‐GPU accelerated framework for fast mapping of high‐resolution human brain connectome. PLoS One, 8, e62789 10.1371/journal.pone.0062789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhong, S. , Jia, Y. , Sun, Y. , Wang, B. , Liu, T. , … Huang, L. (2016). Disrupted resting‐state functional connectivity in nonmedicated bipolar disorder. Radiology, 280, 529–536. [DOI] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & Yong, H. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , & Zang, Y. F. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. , Sui, J. , Kiehl, K. A. , Pearlson, G. , & Calhoun, V. D. (2013). State‐related functional integration and functional segregation brain networks in schizophrenia. Schizophrenia Research, 150, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Li, Z. , Huang, J. , Yan, C. , Dazzan, P. , Pantelis, C. , … Chan, R. C. (2014). Neurological soft signs are not "soft" in brain structure and functional networks: Evidence from ALE meta‐analysis. Schizophrenia Bulletin, 40, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distance strength values in low (≤12 years, HC=59, HR=51, SZ=106) and high (>12 years, HC=151, HR=38, SZ=31) education groups.


