Abstract
The knowledge of the size of our own body parts is essential for accurately moving in space and efficiently interact with objects. A distorted perceptual representation of the body size often represents a core diagnostic criterion for some psychopathological conditions. The metric representation of the body was shown to depend on somatosensory afferences: local deafferentation indeed causes a perceptual distortion of the size of the anesthetized body part. A specular effect can be induced by altering the cortical map of body parts in the primary somatosensory cortex. Indeed, the present study demonstrates, in healthy adult participants, that repetitive Transcranial Magnetic Stimulation to the somatosensory cortical map of the hand in both hemispheres causes a perceptual distortion (i.e., an overestimation) of the size of the participants' own hand (Experiments 1–3), which does not involve other body parts (i.e., the foot, Experiment 2). Instead, the stimulation of the inferior parietal lobule of both hemispheres does not affect the perception of the own body size (Experiment 4). These results highlight the role of the primary somatosensory cortex in the building up and updating of the metric of body parts: somatosensory cortical activity not only shapes our somatosensation, it also affects how we perceive the dimension of our body.
Keywords: body representation, body size, inferior parietal lobule, somatosensory cortex, TMS
1. INTRODUCTION
Neuropsychological evidence suggests the existence of a plethora of conscious and unconscious body representations in the human brain (Berlucchi & Aglioti, 1997, 2010; De Vignemont, 2010; Gallagher, 2005; Haggard & Wolpert, 2005; Pitron & De Vignemont, 2017; Vallar & Rode, 2009). These different mental body representations (MBRs; Miller, Longo, & Saygin, 2016) include the knowledge of the shape and size of body parts, their location in space, and their integration into a representation of the whole body.
The primary somatosensory cortex (S1) is a main neural node of the cortical network representing the body, as acknowledged since the pioneering studies of Penfield and Rasmussen (1950). The somatosensory homunculus appears as a straightforward depiction of the way in which body parts are represented at the cortical level (Harding‐Forrester & Feldman, 2018). As argued by Longo (2015), body representations underlying somatosensory cortical processing are intrinsically related to the representation of body size and shape, sometimes mirroring the distortions that feature the somatosensory homunculus.
The link between somatosensation and body image is well‐exemplified by the occurrence of perceptual distortions of the human body size that can be produced by local anesthesia or cutaneous stimulation (Gandevia & Phegan, 1999): in healthy individuals, the reduction of afferent inputs, induced by peripheral nerve block or local anesthesia, changes the perceived size of the anesthetized body part; a complementary, although less reliable, effect can be induced by repetitive cutaneous stimulation (Gandevia & Phegan, 1999). In line with the somatotopic organization of the contralateral body surface in S1, the effect of the deafferentation is body‐part specific: following the anesthesia of the right thumb, the visual representation of its size is perceived as enlarged, as well as that of the lips, neighboring the representation of the thumb in the somatosensory homunculus. Instead, the size of the index fingers of both hands, and that of the left thumb, are unaffected by anesthesia of the right thumb.
This evidence suggests that tactile inputs contribute to the building up and updating of the internal representations of one's own body, including the visual appreciation of its size (Serino & Haggard, 2010; Tamé, Braun, Holmes, Farnè, & Pavani, 2016; Vallar & Rode, 2009); this occurs notwithstanding the absence of peripheral receptors directly coding the size and shape of body parts (Harding‐Forrester & Feldman, 2018; Kaas, Qi, & Stepniewska, 2018). Hence, the amount of tactile information transmitted from the body to the cortex can directly affect MBRs (Serino & Haggard, 2010). If this is the case, the opposite might also occur: alterations or dysfunctions of the somatosensory cortical maps should influence the metric representation of body parts. However, this hypothesis still needs empirical demonstration.
To address this issue, we performed a series of experiments, aimed at modulating the representation of a body part in S1, namely the hand, by using repetitive transcranial magnetic stimulation (rTMS; Bolognini & Miniussi, 2018), to assess whether and how short‐term, reversible, changes at the level of the central somatosensory maps could alter the perceptual representation of the size of the own hand. We also assessed the selectivity of the effect with respect to the somatotopy of the cortical representation of body parts (Experiments 1 and 2), and the existence of a hemispheric specialization (Experiment 3). Finally, in the last experiment (Experiment 4) the selectivity of the contribution of S1 to the representation of body size was assessed by interfering with the activity of the inferior parietal lobules (IPL) (Caspers & Zilles, 2018).
We choose to compare the effects of interfering with the activity of S1 and of the IPL since the IPL, of both the left and the right hemispheres, is involved in the multisensory representation of the body (Bolognini & Maravita, 2011; Maravita, Spence, & Driver, 2003), as well as in the so‐called “superficial schema,” which mediates the localization of somatic sensations on the body surface (Buxbaum & Coslett, 2001; Felician et al., 2009; Head & Holmes, 1911; Longo & Haggard, 2010; Serino & Haggard, 2010; Vallar & Papagno, 2003). Therefore, the stimulation of the IPL would allow verifying whether the perceptual representation of the size of the own body parts also relies on non‐primary somatosensory higher‐order posterior parietal cortices, which may work in concert with the lower‐level processing of S1.
2. EXPERIMENT 1—MODULATION OF METRIC REPRESENTATION OF THE BODY BY RIGHT S1 rTMS
In this experiment, rTMS was applied over the hand representation of S1 of the right hemisphere; perceptual distortions of the size of the contralateral (left) and of the ipsilateral (right) hands were assessed with a two‐forced choice visual task. The participants's task was to report whether a picture showing their own hand, whose dimension varied, being bigger or smaller than the real one, matched the “size” of their own hand, as actually felt.
2.1. Materials and methods
2.1.1. Participants
Twenty neurologically healthy participants (12 females; 18 right‐handed; mean age ± SD = 24.3 ± 2.9 years; range = 20–33 years) participated in Experiment 1. All participants had normal or corrected‐to‐normal vision. Handedness was assessed by a standard questionnaire (Oldfield, 1971). Participants were naïve both to the experimental procedure and to the purpose of the study. They gave their written informed consent to take part in the study, which was approved by the local Ethical Committee of the University of Milano‐Bicocca, and conducted in line with the Declaration of Helsinki. Exclusion criteria included history of neurological and psychiatric disorders, and contraindications to TMS (Rossi, Hallett, Rossini, & Pascual‐Leone, 2009) and were assessed with a questionnaire before the first experimental session.
2.1.2. Hand size task
The hand size task (HST) was a two‐forced‐choice task developed to assess the perceptual estimation of participants' own hand size. In a dimly‐illuminated room, participants comfortably sat in an armchair in front of the PC screen at a distance equal to their forearm. Stimuli were colored pictures of the participants' left and right hands, seen from the egocentric perspective, taken with a digital camera (ASUS Go 5” HD) before administering the task. In order to prevent shape distortions, each hand picture was acquired by using a wooden box (length = 60 cm, height = 20 cm, width = 30 cm), placing the digital camera above the upper side of the box (open to the view), in the same position for each participant. Thus, pictures were taken at the same distance (i.e., 20 cm) for all participants, with the same zoom settings. Then, each photograph was scaled by using the GIMP software, so that the experimental stimulus was of the real size of the participant's hand (i.e., same size trials, 0% of change respect to the participant's hand size), or could be 3, 6, 9, 12, 15, 18% smaller (−) or bigger (+) (i.e., different size trials) than the participant's real hands, for a total of 13 hand dimensions (see Figure 1a). The different hand dimensions (i.e., smaller or bigger than the real size) were online created by E‐Prime Software (Psychology Software Tools Inc., Pittsburgh, PA), trial‐by trial during the task. Each hand stimulus was presented for 1,500 ms, followed by a central fixation (white cross) presented on a black screen (see Figure 1b for the experimental timeline). The participants' task was to indicate whether the viewed hand matched («Same» response) or not («Different» response) the size of their own hand; participants were instructed to give their response, as accurately and fastly as possible, by pressing the right buttom of the PC mouse (using their right hand, the ones ipsilateral to the rTMS side) if they judged the viewed hand as of the same size of their own hand, or the left button of the PC mouse if they considered the seen hand of a different size. At variance with previous studies using a similar task (e.g., Gandevia & Phegan, 1999; Longo & Haggard, 2012a, 2012b; Longo, Long, & Haggard, 2012), participants had a limited time for responding: this variation was introduced in order to force a “first‐hand” judgment, hence limiting the chance of adopting more cognitive strategies, and as well in consideration of the short‐living after effects of the rTMS (about 15‐min following a 15 min train of rTMS at 1‐Hz; see e.g., Bolognini & Miniussi, 2018; Chen, Friedman, & Roe, 2003; Knecht, Ellger, Breitenstein, Bernd Ringelstein, & Henningsen, 2003).
Figure 1.
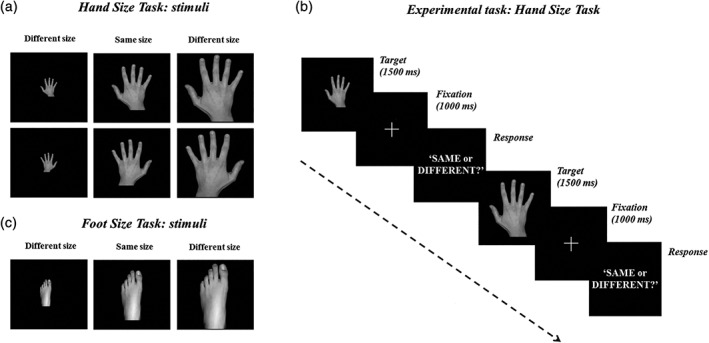
Experiment 1. (a) Hand size task—HST. Stimuli were pictures of the participant's left and right hands with different sizes, with respect to the participant's individual hand: 0% (same size trial), smaller or bigger by 3, 6, 9, 12, 15 or 18% (different size trial). (b) In each trial, a hand picture (target) was presented; the participants' task was to judge whether the size of the viewed hand matched («Same» response) or not («Different» response) the size of their own (out‐of‐view) hand (2 forced‐choice task). The task was performed before (baseline), and after 15 min of 1‐Hz rTMS. (c) Foot size task—FST. In the FST (Experiment 2), the participants' task was identical to that of the HST, but concerned their own foot. rTMS, repetitive Transcranial Magnetic Stimulation
Pictures of the participant's left and right hands were presented in two separate blocks (AB‐BA order, counterbalanced across participants); in both blocks, participants were instructed to focus on the felt size of their own left hand (out‐of‐view, hand contralateral to rTMS side). In each block, 16 trials were presented for each of the 13 hand sizes, for a total of 208 trials. Each block lasted ∼8 min, for a total duration of the procedure of ∼16 min. Stimuli presentation and randomization were computer controlled by the E‐Prime software (Psychology Software Tools Inc., Pittsburgh, PA), used to run the task and to record the participants' responses.
Before the experiment, a training session was performed to allow participants to familiarize with the task. During the experiment, the participants' left and right hands (as well as the PC mouse used for responding) were kept out‐of‐view, hidden under a wooden panel, in order to prevent an online size matching of the hands.
The HST was administered before (baseline) and after the application of 1‐Hz rTMS (see below).
2.1.3. TMS protocol
A Magstim Super Rapid2 transcranial magnetic stimulator (Magstim Co. Ltd, Whitland, UK) with a figure‐of‐eight‐shaped coil (Ø = 70 mm) for focal cortical stimulation was used to deliver biphasic 1‐Hz repetitive TMS (rTMS). rTMS protocol lasted for 15 min, delivering a total of 900 pulses. The TMS intensity was set at 110% of the individual resting motor threshold (rMT, mean = 51 ± 6.52%, range = 42–67% of the maximal stimulator output), defined as the minimum intensity of the TMS stimulator able to elicit five out of ten detectable motor twitches in the contralateral hand (Rossi et al., 2009). The rMT was assessed targeting the optimal scalp position for inducing, with the lowest stimulation intensity, motor twitches in the left hand, by targeting the right primary motor cortex (M1) with single TMS pulses.
In Experiment 1, the coil was positioned over the S1 hand map in the right hemisphere. We firstly used an anatomical procedure to localize the hand area in S1, placing the coil 2 cm backward from the M1 hotspot (e.g., Avenanti, Bolognini, Maravita, & Aglioti, 2007; Bolognini, Rossetti, Convento, & Vallar, 2013; Fiorio & Haggard, 2005; Harris, Miniussi, Harris, & Diamond, 2002).
Worth mentioning, recent evidence indicating that the S1‐hand map is located ∼2 cm lateral and ∼0.5 cm posterior to the M1‐hand scalp location, at least when the index finger map is localized (Holmes et al., 2019; Holmes & Tamè, 2019; Tamè & Holmes, 2016). However, in the present study we aimed at targeting the hand in S1 (in line with the stimuli presented in the HST).
In addition to the anatomical localization approach, given the imprecision and variability of a mere anatomical localization of S1, we also used a Neuronavigation System, and functional criterion (reduction of tactile sensitivity at the hand palm). In particular, the SofTaxic Evolution navigator system (Version 1.0, http://www.emsmedical.net; see for instance, Bolognini, Rossetti, Maravita, & Miniussi, 2011) was used to reconstruct a virtual volume of each participant's brain. This software allows creating from a template an MRI image of the cerebral cortex in Talairach coordinates, by means of a warping procedure. Parameters for warping the template image were estimated on the basis of four digitized skull landmarks (i.e., nasion, inion, and the right/left preauricular points), and 50 uniformly distributed points mapped on the participant's scalp, with a mean error of 2.11 mm and a SD of 2.04 mm. Digitalization and neuronavigation were achieved via a graphic user interface and a 3D optical digitizer (NDI, Polaris Vicra). For each participant, the location of S1 was identified following the Talairach coordinates x = 47, y = −32, z = 59 on the MRI template and using a 3D virtual reconstruction of the participant's brain. The coordinates of S1 were derived from previous functional Magnetic Resonance Imaging (fMRI) studies (e.g., Boakye, Huckins, Szeverenyi, Taskey, & Hodge Jr., 2000), and had already been used in previous TMS studies targeting the S1 hand area (e.g., Bolognini et al., 2011; Bolognini, Olgiati, Rossetti, & Maravita, 2010; Bolognini, Rossetti, Fusaro, Vallar, & Miniussi, 2014; Pisoni, Romero Lauro, Vergallito, Maddaluno, & Bolognini, 2018). In previous TMS studies (see, e.g., Bolognini, Papagno, Moroni, & Maravita, 2010, Bolognini et al., 2011, Bolognini et al., 2014; Rossetti, Miniussi, Maravita, & Bolognini, 2012), the same Talairach coordinates have been shown to be associated to functional effects, including paraesthesia or induction of tactile extinction by single‐pulse TMS to S1. During the stimulation, the correct and stable position of the coil was monitored online with the same neuronavigation system, and the coil was kept tangential to the scalp, with the handle pointing laterally 45° away from the mid‐sagittal line. This neuronavigation procedure has been used in several previous studies (e.g., Bolognini et al., 2014; Carducci & Brusco, 2012; Pisoni et al., 2018; Tecchio et al., 2014).
Moreover, for each participant, the effective modulation of the S1 hand representation by 1‐Hz rTMS was further checked by using a functional method, namely by administering a 2‐point discrimination task (2PDT), to assess changes in tactile sensitivity before and after the rTMS protocol (Kennett, Taylor‐Clarke, & Haggard, 2001; Tegenthoff et al., 2005). This ensured an appropriate, functionally‐based, localization of S1 hand area.
During the 2PDT, participants were blindfolded and their left hand, contralateral to the rTMs site, was touched on the palm of left hand with 1 or with 2 points using a 2‐point discriminator (Touch Test® Two‐Point Discriminator, North Coast Medical & Rehabilitation Products). Four pairs of pins, separated by 7, 8, 9, and 10 mm, as well as a single pin representing the single touch condition, were used. Participant were required to report whether 1 or 2 pins were perceived. A total of 40 stimuli were given, 10 for each distance for a total duration of ∼5 min. The 2PDT was administered before and after 1‐Hz rTMS, along with the HST (experimental task); the order of the two tasks was randomized and counterbalanced across participants (AB‐BA). The comparison of the participants' performance at the 2PDT, showed a significant decrement of tactile sensitivity after 1‐Hz rTMS over right S1, as compared to the baseline (baseline = 80% ± .06 vs. post‐rTMS = 70% ± .14, t19 = 2.92, p = .01), confirming the effective stimulation of the somatosensory hand area in S1.
2.1.4. Statistical analyses
The participant's performance at the HST was analyzed with the statistical program R (R Development Core Team, 2008). Responses were entered as dependent binomial variable, coding the «Same» responses as 1 and the «Different» responses as 0. Data were submitted to a series of generalized mixed effects models (Baayen, Davidson, & Bates, 2008), using the “lme4” package (version 1.1–5, Bates, Maechler, Bolker, & Walker, 2015). First, we assessed if the inclusion of fixed effects or interactions contributed to the model goodness‐of‐fit. This was tested by likelihood ratio tests (LRT), including only effects, which significantly increased the model goodness‐of‐fit (Gelman & Hill, 2006). The fixed factors were Time (factorial, two levels: baseline vs. post‐rTMS), Hand Laterality (factorial, two levels: right vs. left hand), and Hand Size (from −18 to +18%, as a continuous independent variable); their interactions were also tested. A by‐subjects random intercept was included. Parameters from the final, best fitting model are reported, including factors' significance level, based on Satterthwaite's degrees of freedom approximation in the “lmerTest” R package (version 2.0–29, Kuznetsova, Brockhoff, & Christensen, 2015).
2.2. Results
The model included the main effects of Time, Hand Laterality, and linear, quadratic, and cubic effects of Hand Size, as well as the interactions: Time × Hand Size, and Hand Laterality × Hand Size (see Table 1 for results of the LRT procedure for model selection, and for the final model's parameters). The model showed significant effects of Time, and of the linear, quadratic, and cubic trends of the main factor Hand Size. Of main relevance, the Time × Hand Size interaction was significant for the linear, quadratic, and cubic (z = 2.34; p = .019) trends of the Hand Size effect (see Table 1). As shown in Figure 2, after rTMS over the right S1, participants overestimated the size of their hand, regardless of which hand was presented (left or right viewed hand) during the HST, increasing their «Same» responses to hands bigger than their real ones (Figure 2).
Table 1.
Results from statistical analysis of Experiment 1
| HST—LRT goodness‐of‐fit test | ||||
| χ2 | df | p | ||
| Time | .54 | 1 | .46 | |
| Hand Laterality | 4.22 | 1 | .04 | |
| Hand Size | 1,681.82 | 3 | <.001 | |
| Time × Hand Size | 16.61 | 3 | <.001 | |
| Time × Hand Laterality | .91 | 1 | .33 | |
| Hand Laterality × Hand Size | 8.15 | 3 | .04 | |
| Time × Hand Laterality × Hand Size | 2.53 | 3 | .46 | |
| HST—Mixed logistic regression | ||||
| B | SE | z value | p | |
| Intercept | −0.35 | .11 | −3.06 | .002 |
| Time | −0.13 | .05 | −2.57 | .01 |
| Hand Laterality | −0.04 | .05 | −0.76 | .44 |
| Hand Size (linear trend) | −0.11 | .006 | −16.48 | <.001 |
| Hand Size (quadratic trend) | −0.004 | .0003 | −12.48 | <.001 |
| Hand Size (cubic trend) | .0002 | .0001 | 7.58 | <.001 |
| Time × Hand Size (linear trend) | −0.02 | .008 | −3.21 | .001 |
| Time × Hand Size (quadratic trend) | .0007 | .0003 | 2.08 | .03 |
| Time × Hand Size (cubic trend) | .0001 | .0001 | 2.34 | .01 |
| Hand Laterality × Hand Size (linear trend) | .01 | .008 | 1.35 | .17 |
| Hand Laterality × Hand Size (quadratic trend) | −0.0001 | .0003 | −0.17 | .86 |
| Hand Laterality × Hand Size (cubic trend) | −0.0001 | .0001 | −0.14 | .88 |
Abbreviations: HST, hand size task; LRT, likelihood ratio tests.
Figure 2.
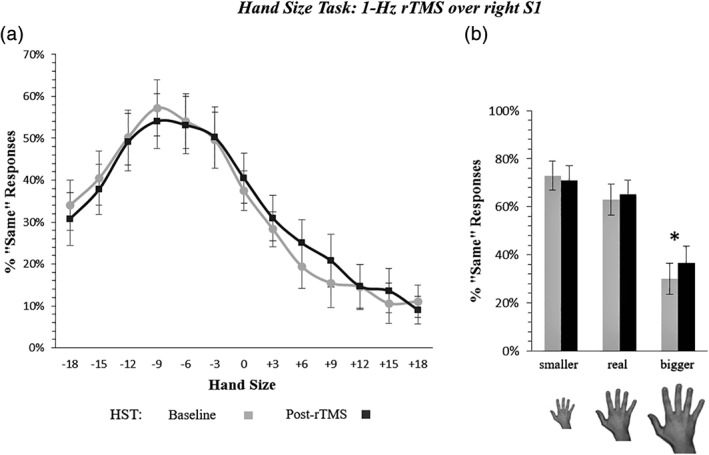
Hand size perceived change by 1‐Hz rTMS of the right S1 hand area (experiment 1). Panel (a) shows the increase of «Same» responses after right S1 rTMS in trials presenting a hand bigger than the participant's real hand, regardless of the hand laterality (Time x Hand Size interaction, p = .01). The x axis = mean percentage of the hand size change, with negative values corresponding to a reduction of the hand size, positive values to an increase; y axis = percentage of «Same» responses. Gray line = participants' performance before 1‐Hz rTMS (baseline); black line = performance after 1‐Hz rTMS (post‐rTMS). Panel (b) recaps the overestimation effect. Error bars represent the standard error of the mean (SEM); * = significant difference (p < .05) between pre‐ and post‐rTMS assessment
3. EXPERIMENT 2—SOMATOTOPIC ORGANIZATION OF BODY'S METRIC PROPERTIES IN S1
Experiment 2 investigated whether the perceptual distortion of the hand size induced by rTMS over the S1‐hand map extended to other body parts, namely the foot, or if it was specific for the body district whose cortical S1 representation was targeted. Critically, S1 cortical map of the foot lies far from the cortical map of the hand, being located in the dorso‐medial surface of S1 (Penfield & Rasmussen, 1950). The prediction was thus made that rTMS to the hand area should not affect the foot cortical somatosensory map, and hence foot size judgements.
3.1. Materials and methods
3.1.1. Participants
Twenty healthy participants (11 females; 18 right‐handed; mean age = 24.7 ± 5.1 years; range = 20–30 years), recruited using the same criteria of Experiment 1, took part in Experiment 2. One participant did not complete the experiment, and therefore was excluded from the analyses. In the final sample (N = 19), the mean individual rMT value was 54% (± 7.2%, range = 41–67%) of the maximal stimulator output.
3.1.2. Experimental paradigm and statistical analyses
Materials, methods, and statistical analyses were identical to those of Experiment 1. The only difference pertained to the experimental tasks, now including the HST with the presentation of the left hand only, and a version of it with foot stimuli (i.e., foot size task, FST). These new stimuli depicted the participants' left foot (see Figure 1c). In both tasks, the size of the stimuli ranged from −15 to +15% (again in steps of 3%; the more extreme dimensions, ±18% were not included, in order to reduce the number of trials). For both tasks, a total of 176 stimuli were given (16 for each distance). The order of the two tasks was randomized and counterbalanced across participants (AB‐BA), with half of participants starting with the HST and the other half starting with the FST.
Changes in tactile sensitivity brought about by rTMS were assessed with the 2PDT on the left hand and on the left foot, contralateral to the rTMS site: a significant decrease of the hand tactile sensitivity was found after 1‐Hz rTMS of S1 hand representation (baseline = 77.19% ± .06 vs. post‐rTMS = 66.75% ± .14, t18 = 2.83; p < .01). Instead, tactile sensitivity at the foot did not change after the stimulation of the S1‐hand map (baseline = 59.47% ± .05 vs. post‐rTMS = 57.36% ± .10, t18 = .93; p = .4).
3.2. Results
With respect to the HST, the best model included the main effects of Time and Hand Size, as well as their interaction (see Table 2 for details). Results showed significant linear, quadratic and cubic trends for the main effect of Hand Size. Furthermore, the interaction between Time, and the linear and cubic (z = 7.19; p < .001) trends for the Hand Size effect, resulted significant, as for Experiment 1. As in the previous experiment, «Same» responses increased for hand stimuli with dimension bigger than the participants' real hand (see Figure 3).
Table 2.
Results from statistical analysis of Experiment 2
| HST—LRT goodness‐of‐fit test | ||||
| χ2 | df | p | ||
| Time | 13.45 | 1 | <.001 | |
| Hand Size | 446.62 | 3 | <.001 | |
| Time × Hand Size | 58.92 | 3 | <.001 | |
| FST—LRT goodness‐of‐fit test | ||||
| χ2 | df | p | ||
| Time | .23 | 1 | .62 | |
| Foot Size | 352.57 | 3 | <.001 | |
| Time × Foot Size | 1.51 | 3 | .67 | |
| HST—Mixed logistic regression | ||||
| B | SE | z value | p | |
| Intercept | −0.11 | .14 | −0.75 | .44 |
| Time | −0.30 | .07 | −3.80 | <.001 |
| Hand Size (linear trend) | −0.75 | .09 | −7.91 | <.001 |
| Hand Size (quadratic trend) | −0.16 | .04 | −3.83 | <.001 |
| Hand Size (cubic trend) | .21 | .04 | 4.31 | <.001 |
| Time × Hand Size (linear trend) | −0.33 | .13 | −2.40 | .01 |
| Time × Hand Size (quadratic trend) | .06 | .06 | .98 | .325 |
| Time × Hand Size (cubic trend) | .21 | .03 | 7.19 | <.001 |
| FST—Mixed logistic regression | ||||
| B | SE | z value | p | |
| Intercept | −0.28 | .16 | −1.70 | .08 |
| Foot Size (linear trend) | −0.79 | .06 | −11.38 | <.001 |
| Foot Size (quadratic trend) | −0.07 | .03 | −2.32 | .02 |
| Foot Size (cubic trend) | .16 | .03 | 4.56 | <.001 |
Abbreviations: FST, foot size task; HST, hand size task; LRT, likelihood ratio tests.
Figure 3.
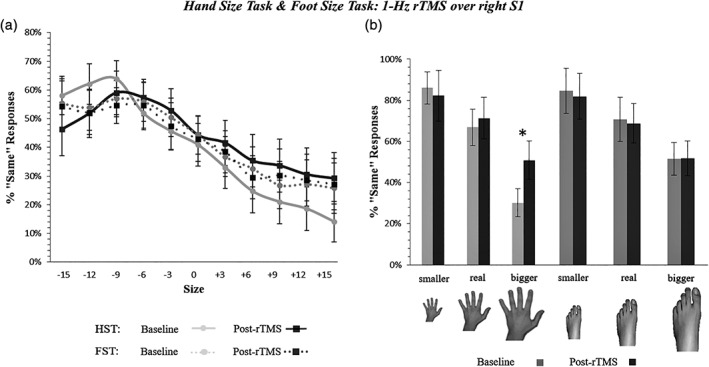
Hand and foot size perceived changes by 1‐Hz rTMS of the S1 hand area (Experiment 2). Panel (a) illustrates the increase of «Same» responses after right S1 rTMS in trials presenting a hand bigger than the participant's real hand (Time x Hand Size interaction, p = .001). Gray continuous and dotted lines = performance before 1‐Hz rTMS (baseline) at the HST and FST, respectively; black continuous and dotted lines = performance after 1‐Hz rTMS at the HST and FST, respectively. See caption of Figure 2 for details (x/y axis). Panel (b) recaps the somatotopic specificity of the overestimation effect. Error bars = SEM. FST, foot size task; HST, hand size task; *significant difference (p < .05) between pre‐ and post‐rTMS assessment
With respect to the FST, the final model included only the main effect of Foot Size, with significant linear, quadratic and cubic trends of the main effect of Foot Size. Importantly, the main effect of Time and its interactions did not reach significance (all p > .63; see Table 2 and Figure 3).
4. EXPERIMENT 3—MODULATION OF METRIC REPRESENTATION OF THE BODY BY LEFT S1 rTMS
Experiment 3 aimed at testing a possible hemispheric asymmetry of S1 in modulating the metric properties of one's own hands, by targeting the hand somatosensory map in the left hemisphere.
4.1. Materials and methods
4.1.1. Participants
Twenty healthy participants (17 females; 18 right‐handed; mean age = 22.5 ± 2.5 years; range = 19–31 years), selected using the same criteria of Experiment 1, took part in Experiment 3. In this experiment, the mean individual rMT value was 52.7% (± 6.4%, range = 43–63%) of the maximal stimulator output.
4.1.2. Experimental paradigm and statistical analyses
Stimuli, procedures, and statistical analyses were identical to Experiment 1, except for the rTMS target, that was the S1 hand representation in the left hemisphere, localized with the SofTaxic Evolution navigator system and following Talairach coordinates (x = −47, y = −32, z = 59, e.g., Boakye et al., 2000). Accordingly, the 2PDT was now delivered to the right hand. Participants performance at the 2PDT showed a significant decrement of right hand tactile sensitivity after 1‐Hz rTMS over the left S1 (baseline = 78.91% ± .78 vs. post‐rTMS = 67.25% ± .67, t19 = 3.88, p = .0009).
4.2. Results
The best model included the main effects of Time, Hand Laterality and Hand Size, as well as the Time × Hand Size and the Time × Hand Laterality interactions (see Table 3). The main effects of Time and Hand Laterality reached significance, as in Experiment 1. Additionally, the main linear, quadratic and cubic trends of the main effect of Hand Size were significant. Crucially, as in Experiment 1, the Time x Hand Size interaction was significant for the linear, quadratic, and cubic (z = 1.98; p = .046) trends of the Hand Size effect, showing effects comparable to those found in Experiment 1, by targeting the right S1: after stimulation of the left S1, participants overestimated the size of their hands, as demonstrated by the increased «Same» responses when the viewed hands was bigger than their real one (see Figure 4).
Table 3.
Results from statistical analysis of Experiment 3
| HST—LRT goodness‐of‐fit test | ||||
| χ2 | df | p | ||
| Time | 9.86 | 1 | .001 | |
| Hand Laterality | 4.21 | 1 | .039 | |
| Hand Size | 1,242.20 | 3 | <.001 | |
| Time × Hand Size | 39.09 | 3 | <.001 | |
| Time × Hand Laterality | 8.55 | 1 | .003 | |
| Hand Laterality × Hand Size | 1.99 | 3 | .57 | |
| Time × Hand Laterality × Hand Size | 4.13 | 3 | .24 | |
| HST—Mixed logistic regression | ||||
| B | SE | z value | p | |
| Intercept | .12 | .13 | .91 | .36 |
| Time | −0.22 | .05 | −4.47 | <.0001 |
| Hand Laterality | .07 | .03 | 2.05 | .04 |
| Hand Size (linear trend) | −0.05 | .005 | −11.32 | <.0001 |
| Hand Size (quadratic trend) | −0.004 | .0002 | −20.59 | <.0001 |
| Hand Size (cubic trend) | .0001 | .0001 | 5.69 | <.0001 |
| Time × Hand Size (linear trend) | −0.03 | .007 | −4.03 | <.0001 |
| Time × Hand Size (quadratic trend) | .0008 | .0003 | 2.62 | .008 |
| Time × Hand Size (cubic trend) | .0001 | .0001 | 1.98 | .04 |
Abbreviations: HST, hand size task; LRT, likelihood ratio tests.
Figure 4.
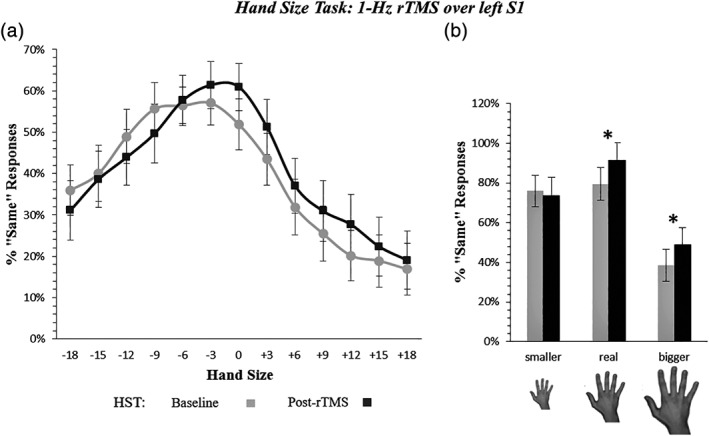
Hand size perceived change by 1‐Hz rTMS of the left S1 hand area (experiment 3). Panel (a) depicts the increase of «Same» responses after left S1 rTMS in trials presenting a hand bigger than the participant's real hand (Time x Hand Size interaction, p = .04). See caption of Figure 2 for details (x/y axis). Panel (b) recaps the overestimation effect. Error bars = SEM; *significant difference (p < .05) between pre‐ and post‐rTMS assessment
However, after stimulation of left S1, but not of right S1 (see Experiments 1 and 2), we found an increase of «Same» responses when the viewed hands mached the size of the participant's hands (i.e., same size trials = 0% of size difference), suggesting an improved recognition of their real hand size. Hence, in order to further verify a possible left–right hemispheric asymmetry of S1 for the estimation of the own hand size, a further analysis was performed, adding as a fix, between‐subjects, factor the Hemisphere over which rTMS was delivered (two levels: left S1 vs. right S1, i.e., Experiment 1 vs. 3 respectively). The LRT test showed significant main effects of Hand Size (χ2 = 3,109.6, Df = 3, p < .001), Time (χ2 = 7.5, Df = 1, p = .006), and Hemisphere (χ2 = 139.5, Df = 1, p < .001). The Hand Size × Hemisphere (χ2 = 152.6, Df = 3, p < .001), and, most importantly, the Hand Size x Time (χ2 = 49.04, Df = 3, p < .001) interactions were significant. The latter interaction effect confirms findings from the previous analyses. The lack of significant Hand Size × Time × Hemisphere (χ2 = 2.52, Df = 3, p = .47), and Time × Hemisphere (χ2 = 1.25, Df = 1, p = .26) interactions rules out the existence of significant differences between rTMS over the left or the right S1 for hand size perception. Rather, overall, participants in Experiments 3 gave more «Same» responses (main effect of Hemisphere), but the rTMS effect did not differ between left and right S1.
5. EXPERIMENT 4—MODULATION OF METRIC REPRESENTATION OF THE BODY BY rTMS OF LEFT AND RIGHT IPL
Experiment 4 aimed at verifying the additional involvement of the IPL of both hemispheres in the observed effects. Indeed, a variety of body representation disturbances occurs after parietal lesions or brain stimulations (Bolognini & Miniussi, 2018), among which distorted awareness of the size of the whole body or of body parts, as well as other forms of spatial size distortion (hyperschematia, see Vallar & Rode, 2009). Furthermore, applying rTMS to other cortical sites could prove the specificity of the effects of interfering with the activity of S1 in bringing about the effects observed in Experiments 1–3.
5.1. Materials and methods
5.1.1. Participants
Twenty healthy participants (15 females; 18 right‐handed; mean age ± SD = 22.3 ± 3.2 years; range = 19–31 years), selected using the same criteria of Experiment 1, entered Experiment 4. One participant could not perform the second rTMS session (see below) and was excluded therefore from statistical analyses. In the final sample, the mean individual rMT value was 55.8% (± 6.2%, range = 43–64%) of the maximal stimulator output for the right IPL, and 55.4% (± 7.2%, range = 42–67%) for the left IPL.
5.1.2. Experimental paradigm and statistical analyses
Stimuli, procedure, and statistical analyses were identical to those of Experiment 1, except for the rTMS target site, which in Experiment 4 was placed over either the right or the left IPL. Adopting a within‐subjects design, participants underwent two rTMS sessions, during which 1‐Hz rTMS was applied over the right or the left IPL. The two experimental sessions were separated by an interval of at least 24 hr, and their order was counterbalanced across participants (AB‐BA; i.e., half of the participants started with the rTMS session over the right IPL, the other half started with the rTMS session over left IPL). The right and left IPL were localized with the SofTaxic Evolution navigator system, following Talairach coordinates (X = ± 40, Y = 52, Z = 44, BA 40) (e.g., Bolognini, Miniussi, Savazzi, Bricolo, & Maravita, 2009). As for stimulation of the S1‐hand area, 1‐Hz rTMS was delivered for 15 min (TMS intensity = 110% of the individual rMT, 900 pulses).
With respect to the statistical analyses, the Hemisphere (right IPL vs. left IPL) was now included among the fixed factors and in interaction with the other effects.
To test possible distant influences of rTMS on S1, we administered the 2PDT. There was no change in tactile sensitivity of the left hand following rTMS of the right IPL (baseline = 80 ± .09%, vs. post‐rTMS = 77.63 ± .17, t18 = .51, p = .6), nor of the right hand following the stimulation of the left IPL (baseline = 78 ± .05%, vs. post‐rTMS = 75 ± .16%, t18 = .72, p = .5). Moreover, there was difference in tactile sensitivity neither between the two baseline sessions (t18 = .82, p = .4), nor between the two post‐TMS sessions (i.e., right vs. left IPL, t18 = .47, p = .6).
5.2. Results
The best‐fitting model for the HST included the main effects of Time, Hand Laterality, Hand Size and Hemisphere, as well as the Time x Hemisphere interaction (see Table 4). The final model showed a main effect of Time, showing an overall increase of «Same» responses after rTMS (38% ± 16.7), as compared with before the stimulation (baseline = 37% ± 13.6), but regardless of the size of the viewed hand. Moreover, we found a significant effect of the linear, quadratic, and cubic trends of Hand Size. The main effect of Hemisphere reached significance, since, in the left IPL sessions, overall participants provided less «Same» responses (36.7 ± 17.2%), than in the right IPL session (38.4 ± 17.6%). Importantly, however, no significant interactions between the factors Time and Hand Size emerged (see Table 4 and Figure 5).
Table 4.
Results from statistical analysis of Experiment 4
| HST—LRT goodness‐of‐fit test | ||||
| χ2 | df | p | ||
| Time | 4.46 | 1 | .03 | |
| Hand Laterality | 15.55 | 1 | <.0001 | |
| Hand Size | 1,559.32 | 3 | <.0001 | |
| Hemisphere | 8.83 | 1 | .002 | |
| Time × Hand Size | 6.77 | 3 | .07 | |
| Time × Hemisphere | 5.54 | 1 | .01 | |
| Hand Size × Hemisphere | 2.36 | 3 | .49 | |
| Time × Hand Size × Hemisphere | 4.97 | 3 | .17 | |
| HST—Mixed logistic regression | ||||
| B | SE | z value | p | |
| Intercept | −0.24 | .18 | −1.35 | .17 |
| Time | −0.11 | .03 | −3.09 | .002 |
| Hand Laterality | .03 | .02 | 1.44 | .14 |
| Hand Size (linear trend) | −1.43 | .03 | −41.58 | <.0001 |
| Hand Size (quadratic trend) | −0.33 | .01 | −21.66 | <.0001 |
| Hand Size (cubic trend) | .35 | .01 | 19.74 | <.0001 |
| Hemisphere | −0.13 | .03 | −3.77 | <.0001 |
| Time × Hemisphere | .09 | .05 | 1.90 | .06 |
Abbreviations: HST, hand size task; LRT, likelihood ratio tests.
Figure 5.
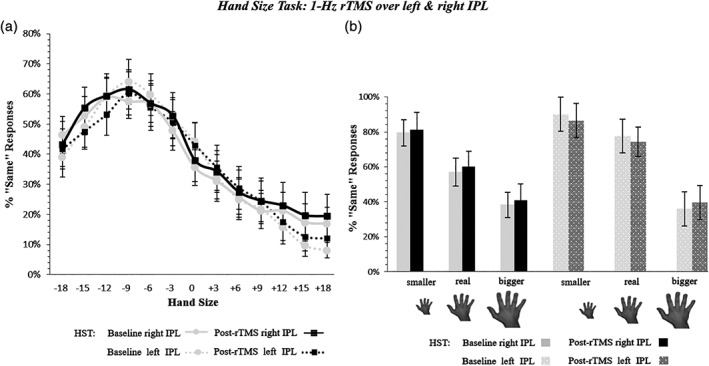
Hand size perceived change by 1‐Hz rTMS of IPL (Experiment 4). Panel (a) illustrates the results showing no change of the perceived size of the hands after the stimulation of both the left and right IPL (Time × Hand Size × Hemisphere interaction, p = .17). Gray continuous and dotted lines = performance before 1‐Hz rTMS over the right and left IPL, respectively. Black continuous and dotted lines = performance after 1‐Hz rTMS over the right and left IPL, respectively. See caption of Figure 2 for details (x/y axis). Panel (b) summarizes the behavioral effect before and after 1‐Hz rTMS. Error bars = SEM. IPL, inferior parietal lobule; *significant difference (p < .05) between pre‐ and post‐rTMS assessment
6. DISCUSSION
In this study, we aimed at investigating whether a re‐size of the metric representation of the body is induced by modulating somatosensory cortical maps with rTMS. Firsly, results show that S1 shapes the metric of body parts: low‐frequency rTMS delivered to the S1 hand area brings about an overestimation of the perceived size of the participant's own hand (Experiment 1). This effect mirrors the phenomenon of macrosomatoagnosia (Frederiks, 1969; Frederiks, 1985), a disorder characterized by the feeling that one or more parts of the body are disproportionately large (Kew, Wright, & Halligan, 1998; Podoll, Mühlbauer, Houben, & Ebel, 1998; Podoll & Robinson, 2000; Vallar & Rode, 2009). Secondly, the effect reflects the somatotopic organization of S1, being body‐part specific: targeting the hand representation in S1 leads to an overestimation of the size of the hand, but not of the foot, (Experiment 2). Thirdly, the stimulation of S1 of both hemispheres affected the visual estimation of the size of both hands, namely both the hand ipsilateral and that contralateral to the stimulated hemisphere (Experiments 1 and 3). Finally, no perceptual distortion of hand size was found after stimulation of either the right or left IPL, although this region holds higher‐order representations of somatosensory information (Huang & Sereno, 2018).
The present results complement and extend the seminal study by Gandevia and Phegan (1999), who showed that the representation of own body size can be affected in a bottom‐up manner by peripheral somatosensory afferents, in a somatotopic‐specific way. In their work, authors speculated that perceptual body size distortions induced by acute anesthesia/deafferentation could be due to a reversible enlargement of S1 neurons' receptive fields, as it occurs in animal after limb amputation or spinal cord transection (Head & Holmes, 1912; Melzack & Bromage, 1973; Merzenich et al., 1983). In human amputees, a shrinking, and retraction of the cortical representation of the phantom limb has been documented (Flor, Nikolajsen, & Staehelin Jensen, 2006; Grüsser et al., 2001), although this phenomenon has been recently questioned by novel evidence, in both human and nonhuman primates, showing that limb amputation does not cause a rearrangement of functional sensory representations; rather, the cortical representation of the limb seems to remain stable despite the loss of the peripheral input (Kikkert et al., 2016; Makin & Bensmaia, 2017; Makin, Scholz, Henderson Slater, Johansen‐Berg, & Tracey, 2015).
The suggestion can be made from our findings is that the perceptual subjective enlargement of the hand is caused by variations of neural activity of S1 by rTMS, which may drive a temporary sort of shrinking of the cortical somatosensory map of the hand. In particular, since low‐frequency rTMS usually has an inhibitory effect on neural activity (Bolognini & Miniussi, 2018), we propose that this kind of stimulation may bring about a functional contraction of the cortical representation of the hand (as acute peripheral hand deafferentation does; e.g., Merzenich et al., 1983; Calford & Tweedale, 1988), which is behaviorally compensated by the perceptual overestimation of its size, as found in the present set of experiments.
This well fits with evidence showing that body parts underrepresented in S1 (Linkenauger et al., 2015; Mancini et al., 2014) are perceptually overestimated. This phenomenon represents a sort of compensatory mechanism by which the perceptual system distorts the experience of a body part's size to a magnitude that compensates for its differences in the somatosensory cortical maps—somatosensory homunculus (Linkenauger et al., 2015; Sadibolova, Ferrè, Linkenauger, & Longo, 2019). Accordingly, an abnormal underrepresentation of the hand in S1, induced by 1‐Hz rTMS, seems to be compensated by an over‐estimation of its size at the perceptual level.
Peripheral deafferentation brings about changes of perceptual size only on the side ipsilateral to the deafferentation (Gandevia & Phegan, 1999). Conversely, in the present experiments rTMS of S1 affects size representation of both the hand contralateral and of the hand ipsilateral to the stimulated hemisphere. These findings broadly agree with the evidence that, in the monkey's somatosensory cortex, at the level of the hand area, some neurons have bilateral and ipsilateral receptive fields (Iwamura, 2000). Recent evidence from humans also shows substantial integration of tactile information from the two hands in S1 (Tamé et al., 2016; Tamè, Pavani, Papadelis, Farnè, & Braun, 2014). Finally, receptive fields may increase in the contralateral homologous cortex after acute deafferentation (Calford & Tweedale, 1990), while unilateral S1 rTMS can affect somatosensory processing in the ipsilateral and in the contralateral hands (Bolognini & Miniussi, 2018; Eshel, Ruff, Spitzer, Blankenburg, & Driver, 2010; Meehan, Linsdell, Handy, & Boyd, 2011; Premji, Ziluk, & Nelson, 2010; Uguisu et al., 2010).
Thus, the emerging view is that human S1 is more than a simple relay for somatosensory inputs from the contralateral side of the body, playing instead a key role in the integration of such inputs from the two sides of the body (Tamè & Holmes, 2016). Additionally, beyond S1, the secondary somatosensory cortex (S2), and Brodmann's area 5 in the posterior parietal cortex (PPC), also receive dense bilateral afferent projections (Forss, Jousmäki, & Hari, 1995; Lin & Forss, 2002; Sakata, Takaoka, Kawarasaki, & Shibutani, 1973). Therefore, a possible account of the present findings is that rTMS delivered to S1, independent of the stimulated hemisphere, may affect size estimation of both the right and the left hand, possibly through both trans‐callosal interactions between the S1s of the two hemispheres, and ipsilateral connections between S1 and S2, which, as noted above, receives dense bilateral afferent projections (Forss et al., 1995). In the light of the distant effects of rTMS through neural connectivity (e.g., Bolognini & Miniussi, 2018), the present findings may also reflect the modulation of broader networks involved in the computation of the own body size, of which nevertheless S1 appears to be a key node (Forss et al., 1995; Lin & Forss, 2002; Sakata et al., 1973).
The present results also demonstrate the role of SI in the somatotopic representation of body size. Indeed, the stimulation of the S1‐hand map brings about an overestimation of the particitants' own hand size, without affecting the perceived size of their own foot. In line with this finding, peripheral changes in the somatosensory input from the thumb do not alter the perceived size of the index finger, although they affect the perceived size of the lips, in keeping with the well‐known plastic changes that may occur across the hand‐face S1 border (Muret et al., 2014; Muret et al., 2016). Hence, the representation of the size of body parts in S1 reflects the topographic map of the body surface (Penfield & Rasmussen, 1950).
In our last experiment (Experiment 4) we assessed for the selective involvement of S1 in the observed effects, by verifying whether perceptual distortion of body parts' size could be induced also by the stimulation of posterior parietal regions, namely the left and right IPL. The IPL of both hemispheres was not found to be involved in this process, suggesting that the estimation of the size of body parts primarily relies on the computation of more elementary somatic signals from body segments in S1. Noteworthy, posterior parietal damage (especially in the right hemisphere) may cause perceptual distortions primarily affecting extra‐personal space (Rode, Chabanat, Revol, & Rossetti, 2018; Rode, Michel, Rossetti, Boisson, & Vallar, 2006; Rode, Revol, Rossetti, & Vallar, 2008). Conversely, body parts' “macrosomatognosia” (Frederiks, 1969; Frederiks, 1985) or “hyperschematia” (Vallar & Rode, 2009) has been described in patients with vestibular dysfunction (Bonnier, 1905; see Vallar & Papagno, 2003, for review), and following lateral medullar damage in the brainstem (Rode et al., 2012), which also causes somatosensory, body‐part specific, impairments, along with a central vestibular dysfunction (Dieterich & Brandt, 2010). It is worth noting that transcranial direct current stimulation (tDCS) of the posterior parietal cortex does not affect telescoping in amputees with a phantom limb (Bolognini et al., 2013; Bolognini et al., 2015; Bolognini, Olgiati, Maravita, Ferraro, & Fregni, 2013), while systematic alterations in the perception of body size occur after spinal cord injury. Critically, in the latter case, patients experiencing phantom sensations may report the feeling of an increased size of the deafferented limb, while a reduction of body part size is generally not reported (Bors, 1951; Conomy, 1973; Evans, 1962; Longo, Mattioni, & Ganea, 2015).
Finally, in all four experiments, in the baseline condition (before rTMS) participants underestimate the perceptual size of their own hands and feet, in accordance with other behavioral evidence documenting the existence of a basal distortion of the perceived hand size in humans (Fuentes, Longo, & Haggard, 2013; Longo, 2015; Longo et al., 2015; Longo & Haggard, 2010), also using template matching tasks similar to that employed in the present study (Longo et al., 2012; Longo & Haggard, 2012a, 2012b).
7. CONCLUSION
The present study provides novel evidence that the perceptual representation of the size of the own body‐parts may be shaped by local changes of activity in the S1 of either hemisphere, induced by rTMS. The mutual relation between body surface, somatosensory processing and abstract representation of one's own body, including its metric properties, dates back to the suggestions of the existence of a “schema” of the body, starting from the end of the XIX century (Vallar & Papagno, 2003 for review). The present results suggest that the perceptual metric representation of the body involves cortical, somatotopically organized, activity, in S1. This conclusion is in line with the suggestion of a distinction between a “somato‐perceptual” representation of the body, built up mainly on external (somatosensory, and also from other modalities, such as vestibular) inputs, and a “somato‐representation,” based on cognitive processes creating a more abstract body knowledge, including semantic features, beliefs, and attitudes related to the body (Longo, Azañón, & Haggard, 2010; see the distinction between “body schema” and “body image,” discussed by Vallar & Papagno, 2003; Head & Holmes, 1911, for the concept of “body schema”; Schilder, 1935, for that of “body image”). In this broader perspective, we then suggest that somato‐perception, and, specifically, its component concerning the perceptual representation of the size of body parts, is essentially based on online computations occurring within somatotopic cortical representations in S1. Representation of body size appears highly dependent upon sensory input processing, and as such, it can be functionally distorted by modulating body‐part maps in S1.
CONFLICT OF INTERESTS
None to declare.
ACKNOWLEDGMENTS
This work was supported by ATE grant (2016‐ATE‐0281) from the University of Milano Bicocca to NB.
Giurgola S, Pisoni A, Maravita A, Vallar G, Bolognini N. Somatosensory cortical representation of the body size. Hum Brain Mapp. 2019;40:3534–3547. 10.1002/hbm.24614
Data Availability Statement:The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information University of Milano Bicocca, Grant/Award Number: 2016‐ATE‐0281
Contributor Information
Serena Giurgola, Email: s.giurgola@campus.unimib.it.
Nadia Bolognini, Email: nadia.bolognini@unimib.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Avenanti, A. , Bolognini, N. , Maravita, A. , & Aglioti, S. M. (2007). Somatic and motor components of action simulation. Current Biology, 17(24), 2129–2135. 10.1016/j.cub.2007.11.045 [DOI] [PubMed] [Google Scholar]
- Baayen, R. H. , Davidson, D. J. , & Bates, D. M. (2008). Mixed‐effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59(4), 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S. (2015). lme4: Linear mixed‐effects models using Eigen and S4. R package version 1.1–7. 2014. Institute for Statistics and Mathematics of WU. https://cran.r-project.org/package=lme4
- Berlucchi, G. , & Aglioti, S. (1997). The body in the brain: Neural bases of corporeal awareness. Trends in Neurosciences, 20(12), 560–564. 10.1016/S0166-2236(97)01136-3 [DOI] [PubMed] [Google Scholar]
- Berlucchi, G. , & Aglioti, S. (2010). The body in the brain rivisited. Experimental Brain Research, 200(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Boakye, M. , Huckins, S. C. , Szeverenyi, N. M. , Taskey, B. I. , & Hodge, C. J., Jr. (2000). Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. Journal of Neurosurgery, 93(5), 774–783. 10.3171/jns.2000.93.5.0774 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , & Maravita, A. (2011). Uncovering multisensory processing through non‐invasive brain stimulation. Frontiers in Psychology, 2, 46 10.3389/fpsyg.2011.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini, N. , & Miniussi, C. (2018). Noninvasive brain stimulation of the parietal lobe for improving neurologic, neuropsychologic, and neuropsychiatric deficits. Handbook of Clinical Neurology, 151, 427–446. 10.1016/B978-0-444-63622-5.00022-X [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Miniussi, C. , Savazzi, S. , Bricolo, E. , & Maravita, A. (2009). TMS modulation of visual and auditory processing in the posterior parietal cortex. Experimental Brain Research, 195(4), 509–517. 10.1007/s00221-009-1820-7 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Olgiati, E. , Maravita, A. , Ferraro, F. , & Fregni, F. (2013). Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain, 154(8), 1274–1280. 10.1016/j.pain.2013.03.040 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Olgiati, E. , Rossetti, A. , & Maravita, A. (2010). Enhancing multisensory spatial orienting by brain polarization of the parietal cortex. European Journal of Neuroscience, 31(10), 1800–1806. 10.1111/j.1460-9568.2010.07211.x [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Papagno, C. , Moroni, D. , & Maravita, A. (2010). Tactile temporal processing in the auditory cortex. Journal of Cognitive Neuroscience, 22(6), 1201–1211. 10.1162/jocn.2009.21267 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Rossetti, A. , Convento, S. , & Vallar, G. (2013). Understanding others' feelings: The role of the right primary somatosensory cortex in encoding the affective valence of others' touch. Journal of Neuroscience, 33(9), 4201–4205. 10.1523/JNEUROSCI.4498-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini, N. , Rossetti, A. , Fusaro, M. , Vallar, G. , & Miniussi, C. (2014). Sharing social touch in the primary somatosensory cortex. Current Biology, 24(13), 1513–1517. 10.1016/j.cub.2014.05.025 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Rossetti, A. , Maravita, A. , & Miniussi, C. (2011). Seeing touch in the somatosensory cortex: A TMS study of the visual perception of touch. Human Brain Mapping, 32(12), 2103–2114. 10.1002/hbm.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini, N. , Spandri, V. , Ferraro, F. , Salmaggi, A. , Molinari, A. C. , Fregni, F. , & Maravita, A. (2015). Immediate and sustained effects of 5‐day Transcranial direct current stimulation of the motor cortex in phantom limb pain. The Journal of Pain, 16(7), 657–665. 10.1016/j.jpain.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Spandri, V. , Olgiati, E. , Fregni, F. , Ferraro, F. , & Maravita, A. (2013). Long‐term analgesic effects of transcranial direct current stimulation of the motor cortex on phantom limb and stump pain: A case report. Journal of Pain and Symptom Management, 46(4), e1–e4. 10.1016/j.jpainsymman.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Bonnier, P. (1905). L'aschématie. Revue Neurologique, 13, 605–609. 10.1016/j.yebeh.2009.09.020 [DOI] [Google Scholar]
- Bors, E. (1951). Phantom limbs of patients with spinal cord injury. Archives of Neurology and Psychiatry, 66(5), 610–631. 10.1001/archneurpsyc.1951.02320110075007 [DOI] [PubMed] [Google Scholar]
- Buxbaum, L. J. , & Coslett, H. B. (2001). Specialised structural descriptions for human body parts: Evidence from autotopagnosia. Cognitive Neuropsychology, 18(4), 289–306. 10.1080/02643290126172 [DOI] [PubMed] [Google Scholar]
- Calford, M. B. , & Tweedale, R. (1988). Immediate and chronic changes in responses of somatosensory cortex in adult flying‐fox after digit amputation. Nature, 332, 446–447. 10.1038/332446a0 [DOI] [PubMed] [Google Scholar]
- Calford, M. B. , & Tweedale, R. (1990). Interhemispheric transfer of plasticity in the cerebral cortex. Science, 249(4970), 805–807. 10.1126/science.2389146 [DOI] [PubMed] [Google Scholar]
- Carducci, F. , & Brusco, R. (2012). Accuracy of an individualized MR‐based head model for navigated brain stimulation. Psychiatry Research: Neuroimaging, 203(1), 105–108. 10.1016/j.pscychresns.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Caspers, S. , & Zilles, K. (2018). Microarchitecture and connectivity of the parietal lobe In Vallar G. & Coslett H. B. (Eds.), Handbook of clinical neurology (Vol. 151, pp. 53–72). Amsterdam: Elsevier; 10.1016/B978-0-444-63622-5.00003-6 [DOI] [PubMed] [Google Scholar]
- Chen, L. M. , Friedman, R. M. , & Roe, A. W. (2003). Optical imaging of a tactile illusion in area 3b of the primary somatosensory cortex. Science, 302(5646), 881–885. 10.1126/science.1087846 [DOI] [PubMed] [Google Scholar]
- Conomy, J. P. (1973). Disorders of body image after spinal cord injury. Neurology, 23(8), 842–850. 10.1212/WNL.23.8.842 [DOI] [PubMed] [Google Scholar]
- De Vignemont, F. (2010). Body schema and body image. Neuropsychologia, 48(3), 669–680. 10.1016/j.neuropsychologia.2009.09.022 [DOI] [PubMed] [Google Scholar]
- Dieterich, M. , & Brandt, T. (2010). Imaging cortical activity after vestibular lesions. Restorative Neurology and Neuroscience, 28(1), 47–56. 10.3233/RNN-2010-0505 [DOI] [PubMed] [Google Scholar]
- Eshel, N. , Ruff, C. C. , Spitzer, B. , Blankenburg, F. , & Driver, J. (2010). Effects of parietal TMS on somatosensory judgments challenge interhemispheric rivalry accounts. Neurospychologia, 48(12), 3470–3481. 10.1016/j.neuropsychologia.2010.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. H. (1962). On disturbance of the body image in paraplegia. Brain, 85, 687–700. 10.1093/brain/85.4.687 [DOI] [Google Scholar]
- Felician, O. , Anton, J. L. , Nazarian, B. , Roth, M. , Roll, J. P. , & Romaiguere, P. (2009). Where is your shoulder? Neural correlates of localizing others' body parts. Neuropsychologia, 47(8–9), 1909–1916. 10.1016/j.neuropsychologia.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Fiorio, M. , & Haggard, P. (2005). Viewing the body prepares the brain for touch: Effects of TMS over somatosensory cortex. European Journal of Neuroscience, 22(3), 773–777. 10.1111/j.1460-9568.2005.04267.x [DOI] [PubMed] [Google Scholar]
- Flor, H. , Nikolajsen, L. , & Staehelin Jensen, T. (2006). Phantom limb pain: A case of maladaptive CNS plasticity? Nature Reviews Neuroscience, 7(11), 873–881. 10.1038/nrn1991 [DOI] [PubMed] [Google Scholar]
- Forss, N. , Jousmäki, V. , & Hari, R. (1995). Interaction between afferent input from fingers in human somatosensory cortex. Brain Research, 685(1–2), 68–76. 10.1016/0006-8993(95)00424-O [DOI] [PubMed] [Google Scholar]
- Frederiks, J. A. M. (1969). Disorders of the body schema In Vinken P. J. & Bruyn G. W. (Eds.), Handbook of clinical neurology. Disorders of speech, perception and symbolic behaviour (Vol. 4, pp. 207–240). Amsterdam: North Holland. [Google Scholar]
- Frederiks, J. A. M. (1985). Disorders of the body schema In Vinken P. J., Bruyn G. W., Klawans H. L., & Frederiks J. A. M. (Eds.), Handbook of clinical neurology. Clinical neuropsychology. Revised series 1 (Vol. 45, pp. 373–393). Amsterdam: Elsevier. [Google Scholar]
- Fuentes, C. T. , Longo, M. R. , & Haggard, P. (2013). Body image distortions in healthy adults. Acta Psychologica, 144(2), 344–351. 10.1016/j.actpsy.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Gallagher, S. (2005). How the body shapes the mind. New York: Oxford University Press; 10.1093/0199271941.001.0001 [DOI] [Google Scholar]
- Gandevia, S. C. , & Phegan, C. M. L. (1999). Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. The Journal of Physiology, 514(2), 609–616. 10.1111/j.1469-7793.1999.609ae.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman, A. , & Hill, J. (2006). Data analysis using regression and multilevel/hierarchical models. NY: Cambridge University Press. [Google Scholar]
- Grüsser, S. , Winter, C. , Mühlnickel, W. , Denke, C. , Karl, A. , Villringer, K. , & Flor, H. (2001). The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience, 102(2), 263–272. 10.1016/S0306-4522(00)00491-7 [DOI] [PubMed] [Google Scholar]
- Haggard, P. , & Wolpert, D. M. (2005). Disorders of body schema In Freund H. J., Jeannerod M., Hallett M., & Leiguarda R. (Eds.), Higher‐order motor disorders: From neuroanatomy and neurobiology to clinical neurology (pp. 261–271). Oxford: Oxford University Press. [Google Scholar]
- Harding‐Forrester, S. , & Feldman, D. E. (2018). Somatosensory maps In Vallar G. & Coslett H. B. (Eds.), Handbook of clinical neurology (Vol. 151, pp. 73–102). Amsterdam: Elsevier; 10.1016/B978-0-444-63622-5.00004-8 [DOI] [PubMed] [Google Scholar]
- Harris, J. A. , Miniussi, C. , Harris, I. M. , & Diamond, M. E. (2002). Transient storage of a tactile memory trace in primary somatosensory cortex. Journal of Neuroscience, 22, 8720–8725. 10.1523/JNEUROSCI.22-19-08720.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, H. , & Holmes, G. (1911). Sensory disturbances from cerebral lesions. Brain, 34(2–3), 102–254. 10.1093/brain/34.2-3.102 [DOI] [Google Scholar]
- Head, H. , & Holmes, G. (1912). Researches into sensory disturbances from cerebral lesions. The Lancet, 179(4612), 144–152. 10.1016/S0140-6736(01)64942-0 [DOI] [Google Scholar]
- Holmes, N. P. , & Tamè, L. (2019). Locating primary somatosensory cortex in human brain stimulation studies: Systematic review and meta‐analytic evidence. Journal of Neurophysiology, 121(1), 152–162. 10.1152/jn.00614.2018 [DOI] [PubMed] [Google Scholar]
- Holmes, N. P. , Tamè, L. , Beeching, P. , Medford, M. , Rakova, M. , Stuart, A. , & Zeni, S. (2019). Locating primary somatosensory cortex in human brain stimulation studies: Experimental evidence. Journal of Neurophysiology, 121(1), 336–344. 10.1152/jn.00641.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. S. , & Sereno, M. I. (2018). Multisensory and sensorimotor maps In Vallar G. & Coslett H. B. (Eds.), The parietal lobe. Handbook of clinical neurology. Vol 151 (1st ed.). Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Iwamura, Y. (2000). Bilateral receptive field neurons and Callosal connections in the somatosensory cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 355(1394), 267–273. 10.1098/rstb.2000.0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas, J. H. , Qi, H. X. , & Stepniewska, I. (2018). The evolution of parietal cortex in primates In Vallar G. & Coslett H. B. (Eds.), Handbook of clinical neurology (Vol. 151, pp. 31–52). Amsterdam: Elsevier; 10.1016/B978-0-444-63622-5.00002-4 [DOI] [PubMed] [Google Scholar]
- Kennett, S. , Taylor‐Clarke, M. , & Haggard, P. (2001). Noninformative vision improves the spatial resolution of touch in humans. Current Biology, 11(15), 1188–1191. 10.1016/S0960-9822(01)00327-X [DOI] [PubMed] [Google Scholar]
- Kew, J. , Wright, A. , & Halligan, P. W. (1998). Somesthetic aura: The experience of “Alice in wonderland”. The Lancet, 351(9120), 1934 10.1016/S0140-6736(05)78619-0 [DOI] [PubMed] [Google Scholar]
- Kikkert, S. , Kolasinski, J. , Jbabdi, S. , Tracey, I. , Beckmann, C. F. , Johansen‐Berg, H. , & Makin, T. R. (2016). Revealing the neural fingerprints of a missing hand. eLife, 5, e15292. 10.7554/eLife15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht, S. , Ellger, T. , Breitenstein, C. , Bernd Ringelstein, E. , & Henningsen, H. (2003). Changing cortical excitability with low‐frequency transcranial magnetic stimulation can induce sustained disruption of tactile perception. Biological Psychiatry, 53(2), 175–179. 10.1016/S0006322302013823 [DOI] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P.B. , & Christensen, R.H.B. (2015). LmerTest: Tests for random and fixed effects for linear mixed effect models. R package, version 2.0–29. http://cran.r-project.org/package=lmerTest.
- Lin, Y. Y. , & Forss, N. (2002). Functional characterization of human second somatosensory cortex by magnetoencephalography. Behavioural Brain Research, 135(1–2), 141–145. 10.1016/S0166-4328(02)00143-2 [DOI] [PubMed] [Google Scholar]
- Linkenauger, S. A. , Wong, H. Y. , Geuss, M. , Stefanucci, J. K. , McCulloch, K. C. , Bülthoff, H. H. , … Proffitt, D. R. (2015). The perceptual homunculus: The perception of the relative proportions of the human body. Journal of Experimental Psychology: General, 144(1), 103–113. 10.1037/xge0000028 [DOI] [PubMed] [Google Scholar]
- Longo, M. R. (2015). Implicit and explicit body representations. European Psychologist, 20(1), 6–15. 10.1027/1016-9040/a000198 [DOI] [Google Scholar]
- Longo, M. R. , Azañón, E. , & Haggard, P. (2010). More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia, 48(3), 655–668. 10.1016/j.neuropsychologia.2009.08.022 [DOI] [PubMed] [Google Scholar]
- Longo, M. R. , & Haggard, P. (2010). An implicit body representation underlying human position sense. PNAS, 107(26), 11727–11732. 10.1073/pnas.1003483107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo, M. R. , & Haggard, P. (2012a). A 2.5‐D representation of the human hand. Journal of Experimental Psychology: Human Perception and Performance, 38(1), 9–13. 10.1037/a0025428 [DOI] [PubMed] [Google Scholar]
- Longo, M. R. , & Haggard, P. (2012b). Implicit body representations and the conscious body image. Acta Psychologica, 141(2), 164–168. 10.1016/j.actpsy.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Longo, M. R. , Long, C. , & Haggard, P. (2012). Mapping the invisible hand: A body model of a phantom limb. Psychological Science, 23(7), 740–742. 10.1177/0956797612441219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo, M. R. , Mattioni, S. , & Ganea, N. (2015). Perceptual and conceptual distortions of implicit hand maps. Frontiers in Human Neuroscience, 9, 656 10.3389/fnhum.2015.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin, T. R. , & Bensmaia, S. J. (2017). Stability of sensory topographies in adult Cortex. Trends in Cognitive Sciences, 21(3), 195–204. 10.1016/j.tics.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin, T. R. , Scholz, J. , Henderson Slater, D. , Johansen‐Berg, H. , & Tracey, I. (2015). Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain, 138(8), 2140–2146. 10.1093/brain/awv161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini, F. , Bauleo, A. , Cole, J. , Lui, F. , Porro, C. A. , Haggard, P. , & Iannetti, G. D. (2014). Whole‐body mapping of spatial acuity for pain and touch. Annals of Neurology, 75(6), 917–924. 10.1002/ana.24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita, A. , Spence, C. , & Driver, J. (2003). Multisensory integration and the body schema: Close to hand and within reach. Current Biology, 13(13), 531–539. 10.1016/S0960-9822(03)00449-4 [DOI] [PubMed] [Google Scholar]
- Meehan, S. K. , Linsdell, M. A. , Handy, T. C. , & Boyd, L. A. (2011). Interhemispheric enhancement of somatosensory cortical excitability through contralateral repetitive transcranial magnetic stimulation. Clinical Neurophysiology, 22(8), 1637–1644. 10.1016/j.clinph.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Melzack, R. , & Bromage, P. R. (1973). Experimental phantom limbs. Experimental Neurology, 39(2), 261–269. 10.1016/0014-4886(73)90228-8 [DOI] [PubMed] [Google Scholar]
- Merzenich, M. M. , Kaas, J. H. , Wall, J. T. , Sur, M. , Nelson, R. J. , & Felleman, D. J. (1983). Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience, 10(3), 639–665. 10.1016/0306-4522(83)90208-7 [DOI] [PubMed] [Google Scholar]
- Miller, L. E. , Longo, M. R. , & Saygin, A. P. (2016). Mental body representations retain homuncular shape distortions: Evidence from Weber's illusion. Consciousness and Cognition, 40, 17–25. 10.1016/j.cognition.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Muret, D. , Daligault, S. , Dinse, H. R. , Delpuech, C. , Mattout, J. , Reilly, K. T. , & Farnè, A. (2016). Neuromagnetic correlates of adaptive plasticity across the hand‐face border in human primary somatosensory cortex. Journal of Neurophysiology, 115(4), 2095–2104. 10.1152/jn.00628.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muret, D. , Dinse, H. R. , Macchione, S. , Urquizar, C. , Farnè, A. , & Reilly, K. (2014). Touch improvement at the hand transfers to the face. Current Biology, 24(16), R736–R737. 10.1016/j.cub.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Penfield, W. , & Rasmussen, T. (1950). The cerebral cortex of man: A clinical study of localization of function. New York: Macmillan. [Google Scholar]
- Pisoni, A. , Romero Lauro, L. J. , Vergallito, A. , Maddaluno, O. , & Bolognini, N. (2018). Cortical dynamics underpinning the self‐other distinction of touch: A TMS‐EEG study. NeuroImage, 178, 475–484. 10.1016/j.neuroimage.2018.05.078 [DOI] [PubMed] [Google Scholar]
- Pitron, V. , & De Vignemont, F. (2017). Beyond differences between the body schema and the body image: Insights from body hallucinations. Consciousness and Cognition, 53, 115–121. 10.1016/j.concog.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Podoll, K. , Mühlbauer, V. , Houben, I. , & Ebel, H. (1998). Hypnagogic hallucinations presenting as macrosomatognosia and microsomatognosia. Fortschritte der Neurologie‐Psychiatrie, 66(8), 338–344. 10.1055/s-2007-995271 [DOI] [PubMed] [Google Scholar]
- Podoll, K. , & Robinson, D. (2000). Macrosomatognosia and microsomatognosia in migraine art. Acta Neurologica Scandinavica, 101(6), 413–416. 10.1034/j.1600-0404.2000.9s334.x [DOI] [PubMed] [Google Scholar]
- Premji, A. , Ziluk, A. , & Nelson, A. J. (2010). Bilateral somatosensory evoked potentials following intermittent theta‐burst repetitive transcranial magnetic stimulation. BMC Neuroscience, 11, 91 10.1186/1471-2202-11-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode, G. , Vallar, G. , Chabanat, E. , Revol, P. , & Rossetti, Y. (2018). What do spatial distortions in patients' drawing after right brain damage teach us about space representation in art? Frontiers in Psychology, 9, 1058 10.3389/fpsyg.2018.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode, G. , Michel, C. , Rossetti, Y. , Boisson, D. , & Vallar, G. (2006). Left size distortion (hyperschematia) after right brain damage. Neurology, 67(10), 1801–1808. 10.1212/01.wnl.0000244432.91915.d0 [DOI] [PubMed] [Google Scholar]
- Rode, G. , Revol, P. , Rossetti, Y. , & Vallar, G. (2008). 3D left hyperschematia after right brain damage. Neurocase, 14(4), 369–377. 10.1080/13554790802389154 [DOI] [PubMed] [Google Scholar]
- Rode, G. , Vallar, G. , Revol, P. , Tilikete, C. , Jacquin‐Courtois, S. , Rossetti, Y. , & Farnè, A. (2012). Facial macrosomatognosia and pain in a case of Wallenberg's syndrome: Selective effects of vestibular and transcutaneous stimulations. Neuropsychologia, 50(2), 245–253. 10.1016/j.neuropsychologia.2011.11.018 [DOI] [PubMed] [Google Scholar]
- Rossetti, A. , Miniussi, C. , Maravita, A. , & Bolognini, N. (2012). Visual perception of bodily interactions in the primary somatosensory cortex. European Journal of Neuroscience, 36(3), 2317–2323. 10.1111/j.1460-9568.2012.08137.x [DOI] [PubMed] [Google Scholar]
- Rossi, S. , Hallett, M. , Rossini, P. M. , & Pascual‐Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadibolova, R. , Ferrè, E. R. , Linkenauger, S. A. , & Longo, M. R. (2019). Distortions of perceived volume and length of body parts. Cortex, 111, 74–86. 10.1016/j.cortex.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Sakata, H. , Takaoka, Y. , Kawarasaki, A. , & Shibutani, H. (1973). Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Research, 64, 85–102. 10.1016/0006-8993(73)90172-8 [DOI] [PubMed] [Google Scholar]
- Schilder, P. (1935). The image and appearance of the human body. New York: International Universities Press. [Google Scholar]
- Serino, A. , & Haggard, P. (2010). Touch and the body. Neuroscience & Biobehavioral Reviews, 34(2), 224–236. 10.1016/j.neubiorev.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Tamé, L. , Braun, C. , Holmes, N. P. , Farnè, A. , & Pavani, F. (2016). Bilateral representations of touch in the primary somatosensory cortex. Cognitive Neuropsychology, 33(1–2), 48–66. 10.1080/02643294.2016.1159547 [DOI] [PubMed] [Google Scholar]
- Tamè, L. , & Holmes, N. P. (2016). Involvement of human primary somatosensory cortex in vibrotactile detection depends on task demands. NeuroImage, 138, 184–196. 10.1016/j.neuroimage.2016.05.056 [DOI] [PubMed] [Google Scholar]
- Tamè, L. , Pavani, F. , Papadelis, C. , Farnè, A. , & Braun, C. (2014). Early integration of bilateral touch in the primary somatosensory cortex. Human Brain Mapping, 36(4), 1506–1523. 10.1002/hbm.22719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio, F. , Cancelli, A. , Cottone, C. , Zito, G. , Pasqualetti, P. , Ghazaryan, A. , … Filippi, M. M. (2014). Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. Journal of Neurology, 261(8), 1552–1558. 10.1016/j.clinph.2015.11.070 [DOI] [PubMed] [Google Scholar]
- Tegenthoff, M. , Ragert, P. , Pleger, B. , Schwenkreis, P. , Förster, A. F. , Nicolas, V. , & Dinse, H. R. (2005). Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biology, 3(11), e362. 10.1371/journal.pbio.0030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguisu, H. , Urushihara, R. , Hosono, Y. , Asanuma, K. , Shimazu, H. , Murase, N. , & Kaji, R. (2010). Very low‐frequency rTMS modulates SEPs over the contralateral hemisphere. The Journal of Medical Investigation, 57(1–2), 109–113. 10.2152/jmi.57.109 [DOI] [PubMed] [Google Scholar]
- Vallar, G. , & Papagno, C. (2003). Pierre Bonnier's (1905) cases of bodily “aschématie” In Code C., Wallesch C.‐W., Joanette Y., & Lecours A. R. (Eds.), Classic cases in neuropsychology (pp. 147–170). Hove, East Sussex: Psychology Press. [Google Scholar]
- Vallar, G. , & Rode, G. (2009). Commentary on Bonnier P. L'aschématie. Rev Neurol (Paris), 1905;13:605‐9. Epilepsy & Behavior, 16(3), 397–400. 10.1016/j.yebeh.2009.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


