Abstract
The study of individuals at high‐altitude (HA) exposure provides an important opportunity for unraveling physiological and psychological mechanism of brain underlying hypoxia condition. However, this has rarely been assessed longitudinally. We aim to explore the cognitive and cerebral microstructural alterations after chronic HA exposure. We recruited 49 college freshmen who immigrated to Tibet and followed up for 2 years. Control group consisted of 49 gender and age‐matched subjects from sea level. Neuropsychological tests were also conducted to determine whether the subjects' cognitive function had changed in response to chronic HA exposure. Surface‐based cortical and subcortical volumes were calculated from structural magnetic resonance imaging data, and tract‐based spatial statistics (TBSS) analysis of white matter (WM) fractional anisotropy (FA) based on diffusion weighted images were performed. Compared to healthy controls, the high‐altitude exposed individuals showed significantly lower accuracy and longer reaction times in memory tests. Significantly decreased gray matter volume in the caudate region and significant FA changes in multiple WM tracts were observed for HA immigrants. Furthermore, differences in subcortical volume and WM integration were found to be significantly correlated with the cognitive changes after 2 years' HA exposure. Cognitive functions such as working memory and psychomotor function were found to be impaired during chronic HA. Differences of brain subcortical volumes and WM integration between HA and sea‐level participants indicated potential impairments in the brain structural modifications and microstructural integrity of WM tracts after HA exposure.
Keywords: cognition, fractional anisotropy, high‐altitude exposure, subcortical volume
1. INTRODUCTION
While encouraged by a series of new policies, an increasing number of adolescents from lowlands immigrate to high altitude (HA) for studying purposes and stay for several years. As of 2006, there were approximately 12 million people living at HA between 2,200 m and 5,200 m on the Qinghai–Tibetan Plateau (Wu & Kayser, 2006), and every year thousands of trekkers and recreational climbers attempt ascents to very high (3,500–5,500 m) or extreme altitudes (>5,500 m) (Gallagher & Hackett, 2004). As one of the most prominent impacts of living in HA area, chronic hypoxia has always been one of the research hot topics of HA‐brain association.
It is well known that long‐term exposure to HA with hypoxia and other physiological stressors, such as hypobaria, cold, ultraviolet rays, and dehydration may lead to cognitive impairments (Virues‐Ortega, Buela‐Casal, Garrido, & Alcazar, 2004). While studies constructed a bridge between hypoxia exposure and cognitive‐executive function, there is mounting evidence that HA residents are tended to experiencing impairments in working memory, attention and learning ability (Wang et al., 2013; Yan, 2014). For example, Davis et al. (2015) reported that HA‐exposure results in cognitive deficits including decreased attention and vigilance, impairment in executive functions and long‐term memory (Davis et al., 2015). Yan, Zhang, Gong, and Weng (2011) found that HA residents had lower accuracy in a verbal‐working memory task, longer reaction times in spatial‐ and verbal‐working memory tasks comparing low‐ and high‐altitude residents (Yan et al., 2011). Furthermore, a study of Go/No Go task reported that a delayed latency of NoGo‐N2 was found in the HA group compared to that of the low‐altitude group (Ma, Wang, Wu, Luo, & Han, 2015). Based on observations associated with an acute ascent to HA areas, cognitive changes have been reported previously (Davis et al., 2015; Ma et al., 2015; Yan et al., 2011). However, cognitive impairments associated with chronic exposure to HA have rarely been assessed longitudinally.
A long‐term damage of individual's cognitive function may increase brain's plasticity changes, resulting in structural and functional reorganization in cognitive‐related brain networks (Fan et al., 2016; Zhang et al., 2010). Surface‐based morphometry (SBM) based on anatomical T1‐weighted magnetic resonance imaging (MRI) and tract‐based spatial statistics (TBSS) based on diffusion tenser imaging (DTI) have been widely used to assess HA resident's structural modifications, respectively (Ghosh et al., 2010; Mills & Tamnes, 2014; Zhang et al., 2012). SBM provides better alignment of cortical landmarks over the whole brain, which used vertex–by‐vertex analysis to explore possible changes in the gray matter (GM) (Mills & Tamnes, 2014). TBSS implements voxel‐wise statistical analysis of the fractional anisotropy (FA) data to examine the alterations in fibrous microstructure properties of white matter (WM) tracts (Zhang et al., 2012). Structural neuroimaging studies demonstrated that individual structural morphologies were affected by hypoxic conditions. Previous studies found that cortical lesions might persist for more than several months after brief episodes of mountain climbing (Fan et al., 2016; Usui et al., 2004). Fan et al. (2016) showed that after 30‐days HA exposure, the GM volumes and cortical surface thickness of healthy subjects significantly increased in a wide spread region, including the precentral gyrus, postcentral gyrus, lateral occipital cortex and temporal pole, and so on(Fan et al., 2016). Young HA immigrant descendants born and raised at HA and adult climbers who spend several times a year during 10 years in mountain climbing have been reported alternations in a number of WM microstructural regions, most prominent in the precentral cortex and parietal cortex (Di Paola, 2008; Zhang et al., 2010). All these studies suggested that microstructural alterations could occur in multiple brain regions because of the interactions between various factors of hypoxia exposure. However, inconsistent structural findings in studies are intriguing, particularly due to the relatively small sample size. Foster et al. observed a GM reduction after a 3‐week exposure to a 5,500‐m altitude, while WM was unchanged (Foster et al., 2015). However, Zhang et al. detected no significant GM changes after mountain climbing (6,206 m) (Zhang et al., 2012), and some studies demonstrated that occupational exposure to hypoxia was associated with subcortical WM hyperintensities (McGuire et al., 2012; Zhang et al., 2012). While numerous MRI studies aiming to detect HA‐brain alterations have examined acute exposure, relatively less work has been done on the basis of chronic plateau environment. One important question to be addressed is the longitudinal comparison with the gender and age matched residents of sea level (SL).
We hypothesized that modified GM and WM volumes and integrity in certain brain regions could exist after chronic HA exposure and these changes may be associated with decreased cognitive functions. To test this hypothesis, we performed a longitudinal study to investigate working memory, psychomotor function and brain structural alterations associated with chronic HA compared to SL group. Brain structural investigation included measurements of cortical and subcortical brain volumes and FA differences. With this effort, we attempted to identify associations among cognitive function, anatomical morphology, and chronic hypoxia, aiming to generate predictive models of chronic HA exposure based on cognitive and anatomical substrates.
2. MATERIALS AND METHODS
2.1. Overall design
The data collection was initiated in July 2014 and the general information is shown in Figure 1. Subjects were recruited for all three sessions in Xi'an (altitude 466 m) China, including baseline investigation, neuropsychological tests, and MRI scans. A series of behavioral data were collected such as HA exposure history, medical history, academic performance, and sociodemographic information including parental education, vocation, and socioeconomic status. The follow‐up investigation and neuro‐psychological measures were performed in June 2015 and May 2016, in Lhasa, Tibet. During the follow‐up investigation, the subjects were required to note the duration of HA exposure and experience in mountain climbing and low‐altitude visits, as well as any medical events that have occurred during the study period.
Figure 1.
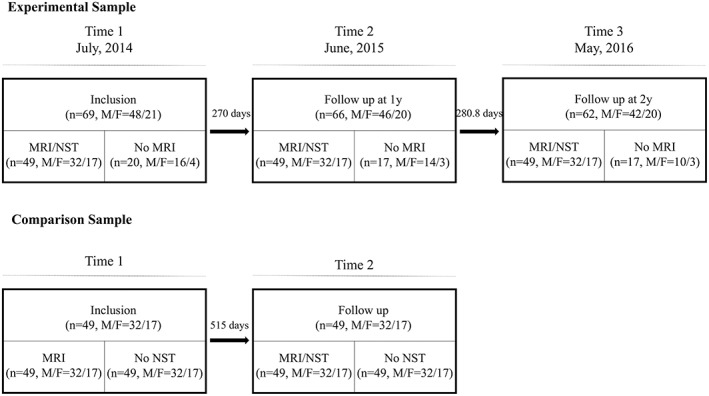
The study includes experimental and control two data sets. The final number of subjects in two groups included 49, respectively. NST, neuropsychology test
A control group of healthy college students was also included in the study. Data were downloaded from the open access Southwest University longitudinal imaging multimodal [SLIM] Brain Data Repository: A Long‐term Test–Retest Sample of Young Healthy Adults in Southwest China. We selected longitudinal MRI data which were gender and age matched with experimental group.
2.2. Participants
Sixty‐nine healthy right‐handed high school graduates (48 males, 21 females; age range 17–20 years) were recruited from Shaanxi Province, who were admitted to Tibet University for a 4‐year higher education period in Lhasa (average altitude: 3,658 m), China. Twenty participants were excluded because of incomplete MRI or questionnaires. Therefore 49 participants (32 males, 17 females; age range 17–20 years) were included in the current study. Participants were screened according to the following criteria: (a) 17–20 years old, (b) altitude of permanent residence is <900 m, (c) no HA exposure (≥2,500 m) in the past, (d) no history of smoking and drug abuse, (e) no history of medications that may affect cognitive function or carbonic anhydrase activity, and (f) no chronic or genetic diseases. All subjects in this study signed the informed consent prior to the experiment on the premise of fully understanding the content of the experiment. This study was approved by the Ethics Committee of the Medical Faculty of Fourth Military Medical University (Registry no. KY20143344‐1) and was conducted in accordance with the ethical principles for medical research involving human subjects as defined in the Declaration of Helsinki.
A control group of 49 healthy college students (32 males, 17 females; age range 17–20 years) was also included in the study. Young adults were screened as eligible for the SLIM if they were university freshman or sophomores and fluent in Chinese. The exclusion criteria included: (a) MRI‐related exclusion criteria, which included claustrophobia, metallic implants, Meniere's Syndrome, and a history of fainting within the previous 6 months; (b) current psychiatric disorders and neurological disorders; (c) the use of psychiatric drugs within the 3 months prior to scanning; (d) pregnancy; and (e) a history of head trauma. The SLIM database was approved by the Research Ethics Committee of the Brain Imaging Center of Southwest University. Informed written consent was obtained from each subject, together with informed written consent from the guardians of the two youngest participants (aged 17 years old) who were their college instructors.
2.3. Neuropsychological tests
The neuropsychology tests (NSTs) consist of the following two tests: (a) verbal memory test, which tests immediate verbal memory (IVBM) and delayed verbal memory (DVBM) by measuring working memory for words and (b) auditory reaction time test, which tests auditory simple reaction time (ASRT) and auditory recognition reaction time test (ACRT) by measuring psychomotor function in response to a simple stimulus and target/distractive stimuli (go/no‐go). All tests were performed using CNS Vital Signs (http://www.cnsvs.com/) (Gualtieri & Johnson, 2006).
2.4. MRI image acquisition
For the HA subjects, the baseline and following‐up MRI images were acquired on a General Electric Discovery MR750 3.0‐T (General Electric Co. Ltd., CT) scanner in Xijing Hospital of the Fourth Military Region. Standard T1‐weighted 3D anatomical data were acquired using echo (3D SPGR) sequence (repetition time = 2,530 ms, echo time = 3.5 ms, flip angle = 7°, resolution matrix = 256 × 256, slices = 192, slice thickness = 1.0 mm, voxel size = 1.0 × 1.0 × 1.0 mm3). For diffusion data, a diffusion‐weighted, single‐shot, spin‐echo, echo‐planar imaging sequence was used to acquire 60 slices of 2 mm thickness in 30 different diffusion directions (b = 900 s/mm2).
For the control group, the baseline and following‐up magnetic resonance images were acquired with a 3.0‐T Siemens Trio MRI scanner (Siemens Medical, Erlangen, Germany) in the Southwest University Center for Brain Imaging. A magnetization‐prepared rapid gradient echo (MPRAGE) sequence was used to acquire high‐resolution T1‐weighted anatomical images (repetition time = 1900 ms, echo time = 2.52 ms, inversion time = 900 ms, flip angle = 9°, resolution matrix = 256 × 256, slices = 176, thickness = 1.0 mm, voxel size = 1.0 × 1.0 × 1.0 mm3). The diffusion data for each subject were obtained using a diffusion‐weighted, single shot, spin‐echo, EPI sequence (TR/TE = 11,000/98 ms, matrix = 128 × 128, field of view [FOV] = 256 × 256 mm, voxel size = 2.0 × 2.0 × 2.0 mm3, 60 axial slices, 2.0 mm slice thickness, b value 1 = 0 s/mm2, b value 2 = 1,000 s/mm2) in 30 directions.
2.5. Surface‐based morphology analysis
Freesurfer was used in this study to investigate the whole brain cortical reconstruction and volumetric segmentation. Freesurfer image analysis suite (version 5.2.0, http://surfer.nmr.mgh.harvard.edu) is a freely available software suite for processing and analyzing (human) brain MRI images. The technical details of the procedures have been described in the previous studies (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000) and validated in a number of publications (Rosas et al., 2002; Salat et al., 2004). Briefly, preprocessing steps included intensity normalization, skull stripping, Talairach transformation, hemispheric separation, and tissue segmentation. The WM/GM border (i.e., white surface) and GM/cerebrospinal fluid border (i.e., pial surface) were identified and transformed into surfaces. Then, the distance between the two surfaces was calculated for every point, for each hemisphere separately. Next, each subject's cortex was anatomically parcellated and each sulcus and gyrus was labeled and aligned to the FreeSurfer's average surface map according to cortical folding patterns and smoothed using a 10‐mm full‐width half‐maximum Gaussian spatial smoothing kernel. The subcortical volumes were obtained from the automated segmentation for the brain structures implemented in Freesurfer. We extracted 40 labels subcortical structures (brain‐stem, caudate, thalamus, pallidum, putamen, hippocampus, and amygdala) from each hemisphere (Fischl et al., 2002).
2.6. Tract‐based spatial statistics and voxel‐based analysis
We analyzed WM properties using the voxel‐wise tract‐based spatial statistics method from the FMRIB Software Library (FSL) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) (Smith et al., 2006). This method identifies a core WM “skeleton” that is anatomically equivalent across participants. By analyzing voxels only from the core WM, it minimizes partial volume effects that occur when more than one tract goes through a voxel (Smith et al., 2006). First, diffusion data were corrected using eddy correction (eddy_correct) and brain‐extracted using BET in FSL (Smith, 2002). FA images were created by fitting a tensor model using DTI fit, and then FA data from all subjects were aligned into a common standard space using the nonlinear registration tool FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b). Next, the mean FA image was created and thinned to create a mean FA skeleton representing the centers of all tracts common to the group. FA images from individual subject were then projected onto this skeleton and the resulting data fed into voxel wise cross‐subject statistics. Significant regions were thickened for visualization using the TBSS fill script in FSL. The Johns Hopkins University ICBM‐DTI‐81 WM labels atlas was used to locate anatomical structures in the MNI152 spaces.
Voxel‐based analysis (VBA) of FA was performed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm)). The VBA was carried out as following: First, register individual FA images of native space to FA template in the MNI space and then apply the resultant warping transformations to write the images of the diffusion metrics into MNI space. The smooth is needed to alleviate the registration error with an 8 mm full width at Gaussian kernel.
2.7. Statistical analysis
Repeated analyses of variance (ANOVA) were performed to assess the changes in the neuropsychological parameters over time. One‐way analyses of covariance (ANCOVA) tests (covariates: age, sex, and baseline volume) were applied to assess the cortical and subcortical volumes differences between HA and SL participants. Pearson correlations were used to assess the correlations of regional cortical volume values with neuropsychological scores. These statistical analyses were performed with SPSS 13.0 Mac. Statistical significance was set at p < .05. Paired t‐tests were conducted separately in experimental and control group to examine FA differences between the 2 years and its correlations with neuropsychological performance changes using nonparametric permutation tool Randomize in FSL.
3. RESULTS
3.1. Demographic information and HA exposure
The procedures for subject enrollment and follow‐up are shown in Figure 1. The average age of the experimental participants (M:27, F:22) was 19.16 ± 0.86 at the baseline timepoint. All subjects completed the 3‐year high school curriculum and their academic performance in the exams were quite similar (513.3 ± 13.6, range: 483–544); therefore, they were admitted to the same college. In the first year, the average cumulative HA exposure time of all the subjects was 270.0 ± 7.4 days (range: 257 d–299 d). In the second year, the cumulative exposure time increased to 280.8 ± 7.3 days (range: 268 d–314 d). The average number of days between the first scan and the second scan were 550 days.
The average age of the control healthy participants (M:27, F:22) was 19.16 ± 0.86 at the baseline timepoint. The average number of days between the first scan and the second scan were 515 days, and these participants only have MRI data.
3.2. Cognitive function impairment
All of the experimental participants who participated in the baseline and follow‐up studies completed the neuropsychological tests. The neuropsychological changes are noted in Table 1.
Table 1.
Descriptive values (mean/SD) and statistics of the altered cognitive performance during high‐altitude exposure
| Exposure time | 0y (14)mean (SD) | 1y (15)mean (SD) | 2y (16)mean (SD) | F | p | ηp 2 |
|---|---|---|---|---|---|---|
| IVBM | 27.918 (1.630) | 27.041 (2.557) | 25.835 (4.195) | 7.193 | .001 | 0.130 |
| DVBM | 27.286 (2.475) | 25.796 (3.285) | 24.959 (4.015) | 9.732 | .000 | 0.169 |
| ASRT | 0.128 (0.082) | 0.292 (0.065) | 0.357 (0.075) | 156.025 | 000 | 0.765 |
| ACRT | 0.392 (0.114) | 0.471 (0.128) | 0.565 (0.188) | 22.498 | .000 | 0.391 |
Abbreviations: ACRT, auditory recognition reaction time; ASRT, auditory simple reaction time; DVBM, delayed verbal memory; IVBM, immediate verbal memory.
3.2.1. Decreased accuracy in the verbal memory/auditory reaction time test
The IVBM significantly varied over the exposure time (F = 7.193, p = .001) by repeated measures ANOVA analyses. However, only the difference between Year 2 and baseline ([I‐J] = − 2.082, p = .004) was statistically significant in the post hoc pair t‐tests. The number of correct responses in the DVBM were significantly different over the exposure time (F = 9.732, p = .000). And the number of correct responses obtained at Year 1 ([I–J] = −1.–90, p = .008) and Year 2 ([I–J] = −2.327, p = .001) were significantly lower than at baseline but not significantly different from each other.
The subjects' ASRT were significantly prolonged after HA exposure (F = 156.025, p = .000). Post hoc analysis showed that the ASRT measured at Year 1 was significantly longer than that at baseline ([I–J] = 0.164, p = .000), while the reaction time surveyed at Year 2 was longer than that at Year 1 ([I–J] = .229, p = .000). Furthermore, the subjects' ACRT also varied over the exposure time (F = 22.498, p = .000). Post hoc analysis showed that the ACRT acquired at Year 1 ([I–J] = 0.099, p = .010) and Year 2 ([I–J] = 0.173, p = .010) were also significantly longer than that at baseline.
3.3. Brain structural alterations: Decreased volume in the caudate
Vertex‐wise/cluster‐forming threshold was set at 4 (p < .0001) and statistical significance was set at p < .05. Results were corrected for multiple comparisons based on Z Monte Carlo permutations with 5,000 iterations using AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). There were no significant differences of cortical volume between the Year 2 and the baseline in both experimental and control group.
Subcortical volumes analyses showed brain structural alterations after chronic HA exposure. Decreased GM volume was found in the caudate after 2 years' HA exposure (one‐way ANCOVA tests, covariates: age, sex, and baseline volume; F(left) = 12.181, p = .001; F(right) = 8.314, p = .005). All subcortical volumes comparison details were shown in Table 2.
Table 2.
Comparison of subcortical volumes between experimental and control group
| Region | Subcortical volume (mm3, SD) | ||||||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | F | p | ηp 2 | |||
| Left | Pre | Post | Pre | Post | |||
| Thalamus | 7,678.7 (878.7) | 7,572.8 (892.6) | 7,452.2 (848.2) | 7,407.1 (831.1) | 0.000 | 1.000 | 0.000 |
| Caudate | 3,891.8 (439.4) | 3,933.8 (429.6) | 3,498.4 (353.4) | 3,486.1 (348.9) | 12.181** | .001 | 0.114 |
| Putamen | 5,562.4 (447.8) | 5,457.0 (522.5) | 5,191.4 (572.1) | 5,180.2 (552.1) | 1.300 | .257 | 0.014 |
| Hippocampus | 4,131.7 (282.8) | 4,128.9 (314.5) | 4,046.6 (342.3) | 4,061.5 (383.3) | 0.009 | .926 | 0.000 |
| Amygdala | 1,676.1 (203.1) | 1,642.8 (215.9) | 1,593.1 (213.8) | 1,618.6 (212.9) | 2.818 | .096 | 0.029 |
| Right | |||||||
| Thalamus | 7,320.4 (741.2) | 7,160.0 (718.1) | 7,097.9(674.5) | 7,078.0 (644.4) | 1.709 | .194 | 0.018 |
| Caudate | 3,974.0 (423.1) | 4,003.4 (441.5) | 3,572.2 (357.5) | 3,558.5 (355.7) | 8.314** | .005 | 0.080 |
| Putamen | 5,598.8 (479.2) | 5,562.8 (510.6) | 5,306.2 (582.5) | 5,277.9 (564.6) | 0.233 | .630 | 0.002 |
| Hippocampus | 4,333.5 (337.1) | 4,320.6 (351.2) | 4,260.4 (412.2) | 4,256.9 (418.0) | 0.000 | 1.000 | 0.000 |
| Amygdala | 1834.7 (213.3) | 1,793.0 (224.8) | 1,708.9 (234.4) | 1,713.9 (233.1) | 0.818 | .368 | 0.009 |
Abbreviations: SD, standard deviation.
p < 0.01.
3.4. Associations between cognitive changes and subcortical volume alterations
To further understand the effects of the chronic HA exposure on brain subcortical volumes, we conducted correlation analyses between before and after exposure behavioral achievements and volume changes of abovementioned subcortical regions.
Subcortical volume alterations in the left‐hippocampus and right‐caudate were found to be positively associated with the accuracy changes in IVBM (r = 0.325, p = .023; r = 0.286, p = .047, Figure 2). Volume alterations in the left‐putamen and right‐thalamus were negatively correlated with the ACRT and ASRT changes (r = −0.302, p = .035; r = −0.384, p = .006, Figure 2).
Figure 2.
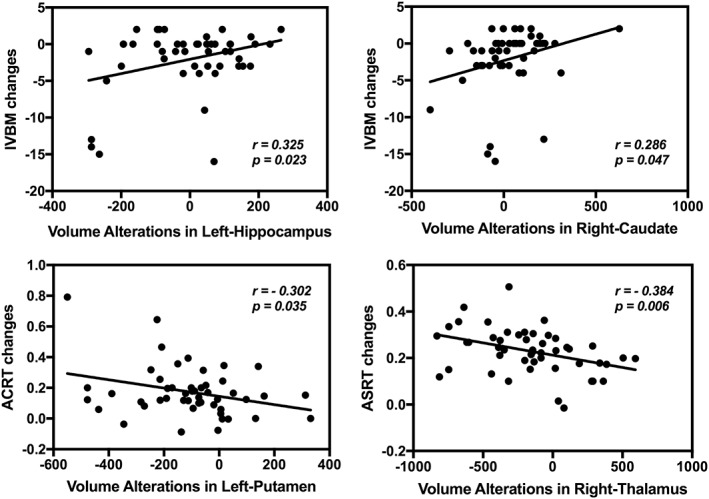
Scatter plots indicating the correlation between the before and after high‐altitude exposure subcortical volume alterations and the corresponding cognitive performance changes (p < .05)
3.5. Fractional anisotropy alterations
3.5.1. HA group
Compared to the pre‐exposure data, FA values after exposure were found to be significantly increased in the regions of right posterior corona radiate (PCR‐R), anterior corona radiate (ACR), splenium of corpus callosum (SCC), and decreased in the regions of superior longitudinal fasciculus (SLF), right anterior limb of internal capsule (ALIC‐R), body of corpus callosum (BCC) (Table 3 and Figure 3). The results of VBA are presented in the Supporting Information.
Table 3.
Main regions showing greater and reduced (clusters >50 voxels) FA in experimental and control groups
| MNI coordinates (peak) | Voxels (mm3) | White matter tract | Corresponding cortical area | FA mean (SD) | p | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Pre | Post | ||||
| Increased FA (EG) | ||||||||
| 26 | −32 | 24 | 3,837 | PCR‐R | Anterior thalamus | 0.526 (0.043) | 0.550 (0.038) | .003 |
| −16 | 30 | −20 | 3,646 | ACR | Inferior fronto‐occipital lobe | 0.305 (0.045) | 0.342 (0.067) | .000 |
| −20 | −51 | 42 | 2,581 | SCC | Cingulate gyrus | 0.580 (0.070) | 0.604 (0.069) | .003 |
| 16 | 37 | −10 | 1,474 | ACR | Inferior fronto‐occipital lobe | 0.565 (0.047) | 0.593 (0.045) | .006 |
| Decreased FA (EG) | ||||||||
| −37 | −8 | 30 | 2084 | SLF‐L | Temporal lobe | 0.521 (0.044) | 0.490 (0.041) | .003 |
| 39 | −24 | 31 | 1,233 | SLF‐R | Temporal lobe | 0.521 (0.059) | 0.496 (0.058) | .002 |
| 18 | 0 | 9 | 494 | ALIC‐R | ALIC | 0.656 (0.030) | 0.618 (0.026) | .008 |
| 14 | −6 | 34 | 492 | BCC | Cingulate gyrus | 0.665 (0.058) | 0.648 (0.061) | .032 |
| Increased FA (CG) | ||||||||
| −7 | −22 | −13 | 339 | ATR‐L | Anterior thalamus | 0.461 (0.061) | 0.502 (0.071) | .045 |
| −29 | −31 | 1 | 313 | ATR‐L | Anterior thalamus | 0.488 (0.039) | 0.536 (0.061) | .034 |
| −39 | −18 | −10 | 298 | SS‐L | Inferior fronto‐occipital lobe | 0.476 (0.055) | 0.508 (0.065) | .035 |
| 44 | −9 | −26 | 290 | ILF‐R | Inferior fronto‐occipital lobe | 0.427 (0.059) | 0.508 (0.071) | .036 |
| 12 | −7 | −9 | 204 | ATR‐R | Anterior thalamus | 0.671 (0.046) | 0.710 (0.051) | .039 |
| 26 | −21 | 0 | 80 | PLIC‐R | PLIC | 0.577 (0.038) | 0.597 (0.038) | .048 |
| 27 | −16 | −7 | 66 | ILF‐R | Inferior fronto‐occipital lobe | 0.397 (0.050) | 0.433 (0.046) | .045 |
Note: Cluster size (p < .05, TFCE corrected) and the location of its peak value in the cluster.
Abbreviations: ACR, anterior corona radiate; ALIC‐R, right anterior limb of internal capsule; ASS‐L, Left sagittal stratum (include inferior longitudinal fasciculus and inferior fronto‐occipital fasciculus); ATR‐R, right anterior thalamic radiation; BCC, body of corpus callosum; CG, control group; EG, experimental group; ILF‐R, right inferior longitudinal fasciculus; PCR‐R, right posterior corona radiate; PLIC‐R, right posterior limb of internal capsule; SCC, splenium of corpus callosum; SD, standard deviation; SLF‐L, left superior longitudinal fasciculus; SLF‐R, right superior longitudinal fasciculus; TR‐L, left anterior thalamic radiation.
Figure 3.
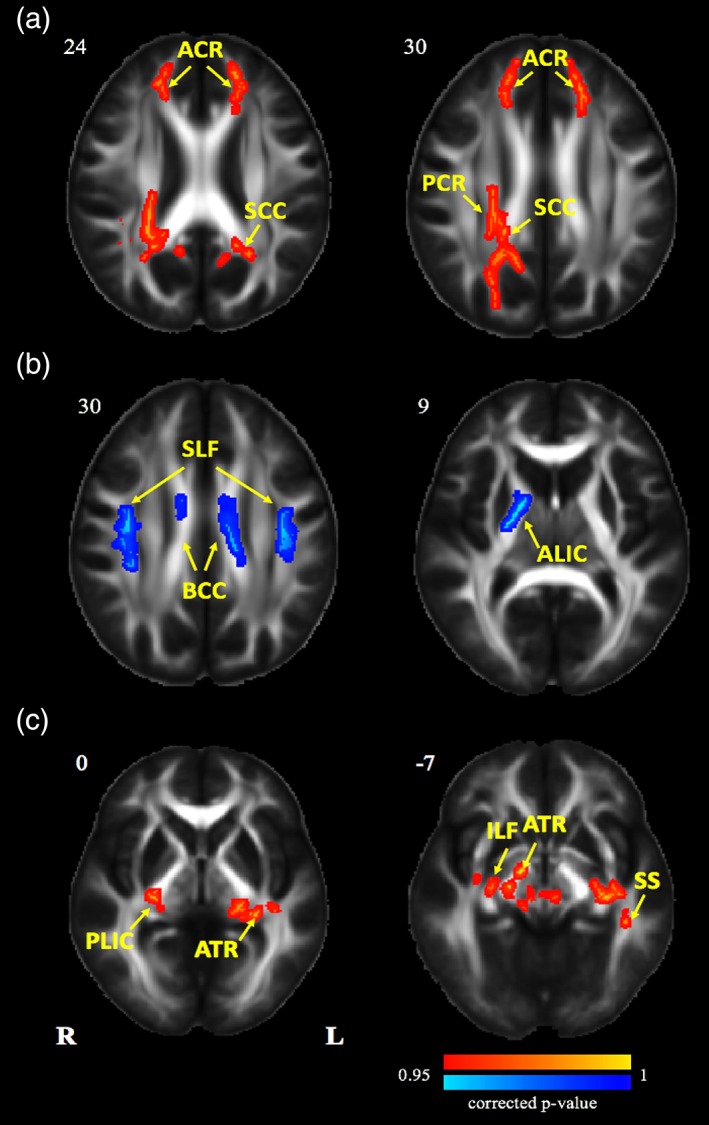
Tract‐based spatial statistics maps are shown the FA differences in experimental and control groups. (a) Red represents regions with significantly increased FA after 2 years' HA exposure; (b) blue represents regions with significantly decreased FA after 2 years' HA exposure; (c) red represents regions with significantly increased FA after nearly 2 years' university life (p < .05, threshold free cluster enhancement (TFCE) corrected). HA, high altitude; FA, fractional anisotropy [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5.2. Control group
Results showed that after 2 years of university life, FA values were significantly increased in the regions of anterior thalamic radiation (ATR), left sagittal stratum (SS‐L), right posterior limb of internal capsule (PLIC‐R), right inferior longitudinal fasciculus (ILF‐R) (Table 3 and Figure 3). The results of VBA are presented in the Supporting Information.
3.6. Significant correlations between cognitive changes and fractional anisotropy alterations
To understand the effects of the chronic HA exposure on structural WM fiber connection, TBSS analyses were also conducted to further develop FA alterations related to behavioral achievements changes in HA group.
Decreased DVBM scores were found to be significantly correlated with FA changes in the ILF‐R (Figure 4).
Figure 4.
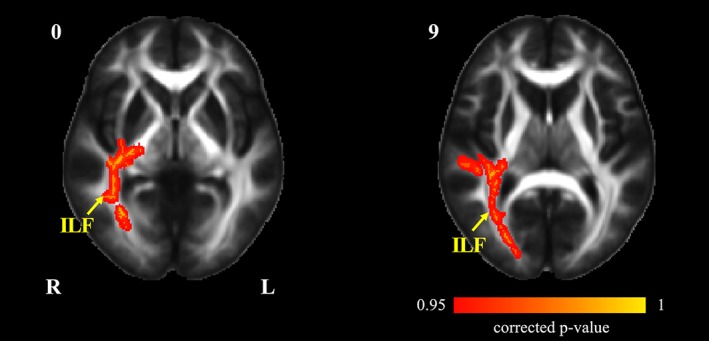
Tract‐based spatial statistics maps are shown the correlation between cognitive changes and FA alterations in HA group. The decreased DVBM scores were significantly correlated with FA in the ILF‐R, inferior longitudinal fasciculus (p < .05, TFCE corrected). DVBM, delayed verbal memory; HA, high altitude; FA, fractional anisotropy; ILF‐R, right inferior longitudinal fasciculus [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The human brain has the lifelong ability to reorganize structural and functional networks to adjust to environmental pressures and physiologic changes (Zatorre, Fields, & Johansen‐Berg, 2012). The possibility of long‐term cerebral sequelae from exposure to severe hypobaric hypoxia has been controversial for decades (Hornbein, 2001). Here, we adopted a longitudinal study on young healthy HA immigrants living in HA for 2 years in order to investigate neuro‐structure basis of the cognitive psychological impairments.
In this study, we recruited subjects under 20 years of age (17–20), who came from less‐developed rural areas and immigrated to Tibet to acquire higher education. People in this age group are more vulnerable to HA hypoxia at the later stage of neurodevelopment, who are forming the main body of Tibet immigrants (Chen et al., 2017). Meanwhile, the cumulative high exposure time of the subjects was nearly 24 months. In order to clarify the structural mechanism of the neuropsychological impairment associated with chronic HA exposure, a longitudinal control group, gender and age‐matched with the experimental group was also included in this study. In summary, this study aim to evaluate the longitudinal changes of cognitive functions and brain structures of HA and SL group, and it might be valuable as a reference for the health maintenance of Tibetan immigrants in China.
The behavioral results revealed that after 2 years' HA‐exposure, subjects' IVBM and DVBM significantly decreased upon HA exposure. The declined verbal memory is consistent with previous studies on hypobaric hypoxia at HA which found a myriad of neurophysiological and neurocognitive dysfunction including vision, memory, language, mood learning, and processing speed (Regard, Oelz, Brugger, & Landis, 1989; Shukitthale et al., 1994; Wilson, Newman, & Imray, 2009). Among these studies, a variety of different types of cognitive assessments have been used, including number‐ and letter‐sequence recognition, word association/generation tasks, and short‐ and long‐term memory. Together with the decreased working memory, both ASRT and ACRT were prolonged among the HA subjects. Previous acute exposure studies using different cognitive tasks have found increased reaction time on acute ascending to HA (Ma et al., 2015), but the opposite result was found with prolonged exposure to HA. Particularly, all of the above cognitive impairments occurred in the early stages of exposure (before Year 1) and continued to decline in subsequent follow‐ups, suggesting that cognitive changes are irreversible under chronic plateau exposure.
From surface‐based morphology analysis, we revealed that there were no significant differences of cortical volume between the Year 2 and the baseline in both experimental and control group. This agrees with previous findings by Kottke et al. (2015), which evaluated the occurrence of structural cerebral changes in a cohort of 38 recreational HA climbers and did not detect structural changes (Kottke et al., 2015). However, some fMRI studies found that compared with the SL individuals, HA young adults who were born and grew up at plateau had decreased volume in the left insula, left inferior parietal gyrus, and right superior parietal gyrus and increased in the left precentral cortex and multiple sites in cerebellar cortex (Usui et al., 2004; Yan, Zhang, Shi, Gong, & Weng, 2010). Here, we suspected the constant GM during prolonged hypoxic exposure could be the result of at least one of the following factors: (a) Neurogenesis directly induced by hypoxia. Adult neocortex such as prefrontal, inferior temporal, and posterior parietal cortex have the capability of neurogenesis (Gould, Reeves, Graziano, & Gross, 1999) and this neurogenesis can be induced (Magavi, Leavitt, & Macklis, 2000). Adolescents are in the late stages of neurodevelopment and hypoxia has been proved to induce adult neurogenesis. (b) Neurogenesis induced by afferent feedback (function‐activated effects). The brain is the source of behavior, but in turn, it is modified by the behaviors it produces. An example is that the increases in gray and WM occurred with learning (Zatorre et al., 2012). As the subjects in this longitudinal study were college students whose primary task was learning and this may partly explain why they maintained stable brain structure even at HA. (c) The interval between our two scans is short, and 2 years may not be enough to change the cortical structures.
After taking gender, age and baseline volume as covariates, brain volume was found to be significantly decreased in the caudate. To the best of our knowledge, this is the first study to provide direct evidence of subcortical GM loss in the caudate after chronic HA exposure. The caudate is involved in the interpretation of individuals' cognitions and emotions on the basis of environmental factor, also a key region for information handling speed, affective and emotional processing (Di Martino et al., 2008; Robinson et al., 2012; Xu et al., 2018). One possible explanation is that cognitive function changes in plateau hypoxia may affect the anatomical morphology of caudate. Involvement of the caudate in chronic hypoxic diseases, such as obstructive sleep apnoea and chronic obstructive pulmonary diseases, is well established (Boero, Ascher, Arregui, Rovainen, & Woolsey, 1999; Kanaan, Farahani, Douglas, LaManna, & Haddad, 2006). Meanwhile, according to clinical studies, the left basal ganglia (including the caudate) appears to be more vulnerable in the intracranial blood supply in the different hemispheres (Benke, Delazer, Bartha, & Auer, 2003); thus, the loss of GM in the left caudate is more than the right side might indicate that the left side is more sensitive to chronic HA hypoxia.
We found that the volume alterations of left‐hippocampus and right‐caudate were positively associated with the accuracy changes in delayed verbal memory. Patient studies of long‐term memory have shown that the hippocampus is critical for recollection (Yonelinas, 2013) and damage to this middle temporal lobe structure may leads to selective deficits in recollection (Baddeley, Jarrold, & Vargha‐Khadem, 2011; Jager et al., 2009). Specifically, various cognitive processes (other than spatial navigational memory) require the left hippocampus more dominantly than the right hippocampus, including temporal sequence memory (Lehn et al., 2009), match–mismatch associative memory (Kumaran & Maguire, 2007) and event memory (Maguire & Frith, 2003). Studies have also implicated the dorsal striatum (caudate and putamen) during the rehearsal of verbal materials, primarily the caudate, may be recruited during working memory tasks (Braver et al., 1997). For example, a previous study reported that the caudate was important for the manipulation of information in working memory (Chai, Abd Hamid, & Abdullah, 2018; Chen et al., 2016). Meanwhile, we also found that volume alterations in the left‐putamen and right‐thalamus were negatively correlated with the auditory recognition reaction time and auditory simple reaction time changes. Putamen, a region associated with working memory and cognitive flexibility, was observed structural connectivity with attention and memory related brain regions (Starr et al., 2011). Volume of thalamus has been found to be associated with recognition in previous study (Gooijers et al., 2016), and our current study provides further evidence for this association. All these findings suggested the potential relationship between the brain anatomical changes and working memory or reaction time, and highlighted the importance of evaluating the cognitive impairment of chronic HA exposure.
FA reflects the structural integrity and geometry of axonal fibers (Gulani and Sundgren, 2006). Greater FA may reflect greater myelination of WM fibers, increased number of myelinated fibers, smaller axonal diameter, or reduced neural branches within MRI voxel (Beaulieu, 2002; Boero et al., 1999; Yan et al., 2011). Whereas reduced FA was associated with local cerebral edema, cerebrospinal fluid, compromised myelin structure, changes in axonal morphologic structure, and altered interaxonal spacing of fiber bundles (Beaulieu, 2002). Increases in anisotropy after HA‐exposure were observed in the basal ganglia, thalamus and prefrontal cortex, which may reflect existence of modulation in the basal‐ganglia‐thalamo‐cortical pathways. The basal ganglia and thalamus have extensive reciprocal connections with the frontal cortex and the anterior cingulate (Graybiel, 2000; Wei & Wang, 2016). These pathways have an important regulatory influence on the cortex and are known to be involved in emotional, behavioral, cognitive, and motor functions as well as in attention, learning and memory (Cummings, 1993; Graybiel, 2000; Herrero, Barcia, & Navarro, 2002; Packard & Knowlton, 2002). WM integrity changes in the BCC and SLF in the HA students were in line with findings of the obstructive sleep apnea patients (Macey et al., 2008) and the congenital central hypoventilation syndrome patients (Patwari et al., 2010), which were proved to be particularly susceptible to hypoxia. Generally, the influence of hypoxic stress, and the organism's response to it, are greater during growth than during adulthood (Boero et al., 1999), and the differences between the highland and lowland natives in their FA are mostly due to adaptations acquired during the developmental period (Frisancho, 1977). One of the most interesting findings of our study is the changes in FA values were symmetric in some regions between the left and right hemispheres in HA students, while the symmetric FA increase in both hemispheres implies the equal impacts of HA on the left and right sides of the brain. Furthermore, we tested the correlation between FA alterations and cognitive achievements, indicating that the ILF‐R impairments of WM may clarify the mechanisms of the decreased DVBM. The ILF extends from the ventral and lateral temporal cortices to the posterior parahippocampal gyrus and is thought to be related to object and face recognition, discrimination, and memory (Schmahmann, 2006). This is in line with previous studies implicating the importance of regions connected by the fronto‐occipital lobe, specifically the frontal cortex, in functions such as trait IQ and cognitive executive function (Hyafil, Summerfield, & Koechlin, 2009; Silton et al., 2010).
Some limitations of the study must be recognized. First, the cognitive changes identified in this longitudinal study were not further confirmed by comparisons with SL group. Second, a 2‐year follow‐up may not be sufficient to assess the dynamic cognitive and anatomical changes that occur upon chronic HA exposure. The following questions remain unclear: whether the changes upon chronic HA exposure are reversible and whether the psychological impairments and neuroimaging changes improve or dissipate over time when the subjects leave the HA environment and return to SL. Third, quality control is really important in a longitudinal study across sites and vendor. MRI images of the HA and SL subjects were acquired on a GE and Siemens Trio scanner, respectively. Although longitudinal comparisons were performed on each group individually, technique differences across sites should be considered. Standard phantom scans over sites and time could further validate our results, but unfortunately, we did not have phantom data because the very beginning of data collection.
5. CONCLUSIONS
We carried out a longitudinal study on young healthy HA and SL populations to assess the cognitive and anatomical changes associated with chronic HA exposure. A series of cognitive functions were identified to be impaired during HA exposure, such as working memory and psychomotor function. Compared with SL residents, our study demonstrated regional GM and WM alterations in adult immigrants who have lived in HA for 2 years. Decreased caudate volume was found after 2 years' HA exposure. Our findings also suggest different WM change patterns between HA and SL participants, indicating the impairments may be existed in the microstructural integrity of WM tracts after HA exposure. Moreover, the cognitive deficits might be attributed to regional GM loss and WM changes. Future research is needed to clarify whether GM loss and WM changes would have recovered to normal after return to SL for a long period of time.
CONFLICT OF INTEREST
The authors declare they have no actual or potential competing financial interests.
Supporting information
Figure S1 Voxel‐based analysis are shown the FA differences in experimental and control groups. (a) Red represents regions with significantly increased FA after two years' high‐altitude exposure; (b) Blue represents regions with significantly decreased FA after two years' high‐altitude exposure; (c) Red represents regions with significantly increased FA after nearly two years' university life (p < 0.05, TFCE corrected)
Table S1 Main regions showing greater and reduced (clusters >50 voxels) FA in experimental and control groups (VBA).
ACKNOWLEDGMENTS
This study was financially supported by the National Science Foundation of China (No. 8133045, 81730053, 81803194, 81502770), the Military Logistics Research Project (No. AWS14L008, AWS16J022, AWS17J013) and the Hundred‐Talent Program, Chinese Academy of Sciences. We thank Southwest University Longitudinal Imaging Multimodal (SLIM) Brain Data Repository for data support.
Chen X, Li H, Zhang Q, et al. Combined fractional anisotropy and subcortical volumetric abnormalities in healthy immigrants to high altitude: A longitudinal study. Hum Brain Mapp. 2019;40:4202–4212. 10.1002/hbm.24696
Xiaoming Chen and Hong Li contributed equally to this study.
Funding information Military Logistics Research Project, Grant/Award Numbers: AWS14L008, AWS16J022, AWS17J013; National Natural Science Foundation of China, Grant/Award Numbers: 8133045, 81502770, 81730053, 81803194; The Hundred‐Talent Program, Chinese Academy of Sciences.
Data Availability: Part of the data used to support the results of this study comes from a publicly available database (Southwest University Longitudinal Imaging Multimodal [SLIM] Brain Data Repository). Another part of the data is not yet available because it involves privacy.
Contributor Information
Jingyuan Chen, Email: jy_chen@fmmu.edu.cn.
Yazhuo Kong, Email: kongyz@psych.ac.cn.
Wenjing Luo, Email: luowenj@fmmu.edu.cn.
DATA AVAILABILITY
Part of the data used to support the results of this study comes from a publicly available database (Southwest University Longitudinal Imaging Multimodal [SLIM] Brain Data Repository). Another part of the data is not yet available because it involves privacy.
REFERENCES
- Andersson, J. L. R. , Jenkinson, M. , & Smith, S. (2007a). Non‐linear optimisation. FMRIB Technical Report TR07JA1.
- Andersson, J. L. R. , Jenkinson, M. , & Smith, S. (2007b). Non‐linear registration, Aka spatial normalisation. FMRIB Technical Report TR07JA2.
- Baddeley, A. , Jarrold, C. , & Vargha‐Khadem, F. (2011). Working memory and the hippocampus. Journal of Cognitive Neuroscience, 23(12), 3855–3861. [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system ‐ a technical review. NMR in Biomedicine, 15(7–8), 435–455. [DOI] [PubMed] [Google Scholar]
- Benke, T. , Delazer, M. , Bartha, L. , & Auer, A. (2003). Basal ganglia lesions and the theory of fronto‐subcortical loops: Neuropsychological findings in two patients with left caudate lesions. Neurocase, 9(1), 70–85. [DOI] [PubMed] [Google Scholar]
- Boero, J. A. , Ascher, J. , Arregui, A. , Rovainen, C. , & Woolsey, T. A. (1999). Increased brain capillaries in chronic hypoxia. Journal of Applied Physiology, 86(4), 1211–1219. [DOI] [PubMed] [Google Scholar]
- Braver, T. S. , Cohen, J. D. , Nystrom, L. E. , Jonides, J. , Smith, E. E. , & Noll, D. C. (1997). A parametric study of prefrontal cortex involvement in human working memory. NeuroImage, 5(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Chai, W. J. , Abd Hamid, A. I. , & Abdullah, J. M. (2018). Working memory from the psychological and neurosciences perspectives: A review. Frontiers in Psychology, 9, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Li, J. Q. , Han, Q. Q. , Lin, J. Z. , Yang, T. H. , Chen, Z. Q. , & Zhang, J. X. (2016). Long‐term acclimatization to high‐altitude hypoxia modifies interhemispheric functional and structural connectivity in the adult brain. Brain and Behavior, 6(9), e00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. M. , Zhang, Q. , Wang, J. Y. , Liu, J. , Zhang, W. B. , Qi, S. , … Luo, W. J. (2017). Cognitive and neuroimaging changes in healthy immigrants upon relocation to a high altitude: A panel study. Human Brain Mapping, 38(8), 3865–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, J. L. (1993). Frontal‐subcortical circuits and human‐behavior. Archives of Neurology, 50(8), 873–880. [DOI] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis ‐ I. segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Davis, J. E. , Wagner, D. R. , Garvin, N. , Moilanen, D. , Thorington, J. , & Schall, C. (2015). Cognitive and psychomotor responses to high‐altitude exposure in sea level and high‐altitude residents of Ecuador. Journal of Physiological Anthropology, 34(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. M. C. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex, 18(12), 2735–2747. [DOI] [PubMed] [Google Scholar]
- Di Paola, M. (2008). Reduced oxygen due to high‐altitude exposure relates to atrophy in motor‐function brain areas (vol 15, pg 1050, 2008). European Journal of Neurology, 15(11), 1256–1256. [DOI] [PubMed] [Google Scholar]
- Fan, C. , Zhao, Y. , Yu, Q. , Yin, W. , Liu, H. , Lin, J. , … Zhang, J. (2016). Reversible brain abnormalities in people without signs of mountain sickness during high‐altitude exposure. Scientific Reports, 6, 33596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Foster, G. E. , Davies‐Thompson, J. , Dominelli, P. B. , Heran, M. K. , Donnelly, J. , duManoir, G. R. , … Sheel, A. W. (2015). Changes in cerebral vascular reactivity and structure following prolonged exposure to high altitude in humans. Physiological Reports, 3(12), e12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho, A. R. (1977). Developmental adaptation to high‐altitude hypoxia. International Journal of Biometeorology, 21(2), 135–146. [DOI] [PubMed] [Google Scholar]
- Gallagher, S. A. , & Hackett, P. H. (2004). High‐altitude illness. Emergency Medicine Clinics of North America, 22(2), 329–355. [DOI] [PubMed] [Google Scholar]
- Ghosh, S. S. , Kakunoori, S. , Augustinack, J. , Nieto‐Castanon, A. , Kovelman, I. , Gaab, N. , … Fischl, B. (2010). Evaluating the validity of volume‐based and surface‐based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. NeuroImage, 53(1), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooijers, J. , Chalavi, S. , Beeckmans, K. , Michiels, K. , Lafosse, C. , Sunaert, S. , & Swinnen, S. P. (2016). Subcortical volume loss in the thalamus, putamen, and Pallidum, induced by traumatic brain injury, is associated with motor performance deficits. Neurorehabilitation and Neural Repair, 30(7), 603–614. [DOI] [PubMed] [Google Scholar]
- Gould, E. , Reeves, A. J. , Graziano, M. S. A. , & Gross, C. G. (1999). Neurogenesis in the neocortex of adult primates. Science, 286(5439), 548–552. [DOI] [PubMed] [Google Scholar]
- Graybiel, A. M. (2000). The basal ganglia. Current Biology, 10(14), R509–R511. [DOI] [PubMed] [Google Scholar]
- Gualtieri, C. T. , & Johnson, L. G. (2006). Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Archives of Clinical Neuropsychology, 21(7), 623–643. [DOI] [PubMed] [Google Scholar]
- Gulani, V. , & Sundgren, P. C. (2006). Diffusion tensor magnetic resonance imaging. J Neuroophthalmol, 26, 51–60. [DOI] [PubMed] [Google Scholar]
- Herrero, M. T. , Barcia, C. , & Navarro, J. M. (2002). Functional anatomy of thalamus and basal ganglia. Childs Nervous System, 18(8), 386–404. [DOI] [PubMed] [Google Scholar]
- Hornbein, T. F. (2001). The high‐altitude brain. Journal of Experimental Biology, 204(18), 3129–3132. [DOI] [PubMed] [Google Scholar]
- Hyafil, A. , Summerfield, C. , & Koechlin, E. (2009). Two mechanisms for task switching in the prefrontal cortex. Journal of Neuroscience, 29(16), 5135–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager, T. , Szabo, K. , Griebe, M. , Bazner, H. , Moller, J. , & Hennerici, M. G. (2009). Selective disruption of hippocampus‐mediated recognition memory processes after episodes of transient global amnesia. Neuropsychologia, 47(1), 70–76. [DOI] [PubMed] [Google Scholar]
- Kanaan, A. , Farahani, R. , Douglas, R. M. , LaManna, J. C. , & Haddad, G. G. (2006). Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. American Journal of Physiology‐Regulatory Integrative and Comparative Physiology, 290(4), R1105–R1114. [DOI] [PubMed] [Google Scholar]
- Kottke, R. , Pichler Hefti, J. , Rummel, C. , Hauf, M. , Hefti, U. , & Merz, T. M. (2015). Morphological brain changes after climbing to extreme altitudes‐‐a prospective cohort study. PLoS One, 10(10), e0141097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran, D. , & Maguire, E. A. (2007). Match‐mismatch processes underlie human hippocampal responses to associative novelty. Journal of Neuroscience, 27(32), 8517–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn, H. , Steffenach, H. A. , van Strien, N. M. , Veltman, D. J. , Witter, M. P. , & Haberg, A. K. (2009). A specific role of the human hippocampus in recall of temporal sequences. Journal of Neuroscience, 29(11), 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. L. , Wang, Y. , Wu, J. H. , Luo, P. , & Han, B. X. (2015). Long‐term exposure to high altitude affects response inhibition in the conflict‐monitoring stage. Scientific Reports, 5(1), 13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey, P. M. , Kumar, R. , Woo, M. A. , Valladares, E. M. , Yan‐Go, F. L. , & Harper, R. M. (2008). Brain structural changes in obstructive sleep apnea. Sleep, 31(7), 967–977. [PMC free article] [PubMed] [Google Scholar]
- Magavi, S. S. , Leavitt, B. R. , & Macklis, J. D. (2000). Induction of neurogenesis in the neocortex of adult mice. Nature, 405(6789), 951–955. [DOI] [PubMed] [Google Scholar]
- Maguire, E. A. , & Frith, C. D. (2003). Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. The Journal of Neuroscience, 23(12), 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, S. A. , Sherman, P. M. , Brown, A. C. , Robinson, A. Y. , Tate, D. F. , Fox, P. T. , & Kochunov, P. V. (2012). Hyperintense white matter lesions in 50 high‐altitude pilots with neurologic decompression sickness. Aviation, Space, and Environmental Medicine, 83(12), 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. L. , & Tamnes, C. K. (2014). Methods and considerations for longitudinal structural brain imaging analysis across development. Developmental Cognitive Neuroscience, 9, 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard, M. G. , & Knowlton, B. J. (2002). Learning and memory functions of the basal ganglia. Annual Review of Neuroscience, 25, 563–593. [DOI] [PubMed] [Google Scholar]
- Patwari, P. P. , Carroll, M. S. , Rand, C. M. , Kumar, R. , Harper, R. , & Weese‐Mayer, D. E. (2010). Congenital central hypoventilation syndrome and the PHOX2B gene: A model of respiratory and autonomic dysregulation. Respiratory Physiology & Neurobiology, 173(3), 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard, M. , Oelz, O. , Brugger, P. , & Landis, T. (1989). Persistent cognitive impairment in climbers after repeated exposure to extreme altitude. Neurology, 39(2), 210–213. [DOI] [PubMed] [Google Scholar]
- Robinson, J. L. , Laird, A. R. , Glahn, D. C. , Blangero, J. , Sanghera, M. K. , Pessoa, L. , … Fox, P. T. (2012). The functional connectivity of the human caudate: An application of meta‐analytic connectivity modeling with behavioral filtering. NeuroImage, 60(1), 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas, H. D. , Liu, A. K. , Hersch, S. , Glessner, M. , Ferrante, R. J. , Salat, D. H. , … Fischl, B. (2002). Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology, 58(5), 695–701. [DOI] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner, R. L. , Snyder, A. Z. , Greve, D. N. , Desikan, R. S. , Busa, E. , … Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , & Pandya, D. N. (2006). Fiber pathways of the brain, New York: Oxford UP. [Google Scholar]
- Shukitthale, B. , Stillman, M. J. , Welch, D. I. , Levy, A. , Devine, J. A. , & Lieberman, H. R. (1994). Hypobaric hypoxia impairs spatial memory in an elevation‐dependent fashion. Behavioral and Neural Biology, 62(3), 244–252. [DOI] [PubMed] [Google Scholar]
- Silton, R. L. , Heller, W. , Towers, D. N. , Engels, A. S. , Spielberg, J. M. , Edgar, J. C. , … Miller, G. A. (2010). The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top‐down attentional control. NeuroImage, 50(3), 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Behrens, T. E. J. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Starr, C. J. , Sawaki, L. , Wittenberg, G. F. , Burdette, J. H. , Oshiro, Y. , Quevedo, A. S. , … Coghill, R. C. (2011). The contribution of the putamen to sensory aspects of pain: Insights from structural connectivity and brain lesions. Brain, 134(7), 1987–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui, C. , Inoue, Y. , Kimura, M. , Kirino, E. , Nagaoka, S. , Abe, M. , … Arai, H. (2004). Irreversible subcortical dementia following high altitude illness. High Altitude Medicine & Biology, 5(1), 77–81. [DOI] [PubMed] [Google Scholar]
- Virues‐Ortega, J. , Buela‐Casal, G. , Garrido, E. , & Alcazar, B. (2004). Neuropsychological functioning associated with high‐altitude exposure. Neuropsychology Review, 14(4), 197–224. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Ke, T. , Zhang, X. , Chen, Y. , Liu, M. , Chen, J. , & Luo, W. (2013). Effects of acetazolamide on cognitive performance during high‐altitude exposure. Neurotoxicology and Teratology, 35, 28–33. [DOI] [PubMed] [Google Scholar]
- Wei, W. , & Wang, X. J. (2016). Inhibitory control in the Cortico‐basal ganglia‐Thalamocortical loop: Complex regulation and interplay with memory and decision processes. Neuron, 92(5), 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. H. , Newman, S. , & Imray, C. H. (2009). The cerebral effects of ascent to high altitudes. Lancet Neurology, 8(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Wu, T. Y. , & Kayser, B. (2006). High altitude adaptation in Tibetans. High Altitude Medicine & Biology, 7(3), 193–208. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Wang, X. , Chen, Z. , Bai, G. , Yin, B. , Wang, S. , … Bai, L. (2018). Longitudinal changes of caudate‐based resting state functional connectivity in mild traumatic brain injury. Frontiers in Neurology, 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, X. D. (2014). Cognitive impairments at high altitudes and adaptation. High Altitude Medicine & Biology, 15(2), 141–145. [DOI] [PubMed] [Google Scholar]
- Yan, X. D. , Zhang, J. X. , Gong, Q. Y. , & Weng, X. C. (2011). Adaptive influence of long term high altitude residence on spatial working memory: An fMRI study. Brain and Cognition, 77(1), 53–59. [DOI] [PubMed] [Google Scholar]
- Yan, X. D. , Zhang, J. X. , Shi, J. F. , Gong, Q. Y. , & Weng, X. C. (2010). Cerebral and functional adaptation with chronic hypoxia exposure: A multi‐modal MRI study. Brain Research, 1348, 21–29. [DOI] [PubMed] [Google Scholar]
- Yonelinas, A. P. (2013). The hippocampus supports high‐resolution binding in the service of perception, working memory and long‐term memory. Behavioural Brain Research, 254, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre, R. J. , Fields, R. D. , & Johansen‐Berg, H. (2012). Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience, 15(4), 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Lin, J. , Sun, Y. , Huang, Y. , Ye, H. , Wang, X. , … Zhang, J. (2012). Compromised white matter microstructural integrity after mountain climbing: Evidence from diffusion tensor imaging. High Altitude Medicine & Biology, 13(2), 118–125. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Yan, X. , Shi, J. , Gong, Q. , Weng, X. , & Liu, Y. (2010). Structural modifications of the brain in acclimatization to high‐altitude. PLoS One, 5(7), e11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Voxel‐based analysis are shown the FA differences in experimental and control groups. (a) Red represents regions with significantly increased FA after two years' high‐altitude exposure; (b) Blue represents regions with significantly decreased FA after two years' high‐altitude exposure; (c) Red represents regions with significantly increased FA after nearly two years' university life (p < 0.05, TFCE corrected)
Table S1 Main regions showing greater and reduced (clusters >50 voxels) FA in experimental and control groups (VBA).
Data Availability Statement
Part of the data used to support the results of this study comes from a publicly available database (Southwest University Longitudinal Imaging Multimodal [SLIM] Brain Data Repository). Another part of the data is not yet available because it involves privacy.


