Abstract
The execution of coordinated hand movements requires complex interactions between premotor and primary motor areas in the two hemispheres. The supplementary motor area (SMA) is involved in movement preparation and bimanual coordination. How the SMA controls bimanual coordination remains unclear, although there is evidence suggesting that the SMA could modulate interhemispheric interactions. With a delayed‐response task, we investigated interhemispheric interactions underlying normal movement preparation and the role of the SMA in these interactions during the delay period of unimanual or bimanual hand movements. We used functional MRI and transcranial magnetic stimulation in 22 healthy volunteers (HVs), and then in two models of SMA dysfunction: (a) in the same group of HVs after transient disruption of the right SMA proper by continuous transcranial magnetic theta‐burst stimulation; (b) in a group of 22 patients with congenital mirror movements (CMM), whose inability to produce asymmetric hand movements is associated with SMA dysfunction. In HVs, interhemispheric connectivity during the delay period was modulated according to whether or not hand coordination was required for the forthcoming movement. In HVs following SMA disruption and in CMM patients, interhemispheric connectivity was modified during the delay period and the interhemispheric inhibition was decreased. Using two models of SMA dysfunction, we showed that the SMA modulates interhemispheric interactions during movement preparation. This unveils a new role for the SMA and highlights its importance in coordinated movement preparation.
Keywords: interhemispheric inhibition, mirror movements, movement preparation, supplementary motor area
1. INTRODUCTION
With training, humans can master highly complex skills ranging from the fluid movements of the virtuoso pianist to the precise life‐saving gestures of the heart surgeon. Most of these complex movements rely on high degree of hand coordination in space and time for which transcallosal mechanisms are essential (Galléa, Popa, Billot, Méneret, Depienne, & Roze, 2011; Gooijers & Swinnen, 2014). Several interconnected cortical areas may influence interhemispheric interactions during the execution of coordinated bimanual movements like the primary motor cortex (M1), dorsal premotor cortex (PMd) as well as the cingulate motor area and posterior parietal cortex (Koeneke, Lutz, Wüstenberg, & Jäncke, 2004; Swinnen, 2002). The communication between the two hemispheres is directly involved during motor execution and interhemispheric interactions are modified even before movement onset, during the preparation period. Motor preparation is broadly defined as the period preceding movement onset and refers to the different steps from decision‐making to movement execution (Bestmann & Duque, 2016; Svoboda & Li, 2018). Delayed response tasks are commonly used to explore the preparation phase. In such paradigms, a first cue informs the subject about the forthcoming movement, while a second cue instructs to move. The “delay period” is the interval between the two cues, during which the subject prepares the movement while remaining immobile, and the “premovement phase” per se is comprised between the instruction to move and movement onset. Many studies focusing on the premovement phase have demonstrated the critical role of interhemispheric inhibition (IHI) and interhemispheric facilitation between the two M1 and between the PMd and the contralateral M1 (Chen & Hallett, 1999; Duque, Hummel et al., 2005; Duque, Mazzocchio, et al., 2005; Duque et al., 2007; Duque et al., 2010; Hinder et al., 2017; Koch et al., 2006; Kroeger et al., 2010; Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000; Liuzzi et al., 2010; Murase, Duque, Mazzocchio, & Cohen, 2004; O'Shea, Sebastian, Boorman, Johansen‐Berg, & Rushworth, 2007). By contrast, there is little insight about the mechanisms and brain regions involved in interhemispheric interactions during the delay period.
The supplementary motor area (SMA), which lies in the medial part of the premotor cortex, has long been known to be involved in movement preparation (Nachev, Kennard, & Husain, 2008; Picard & Strick, 1996) and bimanual coordination (Donchin et al., 2002; Duque, Davare, et al., 2010; Immisch, Waldvogel, van Gelderen, & Hallett, 2001; Kermadi, Liu, Tempini, Calciati, & Rouiller, 1998; Sadato, Yonekura, Waki, Yamada, & Ishii, 1997; Stephan et al., 1999; Toyokura, Muro, Komiya, & Obara, 1999; Uhl et al., 1996; Ullén, Forssberg, & Ehrsson, 2003). When SMA activity is transiently altered, the capacity to produce asymmetric hand movements is impaired (Chan & Ross, 1988; Chouinard & Paus, 2010; Obhi, Haggard, Taylor, & Pascual‐Leone, 2002; Serrien, Strens, Oliviero, & Brown, 2002; Stephan et al., 1999; Steyvers et al., 2003). Unilateral resection of the SMA elicits persistent and striking deficits in complex motor functions requiring high speed, great skill, or bimanual synergy (Krainik et al., 2001; Krainik et al., 2004; Laplane, Talairach, Meininger, Bancaud, & Orgogozo, 1977; Liu, Lai, & Qu, 2004). However, how the SMA influences bimanual coordination is still unclear. Some evidence suggests that the SMA could modulate interhemispheric interactions during coordinated hand movements. The caudal portion of SMA (SMA proper) projects directly to M1 in both hemispheres (Liu, Morel, Wannier, & Rouiller, 2002; Luppino, Matelli, Camarda, & Rizzolatti, 1993; Rouiller et al., 1994). In monkeys, unilateral lesion of the SMA is associated with a trend to perform more symmetric bimanual movements, whereas section of the corpus callosum immediately abolishes this deficit (Brinkman, 1984). Connectivity analysis suggests that the SMA could modulate M1–M1 interactions during movement execution (Sarfeld et al., 2012; Serrien et al., 2002; Siebner et al., 2001). Taking advantage of a pathological model involving patients with congenital mirror movements (CMMs), we brought another clue supporting this hypothesis. These patients are unable to perform asymmetric hand movements and had abnormal interhemispheric coupling between SMA proper and M1 during movement execution (Gallea et al., 2013; Galléa et al., 2011; Welniarz, Dusart, Gallea, & Roze, 2015). In addition, two previous electroencephalography (EEG) study showed altered cortical premovement potentials in CMM patients (Cohen et al., 1991; Franz & Fu, 2017), suggesting abnormal SMA function during movement preparation.
Our goal was to investigate the interhemispheric interactions underlying normal movement preparation as well as the role of the SMA proper in these interactions. We hypothesized that the SMA proper might modulate interhemispheric interactions according to whether or not hand coordination was required (unimanual vs. bimanual movement). We used a delayed response task to explore the preparation of right unimanual and bimanual movements. First, with dynamic causal modeling (DCM), we analyzed SMA proper intrahemispheric and interhemispheric connectivity as well as M1–M1 connectivity during the delay period preceding unimanual or bimanual movements. Second, using dual‐site transcranial magnetic stimulation (TMS), we assessed the excitability of transcallosal pathways from motor/premotor areas in the right hemisphere (PMd, SMA proper, and M1) to the left M1. These analyses were first performed in healthy volunteers (HVs; n = 22), and then in two models of SMA dysfunction: (a) in the same group of HVs after transient disruption of the right SMA proper by continuous transcranial magnetic theta‐burst stimulation (cTBS); (b) in a group of patients with CMM (n = 22), a disorder associated with defective SMA function and interhemispheric coupling. According to our hypothesis, interhemispheric interactions should be modulated depending on whether or not hand coordination is required in HVs, but not after SMA disruption or in CMM patients.
2. MATERIAL AND METHODS
2.1. Subjects and experimental groups
Then, 22 right‐handed HVs and 22 CMM patients were matched for age, gender, and handedness. All the participants had a neurological examination conducted by a trained movement‐disorders neurologist (A.M. or E.R.), focusing on the phenomenology and history of mirror movements. The severity of mirror movements was evaluated with the Woods and Teuber rating scale (Table 1, (Woods & Teuber, 1978)) and quantitatively assessed with the lateralization index. This index was calculated from the electromyographic (EMG) signals recorded from left and right homologous hand muscles during the execution of unimanual movements (see statistical analysis of behavioral data, EMG mirror movement score in Supporting Information). All the participants gave their written informed consent and the protocol was approved by the Ile‐de‐France 6 ethics committee (2013‐A00616‐39).
Table 1.
Demographics and characteristics of the CMM patients. The severity of mirror movements was evaluated with the WT rating scale and quantitatively assessed with the lateralization index. This index was calculated from the electromyographic signals recorded from left and right homologous hand muscles during the execution of unimanual movements. As the EMG was recorded during the fMRI, the laterality index was not calculated for the three patients that did not perform the MRI experiment
| Subject | Age | Sex | Laterality | Family | Score WT | Laterality index |
|---|---|---|---|---|---|---|
| Patient 1 | 45 | F | Right handed | Family 1 | 1 | 0.51 |
| Patient 2 | 20 | H | Right handed | Family 1 | 3 | 0.3 |
| Patient 3 | 34 | F | Right handed | Family 2 | 3 | 0.08 |
| Patient 4 | 63 | H | Right handed | Family 2 | 3 | 0.11 |
| Patient 5 | 56 | H | Right handed | Family 2 | 1 | 0.08 |
| Patient 6 | 18 | H | Right handed | Family 2 | 2 | 0.45 |
| Patient 7 | 41 | H | Right handed | Family 3 | 2 | 0.47 |
| Patient 8 | 44 | F | Right handed | Family 3 | 2 | 0.57 |
| Patient 9 | 30 | F | Right handed | Family 4 | 3 | |
| Patient 10 | 42 | H | Right handed | Family 5 | 2 | |
| Patient 11 | 24 | F | Right handed | Family 6 | 2.5 | 0.46 |
| Patient 12 | 35 | H | Right handed | Sporadic | 3 | 0.12 |
| Patient 13 | 42 | H | Right handed | Sporadic | 3 | 0.55 |
| Patient 14 | 41 | F | Right handed | Sporadic | 3.5 | 0.54 |
| Patient 15 | 43 | F | Right handed | Sporadic | 2 | 0.53 |
| Patient 16 | 44 | H | Right handed | Sporadic | 2 | |
| Patient 17 | 27 | F | Right handed | Sporadic | 2 | 0.87 |
| Patient 18 | 36 | F | Right handed | Sporadic | 2 | 0.71 |
| Patient 19 | 46 | H | Right handed | Sporadic | 2 | 0.78 |
| Patient 20 | 27 | F | Right handed | Sporadic | 3 | 0.57 |
| Patient 21 | 32 | F | Right handed | Sporadic | 3 | 0.67 |
WT = Woods and Teuber scale: 0, no clear imitative movement; 1, barely discernible repetitive movement; 2, slight mirror movements, or stronger, but briefer, repetitive movements; 3, strong and sustained repetitive movement; 4, movement equal to that expected for the intended hand.
2.2. Organization of the protocol
The HVs had five visits. The first and last visits (A and E) were dedicated to the fMRI task, which was performed before and after cTBS or SHAM stimulation of the right SMA proper in a random order across the subjects (Figure 1a). Visit B to Visit D (1 day for each of the three tested circuits, see below) were dedicated to the TMS protocol, which was performed before and within 30 min after cTBS stimulation of the right SMA proper (Figure 1a). The TMS protocol consisted in testing three circuits (M1right–M1left; SMAright–M1left; PMdright–M1left) in random order across the subjects. The minimal interval between two visits was 1 week, to allow complete washout of the cTBS effects. The order of the visits was randomized across the subjects.
Figure 1.
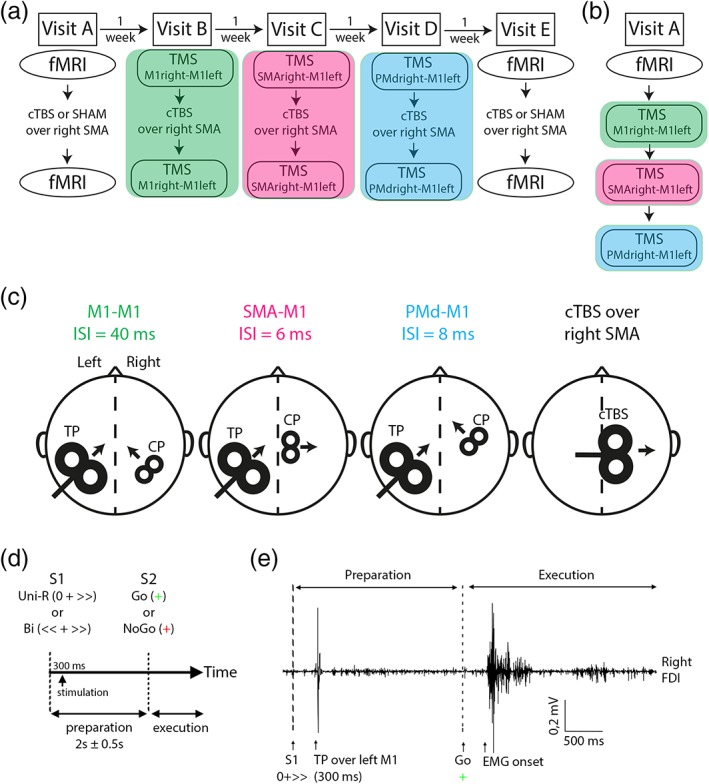
Experimental procedures. (a) Flowchart of the protocol for healthy volunteers (HVs). (b) Flowchart of the protocol for congenital mirror movement patients. (c) Protocol setup of the paired‐pulse transcranial magnetic stimulation (TMS). The test pulse (TP) occurred 300 ms after S1 appearance. The conditioning pulse (CP) occurred at different delays depending on the stimulated area on the right hemisphere (interstimulus interval [ISI]). Protocol setup of the continuous theta‐burst stimulation (cTBS/SHAM) procedure that was only applied in HVs. (d) Schematic representation of the delayed response task. (e) the timing of the TP is represented with an example of the EMG trace recorded in the right FDI of a HVs during the delayed‐response task involving the preparation and execution of a unimanual right hand movement. Bi = bimanual; Uni‐R = unimanual‐right [Color figure can be viewed at http://wileyonlinelibrary.com]
The patient had only one visit, during which they performed the fMRI experiment in the morning, and the TMS (testing of the three circuits described previously) in the afternoon (Figure 1b). Transient SMA proper disruption with cTBS was not performed in patients, and thus there was no need to allow intervals between the experiments.
2.3. Behavioral paradigm
To study movement preparation, we adapted a previously described delayed‐response task (Kroeger et al., 2010) (see Figure 1d). A first visual cue (S1) informed the subjects to respond with the right hand alone (UNI) or with both hands (BI). Following S1 presentation, the subjects had to prepare the movement but remained motionless. A second cue (S2) instructed the subjects either to react (Go) or to withhold the prepared movement (NoGo). In Go trials, the subjects had to tap the finger motor sequence 2‐4‐3 (2: index finger, 3: middle finger, 4: ring finger) with the right hand alone (UNI) or with both hands in a mirrored fashion (BI) on a keyboard compatible with the magnetic environment (one keyboard for each hand) as fast and precisely as possible after S2. Previous studies showed that motor sequence tasks lead to strong SMA activation (Hardwick, Rottschy, Miall, & Eickhoff, 2013; Solopchuk, Alamia, Dricot, Duque, & Zénon, 2017). S2 was a Go signal in 75% of the cases, and a NoGo signal in 25% of the cases. This procedure allowed the attention level to be maintained while minimizing anticipatory responses. Following S2, the subjects had 3 s to respond before the next intertrial interval. The time interval between S1 and S2 ranged between 1.5 and 2.5 s (mean centered on 2 s, jitter of ±0.5 s), and the intertrial interval ranged between 3 and 5 s (mean centered on 4 s, jitter of ±1 s). This experimental design allowed us to isolate the delay period, between S1 and S2, when participants were motionless but were preparing the movement according to S1 (unimanual preparation [UNIP]; bimanual preparation [BIP]). The average duration of a trial was 9 s, which is sufficient to avoid a significant contribution of the BOLD response during motor execution to the delay period of the next trial. The order of conditions was randomized between runs and participants. Participants were familiarized with the task before the first fMRI recording. They were trained during 20 trials outside of the scanner.
2.4. Data acquisition and procedure
2.4.1. Behavioral data
Motor errors (missed or wrong key presses, anticipated key presses before S2 occurrence) were recorded. The averaged reaction time (first key press after S2 occurrence for UNI, average of the first key press of each hand after S2 occurrence for BI) was calculated for each condition.
2.4.2. MRI
The MRI device was a Siemens 3T MAGNETOM Verio with a 32‐channel head coil. Echo planar images were acquired by multiband imaging (TE = 30 ms, TR = 1.31 s, flip angle = 69°, voxel size = 2 × 2 × 2 mm3, 60 slices, MB factor = 4). The participants performed the task previously described, with the two keyboards placed on their upper thighs. Two event‐related conditions of motor preparation of interest were defined, UNIP and BIP (see Supporting Information for details of the global linear model). Participants performed three runs, with 16 repetitions of each condition per run, leading to 48 repetitions of each condition in total. EMG activity was recorded bilaterally from agonist and antagonist muscles controlling the fingers (extensor common digitorum and flexor digitorum) during the fMRI recordings using a magnetic electrodes (reference electrode on the metacarpophalangeal joint). EMG signals were specifically filtered to remove the artifacts related to the gradient, and were stored offline for further analysis (Signal 5.02; CED Ltd., Cambridge, UK).
2.4.3. Study of ipsilateral motor‐evoked potentials in CMM patients
We used single pulse TMS to investigate how neural signals propagate along the corticospinal tract. EMG signals were recorded bilaterally from the first dorsal interosseous (FDI) muscles. Motor‐evoked potentials (MEPs) induced by single monophasic pulses delivered with a figure‐of‐eight coil connected to a Magstim 200 (Magstim, Dyfed, UK) were recorded from electromyographic signals. For M1 stimulation, the coil was placed tangentially over the cortical representations of the hand areas, with the handle pointing backward, 45° from the midline, so that a posterior–anterior current was induced in the left M1. Between 30 and 60 MEPs evoked by calibrated stimulation (1.3× the resting motor threshold) of the dominant hemisphere were recorded bilaterally in the FDI muscles to compare the frequency, latency, and amplitude of the normal contralateral MEPs with those of any mirror MEPs recorded in the hand ipsilateral to the stimulation site.
2.4.4. Study of interhemispheric interactions
EMG activity was recorded bilaterally from the FDI muscle, (active electrode over the motor point and reference electrode on the metacarpophalangeal joint) with disposable surface Ag/AgCl electrodes (Kendall, Covidien). The FDI is a prime mover muscle required for the index finger involved in task performed by the participants. Responses were amplified (1,000×) and filtered (10–1,000 Hz; Digitimer D360; Digitimer Ltd., Welwyn Garden City, UK), then digitally transformed at a sampling rate of 2000 Hz (CED Power 1401; CED Ltd., Cambridge, UK), and stored offline for further analysis (Signal 5.02; CED Ltd.).
We tested different interhemispheric interactions directed toward the left M1. TMS test pulses (TPs) were applied to the FDI hotspot in the left M1. They were preceded or not by conditioning TMS pulses (CP) targeting cortical areas in the right hemisphere (FDI hotspot in right M1, SMA proper or PMd, see Figure 1c for head/coil representation). TMS pulses were delivered through two figure‐of‐eight coils (70‐ and 25‐mm coils for TP and CP, respectively) connected to two Magstim 200 units delivering monophasic current waveforms (Magstim). For M1 stimulation, the coils were placed tangentially over the cortical representations of the hand areas, with the handle pointing backward, 45° from the midline, so that a posterior–anterior current was induced in the corresponding M1. The target in SMA proper was defined using the subjects' individual anatomical images. The SMA proper target was marked on the MRI on the midline and posterior to a vertical line from the anterior commissure perpendicular to the anteroposterior commissure line in the sagittal place, which is a standardized separator for the SMA proper and the pre‐SMA (Picard & Strick, 1996; Vorobiev, Govoni, Rizzolatti, Matelli, & Luppino, 1998). The center of the coil was positioned over the target and the coil oriented so that the induced current was directed from the midline toward the targeted right SMA (Arai, Lu, Ugawa, & Ziemann, 2012). For stimulation of the right PMd, the coil was positioned anterior to the right M1 hotspot, at a distance corresponding to 8% of the distance from nasion to inion (typically around 3 cm) (Koch et al., 2006; Kroeger et al., 2010; Liuzzi et al., 2010). All the stimulation sites were stored in a MRI‐based neuronavigation system (Brainsight, Rogue Research, Montreal, Canada) fed with the subjects' individual MRIs, allowing us to maintain same stimulation conditions throughout the experimental sessions. Measurements of the FDI active and resting motor thresholds (AMT, active motor threslhold and RMT, resting motor threshold) were done in each hemisphere according to the standard procedure (Rothwell et al., 1999).
At baseline, we measured the amplitude of the MEPs triggered by TPs delivered over the left M1 at 1.3 × RMT (A MEP[TP]). To measure interhemispheric interactions, TPs were preceded by CPs delivered as follows: (a) to the right M1, 40 ms before the TP (1.2 × RMT: long IHI) (Reis et al., 2008); (b) to the right SMA proper, 6 ms before the TP (1.4 × AMT) (Arai et al., 2012); or (c) to the right PMd, 8 ms before the TP (1.1 × RMT) (Kroeger et al., 2010). The amplitude of MEPs evoked by CP + TP are noted A MEP[CP + TP]. The interstimulus interval (ISI) and the intensity of the CP were chosen according to previous results. Several studies reported a robust interhemispheric inhibition between the PMd and the contralateral M1 for short ISI (8–10 ms) and subthreshold CP (Koch et al., 2006; Kroeger et al., 2010; Liuzzi et al., 2010; Reis et al., 2008). For SMA–M1 stimulation, we used the results from a study that tested intrahemispheric interactions between SMA and M1: the authors reported a facilitation at short ISI (6 ms) for subthreshold CP (Arai et al., 2012). Finally, for M1–M1 interactions, the long IHI (ISI = 40 ms) as been widely used to test interhemispheric interactions (Gallea et al., 2013; Reis et al., 2008). The short IHI (ISI = 10 ms) was not measured because, in CMM patients, the presence of bilateral MEP responses to TMS of each hemisphere makes it not reliable (Gallea et al., 2013).
TMS measurements were performed at rest and during the task previously described. During the task, TMS stimulations (TP alone or CP + TP) were delivered during the delay period, 300 ms after S1 (see Figure 1e for the timing of TP during the task performance). For each circuit (M1right–M1left; SMAright–M1left; PMdright–M1left), the outcome measures was the ratio: mean of 20 A MEP[CP + TP]/mean of 20 A MEP[TP] at rest, and mean of 15 A MEP[CP + TP]/mean of 15 A MEP[TP] during the task.
2.4.5. Verification of motor activity during the task
To verify that the participants performed the motor task correctly, and in particular to check that they remained motionless during the delay period, their hand movements were video recorded within the MRI. During the TMS experiments, the experimenters checked the hand movements and EMG traces of the two FDIs.
2.4.6. Transient disruption of SMA proper
We used cTBS to interfere with the activity of the right SMA proper in HVs: a total of 600 pulses were delivered at an intensity of 0.9 × AMT, in bursts of three pulses at 50 Hz, with bursts repeated at a frequency of 5 Hz for a total duration of 40 s (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). We used a 70 mm figure‐of‐eight coil that was positioned as previously described, so that the induced current was directed from the midline toward the targeted right SMA. This configuration (coil positioning and stimulation intensity) has been previously used to successfully disrupt SMA activity (Solopchuk et al., 2017; Zénon, Sidibé, & Olivier, 2015). TMS or fMRI recordings were performed before and within 30 min after cTBS or SHAM stimulation of the right SMA proper (see Figure 1a for the organization of the flowchart, and Figure 1c for the head/coil representation). The SHAM stimulation was performed with a special coil (SHAM coil) that delivered low intensity stimulations. We chose to inhibit the right SMA because we wanted to assess the transcallosal effects of the SMA on the active (left) M1.
2.5. Statistical analysis
2.5.1. Behavioral data
The EMG signals recorded during the fMRI session were quantified and a lateralization index reflecting the mirror movements severity score was calculated (see Supporting Information and Figure S1a). For both MRI and TMS experiments, we measured the RT to initiate the first button press in each task for each participant. The RT after the stimulation (SHAM or cTBS) was normalized by the RT before stimulation (normalized RT = Post/Pre). We also measured the number of premature responses occurring before S2 appearance. Premature responses were voluntary movements that the participant failed to withhold until the imperative stimulus appearance. In the first model of SMA dysfunction, we considered the effects of SMA disruption in healthy subjects on the RTs and the number of premature responses. To do so, a repeated measure 2 × 2 × 2 ANOVA (Procedure: cTBS, SHAM; Session: Pre, Post; Condition: UNI, BI) was performed for the normalized RT and the number of premature responses. In the second model of SMA dysfunction, we compared RTs, number of premature responses and the laterality index between healthy subjects and CMM patients. To this aim, the RT and number of premature responses were entered into a repeated measure 2 × 2 ANOVA design (Group as categorical predictor: CMM, HVs; condition as the factor with repeated measures: UNI, BI). Main effects and interaction effects were considered significant at p < 0.05. When these effects were significant, post hoc t tests were performed.
2.5.2. Functional MRI activation
Data were processed and analyzed with statistical parametric mapping software (SPM8) (see Supporting Information for more details). Using individual global linear models, contrasts (UNIP or BIP vs. implicit baseline) were defined to obtain individual Z‐score maps over the whole brain. First, we tested whether cTBS over the SMA affected BOLD signal amplitude during the delay period in the HVs. We used a 2 × 2 × 2 ANOVA (Procedure: cTBS, SHAM; Session: Pre, Post; Condition: UNIP, BIP). Second, we tested the hypothesis of a group difference (CMM patients vs. healthy controls) in activation of networks involved in the transmission of motor plans during the delay period. The individual contrasts in each group were entered separately in a 2 × 2 ANOVA (Group: CMM patients, HVs; Condition: UNIP, BIP). All ANOVA results were considered significant at p < 0.05 with family‐wise error (FWE) correction for multiple comparisons over the whole brain for main effects, and for interaction effects. Using a two‐sample t test in an exploratory analysis, we tested the effect of age as covariate of interest on brain activation involved in the delay period. We entered the main effect of motor preparation separately for each group (individual F tests of both unimanual and bimanual movement preparation), adding two new columns (the first with the age of HVs, the second with the age of patients). We then defined an F contrast (interaction) to test for any region in the whole brain where the BOLD versus age effect is different for patients versus controls (results considered significant at p < 0.001 uncorrected voxel wise, with FWE correction at the level of the cluster).
2.5.3. Effective MRI connectivity
We tested for differences in corticocortical connectivity during the delay period between stimulation procedures (cTBS, SHAM) and between patients and HVs. We investigated effective connectivity in brain networks involved in the transmission of motor plans by means of DCM, 2010. The network included bilateral SMA proper and M1 (Gallea et al., 2013; Grefkes, Eickhoff, Nowak, Dafotakis, & Fink, 2008) using fully connected models (intrinsic or A parameter). Regions of interest (ROIs) and models are presented in Figure S2. ROIs were identified at the individual level to take into account the individual anatomy of the hand knob of M1 (Yousry et al., 1997), at a voxel‐wise threshold of p < 0.001 (uncorrected for multiple comparisons) and a cluster‐wise threshold of p < 0.05 (FWE corrected for multiple comparisons). Note that this threshold can be used for ROI analyses with strong priori hypotheses (Fallon, Chiu, Nurmikko, & Stancak, 2016), especially when greater anatomical precision is needed. ROIs of the SMA proper network included the left and right SMA proper, as well as the left and right M1 hand area. These ROIs were defined as spheres (3 mm radius for better spatial specificity) centered on the global maximum of each of the four areas for the average effect of condition (see Table S1). We verified that the average effect of condition, taking into account both unimanual and bimanual conditions, systematically showed the involvement of left and right M1 and SMA proper in all participants. Time series were extracted from global maxima using the first eigenvariate vector. Different DCM models were compared using Bayesian Model Selection (Stephan & Friston, 2010) to isolate the model with the best fit to the data (Figure S3). Bayesian Model Averaging (weighted according to the level of data fit) was used to compute group differences between HVs with SHAM and real cTBS of the SMA proper and between patients and HVs (see Supporting Information for statistical details). The threshold of significance for Bayesian statistics of procedure (Post cTBS vs. Pre cTBS) or group (patients vs. HVs) difference was set a p > 0.95 corrected for multiple comparisons. Finally, we tested whether SMA could drive interhemispheric connectivity between homologous left and right M1 during UNIP and/or BIP, differently in healthy subjects than in patients. To this end, we evaluated the correlation between individual values of SMA connectivity strength and M1 to M1 connectivity strength (B parameters), separately during UNIP and BIP in HVs and patients.
2.5.4. Transcranial magnetic stimulation
To test the effect of a conditioning stimulation delivered on the right hemisphere (M1right, PMdright, or SMAright) on the amplitude of the MEP evoked in the right FDI by the TP, the values A MEP[TP] and A MEP[CP + TP] were compared with paired Wilcoxon tests. To compare the interhemispheric interactions at rest in HVs before and after transient SMA proper disruption on the one hand, and between CMM patients and HVs on the other hand, the ratios A MEP[CP + TP]/A MEP[TP] for each circuit were compared with Wilcoxon tests. To test whether cTBS over the SMA proper affected interhemispheric interactions during the delay period in the HVs, we then used a repeated measure 2 × 2 ANOVA (Session: Pre, Post; Condition: UNIP, BIP). To test interhemispheric interactions in CMM patients during the delay period, the ratios A MEP[CP + TP]/A MEP[TP] obtained during the delay period were entered separately for each circuit in a 2 × 2 ANOVA (Group: CMM patients, HVs; Condition: UNIP, BIP).
2.5.5. Correlations between fMRI, TMS findings, and behavioral performance
To test whether fMRI and TMS findings were related in CMM patients, we looked at correlations between fMRI connectivity measures and IHI values during the delay period. In addition, we tested whether fMRI and TMS findings would correlate with the motor performance (RT) and the severity of mirror movement measured with the laterality index (see Supporting Information). Depending on the normality of the data distribution, we used a Pearson correlation test or a Spearman correlation test (considered significant at p < 0.05 after correction for multiple comparisons).
3. RESULTS
3.1. Patients and behavioral data
3.1.1. Rationale and summary
Clinical characteristics and task‐related performances were assessed in all participants (CMM patients and HVs) to quantify the severity of mirror movements and behavioral performance. The results show that: (a) CMM patients have abnormal bilateral involvement of hand muscles during unimanual right hand movements which is not seen in controls; (b) CMM patients are slower to initiate their motor responses as compared to controls irrespective of whether hand coordination was needed or not; and (c) SMA proper transient disruption does not alter the RT of HVs.
3.1.2. Patients
Among 22 CMM patients recruited in the protocol, one patient did not show clear mirror movements during the performance of the task and was removed from the study. The characteristics of the patients are detailed in Table 1. One patient had contraindications to perform the TMS study (history of an isolated seizure: Patient 4), so that 20 patients participated in the TMS protocol. For the fMRI study, three patients had contraindications to perform an MRI (one was a welder with possible metallic foreign body in the eye area, another had an intrauterine contraceptive device whose MRI compatibility was unknown, the last being claustrophobic: Patients 9, 10, and 12). Therefore, only 18 patients were included in the MRI part of the protocol. Consequently, 20 and 18 age and gender matched HVs were considered for the TMS and fMRI analyses, respectively. For the experiments involving cTBS over SMA proper performed only in HVs, the whole population was included.
3.1.3. Behavioral data
The results of the ANOVA on the EMG lateralization index showed a main effect of Group (F 1,38 = 6.49, p = 0.01), a main effect of Condition (F 1,38 = 120.96, p < 0.001) and an interaction Group × Condition (F 1,38 = 7.70, p = 0.007). Post hoc t‐test revealed that CMM patients had bilateral involvement of hand muscles during the UNI condition (EMG lateralization index: T 1,38 = 7.84, p = 0.001, Figure S1).
For premature responses, there were no significant Procedure, Condition, or Session difference when we compared the HVs before and after SMA proper disruption (effect of Procedure: p = 0.35; Session: p = 0.28; Condition: p = 0.57). There was no group or condition difference when we compared the CMM patients with HVs (main effects of Group: p = 0.07; Condition: p = 0.35).
The normalized RT Post/RT Pre was not different after SHAM and cTBS during UNI and BI conditions (Procedure: p = .84; Condition: p = .51; interaction Procedure × Condition: p = 0.87; Figure 2a). When we compared the CMM patients with HVs, patients were slower than healthy controls to initiate their motor responses (main effect of Group: F 1,38 = 6.03; p = 0.02; Figure 2b) during both UNI and BI conditions (no significant Group × Condition interaction effect, F 1,38 = 1.61, p = 0.21; effect of Condition, F 1,38 = 5.27, p = 0.03).
Figure 2.
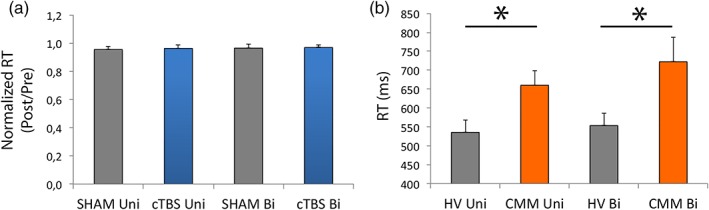
Motor responses are delayed in congenital mirror movement (CMM) patients. (a) Main effect of procedure on the normalized reaction time (RT) in healthy volunteers (HVs): The procedure (SHAM or continuous theta‐burst stimulation [cTBS]) had no effect on the normalized RT (post/pre) during UNI and BI conditions. (b) Main effect of group on RT: Patients (CMM) were slower than HVs to execute the first button press of the sequence for the UNI and BI conditions [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Magnetic resonance imaging
3.2.1. Rationale and summary
We test the hypothesis that in HVs, interhemispheric interactions are modulated during the delay period depending on whether or not hand coordination is required. We then analyze whether these interhemispheric interactions are altered after SMA disruption in HVs or in CMM patients, two models of SMA dysfunction. First, with brain activation analysis, we identified brain areas that were abnormally involved during the delay period of unilateral or bilateral hand movements in CMM patients. This analysis showed an abnormal activation of right M1 during unimanual right hand movements in CMM patients compared to healthy controls. In addition, activation in the left SMA proper during the delay period increased with age in CMM patients whereas it was independent of age in HVs. Second, with dynamic causal modeling (DCM), we analyzed SMA proper intrahemispheric and interhemispheric connectivity as well as M1–M1 connectivity during the delay period preceding unimanual or bimanual movements. This analysis showed that in HVs, interhemispheric connectivity during the delay period was modulated according to whether or not hand coordination was required for the forthcoming movement. In HVs following SMA disruption and in CMM patients, interhemispheric connectivity was modified during the delay period compared to HVs.
3.2.2. Activation analysis
The subject performed the delayed‐response task in the MRI, and the MRI analysis was focused on the delay period. The cTBS on the right SMA proper did not affect BOLD signal amplitude in the cortical motor/premotor areas during the delay period (no effect of Procedure, no Procedure × Session or Procedure × Session × Condition interaction). We observed a main effect of Condition: the activation of the right motor network was larger during the delay period for bimanual movements as compared to right unimanual movements (Table 2). We also observed a main effect of Session: the activation of the right superior parietal lobule and bilateral precuneus was larger during the prestimulation compared to the poststimulation sessions (Table 2). When we compared the CMM patients with HVs, consistently with the previous results, motor activation in the right hemisphere was enhanced during the delay period for bimanual movements as compared to right unimanual movements, similarly in the two groups (positive effect of Condition; Figure 3a, Table 3). In addition, there was a Group × Condition interaction due to greater activation of the right M1 in CMM patients compared to HVs during UNIP (Table 3). Thus, abnormal activation of the ipsilateral M1 that has been reported during motor execution in CMM patients is also present during movement preparation. This suggests that this abnormal cortical activation is not a consequence of mirror movements, but might rather contribute to the inability of these patients to produce purely unimanual movements. Finally, there was an interaction between age and group in the left SMA proper only (coordinates [x, y, z]: −10, −14, 76; cluster volume = 36 voxels; F value = 14.17; threshold of significance: p < 0.001 with small volume correction; Figure 3b). Delay period‐related activation in this area increased with age in CMM patients whereas it was independent of age in HVs (Figure 3b).
Table 2.
Comparison of BOLD response during movement preparation before and after SMA‐proper stimulation with cTBS or SHAM in HVs. MNI coordinates were derived from a 2 × 2 × 2 ANOVA procedure (cTBS; SHAM) × session (before stimulation; after stimulation) × condition (unimanual right; bimanual). Contrasts for the main effect of procedure, session, and condition were thresholded at a corrected threshold of p < 0.05 at the level of the whole brain
| Contrast/anatomical location | MNI coordinates | Z score (F score) | kE |
|---|---|---|---|
| Effect of Condition: right precentral | 36–22–54 | Inf (789.3) | 7,762 |
| Right SMA | 8–22–52 | Inf (314.82) | |
| Left cerebellum | −16–48–20 | Inf (310.19) | 898 |
| Right thalamus | 18–20 6 | Inf (214.48) | 2,369 |
| Right frontal inferior | 58–10–28 | 6.75 (54.36) | 220 |
| Effect of Procedure: — | – | – | – |
| Effect of Session : right parietal inferior | 44–56–52 | 5.14 (30.31) | 52 |
| Left precuneus | ‐4–64–54 | 5.11 (29.93) | 96 |
CMM = congenital mirror movement; cTBS = continuous theta‐burst stimulation; HVs = healthy volunteers; SMA = supplementary motor area.
Figure 3.
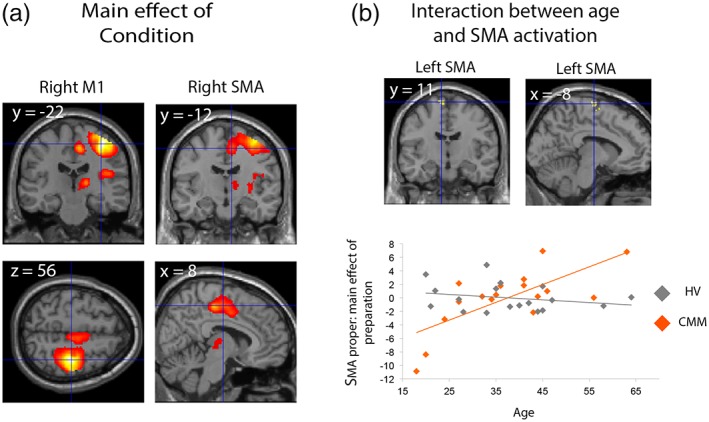
Brain activation during the delay period in healthy volunteers (HVs) and congenital mirror movement (CMM) patients. (a) Anatomical location of brain areas that are more activated during BIP (preparation of bimanual movement) compared to unimanual preparation (preparation of unimanual movement; positive effect of condition). (b) The interaction between age and group during motor preparation showed the sole involvement of the left supplementary motor area proper (upper panel). Whereas motor preparation‐related activation in this area increased with age in CMM patients whereas it was independent from age in HVs (bottom panel) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Comparison of BOLD response during right unimanual or bimanual movement preparation in CMM patients and HVs
| Contrast/anatomical location | MNI coordinates | Z score (F score) | kE |
|---|---|---|---|
| Effect of Condition | |||
| Right precentral | 36–22–56 | Inf (203.75) | 3,714 |
| Right SMA | 8–12–52 | 6.85 (72.36) | |
| Left cerebellum | −14–48–22 | Inf (137.51) | 648 |
| Right thalamus | 16–20–8 | 7.6 (97.56) | 259 |
| Right Rolandic operculum | 46–18–18 | 7.48 (92.86) | 546 |
| Effect of Group: — | – | – | – |
| Interaction Group × Condition | |||
| Right precentral | 36–18–52 | 4.54 (26.37) | 2 |
CMM = congenital mirror movement; HVs = healthy volunteers; SMA = supplementary motor area. MNI coordinates were derived from a 2 × 2 ANOVA Group (CMM patients; HV) × Condition (unimanual right; bimanual). p < 0.05 corrected at the whole brain level. We then used pairwise t test to compare the activations between CMM patients and HV during the preparation of unimanual and bimanual movements.
3.2.3. Connectivity analysis
Information on the Bayesian models are presented in Supporting Information. For both HVs and CMM patients, the data fitted better with models including interhemispheric connections (Figures S2 and S3). First, we analyzed the effect of Condition (UNIP or BIP) separately in HVs and in CMM patients based on Bayesian model averaging (Figure 4a; Table 4). In HVs, during the delay period of UNIP, intrahemispheric (SMAleft–M1left) and interhemispheric (SMAleft–SMAright, SMAleft–M1right, M1left–M1right) connectivities were negative. During the delay period of BIP, the intrahemispheric connection remained negative while interhemispheric connections became positive (Figure 4a, left panel; Table 4). In CMM patients, SMAleft–M1left and M1left–M1right connectivities were positive during UNIP and negative during BIP. SMAleft–SMAright connectivity was negative irrespective of the movement being prepared. Last, similarly to HVs, SMAleft–M1right connectivity was negative during UNIP and positive during BIP (Figure 4a, middle panel; Table 4). When HVs were compared to CMM patients, SMAleft–SMAright connectivity was more negative in HVs during UNIP. This connection turned positive during BIP in HVs while it remained negative in CMM patients, although the group difference was not significant. The drive M1left–M1right was of opposite sign between HVs and CMM patients during both UNIP and BIP conditions: in CMM patients, M1left–M1right connectivity was positive during the delay period of unimanual movements, while it was negative for bimanual movements (Figure 4a, Table 5). In addition, M1right–M1left connectivity was more positive in CMM patients as compared to HVs during BIP. Finally, SMAleft–M1left and SMAleft–M1right connectivities did not significantly differ between the two groups during UNIP and BIP.
Figure 4.
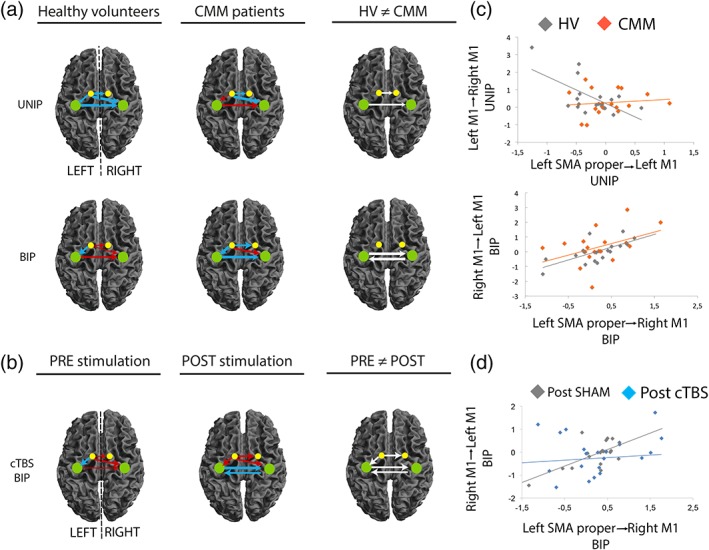
Supplementary motor area (SMA) connectivity network during the delay period. (a) SMA network connectivity between unimanual preparation (UNIP) and bimanual preparation (BIP) in healthy volunteers (HVs) and in congenital mirror movement (CMM) patients. The right column shows the connections that are significantly different between HVs and CMM patients during UNIP and BIP. (b) SMA proper network modulatory connectivity in HVs during BIP (preparation of bimanual movement) was affected by transient SMA disruption but not by the SHAM stimulation. The right column shows the connections that are significantly different between pre and post continuous theta‐burst stimulation (cTBS) sessions for the BIP condition. (c) During UNIP (preparation of unimanual movement), connectivity between left SMA proper and left M1 correlated with the connectivity between left M1 and right M1 in healthy controls (gray plot), but not in patients (orange plot). During BIP, connectivity between left SMA proper and right M1 correlated with the connectivity between right M1 and left M1 in healthy controls (gray), and showed the same tendency in patients (orange, p = 0.08). (d) During BIP, connectivity between left SMA proper and right M1 correlated with the connectivity between right M1 and left M1 in healthy controls after SHAM but not cTBS stimulation. The colors of the arrows represent connections showing a significant positive (red) or negative (blue) drive in each group. Arrow thickness represents the connectivity strength (larger arrows represent greater connectivity toward negative or positive values). White arrows indicate connections that are significantly different between HVs and CMM patients (a) or in HVs before and after cTBS on the right SMA (b). The colored dots indicate the SMA proper (yellow) and M1 (green) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Condition differences of DCM connectivity strength within group. Significant differences are marked with a star, threshold of significance considered at Pp > 0.95 corresponding to 5% of chance of false discovery rate (Pp = posterior probability). UNIP and BIP correspond to values of modulatory connectivity (B parameter of the DCM matrix) specific to each condition. Effect size corresponds to Cohen's d ([M2–M1]/SD pooled), where M1 and M2 are the average value for each group or condition, and SD pooled = √([SD 1 2 + SD 2 2]/2)
| SMA proper left → M1 left | SMA proper left → M1 right | SMA proper left → SMA proper right | M1 left → M1 right | |
|---|---|---|---|---|
| HV | ||||
| UNIP | −0.24 ± 0.10 | −0.57 ± 0.10 | −0.41 ± 0.09 | −0.59 ± 0.11 |
| BIP | −0.17 ± 0.14 | 0.03 ± 0.14 | 0.01 ± 0.11 | 0.32 ± 0.12 |
| Pp (effect size) | 0.66 | 0.99* (−4.93) | 0.99* (−4.18) | 0.95* (−7.91) |
| CMM | ||||
| UNIP | 0.04 ± 0.10 | −0.51 ± 0.11 | −0.11 ± 0.11 | 0.25 ± 0.11 |
| BIP | −0.48 ± 0.15 | 0.21 ± 0.14 | −0.13 ± 0.12 | −0.40 ± 0.12 |
| Pp (effect size) | 0.99* (−4.08) | 1* (−5.72) | 0.57 | 1* (−5.65) |
CMM: patients with congenital mirror movements; DCM = dynamic causal modeling; HVs = healthy volunteers; SMA = supplementary motor area; UNIP = unimanual preparation.
Table 5.
Group, procedure, and session differences of DCM connectivity strength. Significant differences are marked with a star, threshold of significance considered at Pp > 0.95 corresponding to 5% of chance of false discovery rate (Pp = posterior probability). Results with gray font are connections that are not significantly different from zero. UNIP and BIP correspond to values of modulatory connectivity (B parameter of the DCM matrix) specific to each condition. Effect size corresponds to Cohen's d ([M2–M1]/SD pooled), where M1 and M2 are the average value for each group or condition, and SD pooled = √([SD 1 2 + SD 2 2]/2)
| SMA proper left → M1 left | SMA proper left → M1 right | SMA proper left → SMA proper right | M1 left → M1 right | M1 right → M1 left | |
|---|---|---|---|---|---|
| UNIP | |||||
| HV | −0.24 ± 0.11 | −0.57 ± 0.11 | −0.40 ± 0.09 | −0.59 ± 0.11 | |
| CMM | 0.04 ± 0.10 | −0.52 ± 0.11 | −0.13 ± 0.11 | 0.26 ± 0.11 | |
| Pp (effect size) | 0.81 | 0.63 | 0.97* (−2.69) | 0.98* (−7.73) | |
| BIP | |||||
| HV | −0.17 ± 0.15 | 0.03 ± 0.15 | 0.01 ± 0.11 | 0.31 ± 0.14 | 0.01 ± 0.13 |
| CMM | −0.48 ± 0.15 | 0.20 ± 0.14 | −0.11 ± 0.12 | −0.39 ± 0.13 | 0.32 ± 0.14 |
| Pp (effect size) | 0.93 | 0.80 | 0.74 | 0.99* (5.18) | 0.95* (−2.29) |
| HV pre‐cTBS | −0.18 ± 0.13 | 0.15 ± 0.14 | 0.07 ± 0.11 | 0.06 ± 0.13 | 0.09 ± 0.12 |
| HV post‐cTBS | 0.22 ± 0.13 | 0.29 ± 0.13 | 0.26 ± 0.10 | −0.43 ± 0.12 | −0.26 ± 0.12 |
| Pp (effect size) | 0.99* | 0.76 | 0.98* (−1.81) | 0.98* (3.91) | 0.98* (2.91) |
| HV pre‐SHAM | −0.16 ± 0.14 | 0.29 ± 0.15 | −0.09 ± 0.12 | 0.16 ± 0.14 | 0.03 ± 0.14 |
| HV post‐SHAM | −0.14 ± 0.15 | 0.24 ± 0.16 | −0.01 ± 0.14 | −0.13 ± 0.16 | 0.04 ± 0.14 |
| Pp | 0.54 | 0.60 | 0.67 | 0.92 | 0.53 |
CMM: patients with congenital mirror movements; cTBS = continuous theta‐burst stimulation; DCM = dynamic causal modeling; HV = healthy volunteers; SMA = supplementary motor area; UNIP = unimanual preparation.
In HVs, as expected, intrahemispheric and interhemispheric connectivity during the delay period for both UNIP and BIP conditions was not modified by SHAM stimulation of the right SMA proper. By contrast, disruption of the right SMA proper with cTBS modified left SMA connectivity during the delay period of BIP only (Figure 4b), as UNIP was not affected. SMAleft–M1left connectivity was negative before the cTBS and turned positive afterward. SMAleft–SMAright connectivity was positive and reinforced after the cTBS, while M1–M1 connectivity in both directions was turned to a strongly negative drive. Detailed statistics and values of connectivity strength are presented in Table 5.
3.2.4. SMA drive onto M1–M1 connectivity
We then tested whether and how individual connectivity values of the left SMA proper were linked to M1 interhemispheric connectivity during the delay period. In HVs during UNIP, the stronger was the intrahemispheric negative drive from left SMA proper to left M1, the greater was the positive interhemispheric drive between left M1 and right M1 (ρ = −0.62, p = 0.006; Figure 4c, gray plot). This was not observed in patients (p = 0.61, Figure 4c, orange plot). During the delay period for bimanual movements, the stronger the interhemispheric positive drive from left SMA proper to the right M1, the stronger was the interhemispheric positive drive between right M1 and left M1 (Figure 4c). This was not significant in CMM patients (p = 0.08, Figure 4c, orange plot). Interestingly, this correlation was found in HVs after the SHAM stimulation (ρ = 0.65, p = 0.002; Figure 4d, gray plot) but not after the cTBS stimulation (ρ = 0.09, p = 0.69; Figure 4d, blue plot). In other words, the SHAM stimulation did not affect the relationship between brain connectivity within the motor network and the behavioral output, while the cTBS did.
Altogether, these results show that during the delay period in HVs: (a) interhemispheric connectivities (SMAleft–SMAright, SMAleft–M1right, M1left–M1right) depend on whether hand coordination will be required for the forthcoming movement or not; (b) intrahemispheric SMAleft–M1left connectivity correlates with interhemispheric M1left–M1right connectivity for unilateral movements, while interhemispheric SMAleft–M1right connectivity correlates with M1right–M1left connectivity for bilateral movements. The functional relevance of the SMA proper in modulating interhemispheric connectivity during hand‐coordinated movements is shown by the modification of interhemispheric interactions during the delay period for bilateral movements—and not for unilateral ones—in HVs following cTBS of the SMA. Finally, we show that SMAleft–SMAright and M1left–M1right interhemispheric connectivities are altered in CMM patients, who are unable to produce asymmetric hand movements.
3.3. Electrophysiological experiments
3.3.1. Rationale and summary
We test the hypothesis that interhemispheric interactions during the delay period are altered after SMA disruption in healthy controls and in CMM patients. Using dual‐site TMS, we assessed the excitability of transcallosal pathways from motor/premotor areas in the right hemisphere (PMd, SMA proper and M1) to the left M1. We showed (a) systematic occurrence of ipsilateral “mirror” MEP in CMM patients following unilateral M1 stimulation with TMS; (b) a decrease of interhemispheric inhibition from the right M1 to the left M1 at rest in CMM patients; (c) a decrease of interhemispheric inhibition from the right M1 to the left M1 during the delay period preceding unilateral right and bimanual movements in HVs following SMA disruption and in CMM patients.
3.3.2. Study of ipsilateral MEPs in CMM patients
In all the 20 patients who participated to the TMS protocol, unilateral stimulation of the left primary motor cortex at rest elicited ipsilateral responses, which were absent in controls (results are detailed in Table S2). This suggested the existence of fast‐conducting corticospinal projections from the hand area of the dominant primary motor cortex to motoneurons on the ipsilateral side of the spinal cord in the patients (Welniarz et al., 2015).
3.3.3. Study of interhemispheric interactions
We first tested interhemispheric interactions at rest. In a first step, we verified in each group whether the amplitude of MEP evoked in the right FDI after a TP over the left M1 alone (A MEP[TP], baseline) differed from the amplitude of MEP after a TP preceded by a CP (A MEP[CP + TP]). Results are reported in the Supporting Information (Results of TMS data) and Figure S4a. We then compared normalized values of the conditioned MEPs (defined as the ratio A MEP[CP + TP]/A MEP[TP]). In HVs at rest, interhemispheric interactions kept unchanged after right SMA proper transient disruption (M1right–M1left: p = 0.3 in Figure 5a left; SMAright–M1left: p = 0.75; PMdright–M1left: p = 0.32 in Figure S4c,e). IHI from M1right to M1left at rest was significantly reduced in CMM patients compared to HVs (p = 0.011 right panel of Figure 5a right). By contrast, interhemispheric interactions at rest did not differ between patients and HVs for the circuits SMAright–M1left (p = 0.88) and PMDright–M1left (p = 0.67; in Figure S4d,f).
Figure 5.
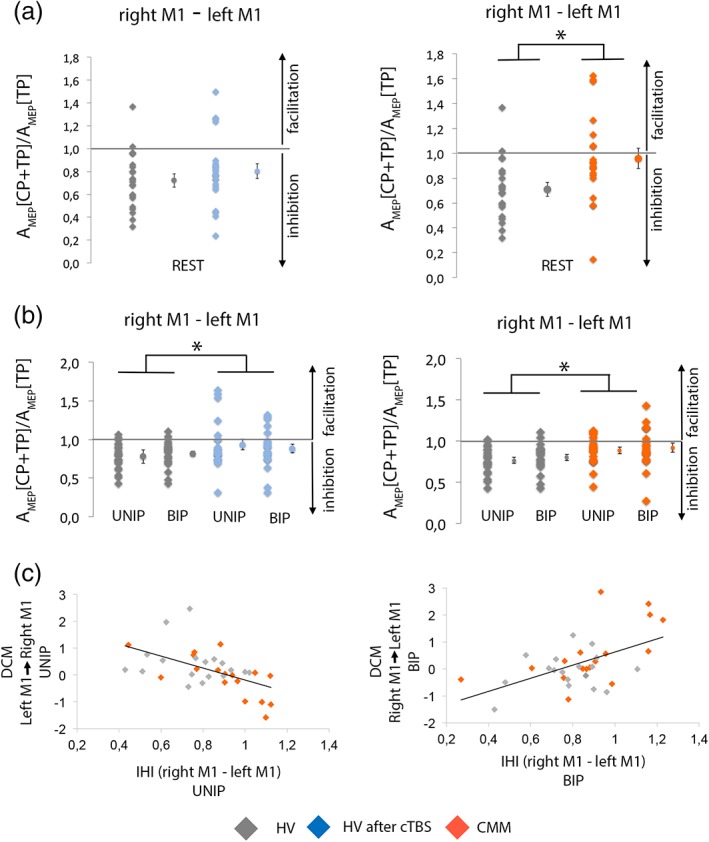
Measure of the interhemispheric interactions at rest and during movement preparation and correlations between fMRI and transcranial magnetic stimulation (TMS) parameters. (a) Interhemispheric inhibition (IHI) at rest in healthy volunteers (HVs) before and after disruption of the supplementary motor area (SMA) proper (left) and in congenital mirror movement (CMM) patients and HVs (right). (b) IHI during preparation of right unimanual or bimanual movement in HVs before and after disruption of the SMA proper (left) and in CMM patients and HVs (right). Results showed that continuous theta‐burst stimulation (cTBS) in the HVs diminished the IHI from right M1 to left M1 during unimanual and bimanual movement preparation but not at rest. The IHI from the right M1 to the left M1 is decreased in CMM patients as compared to HVs both at rest and during movement preparation. Individual data are presented as dot plots alongside the mean and SEM. (c) In HVs and CMM patients, lower connectivity between left M1 and right M1 during unimanual preparation (UNIP) correlates with the degree of IHI during UNIP (left). Greater connectivity between right M1 and left M1 during BIP correlates with the degree of IHI during BIP (right) [Color figure can be viewed at http://wileyonlinelibrary.com]
We then tested interhemispheric interactions during the delay period, 300 ms after the presentation of the first cue (see Figure 1d–e for the timing of the experiment). In HVs, a CP delivered on the M1right resulted in decreased amplitude of the MEP evoked in the right FDI by the TP of M1left (A MEP[TP]) during both UNIP (p < 0.001) and BIP (p < 0.001). A conditioning stimulation delivered on PMdright or SMAright did not change the A MEP[TP] during UNIP or BIP (p > 0.05, data not shown). The level of IHI from M1right to M1left was similar during UNIP and BIP (p = 0.24, data not shown). Disruption of the right SMA proper with cTBS in HVs led to diminish the inhibition from M1right to M1left during both UNIP and BIP (effect of Session, F 1,21 = 4.83, p = 0.039; Figure 5b, left panel; no effect of Condition: F 1,21 = 0.02, p = 0.88; no interaction Session × Condition: F 1,21 = 0.78, p = 0.39), whereas it was ineffective for the circuits SMAright–M1left (p = 0.941) and PMdright–M1left (p = 0.158; in Figure S4c,e). When comparing CMM patients with HVs, CMM patients had less interhemispheric inhibition from M1right to M1left than HVs during UNIP and BIP (effect of Group: F 1,38 = 7.95, p = 0.008; right panel of Figure 5b; no effect of Condition: F 1,38 = 0.68, p = 0.41; no interaction Group Condition: F 1,38 = 0, p = 0.99). Interhemispheric interactions during the delay period did not differ between patients and HVs for the circuits SMAright–M1left (p = 0.644) and PMdright–M1left (p = 0.908; in Figure S4d,f).
3.4. Correlations between fMRI, TMS findings, and behavioral performance
In HVs and in patients, M1–M1 connectivity measured with DCM was correlated to the degree of IHI (Figure 5c). During the delay period for unimanual right hand movement, stronger was the negative drive from the left M1 to the right M1, weaker was the level of IHI from the right M1 to the left M1 (ρ = −0.54, p = 0.0009; Figure 5c, left). Interestingly, during the preparation of bimanual movement, the drive from the right M1 to the left M1 was positively correlated to the IHI measured from the right M1 to the left M1 (ρ = 0.53, p = 0.001; Figure 5c right). A negative drive between the two M1 was associated with a strong IHI, while a positive drive was associated with weak IHI.
To summarize, while transient SMA proper disruption did not affect IHI at rest, it decreased it during the delay period irrespective of whether or not the movement involved hand coordination. CMM patients had a decrease of IHI both at rest and during the delay period compared to healthy controls, whereas the interactions between right secondary motor areas and left M1 were unchanged. Finally, the degree of IHI correlated with M1–M1 connectivity.
4. DISCUSSION
We used fMRI and TMS during a delayed response task to investigate interhemispheric interactions during movement preparation and to clarify the potential role of the SMA proper in modulating these interactions. Studying both normal movement preparation and different models of SMA dysfunction, we provide evidence that the SMA proper modulates interhemispheric interactions during movement preparation. Our data further demonstrate that the SMA proper is involved in the preparation of coordinated hand movements.
We provide a comprehensive description of the SMA proper network involved in unimanual and bimanual movement preparation in HVs. SMA proper participates in shaping appropriate motor plan in a task specific manner during motor execution (Ball et al., 1999; Grefkes et al., 2008; Huang et al., 2005; Sarfeld et al., 2012). Accordingly in HVs, SMA proper interhemispheric connectivity was specifically modulated during movement preparation according to whether or not hand coordination was required for the forthcoming movement. The drive from the left SMA proper to the right SMA proper and right M1 was negative during the preparation of unimanual right hand movements and positive during the preparation of bimanual movements. Similarly, the drive from the left M1 to the right M1 was negative during the preparation of unimanual right hand movements and positive during the preparation of bimanual movements. This fits with the view of strong motor representation in the left hemisphere (Haaland, 2006; Haaland, Prestopnik, Knight, & Lee, 2004; Rushworth & Taylor, 2007) that would lead to strong inhibition of the right hemisphere to suppress the left hand motor representation during right hand movement (Beaulé, Tremblay, & Théoret, 2012; Grefkes et al., 2008; Klein, Duque, Labruna, & Ivry, 2016). By contrast, the SMA proper would exert a positive drive on both hemispheres when hand coordination is required. SMA proper intrahemispheric connectivity was negative, irrespective of the movement type being prepared. Intrahemispheric connectivity might be tuned later than interhemispheric connectivity, closer to the movement onset. These results converge with those of previous studies that explored the network connectivity changes during movement execution: unimanual movements were associated with reduced neuronal coupling toward the ipsilateral hemisphere by transcallosal inhibition, while bimanual movements were associated with increased intrahemispheric connectivity and transcallosal coupling (Grefkes et al., 2008). The new finding here is that dynamic modulation of the SMA proper network is also at stake during movement preparation.
Our data also bring evidence that SMAleft–M1left intrahemispheric connectivity influences interhemispheric M1left–M1right connectivity during the delay period for unilateral movements, while SMAleft–M1right interhemispheric connectivity influences with M1right–M1left connectivity during the delay period for bilateral movements. This emphasizes the key role of the SMA proper in modulating M1–M1 connectivity during movement preparation. We also showed that M1–M1 connectivity was closely linked to the level of IHI. Links between the IHI and the BOLD response have been previously explored (Brocke, Schmidt, Irlbacher, Cichy, & Brandt, 2008; Gallea et al., 2013; Sarfeld et al., 2012). The strength of IHI correlated to the BOLD signal in M1. Our study shows for the first time a correlation between the IHI level and M1–M1 connectivity during motor preparation. This suggests that IHI not only depends on the intrinsic properties of the neuronal circuits at the stimulation sites, but also on interactions with other areas from the motor system.
Using dual‐site TMS in HVs, we showed that the right PMd and SMA proper did not influence the excitability of the left M1 during the delay period. This is in contrast with our results at rest, showing a significant decrease of the left M1 excitability following a conditioning stimulation on the right PMd, as previously shown (Reis et al., 2008). Several studies using short ISIs (between 8 and 10 ms) reported opposite effects (facilitation or inhibition) of PMd conditioning on the contralateral M1 during movement preparation depending on the type of task (simple RT task, choice RT task, and delayed response task), the timing of stimulation and the direction of the tested interaction (toward the dominant or nondominant M1) (Koch et al., 2006; Kroeger et al., 2010; Liuzzi et al., 2010). In a similar delayed response task, a previous study found a significant inhibition between the PMd and the contralateral M1, 300 ms after the beginning of the delay period. However, in this study, the PMd–M1 interaction was directed toward the nondominant hemisphere, and a significant inhibition was found only during the preparation of nondominant hand movements. In our study, the fact that we assessed the PMd–M1 interaction directed toward the dominant hemisphere during the preparation of dominant hand movements may explain this discrepancy. A recent study highlighted the complexity of right PMd structure and the existence of different functional subregions (Genon et al., 2017). It is thus possible that the lack of effect of PMd stimulation during movement preparation was due to the stimulation of different PMd subregions in the different subjects during our experiments. Concerning SMA–M1 interactions, it is possible that the lack of effect we observed was due to the stimulation parameters we used. Indeed, short ISI (6 ms) has been previously used to assess intrahemispheric SMA–M1 interactions (Arai et al., 2012). A recent study (published after the beginning of our experiments) reported significant M1 inhibition following contralateral SMA stimulation at longer ISI (40 ms) (Fiori et al., 2017). By contrast, there was an inhibition from right M1 to left M1 targeting the active hemisphere during the delay period of unimanual right or bimanual movement. The level of inhibition did not depend on whether hand coordination was required or not (unimanual or bimanual). Later, during the premovement phase preceding unimanual movements, the IHI directed toward the active M1 (contralateral to the moving hand) is progressively lifted and turns into facilitation, while the IHI toward the ipsilateral M1 remains constant (Duque, Hummel et al., 2005; Duque et al., 2007; Hinder et al., 2017; Liuzzi et al., 2010; Murase et al., 2004). During the delay period the amplitude of the IHI toward the active hemisphere is modulated by the skillfulness required for the forthcoming unimanual tasks (Wischnewski et al., 2016). Similarly, the preparation of complex bimanual movement is associated with a decreased IHI as compared to simple bimanual movements (Fujiyama, Van Soom, Rens, Gooijers et al., 2016; Fujiyama, Van Soom, Rens, Cuypers et al., 2016). Altogether, previous data show that interhemispheric interactions targeting the active hemisphere are modulated during motor preparation depending on the movement complexity and accuracy. In our study, the IHI measured during the delay period was not modulated according to whether or not hand coordination was required for the forthcoming movement. This in sharp contrast with our DCM analysis showing that M1–M1 connectivity was selectively modulated depending on the type of the forthcoming movement. Several points can explain this discrepancy. First, while M1–M1 connectivity was assessed in both directions, the IHI was only assessed from right to left M1. The left M1 was active in both conditions (unimanual and bimanual), which may explain why IHI was not modulated according to the type of movement. Second, while M1–M1 connectivity measured with DCM was estimated over the whole duration of the delay period, IHI was measured at a specific and early time point of the delay period (300 ms after the first cue). Finally, it might be that the measures provided by the two methods rely on different interhemispheric mechanisms. In humans, IHI relies on excitatory transcallosal neurons contacting inhibitory interneurons in the receiving hemispheres (Chen, 2004; Daskalakis, Christensen, Fitzgerald, Roshan, & Chen, 2002; Irlbacher, Brocke, Mechow, & Brandt, 2007; Reis et al., 2008). By contrast, the neurophysiological substrates underlying the parameters obtained from DCM are not as straightforward. DCM represents the effect that a brain region has on another, but this effect is probably the net outcome of both direct, indirect connections, and of complex subcortical loops (Grefkes et al., 2008). It is thus possible that the M1–M1 connectivity measured with DCM encompasses the IHI assessed with TMS as well as other interhemispheric connections.
After exploring the interhemispheric interactions underlying normal movement preparation in HVs, we aimed at understanding the role of the SMA proper in these interactions. To do so, we assessed whether SMA dysfunction impacted interhemispheric interactions during motor preparation.
In HVs, we aimed at disrupting the right SMA proper with cTBS to evaluate the modifications of the transcallosal pathways directed toward the active (left) M1. Inhibitory stimulation of the right SMA proper only modified the connectivity measured with DCM, while the fMRI BOLD signal and the behavioral performances were unaffected. Two lines of explanation may account for this observation. First, the use a simple motor sequence could explain why we did not observe an impact on the behavioral performance. Indeed, while our finger sequence was sufficient to induce SMA activation, it has been previously reported that SMA perturbation impacts complex but not simple motor tasks (Gerloff, Corwell, Chen, Hallett, & Cohen, 1997; Serrien et al., 2002). Second, this might be due to the fact that we used subthreshold stimulation intensity (0.9 × AMT) to stimulate the right SMA proper. Using the same stimulation parameters, a recent study reported an alteration of cortical sequence representation following SMA disruption while behavioral performance was unchanged (Solopchuk et al., 2017). Another study showed that cTBS over M1 using subthreshold intensities did not modify brain activation, but rather modulated functional connectivity (Steel et al., 2016). In the same way, high frequency repetitive TMS delivered at subthreshold intensities fails to trigger changes in the BOLD signal at the stimulated site but rather modulates corticocortical connections (Bestmann, Baudewig, Siebner, Rothwell, & Frahm, 2003; Bestmann, Baudewig, Siebner, Rothwell, & Frahm, 2004; Bestmann, Baudewig, Siebner, Rothwell, & Frahm, 2005; Bestmann et al., 2008). While higher stimulation intensities could produce greater effects, it could also lead to unspecific disruption of the neighboring regions (Solopchuk et al., 2017). The lack of modification of the BOLD signal and of the behavioral performance does not imply that the SMA stimulation was ineffective. It rather suggests that cTBS had a subtle effect that only impacted the network connectivity. Indeed, the DCM analysis revealed that, during the delay period, disruption of the right SMA proper in HVs strongly altered the interhemispheric interactions. Importantly, this alteration was observed only during bimanual movements preparation (and not for unimanual movements), in keeping with the involvement of SMA in bimanual coordination (Donchin et al., 2002; Duque, Davare, et al., 2010; Immisch et al., 2001; Kermadi et al., 1998; Sadato et al., 1997; Stephan et al., 1999; Toyokura et al., 1999; Uhl et al., 1996; Ullén et al., 2003). Right SMA disruption caused the connectivity from the left SMA proper to the left M1 and right SMA proper to become positive and the connectivity between the two M1 to become more negative. Using TMS, we showed that PMdright–M1left and SMAright–M1left interactions remained unchanged after right SMA disruption. On the contrary, IHI from right M1 to left M1 was reduced during the delay period following disruption of the right SMA proper, regardless of the forthcoming movement (unimanual or bimanual). Most studies that have investigated the role of IHI during movement preparation focused on the premovement phase, and the processes at stake during the delay period remain elusive. Single pulse TMS studies consistently reported a decreased excitability of the active and nonactive M1 during the delay period (Bestmann & Duque, 2016; Duque, Lew, Mazzocchio, Olivier, & Ivry, 2010; Duque & Ivry, 2009; Klein et al., 2016; Kroeger et al., 2010; Lebon et al., 2018; Wilhelm, Quoilin, Petitjean, & Duque, 2016). This early inhibition is thought to reflect two distinct processes (Bestmann & Duque, 2016): “competition resolution” represents the inhibition of the nonselected hand, while “impulse control” may prevent the movement from being executed prematurely. In our study, the IHI was toward the active M1 and was independent of the type of movement being prepared (unimanual or bimanual), and may thus participate to a generic inhibition (Greenhouse, Sias, Labruna, & Ivry, 2015). This is consistent with a previous study showing that changes in IHI occurred independently of changes in corticospinal tract excitability (measured with single pulse TMS) during the premovement phase (Hinder et al., 2017). Altogether, this suggests that, in physiological conditions, SMA proper is essential for bimanual movement preparation as it modulates the interactions between the two M1. While the cTBS was performed on the right SMA proper, it is the connectivity of the left SMA proper that was affected. This suggests that the right SMA proper does not directly influence the left M1 during unimanual right or bimanual movement preparation. This hemispheric asymmetry has been reported by previous studies, showing a dominance of the left hemisphere for hand movements (Haaland, 2006; Haaland & Harrington, 1996; Rogers, Carew, & Meyerand, 2004). In particular, it was previously shown that the effective connectivity of the right SMA with the sensorimotor cortex was not modulated depending on the laterality (right hand or left hand) of the movement. By contrast, the connectivity of the left SMA was modulated depending on the movement's laterality, suggesting a left‐hemisphere‐dominant control of unilateral finger movement (Rogers et al., 2004). This could also be an indirect consequence of the connectivity changes between the two SMA proper. Last because of its location very close to the midline, we cannot exclude the possibility that the left SMA proper was directly stimulated during the cTBS procedure.
We then conducted the same experiments in CMM patients, who are unable to perform asymmetric hand movements and have abnormal interhemispheric coupling between SMA proper and M1 during movement execution (Gallea et al., 2013). First, with fMRI, we showed abnormal activation of the ipsilateral M1 during unilateral movement preparation. Abnormal activation of the ipsilateral M1 during unimanual movement execution has been previously reported in CMM patients (Gallea et al., 2013; Welniarz et al., 2015). However, it was not clear whether this abnormal activation was a cause or a sensory consequence of mirror movements. Our results suggest that abnormal cortical activation is not a consequence of mirror movements, as it is present during movement preparation, but might rather contribute to the inability of these patients to produce purely unimanual movements. With DCM, we showed that SMAleft–SMAright interhemispheric connectivity during the delay period in CMM patients was not specific of the characteristics of the forthcoming movement. This confirms that SMA function is also abnormal during movement preparation in CMM patients, as suggested by two previous EEG studies (Cohen et al., 1991; Franz & Fu, 2017). Furthermore, while M1left–M1right connectivity turned from negative to positive when comparing unimanual right and bimanual movement preparation in HVs, it was the opposite in CMM patients: M1left–M1right connectivity was positive during the delay period of unimanual right hand movements, while it was negative for bimanual movements. In the same patients, IHI from the right M1 to left M1 measured with TMS was decreased both at rest and during the delay period of unimanual and bimanual movements. This suggests that in CMM patients, abnormal SMA proper function strongly interferes with M1–M1 interhemispheric interactions. Abnormal interhemispheric communication resulting in bilateral M1 activation has been identified as one of the mechanisms responsible for mirror movements (Galléa et al., 2011; Welniarz et al., 2015). In this regard, abnormal SMA proper function in these patients contributes to the generation of mirror movements. Although we did not observe a significant increase of premature responses in CMM patients, their RTs were longer as compared to controls. This is consistent with recent results suggesting that inhibition of the active M1 during movement preparation is not only related to the suppression of premature motor response, but rather contributes to successful movement preparation (Hannah, Cavanagh, Tremblay, Simeoni, & Rothwell, 2018). In this later study, stronger inhibition during movement preparation was associated with faster RTs. This would explain the slower reactions times in CMM patients, in whom IHI is decreased during the delay period. This suggests that the SMA proper could be involved in movement preparation by presetting interhemispheric interactions and M1 excitability to release the appropriate motor response in a timely manner. This process has previously been associated with the anterior part of the SMA or pre SMA (Akkal, Escola, Bioulac, & Burbaud, 2004; Aron, Behrens, Smith, Frank, & Poldrack, 2007; Halsband, Ito, Tanji, & Freund, 1993).
The defects in CMM patients were broader and not as specific as those observed in HVs following SMA proper disruption. Indeed, IHI was decreased both at rest and during movement preparation in CMM patients, and their RT was longer as compared to controls. By contrast, in HVs after SMA proper disruption, IHI was decreased only during the delay period (and not at rest) and their RTs were not significantly modified by the stimulation. This suggests that SMA dysfunction may not be the sole explanation for the defects observed in CMM patients, and that some of the abnormalities of movement preparation could be a consequence of mirror movements rather than a primary defect of the cortical motor system. Indeed, two main non exclusive mechanisms may account for mirror movements: (a) corticospinal tract abnormality leading to bilateral downstream transmission of the motor command, and (b) abnormal interhemispheric communication resulting in bilateral activation of primary motor areas (Gallea et al., 2013; Welniarz et al., 2015; Welniarz, Dusart, & Roze, 2017). We demonstrated that abnormal SMA proper function could contribute, at least in part, to abnormal interhemispheric communication in CMM patients. However, other mechanisms could be responsible for altered interhemispheric interactions in these patients. We previously showed that CMM patients had specific structural abnormalities of the transcallosal tract connecting the primary motor and areas in the two hemispheres (Gallea et al., 2013). These structural defects could explain why the IHI is decreased at rest in patients but not in HVs following SMA proper disruption. In addition, we showed that CMM patients have both abnormal corticospinal tract projections and defective interhemispheric interactions. This explains why SMA proper disruption in HVs is not sufficient to reproduce the entire physiopathology of CMM patients. Concerning the RT, it is possible that the corticospinal tract abnormalities observed in patients are responsible for the increased response latency. The systematic occurrence of bimanual symmetric movements in these patients could in turn modify the functional organization of the cortical motor system, as previously suggested (Gallea et al., 2013). We thus hypothesize that some of the defects that we observed in patients are not a direct consequence of SMA proper dysfunction, but rather reflect a global reorganization of the cortical motor system that is induced by the systematic occurrence of mirror movements. In CMM patients, the strength of SMA proper activation during movement preparation is correlated to the patient's age, and not to the severity of mirror movements. This suggests that SMA proper activation strength is not linked to the severity of the mirror movements, but rather reflects the duration of the condition. In other words, greater SMA proper activation in patients might not be the cause of mirror movements, but would rather be their consequence. In CMM patients, the various aspects of SMA proper dysfunction might thus have different origins. On the one hand, abnormal SMA connectivity results in altered M1–M1 interactions, therefore contributing to the generation of mirror movements. On the other hand, it seems that abnormal SMA proper activation could be a consequence, and not a cause, of mirror movements.
5. CONCLUSION
Our data converge to demonstrate that the SMA proper modulates interhemispheric interactions during movement preparation. SMA tuning over M1–M1 interactions was already suggested by previous studies (Sarfeld et al., 2012; Serrien et al., 2002; Siebner et al., 2001). Our strength is to provide converging data from the study of both normal movement preparation and different models of SMA dysfunction with multimodal experiments. We showed that during normal movement preparation, SMA proper interhemispheric connectivity was tuned according to whether or not hand coordination was required for the forthcoming movement and that SMA–M1 connectivity influenced M1–M1 connectivity. In addition, we disclosed impaired interhemispheric interactions during movement preparation in two different models of SMA dysfunction. In both HVs after transient disruption of the SMA proper and in CMM patients, we observed a modification of the interhemispheric interactions measured with DCM and a decreased IHI during the delay period. The functional relevance of the SMA proper for the preparation of hand‐coordinated movements is shown by the modification of interhemispheric interactions during the delay period for bilateral movements—and not for unilateral ones—in HVs following disruption of the SMA proper. Our data provide new insights into the neurophysiological role of SMA proper during movement preparation and outlines its importance for coordinated hand movements.
Supporting information
Appendix S1 Supporting Information
Figure S1 Results of the EMG analysis related to MM severity. (a) Display of EMG recorded inside the MRI scanner during the task performance of a representative healthy volunteer. (b) Display of EMG recorded inside the MRI scanner during the task performance of a representative congenital mirror movement patient. (c) Result of the group analysis performed on the EMG laterality index taken as indicative of MM severity
Figure S2 Definition of the dynamic causal modeling models for supplementary motor area proper network. Red arrows represent the connections that were modulated by unimanual preparation and bimanual preparation conditions
Figure S3 Results of the BMS analysis for the healthy controls before and after supplementary motor area (SMA) inactivation and congenital mirror movement patients. Numbers refers to the ones in Figure S2. The graphs represent the posterior probability of the winning model for the SMA proper network. Simple models family includes models without interhemispheric connections (Models 1, 2, 5, and 6) and complex models family includes models with interhemispheric interactions (Models 3, 4, 7, and 8)
Figure S4 Additional transcranial magnetic stimulation results. (a) Effect of a CP delivered on the right M1 or right supplementary motor area (SMA) or right dorsal premotor cortex (PMd) on the amplitude of the motor‐evoked potential (MEP) evoked by stimulation of the left M1 at rest. A CP on the right M1 significantly reduced the MEP amplitude in HVs before and after SMA proper inhibition but not in congenital mirror movement (CMM) patients. A CP delivered on the right SMA did not affect the MEP amplitude in any group. A CP delivered on the right PMd resulted in smaller MEP amplitude only in HVs before SMA proper inhibition. (b) Amplitude of the TP alone in each group at rest and during the preparation of right unimanual movement. In all groups, there was a significant increase of the TP amplitude during the task as compared to the rest condition. (c, d) Interhemispheric interactions involving the right SMA at rest (left panel) and during motor preparation (right panel) in HVs before and after inhibition of the SMA proper (gray and blue plots) and in CMM patients and HVs (gray and orange plots). (e, f) Interhemispheric interactions involving the right PMd at rest (left panel) and during motor preparation (right panel) in HVs before and after inhibition of the SMA proper (gray and blue plots) and in CMM patients and HVs (gray and orange plots). Individual data are presented as dot plots alongside the mean and SEM. CP = conditioning pulse; HV = healthy volunteers; ISI = interstimulus interval; TP = test pulse.
ACKNOWLEDGMENTS
The study was supported by Fondation Desmarest, INSERM (COSSEC), Merz‐Pharma, Elivie, Djillali Mehri.
Welniarz Q, Gallea C, Lamy J‐C, et al. The supplementary motor area modulates interhemispheric interactions during movement preparation. Hum Brain Mapp. 2019;40:2125–2142. 10.1002/hbm.24512
Funding information Fondation Desmarest; Institut National de la Santé et de la Recherche Médicale; IP‐santé; Merz‐Pharma; Agence Nationale de la Recherche (ANR‐18‐CE16‐0005‐01)
REFERENCES
- Akkal, D. , Escola, L. , Bioulac, B. , & Burbaud, P. (2004). Time predictability modulates pre‐supplementary motor area neuronal activity. Neuroreport, 15, 1283–1286. [DOI] [PubMed] [Google Scholar]
- Arai, N. , Lu, M.‐K. , Ugawa, Y. , & Ziemann, U. (2012). Effective connectivity between human supplementary motor area and primary motor cortex: A paired‐coil TMS study. Experimental Brain Research, 220, 79–87. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Behrens, T. E. , Smith, S. , Frank, M. J. , & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience, 27, 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, T. , Schreiber, A. , Feige, B. , Wagner, M. , Lücking, C. H. , & Kristeva‐Feige, R. (1999). The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. NeuroImage, 10, 682–694. [DOI] [PubMed] [Google Scholar]
- Beaulé, V. , Tremblay, S. , & Théoret, H. (2012). Interhemispheric control of unilateral movement. Neural Plasticity, 2012, 627816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann, S. , Baudewig, J. , Siebner, H. R. , Rothwell, J. C. , & Frahm, J. (2003). Subthreshold high‐frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI‐TMS. NeuroImage, 20, 1685–1696. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , Baudewig, J. , Siebner, H. R. , Rothwell, J. C. , & Frahm, J. (2004). Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. The European Journal of Neuroscience, 19, 1950–1962. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , Baudewig, J. , Siebner, H. R. , Rothwell, J. C. , & Frahm, J. (2005). BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. NeuroImage, 28, 22–29. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , & Duque, J. (2016). Transcranial magnetic stimulation: Decomposing the processes underlying action preparation. The Neuroscientist, 22, 392–405. [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , Ruff, C. C. , Blankenburg, F. , Weiskopf, N. , Driver, J. , & Rothwell, J. C. (2008). Mapping causal interregional influences with concurrent TMS‐fMRI. Experimental Brain Research, 191, 383–402. [DOI] [PubMed] [Google Scholar]
- Brinkman, C. (1984). Supplementary motor area of the monkey's cerebral cortex: Short‐ and long‐term deficits after unilateral ablation and the effects of subsequent callosal section. The Journal of Neuroscience, 4, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke, J. , Schmidt, S. , Irlbacher, K. , Cichy, R. M. , & Brandt, S. A. (2008). Transcranial cortex stimulation and fMRI: Electrophysiological correlates of dual‐pulse BOLD signal modulation. NeuroImage, 40, 631–643. [DOI] [PubMed] [Google Scholar]
- Chan, J. L. , & Ross, E. D. (1988). Left‐handed mirror writing following right anterior cerebral artery infarction: Evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology, 38, 59–63. [DOI] [PubMed] [Google Scholar]
- Chen, R. (2004). Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research, 154, 1–10. [DOI] [PubMed] [Google Scholar]
- Chen, R. , & Hallett, M. (1999). The time course of changes in motor cortex excitability associated with voluntary movement. The Canadian Journal of Neurological Sciences, 26, 163–169. [DOI] [PubMed] [Google Scholar]
- Chouinard, P. A. , & Paus, T. (2010). What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Frontiers in Human Neuroscience, 4, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. G. , Meer, J. , Tarkka, I. , Bierner, S. , Leiderman, D. B. , Dubinsky, R. M. , … Hallett, M. (1991). Congenital mirror movements. Abnormal organization of motor pathways in two patients. Brain, 114(Pt. 1B), 381–403. [DOI] [PubMed] [Google Scholar]
- Daskalakis, Z. J. , Christensen, B. K. , Fitzgerald, P. B. , Roshan, L. , & Chen, R. (2002). The mechanisms of interhemispheric inhibition in the human motor cortex. Journal of Physiology (London), 543, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin, O. , Gribova, A. , Steinberg, O. , Mitz, A. R. , Bergman, H. , & Vaadia, E. (2002). Single‐unit activity related to bimanual arm movements in the primary and supplementary motor cortices. Journal of Neurophysiology, 88, 3498–3517. [DOI] [PubMed] [Google Scholar]
- Duque, J. , Davare, M. , Delaunay, L. , Jacob, B. , Saur, R. , Hummel, F. , … Olivier, E. (2010). Monitoring coordination during bimanual movements: Where is the mastermind? Journal of Cognitive Neuroscience, 22, 526–542. [DOI] [PubMed] [Google Scholar]
- Duque, J. , Hummel, F. , Celnik, P. , Murase, N. , Mazzocchio, R. , & Cohen, L. G. (2005). Transcallosal inhibition in chronic subcortical stroke. NeuroImage, 28, 940–946. [DOI] [PubMed] [Google Scholar]
- Duque, J. , & Ivry, R. B. (2009). Role of corticospinal suppression during motor preparation. Cerebral Cortex, 19, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque, J. , Lew, D. , Mazzocchio, R. , Olivier, E. , & Ivry, R. B. (2010). Evidence for two concurrent inhibitory mechanisms during response preparation. The Journal of Neuroscience, 30, 3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque, J. , Mazzocchio, R. , Dambrosia, J. , Murase, N. , Olivier, E. , & Cohen, L. G. (2005). Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cerebral Cortex, 15, 588–593. [DOI] [PubMed] [Google Scholar]
- Duque, J. , Murase, N. , Celnik, P. , Hummel, F. , Harris‐Love, M. , Mazzocchio, R. , … Cohen, L. G. (2007). Intermanual differences in movement‐related interhemispheric inhibition. Journal of Cognitive Neuroscience, 19, 204–213. [DOI] [PubMed] [Google Scholar]
- Fallon, N. , Chiu, Y. , Nurmikko, T. , & Stancak, A. (2016). Functional connectivity with the default mode network is altered in fibromyalgia patients. PLoS One, 11, e0159198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori, F. , Chiappini, E. , Candidi, M. , Romei, V. , Borgomaneri, S. , & Avenanti, A. (2017). Long‐latency interhemispheric interactions between motor‐related areas and the primary motor cortex: A dual site TMS study. Scientific Reports, 7, 14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz, E. A. , & Fu, Y. (2017). Pre‐movement planning processes in people with congenital mirror movements. Clinical Neurophysiology, 128, 1985–1993. [DOI] [PubMed] [Google Scholar]
- Fujiyama, H. , Van Soom, J. , Rens, G. , Cuypers, K. , Heise, K.‐F. , Levin, O. , & Swinnen, S. P. (2016). Performing two different actions simultaneously: The critical role of interhemispheric interactions during the preparation of bimanual movement. Cortex, 77, 141–154. [DOI] [PubMed] [Google Scholar]
- Fujiyama, H. , Van Soom, J. , Rens, G. , Gooijers, J. , Leunissen, I. , Levin, O. , & Swinnen, S. P. (2016). Age‐related changes in frontal network structural and functional connectivity in relation to bimanual movement control. The Journal of Neuroscience, 36, 1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallea, C. , Popa, T. , Hubsch, C. , Valabregue, R. , Brochard, V. , Kundu, P. , … Roze, E. (2013). RAD51 deficiency disrupts the corticospinal lateralization of motor control. Brain, 136, 3333–3346. [DOI] [PubMed] [Google Scholar]
- Galléa, C. , Popa, T. , Billot, S. , Méneret, A. , Depienne, C. , & Roze, E. (2011). Congenital mirror movements: A clue to understanding bimanual motor control. Journal of Neurology, 258, 1911–1919. [DOI] [PubMed] [Google Scholar]
- Genon, S. , Li, H. , Fan, L. , Müller, V. I. , Cieslik, E. C. , Hoffstaedter, F. , … Eickhoff, S. B. (2017). The right dorsal premotor mosaic: Organization, functions, and connectivity. Cerebral Cortex, 27, 2095–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff, C. , Corwell, B. , Chen, R. , Hallett, M. , & Cohen, L. G. (1997). Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain, 120(Pt. 9), 1587–1602. [DOI] [PubMed] [Google Scholar]
- Gooijers, J. , & Swinnen, S. P. (2014). Interactions between brain structure and behavior: The corpus callosum and bimanual coordination. Neuroscience and Biobehavioral Reviews, 43, 1–19. [DOI] [PubMed] [Google Scholar]
- Greenhouse, I. , Sias, A. , Labruna, L. , & Ivry, R. B. (2015). Nonspecific inhibition of the motor system during response preparation. The Journal of Neuroscience, 35, 10675–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes, C. , Eickhoff, S. B. , Nowak, D. A. , Dafotakis, M. , & Fink, G. R. (2008). Dynamic intra‐ and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage, 41, 1382–1394. [DOI] [PubMed] [Google Scholar]
- Haaland, K. Y. (2006). Left hemisphere dominance for movement. The Clinical Neuropsychologist, 20, 609–622. [DOI] [PubMed] [Google Scholar]
- Haaland, K. Y. , & Harrington, D. L. (1996). Hemispheric asymmetry of movement. Current Opinion in Neurobiology, 6, 796–800. [DOI] [PubMed] [Google Scholar]
- Haaland, K. Y. , Prestopnik, J. L. , Knight, R. T. , & Lee, R. R. (2004). Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain, 127, 1145–1158. [DOI] [PubMed] [Google Scholar]
- Halsband, U. , Ito, N. , Tanji, J. , & Freund, H. J. (1993). The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain, 116(Pt. 1), 243–266. [DOI] [PubMed] [Google Scholar]
- Hannah, R. , Cavanagh, S. E. , Tremblay, S. , Simeoni, S. , & Rothwell, J. C. (2018). Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. The Journal of Neuroscience, 38, 1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder, M. R. , Puri, R. , Kemp, S. , Waitzer, S. , Reissig, P. , Stöckel, T. , & Fujiyama, H. (2017). Distinct modulation of interhemispheric inhibitory mechanisms during movement preparation reveals the influence of cognition on action control. Cortex, 99, 13–29. [DOI] [PubMed] [Google Scholar]
- Huang, Y.‐Z. , Edwards, M. J. , Rounis, E. , Bhatia, K. P. , & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45, 201–206. [DOI] [PubMed] [Google Scholar]
- Immisch, I. , Waldvogel, D. , van Gelderen, P. , & Hallett, M. (2001). The role of the medial wall and its anatomical variations for bimanual antiphase and in‐phase movements. NeuroImage, 14, 674–684. [DOI] [PubMed] [Google Scholar]
- Irlbacher, K. , Brocke, J. , Mechow, J. V. , & Brandt, S. A. (2007). Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clinical Neurophysiology, 118, 308–316. [DOI] [PubMed] [Google Scholar]
- Kermadi, I. , Liu, Y. , Tempini, A. , Calciati, E. , & Rouiller, E. M. (1998). Neuronal activity in the primate supplementary motor area and the primary motor cortex in relation to spatio‐temporal bimanual coordination. Somatosensory & Motor Research, 15, 287–308. [DOI] [PubMed] [Google Scholar]
- Klein, P.‐A. , Duque, J. , Labruna, L. , & Ivry, R. B. (2016). Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. NeuroImage, 125, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, G. , Franca, M. , Del Olmo, M. F. , Cheeran, B. , Milton, R. , Alvarez Sauco, M. , & Rothwell, J. C. (2006). Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. The Journal of Neuroscience, 26, 7452–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeneke, S. , Lutz, K. , Wüstenberg, T. , & Jäncke, L. (2004). Bimanual versus unimanual coordination: What makes the difference? NeuroImage, 22, 1336–1350. [DOI] [PubMed] [Google Scholar]
- Krainik, A. , Duffau, H. , Capelle, L. , Cornu, P. , Boch, A.‐L. , Mangin, J.‐F. , … Lehéricy, S. (2004). Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology, 62, 1323–1332. [DOI] [PubMed] [Google Scholar]
- Krainik, A. , Lehéricy, S. , Duffau, H. , Vlaicu, M. , Poupon, F. , Capelle, L. , … Marsault, C. (2001). Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology, 57, 871–878. [DOI] [PubMed] [Google Scholar]
- Kroeger, J. , Bäumer, T. , Jonas, M. , Rothwell, J. C. , Siebner, H. R. , & Münchau, A. (2010). Charting the excitability of premotor to motor connections while withholding or initiating a selected movement. The European Journal of Neuroscience, 32, 1771–1779. [DOI] [PubMed] [Google Scholar]
- Laplane, D. , Talairach, J. , Meininger, V. , Bancaud, J. , & Orgogozo, J. M. (1977). Clinical consequences of corticectomies involving the supplementary motor area in man. Journal of the Neurological Sciences, 34, 301–314. [DOI] [PubMed] [Google Scholar]
- Lebon, F. , Ruffino, C. , Greenhouse, I. , Labruna, L. , Ivry, R. B. , & Papaxanthis, C. (2018). The neural specificity of movement preparation during actual and imagined movements. Cerebral Cortex, 29, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani, L. , Cohen, L. G. , Wassermann, E. M. , Ikoma, K. , & Hallett, M. (2000). Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain, 123(Pt. 6), 1161–1173. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Morel, A. , Wannier, T. , & Rouiller, E. M. (2002). Origins of callosal projections to the supplementary motor area (SMA): A direct comparison between pre‐SMA and SMA‐proper in macaque monkeys. The Journal of Comparative Neurology, 443, 71–85. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Lai, J. , & Qu, Y. (2004). Surgical treatment of gliomas involving the supplementary motor area in the superior frontal gyrus. Zhonghua Wai Ke Za Zhi, 42, 781–783. [PubMed] [Google Scholar]
- Liuzzi, G. , Hörniss, V. , Hoppe, J. , Heise, K. , Zimerman, M. , Gerloff, C. , & Hummel, F. C. (2010). Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cerebral Cortex, 20, 1323–1331. [DOI] [PubMed] [Google Scholar]
- Luppino, G. , Matelli, M. , Camarda, R. , & Rizzolatti, G. (1993). Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. The Journal of Comparative Neurology, 338, 114–140. [DOI] [PubMed] [Google Scholar]
- Murase, N. , Duque, J. , Mazzocchio, R. , & Cohen, L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55, 400–409. [DOI] [PubMed] [Google Scholar]
- Nachev, P. , Kennard, C. , & Husain, M. (2008). Functional role of the supplementary and pre‐supplementary motor areas. Nature Reviews. Neuroscience, 9, 856–869. [DOI] [PubMed] [Google Scholar]
- Obhi, S. S. , Haggard, P. , Taylor, J. , & Pascual‐Leone, A. (2002). rTMS to the supplementary motor area disrupts bimanual coordination. Motor Control, 6, 319–332. [DOI] [PubMed] [Google Scholar]
- O'Shea, J. , Sebastian, C. , Boorman, E. D. , Johansen‐Berg, H. , & Rushworth, M. F. S. (2007). Functional specificity of human premotor‐motor cortical interactions during action selection. The European Journal of Neuroscience, 26, 2085–2095. [DOI] [PubMed] [Google Scholar]
- Picard, N. , & Strick, P. L. (1996). Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex, 6, 342–353. [DOI] [PubMed] [Google Scholar]
- Reis, J. , Swayne, O. B. , Vandermeeren, Y. , Camus, M. , Dimyan, M. A. , Harris‐Love, M. , … Cohen, L. G. (2008). Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. Journal of Physiology (London), 586, 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, B. P. , Carew, J. D. , & Meyerand, M. E. (2004). Hemispheric asymmetry in supplementary motor area connectivity during unilateral finger movements. NeuroImage, 22, 855–859. [DOI] [PubMed] [Google Scholar]
- Rothwell, J. C. , Hallett, M. , Berardelli, A. , Eisen, A. , Rossini, P. , & Paulus, W. (1999). Magnetic stimulation: Motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology. Supplement, 52, 97–103. [PubMed] [Google Scholar]
- Rouiller, E. M. , Babalian, A. , Kazennikov, O. , Moret, V. , Yu, X. H. , & Wiesendanger, M. (1994). Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research, 102, 227–243. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. S. , & Taylor, P. C. J. (2007). A paradoxical role for inhibition in initiation. Neuron, 54, 669–670. [DOI] [PubMed] [Google Scholar]
- Sadato, N. , Yonekura, Y. , Waki, A. , Yamada, H. , & Ishii, Y. (1997). Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. The Journal of Neuroscience, 17, 9667–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfeld, A.‐S. , Diekhoff, S. , Wang, L. E. , Liuzzi, G. , Uludağ, K. , Eickhoff, S. B. , … Grefkes, C. (2012). Convergence of human brain mapping tools: Neuronavigated TMS parameters and fMRI activity in the hand motor area. Human Brain Mapping, 33, 1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien, D. J. , Strens, L. H. A. , Oliviero, A. , & Brown, P. (2002). Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neuroscience Letters, 328, 89–92. [DOI] [PubMed] [Google Scholar]
- Siebner, H. , Peller, M. , Bartenstein, P. , Willoch, F. , Rossmeier, C. , Schwaiger, M. , & Conrad, B. (2001). Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: A glucose metabolic PET study. Human Brain Mapping, 12, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solopchuk, O. , Alamia, A. , Dricot, L. , Duque, J. , & Zénon, A. (2017). cTBS disruption of the supplementary motor area perturbs cortical sequence representation but not behavioural performance. NeuroImage, 163, 34–40. [DOI] [PubMed] [Google Scholar]
- Steel, A. , Song, S. , Bageac, D. , Knutson, K. M. , Keisler, A. , Saad, Z. S. , … Wilkinson, L. (2016). Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex, 74, 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, K. E. , & Friston, K. J. (2010). Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, K. M. , Binkofski, F. , Halsband, U. , Dohle, C. , Wunderlich, G. , Schnitzler, A. , … Freund, H. J. (1999). The role of ventral medial wall motor areas in bimanual co‐ordination. A combined lesion and activation study. Brain, 122(Pt. 2), 351–368. [DOI] [PubMed] [Google Scholar]
- Steyvers, M. , Etoh, S. , Sauner, D. , Levin, O. , Siebner, H. R. , Swinnen, S. P. , & Rothwell, J. C. (2003). High‐frequency transcranial magnetic stimulation of the supplementary motor area reduces bimanual coupling during anti‐phase but not in‐phase movements. Experimental Brain Research, 151, 309–317. [DOI] [PubMed] [Google Scholar]
- Svoboda, K. , & Li, N. (2018). Neural mechanisms of movement planning: Motor cortex and beyond. Current Opinion in Neurobiology, 49, 33–41. [DOI] [PubMed] [Google Scholar]
- Swinnen, S. P. (2002). Intermanual coordination: From behavioural principles to neural‐network interactions. Nature Reviews. Neuroscience, 3, 348–359. [DOI] [PubMed] [Google Scholar]
- Toyokura, M. , Muro, I. , Komiya, T. , & Obara, M. (1999). Relation of bimanual coordination to activation in the sensorimotor cortex and supplementary motor area: Analysis using functional magnetic resonance imaging. Brain Research Bulletin, 48, 211–217. [DOI] [PubMed] [Google Scholar]
- Uhl, F. , Kornhuber, A. W. , Wartberger, P. , Lindinger, G. , Lang, W. , & Deecke, L. (1996). Supplementary motor area in spatial coordination of bilateral movements: A new aspect to “the SMA debate”? Electroencephalography and Clinical Neurophysiology, 101, 469–477. [DOI] [PubMed] [Google Scholar]
- Ullén, F. , Forssberg, H. , & Ehrsson, H. H. (2003). Neural networks for the coordination of the hands in time. Journal of Neurophysiology, 89, 1126–1135. [DOI] [PubMed] [Google Scholar]
- Vorobiev, V. , Govoni, P. , Rizzolatti, G. , Matelli, M. , & Luppino, G. (1998). Parcellation of human mesial area 6: Cytoarchitectonic evidence for three separate areas. The European Journal of Neuroscience, 10, 2199–2203. [DOI] [PubMed] [Google Scholar]
- Welniarz, Q. , Dusart, I. , Gallea, C. , & Roze, E. (2015). One hand clapping: Lateralization of motor control. Frontiers in Neuroanatomy, 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welniarz, Q. , Dusart, I. , & Roze, E. (2017). The corticospinal tract: Evolution, development, and human disorders. Developmental Neurobiology, 77, 810–829. [DOI] [PubMed] [Google Scholar]
- Wilhelm, E. , Quoilin, C. , Petitjean, C. , & Duque, J. (2016). A double‐coil TMS method to assess corticospinal excitability changes at a near‐simultaneous time in the two hands during movement preparation. Frontiers in Human Neuroscience, 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski, M. , Kowalski, G. M. , Rink, F. , Belagaje, S. R. , Haut, M. W. , Hobbs, G. , & Buetefisch, C. M. (2016). Demand on skillfulness modulates interhemispheric inhibition of motor cortices. Journal of Neurophysiology, 115, 2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, B. T. , & Teuber, H. L. (1978). Mirror movements after childhood hemiparesis. Neurology, 28, 1152–1157. [DOI] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Buettner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120(Pt. 1), 141–157. [DOI] [PubMed] [Google Scholar]
- Zénon, A. , Sidibé, M. , & Olivier, E. (2015). Disrupting the supplementary motor area makes physical effort appear less effortful. The Journal of Neuroscience, 35, 8737–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Figure S1 Results of the EMG analysis related to MM severity. (a) Display of EMG recorded inside the MRI scanner during the task performance of a representative healthy volunteer. (b) Display of EMG recorded inside the MRI scanner during the task performance of a representative congenital mirror movement patient. (c) Result of the group analysis performed on the EMG laterality index taken as indicative of MM severity
Figure S2 Definition of the dynamic causal modeling models for supplementary motor area proper network. Red arrows represent the connections that were modulated by unimanual preparation and bimanual preparation conditions
Figure S3 Results of the BMS analysis for the healthy controls before and after supplementary motor area (SMA) inactivation and congenital mirror movement patients. Numbers refers to the ones in Figure S2. The graphs represent the posterior probability of the winning model for the SMA proper network. Simple models family includes models without interhemispheric connections (Models 1, 2, 5, and 6) and complex models family includes models with interhemispheric interactions (Models 3, 4, 7, and 8)
Figure S4 Additional transcranial magnetic stimulation results. (a) Effect of a CP delivered on the right M1 or right supplementary motor area (SMA) or right dorsal premotor cortex (PMd) on the amplitude of the motor‐evoked potential (MEP) evoked by stimulation of the left M1 at rest. A CP on the right M1 significantly reduced the MEP amplitude in HVs before and after SMA proper inhibition but not in congenital mirror movement (CMM) patients. A CP delivered on the right SMA did not affect the MEP amplitude in any group. A CP delivered on the right PMd resulted in smaller MEP amplitude only in HVs before SMA proper inhibition. (b) Amplitude of the TP alone in each group at rest and during the preparation of right unimanual movement. In all groups, there was a significant increase of the TP amplitude during the task as compared to the rest condition. (c, d) Interhemispheric interactions involving the right SMA at rest (left panel) and during motor preparation (right panel) in HVs before and after inhibition of the SMA proper (gray and blue plots) and in CMM patients and HVs (gray and orange plots). (e, f) Interhemispheric interactions involving the right PMd at rest (left panel) and during motor preparation (right panel) in HVs before and after inhibition of the SMA proper (gray and blue plots) and in CMM patients and HVs (gray and orange plots). Individual data are presented as dot plots alongside the mean and SEM. CP = conditioning pulse; HV = healthy volunteers; ISI = interstimulus interval; TP = test pulse.


