Abstract
This study investigated volumetric brain changes and cognitive performance in premenopausal and postmenopausal patients treated for early‐stage breast cancer. Participants underwent elaborate neurocognitive assessments (neuropsychological testing, cognitive failure questionnaire, and high‐resolution T1‐weighted structural MRI) before and after chemotherapy. Volumetric brain changes were estimated, using longitudinal deformation‐based morphometry, and correlated with cognitive changes. In total, 180 women participated in this study, of whom 72 patients with breast cancer had received adjuvant chemotherapy (C+), 49 patients did not receive chemotherapy (C−), and 59 healthy controls (HC). The population was categorized into two age groups: A young group who were premenopausal and younger than 52 years at baseline (n = 55C+/32C−/41HC), and an older group who were postmenopausal and older than 60 years (n = 17C+/17C−/18HC). Cognitive impairment occurred after chemotherapy in both young and older patients, although older patients showed more decline in processing speed (Trail making test b). White matter volume expansion was observed after chemotherapy, only significantly present in the younger subgroup of patients. In patients not treated with chemotherapy, diffuse gray and white matter volume reduction was observed. Less white matter expansion concurred with more cognitive decline (r > .349, p < .05). In conclusion, we found age‐dependent cognitive decline and white matter volume changes in patients with breast cancer after chemotherapy, which could possibly be linked to neuroinflammatory processes. White matter expansion after chemotherapy, more pronounced in premenopausal patients, correlated with less cognitive decline. This suggests such expansion to be age‐dependent, possibly caused by a protective response in the younger brain to chemotherapy‐induced neurotoxicity.
Keywords: age, brain volume, cancer, chemobrain, chemotherapy, cognition, deformation‐based morphometry, longitudinal, menopause, quality of life
1. INTRODUCTION
Eight percent of women in developed countries are confronted with breast cancer during their lifetime (Torre et al., 2015). The last 25 years, improvements in systemic treatment and screening have drastically improved survival rates (Kaplan, Malmgren, Atwood, & Calip, 2015). However, up to 70% of chemotherapy‐treated patients experience cognitive problems, frequently affecting memory, executive function, processing speed, and attention. This can have a lasting impact on the patient's quality of life (Janelsins, Kesler, Ahles, & Morrow, 2014; Wefel, Kesler, Noll, & Schagen, 2015).
The pathophysiology of chemotherapy‐induced cognitive impairment remains largely unknown (Ahles & Saykin, 2007a; Janelsins et al., 2014; Wefel et al., 2015). Multiple hypotheses of (in)direct pathways have been proposed, including neuroinflammation, neurotoxicity (Han et al., 2008), oxidative stress, hormonal dysregulation, and genetic predisposition (Ahles & Saykin, 2007a; Janelsins et al., 2014).
Despite age being a well‐known risk factor for the incidence of cancer (DeSantis et al., 2016) and neurodegenerative processes (Healey, Campbell, & Hasher, 2008), few studies have explored interactions of age and cognitive functioning after cancer treatment (Ahles, Saykin, McDonald, et al., 2010; Schilder, Seynaeve, Beex, et al., 2010). Ahles et al. (2010) found older patients to perform worse on processing speed measurements. Similarly, Schilder et al. (2010) found older patients treated with endocrine treatment scored lower on more cognitive domains than younger patients. Both studies suggest age to amplify the cognitive effects of chemotherapy (Ahles et al., 2010) and hormonal therapy (Schilder et al., 2010). Hypothesized underlying mechanisms include a weakened blood–brain‐barrier, stronger inflammatory reactions, coinciding neurodegeneration, delayed neural repair, and hormonal changes (Mandelblatt, Hurria, McDonald, et al., 2013). Assessing cognitive impact of cancer treatment in elderly, especially when frailty co‐occurs, is of high importance as therapy could accelerate cognitive aging and increase the risk of dementia (Lange, Rigal, Clarisse, et al., 2014).
In an attempt to unravel the underlying neural correlates, multiple studies have investigated morphometric brain changes after chemotherapy using voxel‐based morphometry (VBM; McDonald & Saykin, 2013). These studies reported lower total brain volume and widespread lower gray matter (GM) density/volume compared to controls (Koppelmans, de Ruiter, van der Lijn, et al., 2012; McDonald & Saykin, 2013). Longitudinal VBM analyses by Lepage, Smith, Moreau, et al. (2014), McDonald, Conroy, Ahles, West, and Saykin (2010), and McDonald, Conroy, Smith, West, and Saykin (2013) found widespread GM density/volume decrease 1 month after chemotherapy compared to baseline, with partial recovery 1 year later. Only a few studies reported white matter (WM) volumetric changes (Inagaki, Yoshikawa, Matsuoka, et al., 2007; McDonald & Saykin, 2013). However, VBM is known to have a low sensitivity in the latter tissue (Kurth, Luders, & Gaser, 2015).
In contrast to VBM, deformation‐based morphometry (DBM) does not rely on tissue segmentation, but on the deformation field derived from image registration, offering a direct method for investigating global volumetric brain changes (Ashburner et al., 1998; Despotović, Goossens, & Philips, 2015; Gaser, Nenadic, Buchsbaum, Hazlett, & Buchsbaum, 2001). Hence, DBM could provide new insights in the neural substrate of aging and chemotherapy‐induced cognitive changes.
Therefore, we used DBM to assess brain volume changes after cancer treatment and its relationship to cognitive performance and age within the context of a longitudinal study, in patients with early‐stage breast cancer. Based on the aforementioned studies, we hypothesized widespread brain volume changes and cognitive decline after chemotherapy, more pronounced in older patients.
2. METHODS
2.1. Participants
A total of 180 women participated in this study, of whom 72 patients with early‐stage breast cancer had received adjuvant chemotherapy (C+), 49 patients did not receive chemotherapy (C−) and 59 healthy controls (HC). The population was recruited into two separate age groups: A young group who were premenopausal and younger than 52 years at baseline (n = 55C+, 32C−, and 41HC), and an older group who were postmenopausal and older than 60 years at baseline (n = 17C+, 17C−, and 18HC). Participants were excluded in case of history of cancer or a neurological/psychiatric condition. All participants were tested and recruited at the university hospitals of Leuven between 2008 and 2014.
Surgery was performed in both C+ and C− patients. Thereafter, C+ were treated with one of two standardized chemotherapy protocols, consisting of either six cycles of FEC (500 mg/m2 Fluorouracil, 100 mg/m2 Epirubicin, and 500 mg/m2 Cyclophosphamide) or three cycles of FEC and three cycles of Docetaxel (100 mg/m2). Chemotherapy cycles were standard administered at 3‐week intervals, except when contra‐indications were present. After the end of chemotherapy in the C+ group or surgery in the C− group, some patients additionally received radiotherapy and/or hormonal therapy.
Baseline assessment (t1) was performed after surgery, before chemo‐ or radiotherapy was initiated. Posttreatment assessment (t2) was conducted after completion of both chemo‐ and radiotherapy (when applicable). Hormonal therapy was already started for some patients at t2. This corresponded to a mean time interval of 5–6 months after end of chemotherapy. HC and C− participants were assessed at matched intervals. The study was approved by the local ethical commission and conducted in accordance with the Declaration of Helsinki. The younger group in this study consists of the populations described in Deprez, Amant, Yigit, et al. (2011) and Deprez, Vandenbulcke, Peeters, et al. (2014) (Table 1), supplemented with 4 C+ patients. However, these publications analyzed cognition and functional/diffusion MRI data.
Table 1.
Overview of the total population size and subdivision into samples
| Young (premenopausal) group 56 C+, 33 C−, and 37 HC | Old (postmenopausal) group 17 C+, 17 C−, and 18 HC | |||||
|---|---|---|---|---|---|---|
| Sample 1a | Sample 2b | Sample 3 | ||||
| Start date | January 17, 2008 | December 28, 2009 | July 29, 2008 | |||
| End date | October 22, 2012 | September 27, 2012 | May 08, 2014 | |||
| Group size | 39 C+, 16 C−, 20 HC | 17 C+, 17 C−, 17 HC | 17 C+, 17 C−, 18 HC | |||
| Available data (after exclusionc) | MRI | 37 C+, 15 C−, 19 HC | MRI | 16 C+, 16 C−, 14 HC | MRI | 17 C+, 14 C−, 16 HC |
| CFQ | 35 C+, 16 C−, 17 HC | CFQ | 17 C+, 17 C−, 17 HC | CFQ | 17 C+, 16 C−, 17 HC | |
| NP | 36 C+, 16 C−, 19 HC | NP | 17 C+, 17 C−, 18 HC | |||
Abbreviations: C−, patients not receiving chemotherapy; C+, patients receiving chemotherapy; CFQ, cognitive failure questionnaire; HC, healthy controls; NP, neuropsychological assessment.
34 C+, 16 C−, and 19 HC described in Deprez et al. (2011) corresponding to 92% of the population in Sample 1.
Population described in Deprez et al. (2014). Initial sample size and start and end dates of data acquisition are described, as well as sample size remaining after exclusion of outlier, missing and artifact‐ridden data.
Exclusions due to missing data, motion artifacts (MRI), or disproportionate time interval between therapy and testing (>5 SD) or between consecutive testing (>6 SD).
All data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2. Neuropsychological assessment
All participants completed the Cognitive Failure Questionnaire (CFQ; Broadbent, Cooper, FitzGerald, & Parkes, 1982) to evaluate self‐reported cognitive functioning, providing five subscales. Anxiety was assessed with the Spielberger State–Trait Anxiety Inventory (STAI; Spielberger, 1985), depressive symptoms with the Beck Depression Inventory (BDI; Bosscher, Koning, & Van Meurs, 1986) and verbal intelligence‐quotient with the Dutch Adult Reading Test (Schmand, Bakker, Saan, & Louman, 1991). More extensive neuropsychological assessments were acquired in a subset of participants (12 tests, 31 outcome measurements), as described previously (Deprez et al., 2011; Table 1).
2.3. Magnetic resonance imaging
Three‐dimensional T1‐weighted images were acquired on a 3 Tesla scanner (Intera; Philips Medical Systems, Best, the Netherlands) with an eight‐channel head coil (TR/TE/TI 9.6/4.6/900 ms, FOV 250 × 250 × 218mm, voxel size 0.98 × 0.98 × 1.20 mm). All scans were acquired on the same scanner with unchanged configuration.
Images were processed using ANTs N4‐bias field correction (Tustison, Avants, Cook, et al., 2010), followed by the longitudinal processing pipeline of the CAT12 (Gaser & Dahnke, 2012) SPM12 toolbox. Jacobian maps were calculated from the deformation matrices using symmetric longitudinal registration (Ashburner & Ridgway, 2013), providing a direct measure of brain volume changes. These maps were smoothed with a Gaussian filter with 8 mm isotropic FWHM. Visual inspection was performed on both raw and registered data to ensure data quality. Images with extensive motion artifacts were excluded.
Regional volume changes were calculated for the correlation analyses with neuropsychological outcome. As volume changes after chemotherapy were most prominent within the WM, the John Hopkins University WM atlas (Mori, Oishi, Jiang, et al., 2008) was applied to calculate the relative volume change within different WM regions.
2.4. Statistical analysis
Statistics of neuropsychological testing and correlation analysis were performed with SPSS version 25. At baseline, one‐way ANOVA was used to assess group differences and two‐way ANOVA to assess age and group interaction effects. Ordinal clinical data were compared between young and old subgroups using Mann–Whitney U‐test. Fisher's exact test was used to compare frequencies. Paired t‐tests were used to assess changes (t1 vs. t2) in neuropsychological performance within each subgroup. We used one‐way RM‐ANOVA to assess time‐by‐group interactions. Similarly, time‐by‐age effects were investigated for each group separately. In all analyses, BDI and IQ score were included as covariates (Pendergrass, Targum, & Harrison, 2018). Statistical significance was assessed at p < .05 with Hochberg correction for multiple outcomes (Blakesley, Mazumdar, Dew, et al., 2009).
We performed a GLM analysis of the Jacobian maps using SPM12 (Friston, Holmes, & Worsley, 1994), including the interactions between age (young vs. old), time (t1 vs. t2) and group (C+, C−, and HC). A pairing factor was included for each subject. BDI and scanner hardware maintenance (<2 months before maintenance of a RF transmitter coil or RF amplifier) were included as covariates (Mcdaniel, 2005; Wang, Wee, Suk, Tang, & Shen, 2015; Webb, Weber, Mundy, & Killgore, 2014). All reported p‐values were family‐wise‐error corrected on cluster level at p < .05, with an uncorrected voxel‐level threshold of p < .001. Two independent neuroradiologists (AR and SS) interpreted the resulting statistical maps.
Explorative Pearson correlations were used to examine the relationship between changes in self‐reported measures of neuropsychological performance and brain volume changes for young and older C+ patients separately. To reduce the number of tests, we only included neuropsychological tests showing a significant time‐by‐group interaction, total CFQ score. Five regions were selected by overlaying the JHU‐atlas with the parametric maps of significant volume changes in both age subgroups and age‐by‐time interactions.
3. RESULTS
3.1. Demographic and medical data
An overview of all participants is included in Table 1. Two young C+ patients were excluded due to a disproportionate time interval (>5 SD above mean) between the last administered chemotherapy and t2 (367 and 392 days, respectively). One young HC was excluded as outlier in time between t1 and t2 of 812 days (>6 SD above mean). Six participants had incomplete imaging data (2C+, 3C−, and 1HC). Because of extensive motion artifacts, six participants were excluded from imaging analysis (2C− and 4HC). Cognitive failure questionnaires were missing for seven participants (3C+, 1C−, and 3HC). Neuropsychological assessment was missing for two young C+ participants.
Table 2 summarizes demographic and medical information. Disease stage was similar for young compared to old patients in both cancer groups. However, C+ patients were more often diagnosed with stage II to III cancer, compared to C− patients. BDI scores were higher in treatment groups than controls but were consistent between the age groups. STAI scores were significantly higher in the old C+ patients than in the young subgroup, with similar trends for C− patients. Most patients received additional radiotherapy (>70%) and hormonal therapy (65%), with radiotherapy being significantly more administered in old than young C− patients. Years of education was slightly higher in both young treatment groups compared to the old subgroups, though verbal IQ was similar between age groups. In HC, IQ was slightly higher (5 points on average) in the older subgroup. Slight test–retest time variations exist (<24 days on average between age subgroups), which were significant for C+ and HC.
Table 2.
Demographics and clinical characteristics of the study population
| C+ (n = 72) | C− (n = 50) | HC (n = 54) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (n = 55) | Old (n = 17) | Young (n = 33) | Old (n = 17) | Young (n = 36) | Old (n = 18) | ||||||||||
| Mean/count | SD | Mean/count | SD | p | Mean/count | SD | Mean/count | SD | p | Mean/count | SD | Mean/count | SD | p | |
| Age at t1 | 43.4 | 5.7 | 63.8 | 3.4 | 43.8 | 5.2 | 63.8 | 4.0 | 42.4 | 5.7 | 62.5 | 2.9 | |||
| Breast cancer stage | |||||||||||||||
| 0 | 0 (0%) | 0 (0%) | 3 (9%) | 0 (0%) | |||||||||||
| I | 8 (15%) | 3 (18%) | 24 (73%) | 11 (65%) | |||||||||||
| II | 35 (64%) | 10 (59%) | 6 (18%) | 6 (35%) | |||||||||||
| III | 12 (22%) | 4 (24%) | 1 | 0 (0%) | 0 (0%) | .161 | |||||||||
| Chemotherapy protocol | |||||||||||||||
| 6× FEC | 16 (29%) | ||||||||||||||
| 3× FEC + 3× Docetaxel | 39 (71%) | 17 (100%) | |||||||||||||
| Antihormone therapy | 38 (69%) | 11 (65%) | .771* | 26 (79%) | 13 (76%) | 1* | |||||||||
| Radiotherapy | 52 (95%) | 16 (94%) | 1* | 23 (70%) | 17 (100%) | .010* | |||||||||
| Days since end of chemotherapy (t2) | 150 | 36 | 183 | 42 | <.001 | ||||||||||
| Depression BDI at t1 | 7.1 | 5.3 | 8.7 | 5.5 | .304 | 5.0 | 4.0 | 6.2 | 4.3 | .383 | 3.3 | 3.2 | 4.1 | 3.4 | .368 |
| Anxiety STAI at t1 | 33.1 | 8.3 | 38.2 | 8.4 | .016 | 33.8 | 7.3 | 37.2 | 9.4 | .196 | 30.8 | 6.3 | 30.2 | 6.7 | .650 |
| Years of education | 15.1 | 1.7 | 13.4 | 1.7 | <.001 | 14.7 | 1.7 | 13.5 | 2.5 | .081 | 15.0 | 1.7 | 14.5 | 2.8 | .886 |
| Verbal IQ | 114.3 | 7.3 | 114.6 | 6.2 | .733 | 113.2 | 6.6 | 111.5 | 10.8 | .695 | 115.6 | 5.1 | 120.4 | 7.2 | .002 |
| Days between t1 and t2 | 271 | 40 | 295 | 41 | .020 | 259 | 81 | 262 | 52 | .413 | 241 | 50 | 218 | 80 | .020 |
Differences between each age cohort are tested with Mann–Whitney U‐test for ordinal data, and Fischer's exact test for frequencies indicated with *. Significant results (p < .05, uncorrected) are indicated in bold typing.
Abbreviations: BDI, Beck Depression Inventory; C+, patients with breast cancer who underwent chemotherapy; C−, patients with breast cancer who did not undergo chemotherapy; FEC, 5−fluorouracil + eprirubicin + cyclophosphamide; HC, healthy controls; STAI, State−Trait Anxiety Inventory.
3.2. Neuropsychological assessment
Neuropsychological results are described in Tables 3 and 4 and Table A1.
Table 3.
Summary of the neuropsychological assessments
| All C+ (n = 53) | All C− (n = 33) | All HC (n = 37) | Group time interaction | Baseline age comparison | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | Paired t test | t1 | t2 | Paired t test | t1 | t2 | Paired t test | RM‐ANOVA | ANOVA | |||||||||
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | F | p | F | p | |
| Attention and concentration | |||||||||||||||||||
| Bourdon‐Wiersma—avg/row (s) | 12.1 | 2.5 | 11.5 | 2.0 | .006 | 12.6 | 2.8 | 12.1 | 2.8 | .038 | 11.4 | 1.7 | 10.8 | 1.7 | .001 | .1 | .886 | 13.3 | <.001 |
| Everyday attention—attentional switching (s) | 3.6 | 1.0 | 3.2 | .9 | .004 | 3.6 | 0.8 | 3.4 | 1.1 | .209 | 3.6 | 0.8 | 3.2 | 0.8 | <.001 | 1.9 | .155 | 15.6 | <.001 |
| WAIS letter number sequencing—total score | 11.0 | 2.1 | 10.0 | 1.8 | <.001 | 10.4 | 2.2 | 10.7 | 2.1 | .239 | 10.6 | 1.9 | 10.8 | 2.2 | .560 | 6.6 | .002 | 6.5 | .012 |
| Executive functioning | |||||||||||||||||||
| Controlled oral word association test—NAK | 43.2 | 11.2 | 41.5 | 11.3 | .020 | 38.2 | 11.2 | 36.2 | 10.3 | .240 | 41.7 | 7.0 | 44.3 | 7.7 | .044 | 2.8 | .064 | 1.5 | .222 |
| Stroop—Interference score (s) | 38.8 | 18.6 | 36.8 | 19.2 | .219 | 35.0 | 18.6 | 33.0 | 14.9 | .208 | 40.4 | 18.6 | 34.4 | 14.4 | .006 | 1.7 | .193 | 10.6 | .001 |
| Memory | |||||||||||||||||||
| AVLT—learning | 56.0 | 10.5 | 53.6 | 9.2 | .008 | 50.8 | 9.0 | 53.1 | 7.8 | .013 | 55.0 | 6.5 | 56.0 | 6.6 | .305 | 5.8 | .004 | 9.3 | .003 |
| RVDLT—learning | 45.2 | 12.0 | 46.9 | 12.0 | .066 | 42.4 | 9.2 | 45.2 | 12.8 | .031 | 45.2 | 11.5 | 49.4 | 11.0 | .006 | .9 | .420 | 20.7 | <.001 |
| Processing speed | |||||||||||||||||||
| WAIS digit symbol | 63.0 | 13.0 | 60.3 | 13.0 | .008 | 58.3 | 11.1 | 58.5 | 13.1 | .861 | 62.2 | 12.4 | 64.2 | 13.7 | .054 | 3.0 | .057 | 55.4 | <.001 |
| 9PEG—dominant hand (s) | 18.2 | 2.2 | 18.9 | 3.0 | .026 | 19.5 | 3.5 | 19.2 | 3.4 | .592 | 18.5 | 3.1 | 17.8 | 2.2 | .065 | 2.4 | .099 | 48.5 | <.001 |
| 9PEG—nondominant hand (s) | 19.4 | 2.7 | 20.3 | 3.0 | .001 | 20.6 | 2.7 | 20.1 | 2.6 | .215 | 19.5 | 2.8 | 19.0 | 2.4 | .130 | 4.1 | .020 | 53.7 | <.001 |
| All C+ (n = 69) | All C− (n = 49) | All HC (n = 51) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self‐reported cognitive complaints: CFQ | |||||||||||||||||||
| Distraction | 9.0 | 3.6 | 10.8 | 4.5 | <.001 | 9.8 | 5.1 | 9.4 | 5.3 | .710 | 9.3 | 2.9 | 9.1 | 3.0 | .393 | 9.0 | <.001 | 12.6 | .001 |
| Names and words finding | 5.4 | 2.1 | 6.0 | 2.3 | .006 | 6.2 | 2.2 | 6.3 | 2.3 | .731 | 5.8 | 2.1 | 5.7 | 2.2 | .781 | 2.0 | .142 | 5.0 | .027 |
| Total score | 33.3 | 12.0 | 38.4 | 14.3 | <.001 | 34.5 | 11.9 | 34.9 | 12.3 | .689 | 34.1 | 8.5 | 33.8 | 10.0 | .762 | 8.1 | <.001 | 3.8 | .053 |
Only tests with significant differences are listed below (p < .05, uncorrected, bold). Higher scores on Auditory Verbal Learning Test (AVLT), controlled oral word association test, Rey Verbal Learning Test (RVDLT), Wechsler Adult Intelligence Scale (WAIS) digit symbol and WAIS letter‐number sequencing indicate better performance. Whereas for Bourdon–Wiersma Dot cancelation test, Everyday Attention test, nine‐hole peg (9PEG), Stroop test, and trail making test lower scores indicate better performance. ANOVA model with Beck Depression Inventory and IQ as covariates. For the neuropsychological tests, false discovery rate (FDR) correction (Hochberg) was performed for 31 outcome measurements. For the cognitive complaints, FDR‐ correction (Hochberg) was performed for five outcome measurements. Tests surviving correction are italicized.
Abbreviations: C+, Patients with breast cancer who underwent chemotherapy; C−, patients with breast cancer who did not undergo chemotherapy; HC, healthy controls.
Table 4.
Neuropsychological assessment of the young and old C+ patients
| Young C+ (n = 36) | Old C+ (n = 17) | Age time interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | Paired t test | t1 | t2 | Paired t test | RM‐ANOVA | ||||||
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | F | p | |
| Attention and concentration | ||||||||||||
| Bourdon–Wiersma—avg/row (s) | 11.8 | 2.8 | 11.2 | 2.0 | .007 | 12.5 | 1.4 | 12.2 | 2.1 | .375 | .3 | .614 |
| Everyday attention—attentional switching (s) | 3.3 | 0.8 | 3.2 | 0.8 | .316 | 4.1 | 1.2 | 3.3 | 1.1 | .003 | 12.5 | <.001 |
| WAIS letter number sequencing—total score | 11.2 | 2.2 | 10.3 | 1.8 | .012 | 10.5 | 1.8 | 9.2 | 1.4 | .004 | .7 | .401 |
| Executive functioning | ||||||||||||
| Stroop—interference score (s) | 34.4 | 18.6 | 31.1 | 12.6 | .019 | 45.2 | 19.0 | 48.9 | 24.7 | .218 | 5.4 | .024 |
| Controlled oral word association test—NAK | 43.3 | 10.7 | 41.9 | 12.0 | .434 | 42.8 | 12.7 | 39.8 | 10.2 | .014 | .6 | .459 |
| Memory | ||||||||||||
| AVLT—learning | 58.8 | 9.4 | 56.6 | 8.0 | .029 | 51.1 | 10.8 | 47.4 | 8.3 | .083 | .7 | .413 |
| RVDLT—learning | 49.1 | 11.4 | 50.2 | 11.4 | .268 | 37.0 | 8.2 | 39.5 | 9.0 | .050 | .1 | .708 |
| Processing speed | ||||||||||||
| WAIS digit symbol | 68.1 | 10.7 | 65.0 | 10.0 | .028 | 51.6 | 9.8 | 49.3 | 12.4 | .151 | .3 | .596 |
| Trail making test b | 59.4 | 19.4 | 55.7 | 16.1 | .257 | 83.2 | 31.6 | 94.1 | 42.6 | .022 | 12.0 | .001 |
| 9PEG—dominant hand (s) | 17.2 | 1.7 | 18.1 | 2.1 | .044 | 19.8 | 2.2 | 20.7 | 3.6 | .280 | .2 | .657 |
| 9PEG—nondominant hand (s) | 18.6 | 2.2 | 19.4 | 2.4 | .003 | 21.2 | 3.0 | 22.3 | 3.5 | .105 | .4 | .538 |
| Young C+ (n = 52) | Old C+ (n = 17) | |||||||||||
| Self‐reported cognitive complaints: CFQ | ||||||||||||
| Distraction | 8.7 | 3.6 | 11.0 | 4.7 | <.001 | 9.9 | 3.6 | 10.5 | 4.2 | .581 | 1.4 | .240 |
| Names and words finding | 5.1 | 2.1 | 5.8 | 2.3 | .010 | 6.4 | 1.9 | 6.8 | 2.0 | .452 | .3 | .594 |
| Total score | 32.5 | 12.4 | 38.8 | 15.4 | <.001 | 35.3 | 10.3 | 37.3 | 10.3 | .354 | 2.1 | .155 |
Only tests with significant differences are listed below (p < .05, uncorrected, bold). Higher scores on Auditory Verbal Learning Test (AVLT), controlled oral word association test, Rey Verbal Learning Test (RVDLT), Wechsler Adult Intelligence Scale (WAIS) digit symbol and WAIS letter‐number sequencing indicate better performance. Whereas for Bourdon–Wiersma Dot cancelation test, Everyday Attention test, nine‐hole peg (9PEG), Stroop test, and trail making test lower scores indicate better performance. ANOVA model with Beck Depression Inventory and IQ as covariates. For the neuropsychological t tests, only tests showing an age‐time interaction (this table) or time effect (Table 3) are included. False discovery rate (FDR) correction (Hochberg) was therefore performed for the resulting 11 outcome measurements. For the neuropsychological RM‐ANOVA, FDR‐correction (Hochberg) was performed for 31 outcome measurements. For the cognitive complaints, FDR‐correction (Hochberg) was performed for five outcome measurements. Tests surviving correction are italicized.
Abbreviation: C+, patients with breast cancer who underwent chemotherapy.
At baseline, no differences in neuropsychological performance or subjective measures between patients and controls were observed, after correction for BDI and IQ. Group‐by‐time interactions revealed C+ patients (n = 53) performed significantly worse after chemotherapy on attention/concentration, memory, and processing speed tests, while both control groups (n = 37HC, 33C−) showed increased performance on the same tests (Table 3).
Main effects of age were found on all neuropsychological test domains at baseline, with older participants (n = 52) performing consistently worse than younger participants (n = 71) (Table 4). No group‐by‐age interactions were found at baseline. When evaluating effects of age on cognitive decline, time‐by‐age interactions revealed older chemotherapy‐treated patients (n = 17) scored lower for processing speed (Trail making test b), while younger patients (n = 36) improved on this domain. A similar trend was seen for executive functioning (Stroop). A time‐by‐age interaction for the Everyday Attention test showed older patients to improve throughout treatment, approaching the average score of younger patients, who did not improve over time. No time‐by‐age interactions were found in C− and HC.
At baseline, a main effect of age was found for the self‐reported subscale of distraction in social situations and names and word‐finding; older participants reported being more distracted (Table 4). Neither group‐by‐age interaction at baseline nor time‐by‐age interaction for the treatment groups separately was found. However, paired t tests revealed younger participants reported more cognitive complaints after chemotherapy compared to baseline, whereas this was not significant for older patients.
3.3. Deformation‐based morphometry
DBM analysis showed widespread WM volume expansion 5–6 months (t2 vs. t1) after end of chemotherapy in C+ patients, accompanied by volume reductions in the ventricles and interhemispheric fissure (Figure 1a. In C− patients, volume decrease was found throughout multiple GM regions, extending into WM. Associated volume increase was found in the dorsal interhemispheric fissure (Figure 1b). No significant changes were detected in HC (Figure 1c).
Figure 1.
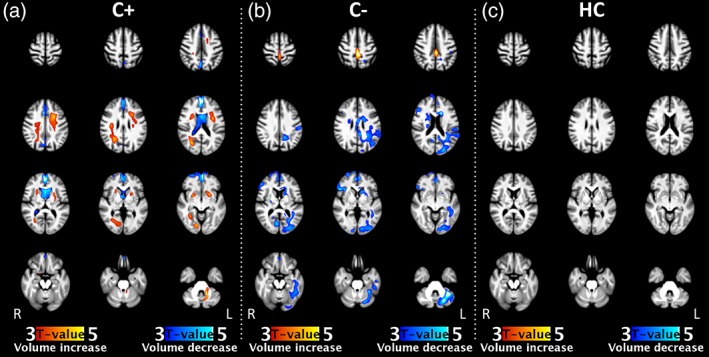
Within‐group volumetric changes of all groups, independent of age, comparing time point t2 with baseline. Brain volume changes were found in both cancer groups (C+: n = 70, C−: n = 45). No effects were found in the healthy control group (HC: n = 49). Statistical maps of volume increase and decrease are thresholded at p < .05, FWE corrected at cluster level, and color‐coded according to t‐value. See Table A2 on statistical values and MNI‐coordinates of significant clusters. C+, patients with breast cancer who underwent chemotherapy; C−, with patients with breast cancer who did not undergo chemotherapy; HC, healthy controls [Color figure can be viewed at http://wileyonlinelibrary.com]
With regard to age effects, widespread WM expansion was observed in the younger C+ group, alongside volume reductions in the CSF of the ventricles, interhemispheric fissure, and multiple cortical sulci, extending into the GM of the medial prefrontal cortex (MPFC) (Figure 2a). In contrast, no significant changes were found in older C+ patients (Figure 2b). Age‐by‐time interactions in the C+ group confirmed more WM expansion in the young compared to the older group, in right frontal and temporal regions, combined with volume reductions in the ventricles and the frontal interhemispheric fissure (Figure 2c). In C− patients, both young and old women (Figure 2d,e) showed volume decreases. In young patients, this was observed in the MPFC, fusiform gyrus, and left cerebellar hemisphere. In older patients, these patterns appeared in the bilateral cingulate cortices, as well as left frontal, parietal, occipital regions, basal ganglia, and anterior corpus callosum. For the younger subgroup, volume increase was found in the dorsal interhemispheric fissure. The age‐by‐time interaction indicated more volume decrease in the older subgroup in the subcortical WM and GM of the left orbitofrontal and middle frontal gyri (Figure 2f).
Figure 2.
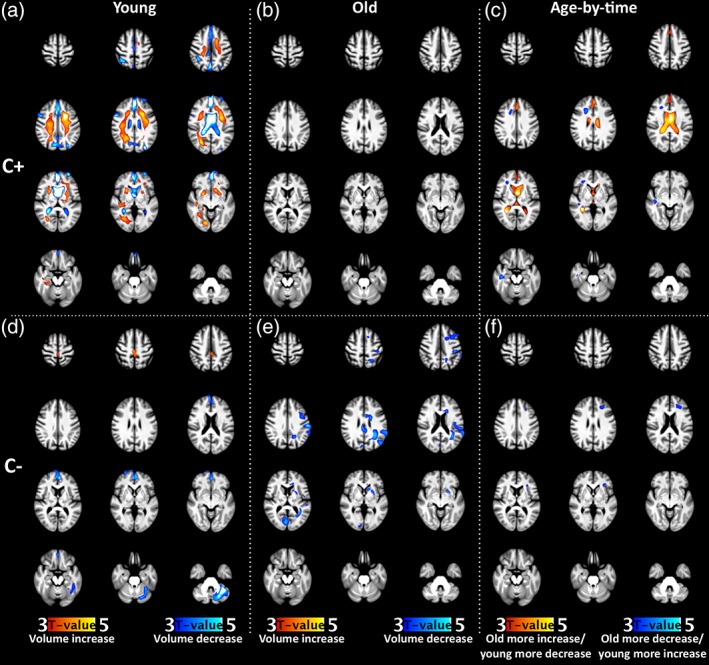
Comparison of the volumetric changes in young and old women after cancer therapy. Changes are shown for C+ (Row 1, a–c) and C− (Row 2, d–f) patients. The first (a, d with n = 53,3) and second (b, e with n = 17,14) column show regions of significant volume increase and decrease over time for the different young (premenopausal) and old (postmenopausal) groups, respectively. The last column (c, f) show regions with a significant time‐by‐age interaction effect for each group. All statistical maps are thresholded at p < .05, FWE corrected at cluster level, and color‐coded according to t‐value. See Table A3 for statistical values and MNI‐coordinates of significant clusters. C+, patients with breast cancer who underwent chemotherapy; C−, patients with breast cancer who did not undergo chemotherapy [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Correlation analysis of neuropsychological testing and WM volume changes
Explorative correlation analyses were performed for C+ patients between change in volume and performance on neuropsychological tests and cognitive complaints. Since only C+ patients showed cognitive decline, correlations were only performed for this group. Regions included: Right/left corona radiata, right/left superior longitudinal fasciculus (SLF), and the right sagittal stratum. In the older subgroup, a correlation was found between decreased verbal memory (AVLT) and more volume reduction in the right corona radiata (r = .484, p = .049, Figure 3b). For young C+ patients, correlations were found between decreased attention/concentration (WAIS letter‐number sequencing) and less volume increase in the right corona radiata (r = .349, p = .040, Figure 3a) and left SLF (r = .354, p = .037, Figure 3c). Cognitive complaints did not correlate with regional WM volume changes in either age groups.
Figure 3.

Correlation of changes in cognitive scores with changes in brain volume of young and old C+ patients. (a, b) Scatter plots of percental longitudinal change in left corona radiate brain volume and WAIS letter‐number sequencing change score in young patients (a) and AVLT learning in old patients (b). (c) Scatter plot of percental longitudinal change in left SLF volume and WAIS letter‐number sequencing change score in young patients. Change scores were calculated as t2 score − t1 score. For the percental volume change, these scores were divided by the baseline volume. A higher change score on the WAIS letter‐number sequencing and AVLT test indicate better performance. Positive brain volume change indicates volume expansion, while negative values indicate decrease in brain volume. AVLT, Auditory Verbal Learning Test, C+, patients with breast cancer who underwent chemotherapy; SLF, superior longitudinal fasciculus; WAIS, Wechsler Adult Intelligence Scale
4. DISCUSSION
This longitudinal study investigated cognitive functioning and brain volume changes after chemotherapy for breast cancer, and its interaction with aging. Cognitive impairment occurred after chemotherapy in both young and older patients, although older patients experienced more processing speed decline. Widespread WM volume expansion was observed 5–6 months after chemotherapy, only significantly present in young patients. In patients with cancer not treated with chemotherapy, diffuse GM/WM volume decreases were observed, with more volume decrease in the left frontal lobe of the older subgroup. Less WM expansion after chemotherapy correlated with cognitive decline.
The findings of decreased attention/concentration, memory, and processing speed after chemotherapy are in concordance with previous longitudinal studies (Mandelblatt et al., 2013; Vitali, Ripamonti, Roila, et al., 2017). Additionally, this study found older patients receiving chemotherapy to be more vulnerable with regard to processing speed. This corroborates the study of Ahles et al. (2010), investigating interactions between age and chemotherapy, which found more processing speed decline in older patients with less cognitive reserve. However, the majority of included patients received additional endocrine treatment, which could also have a cognitive impact (Schilder et al., 2010). Improvement on the Everyday Attention test only in older patients could be explained by a ceiling effect, since younger patients reached the same posttherapy average score as healthy volunteers (Chan, Lai, & Robertson, 2006). Nevertheless, younger patients reported more subjective cognitive decline. This suggests daily‐life experienced cognition depends on age‐modulated social expectations, which can be difficult to capture with objective tests. While no interactions between age and cognitive complaints have been found in an earlier study using a different questionnaire (Ahles et al., 2010), this finding adds to current recognition of young, highly educated women reporting persistent cognitive changes after chemotherapy (Vardy, Rourke, & Tannock, 2007).
To the best of our knowledge, this study is the first to observe WM expansion shortly after adjuvant chemotherapy in patients. However, a recent study by Winocur, Berman, Nguyen, et al. (2018), using a mouse model of breast cancer, showed chemotherapy to cause frontal brain volume enlargement. Moreover, earlier diffusion tensor imaging studies in this population reported widespread increased mean diffusivity and decreased fractional anisotropy in WM (Deprez et al., 2011; Menning, de Ruiter, Veltman, et al., 2017). A potential mechanism underlying such microstructural and volumetric changes could be edema formation, as a chemotherapy‐induced neuroinflammatory mechanism (Ahles & Saykin, 2007b). However, further research is necessary to confirm this hypothesis.
The chemotherapeutic agents used in our sample are known to have (in)direct neurotoxic effects. 5‐Fluorouracil, for example, is known to cross the blood–brain barrier by simple diffusion (Bourke, West, Chheda, & Tower, 1973) and causes WM changes in mice (Han et al., 2008). Besides direct toxic mechanisms, indirect processes such as inflammatory responses can also affect neural integrity, which was evidenced in rodents for multiple chemotherapeutic agents administered in our population (Seigers et al., 2016).
The lack of significant WM volume increase in old C+ patients suggests an interaction between neural changes induced by aging, hormonal changes, and chemotherapy. There are multiple aging candidate mechanisms for modulating this WM expansion: A primed proinflammatory state in the brain (Grebenciucova & Berger, 2017a; Raj, Yin, Breur, et al., 2017a; Salvadores, Sanhueza, Manque, & Court, 2017), delayed recruitment of microglia (Damani et al., 2011), and phagocytic cells (Zhao, Li, & Franklin, 2006) to the injured site, age‐related neurodegeneration (Salvadores et al., 2017), changes in brain viscoelasticity (Sack, Beierbach, Wuerfel, et al., 2009), blood–brain‐barrier changes (Mandelblatt et al., 2013), CSF changes slowing down excretion of neurotoxic metabolites (Simon & Iliff, 2016), and poorer tissue regeneration (Salvadores et al., 2017). This multitude of interacting processes could result in inefficient and/or delayed responses to neurotoxic effects of chemotherapy. Additionally, the different menopausal status in both age groups could have played a role. For example, estrogen is known to have a neuroprotective function (Arevalo, Azcoitia, & Garcia‐Segura, 2015; Mandelblatt et al., 2013). Moreover, given the well‐known proinflammatory state present in the aged brain (Grebenciucova & Berger, 2017b; Raj, Yin, Breur, et al., 2017b; Salvadores et al., 2017), it is unlikely the reduced volumetric response in the older C+ subgroup was caused by decreased neuroinflammation. Rather, the different volumetric brain patterns are presumably caused by a different progression of neuroinflammation with age, combined with an altered response to neuroinflammation in the aged brain.
In C+ patients, less pronounced WM swelling in either SLF or corona radiata correlated with more cognitive decline after chemotherapy; for attention and processing‐speed domains in young patients and memory in old patients. This result adds to the hypothesis of the observed WM enlargement as a “protective” response to neurotoxicity, which is more pronounced in young patients. Both WM regions found in this correlation analysis are known to be involved in higher cognitive functioning. The SLF, a long association tract between frontal and parietal cortices, is known to be linked with processing speed in healthy volunteers (Turken et al., 2008) and attention in MS patients (Van et al., 2010). Likewise, the corona radiata has been linked to attention, executive functioning, and memory domains (Kraus et al., 2007). Previous studies from our group on a subset of this data revealed correlations between microstructural WM changes of the corona radiata and SLF with attention and verbal memory tests (Deprez et al., 2011).
The GM volume decrease found in the C− group could reflect neural changes after cancer therapy, apart from chemotherapy. These changes can be attributed to a combination of other cancer therapies (radiotherapy (Ahles & Root, 2018), hormonal therapy (Boele, Schilder, de Roode, Deijen, & Schagen, 2015; Seliktar, Polek, Brooks, & Hardie, 2015)), cancer itself (Winocur et al., 2018), and interactions with anxiety (Andreotti, Root, Ahles, McEwen, & Compas, 2015), stress (Andreotti et al., 2015), and fatigue (Menning, de Ruiter, Veltman, et al., 2015). Specifically, a rodent study found tumorigenic mice to have decreased regional brain volume, while control mice treated with chemotherapy (5‐Fluorouracil and methotrexate) showed increased volume (Winocur et al., 2018). This preclinical observation suggests chemotherapy‐induced effects to potentially mask cancer‐induced GM decrease. Moreover, as DBM, compared to VBM, is less sensitive to mesoscopic volume changes (Ashburner et al., 1998), previously observed GM decrease (Lepage et al., 2014; McDonald et al., 2010, 2013) might be missed. This might partially explain the discrepancy between our findings and previous studies (Lepage et al., 2014; McDonald et al., 2010, 2013).
Some limitations of this study need to be mentioned. First, the sample size of the older subgroup remained limited, especially when compared to the younger subgroup. Still, the observed interaction effects between age and patient groups took different sample sizes into account and confirmed the observed within‐group differences. Second, breast cancer stage was significantly different between the patients treated with (C+) and without (C−) chemotherapy in both age groups. Although our results point to chemotherapy‐induced cognitive changes, the observed responses could still be a combinational effect of cancer‐induced changes, hormonal therapy, and chemotherapy. More specifically, as depicted in Table 2, the majority of C+ and C− also receive hormonal treatment. Although, at t2, hormonal treatment was only received for a short period of time, this might have modulated the observed effects. Moreover, by design of the study, both age groups differed in age as well as menopausal status. Consequently, further research is needed to distinguish the impact of both underlying factors. Next, slight test–retest variations were observed between groups. However, when we added this variation as a covariate in the imaging analysis, our results remained consistent. Finally, future studies should investigate metabolic and physiological changes after chemotherapy more directly and during a longer follow‐up, to confirm the hypothesis of neuroinflammation induced WM expansion, modulated by aging and hormonal changes.
To the best of our knowledge, this study is the first reporting an age‐modulated volumetric brain response to chemotherapy and its relation to cognition. We observed widespread WM enlargement after chemotherapy, only significantly present in the younger brain, suggested as a response to chemotherapy‐induced neuroinflammation. Patients with breast cancer who did not receive chemotherapy showed GM volume reduction, indicating a potential impact of cancer itself and/or the therapies apart from chemotherapy on neurodegeneration. Older chemotherapy patients showed to be more vulnerable to cognitive decline than their younger counterparts, while showing no WM enlargement after chemotherapy. Less WM expansion correlated with more cognitive decline, suggesting this volume response to originate from a protective mechanism against the neural impact of chemotherapy.
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The authors wish to acknowledge Frederik De Keyzer and Annouschka Laenen for giving advice on the statistical analysis. This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme (European Research Council, grant no 647047), Foundation against cancer (Stichting tegen kanker, grants no. 2014‐152 and no. 2010‐201) and Research Foundation Flanders (FWO, grant no. G.0480.10N). F.A. and S.S. are senior clinical researchers for the FWO. J.B. is an aspirant researcher for FWO.
Appendix 1.
1.1.
Table A1.
Neuropsychological assessment of the C− patients and healthy controls
| Young C− (n = 16) | Old C− (n = 17) | Young HC (n = 18) | Old HC (n = 18) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | Paired t test | t1 | t2 | Paired t test | t1 | t2 | Paired t test | t1 | t2 | Paired t test | |||||||||
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| Attention and concentration | ||||||||||||||||||||
| Bourdon–Wiersma ‐ avg/row (s) | 11.3 | 1.3 | 10.6 | 1.1 | .013 | 13.8 | 3.4 | 13.5 | 3.3 | .448 | 10.4 | 1.3 | 10.2 | 1.7 | .286 | 12.4 | 1.4 | 11.5 | 1.3 | <.001 |
| Everyday attention—attentional switching (s) | 3.4 | 0.8 | 3.0 | 0.8 | .044 | 3.8 | 0.9 | 3.8 | 1.2 | .990 | 3.3 | 0.7 | 2.9 | 0.5 | <.001 | 3.9 | 0.7 | 3.6 | 0.9 | .086 |
| WAIS letter number sequencing—total score | 11.1 | 2.3 | 11.5 | 1.8 | .343 | 9.8 | 2.1 | 10.0 | 2.2 | .509 | 11.0 | 2.2 | 11.7 | 2.0 | .235 | 10.2 | 1.4 | 9.9 | 2.1 | .231 |
| Executive functioning | ||||||||||||||||||||
| Stroop—interference score (s) | 30.4 | 10.0 | 27.2 | 8.0 | .030 | 39.3 | 23.6 | 38.4 | 17.8 | .747 | 32.8 | 11.4 | 26.7 | 11.1 | .008 | 48.5 | 21.5 | 42.5 | 13.1 | .218 |
| Controlled oral word association test—NAK | 41.1 | 12.2 | 38.0 | 10.5 | .333 | 35.5 | 9.7 | 34.5 | 10.1 | .528 | 41.7 | 8.7 | 44.2 | 8.0 | .194 | 41.7 | 6.8 | 44.4 | 6.1 | .132 |
| Memory | ||||||||||||||||||||
| AVLT—learning | 53.2 | 9.2 | 54.8 | 8.4 | .244 | 48.5 | 8.3 | 51.6 | 7.1 | .023 | 55.4 | 6.4 | 57.1 | 6.0 | .078 | 54.6 | 6.8 | 54.8 | 7.3 | .899 |
| RVDLT—learning | 46.9 | 8.4 | 51.9 | 12.3 | .011 | 38.1 | 11.4 | 38.9 | 11.4 | .644 | 47.2 | 11.3 | 51.9 | 10.9 | .067 | 43.1 | 11.7 | 46.8 | 10.8 | .032 |
| Processing speed | ||||||||||||||||||||
| WAIS digit symbol | 63.4 | 9.6 | 64.5 | 12.3 | .569 | 53.2 | 10.2 | 52.6 | 11.2 | .719 | 69.1 | 11.4 | 72.9 | 11.7 | .014 | 54.8 | 8.9 | 54.9 | 8.7 | .935 |
| Trail making test b | 59.4 | 13.2 | 55.0 | 15.3 | .127 | 96.1 | 40.8 | 86.8 | 39.8 | .213 | 56.3 | 15.0 | 54.4 | 22.2 | .698 | 74.8 | 23.0 | 67.8 | 20.5 | .152 |
| 9PEG—dominant hand (s) | 17.5 | 1.3 | 17.1 | 1.7 | .169 | 21.2 | 3.9 | 21.0 | 3.5 | .927 | 16.8 | 2.4 | 16.5 | 1.3 | .317 | 20.2 | 3.0 | 19.1 | 2.0 | .123 |
| 9PEG—nondominant hand (s) | 18.9 | 2.0 | 18.6 | 1.9 | .598 | 22.1 | 2.5 | 21.4 | 2.5 | .263 | 17.8 | 1.5 | 17.3 | 1.2 | .177 | 21.2 | 2.8 | 20.6 | 2.2 | .363 |
| Young C− (n = 32) | Old C− (n = 16) | Young HC (n = 32) | Old HC (n = 17) | |||||||||||||||||
| Self‐reported cognitive complaints: CFQ | ||||||||||||||||||||
| Distraction | 8.6 | 3.7 | 8.6 | 3.8 | .967 | 12.1 | 6.9 | 11.5 | 7.1 | .446 | 8.5 | 2.4 | 8.5 | 2.8 | 1.00 | 10.8 | 3.3 | 10.0 | 3.2 | .581 |
| Names and words finding | 6.0 | 2.2 | 6.1 | 2.4 | .936 | 6.6 | 2.2 | 6.9 | 2.0 | .615 | 5.7 | 2.3 | 5.7 | 2.4 | .917 | 6.0 | 1.6 | 5.8 | 1.8 | .450 |
| Total score | 33.5 | 11.7 | 34.1 | 11.0 | .732 | 37.1 | 12.3 | 37.6 | 14.2 | .845 | 32.7 | 8.0 | 33.4 | 9.6 | .569 | 36.9 | 9.5 | 34.8 | 10.2 | .231 |
Only tests with significant differences for the C+ group (chemotherapy treated patients) are listed below (p < .05, uncorrected, bold). Higher scores on Auditory Verbal Learning Test (AVLT), controlled oral word association test, Rey Verbal Learning Test (RVDLT), Wechsler Adult Intelligence Scale (WAIS) digit symbol, and WAIS letter‐number sequencing indicate better performance. Whereas for Bourdon–Wiersma Dot cancelation test, Everyday Attention test, nine‐hole peg (9PEG), Stroop test, and trail making test, lower scores indicate better performance. For the neuropsychological tests, False Discovery Rate (FDR) correction (Hochberg) was performed for 31 outcome measurements. For the cognitive complaints, FDR‐correction was performed for five outcome measurements. Tests surviving correction are italicized.
Abbreviations: C−, patients with breast cancer who did not undergo chemotherapy; HC, healthy controls.
Table A2.
MNI coordinates, cluster extent, and statistical values of significant clusters from the DBM analysis described in Figure 1
| Set level | Cluster level | Peak level | MNI coordinates (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | p | c | p FWE | q FDR | K E | p uncorr | p FWE | q FDR | T | p uncorr | x | y | z |
| C+: t1 − t2 | .000 | 3 | .000 | .000 | 6,554 | .000 | .000 | .001 | 6.23 | .000 | 4.5 | 54 | 18 |
| .002 | .011 | 5.58 | .000 | ‐3 | 60 | −6 | |||||||
| .003 | .013 | 5.44 | .000 | −3 | 51 | 19.5 | |||||||
| .000 | .000 | 5,380 | .000 | .013 | .023 | 5.12 | .000 | 1.5 | 16.5 | 13.5 | |||
| .021 | .024 | 4.99 | .000 | 7.5 | 4.5 | 4.5 | |||||||
| .079 | .063 | 4.64 | .000 | −9 | 21 | 9 | |||||||
| .013 | .025 | 1,102 | .002 | .068 | .058 | 4.68 | .000 | 3 | −63 | 48 | |||
| .590 | .329 | 3.92 | .000 | 7.5 | −72 | 43.5 | |||||||
| C+: t2 − t1 | .000 | 4 | .023 | .019 | 956 | .003 | .007 | .041 | 5.26 | .000 | −13.5 | −49.5 | −34.5 |
| .532 | .282 | 3.97 | .000 | −16.5 | −34.5 | −37.5 | |||||||
| .000 | .000 | 5,928 | .000 | .110 | .142 | 4.54 | .000 | 27 | −66 | 19.5 | |||
| .185 | .142 | 4.38 | .000 | 28.5 | −58.5 | −3 | |||||||
| .225 | .142 | 4.31 | .000 | 13.5 | −78 | −7.5 | |||||||
| .000 | .000 | 5,267 | .000 | .133 | .142 | 4.48 | .000 | −22.5 | 9 | 37.5 | |||
| .200 | .142 | 4.35 | .000 | −27 | 1.5 | 15 | |||||||
| .259 | .144 | 4.26 | .000 | −22.5 | 9 | −7.5 | |||||||
| .007 | .008 | 1,269 | .001 | .234 | .142 | 4.30 | .000 | 25.5 | 10.5 | 15 | |||
| .267 | .144 | 4.25 | .000 | 22.5 | 4.5 | −6 | |||||||
| .769 | .436 | 3.76 | .000 | 22.5 | 19.5 | 1.5 | |||||||
| C−: t1 − t2 | .000 | 5 | .000 | .000 | 27,444 | .000 | .001 | .021 | 5.76 | .000 | −34.5 | −48 | −36 |
| .003 | .022 | 5.46 | .000 | −16.5 | 19.5 | 18 | |||||||
| .010 | .048 | 5.18 | .000 | −24 | −70.5 | −36 | |||||||
| .000 | .000 | 2,972 | .000 | .004 | .022 | 5.43 | .000 | 9 | 73.5 | 4.5 | |||
| .027 | .075 | 4.93 | .000 | 6 | 51 | 3 | |||||||
| .063 | .116 | 4.70 | .000 | −4.5 | 55.5 | 1.5 | |||||||
| .000 | .001 | 2,125 | .000 | .023 | .075 | 4.97 | .000 | 48 | 24 | −1.5 | |||
| .092 | .130 | 4.59 | .000 | 34.5 | 22.5 | 0 | |||||||
| .198 | .157 | 4.35 | .000 | 51 | −37.5 | 9 | |||||||
| .017 | .025 | 1,033 | .002 | .058 | .114 | 4.72 | .000 | 7.5 | −72 | 13.5 | |||
| .382 | .205 | 4.12 | .000 | 9 | 57 | 18 | |||||||
| .536 | .272 | 3.97 | .000 | 13.5 | −91.5 | 3 | |||||||
| .010 | .019 | 1,171 | .001 | .568 | .280 | 3.94 | .000 | 22.5 | −10.5 | 21 | |||
| .626 | .294 | 3.89 | .000 | 18 | 3 | 18 | |||||||
| .967 | .663 | 3.45 | .000 | 22.5 | −4.5 | 28.5 | |||||||
| C−: t2 − t1 | .067 | 1 | .001 | .001 | 1,920 | .000 | .003 | .004 | 5.46 | .000 | −3 | −33 | 52.5 |
Statistics are performed at three levels: Set level (indicating chance of finding this number of clusters), cluster level, and peak level. Significance in this study was assessed on cluster level for a Bonferroni‐correct p < .05.
Abbreviations: c, number of clusters found at p < .001; C−, patients with breast cancer who did not undergo chemotherapy; C+, patients breast cancer who underwent chemotherapy; HC, healthy controls; K E, Cluster extent (in voxels); p FWE, Bonferroni corrected p; q FDR, false‐discovery rate corrected q; t1, timepoint 1; t2, timepoint 2.
Table A3.
MNI coordinates, cluster extent, and statistical values of significant clusters from the DBM analysis described in Figure 2
| Set level | Cluster level | Peak level | MNI coordinates (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | p | c | p FWE | q FDR | K E | p uncorr | p FWE | q FDR | T | p uncorr | x | y | z |
| C+young: t1 − t2 | .000 | 6 | .000 | .000 | 10,044 | .000 | .000 | .000 | 6.91 | .000 | 4.5 | 52.5 | 16.5 |
| .000 | .000 | 6.27 | .000 | −3 | 60 | −7.5 | |||||||
| .000 | .000 | 6.15 | .000 | −3 | 51 | 19.5 | |||||||
| .000 | .000 | 10,557 | .000 | .000 | .000 | 6.61 | .000 | 0 | 7.5 | 18 | |||
| .000 | .000 | 6.25 | .000 | 1.5 | 4.5 | 7.5 | |||||||
| .000 | .001 | 5.91 | .000 | −9 | 19.5 | 10.5 | |||||||
| .049 | .041 | 774 | .007 | .000 | .001 | 5.96 | .000 | −30 | −79.5 | 30 | |||
| .029 | .036 | 905 | .004 | .003 | .003 | 5.49 | .000 | 34.5 | −49.5 | 51 | |||
| .664 | .244 | 3.86 | .000 | 43.5 | −51 | 63 | |||||||
| .000 | .001 | 2,204 | .000 | .004 | .004 | 5.41 | .000 | 3 | −67.5 | 46.5 | |||
| .022 | .014 | 4.98 | .000 | 9 | −72 | 40.5 | |||||||
| .462 | .184 | 4.04 | .000 | −1.5 | −70.5 | 31.5 | |||||||
| .049 | .041 | 775 | .007 | .005 | .005 | 5.33 | .000 | 52.5 | 10.5 | 30 | |||
| C+young: t2 − t1 | .001 | 2 | .000 | .000 | 11,516 | .000 | .002 | .021 | 5.54 | .000 | −21 | 9 | 37.5 |
| .024 | .030 | 4.96 | .000 | −25.5 | 1.5 | 15 | |||||||
| .029 | .030 | 4.91 | .000 | −31.5 | 1.5 | 21 | |||||||
| .000 | .000 | 15,781 | .000 | .025 | .030 | 4.95 | .000 | 27 | −64.5 | 18 | |||
| .027 | .030 | 4.93 | .000 | 15 | −76.5 | −9 | |||||||
| .075 | .057 | 4.65 | .000 | 22.5 | −54 | 30 | |||||||
| C+: (t2 − t1)o − (t2 − t1)y | .006 | 2 | .000 | .000 | 10,612 | .000 | .001 | .005 | 5.84 | .000 | 28.5 | −51 | 10.5 |
| .004 | .010 | 5.42 | .000 | −1.5 | 1.5 | 18 | |||||||
| .004 | .010 | 5.41 | .000 | 0 | 3 | 9 | |||||||
| .000 | .001 | 2,137 | .000 | .218 | .136 | 4.32 | .000 | −3 | 43.5 | 28.5 | |||
| .418 | .230 | 4.08 | .000 | −3 | 30 | 39 | |||||||
| .542 | .274 | 3.97 | .000 | −4.5 | 67.5 | 18 | |||||||
| C+: (t1 − t2)o − (t1 − t2)y | .005 | 2 | .032 | .024 | 879 | .004 | .315 | .434 | 4.19 | .000 | 43.5 | −16.5 | −18 |
| .773 | .434 | 3.75 | .000 | 43.5 | −19.5 | −6 | |||||||
| .005 | .008 | 1,353 | .001 | .539 | .434 | 3.97 | .000 | 19.5 | 19.5 | 27 | |||
| .899 | .519 | 3.60 | .000 | 30 | 36 | 4.5 | |||||||
| C−young: t1 − t2 | .001 | 2 | .000 | .000 | 6,886 | .000 | .000 | .001 | 6.33 | .000 | −33 | −46.5 | −37.5 |
| .007 | .020 | 5.27 | .000 | −21 | −69 | −31.5 | |||||||
| .011 | .023 | 5.15 | .000 | −27 | −48 | −45 | |||||||
| .000 | .000 | 3,083 | .000 | .004 | .015 | 5.44 | .000 | −4.5 | 49.5 | 12 | |||
| .016 | .023 | 5.06 | .000 | 9 | 73.5 | 4.5 | |||||||
| .016 | .023 | 5.06 | .000 | 4.5 | 52.5 | 3 | |||||||
| C−young: t2 − t1 | .345 | 1 | .025 | .025 | 936 | .004 | .292 | .447 | 4.22 | .000 | 3 | −24 | 57 |
| .554 | .447 | 3.95 | .000 | −1.5 | −30 | 54 | |||||||
| .571 | .447 | 3.94 | .000 | −3 | −36 | 60 | |||||||
| C−old: t1 − t2 | .000 | 8 | .005 | .008 | 1,354 | .001 | .013 | .155 | 5.11 | .000 | −57 | −21 | 39 |
| .322 | .368 | 4.19 | .000 | −40.5 | −28.5 | 48 | |||||||
| .008 | .010 | 1,223 | .001 | .054 | .204 | 4.74 | .000 | 4.5 | −78 | 12 | |||
| .625 | .388 | 3.89 | .000 | 9 | −79.5 | 33 | |||||||
| .746 | .435 | 3.78 | .000 | 10.5 | −67.5 | 10.5 | |||||||
| .040 | .033 | 824 | .006 | .056 | .204 | 4.73 | .000 | 3 | −24 | 31.5 | |||
| .485 | .388 | 4.02 | .000 | −4.5 | −34.5 | 31.5 | |||||||
| .000 | .000 | 3,751 | .000 | .088 | .210 | 4.60 | .000 | −28.5 | −45 | 28.5 | |||
| .114 | .226 | 4.53 | .000 | −33 | −52.5 | 15 | |||||||
| .231 | .300 | 4.30 | .000 | −46.5 | −70.5 | 28.5 | |||||||
| .000 | .001 | 2,205 | .000 | .146 | .226 | 4.45 | .000 | −63 | −42 | 24 | |||
| .500 | .388 | 4.00 | .000 | −55.5 | −30 | 19.5 | |||||||
| .615 | .388 | 3.90 | .000 | −39 | −13.5 | 18 | |||||||
| .001 | .002 | 1,834 | .000 | .223 | .300 | 4.31 | .000 | −46.5 | 18 | 51 | |||
| .567 | .388 | 3.94 | .000 | −39 | 4.5 | 40.5 | |||||||
| .776 | .451 | 3.75 | .000 | −16.5 | 9 | 49.5 | |||||||
| .018 | .018 | 1,019 | .003 | .396 | .376 | 4.10 | .000 | −18 | 0 | 28.5 | |||
| .626 | .388 | 3.89 | .000 | −18 | 21 | 18 | |||||||
| .686 | .390 | 3.84 | .000 | −6 | 4.5 | 28.5 | |||||||
| .050 | .037 | 769 | .007 | .396 | .376 | 4.10 | .000 | −22.5 | 10.5 | −4.5 | |||
| .512 | .388 | 3.99 | .000 | −12 | 15 | 1.5 | |||||||
| C−: (t1 − t2)o − (t1 − t2)y | .067 | 1 | .012 | .032 | 1,137 | .002 | .889 | .870 | 3.61 | .000 | −30 | 28.5 | 1.5 |
| .933 | .870 | 3.54 | .000 | −25.5 | 33 | 25.5 | |||||||
| .999 | .952 | 3.16 | .001 | −16.5 | 31.5 | 13.5 | |||||||
Statistics are performed at three levels: Set level (indicating chance of finding this number of clusters), cluster level, and peak level. Significance in this study was assessed on cluster level for a Bonferroni‐correct p < .05.
Abbreviations: c, number of clusters found at p < .001; C−, patients with breast cancer who did not undergo chemotherapy; C+, patients breast cancer who underwent chemotherapy; HC, healthy controls; K E, cluster extent (in voxels); o, old, postmenopausal subgroup; p FWE, Bonferroni corrected p; q FDR, false discovery rate corrected q; t1, timepoint 1; t2, timepoint 2; y, young, premenopausal subgroup.
Blommaert J, Schroyen G, Vandenbulcke M, et al. Age‐dependent brain volume and neuropsychological changes after chemotherapy in breast cancer patients. Hum Brain Mapp. 2019;40:4994–5010. 10.1002/hbm.24753
Parts of this work have been presented at the 2017 yearly conference of the European Society for Magnetic Resonance in Medicine and Biology (ESMRMB).
Funding information Fonds Wetenschappelijk Onderzoek, Grant/Award Number: G.0480.10N; H2020 European Research Council, Grant/Award Number: 647047; Stichting Tegen Kanker, Grant/Award Numbers: 2010‐201, 2014‐152
Contributor Information
Jeroen Blommaert, Email: jeroen.blommaert@kuleuven.be.
Sabine Deprez, Email: sabine.deprez@uzleuven.be.
REFERENCES
- Ahles, T. A. , & Root, J. C. (2018). Cognitive effects of Cancer and Cancer treatments. Annual Review of Clinical Psychology, 14(1), 084903 10.1146/annurev-clinpsy-050817-084903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles, T. A. , & Saykin, A. J. (2007a). Candidate mechanisms for chemotherapy‐induced cognitive changes. Nature Reviews. Cancer, 7(3), 192–201. 10.1038/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles, T. A. , & Saykin, A. J. (2007b). Candidate mechanisms for chemotherapy‐induced cognitive changes. Nature Reviews. Cancer, 7(3), 192–201. 10.1038/nrc2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles, T. A. , Saykin, A. J. , McDonald, B. C. , Li, Y. , Furstenberg, C. T. , Hanscom, B. S. , … Kaufman, P. A. (2010). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology, 28(29), 4434–4440. 10.1200/JCO.2009.27.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti, C. , Root, J. C. , Ahles, T. A. , McEwen, B. S. , & Compas, B. E. (2015). Cancer, coping, and cognition: A model for the role of stress reactivity in cancer‐related cognitive decline. Psycho‐Oncology, 24(6), 617–623. 10.1002/pon.3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo, M.‐A. , Azcoitia, I. , & Garcia‐Segura, L. M. (2015). The neuroprotective actions of oestradiol and oestrogen receptors. Nature Reviews. Neuroscience, 16(1), 17–29. 10.1038/nrn3856 [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , Hutton, C. , Frackowiak, R. , Johnsrude, I. , Price, C. , & Friston, K. (1998). Identifying global anatomical differences: Deformation‐based morphometry. Human Brain Mapping, 6(5–6), 348–357. 10.1002/(SICI)1097-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Ridgway, G. R. (2013). Symmetric diffeomorphic modeling of longitudinal structural MRI. Frontiers in Neuroscience, 6, 1–19. 10.3389/fnins.2012.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakesley, R. E. , Mazumdar, S. , Dew, M. A. , Houck, P. R. , Tang, G. , Reynolds, C. F., 3rd , & Butters, M. A. (2009). Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology, 23(2), 255–264. 10.1037/a0012850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele, F. W. , Schilder, C. M. T. , de Roode, M.‐L. , Deijen, J. B. , & Schagen, S. B. (2015). Cognitive functioning during long‐term tamoxifen treatment in postmenopausal women with breast cancer. Menopause, 22(1), 17–25. 10.1097/GME.0000000000000271 [DOI] [PubMed] [Google Scholar]
- Bosscher, R. J. , Koning, H. , & Van Meurs, R. (1986). Reliability and validity of the Beck depression inventory in a Dutch college population. Psychological Reports, 58(3), 696–698. 10.2466/pr0.1986.58.3.696 [DOI] [PubMed] [Google Scholar]
- Bourke, R. S. , West, C. R. , Chheda, G. , & Tower, D. B. (1973). Kinetics of entry and distribution of 5‐fluorouracil in cerebrospinal fluid and brain following intravenous injection in a primate. Cancer Research, 33(7), 1735–1746. [PubMed] [Google Scholar]
- Broadbent, D. E. , Cooper, P. F. , FitzGerald, P. , & Parkes, K. R. (1982). The cognitive failures questionnaire (CFQ) and its correlates. The British Journal of Clinical Psychology, 21(1), 1–16. 10.1111/j.2044-8260.1982.tb01421.x [DOI] [PubMed] [Google Scholar]
- Chan, R. C. K. , Lai, M. K. , & Robertson, I. H. (2006). Latent structure of the test of everyday attention in a non‐clinical Chinese sample. Archives of Clinical Neuropsychology, 21(5), 477–485. 10.1016/j.acn.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Damani, M. R. , Zhao, L. , Fontainhas, A. M. , Amaral, J. , Fariss, R. N. , & Wong, W. T. (2011). Age‐related alterations in the dynamic behavior of microglia. Aging Cell, 10(2), 263–276. 10.1111/j.1474-9726.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez, S. , Amant, F. , Yigit, R. , Porke, K. , Verhoeven, J. , Van den Stock, J. , … Sunaert, S. (2011). Chemotherapy‐induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human Brain Mapping, 32(3), 480–493. 10.1002/hbm.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez, S. , Vandenbulcke, M. , Peeters, R. , Emsell, L. , Smeets, A. , Christiaens, M. R. , … Sunaert, S. (2014). Longitudinal assessment of chemotherapy‐induced alterations in brain activation during multitasking and its relation with cognitive complaints. Journal of Clinical Oncology, 32(19), 2031–2038. 10.1200/JCO.2013.53.6219 [DOI] [PubMed] [Google Scholar]
- DeSantis, C. E. , Fedewa, S. A. , Goding Sauer, A. , Kramer, J. L. , Smith, R. A. , & Jemal, A. (2016). Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians, 66(1), 31–42. 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- Despotović, I. , Goossens, B. , & Philips, W. (2015). MRI segmentation of the human brain: Challenges, methods, and applications. Computational and Mathematical Methods in Medicine, 2015, 1–23. 10.1155/2015/450341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. , Holmes, A. , & Worsley, K. (1994). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2(4), 189–210. [Google Scholar]
- Gaser C, Dahnke R. CAT—A computational anatomy toolbox for the analysis of structural MRI data In: HBM Conference 2012, Geneva. Vol. 32; 2012:7743.
- Gaser, C. , Nenadic, I. , Buchsbaum, B. R. , Hazlett, E. A. , & Buchsbaum, M. S. (2001). Deformation‐based Morphometry and its relation to conventional Volumetry of brain lateral ventricles in MRI. NeuroImage, 13(6), 1140–1145. 10.1006/nimg.2001.0771 [DOI] [PubMed] [Google Scholar]
- Grebenciucova, E. , & Berger, J. R. (2017a). Immunosenescence: The role of aging in the predisposition to Neuro‐infectious complications arising from the treatment of multiple sclerosis. Current Neurology and Neuroscience Reports, 17(8), 61 10.1007/s11910-017-0771-9 [DOI] [PubMed] [Google Scholar]
- Grebenciucova, E. , & Berger, J. R. (2017b). Immunosenescence: The role of aging in the predisposition to neuro‐infectious complications arising from the treatment of multiple sclerosis. Current Neurology and Neuroscience Reports, 17(8), 61 10.1007/s11910-017-0771-9 [DOI] [PubMed] [Google Scholar]
- Han, R. , Yang, Y. M. , Dietrich, J. , Luebke, A. , Mayer‐Pröschel, M. , & Noble, M. (2008). Systemic 5‐fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. Journal of Biology, 7(4), 12 10.1186/jbiol69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey, M. K. , Campbell, K. L. , & Hasher, L. (2008). Cognitive aging and increased distractibility: Costs and potential benefits. Progress in Brain Research, 169, 353–363. 10.1016/S0079-6123(07)00022-2 [DOI] [PubMed] [Google Scholar]
- Inagaki, M. , Yoshikawa, E. , Matsuoka, Y. , Sugawara, Y. , Nakano, T. , Akechi, T. , … Uchitomi, Y. (2007). Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer, 109(1), 146–156. 10.1002/cncr.22368 [DOI] [PubMed] [Google Scholar]
- Janelsins, M. C. , Kesler, S. R. , Ahles, T. A. , & Morrow, G. R. (2014). Prevalence, mechanisms, and management of cancer‐related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. 10.3109/09540261.2013.864260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, H. G. , Malmgren, J. A. , Atwood, M. K. , & Calip, G. S. (2015). Effect of treatment and mammography detection on breast cancer survival over time: 1990‐2007. Cancer, 121(15), 2553–2561. 10.1002/cncr.29371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans, V. , de Ruiter, M. B. , van der Lijn, F. , Boogerd, W. , Seynaeve, C. , van der Lugt, A. , … Schagen, S. B. (2012). Global and focal brain volume in long‐term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Research and Treatment, 132(3), 1099–1106. 10.1007/s10549-011-1888-1 [DOI] [PubMed] [Google Scholar]
- Kraus, M. F. , Susmaras, T. , Caughlin, B. P. , Walker, C. J. , Sweeney, J. A. , & Little, D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain, 130(10), 2508–2519. 10.1093/brain/awm216 [DOI] [PubMed] [Google Scholar]
- Kurth, F. , Luders, E. , & Gaser, C. (2015). Voxel‐based morphometry. Brain Mapping: An Encyclopedic Reference, 1, 345–349. 10.1016/B978-0-12-397025-1.00304-3 [DOI] [Google Scholar]
- Lange, M. , Rigal, O. , Clarisse, B. , Giffard, B. , Sevin, E. , Barillet, M. , … Joly, F. (2014). Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer Treatment Reviews, 40(6), 810–817. 10.1016/j.ctrv.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Lepage, C. , Smith, A. M. , Moreau, J. , Barlow‐Krelina, E. , Wallis, N. , Collins, B. , … Scherling, C. (2014). A prospective study of grey matter and cognitive function alterations in chemotherapy‐treated breast cancer patients. Springerplus, 3(1), 444 10.1186/2193-1801-3-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt, J. S. , Hurria, A. , McDonald, B. C. , Saykin, A. J. , Stern, R. A. , VanMeter, J. W. , … Thinking and Living With Cancer Study . (2013). Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Seminars in Oncology, 40(6), 709–725. 10.1053/j.seminoncol.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdaniel, M. A. (2005). Big‐brained people are smarter: A meta‐analysis of the relationship between in vivo brain volume and intelligence. Intelligence, 33(4), 337–346. 10.1016/j.intell.2004.11.005 [DOI] [Google Scholar]
- McDonald, B. C. , Conroy, S. K. , Ahles, T. A. , West, J. D. , & Saykin, A. J. (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Research and Treatment, 123(3), 819–828. 10.1007/s10549-010-1088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, B. C. , Conroy, S. K. , Smith, D. J. , West, J. D. , & Saykin, A. J. (2013). Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain, Behavior, and Immunity, 30, S117–S125. 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, B. C. , & Saykin, A. J. (2013). Alterations in brain structure related to breast cancer and its treatment: Chemotherapy and other considerations. Brain Imaging and Behavior, 7(4), 374–387. 10.1007/s11682-013-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning, S. , de Ruiter, M. B. , Veltman, D. J. , Koppelmans, V. , Kirschbaum, C. , Boogerd, W. , … Schagen, S. B. (2015). Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—The role of fatigue. NeuroImage Clinical, 7, 547–554. 10.1016/j.nicl.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning, S. , de Ruiter, M. B. , Veltman, D. J. , Boogerd, W. , Oldenburg, H. S. A. , Reneman, L. , & Schagen, S. B. (2017). Changes in brain white matter integrity after systemic treatment for breast cancer: A prospective longitudinal study. Brain Imaging and Behavior, 12(2), 324–334. 10.1007/s11682-017-9695-x [DOI] [PubMed] [Google Scholar]
- Mori, S. , Oishi, K. , Jiang, H. , Jiang, L. , Li, X. , Akhter, K. , … Mazziotta, J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40(2), 570–582. 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass, J. C. , Targum, S. D. , & Harrison, J. E. (2018). Cognitive impairment associated with Cancer: A brief review. Innovations in Clinical Neuroscience, 15(1–2), 36–44. [PMC free article] [PubMed] [Google Scholar]
- Raj, D. , Yin, Z. , Breur, M. , Doorduin, J. , Holtman, I. R. , Olah, M. , … Boddeke, E. (2017a). Increased white matter inflammation in aging‐ and Alzheimer's disease brain. Frontiers in Molecular Neuroscience, 10(June), 1–18. 10.3389/fnmol.2017.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, D. , Yin, Z. , Breur, M. , Doorduin, J. , Holtman, I. R. , Olah, M. , … Boddeke, E. (2017b). Increased white matter inflammation in aging‐ and Alzheimer's disease brain. Frontiers in Molecular Neuroscience, 10(June), 1–18. 10.3389/fnmol.2017.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, I. , Beierbach, B. , Wuerfel, J. , Klatt, D. , Hamhaber, U. , Papazoglou, S. , … Braun, J. (2009). The impact of aging and gender on brain viscoelasticity. NeuroImage, 46(3), 652–657. 10.1016/J.NEUROIMAGE.2009.02.040 [DOI] [PubMed] [Google Scholar]
- Salvadores, N. , Sanhueza, M. , Manque, P. , & Court, F. A. (2017). Axonal degeneration during aging and its functional role in neurodegenerative disorders. Frontiers in Neuroscience, 11, 451 10.3389/fnins.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder, C. M. , Seynaeve, C. , Beex, L. V. , Boogerd, W. , Linn, S. C. , Gundy, C. M. , … Schagen, S. B. (2010). Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of Clinical Oncology, 28(8), 1294–1300. 10.1200/JCO.2008.21.3553 [DOI] [PubMed] [Google Scholar]
- Schmand, B. , Bakker, D. , Saan, R. , & Louman, J. (1991). The Dutch Reading test for adults: A measure of premorbid intelligence level. Tijdschrift voor Gerontologie en Geriatrie, 22(1), 15–19. [PubMed] [Google Scholar]
- Seigers, R. , Loos, M. , Van, T. O. , Boogerd, W. , Smit, A. B. , & Schagen, S. B. (2016). Neurobiological changes by cytotoxic agents in mice. Behavioural Brain Research, 299, 19–26. 10.1016/j.bbr.2015.10.057 [DOI] [PubMed] [Google Scholar]
- Seliktar, N. , Polek, C. , Brooks, A. , & Hardie, T. (2015). Cognition in breast cancer survivors: Hormones versus depression. Psycho‐Oncology, 24(4), 402–407. 10.1002/pon.3602 [DOI] [PubMed] [Google Scholar]
- Simon, M. J. , & Iliff, J. J. (2016). Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochimica et Biophysica Acta, 1862(3), 442–451. 10.1016/j.bbadis.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. (1985). Assessment of state and trait anxiety: Conceptual and methodological issues. Southern Psychologist, 2, 6–16. [Google Scholar]
- Torre, L. A. , Bray, F. , Siegel, R. L. , Ferlay, J. , Lortet‐Tieulent, J. , & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- Turken, A. , Whitfield‐Gabrieli, S. , Bammer, R. , Baldo, J. V. , Dronkers, N. F. , & Gabrieli, J. D. E. (2008). Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage, 42(2), 1032–1044. 10.1016/j.neuroimage.2008.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison, N. J. , Avants, B. B. , Cook, P. A. , Zheng, Y. , Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 Bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke, W. , Nagels, G. , Leemans, A. , Vandervliet, E. , Sijbers, J. , & Parizel, P. M. (2010). Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. Journal of Magnetic Resonance Imaging, 31(6), 1492–1498. 10.1002/jmri.22198 [DOI] [PubMed] [Google Scholar]
- Vardy, J. , Rourke, S. , & Tannock, I. F. (2007). Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. Journal of Clinical Oncology, 25(17), 2455–2463. 10.1200/JCO.2006.08.1604 [DOI] [PubMed] [Google Scholar]
- Vitali, M. , Ripamonti, C. I. , Roila, F. , Proto, C. , Signorelli, D. , Imbimbo, M. , … Lo Russo, G. (2017). Cognitive impairment and chemotherapy: A brief overview. Critical Reviews in Oncology/Hematology, 118, 7–14. 10.1016/j.critrevonc.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wee, C.‐Y. , Suk, H.‐I. , Tang, X. , & Shen, D. (2015). MRI‐based intelligence quotient (IQ) estimation with sparse learning. PLoS One, 10(3), e0117295 10.1371/journal.pone.0117295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, C. A. , Weber, M. , Mundy, E. A. , & Killgore, W. D. S. (2014). Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: A voxel‐based morphometric analysis. Psychological Medicine, 44(13), 2833–2843. 10.1017/S0033291714000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel, J. S. , Kesler, S. R. , Noll, K. R. , & Schagen, S. B. (2015). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer‐related cognitive impairment in adults. CA: A Cancer Journal for Clinicians, 65(2), 123–138. 10.3322/caac.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur, G. , Berman, H. , Nguyen, M. , Binns, M. A. , Henkelman, M. , van Eede, M. , … Tannock, I. F. (2018). Neurobiological mechanisms of chemotherapy‐induced cognitive impairment in a transgenic model of breast Cancer. Neuroscience, 369, 51–65. 10.1016/J.NEUROSCIENCE.2017.10.048 [DOI] [PubMed] [Google Scholar]
- Zhao, C. , Li, W.‐W. , & Franklin, R. J. M. (2006). Differences in the early inflammatory responses to toxin‐induced demyelination are associated with the age‐related decline in CNS remyelination. Neurobiology of Aging, 27(9), 1298–1307. 10.1016/J.NEUROBIOLAGING.2005.06.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available from the corresponding author upon reasonable request.


