Abstract
Colon cancer is a common malignancy, and its morbidity and mortality have been increasing in recent years in China. Shikonin (Shi), a naturally occurring naphthoquinone, exhibits anticancer activity. However, the mechanisms of action of Shi remain unclear. The aim of the present study was to investigate the antitumor mechanism of Shi in colon cancer cells. The effects of different Shi concentrations on the viability of colon cancer cells using MTT, colony formation and wound-healing assays were assessed. Western blot analysis was performed to detect the expression of LC3-II, p62. Shi effectively suppressed viability and cell migration, and induced autophagy in colon cancer cells. Yes-associated protein (YAP) increases cell viability, and inhibits cell apoptosis and cell contact. Expression of YAP is downregulated by Shi. The cytotoxic effects of Shi were further investigated on YAP overexpression and on YAP knockout cell lines. The findings revealed that Shi suppressed the viability and induced autophagy of colon cancer cells. Additionally, YAP expression reversed the effects of Shi. The results of the present study suggest that Shi may be a promising anticancer treatment for colon cancer, and YAP may be a potential diagnostic marker for colon cancer.
Keywords: colon cancer, shikonin, autophagy, yes-associated protein
Introduction
Cancer is the second largest cause of death in the world (1). Colon cancer is the fourth leading cause of all cancer-associated deaths worldwide. With increases in population size and age, the incidence rate of colorectal cancer is increasing year by year in China (2). In 2014, an estimated 71,830 men and 65,000 women were diagnosed with colorectal cancer and 26,270 men and 24,040 women died of the disease (3). At present, the treatments for colon cancer include surgical resection, radiation therapy and adjuvant chemotherapy. Radiotherapy and chemotherapy have well-established side effects in patients with colon cancer (4). Therefore, searching for effective drugs with fewer side effects remains a major task for researchers.
Hippo signaling pathways are important for maintaining organ size and tumor development (5). Yes-associated protein (YAP) is a cancer-associated oncogene and is one of the Hippo signaling pathway downstream signaling molecules (6). YAP can induce cell proliferation, restrain cell apoptosis, reduce cell contact inhibition and accelerate cancer cell transformation. Wang and Tang (7) reported that the protein expression levels of YAP in liver cancer were upregulated. Shikonin (Shi), which is extracted from Lithospermum erythrorhizon, has been used to treat burns, sore throats, measles and other ailments in China since 1963 (8). Recent research has shown that Shi has antitumor potential in various types of cancer cell lines, including pancreatic cancer, non-small cell lung cancer (9,10).
The aim of the present study was to assess the proliferation, cell migration and autophagy in vitro to investigate the effects of Shi on human colon HCT116 and SW620 cells, and to assess whether YAP was a target of Shi in these cell lines. The results showed that Shi inhibited proliferation, induced autophagy and inhibited migration of human colon cells. Additionally, Shi reduced the expression of YAP, whereas YAP overexpression reversed the effects of Shi on colon cancer. The results suggest that Shi may potentially be used as a treatment due to its ability to reduce YAP expression and reverse autophagy of colon cancer cells.
Materials and methods
Reagents
Shi was purchased from the Shanghai Technology Institute of Yuanye. The chemical structure of Shi is shown in Fig. 1.
Figure 1.
Chemical structure of shikonin.
Cell culture
The colon cancer cell lines, HCT116 and SW620, were purchased from the American Type Culture Collection. HCT116 and SW620 cells were cultured in McCoy's 5A and L-15 medium, respectively, containing 10% FBS (HyClone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100 mg/ml streptomycin (both Amresco; VWR International, LLC). All cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
MTT assay
Both HCT116 and SW620 cells (8×103 cells/well) were seeded in 96-well plates. Shi (0, 1, 2.5, 5, 7.5 and 10 µM) was added to the cells at the different concentrations stated and incubated for 24 and 48 h. Subsequently, MTT solution (10 mg/ml) was added. Cells were cultured for a further 2 h at 37°C, and 100 µl DMSO was added. The optical density of each well was measured at 570 nm and the inhibition rate was calculated using the following equation: Inhibition rate (%)=(average A570 of the control group-average A570 of the experimental group)/(average A570 of the control group-average A570 of the blank group) ×100. All MTT assays were repeated at least three times. The control group was the cells treated with DMSO alone, and the blank group was the wells without cells added.
Colony formation assay
HCT116 and SW620 cells were cultured in a 6-well plate at a density of 1×103 cells/well, and treated with Shi (0, 5 or 10 µM). After three weeks, cells were stained with crystal violet staining solution for 30 min at room temperature (Beyotime Institute of Biotechnology) and the visible colonies were counted under an optical light microscope at ×4 magnification (IX70; Olympus Corporation).
Wound-healing assay
HCT116 cells were cultured in McCoy's 5A medium and SW620 cells were cultured in L-15 without FBS. The cells were cultured in 6-well plates and grown to 100% confluence. A 200 µl pipette was used to create the wound, then PBS was used to wash the cell debris, and serum-free medium was added. Cells were treated with 10 µM Shi, and wound closure was observed using an inverted light microscope at a ×4 magnification imaged using a digital camera. The extent of wound healing is defined as the ratio of the difference of wound area. All the experiments were repeated three times.
Transfection
The HCT116 and SW620 cells (3×105 cells/well) were grown in 6-well plates, treated with various concentrations of Shi and transfected with YAP cDNA or YAP small interfering (si)RNA or empty vector using lipofectamine® 3000 according to the manufacturer's protocol. YAP siRNA oligonucleotides were purchased from GenePharma (Shanghai, China) and the sequences were; forward, 5′-GGUGAUACUAUCAACCAAATT-3′; and reverse, 5′-UUUGGUUGAUAGUAUCACCTT-3′. After 48 h of transfection with the lentiviral vectors (Biofeng, Guangzhou, China), successfully transfected cell lines were selected for using puromycin (Santa Cruz Biotechnology, Inc.) for 21 days.
Western blot analysis
Cells were harvested using RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein concentration was determined using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology). Soluble proteins were extracted from the cell lysate using 150 mM NaCl, 50 mM Tris, pH 8.0, 30% Acrylamide-Bis-acrylamide, 10% SDS, 1 mM PMSF, 1 mM sodium vanadate (Beyotime, Shanghai, China). A total of 25 µg protein were loaded into 10 or 15% SDS gels and resolved using SDS-PAGE. Subsequently, resolved proteins were transferred to PVDF membranes (EMD Millipore) and blocked in 5% skimmed milk for 2 h at 37°C. The membranes were incubated with primary antibodies at 4°C overnight and then washed with TBS-Tween-20. The primary antibodies used were: Anti-GAPDH (1:1,000; cat. no. AF0006; Beyotime Institute of Biotechnology); anti-LC3 (1:1,000; cat. no. 3868T; CST Biological Reagents Co., Ltd.); anti-P62 (1:1,000; cat. no. no. 5114T; CST Biological Reagents Co., Ltd.); anti-YAP (1:1,000; cat. no. 14074; CST Biological Reagents Co., Ltd.), anti-phospho-(p-)YAP (S127) (1:1,000; cat. no. 4911; CST Biological Reagents Co., Ltd.). Subsequently, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:5,000; cat. nos. E030120-01 and E030110-01; EarthOx Life Sciences) at room temperature for 1 h. Signals were visualized using a SuperSignal® West Pico Trial kit (Pierce; Thermo Fisher Scientific, Inc.). ImageJ 1.43U (National Institutes of Health) was used for densitometry analysis.
Statistical analysis
In the present study, all experiments were repeated at least three times and the results were analyzed using GraphPad Prism v5.0 (GraphPad Software, Inc.) and SPSS v17.0 (SPSS, Inc.). Data are presented as the mean ± standard deviation. Results were analyzed using an unpaired Student's t-test or one-way ANOVA followed by a Bonferroni's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
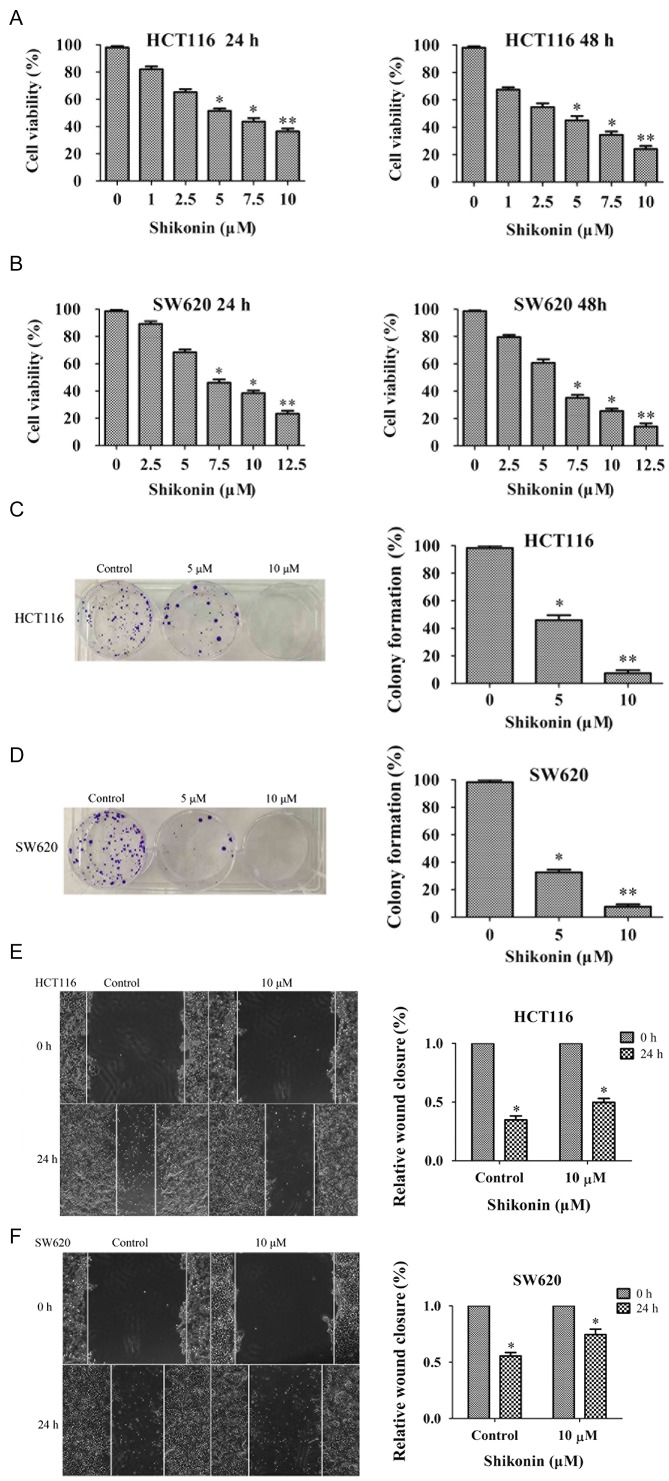
Shi decreases the viability and suppresses migration of HCT116 and SW620 cells
Shi is a natural plant extract which exhibits antiproliferative effects on cancer cell growth (8). The chemical structure of Shi is shown in Fig. 1. The effect of Shi on HCT116 and SW620 cells was investigated using an MTT assay, and the results showed that as Shi concentration or treatment time was increased, cell viability of HCT116 and SW620 cells appeared to be reduced (Fig. 2A and B). Colony formation assays showed a significant decrease in colony formation following Shi treatment and this appeared to be dose-dependent (Fig. 2C and D). Subsequently wound-healing assays were performed to examine the effects of Shi on colon cancer cells migration. When the cell density reached 100%, wounds were created with a pipette tip, and 10 µM Shi was added. After 24 h, wound closure in the HCT116 and SW620 cells was ~35 and 55%, respectively. Shi treatment significantly slowed wound closure in HCT116 (50%) and SW620 (80%) cells compared with the respective control (Fig. 2E and F). All these results showed that Shi exhibited cytotoxic effects on HCT116 and SW620 cells.
Figure 2.
Shi inhibits cell growth and the migration of colon cancer cells. The inhibitory effects of Shi on (A) HCT116 and (B) SW620 cells using MTT assay after treatment with Shi for 24 and 48 h, respectively. Colony formation ability of (C) HCT116 and (D) SW620 cells treated with Shi at the indicated concentrations. (E) HCT116 and (F) SW620 cells were assessed using a wound-healing assay and the relative wound closure was calculated compared with the respective 0 h time point. *P<0.05, **P<0.01vs. control DMSO group. Shi, shikonin.
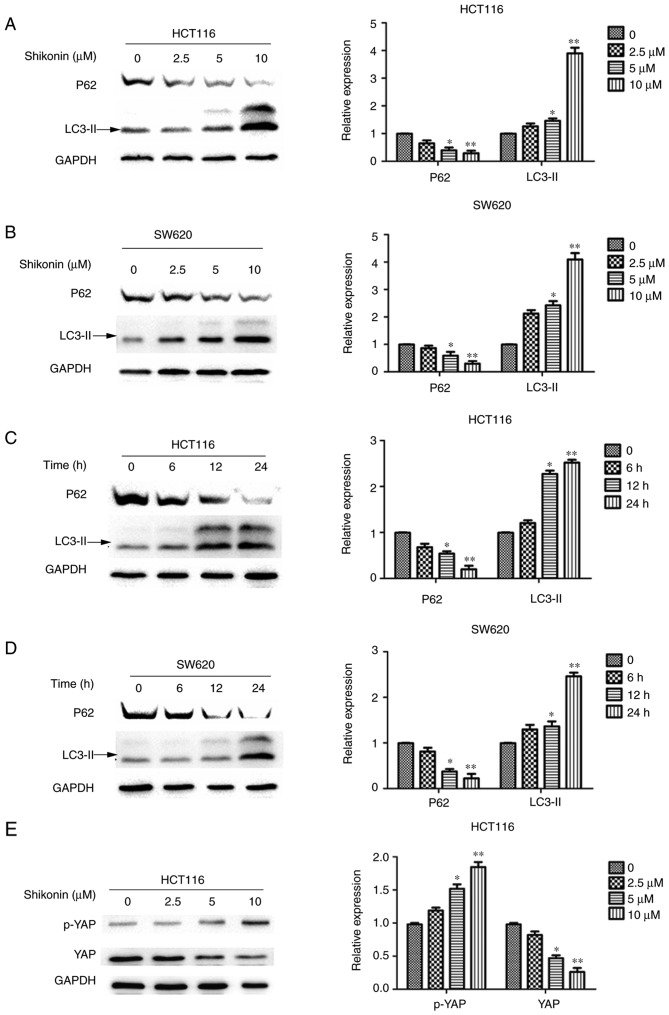
Shi induces autophagy and reduces YAP expression in HCT116 and SW620 cells
Increasing evidence suggests that autophagy is an important mechanism of death in cancer cells and may be of importance in the development of tumors (11). To evaluate whether autophagy was involved in Shi-induced cell death, the expression levels of LC3-II and p62 were evaluated in Shi-treated cells. HCT116 and SW620 cells were treated with 0, 2.5, 5 or 10 µM Shi for 24 h, or with 10 µM Shi for 0, 6, 12 or 24 h. After autophagy begins, the C-terminal LC3 is cleaved to produce the LC3II protein, which then translocates to autophagosomes (12). The expression of LC3-II was detected using western blot analysis and the results suggested that increasing Shi concentrations resulted in significantly upregulated LC3-II expression (Fig. 3A-D). The degradation of p62 (also known as sequestosome 1) is considered an accurate indicator of autophagy. Therefore, the expression of p62 in colon cancer cells treated with Shi was investigated. As the concentration of Shi used or the treatment time was increased, degradation of P62 became more notable in HCT116 and SW620 cells and the difference was significant compared with the control group (P<0.05) (Fig. 3A-D). In conclusion, these findings suggest that Shi induced autophagy in colon cancer cells. In addition, the changes in expression of YAP was also studied. The expression of YAP in HCT116 and SW620 cells treated with Shi was downregulated as the concentration or treatment with Shi was increased, and the difference was significant compared with the control group (P<0.05; Fig. 3E-H). Meanwhile, the serine127 phosphorylation of YAP in HCT116 and SW620 cells was also detected (Fig. 3E-H), suggesting that Shi could upregulate YAP phosphorylation. All these data concluded that Shi decreased the protein expression of YAP.
Figure 3.
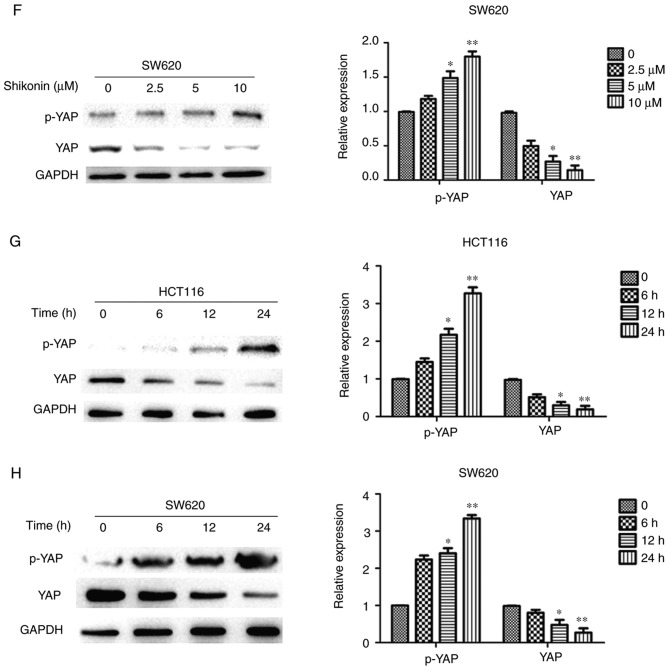
Shi induces autophagy and reduces YAP expression in HCT116 and SW620 cells. The expression LC3-II and p62 were determined using western blot analysis in (A) HCT116 and (B) SW620 cells treated with an increasing concentration of Shi for 24 h and in (C) HCT116 and (D) SW620 cells treated with 10 µM Shi for 6, 12 and 24 h. YAP and p-YAP expression was determined using western blot analysis in (E) HCT116. YAP and p-YAP expression was determined using western blot analysis. (F) SW620 cells treated with an increasing concentration of Shi for 24 h and in (G) HCT116 and (H) SW620 cells treated with 10 µM Shi for 6, 12 and 24 h. *P<0.05, **P<0.01, compared with control. Shi, shikonin; p, phosphorylated; YAP, yes-associated protein.
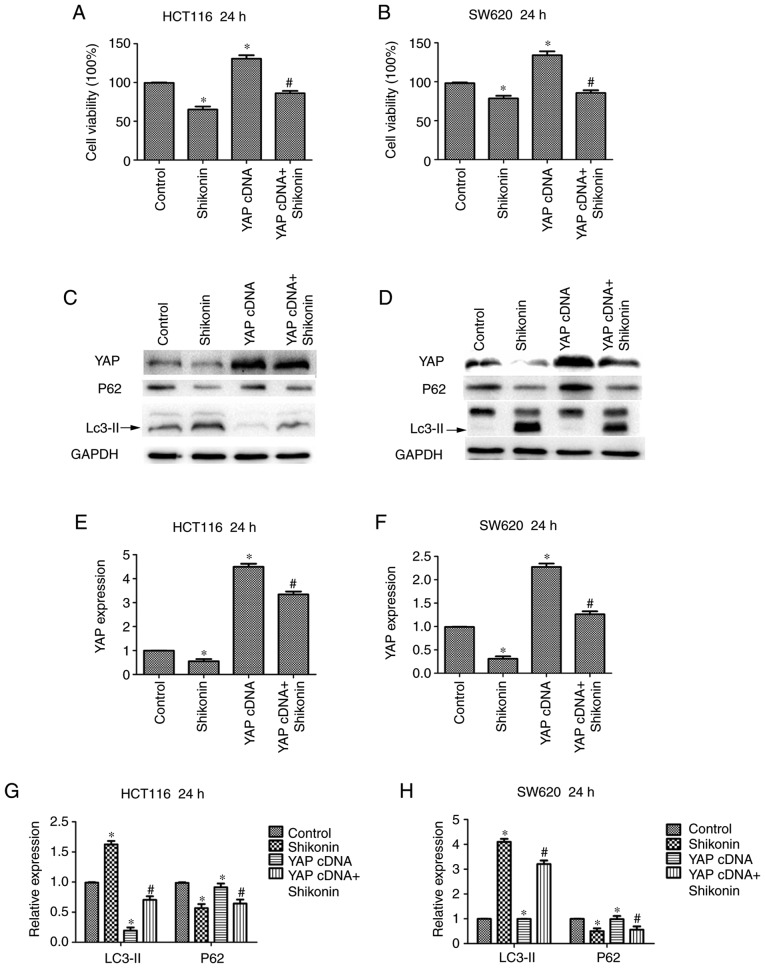
Overexpression of YAP antagonizes the inhibitory effect of Shi in HCT116 and SW620 cells
To detect whether YAP serves a critical role in the inhibitory effect of Shi on colon cancer, HCT116 and SW620 cells were transfected with the YAP-specific cDNA and subsequently treated with 10 µM Shi for 24 h (Fig. 4A and B). The results showed that overexpression of YAP promoted colon cancer cell growth and reversed the inhibition of Shi on cell growth to a certain extent. In addition, as shown in the Fig. 4C and D, YAP overexpression decreased the percentage of autophagy in HCT116 cells and SW620 cells. Additionally, LC3II protein expression levels were increased and p62 levels were decreased (Fig. 4C and D) in both cell lines. Densitometry analysis of LC3-II, P62 and YAP expression levels are shown in Fig. 4E-H. These results showed that the anticancer activity of Shi is partly caused by the inactivation of YAP.
Figure 4.
Shi reverses the influence of overexpressed YAP in HCT116 and SW620 cells. MTT assay was used to detect the effect of YAP overexpression in combination with 10 µM Shi on (A) HCT116 and (B) SW620 cell growth. YAP, LC3-II and p62 expression levels were determined using western blot analysis in (C) HCT116 and (D) SW620 cells transfected with YAP cDNA or treated with Shi. (E-H) Densitometry analysis of LC3-II, p62 and YAP. *P<0.05 compared with control. #P<0.05, compared with YAP cDNA transfection. Shi, shikonin; YAP, yes-associated protein.
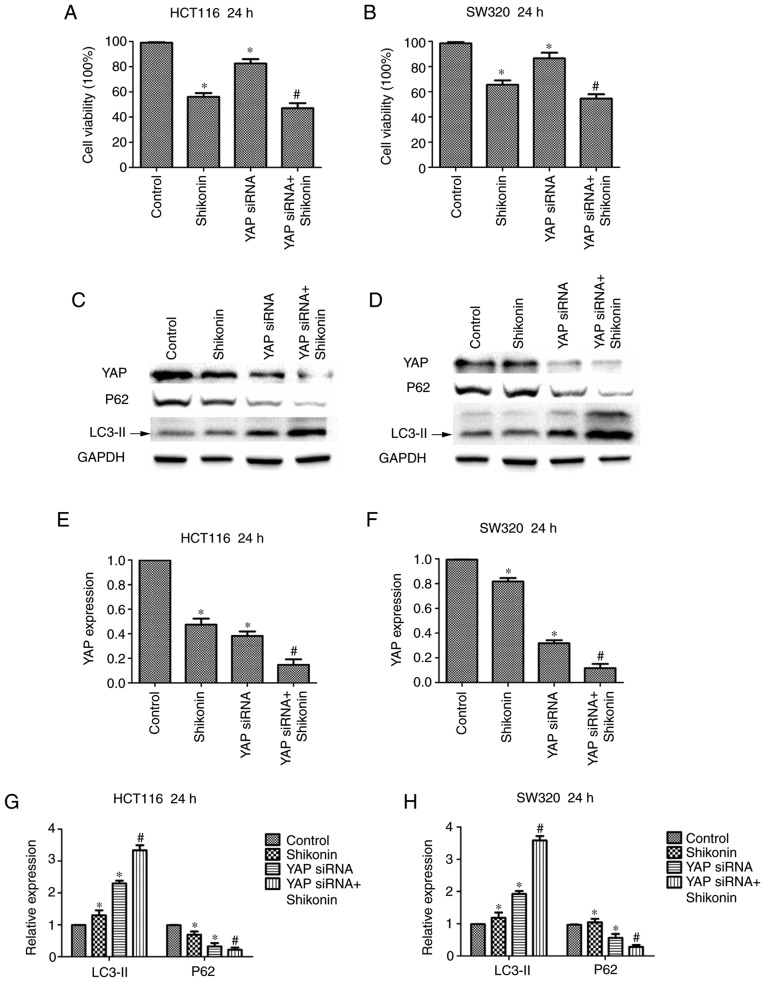
Knockdown of YAP increases sensitivity of colon cancer cells to Shi
To investigate whether the cytotoxicity of Shi is related to the regulation of YAP, viability assays were performed in HCT116 and SW620 cells. The HCT116 and SW620 cells were transfected with YAP-specific siRNA. The results show that downregulation of YAP decreased cell viability compared with the control group and caused colon cancer cells to become more sensitive to Shi. Shi and YAP siRNA only compared with control group and YAP siRNA + Shi group compared with YAP siRNA group (Fig. 5A and B). Moreover, it was found that the expression of LC3-II increased and expression of p62 decreased in the Shi and YAP siRNA only group compared with the control group, and in the YAP siRNA + Shi group compared with YAP siRNA group (Fig. 5C and D). Therefore Shi and YAP siRNA showed stronger induction of autophagy compared with siRNA transfection or Shi alone. Densitometry analysis of LC3-II, p62 and YAP expression levels are presented in Fig. 5E-H. The results showed that the synergistic effects of YAP-specific siRNA and Shi increased Shi-induced autophagy in colon cancer cells.
Figure 5.
Knockdown of YAP increases sensitivity of HCT116 and SW620 cells to Shi. The effect of YAP downregulation in combination with 10 µM Shi on (A) HCT116 and (B) SW620 cell growth. YAP, LC3-II and p62 protein expression was measured using western blot analysis in (C) HCT116 and (D) SW620 cells transfected with YAP siRNA and treated with Shi. (E-H) Densitometry analysis of LC3-II, p62 and YAP. *P<0.05, compared with control. #P<0.05, compared with YAP siRNA transfection. Shi, shikonin; YAP, yes-associated protein.
Discussion
A number of novel anticancer drugs are extracted from plants (13), and drugs obtained from plants have shown potential antitumor effects. Shi, which is a type of naphthoquinone extracted from the herbal plant Lithospermum erythrorhizon, and is considered to have anti-inflammatory and antivirus effects (14). In a previous colon cancer study, it was reported that Shi increased apoptosis in cisplatin-induced cancer cells (15). The effectiveness and safety of Shi for colon cancer requires further investigation. In the present study, Shi suppressed the viability and migration, and induced autophagy of the human HCT116 and SW620 colon cancer cell lines. The growth and migration of HCT116 and SW620 cells was significantly inhibited by Shi based on the results of the MTT, colony formation and wound-healing assays. Meanwhile, cell autophagy appeared to be increased by Shi in a dose- and time-dependent manner. These results strongly indicate that Shi has chemotherapeutic potential to treat patients with colon cancer.
Autophagy is a highly conserved cellular process and a biologically programmed form of cell death (16). Shi and Cao (9) reported that Shi could induce autophagy in the pancreatic BXPC3 cell line. In autophagy, LC3-II serves a role in the formation of autophagosomes and p62 is an autophagy marker (17). In the present study, the protein expression levels of LC3-II and p62 were altered resulting in activation of autophagy in cells treated with Shi. It has been demonstrated that Shi could induce autophagy in HCT116 and SW620 cells.
In 1995, the Hippo signaling pathway was first described (18). YAP is an effector of the Hippo-YAP signaling pathway in mammals, and can reduce excessive proliferation of cancer cells (19). Reduced expression of YAP is associated with autophagy and differentiation in carcinoma cells (20). Activation of YAP is involved in the development in cancer of the liver and the lung cancer amongst others (21). Therefore YAP has become a novel marker for cancer treatment (22,23). Phosphorylated YAP is sequestered in the cytoplasm by binding to 14-3-3 proteins thus; YAP cannot enter the nucleus, resulting in inhibition of its activity (24). In the present study, the expression of YAP was significantly reduced, and YAP phosphorylation was upregulated in colon cancer cells treated with Shi. Autophagy of colon cancer cells was related to the change in expression of YAP, suggesting that autophagy and YAP may be associated with the cytotoxic effects of Shi on colon cancer cells.
In conclusion, Shi is an effective suppressor of cell survival and invasion as well as an inducer of autophagy in colon cancer cells. In addition, Shi was found to reduce the expression of YAP and reverse the effects of YAP on colon cancer cells. Therefore YAP may be used as a potential diagnostic marker of colon cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Technology Foundation of China (Beijing, China; grant no. 81428016) and the Key Program for Science and Technology Development of Beijing (Beijing, China; grant no. Z151100003915073).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ drafted the manuscript. JZ and LZ performed experiments and data analysis. WS and BL performed the statistical analysis. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jouffret L, Turrini O, Ewald J, Moutardier V, Iovanna JL, Delpero JR. Long-term survivors after pancreatectomy for cancer: The TNM classification is outdated. ANZ J Surg. 2015;85:860–864. doi: 10.1111/ans.13277. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA. Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol. 2014;3:176–184. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SP, Wang LH. Disease implication of hyper-Hippo signalling. Open Biol. 2016;6 doi: 10.1098/rsob.160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Liu P, Zhou X, Wang T, Feng X, Sun YP, Xiong Y, Yuan HX, Guan KL. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res. 2017;77:2413–2423. doi: 10.1158/0008-5472.CAN-16-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YP, Tang DX. Expression of Yes-associated protein in liver cancer and its correlation with clinicopathological features and prognosis of liver cancer patients. Int J Clin Exp Med. 2015;8:1080–1086. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Xie J, Pan Q, Wang B, Hu D, Hu X. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PLoS One. 2013;8:e52706. doi: 10.1371/journal.pone.0052706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S, Cao H. Shikonin promotes autophagy in BXPC-3 human pancreatic cancer cells through the PI3K/Akt signaling pathway. Oncol Lett. 2014;8:1087–1089. doi: 10.3892/ol.2014.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Hwang KE, Park DS, Oh SH, Jun HY, Yoon KH, Jeong ET, Kim HR, Kim YS. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. J Transl Med. 2017;15:123. doi: 10.1186/s12967-017-1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian HR, Yang Y. Functional role of autophagy in gastric cancer. Oncotarget. 2016;7:17641–17651. doi: 10.18632/oncotarget.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Jiang Hda G, Peng Y, Li SS. Comparative study on enantiomeric excess of main akannin/shikonin derivatives isolated from the roots of three endemic Boraginaceae plants in China. Biomed Chromatogr. 2011;25:1067–1075. doi: 10.1002/bmc.1570. [DOI] [PubMed] [Google Scholar]
- 15.He G, He G, Zhou R, Pi Z, Zhu T, Jiang L, Xie Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem Biophys Res Commun. 2016;469:1075–1082. doi: 10.1016/j.bbrc.2015.12.100. [DOI] [PubMed] [Google Scholar]
- 16.Petherick KJ, Williams AC, Lane JD, Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik K, Paraskeva C, Greenhough A. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013;32:1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye WC, Zhang DM, Chen ZS. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Zeng W, Wang S, Zhao X, Guo Y, Yu P, Yin X, Liu C, Huang T. A potential role for the Hippo pathway protein, YAP, in controlling proliferation, cell cycle progression, and autophagy in BCPAP and KI thyroid papillary carcinoma cells. Am J Transl Res. 2017;9:3212–3223. [PMC free article] [PubMed] [Google Scholar]
- 21.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K, Wang K, Wang X, Chang LS, He D, Gu P. Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int J Mol Sci. 2014;15:15173–15187. doi: 10.3390/ijms150915173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oku Y, Nishiya N, Shito T, Yamamoto R, Yamamoto Y, Oyama C, Uehara Y. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio. 2015;5:542–549. doi: 10.1016/j.fob.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.