Abstract
As a malignant tumor with poor prognosis, accurate and effective treatment of non-small cell lung cancer (NSCLC) is crucial. To predict overall survival in patients with stage II and III NSCLC, a nomogram was constructed using data from the Surveillance, Epidemiology and End Results database. Eligible patients with NSCLC with available clinical information diagnosed between January 1, 2010 and November 31, 2015 were selected from the database, and the data were randomly divided into a training set and a validation set. Univariate and multivariate Cox regression analyses were used to identify prognostic factors with a threshold of P<0.05, and a nomogram was constructed. Harrell's concordance indexes and calibration plots were used to verify the predictive power of the model. Risk group stratification by stage was also performed. A total of 15,344 patients with stage II and III NSCLC were included in the study. The 3- and 5-year survival rates were 0.382 and 0.278, respectively. The training and validation sets comprised 10,744 and 4,600 patients, respectively. Age, sex, race, marital status, histology, grade, Tumor-Node-Metastasis T and N stage, surgery type, extent of lymph node dissection, radiation therapy and chemotherapy were identified as prognostic factors for the construction of the nomogram. The nomogram exhibited a clinical predictive ability of 0.719 (95% CI, 0.718–0.719) in the training set and 0.721 (95% CI, 0.720–0.722) in the validation set. The predicted calibration curve was similar to the standard curve. In addition, the nomogram was able to divide the patients into groups according to stage IIA, IIB, IIIA, and IIIB NSCLC. Thus, the nomogram provided predictive results for stage II and III NSCLC patients and accurately determined the 3- and 5-year overall survival of patients.
Keywords: nomogram, non-small cell lung cancer, SEER, prognosis
Introduction
According to a large-scale survey of 36 types of cancer spanning 185 countries in 2018, lung cancer ranks first in the global cancer incidence (11.6%) and mortality rates (18.4%) (1). In China, lung cancer also accounts for the highest cancer-related morbidity and mortality (2). Among all pathological types of lung cancer, non-small cell lung cancer (NSCLC) accounts for ~80% of cases, making it the most common histological type, with a 5-year survival rate of only 15%. Patients with lung cancer are usually diagnosed at an advanced stage (3). However, with the widespread application of low-dose helical computed tomography (CT) in clinical practice, early- or intermediate-stage NSCLC is being diagnosed in an increasing number of asymptomatic patients (4). For patients with early- or intermediate-stage lung cancer, surgery is the first and most effective treatment method, as well as the only method that can cure NSCLC. However, the influence of different surgical methods on the prognosis of patients is still controversial (5–7).
The concept of precision medicine (8) has facilitated the development of medical methodology specific to the patient. In contrast to the original ‘one size fits all’ approach, medical professionals can adjust the treatment according to subtle differences in patients to maximize the outcome. Based on this, the latest (8th) edition of the Tumor-Node-Metastasis (TNM) staging system can more accurately predict the prognosis of lung cancer patients (9). However, it is necessary to combine variables such as age, sex, degree of tumor differentiation and treatment to further predict the survival rate of patients with lung cancer, as predicting the prognosis of patients exclusively by TNM stage of NSCLC is insufficient.
Nomograms are based on multifactor regression analyses and integrate multiple predictive indexes with visual graphics, which makes the results of the prediction model readable and the prognosis of patients easy to evaluate (10). Despite a number of nomograms based on large samples for predicting the prognosis of patients with lung cancer, limited studies have been performed on stage II or III NSCLC. Therefore, the present study aimed to construct a nomogram based on early (11,12) and advanced (13) lung cancer using large-scale data obtained from the Surveillance, Epidemiology and End Results (SEER) database containing information on the clinical characteristics and patient survival.
Materials and methods
Data collection
Data were extracted from the SEER database (https://seer.cancer.gov) by SEER*Stat Software (version 8.3.5; http://seer.cancer.gov/data-software/), and the Incidence SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying), were selected for analysis. Data were limited to patients diagnosed with stage II and III lung cancer between January 1, 2010 to November 31, 2015. The retrieval formula based on the inclusion criteria displayed in the software was {Site and Morphology. CS Schema v0204+}=‘Lung’ AND {Stage-American Joint Committee on Cancer (AJCC). Derived AJCC Stage Group, 7th ed. (2010+)} =‘II’,‘IIA’,‘IIA1’,‘IIA2’,‘IIB’,‘IIC’,‘III’,‘IIIA’,‘IIIB’,‘IIIC’,‘IIIC1’,‘IIIC2’ AND {Race, Sex, Year Dx, Registry, County, Year of diagnosis}=‘2010’,‘2011’,‘2012’,‘2013’,‘2014’,‘2015’. The clinical information of the patients included patient ID, age at diagnosis, diagnostic confirmation, race (Caucasian, African-American or other), sex, marital status at diagnosis, histologic type, grade, primary site and laterality, AJCC stage, T stage, N stage, surgery at primary site, scope of regional lymph node surgery, radiation therapy, chemotherapy, survival months, vital status, first malignant primary indicator and sequence number.
Once the preliminary data were obtained, patients were excluded based on the following exclusion criteria: i) Clinical information of the patient was missing; ii) diagnostic confirmation was not consistent with positive histology, such as only clinical diagnosis or direct visualization without microscopic confirmation; iii) patient was <18 years; iv) survival time was <1 month; v) the meaning of the patient's relevant code was unclear or had other meaning that could not be included in the study; for example, in a surgical procedure, 00 refers to no surgery, 21 refers to wedge resection, 22 refers to segmental resection, and 31 refers to lobectomy, and patients with additional codes, such as 41–49, were excluded; vi) the data point applied to a small number of patients; for example, only 23 patients underwent pneumonectomy and were thus excluded; vii) the patient had more than one primary tumor.
The data were divided into two groups based on patient age (≤60 and >60 years). In addition, regarding marital status, patients who were widowed, divorced, unmarried or domestic partners and single (unmarried) were all considered unmarried. In terms of pathological types, only four pathological tissue types were included: Adenocarcinoma (ADC), squamous cell carcinoma (SCC), large cell carcinoma (LCC) and adenosquamous carcinoma (ASC). Regarding radiotherapy, patients were divided into two groups: Yes and no, where beam radiation, radioactive implants, and radioisotopes were all considered as ‘yes’. The remaining clinical information was determined via the specific meaning of the code and the specific output of the software.
Statistical analysis
The random allocation method was used to divide the data into a training set and a validation set at a 7:3 ratio. Median survival time with 95% confidence intervals (CIs) for the two groups was determined using the Kaplan-Meier method. In the training cohort, unadjusted univariate Cox regression analysis was used for all variables included in the study. P<0.05 was considered to indicate a statistically significant difference. Factors with statistical significance according to the results of the unadjusted univariate Cox regression analysis were included in the multivariate Cox regression analysis to identify independent risk factors. These independent risk factors were used to construct a nomogram using R software version 3.5.1 (64 bit; http://www.r-project.org) using the rms (14) and survival packages (https://www.rdocumentation.org/packages/survival/versions/2.42-3). The nomogram used 3- and 5-year overall survival (OS) as end points. Harrell's concordance indexes (C-indexes) (15) and calibration curves were used to verify the predicted effect of the nomogram. The training set was used for internal validation, and the validation set was used for external validation. Bootstraps of 1,000 resamples were used for analysis. In addition, to validate the ability of the nomogram to discriminate patients with different TNM stages, patients in the validation set were assigned into four groups according to the quartiles of their prognostic scores. The Kaplan-Meier method was used to estimate overall survival rate in the four groups, and the differences were evaluated using the log-rank test with a threshold of P<0.05.
Results
Patient clinicopathological characteristics
According to the inclusion and exclusion criteria, 15,344 patients with stage II and III NSCLC were included in the study. Among them, 3,261 patients had stage IIA, 2,865 had stage IIB, 6,851 had stage IIIA and 2,367 had stage IIIB NSCLC. The mean age was 68.41 (range, 15–101 years) years. The patients were divided into two random groups at a ratio of 7:3 to form the training and validation sets, comprising 10,744 and 4,600 patients, respectively. The median survival was 22.00 months and the 3- and 5-year survival rates were 0.638 and 0.512 for the training set. The median survival was 23.50 months and the 3- and 5-year survival rates were 0.666 and 0.551 for the validation set. The clinical characteristics and survival information of the two sets are presented in Tables I and II.
Table I.
Clinical characteristics of the training set.
| OS, months | OS, % | |||||
|---|---|---|---|---|---|---|
| Characteristic | No. of patients | Median | 95% CI | 3-year | 5-year | Log-rank P-value |
| Age | ||||||
| ≤60 | 2,360 | 34 | 30.61–37.39 | 48.5±1.2 | 39.4±1.4 | <0.001 |
| >60 | 8,384 | 21 | 21.13–21.87 | 35.8±0.6 | 25.4±0.7 | |
| Sex | ||||||
| Male | 5,929 | 19 | 18.06–19.94 | 34.1±0.7 | 25.9±0.8 | <0.001 |
| Female | 4,815 | 29 | 27.22–30.78 | 43.3±0.9 | 31.7±1.0 | |
| Marital status | ||||||
| Married | 5,896 | 25 | 23.67–26.33 | 34.1±0.7 | 25.3±0.9 | <0.001 |
| Unmarried | 4,848 | 21 | 19.83–22.16 | 36.1±0.8 | 25.0±1.0 | |
| Race | ||||||
| Caucasian | 8,623 | 25 | 23.67–26.33 | 37.7±0.6 | 28.2±0.7 | <0.001 |
| African-American | 1,274 | 21 | 19.83–22.17 | 39.7±1.6 | 27.1±2.0 | |
| Other | 847 | 23 | 22.15–23.87 | 46.2±2.1 | 34.3±2.4 | |
| Histology | ||||||
| ADC | 5,552 | 32 | 30.17–33.83 | 46.1±0.8 | 33.9±1.0 | <0.001 |
| SCC | 4,784 | 16 | 15.12–16.87 | 29.6±0.8 | 22.2±0.9 | |
| LCC | 270 | 22 | 17.86–26.14 | 37.0±3.5 | 26.8±4.2 | |
| ASC | 138 | 17 | 13.13–20.87 | 78.4±3.6 | 61.2±4.3 | |
| Grade | ||||||
| I | 765 | 31 | 26.10–35.89 | 46.4±2.2 | 30.2±2.9 | <0.001 |
| II | 4,379 | 26 | 24.43–27.56 | 41.6±0.9 | 31.1±1.0 | |
| III | 5,441 | 20 | 18.96–21.04 | 35.3±0.8 | 26.2±0.9 | |
| IV | 159 | 17 | 12.84–21.16 | 34.7±4.3 | 29.8±4.6 | |
| AJCC (7th) T stage (45) | ||||||
| T1 | 1,324 | 32 | 27.81–36.20 | 47.1±1.6 | 34.2±2.1 | <0.001 |
| T2 | 3,996 | 27 | 25.24–28.76 | 42.0±0.9 | 31.0±1.1 | |
| T3 | 3,419 | 23 | 21.53–24.47 | 38.5±1.0 | 29.3±1.1 | |
| T4 | 2,005 | 14 | 12.90–15.10 | 26.3±1.2 | 17.9±1.3 | |
| AJCC (7th) N stage (45) | ||||||
| N0 | 3,362 | 29 | 26.56–31.44 | 45.3±1.0 | 34.2±1.2 | <0.001 |
| N1 | 2,293 | 35 | 31.84–38.16 | 48.6±1.3 | 36.9±1.5 | |
| N2 | 4,323 | 18 | 17.03–18.97 | 31.1±0.8 | 21.9±0.9 | |
| N3 | 766 | 13 | 11.91–14.08 | 22.0±1.8 | 13.8±2.0 | |
| Primary site | ||||||
| Main bronchus | 302 | 12 | 9.78–14.21 | 33.9±0.9 | 25.4±1.0 | <0.001 |
| Upper lobe | 6,650 | 24 | 22.86–25.13 | 39.4±0.7 | 30.2±0.8 | |
| Middle lobe | 460 | 28 | 23.24–32.76 | 43.6±2.8 | 31.9±3.4 | |
| Lower lobe | 3,332 | 23 | 21.49–24.51 | 37.9±1.0 | 26.6±1.2 | |
| Laterality | ||||||
| Left | 4,448 | 23 | 21.64–24.37 | 38.9±0.9 | 29.4±1.0 | 0.346 |
| Right | 6,296 | 23 | 21.85–24.15 | 38.5±0.7 | 27.9±0.8 | |
| Surgery | ||||||
| None | 5,757 | 13 | 12.49–13.51 | 20.2±0.7 | 12.2±0.7 | <0.001 |
| Wedge resection | 351 | 30 | 24.78–35.22 | 45.9±3.2 | 35.6±3.7 | |
| Segmental resection | 114 | 41 | 28.39–53.61 | 52.5±5.9 | 37.6±7.2 | |
| Lobectomy | 4,522 | 56 | 51.17–60.83 | 60.4±0.9 | 47.9±1.1 | |
| Lymph node dissection | ||||||
| None | 5,652 | 13 | 12.49–13.51 | 20.2±0.7 | 12.2±0.7 | <0.001 |
| 1–3 | 651 | 40 | 33.07–46.93 | 45.9±3.2 | 35.6±3.7 | |
| ≥4 | 4,441 | 53 | 48.87–23.87 | 60.4±0.9 | 47.9±1.1 | |
| Radiation | ||||||
| No | 5,152 | 19 | 18.17–19.83 | 33.9±0.9 | 25.4±1.0 | <0.001 |
| Yes | 5,592 | 29 | 27.12–30.88 | 45.1±0.8 | 35.5±0.9 | |
| Chemotherapy | ||||||
| No | 4,185 | 18 | 16.81–19.19 | 33.9±0.9 | 25.4±1.0 | <0.001 |
| Yes | 6,559 | 27 | 25.73–28.27 | 41.6±0.7 | 30.5±0.9 | |
OS, overall survival; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ASC, adenosquamous carcinoma; AJCC (7th), American Joint Committee on Cancer.
Table II.
Clinical characteristics of the validation set.
| OS, months | OS, % | |||||
|---|---|---|---|---|---|---|
| Characteristic | No. of patients | Median | 95% CI | 3-year | 5-year | Log-rank P-value |
| Age | ||||||
| ≤60 | 1,041 | 30 | 24.54–34.54 | 45.7±1.8 | 34.6±2.1 | <0.001 |
| >60 | 3,559 | 21 | 19.68–22.31 | 34.6±1.0 | 23.7±1.1 | |
| Sex | ||||||
| Male | 2,507 | 20 | 18.77–21.22 | 31.7±1.1 | 22.3±1.2 | <0.001 |
| Female | 2,093 | 28 | 25.31–30.69 | 43.9±1.3 | 31.3±1.6 | |
| Marital status | ||||||
| Married | 2,519 | 26 | 24.01–27.98 | 41.8±1.2 | 30.0±1.3 | <0.001 |
| Unmarried | 2,081 | 20 | 18.44–21.55 | 31.5±1.2 | 21.5±1.4 | |
| Race | ||||||
| Caucasian | 3,713 | 22 | 20.68–23.32 | 36.8±0.9 | 26.1±1.1 | 0.035 |
| African-American | 547 | 22 | 19.31–24.68 | 35.2±2.5 | 26.3±2.7 | |
| Other | 340 | 31 | 25.94–36.05 | 44.2±3.4 | 25.4±3.9 | |
| Histology | ||||||
| ADC | 2,420 | 31 | 28.66–33.33 | 41.8±1.2 | 30.0±1.3 | <0.001 |
| SCC | 2,026 | 16 | 14.69–17.30 | 28.1±1.2 | 19.1±1.3 | |
| LCC | 103 | 30 | 18.56–41.43 | 43.9±5.8 | 33.4±6.1 | |
| ASC | 31 | 15 | 7.23–22.77 | 31.5±1.2 | 21.5±1.4 | |
| Grade | ||||||
| I | 370 | 41 | 30.81–51.18 | 52.0±3.1 | 40.1±3.9 | <0.001 |
| II | 1,837 | 26 | 23.79–28.20 | 40.7±1.4 | 27.4±1.6 | |
| III | 2,334 | 19 | 17.62–20.37 | 32.2±1.2 | 23.0±1.2 | |
| IV | 59 | 20 | 9.21–30.78 | 37.2±7.1 | 30.1±7.4 | |
| AJCC (7th) T stage (45) | ||||||
| T1 | 601 | 37 | 28.94–45.05 | 50.9±2.4 | 35.1±3.0 | <0.001 |
| T2 | 1,718 | 25 | 22.64–27.35 | 39.6±1.4 | 27.3±1.6 | |
| T3 | 1,444 | 23 | 21.08–24.91 | 36.5±1.5 | 26.0±1.7 | |
| T4 | 837 | 13 | 11.45–14.54 | 23.4±1.8 | 16.0±1.9 | |
| AJCC (7th) N stage (45) | ||||||
| N0 | 1,433 | 30 | 26.09–33.90 | 45.6±1.6 | 35.4±1.9 | <0.001 |
| N1 | 990 | 34 | 29.35–38.46 | 48.7±1.9 | 32.3±2.3 | |
| N2 | 1,848 | 18 | 16.68–19.32 | 27.9±1.2 | 18.6±1.3 | |
| N3 | 329 | 13 | 10.62–15.37 | 19.7±2.8 | 4.5±3.5 | |
| Primary site | ||||||
| Main bronchus | 118 | 14 | 9.43–18.57 | 18.1±4.4 | 12.1±5.7 | <0.001 |
| Upper lobe | 2,869 | 22 | 20.46–23.53 | 37.0±1.1 | 25.4±1.2 | |
| Middle lobe | 181 | 22 | 17.03–26.96 | 36.2±4.3 | 32.2±4.7 | |
| Lower lobe | 1,432 | 25 | 22.55–27.48 | 39.2±1.6 | 28.6±1.8 | |
| Laterality | ||||||
| Left | 1,913 | 23 | 20.98–25.01 | 37.7±1.3 | 26.2±1.5 | 0.932 |
| Right | 2,687 | 23 | 21.48–24.51 | 36.8±1.1 | 26.4±1.2 | |
| Surgery | ||||||
| None | 2,433 | 13 | 12.23–13.77 | 17.9±1.0 | 9.4±1.0 | <0.001 |
| Wedge resection | 163 | 35 | 26.19–43.80 | 47.8±5.0 | 30.7±6.5 | |
| Segmental resection | 45 | 31 | 24.01–37.98 | 46.9±9.1 | 37.5±9.4 | |
| Lobectomy | 1,959 | 50 | 44.69–55.03 | 60.0±1.3 | 45.3±1.6 | |
| Lymph node dissection | ||||||
| None | 2,398 | 13 | 12.20–13.79 | 18.3±1.0 | 10.1±1.0 | <0.001 |
| 1–3 | 306 | 35 | 29.49–40.51 | 46.5±3.4 | 29.2±4.2 | |
| ≥4 | 1,896 | 50 | 44.13–55.87 | 59.5±1.4 | 45.4±1.7 | |
| Radiation | ||||||
| No | 2,176 | 19 | 17.75–20.25 | 28.6±1.2 | 19.3±1.2 | <0.001 |
| Yes | 2,424 | 30 | 27.34–32.65 | 44.9±1.2 | 32.7±1.4 | |
| Chemotherapy | ||||||
| No | 1,812 | 18 | 16.04–19.96 | 33.9±1.3 | 24.9±1.5 | <0.001 |
| Yes | 2,788 | 25 | 23.32–26.67 | 39.3±1.1 | 27.3±1.3 | |
OS, overall survival; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ASC, adenosquamous carcinoma; AJCC (7th), American Joint Committee on Cancer.
Cox regression analysis
The following factors were included in the univariate Cox regression analysis: Age (≤60 vs. >60), race (Caucasian vs. African-American vs. other), sex (male vs. female), marital status (married vs. unmarried), histological type (ADC vs. SCC vs. LCC vs. ADC), grade (well differentiated, grade I vs. moderately differentiated, grade II vs. poorly differentiated, grade III vs. undifferentiated or anaplastic, grade IV), primary site (main bronchus vs. upper lobe vs. middle lobe vs. lower lobe), latency (left vs. right), T stage (T1 vs. T2 vs. T3 vs. T4), N stage (N0 vs. N1 vs. N2 vs. N3), surgery at primary site (none vs. wedge resection vs. segmental resection vs. lobectomy), scope of regional lymph node surgery (none vs. 1–3 regional lymph nodes removed vs. ≥4 regional lymph nodes removed), radiation therapy (yes vs. no) and chemotherapy (yes vs. no). The results of the univariate Cox regression analysis indicated no significant difference regarding laterality (P=0.353). The remaining prognostic factors with P<0.05 were included in the multivariate Cox regression analysis. The results demonstrated that all included factors, with the exception of the primary site, were independent prognostic factors and were thus included in the construction of the nomogram (Table III).
Table III.
Results of the univariate and multivariate Cox regression analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Age | ||||||
| ≤60 | Reference | Reference | ||||
| >60 | 1.479 | 1.383–1.581 | <0.001 | 1.293 | 1.208–1.205 | <0.001 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.762 | 0.723–0.804 | <0.001 | 0.770 | 0.728–0.813 | <0.001 |
| Marital status | ||||||
| Married | Reference | Reference | ||||
| Unmarried | 1.167 | 1.108–1.229 | <0.001 | 1.103 | 1.043–1.165 | <0.001 |
| Race | ||||||
| Caucasian | Reference | Reference | ||||
| African-American | 0.987 | 0.911–1.069 | 0.745 | 0.893 | 0.822–0.968 | 0.007 |
| Other | 0.804 | 0.725–0.890 | <0.001 | 0.825 | 0.744–0.914 | <0.001 |
| Histology | ||||||
| ADC | Reference | Reference | ||||
| SCC | 1.651 | 1.566–1.741 | <0.001 | 1.210 | 1.143–1.281 | <0.001 |
| LCC | 1.261 | 1.068–1.488 | 0.006 | 1.367 | 1.157–1.614 | <0.001 |
| ASC | 1.369 | 1.090–1.720 | 0.007 | 1.031 | 0.796–1.336 | 0.816 |
| Grade | ||||||
| I | Reference | Reference | ||||
| II | 1.159 | 1.036–1.297 | 0.010 | 1.140 | 1.016–1.280 | 0.025 |
| III | 1.423 | 1.274–1.589 | <0.001 | 1.290 | 1.152–1.445 | <0.001 |
| IV | 1.490 | 1.184–1.874 | 0.001 | 1.387 | 1.073–1.792 | 0.013 |
| AJCC (7th) T stage (45) | ||||||
| T1 | Reference | Reference | ||||
| T2 | 1.170 | 1.069–1.281 | 0.001 | 1.094 | 0.997–1.199 | 0.056 |
| T3 | 1.333 | 1.216–1.461 | <0.001 | 1.275 | 1.158–1.405 | <0.001 |
| T4 | 1.900 | 1.725–2.092 | <0.001 | 1.387 | 1.247–1.530 | <0.001 |
| AJCC (7th) N stage (45) | ||||||
| N0 | Reference | Reference | ||||
| N1 | 0.864 | 0.799–0.935 | <0.001 | 1.248 | 1.147–1.358 | <0.001 |
| N2 | 1.442 | 1.354–1.535 | <0.001 | 1.478 | 1.379–1.584 | <0.001 |
| N3 | 1.915 | 1.733–2.116 | <0.001 | 1.501 | 1.349–1.668 | <0.001 |
| Primary site | ||||||
| Main bronchus | Reference | Reference | ||||
| Upper lobe | 0.559 | 0.488–0.641 | <0.001 | 0.887 | 0.773–1.019 | 0.092 |
| Middle lobe | 0.499 | 0.414–0.602 | <0.001 | 0.893 | 0.739–1.079 | 0.244 |
| Lower lobe | 0.582 | 0.505–0.669 | <0.001 | 1.022 | 0.885–1.180 | 0.762 |
| Laterality | ||||||
| Left | Reference | NS | ||||
| Right | 1.025 | 0.973–1.081 | 0.353 | NS | ||
| Surgery | ||||||
| None | Reference | Reference | ||||
| Wedge resection | 0.446 | 0.381–0.522 | <0.001 | 0.488 | 0.409–0.582 | <0.001 |
| Segmental resection | 0.392 | 0.292–0.526 | <0.001 | 0.453 | 0.330–0.622 | <0.001 |
| Lobectomy | 0.300 | 0.283–0.318 | <0.001 | 0.389 | 0.334–0.452 | <0.001 |
| Lymph node dissection | ||||||
| None | Reference | Reference | ||||
| 1–3 | 0.395 | 0.350–0.447 | <0.001 | 0.815 | 0.697–0.953 | 0.010 |
| ≥4 | 0.312 | 0.294–0.331 | <0.001 | 0.742 | 0.641–0.857 | <0.001 |
| Radiation | ||||||
| No | Reference | Reference | ||||
| Yes | 0.751 | 0.712–0.791 | <0.001 | 0.756 | 0.710–0.805 | <0.001 |
| Chemotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.706 | 0.670–0.744 | <0.001 | 0.632 | 0.596–0.670 | <0.001 |
ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ASC, adenosquamous carcinoma; AJCC, American Joint Committee on Cancer; NS, not significant.
Construction and validation of the nomogram
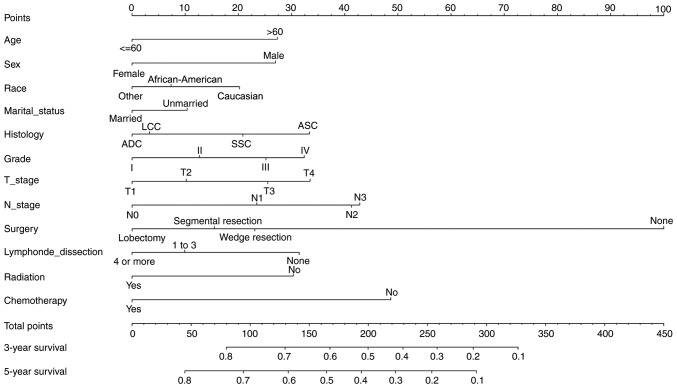
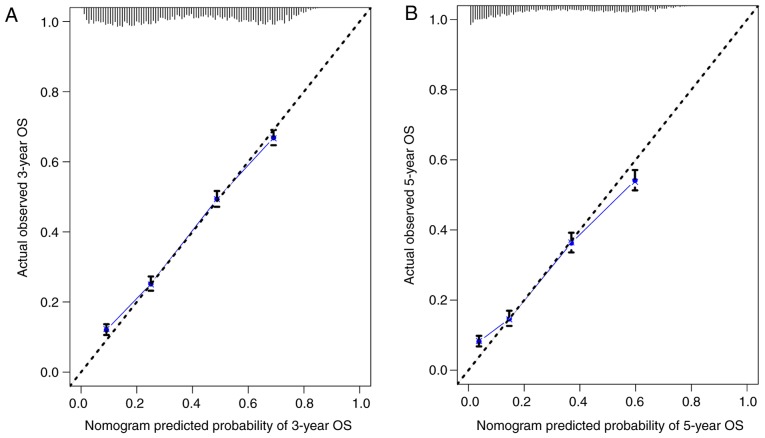
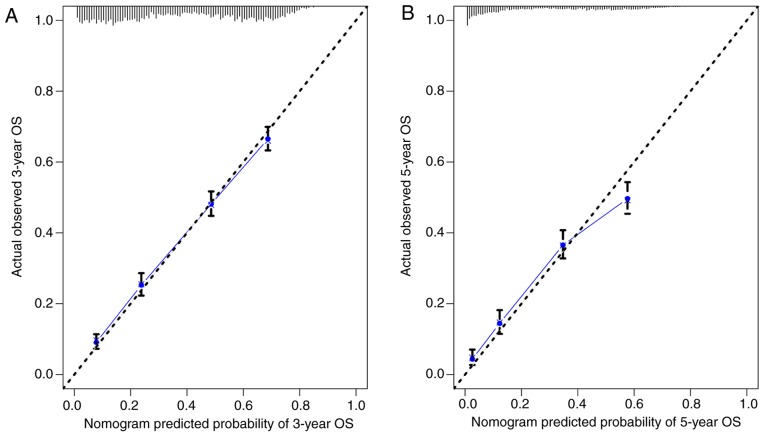
The nomogram comprised 12 prognostic factors: Age, sex, race, marital status, histological type, grade, T stage, N stage, surgery type, extent of lymph node dissection, radiation therapy and chemotherapy. Surgery, especially lobectomy, exhibited the strongest impact on prognosis among all factors; chemotherapy also served an important role (Fig. 1). Marriage had a relatively small effect on prognosis. The effects of other factors on prognosis were moderate. A total score was calculated by adding up the scores of each factor according to the different characteristics. The 3- and 5-year survival rates were estimated by drawing a straight line from the total score on the nomogram. The C-index calculated by the bootstrap self-sampling method was 0.719 (95% CI, 0.718–0.719) in the training set and 0.721 (95% CI, 0.720–0.722) in the validation set, indicating good predictability of the nomogram. In addition, the calibration curve was similar to the standard curve in predicting the 3- and 5-year survival rates of patients from the training set and validation set, indicating good predictive ability of the nomogram (Figs. 2 and 3).
Figure 1.
Nomogram for predicting the 3- and 5-year overall survival of patients with stage II and III non-small cell lung cancer. ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; ASC, adenosquamous carcinoma.
Figure 2.
(A) Calibration curve of the nomogram for predicting the 3-year OS rates of patients with stage II and III NSCLC from the training set. (B) Calibration curve of the nomogram for predicting the 5-year OS rates of patients with stage II and III NSCLC from the training set. OS, overall survival; NSCLC, non-small cell lung cancer.
Figure 3.
(A) Calibration curve of the nomogram for predicting the 3-year OS rates of patients with stage II and III NSCLC from the validation set. (B) Calibration curve of the nomogram for predicting the 5-year OS rates of patients with stage II and III NSCLC from the validation set. OS, overall survival; NSCLC, non-small cell lung cancer.
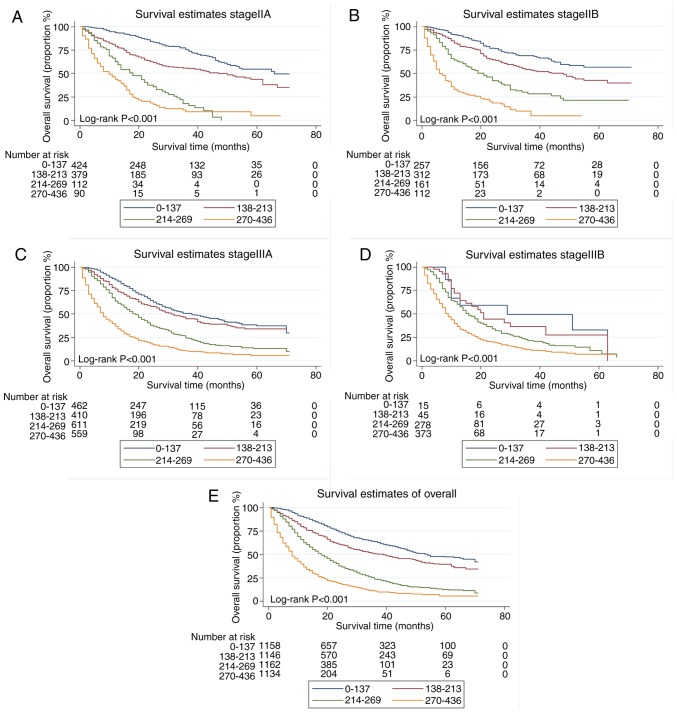
Risk stratification
The total score for each patient in the training set was calculated, and the scores divided into quartiles (0–137, 138–213, 214–269 and 270–436) to represent different outcomes. A statistically significant difference in survival was identified among patients with stage IIA, IIB, IIIA and IIIB NSCLC when the quartiles of scores were applied to divide the patients in the validation set (all P<0.001; Fig. 4).
Figure 4.
Risk group stratification within stage (A) IIA, (B) IIB, (C) IIIA and (D) IIIB, as well as (E) all patients.
Discussion
To the best of our knowledge, this is the first large-scale clinical retrospective study that used the SEER database to construct a nomogram to predict survival rates for patients with stage II and III NSCLC. In this study, a total of 15,344 patients were included following rigorous screening, and 12 risk factors that significantly affected prognosis were determined by the Cox regression method. A nomogram was constructed based on these 12 risk factors. The C-index and the graphical calibration method were used for internal validation, which suggested that the nomogram exhibited a good predictive ability. The nomogram demonstrated that the survival of patients with NSCLC was affected by multiple factors, especially the treatment strategy. The nomogram also accurately predicted the prognosis of different risk groups, including patients with stage IIA, IIB, IIIA and IIIB NSCLC. Compared with traditional TNM staging, the model established in the present study combined more clinical information to determine the prognosis of patients more accurately and guide the treatment strategy.
Demographic factors of the patients in the present study, such as age, sex and race, had a moderate influence on prognosis, and marital status only had a minor effect. In elderly patients with NSCLC, the aging of organs coupled with a decrease in immune function leads to a high possibility of tumor recurrence. Elderly patients with NSCLC exhibit low tolerance to surgery, radiotherapy and chemotherapy, and therefore, their compliance to anticancer treatment may be poor. Additionally, elderly patients may often suffer from other conditions, and thus their survival rate is reduced (16). Through follow-up of 14,578 postoperative patients with NSCLC between January 2009 and January 2014 in multiple centers, Dziedzic et al (17) demonstrated that the risk of tumor recurrence and metastasis increased with age. In a retrospective study involving 33,919 patients with lung cancer from Taiwan, China, Wang et al (18) reported that age >65 years was an independent risk factor for prognosis. Thus, age is associated with the prognosis of patients with lung cancer.
A recent large-scale epidemiological survey revealed that the incidence and mortality rates of female patients with lung cancer are increasing (1). Chang et al (19) retrospectively analyzed 2,770 patients with stage I and II NSCLC and demonstrated that the 5-year survival rate of female patients with lung ADC was higher compared with that of male patients, whereas no significant differences were observed in the 5-year survival rate of patients with non-adenocarcinoma. However, another study has reported the opposite result, i.e. that sex is not a risk factor for patients with NSCLC (17). Although the mechanism by which sex affects the prognosis of patients with lung cancer requires further study, male and female patients exhibit distinctive clinical and biological characteristics, such as the likelihood of ADC. In addition, mutations in the epidermal growth factor receptor gene are often identified in female patients (17). Results for non-smoking patients with lung ADC also indicated that female patients may more likely benefit from targeted therapy compared with male patients (20).
In terms of marital status, Merrill et al (21) used a sample of 779,978 male and 1,032,868 female patients from the SEER database and reached the opposite conclusion from that of the present study; marriage was beneficial for only non-fatal cancers such as breast, colorectal and kidney cancer, but did not improve the 5-year survival rate for patients with lung or liver cancer. However, another study that used the SEER database reported that among patients with NSCLC, married individuals exhibited higher overall and tumor-specific survival rates compared with unmarried individuals (22). A multicenter trial (23) has demonstrated that married patients with cancer experience less psychological distress and receive better social support compared with unmarried patients, which may explain why marriage improved the prognosis of patients with lung cancer. Race is also strongly associated with the prognosis of lung cancer, although this association remains controversial. An epidemiological survey of 38 states in the United States that included 80% of the population revealed that African-American patients exhibited lower survival rates compared with Caucasian patients (24). Another retrospective analysis, spanning 10 years in the United States, suggested that racial differences in lung cancer mortality were due to differences in access to health care and provision of the recommended treatment (25). In a retrospective study from Florida, the authors noted that Asian patients had higher survival rates compared with Caucasian and African-American patients following adjustment for certain confounding factors, such as economic status (26). In addition, the results of the present study suggested that other populations, including Asians, exhibited the best prognosis.
In the present study, the results of the traditional TNM staging of lung cancer were the same as those of NSCLC. Prognosis became progressively worse with increasing T/N stages, and the histopathological type and degree of tumor differentiation also determined the prognosis of patients with lung cancer. Regarding differentiation, poorly differentiated tumors have a stronger ability to invade and metastasize and are highly malignant; the results of the present study also demonstrated that a low degree of tumor differentiation in patients with NSCLC was associated with a low survival rate. There are different views regarding the ability of TNM stage to reflect accurately the prognosis of patients. For example, three retrospective studies from Asia and Europe suggested that patients with different AJCC stages had no statistical difference in prognosis, therefore it was not advisable to rely solely on AJCC stages to determine prognosis, as there are numerous remaining factors affecting the survival of patients with NSCLC (27–29). A previous study confirmed that gene mutation is one of the possible reasons for the difference in prognosis between lung SCC and ADC (30). However, in further studies on NSCLC, more attention should be paid to the pathological types and differentiation degree. For example, the AJCC has advocated that researchers focus on the influence of different pathological types on prognosis in esophageal cancer (31).
For patients with NSCLC, appropriate treatment such as surgery or drug therapy should be selected based on the clinical situation of the patients, in order to obtain the optimal prognosis.. In theory, cancer can be cured if drugs that completely eradicate cancer cells were discovered; currently, the ability of drugs to cure cancer is limited to several types of malignant tumors. For the majority of malignant tumors, finding a cure is more likely in the early stages of disease, when the tumor has not spread and can be surgically removed. The present study demonstrated that patients with >4 groups lymph node metastasis, lobectomy in combination with lymph node dissection was associated with the best prognosis. Speicher et al (32) have reported that surgical lobectomy significantly prolonged long-term survival in a follow-up study of 39,403 patients with lung cancer. A meta-analysis by Zhang et al (33) also revealed that lobectomy resulted in better prognosis compared with segmental lung resection in patients with stage I NSCLC, and that age and tumor size should not be considered limiting factors for lobectomy. In addition, the latest National Comprehensive Cancer Network guidelines recommend lobectomy as the first-choice treatment for patients with stage II and III NSCLC with good lung reserve function that can tolerate surgery (34). For NSCLC, lymph node metastasis, especially mediastinal lymph node metastasis, is an independent risk factor for poor prognosis (35). Lymph node dissection has also been demonstrated to significantly improve prognosis (36). In 1996, the International Association for the Study of Lung Cancer presented the concept of systematic lymph node dissection (SLD), with lobectomy combined with SLD identified as the standard surgical method for NSCLC (37). In 2006, the guidelines of the European Society of Thoracic Surgeons defined the scope of SLD as the resection of at least six groups of lymph nodes, including >3 ipsilateral mediastinal and subcarinal lymph nodes. The complete dissection of mediastinal lymph nodes and surrounding adipose tissue is required (38). According to the standards of the Japan Lung Cancer Society, Adachi et al (39) defined lymph node dissection as: i) The removal of at ≥3 hilar and intrapulmonary lymph nodes; ii) the resection of ≥3 mediastinal lymph nodes; or iii) the removal of ≥6 lymph nodes. Similarly, the results of the present study demonstrated that the dissection of ≥4 groups of lymph nodes significantly improved patient survival.
With the emergence of targeted therapy and immunotherapy, the survival advantage of patients receiving conventional platinum-based adjuvant chemotherapy as the standard treatment is moderate, especially for patients with stage II or III NSCLC (40). However, a large retrospective study has also reported that postoperative adjuvant chemotherapy is essential for improving the prognosis of patients (41). Radiotherapy is also a standard treatment for NSCLC. A large-scale retrospective analysis using the SEER database concluded that preoperative radiotherapy can significantly improve the survival rate of patients with IIIA/N2 NSCLC (42). However, radiotherapy results in numerous side effects, such as skin ulcers or severe reactions including radiological pneumonia (43). Increasing the dose of radiation appears to improve the prognosis of patients who only receive radiation therapy, but decrease survival in patients who receive a combination of radiotherapy and chemotherapy (44). Therefore, the selection of anticancer treatment strategy for patients should be combined with their clinicopathological data.
The present study had several limitations. First, due to the limited information available in the SEER database, smoking history, radiotherapy dose, specific chemotherapy regimen, surgical methods (open or endoscopic surgery) and additional clinical information could not be obtained, which may have affected the results. Second, the T/N staging was based on the 7th edition of the AJCC staging system. Although the 7th edition TN stage and tumor size were available, the tumor invasion degree information was not included, and thus the AJCC staging results could not be converted to the 8th edition. For example, a 600 mm T3 lung cancer record from the SEER database should be classified as T4 according to the 8th edition of AJCC staging if it extends to the diaphragm. Finally, the patients with NSCLC in the SEER database were all from the United States, and although patients of different races were included, the cohort may not be representative of patients worldwide.
In conclusion, a nomogram combining substantial demographic, pathological and treatment data to predict OS for patients with stage II and III NSCLC was established and validated using a population-based study from the SEER database. Well-designed trials are needed to improve this nomogram.
Table IV.
Scores of every subgroup within each variable.
| Variable | Points | Variable | Points |
|---|---|---|---|
| Age | Sex | ||
| ≤60 | 0 | Male | 27 |
| >60 | 27 | Female | 0 |
| Chemotherapy | Radiation | ||
| No | 49 | No | 30 |
| Yes | 0 | Yes | 0 |
| AJCC (7th) T stage (45) | AJCC (7th) Nstage(45) | ||
| T1 | 0 | N0 | 0 |
| T2 | 10 | N1 | 23 |
| T3 | 22 | N2 | 41 |
| T4 | 33 | N3 | 43 |
| Race | Extentoflymph nodedissection | ||
| Caucasian | 20 | None | 31 |
| African-American | 7 | 1–3 | 10 |
| Other | 0 | ≥4 | 0 |
| Histology | Grade | ||
| AC | 0 | I | 0 |
| SC | 21 | II | 13 |
| ASC | 33 | III | 25 |
| LCC | 3 | IV | 33 |
| Surgery | Marital status | ||
| None | 100 | Married | 0 |
| Wedgeresection | 23 | Unmarried | 10 |
| Segmentalresection | 15 | ||
| Lobectomy | 0 |
ADC, adenocarcinoma; SCC, squamouscellcarcinoma; LCC, largecellcarcinoma; ASC, adenosquamous carcinoma; AJCC, American Joint Committeeon Cancer.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YL and XW conceived of and designed the study, acquired and analyzed the data and wrote the manuscript. PZ, GY, XF and CH revised the manuscript and analyzed the data. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Chen Q, Guo L, Cao X, Sun X, Chen W, He J. Incidence and mortality of lung cancer in China, 2008–2012. Chin J Cancer Res. 2018;30:580–587. doi: 10.21147/j.issn.1000-9604.2018.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team. Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorenstein LA, Sonett JR. The surgical management of stage I and stage II lung cancer. Surg Oncol Clin N Am. 2011;20:701–720. doi: 10.1016/j.soc.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Filosso PL, Rena O, Donati G, Casadio C, Ruffini E, Papalia E, Oliaro A, Maggi G. Bronchial carcinoid tumors: Surgical management and long-term outcome. J Thorac Cardiovasc Surg. 2002;123:303–309. doi: 10.1067/mtc.2002.119886. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Xiao W, Xie T, He J, Han Y, Zhu J. Surgical treatment for lung cancer in the elderly. Zhongguo Fei Ai Za Zhi. 2007;10:34–36. doi: 10.3779/j.issn.1009-3419.2007.01.08. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 8.König IR, Fuchs O, Hansen G, von Mutius E, Kopp MV. What is precision medicine? Eur Respir J. 2017;50:1700391. doi: 10.1183/13993003.00391-2017. [DOI] [PubMed] [Google Scholar]
- 9.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, Rice T, Suzuki K, Thomas CF, Jr, Travis WD, et al. The IASLC lung cancer staging project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003. doi: 10.1097/JTO.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 10.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Li X, Shi J, Fu H, Yang H, Liang Z, Xiong H, Wang H. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res. 2018;10:6611–6626. doi: 10.2147/CMAR.S182458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Zhang Y, Qiu Z, Chen G, Hong S, Chen X, Zhang Z, Huang Y, Zhang L. Nomogram to predict cause-specific mortality in patients with surgically resected stage I non-small-cell lung cancer: A competing risk analysis. Clin Lung Cancer. 2018;19:e195–e203. doi: 10.1016/j.cllc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Ren Z, Wen J, Wang B, Hou X, Xue Z, Chu X. Construction of a nomogram predicting the overall survival of patients with distantly metastatic non-small-cell lung cancer. Cancer Manag Res. 2018;10:6143–6156. doi: 10.2147/CMAR.S183878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank E, Harrel J, (homepage on the Internet) Rms: Regression Modeling Strategies. http://CRAN.Rproject.org/packagerms. [Feb 19;2019 ];R Package Version 3.4–0.
- 15.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, Stinchcombe TE. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziedzic DA, Rudzinski P, Langfort R, Orlowski T, Polish Lung Cancer Study Group (PLCSG) Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17:e157–e167. doi: 10.1016/j.cllc.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: A population-based cancer registry. J Thorac Oncol. 2013;8:1128–1135. doi: 10.1097/JTO.0b013e31829ceba4. [DOI] [PubMed] [Google Scholar]
- 19.Chang JW, Asamura H, Kawachi R, Watanabe S. Gender difference in survival of resected non-small cell lung cancer: Histology-related phenomenon? J Thorac Cardiovasc Surg. 2009;137:807–812. doi: 10.1016/j.jtcvs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 21.Merrill RM, Johnson E. Benefits of marriage on relative and conditional relative cancer survival differ between males and females in the USA. J Cancer Surviv. 2017;11:578–589. doi: 10.1007/s11764-017-0627-y. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: An analysis of 70006 patients in the SEER database. Oncotarget. 2017;8:103518–103534. doi: 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varlotto JM, Voland R, McKie K, Flickinger JC, DeCamp MM, Maddox D, Rava P, Fitzgerald TJ, Graeber G, Rassaei N, et al. Population-based differences in the outcome and presentation of lung cancer patients based upon racial, histologic, and economic factors in all lung patients and those with metastatic disease. Cancer Med. 2018;7:1211–1220. doi: 10.1002/cam4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards TB, Henley SJ, Puckett MC, Weir HK, Huang B, Tucker TC, Allemani C. Lung cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):S5079–S5099. doi: 10.1002/cncr.31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11:1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Tannenbaum SL, Koru-Sengul T, Zhao W, Miao F, Byrne MM. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014;20:237–245. doi: 10.1097/PPO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 27.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et al. A Japanese lung cancer registry study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 28.Cho BC, DE Pas T, Kalofonos H, Wang Q, Ramlau R, Cheng Y, Vitiello F, Laisaar T, Vallières E, Kubisa B, et al. Prognostic factors in early-stage NSCLC: Analysis of the placebo group in the MAGRIT study. Anticancer Res. 2019;39:1403–1409. doi: 10.21873/anticanres.13255. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Sun Z, Yang F, Sui X, Liu T, Wang J. Primary tumour resection in non-small-cell lung cancer patients with ipsilateral pleural dissemination (M1a): A population-based study. Eur J Cardiothorac Surg. 2019;55:1121–1129. doi: 10.1093/ejcts/ezy439. [DOI] [PubMed] [Google Scholar]
- 30.Meng F, Zhang L, Ren Y, Ma Q. The genomic alterations of lung adenocarcinoma and lung squamous cell carcinoma can explain the differences of their overall survival rates. J Cell Physiol. 2019;234:10918–10925. doi: 10.1002/jcp.27917. [DOI] [PubMed] [Google Scholar]
- 31.Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 32.Speicher PJ, Gu L, Gulack BC, Wang X, D'Amico TA, Hartwig MG, Berry MF. Sublobar resection for clinical stage IA non-small-cell lung cancer in the United States. Clin Lung Cancer. 2016;17:47–55. doi: 10.1016/j.cllc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Sun Y, Wang R, Ye T, Zhang Y, Chen H. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol. 2015;111:334–340. doi: 10.1002/jso.23800. [DOI] [PubMed] [Google Scholar]
- 34.NCCN Guidelines Insights (homepage on the Internet), corp-author Non-Small Cell Lung Cancer, Version 3. www.nccn.org/patients. [Feb 19;2019 ];2019
- 35.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138–155. doi: 10.3322/caac.21390. [DOI] [PubMed] [Google Scholar]
- 36.Hughes MJ, Chowdhry MF, Woolley SM, Walker WS. In patients undergoing lung resection for non-small cell lung cancer, is lymph node dissection or sampling superior? Interact Cardiovasc Thorac Surg. 2011;13:311–315. doi: 10.1510/icvts.2011.268979. [DOI] [PubMed] [Google Scholar]
- 37.Sugi K, Nawata K, Fujita N, Ueda K, Tanaka T, Matsuoka T, Kaneda Y, Esato K. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg. 1998;22:290–295. doi: 10.1007/s002689900384. [DOI] [PubMed] [Google Scholar]
- 38.Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, Zielinski M, Lerut T, Weder W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787–792. doi: 10.1016/j.ejcts.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Adachi H, Sakamaki K, Nishii T, Yamamoto T, Nagashima T, Ishikawa Y, Ando K, Yamanaka K, Watanabe K, Kumakiri Y, et al. Lobe-specific lymph node dissection as a standard procedure in surgery for non-small cell lung cancer: A propensity score matching study. J Thorac Oncol. 2017;12:85–93. doi: 10.1016/j.jtho.2016.08.127. [DOI] [PubMed] [Google Scholar]
- 40.Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18:63–70. doi: 10.1080/14737140.2018.1409624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, Kim AW, Detterbeck FC, Blasberg JD, Boffa DJ. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–619. doi: 10.1001/jamaoncol.2016.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, Wang H, Song X, Yue J, Yu J. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non-small-cell lung cancer patients: A propensity score matching-based analysis from surveillance, epidemiology, and end results database. Cancer Med. 2018;7:4354–4360. doi: 10.1002/cam4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benveniste MF, Gomez D, Carter BW, Betancourt Cuellar SL, Shroff GS, Benveniste APA, Odisio EG, Marom EM. Recognizing radiation therapy-related complications in the chest. Radiographics. 2019;39:344–366. doi: 10.1148/rg.2019180061. [DOI] [PubMed] [Google Scholar]
- 44.Bradley J, Hu C. Learning from trials on radiation dose in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;96:748–750. doi: 10.1016/j.ijrobp.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th edition. Springer; New York, NY: 2009. AJCC Cancer Staging Manual. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.