Abstract
In this article, we used High Angular Resolution Diffusion Imaging (HARDI) with advanced anatomically constrained particle filtering tractography to investigate the role of the arcuate fasciculus (AF) and the middle longitudinal fasciculus (MdLF) in speech perception in noise in younger and older adults. Fourteen young and 15 elderly adults completed a syllable discrimination task in the presence of broadband masking noise. Mediation analyses revealed few effects of age on white matter (WM) in these fascicles but broad effects of WM on speech perception, independently of age, especially in terms of sensitivity and criterion (response bias), after controlling for individual differences in hearing sensitivity and head size. Indirect (mediated) effects of age on speech perception through WM microstructure were also found, after controlling for individual differences in hearing sensitivity and head size, with AF microstructure related to sensitivity, response bias and phonological priming, and MdLF microstructure more strongly related to response bias. These findings suggest that pathways of the perisylvian region contribute to speech processing abilities, with relatively distinct contributions for the AF (sensitivity) and MdLF (response bias), indicative of a complex contribution of both phonological and cognitive processes to age‐related speech perception decline. These results provide new and important insights into the roles of these pathways as well as the factors that may contribute to elderly speech perception deficits. They also highlight the need for a greater focus to be placed on studying the role of WM microstructure to understand cognitive aging.
Keywords: cognitive aging, diffusion MRI, HARDI, hearing, language, normal aging, speech discrimination, white matter
1. INTRODUCTION
One of the most common complaints of elderly adults is a difficulty in perceiving speech in the presence of background noise. In healthy young and older adults, the perception of consonants in noise is more affected than the perception of vowels (Gelfand, Piper, & Silman, 1985, 1986; Meyer, Dentel, & Meunier, 2013). Whether different types of consonants are more difficult to perceive than others remains uncertain. Meyer et al. showed that the identification of specific fricative consonants presented in noise, such as /f/, were among those most often confused (Meyer et al., 2013). Others found that the ability to identify temporal cues in fricative sounds does decline with age (Gordon‐Salant, Yeni‐Komshian, Fitzgibbons, & Barrett, 2006).
Traditionally, age‐related speech perception deficits have been ascribed to presbycusis, the biological aging of the peripheral hearing system (Gates & Mills, 2004; Mazelová, Popelar, & Syka, 2003) which manifests itself as a loss of sensitivity to all sound, but, most notably, to high‐frequency sounds. Presbycusis is estimated to affect more than 60% of individuals aged over 70 years (Feder, Michaud, Ramage‐Morin, McNamee, & Beauregard, 2015; Lin, Thorpe, Gordon‐Salant, & Ferrucci, 2011). Yet, deficits in elderly speech perception are present even when elderly participants have normal hearing (Gordon‐Salant & Fitzgibbons, 1993; Tun, 1998; Tun & Wingfield, 1999; Wong et al., 2009); when they are compared to younger participants with equally poor hearing (Frisina & Frisina, 1997; Jerger, 1992); or when statistical (Bilodeau‐Mercure, Lortie, Sato, Guitton, & Tremblay, 2015) or experimental (Moore, Peters, & Stone, 1999) adjustments are made to control for the increased auditory thresholds of the older group. This suggests that factors in addition to the decreasing sensitivity of the peripheral auditory system are contributing to the speech in noise difficulties such as a central processing deficit related to brain aging.
In addition to the peripheral hearing system, the human brain also undergoes numerous morphological changes with age, including a reduction in total gray matter volume, increased cerebrospinal fluid volume and, of most interest to the current study, significant white matter (WM) changes including malformations of the myelin sheath and a notable loss of myelinated fibers (Gunning‐Dixon, Brickman, Cheng, & Alexopoulos, 2009; Guttmann et al., 1998; Marner, Nyengaard, Tang, & Pakkenberg, 2003; Pakkenberg & Gundersen, 1997). Degeneration of this kind is known to impair conduction of neural signals throughout the brain resulting in slower or incomplete transmission of neural impulses (Bartzokis, 2004). Such changes in WM properties in old age have repeatedly been linked with declines in cognitive abilities (Bennett & Madden, 2014; Madden et al., 2012) especially on tasks requiring speeded responses (Andrews‐Hanna et al., 2007; Charlton et al., 2006; O'Sullivan et al., 2001). Diffusion‐weighted magnetic resonance imaging (dMRI) provides a means to study, noninvasively, the WM fascicles of the human brain, and to investigate the types of changes that occur in these structures throughout the lifespan as well as associated behavioral consequences (Descoteaux, 2015; Lebel et al., 2012).
Although different approaches to dMRI exist (Daducci et al., 2014), one of the most common approaches is to compute the diffusion tensor, from which measures such as Fractional Anisotropy (FA), Axial Diffusivity (AD), Radial Diffusivity (RD), and Mean Diffusivity (MD) can be extracted to quantify microstructural properties of WM (Le Bihan et al., 2001; Soares Jé, Marques, Alves, & Sousa, 2013). Supporting Information 1 provides a brief description of the main diffusion tensor imaging (DTI) metrics (FA, AD, MD, RD). However, DTI metrics present some limitations. The most important of these limits is the inability of the tensor model to resolve complex fiber configurations, such as crossing (X), kissing (>> <<) and highly curved (>>) fibers within a voxel (e.g., Alexander, Barker, & Arridge, 2002; Alexander, Hasan, Lazar, Tsuruda, & Parker, 2001; Frank, 2002). With a recent study estimating that between 60 and 90% of the WM voxels in the human brain contains complex fiber configurations (Jeurissen, Leemans, Tournier, Jones, & Sijbers, 2013), and with additional research showing that FA, AD, RD, and MD are affected by this heterogeneity (Alexander et al., 2001; Tournier, Mori, & Leemans, 2011; Wheeler‐Kingshott & Cercignani, 2009), an alternative analysis model is needed. High angular resolution diffusion imaging (HARDI) tractography combined to non‐DTI reconstruction methods provide alternative strategies that are more robust to complex fiber architectures (Raffelt et al., 2012).
One such non‐DTI model focuses on estimating Fiber Orientation Distribution functions (fODF) instead of diffusion tensors. FODF—the continuous distribution of fiber orientations within the voxel—are obtained using spherical deconvolution (Tournier, Calamante, & Connelly, 2007; Tournier, Calamante, Gadian, & Connelly, 2004) and can be used for tractography. Apparent Fiber Density (AFD) is a measure based on fODF amplitude that provides information about the fraction of space occupied by a fiber bundle (Raffelt et al., 2012), a measure that is age sensitive (Mito et al., 2018). Another measure extracted from fODF is the Number of Fiber Orientations (NuFO) within each voxel. This measure provides information about WM complexity (Dell'Acqua, Simmons, Williams, & Catani, 2013). Supporting Information 1 provides a description of these two non‐DTI measures.
The overall goal of this study was to examine whether WM microstructure contributes to speech perception abilities, focusing on two long association fascicles associated of the perisylvian area: the Arcuate Fasciculus (AF) and the Middle Longitudinal Fasciculus (MdLF).
The AF is well‐known for its contribution to speech and language functions. First described in 1809 by Reil and colleagues (Reil, 1809), the AF connects the posterior superior temporal cortex (pSTC) to the inferior frontal gyrus (IFG)/ventral premotor cortex (PMv) forming a “dorsal language stream,” which is believed to be involved in language processing, sensorimotor mapping and phonological processing (Brauer, Anwander, Perani, & Friederici, 2013; Saur et al., 2008). Despite its central role for the neurobiology of language, the course, origins, terminations, and number of subcomponents of the AF remain contentious and several models of AF have been proposed (for a review see Dick, Bernal, & Tremblay, 2014; Dick & Tremblay, 2012). One of the dominant AF models proposes that the AF consists of two segments: direct and indirect (Catani, Jones, & ffytche, 2005). The direct segment arches around the lateral fissure and connects the pSTC to the IFG, middle frontal gyrus (MFG) and PMv. The indirect segment is divided into two components, a posterior AF segment that connects STC to inferior parietal regions, and an anterior segment that connects inferior parietal regions to IFG/MFG and PMv (Catani, Jones, & ffytche, 2005; Catani & Thiebaut de Schotten, 2012; Thiebaut et al., 2011; Weiner, Yeatman, & Wandell, 2016). Other models of the AF connectivity have also been proposed. For example, a dual‐pathway architecture in the dorsal language system was recently proposed (Berwick, Friederici, Chomsky, & Bolhuis, 2013; Brauer et al., 2013). According to this model, the connections from pSTC to IFG and PMv would mature at distinct rates and support distinct functions, with the pSTC‐IFG involved in processing higher‐order aspects of language (e.g.,syntax) and the pSTC‐PMv involved in sensory‐motor mapping and phonological processes for speech. While the specific number of components in AF and their functions remains uncertain, clearly, the AF is a major pathway for language, although there remain important questions about its structure and functions. Although limited, there is some evidence to suggest that the AF undergoes changes in FA in normal aging (Voineskos et al., 2010; Voineskos et al., 2012). Given the importance of the AF for speech/language, it is expected that any change in its microstructure will affect speech and language processing.
The MdLF is, like the AF, a long perisylvian association pathway. However, it has received much less attention than the AF and its functions are less well understood. The MdLF runs through the entire STC. It was first discovered in the macaque using autoradiographic techniques (Seltzer & Pandya, 1984), and several recent studies have confirmed its presence in the human brain using diffusion MRI (e.g., Makris et al., 2009; Makris et al., 2013; Makris & Pandya, 2009; Menjot de Champfleur et al., 2013) and fiber dissection (Maldonado et al., 2013). It was recently proposed that the MdLF is divided into two components: a ventral segment linking the inferior parietal lobule (IPL) to the temporal pole and a more caudal segment linking the superior parietal lobule (SPL) to the temporal pole (Makris et al., 2013; Makris, Preti, Asami, et al., 2013). Though sometimes seen as forming part of the ventral language streams (Saur et al., 2008), involved in mapping auditory speech sounds to meaning, the specific role of the two MdLF segments remains unclear. Indeed, it has been reported that electrostimulation of this fascicle does not elicit semantic paraphasias, and that electrostimulation and resection of the anterior part of MdLF have no impact on picture naming (De Witt Hamer, Moritz‐Gasser, Gatignol, & Duffau, 2010). It has been proposed that based on its connectivity, the MdLF could play a role in audiovisual integration (Wang et al., 2013) or attention (Steffens, Wang, Manning, & Pearlson, 2017). For example, a correlation was found between poor attention and the microstructure of MdLF in patients with schizophrenia, suggesting a role for the MdLF in attention (Steffens et al., 2017). No study has investigated the changes that occur in the MdLF in normal aging, yet this could contribute to understanding its roles. Clearly, additional data is needed to understand the functions of this pathway.
As such, the overall goal of this study was to advance current knowledge of the neurobiological foundation of speech perception in noise in adulthood. The main objective of the study was to determine whether the macrostructure and the microstructure of the AF and MdLF affects speech perception performance for consonants with different manners of articulation (fricative and stop consonants), and whether age‐related changes in these tracts contribute to age‐related decline in speech perception in noise. A secondary aim was to confirm the presence of the MdLF, using dMRI with advanced anatomically constrained particle filtering tractography (PFT) algorithms robust to crossing fibers and partial volume effects. Our main hypotheses were that (a) speech perception performance would decline with age (b) because speech perception relies on phonological processing, the structure of the AF would be associated with speech perception performance, especially in terms of sensibility (d′), and (c) because of its potential role in cognition/attention and language processing, we hypothesized that the structure of the MdLF would also affect speech perception, particularly in terms of response bias.
2. METHODS
2.1. Participants
A total of 32 participants were initially recruited for this study; three subjects were removed from the final statistical analyses due to technical faults that caused a loss of data. The 29 remaining participants were divided into two subgroups; a younger age group contained 14 participants (M = 29.5 ± 10.49; range: 19–46 years; 5 females) and an older age group contained 15 participants (M = 71 ± 5.85; range: 65–84 years; 3 females). Both groups were matched on distribution of gender (χ2 (1) = 0.895, p = .43, Cramer's V = 0.18). All participants were native speakers of Canadian French, were right‐handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971), had normal or corrected to normal vision and were highly educated (Younger: M = 16.92 ± 2.34 years; Older: M = 16.4 ± 4.6 years). No participants reported a history of language, speech, neurological or psychiatric disorders. Neither group showed any sign of depression as assessed by the Geriatric Depression Scale (Yesavage et al., 1983), or mild cognitive impairment using the Montreal Cognitive Assessment (Nasreddine et al., 2005). A summary of the participant data is outlined in Table 1. The study was approved by the Comité d'Éthique de la Recherche, Institut Universitaire en Santé Mentale de Québec (# 360–2014).
Table 1.
Participants' characteristics
| Age group | Younger (N = 14; 5F) | Older (N = 15; 3F) | ||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| Age | 29.43 | 10.49 | 19–46 | 71.93 | 5.85 | 65–84 |
| Handedness | 83.78 | 18.27 | 50–100 | 96.24 | 8.87 | 68.42–100 |
| Education (years) | 16.92 | 2.34 | 13–21 | 16.40 | 4.60 | 10–30 |
| GDS (/30) | 3.79 | 3.68 | 0–12 | 2.53 | 3.07 | 0–8 |
| MOCA (/30) | 28.57 | 1.09 | 27–30 | 27.47 | 1.68 | 25–30 |
| Right ear PTA | 4.95 | 9.58 | 1–37 | 13.76 | 8.21 | 3–32 |
| Left ear PTA | 3.00 | 8.24 | 4–29 | 15.64 | 9.74 | 2–43 |
| Right ear 6 KHz | 8.79 | 7.81 | 4–20 | 44.67 | 23.72 | 11–90 |
| SRT | 27.04 | 8.56 | 15–52 | 41.50 | 7.74 | 30–55 |
| Health (/7) | 4.78 | 1.069 | 2–6 | 5.4 | 1.00 | 3–7 |
Note. M = Mean; SD = standard deviation of the mean; N = number of participants per group; F = number of female participants; MoCA = Montreal Cognitive Assessment scale. The MOCA is a short cognitive test that is scored on a 30‐point scale. Higher scores indicate better cognitive functions. GDS = Geriatric Depression Screening Scale. The GDS includes 30 questions. Each “negative” answer is worth one point; thus, a higher score indicates a more depressed state. For example, question one asks whether the person is globally satisfied with his/her life. A “no” answer is worth one point, whereas a “yes” answer is worth no points. Participants with scores between 0 and 9 are considered normal, while scores between 10 and 19 indicate a depression, and 20–30 indicate a severe depression. PTA = pure tone average, measured in dB. Normal hearing should range between 0 and −10 dB. All participants had a hearing that ranged between normal and mild hearing loss, which is normal in elderly populations. Right ear 6 KHz = hearing threshold at 6 KHz in the right ear. SRT = Speech Reception Threshold in dB. SRT represents the lowest sound intensity level at which participants are able to correctly identify 50% of monosyllabic words. Health = self‐reported general health status on a scale of 0 to 7, with 0 being lowest health level and 7 the maximal one.
2.2. Procedure
Participants underwent audiological and cognitive testing and they completed a speech perception task. All procedures took place in a double‐walled soundproof room. The session had duration of approximately 2 to 3 hr and included several breaks. MRI images were acquired on a separate day.
2.2.1. Audiological assessment
Audiometric evaluations were performed in a double‐walled soundproof room. It consisted of three parts: (a) Pure tone audiometry using a clinical audiometer (AC40, Interacoustic) with each ear tested separately, at the following frequencies: .25, .5, 1, 2, 3, 4, 6, 8, 10 kHz. For each participant, a standard pure tone average (PTA: average of thresholds at .5, 1 and 2 kHz) was computed for the left and right ear (Stach, 2010). (b) A speech recognition threshold (SRT) test was used to assess the lowest sound intensity level at which participants were able to correctly identify 50% of monosyllabic words. (c) Distortion product otoacoustic emissions recording (DPOAEs) provides an objective measure of cochlear outer hair cell function through the measurement of cochlear acoustic emissions.
2.3. Speech perception task
An auditory discrimination task was used to evaluate speech perception skills. Three sixty unique pairs of syllables were presented, one at a time, at an individually adjusted intensity. Participants were asked to determine if the syllables were identical or different. The syllables were presented 200 ms apart to minimize working memory demand. The presentation of the second syllable was followed by a question mark cueing participant to respond. Participants were asked to answer as quickly as possible using a response box (RB‐840 model, Cedrus, San Pedro, California). All stimuli were presented using Presentation Software (Neurobehavioral System, CA) through high quality headphones (DT 770 Pro, Beyerdynamic Inc.), while participants were comfortably seated in a soundproof room. The pairing of the responses and button on the response box was counterbalanced across participants. Unlike speech audiometry, which determines the minimal sound intensity necessary for a person to hear a word correctly, speech discrimination evaluates sensitivity to the phonetic details of native speech sounds. Unlike identification tasks, discrimination does not require explicit categorical judgment.
One eighty pairs (50%) were identical (/fe/ vs. /fe/), 60 pairs had different fricative consonants (/fe/ vs. /se/), 60 had different stop consonants (/pe/ vs. /ge/), and 60 had different vowels (/f/ vs. /fe/). The syllables that were used to create the pairs were 48 different syllables recorded by three native adult male French speakers (See Supporting Information 2 for more details). Participants were asked to ignore the speaker and focus on the consonant and vowel that were presented. Syllables were used instead of words to avoid lexical/semantic effects, which may mask perceptual difficulties. All syllables were peak amplitude normalized across all speakers using Pratt to a mean intensity of 70 dB HL. The syllables had an average duration (M ±SD) of 350 ±0.05 ms. Intelligibility was manipulated by adding pink noise to the syllables to reach a dB signal‐noise ratio (SNR) of either 15 (mid) or −5 (low intelligibility) according to the following formula: dB SNR = 10log10 (Pressuresignal/Pressurenoise), as initially described by (Wong, Uppunda, Parrish, & Dhar, 2008). In the high intelligibility condition, syllables were presented in the absence of noise.
Response accuracy was analyzed within the framework of signal detection theory. Specifically, d‐prime (d′) and criterion (c) were calculated. D‐prime is defined as the ability to accurately discriminate between target (identical syllable pairs) and nontarget trials whereas criterion (c) is defined as the tendency to choose one response over the other, that is, response bias (Macmillan & Creelman, 1990). If c = 0, then participants do not have a tendency to choose one response over the other (i.e., no bias). A negative value of c indicates a bias toward responding “yes” whereas a positive value of c represents a bias towards responding “no.” In addition, a measure of facilitation operationalized as repetition priming (measured in milliseconds) was also computed. Repetition priming is the facilitation in cognitive processing that occurs as a consequence of repeated exposure to a stimulus (here identical syllable pairs); it manifests itself behaviorally by a decrease in reaction times (Logan, 1990; Schacter & Buckner, 1998). This behavioral measure was used to evaluate speed of processing in the speech domain.
2.4. MR image acquisition
The data were acquired on a whole‐body Philips 3.0 Tesla Achieva TX at the Clinic IRM Québec‐Mailloux in Québec City. Structural MR images were acquired with 3D T1‐weighted MPRAGE sequence (TR = 8.2 ms, TE = 3.7 ms, FoV = 250 mm, flip angle = 8°, 256 × 256 matrix, 180 slices/volume, no gap, 1 mm3). HARDI were collected to allow for analysis of age differences in WM (TR = 8.5 ms; TE = 76.7 ms; b = 1,500 s/m2, 60 directions, 128 slices/volume, no gap, 1.8 mm3). Single shot EPI BOLD functional images and Susceptibility Weighted images were also collected for each participant as part of a separate experiment. Throughout the procedure, each participant's head was immobilized using a set of cushions and pads. The complete image acquisition protocol (all four sequences) lasted no more than 45 min per participant.
2.5. Image processing
All diffusion weighted images were visually inspected by two of the authors (DKH, ID) for the presence of artifacts with particular focus on ‘striping’ due to its frequent occurrence. If such artifacts were present, the corresponding directions were removed (13 participants had at least one direction removed; M = 4, SD = 3.24) before continuing with preprocessing (Sharman et al., 2011). Next, susceptibility artifacts (caused by magnetic field fluctuations that occur at the boundary between substances with different magnetic sensitivities and result in image distortions) were corrected using the FSL TOPUP procedure (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TOPUP; (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004). Data were also preprocessed for eddy currents and subject motion using the FSL eddy tool (Andersson & Sotiropoulos, 2016). Finally, images were denoised to improve the signal to noise ratio and thus aid subsequent processing using a Non‐Local Means filter robust to Rician noise implemented using DIPY (Descoteaux, Wiest‐Daesslé, Prima, Barillot, & Deriche, 2008; Garyfallidis et al., 2014). All processing was performed in each participants' native anatomical space. Using FreeSurfer (v5.3.0), the T1 images were segmented into gray and WM and parceled. The resulting WM cortical parcellation images were registered to individual diffusion scans to preserve the correct orientation of diffusion‐weighted images. Eigenvalues reflecting perpendicular and parallel diffusion, also called axial and (AD‐RD) respectively, MD and FA were calculated from the diffusion tensor (Basser, Mattiello, & Lebihan, 1994) using a nonlinear least squares model, as implemented in DIPY (Garyfallidis et al., 2014).
Fiber tractography was performed on the field of fiber orientation distribution function (fODF) (Descoteaux, Deriche, Knosche, & Anwander, 2009; Tournier et al., 2007), of spherical harmonics order eight, as implemented in DIPY. The T1 anatomy was registered with the symmetric image normalization registration algorithm available in ANTS (Avants, Tustison, & Song, 2011). Then, the white/gray matter interface was extracted using the FSL fast command and probabilistic PFT (with anatomical priors) was run with 3 seeds per voxel of the WM/gray matter interface leading to full brain tractograms with approximately 1 million streamlines per participant (Girard, Whittingstall, Deriche, & Descoteaux, 2014a). Default parameters were used as optimized in Girard, Whittingstall, Deriche, and Descoteaux (2014a). This streamline‐based PFT seeded from the WM/gray matter interface has been shown to reduce tractography length and size biases and limitations (Girard et al., 2014a) in making sure that streamlines connect cortical or subcortical areas, or exit the brainstem, and do not stop in anatomically invalid areas such as WM or cortico‐spinal fluid (ventricles). Hence, the streamline count is less biased by the shape, length, and volume of bundles.
The White Matter Query Language (WMQL) (Wassermann et al., 2013, 2016) was used in conjunction with the FreeSurfer Desikan atlas (Desikan et al., 2006) to define the three segments of the AF and the two segments of the MdLF in both hemispheres. In addition, a control fasciculus, the bilateral uncinate fasciculus (UF), was also added to test for the specificity of potential relationships between age, speech perception and the fasciculi of interest (AF, MdLF). The WMQL adopts a text‐based, human‐readable approach to defining WM fascicles. Use of this language reduces the amount of variance introduced into the data when drawing regions of interest directly onto anatomical or diffusion scans. Additionally, this language allows for direct comparison between datasets through simple application of the same written definition (for the definitions see Supporting Information 3). Finally, once extracted, these WM bundles were processed through an additional pipeline as described in (Cousineau et al., 2017), available as part of the Sherbrooke Connectivity Imaging Lab (SCIL) python toolbox (SCILPY), with elimination of spurious fascicles using a pruning and outlier‐rejection step (Cote et al., 2013) after which the means and standard deviations of each metric were computed. Each fascicle of each participant was observed with MI‐Brain software (https://www.imeka.ca/mi-brain) to determine a weight interval to remove the spurious fascicles (Rheault, Houde, Goyette, Morency, & Descoteaux, 2016). A mean of intervals was calculated and the final fascicles were extracted.
2.6. Statistical analyzes
Linear mixed model (LMM) analyses were conducted in SPSS Version 25 for Mac (IBM), separately for each dependent variable (d′, C, repetition priming), with Consonant type (fricative, stop) as within‐subject (repeated) fixed factors, Age group as a between‐subject categorical fixed factor, and hearing, operationalized as hearing thresholds at 6 K Hz in the right ear, was used as continuous between‐subject covariates to control for individual differences in hearing. Participants were included as a random factor in the model.
Next, to address the main objective of the study, which was to determine whether the macrostructure (volume) and the microstructure of the AF and MdLF affects speech perception performance in adulthood, and whether age‐related changes in these tracts contribute to age‐related decline in speech perception in noise, a series of simple mediation analyses were conducted using ordinary least squares path analysis. Prior to running the mediation analyses, the data were visually inspected using histograms and boxplots in SPSS. Outliers were removed. The Shapiro–Wilk test of normality was used to verify normality of the distribution for each diffusion metric and each group (p > .05). The Levene's test for equality of variances was used to verify the homogeneity of variances across groups, the (p > .05). If one of these assumptions was not met, the data were transformed. For AF, Nufo (cubic), AFD max (cubic), AFD total (squared) and Volume (square root) were transformed. For MdLF, AFD max (cubic), AFD total (squared) and the volume (square root) was transformed and the normality and homogeneity were verified again. Finally, for UF only NuFO (squared) and AFD total (squared) were transformed.
The mediation analyses were conducted using PROCESS macro version 2.16 (model #4) for SPSS (Hayes, 2013; Preacher & Hayes, 2004, 2008). In this model, Age was used as the continuous predictor variable (X), speech perception scores were used as dependent variables (Y) and diffusion metrics were used as continuous mediators (M). In all analyses, hearing was used as continuous between‐subject covariates to control for individual differences in hearing. To control for individual differences in head size, the proportion approach was adopted (O'Brien et al., 2011), whereby each diffusion metric value for a tract was divided by the diffusion metric of total brain (adjusted value = [tract value/total brain value]). A bootstrapping approach was used to test for the significance of the indirect effects (ab) (p < .05, using bias‐corrected bootstrapping with 20,000 samples). Bootstrapping involves the repeated extraction of samples, with replacement, from a data set and the estimation of the indirect effect in each resampled data set.
To correct for multiple comparisons, we applied a false discovery rate (FDR) procedure (Benjamini & Hochberg, 1995) to the tests of direct effects (c′) and b‐paths. For the purpose of FDR correction, a family, that is, the smallest set of items of inference in an analysis, was defined as all the tests conducted in one segment (e.g., NuFO in the Left Anterior AF) (N = 6, one for each behavioral measure). The a‐paths, which test for the effect of age on WM, were only tested once per tract and were therefore left uncorrected. The indirect effects (ab) are bootstrap‐corrected; no additional correction was applied.
3. RESULTS
3.1. Speech perception
The LMM analyses revealed a significant interaction between Age group and Consonant type for sensitivity (F [1, 24,433] = 5,929, p = .023), with a significant effect of Age group only for the fricatives. Older adults exhibited a lower sensitivity (M = 1.08, SE = 0.19) than younger adults (M = 1,638, SE = 0.35). Although a similar pattern was found for Priming, it did not reach statistical significance (F [1, 24,433] = .130, p = .722). Older adults exhibited a lower priming (M = .288, SE = 0.079) than younger adults (M = .129, SE = 0.046) for the fricatives. There was no Age group by Consonant interaction for criterion (F [1, 24,389] = 2,402, p = .134). The behavioral data are illustrated in Figure 1, and descriptive statistics are provided in Table 2.
Figure 1.
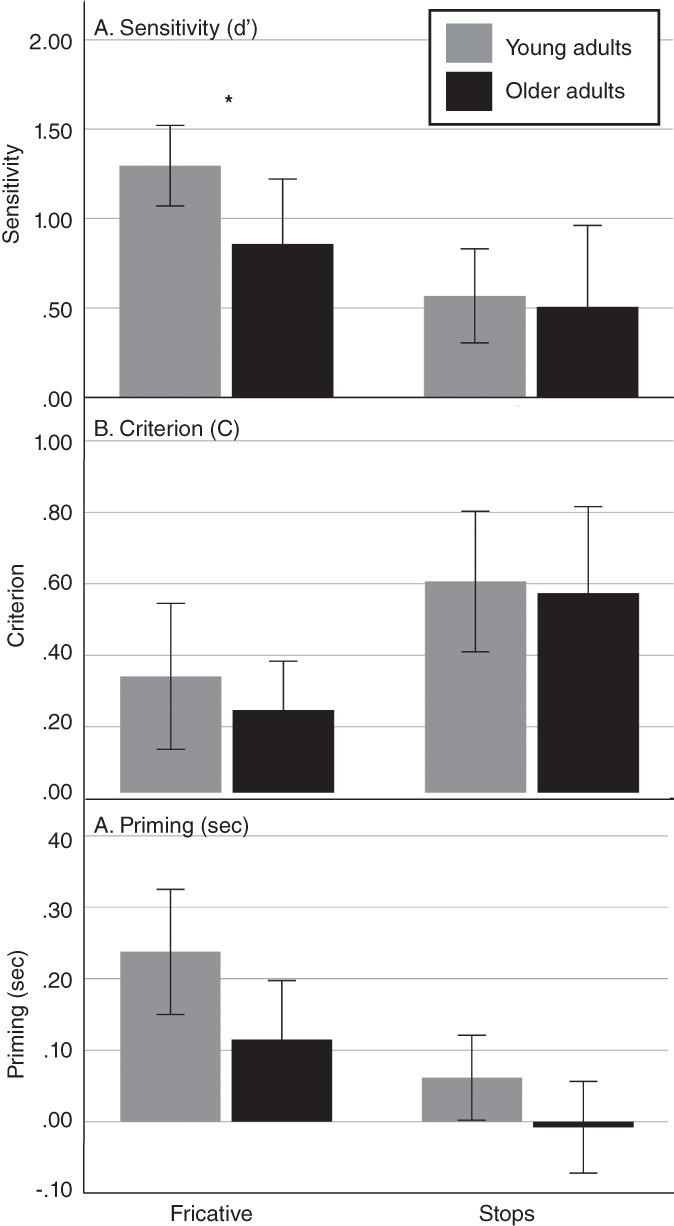
Results of the linear mixed model analyses shown separately for the younger and older adults, at each level of the consonant variable. (a) Sensitivity (d′). (b) Criterion (C), (c) priming in seconds. The error bars represent the confidence interval of the group mean. The asterisk indicates statistical significance (p ≤ .05)
Table 2.
Descriptive statistics for the behavioral measures computed from the discrimination task
| Behavioral metrics | Young N = 14 | Older N = 15 | Overall N = 29 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | 95% CI [LCI, UCI] | M | SD | 95% CI [LCI, UCI] | M | SD | 95% CI [LCI, UCI] | |
| Priming fricatives | 0.06 | 0.10 | [.002, .12] | −0.01 | 0.11 | [−.07, .05] | 0.03 | 0.11 | [−.13, .29] |
| Priming stops | 0.28 | 0.22 | [.16, 41] | 0.14 | 0.17 | [.05, 24] | 0.21 | 0.21 | [−.02, .07] |
| C fricatives | 0.34 | 0.35 | [0.14, 0.55] | 0.25 | 0.25 | [0.11, 0.38] | 0.29 | 0.30 | [0.18, 0.41] |
| C stops | 0.61 | 0.34 | [0.41, 0.80] | 0.50 | 0.48 | [0.24, 0.77] | 0.55 | 0.42 | [0.40, 0.71] |
| d’ fricatives | 1.30 | 0.39 | [1.07, 1.52] | 0.86 | 0.65 | [0.50, 1.22] | 1.07 | 0.58 | [0.85, 1.29] |
| d’ stops | 0.43 | 0.65 | [0.06, 0.81] | 0.51 | 0.82 | [0.05, 0.96] | 0.47 | 0.73 | [0.19, 0.75] |
Note. M = Mean; SD = standard deviation of the mean; N = number of participants per group or total; 95%CI: confidence interval at 95%. LCI: lower bound of the CI, UCI: upper bound of CI.
As part of the mediation analysis framework described in section 3.2, we tested the effects of Age as a continuous factor on Speech perception, holding WM and hearing constant. The results show that the direct effect of Age on Priming for the fricatives was significant in a large number of models. In all these analyses, the effect of Age on speech perception was negative, indicative of lower priming. These effects are reported in Supporting Information 4.
3.2. Relationship between WM, speech perception and age
Figures 2 and 3 illustrate the average AF and MdLF tracts, respectively. The individual pathways are illustrated in Supporting Information 5 and descriptive statistics are provided in Supporting Information 6. In all but two participants (one young and one older), all fascicles were found. In the two participants with missing data, only the direct segment of the AF was missing (in both hemispheres for one and only in the left hemisphere for the other). The missing data was replaced by the age group average of each metric for the mediation analyses. The mediation analyses are detailed in the next paragraph, pathway by pathway (a, b, ab).
Figure 2.
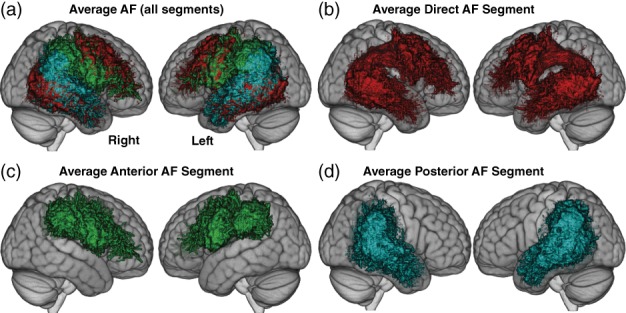
Average AF segments displayed on the linear ICBM average brain (ICBM152) stereotaxic registration model. (a) the three segments are shown on the left and right hemisphere; (b) the direct segment (red); (c) the indirect anterior segment (green) and (d) the indirect posterior segment (cyan)
Figure 3.
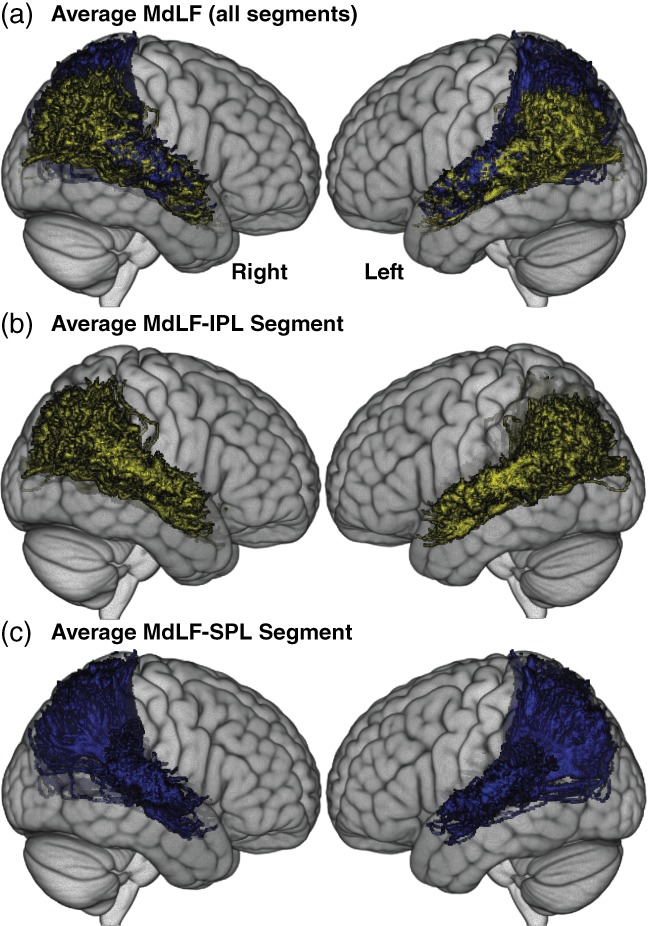
Average MdLF segments displayed on the linear ICBM average brain (ICBM152) stereotaxic registration model. (a) the two segments are shown on the left and right hemisphere; (b) the MdLF‐IPL (yellow) and (c) the MdLF‐SPL (blue). AF = arcuate fasciculus. MdLF = middle longitudinal fasciculus. UF = uncinate fasciculus
3.2.1. Aging of AF and MdLF (a pathways)
Holding hearing constant, the mediation analysis revealed limited age effects on the AF and MdLF (significant a‐paths), most of which in AF, and mainly positive. These effects are detailed in Supporting Information 7. Negative effects of age (decline) were found in the volume of the left posterior AF (a = 0.0007), MD of the left direct AF (a = 0.0042) (that is, an increase in MD) and FA of the right MdLF‐IPL (a = −0.0019). Positive age effects were more widespread and included an age‐related increase in the volume of the right direct AF (a = 0.0014), AFD max of the right direct AF (a = 0.0091), NuFO of the left direct AF (a = 0.0040), NuFO of the right MdLF‐IPL (a = 0.0012), and AFD total of the right MdLF‐SPL (a = 0.0019).
3.2.2. Age‐independent impact of WM on speech perception (b pathways)
There were several instances of an age independent effect of WM on speech perception, holding hearing constant. These effects are detailed in Supporting Information 8A and 8B. The majority (62%) of these effects were positive, meaning that, two people of the same age that differed in one unit of WM also differed on speech perception in noise, with higher WM value associated with better speech perception in noise, holding hearing constant. Better sensitivity to speech sounds, in particular, was associated with higher AD in all tracts. More positively biased responses (i.e., higher criterion) were associated mainly with higher ODF metric values (AFD total, AFD max, NuFO) in the MdLF and, to a lesser extent, with lower values in the left posterior AF and right MdLF‐SPL, meaning that a negative bias (tendency to say “same”) was associated with higher value in these segments. Finally, greater priming was associated mainly with higher ODF metric values (AFD total, NuFO) in the MdLF and, to a lesser extent, also in the AF.
3.2.3. Indirect effect of age on speech perception through WM (ab)
We found indirect effects of Age on speech perception through WM in the bilateral AF (all three segments) and right MdLF (IPL and SPL segments). These effects are detailed in Figure 4 (AF) and 5 (MdLF). There was no indirect effect of Age on Speech perception through the structure of the UF. Most indirect effects were positive (70%), meaning that aging was likely to lead to better speech perception (mainly in terms of sensitivity and criterion) as a result of the positive effect of Age on WM value, which, in turn, affected speech perception performance positively. In the right MdLF‐IPL (Figure 5b), the indirect effect, albeit positive, has a different meaning. There, aging was likely to lead to better speech perception (sensitivity for the stops) as a result of the negative effect of Age on FA, which, in turn, affected speech perception performance negatively (higher FA associated with worse sensitivity). In two other cases, in the AF (Figure 4c,f) the indirect effects, albeit positive, also have a different meaning because they involve MD. A higher value of MD indicates a decline in the microstructure of the WM. For these two cases, aging was likely to lead to better speech perception (sensitivity for the fricatives) as a result of the negative effect of Age on MD, which, in turn, affected speech perception performance positively (higher MD [i.e., decline] associated with better sensitivity).
Figure 4.
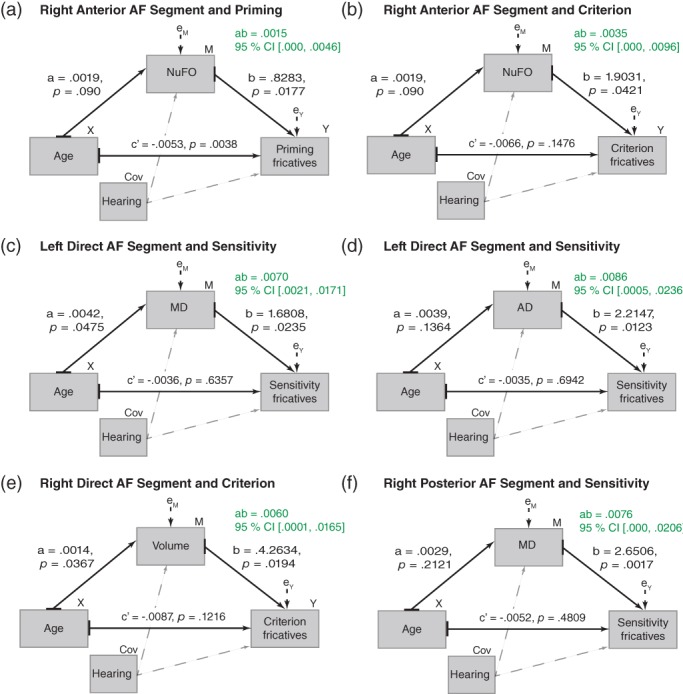
Structural path diagrams of the indirect effects of age on speech perception through white matter in AF. Each of the six indirect effects found in AF is illustrated separately. Unstandardized coefficients are provided with probability value (for the a‐path, b‐path, c‐path, and c′ path). The bootstrapped 95% confidence interval is provided for the indirect effects
Figure 5.
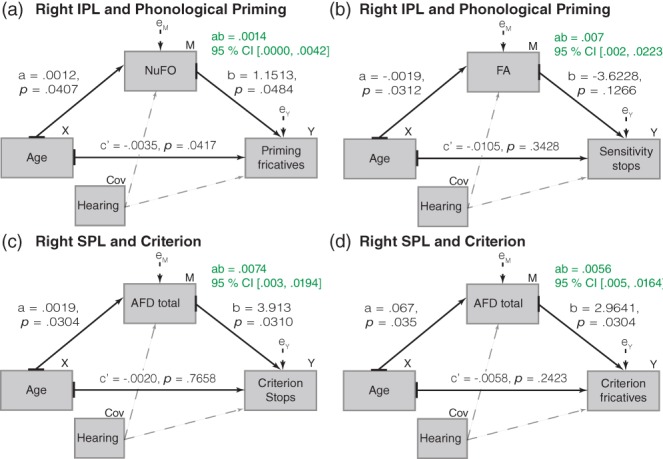
Structural path diagrams of the indirect effects of age on speech perception through white matter in MdLF. Each of the four indirect effects found in MdLF is illustrated separately. Unstandardized coefficients are provided with probability value (for the a‐path, b‐path, c‐path, and c′ path). The bootstrapped 95% confidence interval is provided for the indirect effects
4. DISCUSSION
In this study, we aimed to advance current knowledge about the relationship between speech perceptions in noise in healthy adults focusing on fiber pathways of the perisylvian region. Our main hypotheses were that (a) speech perception performance would decline with age, (b) because speech perception relies on phonological processing, the microstructure of the AF would be associated with speech perception performance, especially in terms of sensibility (d′) and phonological repetition priming, and (c) because of its potential role in cognition/executive processes, we also hypothesized that the microstructure of the MdLF would also affect speech perception, especially in terms of response bias (C), the most cognitive component of speech discrimination.
To gain a broader understanding of the mechanisms that contribute to age differences in speech perception in noise, we measured speech perception using three different behavioral measures that provide information about the sensitivity to differences in speech sounds (d′), but also response bias (also referred to as the internal decision criteria and phonological processing (phonological repetition priming). Based on available evidence, we expected that sensitivity and phonological processing would be most affected by aging. Our behavioral analyses indicated that sensitivity was the most affected of all measures, followed by phonological priming. There were also indirect effects of age on response bias through WM integrity in the right MdLF and right AF. Indirect age effects on Sensitivity and Priming through WM were also found. These results are discussed in the following paragraphs.
4.1. Aging of speech perception
The results of the LMM analyses show that after controlling for hearing acuity, older adults are less sensitive to phonetic details in low intelligibility situations, operationalized as differences in sensitivity (d′), for fricative consonants. The finding of an age difference in sensitivity is consistent with prior studies that have shown an age‐related decline in the sensitivity to phonetic details even in the absence of noise (Harkrider, Plyler, & Hedrick, 2005; Nilsson, Soli, & Sullivan, 1994; Plomp & Mimpen, 1979; Strouse, Ashmead, Ohde, & Grantham, 1998; Tremblay, Piskosz, & Souza, 2002, 2003). Whether this difficulty reflects a central auditory processing deficit or a speech‐specific deficit that could be related to a decline in phonological processing remains to be determined. The finding of indirect effects of age on sensitivity through the left direct and right posterior segments of the AF suggests that this decline may be related to speech‐related mechanisms that are supported by the AF, which is involved in mapping sound to actions (see Section 4.2).
Interestingly, when using more complex statistical models that also included individual differences in WM, additional direct and indirect effects of age emerged. Indeed, when WM was entered in the mediation models, indirect effects of age on response bias (C) were found. While sensitivity is related to the sensory processes and is untainted by response bias, criterion is a measure of the decision process that reflects potential bias towards responding “same” or “different” that is uncoloured by sensitivity. A value of 0 indicates the absence of a response bias, while a positive criterion indicates a liberal approach (i.e., a tendency to declare the stimuli to be the same) and a negative value indicates a conservative approach (i.e., a tendency to declare the stimuli to be different). Our results show that older participants in our study had a more liberal approach to the task, especially for the fricative consonants, a finding that is consistent with a prior study that showed less strict decision criterion in older adults in a speech perception task (Gordon‐Salant, 1986). The finding of age differences in criterion is also consistent with studies that have shown that the reliance on acoustic and temporal cues changes with age (e.g., Strouse et al., 1998; Toscano & Lansing, 2017; Tremblay et al., 2002, 2003), with a decreased ability to interpret certain acoustic cues in older adults. Age differences in decision criterion may thus reflect the reliance on different cues or a different weighting of the importance of making different kinds of mistakes (saying the stimuli are identical when they are not). It may also reflect a more global change in cognitive strategy (less cautious decision‐making process) or even personality.
Lastly, the examination of the direct effect of Age on Speech perception controlling for WM and hearing revealed age‐related decline in phonological repetition priming. Repetition priming is the facilitation in cognitive processing that occurs as a consequence of repeated exposure to a stimulus, in this case auditory syllables (Schacter & Buckner, 1998). It is also referred to as implicit memory. Though it has been suggested that implicit memory processes are age‐invariant (for a review see Fleischman, 2007), phonological processes are known to decline with age, which is shown by increased word retrieval failures, such as the tip‐of‐the‐tongue phenomenon (Brown & Nix, 1996; Burke, MacKay, Worthley, & Wade, 1991; Heine, Ober, & Shenaut, 1999; Rastle & Burke, 1996). The current finding of an age‐related reduction in phonological repetition priming is, therefore, consistent with the literature on the aging of phonological processes and suggest that deterioration of phonological processing mechanisms may be a contributing factor to age‐related speech perception difficulties.
In sum, we found that the discrimination of fricative consonants, in terms of sensitivity, criterion and priming, was more affected by aging than the discrimination of stop consonants. These findings add to the literature on age differences in the perception of consonants. They are consistent with the results of Gordon‐Salant et al., who found that the ability to identify temporal cues in fricative sounds declines with age, even in the absence of noise (Gordon‐Salant et al., 2006). Our results are also consistent with a study by Meyer et al., in which identification of certain fricative consonants presented in noise, such as /f/, were amongst those most often confused (Meyer et al., 2013). That study also reported that voiceless consonants, in general, were more often confused than voiced consonants. In the present study, we did not distinguish between voiced and voiceless fricative consonants. Our results nevertheless show that, overall, manner of articulation appears to be an important factor affecting speech perception in aging, with fricative consonants being more vulnerable to aging than stop consonants.
Taken together, our findings suggest that age differences in speech processing in noise are complex, involving differences at the level of sensory/phonological processes and decision process, and strongly affected by individual differences in AF and MdLF. The relationship between speech perception and WM in these pathways is described in Sections 4.2 and 4.3.
4.2. Speech discrimination and the AF
Consistent with our hypothesis, the structural properties of all three segments of the AF have an impact on speech perception in noise. To the best of our knowledge, this is the first study to investigate the role of the AF and its subcomponents in speech perception in noise in a healthy adult population. We found that the structure of all three segments of the AF was relevant to the processing of speech sounds in noise, especially in terms of sensitivity to phonetic details, operationalized as sensitivity (d′).
In contemporary literature, the AF is usually regarded as consisting of at least two segments. According to one dominant model, AF is composed of a direct and an indirect segment, with the indirect segment composed of two segments: anterior and posterior (Catani et al., 2005; Catani & Mesulam, 2008). Catani and all have proposed that the direct segment is involved with phonological processing, while the anterior segment is involved in articulation and the posterior segment in auditory comprehension (Catani et al., 2005). Others have proposed that AF contains two distinct fiber pathways, one connecting pSTC to IFG and the other connecting pSTC to PMv (Berwick et al., 2013), with the pSTC‐PMv involved in sensory‐motor mapping and phonological processing for speech.
Using the 3‐component model of AF, here we found that the structure of all three segments of AF was relevant to the processing of speech sounds in noise, in particular in terms of sensitivity to phonetic details. The AF appears to be instrumental in mapping degraded auditory speech input to speech representations at all ages. According to the DIVA model of speech production (Guenther, 1994, 1995; Guenther, Ghosh, & Tourville, 2006), speech sounds are represented in the PMv, one of the termination sites of AF. This supports the notion of phonological processing within AF. A recent fMRI study using multi‐voxel pattern analysis (MVPA) found sensitivity to place of articulation during passive listening of syllables in several brain regions including the posterior IFG and PMv (Correia, Jansma, & Bonte, 2015). Transcranial magnetic stimulation (TMS) to PMv has a strong impact on speech perception when intelligibility is low (Meister, Wilson, Deblieck, Wu, & Iacoboni, 2007), but not when it is high (Sato, Tremblay, & Gracco, 2009), suggesting that this region is sensitive to the quality of the auditory speech signal and that it could play a causal role in speech perception. TMS has also revealed that the PMv can influence speech sound categorization (Grabski, Tremblay, Gracco, Girin, & Sato, 2013). Moreover, an fMRI study has shown that correct identification of phonemes presented in noise is associated with increased activation in PMv relative to trials in which phonemes are incorrectly identified (Callan, Callan, Gamez, Sato, & Kawato, 2010). Increased PMv activation was also seen for time‐compressed speech (Adank & Devlin, 2010). Stimulation of the adjacent posterior IFG was also found to disrupt performance during phonological tasks, suggesting a role for this region in phonological processes (e.g., Gough, Nobre, & Devlin, 2005; Hartwigsen et al., 2010). Taken together, these results support a role for the PMv/posterior IFG in speech processing, particularly in difficult listening conditions (distorted speech or presence of background noise) or when a challenging phonological task is performed.
A role for the AF in phonological processes is thus consistent with prior evidence. For example, direct stimulation of AF in awake neurosurgical patients produces phonological paraphasias (Maldonado, Moritz‐Gasser, & Duffau, 2011; Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007). The microstructural properties of AF are also linked with phonological awareness (Yeatman et al., 2011), reading ability in children (Deutsch et al., 2005; Niogi & McCandliss, 2006) and pseudoword language learning in adults (López‐Barroso et al., 2013).
The AF could also be involved more specifically in phonological working memory during speech perception, consistent with a study that showed that phonological awareness is related to FA of the direct AF segment in adults with and without dyslexia (Vandermosten et al., 2012). Interestingly, the segment that was most related to our measure of phonological priming was the anterior AF, which connects the IPL to IFG/PMv, which have also been implicated in articulatory rehearsal mechanisms (e.g., Fegen, Buchsbaum, & D'Esposito, 2015; Romero, Walsh, & Papagno, 2006). Articulatory rehearsal serves to refresh memory traces by way of subvocal speech allowing the traces to remain in working memory for longer periods of time.
In addition to numerous age‐independent effects, we also found indirect effects of Age on Speech perception through the microstructure of AF. Here the effects were less specific and affected sensitivity, priming and criterion. This suggests that age‐related changes to AF may have a global impact on speech perception in noise. It also suggests that phonological processing and phonological working memory may be part of the etiology of speech difficulties in aging. We hypothesized that indirect effects would be generalized and negative; instead we found that age effects on AF were limited, and slightly positive, meaning that aging was likely to lead to better speech perception (mainly in terms of sensitivity), at least in our sample of healthy highly educated adults, as a result of the positive effect of Age on AF, which, in turn, affected speech perception performance positively. The neurophysiology of aging and WM is discussed in Section 4.4.
Taken together, our results are suggestive of a relationship between the microstructure of AF and speech perception in noise, predominantly in terms of sensitivity. While our results may suggest a slightly more important contribution of the anterior tract to phonological working memory, additional studies comparing different types of speech tasks, such a perceptual and motor tasks, are needed to refine current understanding of the role of each segment on speech functions in adulthood and in aging. Studies are also needed to compare the ability for different AF models (e.g., Berwick et al., 2013; Catani et al., 2005) to account for age‐related changes in speech and language functions across the lifespan, which is beyond the scope of the current investigation.
4.3. Speech processing and the MdLF
A novel and important finding of this study is that the structural integrity of the two components of the MdLF impacts speech perception in noise, especially in terms of sensitivity and response bias (C), in an age‐independent manner. In addition, the structure of the right MdLF‐SPL is related to response bias in an age dependent manner.
The MdLF is a long association pathway that runs through the superior temporal cortex. It was first discovered in the macaque monkey using autoradiographic techniques (Seltzer & Pandya, 1984), but several recent studies have confirmed its presence in the human brain (Burks et al., 2017; Maldonado et al., 2013). Because of its overall trajectory, the left MdLF, as a whole, is sometimes regarded as forming part of the ventral language streams (Saur et al., 2008) which is involved in mapping auditory speech sounds to meaning. However, like AF, the anatomy of MdLF has undergone some revisions since the introduction of diffusion MRI techniques. From its original description as a single track, the MdLF has been proposed to be divided into two components, with a first segment connecting the anterior temporal lobe to the IPL through a ventrolateral trajectory, and another segment, more caudal and running steeper, connecting the anterior temporal lobe to the SPL through a dorsomedial trajectory (Makris, Preti, Asami, et al., 2013; Makris, Preti, Wassermann, et al., 2013).
Evidence for a role for MdLF in speech and language is limited, mainly because the tract has not been investigated extensively yet. Using direct electrical stimulation of left MdLF on eight awake patients, De Witt and colleagues were unable to interfere with a picture‐naming task. Moreover, following resection of MdLF, no permanent language deficits were found (De Witt Hamer et al., 2010). Yet, given its connectivity, it is likely that MdLF has a role in cognition even if not as a principle pathway, as suggested by Burks et al. (Burks et al., 2017). Because of its connectivity with the IPL, which is part of the ventral attention network (Corbetta, Patel, & Shulman, 2008; Vallesi, McIntosh, & Stuss, 2011), a right‐hemisphere lateralised network involved in attention, in terms of stimulus‐driven attentional (e.g., reorienting) responses (Corbetta et al., 2008; Katsuki & Constantinidis, 2014; Vossel, Geng, & Fink, 2014), the MdLF‐IPL could be supporting attentional functions. In support of this notion, a recent study reported a correlation between poor attention and the microstructure of MdLF in patients with schizophrenia, suggesting a role for the MdLF in attention (Steffens et al., 2017). The MdLF‐SPL, in contrast, is part of the dorsal attention network (DAN), which is involved in top‐down bias of attentional resources (Corbetta et al., 2008). The right SPL was found to be modulated by speech intelligibility in previous studies (Bilodeau‐Mercure et al., 2015; Bishop & Miller, 2009). In a study from our group, we found that this modulation was age dependent (Bilodeau‐Mercure et al., 2015), suggestive of a transformation in the role of DAN to the processing of speech in noise throughout adulthood, which could contribute to compensating for age‐related decline occurring within the auditory and language networks. The current finding of a positive indirect effect of age on speech processing through the integrity of the MdLF‐SPL tract, combined with the direct relationship between the tract's integrity and speech processing, especially in terms of response bias, suggests a role for this tract in supporting speech processing, possibly through changes in attentional biases.
4.4. Aging of Perisylvian white matter pathways
One of the main findings of the present study is that the microstructure of AF and MdLF did not massively decline with age, at least in our sample of well‐educated individuals. Indeed, participants in our study had an average of 16.6 years of education (SD = 3.62 years) and 76% had 15 or more years of education, making this sample relatively well educated compared to their peers. For comparison, only 30% of the Canadian population attained at least 15 years of education (i.e.,university level) in 2011 (https://www12.statcan.gc.ca). Further, in our sample, education level did not vary as a function of age (r 2 = .006, β = −.012, p = .692). Moreover, participants in our study self‐evaluated their general health level as high (5,103 ±1.06, on a scale of 1 to 7), and this evaluation did not vary as a function of age (r 2 = .106, β = .05, p = .084). Finally, their attitude towards life, as measured by the GDS, was very positive (mean of 3.14 on a possibility of 30, with scores between 0 and 9 being considered normal). GDS scores also did not vary with age (r 2 = .03, β = −.025, p = .369).
While aging of WM is a well‐established phenomenon, with the literature on the normal aging of WM in humans and animals showing several types of ultrastructural alterations in myelin and axons (for a review, see Liu et al., 2017), to the best of our knowledge, only a few studies have examined aging of AF in a healthy population (Voineskos et al., 2010; Voineskos et al., 2012) and none has examined MdLF.
In the present study, negative age‐related microstructural changes took the form of lower FA in the right MdLF‐IPL, lower volume in the left posterior AF segment and higher MD in the left direct AF segment. Age‐related decline in FA is a well‐established phenomenon (e.g., Gunning‐Dixon et al., 2009). Though the relationship between specific microstructural and ultrastructural properties of WM fibers and diffusion MRI metrics have not yet been fully elucidated, changes in diffusivity in the WM is consistent with evidence of a decline in the ultrastructure of both myelin and axons. A study combining Diffusion MRI and light microscopy techniques in intact rat brains, showed that FA is sensitive to myelination in WM regions with coherent fiber orientations (low fiber crossing) and low fiber dispersion (Chang et al., 2017). A relationship was also found between low FA in young adult and elderly adults and increased pulsativity index, a marker of cerebrovascular small vessel disease (Fleysher et al., 2017; Jolly et al., 2013) that signals a decrease in arterial compliance which may be related to arteriosclerosis.
In addition to lower FA, higher MD was also found in older adults in the left direct AF segment. MD indexes the average rate of diffusion in WM fibers, which depends on the density of physical barriers such as cellular membranes and the distribution of water molecules between cellular compartments. Increased MD has been reported in patients with reduced membrane density (Sen & Basser, 2005) such as tissue degeneration following injury (Concha, Gross, Wheatley, & Beaulieu, 2006). Although more research combining microscopy techniques and diffusion MRI are needed, it is possible that an increase in MD in aging is a marker of deteriorated cellular membrane in WM fibers of the AF and MdLF.
In addition to demonstrating a limited decline in AF and MdLF, our results suggest relative gain in WM integrity with age in these tracts, particularly in the bilateral AF. Though speculative, it is possible that these results demonstrate a form of age‐dependent plasticity. There is growing empirical evidence that experience can alter WM structure throughout the entire lifespan. Learning, physical exercise, sleep and even social experience have been shown to impact myelination in adulthood (for a review, see Sampaio‐Baptista & Johansen‐Berg, 2017). In our sample, education, general health status and mood were not related to age, and therefore cannot explain the WM gains that were found. However, we did not measure lifestyle factors, which could contribute to explaining these effects. Future studies on normal aging such as this one should strive to characterize their sample as thoroughly as possible to tease apart age effects from those related to experience‐dependent plasticity in brain structure, and more precisely WM structure, such as the amount and quality of social interactions, general quality of life, amount of cognitive activity, but also strive to collect data from more diverse social‐economic backgrounds.
5. LIMITATIONS
This study presents a number of limitations worth discussing. First, cross‐sectional designs cannot be used to determine if age differences are the result of natural changes that occur over the course of a lifetime, or if they result from secular and historical differences between cohorts, such as changes in nutritional habits between generations (Carlson & Morrison, 2009; Robinson, Schmidt, & Teti, 2008). However, it has been suggested that such cohort effects are likely to have less of an impact when the dependent variable is of a basic biological nature (Miller, 1998) as is the case here. Another limitation to this work is the small sample consisting mainly of highly educated individuals. Though this limitation does constraints the external validity of our results, we believe our findings provide important preliminary evidence for a role for the AF and MdLF in speech processing in noise. The validity of these preliminary results is strengthened by a thorough procedure for the analysis of diffusion data and tractography, our demonstration of our ability to track each participant's fasciculi, a very strict verification of the normality and homogeneity of the distribution of each variable, the illustration of individual data (Supporting Information 8), and the use of bias‐corrected bootstrap confidence intervals for the indirect effects.
6. CONCLUSIONS
By examining the macrostructural and microstructural properties of two important fiber pathways of the perisylvian regions, using robust tractography methods, our approach allows for an integrative and anatomically informed investigation of wm fascicles involved in speech perception in noise. Our findings reveal that pathways of the perisylvian region contribute to speech processing abilities in an age‐independent and in an age‐dependent fashion, with relatively distinct contributions for AF (sensitivity) and MdLF (response bias), suggestive of a complex contribution of WM in terms of phonological and cognitive processes to speech perception. Importantly, our findings suggest that individual differences in tract microstructure have a stronger impact on speech perception than age alone. It will be important to replicate these preliminary findings and continue investigate the specific roles of these two pathways in speech processing using a larger and more socioeconomically diverse sample, and more complex statistical models taking into consideration additional factors such as education and cognitive abilities.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENTS
This study was supported by research funds from the Québec Bio‐imaging network to PT, MD, and ASD, and from the Fonds de la Recherche en Santé du Québec (FRQ‐S, #27170) to PT, who also holds a Career Awards from the “Fonds de Recherche du Québec – Santé” (FRQS). PT and MD are supported by NSERC Discovery Grants and MD is supported by institutional research Chair in NeuroInformatics. DK‐H was supported by a UCL Bogue Fellowship. Technical support for protocol development and data acquisition was provided by the “Consortium d'imagerie en neuroscience et santé mentale de Québec” (CINQ) via a platform support grant (#3456) from the Brain Canada Foundation to PT. Thanks to all of the individuals who participated in this study, and to A.‐M. Audet, and C. Ouellet for their help with participant recruitment and testing.
Tremblay P, Perron M, Deschamps I, et al. The role of the arcuate and middle longitudinal fasciculi in speech perception in noise in adulthood. Hum Brain Mapp. 2019;40:226–241. 10.1002/hbm.24367
Funding information Fonds de la Recherche en Santé du Québec, Grant/Award Number: 27170, 35016; Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: Discovery Grant (#195812603); Québec Bio‐imaging network, Grant/Award Number: Pilot project grant #5886; Brain Canada Foundation, Grant/ Award Number: Platform support grant (#3456)
REFERENCES
- Adank, P. , & Devlin, J. T. (2010). On‐line plasticity in spoken sentence comprehension: Adapting to time‐compressed speech. NeuroImage, 49(1), 1124–1132. 10.1016/j.neuroimage.2009.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, A. L. , Hasan, K. M. , Lazar, M. , Tsuruda, J. S. , & Parker, D. L. (2001). Analysis of partial volume effects in diffusion‐tensor MRI. Magnetic Resonance in Medicine, 45(5), 770–780. 10.1002/mrm.1105 [DOI] [PubMed] [Google Scholar]
- Alexander, D. C. , Barker, G. J. , & Arridge, S. R. (2002). Detection and modeling of non‐Gaussian apparent diffusion coefficient profiles in human brain data. Magnetic Resonance in Medicine, 48(2), 331–340. 10.1002/mrm.10209 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Snyder, A. Z. , Vincent, J. L. , Lustig, C. , Head, D. , Raichle, M. E. , & Buckner, R. L. (2007). Disruption of large‐scale brain Systems in Advanced Aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. , & Song, G. (2011). Advanced Normalization Tools (ANTS) Release 1.5. Penn Image Computing And Science Laboratory University of Pennsylvania. http://stnava.github.io/ANTs/
- Bartzokis, G. (2004). Age‐related myelin breakdown: A developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging, 25(1), 5–18. 10.1016/j.neurobiolaging.2003.03.001 [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , Mattiello, J. , & Lebihan, D. (1994). Estimation of the effective self‐diffusion tensor from the NMR spin Echo. Journal of Magnetic Resonance, 103, 247–254. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 57(1), 289–300. [Google Scholar]
- Bennett, I. J. , & Madden, D. J. (2014). Disconnected aging: Cerebral white matter integrity and age‐related differences in cognition. Neuroscience, 276, 187–205. 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick, R. C. , Friederici, A. D. , Chomsky, N. , & Bolhuis, J. J. (2013). Evolution, brain, and the nature of language. Trends in Cognitive Sciences, 17(2), 89–98. 10.1016/j.tics.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Bilodeau‐Mercure, M. , Lortie, C. L. , Sato, M. , Guitton, M. J. , & Tremblay, P. (2015). The neurobiology of speech perception decline in aging. Brain Structure & Function, 220(2), 979–997. 10.1007/s00429-013-0695-3 [DOI] [PubMed] [Google Scholar]
- Bishop, C. W. , & Miller, L. M. (2009). A multisensory cortical network for understanding speech in noise. Journal of Cognitive Neuroscience, 21(9), 1790–1805. 10.1162/jocn.2009.21118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer, J. , Anwander, A. , Perani, D. , & Friederici, A. D. (2013). Dorsal and ventral pathways in language development. Brain and Language, 127(2), 289–295. 10.1016/j.bandl.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Brown, A. , & Nix, L. (1996). Age‐related changes in the tip‐of‐the‐tongue experience. The American journal of psychology, 109(1), 79–91. [PubMed] [Google Scholar]
- Burke, D. M. , MacKay, D. G. , Worthley, J. S. , & Wade, E. (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579. [Google Scholar]
- Burks, J. D. , Boettcher, L. B. , Conner, A. K. , Glenn, C. A. , Bonney, P. A. , Baker, C. M. , … Sughrue, M. E. (2017). White matter connections of the inferior parietal lobule: A study of surgical anatomy. Brain and Behavior: A Cognitive Neuroscience Perspective, 7(4), e00640. 10.1002/brb3.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan, D. , Callan, A. , Gamez, M. , Sato, M. A. , & Kawato, M. (2010). Premotor cortex mediates perceptual performance. NeuroImage, 51(2), 844–858. doi:S1053‐8119(10)00195‐3 [pii]. 10.1016/j.neuroimage.2010.02.027 [DOI] [PubMed] [Google Scholar]
- Carlson, M. D. A. , & Morrison, R. S. (2009). Study design, precision, and validity in observational studies. Journal of Palliative Medicine, 12(1), 77–82. 10.1089/jpm.2008.9690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , & Ffytche, D. H. (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57, 8–16. [DOI] [PubMed] [Google Scholar]
- Catani, M. , & Mesulam, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , & Thiebaut de Schotten, M. (2012). Atlas of human brain connections. New York: Oxford University Press. [Google Scholar]
- Chang, E. H. , Argyelan, M. , Aggarwal, M. , Chandon, T. S. , Karlsgodt, K. H. , Mori, S. , & Malhotra, A. K. (2017). The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two‐photon microscopy of CLARITY intact brains. NeuroImage, 147, 253–261. 10.1016/j.neuroimage.2016.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton, R. A. , Barrick, T. R. , McIntyre, D. J. , Shen, Y. , O'Sullivan, M. , Howe, F. A. , … Markus, H. S. (2006). White matter damage on diffusion tensor imaging correlates with age‐related cognitive decline. Neurology, 66(2), 217–222. 10.1212/01.wnl.0000194256.15247.83 [DOI] [PubMed] [Google Scholar]
- Concha, L. , Gross, D. W. , Wheatley, B. M. , & Beaulieu, C. (2006). Diffusion tensor imaging of time‐dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage, 32(3), 1090–1099. 10.1016/j.neuroimage.2006.04.187 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia, J. M. , Jansma, B. M. , & Bonte, M. (2015). Decoding articulatory features from fMRI responses in dorsal speech regions. The Journal of Neuroscience, 35(45), 15015–15025. 10.1523/JNEUROSCI.0977-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, M. A. , Girard, G. , Bore, A. , Garyfallidis, E. , Houde, J. C. , & Descoteaux, M. (2013). Tractometer: Towards validation of tractography pipelines. Medical Image Analysis, 17(7), 844–857. 10.1016/j.media.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Cousineau, M. , Jodoin, P. M. , Morency, F. C. , Rozanski, V. , Grand'Maison, M. , Bedell, B. J. , & Descoteaux, M. (2017). A test‐retest study on Parkinson's PPMI dataset yields statistically significant white matter fascicles. Neuroimage Clinical, 16, 222–233. 10.1016/j.nicl.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci, A. , Canales‐Rodrı, E. J. , Descoteaux, M. , Garyfallidis, E. , Gur, Y. , Lin, Y.‐C. , … Ramirez‐Manzanares, A. (2014). Quantitative comparison of reconstruction methods for intra‐voxel fiber recovery from diffusion MRI. IEEE Transactions on Medical Imaging, 33(2), 384–399. [DOI] [PubMed] [Google Scholar]
- De Witt Hamer, P. C. , Moritz‐Gasser, S. , Gatignol, P. , & Duffau, H. (2010). Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Human Brain Mapping, 32, 962–973. 10.1002/hbm.21082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua, F. , Simmons, A. , Williams, S. C. , & Catani, M. (2013). Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true‐tract specific index to characterize white matter diffusion. Human Brain Mapping, 34(10), 2464–2483. 10.1002/hbm.22080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux, M. (2015). High Angular Resolution Diffusion Imaging (HARDI). In J. Webster (Ed.), Wiley Encyclopedia of Electrical and Electronics Engineering (EEEE) (pp. 1–25), John Wiley & Sons, Inc. [Google Scholar]
- Descoteaux, M. , Deriche, R. , Knosche, T. R. , & Anwander, A. (2009). Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Transactions on Medical Imaging, 28(2), 269–286. 10.1109/TMI.2008.2004424 [DOI] [PubMed] [Google Scholar]
- Descoteaux, M. , Wiest‐Daesslé, N. , Prima, S. , Barillot, C. , & Deriche, R. (2008). Impact of Rician adapted non‐local means filtering on HARDI. In Metaxas D., Axel L., Fichtinger G., & Székely G. (Eds.), Medical Image Computing and Computer‐Assisted Intervention – MICCAI 2008: 11th International Conference, New York, NY, USA, September 6–10, 2008, proceedings, part II (pp. 122–130). Berlin, Heidelberg: Springer Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Deutsch, G. K. , Dougherty, R. F. , Bammer, R. , Siok, W. T. , Gabrieli, J. D. , & Wandell, B. (2005). Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex, 41(3), 354–363. [DOI] [PubMed] [Google Scholar]
- Dick, A. S. , Bernal, B. , & Tremblay, P. (2014). The language connectome: New pathways, new concepts. The Neuroscientist, 20(5), 453–467. 10.1177/1073858413513502 [DOI] [PubMed] [Google Scholar]
- Dick, A. S. , & Tremblay, P. (2012). Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain, 135(Pt 12), 3529–3550. 10.1093/brain/aws222 [DOI] [PubMed] [Google Scholar]
- Feder, K. , Michaud, D. , Ramage‐Morin, P. , McNamee, J. , & Beauregard, Y. (2015). Prevalence of hearing loss among Canadians aged 20 to 79: Audiometric results from the 2012/2013 Canadian health measures survey. Health Reports, 26(7), 18–25. [PubMed] [Google Scholar]
- Fegen, D. , Buchsbaum, B. R. , & D'Esposito, M. (2015). The effect of rehearsal rate and memory load on verbal working memory. NeuroImage, 105, 120–131. 10.1016/j.neuroimage.2014.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman, D. A. (2007). Repetition priming in aging and Alzheimer's disease: An integrative review and future directions. Cortex, 43(7), 889–897. [DOI] [PubMed] [Google Scholar]
- Fleysher, R. , Lipton, M. L. , Noskin, O. , Rundek, T. , Lipton, R. , & Derby, C. A. (2017). White matter structural integrity and trans‐cranial Doppler blood flow pulsatility in normal aging. Magnetic Resonance Imaging, 47, 97–102. 10.1016/j.mri.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, L. R. (2002). Characterization of anisotropy in high angular resolution diffusion‐weighted MRI. Magnetic Resonance in Medicine, 47(6), 1083–1099. 10.1002/mrm.10156 [DOI] [PubMed] [Google Scholar]
- Frisina, D. R. , & Frisina, R. D. (1997). Speech recognition in noise and presbycusis: Relations to possible neural mechanisms. Hearing Research , 106(1–2), 95‐104. doi: 10.1016/S0378-5955(97)00006‐3 [DOI] [PubMed] [Google Scholar]
- Garyfallidis, E. , Brett, M. , Amirbekian, B. , Rokem, A. , Van Der Walt, S. , Descoteaux, M. , & Nimmo‐Smith, I. (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8, 1–17. 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, G. A. , & Mills, J. H. (2004). Presbycusis. The Lancet , 366(9491), 1111–1120. doi: 10.1016/S0140-6736(05)67423‐5 [DOI] [PubMed] [Google Scholar]
- Gelfand, S. A. , Piper, N. , & Silman, S. (1985). Consonant recognition in quiet as a function of aging among normal hearing subjects. The Journal of the Acoustical Society of America, 78(4), 1198–1206. [DOI] [PubMed] [Google Scholar]
- Gelfand, S. A. , Piper, N. , & Silman, S. (1986). Consonant recognition in quiet and in noise with aging among normal hearing listeners. The Journal of the Acoustical Society of America, 80(6), 1589–1598. [DOI] [PubMed] [Google Scholar]
- Girard, G. , Whittingstall, K. , Deriche, R. , & Descoteaux, M. (2014a). Towards quantitative connectivity analysis: Reducing tractography biases. NeuroImage, 98, 266–278. 10.1016/j.neuroimage.2014.04.074 [DOI] [PubMed] [Google Scholar]
- Gordon‐Salant, S. (1986). Effects of aging on response criteria in speech‐recognition tasks. Journal of Speech and Hearing Research, 29(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Gordon‐Salant, S. , & Fitzgibbons, P. J. (1993). Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech, Language, and Hearing Research, 36(6), 1276–1285. 10.1044/jshr.3606.1276 [DOI] [PubMed] [Google Scholar]
- Gordon‐Salant, S. , Yeni‐Komshian, G. H. , Fitzgibbons, P. J. , & Barrett, J. (2006). Age‐related differences in identification and discrimination of temporal cues in speech segments. The Journal of the Acoustical Society of America, 119(4), 2455–2466. [DOI] [PubMed] [Google Scholar]
- Gough, P. M. , Nobre, A. C. , & Devlin, J. T. (2005). Dissociating linguistic processes in the left inferior frontal cortex with Transcranial magnetic stimulation. Journal of Neuroscience, 25(35), 8010–8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski, K. , Tremblay, P. , Gracco, V. L. , Girin, L. , & Sato, M. (2013). A mediating role of the auditory dorsal pathway in selective adaptation to speech: A state‐dependent transcranial magnetic stimulation study. Brain Research, 1515, 55–65. 10.1016/j.brainres.2013.03.024 [DOI] [PubMed] [Google Scholar]
- Guenther, F. H. (1994). A neural network model of speech acquisition and motor equivalent speech production. Biological Cybernetics, 72(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Guenther, F. H. (1995). Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychological Review, 102(3), 594–621. [DOI] [PubMed] [Google Scholar]
- Guenther, F. H. , Ghosh, S. S. , & Tourville, J. A. (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96(3), 280–301. doi:S0093‐934X(05)00115‐X [pii]. 10.1016/j.bandl.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon, F. M. , Brickman, A. M. , Cheng, J. C. , & Alexopoulos, G. S. (2009). Aging of cerebral white matter: A review of MRI findings. International Journal of Geriatric Psychiatry, 24(2), 109–117. 10.1002/gps.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann, C. R. G. , Jolesz, F. A. , Kikinis, R. , Killiany, R. J. , Moss, M. B. , Sandor, T. , & Albert, M. S. (1998). White matter changes with normal aging. Neurology, 50(4), 972–978. 10.1212/wnl.50.4.972 [DOI] [PubMed] [Google Scholar]
- Harkrider, A. W. , Plyler, P. N. , & Hedrick, M. S. (2005). Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clinical Neurophysiology, 116(9), 2153–2164. 10.1016/j.clinph.2005.05.016 [DOI] [PubMed] [Google Scholar]
- Hartwigsen, G. , Price, C. J. , Baumgaertner, A. , Geiss, G. , Koehnke, M. , Ulmer, S. , & Siebner, H. R. (2010). The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: Evidence from dual‐site TMS. Neuropsychologia, 48(10), 3155–3163. doi:S0028‐3932(10)00271‐X [pii]. 10.1016/j.neuropsychologia.2010.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach. New York, London: Guilford Press. [Google Scholar]
- Heine, M. K. , Ober, B. A. , & Shenaut, G. K. (1999). Naturally occurring and experimentally induced tip‐of‐the‐tongue experiences in three adult age groups. Psychology and Aging, 14(3), 445–457. [DOI] [PubMed] [Google Scholar]
- Jerger, J. (1992). Can age‐related decline in speech understanding be explained by peripheral hearing loss? Journal of the American Academy of Audiology, 3(1), 33–38. [PubMed] [Google Scholar]
- Jeurissen, B. , Leemans, A. , Tournier, J. D. , Jones, D. K. , & Sijbers, J. (2013). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34(11), 2747–2766. 10.1002/hbm.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly, T. A. , Bateman, G. A. , Levi, C. R. , Parsons, M. W. , Michie, P. T. , & Karayanidis, F. (2013). Early detection of microstructural white matter changes associated with arterial pulsatility. Frontiers in Human Neuroscience, 7, 782 10.3389/fnhum.2013.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki, F. , & Constantinidis, C. (2014). Bottom‐up and top‐down attention: Different processes and overlapping neural systems. The Neuroscientist, 20(5), 509–521. 10.1177/1073858413514136 [DOI] [PubMed] [Google Scholar]
- Le Bihan, D. , Mangin, J. F. , Poupon, C. , Clark, C. A. , Pappata, S. , Molko, N. , & Chabriat, H. (2001). Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging, 13(4), 534–546. [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Lin, F. R. , Thorpe, R. , Gordon‐Salant, S. , & Ferrucci, L. (2011). Hearing loss prevalence and risk factors among older adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 66A(5), 582–590. 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Yang, Y. , Xia, Y. , Zhu, W. , Leak, R. K. , Wei, Z. , … Hu, X. (2017). Aging of cerebral white matter. Ageing Research Reviews, 34, 64–76. 10.1016/j.arr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, G. D. (1990). Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology, 22(1), 1–35. 10.1016/0010-0285(90)90002-L [DOI] [Google Scholar]
- López‐Barroso, D. , Catani, M. , Ripollés, P. , Dell'Acqua, F. , Rodríguez‐Fornells, A. , & de Diego‐Balaguer, R. (2013). Word learning is mediated by the left arcuate fasciculus. Proceedings of the National Academy of Sciences of the United States of America, 110(32), 13168–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan, N. A. , & Creelman, C. D. (1990). Response bias: Characteristics of detection theory, threshold theory, and" nonparametric" indexes. Psychological Bulletin, 107(3), 401–413. [Google Scholar]
- Madden, D. J. , Bennett, I. J. , Burzynska, A. , Potter, G. G. , Chen, N.‐k. , & Song, A. W. (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease, 1822(3), 386–400. 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , & Pandya, D. N. (2009). The extreme capsule in humans and rethinking of the language circuitry. Brain Structure and Function, 213, 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , Papadimitriou, G. M. , Kaiser, J. R. , Sorg, S. , Kennedy, D. N. , & Pandya, D. N. (2009). Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cerebral Cortex, 19(4), 777–785. 10.1093/cercor/bhn124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , Preti, M. G. , Asami, T. , Pelavin, P. , Campbell, B. , Papadimitriou, G. M. , … Kubicki, M. (2013). Human middle longitudinal fascicle: Variations in patterns of anatomical connections. Brain Structure & Function, 218(4), 951–968. 10.1007/s00429-012-0441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , Preti, M. G. , Wassermann, D. , Rathi, Y. , Papadimitriou, G. M. , Yergatian, C. , … Kubicki, M. (2013). Human middle longitudinal fascicle: Segregation and behavioral‐clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi‐tensor tractography. Brain Imaging and Behavior, 7(3), 335–352. 10.1007/s11682-013-9235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, I. L. , de Champfleur, N. M. , Velut, S. , Destrieux, C. , Zemmoura, I. , & Duffau, H. (2013). Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. Journal of Anatomy, 223(1), 38–45. 10.1111/joa.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, I. L. , Moritz‐Gasser, S. , & Duffau, H. (2011). Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Structure & Function, 216(3), 263–274. 10.1007/s00429-011-0309-x [DOI] [PubMed] [Google Scholar]
- Mandonnet, E. , Nouet, A. , Gatignol, P. , Capelle, L. , & Duffau, H. (2007). Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain, 130(Pt 3), 623–629. 10.1093/brain/awl361 [DOI] [PubMed] [Google Scholar]
- Marner, L. , Nyengaard, J. R. , Tang, Y. , & Pakkenberg, B. (2003). Marked loss of myelinated nerve fibers in the human brain with age. The Journal of Comparative Neurology, 462(2), 144–152. 10.1002/cne.10714 [DOI] [PubMed] [Google Scholar]
- Mazelová, J. , Popelar, J. , & Syka, J. (2003). Auditory function in presbycusis: Peripheral vs. central changes. Experimental Gerontology , 38(1–2), 87–94. doi: 10.1016/S0531-5565(02)00155‐9 [DOI] [PubMed] [Google Scholar]
- Meister, I. G. , Wilson, S. M. , Deblieck, C. , Wu, A. D. , & Iacoboni, M. (2007). The essential role of premotor cortex in speech perception. Current Biology, 17, 1692–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menjot de Champfleur, N. , Lima Maldonado, I. , Moritz‐Gasser, S. , Machi, P. , Le Bars, E. , Bonafe, A. , & Duffau, H. (2013). Middle longitudinal fasciculus delineation within language pathways: A diffusion tensor imaging study in human. European Journal of Radiology, 82(1), 151–157. 10.1016/j.ejrad.2012.05.034 [DOI] [PubMed] [Google Scholar]
- Meyer, J. , Dentel, L. , & Meunier, F. (2013). Speech recognition in natural background noise. PLoS One, 8(11), e79279. 10.1371/journal.pone.0079279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. A. (1998). Developmental research methods (2nd ed.). Englewood Cliffs, NJ, US: Prentice‐Hall, Inc. [Google Scholar]
- Mito, R. , Raffelt, D. , Dhollander, T. , Vaughan, D. N. , Tournier, J. D. , Salvado, O. , … Connelly, A. (2018). Fibre‐specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain, 141, 888–902. 10.1093/brain/awx355 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. , Peters, R. W. , & Stone, M. A. (1999). Benefits of linear amplification and multichannel compression for speech comprehension in backgrounds with spectral and temporal dips. The Journal of the Acoustical Society of America, 105(1), 400–411. [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nilsson, M. , Soli, S. D. , & Sullivan, J. A. (1994). Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. The Journal of the Acoustical Society of America, 95(2), 1085–1099. [DOI] [PubMed] [Google Scholar]
- Niogi, S. N. , & McCandliss, B. D. (2006). Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia, 44(11), 2178–2188. 10.1016/j.neuropsychologia.2006.01.011 [DOI] [PubMed] [Google Scholar]
- O'Brien, L. M. , Ziegler, D. A. , Deutsch, C. K. , Frazier, J. A. , Herbert, M. R. , & Locascio, J. J. (2011). Statistical adjustments for brain size in volumetric neuroimaging studies: Some practical implications in methods. Psychiatry Research, 193(2), 113–122. 10.1016/j.pscychresns.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–112. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, M. , Jones, D. K. , Summers, P. E. , Morris, R. G. , Williams, S. C. , & Markus, H. S. (2001). Evidence for cortical "disconnection" as a mechanism of age‐related cognitive decline. Neurology, 57(4), 632–638. [DOI] [PubMed] [Google Scholar]
- Pakkenberg, B. , & Gundersen, H. J. G. (1997). Neocortical neuron number in humans: Effect of sex and age. The Journal of Comparative Neurology, 384(2), 312–320. [DOI] [PubMed] [Google Scholar]
- Plomp, R. , & Mimpen, A. M. (1979). Speech‐reception threshold for sentences as a function of age and noise level. The Journal of the Acoustical Society of America, 66(5), 1333–1342. [DOI] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36(4), 717–731. 10.3758/bf03206553 [DOI] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. 10.3758/brm.40.3.879 [DOI] [PubMed] [Google Scholar]
- Raffelt, D. , Tournier, J. D. , Rose, S. , Ridgway, G. R. , Henderson, R. , Crozier, S. , … Connelly, A. (2012). Apparent fibre density: A novel measure for the analysis of diffusion‐weighted magnetic resonance images. NeuroImage, 59(4), 3976–3994. 10.1016/j.neuroimage.2011.10.045 [DOI] [PubMed] [Google Scholar]
- Rastle, K. G. , & Burke, D. M. (1996). Priming the tip of the tongue: Effects of prior processing on word retrieval in young and older adults. Journal of Memory and Language, 35(4), 586–605. [Google Scholar]
- Reil, J. (1809). Die Sylvische Grube oder das Thal, das gestreifte grobe hirnganglium, dessen kapsel und die seitentheile des grobn gehirns. Archives of Physiology, 9, 195–208. [Google Scholar]
- Rheault, F. , Houde, J.‐C. , Goyette, N. , Morency, F. , & Descoteaux, M . (2016). MI‐brain, a software to handle tractograms and perform interactive virtual dissection. Paper presented at the Proceeding of: Breaking the barriers of diffusion MRI (ISMRM workshop), Lisbon, Portugal. Available at: https://www.ismrm.org/workshops/Diffusion16/program.htm [Google Scholar]
- Robinson, K. , Schmidt, T. , & Teti, D. M. (2008). Issues in the Use of Longitudinal and Cross‐Sectional Designs In Douglas M. T. (Ed.), Handbook of Research Methods in Developmental Science (pp. 1–20). Hoboken, New Jersey: Blackwell Publishing Ltd. [Google Scholar]
- Romero, L. , Walsh, V. , & Papagno, C. (2006). The neural correlates of phonological short‐term memory: A repetitive transcranial magnetic stimulation study. Journal of Cognitive Neuroscience, 18(7), 1147–1155. 10.1162/jocn.2006.18.7.1147 [DOI] [PubMed] [Google Scholar]
- Sampaio‐Baptista, C. , & Johansen‐Berg, H. (2017). White matter plasticity in the adult brain. Neuron, 96(6), 1239–1251. 10.1016/j.neuron.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, M. , Tremblay, P. , & Gracco, V. L. (2009). A mediating role of the premotor cortex in phoneme segmentation. Brain and Language, 111(1), 1–7. 10.1016/j.bandl.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Kreher, B. W. , Schnell, S. , Kümmerer, D. , Kellmeyer, P. , Vry, M.‐S. , … Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the USA, 105(46), 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter, D. L. , & Buckner, R. L. (1998). Priming and the brain. Neuron, 20(2), 185–195. [DOI] [PubMed] [Google Scholar]
- Seltzer, B. , & Pandya, D. N. (1984). Further observations on parieto‐temporal connections in the rhesus monkey. Experimental Brain Research, 55(2), 301–312. [DOI] [PubMed] [Google Scholar]
- Sen, P. N. , & Basser, P. J. (2005). A model for diffusion in white matter in the brain. Biophysical Journal, 89(5), 2927–2938. 10.1529/biophysj.105.063016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman, M. A. , Cohen‐Adad, J. , Descoteaux, M. , Messe, A. , Benali, H. , & Lehericy, S. (2011). Impact of outliers on diffusion tensor and Q‐ball imaging: Clinical implications and correction strategies. Journal of Magnetic Resonance Imaging, 33(6), 1491–1502. 10.1002/jmri.22577 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Soares, J. M. , Marques, P. , Alves, V. , & Sousa, N. (2013). A hitchhiker's guide to diffusion tensor imaging. Front Neurosci, 7, 1–14. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach, B. A. (2010). Clinical audiology: An introduction. Clifton Park, NY, USA: Delmar. [Google Scholar]
- Steffens, D. C. , Wang, L. , Manning, K. J. , & Pearlson, G. D. (2017). Negative affectivity, aging, and depression: Results from the neurobiology of late‐life depression (NBOLD) study. The American Journal of Geriatric Psychiatry, 25, 1135–1149. 10.1016/j.jagp.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse, A. , Ashmead, D. H. , Ohde, R. N. , & Grantham, D. W. (1998). Temporal processing in the aging auditory system. The Journal of the Acoustical Society of America, 104(4), 2385–2399. [DOI] [PubMed] [Google Scholar]
- Thiebaut de S.M., Ffytche, D. H. , Bizzi, A. , Dell'Acqua, F. , Allin, M. , Walshe, M. , . . . Catani, M. (2011). Atlasing location, asymmetry and inter‐subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage , 54(1), 49–59. doi: 10.1016/j.neuroimage.2010.07.055 [DOI] [PubMed] [Google Scholar]
- Toscano, J. C. , & Lansing, C. R. (2017). Age‐related changes in temporal and spectral cue weights in speech. Lang Speech, 1–19. 10.1177/0023830917737112 [DOI] [PubMed] [Google Scholar]
- Tournier, J. D. , Calamante, F. , & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. NeuroImage, 35(4), 1459–1472. 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Tournier, J. D. , Calamante, F. , Gadian, D. G. , & Connelly, A. (2004). Direct estimation of the fiber orientation density function from diffusion‐weighted MRI data using spherical deconvolution. NeuroImage, 23(3), 1176–1185. 10.1016/j.neuroimage.2004.07.037 [DOI] [PubMed] [Google Scholar]
- Tournier, J.‐D. , Mori, S. , & Leemans, A. (2011). Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine, 65(6), 1532–1556. 10.1002/mrm.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, K. L. , Piskosz, M. , & Souza, P. (2002). Aging alters the neural representation of speech cues. Neuroreport, 13(15), 1865–1870. [DOI] [PubMed] [Google Scholar]
- Tremblay, K. L. , Piskosz, M. , & Souza, P. (2003). Effects of age and age‐related hearing loss on the neural representation of speech cues. Clinical Neurophysiology, 114(7), 1332–1343. [DOI] [PubMed] [Google Scholar]
- Tun, P. A. (1998). Fast noisy speech: Age differences in processing rapid speech with background noise. Psychology and Aging, 13(3), 424–434. 10.1037/0882-7974.13.3.424 [DOI] [PubMed] [Google Scholar]
- Tun, P. A. , & Wingfield, A. (1999). One voice too many: Adult age differences in language processing with different types of distracting sounds. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 54(5), P317–P327. [DOI] [PubMed] [Google Scholar]
- Vallesi, A. , McIntosh, A. R. , & Stuss, D. T. (2011). Overrecruitment in the aging brain as a function of task demands: Evidence for a compensatory view. Journal of Cognitive Neuroscience, 23(4), 801–815. 10.1162/jocn.2010.21490 [DOI] [PubMed] [Google Scholar]
- Vandermosten, M. , Boets, B. , Poelmans, H. , Sunaert, S. , Wouters, J. , & Ghesquière, P. (2012). A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain, 135(3), 935–948. 10.1093/brain/awr363 [DOI] [PubMed] [Google Scholar]
- Voineskos, A. N. , Lobaugh, N. J. , Bouix, S. , Rajji, T. K. , Miranda, D. , Kennedy, J. L. , … Shenton, M. E. (2010). Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain, 133(Pt 5), 1494–1504. 10.1093/brain/awq040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos, A. N. , Rajji, T. K. , Lobaugh, N. J. , Miranda, D. , Shenton, M. E. , Kennedy, J. L. , … Mulsant, B. H. (2012). Age‐related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiology of Aging, 33(1), 21–34. 10.1016/j.neurobiolaging.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel, S. , Geng, J. J. , & Fink, G. R. (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. The Neuroscientist, 20(2), 150–159. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Fernandez‐Miranda, J. C. , Verstynen, T. , Pathak, S. , Schneider, W. , & Yeh, F. C. (2013). Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cerebral Cortex, 23(10), 2347–2356. 10.1093/cercor/bhs225 [DOI] [PubMed] [Google Scholar]
- Wassermann, D. , Makris, N. , Rathi, Y. , Shenton, M. , Kikinis, R. , Kubicki, M. , & Westin, C.‐F. (2013). On describing human white matter anatomy: The white matter query language. Med Image Comput Comput Assist Interv., 16(01), 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann, D. , Makris, N. , Rathi, Y. , Shenton, M. , Kikinis, R. , Kubicki, M. , & Westin, C.‐F. (2016). The white matter query language: A novel approach for describing human white matter anatomy. Brain Structure and Function, 221, 1–17. 10.1007/s00429-015-1179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, K. S. , Yeatman, J. D. , & Wandell, B. A. (2016). The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex, 97, 274–276. 10.1016/j.cortex.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler‐Kingshott, C. A. M. , & Cercignani, M. (2009). About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine, 61(5), 1255–1260. 10.1002/mrm.21965 [DOI] [PubMed] [Google Scholar]
- Wong, P. C. , Jin, J. X. , Gunasekera, G. M. , Abel, R. , Lee, E. R. , & Dhar, S. (2009). Aging and cortical mechanisms of speech perception in noise. Neuropsychologia, 47(3), 693–703. 10.1016/j.neuropsychologia.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. C. M. , Uppunda, A. K. , Parrish, T. B. , & Dhar, S. (2008). Cortical mechanisms of speech perception in noise. Journal of Speech, Language, and Hearing Research, 51(4), 1026–1041. 10.1044/1092-4388(2008/075) [DOI] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Rykhlevskaia, E. , Sherbondy, A. J. , Deutsch, G. K. , Wandell, B. A. , & Ben‐Shachar, M. (2011). Anatomical properties of the Arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience, 23(11), 3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. , & Leirer, V. O. (1983). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.


