Abstract
The human gustatory cortex analyzes the chemosensory properties of tastants, particularly the quality, intensity, and affective valence, to determine whether a perceived substance should be ingested or rejected. Among previous studies, the spatial distribution of taste intensity‐related activations within the human insula has been scarcely addressed. To spatially characterize a specialized or distributed nature of the cortical responses to taste intensities, a functional magnetic resonance imaging study was performed at 3 T in 44 healthy subjects where sweet and bitter tastants were administered at five increasing concentrations and cortex‐based factorial and parametric analyses were performed. Two clusters in the right middle‐posterior and left middle insula were found specialized for taste intensity processing, exhibiting a highly nonlinear profile across concentrations. Multiple clusters were found activated by sweet and bitter taste stimuli at most concentrations, in the anterior, middle‐posterior, and inferior portion of the bilateral insula. Across these clusters, respectively, for the right and left insula, a superior‐to‐inferior and an anterior‐to‐posterior spatial gradient for high‐to‐low concentrations were observed for the most responsive intensity of both tastes. These findings may gather new insights regarding how the gustatory cortex is spatially organized during the perceptual processing of taste intensity for two basic tastants.
Keywords: taste, intensity, primary gustatory cortex, spatial distribution, sweet, bitter, fMRI
1. INTRODUCTION
The human gustatory system is evolutionary organized to analyze and code the tastants, based on their chemosensory and somatosensory features, as well as their hedonic properties, to promptly determine whether the carrier substances should be ingested, because of their energetic or nutritive content, or, rather, avoided and rejected, due to possible contamination and potential poisoning effects (Gutierrez & Simon, 2011; Jezzini, Mazzucato, La Camera, & Fontanini, 2013; Yeomans, 1998).
It is well established that humans can discriminate at least five basic tastes (sweet, bitter, sour, salty, and umami), and that the quality of a taste is assigned with a hedonic value which determines selective behaviors (Wang et al., 2018). However, during the processing of taste features, the gustatory system also needs to distinguish the intensity (or concentration) of a given tastant. In fact, when increasing or decreasing the intensity of a tastant, the perceived expected quantity may possibly change the predictable consequences of the ingestion itself. Thereby, the intensity information should be also analyzed by the gustatory system and eventually coded during taste perception (Scott, 2004). However, the processing of taste stimuli is not only determined by the physical characteristics of the presented stimuli, but also by other cooccurrent factors such as expectation (Wilton, Stancak, Giesbrecht, Thomas, & Kirkham, 2018) and subjective motivation (Wegman, van Loon, Smeets, Cools, & Aarts, 2018). For instance, taste‐expectation interactions have been highlighted in several previous studies (Nitschke et al., 2006; Sarinopoulos, Dixon, Short, Davidson, & Nitschke, 2006), and it has also been previously shown that top‐down information processes can be elicited by taste stimuli specifically affecting the response of the primary gustatory cortex (Wilton et al., 2018).
The cortical processes underlying the intensity‐related perception of taste have been scarcely investigated in humans. Particularly, a detailed spatial pattern of intensity‐related activations within the human insular cortex, which is the putative location of the primary gustatory cortex (Small, 2010), in response to parametrically varied intensities of a taste stimulus, is currently lacking in the literature and some relevant inconsistencies were noted among previous reports. For example, among previous human studies using functional magnetic resonance imaging (fMRI) with taste stimulation, a work by Dalenberg, Hoogeveen, Renken, Langers, and ter Horst (2015) has provided a detailed factorial analysis of the insular responses to taste quality, concentration, and affective valence, demonstrating that the left insula is more specialized for detecting the quality of a tastant and its affective valence, independently of the concentration, whereas the right insula, and particularly the dysgranular anterior part of it, is more sensitive to intensity variations albeit according to a nonlinear response. However, for what concerns the nonlinear profile of the responses to taste intensity no further details and no definitive interpretations were provided. More recently, the study of Yeung, Tanabe, Suen, and Goto (2016) demonstrated that the processing of taste intensity in the right hemisphere may also exert a top‐down stimulatory effect from the middle insula to the thalamic activity, which might in turn modulate the response to taste intensity via a psycho‐physiological interaction.
In addition, the pilot study of Prinster et al. (2017) has suggested the possibility that the right insular cortex, while not being necessarily specialized for detecting the quality of each basic tastant, may still exhibit a different spatial distribution of neural activation in relation to the perceived quality of a tastant, albeit the possible modulating effect of concentration was not investigated.
The lateralized specialization of the right hemisphere for the concentration‐related processing was partly inconsistent with two previous studies, respectively from Small et al. (2003) and Spetter, Smeets, de Graaf, and Viergever (2010), reporting evidence that: (a) both the left and right middle insula regions can be sensitive to taste intensity (irrespective of valence) (Small et al., 2003), and (b) the bilateral middle insula activation linearly increases across four different concentrations (Spetter et al., 2010), whereas the findings in Dalenberg et al. (2015) highlighted a nonlinear (quadratic) trend of the functional activity in the right insula in response to the varying concentrations. On the other hand, some electrophysiological studies have been performed in animals, analyzing the neuronal firing rates in the insula after the injection of tastes at different concentrations, which reported both monotonically increasing (Scott, Plata‐Salamán, Smith‐Swintosky, & Giza, 1991) and far more complex (nonlinear) changes (Stapleton, Lavine, Wolpert, Nicolelis, & Simon, 2006) of firing rates with the intensity of taste.
To possibly shed more light about the effects of taste intensity on the neural activity within the human insular cortex, the aim of the present study was to analyze and report the detailed spatial distribution of neural activity elicited by the perception of five different concentrations by using fMRI in 44 normal subjects. Similar to Small et al. (2003), we administered two tastes with opposite valence (sweet and bitter). Similar to Dalenberg et al. (2015), we used five different concentrations of each tastant, which were carefully chosen after preliminary offline tests on a separate cohort of subjects in such a way that, besides the correct detection of the expected quality and valence, all parametrically increased concentrations were reported as linearly increasing intensity of taste perception during random injections. To maximally reduce the duration of the tasting phase and to minimize the impact of tongue movements during taste perception (Marciani et al., 2006), an event‐related stimulation paradigm was designed by relying on the rapid injection (600 ms) of small volumes (1 mL) of tastants. Moreover, to attain statistical assumptions preserving as much as possible the intrinsic spatial resolution of the original fMRI data in determining the cortical distribution of intensity‐related taste activations, only modest spatial smoothing with a kernel size of 4 mm full width at half maximum (FWHM) was applied to the fMRI images. Finally, to maximize the spatial correspondence of the insular cortex across all individual brains, a high resolution cortical registration method was applied.
2. MATERIALS AND METHODS
2.1. Participants
Forty‐eight healthy adult volunteers (mean age ± SD = 25 ± 4 years) were enrolled in this study on the basis of a written informed consent. Participation was in accordance with the requirements of the local ethical committee at the University Hospital “San Giovanni Di Dio e Ruggi D'Aragona” of Salerno. Included participants were right handed, nonsmoker and without any history of taste, smell, neurological, and psychiatric disorders. Participants using any form of medication that possibly affected taste perception (i.e., gastrointestinal complaints, dry mouth, nausea, and taste disturbance) were not enrolled in the study. Participants were instructed not to eat or drink during 2 h prior to the scanning session (only plain water allowed). The eating behavior of each participant was assessed with the “PREDIMED test” (Martınez‐Gonzalez et al., 2012).
2.2. Experimental procedure
In order to choose an optimal set of increasing concentrations, we enrolled a sample of 13 young healthy subjects (mean age ± SD = 25 ± 6 years) to perform a preliminary offline study consisting on a visual assessment score (VAS) test. This test was organized as two separate sessions for sweet and bitter tastes. In these sessions, each subject received 1 mL of tastant with a concentration randomly chosen among eight prepared concentrations followed by 1 mL of water for rewash. Prior to the examination, using a category scale (Lawless & Heymann, 2010), each subject was instructed to report a score between 0 and 10 mm, respectively, for very low perceived intensity and very high perceived intensity with respect to a neutral solution (water). The injection of all the concentrations was performed with a homemade gustometer (Canna et al., 2018) and each concentration was presented twice. For each repetition, the order of the concentrations was randomly shuffled.
The starting sets of solutions (prepared in water) included 50, 60, 117, 245, 447, 658, 800, and 976 mM of sucrose for the sweet taste and 0.05, 0.06, 0.12, 0.25, 0.50, 0.75, 1.00, and 1.25 mM of quinine hydrochloride for the bitter taste. To establish the final set of five concentrations for the fMRI experiment, for each taste separately, a two‐way analysis of variance (2w‐ANOVA) model was defined using concentration and repetition as within‐subject factors and post hoc pair‐wise comparisons were performed to discard those consecutive concentrations that did not yield a significant difference in the intensity perception (p > .05).
In order to determine the positive and negative valence of each taste at the concentrations selected for the fMRI experiment, we enrolled another group of 30 healthy subjects (mean age ± SD = 21 ± 3 years) to perform the VAS test twice with different valence prompting. Namely, the test was performed in two separate sessions for sweet and bitter tastes, consisted on the injection of 1 mL of tastant with randomly chosen concentration, followed by 1 mL of water for rewash, and, after tastant injection, participants were asked to report a score between 0 and 10 mm for both the perceived pleasant (positive) and the perceived unpleasant (negative) valence in relation to each concentration.
The taste solutions for both the offline and MRI acquisition were prepared the day before the examinations and stored at controlled low temperature (4 °C) for the night. Two hours before the scanning, they were stored at room temperature (~20°) to keep the same starting condition for all subjects. Solutions were brought to the site of experiment in a polystyrene box and the filling of the tubes for all the experiments were carried out 10 min before starting the acquisitions.
All subjects enrolled for the fMRI experiment were asked to rate the selected intensities, while a subgroup of 24 subjects were also asked to rate the pleasantness and unpleasantness related to each selected taste and concentration using the same VAS test.
The offline tests were performed the day before the fMRI experiment with the same gustometer used for the fMRI acquisition, albeit only one repetition of the stimuli was presented. In addition, during these offline tests, we were able to preliminarily determine whether subjects were able to discriminate the selected concentrations of sweet and bitter solutions.
Finally, prior to the scanning session, we instructed all enrolled subjects for the fMRI experiment. First of all, we specifically trained each subject to swish and immediately swallow after each taste and rewashing injection. Then, we also asked each subject to: (a) passively taste all the stimuli, without trying to actively discriminate the incoming concentrations that were always presented in a shuffled order (six times for each concentration) and (b) stay awake for the entire scanning session.
Although we injected small quantities of taste for each concentration (1 mL), after the two sessions of sweet and bitter taste experiment, we also asked subjects whether tastants were perceived at different intensities across all the stimulus presentations.
2.3. MRI data acquisition
MRI image data sets were acquired on a 3 T MRI scanner (MAGNETOM Skyra, Siemens, Erlangen Germany) equipped with a 20‐channel radio‐frequency receive head coil. The imaging protocol consists of two identical fMRI sessions, the first of which was performed during the morning (between 9:00 and 12:00 a.m.) and the other during the afternoon (between 3:00 and 6:00 p.m.). During the first session, we used sweet taste solutions while in the second session we used bitter taste (more detailed are provided below). Each session included a three‐dimensional anatomical T1‐weighted magnetization prepared rapid gradient echo sequence with repetition time/echo time (TR/TE) = 2400/2.25 ms, resolution = 1 mm isotropic, matrix size = 256 × 256, 192 slices, a gradient‐echo echo‐planar imaging (GRE‐EPI) with a multiband (MB) factor of 4 (MB‐EPI (Feinberg et al., 2010; Moeller et al., 2011; Xu et al., 2013), TE = 30 ms, TR = 1,000 ms, matrix size = 96 × 96, voxel size = 2.5 × 2.5 × 2.5 mm3, 60 slices, 910 dynamic scans, direction of phase encoding acquisition anterior–posterior. The same GRE‐EPI series was repeated two more times with only five dynamic scans and opposite (anterior–posterior, posterior–anterior) phase encoding directions for the purpose to correct GRE‐EPI image distortions (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004). Each scanning session was 20 min long: 15 min for functional acquisition, 5 min for anatomical acquisition.
Synchronization between fMRI acquisition and stimulus delivery was controlled using Presentation software (Neurobehavioral Systems, Inc., Berkeley, CA, http://www.neurobs.com).
Stimulus delivery was performed with our custom‐built gustometer based on an Arduino microcontroller (Canna et al., 2018). The device controls six peristaltic micropumps for the rapid injection of small volumes of solution with a range of flow rates from 0 to 200 mL/min. The digital communication between the stimulation computer and the microcontroller is hosted by a serial port interface. Each micropump is connected to a polyvinylchloride tube (inner and outer diameters equal to 1/16 and 1/8 in, respectively), that ends to a custom‐made mouthpiece leaning on the mouth of the subject.
Our stimulation protocol has been designed as a time‐resolved event‐related protocol, during which we injected 1 mL of taste in 600 ms. The flow rate of each micropump was set to 100 mL/min. The picture describing a single injection phase of the experimental protocol is shown in Figure 1; it contains four different stimuli: two visual cues anticipating two taste injections, respectively, of taste and of water for rewashing. A single injection phase is repeated five times to deliver all of the taste concentrations. This latter block of injections of all the concentrations is repeated six times and during each repetition, the order of concentrations was shuffled.
Figure 1.
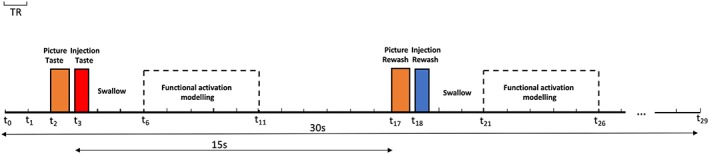
Representation of one block containing the timings of the visual (orange) and taste/rewash injections (red/blue) events. The dashed blocks represent the intervals when the fMRI data responses are modeled. In particular, t2 is the starting time of the taste‐related visual cue: the word “taste” is presented on a video display unit for 800 ms; t3 is the starting time of the taste injection: the pump of the gustometer containing taste is activated for 600 ms and 1 mL of solution with a specific taste is delivered; t17 is the starting time of the rewash‐related visual cue: the word “rewash” is presented on a video display unit for 800 ms; t18 is the starting time of the water‐injection: the pump of the gustometer containing water is activated for 600 ms; the times between t6 and t11 as well as between t21 and t26 are the intervals of the functional activation modeling (delays analyzed in the deconvolution GLM) for both taste and rewashing phases. The swallowing events start after taste and rewash injections (t3 + 600 ms and t18 + 600 ms). fMRI, functional magnetic resonance imaging; GLM, general linear model [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. MRI data processing
Image data preparation, (part of) preprocessing, and statistical analysis were performed in BrainVoyager, (Brain Innovation, Maastricht, The Netherlands, http://www.brainvoyager.com). GRE‐EPI image distortion correction was performed using the tool TOPUP from the FMRI Software Library (FSL; http://fsl.fmrib.ox.ac.uk/fsl). Additional analyses were performed using custom scripts written in MATLAB R2016a (The MathWorks Inc., Natick, MA, http://www.themathworks.com).
For each individual subject, the anatomical T1w images of the first and second sessions were skull stripped and corrected for intensity inhomogeneities. The T1w images from the first scan were transformed to Talairach space. The T1w images from the second scan were aligned to the AC (anterior commissure)‐PC (posterior commissure) transformed T1w images of the first scan via a six‐parameter affine transformation and then the same Talairach transformation was applied. In Talairach space, the images of the two scans were averaged, yielding an anatomical image with higher signal to noise. The average T1w image was then used for segmentation and cortical surface reconstruction, yielding the initial surface meshes for the cortex‐based alignment (CBA) procedure (Frost & Goebel, 2012).
In order to perform a cortex‐based data analysis, the gray/white matter boundary was segmented using the automatic region‐growing methods based on the analysis of intensity histogram, as implemented in BrainVoyager. Morphological operations were used to smooth the borders of the segmented data and to separate the left from the right hemisphere. The obtained segmented hemispheres were then submitted to a “bridge removal” procedure, which ensures the reconstruction of maximally topologically correct mesh representations (Kriegeskorte & Goebel, 2001). Segmented images were also carefully inspected, and, when needed, manually corrected to avoid residual topological errors and further improve mesh reconstructions. The borders of the segmented hemispheres were tessellated along the border to produce a (folded) surface mesh of each hemisphere which was morphed to an inflated mesh while keeping the link to the original reference (folded) mesh and to guarantee the correct localization of functional data. We used all default parameter settings of the CBA procedure and, particularly, chose the “moving target” approach, according to which, all individual brains are iteratively aligned to a dynamically updated average brain only based on the cortical folding patterns. A folding pattern represents the alternation of gyri and sulci of the brains and can be quantitatively assessed by calculating the curvature values from the shape analysis of the surface mesh. During the CBA procedure, the algorithm essentially aligns as much as possible corresponding gyri and sulci among individual brains with the result of reducing the anatomical intersubject variability (Frost, Esposito, & Goebel, 2014; Frost & Goebel, 2012; Goebel, Esposito, & Formisano, 2006). The CBA procedure is identically repeated for each separate hemisphere, allowing the mapping of the individual cortical surface mesh vertices to an average cortical surface mesh.
2.5. FMRI data analysis
MB‐EPI time series from both sessions were corrected for the different slice scan acquisition times using a cubic spline interpolation procedure and for movement artifacts by a rigid realignment of all the volumes to the first volume based on a Levenberg–Marquardt algorithm, optimizing three translation and three rotation parameters on a resampled version of each image. The estimated motion parameters were carefully inspected to control that no excessive residual motion was present (more than 2 mm in head translation or more than 2° in head rotation, see Figure S1). In order to test the possible synchronization between the swallowing phases and the head movements, for each subject, the swallowing phase signals (modeled as a boxcar function in the intervals of 2 s after each taste and rewashing injections) were considered as predictor of the motion trend in a general linear model (GLM) analysis of the estimated motion parameters.
The head motion‐corrected time series were then exported to NIFTI image format for the GRE‐EPI distortion correction which was performed with the TOPUP tool of FSL (Andersson et al., 2003; Smith et al., 2004). Then, the fMRI time series were filtered in the temporal domain using a high‐pass filter with cutoff set to 0.008 Hz to reduce linear and nonlinear trends in the time courses and were spatially smoothed with a 4 mm FWHM Gaussian kernel. Finally, all images were transformed to Talairach space, with a resampling to 2 mm isotropic voxels using the trilinear interpolation to achieve better accuracy/precision in the registration between functional and anatomical data set before the normalization and to minimize as much as possible the interpolation errors during the normalization procedure (Mahmoudzadeh & Kashou, 2013). Thus, the folded cortex meshes were used to sample the functional data at each vertex resulting in one mesh time course data set for each scan and each subject.
In order to focus the fMRI data analysis on the insular cortex, two 1 mm resolution volume masks covering the left and right insulae were imported in Talairach space from the Harvard Oxford atlas (originally available in the Montreal Neurological Institute space from the FSL). The volume masks were projected from the volume space to the target brain mesh resulting in two cortical patches of interest (POIs) on this mesh. To ensure that no insular regions were missing in the projection, a slight dilation of three vertices was applied to each POI.
The first and second level analyses were performed separately for the left and right insular POIs. For the first level analysis, a single study deconvolution‐based GLM was applied to the mesh time courses with “stick” predictors defined over each interval of 13 s (corresponding to 13 time points) from the time of injection. In this way, we defined 13 predictors of interest for each concentration of each tastant covering the taste perception response phase (before the injection of plain water for rewashing). Additional predictors (of no interest) were added to the GLM to model the responses during the rewash phases. The six motion parameters were also added in the GLM model as confound predictors. A correction for serial correlation was applied using a fit–refit procedure with a second‐order autoregressive model applied to the GLM residuals. Z‐transformation was applied to the time courses before the GLM fitting. For the second‐level analysis, a random‐effects (RFX) GLM was applied and the main effects of all five predictors corresponding to the five delays between 4 and 8 s (i.e., around the expected peak of the BOLD response) were combined into one single contrast per concentration and per taste, and mapped onto the target mesh. A 2w‐ANOVA was also performed, considering taste and concentration as within‐subject factors with, respectively, two (sweet, bitter) and five (all concentrations) levels. A statistical threshold was applied to the F‐ and t‐maps, which protected against false‐positive clusters at 5% (cluster‐level corrected for multiple comparisons over the mesh after 1,000 Monte Carlo simulations (Forman et al., 1995), performed with the tool implemented in BrainVoyager, and cluster‐forming threshold set to p = .001 according to Eklund, Nichols, and Knutsson (2016).
To display the cortical distribution of concentrations to which the insular regions are most responsive, for the two tastes separately, we calculated the group‐level maps of most responsive concentrations (Prinster et al., 2017) by assigning to each mesh vertex a different color code in relation to the specific concentration yielding the highest response as quantified by the t‐score from the concentration‐specific contrast in the RFX–GLM analysis (blue: first concentration, light blue: second concentration, green: third concentration, yellow: fourth concentration, and red: fifth concentration). Albeit only descriptive, the maps of most responsive concentrations allow to give an idea about if (and how) the maximal responses among the different concentrations tend to be spatially organized within the insular regions according to a spatial gradient from low to high concentrations, and to eventually suggest the direction of this gradient. To preserve as much as possible the spatial details, maps of most responsive concentrations were examined both with and without spatial smoothing.
3. RESULTS
3.1. Behavioral results
From the offline test performed on 13 subjects (not enrolled in the fMRI experiment), Figure S2 shows the trends of VAS scores across eight preliminary chosen concentrations for sweet and bitter tastes. Particularly, for both tastes, the intensity perceived by the group of subjects clearly exhibits an S‐shaped curve that refers to the psychophysical function described in the model of Beidler (1961) that reports an initial flat portion of low‐perceived intensities, an intermediate step rise, and a final flat region where the solutions' concentration saturates the intensity perception.
Thereby, from the initial set of concentrations, the following five were chosen for the fMRI experiments: sweet 50, 117, 245, 447, and 658 mM and for bitter 0.06, 0.25, 0.50, 0.75, and 1.25 mM. The t and p values of the significant statistical tests for the pair‐wise comparisons of consecutive concentrations are reported in Table 1. When comparing the ratings obtained by the offline test in the group of the enrolled subjects for the fMRI experiment, all the consecutive pairs of the selected concentrations showed significant differences (Table 2).
Table 1.
Results from the offline VAS test performed with sweet (a) and bitter (b) tastes in the group of subjects not enrolled in the fMRI experiment
| Concentrations | t (df = 24) | p | |
|---|---|---|---|
| a | |||
| Sweet concentrations | 1–3 | 2.45 | .02 |
| 3–4 | 3.41 | .001 | |
| 4–5 | 5.4 | <.001 | |
| 5–6 | 3.16 | .003 | |
| b | |||
| Bitter concentrations | 2–4 | 4.36 | <.001 |
| 4–5 | 3.19 | .002 | |
| 5–6 | 3.28 | .002 | |
| 6–8 | 3.86 | <.001 | |
Abbreviations: fMRI = functional magnetic resonance imaging; VAS = visual assessment score.
Note. Results from the offline VAS test with sweet (a) and bitter (b) tastes reporting the significant effects of concentrations in the group of thirteen subjects not enrolled in the fMRI experiment. The t and p values of the significant statistical tests for the pair‐wise comparisons are reported; df represents the degree of freedom of the t statistics.
Table 2.
Results from the offline VAS test performed with sweet (a) and bitter (b) tastes in the group of subjects enrolled in the fMRI experiment
| Concentrations | t (df = 86) | p | |
|---|---|---|---|
| a | |||
| Sweet concentrations | 1–2 | 3.15 | .002 |
| 2–3 | 5.02 | <.001 | |
| 3–4 | 4.55 | <.001 | |
| 4–5 | 2.9 | .004 | |
| b | |||
| Bitter concentrations | 1–2 | 2.76 | .007 |
| 2–3 | 2.73 | .007 | |
| 3–4 | 3.63 | <.001 | |
| 4–5 | 3.4 | .001 | |
Abbreviations: fMRI = functional magnetic resonance imaging; VAS = visual assessment score.
Note. Results from the offline VAS test with the sweet (a) and bitter (b) selected concentrations in the group of subjects enrolled in the fMRI experiment. The t and p values of the significant statistical tests for the pair‐wise comparisons of consecutive concentrations are reported, df represents the degree of freedom of the t statistics.
From the offline test performed on a different group of 30 subjects to assess the taste pleasantness and unpleasantness perceived from the selected concentrations, we obtained for sweet taste a significant ascending linear trend for the positive valence (R 2 = .252, p < .001) but not for the negative valence (R 2 = .003, p > .05). For bitter taste, we obtained a significant descending linear trend for the positive valence (R 2 = .066, p = .002) and a significant ascending linear trend for negative valence (R 2 = .163, p < .001). These results are shown in the Figure S3.
3.2. FMRI results
Four of the forty‐eight subjects were excluded from the image analysis because of excessive movements during fMRI scanning. In none of 44 subjects enrolled for the fMRI experiment, the swallowing phases significantly predicted an increase of the head movements (p > .05), suggesting a lack of systematic synchronization between the swallowing and the head movements of the subjects.
Table 3 summarizes the demographic characteristics of our final group of study. At the end of the functional scans, all subjects reported that they were able to clearly perceive the variability in the intensity of the taste perception and none reported to be fallen asleep during the acquisitions.
Table 3.
Demographic distribution of subjects included in the fMRI data analysis
| Age (mean ± SD) | BMI (mean ± SD) | Sex | PREDIMED results (mean ± SD) |
|---|---|---|---|
| 25 ± 4 | 23 ± 2.7 | 25 M, 19 F | 8.1 ± 1.9 |
Abbreviation: fMRI = functional magnetic resonance imaging.
Note. Demographic table. Data are referred to the 44 subjects included in the data analysis.
Figure 2a,b shows the correlations between the intensity ratings collected from the selected subjects (included in the fMRI data analysis) and the concentrations used in the fMRI experiment. A linear trend is significant for both sweet and bitter tastes (sweet: R 2 = .58 and p < .001, bitter: R 2 = .47 and p < .001). Figure 2 also shows the pleasantness (c, d) and unpleasantness values (e, f) reported by a subgroup of 24 subjects enrolled in the fMRI experiment for the sweet and bitter tastes. Particularly, for sweet taste, we obtained a significant linear trend for the positive valence (R 2 = .33, p < .001) but not for the negative valence (R 2 = .02, p > .05). For bitter taste, we obtained a descending linear trend for the positive valence (R 2 = .03, p = .05) and an ascending linear trend for negative valence (R 2 = .25, p < .001).
Figure 2.
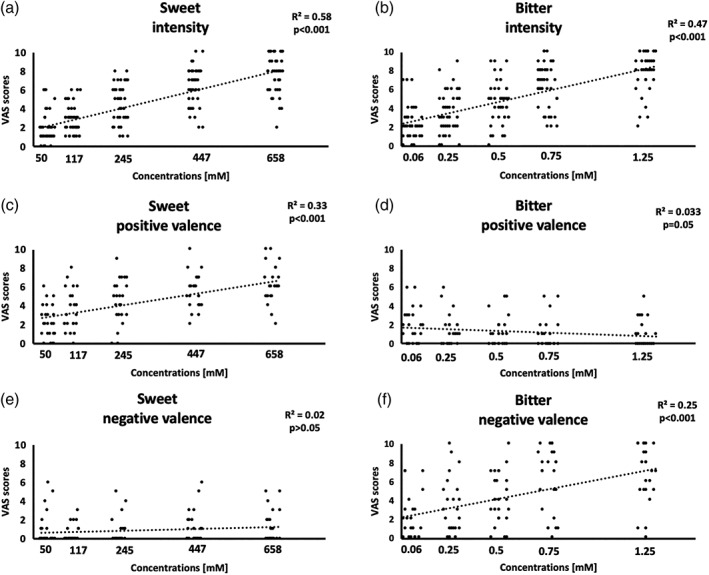
(a,b) Scatterplots of the visual assessment score (VAS) scores and correlation values between the intensity ratings and concentrations for both sweet (a) and bitter (b) tastes obtained by the 44 enrolled subjects. (c–f) Scatterplots of the VAS scores for the positive and negative valences of sweet (c,e) and bitter (d,f) tastes obtained by a subgroup of 24 enrolled subjects. For each graph, the corresponding linear fitting, R 2 and p values are reported
The analysis of the main effects of taste at all concentrations within the right insular cortex revealed the presence of three compact clusters located in the anterior, middle‐posterior, and inferior insula (Figure 3, first row). However, when displaying the contrasts across different concentrations, the extensions of these clusters were dramatically reduced for the third and fifth concentrations for both tastes (Figure 3, second and third rows).
Figure 3.
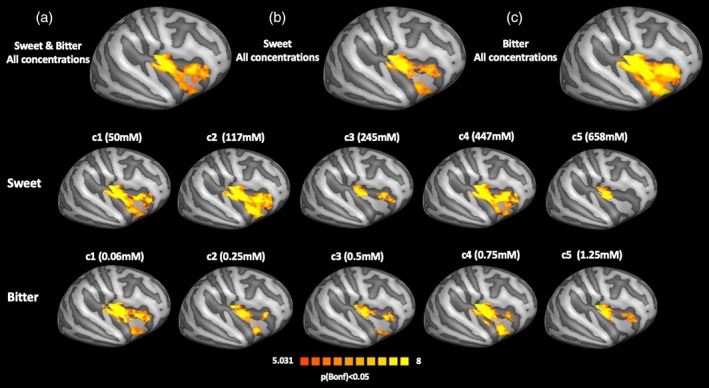
Main effects analysis in the right insular cortex. First row: (a) main effect of all concentrations of sweet and bitter tastes; (b) main effects of all concentrations of sweet taste; and (c) main effects of all concentrations of bitter taste. Second row: main effects of sweet taste for each concentration taken separately. Third row: main effects of bitter taste for each concentration taken separately [Color figure can be viewed at http://wileyonlinelibrary.com]
In the left insular cortex, the same analysis of the main effects of taste at all concentrations also revealed the presence of three compact clusters located in the anterior, middle‐posterior, and inferior insula (Figure 4, first row). When displaying the contrasts across different concentrations, the extensions of these clusters were dramatically reduced for the third and fifth concentrations for both tastes (Figure 4, second and third rows).
Figure 4.
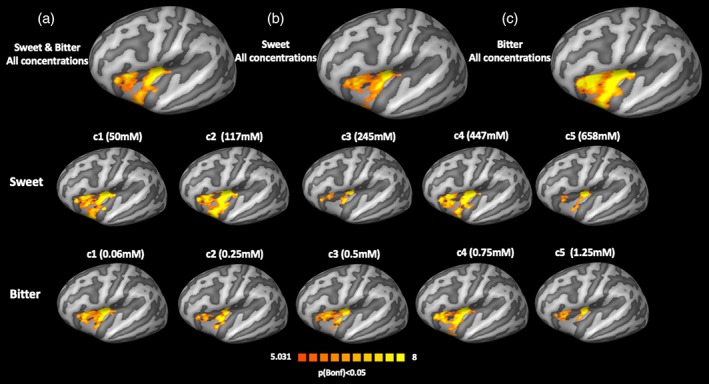
Main effects analysis in the left insular cortex. First row: (a) main effect of all concentrations of sweet and bitter tastes; (b) main effects of all the concentrations of sweet taste; and (c) main effects of all concentrations of bitter taste. Second row: main effects of sweet taste for each concentration taken separately. Third row: main effects of bitter taste for each concentration taken separately [Color figure can be viewed at http://wileyonlinelibrary.com]
When jointly modeling taste quality and concentration (2w‐ANOVA) in the right hemisphere, we obtained a statistically significant effect of concentration in the middle‐posterior portion of the insula (Figure 5), but no statistically significant effects of taste or taste by concentration interaction.
Figure 5.
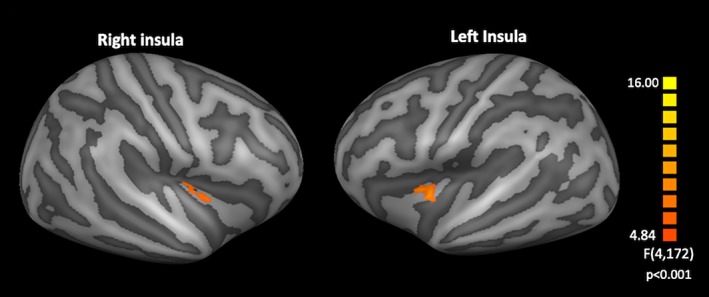
Effect of concentration obtained by the two‐way analysis of variance analysis in the right and left insulae with a statistical threshold of p < .05 cluster corrected using Monte Carlo simulations with a cluster threshold forming p = .001 [Color figure can be viewed at http://wileyonlinelibrary.com]
This cluster (cluster size 152 mm3) was also significant after false discovery rate (FDR) correction at both q < 0.05 and q < 0.01, Figure S4.
In the left hemisphere, we did not obtain statistically significant effects of taste and of the interaction between taste and concentration, but the concentration factor produced a cluster (cluster size 156 mm3) of statistically significant effects within the middle insula, as shown in Figure 5.
Thus, cluster also survived the FDR correction (with both q < 0.05 and q < 0.01, Figure S5).
A post hoc analysis of mean regional beta values from the above clusters was performed using a paired t test. In the right insula, for sweet taste, we found a significant difference between the second and third concentrations (p = .002), between the third and fourth concentrations (p < .001), and between the fourth and fifth concentrations (p < .001); for bitter taste, we obtained a significant difference between the first and second concentrations (p < .001), between the third and fourth concentrations (p = .006), and between the fourth and fifth concentrations (p = .008). In the left insula, for sweet taste, we found a significant difference between the first and second concentrations (p = .007), between the second and third concentrations (p < .001), between the third and fourth concentrations (p < .001), and between the fourth and fifth concentrations (p < .001); for bitter taste, we obtained a significantly difference between the third and fourth concentrations (p = .03), and between the fourth and fifth concentrations (p = .008). In Figure 6, we report the bar graph (with SE) of the mean beta values in these clusters across the right and left insulae and for both sweet and bitter tastes, as well as the mean beta values for each subject and the linear and quadratic fits (with R 2). For both sweet and bitter tastes, no linear and quadratic trends were found significant (R 2 1 st sweet = .0037, R 2 2 nd sweet = .004, R 2 1 st bitter = .0028, R 2 2 nd bitter = .0031, p > .05 for the right insula and R 2 1 st sweet = .0388, R 2 2 nd sweet = .042, R 2 1 st bitter = .0122, R 2 2 nd bitter = .0129, p > .05 for the left insula).
Figure 6.

Left side: bar graph with SE of the mean beta values in the right middle‐posterior and left middle insulae. Right side: mean beta values for each subject for sweet and for bitter tastes with corresponding polynomial fitting curves and R 2 (blue: first order and red: second order) [Color figure can be viewed at http://wileyonlinelibrary.com]
In order to relate the responses observed in the above clusters to taste valence, for the subgroup of 24 subjects that also provided the valence ratings, Spearman correlations were computed between the mean beta values and the reported (offline) VAS scores representing the positive and negative valences of the selected concentrations of sweet and bitter tastes. No significant correlations were found for both the positive and negative valences of sweet and bitter tastes in the two clusters showing significant concentration effects. Therefore, we further tested whether the concentration effect would remain significant after including the positive (for sweet) and the negative (for bitter) reported valence scores as a continuous variable in an analysis of covariance (ANCOVA) model of the same beta values across the five concentration levels. From this analysis, while the valence scores did not yield any significant effects on the insular activation (p > .05), the concentration effect remained significant in both the left and right insular clusters (F = 3.67, p < .01, and F = 4.06, p < .01, respectively). In the right insular cluster, the interaction between concentration and valence was also significant (F = 3.00, p < .02).
To descriptively assess the continuous spatial distribution of the insular activation across all concentrations, group‐level maps of most responsive concentrations were calculated to represent the most responsive taste intensity within and across all the significantly activated clusters. In Figure 7, we report the maps of most responsive concentrations with and without spatial smoothing, in the latter case to represent the maximal response of all insular vertices with the highest possible spatial specificity.
Figure 7.
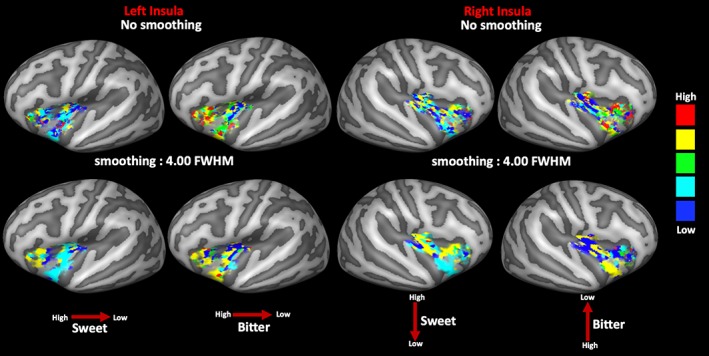
Group‐based map of most responsive concentration computed in the regions of the significant main effects within the left (left side) and right (right side) insular cortices of sweet and bitter tastes (respectively, left and right columns) with and without spatial smoothing. Red arrows indicate the direction of the spatial gradient (high vs. low concentrations) [Color figure can be viewed at http://wileyonlinelibrary.com]
In the left insula, for sweet taste (Figure 7, first column), the lower concentrations appeared more represented across all clusters with significant main effects, although an anterior–posterior spatial gradient for high‐to‐low concentrations is visible from the anterior to the posterior side of the insular cortex. For the bitter taste (Figure 7, second column), a similar anteroposterior spatial gradient for high‐to‐low concentrations was observed.
In the right insula, for the sweet taste (Figure 7, third column), the spatial change of the most responsive concentrations suggests the presence of a superior–inferior spatial gradient for the high‐to‐low concentrations extending from the superior cluster (higher concentrations more responsive than lower concentrations) toward the inferior cluster (lower concentrations more responsive than higher concentrations). For the bitter taste (Figure 7, forth column), an inverse gradient was observed with the higher concentrations more responsive in the inferior cluster and the lower concentrations more responsive in the superior clusters.
Finally, in order to assess how much information from the above spatial patterns was actually present in each single individual, single‐subject maps of most responsive concentrations were also generated (from the first‐level GLM fits). To quantify the adherence of each single subject to the group map of most responsive concentrations, the spatial correlation coefficient (Spearman correlation) between the individual and the group‐level maps of most responsive concentrations was calculated and reported for all subjects in Tables 4 and 5 (respectively, for right and left insula). These data show that at least 70% of subjects exhibited a significant spatial correlation (p < .05) in both hemispheres.
Table 4.
Spearman correlations results in the right insula
| Subjects | Sweet | Bitter | ||
|---|---|---|---|---|
| Rho | p | Rho | p | |
| 1 | 0.30 | <.001 | 0.11 | <.001 |
| 2 | 0.32 | <.001 | 0.17 | <.001 |
| 3 | 0.19 | <.001 | 0.14 | <.001 |
| 4 | 0.24 | <.001 | 0.22 | <.001 |
| 5 | 0.13 | <.001 | 0.18 | <.001 |
| 6 | 0.28 | <.001 | 0.30 | <.001 |
| 7 | 0.16 | <.001 | 0.17 | <.001 |
| 8 | 0.23 | <.001 | 0.19 | <.001 |
| 9 | 0.21 | <.001 | 0.13 | <.001 |
| 10 | 0.25 | <.001 | 0.15 | <.001 |
| 11 | 0.19 | <.001 | 0.16 | <.001 |
| 12 | 0.14 | <.001 | 0.18 | <.001 |
| 13 | 0.12 | <.001 | 0.23 | <.001 |
| 14 | 0.11 | <.001 | 0.21 | <.001 |
| 15 | 0.20 | <.001 | 0.27 | <.001 |
| 16 | 0.17 | <.001 | 0.27 | <.001 |
| 17 | 0.19 | <.001 | 0.13 | <.001 |
| 18 | 0.23 | <.001 | 0.12 | <.001 |
| 19 | 0.20 | <.001 | 0.25 | <.001 |
| 20 | 0.13 | <.001 | 0.17 | <.001 |
| 21 | 0.21 | <.001 | 0.15 | <.001 |
| 22 | 0.19 | <.001 | 0.28 | <.001 |
| 23 | 0.21 | <.001 | 0.10 | <.001 |
| 24 | 0.23 | <.001 | 0.17 | <.001 |
| 25 | 0.20 | <.001 | 0.16 | <.001 |
| 26 | 0.20 | <.001 | 0.23 | <.001 |
| 27 | 0.22 | <.001 | 0.24 | <.001 |
| 28 | 0.23 | <.001 | 0.14 | <.001 |
| 29 | 0.15 | <.001 | 0.28 | <.001 |
| 30 | 0.23 | <.001 | 0.25 | <.001 |
| 31 | 0.15 | <.001 | 0.19 | <.001 |
| 32 | 0.26 | <.001 | 0.18 | <.001 |
| 33 | 0.25 | <.001 | 0.13 | <.001 |
| 34 | 0.21 | <.001 | 0.17 | <.001 |
| 35 | 0.15 | <.001 | 0.26 | <.001 |
| 36 | 0.16 | <.001 | 0.17 | <.001 |
| 37 | 0.05 | .00 | 0.19 | <.001 |
| 38 | 0.18 | <.001 | 0.29 | <.001 |
| 39 | 0.28 | <.001 | 0.25 | <.001 |
| 40 | 0.12 | <.001 | 0.25 | <.001 |
| 41 | 0.21 | <.001 | 0.11 | <.001 |
| 42 | 0.28 | <.001 | 0.15 | <.001 |
| 43 | 0.22 | <.001 | 0.17 | <.001 |
| 44 | 0.05 | .00 | 0.21 | <.001 |
Note. Spearman correlation between individual and group‐level maps of most responsive concentrations in the right insula for sweet and bitter tastes. The correlation coefficients (rho) and the p values are reported.
Table 5.
Spearman correlations results in the left insula
| Subjects | Sweet | Bitter | ||
|---|---|---|---|---|
| Rho | p | Rho | p | |
| 1 | −0.03 | .18 | 0.07 | .00 |
| 2 | 0.02 | .26 | 0.07 | .00 |
| 3 | 0.18 | <.001 | 0.05 | .01 |
| 4 | 0.05 | .01 | 0.07 | .00 |
| 5 | 0.13 | <.001 | 0.10 | <.001 |
| 6 | 0.03 | .14 | 0.05 | .01 |
| 7 | 0.03 | .08 | 0.13 | <.001 |
| 8 | 0.01 | .62 | −0.09 | <.001 |
| 9 | 0.10 | <.001 | −0.03 | .18 |
| 10 | 0.17 | <.001 | 0.01 | .55 |
| 11 | 0.08 | <.001 | −0.02 | .37 |
| 12 | 0.01 | .63 | 0.35 | <.001 |
| 13 | 0.09 | <.001 | 0.19 | <.001 |
| 14 | −0.02 | .30 | 0.02 | .42 |
| 15 | 0.13 | <.001 | 0.20 | <.001 |
| 16 | 0.14 | <.001 | 0.13 | <.001 |
| 17 | 0.18 | <.001 | −0.12 | <.001 |
| 18 | 0.27 | <.001 | 0.09 | <.001 |
| 19 | 0.15 | <.001 | 0.11 | <.001 |
| 20 | 0.17 | <.001 | 0.03 | .08 |
| 21 | 0.03 | .12 | 0.09 | <.001 |
| 22 | 0.13 | <.001 | 0.23 | <.001 |
| 23 | 0.04 | .06 | −0.09 | <.001 |
| 24 | 0.11 | <.001 | 0.04 | .02 |
| 25 | 0.08 | <.001 | −0.01 | .43 |
| 26 | 0.05 | .01 | 0.16 | <.001 |
| 27 | 0.17 | <.001 | −0.02 | .25 |
| 28 | 0.04 | .04 | −0.04 | .02 |
| 29 | 0.13 | <.001 | −0.04 | .06 |
| 30 | 0.17 | <.001 | 0.27 | <.001 |
| 31 | 0.12 | <.001 | 0.01 | .55 |
| 32 | 0.06 | .00 | 0.24 | <.001 |
| 33 | −0.02 | .42 | −0.13 | <.001 |
| 34 | 0.15 | <.001 | 0.04 | .05 |
| 35 | 0.05 | .01 | 0.05 | .01 |
| 36 | 0.10 | <.001 | −0.02 | .24 |
| 37 | 0.12 | <.001 | 0.12 | <.001 |
| 38 | 0.06 | .00 | 0.27 | <.001 |
| 39 | 0.18 | <.001 | 0.26 | <.001 |
| 40 | 0.04 | .06 | 0.02 | .41 |
| 41 | 0.10 | <.001 | 0.16 | <.001 |
| 42 | 0.10 | <.001 | 0.19 | <.001 |
| 43 | 0.18 | <.001 | −0.01 | .70 |
| 44 | 0.14 | <.001 | 0.08 | <.001 |
Note. Spearman spatial correlation between individual and group‐level maps of most responsive concentrations in the left insula for sweet and bitter tastes. The correlation coefficients (rho) and the p values are reported.
4. DISCUSSION
In this study, by using fMRI, we have analyzed the cortical processes elicited by the perception of five parametrically increasing intensities of a pleasant (sweet) and an unpleasant (bitter) taste stimulus in the human insular cortex. This experiment principally aimed at disclosing whether the insular cortex, which is the putative cortical location of primary taste perception, includes clusters with a robust specialization for the intensity processing of these tastants, and whether a spatial gradient of most responsive concentrations exists among the insular responses to the varying intensity of the taste perception. To maximize the spatial information in the cortical responses, we acquired 3 T fMRI data at relatively high spatial resolution (2.5 mm isotropic). Moreover, we used a high resolution cortical registration method to improve the anatomical correspondence across individual cortices (Prinster et al., 2017). We found that (a) both the left and the right insular cortices include a specialized cluster for processing taste intensities independently of the quality and valence of the tastant; (b) the spatial distribution of the highest responses across the main effects of the insular activation delineates, respectively, for the right and left insula, a superior–inferior and an anterior–posterior spatial gradient for high‐to‐low most responsive concentrations.
All the insular regions activated during taste perception were perfectly overlapping with those reported in two recent meta‐analyses of taste perception in humans (Yeung, Goto, & Leung, 2017; Yeung, Goto, & Leung, 2018) covering several gustatory fMRI studies based on the injection of basic taste solutions to healthy subjects. However, when assessing the main effects at some specific concentrations, some of the clusters were reduced depending on the intensity of the tastant, suggesting the presence of intensity‐related effects in the activation patterns. For example, reduced clusters of activations were found for the third and fifth concentrations for both the right and left insulae, suggesting that: (a) a nonlinear relationship is likely to exist between concentration and neural activation in some clusters and (b) a spatial gradient of most responsive concentrations might exist in the spatial distribution of the insular responses to the taste intensity.
To possibly isolate the presence of compact clusters with taste or intensity specialization, a full factorial (two tastes × five concentrations) analysis was performed. From this analysis, two specialized clusters for the processing of taste intensity were obtained in the middle‐posterior part of the right insula and in the middle part of the left insula, whereas no quality or intensity‐by‐quality interaction effects were detected in both the right and left insula.
The lack of specialized effects for taste quality, in both the left and right hemispheres, could be possibly ascribed to the use of small acquisition voxels which might have reduced the sensitivity of our analysis, in favor of a possibly higher specificity in the computation of the maps of most responsive concentrations, for which we present results obtained with and without applying a (modest) spatial smoothing. Future works using ultrahigh field fMRI are needed to increase the contrast‐to‐noise of the analysis without sacrificing the specificity of the spatial analysis.
The finding of a significant effect of taste intensity in the bilateral insular cortices is in agreement with previous studies (Small et al., 2003) and (Spetter et al., 2010) but is seemingly contrasting with the finding of Dalenberg et al. (2015) of a right insula more specialized for taste intensity processing and a left insula more specialized for taste quality processing. On the other hand, the factorial modeling in Dalenberg et al. (2015) was performed on the first two principle components of the group‐level data over the entire insula and not at each individual brain location (e.g., voxel, or vertex in our case). As a consequence, no clusters with specialized intensity‐related function or maps of most responsive concentrations could be reported to respectively address the location and distribution of taste intensity rankings over the insula. Thus, the existence of a spatially distributed processing for the taste intensity responses in the right insula was not excluded by this previous study and, actually, the presence of such a spatial code might be postulated on the basis of the taste intensity maps of most responsive concentrations that we show in addition to the statistical analyses in both the right and left insula. In fact, from this analysis, we observed a changing local response from high to low concentrations in both the right and left insula with an intriguing orthogonal pattern between sweet and bitter tastes in the two hemispheres: for sweet and bitter tastes, a superior–inferior and a complementary inferior–superior spatial gradient for high‐to‐low concentrations was evident in the right insula; for both sweet and bitter taste, a similar anterior–posterior spatial gradient for high‐to‐low concentrations was more evident in the left insula. In both cases, these spatial distributions would be compatible with the idea that the processing of taste intensity could be configured for a spatially distributed processing code, as previously discussed in some other works (see Jones, Fontanini, & Katz, 2006; Simon, De Araujo, Gutierrez, & Nicolelis, 2006). In addition, in vivo imaging studies of neuronal activity in rats have also reported different spatial patterns of activation in response to appetitive (saccharin) and aversive (quinine) tastes (Accolla & Carleton, 2008; Yiannakas & Rosenblum, 2017). In particular, the evidence for distributed gustatory processing in the primary gustatory cortex was gathered by the fact that taste identity, palatability and, in particular, intensity were more efficiently decoded when the activity of different spatially localized neuronal populations were taken into account (Jones et al., 2006; Katz, Simon, & Nicolelis, 2002).
Previous studies had also highlighted that specific regions of the insula are responsible for processing at the same time several and, sometimes opposite, aspects of taste perception. This could be particularly the case of the anterior insula that is engaged in the processing of food rewards (Monteleone et al., 2017; Sescousse, Caldú, Segura, & Dreher, 2013; Van der Laan, de Ridder, Viergever, & Smeets, 2011), pleasantness (Dalenberg, Weitkamp, Renken, Nanetti, & Ter Horst, 2017; Small, 2010) as well as in the experiencing of aversive smells (Wicker et al., 2003), anticipation of aversive stimuli (Nitschke et al., 2006) and aversive taste learning (Bermudez‐Rattoni, 2014). Similarly, the middle insula is known to be involved in several mechanisms, including the simple response to oral somatosensory stimuli (De Araujo & Rolls, 2004; Rolls, 2016). However, the bilateral middle insula has been also reported as specialized for taste intensity (Small et al., 2003; Spetter et al., 2010) with the middle‐posterior part being specifically more activated by unpleasant tastes causing aversive reactions (Nitschke et al., 2006; O'Doherty, Rolls, Francis, Bowtell, & McGlone, 2001). This latter aspect could also explain the similarities and differences revealed by the maps of most responsive concentrations between the two tastes and the two hemispheres. In fact, comparing the sweet‐ and bitter‐related spatial patterns of most responsive concentrations, while a complementary superior‐to‐inferior spatial pattern was observed in the right insula, a similar anteroposterior spatial pattern was observed in the left insula.
However, the two hemispheric patterns appear more similar for the anterior clusters for the sweet taste (where the high concentrations are most responsive) and more different for the middle‐posterior clusters. In fact, for the middle‐posterior clusters, we found that the lower concentrations determined the highest response for both tastes only in the left hemisphere whereas the same distribution was visible only for bitter taste in the right hemisphere. This aspect is consistent with the recent pilot study (Prinster et al., 2017), where the differential distribution of the highest responses has been observed for sweet and bitter tastes in comparison to all five basic tastes across the right insular cortex. In this previous study, it was indeed found that sweet and bitter tastes determined the highest response (across tastes) in the middle‐posterior and inferior insula whereas the other basic tastes (sour, umami, and salty) determined the highest responses in the anterior part of the right insula.
Given the merely descriptive nature of this analysis, an additional spatial consistency analysis of these patterns across all individual subjects was also performed. Despite the high variability in the obtained spatial distributions, more prominent for the bitter taste, the similarity between each individual map and the group map of most responsive concentrations was high for most subjects (> = 70% for bitter and 100% for sweet) and for both tastants.
In both hemispheres, these patterns even encompass a specialized cluster in the (right) middle‐posterior and in the (left) middle insula where the intensity factor becomes statistically significant in the 2w‐ANOVA. In these regions, we observed a highly nonlinear trend of the responses to the concentration of both sweet and bitter tastants.
More specifically, the activation responses were lower at the third and fifth concentrations, for both sweet and bitter tastes. Not even a quadratic trend was significantly explaining the measured responses as observed in Dalenberg et al. (2015) for the principle component scores of the entire right insular cortex, whereas two other human studies (Kobayakawa, Saito, Gotow, & Ogawa, 2008) and (Spetter et al., 2010) reported a linearly increasing response across four different intensities of sweet in the left middle insula. However, since not all previous studies have formally tested for specific nonlinear associations, it is not straightforward to compare the present findings with all results in previous reports.
Several studies performed in animals have reported a complex nonmonotonic insular response to taste intensity. For example, while in some electrophysiological studies [as in Scott et al. (1991)], a monotonic increase of firing rates in the insula neurons with increasing tastant concentration has been observed, far more complex (nonlinear) trends emerged when other aspects, such as the tasting phase (e.g., the number of licking cycles before ingestion), were taken into account (Stapleton et al., 2006), thereby gustatory neurons were found to respond more to sucrose (sweet) tastants of higher or lower concentrations depending on the latency of the observation. Thus, changes of functional activation in response to taste intensity may be influenced by the exact number and latencies of the different tasting phases occurring when the subject holds the taste in mouth for a given period. In our experiment, although we tried to minimize the duration of the tasting period by implementing a rapid taste injection (600 ms) of very small amounts of solution (1 mL), we substantially failed to obtained a linear relationship between cortical activation and taste intensity, probably due to the impossibility to control the exact number of tasting cycles occurring at each concentration.
On the other hand, it has been also reported that the primary gustatory cortex response may change in relation to the level of attention paid by subjects to the taste intensity or pleasantness (Bender, Veldhuizen, Meltzer, Gitelman, & Small, 2009; Nitschke et al., 2006; Veldhuizen, Bender, Constable, & Small, 2007; Wang & Spence, 2017). Thus, although our paradigm did not require subjects to focus on stimuli and give any (online) scores about both the intensity and pleasantness of the stimuli during the fMRI experiment, we cannot exclude that systematic top‐down effects have come into play and contributed to modulating the responses across different concentrations as previously demonstrated in terms of functional connectivity by Yeung et al. (2016), or in terms of functional effects of food motivation by Wegman et al. (2018).
The ratings of perceived intensity obtained by the offline VAS tests exhibited a highly significant linear relation with the concentration levels, for both the sweet and bitter tastes, that is reflected neither in the left nor in the right insular response to taste intensities. A recent study of Hwang et al. (2019) also reported a lack of association between the perceived intensity of sweet and bitter tastes with the insular cortical volume.
In psychophysics research on taste, however, it has been assessed that taste perceived intensities (or intensity ratings) and tastant concentrations hold a relationship expressed by a power law function of stimulus concentration with an exponential factor that explains how the magnitude of the subjective response increases with the stimulus concentrations (Moskowitz, 1970; Stevens, 1969). Particularly, for sour and bitter tastes, this exponent has been assessed to be approximately equal to 1 indicating that the intensity of these two tastes is linearly related to their concentration. Contrariwise, for sweet and salty tastes, this value is about 1.3 and 1.4, respectively, suggesting that the strength of the perceived intensity more rapidly increases with the stimulus concentration (Moskowitz, 1970). An alternative psychophysical model that describes the relationship between the concentration of a taste stimulus and the reported subjective rates is based on an S‐shaped curve with an initial flat portion, an intermediate step rise and another flat region representing saturation regions (Beidler, 1961). In our sample of 13 subjects who performed the offline test on the intensity perception over a more extended range of concentrations, the trend of the perceived intensities across the eight preliminary chosen concentrations was consistent with the S‐shaped profile (Beidler, 1961). However, to establish the final set of five concentrations for the fMRI experiment, we considered a linear ANOVA model and discarded those concentrations that did not yield a significant difference in the intensity perception from the previous or next concentration in the pair‐wise comparisons between consecutive concentrations. Thus, the selected concentrations were expected (and, then, verified) to produce a significant linear trend in the perceived intensity also in the enrolled subjects for the fMRI experiment.
On these premises, the observed discrepancy between the linearity of the subjective ratings and the nonlinearity of the observed brain responses should be thus attributed to other neural effects elicited by the stimuli, the size of which is not proportionally reflected in the ratings. For example, some top‐down modulating effects may systematically come into play at some, but not all, concentrations, causing a different amplitude of the insular response. This could be particularly the case of the low and high concentrations (where we found high and low insular responses, respectively) that may have determined some selective attentional tasks, respectively, to taste intensities and/or pleasantness, that directly or through the influence of other brain regions might have influenced the insular response, as demonstrated in the work of Luo, Ge, Grabenhorst, Feng, and Rolls (2013). Therefore, future studies expressly focused on the presence of top‐down or other psychophysiological interaction effects (e.g., by stimulating a selective attention to taste features) on the insular profile of responses to increasing concentrations, are needed to definitively verify this possible interpretation.
A specific focus is needed to disclose the possible effects of the affective valence of tastes as actually perceived by the subjects on the observed insular responses. Our experiment was specifically designed to avoid subjects to give any feedback in terms of the perceived pleasantness or unpleasantness of the stimuli in order to minimize (or limit) the activation of additional cognitive mechanisms likely required during an active evaluation or decision task triggered by the taste perception (Bender et al., 2009; Nitschke et al., 2006; Veldhuizen et al., 2007; Wang & Spence, 2017). Therefore, we cannot exclude that the effects observed in the two clusters across the left and right insula could be partly related to the perceived valence. Nonetheless, at least in a subgroup of 24 subjects, not only the VAS scores for the perceived intensities, but also the same VAS scores for the pleasantness/unpleasantness related to each concentration, were considered and there was no correlation with the neural response obtained in the left and right insular clusters. In fact, as expected, the perceived valence showed an increasing linear trend for the positive ratings of the sweet taste and an increasing linear trend for the negative ratings of the bitter taste and the fitted profiles were perfectly overlapping with those obtained in the more extended group of subjects performing the same offline VAS test. Moreover, when further evaluating the possible effects of both taste concentration level and valence rating on the same insular responses with an ANCOVA model, while the valence scores did not yield any significant effects, the concentration effect remained significant for both the left and right insular clusters. In addition, the covariance of neural activity in the right insular cluster with valence ratings disclosed a significant valence by concentration interaction, which may further contribute to explain the observed nonlinear relationship between neural activation and tastant concentration levels in terms of valence. For example, as previously mentioned, recent works have shown how the responses in the primary gustatory cortex may change in relation to the level of attention paid by subjects to both the taste intensity and pleasantness (Bender et al., 2009; Nitschke et al., 2006; Veldhuizen et al., 2007; Wang & Spence, 2017). Therefore, considering that the VAS scores were collected offline, and not during the image acquisition, it was not possible to completely isolate the effect of intensity from any other possible effects (directly or indirectly) related to a potentially valence‐specific processing of the gustatory stimulus.
Another possible limitation of the present study pertains to the use of very short stimulus events in the experimental design. While this solution has the previously highlighted advantages in terms of spatial and temporal specificity of the analysis, it is not possible to robustly assess from the present data the responses of the whole‐brain functional connectome and, for example, determine how (and to what extent) several brain regions would be consistently coactivated in response to the gustatory stimulation. Future studies using bigger amounts of tastant (to induce prolonged gustatory responses over longer intervals), as well as bigger functional voxels (> = 3 mm) to gather a higher signal to noise ratio (SNR) and a whole brain coverage, will be extremely important to further advance our understanding of the complex nature of the insular response to taste intensity as well as the role of other modulating effects eventually operated from/to other brain regions within (or outside) the gustatory pathway.
In conclusion, we have reported that the processing of taste intensity activates multiple regions within the bilateral insular cortex including two clusters in the middle portions determining a significant effect of taste concentrations. The difficulty in localizing specialized clusters for parametric taste intensity processing at the used spatio‐temporal scales was possibly addressed and explained by the existence of spatially continuous cortical processes, whereby the response of a most responsive intensity appears distributed over smaller clusters according to a superior–inferior and to an anterior–posterior spatial gradient for high‐to‐low concentration changes. Future investigations at ultrahigh magnetic fields are needed to ultimately disclose whether compact insular clusters exist and can be targeted to parametrically control the intensity of the perceived taste or whether the suppression of specific confounders would help reducing the intersubject variability in the spatial distribution of the taste intensity‐related responses.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
5.
Supporting information
Figure S1 Distribution of the maximum values of motion parameters (respectively for translations TX, TY and TZ and for rotations RX, RY and RZ) during both sweet and bitter related tasks.
Figure S2: Trend of VAS scores for the sweet (a) and the bitter (b) tastes for each of the two repetitions of taste injection performed during the offline test in the group of thirteen subjects not enrolled in the fMRI experiment.
Figure S3: Scatterplots of VAS scores for the positive and negative valences of sweet (a and c) and bitter tastes (b and d), with the corresponding linear fitting, R‐square and P‐values in the group of thirty subjects not enrolled in the fMRI experiment.
Figure S4: Effect of concentration obtained by the 2w‐ANOVA analysis in the right insula, left side FDR corrected with q < 0.05, right side FDR corrected with q < 0.01.
Figure S5: Effect of concentration obtained by the 2w‐ANOVA analysis in the left insula, left side FDR corrected with q < 0.05, right side FDR corrected with q < 0.01.
ACKNOWLEDGMENTS
The authors would like to thank Michele Fratello for the useful suggestions about data analysis.
Canna A, Prinster A, Cantone E, et al. Intensity‐related distribution of sweet and bitter taste fMRI responses in the insular cortex. Hum Brain Mapp. 2019;40:3631–3646. 10.1002/hbm.24621
Data Availability Statement: Relevant data and codes used to generate results of this study are available from the corresponding author upon request.
DATA AVAILABILITY
Relevant data and codes used to generate results of this study are available from the corresponding author upon request.
REFERENCES
- Accolla, R. , Carleton, A. (2008): Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proceedings of the National Academy of Sciences of the United States of America, 105:4010–4015. http://www.pnas.org/cgi/doi/10.1073/pnas.0708927105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20, 870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Beidler, L. M. (1961). Biophysical approaches to taste. American Scientist, 49, 421–431. [PubMed] [Google Scholar]
- Bender, G. , Veldhuizen, M. G. , Meltzer, J. A. , Gitelman, D. R. , & Small, D. M. (2009). Neural correlates of evaluative compared with passive tasting. European Journal of Neuroscience, 30, 327–338. 10.1111/j.1460-9568.2009.06819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez‐Rattoni, F. (2014). The forgotten insular cortex: Its role on recognition memory formation. Neurobiology of Learning and Memory, 109, 207–216. 10.1016/j.nlm.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Canna, A. , Prinster, A. , Fratello, M. , Puglia, L. , Magliulo, M. , Cantone, E. , … Esposito, F. (2018). A low‐cost open‐architecture taste delivery system for gustatory fMRI and BCI experiments article. Journal of Neuroscience Methods, 311, 1–12. 10.1016/j.jneumeth.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Dalenberg, J. R. , Hoogeveen, H. R. , Renken, R. J. , Langers, D. R. M. , & ter Horst, G. J. (2015). Functional specialization of the male insula during taste perception. NeuroImage, 119, 210–220. 10.1016/j.neuroimage.2015.06.062 [DOI] [PubMed] [Google Scholar]
- Dalenberg, J. R. , Weitkamp, L. , Renken, R. J. , Nanetti, L. , & Ter Horst, G. J. (2017). Flavor pleasantness processing in the ventral emotion network. PLoS One, 12, 1–20. 10.1371/journal.pone.0170310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo, I.E. , Rolls, E.T. (2004): Representation in the human brain of food texture and oral fat. The Journal of Neuroscience, 24:3086–3093. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T.E. , Knutsson, H. (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28): 7900–7905. http://www.pnas.org/cgi/doi/10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg, D. A. , Moeller, S. , Smith, S. M. , Auerbach, E. , Ramanna, S. , Glasser, M. F. , … Yacoub, E. (2010). Multiplexed echo planar imaging for sub‐second whole brain fmri and fast diffusion imaging. PLoS One, 5(12), 1–11. 10.1371/journal.pone.0015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman, S. D. , Cohen, J. D. , Fitzgerald, M. , Eddy, W. F. , Mintun, M. A. , & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magnetic Resonance in Medicine, 33, 636–647. 10.1002/mrm.1910330508 [DOI] [PubMed] [Google Scholar]
- Frost, M. A. , Esposito, F. , & Goebel, R. (2014). Improved correspondence of resting‐state networks after macroanatomical alignment. Human Brain Mapping, 35, 673–682. 10.1002/hbm.22191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, M. A. , & Goebel, R. (2012). Measuring structural‐functional correspondence: Spatial variability of specialised brain regions after macro‐anatomical alignment. NeuroImage, 59, 1369–1381. 10.1016/j.neuroimage.2011.08.035 [DOI] [PubMed] [Google Scholar]
- Goebel, R. , Esposito, F. , & Formisano, E. (2006). Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Human Brain Mapping, 27, 392–401. 10.1002/hbm.20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, R. , & Simon, S. A. (2011). Chemosensory processing in the taste—Reward pathway. Flavour and Fragrance Journal, 26, 231–238. 10.1002/ffj.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, L. D. , Strike, L. T. , Couvy‐Duchesne, B. , de Zubicaray, G. I. , McMahon, K. , Breslin, P. A. S. , … Wright, M. J. (2019). Associations between brain structure and perceived intensity of sweet and bitter tastes. Behavioural Brain Research, 363, 103–108. 10.1016/j.bbr.2019.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzini, A. , Mazzucato, L. , La Camera, G. , & Fontanini, A. (2013). Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. The Journal of Neuroscience, 33, 18966–18978. 10.1523/JNEUROSCI.2974-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, L. M. , Fontanini, A. , & Katz, D. B. (2006). Gustatory processing: A dynamic systems approach. Current Opinion in Neurobiology, 16, 420–428. 10.1016/j.conb.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Katz, D. B. , Simon, S. , & Nicolelis, A. M. L. (2002). Taste‐specific neuronal ensembles in the gustatory cortex of awake rats. The Journal of Neuroscience, 22(5), 1850–1857. 10.1523/JNEUROSCI.22-05-01850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa, T. , Saito, S. , Gotow, N. , & Ogawa, H. (2008). Representation of salty taste stimulus concentrations in the primary gustatory area in humans. Chemosensory Perception, 1, 227–234. 10.1007/s12078-008-9030-4 [DOI] [Google Scholar]
- Kriegeskorte, N. , & Goebel, R. (2001). An efficient algorithm for topologically correct segmentation of the cortical sheet in anatomical MR volumes. NeuroImage, 14, 329–346. 10.1006/nimg.2001.0831 [DOI] [PubMed] [Google Scholar]
- Lawless, H. , & Heymann, H. (2010). Sensory evaluation of food: Principles and practices (2nd ed.). New York, NY: Springer; 10.1007/978-1-4419-6488-5 [DOI] [Google Scholar]
- Luo, Q. , Ge, T. , Grabenhorst, F. , Feng, J. , & Rolls, E. T. (2013). Attention‐dependent modulation of cortical taste circuits revealed by granger causality with signal‐dependent noise. PLoS One, 9, e1003265 10.1371/journal.pcbi.1003265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh, A. P. , & Kashou, N. H. (2013). Evaluation of interpolation effects on upsampling and accuracy of cost functions‐based optimized automatic image registration. International Journal of Biomedical Imaging, 2013, 1–19. 10.1155/2013/395915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani, L. , Pfeiffer, J. C. , Hort, J. , Head, K. , Bush, D. , Taylor, A. J. , … Gowland, P. A. (2006). Improved methods for fMRI studies of combined taste and aroma stimuli. Journal of Neuroscience Methods, 158, 186–194. 10.1016/j.jneumeth.2006.05.035 [DOI] [PubMed] [Google Scholar]
- Martınez‐Gonzalez, M. A. , Corella, D. , Salas‐Salvado, J. , Ros, E. , Covas, M. I. , Fiol, M. , … Estruch, R. (2012). Cohort profile: Design and methods of the PREDIMED study. International Journal of Epidemiology, 41, 377–385. 10.1093/ije/dyq250 [DOI] [PubMed] [Google Scholar]
- Moeller, S. , Yacoub, E. , Olman, C. A. , Auerbach, E. , Strupp, J. , Harel, N. , & Uğurbil, K. (2011). Multiband multislice GE‐EPI at 7 Tesla, with 16‐fold acceleration using partial parallel imaging with application to high spatial and temporal whole‐brain FMRI. Magnetic Resonance in Medicine, 63, 1144–1153. 10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone, A. M. , Monteleone, P. , Esposito, F. , Prinster, A. , Volpe, U. , Cantone, E. , … Maj, M. (2017). Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: An fMRI study. Journal of Psychiatric Research, 90, 94–101. 10.1016/j.jpsychires.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Moskowitz, H. R. (1970). Taste intensity as a function of stimulus concentration and solvent viscosity. Journal of Texture Studies, 1, 502–510. [DOI] [PubMed] [Google Scholar]
- Nitschke, J. B. , Dixon, G. E. , Sarinopoulos, I. , Short, S. J. , Cohen, J. D. , Smith, E. E. , … Davidson, R. J. (2006). Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature Neuroscience, 9(3), 435–442. 10.1038/nn1645 [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. , Rolls, E.T. , Francis, S. , Bowtell, R. , McGlone, F. (2001): Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology, 85:1315–1321. http://www.physiology.org/doi/10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Prinster, A. , Cantone, E. , Verlezza, V. , Magliulo, M. , Sarnelli, G. , Iengo, M. , … Esposito, F. (2017). Cortical representation of different taste modalities on the gustatory cortex: A pilot study. PLoS One, 12(12), e0190164 10.1371/journal.pone.0190164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls, E. T. (2016). Functions of the anterior insula in taste, autonomic, and related functions. Brain and Cognition, 110, 4–19. 10.1016/j.bandc.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Sarinopoulos, I. , Dixon, G. E. , Short, S. J. , Davidson, R. J. , & Nitschke, J. B. (2006). Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: Implications for placebo. Brain, Behaviour, and Immunity, 20, 120–132. 10.1016/j.bbi.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Scott, K. (2004). The sweet and the bitter of mammalian taste. Current Opinion in Neurobiology, 14, 423–427. 10.1016/j.conb.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Scott, T. R. , Plata‐Salamán, C. R. , Smith‐Swintosky, V. L. , & Giza, B. K. (1991). Gustatory neural coding in the monkey cortex: Stimulus intensity. Journal of Neurophysiology, 65(1), 76–86. [DOI] [PubMed] [Google Scholar]
- Sescousse, G. , Caldú, X. , Segura, B. , & Dreher, J. C. (2013). Processing of primary and secondary rewards: A quantitative meta‐analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37, 681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Simon, S. A. , De Araujo, I. E. , Gutierrez, R. , & Nicolelis, M. A. L. (2006). The neural mechanisms of gustation: A distributed processing code. Nature Reviews. Neuroscience, 7, 890–901. 10.1038/nrn2006 [DOI] [PubMed] [Google Scholar]
- Small, D. M. (2010). Taste representation in the human insula. Brain Structure and Function, 214, 551–561. 10.1007/s00429-010-0266-9 [DOI] [PubMed] [Google Scholar]
- Small, D. M. , Gregory, M. D. , Mak, Y. E. , Gitelman, D. , Mesulam, M. M. , & Parrish, T. (2003). Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron, 39, 701–711. 10.1016/S0896-6273(03)00467-7 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, 208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Spetter, M. S. , Smeets, P. A. M. , de Graaf, C. , & Viergever, M. A. (2010). Representation of sweet and salty taste intensity in the brain. Chemical Senses, 35, 831–840. 10.1093/chemse/bjq093 [DOI] [PubMed] [Google Scholar]
- Stapleton, J.R. , Lavine, M.L. , Wolpert, R.L. , Nicolelis, M.A.L. , Simon, S.A. (2006): Rapid taste responses in the gustatory cortex during licking. The Journal of Neuroscience, 26:4126–4138. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, S. S. (1969). Sensory scales of taste intensity. Perception & Psychophysics, 6(5), 302–308. 10.3758/BF03210101 [DOI] [Google Scholar]
- Van der Laan, L. N. , de Ridder, D. T. D. , Viergever, M. A. , & Smeets, P. A. M. (2011). The first taste is always with the eyes: A meta‐analysis on the neural correlates of processing visual food cues. NeuroImage, 55, 296–303. 10.1016/j.neuroimage.2010.11.055 [DOI] [PubMed] [Google Scholar]
- Veldhuizen, M. G. , Bender, G. , Constable, R. T. , & Small, D. M. (2007). Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chemical Senses, 32, 569–581. 10.1093/chemse/bjm025 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Gillis‐Smith, S. , Peng, Y. , Zhang, J. , Chen, X. , Salzman, C. D. , … Zuker, C. S. (2018). The coding of valence and identity in the mammalian taste system. Nature, 558, 127–131. 10.1038/s41586-018-0165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. J. , & Spence, C. (2017). “A sweet smile”: The modulatory role of emotion in how extrinsic factors influence taste evaluation. Cognition and Emotion, 32(5), 1052–1061. 10.1080/02699931.2017.1386623 [DOI] [PubMed] [Google Scholar]
- Wegman, J. , van Loon, I. , Smeets, P. A. M. , Cools, R. , & Aarts, E. (2018). Top‐down expectation effects of food labels on motivation. NeuroImage, 173, 13–24. 10.1016/j.neuroimage.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Wicker, B. , Keysers, C. , Plailly, J. , Royet, J. P. , Gallese, V. , & Rizzolatti, G. (2003). Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron, 40, 655–664. 10.1016/S0896-6273(03)00679-2 [DOI] [PubMed] [Google Scholar]
- Wilton, M. , Stancak, A. , Giesbrecht, T. , Thomas, A. , & Kirkham, T. (2018). Intensity expectation modifies gustatory evoked potentials to sweet taste: Evidence of bidirectional assimilation in early perceptual processing. Psychophysiology, 56, e13299 10.1111/psyp.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Moeller, S. , Auerbach, E. J. , Strupp, J. , Smith, S. M. , Feinberg, D. A. , … Ugurbil, K. (2013). Evaluation of slice accelerations using multiband echo planar imaging at 3 Tesla. NeuroImage, 14, 384–399. 10.1016/j.neuroimage.2013.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans, M.R. (1998): Taste, palatability and the control of appetite. The Proceedings of the Nutrition Society, 57:609–615. http://www.journals.cambridge.org/abstract_S0029665198000925. [DOI] [PubMed] [Google Scholar]
- Yeung, A. W. K. , Goto, T. K. , & Leung, W. K. (2017). Basic taste processing recruits bilateral anteroventral and middle dorsal insulae: An activation likelihood estimation analysis of fMRI studies. Brain and Behaviour, 7, 1–12. 10.1002/brb3.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, A. W. K. , Goto, T. K. , & Leung, W. K. (2018). Affective value, intensity and quality of liquid tastants/food discernment in the human brain: An activation likelihood estimation meta‐analysis. NeuroImage, 169, 189–199. 10.1016/j.neuroimage.2017.12.034 [DOI] [PubMed] [Google Scholar]
- Yeung, A. W. K. , Tanabe, H. C. , Suen, J. L. K. , & Goto, T. K. (2016). Taste intensity modulates effective connectivity from the insular cortex to the thalamus in humans. NeuroImage, 135, 214–222. 10.1016/j.neuroimage.2016.04.057 [DOI] [PubMed] [Google Scholar]
- Yiannakas, A. , Rosenblum, K. (2017). The insula and taste learning. Frontiers in Molecular Neuroscience, 10: 1–24. http://journal.frontiersin.org/article/10.3389/fnmol.2017.00335/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distribution of the maximum values of motion parameters (respectively for translations TX, TY and TZ and for rotations RX, RY and RZ) during both sweet and bitter related tasks.
Figure S2: Trend of VAS scores for the sweet (a) and the bitter (b) tastes for each of the two repetitions of taste injection performed during the offline test in the group of thirteen subjects not enrolled in the fMRI experiment.
Figure S3: Scatterplots of VAS scores for the positive and negative valences of sweet (a and c) and bitter tastes (b and d), with the corresponding linear fitting, R‐square and P‐values in the group of thirty subjects not enrolled in the fMRI experiment.
Figure S4: Effect of concentration obtained by the 2w‐ANOVA analysis in the right insula, left side FDR corrected with q < 0.05, right side FDR corrected with q < 0.01.
Figure S5: Effect of concentration obtained by the 2w‐ANOVA analysis in the left insula, left side FDR corrected with q < 0.05, right side FDR corrected with q < 0.01.
Data Availability Statement
Relevant data and codes used to generate results of this study are available from the corresponding author upon request.


