Abstract
The aim of the present study was to assess the blood the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and monocyte-lymphocyte ratio (MLR) as prognostic factors in breast cancer (BC) patients. A retrospective analysis of 436 BC patients who were treated at COI (Gliwice, Poland) between January 2005 and June 2018 was performed. The prognostic value [overall survival (OS)] of the pre-treatment PLR, NLR and MLR was assessed by univariate and multivariate analysis. The 5-year OS was lower in the NLR >2.65 compared with that in the NLR≤2.65 group (82.5 vs. 89.6%; P=0.053), and significantly lower in the subgroup of triple-negative breast cancer (TNBC; 70.3 vs. 89.3%; P=0.034) and in patients whose tumors had an estrogen receptor-negative [ER(−)] status (66.6 vs. 83.6%; P=0.018). The 5-year OS was lower in patients with PLR >190.9 compared with that in the PLR≤190.9 group (78.7 vs. 89.4%; P=0.020). A poor OS rate associated with an elevated PLR was also observed in the subgroups with TNBC (68.2 vs. 88.5%; P=0.032) and with ER(−) status tumors (57.7 vs. 83.6%, P=0.002). An elevated MLR (>0.28) was not associated with OS time (P=0.830). Multivariate analysis revealed that the NLR and PLR were insignificant negative prognostic factors, except for the subgroup of patients with ER(−) tumors, where an elevated NLR [hazard ratio (HR)=2.40; 95% confidence interval (CI): 1.20–4.80; P=0.013] and a higher PLR (HR=2.51; 95%CI: 1.23–5.14; P=0.012) were independent prognostic factors for poor OS together with lymph node metastasis ((HR=5.47; 95%CI: 2.46–12.15; P=0.0001 and HR=4.82; 95% CI: 2.15–10.78; P=0.0001), respectively. The present results revealed that an elevated NLR (>2.65) and PLR (>190.9) are associated with poor OS in BC patients. In the ER(−) subgroup of patients, an elevated NLR and PLR were significant independent prognostic factors. However, the MLR did not affect OS.
Keywords: breast cancer, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, monocyte-lymphocyte ratio, overall survival
Introduction
Breast cancer (BC) is a common malignancy in women. In the Silesian region of Poland, the BC-associated morbidity was reported to be 21% of cancer cases in females in the year 2013. Cancer-associated mortality has been reported in 15% of BC patients. Traditional prognostic factors in BC patients are metastases in lymph/axillary nodes, tumor size, tumor grade (histologic or nuclear), vessel infiltration, the estrogen receptor (ER) and progesterone receptor (PR) status, and HER2 overexpression (1).
Inflammation impacts each step of tumorigenesis, including tumor initiation, promotion and metastatic progression (2). Biomarkers including the neutrophil, lymphocyte and platelet count, as well as the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and monocyte-lymphocyte ratio (MLR) are indices of inflammation (3). They have been reported to be prognostic factors in several types of solid tumor. The NLR is defined as neutrophil count divided by lymphocyte count. The prognostic value of the NLR has been confirmed in patients with colorectal cancer (4), hepatocellular carcinoma (5), BC (6), bladder cancer (7), lung cancer (8), pancreatic cancer (9), prostate cancer (10) and renal cell cancer (RCC) (11–13). The PLR is defined as the platelet count divided by the lymphocyte count. The prognostic value of PLR has been studied in patients with various cancer types (14), including gastric cancer (15), colorectal cancer (16), hepatocellular carcinoma (17), ovarian cancer (18), non-small cell lung cancer (19), pancreatic cancer (20), prostate cancer and RCC (21–24).
The LMR is the determined by dividing the lymphocyte count by the monocyte count in the blood. In turn, the MLR is the monocyte count divided by the lymphocyte count in the blood. The prognostic value of the LMR or MLR has been reported in patients with pulmonary squamous cell carcinoma (lung cancer) (25), hepatocellular carcinoma (26), colorectal cancer (27), endometrial cancer (28), pancreatic cancer (29), gastric cancer (30) and ovarian cancer (31). An elevated pre-treatment LMR was reported as a significant positive prognostic factor for patients with locally advanced BC. According to univariate and multivariate Cox regression analyses, elevated LMR levels (≥4.25) were significantly associated with a favorable prognosis regarding disease-free survival (DFS) (32). In line with this, a low pre-operative LMR was reported to be a poor prognostic factor for BC patients (33). A prognostic role of the NLR in BC patients has been determined by certain studies (6,34). A higher pre-treatment peripheral NLR was identified as a significant and independent poor prognostic factor for BC and TNBC (34). Certain meta-analyses have reported that the PLR may be a prognostic factor in BC patients. Zhu et al (35), have demonstrated that a high PLR was associated with worse overall survival (OS) and DFS in BC patients.
The aim of the present study was to evaluate the prognostic value of the PLR, NLR and the MLR in BC patients.
Patients and methods
Patients
The medical records and laboratory results of 436 BC patients who were diagnosed and treated at the MSC Memorial Cancer Centre and Institute of Oncology, Gliwice Branch (Gliwice, Poland) from January 2005 to June 2018 were reviewed. The median age of the patients was 52.5 years (range, 25.2–78.3 years). All of the patients were women and had a good overall performance status (ZUBROD 0–1) (normal activity or symptomatic and ambulatory, cares for self) (36). All patients provided written informed consent regarding the use of their biological material for clinical research (all were routine laboratory analyses). The blood cell parameters were determined at the baseline, before first treatment. Treatment strategies are showed in Table I. In retrospective analysis, patients with PLR (>190.9) (P=0.026) and NLR (>2.65) (P=0.025) significantly more often had received chemotherapy regiments with taxanes. Similarly, patients with elevated PLR (P=0.0001) and NLR (P=0.042) more frequently had no surgery. In contrary, women with lower PLR (P=0.006), NLR (P=0.015) or MLR (P=0.012) were more frequently treated with hormonotherapy. In our study, there was reported no association between radiotherapy and PLR (P=0.359), NLR (P=0.981) or MLR (P=0.225).
Table I.
Treatment strategy.
| NLR, n (%) | PLR, n (%) | MLR, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | All groups (n=436) | NLR≤2.65 (n=346) | NLR>2.65 (n=90) | P-value | PLR ≤190.9 (n=382) | PLR >190.9 (n=54) | P-value | MLR ≤0.28 (n=275) | MLR>0.28 (n=159) | P-value |
| Chemotherapy | 0.0498 | 0.088 | 0.627 | |||||||
| No | 45 (10) | 41 (12) | 4 (4) | 43 (11) | 2 (4) | 30 (11) | 15 (9) | |||
| Yes | 391 (90) | 305 (88) | 86 (96) | 339 (89) | 52 (96) | 245 (89) | 144 (91) | |||
| Chemotherapy regimen | ||||||||||
| Total n for all chemotherapy patients | 391 | 305 | 86 | 339 | 52 | 245 | 144 | |||
| AC FAC | 302 (77) | 241 (79) | 61 (71) | 0.025 | 265 (78) | 37 (71) | 0.026 | 193 (79) | 108 (75) | 0.441 |
| AC + taxanes | 80 (20) | 56 (18) | 24 (28) | 66 (19) | 14 (27) | 47 (19) | 33 (23) | |||
| CMF | 8 (2) | 8 (3) | 0 | 8 (2) | 0 | 5 (2) | 2 (1) | |||
| Other | 1 (0) | 0 | 1 (1) | 0 | 1 (2) | 0 | 1 (1) | |||
| Local treatment | 0.042 | 0.0001 | 0.153 | |||||||
| Mastectomy | 278 (64) | 224 (65) | 54 (60) | 246 (64) | 32 (59) | 177 (64) | 99 (62) | |||
| Breast conservation surgery | 130 (30) | 105 (30) | 25 (28) | 119 (31) | 11 (20) | 85 (31) | 45 (28) | |||
| Without surgery | 28 (6) | 17 (5) | 11 (12) | 17 (4) | 11 (20) | 13 (5) | 15 (9) | |||
| Hormonotherapy | 0.015 | 0.006 | 0.012 | |||||||
| No | 149 (34) | 108 (31) | 41 (46) | 121 (32) | 28 (52) | 82 (30) | 67 (42) | |||
| Yes | 287 (66) | 238 (69) | 49 (54) | 261 (68) | 26 (48) | 193 (70) | 92 (58) | |||
| Radiotherapy | 0.981 | 0.359 | 0.225 | |||||||
| No | 111 (25) | 88 (25) | 23 (26) | 100 (26) | 11 (20) | 75 (27) | 35 (22) | |||
| Yes | 325 (75) | 258 (75) | 67 (74) | 282 (74) | 43 (80) | 200 (73) | 124 (78) | |||
Data are presented as n (%). AC, Adriamycin (or doxorubicin; 60 mg/m2) and Cyclophosphamide (600 mg/m2) treatment; FAC, Fluorouracil (500 mg/m2), Adriamycin (or doxorubicin; 50 mg/m2) and Cyclophosphamide (500 mg/m2) treatment; CMF, Cyclophosphamide (100 mg/m2), Methotrexate (40 mg/m2) and Fluorouracil (600 mg/m2) treatment.
Patients underwent clinical follow-up examinations every three months in the first two years, then every six months until the fifth year after diagnosis and every year thereafter. The inclusion criteria were as follows: BC confirmed by microscopic examination, performance status of ZUBROD 0–1, an age of >18 years, and renal and liver function as well as bone marrow parameters within the normal ranges. The data, including the age at diagnosis, menopausal status, treatment strategy, disease stage according to the Tumor-Nodes-Metastasis classification, tumor histology, estrogen (ER) and progesterone (PR) status, as well as the presence of HER2 overexpression and contralateral BC, were gathered from hospital records and pathology reports. The analysis of the patients' medical records was performed according to national law regulations. The clinicopathological characteristics of the patients are presented in Table II.
Table II.
Patient clinicopathological characteristics.
| NLR | PLR | MLR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All groups (n=436) | NLR≤2.65 (n=346) | NLR>2.65 (n=90) | P-value | PLR ≤190.9 (n=382) | PLR >190.9 (n=54) | P-value | MLR ≤0.28 (n=275) | MLR>0.28 (n=159) | P-value |
| Age, median (Q1-Q3) | 52.5 (44.2–60.8) | 53.5 (45.0–61.0) | 47.7 (41.3–59.5) | 0.021 | 53.4 (44.9–61.1) | 47.7 (42.6–57.0) | 0.022 | 53.5 (45.0–60.7) | 50.8 (43.1–61.7) | 0.002 |
| Menopausal status | 0.021 | 0.035 | 0.209 | |||||||
| Pre- | 224 (51%) | 168 (49%) | 56 (62%) | 189 (49%) | 35 (65%) | 135 (49%) | 88 (55%) | |||
| Post | 212 (49%) | 178 (51%) | 34 (38%) | 193 (51%) | 19 (35%) | 140 (51%) | 71 (45%) | |||
| Tumor size | 0.187 | 0.146 | 0.186 | |||||||
| T1-T2 | 333 (76%) | 269 (78%) | 64 (71%) | 296 (77%) | 37 (69%) | 216 (79%) | 116 (73%) | |||
| T3-T4 | 103 (24%) | 77 (22%) | 26 (29%) | 86 (23%) | 17 (31%) | 59 (21%) | 43 (27%) | |||
| Lymph node status | 0.803 | 0.564 | 0.345 | |||||||
| Negative | 242 (56%) | 191 (55%) | 51 (57%) | 214 (56%) | 28 (52%) | 148 (54%) | 93 (58%) | |||
| Positive | 194 (44%) | 155 (45%) | 39 (43%) | 168 (44%) | 26 (48%) | 127 (46%) | 66 (42%) | |||
| Tumor grade | 0.062 | 0.020 | 0.783 | |||||||
| G1-G2 | 265 (61%) | 218 (63%) | 47 (52%) | 240 (63%) | 25 (46%) | 168 (61%) | 95 (60%) | |||
| G3 | 171 (39%) | 128 (37%) | 43 (48%) | 142 (37%) | 29 (54%) | 107 (39%) | 64 (40%) | |||
| Estrogen receptor | 0.008 | 0.005 | 0.031 | |||||||
| ER(−) | 152 (35%) | 110 (32%) | 42 (47%) | 124 (32%) | 28 (52%) | 86 (31%) | 66 (42%) | |||
| ER(+) | 284 (65%) | 236 (68%) | 48 (53%) | 258 (68%) | 26 (48%) | 189 (69%) | 93 (58%) | |||
| Molecular subtype | 0.242 | 0.028 | 0.580 | |||||||
| Luminal | 171 (39%) | 142 (41%) | 29 (32%) | 157 (41%) | 14 (26%) | 112 (41%) | 58 (36%) | |||
| HER2 positive | 179 (41%) | 140 (40%) | 39 (43%) | 156 (41%) | 23 (43%) | 112 (41%) | 66 (42%) | |||
| Triple negative | 86 (20%) | 64 (18%) | 22 (24%) | 69 (18%) | 17 (31%) | 51 (19%) | 35 (22%) | |||
| WBC (109 cells/l) | 6.38 (5.45–7.69) | 6.18 (5.27–7.30) | 7.64 (6.16–8.97) | 0.0001 | 6.49 (5.49–7.70) | 5.70 (5.11–6.73) | 0.017 | 6.22 (5.30–7.45) | 6.67 (5.65–8.27) | 0.002 |
| Neutrophil count (109 cells/l) | 3.72 (2.96–4.62) | 3.42 (2.79–4.16) | 5.30 (4.22–6.52) | 0.0001 | 3.69 (2.96–4.61) | 3.78 (2.96–4.76) | 0.314 | 3.41 (2.80–4.31) | 4.07 (3.37–5.35) | 0.0001 |
| Lymphocyte (109 cells/l) | 1.92 (1.60–2.28) | 2.01 (1.69–2.41) | 1.51 (1.31–1.82) | 0.0001 | 1.99 (1.68–2.36) | 1.42 (1.11–1.65) | 0.0001 | 2.08 (1.72–2.45) | 1.69 (1.47–1.95) | 0.0001 |
| Monocyte (109 cells/l) | 0.50 (0.40–0.60) | 0.49 (0.39–0.58) | 0.54 (0.42–0.66) | 0.023 | 0.50 (0.41–0.60) | 0.42 (0.35–0.55) | 0.013 | 0.45 (0.36–0.52) | 0.59 (0.50–0.71) | 0.0001 |
| Platelet (109 cells/l) | 252.0 (217.0–292.0) | 250.0 (215.0–290.0) | 260.5 (223.0–295.0) | 0.199 | 248.0 (213.0–281.0) | 317.5 (272.0–367.0) | 0.0001 | 249.0 (215.0–288.0) | 260.0 (221.0–295.0) | 0.123 |
Data are presented as either n (%) or the median (Q1-Q3), as indicated. NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; MLR, monocyte to lymphocyte ratio; T, tumor size; G, tumor grade; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
The prognostic value (regarding OS) of various laboratory parameters, including the PLR, NLR and MLR, was assessed based on univariate and multivariate analysis. The cut-off values were determined using receiver operating characteristic curves. Based on the cut-off values determined, the NLR was considered as ‘elevated’ at >2.65, the MLR value was ‘elevated’ at >0.28 and the PLR was considered ‘elevated’ at >190.9.
Statystical analysis
Statistical analysis was performed using Dell Statistica v.13 software. The frequency of the appearance of side effects was denoted. Qualitative features were presented as the percentage of their occurrence and evaluated with Fisher's test and the Chi-squared test with Yates correction. Continuous data were expressed as the median (first quartile-third quartile) and the significance of differences was identified using the Mann-Whitney U-test. Survival curves were obtained using the Kaplan-Meier method and the log-rank test was performed to determine the significance of differences in survival between subgroups. The relative risk of death was estimated as hazard ratios (HRs) using the Cox proportional hazard regression. NLR and PLR were re-evaluated in multivariate analyses adjusted significant BC prognostic factors. P<0.05 was considered to indicate a statistically significant difference.
Results
Follow-up
The median duration of follow-up was 71 months (range, 3–156 months). The 5- and 10-year OS rates were 88.1 and 80.2%, respectively.
Patients characteristics according to NLR
Patients with an NLR of >2.65 were more frequently of younger age (median 47.7 vs. 53.5 years, P=0.021) and more frequently had a negative ER(−) status (47 vs. 32%, P=0.008) in comparison with the NLR ≤2.65 subgroup. There was no difference between the NLR >2.65 and ≤2.65 groups with regard to tumor size (78 vs. 71%; P=0.187), negative lymph node status (57 vs. 55%; P=0.803) and BC subtype (P=0.242; Table II).
Prognostic value of an elevated NLR
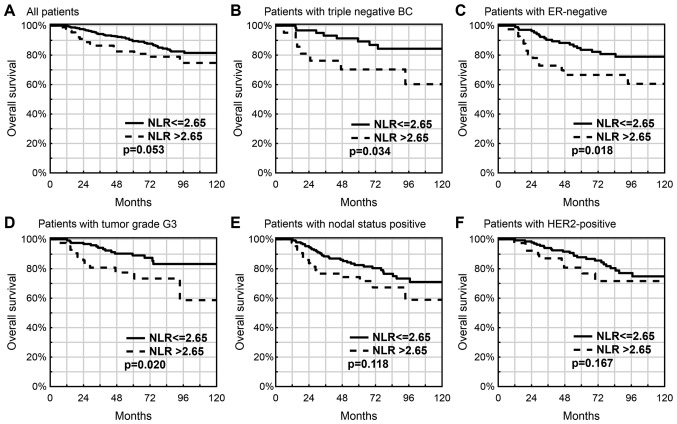
The 5-year OS in the NLR >2.65 subgroup was lower compared with that in the NLR ≤2.65 subgroup (82.5 vs. 89.6%; P=0.053; Fig. 1A), particularly in those patients with triple-negative breast cancer (TNBC; 70.3 vs. 89.3%; P=0.034; Fig. 1B), in patients with ER(−) status tumors (66.6 vs. 83.6%; P=0.018; Fig. 1C) or with a higher tumor grade of G3 (77.4 vs. 89.0%; P=0.020; Fig. 1D). Similar but insignificant association was observed in subgroups with lymph node metastases (74.3 vs. 82.6%; P=0.118; Fig. 1E) and HER2 overexpression (80.8 vs. 87.8%; P=0.167; Fig. 1F).
Figure 1.
Prognostic value of an elevated NLR in breast cancer patients. (A) All patients (P=0.053), (B) patients with triple negative BC (P=0.034), (C) patients with ER-negative (P=0.018), (D) patients with tumor grade G3 (P=0.020), (E) patients with nodal status positive (P=0.118) and (F) patients with HER2-positive (P=0.167). NLR, neutrophil-lymphocyte ratio; BC, breast cancer; ER, estrogen receptor.
Patients characteristics according to PLR
Patients with a high PLR (>190.9) more frequently had a higher histological tumor grade of G3 (54 vs. 37%; P=0.020), an ER(−) status (52 vs. 32%; P=0.005) and TNBC (31 vs. 18%; P=0.028) in comparison with those with a low PLR (Table II).
Prognostic value of an elevated PLR
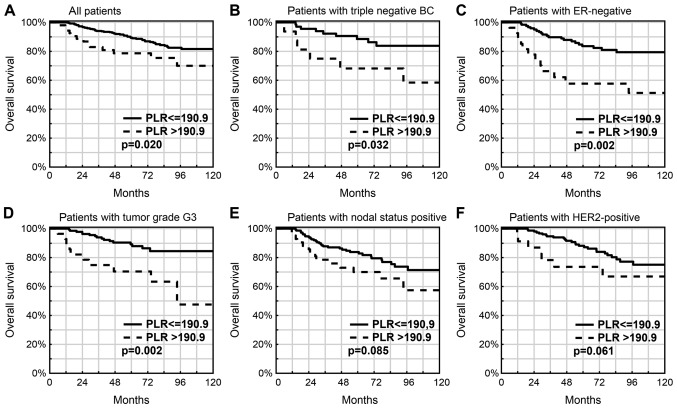
The 5-year OS was lower in patients with a PLR of >190.9 compared with that in patients with a PLR of ≤190.9 (78.7 vs. 89.4%; P=0.020; Fig. 2A). A PLR of >190.9 was also associated with a worse OS rate in the subgroups with TNBC (68.2 vs. 88.5%; P=0.032; Fig. 2B), ER(−) status tumors (57.7 vs. 83.6%; P=0.002; Fig. 2C) or tumors with a higher histological grade of G3 (70.4 vs. 89.2%; P=0.002; Fig. 2D), lymph node metastases (70.0 vs. 83.8%; P=0.085; Fig. 2E), tumors with HER2 overexpression (73.7 vs. 88.2%; P=0.061; Fig. 2F) and the non-Luminal BC subtype (43.6 vs. 74.8%; P=0.018) and the presence of BRCA mutation (61.4 vs. 81.6%; P=0.058).
Figure 2.
Prognostic value of an elevated PLR in breast cancer patients. (A) All patients (P=0.020), (B) patients with triple negative BC (P=0.032), (C) patients with ER-negative (P=0.002), (D) patients with tumor grade G3 (P=0.002), (E) patients with nodal status positive (P=0.085) and (F) patients with HER2-positive (P=0.061). PLR, platelet-lymphocyte ratio; BC, breast cancer; ER, estrogen receptor.
Patients characteristics according to MLR
Patients with an elevated MLR (>0.28) more frequently had an ER(−) status (42 vs. 31%; P=0.031) compared with those with a lower MLR. There was no difference between the high and low MLR groups regarding the tumor size (73 vs. 76%; P=0.186), the presence of lymph node metastases (58 vs. 56%; P=0.345) and the frequency of a histological tumor grade G3 (40 vs. 39%; P=0.783; Table II).
Prognostic value of an elevated MLR
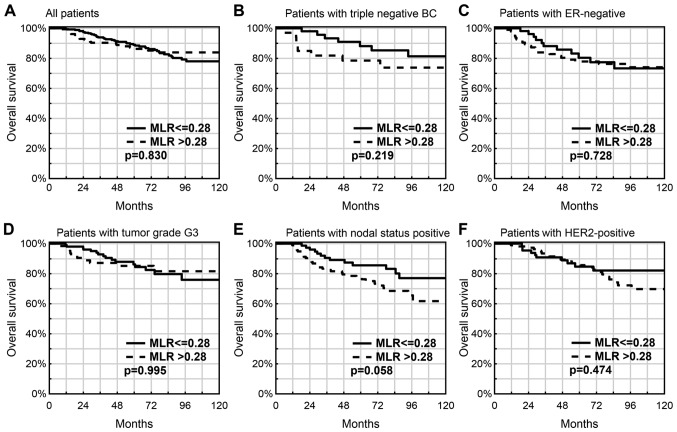
In the cohort of the present study, an ‘elevated’ MLR (>0.28) was not associated with OS time (P=0.830; Fig. 3A), also not in the subgroups with TNBC (P=0.219; Fig. 3B), ER(−) (P=0.453; Fig. 3C), G3 (P=0.995; Fig. 2D) and HER2 overexpression (P=0.474; Fig. 3F). However, a worse OS rate was observed in patients with lymph node metastases and an ‘elevated’ MLR (77.5 vs. 85.6%; P=0.058; Fig. 2E).
Figure 3.
Prognostic value of an elevated MLR in breast cancer patients. (A) All patients (P=0.830), (B) patients with triple negative BC (P=0.219), (C) patients with ER-negative (P=0.728), (D) patients with tumor grade G3 (P=0.995), (E) patients with nodal status positive (P=0.058) and (F) patients with HER2-positive (P=0.474). MLR, monocyte-lymphocyte ratio; BC, breast cancer; ER, estrogen receptor.
Univariate and multivariate analysis
Univariate Cox regression analyses of OS showed prognostic significance for factors such as patient's age [hazard ratio (HR)=1.03; 95% confidence interval (CI): 1.00–1.05; P=0.018], tumor size (T3-4 vs. T1-2, HR=2.75; 95% CI: 1.69–4.48; P=0.0001), the presence of lymph node metastases (N+ vs. N0, (HR=3.74; 95% CI: 2.17–6.46; P=0.0001), estrogen receptor status (ER(+) vs. ER(−), HR=0.51; 95% CI: 0.32–0.83; P=0.007) and PLR (PLR>190.9 vs. ≤190.9, HR=2.02; 95% CI: 1.12–3.65; P=0.020). Factors such as menopausal status, tumor grade, HER2 overexpression, NLR and MLR were not statistically significant (Table III).
Table III.
Univariate and multivariate analysis in all breast cancer patients.
| Univariate analysis | NLR Multivariate analysis | PLR Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value |
| Age | 1.03 (1.00–1.05) | 0.018 | 1.03 (1.01–1.06) | 0.005 | 1.03 (1.01–1.06) | 0.006 |
| Status postmenopausal vs. pre | 1.36 (0.83–2.22) | 0.218 | ||||
| T3-T4 vs. T1-T2 | 2.75 (1.69–4.48) | 0.0001 | 1.97 (1.17–3.31) | 0.010 | 1.97 (1.17–3.30) | 0.010 |
| N+ vs. N0 | 3.74 (2.17–6.46) | 0.0001 | 3.65 (2.09–6.37) | 0.0001 | 3.56 (2.03–6.23) | 0.0001 |
| G3 vs. G1-G2 | 1.27 (0.77–2.09) | 0.344 | ||||
| ER(+) vs. ER(−) | 0.51 (0.32–0.83) | 0.007 | 0.53 (0.32–0.89) | 0.016 | 0.54 (0.32–0.91) | 0.021 |
| HER2 positive vs. HER2 negative | 1.56 (0.97–2.54) | 0.069 | ||||
| NLR>2.65 vs. NLR≤2.65 | 1.70 (1.00–2.90) | 0.050 | 1.58 (0.92–2.72) | 0.100 | ||
| PLR >190.9 vs. PLR ≤190.9 | 2.02 (1.12–3.65) | 0.020 | 1.55 (0.83–2.88) | 0.170 | ||
| MLR>0.28 vs. MLR ≤0.28 | 0.94 (0.56–1.58) | 0.829 | ||||
NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; HR, hazard ratio; CI, confidence interval; T, tumor size; N, node; G, tumor grade; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; MLR, monocyte to lymphocyte ratio.
Multivariate analysis revealed that the NLR and PLR are insignificant negative prognostic factors in all BC patients (Table III). Negative prognostic factors were: Patients age, tumor size and lymph node metastases. In contrary, positive prognostic factor was positive steroid receptor status (ER+). However, analysis of the subgroup of patients with ER(−) tumors indicated that a higher NLR (HR=2.40; 95% CI: 1.20–4.80; P=0.013) and a higher PLR (HR=2.51; 95% CI: 1.23–5.14; P=0.012) were independent prognostic factors for a lower OS together with metastatic lymph nodes (HR=5.47; 95% CI: 2.46–12.15; P=0.0001 and HR=4.82; 95% CI: 2.15–10.78; P=0.0001, respectively; Table IV).
Table IV.
Multivariate analysis of the subgroup of patients with ER negative and grade G3 tumors.
| NLR Multivariate analysis | PLR Multivariate analysis | |||
|---|---|---|---|---|
| Patient group | HR (95%CI) | P-value | HR (95%CI) | P-value |
| Patients with ER negative | ||||
| N+ vs. N0 | 5.47 (2.46–12.15) | 0.0001 | 4.82 (2.15–10.78) | 0.0001 |
| NLR >2.65 vs. ≤2.65 | 2.40 (1.20–4.80) | 0.013 | – | – |
| PLR >190.9 vs. ≤190.9 | – | – | 2.51 (1.23–5.14) | 0.012 |
| Patients with tumor grade G3 | ||||
| T3-T4 vs. T1-T2 | – | – | 1.99 (0.88–4.49) | 0.098 |
| N+ vs. N0 | 4.04 (1.73–9.40) | 0.001 | 3.53 (1.51–8.25) | 0.004 |
| ER(+) vs. ER(−) | 0.28 (0.12–0.68) | 0.005 | 0.37 (0.15–0.94) | 0.036 |
| NLR >2.65 vs. ≤2.65 | 2.14 (0.97–4.68) | 0.058 | – | – |
| PLR >190.9 vs. ≤190.9 | – | – | 2.61 (1.15–5.89) | 0.021 |
NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; HR, hazard ratio; CI, confidence interval; T, tumor size; N, node; ER, estrogen receptor.
Discussion
In this retrospective study, we reported the prognostic value of the NLR, PLR and MLR in BC patients. The influence of the NLR, MLR and PLR on the survival time (OS or DFS) of BC patients has been investigated in numerous studies (6,34,35).
In the present study, no association between an elevated MLR (>0.28) and the OS time was identified (P=0.830), also not in the subgroups with TNBC (P=0.219) and ER(−) status (P=0.453). An elevated pre-treatment (prior to neoadjuvant chemotherapy) peripheral blood LMR was reported to be a significantly favorable prognostic factor for patients with locally advanced BC. Univariate and multivariate analysis confirmed that a higher LMR (≥4.25) was significantly associated with favorable DFS (P = 0.009 and P = 0.011, respectively). In addition, univariate analysis revealed an increased probability of DFS in patients with a higher lymphocyte count (≥1.5×109/l). However, a lower monocyte count (<0.4×109/l) was associated with a significantly better prognosis regarding DFS (P = 0.010) (32). The pre-operative LMR (prior to neoadjuvant chemotherapy) as a prognostic factor in BC patients was also analyzed in a meta-analysis by Hu et al (33), revealing that a low LMR was significantly associated with a worse prognosis regarding OS (HR=0.65; 95% CI: 0.47–0.90; P=0.009) and DFS (HR=0.60; 95% CI: 0.49–0.74; P<0.001). Subgroup analyses indicated that a low LMR had a negative impact on the prognosis regarding OS in Asian populations with triple-negative BC without metastases. However, no association between a low LMR and clinicopathological factors was identified (33). Our data did not confirmed above mentioned results. In our study MLR was not prognostic factor according to OS, also in subgroup analysis. However, we did not analyze DFS.
In the present study, a higher NLR was associated with a lower 5-year OS rate, particularly in the subgroup of TNBC (P=0.034), in patients with ER(−) status tumors (P=0.018) and in patients with G3 (P=0.020). Similar but insignificant association was observed in subgroups with lymph node metastases (P=0.118) and HER2 overexpression (P=0.167). In a previous study, Chen et al (6) suggested that a higher NLR may be a prognostic factor regarding OS with an HR of 2.28 (95% CI: 1.08–4.80; Pheterogeneity<0.001), particularly in Caucasian populations (HR=4.53; 95% CI: 3.11–6.60; Pheterogeneity=0.096). An elevated NLR was also associated with a high risk regarding DFS (HR=1.38; 95% CI=1.09–1.74; Pheterogeneity=0.050) (36). In an analysis conducted by Jia et al (34), a higher pre-treatment level of NLR (before neo-adjuvant chemotherapy) was identified as a significant and independent poor prognostic factor for BC patients, particularly in the TNBC subgroup. The higher NLR was a better prognostic factor in comparison to a lower LMR. Univariate analysis indicated that a lower NLR (≤2.0) and a higher LMR (>4.8) were significantly associated with a better DFS in TNBC patients (P=0.007 and 0.011, respectively). By contrast, in other molecular BC subtypes (luminal subtype: ER+ and/or PR+ and HER2-; HER2-positive subtype: HER2+), no significant association between the NLR or the LMR with survival (DFS or OS) was identified (34). Our study support the results of previous dates. We confirm NLR to be negative prognostic factor, especially for subgroups with TNBC, ER negative status or G3 tumors. In study conducted by Li et al (37) NLR in healthy people was positively associated with age. There was reported the highest NLR in the eldest age group. In contrary, the youngest age group had the lowest NLR. NLR was also slightly positively associated with blood pressure, and BMI (P<0.001). In our group, patients with an NLR of >2.65 were more frequently of younger age (median 47.7 vs. 53.5 years, P=0.021).
Another hematological parameter examined as a prognostic factor in BC patients is the PLR. In the present study, a lower 5-year OS in patients with PLR>190.9 in comparison with those with PLR≤190.9 was observed, particularly in the subgroup with TNBC (P=0.032) and in those patients with ER(−) status tumors (P=0.002) and in those patients with G3 (P=0.002). A meta-analysis conducted by Zhu et al (35), revealed that the PLR is an unfavorable prognostic factor in BC patients. In that study, a higher PLR was associated with a worse OS (HR=1.55; 95% CI: 1.07–2.25; P=0.022) and DFS (HR=1.73; 95% CI: 1.3–2.3; P<0.001) in BC patients. An elevated PLR was associated with worse OS in Asian populations and with poor DFS in Asian as well as non-Asian subgroups. In addition, PLR was identified as a significant prognostic factor for OS (HR=1.78; 95% CI=1.06–2.99; P=0.03) and DFS in patients who receive chemotherapy (HR=2.6; 95% CI=1.47–4.61; P=0.001). Furthermore, the study reported an association between PLR and the presence of HER-2 overexpression (odds ratio=1.48; 95% CI: 1.2–1.83; P<0.001) (35). Results of our study confirm the role of elevated PLR as a negative prognostic factor in BC patients, particularly in the subgroups with TNBC, ER(−) status tumors or tumors with a higher histological grade of G, lymph node metastases, tumors with HER2 overexpression and the non-Luminal BC subtype and the presence of BRCA mutation.
An elevated pre-treatment NLR (>2.65) (insignificantly) and PLR (>190.9) (significantly) was associated with a worse prognosis regarding OS in BC patients. In univariate analysis higher NLR and PLR were significantly negative prognostic factors for subgroups such as: TNBC, ER(−) and with a higher tumor grade of G3. However, the MLR did not affect OS. In multivariate analyses in the ER(−) subgroup of patients, an elevated NLR and PLR were significant independent prognostic factors.
Acknowledgements
The authors would like to thank Dr. Jolanta Mrochem-Kwarciak (Analytics and Clinical Biochemistry Department) for the laboratory assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
JH analyzed and interpreted the patients' data and was a major contributor in writing the manuscript. ZK performed the statistical analyses, and analyzed and interpreted the data.
Ethics approval and consent to participate
At the time of venous blood collection for genetic diagnostic testing, all patients provided written informed consent. The present study analyzed the results of these genetic diagnostic tests retrospectively.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bundred NJ. Prognostic and predictive factors in breast cancer. Cancer Treat Rev. 2001;27:137–142. doi: 10.1053/ctrv.2000.0207. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutterer GC, Stoeckigt C, Stojakovic T, Jesche J, Eberhard K, Pummer K, Zigeuner R, Pichler M. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. UrolOncol. 2014;32:1041–1048. doi: 10.1016/j.urolonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 5.Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Deng Q, Pan Y, He B, Ying H, Sun H, Liu X, Wang S. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio. 2015;5:502–507. doi: 10.1016/j.fob.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: A systematic review and meta-analysis. Int J Clin Oncol. 2017;22:817–825. doi: 10.1007/s10147-017-1171-5. [DOI] [PubMed] [Google Scholar]
- 8.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16:434–440. doi: 10.1016/j.pan.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Zhu X, Zhao X, Li XF, Xu R. Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis in prostate cancer: A systematic review and meta-analysis. PLoS One. 2016;11:e0158770. doi: 10.1371/journal.pone.0158770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semeniuk-Wojtaś A, Lubas A, Stec R, Syryło T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: A systemic review and meta-analysis. Clin Genitourin Cancer. 2018;16:e685–e693. doi: 10.1016/j.clgc.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A meta-analysis. BMJ Open. 2015;5:e006404. doi: 10.1136/bmjopen-2014-006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YH, Ku JH, Kwak C, Kim HH. Post-treatment neutrophil-to-lymphocyte ratio in predicting prognosis in patients with metastatic clear cell renal cell carcinoma receiving sunitinib as first line therapy. Springerplus. 2014;3:243. doi: 10.1186/2193-1801-3-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z, Xu W, Cheng H, Shen W, Ying J, Cheng F, Xu W. The prognostic role of the platelet-lymphocytes ratio in gastric cancer: A meta-analysis. PLoS One. 2016;11:e0163719. doi: 10.1371/journal.pone.0163719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837. doi: 10.1097/MD.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Si G, Zhu F, Hui J, Cai S, Huang C, Cheng S, Fathy AH, Xiang Y, Li J. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2017;8:22854–22862. doi: 10.18632/oncotarget.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–273. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi F, Gu Y, Chen S, Liu Y, Yin W, Zhang Y, Cao B. Impact of the pretreatment or posttreatment NLR and PLR on the response of first line chemotherapy and the outcomes in patients with advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2018;21:481–492. doi: 10.3779/j.issn.1009-3419.2018.06.02. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: A comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. 2018;5:1899–1908. doi: 10.2147/OTT.S154162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Liu Y, Zhang N, Li X, Xin P, Bi J, Kong Ch. Prognostic role of pretreatment platelet to lymphocyte ratio in urologic cancer. Oncotarget. 2017;8:70874–70882. doi: 10.18632/oncotarget.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sciarra A, Gentilucci A, Salciccia S, Pierella F, Del Bianco F, Gentile V, Silvestri I, Cattarino S. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: A critical review. J Inflamm (Lond) 2016;13:35. doi: 10.1186/s12950-016-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunduz S, Mutlu H, Tural D, Yıldız Ö, Uysal M, Coskun HS, Bozcuk H. Platelet to lymphocyte ratio as a new prognostic for patients with metastatic renal cell cancer. Asia Pac J Clin Oncol. 2015;11:288–292. doi: 10.1111/ajco.12358. [DOI] [PubMed] [Google Scholar]
- 24.Park TJ, Cho YH, Chung HS, Hwang EC, Jung SH, Hwang JE, Bae WK, Kim JW, Heo SH, Hur YH, et al. Prognostic significance of platelet-lymphocyte ratio in patients receiving first-line tyrosine kinase inhibitors for metastatic renal cell cancer. Springerplus. 2016;5:1889. doi: 10.1186/s40064-016-3592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minami S, Ihara S, Komuta K. Pretreatment lymphocyte to monocyte ratio as a prognostic marker for advanced pulmonary squamous cell carcinoma treated with chemotherapy. J Clin Med Res. 2018;10:657–664. doi: 10.14740/jocmr3490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang YT, Jiang JH, Yang HJ, Wu ZJ, Xiao ZM, Xiang BD. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival compared to established biomarkers in HCC patients undergoing liver resection. Sci Rep. 2018;8:2535. doi: 10.1038/s41598-018-20199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, Hu T, Zheng E, Deng X, Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: An up-to-date meta-analysis. Medicine (Baltimore) 2017;96:e7051. doi: 10.1097/MD.0000000000007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimyon Cömert G, Türkmen O, Kar İ, Sınacı S, Yılmaz Ergani S, Karalök A, Başaran D, Turan T. Independent predictors of survival in endometrium cancer: Platelet-to-lymphocyte ratio and platelet/neutrophil/monocyte-to-lymphocyte ratio. J Turk Ger Gynecol Assoc. 2018;19:78–86. doi: 10.4274/jtgga.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Tao L, Zhang L, Xiu D. Prognostic role of lymphocyte to monocyte ratio for patients with pancreatic cancer: A systematic review and meta-analysis. Onco Targets Ther. 2017;10:3391–3397. doi: 10.2147/OTT.S142022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int J Surg. 2018;50:67–71. doi: 10.1016/j.ijsu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Xiang J, Zhou L, Li X, Bao W, Chen T, Xi X, He Y, Wan X. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017;10:33–39. doi: 10.1016/j.tranon.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni XJ, Zhang XL, Ou-Yang QW, Qian GW, Wang L, Chen S, Jiang YZ, Zuo WJ, Wu J, Hu X, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9:e111886. doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu RJ, Liu Q, Ma JY, Zhou J, Liu G. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: A meta-analysis. Clin Chim Acta. 2018;84:1–6. doi: 10.1016/j.cca.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen K, Su F. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One. 2015;10:e0143061. doi: 10.1371/journal.pone.0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Si W, Sun Q, Qin B, Zhao W, Yang J. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget. 2017;8:1023–1030. doi: 10.18632/oncotarget.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West HJ, Jin JO. Performance status in patients with cancer. JAMA Oncol. 2015;1:998. doi: 10.1001/jamaoncol.2015.3113. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Chen Q, Luo X, Hong J, Pan K, Lin X, Liu X, Zhou L, Wang H, Xu Y, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal. 2015;29:437–443. doi: 10.1002/jcla.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.