Abstract
Changes in brain morphology are hypothesized to be an underlying process that drive the widespread pain and motor impairment in patients with chronic neck pain. However, no earlier research assessed whole‐brain cortical morphology in these patients. This case–control study assesses group‐differences in whole‐brain morphology between female healthy controls (HC; n = 34), and female patients with chronic idiopathic neck pain (CINP; n = 37) and whiplash‐associated disorders (CWAD; n = 39). Additionally, the associations between whole‐brain morphology and motor performance including balance, strength, and neuromuscular control were assessed. Cortical volume, thickness, and surface area were derived from high resolution T1‐weighted images. T2*‐weighted images were obtained to exclude traumatic brain injury. Vertex‐wise general‐linear‐model‐analysis revealed cortical thickening in the left precuneus and increased volume in the left superior parietal gyrus of patients with CINP compared to HC, and cortical thickening of the left superior parietal gyrus compared to HC and CWAD. Patients with CWAD showed a smaller cortical volume in the right precentral and superior temporal gyrus compared to HC. ANCOVA‐analysis revealed worse neuromuscular control in CWAD compared to HC and CINP, and in CINP compared to HC. Patients with CWAD showed decreased levels of strength and sway area compared to CINP and HC. Partial correlation analysis revealed significant associations between the volume of the precentral gyrus, and neuromuscular control and strength together with an association between the volume of the superior temporal gyrus and strength. Our results emphasize the role of altered gray matter alterations in women with chronic neck pain, and its association with pain and motor impairment.
Keywords: chronic pain, FreeSurfer, idiopathic neck pain, primary motor cortex, surface‐based morphology, whiplash‐associated disorder
1. INTRODUCTION
Magnetic resonance imaging (MRI) research has identified morphological gray matter alterations in different chronic musculoskeletal pain conditions such as chronic back pain, fibromyalgia, and osteoarthritis (Baliki, Schnitzer, Bauer, & Apkarian, 2011; Cagnie et al., 2014; Kregel et al., 2015; May, 2011; Smallwood et al., 2013). Surprisingly, the amount of brain research in chronic neck pain is limited (Coppieters et al., 2017, 2018), although chronic neck pain will affect 3% of all people (Breivik, Collett, Ventafridda, Cohen, & Gallacher, 2006; Fejer, Kyvik, & Hartvigsen, 2006; Phillips, 2009; Reid et al., 2011), making it the 6th leading cause of disability world‐wide (Vos et al., 2017).
The majority of these patients suffers from nonspecific pain, which is often classified into subgroups of patients with chronic traumatic neck pain (whiplash‐associated disorders; CWAD) and patients with chronic nontraumatic neck pain (idiopathic neck pain; CINP) (Guzman et al., 2009). Both groups show shared signs of motor impairment, which often consist of impairment in postural control (De Pauw et al., 2018; Ruhe, Fejer, & Walker, 2011; Silva & Cruz, 2013), neuromuscular control (De Pauw et al., 2018; Falla, 2004; Meisingset et al., 2015), and strength (Cagnie, Cools, De Loose, Cambier, & Danneels, 2007; De Pauw et al., 2018; Meisingset et al., 2015; Pearson, Reichert, De Serres, Dumas, & Côté, 2009). In contrast, signs of central hyperexcitability (Malfliet et al., 2015), cognitive impairment, and emotional impairment (Coppieters et al., 2015) have only been identified in patients with CWAD. However, some have questioned the need to distinguish both groups as observed differences are often small (Anstey, Kongsted, Kamper, & Hancock, 2016).
In (chronic) neck pain, researchers have formerly attempted to uncover the neural substrate of pain using a myriad of medical imaging techniques including magnetic resonance imaging (MRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT), which has led to only a little increase in knowledge (De Pauw et al., 2017). Most of these studies focused on macrostructural alterations or clinical signs, but were unable to demonstrate findings of edema and/or lesions (Borchgrevink et al., 1997; Karlsborg et al., 1998). Only in post‐traumatic headache, subtle changes in the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) were reported (Obermann et al., 2009). Two recent studies by Coppieters et al. (2017, 2018) assessed a restricted set of brain regions that were involved in pain processing and cognition. Their results showed a selective thinning in the left precuneus, together with a decrease in gray matter volume in the right superior parietal cortex and left cingulate cortex in patients with CINP compared to CWAD. Additionally, a decrease in the right lateral orbitofrontal cortex, left supramarginal cortex, and left posterior cingulate cortex was revealed in patients with CWAD. These changes did furthermore correlate with cognitive performance and pain processing.
Originally, it was believed that a static number of brain regions was involved in the human pain‐experience, that is, the so‐called “pain matrix” (PM) (May, 2008; Melzack, 1990; Ploghaus et al., 1999). Indeed, Wager et al. (2013) were able to synthesize a neurological signature for pain in response to heat with a 93% sensitivity and specificity. Brain regions involved in this signature are the thalamus, posterior and anterior insulae, secondary somatosensory cortex, anterior cingulate cortex, and periaqueductal gray matter. The functional response of this signature furthermore highly correlated with the reported level of pain. There are however almost no specific sites exclusively for pain responses (Garcia‐Larrea & Peyron, 2013; Kucyi & Davis, 2017), as many of these apparent pain‐related regions are also involved in the cognitive‐evaluative and motivational‐affective dimension of pain (Garcia‐Larrea & Peyron, 2013; Kucyi & Davis, 2017). For example, a perceptual set of regions is activated in contexts beyond pain, but this set is nevertheless important in making pain a conscious experience (Garcia‐Larrea & Peyron, 2013). More recently, research has emphasized the complex temporal and spatial coding that derives pain, that is, the dynamic pain connectome (Kucyi & Davis, 2015, 2017). This dynamic pain connectome considers the interaction between the overall intrinsic activity of the brain and the ongoing activity (e.g., induced by pain). This concept furthermore emphasizes the individual‐to‐disorder‐specific character of brain fluctuations in the presence of pain (Farmer, Baliki, & Apkarian, 2012). Similarly to these functional alterations, longlasting pain results in structural brain alterations in different regions such as the cingular cortex, insular cortex, temporal lobe, frontal cortex, prefrontal cortex, thalamus/basal ganglia, motor cortex, brain stem, and dorsolateral prefrontal cortex (May, 2011). Unlike the initial idea of the static PM, changes in gray matter morphology are similarly noticeably diverse across different pain syndromes (Baliki et al., 2011; Baliki, Geha, Apkarian, & Chialvo, 2008; Smallwood et al., 2013). Fortunately, these changes do not reflect permanent brain damage since some authors observed a normalization after therapy or after cessation of the pain (Kucyi & Davis, 2017; May, 2011).
The most common technique implemented in the assessment of cortical/gray matter changes is voxel‐based morphometry (VBM), a straightforward approach that involves voxel‐wise comparison of local concentrations of gray matter between different groups (Ashburner & Friston, 2000). Surface‐based morphometry (SBM) is a more complex approach with some major advantages over VBM, such as the improved reduction of intersubject variability (Argall, Saad, & Beauchamp, 2006), and the provision of more specific information on the nature of the observed morphological alteration (expressed as a change in cortical thickness, cortical area, or cortical volume) (Fischl, 2012). Cortical volume has been expressed as a function between cortical area and cortical thickness, of which only cortical area correlates with gray matter volume (Winkler et al., 2010). Previous research has shown this nonuniformity across morphological measures, and in some specific disorders or processes, only one measure is affected (Lemaitre et al., 2012). However, most of the previously conducted studies in (chronic neck) pain involve regions of interest (ROI)‐based approaches, based on a priori knowledge of regions that are known to be involved in the PM. However, it is likely that more and/or other regions are involved in the process of chronic neck pain.
So far, only two studies have investigated the existence of neuroplastic changes in patients with chronic neck pain, albeit only in a selective set of regions and only with regard to cortical volume and thickness. In addition, information on the association between morphological alterations and motor impairment observed in these patients is currently lacking. Therefore, the aims of this study are (a) to assess the presence of brain morphological alterations based on a whole‐brain SBM approach in patients with chronic traumatic and nontraumatic neck pain and (b) to assess the association between morphological alterations and motor impairments in these patients.
2. METHODS
2.1. Participants
Participants (n = 110) were female, Dutch native speakers aged between 18 and 65 years, who were recruited via internet, flyers, and posters. Inclusion criteria for patients with CWAD and CINP were persistent neck pain (>3 months) with an average pain intensity of more than 3/10 on the Verbal Numeric Rating Scale (VNRS), mild/moderate to severe pain‐related disability (≥10/50 on the Neck Disability Index [NDI]) (Vernon, 2008), and stability of pain medication for at least 4 weeks prior to study participation. Patients with CWAD were only included if they were classifiable as WAD II A, B, or C according to the modified Quebec Task Force Scale (Spitzer et al., 1995; Sterling, 2004), and if they did not report loss of consciousness during or after the trauma to exclude possible mild TBI patients. Healthy pain‐free women (HC) could only participate if they were pain‐free on the test day (VNRS < 2/10), had no history of neck‐shoulder‐arm pain for more than eight consecutive days during the last year (average VNRS ≥ 2/10), a score of less than 8 out of 50 on the NDI, no medical consultation for neck‐shoulder‐arm pain during the last year and no history of a whiplash trauma.
General exclusion criteria for all study groups were psychiatric illness, neurologic, metabolic, cardiovascular disorders, inflammatory conditions, fibromyalgia, chronic fatigue syndrome, and a history of neck or shoulder girdle surgery. Furthermore, pregnant women and women 1 year postnatal were excluded. All participants were asked to stop intake of nonopioid analgesics 48 hours prior to study participation. In addition, participants were asked not to undertake heavy physical exertion, and to refrain from consuming alcohol, caffeine, and nicotine on the day of testing. If micro‐hemorrhage were observed, participants were as well excluded. Ethical clearance was received from the Ghent University Hospital ethical committee under registration number EC/2013/1053. Written informed consent was obtained from each participant prior to participation.
2.2. Self‐reported symptoms
Participants scored their neck pain intensity on a VNRS, a usable and valid pain rating scale (Hjermstad et al., 2011), with scores ranging from 0 to 10, with 0 reflecting “no pain” and 10 reflecting “the worst pain imaginable.” Self‐reported disability was assessed with the Dutch version of the NDI (Vernon, 2008), which has demonstrated high reliability and validity (Ailliet, Rubinstein, De Vet, Van Tulder, & Terwee, 2015; Jorritsma, de Vries, Dijkstra, Geertzen, & Reneman, 2012). The scale includes 10 items: pain intensity, personal care, lifting, reading, headache, concentration, work, driving, sleeping, and recreation, whereby each of item has six response categories ranging from 0 to 5 (with 0 “no disability” and 5 “excessive disability”), resulting in a total score ranging up to 50. Higher scores indicate increased self‐reported disability (Vernon, 2008).
2.3. Motor performance
Three motor domains were included: postural, strength, and neuromuscular control.
Strength was measured using a Hand‐held dynamometer (MicroFET 2, Hoggan Health Industries Inc., Biometrics, The Netherlands), an apparatus with a good intertester and intratester reliability (Strimpakos & Oldham, 2001). The subject was seated with the thorax stabilized. Places of resistance were the forehead (frontal bone), the occiput, and just above the left and right ear (parietal bone) for flexion (M. Sternocleidomastoideus, M. Longus Colli, M. Longus Capitis, M. Rectus Capitis anterior & Mm. Scaleni), extension (M. Trapezius, M. Levator Scapulae, M. Splenius Capitis, M. Semispinalis capitis, M. Splenius Cervicis), and left and right side bending respectively (M. Sternocleidomastoid, M. Trapezius, M. Levator Scapulae). Patients were asked to perform three consecutive trials with a 10 s rest‐interval. The maximum of three strength‐measures was included in the final dataset. All measurements were recorded in Newton (N) with a threshold of 3.6N and a sensitivity of 0.4N.
Postural control was assessed with an AMTI ACG portable forceplate (50 cm × 50 cm) (Advanced Medical Technology, Inc., Watertown, MA), which was connected to the standard amplifier to record changes in displacement of the Center of Pressure (CoP). Before each trial, the force plate was calibrated. The subject was placed on a firm surface, feet placed at hip width, and eyes closed to measure postural control under high sensory load. CoP‐data was acquired via three consecutive measurements of 90 s using a sampling frequency of 100 Hz to obtain reliable results (Ruhe, Fejer, & Walker, 2010). Using MATLAB R2015a (MathWorks, Inc.), the raw data were filtered using a fourth order low pass digital Butterworth filter with a cut‐off frequency of 5 Hz. Afterwards the following CoP parameters were computed: mean sway velocity (cm2/s), and the 95% confidence ellipse area (cm2) (Silva & Cruz, 2013). A higher area often reflects a higher sway, indicating a worse postural control strategy.
Neuromuscular control, which reflects the capability to spontaneously contract a specific muscle, was assessed with the craniocervical flexion test (CCFT) and the scapular holding test (SHT). The CCFT has been proved to be a valid (Cagnie et al., 2008; Falla, Jull, Dall'Alba, Rainoldi, & Merletti, 2003) and reliable (Wing Chiu, Hung Law, & Fai Chiu, 2005) test in patients with and without neck pain. No data is currently available for the reliability or validity of the SHT. For both tests a form with specific criteria assessing neuromuscular control, movement control and endurance was constructed resulting in a score ranging from 0 to 10 with a lower score indicating more neuromuscular impairment. Besides, the endurance of the cervical flexor muscles was measured using the protocol described by Olson, Millar, Dunker, Hicks, and Glanz (2006), who obtained a high intertester and intratester reliability. A more detailed description can be found in appendix.
2.4. Imaging protocol
A Siemens 3T TrioTim scanner and a standard 32‐channel head coil was used for MRI data acquisition. High‐resolution whole‐brain T1‐weighted anatomical scans were obtained with a 3D‐T1 MPRAGE sequence (voxel size = 1.00 × 1.00 × 1.00 mm3, TR = 2,250 ms, TE = 4.18 ms, flip angle = 3°, 176 coronal slices, FoV‐matrix = 256 × 256 mm3, and a TA = 5.14 min). In addition, high‐resolution whole‐brain T2*‐weighted images were obtained to assess potential micro‐hemorrhage in participants (voxel size of 1.00 × 0.70 × 3.00 mm3, TR of 839 ms, TE of 18.6 ms, flip angle of 20°, 33 transversal slices, FoV of 230 × 230 × 230 mm3, and a TA of 3.48 min).
2.5. Cortical measures
Cortical volume, thickness, and area were all estimated using the FreeSurfer software version 5.3.0 (http://surfer.nmr.mgh.harvard.edu). Full details on the implemented methods can be found elsewhere (see Fischl, 2012). Preprocessing included intensity normalization, removal of nonbrain tissue, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, tessellation of the gray matter white matter boundary, automated topology correction (Fischl, Liu, & Dale, 2001; Ségonne, Pacheco, & Fischl, 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. The data was registered to a spherical atlas which is based on individual cortical folding patterns to match cortical geometry across participants (Fischl, Sereno, Tootell, & Dale, 1999), the cerebral cortex is parcellated into units with respect to gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004). A variety of surface based metrics are obtained including cortical thickness, cortical volume, and cortical surface area. The maps produced are not restricted to the voxel resolution of the original data thus are capable of inferring submillimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). To compensate for topographical heterogeneity among different participants, a 10 mm full‐width half‐maximum (FWHM) Gaussian spatial smoothing kernel was applied to the data prior to statistical analysis. All data went through visual inspection of the pial and white surface boundaries, to assess potential surface artifacts. Only participants with a valid surface estimation and without any artifacts were included for further statistical analysis. No manual corrections were performed to the FreeSurfer output (Wenger et al., 2014).
2.6. Statistical analysis
2.6.1. Motor performance
To tackle multicollinearity in the included motor performance dataset, this dataset was first divided into three functional motor domains, all containing several tests which were highly correlated: strength (including flexion, extension, side bending left and right), postural control (including sway area and velocity), and neuromuscular control (including flexor endurance, CCFT, and SHT). These domains allow for a clinical interpretation of the data and were defined as such. For strength and neuromuscular control, an index was calculated based on the average of the scaled variables.
For each motor domain, a general linear model was estimated including group, and age as a covariate. To assess significance of included variables an F‐test (type III Sums of Squares) was performed with a significance level of α = .05. Each model was assessed on linearity, homoscedasticity, and normality of the error terms. When these assumptions were met, the model was deemed appropriate for post hoc comparison to assess between‐group differences, corrected for multiple comparison with the Tukey's Honest Significant Difference (Rice, 1989). If not, a nonparametric alternative, that is, a Kruskal–Wallis test with pairwise Wilcoxon‐tests, was applied. All analyses were performed in R (version 3.3.0; R Core Team, 2017) with the package “stats.”
2.6.2. Cortical morphology
Group differences for volume, area, and thickness were analyzed in FreeSurfer by estimating a General Linear Model (GLM) at each vertex with each group, and age as covariate. After estimation, pairwise t‐contrasts were used to test between‐group differences at each vertex at a significance level α = .05. Data was Bonferroni‐corrected for analysis in both hemispheres to keep the family wise error rate (FWER) < 0.05 and subjected to cluster‐wise correction for multiple comparison at a cluster‐based significance level of κ = 0.05 using a Z Monte Carlo simulation with 5,000 iterations. For each cluster, the p‐value equals the probability of seeing a maximum cluster of that particular size or larger during the simulation. Information on cluster‐wise p‐values with their corresponding 90% confidence interval was reported alongside the estimates for brain morphology in each group.
2.6.3. Associations
Associations between brain morphology and clinical variables, including motor performance, disability, self‐reported pain (NRS), and pain duration, were analyzed in brain regions with a significant observable group difference. Besides, the associations between these clinical variables and self‐reported pain were analyzed. Bivariate associations were plotted to judge the linearity of the associations, and if necessary variables were transformed. A partial Pearson‐correlation coefficient corrected for age was calculated (ρ) and tested at a significance level of .05 (α). Additionally we corrected for multiple comparison by applying a sequential Bonferroni‐correction for the set of five regions (Abdi, 2010). Both the overall and group‐specific correlations were tested at an alpha level of .05.
3. RESULTS
3.1. Demographics and clinical characteristics
In total, 110 patients and healthy controls participated in this study, of which 39 were classified as patients with CWAD, 37 as patients with CINP, and 34 as HC. A description of the demographics of participants in each group is tabulated in Table 1. None of the participants showed signs that were indicative for micro‐hemorrhage. Groups were comparable for BMI (H(2) = 2.15, p = .34), but not for age (H(2) = 8.279, p = .016). Patient‐groups showed no significant difference in pain duration (Z = 552.5, p = .92). In contrast, a significantly higher degree of disability (W = 226, p < .001) was reported by patients with CWAD. Of the T1‐images only 95 (86.4%) were retained for further analysis after analyzing the FreeSurfer‐output on surface deformations and segmentation errors, of which 35 were labeled as CWAD, 31 as CINP, and 29 as HC. Some participants did not complete the entire motor performance test battery, and were therefore excluded for the subanalysis of associations (Table 2). The main reasons for noncompletion were dizziness, and an increased sensation of pain during testing.
Table 1.
Demographic variables
| HC (n = 29) | INP (n = 31) | WAD (n = 35) | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | p‐value | |
| Age (years) | 30.54 (13.10) | 19–62 | 37.77 (12.80) | 19–63 | 37.74 (12.15) | 22–60 | .016 * |
| BMI (kg/m 2 ) | 21.79 (2.02) | 18.07–26.75 | 22.62 (2.76) | 18.34–29.07 | 22.20 (4.01) | 16.65–32.05 | .48* |
| Pain duration (months) | NA | 84.38 (78.69) | 4–288 | 99.73 (98.61) | 6–444 | .54* | |
| NDI | NA | 16.62 (4.78) | 10–27 | 22.70 (6.68) | 10–37 | <.001 * | |
| Total gray matter volume (cm 3 ) | 633.49 (46.66) | 538.76–699.18 | 617.75 (55.07) | 507.35–735.10 | 600.51 (40.28) | 515.51–683.42 | .18† |
Abbreviations: n, sample size; SD, sample standard deviation; HC, healthy controls; INP, idiopathic neck pain; WAD, whiplash‐associated disorders; NA, not available.
† p‐value resulting from an ANCOVA with groups as fixed factors and age as covariate;* p‐value resulting from nonparametric Kruskal–Wallis or Wilcoxon Test.
Table 2.
Between‐group differences in motor performance
| Variables | F‐value (df) | Significance | Post hoc comparison | |||
|---|---|---|---|---|---|---|
| Comparison | Estimated difference (95%‐CI) | p‐value | ||||
|
Neuromuscular control (n = 100) |
Group Age |
17.23 (2) 5.00 (1) |
<.001 .028 |
WAD – INP | −0.48 [−0.82; −0.14] | .003 |
| HC ‐ INP | 0.39 [0.03; 0.74] | .029 | ||||
| HC ‐ WAD | 0.87 [0.50; 1.23] | <.001 | ||||
|
Sway velocity (n = 84) |
Group Age |
1.90 (2) 8.79 (1) |
.156 .004 |
WAD ‐ INP | 0.05 [−0.13; 0.24] | .773 |
| HC ‐ INP | −0.10 [−0.29; 0.09] | .421 | ||||
| HC ‐ WAD | −0.15 [−0.35; 0.04] | .152 | ||||
|
Sway area (n = 84) |
Group Age |
7.44 (2) 6.43 (1) |
.001 .013 |
WAD ‐ INP | 1.19 [0.03; 2.34] | .042 |
| HC ‐ INP | −0.70 [−1.87; 0.46] | .324 | ||||
| HC ‐ WAD | −1.89 [−3.10; −0.69] | <.001 | ||||
|
Strength (n = 100) |
Group Age |
22.17 (2) 10.36 (1) |
<.001 .002 |
WAD ‐ INP | −0.84 [−1.26; −0.43] | <.001 |
| HC ‐ INP | 0.30 [−0.13; 0.74] | .218 | ||||
| HC ‐ WAD | 1.15 [0.71; 1.59] | <.001 | ||||
Abbreviations: n, sample size; df, degrees of freedom; 95%‐CI, 95% confidence interval; HC, healthy controls; INP, idiopathic neck pain; WAD, whiplash‐associated disorders.
3.2. Motor performance
As shown in Table 2, the F‐test for group differences achieved significance for strength, sway area, and neuromuscular control, but not for sway velocity. Patients suffering from CWAD performed worse on neuromuscular control, strength, and sway area compared to HC, and on neuromuscular control, sway area and strength compared to CINP. In contrast, patients with CINP only performed worse compared to HC on neuromuscular control.
3.3. Cortical morphology
Results on group comparisons are depicted in Figure 1, and a detailed summary can be found in Table 3. Total cortical gray matter was not observed to be significantly different between the included groups. Patients with CINP showed an increase in cortical thickness in the left precuneus compared to HC, and in the left superior parietal gyrus compared to HC and patients with CWAD. Similarly, patients with CINP exhibited a greater amount of cortical volume in the left superior parietal gyrus compared to HC. Patients with CWAD featured a smaller amount of cortical volume in the right precentral and superior temporal gyrus compared to HC. No significant group differences were observed for surface area. Figure 2 visualizes the clusters of brain areas with identified between‐group differences.
Figure 1.
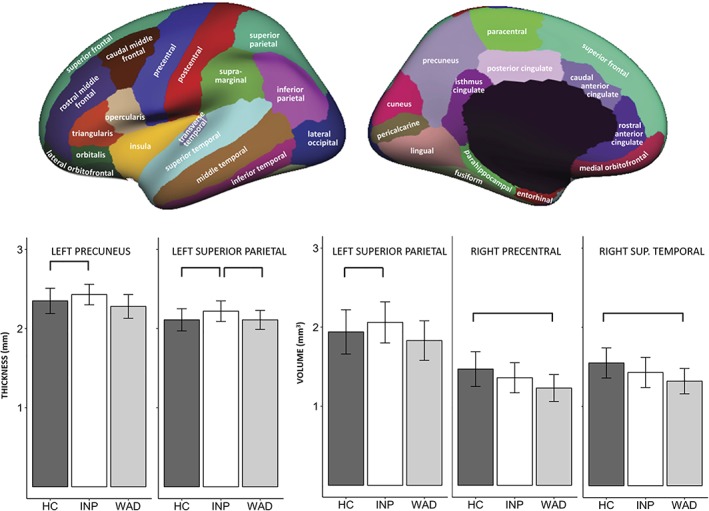
Between‐group comparison of brain morphology. Significant differences based on cluster‐wise p‐values were amended with *. Lateral and medial view of cortical parcellation (Desikan, Rahul S., et al. “An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest.” Neuroimage 31.3 (2006): 968–980) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Between‐group differences in cortical morphology
| Mean (SD) | MNI coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| WAD (n = 31) | INP (n = 35) | HC (n = 29) | Size (mm2) | X | Y | Z | Annotation | CWP (90%‐CI) | ES |
| Thickness (mm) | |||||||||
| 2.28 (0.15) | 2.43 (0.13) | 2.35 (0.16) | 914.62 | −7.7 | −70.9 | 37.9 | Precuneus (lh) | .023 (.020; .026) | 0.55 |
| 2.07 (0.13) | 2.20 (0.14) | 2.09 (0.15) | 876.29 | −22.9 | −80.1 | 17.7 | Superiorparietal (lh) | .032 (.029; .035) | 0.76 |
| 2.15 (0.11) | 2.23 (0.12) | 2.12 (0.13) | 2,317.30 | −19.7 | −87.0 | 15.3 | Superiorparietal (lh) | <.001 (.0001; .00004) | 0.69 |
| Volume (cm 3 ) | |||||||||
| 1.83 (0.25) | 2.06 (0.26) | 1.94 (0.28) | 931.76 | −13.9 | −86.5 | 32.8 | Superiorparietal (lh) | .040 (.036; .043) | 0.45 |
| 1.23 (0.17) | 1.36 (0.19) | 1.47 (0.22) | 954.82 | 55.4 | −2.9 | 35.6 | Precentral (rh) | .046 (.042; .050) | 1.23 |
| 1.32 (0.16) | 1.43 (0.19) | 1.55 (0.19) | 1,065.20 | 64.5 | −37.3 | 14.5 | Superiortemporal (rh) | .021 (.019; .024) | 1.32 |
Notes: Size: estimated size of the cluster; Annotation: Desikan et al. (2006); Cave: significance was based on cluster‐wise p‐values
Abbreviations: SD, sample standard deviation; HC, healthy controls; INP, idiopathic neck pain; WAD, whiplash‐associated disorders; CWP, cluster‐wise p‐value with 90%‐CI for the comparison between groups marked in gray; ES, effect size; lh, left hemisphere; n, sample size; rh, right hemisphere.
Figure 2.
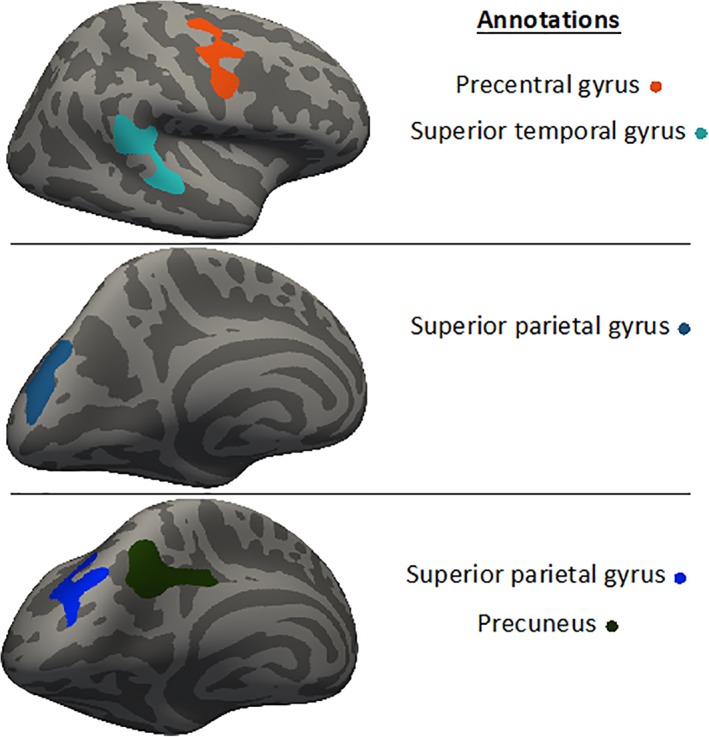
Visualization of brain‐clusters with identified between‐group differences [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Associations between cortical brain morphology and clinical variables
As shown in Figure 3, a significant overall correlation was found between the volume of the precentral gyrus and both neuromuscular control (r(97) = .23, p = .02) and strength (r(97) = .22, p = .02), for which a better performance on neuromuscular control or strength is associated with an increase in cortical volume. An increased volume of the superior temporal gyrus was significantly associated over all groups with greater strength (r(97) = .27, p = .006). A higher degree of self‐reported pain correlated furthermore significantly over all groups with a decrease in cortical volume of the precentral (r(97) = −.25, p = .04) and superior temporal gyrus (r(97) = −.30, p = .01). Furthermore, a group‐specific association between self‐reported pain and volume of the superior temporal gyrus was identified (r(97) = −.44, p = .01). After correcting for multiple comparison by the sequential Bonferroni‐correct only the association between the volume of the superior temporal gyrus and strength together with the association between self‐reported pain and the volume of the precentral was formally identified as significant.
Figure 3.
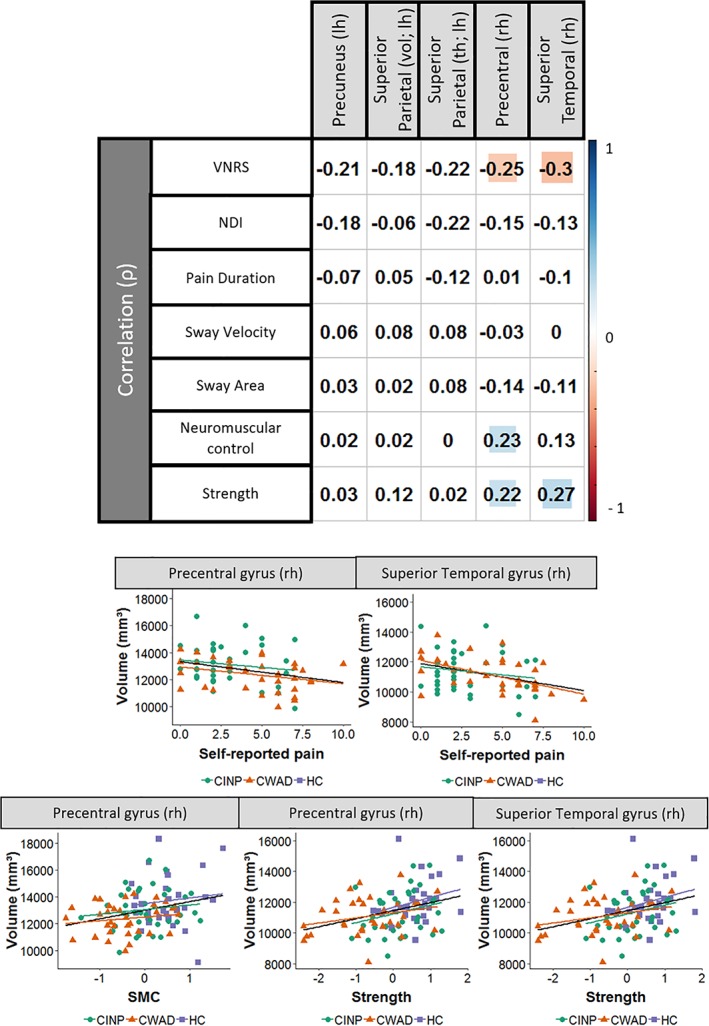
Correlation‐plot with scatter plot. Significant correlations are highlighted in blue (positive) or red (negative). Rh: right hemisphere; lh: left hemisphere; ρ: Pearson correlation; VNRS, verbal numeric rating scale; HC, healthy controls; INP, idiopathic neck pain; WAD, whiplash‐associated disorders [Color figure can be viewed at http://wileyonlinelibrary.com]
Last, a higher degree of self‐reported pain was overall associated with a higher degree of self‐reported disability (r(97) = .56, p < .001), and a lower score on neuromuscular control (r(97) = −.39, p < .001) and strength (r(97) = −.42, p < .001). No significant overall correlations were found between brain morphology and disability, nor between brain morphology and pain duration. We were furthermore unable to reveal other then aforementioned group‐specific associations between the included variables.
4. DISCUSSION
In this study, we investigated gray matter morphology in a population of healthy pain‐free controls, patients with chronic nonspecific traumatic neck pain and patients with nonspecific nontraumatic neck pain using a whole‐brain surface‐based morphometry approach. Additionally, we assessed the association between gray matter morphology and different clinical parameters including motor performance and self‐reported pain. Our results indicate that changes in gray matter morphology occur in patients with chronic nonspecific neck pain compared to healthy pain‐free controls. More specifically, these gray matter alterations were different across the included patient‐subgroups. Regions related to chronic neck pain were the precuneus, superior parietal gyrus, superior temporal gyrus, and precentral gyrus. Last, we were able to demonstrate an overall association between gray matter morphology and self‐reported pain, neuromuscular control and strength.
The observed motor impairments in patients compared to HC were observed in a larger amount in patients with CWAD compared to CINP. Patients with CWAD do not only show impairments in neuromuscular control, but also in strength and postural control as opposed to the group of CINP. This is in agreement with the previous results from Woodhouse and Vasseljen (2008), who observed a more rigid movement pattern among patients with CINP and CWAD compared to HC. The observed rigidity could result in an impaired postural control (Ruhe et al., 2011; Silva & Cruz, 2013), which was the case for patients with CWAD. Impairments in strength of patients with CWAD compared to HC are probably strongly associated with self‐reported pain, as reported by Ylinen et al. (2004) and confirmed by our results. This could indicate that the amount of strength measured in patients could reflect the patients' ability to bear strain. We observed no impairment in postural control and strength in patients with CINP compared to HC, which might correspond with the less distinct clinical presentation of these patients. Patients with CINP de facto feature a significantly lesser degree of pain and disability (NDI). Together, the high variability in symptoms and the lesser degree of impairment compared to CWAD (Falla, 2004; Field, Treleaven, & Jull, 2008; Sjölander, Michaelson, Jaric, & Djupsjöbacka, 2008; Stanton, Leake, Chalmers, & Moseley, 2016; Woodhouse & Vasseljen, 2008) could explain the absence of significance in CINP for strength and postural control compared to HC.
Based on the whole‐brain vertex‐wise statistical approach four important regions, namely the precuneus, the superior parietal gyrus, the precentral gyrus, and the superior temporal gyrus, showed a significant group difference. Although, none of these regions were described in the original PM (Ploghaus et al., 1999), they were reported in more recent pain‐related research (Garcia‐Larrea & Peyron, 2013; Hardwick, Rottschy, Miall, & Eickhoff, 2013; Wager et al., 2013). The selective increase of gray matter in the superior parietal gyrus and precuneus in patients with CINP compared to healthy controls and CWAD is partly in accordance with recent publications on cortical alterations in patients with chronic neck pain (Coppieters et al., 2017, 2018). Unlike these earlier studies, we were able to demonstrate a selective cortical thickening of the left superior parietal gyrus in patients with CINP compared to CWAD and HC. Furthermore, we were able to show a regional decrease in gray matter volume of the precentral and superior temporal gyrus in CWAD compared to HC. We did however not identify differences in the posterior cingulate, orbitofrontal or supramarginal cortex. The differences in outcome might relate to several reasons. First of all, a whole‐brain analysis might uncover regions that are affected beyond the theoretically hypothesized regions of interest (Giuliani, Calhoun, Pearlson, Francis, & Buchanan, 2005). Moreover, voxel‐ or vertex‐wise approaches might uncover changes in subparts of some specific brain regions. In contrast, regional differences might disappear in ROI‐based approaches, as the estimates for gray matter morphology are averaged over a certain region (Giuliani et al., 2005).
The precuneus and superior parietal gyrus are both part of a set of regions associated with the neurologic signature of pain (Wager et al., 2013). Interestingly, the precuneus is identified as a functional core of the default‐mode network, and acts as an antinociceptive region (Davis & Moayedi, 2013; Utevsky, Smith, & Huettel, 2014). The superior parietal gyrus as a part of the parietofrontal network has been related to the perceptual matrix of pain (Garcia‐Larrea & Peyron, 2013), but its exact function in pain remains partly unknown.
Additionally, we showed a decrease in gray matter volume of the precentral and superior temporal gyrus in patients with CWAD compared to HC, which is in accordance with other studies in chronic pain (Smallwood et al., 2013). The precentral gyrus is not only involved in the sensation of pain (Garcia‐Larrea & Peyron, 2013), but is also part of the motor cortex. Previous research shows that in chronic musculoskeletal pain this region undergoes functional and structural changes that were linked to pain (Flor, Braun, Elbert, & Birbaumer, 1997; Smallwood et al., 2013), and to alterations of paraspinal muscle activity (Hodges, Tsao, & Danneels, 2011). Furthermore, we observed a significant overall association between the volume of the precentral gyrus and both neuromuscular control and strength. The decreased scores in strength and neuromuscular control might in part relate to the decreased amount of cortical volume in the right precentral gyrus observed in patients. Despite finding an overall association, we were unable to identify group‐specific associations. The precentral gyrus contains information on muscle synergies and plays an important role in movement‐dissociations (e.g., separate finger movements) and movement‐variability reduction to enable improvement of skilled performance (Hardwick et al., 2013; Rizzolatti & Luppino, 2001). Patients suffering from pain partially lose the ability to dissociate movements, which results in more rigid movement patterns (Falla, 2004; Hodges et al., 2011; Hodges & Tucker, 2011; Meisingset et al., 2015).
Unlike the previously mentioned regions, the superior temporal gyrus has largely been ignored in previous pain studies, due to the implementation of mainly ROI‐based approaches. Activity of the superior temporal gyrus has however been associated with efference copy (Leube et al., 2003), a process responsible for monitoring mismatches between predicted and actual sensation. The superior temporal gyrus might be involved in pain due to mismatches between pain expectation and perception (Smallwood et al., 2013). Interestingly, we observed an association between the volume of the superior temporal gyrus and strength. Again, no group‐specific associations were identified. The superior temporal gyrus has a high connectivity pattern with the primary sensory cortex (Cavanna & Trimble, 2006), and plays an important role in the integration of somatosensory, visual, and auditory information (Karnath, 2001). This observation is furthermore in accordance with previous results from Freitag, Greenlee, Wachter, Ettlin, and Radue (2001), who demonstrated a decreased activity of the superior temporal gyrus in patients with CWAD that were subjected to a visual task in the scanner. This decrease of volume was however not observed in patients with CINP compared to HC.
Only alterations in cortical volume and cortical thickness, but not in surface area were identified. Considering cortical volume is a product of cortical thickness and cortical area, a reduction in cortical volume may reflect either reduced thickness, reduced area, or both. These two constituent components of cortical volume result from well‐differentiated ontogenic stages during corticogenesis and appear to have independent genetic etiologies (Panizzon et al., 2009). At birth, surface area is determined by the number of cortical columns and cortical thickness by the number of cells within a column (Rakic, 1988). Interestingly, aging and brain disorders more often result in cortical thinning, and only in the minority of cases changes in cortical volume are attributed to decreased surface area (Hanford, Nazarov, Hall, & Sassi, 2016; Lerch et al., 2004; Rimol et al., 2012). The exact histological nature of these changes remains however uncertain (Panizzon et al., 2009; Winkler et al., 2010). Moreover, the mechanism by which gray matter changes in either direction is still unknown (Smallwood et al., 2013).
Nevertheless, the observed difference between patient‐subgroups might relate to the presence of central sensitization, which is only a feature in patients with CWAD (Coppieters et al., 2017, 2018; Malfliet et al., 2015). Central sensitization has furthermore been linked to brain alterations (Coppieters et al., 2016). Another hypothesis that has been postulated is that the decrease of gray matter observed in patients with CWAD—in contrast to patients with CINP—might relate to the traumatic history (Coppieters et al., 2018). However, as opposed to patients suffering from mild traumatic brain injury (Koerte, Hufschmidt, Muehlmann, Lin, & Shenton, 2016), no signs of micro‐hemorrhages, nor a general decrease in gray matter volume were found. Instead, the decrease in gray matter of the precentral gyrus was linked to an increase in self‐reported pain, and a decrease in neuromuscular performance and strength, and a similar association was shown for the superior temporal gyrus.
Possible explanations for the lack of significance between patients with CINP and HC may be the insensitivity of the applied scanning‐protocol to detect these potentially more subtle differences or such differences could be nonexistent in this specific patient‐group. Another reason concerning not being able to detect group differences between patients with CINP and HC might be attributed to power. Although whole‐brain analysis has some major advantages, it comes at the cost of reduced power and thus requires a large patient‐sample (Cremers, Wager, & Yarkoni, 2017). The absence of significant associations between brain morphology and postural control might be attributed to the complexity of the system that is responsible for postural steadiness (Treleaven, 2008). Postural steadiness is the result of a complex interaction between multiple systems, of which the brain might only play a limited role. Unlike the overall associations, almost no group‐specific associations were identified. This might be attributed to several reasons, including a lack of power to detect a correlation within each group or the between‐group differences may artificially drive the observed correlations.
4.1. Strengths and limitations
This study is the first to provide evidence for neuroplastic alterations in patients suffering from chronic neck pain using a whole‐brain SBM approach based on a sample of 95 participants. These results might serve as a basis for further longitudinal research, which is necessary to analyze the causal relation between neuroplastic changes and chronic neck pain. Only macrostructural alterations in brain morphology were analyzed in this study, the histological nature of gray matter alterations varies from a simple decrease in cell size, neural or glial apoptosis, a decrease in spine density to changes in blood flow or interstitial fluids (May, 2011), and should be explored further to provide a profound understanding of the pathophysiological processes at the rot of the observed macrostructural changes. This study only included women, which makes it inappropriate to infer conclusions on neuroplastic alterations in men. We were unable to find group‐specific associations between brain morphology and motor performance. Besides, the size of the observed partial Pearson correlation is rather small. Some associations were furthermore only found at a significance level of .05, emphasizing the exploratory character of the identified associations. These correlations were however corrected for age, which is considered an important confounding factor. More research will however still be necessary to make final conclusions on the extent of the link between brain morphology and motor impairment. Only including women allows to interpret observed changes without risk for a confounding effect of gender. Future studies should aim at including a combined whole‐brain vertex‐wise and ROI‐driven approach that targets at the analysis of all constituent components of cortical volume (Giuliani et al., 2005; Rajtmajer, Roy, Albert, Molenaar, & Hillary, 2015).
5. CONCLUSION
In conclusion, we revealed a selective thickening of the precuneus and superior parietal gyrus in CINP compared to HC, regions known to be involved in pain processing. Patients with CWAD exhibit a decrease in gray matter volume of the precentral and superior temporal gyrus compared to HC. The identified regions are involved in pain and motor performance. Furthermore, a decrease in volume of the superior parietal and precentral gyrus was associated with decreased motor performance for neuromuscular control and strength. The presence of structural brain alterations in patients with chronic neck pain should thus be emphasized when working with patients with chronic neck pain.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We would like to thank all the volunteers that participated in our study, neurologist Karel Deblaere, Ph.D. for analyzing the MRI‐scans of all the participants, and the GifMI institute for providing all equipment and the expertise to integrate the MRI‐protocol in this study. In addition, we are grateful to the HPC‐infrastructure (as part of the VSC‐center; https://www.vscentrum.be/) for providing the necessary computational power.
APPENDIX 1.
| Neuromuscular control: CCFT |
| The first part of the scoring process for the CCFT consisted of the original test as described by Jull, O'Leary, and Falla (2008) resulting in a score ranging from 0 to 4 (22–30 mmHg) with the aid of a stabilizer‐cuff (Chattanooga Stabilizer Group Inc., Hoxson, TN). In addition, patients were asked to perform the same movement five consecutive times while trying to reach the level of 26 mmHg. A score ranging from 0 (unable) to 4 (excellent) was given based on fluency, respiration, compensation of superficial muscles, and under or overshooting of the targeted pressure. Thereafter, a score ranging from 0 to 2 was given based on the endurance in which patients were asked to hold a normative pressure of 26 mmHg for 10 s during 10 consecutive trials. The score was calculated as the amount of successful repetitions multiplied by 0.2. |
| Neuromuscular control: SHT |
| The neuromuscular capacity of scapulothoracic muscles was assessed using the scapular holding test (SHT), performed at the dominant painful side. Participants were positioned prone with their head in a neutral position and arms besides their thorax. The first part of the form assesses compensatory movements (elevation, retraction, downwards rotation, tipping, or internal rotation of the scapula), and the quality of contraction of the lower trapezius muscle after the examiner instructed the patient to keep the scapula in this optimal position (Jull, Sterling, Falla, Treleaven, & O'Leary, 2008), resulting in a score ranging from 0 to 4. Afterwards, patients were asked to perform the same movement five consecutive times trying to reach the scapular setting. The performance of these trials was assessed on fluency, compensatory movements, and under or overshooting from the targeted position, resulting in a score ranging from 0 (worse) to 4 (best). Last, a score ranging from 0 to 2 was given based on the endurance in which Participants were asked to hold the scapular setting for 10 s during 10 consecutive trials. The score was calculated as the amount of successful repetitions multiplied by 0.2. |
| Neuromuscular control: Endurance cervical flexors |
| Participants laid supine in a hook‐lying position, hands resting on their abdomen, and were asked to slightly raise the head allowing the tester to slide the widths of the index and middle finger of one hand, one atop the other, under the participant's head at the most posterior aspect of the occiput. The participant was then asked to rest his head on the examiner's fingers. Next, the subject was directed to perform a craniocervical flexion and raise the head just off the tester's fingers resulting in a cervical flexion and hold this position as long as possible. During the test, the examiner gently moved his/her fingers from side to side under the subject's head, providing a tactile cue for maintaining proper head position. Timing of the duration of the trial started after the subject raises the head off the tester's fingers, and ended when one of the following four criteria were met: (a) the subject experiences pain and is unwilling to continue; (b) the subject is unwilling to continue; (c) the examiner determines that the subject loses chin tuck; and (d) the examiner determines that the subject raised the head (flexes the neck while still in chin tuck) such that the tester's fingers no longer maintain contact. |
De Pauw R, Coppieters I, Caeyenberghs K, et al. Associations between brain morphology and motor performance in chronic neck pain: A whole‐brain surface‐based morphometry approach. Hum Brain Mapp. 2019;40:4266–4278. 10.1002/hbm.24700
REFERENCES
- Abdi, H. (2010). Holm's sequential Bonferroni procedure. Encyclopedia of Research Design, 1(8), 1–8. [Google Scholar]
- Ailliet, L. , Rubinstein, S. M. , De Vet, H. C. W. , Van Tulder, M. W. , & Terwee, C. B. (2015). Reliability, responsiveness and interpretability of the neck disability index‐Dutch version in primary care. European Spine Journal, 24(1), 88–93. [DOI] [PubMed] [Google Scholar]
- Anstey, R. , Kongsted, A. , Kamper, S. , & Hancock, M. J. (2016). Are people with whiplash‐associated neck pain different from people with nonspecific neck pain? Journal of Orthopaedic & Sports Physical Therapy, 46(10), 894–901. [DOI] [PubMed] [Google Scholar]
- Argall, B. D. , Saad, Z. S. , & Beauchamp, M. S. (2006). Simplified intersubject averaging on the cortical surface using SUMA. Human Brain Mapping, 27(1), 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2000). Voxel‐based morphometry—The methods. NeuroImage, 11(6), 805–821. [DOI] [PubMed] [Google Scholar]
- Baliki, M. N. , Geha, P. Y. , Apkarian, A. V. , & Chialvo, D. R. (2008). Beyond feeling: Chronic pain hurts the brain, disrupting the default‐mode network dynamics. The Journal of Neuroscience, 28(6), 1398–1403. 10.1523/JNEUROSCI.4123-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki, M. N. , Schnitzer, T. J. , Bauer, W. R. , & Apkarian, A. V. (2011). Brain morphological signatures for chronic pain. PLoS One, 6(10), e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchgrevink, G. , Smevik, O. , Haave, I. , Haraldseth, O. , Nordby, A. , & Lereim, I. (1997). MRI of cerebrum and cervical columna within two days after whiplash neck sprain injury. Injury, 28(5–6), 331–335. [DOI] [PubMed] [Google Scholar]
- Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , & Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain, 10(4), 287–333. [DOI] [PubMed] [Google Scholar]
- Cagnie, B. , Cools, A. , De Loose, V. , Cambier, D. , & Danneels, L. (2007). Differences in isometric neck muscle strength between healthy controls and women with chronic neck pain: The use of a reliable measurement. Archives of Physical Medicine and Rehabilitation, 88(11), 1441–1445. [DOI] [PubMed] [Google Scholar]
- Cagnie, B. , Coppieters, I. , Denecker, S. , Six, J. , Danneels, L. , & Meeus, M. (2014). Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Seminars in Arthritis and Rheumatism, 44(1), 68–75. 10.1016/j.semarthrit.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Cagnie, B. , Dickx, N. , Peeters, I. , Tuytens, J. , Achten, E. , Cambier, D. , & Danneels, L. (2008). The use of functional MRI to evaluate cervical flexor activity during different cervical flexion exercises. Journal of Applied Physiology (Bethesda, MD: 1985), 104(1), 230–235. 10.1152/japplphysiol.00918.2007 [DOI] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. [DOI] [PubMed] [Google Scholar]
- Coppieters, I. , De Pauw, R. , Caeyenberghs, K. , Danneels, L. , Kregel, J. , Pattyn, A. , … Cagnie, B. (2017). Decreased regional grey matter volume in women with chronic whiplash‐associated disorders: Relationships with cognitive deficits and disturbed pain processing. Pain Physician, 20(7), e1025–e1051. [PubMed] [Google Scholar]
- Coppieters, I. , De Pauw, R. , Caeyenberghs, K. , Lenoir, D. , Deblaere, K. , Genbrugge, E. , … Cagnie, B. (2018). Differences in white matter structure and cortical thickness between patients with traumatic and idiopathic chronic neck pain: Associations with cognition and pain modulation? Human Brain Mapping, 39, 1721–1742. 10.1002/hbm.23947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters, I. , Ickmans, K. , Cagnie, B. , Nijs, J. , De Pauw, R. , & Meeus, M. (2015). Cognitive performance is related to central sensitization in patients with chronic whiplash‐associated disorders and fibromyalgia: A case‐control study. Pain Science in Motion Congress. [PubMed]
- Coppieters, I. , Meeus, M. , Kregel, J. , Caeyenberghs, K. , De Pauw, R. , Goubert, D. , & Cagnie, B. (2016). Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: A systematic review. Journal of Pain, 17(9), 949–962. 10.1016/j.jpain.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Cremers, H. R. , Wager, T. D. , & Yarkoni, T. (2017). The relation between statistical power and inference in fMRI. PLoS One, 12(11), e0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, K. D. , & Moayedi, M. (2013). Central mechanisms of pain revealed through functional and structural MRI. Journal of Neuroimmune Pharmacology, 8(3), 518–534. 10.1007/s11481-012-9386-8 [DOI] [PubMed] [Google Scholar]
- De Pauw, R. , Coppieters, I. , Meeus, M. , Caeyenberghs, K. , Danneels, L. , & Cagnie, B. (2017). Is traumatic and non‐traumatic neck pain associated with brain alterations? – A systematic review. Pain Physician, 20(4), 245–260. [PubMed] [Google Scholar]
- De Pauw, R. , Coppieters, I. , Palmans, T. , Danneels, L. , Meeus, M. , & Cagnie, B. (2018). Motor impairment in patients with chronic neck pain: Does the traumatic event play a significant role? A case‐control study. Spine Journal, 18, 1406–1416. 10.1016/j.spinee.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Ségonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Hyman, B. T. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Falla, D. (2004). Unravelling the complexity of muscle impairment in chronic neck pain. Manual Therapy, 9(3), 125–133. [DOI] [PubMed] [Google Scholar]
- Falla, D. , Jull, G. , Dall'Alba, P. , Rainoldi, A. , & Merletti, R. (2003). An electromyographic analysis of the deep cervical flexor muscles in performance of craniocervical flexion. Physical Therapy, 83(10), 899–906. [PubMed] [Google Scholar]
- Farmer, M. A. , Baliki, M. N. , & Apkarian, A. V. (2012). A dynamic network perspective of chronic pain. Neuroscience Letters, 520(2), 197–203. 10.1016/j.neulet.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejer, R. , Kyvik, K. O. , & Hartvigsen, J. (2006). The prevalence of neck pain in the world population: A systematic critical review of the literature. European Spine Journal, 15(6), 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, S. , Treleaven, J. , & Jull, G. (2008). Standing balance: A comparison between idiopathic and whiplash‐induced neck pain. Manual Therapy, 13(3), 183–191. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Liu, A. , & Dale, A. M. (2001). Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Sereno, M. I. , Tootell, R. B. H. , & Dale, A. M. (1999). High‐resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , van der Kouwe, A. , Destrieux, C. , Halgren, E. , Ségonne, F. , Salat, D. H. , … Kennedy, D. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Flor, H. , Braun, C. , Elbert, T. , & Birbaumer, N. (1997). Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neuroscience Letters, 224(1), 5–8. [DOI] [PubMed] [Google Scholar]
- Freitag, P. , Greenlee, M. W. , Wachter, K. , Ettlin, T. M. , & Radue, E. W. (2001). fMRI response during visual motion stimulation in patients with late whiplash syndrome. Neurorehabilitation and Neural Repair, 15(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea, L. , & Peyron, R. (2013). Pain matrices and neuropathic pain matrices: A review. Pain, 154(Suppl), S29–S43. 10.1016/j.pain.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Giuliani, N. R. , Calhoun, V. D. , Pearlson, G. D. , Francis, A. , & Buchanan, R. W. (2005). Voxel‐based morphometry versus region of interest: A comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophrenia Research, 74(2–3), 135–147. [DOI] [PubMed] [Google Scholar]
- Guzman, J. , Hurwitz, E. L. , Carroll, L. J. , Haldeman, S. , Côté, P. , Carragee, E. J. , … Hogg‐Johnson, S. (2009). A new conceptual model of neck pain: Linking onset, course, and care: The bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Journal of Manipulative and Physiological Therapeutics, 32(2), S17–S28. [DOI] [PubMed] [Google Scholar]
- Hanford, L. C. , Nazarov, A. , Hall, G. B. , & Sassi, R. B. (2016). Cortical thickness in bipolar disorder: A systematic review. Bipolar Disorders, 18(1), 4–18. [DOI] [PubMed] [Google Scholar]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjermstad, M. J. , Fayers, P. M. , Haugen, D. F. , Caraceni, A. , Hanks, G. W. , Loge, J. H. , … Kaasa, S. (2011). Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. Journal of Pain and Symptom Management, 41(6), 1073–1093. 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Hodges, P. W. , Tsao, H. , & Danneels, L. (2011). Smudging of the motor cortex representation of the paraspinal muscles in low back pain. Journal of Orthopaedic & Sports Physical, 41(1), A27. [Google Scholar]
- Hodges, P. W. , & Tucker, K. (2011). Moving differently in pain: A new theory to explain the adaptation to pain. Pain, 152(3 Suppl), S90–S98. [DOI] [PubMed] [Google Scholar]
- Jorritsma, W. , de Vries, G. E. , Dijkstra, P. U. , Geertzen, J. H. B. , & Reneman, M. F. (2012). Neck pain and disability scale and neck disability index: Validity of Dutch language versions. European Spine Journal, 21(1), 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull, G. A. , O'Leary, S. P. , & Falla, D. L. (2008). Clinical assessment of the deep cervical flexor muscles: The craniocervical flexion test. Journal of Manipulative and Physiological Therapeutics, 31(7), 525–533. [DOI] [PubMed] [Google Scholar]
- Jull, G. , Sterling, M. , Falla, D. , Treleaven, J. , & O'Leary, S. (2008). Whiplash, Headache, and Neck Pain (pp. 101–115). Philadelphia, PA: Elsevier. [Google Scholar]
- Karlsborg, M. , Smed, A. , Jespersen, H. F. , Stephensen, S. L. , Cortsen, M. E. , Jennum, P. J. , … Werdelin, L. M. (1998). Whiplash injury syndrome. A prospective study of 39 patients with whiplash injury. Ugeskrift for Laeger, 160(43), 6211–6215. [PubMed] [Google Scholar]
- Karnath, H.‐O. (2001). New insights into the functions of the superior temporal cortex. Nature Reviews Neuroscience, 2(8), 568–576. [DOI] [PubMed] [Google Scholar]
- Koerte, I. K. , Hufschmidt, J. , Muehlmann, M. , Lin, A. P. , & Shenton, M. E. (2016). Translational Research in Traumatic Brain Injury Laskowitz, D. and Grant, G. In Frontiers in Neuroscience. Boca Raton (FL): CRC Press. [Google Scholar]
- Kregel, J. , Meeus, M. , Malfliet, A. , Dolphens, M. , Danneels, L. , Nijs, J. , & Cagnie, B. (2015). Structural and functional brain abnormalities in chronic low back pain: A systematic review. Seminars in Arthritis and Rheumatism, 45(2), 229–237. 10.1016/j.semarthrit.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2015). The dynamic pain connectome. Trends in Neurosciences, 38(2), 86–95. 10.1016/j.tins.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2017). The neural code for pain: From single‐cell electrophysiology to the dynamic pain connectome. The Neuroscientist, 23(4), 397–414. 10.1177/1073858416667716 [DOI] [PubMed] [Google Scholar]
- Kuperberg, G. R. , Broome, M. R. , McGuire, P. K. , David, A. S. , Eddy, M. , Ozawa, F. , … van der Kouwe, A. J. W. (2003). Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry, 60(9), 878–888. [DOI] [PubMed] [Google Scholar]
- Lemaitre, H. , Goldman, A. L. , Sambataro, F. , Verchinski, B. A. , Meyer‐Lindenberg, A. , Weinberger, D. R. , & Mattay, V. S. (2012). Normal age‐related brain morphometric changes: Nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging, 33(3), 617–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch, J. P. , Pruessner, J. C. , Zijdenbos, A. , Hampel, H. , Teipel, S. J. , & Evans, A. C. (2004). Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cerebral Cortex, 15(7), 995–1001. [DOI] [PubMed] [Google Scholar]
- Leube, D. T. , Knoblich, G. , Erb, M. , Grodd, W. , Bartels, M. , & Kircher, T. T. J. (2003). The neural correlates of perceiving one's own movements. NeuroImage, 20(4), 2084–2090. [DOI] [PubMed] [Google Scholar]
- Malfliet, A. , Kregel, J. , Cagnie, B. , Kuipers, M. , Dolphens, M. , Roussel, N. , … Nijs, J. (2015). Lack of evidence for central sensitization in idiopathic, non‐traumatic neck pain: A systematic review. Pain Physician, 18(3), 223–235. [PubMed] [Google Scholar]
- May, A. (2008). Chronic pain may change the structure of the brain. Pain, 137(1), 7–15. [DOI] [PubMed] [Google Scholar]
- May, A. (2011). Structural brain imaging: A window into chronic pain. The Neuroscientist, 17(2), 209–220. [DOI] [PubMed] [Google Scholar]
- Meisingset, I. , Woodhouse, A. , Stensdotter, A.‐K. , Stavdahl, Ø. , Lorås, H. , Gismervik, S. , … Vasseljen, O. (2015). Evidence for a general stiffening motor control pattern in neck pain: A cross sectional study. BMC Musculoskeletal Disorders, 16(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack, R. (1990). Phantom limbs and the concept of a neuromatrix. Trends in Neurosciences, 13(3), 88–92. [DOI] [PubMed] [Google Scholar]
- Obermann, M. , Nebel, K. , Schumann, C. , Holle, D. , Gizewski, E. R. , Maschke, M. , … Katsarava, Z. (2009). Gray matter changes related to chronic posttraumatic headache. Neurology, 73(12), 978–983. 10.1212/WNL.0b013e3181b8791a [DOI] [PubMed] [Google Scholar]
- Olson, L. E. , Millar, A. L. , Dunker, J. , Hicks, J. , & Glanz, D. (2006). Reliability of a clinical test for deep cervical flexor endurance. Journal of Manipulative and Physiological Therapeutics, 29(2), 134–138. [DOI] [PubMed] [Google Scholar]
- Panizzon, M. S. , Fennema‐Notestine, C. , Eyler, L. T. , Jernigan, T. L. , Prom‐Wormley, E. , Neale, M. , … Kremen, W. S. (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, I. , Reichert, A. , De Serres, S. J. , Dumas, J.‐P. , & Côté, J. N. (2009). Maximal voluntary isometric neck strength deficits in adults with whiplash‐associated disorders and association with pain and fear of movement. Journal of Orthopaedic & Sports Physical Therapy, 39(3), 179–187. [DOI] [PubMed] [Google Scholar]
- Phillips, C. J. (2009). The cost and burden of chronic pain. Reviews in Pain, 3(1), 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus, A. , Tracey, I. , Gati, J. S. , Clare, S. , Menon, R. S. , Matthews, P. M. , & Rawlins, J. N. P. (1999). Dissociating pain from its anticipation in the human brain. Science, 284(5422), 1979–1981. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Vienna, Austria. Retrieved from https://www.r-project.org/
- Rajtmajer, S. M. , Roy, A. , Albert, R. , Molenaar, P. , & Hillary, F. G. (2015). A voxelwise approach to determine consensus regions‐of‐interest for the study of brain network plasticity. Frontiers in Neuroanatomy, 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic, P. (1988). Specification of cerebral cortical areas. Science, 241(4862), 170–176. [DOI] [PubMed] [Google Scholar]
- Reid, K. J. , Harker, J. , Bala, M. M. , Truyers, C. , Kellen, E. , Bekkering, G. E. , & Kleijnen, J. (2011). Epidemiology of chronic non‐cancer pain in Europe: Narrative review of prevalence, pain treatments and pain impact. Current Medical Research and Opinion, 27(2), 449–462. [DOI] [PubMed] [Google Scholar]
- Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution, 43(1), 223–225. [DOI] [PubMed] [Google Scholar]
- Rimol, L. M. , Nesvåg, R. , Hagler, D. J. , Bergmann, Ø. , Fennema‐Notestine, C. , Hartberg, C. B. , … Dale, A. M. (2012). Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biological Psychiatry, 71(6), 552–560. 10.1016/j.biopsych.2011.11.026 [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. , & Luppino, G. (2001). The cortical motor system. Neuron, 31(6), 889–901. [DOI] [PubMed] [Google Scholar]
- Rosas, H. D. , Liu, A. K. , Hersch, S. , Glessner, M. , Ferrante, R. J. , Salat, D. H. , … Fischl, B. (2002). Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology, 58(5), 695–701. [DOI] [PubMed] [Google Scholar]
- Ruhe, A. , Fejer, R. , & Walker, B. (2010). The test‐retest reliability of centre of pressure measures in bipedal static task conditions‐‐a systematic review of the literature. Gait & Posture, 32(4), 436–445. 10.1016/j.gaitpost.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Ruhe, A. , Fejer, R. , & Walker, B. (2011). Altered postural sway in patients suffering from non‐specific neck pain and whiplash associated disorder‐a systematic review of the literature. Chiropractic & Manual Therapies, 19(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner, R. L. , Snyder, A. Z. , Greve, D. N. , Desikan, R. S. R. , Busa, E. , … Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. [DOI] [PubMed] [Google Scholar]
- Ségonne, F. , Pacheco, J. , & Fischl, B. (2007). Geometrically accurate topology‐correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518–529. [DOI] [PubMed] [Google Scholar]
- Silva, A. G. , & Cruz, A. L. (2013). Standing balance in patients with whiplash‐associated neck pain and idiopathic neck pain when compared with asymptomatic participants: A systematic review. Physiotherapy Theory and Practice, 29(1), 1–18. 10.3109/09593985.2012.677111 [DOI] [PubMed] [Google Scholar]
- Sjölander, P. , Michaelson, P. , Jaric, S. , & Djupsjöbacka, M. (2008). Sensorimotor disturbances in chronic neck pain—Range of motion, peak velocity, smoothness of movement, and repositioning acuity. Manual Therapy, 13(2), 122–131. [DOI] [PubMed] [Google Scholar]
- Smallwood, R. F. , Laird, A. R. , Ramage, A. E. , Parkinson, A. L. , Lewis, J. , Clauw, D. J. , … Eickhoff, S. B. (2013). Structural brain anomalies and chronic pain: A quantitative meta‐analysis of gray matter volume. The Journal of Pain, 14(7), 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, W. O. , Skovron, M. L. , Salmi, L. R. , Cassidy, J. D. , Duranceau, J. , Suissa, S. , & Zeiss, E. (1995). Scientific monograph of the Quebec Task Force on Whiplash‐Associated Disorders: Redefining “whiplash” and its management. Spine (Phila Pa 1976), 20(8 Suppl), 1s–73s. [PubMed] [Google Scholar]
- Stanton, T. R. , Leake, H. B. , Chalmers, K. J. , & Moseley, G. L. (2016). Evidence of impaired proprioception in chronic, idiopathic neck pain: Systematic review and meta‐analysis. Physical Therapy, 96(6), 876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling, M. (2004). A proposed new classification system for whiplash associated disorders—Implications for assessment and management. Manual Therapy, 9(2), 60–70. [DOI] [PubMed] [Google Scholar]
- Strimpakos, N. , & Oldham, J. A. (2001). Objective measurements of neck function. A critical review of their validity and reliability. Physical Therapy Reviews, 6(1), 39–51. [Google Scholar]
- Treleaven, J. (2008). Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Manual Therapy, 13(1), 2–11. [DOI] [PubMed] [Google Scholar]
- Utevsky, A. V. , Smith, D. V. , & Huettel, S. A. (2014). Precuneus is a functional core of the default‐mode network. Journal of Neuroscience, 34(3), 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon, H. (2008). The Neck Disability Index: State‐of‐the‐art, 1991‐2008. Journal of Manipulative and Physiological Therapeutics, 31(7), 491–502. 10.1016/j.jmpt.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Vos, T. , Abajobir, A. A. , Abbafati, C. , Abbas, K. M. , Abate, K. H. , Abd‐Allah, F. , … Murray, C. J. L. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 390(10100), 1211–1259. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Atlas, L. Y. , Lindquist, M. A. , Roy, M. , Woo, C.‐W. , & Kross, E. (2013). An fMRI‐based neurologic signature of physical pain. New England Journal of Medicine, 368(15), 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger, E. , Mårtensson, J. , Noack, H. , Bodammer, N. C. , Kühn, S. , Schaefer, S. , … Lövdén, M. (2014). Comparing manual and automatic segmentation of hippocampal volumes: Reliability and validity issues in younger and older brains. Human Brain Mapping, 35(8), 4236–4248. 10.1002/hbm.22473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing Chiu, T. T. , Hung Law, E. Y. , & Fai Chiu, T. H. (2005). Performance of the craniocervical flexion test in subjects with and without chronic neck pain. Journal of Orthopaedic & Sports Physical Therapy, 35(9), 567–571. [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Kochunov, P. , Blangero, J. , Almasy, L. , Zilles, K. , Fox, P. T. , … Glahn, D. C. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage, 53(3), 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse, A. , & Vasseljen, O. (2008). Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskeletal Disorders, 9(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen, J. , Takala, E. , Kautiainen, H. , Nykänen, M. , Häkkinen, A. , Pohjolainen, T. , … Airaksinen, O. (2004). Association of neck pain, disability and neck pain during maximal effort with neck muscle strength and range of movement in women with chronic non‐specific neck pain. European Journal of Pain, 8(5), 473–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


