Abstract
Mismatch responses reflect neural mechanisms of early cognitive processing in the auditory domain. Disturbances of these mechanisms on multiple levels of neural processing may contribute to clinical symptoms in major depression (MD). A functional magnetic resonance imaging (fMRI) study was conducted to identify neurobiological foundations of altered mismatch processing in MD. Twenty‐five patients with major depression and 25 matched healthy individuals completed an auditory mismatch paradigm optimized for fMRI. Brain activity during mismatch processing was compared between groups. Moreover, seed‐based connectivity analyses investigated depression‐specific brain networks. In patients, mismatch processing was associated with reduced activation in the right auditory cortex as well as in a fronto‐parietal attention network. Moreover, functional coupling between the right auditory cortex and frontal areas was reduced in patients. Seed‐to voxel analysis on the whole‐brain level revealed reduced connectivity between the auditory cortex and the thalamus as well as posterior cingulate. The present study indicates deficits in sensory processing on the level of the auditory cortex in depression. Hyposensitivity in a fronto‐parietal network presumably reflects altered attention mechanisms in depression. The observed impairments may contribute to psychopathology by reducing the ability of the affected individuals to orient attention toward important environmental cues.
Keywords: attention mechanisms, depression, mismatch processing, neuroimaging, sensory information processing
1. INTRODUCTION
Cognitive deficits in depression have recently gained more attention, presumably contributing to social dysfunctions (Bortolato et al., 2016; Jaeger, Berns, Uzelac, & Davis‐Conway, 2006; Murrough, Iacoviello, Neumeister, Charney, & Iosifescu, 2011). Indeed, neuropsychological studies indicate cognitive disturbances in depression, including deficits in executive functions, memory and attention (Lee, Hermens, Porter, & Redoblado‐Hodge, 2012; McDermott & Ebmeier, 2009; Millan et al., 2012). These impairments may be mediated by dysfunction of the prefrontal lobe, which has been widely discussed as a neural correlate of depressive symptomatology (Ottowitz, Tondo, Dougherty, & Savage, 2002; Price & Drevets, 2010). The neural mechanisms underlying altered cognitive processing in depression are poorly understood. Fitzgerald (2013) suggests that impairments are present already at the level of early sensory information processing. Accordingly, studies show diminished visual contrast gain and reduced auditory pitch identification in patients with depression (Bubl, Kern, Ebert, Bach, & Tebartz Van Elst, 2010; Schwenzer, Zattarin, Grözinger, & Mathiak, 2012). So far, sensory processing deficits in depression have gained less attention compared to aspects of higher cognitive processing levels such as emotion regulation and attentional biases. As a result, neural correlates of impaired sensory processing in depression remain elusive.
Mismatch paradigms can be implemented to study sensory processing in the auditory domain. A mismatch response is elicited by infrequent sounds (deviants) in an array of frequent sounds (standards). Mismatch responses have been predominantly investigated in electroencephalography (EEG) by the mismatch negativity (MMN). The MMN is an early event‐related potential (ERP). According to predictive coding models, the mismatch negativity is a prediction error signal resulting from a mismatch between bottom‐up auditory input and top‐down predictions based on a memory trace that reflects recent auditory information (Garrido, Kilner, Stephan, & Friston, 2009). Mismatch processing has been linked to a fronto‐temporal network (Molholm, Martinez, Ritter, Javitt, & Foxe, 2005; Opitz, Rinne, Mecklinger, von Cramon, & Schröger, 2002). Whereas temporal areas are associated with the detection of change in the auditory landscape, frontal brain regions may reflect a switch in attention caused by unexpected auditory information (Alho, 1995; Näätänen & Michie, 1979).
In the context of mental disorders, mismatch paradigms have been considered a powerful tool to investigate auditory cognition and associated impairments for a wide range of neuropsychiatric or degenerative brain disorders (e.g., Ahveninen et al., 1999; see Näätänen, Sussman, Salisbury, & Shafer, 2014 for review). As markers for biological dysfunctions in various mental disorders, mismatch responses detect deficits in core cognitive functions such as attention mechanisms and working memory that have been shown to be altered in mental disorders (Ahveninen et al., 1999; Bonetti et al., 2018; Hahn et al., 2012; Potkin et al., 2009).
EEG and MEG studies in patients with depression yielded inconsistent findings. Takei and colleagues (Takei et al., 2009) found reduced magnetic global field power of the mismatch response in patients. Deficits in mismatch processing were confirmed in subsequent investigations (Chen et al., 2015; Qiao et al., 2013). For instance, Pang et al. (2014) found impaired MMN to sad syllables, whereas happy, angry, and neutral deviants were not processed differently in patients compared to healthy individuals. In contrast, other studies reported increased MMN amplitudes in patients with depression (He et al., 2010; Kähkönen et al., 2007) as well as an association between the risk for depression and higher MMN amplitudes in a subclinical sample (Bonetti, Haumann, Vuust, Kliuchko, & Brattico, 2017). Mu et al. (2016) investigated mismatch responses to musical features and found increased timbre‐MMN in depression but no differences for the remaining five sound features. Finally, other studies did not find any differences in mismatch processing (Umbricht et al., 2003). Although electrophysiology enables us to precisely investigate temporal sequencing of information processing, the spatial resolution of this technique is less than ideal. It is plausible that the heterogeneous findings of EEG and MEG can be partly explained by different sensitivities of the methods for the detection of frontal and temporal mismatch sources.
The neural networks underlying mismatch processing can be localized more precisely by fMRI (Gaebler et al., 2015; Mathiak et al., 2002). To identify dysfunctional brain networks associated with mismatch processing in depression, we implemented a mismatch paradigm that showed increased sensitivity to impairments in schizophrenia in fMRI and MEG (Gaebler et al., 2015; Thönnessen et al., 2008).
First, we hypothesized that our paradigm is suitable to detect activation in brain networks that have previously been associated with mismatch processing in both, healthy individuals and patients with major depression (MD). Second, we expected altered neural activation patterns associated with mismatch processing in MD compared to healthy individuals. We hypothesized that impairments are most pronounced in frontal areas reflecting the key role of this region in depression. Third, we explored group differences in task‐dependent and independent connectivity.
To our knowledge this is the first fMRI study investigating neural correlates of auditory mismatch processing in MD.
2. METHOD
2.1. Participants
Twenty‐five patients diagnosed with MD (age 37.9 ± 11; 8 females) and twenty‐five healthy individuals matched for age and gender (age 36.8 ± 10.7; 8 females) participated in the present study. Expert diagnosis of psychiatric illness was based on ICD‐10 criteria and confirmed by the German version of the structured clinical interview for assessment of DSM‐IV‐TR criteria (SCID‐I; American Psychiatric Association, 2000). Inclusion criteria for the patients were an acute depressive episode, a minimum of mild depressive symptomatology according to the Beck depression inventory‐II (BDI‐II score > 14) and no history of manic or mixed episodes, acute substance abuse, psychotic symptoms, and severe neurological disorders. Patients were recruited from the psychiatric wards of the Department of Psychiatry, Psychotherapy, and Psychosomatics, University Hospital Aachen. Healthy individuals had no history of neurological or psychiatric disorders. Contraindications for MRI and hearing impairments served as exclusion criteria for all participants. The study was conducted according to the declaration of Helsinki and approved by the local Ethics Committee of the RWTH Aachen University Hospital (EK 216/11). All participants provided written informed consent prior to participation and after receiving a complete description of the study. Healthy individuals were recruited from the same pool of a previous study (Gaebler et al., 2015) resulting in an overlap of 12 participants in both samples.
2.2. Assessment and tests
All participants provided demographic data on age, gender, educational level, and parental education. Self‐ and other‐rated depressive symptomatology was assessed by the BDI‐II and the 21‐item version of the Hamilton rating scale for depression (HAM‐D). The level of social, occupational, and psychological functioning was evaluated by the global assessment of functioning scale (GAF). All participants completed version B of the Trail‐making task (TMT‐B) to assess executive functions, speed of processing and mental flexibility.
2.3. Experimental paradigm
Previous studies have shown that mismatch responses can be investigated with fMRI (Kircher et al., 2004; Molholm et al., 2005). We applied a modified version of the optimum mismatch design, which was first introduced by Näätänen, Pakarinen, Rinne, and Takegata (2004) and that has recently been adapted for fMRI application (Gaebler et al., 2015). This paradigm is sensitive for the detection of temporal and frontal activation associated with mismatch processing in healthy and clinical populations and was found to be specifically suitable for the detection of pathophysiological changes in schizophrenia (Gaebler et al., 2015; Thönnessen et al., 2008). All participants were instructed to watch a silent movie (Koyaanisqatsi, Godfrey Reggio, IRE Productions, NM, 1982) and to ignore the auditory stimulation. This constitutes a substantial advantage of the implemented design in the context of psychiatric disorders. The task demands are minimal, rendering distortion of the results by motivational factors less likely (Näätänen, 1995).
In a block design, eight standard blocks and eight deviant blocks were presented alternately. Each block comprised 60 stimuli and lasted 30 s resulting in a total length of the paradigm of 8 min (see Figure 1). Standard blocks only comprised standard sounds that were synthesized by three sinusoidal partials at 500, 1,000, and 1,500 Hz and lasted for 100 ms. The sounds were presented binaurally via headphones at 90 dB with the last two of three partials being 3 dB and 6 dB softer, respectively. A sound threshold of 90 dB has previously been shown to be suitable to ensure audibility in the MRI scanner at a comfortable listening level (Molholm et al., 2005). In deviant blocks, standard and deviant sounds were presented in alternating fashion—with the restriction that two deviants of the same category never followed each other. The following five deviant types were implemented: (a) amplitude—10 dB or softer than the standard sound; (b) duration—50 ms shorter or 200 ms longer compared to the standard sound; (c) frequency—33% higher (667, 1,333, and 2,000 Hz) or 33% lower (333, 667, and 1,000 Hz) than the standard sound; (d) location—an interaural time (1 ms) and amplitude (3 dB) difference in favor of the left or right auditory channel was introduced rendering the perceived sound source as 90° to the left or right side (standard sounds were perceived in the center); and (e) gap—a pause of 25 or 50 ms was inserted in the middle of the standard sound. Apart from these alterations, all features of the deviant sounds were constructed in parallel to the standard sounds. Each deviant type was presented with a probability of 10% (i.e., the respective stimulus feature was presented in 90% of the trials in its standard configuration). The stimuli were generated with the Matlab 2011b software (Mathworks, MA) and presented with a stimulus onset asynchrony of 500 ms.
Figure 1.
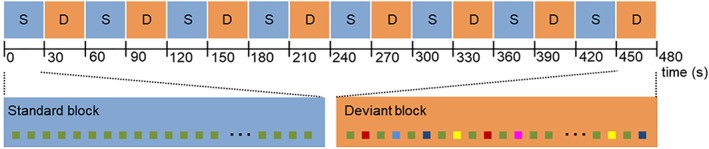
Optimum mismatch paradigm. Eight standard blocks “S” (standard stimuli) and eight deviant blocks “D” (standard and deviant stimuli) were presented in an 8 min scanning session. Standard and deviant blocks were presented alternately and lasted 30s [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. MRI data acquisition
The MRI scanning was performed using a 3.0 Tesla Tim Trio Scanner (Siemens Medical Systems, Erlangen, Germany) equipped with a 12‐channel head coil. The T2*‐weighted images were obtained using echo‐planar imaging with the following parameters: repetition time (TR) = 1,800 ms, echo time (TE) = 36 ms, flip angle = 77° and matrix size 64 × 64. Images were acquired with 26 transverse slices in interleaved order (voxel size 3 × 3 × 4 mm3; gap = 0.5 mm; field of view = 192 × 192mm2). The first five volumes were discarded to account for T1‐saturation effects. All participants were instructed to minimize movement inside the scanner and to keep eyes open.
2.5. Data analysis
Preprocessing of imaging data and statistical analysis were carried out using SPM12 software (Wellcome Trust Center for Neuroimaging, London, UK) implemented in Matlab R2012b. Preprocessing included slice‐time correction, motion correction, and transformation into MNI template space to allow for group analysis. A 128 s high‐pass filter was used to remove low‐frequency drifts. Spatial smoothing was performed using a 6 mm full‐width at half maximum Gaussian kernel. Head motion parameters and their temporal derivatives were included in the linear model to reduce motion artifacts.
At the single subject level, whole‐brain general linear model (GLM) analysis modeled the deviant blocks as the predictor of interest and the standard blocks as high‐level baseline. At the group level, beta values represented baseline‐corrected mismatch responses and entered the analysis of variance. The F‐test assessed brain network activation across all participants (effect of interest). Two one‐sample t‐tests were carried out to investigate the general mismatch response (deviant > baseline) for patients with depression and healthy individuals.
Finally, the independent samples t‐test ascertained differences in mismatch processing between the two groups (HC > MD). According to the recommendations by Woo, Krishnan, and Wager (2014), cluster‐extent based thresholding was performed in two steps: (a) a primary threshold of p < .001 on voxel‐level defined suprathreshold voxels and (b) a cluster‐level extent threshold controlling the family‐wise error rate (FWE; pFWE < .05) was estimated applying random field theory (RFT). While the Bonferroni correction assumes independency of observations, RFT corrections account for the spatial correlations in neuroimaging data.
The auditory cortex is considered the primary source of the auditory mismatch response. Numerous studies point towards a (bilateral) involvement of the primary and/or secondary auditory cortex in mismatch processing (Gaebler et al., 2015; Kircher et al., 2004; Mathiak et al., 2002). Therefore, a region of interest (ROI) analysis investigated differences between patients with depression and healthy individuals in the auditory cortex. ROIs were defined based on the automatic anatomical labeling (AAL) atlas and included superior temporal gyrus (STG) and Heschl's gyrus of the left or right hemisphere, respectively. The mean beta values of the ROIs were extracted and subjected to independent‐samples t‐tests. Results were Bonferroni corrected for multiple comparisons.
Using the same ROIs, Pearson product moment correlation coefficients were calculated to assess the relationship between the extracted beta values in the left and right auditory cortex and symptom severity such as indicated by BDI‐II, HRSD, and GAF scores as well as dosage of medication, the age of illness onset and cognitive functions (TMT‐B) in patients. Additionally, mean beta‐weights of clusters that revealed significant group differences in the whole brain analysis (i.e., left IFG and IPL) were extracted to investigate the association between blood‐oxygen‐level‐dependent (BOLD) activation patterns and the same variables in patients with depression as well as a correlation with age in all participants. The statistical package for the social sciences (SPSS, IBM, Armonk, NY) version 20.0 was used for computation of the correlation coefficients, investigation of group differences and analysis of the descriptive data.
Connectivity analysis. In order to assess group differences in task‐dependent and task‐independent functional connectivity, we conducted a psychophysiological interaction (PPI) analysis in a ROI‐to‐ROI and a seed‐to‐voxel approach. For both approaches, the bilateral auditory cortex served as the seed region, which was defined according to the AAL atlas (encompassing STG and Heschl's gyrus).
Within the framework of PPI, task‐dependent connectivity reflects changes in network connectivity that depend on the task. Accordingly, it was assessed if synchronization between brain regions is higher during mismatch compared to baseline blocks. Task independent connectivity represents network connectivity irrespective of the task modulation. In this context, the time series of a target region (i.e., a ROI in the ROI‐to ROI‐approach or any voxel in the seed‐to voxel‐approach) is regressed on three explanatory variables: (a) the time series of the seed regions estimating connectivity irrespective of the experimental condition, (b) the HRF‐convolved task time course representing the main effect of the experimental condition, and (c) the element by element product of both regressors estimating the interaction of task condition and connectivity (i.e., task‐dependent connectivity). Accordingly, the time series of the target region represents the dependent variable in the regression.
The ROI‐to‐ROI approach assessed connectivity between the bilateral auditory cortex (seed region) and left IFG as well as left IPL as target regions. All regions were defined according to the AAL atlas and selected based on their proposed role in mismatch processing as well as the observed group differences. The average time series of the ROI signals were extracted and normalized. Independent samples t‐tests investigated differences in task‐dependent and task‐independent connectivity between the patients with depression and healthy individuals.
The seed‐to‐voxel approach assessed connectivity between the bilateral auditory cortex and all other voxels in the brain. Again, independent samples t‐test analyses were carried out to assess differences between healthy individuals and patients in task‐dependent connectivity and task‐independent connectivity on whole‐brain level. In this approach, data were corrected for multiple testing using a primary threshold of p < .001 on voxel‐level combined with a cluster‐level threshold (pFWE < .05).
3. RESULTS
3.1. Demographic and behavioral data
Patients with depression and healthy individuals were matched for age and gender (8 females, 17 males in each group). The groups did not differ significantly regarding own and parental educational level (see Table 1 for detailed sample characteristics). Elapsed time since the first diagnosed depressive episode in the present sample ranged from less than a year to 26 years (mean duration in years: 5.9 ± 6.8). Patients experienced mild to moderate depressive symptomatology and serious functional impairments as assessed by the HRSD, BDI‐II, and GAF. Apart from two patients who did not receive psychopharmacological treatment, all patients received antidepressant medication. Seven patients additionally took antipsychotic medication, mainly as an augmentative treatment. A significantly higher number of participants in the clinical sample were smokers (48% compared to 8% in the control group; χ 2[1] = 9.92, p = .002). Groups did not significantly differ in handedness (χ 2[2] = 3.19, p = .2).
Table 1.
Sample characteristics
| MD | HC | |||||
|---|---|---|---|---|---|---|
| (N = 25) | (N = 25) | Comparison | ||||
| Mean | SD | Mean | SD | t(48) | p | |
| Age (years) | 37.9 | 11.0 | 37.8 | 10.7 | .05 | .96 |
| Education (years) | 14.1 | 2.9 | 15.5 | 2.6 | −1.81 | .08 |
| Parental education (years)a | 12.5 | 3.2 | 13.4 | 2.6 | .28 | .28 |
| TMT‐B (in seconds)b | 47.7 | 19.7 | 41.9 | 14.8 | 1.13 | .26 |
| Clinical characteristics | Mean | SD | ||||
| BDI‐II | 26.2 | 8.3 | ||||
| HAM‐D | 17.0 | 7.1 | ||||
| GAF | 46.1 | 10.0 | ||||
| Age of onset (years) | 30.5 | 12.7 | ||||
| Antidepressantsc (N = 23) | 171.3 | 87.9 | ||||
| Antipsychoticsc (N = 7) | 12.0 | 22.7 | ||||
Abbreviations: TMT‐B, trail‐making task, version B; BDI‐II, beck depression inventory‐II; HAM‐D, Hamilton depression rating scale; GAF, global assessment of functioning.
Two data points were missing in the patient group (4%).
Three extreme data points were excluded from analysis in the patient (2%) and control group (4%); extreme cases were defined as data points deviating more than 3SD from the mean.
Medication is reported as percentage of the defined daily dose.
3.2. Brain mapping
Mismatch processing was associated with extensive bilateral activation clusters encompassing the auditory cortex (bilateral STG and Heschl's gyrus) in both groups. Clusters extended into bilateral insula and, in healthy individuals only, into bilateral IPL. Furthermore, mismatch processing in healthy individuals was associated with wide‐spread activation patterns including a cluster comprising the left IFG pars triangularis and the middle frontal gyrus as well as clusters in the right precentral gyrus, the medial superior frontal lobe, and one cluster extending from the precuneus to the mid‐cingulate cortex. The effect of interest analysis in all participants revealed an additional cluster in the occipital lobe (Figure 2; see Table 2 for a list of activation clusters).
Figure 2.
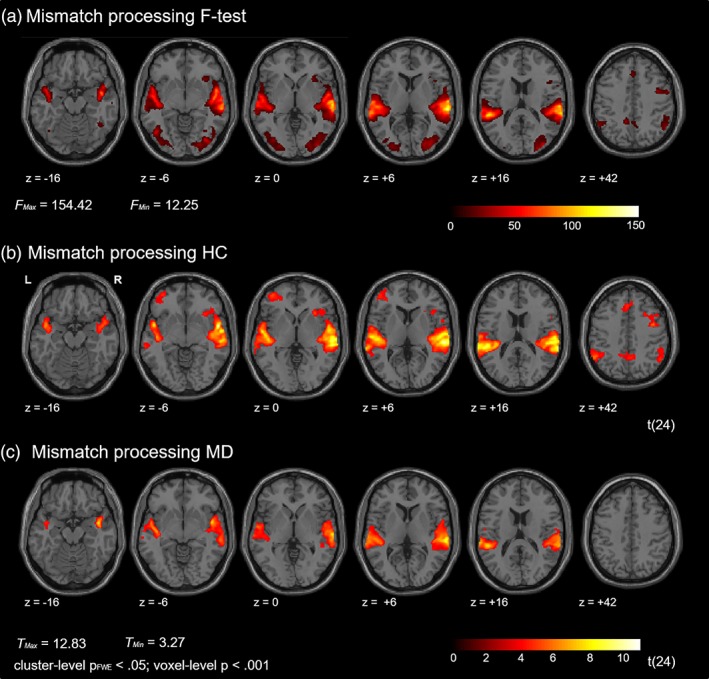
Mismatch processing. (a) The F‐contrast (effect of interest) revealed activation clusters in multiple brain regions, including bilateral auditory cortex and the occipital lobe. (b) Auditory mismatch processing was associated with widespread activation in brain networks including bilateral auditory cortex and fronto‐parietal brain regions in healthy controls (HC). (c) In patients with major depression (MD) activation cluster emerged in bilateral auditory cortex [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain activation associated with mismatch processing
| All (F‐Test) | HC (deviant > baseline) | MD (deviant > baseline) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI coordinates | F | MNI coordinates | T | MNI coordinates | T | |||||||||||
| Cluster | Brain region | x | y | z | (d = 24) | k E | x | y | z | (d = 24) | k E | x | y | z | (d = 28) | k E |
| 1 | Right STG/Heschl's gyrusa | 60 | −24 | 12 | 154.42 | 5,178 | 60 | −30 | 4 | 11.27 | 6,165 | 64 | −28 | 6 | 9.01 | 3,287 |
| 2 | Left STG/Heschl's gyrusa | −52 | −38 | 14 | 125.79 | 4,592 | −48 | −36 | 12 | 11.18 | 4,979 | −62 | −32 | 12 | 9.09 | 2,276 |
| IPL | −52 | −52 | 42 | 6.17 | ||||||||||||
| 3 | Right inferior occipital lobe | 36 | −78 | −6 | 43.77 | 2,283 | ||||||||||
| 4 | Left inferior temporal lobe | −46 | −64 | 6 | 43.22 | 1,105 | ||||||||||
| 5 | Right precentral/middle FG | 40 | 0 | 42 | 30.29 | 332 | 50 | 2 | 48 | 6.58 | 561 | |||||
| 6 | Left IFG, pars triangularis | −38 | 50 | 0 | 5.33 | 591 | ||||||||||
| 7 | Right inferior parietal lobe | 50 | −58 | 48 | 30.06 | 604 | ||||||||||
| 8 | Precuneus/middle cingulum | −12 | −48 | 42 | 28.78 | 267 | 6 | −54 | 44 | 4.72 | 258 | |||||
| 9 | Inferior opercula frontal lobe | 32 | 22 | −6 | 22.59 | 295 | ||||||||||
| 10 | SMA / superior medial frontal lobe |
0 18 |
28 28 |
42 50 |
21.85 20.75 |
137 140 |
−4 | 24 | 46 | 5.32 | 261 | |||||
| 11 | Left SMA | −10 | 0 | 68 | 21.21 | 182 | ||||||||||
| C | Right middle frontal lobe | 40 | 38 | 28 | 20.77 | 180 | ||||||||||
Cluster‐level pFWE < .05; voxel‐level p < .001.
Cluster extends to inferior parietal lobe (IPL) in healthy individuals. STG, superior temporal gyrus; FG, frontal gyrus; IFG, inferior frontal gyrus.
Confirming our main hypothesis, a significant group difference emerged. More precisely, patients showed less activation during mismatch processing relative to healthy individuals in a prefrontal cluster including the left IFG, pars triangularis. Furthermore, patients exhibited less activation in the left IPL encompassing the supramarginal gyrus. No other group differences emerged—in particular in the auditory cortex at the corrected mapping threshold (Figure 3; see Table 3 for a list of activation clusters).
Figure 3.
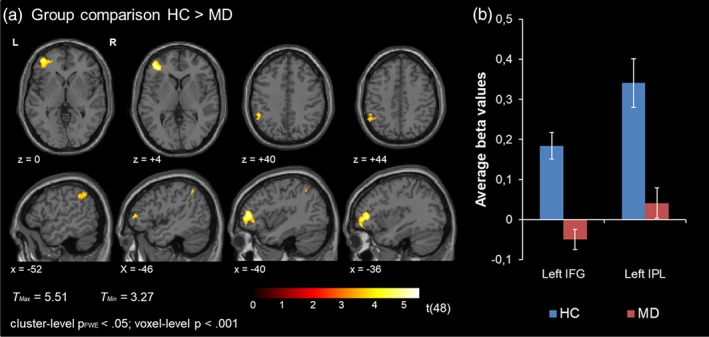
(a) Group comparison. Significant group differences emerged in the left IFG and the left IPL. HC showed higher BOLD activation relative to patients with MD. (b) Extracted mean beta values from the left IFG and left IPL in patients and HC [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Group comparison
| HC > MD | ||||||
|---|---|---|---|---|---|---|
| MNI coordinates | T | |||||
| Cluster | Brain region | x | y | z | (d = 28) | k E |
| 1 | Left IFG, pars triangularis | −38 | 42 | 6 | 5.51 | 425 |
| 2 | Left IPL | −50 | −44 | 42 | 4.02 | 144 |
Cluster‐level pFWE < .05; voxel‐level p < .001.
Notably, the anatomical ROI analysis revealed group differences at the level of the right auditory cortex indicating higher activation in healthy individuals in this brain region (t[48] = −2.65, p = .01). The comparison between groups for the left auditory cortex did not reach significance (left auditory cortex: t[48] = −1.94, p = .06; see Figure 4).
Figure 4.
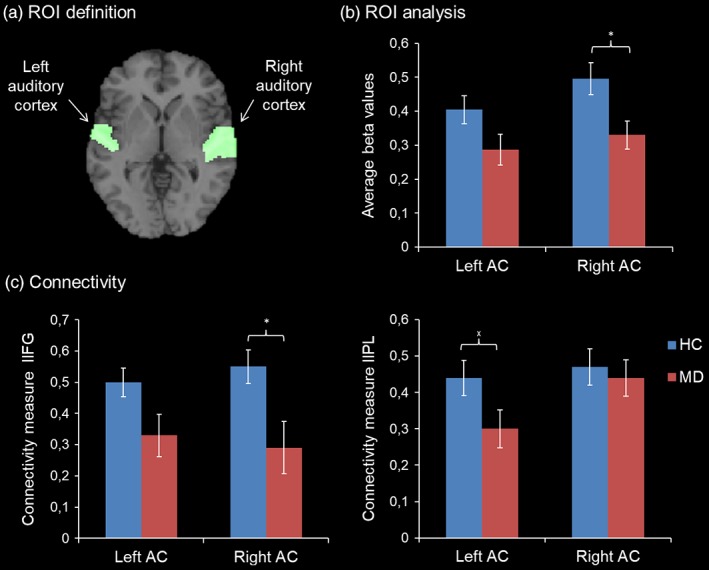
ROI analysis. (a) The ROIs were selected based on the AAL atlas and comprised bilateral STG and Heschl's gyri. (b) HC showed a higher BOLD response compared to patients with MD in the right auditory cortex only (*p < .05). (c) Connectivity analysis revealed reduced coupling between the left IFG and right auditory cortex in patients (left panel; *p < .05) and nominal significant group differences in connectivity between the left IPL and the left auditory cortex (right panel: x p < .05). Standard errors are indicated by the white error bars [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Brain behavior relationship
The extracted mean beta values from the ROIs in the auditory cortex, IFG, and IPL were not significantly associated with symptom severity (BDI‐II, HRSD, and GAF scores), age of onset or dosage of antidepressant medication as well as cognitive functioning when corrected for multiple comparisons (all p > .1). Furthermore, no significant association emerged between the extracted beta weights in the left IFG and IPL and age across all subjects (IFG p = .09; IPL p = .28).
3.4. Connectivity analysis
To assess functional connectivity and its modulation by the paradigm, a PPI analysis was conducted. The analysis revealed a significantly higher synchronization between the right auditory cortex and the left IFG in healthy individuals (.55 ± .23) compared to patients with depression (.29 ± .42; t[48] = 2.78, p = .008), irrespective of the task modulation. Furthermore, nominal significant differences between groups emerged with regard to the association between the left auditory cortex and the left IPL (t[48] = 2.03, p = .048). For the left auditory cortex and the left IFG as well as the left IFG and the left IPL, this analysis detected no significant differences between groups (t(48) = 1.94, p = .059; t(48) = −.70, p > .1). Only the association between the right auditory cortex and the left IFG survived Bonferroni correction for multiple comparisons. Task‐dependent functional connectivity between the selected ROIs did not yield any significant differences between groups (all p > .1).
On the whole‐brain level, task‐based connectivity did not differ significantly between groups at the corrected threshold. However, group differences in task‐independent connectivity emerged in the right thalamus and left posterior cingulum (Figure 5, for a list of activation clusters see Table 4).
Figure 5.
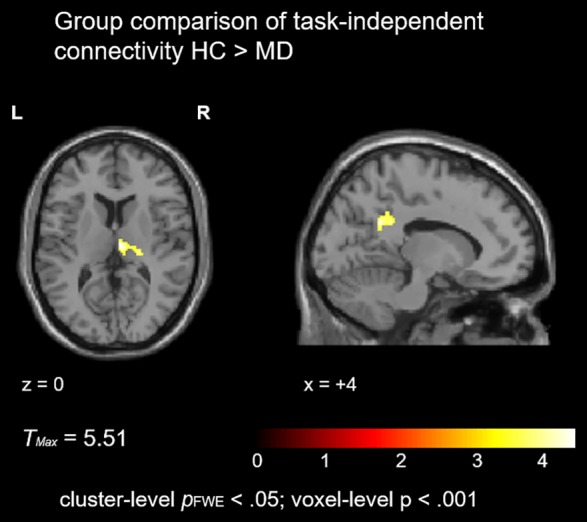
Task‐independent connectivity. Patients with MD exhibited reduced task‐independent connectivity between the bilateral auditory cortex and the right thalamus as well as left posterior cingulum [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Task‐independent connectivity
| HC > MD | ||||||
|---|---|---|---|---|---|---|
| MNI coordinates | T | |||||
| Cluster | Brain region | x | y | z | (d = 28) | k E |
| 1 | Right thalamus | 8 | −18 | 10 | 4.67 | 190 |
| 2 | Left posterior cingulum | −12 | −50 | 28 | 4.66 | 226 |
Cluster‐level pFWE < .05; voxel‐level p < .001.
4. DISCUSSION
The present study investigated auditory mismatch processing in 25 patients with depression and 25 healthy individuals using fMRI. Mismatch processing was associated with widespread brain activation in healthy individuals including bilateral auditory cortex as well as frontal and parietal brain regions. Group comparisons revealed lower levels of activation in the left IFG and IPL in patients, possibly reflecting altered functionality of a fronto‐parietal attention network. Additionally, a ROI analysis revealed reduced activation in the right auditory cortex in patients. Connectivity analysis confirmed reduced coupling between the left IFG and right auditory cortex. On the whole‐brain level, synchronization between the auditory cortex and the thalamus/posterior cingulate was reduced in patients. To our knowledge this is the first fMRI study that examined mismatch processing in patients with depression.
Our findings are in line with previous imaging studies that revealed activation related to mismatch processing in bilateral STG (Doeller et al., 2003; Kircher et al., 2004; Mathiak et al., 2002; Molholm et al., 2005) as well as EEG and MEG studies that locate the main generator of the MMN in the temporal lobes (Alho, 1995; Molholm et al., 2005). Importantly, Molholm et al. (2005) showed involvement of a fronto‐parietal network in mismatch processing as well as the precuneus, superior frontal areas, and the right middle frontal gyrus encompassing the supplementary motor area in response to different deviant types. The activation pattern largely resembles our findings. The fronto‐parietal activation was interpreted by the authors as reflecting attention switching mechanisms that were elicited by the deviant stimuli. In a similar vein, frontal activation during mismatch processing has previously been described as reflecting an orienting response (Rinne, Alho, Ilmoniemi, Virtanen, & Näätänen, 2000). At the cellular level, animal studies have documented neural responses to auditory stimulation in the ventrolateral prefrontal cortex, which may play an important role in auditory working memory (for review see Plakke & Romanski, 2016). Indeed, multiple studies have confirmed the involvement of the right IFG in auditory change detection in humans (Doeller et al., 2003; Opitz et al., 2002). In parallel to our results, Müller, Jüptner, Jentzen, and Müller (2002) found involvement of the left IFG in mismatch processing. Differences in the observed activation foci may depend on physical stimulus characteristics. For example, frontal activation may be feature dependent, with a higher responsivity of the left IFG to duration deviants and the right IFG to frequency deviants (Molholm et al., 2005). The auditory stimulation in the present study was complex, involving five different types of deviants. Mismatch designs that allow for a differentiation between deviant types may help to gain further insights on feature‐specific modulations of mismatch processing.
In the context of depression, sensory processing deficits have for a long time not been considered characteristic for the psychopathology and were largely neglected as a field of study. Now, converging evidence suggests that auditory information processing may be impaired in depression. For example, patients show reduced auditory pitch identification (Schwenzer et al., 2012) and disturbed information processing in the auditory domain (Qiao et al., 2013; Takei et al., 2009; Tollkötter, Pfleiderer, Sörös, & Michael, 2006). Indeed, the ROI analysis revealed reduced activation in the right auditory cortex presumably reflecting impaired sensory processing on the level of the auditory cortex in patients. Accordingly, our results add to a growing body of evidence suggesting impaired sensory processing in depression (Fitzgerald, 2013). So far, it is not clear if these deficits are trait or state dependent. The lack of an association between symptom severity and the extracted beta weights in frontal, parietal, and temporal regions indicates that impairment may be trait rather than state dependent. This is in line with results of an EEG study by Qiao et al. (2013). The authors did not find an association between MMN amplitudes and symptom severity and suggested that the observed impairments are a trait marker of depression.
Traditionally, auditory change detection is conceptualized as a cortical phenomenon. Challenging this view, it has been suggested that deviance detection originates at earlier stages in the auditory hierarchy, involving subcortical structures such as the thalamus and inferior colliculus (Escera, Leung, & Grimm, 2014; Kraus, McGee, Littman, Nicol, & King, 1994). Notably, patients with MD showed reduced connectivity compared to healthy individuals between the auditory cortex and the thalamus, which may further contribute to reduced mismatch processing on the cortical level. However, at this point, this perspective is speculative and needs further investigation.
Apart from activation in response to deviants in the temporal lobe, deficits in mismatch processing were most pronounced in frontal and parietal brain regions. Previous EEG and MEG studies associated deficits in mismatch processing with frontal dysfunction in patients with depression (Chen et al., 2015; He et al., 2010; Kähkönen et al., 2007; Qiao et al., 2013). Aberrant mismatch responses have been interpreted in terms of deficient attention switching following the presentation of a deviant (Qiao et al., 2013). In a similar vein, the involvement of a fronto‐parietal network may reflect an orienting response in healthy individuals, indicating a shift in attention toward the salient stimulus (Molholm et al., 2005; Rinne et al., 2000). The observed hyposensitivity of frontal and parietal areas in patients with depression during mismatch processing suggests that these mechanisms—that is, a shift in attention in response to novel auditory information—may be impaired in this patient cohort.
Furthermore, a desynchronization between temporal and fronto‐parietal processing nodes—as suggested by the connectivity analysis—may contribute to the observed fronto‐temporal hypoactivation in depressed patients. In this vein, Chen et al. (2015) suggest that the neurophysiological transmission from mismatch detection to the subsequent orienting response may be disrupted in patients with depression. Our finding of a weakened fronto‐temporal connectivity supports this notion.
Functionally, mismatch processing is thought to enable an individual to orient towards important stimuli in the environment (Belger, Yucel, & Donkers, 2012). Impairments in mismatch processing may impact social, occupational, and psychological functioning of an individual by diminishing detection and orientation toward salient (social) environmental cues leading to decreased levels of global functioning (Javitt & Freedman, 2015; Light et al., 2015). Naismith et al. (2012) report an association between reduced MMN amplitudes and self‐rated functional disability. Accordingly, patients showed serious impairment in social, occupational, and psychological functioning in our study. Furthermore, a meta‐analysis of resting‐state functional connectivity in depression revealed reduced connectivity in a fronto‐parietal network that has been associated with attention and emotion regulation (Kaiser, Andrews‐Hanna, Wager, & Pizzagalli, 2015). The authors interpret their results as an indicator of impaired cognitive control leading to a bias for self‐referential thinking at the expense of attention to external stimuli. Alterations in sensory information processing such as indicated by the present study may contribute to a self‐referential thinking style by reducing the likelihood of disengaging from internal thought processes because of salient external cues. Fitzgerald (2013) suggests that impaired sensory processing may lead to less efficient activation of reward systems reducing the likelihood of the detection of salient information and subsequent positive emotional responses. Indeed, a core symptom of depression is a loss of interest in previously enjoyable activities and deficits in visual information processing in depression diminished after successful psychopharmacological treatment (Bubl et al., 2010; Bubl, Ebert, Kern, van Elst, & Bach, 2012). Taken together, sensory deficits such as detected by mismatch paradigms and impaired attention mechanisms may considerably contribute to depressive psychopathology.
5. LIMITATIONS
Due to the low temporal resolution of the BOLD‐response we were not able to differentiate between early and late components of mismatch processing. Our study aimed at identifying dysfunctional brain networks involved in mismatch processing in depression. To disentangle time locked contributions of brain regions, combined EEG and fMRI investigations are warranted, allowing for an independent investigation of the neural representation and integrity of mismatch detection and attention mechanisms as well as the functional interrelationship of both processes. In a similar vein, the design of the current study is not sensitive to event‐related changes in response to the deviant tones.
Most patients received drug treatment. A previous investigation (Tollkötter et al., 2006) highlights the impact of psychopharmacological modulation on mismatch processing, implying the need to take it into account as a possible confound. In healthy individuals, the administration of serotonin reuptake inhibitors (SSRIs) has been shown to increase the MMN amplitude (Wienberg, Glenthoj, Jensen, & Oranje, 2010). Accordingly, the present results may underestimate deficits in sensory processing in unmedicated patients with depression. However, generalization of drug effects in healthy individuals to clinical populations is clearly limited. Here, we did not find an association between the dosage of antidepressants and brain activation. Studies comparing unmedicated first episode patients to medicated patients are warranted.
Finally, it has to be investigated whether the observed fronto‐parietal deficits in mismatch processing are specific for patients with depression. However, in light of recent dimensional approaches in contrast to the traditional categorical classification systems of psychiatric disorders this aspect may be of less importance. The research domain criteria (RDoC) include deviance detection as one element of the domain “cognitive systems”. The investigation of neural correlates of mismatch responses with highly standardised procedures integrating neutral stimuli such as in the present study may become highy relevant in the implementation of dimensional approaches.
6. CONCLUSION
Our findings highlight the feasibility of mismatch designs to investigate sensory information processing in this patient cohort. Mismatch processing was associated with reduced activation in temporal and fronto‐parietal brain regions as well as reduced connectivity within a fronto‐temporal network. The observed brain activation patterns presumably reflect an impaired transmission between mismatch detection and the subsequent orienting response. A decreased ability to orient toward salient environmental cues may have a negative impact on global functioning of the affected individuals and may considerably contribute to psychopathology.
ACKNOWLEDGMENTS
This study was supported by the German Research Foundation (DFG IRTG 2150, MA 2631/6‐1); the Federal Ministry for Education and Research (BMBF; APIC: 01EE1405 A‐C). We thank the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research at the RWTH Aachen University for technical support. The authors report no further financial disclosures or potential conflicts of interest.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Zweerings J, Zvyagintsev M, Turetsky BI, et al. Fronto‐parietal and temporal brain dysfunction in depression: A fMRI investigation of auditory mismatch processing. Hum Brain Mapp. 2019;40:3657–3668. 10.1002/hbm.24623
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Number: APIC: 01EE1405 A‐C; Deutsche Forschungsgemeinschaft, Grant/Award Number: DFG IRTG 2150; MA 2631/6‐1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ahveninen, J. , Jaaskelainen, I. P. , Pekkonen, E. , Hallberg, A. , Hietanen, M. , Makela, R. , … Sillanaukee, P. (1999). Suppression of Mismatch Negativity by Backward Masking Predicts Impaired Working‐Memory Performance in Alcoholics. Alcoholism: Clinical and Experimental Research, 23(9), 1507–1514. 10.1111/j.1530-0277.1999.tb04674.x [DOI] [PubMed] [Google Scholar]
- Alho, K. (1995). Cerebral Generators of Mismatch Negativity (MMN) and Its Magnetic Counterpart (MMNm) Elicited by Sound Changes. Ear and Hearing, 16(1), 38–51. 10.1097/00003446-199502000-00004 [DOI] [PubMed] [Google Scholar]
- Belger, A. , Yucel, G. H. , & Donkers, F. C. L. (2012). In Search of Psychosis Biomarkers in High‐risk Populations: Is the Mismatch Negativity the One We've Been Waiting for? Biological Psychiatry, 71(2), 94–95. 10.1016/j.biopsych.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Bonetti, L. , Haumann, N. T. , Brattico, E. , Kliuchko, M. , Vuust, P. , Särkämö, T. , & Näätänen, R. (2018). Auditory sensory memory and working memory skills: Association between frontal MMN and performance scores. Brain Research, 1700, 86–98. 10.1016/j.brainres.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Bonetti, L. , Haumann, N. T. , Vuust, P. , Kliuchko, M. , & Brattico, E. (2017). Risk of depression enhances auditory Pitch discrimination in the brain as indexed by the mismatch negativity. Clinical Neurophysiology, 128(10), 1923–1936. 10.1016/j.clinph.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Bortolato, B. , Miskowiak, K. W. , Köhler, C. A. , Maes, M. , Fernandes, B. S. , Berk, M. , & Carvalho, A. F. (2016). Cognitive remission: A novel objective for the treatment of major depression? BMC Medicine, 14(1), 1–18. 10.1186/s12916-016-0560-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubl, E. , Ebert, D. , Kern, E. , van Elst, L. T. , & Bach, M. (2012). Effect of antidepressive therapy on retinal contrast processing in depressive disorder. British Journal of Psychiatry, 201(2), 151–158. 10.1192/bjp.bp.111.100560 [DOI] [PubMed] [Google Scholar]
- Bubl, E. , Kern, E. , Ebert, D. , Bach, M. , & Tebartz Van Elst, L. (2010). Seeing gray when feeling blue? Depression can be measured in the eye of the diseased. Biological Psychiatry, 68(2), 205–208. 10.1016/j.biopsych.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Zhang, Y. , Wei, D. , Wu, X. , Fu, Q. , Xu, F. , … Zhang, Z. (2015). Neurophysiological handover from MMN to P3a in first‐episode and recurrent major depression. Journal of Affective Disorders, 174, 173–179. 10.1016/j.jad.2014.11.049 [DOI] [PubMed] [Google Scholar]
- Doeller, C. F. , Opitz, B. , Mecklinger, A. , Krick, C. , Reith, W. , & Schröger, E. (2003). Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. NeuroImage, 20(2), 1270–1282. [DOI] [PubMed] [Google Scholar]
- Escera, C. , Leung, S. , & Grimm, S. (2014). Deviance Detection Based on Regularity Encoding Along the Auditory Hierarchy: Electrophysiological Evidence in Humans. Brain Topography, 27(4), 527–538. 10.1007/s10548-013-0328-4 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. J. (2013). Gray colored glasses: Is major depression partially a sensory perceptual disorder? Journal of Affective Disorders, 151(2), 418–422. 10.1016/j.jad.2013.06.045 [DOI] [PubMed] [Google Scholar]
- Gaebler, A. J. , Mathiak, K. , Koten, J. W. , König, A. A. , Koush, Y. , Weyer, D. , … Zvyagintsev, M. (2015). Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain, 138(5), 1410–1423. 10.1093/brain/awv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, M. I. , Kilner, J. M. , Stephan, K. E. , & Friston, K. J. (2009). The mismatch negativity: A review of underlying mechanisms. Clinical Neurophysiology, 120(3), 453–463. 10.1016/j.clinph.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, B. , Hollingworth, A. , Robinson, B. M. , Kaiser, S. T. , Leonard, C. J. , Beck, V. M. , … Gold, J. M. (2012). Control of working memory content in schizophrenia. Schizophrenia Research, 134(1), 70–75. 10.1016/j.schres.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Chai, H. , Zheng, L. , Yu, W. , Chen, W. , Li, J. , … Wang, W. (2010). Mismatch negativity in treatment‐resistant depression and borderline personality disorder. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 34(2), 366–371. 10.1016/j.pnpbp.2009.12.021 [DOI] [PubMed] [Google Scholar]
- Jaeger, J. , Berns, S. , Uzelac, S. , & Davis‐Conway, S. (2006). Neurocognitive deficits and disability in major depressive disorder. Psychiatry Research, 145(1), 39–48. 10.1016/j.psychres.2005.11.011 [DOI] [PubMed] [Google Scholar]
- Javitt, D. C. , & Freedman, R. (2015). Sensory Processing Dysfunction in the Personal Experience and Neuronal Machinery of Schizophrenia. American Journal of Psychiatry, 172(1), 17–31. 10.1176/appi.ajp.2014.13121691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen, S. , Yamashita, H. , Rytsälä, H. , Suominen, K. , Ahveninen, J. , & Isometsä, E. (2007). Dysfunction in early auditory processing in major depressive disorder revealed by combined MEG and EEG. Journal of Psychiatry & Neuroscience : JPN, 32(5), 316–322. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/17823647 [PMC free article] [PubMed] [Google Scholar]
- Kaiser, R. H. , Andrews‐Hanna, J. R. , Wager, T. D. , & Pizzagalli, D. A. (2015). Large‐scale network dysfunction in major depressive disorder: A meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry, 72(6), 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, T. T. , Rapp, A. , Grodd, W. , Buchkremer, G. , Weiskopf, N. , Lutzenberger, W. , … Mathiak, K. (2004). Mismatch responses in schizophrenia: a combined fMRI and whole head MEG study. The American Journal of Psychiatry, 161(2), 294–304. 10.1176/appi.ajp.161.2.294 [DOI] [PubMed] [Google Scholar]
- Kraus, N. , McGee, T. , Littman, T. , Nicol, T. , & King, C. (1994). Nonprimary auditory thalamic representation of acoustic change. Journal of Neurophysiology, 72(3), 1270–1277. 10.1152/jn.1994.72.3.1270 [DOI] [PubMed] [Google Scholar]
- Lee, R. S. C. , Hermens, D. F. , Porter, M. A. , & Redoblado‐Hodge, M. A. (2012). A meta‐analysis of cognitive deficits in first‐episode Major Depressive Disorder. Journal of Affective Disorders, 140(2), 113–124. 10.1016/j.jad.2011.10.023 [DOI] [PubMed] [Google Scholar]
- Light, G. A. , Swerdlow, N. R. , Thomas, M. L. , Calkins, M. E. , Green, M. F. , Greenwood, T. A. , … Turetsky, B. I. (2015). Validation of mismatch negativity and P3a for use in multi‐site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS‐2. Schizophrenia Research, 163(1–3), 63–72. 10.1016/j.schres.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak, K. , Rapp, A. , Kircher, T. T. J. , Grodd, W. , Hertrich, I. , Weiskopf, N. , … Ackermann, H. (2002). Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole‐head magnetoencephalography. Human Brain Mapping, 16(3), 190–195. 10.1002/hbm.10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, L. M. , & Ebmeier, K. P. (2009). A meta‐analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- Millan, M. J. , Agid, Y. , Brüne, M. , Bullmore, E. T. , Carter, C. S. , Clayton, N. S. , … Young, L. J. (2012). Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nature Reviews Drug Discovery, 11(2), 141–168. 10.1038/nrd3628 [DOI] [PubMed] [Google Scholar]
- Molholm, S. , Martinez, A. , Ritter, W. , Javitt, D. C. , & Foxe, J. J. (2005). The neural circuitry of pre‐attentive auditory change‐detection: an fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex (New York, N.Y. : 1991), 15(5), 545–551. 10.1093/cercor/bhh155 [DOI] [PubMed] [Google Scholar]
- Mu, Z. , Chang, Y. , Xu, J. , Pang, X. , Zhang, H. , Liu, X. , … Wan, Y. (2016). Pre‐attentive dysfunction of musical processing in major depressive disorder: A mismatch negativity study. Journal of Affective Disorders, 194, 50–56. 10.1016/j.jad.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Müller, B. W. , Jüptner, M. , Jentzen, W. , & Müller, S. P. (2002). Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: A PET study. NeuroImage, 17(1), 231–239. 10.1006/nimg.2002.1176 [DOI] [PubMed] [Google Scholar]
- Murrough, J. W. , Iacoviello, B. , Neumeister, A. , Charney, D. S. , & Iosifescu, D. V. (2011). Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiology of Learning and Memory, 96(4), 553–563. 10.1016/j.nlm.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Näätänen, R. (1995). The mismatch negativity: A powerful tool for cognitive neuroscience. Ear and Hearing, 16(1), 6–18. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/7774770 [PubMed] [Google Scholar]
- Näätänen, R. , & Michie, P. T. (1979). Early selective‐attention effects on the evoked potential: a critical review and reinterpretation. Biological Psychology, 8(2), 81–136. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/465623 [DOI] [PubMed] [Google Scholar]
- Näätänen, R. , Pakarinen, S. , Rinne, T. , & Takegata, R. (2004). The mismatch negativity (MMN): towards the optimal paradigm. Clinical Neurophysiology, 115(1), 140–144. 10.1016/j.clinph.2003.04.001 [DOI] [PubMed] [Google Scholar]
- Näätänen, R. , Sussman, S. E. , Salisbury, D. , & Shafer, V. L. (2014). Mismatch Negativity (MMN) as an Index of Cognitive Dysfunction. Brain Topography, 27(4), 451–466. 10.1007/s10548-014-0374-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith, S. L. , Mowszowski, L. , Ward, P. B. , Diamond, K. , Paradise, M. , Kaur, M. , … Hermens, D. F. (2012). Reduced temporal mismatch negativity in late‐life depression: An event‐related potential index of cognitive deficit and functional disability? Journal of Affective Disorders, 138(1–2), 71–78. 10.1016/j.jad.2011.12.028 [DOI] [PubMed] [Google Scholar]
- Opitz, B. , Rinne, T. , Mecklinger, A. , von Cramon, D. Y. , & Schröger, E. (2002). Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. NeuroImage, 15(1), 167–174. 10.1006/nimg.2001.0970 [DOI] [PubMed] [Google Scholar]
- Ottowitz, W. E. , Tondo, L. , Dougherty, D. D. , & Savage, C. R. (2002). The neural network basis for abnormalities of attention and executive function in major depressive disorder: Implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry, 10(2), 86–99. 10.1080/10673220216210 [DOI] [PubMed] [Google Scholar]
- Pang, X. , Xu, J. , Chang, Y. , Tang, D. , Zheng, Y. , Liu, Y. , & Sun, Y. (2014). Mismatch Negativity of Sad Syllables Is Absent in Patients with Major Depressive Disorder. PLoS ONE, 9(3), e91995. 10.1371/journal.pone.0091995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke, B. , & Romanski, L. M. (2016). Neural circuits in auditory and audiovisual memory. Brain Research, 1640, 278–288. 10.1016/j.brainres.2015.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin, S. G. , Turner, J. A. , Brown, G. G. , McCarthy, G. , Greve, D. N. , Glover, G. H. , … Lim, K. O. (2009). Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophrenia Bulletin, 35(1), 19–31. 10.1093/schbul/sbn162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, J. L. , & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Z. , Yu, Y. , Wang, L. , Yang, X. , Qiu, X. , Zhang, C. , … Yang, Y. (2013). Impaired pre‐attentive change detection in major depressive disorder patients revealed by auditory mismatch negativity. Psychiatry Research: Neuroimaging, 211(1), 78–84. 10.1016/j.pscychresns.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Rinne, T. , Alho, K. , Ilmoniemi, R. J. , Virtanen, J. , & Näätänen, R. (2000). Separate time behaviors of the temporal and frontal mismatch negativity sources. NeuroImage, 12(1), 14–19. 10.1006/nimg.2000.0591 [DOI] [PubMed] [Google Scholar]
- Schwenzer, M. , Zattarin, E. , Grözinger, M. , & Mathiak, K. (2012). Impaired pitch identification as a potential marker for depression. BMC Psychiatry, 12(1), 32 10.1186/1471-244X-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei, Y. , Kumano, S. , Hattori, S. , Uehara, T. , Kawakubo, Y. , Kasai, K. , … Mikuni, M. (2009). Preattentive dysfunction in major depression: A magnetoencephalography study using auditory mismatch negativity. Psychophysiology, 46(1), 52–61. 10.1111/j.1469-8986.2008.00748.x [DOI] [PubMed] [Google Scholar]
- Thönnessen, H. , Zvyagintsev, M. , Harke, K. C. , Boers, F. , Dammers, J. , Norra, C. , & Mathiak, K. (2008). Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. A combined EEG and MEG study. Biological Psychology, 77(2), 205–216. 10.1016/j.biopsycho.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Tollkötter, M. , Pfleiderer, B. , Sörös, P. , & Michael, N. (2006). Effects of antidepressive therapy on auditory processing in severely depressed patients: A combined MRS and MEG study. Journal of Psychiatric Research, 40(4), 293–306. 10.1016/j.jpsychires.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Umbricht, D. , Koller, R. , Schmid, L. , Skrabo, A. , Grübel, C. , Huber, T. , & Stassen, H. (2003). How specific are deficits in mismatch negativity generation to schizophrenia? Biological Psychiatry, 53(12), 1120–1131. 10.1016/S0006-3223(02)01642-6 [DOI] [PubMed] [Google Scholar]
- Wienberg, M. , Glenthoj, B. Y. , Jensen, K. S. , & Oranje, B. (2010). A single high dose of escitalopram increases mismatch negativity without affecting processing negativity or P300 amplitude in healthy volunteers. Journal of Psychopharmacology (Oxford, England), 24(8), 1183–1192. 10.1177/0269881109102606 [DOI] [PubMed] [Google Scholar]
- Woo, C.‐W. , Krishnan, A. , & Wager, T. D. (2014). Cluster‐extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


