Abstract
Essential tremor (ET) is a neurological disease with both motor and nonmotor manifestations; however, little is known about its underlying brain basis. Furthermore, the overall organization of the brain network in ET remains largely unexplored. We investigated the topological properties of brain functional network, derived from resting‐state functional magnetic resonance imaging (MRI) data, in 23 ET patients versus 23 healthy controls. Graph theory analysis was used to assess the functional network organization. At the global level, the functional network of ET patients was characterized by lower small‐worldness values than healthy controls—less clustered functionality of the brain. At the regional level, compared with the healthy controls, ET patients showed significantly higher values of global efficiency, cost and degree, and a shorter average path length in the left inferior frontal gyrus (pars opercularis), right inferior temporal gyrus (posterior division and temporo‐occipital part), right inferior lateral occipital cortex, left paracingulate, bilateral precuneus bilaterally, left lingual gyrus, right hippocampus, left amygdala, nucleus accumbens bilaterally, and left middle temporal gyrus (posterior part). In addition, ET patients showed significant higher local efficiency and clustering coefficient values in frontal medial cortex bilaterally, subcallosal cortex, posterior cingulate cortex, parahippocampal gyri bilaterally (posterior division), right lingual gyrus, right cerebellar flocculus, right postcentral gyrus, right inferior semilunar lobule of cerebellum and culmen of vermis. Finally, the right intracalcarine cortex and the left orbitofrontal cortex showed a shorter average path length in ET patients, while the left frontal operculum and the right planum polare showed a higher betweenness centrality in ET patients. In conclusion, the efficiency of the overall brain functional network in ET is disrupted. Further, our results support the concept that ET is a disorder that disrupts widespread brain regions, including those outside of the brain regions responsible for tremor.
Keywords: biomarker, essential tremor, functional connectivity, graph theory, MRI, resting state
1. INTRODUCTION
The current view of essential tremor (ET), one of the most common adult neurological disorders (Benito‐León, 2009; Louis & Ferreira, 2010), is that it may be a family of diseases, unified by the presence of kinetic tremor, and characterized by etiological, clinical and pathological heterogeneity (Benito‐León, 2014; Benito‐León & Louis, 2006; Benito‐León & Louis, 2007; Benito‐León & Louis, 2011). Aside from classic motor manifestations, ET is now considered a heterogeneous entity associated with a number of nonmotor manifestations, (Sengul et al., 2015; Sengul, Sengul, Sural, Bakim, & Forta, 2015) including cognitive disorders (Benito‐León, Louis, Bermejo‐Pareja, & Neurological Disorders in Central Spain Study Group, 2006a; Benito‐León, Louis, Bermejo‐Pareja, & Neurological Disorders in Central Spain Study Group et al., 2006b; Benito‐León, Louis, Mitchell, & Bermejo‐Pareja, 2011; Bermejo‐Pareja et al., 2007; Cersonsky et al., 2018; Collins et al., 2017), depressive symptoms (Louis et al., 2007), personality changes (Thenganatt & Louis, 2012), changes in sleep patterns (Benito‐León, Louis, & Bermejo‐Pareja, 2013; Gerbin, Viner, & Louis, 2012), changes in visual reaction time (Jiménez‐Jiménez et al., 2010), and hearing impairment (Benito‐León et al., 2007; Ondo, Sutton, Dat Vuong, Lai, & Jankovic, 2003).
Clinical and neuroimaging data suggest that ET motor symptoms could arise from cerebello‐thalamo‐cortical network abnormalities (Benito‐León, 2014; Benito‐León & Louis, 2006; Benito‐León & Louis, 2007; Benito‐León & Louis, 2011). Several studies have also demonstrated the presence of tremor‐related activity in cerebral cortical structures (Ibanez et al., 2014; Raethjen, Govindan, Kopper, Muthuraman, & Deuschl, 2007). In line with this, changes in the integrity of frontal and parietal areas involved in movement sequencing might be associated with tremorgenic activity in ET (Benito‐León et al., 2019), similar to that suggested for Parkinson's disease (Benito‐León et al., 2018). Notwithstanding, very little is known about the underlying causes and alterations in brain networks, mainly those involved in nonmotor manifestations in ET, and, hence, further study is needed.
There are fewer neuroimaging studies that have examined functional alterations in ET patients (Louis, Huang, Dyke, Long, & Dydak, 2014), especially those which acquired data during resting state conditions (i.e., with participants awake, but relaxed and not involved in any task). Resting‐state functional MRI (rs‐fMRI) is a suitable neuroimaging modality for the evaluation of many neurological and psychiatric diseases (Barkhof, Haller, & Rombouts, 2014; Benito‐León et al., 2016; Hacker, Perlmutter, Criswell, Ances, & Snyder, 2012). To date, only a few studies have explored connectivity alterations in ET, and those that did were mainly focused on the cerebello‐thalamo‐cortical network, which is mainly related to motor symptoms (Buijink et al., 2015; Caligiuri et al., 2017; Mueller et al., 2017), or on the study of surgical therapeutic applications (Akram et al., 2018; Tuleasca et al., 2018). In a recent study, ET patients showed increased connectivity in resting‐state networks involved in cognitive processes and decreased connectivity in the cerebellum and visual networks (Benito‐León et al., 2015). Changes in network integrity were associated not only with ET severity and ET duration, but also with cognitive ability. (Benito‐León et al., 2015) Further, in at least three networks (default mode network and frontoparietal networks), increased connectivity was associated with worse performance on different cognitive domains and depressive symptoms (Benito‐León et al., 2015).
Neither seed‐based functional connectivity nor the independent component analysis approach, which are the most widely used rs‐fMRI analysis techniques, can completely characterize the brain functional network (Calhoun & de Lacy, 2017; Joel, Caffo, van Zijl, & Pekar, 2011), which is in turn dynamic, as it provides support for several cognitive and emotional processes (Carhart‐Harris & Friston, 2010; Deco, Jirsa, & McIntosh, 2011) that might be altered in ET. For that reason, the graph theory approach has been recently applied to data obtained from rs‐fMRI, to characterize the functional connectivity within the whole‐brain network (Medaglia, 2017; Wang, Zuo, & He, 2010) with a moderate to high reliability (Braun et al., 2012). This technology has enabled the characterization of the human brain as a highly efficient large‐scale network consisting of nodes or vertices (i.e., brain regions) and pair‐wise edges (i.e., functional connectivity) which tend to a highly clustered organization also known as “small‐world” network (Medaglia, 2017; Wang et al., 2010). Lower small‐worldness values are related with worse performance in neuropsychological tasks (Douw et al., 2011; Langer, von Bastian, Wirz, Oberauer, & Jancke, 2013) and are commonly found in a wide variety of diseases, such as schizophrenia or epilepsy, when comparing with healthy control groups (Lynall et al., 2010; Vlooswijk et al., 2011). Graph theory analysis also provides regional metrics to define the connectivity properties of particular brain regions, such as their connections with the rest of the brain, the length of these connections or the relationship with their nearest regions (Wang, Li, Metzak, He, & Woodward, 2010). Unlike previous techniques, graph theory approach permits one to not only visualize the overall connectivity pattern among all the brain regions, but also to quantitatively characterize the global organization (Medaglia, 2017; Wang et al., 2010) providing a novel insight into the biological mechanisms of many neurological diseases, such as mild cognitive impairment and Alzheimer's disease (Khazaee, Ebrahimzadeh, & Babajani‐Feremi, 2016), epilepsy (Chiang, Stern, Engel Jr., Levin, & Haneef, 2014), Huntington's disease (Gargouri et al., 2016), and Parkinson's disease (Gottlich et al., 2013; Sreenivasan et al., 2019; Zhang, Liu, Chen, Liu, & Wang, 2015), among others. Only one previous study applied graph theory analysis to longitudinal rs‐fMRI data in eight ET patients before and after a focal lesion in the ventralis intermedius nucleus of the thalamus, using MRI‐guided high‐intensity focused ultrasound. (Jang, Park, Chang, Pae, & Chang, 2016) Thalamotomy led to changes in both the global brain network and motor circuit (Jang et al., 2016). However, the fact that the sample size was small (N = 8) as well as selection factors (e.g., as surgical patients, the ET patients included in the study had a more severe form of the disease) does not permit one to draw reliable conclusions. Hence, the topological organization of whole‐brain functional networks in ET patients remains largely unknown. The identification of specific abnormalities of the efficiency of the brain functional network at the global and regional levels in ET could provide novel insights into the understanding of underlying pathological mechanisms of this entity.
Given the relationship between Parkinson's disease and ET (Benito‐León et al., 2009; LaRoia & Louis, 2011; Louis & Ottman, 2013; Tarakad & Jankovic, 2018), we hypothesized that in ET patients the topological organization of whole‐brain functional networks is disrupted, and that these alterations may be associated with motor and nonmotor manifestations (i.e., cognitive dysfunction). To test this hypothesis, rs‐fMRI data were acquired from both ET patients and healthy controls. Graph theory was subsequently used to investigate the differences in the global and regional topological properties between both groups. Furthermore, we tested correlations between the altered graph theory metrics and clinical variables (e.g., duration and severity of tremor) and neuropsychological tests, and searched for potential topological features that may serve as neuroimaging biomarkers for ET.
2. METHODS
All procedures were approved by the ethical standards committees on human experimentation at the University Hospital “12 de Octubre” (Madrid). Written (signed) informed consent was obtained from all enrollees.
2.1. Participants
ET patients were consecutively recruited from October 2012 to July 2013 from the outpatient neurology clinic of the University Hospital “12 de Octubre” in Madrid (Spain). A 20 min, semistructured, tremor interview was conducted in which demographic information and data on tremor (e.g., duration) were collected. Two neurologists with expertise in movement disorders (J.B.‐L. and J.P.R.), who were blinded to the MRI results, examined the patients and used the Fahn–Tolosa–Marìn tremor rating scale to assign a total tremor score (range = 0–144) (Jankovic & Tolosa, 1988).
Diagnoses of ET were assigned by the two neurologists using the Consensus Statement on Tremor by the Movement Disorder Society (Deuschl, Bain, & Brin, 1998).
ET patients with a history of stroke, epilepsy, or head trauma were excluded from the study. Furthermore, based on a detailed clinical mental status examination, we excluded ET patients with dementia, using the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV criteria (American Psychiatric, 1994) or mild cognitive impairment, using Peterson/Winblad criteria (Petersen & Morris, 2005; Winblad et al., 2004) (impairment on one neuropsychological score defined as at least 1.5 SD below normative expectations).
Healthy controls were recruited either from relatives or friends of the health professionals working at the University Hospital “12 de Octubre” of Madrid (Spain) or among the relatives of patients who came to the neurological clinics for reasons other than ET (e.g., headache, dizziness). Enrolment at clinics was consecutive, beginning in October 2012. Controls were age‐frequency matched to the ET patients. None of the controls had a history of a neurological disorder (e.g., ET, Parkinson's disease, dementia) and none had received a neurological diagnosis from a physician. None reported having a first‐degree or second‐degree relative with ET or Parkinson's disease. Each control was examined by two neurologists (J.B.‐L. and J.P.R.), who were blinded to the MRI results, to further rule out any neurological conditions, including dementia, using DSM‐IV criteria,(American Psychiatric, 1994), mild cognitive impairment, using Peterson/Winblad criteria (Petersen & Morris, 2005; Winblad et al., 2004), Parkinson's disease or any other movement disorders.
2.2. Neuropsychological assessment
All participants underwent a detailed neuropsychological assessment covering the domains of attention (Direct digit span and Digit Symbol‐‐Coding both from WAIS‐III), executive functions (Stroop Color–Word Trial, Frontal Assessment Battery, WAIS‐III Similarities subtest, Indirect Digit Span test from the WAIS‐III, and the Controlled Oral Word Association Test), visuospatial and visual functions (Brief Visuospatial Memory Test‐Revised, Benton Judgment of Line Orientation Test, and the Hooper Visual Organization Test), verbal memory (WMS‐III Word List), and language (Boston Naming Test and total number of animals as possible in 1 min) (Benito‐León et al., 2015; Puertas‐Martin et al., 2016). In addition, severity of depressive symptoms was measured by the original 17‐item version of the Hamilton Depression Rating Scale (Hamilton, 1967). For the current study, we applied the Bech criteria to classify depressive symptoms as minor depression or dysthymia (score ranging from 8 to 14), and moderate or severe depression (≥15) (Bech, 1993).
Testing was performed by a trained neuropsychologist using standardized procedures. The entire neuropsychological battery took approximately 1.5 hr to administer. The tests, which have been described elsewhere, (Bermejo‐Pareja & Puertas‐Martín, 2012) were selected in part to avoid the effects of any hand tremor because they made, in general, minimal demands on motor processes. Raw scores were transformed into z scores based on the mean and SD values from healthy controls. Higher z scores indicate better performance.
To simplify the neuropsychological assessment for subsequent correlation with the graph theory metrics, we reduced the tests results into a reduced number of composites results (Ackerman & Cianciolo, 2000). All tests results were z‐standardized. Then, a factorial analysis (principal components analysis) of these z‐scores, excluding the 17‐item version of the Hamilton Depression Rating Scale score that constituted a factor by itself, revealed four factors grouping these tests. The cognitive domain of tests was also considered, and then the first factor was divided into three, obtaining the six following composites (Table 1): attention (Direct digit span and digit‐symbol coding both from WAIS‐III), executive functions (Stroop Color–Word Trial, Frontal Assessment Battery, WAIS‐III Similarities subtest and Indirect Digit Span test from the WAIS‐III), visuospatial and visual functions (learning list and delayed recall from the Brief Visuospatial Memory Test‐Revised, and the Hooper Visual Organization Test), recognition (recognition from the WMS‐III, recognition from the Brief Visuospatial Memory Test‐Revised), verbal memory (learning list, immediate recall and delayed recall from the WMS‐III) and language (Boston Naming Test, total number of animals as possible in 1 min, and Controlled Oral Word Association Test). The 17‐item version of the Hamilton Depression Rating Scale score was used individually. The Benton Judgment of Line Orientation Test was excluded because it did not correlate with any factor. The composite score was then used as a continuous variable in partial correlation analyses with the network metrics, controlling the effect of sex and age.
Table 1.
Comparison of demographic, clinical, and cognitive domains of essential tremor patients versus healthy controls
| patients (N = 23) | Healthy controls (N = 23) | p value | |
|---|---|---|---|
| Age in years | 63.3 (68.0) ± 13.4 | 61.1 (62.0) ± 13.1 | .409a |
| Sex (female) | 12 (52.2%) | 13 (56.5%) | .767 |
| Educational level | .162 | ||
| Can read and write | 8 (34.8%) | 4 (17.4%) | |
| Primary studies | 8 (34.8%) | 5 (21.7%) | |
| Secondary studies | 5 (21.7%) | 7 (30.4%) | |
| University studies | 2 (8.7%) | 7 (30.4%) | |
| Dominant hand | .368 | ||
| Right handedness | 22 (50%) | 22 (50%) | |
| Left handedness | 1 (100%) | 0 (0%) | |
| Mixed handedness | 0 (0%) | 1 (100%) | |
| Tremor duration, years | 22.9 (20.0) ± 16.5 | – | |
| Fahn–Tolosa–Marin tremor rating scale score | 30.3 (30.5) ± 15.3 | – | |
| Cognitive domains | |||
| Attention | |||
| Direct Digit Span test from the WAIS‐III | 5.6 (5.0) ± 1.4 | 5.9 (6.0) ± 1.3 | .425a |
| WAIS‐III Digit Symbol—Coding subtest | 33.0 (27.0) ± 17.4 | 53.3 (50.0) ± 19.4 | .010b |
| Executive functions | |||
| Stroop Color–Word Trial | 26.4 (27.5) ± 13.3 | 33.4 (38.0) ± 12.1 | .070b |
| Frontal Assessment Battery | 15.4 (16.0) ± 2.9 | 16.8 (17.0) ± 1.0 | .040a |
| WAIS‐III Similarities subtest | 16.2 (16.0) ± 6.3 | 18.2 (18.0) ± 5.6 | .264b |
| Indirect Digit Span test from the WAIS‐III | 3.8 (4.0) ± 1.2 | 4.3 (4.0) ± 1.1 | .094a |
| Visuospatial and visual functions | |||
| Brief Visuospatial Memory Test‐Revised | |||
| Learning total | 23.0 (22.0) ± 9.6 | 27.6 (28.0) ± 6.7 | .080b |
| Delayed free recall trial | 8.5 (10.5) ± 3.6 | 10.1 (11.0) ± 2.4 | .181a |
| Hooper Visual Organization Test | 35.8 (36.0) ± 9.4 | 40.9 (39.0) ± 8.7 | .060b |
| Recognition | |||
| Recognition trial (BVMT‐R) | 11.4 (12.0) ± 0.9 | 11.8 (12.0) ± 0.5 | .114a |
| Recognition (WMS‐III) | 21.7 (22.0) ± 2.1 | 22.3 (22.0) ± 1.4 | .395a |
| Verbal memory | |||
| WMS‐III Word List | |||
| Learning list | 28.3 (28.0) ± 5.6 | 29.0 (28.0) ± 6.4 | .698b |
| Immediate recall | 6.3 (6.0) ± 2.4 | 6.9 (7.0) ± 2.3 | .386b |
| Delayed recall | 5.5 (6.0) ± 2.6 | 6.8 ± (7.0) 2.3 | .080b |
| Language | |||
| Boston Naming Test | 44.7 (44.0) ± 11.7 | 52.6 (53.0) ± 5.2 | .006b |
| Total number of animals as possible in 1 min | 18.7 (17.0) ± 8.4 | 21.5 (21.0) ± 6.8 | .090a |
| Controlled Oral Word Association Test | 26.8 (28.0) ± 13.6 | 37.0 (39.0) ± 13.1 | .010b |
| Depressive symptoms | |||
| 17‐Item Hamilton Depression Rating Scale total score | 6.8 (7.0) ± 5.0 | 5.8 (5.0) ± 5.0 | .541b |
Note. Mean (median) ± SD and frequency (%) are reported.
Abbreviations: ET, essential tremor; WAIS‐III, Wechsler Adult Intelligence Scale‐Third Edition; WMS‐III, Wechsler Memory Scale‐Third Edition.
Mann–Whitney U test.
Student's t test were used for comparisons of continuous data, and chi‐square test for proportions.
2.3. MRI procedure
Patients and healthy controls were positioned in the scanner and immobilized with a custom‐fit blue bag vacuum mold (Medical Intelligence, Inc.) to prevent image artifacts. Earplugs and noise‐reduction headphones were used to attenuate scanner noise. A strict criterion for head movement assessment was adopted (maximal absolute head movement less than 1.0 mm and 1.0° in the x, y, and z directions). Neither ET patients nor healthy controls were excluded from the analysis due to this criterion.
Images were acquired on a General Electric Sigma 3 T MR Scanner (General Electric Healthcare, Fairfield, CT) using a whole‐body radiofrequency coil for signal excitation and quadrature eight‐channel coil for reception. For the structural image, a high‐resolution, three‐dimensional T1‐weighted spoiled gradient echo was acquired (repetition time [TR] = 9.2 ms, echo time [TE] = 4.128 ms, TI = 500 ms, field of view = 240 mm, acquisition matrix = 240 × 240, slice thickness = 1 mm, full brain coverage, resolution = 1 × 1 × 1 mm3, flip angle = 120°, and 166 sagittal slices).
Resting‐state fMRI data consisted of 120 volumes of a repeated gradient‐echo echo planar imaging T2*‐weighted sequence (TR = 3 s, TE = 28 ms, voxel dimensions = 2.7 × 2.7 × 2.8 mm3, 39 oblique slices aligned to Anterior and Posterior Commissures, flip angle = 90°, and six dummy scans). During the functional acquisition (total run = 6 min), participants were instructed to relax with their eyes closed and not to engage in cognitive or motor activities.
All volumes were visually reviewed and manually reoriented. Preprocessing was performed using CONN (available at https://www.nitrc.org/projects/conn) and SPM12 (available at: http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The preprocessing pipeline included: fieldmap correction, realign and unwarp, slice‐timing correction, denoising (CompCor and Artifact Removal Tool), normalization to Montreal Neurological Institute 152 space, and smoothing (Full Width at Half Maximum 8 mm).
2.4. Graph theory analysis
To define the network nodes, the whole brain data of each participant were parceled into 91 cortical areas and 15 subcortical areas from the FMRIB Software Library's Harvard‐Oxford atlas (available at http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) and 26 cerebellar areas from the Anatomical Automatic Labeling atlas (Tzourio‐Mazoyer et al., 2002). The bivariate Pearson correlation of BOLD timeseries between every pair of regions of interest (ROIs) defined by the parcellation was computed for each participant. The scrubbing regressors, provided by CompCor, Artifact Removal Tool, and the motion parameters estimated for each participant, were included as covariates. Due to the large amount of comparisons between groups that the graph theory analysis implies, and the fact that all the metrics in a node are highly interrelated, the correction for multiple comparisons in analysis requires a special treatment. An option is to apply a correction over the graph prior to network analysis, thresholding the connectivity matrixes. The thresholding method has proved capable of reducing the false positives resulting of the comparison of low correlated nodes between groups (Drakesmith et al., 2015). For this reason, the correlation previously obtained for all participants were binarized, thresholding them over increasing correlation values in steps of 0.1, starting from 0.1. The maximum threshold selected was the value for which every node remained connected with at least one other node (in our case ≤0.17). Thus, within the maximum threshold, every node has at least one correlation of 0.17 or higher with another node and no node becomes isolated. To simplify the interpretation of the results, the thresholds are always positive, and therefore the resulting binarized matrixes represent only positive correlations between nodes. The resulting 17 binarized connectivity matrices for each subject constituted the graphs in the subsequent analyses.
To understand the functional characteristics of the network, several metrics were explored for each graph. Specifically, global efficiency (GE), local efficiency (LE), betweenness centrality (BC), cost, average path length (APL), clustering coefficient (CC), and degree were calculated using CONN. Additionally, the small‐worldness coefficient was calculated using in‐house software developed in MATLAB (MathWorks Inc., Natick, Massachusetts, USA). This metric is the result of dividing the γ clustering coefficient by the λ average path length coefficient, where γ = Cp/Cprand and λ = Lp/Lprand (Cp and Lp are the average of the clustering coefficients and APL coefficients over all nodes respectively, and Cprand and Lprand are the same averages, but calculated from a matched random network). This random network was created using the Brain Connectivity Toolbox (available at https://www.nitrc.org/projects/bct/), always preserving the degree of the original network and randomly rewiring each pair of nodes when possible. The definitive γ and λ were the result of averaging 50 repetitions of each parameter with different random networks each time. After that amount of repetitions, the coefficient values remained stable.
2.5. Sample size and statistical analyses
In several recent publications on suitable sample sizes for functional neuroimaging studies using graph theory analysis, it has been found that a group size of approximately 20 is sufficient (Gargouri et al., 2016; Sreenivasan et al., 2019).
Statistical analyses were conducted using R (available at https://www.r-project.org/). Mean scores (age and neuropsychological variables) were compared using Student's t tests for continuous and normally distributed data, and Mann–Whitney U test for nonnormally distributed data, where appropriate, while a chi‐squared test was used to compare the sex and educational level distribution between the two groups.
The differences between groups for each graph theory metric (GE, LE, BC, cost, APL, CC, and degree) were analyzed through univariate analyses of covariance, including age, sex, dominant handedness, and educational level as covariates. The educational level was coded from 1 to 4 (1—under primary education; 2—primary education; 3—secondary education; and 4—university studies). Levene's test was used to explore the homoscedasticity of the factors to meet the analysis of variance assumptions. Marginal means estimation informed about the direction of the differences. Bonferroni correction for multiple comparisons was applied for the seven metrics contrasts of each ROI and threshold (correction over seven tests) to diminish the Type I error of simple effects. Every ROI and the whole network were analyzed through the 17 correlation thresholds previously determined (0.01–0.17). An independent samples t test was used to compare the small‐worldness between groups.
Finally, the partial correlation coefficient was used to analyze the correlations between the ROIs that showed intergroup differences and both the cognitive domains (neuropsychological assessment composites) and the clinical variables (duration and severity of disease) in ET patients, controlling the effect of age and sex.
3. RESULTS
3.1. Clinical and neuropsychological testing results
Of the 26 ET patients who were initially eligible, two had ET with dystonic features after reviewing their videotapes by a senior expert in movement disorders (E.D.L) and were therefore excluded. One was excluded from the final analyses because he developed incident Parkinson's disease during follow‐up. None of the patients and healthy controls were excluded because of neurological comorbidities, including dementia or mild cognitive impairment, or structural abnormalities on conventional MRI images. According to Fazekas visual rating scale, all participants had Fazekas score ≤1 (i.e., normal in the elderly) (Fazekas, Chawluk, Alavi, Hurtig, & Zimmerman, 1987).
The final sample included 23 ET patients (12 women and 11 men) and 23 healthy controls (13 women and 10 men). No significant differences between both groups in demographic variables (age, sex, handedness, and educational level) were observed (Table 1).
The mean tremor duration was 22.9 ± 16.5 and the mean total tremor score was 29.3 ± 15.7 (median 30) (Table 1). In addition to arm tremor, rest tremor was also present on tremor examination in six (26.1%) ET patients. Further, head tremor was also present in seven (30.4%) ET patients. In these seven patients, the head tremor was intermittent, and it took place in the “yes–yes” direction.” All the ET patients had a normal [(123) I]FP‐CIT single photon emission computed tomography scan. Fifteen (65.2%) were taking one or more anti‐tremor medications (six propranolol, one primidone, one clonazepam, one clonazepam and propranolol, one gabapentin and primidone, one primidone and propranolol, one zonisamide and primidone, one zonisamide and propranolol, one zonisamide, primidone, and alprazolam, and one zonisamide, primidone, and propranolol).
The results of neuropsychological performance are listed in Table 1. As expected, ET patients' cognitive performance was significantly worse than that of the healthy controls, mainly on attention, executive functions, and language. No participant met criteria for mild cognitive impairment. Of the 23 ET patients, 15 had no depression (65.2%), six (26.1%) had dysthymia or moderate depression and two (8.7%) had severe depression versus 16 without depression (69.6%), seven (30.4%) with dysthymia or moderate depression and none (0.0%) with severe depression in the healthy control group (p = .348, Fisher exact test).
3.2. Global graph topological analyses
Analyses of covariance showed intergroup significant differences in the first three levels of correlation (0.01–0.03). Pairwise comparisons of the marginal means showed differences in BC and APL (healthy controls > ET patients; p < .05 Bonferroni corrected), and GE, cost, and degree (ET patients > healthy controls; p < .05 Bonferroni corrected) (Table 2). Sex was the only covariate that showed a significant effect over most dependent variables. No other significant effects or interactions were found.
Table 2.
Results for the global network
| Correlation threshold | Metric | Healthy controls | ET patients | p value |
|---|---|---|---|---|
| 0.01 | GE | 0.786 ± 0.020 | 0.796 ± 0.022 | .043 a |
| LE | 0.837 ± 0.018 | 0.845 ± 0.019 | .063 | |
| BC | 0.003 ± 0.000 | 0.003 ± 0.000 | .043 b | |
| Cost | 0.571 ± 0.041 | 0.592 ± 0.044 | .043 a | |
| APL | 1.429 ± 0.041 | 1.408 ± 0.044 | .043 b | |
| CC | 0.675 ± 0.036 | 0.690 ± 0.039 | .063 | |
| Degree | 93.111 ± 6.655 | 96.461 ± 7.216 | .043 a | |
| 0.03 | GE | 0.769 ± 0.021 | 0.779 ± 0.022 | .048 a |
| LE | 0.828 ± 0.018 | 0.835 ± 0.020 | .074 | |
| BC | 0.003 ± 0.000 | 0.003 ± 0.000 | .048 b | |
| Cost | 0.538 ± 0.042 | 0.558 ± 0.045 | .048 a | |
| APL | 1.462 ± 0.042 | 1.442 ± 0.045 | .048 b | |
| CC | 0.655 ± 0.037 | 0.671 ± 0.040 | .074 | |
| Degree | 87.661 ± 6.810 | 91.009 ± 7.318 | .048 a | |
| 0.05 | GE | 0.752 ± 0.021 | 0.762 ± 0.023 | .059 |
| LE | 0.819 ± 0.018 | 0.826 ± 0.020 | .092 | |
| BC | 0.003 ± 0.000 | 0.003 ± 0.000 | .059 | |
| Cost | 0.505 ± 0.042 | 0.524 ± 0.046 | .060 | |
| APL | 1.496 ± 0.042 | 1.476 ± 0.046 | .059 | |
| CC | 0.637 ± 0.037 | 0.652 ± 0.041 | .092 | |
| Degree | 82.242 ± 6.840 | 85.452 ± 7.496 | .060 | |
| 0.07 | GE | 0.736 ± 0.021 | 0.745 ± 0.024 | .070 |
| LE | 0.810 ± 0.019 | 0.817 ± 0.021 | .107 | |
| BC | 0.003 ± 0.000 | 0.003 ± 0.000 | .071 | |
| Cost | 0.471 ± 0.042 | 0.490 ± 0.047 | .070 | |
| APL | 1.529 ± 0.042 | 1.510 ± 0.047 | .071 | |
| CC | 0.620 ± 0.038 | 0.634 ± 0.042 | .108 | |
| Degree | 76.827 ± 6.894 | 79.933 ± 7.664 | .070 | |
| 0.09 | GE | 0.719 ± 0.021 | 0.728 ± 0.024 | .075 |
| LE | 0.802 ± 0.020 | 0.808 ± 0.021 | .120 | |
| BC | 0.003 ± 0.000 | 0.003 ± 0.000 | .075 | |
| Cost | 0.438 ± 0.042 | 0.457 ± 0.047 | .075 | |
| APL | 1.562 ± 0.043 | 1.544 ± 0.047 | .075 | |
| CC | 0.603 ± 0.039 | 0.616 ± 0.043 | .121 | |
| Degree | 71.466 ± 6.897 | 74.518 ± 7.685 | .075 | |
| 0.11 | GE | 0.703 ± 0.021 | 0.711 ± 0.023 | .100 |
| LE | 0.794 ± 0.020 | 0.800 ± 0.022 | .133 | |
| BC | 0.004 ± 0.000 | 0.004 ± 0.000 | .103 | |
| Cost | 0.407 ± 0.043 | 0.424 ± 0.047 | .099 | |
| APL | 1.595 ± 0.043 | 1.578 ± 0.047 | .103 | |
| CC | 0.588 ± 0.040 | 0.601 ± 0.044 | .135 | |
| Degree | 66.283 ± 6.977 | 69.049 ± 7.636 | .099 | |
| 0.13 | GE | 0.687 ± 0.022 | 0.695 ± 0.024 | .110 |
| LE | 0.786 ± 0.021 | 0.792 ± 0.023 | .150 | |
| BC | 0.004 ± 0.000 | 0.004 ± 0.000 | .109 | |
| Cost | 0.375 ± 0.043 | 0.392 ± 0.047 | .110 | |
| APL | 1.630 ± 0.045 | 1.613 ± 0.048 | .109 | |
| CC | 0.574 ± 0.041 | 0.586 ± 0.045 | .152 | |
| Degree | 61.172 ± 7.042 | 63.837 ± 7.667 | .110 | |
| 0.15 | GE | 0.671 ± 0.022 | 0.679 ± 0.024 | .118 |
| LE | 0.779 ± 0.021 | 0.785 ± 0.023 | .152 | |
| BC | 0.004 ± 0.000 | 0.004 ± 0.000 | .116 | |
| Cost | 0.345 ± 0.042 | 0.361 ± 0.047 | .120 | |
| APL | 1.667 ± 0.046 | 1.649 ± 0.050 | .116 | |
| CC | 0.560 ± 0.041 | 0.573 ± 0.046 | .156 | |
| Degree | 56.242 ± 6.849 | 58.827 ± 7.684 | .120 | |
| 0.17 | GE | 0.654 ± 0.022 | 0.662 ± 0.024 | .127 |
| LE | 0.772 ± 0.021 | 0.778 ± 0.024 | .161 | |
| BC | 0.004 ± 0.000 | 0.004 ± 0.000 | .119 | |
| Cost | 0.316 ± 0.041 | 0.331 ± 0.046 | .134 | |
| APL | 1.708 ± 0.049 | 1.690 ± 0.052 | .119 | |
| CC | 0.548 ± 0.041 | 0.560 ± 0.046 | .168 | |
| Degree | 51.548 ± 6.761 | 53.977 ± 7.555 | .134 |
Note. Values are expressed in means ± SD from the analysis of covariance. Every threshold follows the same contrast direction pattern. Significant values are in bold font.
Abbreviations: APL, average path length; BC, betweenness centrality; ET, Essential tremor; GE, global efficiency; LE, local efficiency.
ET patients > healthy controls.
Healthy controls > ET patients.
The independent‐samples t test revealed intergroup differences (healthy controls > ET patients; p < .01) in small‐worldness (Figure 1).
Figure 1.
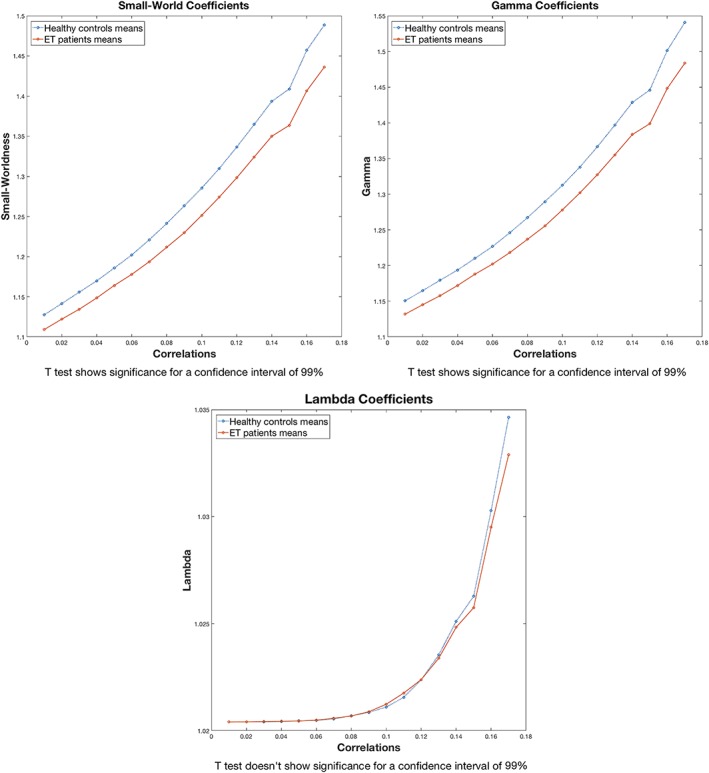
Small‐worldness, Gamma and Lambda coefficients. Small‐worldness coefficients are obtained dividing the Gamma coefficients by the Lambda coefficients. Each line represents the coefficients by group. The significance of two sample T‐tests between groups (alpha = 0.01) are displayed below each graph [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Regional graph topological analyses
Table 3 shows the significant differences between both groups in each ROI at a 0.17 correlation threshold. Figure 2 shows the mean binary adjacency matrixes in healthy controls and ET patients at a correlation threshold of 0.17. Some metrics were consistently significant at a lower correlation, such as 0.15, and were included as interesting ROIs (highlighted with “a” in Table 3).
Table 3.
Regional graph topological analyses
| ROI | Global efficiency | Local efficiency | Betweenness centrality | Cost | Average path length | Clustering coefficient | Degree | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| Inferior frontal gyrus, pars opercularis | E>H | E>H | H>E | E>H | ||||||||||
| Middle temporal gyrus, posterior part | E>Ha | E>Ha | H>Ea | E>Ha | ||||||||||
| Inferior temporal gyrus, posterior division | E>H | E>H | H>E | E>H | ||||||||||
| Inferior temporal gyrus, temporo‐occipital part | E>H | E>H | E>H | |||||||||||
| Postcentral gyrus | E>Ha | E>Ha | ||||||||||||
| Superior lateral occipital cortex | E>H | H>Eb | E>H | |||||||||||
| Inferior lateral occipital cortex | E>H | E>H | H>E | E>H | ||||||||||
| Intracalcarine cortex | H>E | |||||||||||||
| Frontal medial cortex | E>H | E>H | ||||||||||||
| Subcallosal cortex | E>H | E>H | ||||||||||||
| Paracingulate gyrus | E>H | E>H | H>E | E>H | ||||||||||
| Posterior cingulate | E>H | E>H | ||||||||||||
| Precuneus | E>H | E>H | H>E | E>H | ||||||||||
| Frontal orbital cortex | H>E | |||||||||||||
| Parahippocampal gyrus, posterior division | E>H | E>H | E>H | E>H | ||||||||||
| Lingual gyrus | E>H | E>H | E>H | H>E | E>H | E>H | ||||||||
| Frontal operculum | E>H | |||||||||||||
| Planum polare | H>E | |||||||||||||
| Pallidum | H>Eb | |||||||||||||
| Hippocampus | E>H | E>H | ||||||||||||
| Amygdala | E>H | E>H | H>E | E>H | ||||||||||
| Nucleus accumbens | E>H | E>H | E>H | E>H | ||||||||||
| Inferior semilunar lobule, caudal part | E>Ha | H>Ea | E>Ha | |||||||||||
| Biventral lobule, rostral part | H>Eb | |||||||||||||
| Cerebellar tonsil | H>Eb | |||||||||||||
| Flocculus | E>H | E>H | ||||||||||||
| Culmen of vermis | E>Ha | E>Ha | ||||||||||||
| Tuber of vermis | H>E | |||||||||||||
Note. E is the essential tremor patients and H is the healthy controls.
Consistent significance at a threshold of 0.15 and lower.
Not significant at lower thresholds.
Figure 2.
Mean binary adjacency matrixes in healthy controls and essential tremor (ET) patients at a correlation threshold of 0.17. The mean values are those present in at least half of the subjects [Color figure can be viewed at http://wileyonlinelibrary.com]
At the regional level, ET patients (vs. healthy controls) showed higher values of GE, cost and degree, and a shorter APL in the left inferior frontal gyrus (pars opercularis), right inferior temporal gyrus (posterior divisionand temporo‐occipital part), right inferior lateral occipital cortex, left paracingulate, precuneus bilaterally, left lingual gyrus, right hippocampus, left amygdala, nucleus accumbens bilaterally, and left middle temporal gyrus (posterior part).
In other ROIs, ET patients showed higher LE and CC than healthy controls. These regions include the frontal medial cortex bilaterally, subcallosal cortex, posterior cingulate cortex, parahippocampal gyri bilaterally (posterior division), right lingual gyrus, right cerebellar floculus, right postcentral gyrus, right inferior semilunar lobule of cerebellum and culmen of vermis. Finally, the right intracalcarine cortex and the left orbitofrontal cortex showed a shorter APL in ET patients, while the left frontal operculum and the right planum polare showed a higher BC in ET patients. Figure 3 shows all the significant ROIs obtained and their interconnections.
Figure 3.
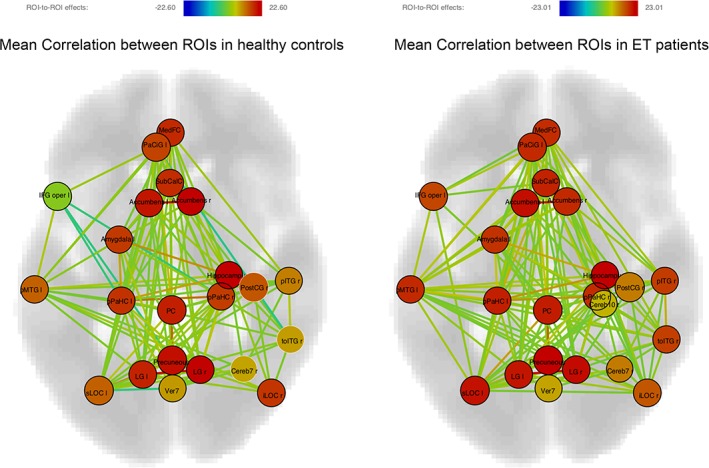
Axial view of the significant regions of interest (ROIs) and their correlations in healthy controls and essential tremor (ET) patients. Node intensity values represent the F statistic over the null hypothesis that all ROIs correlations are equal to 0. Edges intensity values represent the T statistic over the null hypothesis that its correlation is equal to 0. False discovery rate correction for multiple comparisons was applied to the significance level for the correlation contrasts between the significant ROIs
The above observed changes in connectivity patterns are mostly involved in the pathogenesis of the several nonmotor features previously described in ET, such as cognitive dysfunction, depression, singular personality, and impaired visual‐motor integration (see Table 4).
Table 4.
Regional graph topological differences and its relationship with the pathogenesis of ET
| Regional graph topological differences | Function | Possible meaning in ET |
|---|---|---|
| Precuneus/posterior cingulate cortex | Disruption of this network may play an important role in developing Alzheimer's disease (Yokoi et al., 2018) | Older‐onset ET is associated not only with higher odds of prevalent Alzheimer's disease (Benito‐León et al., 2006a), but also with an increased risk of Alzheimer's disease (Bermejo‐Pareja et al., 2007) |
| Frontal operculum | This is a key node in a network for exerting control over cognitive processes (Higo, Mars, Boorman, Buch, & Rushworth, 2011) | Cognitive deficits have been described in ET (Benito‐León et al., 2006b). |
| Right hippocampus and the parahippocampal gyri | Involved in episodic memory (Ekstrom & Bookheimer, 2007) | Memory deficits have been described in ET (Benito‐León et al., 2006b) |
| Left inferior frontal gyrus (pars opercularis) | The posterior part of the inferior frontal gyrus, traditionally referred to as Broca's area, is thought to play a critical role in processing both the phonological aspects and meanings of words, and hence is crucial for language production and comprehension (Gabrieli, Poldrack, & Desmond, 1998) | In population‐based studies and clinical series (Benito‐León et al., 2006b; Puertas‐Martin et al., 2016), verbal fluency has been found to be impaired in ET patients |
| Nucleus accumbens bilaterally | The nucleus accumbens is related with the impulsivity associated with drug addictions (Dalley et al., 2007) and with depression (Francis & Lobo, 2017) | Personality characteristics have been observed in ET; patients are more prone to a personality characterized by greater higher harm avoidance (Thenganatt & Louis, 2012). ET patients also have an increased odds and risk of depression (Louis et al., 2007) |
| Left amygdala | This is a key brain structure regulating different aspects of emotional processing, including recognition of emotional expression in faces, as well as in mediating emotional interference during tasks requiring cognitive resources (Adolphs & Spezio, 2006) | An inverse relation has been described between facial emotion recognition and tremor severity in ET patients (Auzou, Foubert‐Samier, Dupouy, & Meissner, 2014). In addition, ET patients did not perform as well in joy and fear recognition, and they had subtle abnormalities in risk detection (Auzou et al., 2014) |
| Left amygdala and several cerebellar regions | Startle hyporeactivity might be mediated by aberrant cerebellar input to the amygdala, which is involved in priming the startle response in emotional contexts (Lafo et al., 2017) | ET patients respond abnormally in terms of startle reactivity to emotional pictures (Lafo et al., 2017) |
| Parahippocampal gyrus, posterior cingulate, and lingual gyrus bilaterally | These brain regions are involved in visual tasks (Yang, Deng, Xing, Xia, & Li, 2015) | Impaired visual‐motor integration in ET has been described (Bares, Lungu, Husarova, & Gescheidt, 2010). Changes in the connectivity of the lingual gyrus, which may be related to internally directed attention (Benedek et al., 2016), correlated negatively with attention domain |
| Right inferior lateral occipital cortex | The extrastriate body, which is well known to respond to visual processing of static and moving human bodies (Astafiev, Stanley, Shulman, & Corbetta, 2004; Lingnau & Downing, 2015), is located in the lateral occipital cortex. Further, the extrastriate body responds during the perception of other people's body parts or during goal‐directed movements of the observer's body parts (Astafiev et al., 2004; Lingnau & Downing, 2015). Finally, the lateral occipitotemporal cortex represents varied aspects of action, ranging from perception of tools and bodies and the way they typically move (Lingnau & Downing, 2015) | Given the involvement of the lateral occipital cortex in the control of movement, its deterioration could plausibly be involved in the genesis of tremor (Serrano et al., 2017) |
Abbreviation: ET, essential tremor.
3.4. Relationships between network characteristics and neuropsychological variables and disease variables (severity and duration of disease) in ET patients
To understand the relationship between the intergroup differences observed in the network topology (see Table 3) and the neuropsychological variables, a correlation analysis was performed in ET patients. These analyses revealed a relationship (p < .05) between the six cognitive composites and the depressive symptoms with the graph theory metrics in the medial frontal cortex, parahippocampal gyrus bilaterally, left lingual gyrus, right planum polare, right pallidum, right hippocampus, right nucleus accumbens, and right cerebellum flocculus (Table 5). No relationship between any graph theory metric and tremor severity or duration was observed.
Table 5.
Matrix of partial correlations among the cognitive domains and the ROIs that showed differences between ET patients versus healthy controls
| ROI | Attention | Executive function | Verbal memory | Recognition | Visuospatial/visual functions | Language | Depressive symptoms | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | |
| Medial frontal cortex | ||||||||||||||
| BC | −.476 | .022 | −.494 | .017 | ||||||||||
| Right parahippocampal gyrus, posterior division | ||||||||||||||
| LE | .563 | .005 | ||||||||||||
| CC | −.431 | .045 | .577 | .004 | ||||||||||
| Left parahippocampal gyrus, posterior division | ||||||||||||||
| LE | −.473 | .023 | .441 | .035 | ||||||||||
| BC | .483 | .019 | ||||||||||||
| CC | −.475 | .022 | .428 | .042 | ||||||||||
| Left lingual gyrus | ||||||||||||||
| LE | −.435 | .038 | ||||||||||||
| BC | .443 | .034 | .454 | .03 | ||||||||||
| CC | −.435 | .038 | ||||||||||||
| Right planum polare | ||||||||||||||
| GE | .436 | .044 | .461 | .027 | ||||||||||
| LE | .451 | .031 | ||||||||||||
| Cost | .428 | .041 | .459 | .028 | ||||||||||
| APL | −.445 | .033 | ||||||||||||
| Degree | .428 | .041 | .459 | .028 | ||||||||||
| CC | .422 | .045 | ||||||||||||
| Right pallidum | ||||||||||||||
| BC | .475 | .022 | ||||||||||||
| Right hippocampus | ||||||||||||||
| BC | −.504 | .014 | ||||||||||||
| Right nucleus accumbens | ||||||||||||||
| BC | .581 | .003 | .497 | .016 | .563 | .005 | .585 | .003 | ||||||
| Right cerebellum flocculus | ||||||||||||||
| LE | −.481 | .02 | ||||||||||||
| CC | −.456 | .029 | ||||||||||||
Abbreviations: APL, average path length; BC, betweenness centrality; ET, essential tremor; GE, global efficiency; LE, local efficiency; ROI, region of interest.
4. DISCUSSION
Our results show that topological organization of whole‐brain functional networks is disrupted in ET. Specifically, ET patients are characterized by lower small‐worldness values of the network—less clustered functionality of the brain—together with altered connectivity patterns in specific systems. The results in the metrics of the global network are only significant in very low thresholds and therefore were discarded. At the regional level, we found the existence of multiple connectivity differences between ET patients and healthy controls in motor and extramotor related areas. Interpreting these data is challenging; nonetheless, each of the observed changes in connectivity patterns can be linked to one of the several nonmotor features previously described in ET, such as cognitive dysfunction, depression, personality style, and impaired visual‐motor integration. The data also support the concept that ET is a disorder that disrupts widespread brain regions, including those outside of the brain regions responsible for tremor (i.e., cerebellum, thalamus, motor cortex). Interestingly, analogous results regarding widespread disruptions have been reported in Parkinson's disease as well (Gottlich et al., 2013; Zhang et al., 2015).
The pathogenesis of ET remains unclear, although clinical and neuroimaging data suggest, as in other tremor disorders, such as orthostatic tremor (Benito‐León & Domingo‐Santos, 2016), that it may be related to the existence of alterations in the cerebello‐thalamo‐cortical network (the so called “tremor network”) (Benito‐León & Louis, 2006; Benito‐León & Louis, 2011). The studies that have provided evidence of involvement of the “tremor network” have however used a different methodology; specifically, they focused on the areas involved in motor symptoms, and measured the tremorgenic activity using surface electromyography (Ibanez et al., 2014; Raethjen et al., 2007). The current study did not restrict its focus to motor‐related symptoms, and we did not acquire additional specific measures other than Fahn‐Tolosa‐Marin scores of severity and duration. Notwithstanding, intergroup differences in some of the areas typically related with motor symptoms in ET, such as cerebellar regions, pallidum, and sensorimotor regions, were found in the regional graph topological analysis. Our results show changes in ET that are widespread and not restricted to the tremor network. Table 4 summarizes the most important findings in this study at the regional level and discusses the potential relationship between each finding and the pathogenesis of ET.
The significant correlations of graph theory metrics with neuropsychological scores provided us some useful information to interpret the functional connectivity differences between healthy controls and ET patients. Of special interest were the changes in connectivity of the posterior parahippocampal gyri (higher LE and CC), a region associated with visuospatial processing and episodic memory, which were positively correlated with depressive symptoms and negatively with visuospatial functions. The involvement of the parahippocampal gyri in the pathogenesis of depressive symptoms in ET is unclear. However, increased connectivity of the posterior cingulate cortex with the parahippocampal gyri has been associated with future depressive symptoms in remitted depressed patients (Zamoscik, Huffziger, Ebner‐Priemer, Kuehner, & Kirsch, 2014). The same pattern appears on the right cerebellum floculus (higher LE and CC) with an inverse correlation between these metrics and the cognitive domain of attention. In other regions, the results were not so clear but were not in conflict with the previous patterns. Overall, it is still too soon to fully understand the neuropsychological implications of the changes in connectivity of these regions, which are altered in ET patients. Maybe some of the alterations are related with neuropsychological compensatory mechanisms, not only with a lower cognitive performance.
The lack of correlation between any graph theory metric and tremor duration or severity merits an explanation. Overall, tremor duration is not a reliable variable since differences in reported age of onset in ET patients may vary widely, and in up to one fifth of patients may be substantial (Louis, 2013). With respect to severity, one should in mind that the Fahn–Tolosa–Marin tremor rating scale is a motor scale (Jankovic & Tolosa, 1988) and most the observed intergroup differences in connectivity were found in extra‐motor areas.
The study was not without limitations. First, the diagnosis of ET was based on clinical criteria and further supported by normal [(123) I]FP‐CIT single photon emission computed tomography scan results. None of the ET patients had postmortem assessments, so that it was not possible to determine whether they had the types of changes that have been reported in ET (Louis, 2016). Second, the study was designed to distinguish ET cases from healthy controls, and we did not include any diseased control groups (e.g., patients with Parkinson's disease or dystonia). Inclusion of such comparison groups in subsequent studies will allow us to determine whether the findings of this study are unique to ET or whether they extend in part or fully to patients with other movement disorders. Nonetheless, the current findings are internally valid, and they provide an initial step towards identifying and delineating an ET signature. Third, there is some evidence that there is clinical heterogeneity in ET and that some subgroups of patients seem to differ from others in selected imaging studies (Caligiuri et al., 2017; Wang et al., 2018). Two such subgroups are the patients with rest or head tremor. While it would have been interesting to have stratified our study group, based on rest and head tremor, the sample size was too small to do so. Fourth, an objective measure of the motor symptoms via surface electromyography would be more able to detect the relationship between motor symptoms and the functional connectivity of the brain than the clinical diagnosis employed. Finally, 15 (65.2%) of the ET patients were assessed while taking stable doses of anti‐tremor medications. As some medications used to treat ET (i.e., propranolol or benzodiazepines) might cause changes in brain networks (Brown et al., 2015; Narayanan et al., 2010), studying patients off medication seems preferable to assess the unmasked effects of the disease. However, in unmedicated ET patients, tremor can cause movement artifacts that may confound neuroimaging findings. Moreover, despite receiving anti‐tremor medications the patients in our sample still showed significant motor impairments (as assessed with the Fahn–Tolosa–Marin tremor rating scale), which indicates that these medications did not reverse the pathogenesis of the disease.
In conclusion, graph theory analysis is a novel and promising approach for the study of ET, as it may provide new insights into the pathophysiology of this entity, mainly in nonmotor symptoms (especially, cognitive symptoms). We found differences between ET patients and healthy controls both at the global and local levels, suggesting that ET is characterized by lower small‐worldness values of the network—less clustered functionality of the brain. At the regional level, we found the existence of multiple connectivity differences between ET patients and healthy controls in motor and extra‐motor‐related areas. Most importantly, these data furthermore support the concept that ET is a disorder that disrupts widespread brain regions, including those outside of the brain regions responsible for tremor (i.e., cerebellum, thalamus, motor cortex). Finally, our results constitute a first step to further explore the neural basis of the nonmotor manifestations in ET.
AUTHOR CONTRIBUTIONS
Dr J.B.L. collaborated in the conception, organization of the research project; data analysis; and the writing of the manuscript first draft and the review and critique of the manuscript. E.S.M. collaborated in the conception, organization of the research project; data analysis; and the writing of the manuscript first draft and the review and critique of the manuscript. Dr H.M. collaborated in the conception, organization of the research project; data analysis; and the review and critique of the manuscript. Dr E.D.L. collaborated in the conception, organization of the research project and the review and critique of the manuscript. Dr J.P.R. collaborated in the conception, organization of the research project and the review and critique of the manuscript. Dr E.R. collaborated in the conception, organization of the research project and the review and critique of the manuscript. Dr N.M. collaborated in the conception, organization of the research project and the review and critique of the manuscript.
CONFLICT OF INTEREST
The authors declare no competing conflict of interest.
ACKNOWLEDGMENTS
Dr J.B.L. is supported by the National Institutes of Health, Bethesda, MD (NINDS #R01 NS39422), the European Commission (grant ICT‐2011‐287739, NeuroTREMOR), the Spanish Ministry of Economy and Competitiveness (grant RTC‐2015‐3967‐1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (FIS PI12/01602 and FIS PI16/00451). Dr E.D.L. has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #R01 NS073872 (principal investigator), and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O'Neil Essential Tremor Research Fund (Yale University). Drs H.M., J.P.R., E.R., and N.M. are supported by the Spanish Ministry of Economy and Competitiveness (grant DPI‐2015‐68664‐C4‐1‐R, NeuroMOD). Drs J.P.R. and E.R. are supported by the European Commission (grant ICT‐2011‐287739, NeuroTREMOR). Dr. E.R. is supported by the project ESSENTIAL (DPI2015‐72638‐EXP). The authors thank Drs Juan Álvarez‐Linera and Juan Antonio Hernández‐Tamames for their assistance to the project.
Benito‐León J, Sanz‐Morales E, Melero H, et al. Graph theory analysis of resting‐state functional magnetic resonance imaging in essential tremor. Hum Brain Mapp. 2019;40:4686–4702. 10.1002/hbm.24730
Funding information Spanish Ministry of Economy and Competitiveness, Grant/Award Number: DPI‐2015‐72638‐EXP; European Commission, Grant/Award Number: ICT‐2011‐287739; Spanish Ministry of Economy and Competitiveness, Grant/Award Number: DPI‐2015‐68664‐C4‐1‐R; Claire O'Neil Essential Tremor Research Fund; Spanish Health Research Agency, Grant/Award Numbers: FIS PI16/00451, FIS PI12/01602; Ministry of Economy and Competitiveness, Grant/Award Number: RTC‐2015‐3967‐1; National Institutes of Health, Grant/Award Numbers: NINDS #R01 NS088257, NINDS #R01 NS073872, NINDS #R01 NS085136, NINDS #R01 NS094607, NINDS #R01 NS39422
DATA AVAILABILITY STATEMENT
The original database from the study can be obtained by contacting the first author (J.B.‐L.).
REFERENCES
- Ackerman, P. L. , & Cianciolo, A. T. (2000). Cognitive, perceptual‐speed, and psychomotor determinants of individual differences during skill acquisition. Journal of Experimental Psychology. Applied, 6, 259–290. [DOI] [PubMed] [Google Scholar]
- Adolphs, R. , & Spezio, M. (2006). Role of the amygdala in processing visual social stimuli. Progress in Brain Research, 156, 363–378. [DOI] [PubMed] [Google Scholar]
- Akram, H. , Dayal, V. , Mahlknecht, P. , Georgiev, D. , Hyam, J. , Foltynie, T. , … Zrinzo, L. (2018). Connectivity derived thalamic segmentation in deep brain stimulation for tremor. NeuroImage: Clinical, 18, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric, A. (1994). Diagnostic and Statistical Manual of Mental Disorders DSM‐IV. Washington, DC: American Psychiatric Association. [Google Scholar]
- Astafiev, S. V. , Stanley, C. M. , Shulman, G. L. , & Corbetta, M. (2004). Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nature Neuroscience, 7, 542–548. [DOI] [PubMed] [Google Scholar]
- Auzou, N. , Foubert‐Samier, A. , Dupouy, S. , & Meissner, W. G. (2014). Facial emotion recognition is inversely correlated with tremor severity in essential tremor. Journal of Neural Transmission (Vienna), 121, 347–351. [DOI] [PubMed] [Google Scholar]
- Bares, M. , Lungu, O. V. , Husarova, I. , & Gescheidt, T. (2010). Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson's disease. Cerebellum, 9, 124–135. [DOI] [PubMed] [Google Scholar]
- Barkhof, F. , Haller, S. , & Rombouts, S. A. (2014). Resting‐state functional MR imaging: A new window to the brain. Radiology, 272, 29–49. [DOI] [PubMed] [Google Scholar]
- Bech, P. (1993). Rating scales for psychopathology, health status, and quality of life: A compendium on documentation in accordance with the DSM‐III‐R and WHO systems. Berlin, Germany: Springer‐Verlag; xiii, 503: ill; 24cm. [Google Scholar]
- Benedek, M. , Jauk, E. , Beaty, R. E. , Fink, A. , Koschutnig, K. , & Neubauer, A. C. (2016). Brain mechanisms associated with internally directed attention and self‐generated thought. Scientific Reports, 6, 22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. (2009). How common is essential tremor? Neuroepidemiology, 32, 215–216. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. (2014). Essential tremor: A neurodegenerative disease? Tremor and Other Hyperkinetic Movements, 4, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , & Domingo‐Santos, A. (2016). Orthostatic tremor: An update on a rare entity. Tremor and Other Hyperkinetic Movements, 6, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , & Louis, E. D. (2006). Essential tremor: Emerging views of a common disorder. Nature Clinical Practice. Neurology, 2, 666–678 quiz 2p following 691. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , & Louis, E. D. (2007). Clinical update: Diagnosis and treatment of essential tremor. Lancet, 369, 1152–1154. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , & Louis, E. D. (2011). Update on essential tremor. Minerva Medica, 102, 417–439. [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , & Bermejo‐Pareja, F. (2013). Short sleep duration heralds essential tremor: A prospective, population‐based study. Movement Disorders, 28, 1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Bermejo‐Pareja, F. , & Neurological Disorders in Central Spain Study Group . (2006a). Elderly‐onset essential tremor is associated with dementia. Neurology, 66, 1500–1505. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Bermejo‐Pareja, F. , & Neurological Disorders in Central Spain Study Group . (2006b). Population‐based case‐control study of cognitive function in essential tremor. Neurology, 66, 69–74. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Bermejo‐Pareja, F. , & Neurological Disorders in Central Spain Study Group . (2007). Reported hearing impairment in essential tremor: A population‐based case‐control study. Neuroepidemiology, 29, 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Bermejo‐Pareja, F. , & Neurological Disorders in Central Spain Study Group . (2009). Risk of incident Parkinson's disease and parkinsonism in essential tremor: A population based study. Journal of Neurology, Neurosurgery, and Psychiatry, 80, 423–425. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Manzanedo, E. , Hernández‐Tamames, J. A. , Álvarez‐Linera, J. , Molina‐Arjona, J. A. , … Sánchez‐Ferro, A. (2016). Resting state functional MRI reveals abnormal network connectivity in orthostatic tremor. Medicine, 95, e4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Mitchell, A. J. , & Bermejo‐Pareja, F. (2011). Elderly‐onset essential tremor and mild cognitive impairment: A population‐based study (NEDICES). Journal of Alzheimer's Disease, 23, 727–735. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Romero, J. P. , Hernández‐Tamames, J. A. , Manzanedo, E. , Álvarez‐Linera, J. , … Rocon, E. (2015). Altered functional connectivity in essential tremor: A resting‐state fMRI study. Medicine, 94, e1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , Serrano, J. I. , Louis, E. D. , Holobar, A. , Romero, J. P. , Povalej‐Brzan, P. , … Rocon, E. (2018). Tremor severity in Parkinson's disease and cortical changes of areas controlling movement sequencing: A preliminary study. Journal of Neuroscience Research, 96, 1341–1352. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Serrano, J. I. , Louis, E. D. , Holobar, A. , Romero, J. P. , Povalej‐Brzan, P. , … Rocon, E. (2019). Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Annals of Clinical Translational Neurology, 6, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo‐Pareja, F. , Louis, E. D. , Benito‐León, J. , & Neurological Disorders in Central Spain Study Group . (2007). Risk of incident dementia in essential tremor: A population‐based study. Movement Disorders, 22, 1573–1580. [DOI] [PubMed] [Google Scholar]
- Bermejo‐Pareja, F. , & Puertas‐Martín, V. (2012). Cognitive features of essential tremor: A review of the clinical aspects and possible mechanistic underpinnings. Tremor and Other Hyperkinetic Movements, 2, 02‐74‐541‐1. 10.7916/D89W0D7W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, U. , Plichta, M. M. , Esslinger, C. , Sauer, C. , Haddad, L. , Grimm, O. , … Meyer‐Lindenberg, A. (2012). Test‐retest reliability of resting‐state connectivity network characteristics using fMRI and graph theoretical measures. NeuroImage, 59, 1404–1412. [DOI] [PubMed] [Google Scholar]
- Brown, G. G. , Ostrowitzki, S. , Stein, M. B. , von Kienlin, M. , Liu, T. T. , Simmons, A. , … Paulus, M. (2015). Temporal profile of brain response to alprazolam in patients with generalized anxiety disorder. Psychiatry Research, 233, 394–401. [DOI] [PubMed] [Google Scholar]
- Buijink, A. W. , van der Stouwe, A. M. , Broersma, M. , Sharifi, S. , Groot, P. F. , Speelman, J. D. , … van Rootselaar, A. F. (2015). Motor network disruption in essential tremor: A functional and effective connectivity study. Brain, 138, 2934–2947. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , & de Lacy, N. (2017). Ten key observations on the analysis of resting‐state functional MR imaging data using independent component analysis. Neuroimaging Clinics of North America, 27, 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri, M. E. , Arabia, G. , Barbagallo, G. , Lupo, A. , Morelli, M. , Nistico, R. , … Quattrone, A. (2017). Structural connectivity differences in essential tremor with and without resting tremor. Journal of Neurology, 264, 1865–1874. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , & Friston, K. J. (2010). The default‐mode, ego‐functions and free‐energy: A neurobiological account of Freudian ideas. Brain, 133, 1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersonsky, T. E. K. , Morgan, S. , Kellner, S. , Robakis, D. , Liu, X. , Huey, E. D. , … Cosentino, S. (2018). Evaluating mild cognitive impairment in essential tremor: How many and which neuropsychological tests? Journal of the International Neuropsychological Society, 24, 1084–1098. [DOI] [PubMed] [Google Scholar]
- Chiang, S. , Stern, J. M. , Engel, J., Jr. , Levin, H. S. , & Haneef, Z. (2014). Differences in graph theory functional connectivity in left and right temporal lobe epilepsy. Epilepsy Research, 108, 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, K. , Rohl, B. , Morgan, S. , Huey, E. D. , Louis, E. D. , & Cosentino, S. (2017). Mild cognitive impairment subtypes in a cohort of elderly essential tremor cases. Journal of the International Neuropsychological Society, 23, 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J. W. , Fryer, T. D. , Brichard, L. , Robinson, E. S. , Theobald, D. E. , Laane, K. , … Robbins, T. W. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315, 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , Jirsa, V. K. , & McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting‐state activity in the brain. Nature Reviews. Neuroscience, 12, 43–56. [DOI] [PubMed] [Google Scholar]
- Deuschl, G. , Bain, P. , & Brin, M. (1998). Consensus statement of the movement disorder society on tremor. Ad hoc scientific committee. Movement Disorders, 13(Suppl 3), 2–23. [DOI] [PubMed] [Google Scholar]
- Douw, L. , Schoonheim, M. M. , Landi, D. , van der Meer, M. L. , Geurts, J. J. , Reijneveld, J. C. , … Stam, C. J. (2011). Cognition is related to resting‐state small‐world network topology: An magnetoencephalographic study. Neuroscience, 175, 169–177. [DOI] [PubMed] [Google Scholar]
- Drakesmith, M. , Caeyenberghs, K. , Dutt, A. , Lewis, G. , David, A. S. , & Jones, D. K. (2015). Overcoming the effects of false positives and threshold bias in graph theoretical analyses of neuroimaging data. NeuroImage, 118, 313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, A. D. , & Bookheimer, S. Y. (2007). Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning & Memory, 14, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas, F. , Chawluk, J. B. , Alavi, A. , Hurtig, H. I. , & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR. American Journal of Roentgenology, 149, 351–356. [DOI] [PubMed] [Google Scholar]
- Francis, T. C. , & Lobo, M. K. (2017). Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biological Psychiatry, 81, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli, J. D. , Poldrack, R. A. , & Desmond, J. E. (1998). The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America, 95, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri, F. , Messe, A. , Perlbarg, V. , Valabregue, R. , McColgan, P. , Yahia‐Cherif, L. , … Lehericy, S. (2016). Longitudinal changes in functional connectivity of cortico‐basal ganglia networks in manifests and premanifest Huntington's disease. Human Brain Mapping, 37, 4112–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbin, M. , Viner, A. S. , & Louis, E. D. (2012). Sleep in essential tremor: A comparison with normal controls and Parkinson's disease patients. Parkinsonism & Related Disorders, 18, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlich, M. , Munte, T. F. , Heldmann, M. , Kasten, M. , Hagenah, J. , & Kramer, U. M. (2013). Altered resting state brain networks in Parkinson's disease. PLoS One, 8, e77336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker, C. D. , Perlmutter, J. S. , Criswell, S. R. , Ances, B. M. , & Snyder, A. Z. (2012). Resting state functional connectivity of the striatum in Parkinson's disease. Brain, 135, 3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1967). Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology, 6, 278–296. [DOI] [PubMed] [Google Scholar]
- Higo, T. , Mars, R. B. , Boorman, E. D. , Buch, E. R. , & Rushworth, M. F. (2011). Distributed and causal influence of frontal operculum in task control. Proceedings of the National Academy of Sciences of the United States of America, 108, 4230–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez, J. , González de la Aleja, J. , Gallego, J. A. , Romero, J. P. , Saíz‐Díaz, R. A. , Benito‐León, J. , & Rocon, E. (2014). Effects of alprazolam on cortical activity and tremors in patients with essential tremor. PLoS One, 9, e93159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, C. , Park, H. J. , Chang, W. S. , Pae, C. , & Chang, J. W. (2016). Immediate and longitudinal alterations of functional networks after thalamotomy in essential tremor. Frontiers in Neurology, 7, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic, J. , & Tolosa, E. (1988). Parkinson's disease and movement disorders. Baltimore, MD: Urban & Schwarzenberg; xii, 499. [Google Scholar]
- Jiménez‐Jiménez, F. J. , Rubio, L. , Alonso‐Navarro, H. , Calleja, M. , Pilo‐de‐la‐Fuente, B. , Plaza‐Nieto, J. F. , … Agundez, J. A. (2010). Impairment of rapid repetitive finger movements and visual reaction time in patients with essential tremor. European Journal of Neurology, 17, 152–159. [DOI] [PubMed] [Google Scholar]
- Joel, S. E. , Caffo, B. S. , van Zijl, P. C. , & Pekar, J. J. (2011). On the relationship between seed‐based and ICA‐based measures of functional connectivity. Magnetic Resonance in Medicine, 66, 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaee, A. , Ebrahimzadeh, A. , & Babajani‐Feremi, A. (2016). Application of advanced machine learning methods on resting‐state fMRI network for identification of mild cognitive impairment and Alzheimer's disease. Brain Imaging and Behavior, 10, 799–817. [DOI] [PubMed] [Google Scholar]
- Lafo, J. A. , Mikos, A. , Mangal, P. C. , Scott, B. M. , Trifilio, E. , Okun, M. S. , & Bowers, D. (2017). Emotion modulation of the startle reflex in essential tremor: Blunted reactivity to unpleasant and pleasant pictures. Parkinsonism & Related Disorders, 34, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, N. , von Bastian, C. C. , Wirz, H. , Oberauer, K. , & Jancke, L. (2013). The effects of working memory training on functional brain network efficiency. Cortex, 49, 2424–2438. [DOI] [PubMed] [Google Scholar]
- LaRoia, H. , & Louis, E. D. (2011). Association between essential tremor and other neurodegenerative diseases: What is the epidemiological evidence? Neuroepidemiology, 37, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau, A. , & Downing, P. E. (2015). The lateral occipitotemporal cortex in action. Trends in Cognitive Sciences, 19, 268–277. [DOI] [PubMed] [Google Scholar]
- Louis, E. D. (2013). Age of onset: Can we rely on essential tremor patients to report this? Data from a prospective, longitudinal study. Neuroepidemiology, 40, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. (2016). Essential tremor: A common disorder of Purkinje neurons? The Neuroscientist, 22, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. , Benito‐León, J. , Bermejo‐Pareja, F. , & Neurological Disorders in Central Spain Study Group . (2007). Self‐reported depression and anti‐depressant medication use in essential tremor: Cross‐sectional and prospective analyses in a population‐based study. European Journal of Neurology, 14, 1138–1146. [DOI] [PubMed] [Google Scholar]
- Louis, E. D. , & Ferreira, J. J. (2010). How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders, 25, 534–541. [DOI] [PubMed] [Google Scholar]
- Louis, E. D. , Huang, C. C. , Dyke, J. P. , Long, Z. , & Dydak, U. (2014). Neuroimaging studies of essential tremor: How well do these studies support/refute the neurodegenerative hypothesis? Tremor and Other Hyperkinetic Movements, 4, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. , & Ottman, R. (2013). Is there a one‐way street from essential tremor to Parkinson's disease? Possible biological ramifications. European Journal of Neurology, 20, 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall, M. E. , Bassett, D. S. , Kerwin, R. , McKenna, P. J. , Kitzbichler, M. , Muller, U. , & Bullmore, E. (2010). Functional connectivity and brain networks in schizophrenia. The Journal of Neuroscience, 30, 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia, J. D. (2017). Graph theoretic analysis of resting state functional MR imaging. Neuroimaging Clinics of North America, 27, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, K. , Jech, R. , Hoskovcova, M. , Ulmanova, O. , Urgosik, D. , Vymazal, J. , & Ruzicka, E. (2017). General and selective brain connectivity alterations in essential tremor: A resting state fMRI study. NeuroImage: Clinical, 16, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, A. , White, C. A. , Saklayen, S. , Scaduto, M. J. , Carpenter, A. L. , Abduljalil, A. , … Beversdorf, D. Q. (2010). Effect of propranolol on functional connectivity in autism spectrum disorder—A pilot study. Brain Imaging and Behavior, 4, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo, W. G. , Sutton, L. , Dat Vuong, K. , Lai, D. , & Jankovic, J. (2003). Hearing impairment in essential tremor. Neurology, 61, 1093–1097. [DOI] [PubMed] [Google Scholar]
- Petersen, R. C. , & Morris, J. C. (2005). Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology, 62, 1160–1163 discussion 1167. [DOI] [PubMed] [Google Scholar]
- Puertas‐Martin, V. , Villarejo‐Galende, A. , Fernandez‐Guinea, S. , Romero, J. P. , Louis, E. D. , & Benito‐León, J. (2016). A comparison study of cognitive and neuropsychiatric features of essential tremor and Parkinson's disease. Tremor and Other Hyperkinetic Movements, 6, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raethjen, J. , Govindan, R. B. , Kopper, F. , Muthuraman, M. , & Deuschl, G. (2007). Cortical involvement in the generation of essential tremor. Journal of Neurophysiology, 97, 3219–3228. [DOI] [PubMed] [Google Scholar]
- Sengul, Y. , Sengul, H. S. , Sural, M. K. , Bakim, B. , & Forta, H. (2015). A comparison between rate of nonmotor symptom development in essential tremor and Parkinson's disease. Acta Neurologica Belgica, 115, 289–294. [DOI] [PubMed] [Google Scholar]
- Sengul, Y. , Sengul, H. S. , Yucekaya, S. K. , Yucel, S. , Bakim, B. , Pazarci, N. K. , & Ozdemir, G. (2015). Cognitive functions, fatigue, depression, anxiety, and sleep disturbances: Assessment of nonmotor features in young patients with essential tremor. Acta Neurologica Belgica, 115, 281–287. [DOI] [PubMed] [Google Scholar]
- Serrano, J. I. , Romero, J. P. , Castillo, M. D. D. , Rocon, E. , Louis, E. D. , & Benito‐León, J. (2017). A data mining approach using cortical thickness for diagnosis and characterization of essential tremor. Scientific Reports, 7, 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan, K. , Mishra, V. , Bird, C. , Zhuang, X. , Yang, Z. , Cordes, D. , & Walsh, R. R. (2019). Altered functional network topology correlates with clinical measures in very early‐stage, drug‐naive Parkinson's disease. Parkinsonism & Related Disorders, 62, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakad, A. , & Jankovic, J. (2018). Essential tremor and Parkinson's disease: Exploring the relationship. Tremor and Other Hyperkinetic Movements, 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenganatt, M. A. , & Louis, E. D. (2012). Personality profile in essential tremor: A case‐control study. Parkinsonism & Related Disorders, 18, 1042–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuleasca, C. , Najdenovska, E. , Regis, J. , Witjas, T. , Girard, N. , Champoudry, J. , … Van De Ville, D. (2018). Ventrolateral motor thalamus abnormal connectivity in essential tremor before and after thalamotomy: A resting‐state functional magnetic resonance imaging study. World Neurosurgery, 113, e453#x2013;e464. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Vlooswijk, M. C. , Vaessen, M. J. , Jansen, J. F. , de Krom, M. C. , Majoie, H. J. , Hofman, P. A. , … Backes, W. H. (2011). Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology, 77, 938–944. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Zuo, X. , & He, Y. (2010). Graph‐based network analysis of resting‐state functional MRI. Frontiers in Systems Neuroscience, 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Lei, D. , Suo, X. , Li, N. , Lu, Z. , Li, J. , … Peng, R. (2018). Resting‐state fMRI study on drug‐naive patients of essential tremor with and without head tremor. Scientific Reports, 8, 10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Li, Y. , Metzak, P. , He, Y. , & Woodward, T. S. (2010). Age‐related changes in topological patterns of large‐scale brain functional networks during memory encoding and recognition. NeuroImage, 50, 862–872. [DOI] [PubMed] [Google Scholar]
- Winblad, B. , Palmer, K. , Kivipelto, M. , Jelic, V. , Fratiglioni, L. , Wahlund, L. O. , … Petersen, R. C. (2004). Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of Internal Medicine, 256, 240–246. [DOI] [PubMed] [Google Scholar]
- Yang, Y. L. , Deng, H. X. , Xing, G. Y. , Xia, X. L. , & Li, H. F. (2015). Brain functional network connectivity based on a visual task: Visual information processing‐related brain regions are significantly activated in the task state. Neural Regeneration Research, 10, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi, T. , Watanabe, H. , Yamaguchi, H. , Bagarinao, E. , Masuda, M. , Imai, K. , … Sobue, G. (2018). Involvement of the precuneus/posterior cingulate cortex is significant for the development of Alzheimer's disease: A PET (THK5351, PiB) and resting fMRI study. Frontiers in Aging Neuroscience, 10, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoscik, V. , Huffziger, S. , Ebner‐Priemer, U. , Kuehner, C. , & Kirsch, P. (2014). Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Social Cognitive and Affective Neuroscience, 9, 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Liu, X. , Chen, J. , Liu, B. , & Wang, J. (2015). Widespread increase of functional connectivity in Parkinson's disease with tremor: A resting‐state FMRI study. Frontiers in Aging Neuroscience, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original database from the study can be obtained by contacting the first author (J.B.‐L.).


