Abstract
Pathophysiological and atrophic changes in the cerebellum have been well‐documented in schizophrenia. Reduction of gray matter (GM) in the cerebellum was confirmed across cognitive and motor cerebellar modules in schizophrenia. Such abnormalities in the cerebellum could potentially have widespread effects on both sensorimotor and cognitive symptoms. In this study, we investigated how reduction change in the cerebellum affects the static and the dynamic functional connectivity (FC) between the cerebellum and cortical/subcortical networks in schizophrenia. Reduction of GM in the cerebellum was confirmed across the cognitive and motor cerebellar modules in schizophrenic subjects. Results from this study demonstrates that the extent of reduction of GM within cerebellum correlated with increased static FCs between the cerebellum and the cortical/subcortical networks, including frontoparietal network (FPN), and thalamus in patients with schizophrenia. Decreased GM in the cerebellum was also associated with a declined dynamic FC between the cerebellum and the FPN in schizophrenic subjects. The severity of patients' positive symptom was related to these structural‐functional coupling score of cerebellum. These findings identified potential cerebellar driven functional changes associated with positive symptom deficits. A post hoc analysis exploring the effect of changed FC within cerebellum, confirmed that a significant positive relationship, between dynamic FCs of cerebellum–thalamus and intracerebellum existed in patients, but not in controls. The reduction of GM within the cerebellum might be associated with modulation of cerebellum–thalamus, and contributes to the dysfunctional cerebellar‐cortical communication in schizophrenia. Our results provide a new insight into the role of cerebellum in understanding the pathophysiological of schizophrenia.
Keywords: cerebellum, cortical–subcortical networks, functional connectivity, temporal variability, voxel‐base morphometry
1. INTRODUCTION
As a complex psychiatric disorder, schizophrenia is often referred to as a “dysconnection” syndrome, where the underlying neurobiology arises from a failure to coordinated action across multiple brain regions (Stephan, Friston, & Frith, 2009). Functional neuroimaging studies have suggested that cerebellum exerts a far‐reaching influence on the coordination of behaviors through its interconnections with the basal ganglia (BG) and much of cortical mantle (Desmond & Fiez, 1998; Ito, 2006; Leggio & Molinari, 2015). Specifically, the cognitive dysmetria hypothesis by Andreasen and colleagues suggests that cerebellum plays an important role in the impaired sequencing and coordination of sensorimotor and mental processes and might underlie many features of the schizophrenia syndrome (Andreasen et al., 1999).
The human cerebellum is a heterogeneous structure and has been anatomically divided into vermal and hemispheric subregions designated from I–X (Schmahmann et al., 1999). In healthy controls, anatomical and functional neuroimaging studies have confirmed that the cerebellum has a distinct functional motor and cognitive module and that it links to cerebral cortex through the BG and the thalamus, respectively (Duan et al., 2015; Middleton & Strick, 1994; Middleton & Strick, 2001). The cerebellar motor module comprises lobules V, VI, VIIb, and VIII, projecting to the motor regions. The cerebellar cognitive module comprising Crus I and II, projects to prefrontal and parietal cortices (Balsters, Laird, Fox, & Eickhoff, 2014). From a functional imaging perspective, cerebellar motor module shows preferential coupling with the cortical sensorimotor network, while the cognitive module is associated with the cognitive and limbic function, including the default, cognitive control, and salience networks (Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011; Habas et al., 2009). Together, cerebellum might play a critical role in coordinating or modulating aspects of cortical activity by a cortico‐cerebellar‐subcortico‐cortical circuit.
Recent studies have demonstrated converging lines of evidence highlighting the structural and functional abnormalities of the cerebellum in subjects with schizophrenia (Lungu et al., 2013; Moberget et al., 2017). Neuropathological studies in humans have documented a reduction in size and density of Purkinje cells in the cerebellum of schizophrenia subjects (Katsetos, Hyde, & Herman, 1997). Moreover, schizophrenic subjects have exhibited differences in gray and white matter probabilities (Laidi et al., 2015; Lee et al., 2007). Also, functional imaging studies add evidence for cerebellar dysfunction in patients with schizophrenia compared with matched healthy controls (Whalley et al., 2004). Importantly, several resting‐state functional connectivity (FC) studies have independently replicated robust disruptions of cerebellum–BG/cortical FC in schizophrenic subjects (Anticevic et al., 2014; Duan et al., 2015; Moberget et al., 2017; Repovs, Csernansky, & Barch, 2011). Furthermore, reduced gray matter (GM) within cerebellum has been identified as a critical factor that drives both changes in task‐based activation and resting state FC networks (James, James, Smith, & Javaloyes, 2004; Loeber, Cintron, & Yurgelun‐Todd, 2001). Establishing the association between focal GM changes and FC changes have received considerable interest in schizophrenia (Edwards et al., 2008; Kim et al., 2007; Zhang et al., 2015). However, the manner in which local GM change in the cerebellum, might influence FC within the cerebellar module, or FC with cortical/subcortical networks, have not been investigated in schizophrenia. Thus, focusing on the functional cerebellar modules could provide an important framework for understanding how changes in cerebellar structure and function may manifest clinical symptom in schizophrenia.
In recent years, resting‐state functional magnetic resonance imaging (rs‐fMRI), has become a popular method for mapping brain function by measuring ongoing spontaneous brain activity and inter‐regional FC (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox & Raichle, 2007). Recent studies have demonstrated that FC is not stationary and changes over time (in minutes) in the resting state (Chang & Glover, 2010; Hansen, Battaglia, Spiegler, Deco, & Jirsa, 2015). Integrating the FC signals over a long time may obscure important dynamic features of network behavior. Specifically, many prevailing neuroimaging studies support the idea that the FC variability appears to be a reliable property and depend in part on functional relationships between neural systems (Hutchison et al., 2013). The results of dynamic FC analysis support and expand current knowledge regarding dysconnectivity in schizophrenia (Damaraju et al., 2014). Furthermore, a recent study has also reported that dynamic FC significantly outperforms the static FC in terms of the classification of schizophrenia and bipolar patients (Rashid et al., 2016). Together, static and dynamic FC approaches can provide complementary information about brain function.
In this study, we sought to determine whether a focal structural change in the cerebellum was associated with changes in the FCs between the cerebellum and large‐scale networks in schizophrenia. We hypothesized that the change of GM in cerebellum would be associated with specific changes in static and dynamic FC of cerebellum–BG/cortical in schizophrenic subjects. We further tested our hypothesis, by determining the relationships between the disease severity with the coupling information from structural and functional features of the cerebellum in schizophrenic patients. We posit that these reflect local structural changes in the cerebellum are an important driving factor in pathological functional changes in schizophrenia.
2. METHODS AND MATERIALS
2.1. Subjects selection
A total of 102 subjects (47 schizophrenia patients and 55 healthy controls) were acquired for this study. The 47 schizophrenia subjects were recruited from the Clinical Hospital of Chengdu Brain Science Institute. The patients were diagnosed using the structured clinical interview for the DSM‐IV axis I disorders—clinical version (SCID‐I‐CV), and all were being treated with medication (e.g., antipsychotics). The illness duration for all the patients was more than 3 years. The psychiatric symptom of schizophrenia was assessed using positive and negative symptom scale (PANSS). A total of 55 right‐handed healthy controls were also recruited to participate in the study. The control subjects were matched with schizophrenic subjects for years of education, age, and gender. The control subjects were screened for a history of medical or neuropsychiatric illness, as well as major neurological or psychiatric illness in their first‐degree relatives. The Ethics Committee of the Clinical Hospital of Chengdu Brain Science Institute in accordance with the Helsinki Declaration approved this study. Written informed consent was obtained from each subject before the beginning of the study.
2.2. Imaging acquisition
Imaging was conducted on a 3T MRI scanner (GE DISCOVERY MR750). During scanning, we used foam padding and ear plugs to reduce head motion and scanner noise, respectively. High‐resolution T1‐weighted images were acquired using a three dimensional fast spoiled gradient echo sequence (repetition time [TR] = 6.008 ms, flip angle [FA] = 9°, matrix = 256 × 256, field of view [FOV] = 256 × 256 mm2, slice thickness = 1 mm, no gap, 152 slices). Subsequently, resting state functional MRI data were acquired using gradient‐echo echo planar imaging sequences (TR = 2000 ms, echo time [TE] = 30 ms, FA = 90°, matrix = 64 × 64, FOV = 240 × 240 mm2, slice thickness/gap = 4 mm/0.4 mm, number of slices = 35), with an eight channel‐phased array head coil. All subjects underwent a 510 s resting state scan to yield 255 volumes. The first five volumes were discarded for the magnetization equilibrium. During rs‐fMRI, all subjects were instructed to have their eyes‐closed and to move as little as possible without falling asleep.
2.3. Voxel‐based morphometry analysis
Voxel‐based morphometry (VBM) was performed on the T1‐weighted images, using SPM8 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/). Before data analysis, all the imaging data sets were checked for artifacts, and the image origins were manually set at the anterior commissure. Subsequently, structural images were processed with spatial normalization to MNI‐space using a diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL), and segmentation into GM, white matter, and cerebrospinal fluid. The segmented GM was Jacobian modulated, and finally, was smoothed with an 8‐mm full‐width at half‐maximum (FWHM) Gaussian kernel. A mask encompassing the entire cerebellum, was created to compare cerebellar GM intensity between two groups. The cerebellum was defined using the atlas of the human cerebellum (Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009). Years of education, age, and gender were regressed out as potential confounding covariates in the general linear model. Then, we established the structural abnormalities between patients and healthy controls through a voxel‐wise statistical parametric mapping (two‐sample t test). The comparison was tested for significant level of p < .05, corrected for multiple comparisons via cluster‐level false discovery rate (FDR) correction.
To quantify reduction in the GM in the cerebellum (pursuant to the VBM statistical tests between two groups), motor and cognitive cerebellum modules were created using cerebellar subregions from the atlas of human brain referenced above. Cerebellar cognitive cluster (CBCc) was defined as bilateral Crus I and Crus II and cerebellar motor cluster (CBCm) was defined as the bilateral lobules V, VI, VIIb, VIIIa, and VIIIb (Balsters et al., 2014; O'Callaghan et al., 2016). Within each cerebellar module, the peak t value corresponding to each subregion was used to create corresponding spheres (radius: 4 mm). All of these structural measurements from each cerebellar subregion were then combined to represent the extent of cerebellar GM intensity within CBCc and CBCm for each subject.
2.4. fMRI preprocessing
Functional imaging data were preprocessed using SPM8 according to a standard pipeline (He et al., 2017) and only briefly described here. Slice time correction, head motion correction, normalization (3 mm * 3 mm * 3 mm) into EPI template, and image smoothing (FWHM 8 mm) were carried out. Any subject who had a maximum translation in any of the orthogonal directions larger than 1.5 mm or rotation larger than 1.5° were excluded from subsequent analysis. Besides, framewise displacement (FD) was evaluated in the two groups as suggested by Power et al. (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). To minimize the effect in fMRI due to linear trend, detrending analysis was performed. Then, sources of nuisance signals were removed from the smoothed images through linear regression (six motion parameters and their first temporal derivative, white matter signal, cerebrospinal fluid signal). The global signal was not regressed out as has been recently suggested in processing the schizophrenia functional data (Yang et al., 2014). Finally, fMRI data were passed through a band‐pass 0.01 Hz–0.08 Hz.
Due to individual subject variability, brain networks obtained from each subject were identified using group‐level spatial independent component analysis (ICA) on smoothed data using GIFT toolbox (http://mialab.mrn.org/software/gift/). We used a higher‐order ICA model (number of components, C = 100) based on previous research, that has demonstrated that this number of components achieves sufficient “functional parcellation” of major brain systems (Allen et al., 2014; Damaraju et al., 2014). The Infomax algorithm was repeated 30 times in ICASSO (http://research.ics.tkk.fi./ica/icasso). Subject‐specific time courses and spatial maps were then reconstructed using spatial–temporal regressions approach for each subject. Subject‐specific time courses were passed through a band‐pass 0.01 Hz–0.08 Hz. As we were primarily interested in exploring the commonly reported altered cortical–subcortical networks in schizophrenia subjects, our study specifically targeted eight major networks of interest: the default model network (DMN), frontoparietal network (FPN), salience network (SN), dorsal attention network (DAN), sensorimotor network (SM), auditory network (AN), visual network (VN), and subcortical (SC). The time courses of components within each network were selected and static and dynamic FC analyses was performed.
2.5. Static and dynamic resting state FC analyses
To assess the static and dynamic FC between the local changed structural cerebellar regions (pursuant to the VBM statistical tests between two groups) and cortical–subcortical networks, a series of steps were performed for each subject. The preprocessed blood oxygen level dependence time courses were extracted within the spheres of CBCc and CBCm and were then averaged to represent the functional time courses of changed structural cerebellar modules for each subject. Time courses of selected components were extracted from the ICA. Then, Pearson correlation coefficients were calculated between the local changed structural region in cerebellum and selected brain components. This correlation coefficient based on whole time‐course was considered as the static FC (rstatic). The resulting values were transformed to approximate a Gaussian distribution using Fisher's r‐to‐z transformation.
We also measured dynamic FC using sliding windows. The time courses were segmented into windows to efficiently capture cognitive status and produce robust results in image acquisitions (Allen et al., 2014; Shirer, Ryali, Rykhlevskaia, Menon, & Greicius, 2012). In the sliding window‐based dynamic FC analysis, the optimal window length is an open area of research. Recent research has indicated a “rule of thumb” that the minimum window length should be no less than 1/f min (Leonardi & Van De Ville, 2015). Thus, the time courses were segmented into 100‐s windows (fmin = 0.01 Hz), sliding the onset of each window by 2 s of data. Within the ith time window, the Pearson correlations (ri) were computed between the changed cerebellum module (seeds) and selected brain components. We then define the dynamic index of a component k as:
where rt is the correlation between seed and the component k over time window t, t = 1,2,…n. Similar to that observed in previous studies (Hindriks et al., 2016; Kang et al., 2011), this dynamic index is an overall metrics of the changes in FC profile around static FC across widows. Large values in dynamic FC indicate more variability in static FC over time. This procedure was repeated to obtain the static and dynamic FC value for the selected brain network for every subject.
For the static or dynamic FC, the years of education, age and gender were regressed out as potential confounding covariates in the general linear model. Then, the statistical analyses were compared between healthy and schizophrenic subjects using a two‐sample t test. False discovery rate correction (p < .05) was used for multiple comparisons. Furthermore, to avoid arbitrary choice of window, we also performed the same processing using other two additional window lengths (i.e., 120 s and 140 s).
2.6. Significance testing of dynamic FC
To test whether the observed temporal fluctuations of FC exhibit significantly greater temporal variability than simple variations around static FC, an appropriate statistical test was carried out. One way to answer it is to construct confidence intervals around the values in the correlation time series (Kang et al., 2011). This question can also be formalized in a statistical hypothesis test whether the observed value of the test statistic falls outside the null distribution (Chang & Glover, 2010; Hindriks et al., 2016; Zalesky, Fornito, Cocchi, Gollo, & Breakspear, 2014). Constructing the null distribution is an appropriate way of creating surrogate data to fit a time series model to the data. One simple way to preserve the static FC was to shuffle the Fourier phases (Prichard & Theiler, 1994). Thus, in this study, we used Fourier‐based surrogate method to generate null hypothesis (Hindriks et al., 2016).
One thousand null data sets were generated using Fourier‐based surrogate method for each time‐varying connectivity to estimate the null hypothesis (absence of dynamic FC). This was performed in a step wise manner as follows: (a) taking the discrete Fourier transformations X1, X2, …, Xn and Y1, Y2, …, Yn of two BOLD signals x and y; (b) multiplying each signal's Fourier transform with a random phase: n = and n = , where ϕ1, …, ϕn is a vector of independent stochastic variables that are uniformly distributed in the interval [0, 2π]. To preserve the static correlation structure, both X and Y are multiplied by the same phases; (c) perform inverse discrete Fourier transformation to n and n to yield randomized of x and of y; (d) a sliding‐window approach was applied to each of the 1,000 surrogate data. The dynamic index was then obtained using the equation referenced above; (e) we obtained two kinds of P‐values for the observed value of the test statistic. First kind of p value for each dynamic FC: calculating the percentile of real dynamic index under the null distribution. For the second kind, we calculated the p value for each cerebellum regions (Hindriks et al., 2016). For every cerebellum region we averaged its real dynamic index across all other components. Then, we estimated the percentile of real dynamic index under the null distribution for each cerebellum. Final, false discovery rate correction (p < .05) was used across all dynamic FC to define greater temporal variability of FC around static FC.
After testing for the dynamic FC for each subject, the probability value (number of significant testing/number of subject) across subjects was then used to establish whether the greater dynamic FC of cerebellar‐regions were consistent across patient and control groups, respectively. The nonparameter statistical analysis (permutation testing) was performed to find the difference of number of significant testing between healthy and schizophrenic groups on time‐varying FC, as well as on the various cerebellum regions.
2.7. Noise confounds
Dynamic FCs are particularly susceptible to noise confounds for rs‐fMRI data, such as scanner drift, head motion (Power et al., 2014), and physiological noise due to respiration and cardiac processes (Chang et al., 2013; Chang & Glover, 2009). In this study, physiological noise signals were estimated with the CompCor procedure (Behzadi, Restom, Liau, & Liu, 2007; Zalesky et al., 2014). To exclude these confounds as possible causes of the results, we tested for relationship between correlation variability time‐course of dynamic FC and estimates of physiological noise as well as head motion, namely, FD. False discovery rate correction (p < .05) was used for these relationships.
2.8. Coupling between cerebellar structural alteration and FC
To investigate the relationship between the structural changes in cerebellum and its FC, we correlated the average GM intensity of each structural alteration of cerebellum module and each static or dynamic FC. For each cortical–subcortical network, the gender, years of education, and age were used as covariates, for both the patients and healthy controls. The association between the two groups was obtained using the permutation testing. Using the correlation in controls as a baseline, negative differences between patients and controls implied a relative loss of the structure–function relationship. While positive differences might reflect a compensatory mechanism.
2.9. Relationship between clinical symptom and cerebellar structural–functional coupling
Furthermore, we evaluated the relationship between the clinical symptom and the structural–functional coupling score in patients with schizophrenia. First, the coupling score (either static or dynamic FC/GM probability) was created by multiplying the static or dynamic FC for each cerebellar module to each network by the inverse value of the atrophied cerebellum module. Then, the partial correlation analysis was performed between coupling score and patients' PANSS score with age, gender, and years of education as covariates.
3. RESULTS
3.1. Demographics and patient clinical characteristics
A total of eight subjects (5 [out of 47] schizophrenic patients and 3 [out of 55] healthy controls) were excluded due to excessive head motion during fMRI scans. Thus, a total of 42 patients with schizophrenia and 52 healthy controls were included in the FC analysis. Demographic information and patient clinical characteristics, such as illness duration and PANSS scores, were presented in Table 1. No significant differences in age, gender, FD, and years of education were observed between the two groups (Table 1).
Table 1.
Demographic and clinical characteristics of the participants
| Patients with schizophrenia | Healthy controls | p | |
|---|---|---|---|
| Gender (male/female) | 26/16 | 29/23 | .55a |
| Age (years) | 42.14 ± 10.67 | 41.48 ± 12.88 | .79b |
| Education level (years) | 11.73 ± 2.85 | 11.12 ± 3.37 | .34b |
| Head motion (FD) | 0.11 ± 0.05 | 0.09 ± 0.05 | .43b |
| Disease duration (years) | 17.31 ± 9.87 | – | – |
| PANSS‐positive score | 12.98 ± 5.52 | – | – |
| PANSS‐negative score | 20.88 ± 6.53 | – | – |
| PANSS‐global score | 27.64 ± 5.24 | – | – |
| PANSS‐total score | 61.50 ± 13.19 | – | – |
Indicated values are shown mean ± standard deviation. PANSS = positive and negative symptom scale.
Indicates the p values from the comparison analysis (chi‐square test).
Indicates the p values from the comparison analysis (two sample t test).
3.2. Comparison of cerebellar GM intensity
Reduced GM probability in bilateral cerebellar lobule VI and left cerebellar Crus I was determined using VBM in patients with schizophrenia (Table 2). The altered clusters are presented in Figure 1. The clusters including left and right lobule VI as belonging to the motor cerebellar module and the left Crus I belonging to cognitive cerebellar module. Thus, CBCc and CBCm were used to represent these cerebellar regions, respectively.
Table 2.
Significant reduction in gray matter in cerebellum for schizophrenic subjects in comparison to healthy controls
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Regions | x | y | z | Peak t‐score | Cluster voxels |
| Left cerebellum VI | −26 | −60 | −28 | 5.632 | 147 |
| Right cerebellum VI | 22 | −62 | −28 | 4.763 | 112 |
| Left cerebellum Crus I | −28 | −74 | −30 | 4.916 | 64 |
Figure 1.
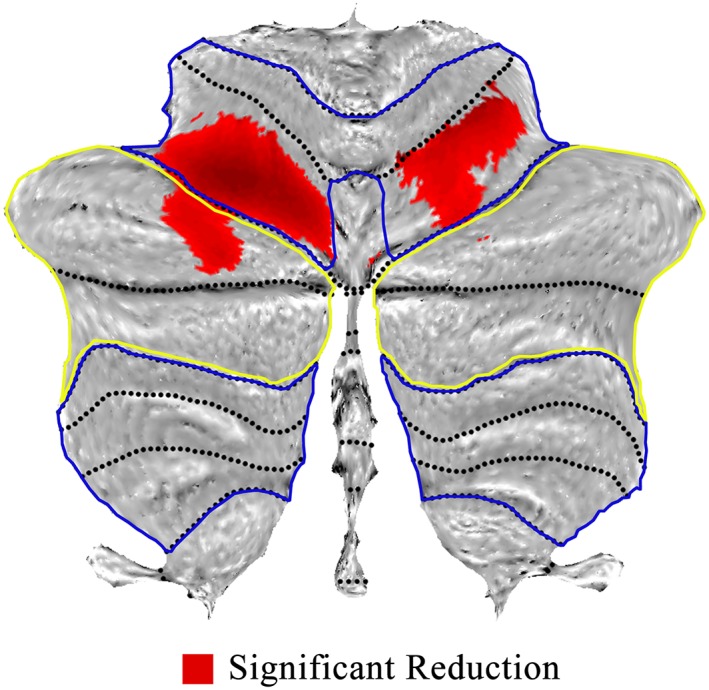
Voxel‐based morphometry showing difference of gray matter within cerebellum for schizophrenia. Areas of significant reduction of gray matter (red) in the motor (within blue circle) and cognitive (within yellow circle) cerebellar territories, for patients versus healthy controls [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Abnormalities of static and dynamic FC in patients
The results of the higher‐order ICA model are depicted in Figure 2 showing the 42 non‐artifactual components. The functional parcellation of cortical and subcortical brain components is similar to those observed in previous higher‐order ICA model decompositions (Allen et al., 2014; Kiviniemi et al., 2009).
Figure 2.
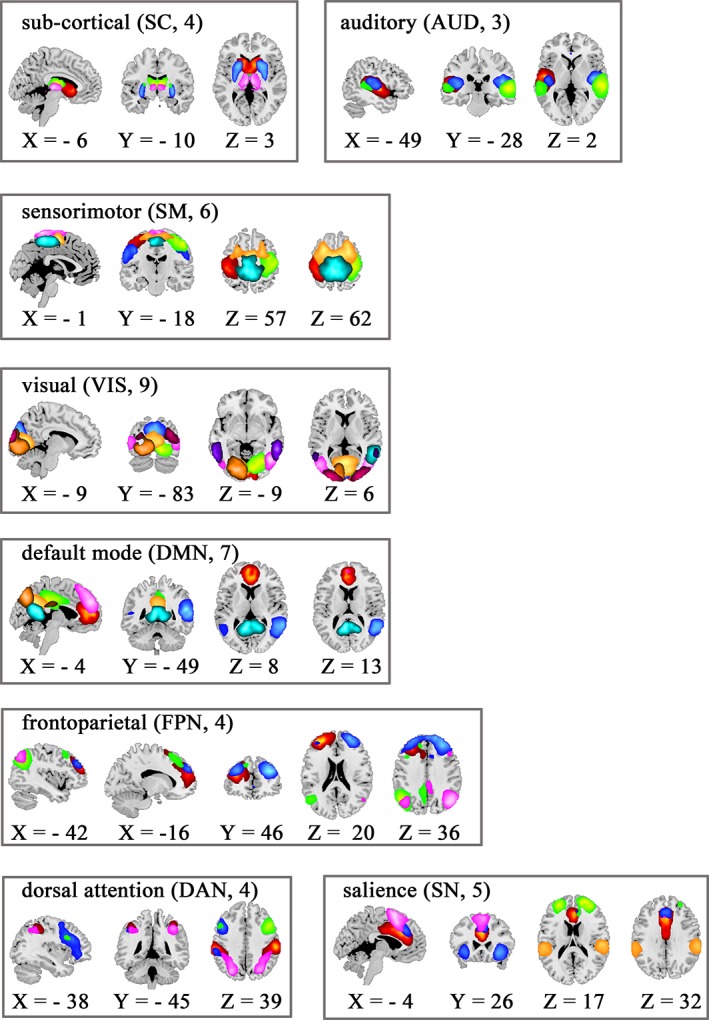
Composite maps of the 42 identified intrinsic connectivity networks, sorted into eight networks. Each color in the composite maps corresponds to a different independent component [Color figure can be viewed at http://wileyonlinelibrary.com]
In the static FC analysis within the cerebellum, patients with schizophrenia showed increased static FC between CBCc and CBCm compared with healthy controls (Figure 3a). Moreover, the increase in the static FCs in schizophrenic subjects were observed between CBCm and several cortical/subcortical networks, including FPN (left and right inferior parietal lobule [IPL]), SM network (left and right postcentral (SI), supplemental motor area [SMA]) and SC (dorsal thalamus [dTHA] and ventral thalamus [vTHA]). In addition, increased static FCs in Schizophrenia subjects were observed between CBCc and cortical/subcortical networks, including SC (dTHA and vTHA) and DMN (ventral medial prefrontal cortex [VMPFC], precuneus [PCUN]).
Figure 3.

The altered static and dynamic functional connectivity in schizophrenia. Within “(a)” red lines denotes increased static functional connectivity in patients compared with healthy controls. Within “(b)” blue lines denote decreased dynamic functional connectivity in patients. CBCc = cerebellar cognitive cluster; CBCm = cerebellar motor cluster; FPN = frontoparietal network; SC = subcortical; DMN = default model network; SM = sensorimotor network; L‐DLPFC = left dorsolateral prefrontal cortex; L/R‐IPL = left/right inferior parietal lobule; dTHA = dorsal thalamus; vTHA = ventral thalamus; VMPFC = ventral medial prefrontal cortex; PCUN = precuneus; L/R‐SI = left/right postcentral; SMA = supplemental motor area [Color figure can be viewed at http://wileyonlinelibrary.com]
The dynamic FC analysis when compared between the healthy controls and schizophrenic subjects exhibited a decrease in dynamic FC between CBCc and CBCm. Schizophrenia subjects also demonstrated decreased dynamic FCs (Figure 3b) between CBCm and cortical/subcortical networks including FPN (left dorsolateral prefrontal cortex [DLPFC]), DMN (PCUN) and SM networks (left and right SI, and SMA). Similar results were also observed for two other window lengths (i.e., 120 s and 140 s).
3.4. Significant dynamic FC and no noise confound
Similar with previous research (Hindriks et al., 2016), the reason for significant greater dynamic FC are twofold. First, across the schizophrenic or healthy subjects, the number of CBCm/CBCc‐component connections for which the null hypothesis of simple dynamic was rejected varied, such as CBCm in patients: between 0 and 7 (mean: 2 [4.7%]; SD:2.0); CBCm in controls: between 0 and 13 (mean: 4 [9.5%]; SD:3.3); CBCc in patients: between 0 and 7 (mean: 3 [7.1%]; SD:2.0); CBCc in controls: between 0 and 11 (mean: 3 [7.1%]; SD:2.8). Low probabilities were also observed for the consistent significant greater dynamic FCs across healthy subjects (CBCm: 4.76%–21.43%, mean: 11.39%; CBCc: 2.38%–26.19%, mean: 13.04%) or schizophrenic subjects (CBCm: 3.85%–30.77%, mean: 14.84%; CBCc: 3.85%–21.15%, mean: 11.59%). Second, the probabilities across subjects could be considerably increased on cerebellar regions by average test analysis including: CBCm in patients/controls: 26.19%/46.15%, CBCc in patients/controls: 28.57%/28.85%.
Lower number of greater dynamic FCs were found in schizophrenic subjects including: CBCm‐left SI (number of patients:1, number of controls: 8, p = .0048), CBCm‐right SI ([number of patients:2, number of controls: 9, p = .0033]), CBCm‐left DLPFC (number of patients:0, number of controls: 6, p = .0061). Moreover, the lower number of dynamic FC on cerebellar region (CBCm, p < .001) was also observed in schizophrenic subjects compared with healthy controls. Furthermore, there are no significant association between head motion or estimated physiological noise and dynamic FC fluctuations for any individual subject. Together, we found the FC between cerebellum and cortical/subcortical networks are significant abnormal dynamic in schizophrenia.
3.5. Abnormal coupling of structure–function
There was no significant coupling of structure–function within the cerebellum for schizophrenia subjects and healthy controls, respectively. The extent of difference in GM probability of cerebellum was differentially correlated with specific impaired FC of cerebellum‐cortical/subcortical network in schizophrenic subjects. A reduction in the negative relationship was observed between the extent of cerebellar difference and relative static FC (CBCm and dTHA, CBCm and IPL, Figure 4a,b) in patients compared with healthy controls. We also found a reduction in positive relationship between the extent of cerebellar difference and relative dynamic FC (CBCm, and left DLPFC, Figure 4c).
Figure 4.
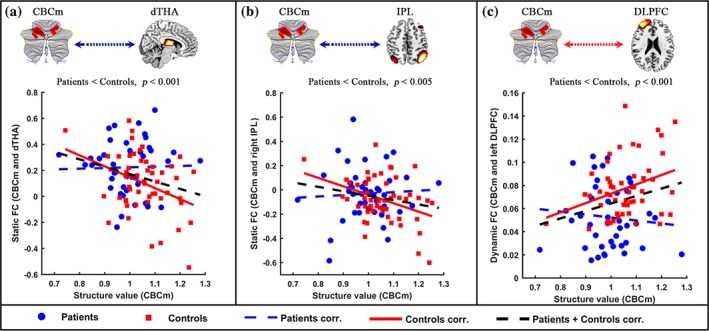
Reduction in structural‐functional relationships between cerebellum and cortical/subcortical networks in schizophrenic subjects. “(a)” denotes a reduction in negative relationship between structure value (CBCm) and static FC (CBCm and dTHA) in patients. ‘“(b)” denotes a reduction in negative relationship between structure value (CBCm) and static FC (CBCm and right IPL) in patients. ‘“(c)” denotes a reduction in positive relationship between structure value (CBCm) and dynamic FC (CBCm and left DLPFC) in patients. Blue node and line denote the patients, red node and line represent the healthy controls. CBCc = cerebellar cognitive cluster; CBCm = cerebellar motor cluster; DLPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobule; dTHA = dorsal thalamus [Color figure can be viewed at http://wileyonlinelibrary.com]
As a post hoc analysis to determine the influence of differences in GM probability within cerebellum to FC of cerebellum–thalamus, in patients with schizophrenia we observed positive relationship (r = .351, p < .01) between the extent of decreased dynamic FC of CBCm–CBCc and CBCm–dTHA. Whereas, this correlation was not apparent in the controls (r = .04, p = .738). The difference between these two correlation coefficients was also significant (p < .05) (Supporting Information Figure S1), suggesting that the effect of reduced GM probability within cerebellum may be associated with the modulation connectivity between CBCm and thalamus.
3.6. Relationship between clinical symptom and coupling of cerebellar structure–function
We observed an inverse correlation between PANSS‐positive score and the coupling score (dynamic FC/GM probability) in patients with schizophrenia. The correlation between CBCm and component within SM (SMA), CBCm and the component within FPN (left DLPFC) were found to be r = −.432, (p < .001, Figure 5a) and r = −.621, (p < .001, Figure 5b), respectively. No other significant correlations were found between the PANSS scores and measures of MRI (i.e., the differences in GM probability within cerebellum, altered static and dynamic FC), suggesting a selective relationship between positive symptom and impaired communication in FC of cerebellum‐cortical/subcortical networks.
Figure 5.
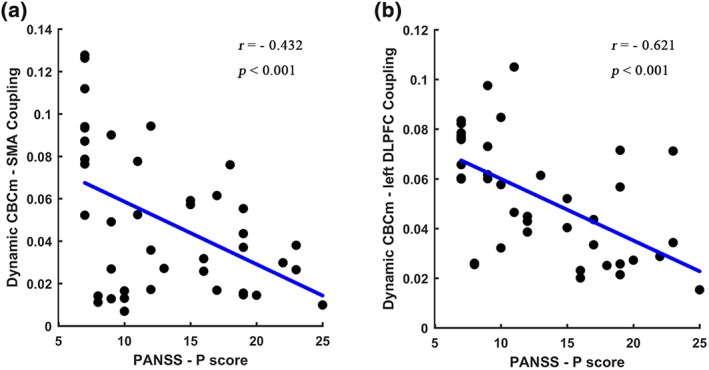
Relationship between positive symptom and structural‐functional coupling score. “(a)” represents a negative correlation between coupling score (structure value in CBCm and dynamic FC between CBCm and supplemental motor area [SMA]) and PANSS‐P score. “(b)” denotes a negative correlation between coupling score (structure value in CBCm and dynamic FC between CBCm and left dorsolateral prefrontal cortex [DLPFC]) and PANSS‐P score. CBCc = cerebellar cognitive cluster; CBCm = cerebellar motor cluster [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
This study combined resting‐state FC and VBM approaches to characterize the cerebellar GM in schizophrenia subjects and its influence on brain FC. Consistent with our hypothesis, significant decrease in GM probability of cerebellum, including regions in CBCm and CBCc territories, were apparent in schizophrenic subjects. These structural changes were accompanied by an increased static FC, a decreased dynamic FC within the cerebellum, and between the cerebellum and several cortical–subcortical networks. Furthermore, the evaluation of structural alteration and the FC with cortical/subcortical networks revealed the structure–function discoupling between CBCm and SC, and FPN in schizophrenia subjects. Aggravated discoupling was also related with the higher severity of positive symptom in schizophrenia subjects. Interestingly, the decreased dynamic FC within cerebellum (between CBCm and CBCc) was positively correlated to the dynamic FC between CBCm to thalamus. These abnormal coupling between difference GM probability within cerebellum and relative static and dynamic connectivity, suggest an important role for intrinsic structural changes in the cerebellum in schizophrenia.
4.1. Reduced GM of cerebellum underlying FC difference within cerebellum
Static FC of patients with schizophrenia demonstrated may serve as the candidate endophenotypes for schizophrenia (Chen et al., 2018; Guo et al., 2015; Stip et al., 2005), suggesting the possibility that the cerebellum overactivity is pathological rather than compensatory. Importantly, recent theories postulate that the cerebellum forms internal models for both motor and cognitive function, allowing for ongoing feedback resulting in well‐time and coordinated behaviors (Ito, 2008; Ramnani, 2006). In schizophrenia subjects, neuropathological studies have reported a reduction in size and density of Purkinje cells in the cerebellum (Katsetos, Hyde, & Herman, 1997), which balance the excitatory and inhibitory tone of cerebellar modulation signals. This is consistent with our results showing significant decreased GM probability in cerebellum (both CBCm and CBCc territories), accompanied by increased static and decreased dynamic FC between them. Altered FC within the cerebellum may be contributing to many deficits associated with the schizophrenia (Bernard & Mittal, 2015). Thus, the altered FC within cerebellum may suggest the dyscoordination in the internal models in schizophrenia and may reflect the underlying pathology associated with schizophrenia. We found that the altered intracerebellar connectivity was not associated with the differences in GM probability within cerebellum in our patient sample, suggesting the effect of cerebellar difference may not only be within the cerebellum.
4.2. Widespread effects of reduced GM within cerebellum on cortical network
This study furthermore demonstrated increased static and decreased dynamic FC of the cerebellum and several important cortical–subcortical networks, including DMN, FPN, SM, and SC. Many of previous studies have indicated that the abnormality in these networks influences the clinical symptom in schizophrenia (Chen et al., 2015; Kambeitz et al., 2015; Stegmayer et al., 2017). The cerebellum is connected to cortical networks, which coordinate or modulate aspects of cortical activity. The cerebellum also acts as a general purpose modulator that detects pattern changes and errors in both movements and thought, and can then provide inhibiting feedback to the cerebral cortex (Seidler et al., 2002).
In schizophrenia subjects, tasks normally performed by the cerebellum are presumed to respond in a flawed manner: instead of modulating, the increased inhibition of the cerebellum may misconnect the information arriving from cortical regions. The output will then be flawed (Andreasen et al., 1999; Picard, Amado, Mouchet‐Mages, Olie, & Krebs, 2008). In addition, in this study, the dynamic FC approach characterizes the temporal variability of FCs, which are not caused by non‐neuronal processes, between the cerebellum and cortical/subcortical networks. Recently, several studies have reported that brain regions involved in regulatory functions show higher dynamic FC, and may reflect flexible and adaptable functional coupling among brain networks (Bray, Arnold, Levy, & Iaria, 2015; Zhang et al., 2016). Decreased dynamic FCs of cerebellum‐cortical/subcortical networks may be related to the low interaction between the cerebellum and cortical/subcortical networks in schizophrenia subjects. Our results also demonstrates that PANSS‐positive symptom in patients is associated with coupling information (dynamic connectivity/GM probability) of several FCs of cerebellum–cortical/subcortical networks, such as cerebellum–thalamus, cerebellum‐FPN, and cerebellum‐SM. Taken together, our results indicate that lower variability and higher inhibiting connectivity of cerebellum–cortical/subcortical may contribute to the pathological interaction of cerebellum–cortical/subcortical network and symptom in schizophrenia. Specifically, the disconnection of the cerebellum in schizophrenia is network‐specific (Chen et al., 2013) and consistent with Chen's study, these significant differences were not found between the cerebellum and VN, AN, and SN through the static or dynamic FC analyses in schizophrenic subjects.
The structural and functional discoupling were observed between decreased GM probability of cerebellum and decreased dynamic FCs of CBCm‐FPN, as well as CBCm‐thalamus in patients with schizophrenia. CBCm was correlated with the sensorimotor, visual, auditory, salience networks and striatum, indicating that this cerebellum region is a functional hub with high connectivity to the cerebral cortex and BG/thalamus (Sang et al., 2012). Several studies have indicated that the cerebellum is involved in nonmotor functions associated with the FPN (Bostan, Dum, & Strick, 2013; Schmahmann, 1996). The disintegration of cerebellar‐FPN circuitry constitutes a key network for a variety of neuropsychological symptoms in schizophrenia (Wagner et al., 2015). Together, our results indicate that decreased GM probability within cerebellum may be an important determinant of alterations in the connectivity between the cerebellum and cortical networks, suggesting a disruption of the cerebellar–thalamus–cortical loop in patients with schizophrenia. Our finding underscores a more causative role for impaired cerebellum–cortical/subcortical connectivity in the pathophysiology of schizophrenia positive symptom.
4.3. Involvement of the difference within cerebellum and FC of cerebellum–thalamus
Recent studies have indicated that the cerebellum and the BG/thalamus are reciprocally connected network concerned with motor and nonmotor behaviors (Akkal, Dum, & Strick, 2007; Middleton & Strick, 2000). These two major systems are likely to interact as part of their normal function. Such interactions imply that the abnormal activity in one system would have important effects on the other system (Bostan et al., 2013; Jiang et al., 2018; O'Doherty, Dayan, Friston, Critchley, & Dolan, 2003). The effect of decreased GM probability in cerebellum in patients, will most likely lead to an altered connectivity, between cerebellum and BG/thalamus, which may be associated with the abnormal internal models of cerebellum. Consistent with these studies, we observed positive relationship between decreased dynamic FC of intracerebellar and dynamic FC of cerebellum–thalamus. The altered FC of cerebellar‐thalamus contribution to schizophrenia seems limited or indirect (Bernard, Goen, & Maldonado, 2017; Losak et al., 2016). Our study found that the altered connectivity in patients, between cerebellar systems and thalamus, suggesting that the focal structural change in cerebellum contribute to pathological cerebellar‐thalamus connectivity. Importantly, the loops that link cerebellum with cortical cortex have been considered to be anatomically connected through thalamus (Dum & Strick, 2003). Thus, altered cerebellar‐thalamus connectivity may play a crucial role in this distributed circuit and contribute to the functional dysfunction between cerebellar and cortical networks in schizophrenia.
4.4. Methodological considerations
While our findings provide new insight into the role of brain FC in understanding the effects of reduced GM of cerebellum in schizophrenia, several methodological issues need to be further addressed. First, a recent study has indicated that there is a limitation in generating the surrogate null hypothesis (Hindriks et al., 2016) during dynamic FC testing, such as constrained surrogate data used in this study or in the studies of Chang and Glover (2010) and Zalesky et al. (2014). Through sample dynamic FC testing the absence of dynamic FC could not imply stationarity of FC. Thus, when dynamic FC is detected using these surrogate data, the only conclusion could be draw is that the FC is nonstationary. Besides, several studies have reported that the relationships between fluctuations of FC and measures derived from electrophysiological recordings as well as behavioral measures (Di & Biswal, 2013; Di & Biswal, 2015; Tagliazucchi, von Wegner, Morzelewski, Brodbeck, & Laufs, 2012). Together, although the absence of dynamic FC cannot be interpreted as dynamic FC because of specific statistical meaning, these observed fluctuations of FC are informative. In this study, differences in dynamic FC was investigated between two groups of subjects, and because there is no known non‐neural factors that are different between the two groups, one can thus ascertain that these differences in dynamic FC are due to neuronal factors. Specifically, because we observed decreased dynamic FC on CBCm in patients, we believe these are due to neuronal factors. Second, dynamic FC was computed using a sliding window correlation approach. Currently, there are no formal consensuses regarding the window length. Final, as noted previously, what dynamic FC represents in terms of neurocognitive functioning is not completely understood. As the field of dynamic FC grows, we may gain a better understanding of how these parameter and properties of brain functioning relates to psychopathology.
5. CONCLUSIONS
In conclusion, by combining resting‐state FC and VBM approaches, our findings reveal the prominent functional and focal structural changes within cerebellum in patients with schizophrenia. Furthermore, we show that the reduced GM within cerebellum has significant ramification for the integrity of cortical and subcortical networks, highlighting the fact that cerebellar abnormalities in schizophrenia are likely to have far‐reaching effects particularly on the pathophysiology of schizophrenia positive symptom. These findings provided new insights into the effects of abnormal cerebellum at the level of large‐scale networks connectivity and might help to design new treatment strategies. Continued understanding of its role in schizophrenia remains an important goal in unraveling the complex, multisystem nature of this disorder.
Supporting information
FIGURE S1 The relationship between dynamic FC of CBCm‐CBCc and CBCm‐ dorsal thalamus in patients with schizophrenia. Blue node and line denote the patients, red node and line represent the healthy controls.
ACKNOWLEDGMENTS
This scientific work was supported by grants from the National Nature Science Foundation of China (grant numbers 81771822, 81861128001, 81471638, 81571759, 81660233), the “111” project of China (grant number B12027), Project of Scientific Expenses of the Ministry of Education (2017PT14) and Sichuan Province Science and Technology Support Project (Nos. 2017SZ0004 and 2017HH0001). We thank Dr. Xi Chen for her helping to collect data. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
He H, Luo C, Luo Y, et al. Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Hum Brain Mapp. 2019;40:517–528. 10.1002/hbm.24391
Funding information National Nature Science Foundation of China, Grant/Award Numbers: 81771822, 81861128001, 81660233, 81571759, 81471638; Sichuan Province Science and Technology Support Project, Grant/Award Numbers: No.2017HH0001No.2017SZ0004, 2017HH0001, 2017SZ0004; Project of Scientific Expenses of the Ministry of Education, Grant/Award Number: 2017PT14; “111” project of China, Grant/Award Number: B12027
Contributor Information
Cheng Luo, Email: chengluo@uestc.edu.cn.
Bharat B. Biswal, Email: bbiswal@gmail.com.
REFERENCES
- Akkal, D. , Dum, R. P. , & Strick, P. L. (2007). Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 27, 10659–10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, N. C. , Nopoulos, P. , O'Leary, D. S. , Miller, D. D. , Wassink, T. , & Flaum, M. (1999). Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biological Psychiatry, 46, 908–920. [DOI] [PubMed] [Google Scholar]
- Anticevic, A. , Cole, M. W. , Repovs, G. , Murray, J. D. , Brumbaugh, M. S. , Winkler, A. M. , … Glahn, D. C. (2014). Characterizing thalamo‐cortical disturbances in schizophrenia and bipolar illness. Cerebral Cortex, 24, 3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters, J. H. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2014). Bridging the gap between functional and anatomical features of cortico‐cerebellar circuits using meta‐analytic connectivity modeling. Human Brain Mapping, 35, 3152–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, J. A. , Goen, J. R. M. , & Maldonado, T. (2017). A case for motor network contributions to schizophrenia symptoms: Evidence from resting‐state connectivity. Human Brain Mapping, 38, 4535–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, J. A. , & Mittal, V. A. (2015). Dysfunctional activation of the cerebellum in schizophrenia: A functional neuroimaging meta‐analysis. Clinical Psychological Science: A Journal of the Association for Psychological Science, 3, 545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine : Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Bostan, A. C. , Dum, R. P. , & Strick, P. L. (2013). Cerebellar networks with the cerebral cortex and basal ganglia. Trends in Cognitive Sciences, 17, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, S. , Arnold, A. E. G. F. , Levy, R. M. , & Iaria, G. (2015). Spatial and temporal functional connectivity changes between resting and attentive states. Human Brain Mapping, 36, 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , Castellanos, A. , Diaz, J. C. , & Yeo, B. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. H. (2009). Relationship between respiration, end‐tidal CO2, and BOLD signals in resting‐state fMRI. NeuroImage, 47, 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. H. (2010). Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage, 50, 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , Metzger, C. D. , Glover, G. H. , Duyn, J. H. , Heinze, H. J. , & Walter, M. (2013). Association between heart rate variability and fluctuations in resting‐state functional connectivity. NeuroImage, 68, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Duan, M. J. , Xie, Q. K. , Lai, Y. X. , Dong, L. , Cao, W. F. , … Luo, C. (2015). Functional disconnection between the visual cortex and the sensorimotor cortex suggests a potential mechanism for self‐disorder in schizophrenia. Schizophrenia Research, 166, 151–157. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Ji, G. J. , Zhu, C. , Bai, X. , Wang, L. , He, K. , … Wang, K. (2018). Neural correlates of auditory verbal hallucinations in schizophrenia and the therapeutic response to theta‐burst transcranial magnetic stimulation. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. L. , Tu, P. C. , Lee, Y. C. , Chen, Y. S. , Li, C. T. , & Su, T. P. (2013). Resting‐state fMRI mapping of cerebellar functional dysconnections involving multiple large‐scale networks in patients with schizophrenia. Schizophrenia Research, 149, 26–34. [DOI] [PubMed] [Google Scholar]
- Damaraju, E. , Allen, E. A. , Belger, A. , Ford, J. M. , McEwen, S. , Mathalon, D. H. , … Calhoun, V. D. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin, 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond, J. E. , & Fiez, J. A. (1998). Neuroimaging studies of the cerebellum: Language, learning and memory. Trends in Cognitive Sciences, 2, 355–362. [DOI] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2013). Modulatory interactions of resting‐state brain functional connectivity. PLoS One, 8, e71163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2015). Dynamic brain functional connectivity modulated by resting‐state networks. Brain Structure & Function, 220, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen, J. , Balsters, J. H. , Flavell, J. , Cussans, E. , & Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage, 46, 39–46. [DOI] [PubMed] [Google Scholar]
- Duan, M. , Chen, X. , He, H. , Jiang, Y. , Jiang, S. , Xie, Q. , … Yao, D. (2015). Altered basal ganglia network integration in schizophrenia. Frontiers in Human Neuroscience, 9, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum, R. P. , & Strick, P. L. (2003). An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. Journal of Neurophysiology, 89, 634–639. [DOI] [PubMed] [Google Scholar]
- Edwards, C. R. , Newman, S. , Bismark, A. , Skosnik, P. D. , O'Donnell, B. F. , Shekhar, A. , … Hetrick, W. P. (2008). Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Research: Neuroimaging, 162, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Guo, W. , Liu, F. , Zhang, Z. , Liu, G. , Liu, J. , Yu, L. , … Zhao, J. (2015). Increased cerebellar functional connectivity with the default‐mode network in unaffected siblings of schizophrenia patients at rest. Schizophrenia Bulletin, 41, 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, C. , Kamdar, N. , Nguyen, D. , Prater, K. , Beckmann, C. F. , Menon, V. , & Greicius, M. D. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29, 8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, E. C. , Battaglia, D. , Spiegler, A. , Deco, G. , & Jirsa, V. K. (2015). Functional connectivity dynamics: Modeling the switching behavior of the resting state. NeuroImage, 105, 525–535. [DOI] [PubMed] [Google Scholar]
- He, H. , Yang, M. , Duan, M. , Chen, X. , Lai, Y. , Xia, Y. , … Yao, D. (2017). Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Frontiers in Neuroscience, 11, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks, R. , Adhikari, M. H. , Murayama, Y. , Ganzetti, M. , Mantini, D. , Logothetis, N. K. , & Deco, G. (2016). Can sliding‐window correlations reveal dynamic functional connectivity in resting‐state fMRI? (vol 127, pg 242, 2016). NeuroImage, 132, 115–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , … Chang, C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, M. (2006). Cerebellar circuitry as a neuronal machine. Progress in Neurobiology, 78, 272–303. [DOI] [PubMed] [Google Scholar]
- Ito, M. (2008). Control of mental activities by internal models in the cerebellum. Nature Reviews. Neuroscience, 9, 304–313. [DOI] [PubMed] [Google Scholar]
- James, A. C. , James, S. , Smith, D. M. , & Javaloyes, A. (2004). Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent‐onset schizophrenia. American Journal of Psychiatry, 161, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. , Luo, C. , Li, X. , Duan, M. , He, H. , Chen, X. , … Yao, D. (2018). Progressive reduction in gray matter in patients with schizophrenia assessed with MR imaging by using causal network analysis. Radiology, 287, 633–642. [DOI] [PubMed] [Google Scholar]
- Kambeitz, J. , Kambeitz‐Ilankovic, L. , Leucht, S. , Wood, S. , Davatzikos, C. , Malchow, B. , … Koutsouleris, N. (2015). Detecting neuroimaging biomarkers for schizophrenia: A meta‐analysis of multivariate pattern recognition studies. Neuropsychopharmacology, 40, 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. , Wang, L. , Yan, C. G. , Wang, J. H. , Liang, X. , & He, Y. (2011). Characterizing dynamic functional connectivity in the resting brain using variable parameter regression and Kalman filtering approaches. NeuroImage, 56, 1222–1234. [DOI] [PubMed] [Google Scholar]
- Katsetos, C. D. , Hyde, T. M. , & Herman, M. M. (1997). Neuropathology of the cerebellum in schizophrenia–An update: 1996 and future directions. Biological Psychiatry, 42, 213–224. [DOI] [PubMed] [Google Scholar]
- Kim, J. J. , Kim, D. J. , Kim, T. G. , Seok, J. H. , Chun, J. W. , Oh, M. K. , & Park, H. J. (2007). Volumetric abnormalities in connectivity‐based subregions of the thalamus in patients with chronic schizophrenia. Schizophrenia Research, 97, 226–235. [DOI] [PubMed] [Google Scholar]
- Kiviniemi, V. , Starck, T. , Remes, J. , Long, X. Y. , Nikkinen, J. , Haapea, M. , … Tervonen, O. (2009). Functional segmentation of the brain cortex using high model order group PICA. Human Brain Mapping, 30, 3865–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidi, C. , d'Albis, M. A. , Wessa, M. , Linke, J. , Phillips, M. L. , Delavest, M. , … Houenou, J. (2015). Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatrica Scandinavica, 131, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. H. , Farrow, T. F. , Parks, R. W. , Newton, L. D. , Mir, N. U. , Egleston, P. N. , … Woodruff, P. W. (2007). Increased cerebellar vermis white‐matter volume in men with schizophrenia. Journal of Psychiatric Research, 41, 645–651. [DOI] [PubMed] [Google Scholar]
- Leggio, M. , & Molinari, M. (2015). Cerebellar sequencing: A trick for predicting the future. Cerebellum, 14, 35–38. [DOI] [PubMed] [Google Scholar]
- Leonardi, N. , & Van De Ville, D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage, 104, 430–436. [DOI] [PubMed] [Google Scholar]
- Loeber, R. T. , Cintron, C. M. , & Yurgelun‐Todd, D. A. (2001). Morphometry of individual cerebellar lobules in schizophrenia. The American Journal of Psychiatry, 158, 952–954. [DOI] [PubMed] [Google Scholar]
- Losak, J. , Huttlova, J. , Lipova, P. , Marecek, R. , Bares, M. , Filip, P. , … Kasparek, T. (2016). Predictive motor timing and the cerebellar vermis in schizophrenia: An fMRI study. Schizophrenia Bulletin, 42, 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu, O. , Barakat, M. , Laventure, S. , Debas, K. , Proulx, S. , Luck, D. , & Stip, E. (2013). The incidence and nature of cerebellar findings in schizophrenia: A quantitative review of fMRI literature. Schizophrenia Bulletin, 39, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science, 266, 458–461. [DOI] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research. Brain Research Reviews, 31, 236–250. [DOI] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (2001). Cerebellar projections to the prefrontal cortex of the primate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 21, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget, T. , Doan, N. T. , Alnaes, D. , Kaufmann, T. , Cordova‐Palomera, A. , Lagerberg, T. V. , … Westlye, L. T. (2017). Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega‐analysis of 983 patients and 1349 healthy controls. Mololecular Psychiatry, 23, 1512–1520. [DOI] [PubMed] [Google Scholar]
- O'Callaghan, C. , Hornberger, M. , Balsters, J. H. , Halliday, G. M. , Lewis, S. J. G. , & Shine, J. M. (2016). Cerebellar atrophy in Parkinson's disease and its implication for network connectivity. Brain, 139, 845–855. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. P. , Dayan, P. , Friston, K. , Critchley, H. , & Dolan, R. J. (2003). Temporal difference models and reward‐related learning in the human brain. Neuron, 38, 329–337. [DOI] [PubMed] [Google Scholar]
- Picard, H. , Amado, I. , Mouchet‐Mages, S. , Olie, J. P. , & Krebs, M. O. (2008). The role of the cerebellum in schizophrenia: An update of clinical, cognitive and functional evidences. Schizophrenia Bulletin, 34, 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard, D. , & Theiler, J. (1994). Generating surrogate data for time‐series with several simultaneously measured variables. Physical Review Letters, 73, 951–954. [DOI] [PubMed] [Google Scholar]
- Ramnani, N. (2006). The primate cortico‐cerebellar system: Anatomy and function. Nature Reviews. Neuroscience, 7, 511–522. [DOI] [PubMed] [Google Scholar]
- Rashid, B. , Arbabshirani, M. R. , Damaraju, E. , Cetin, M. S. , Miller, R. , Pearlson, G. D. , & Calhoun, V. D. (2016). Classification of schizophrenia and bipolar patients using static and dynamic resting‐state fMRI brain connectivity. NeuroImage, 134, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs, G. , Csernansky, J. G. , & Barch, D. M. (2011). Brain network connectivity in individuals with schizophrenia and their siblings. Biological Psychiatry, 69, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang, L. , Qin, W. , Liu, Y. , Han, W. , Zhang, Y. T. , Jiang, T. Z. , & Yu, C. S. (2012). Resting‐state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage, 61, 1213–1225. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. (1996). From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping, 4, 174–198. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Doyon, J. , McDonald, D. , Holmes, C. , Lavoie, K. , Hurwitz, A. S. , … Petrides, M. (1999). Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage, 10, 233–260. [DOI] [PubMed] [Google Scholar]
- Seidler, R. D. , Purushotham, A. , Kim, S. G. , Ugurbil, K. , Willingham, D. , & Ashe, J. (2002). Cerebellum activation associated with performance change but not motor learning. Science, 296, 2043–2046. [DOI] [PubMed] [Google Scholar]
- Shirer, W. R. , Ryali, S. , Rykhlevskaia, E. , Menon, V. , & Greicius, M. D. (2012). Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cerebral Cortex, 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer, K. , Bohlhalter, S. , Vanbellingen, T. , Federspiel, A. , Wiest, R. , Muri, R. M. , … Walther, S. (2017). Limbic interference during social action planning in schizophrenia. Schizophrenia Bulletin, 44, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, K. E. , Friston, K. J. , & Frith, C. D. (2009). Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self‐monitoring. Schizophrenia Bulletin, 35, 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stip, E. , Fahim, C. , Liddle, P. , Mancini‐Marie, A. , Mensour, B. , Bentaleb, L. A. , & Beauregard, M. (2005). Neural correlates of sad feelings in schizophrenia with and without blunted affect. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 50, 909–917. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi, E. , von Wegner, F. , Morzelewski, A. , Brodbeck, V. , & Laufs, H. (2012). Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Frontiers in Human Neuroscience, 6, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, G. , De la Cruz, F. , Schachtzabel, C. , Gullmar, D. , Schultz, C. C. , Schlosser, R. G. , … Koch, K. (2015). Structural and functional dysconnectivity of the fronto‐thalamic system in schizophrenia: A DCM‐DTI study. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 66, 35–45. [DOI] [PubMed] [Google Scholar]
- Whalley, H. C. , Simonotto, E. , Flett, S. , Marshall, I. , Ebmeier, K. P. , Owens, D. G. , … Lawrie, S. M. (2004). fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain, 127, 478–490. [DOI] [PubMed] [Google Scholar]
- Yang, G. J. , Murray, J. D. , Repovs, G. , Cole, M. W. , Savic, A. , Glasser, M. F. , … Anticevic, A. (2014). Altered global brain signal in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 111, 7438–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , Cocchi, L. , Gollo, L. L. , & Breakspear, M. (2014). Time‐resolved resting‐state brain networks. Proceedings of the National Academy of Sciences of the United States of America, 111, 10341–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Cheng, W. , Liu, Z. , Zhang, K. , Lei, X. , Yao, Y. , … Feng, J. (2016). Neural, electrophysiological and anatomical basis of brain‐network variability and its characteristic changes in mental disorders. Brain, 139, 2307–2321. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Zheng, J. J. , Fan, X. D. , Guo, X. F. , Guo, W. B. , Yang, G. , … Lv, L. X. (2015). Dysfunctional resting‐state connectivities of brain regions with structural deficits in drug‐naive first‐episode schizophrenia adolescents. Schizophrenia Research, 168, 353–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 The relationship between dynamic FC of CBCm‐CBCc and CBCm‐ dorsal thalamus in patients with schizophrenia. Blue node and line denote the patients, red node and line represent the healthy controls.


