Abstract
Depression is the leading cause of years lost due to disability worldwide. Still, the mechanisms underlying its development are not well understood. This study aimed to evaluate white‐matter properties associated with depressive symptomatology in young adulthood and their developmental origins. Diffusion tensor imaging and assessment of depressive symptomatology were conducted in 128 young adults (47% male, age 23–24) from a prenatal birth cohort (European Longitudinal Study of Pregnancy and Childhood). For a subset of these individuals, the database included information on prenatal stress (n = 93) and depressive symptoms during adolescence (assessed repeatedly at age 15 and 19). Depressive symptoms in young adulthood were associated with lower fractional anisotropy in the left and right cingulum and higher fractional anisotropy in the right corticospinal tract and superior longitudinal fasciculus. Further analyses revealed that prenatal stress and depressive symptomatology during adolescence were independent predictors of altered white‐matter properties in the cingulum in young adulthood. We conclude that typically developing young adults with more depressive symptoms already exhibit tract‐specific alterations in white‐matter properties and that prenatal stress and depressive symptomatology during adolescence might contribute to their development.
Keywords: adolescence, cohort studies, depression, diffusion tensor imaging, life stress, prenatal development, white matter
1. INTRODUCTION
Depression is the leading cause of years lost due to disability worldwide (Ustun, Ayuso‐Mateos, Chatterji, Mathers, & Murray, 2004). It has an early onset, the lifelong trajectory of recurrent episodes with increasing severity, and is known for progressive treatment resistance (Kessler et al., 2005; Moylan, Maes, Wray, & Berk, 2013). The lifetime prevalence of depression is up to 17% (Kessler et al., 2005). Still, the developmental origins of this common and debilitating disease are poorly understood (Kendler, Karkowski, & Prescott, 1999).
Growing evidence supports disturbed neural connectivity in the pathogenesis of depression. Structural magnetic resonance imaging (MRI) studies reported an association between higher rate and severity of white‐matter hyperintensities and more depressive symptoms (Coffey et al., 1993; Coffey, Figiel, Djang, Saunders, & Weiner, 1989; de Groot et al., 2000; Howard et al., 1993; Lenze, Cross, McKeel, Neuman, & Sheline, 1999; Rabins, Pearlson, Aylward, Kumar, & Dowell, 1991; Zubenko et al., 1990). White‐matter hyperintensities were also associated with lower rates of treatment response in both younger (Iosifescu et al., 2006) and older (Gunning‐Dixon et al., 2010) patients with major depressive disorder (MDD). Further research using diffusion tensor imaging (DTI) provided evidence for white‐matter tract disruptions in MRI hyperintensities (vs. normal tissue) (Taylor et al., 2001). MRI hyperintensities were characterized by higher apparent diffusion coefficient (ADC) and lower anisotropy (Taylor et al., 2001). Subsequently, numerous studies used DTI and reported abnormalities in fractional anisotropy (FA) in young, middle‐aged and older adults with MDD (Chen et al., 2017; Kieseppa et al., 2010; Liao et al., 2013; Ma et al., 2007; Shimony et al., 2009). Hoogenboom et al. (2014) showed that abnormalities in the structural properties of white‐matter tracts underlie persistent, treatment‐resistant depression. While lower FA among MDD patients is relatively consistent and has been also linked to depression severity and illness duration (Chen et al., 2016; Zou et al., 2008), the specific locations of these FA reductions are rather inconsistent (Chen et al., 2016). Lower FA among MDD patients vs. controls was reported in the cingulate cortex (Kieseppa et al., 2010; Zhu et al., 2011), dorsolateral prefrontal cortex (Blood et al., 2010; Korgaonkar et al., 2011), corpus callosum (Kieseppa et al., 2010), superior longitudinal fasciculus (Wu et al., 2011) as well as other regions. A meta‐analysis of diffusion tensor imaging studies in MDD patients vs. healthy controls reported reductions in FA in the left superior longitudinal fasciculus (Murphy & Frodl, 2011). Importantly, Ghazi Sherbaf, Same, Ashraf‐Ganjouei, and Aarabi (2018), conducted the first DTI study to assess minor forms of depression and demonstrated that depressive symptoms and the underlying white‐matter alterations exist in a continuum. Therefore, studying white‐matter alterations in members of a birth cohort presenting subthreshold forms of depression might offer relevant insights into the developmental origins of the disease.
Prenatal stress (Goldstein, Handa, & Tobet, 2014; Mareckova et al., 2018) and depression during adolescence (Copeland, Wolke, Shanahan, & Costello, 2015) are two of the most important predictors of depression in later life. Longitudinal data from the Great Smoky Mountains study demonstrated that participants with a psychiatric disorder during adolescence had 5.8 higher odds of developing psychiatric problems in adulthood and that participants with subthreshold psychiatric problems in their adolescence had three times higher odds of adverse outcomes in adulthood (Copeland et al., 2015). According to the prenatal stress model, stress during gestation programs functioning of the hypothalamic–pituitary–adrenal (HPA) axis (Bilbo & Schwarz, 2009; Bock, Rether, Groger, Xie, & Braun, 2014; Entringer, Kumsta, Hellhammer, Wadhwa, & Wust, 2009; Weinstock, 2008; Yong Ping et al., 2015) in the offspring and, in turn, subsequent emotional development and long‐term psychiatric vulnerability (Goldstein et al., 2014). This developmental model has been supported by animal research as well as a number of epidemiological studies in humans. Preclinical research on prenatally stressed animals demonstrated increased anxiety and depressive‐like behaviors (Babri, Doosti, & Salari, 2014; Enayati et al., 2012; Khan et al., 2014; Lin & Wang, 2014; Markham & Koenig, 2011; Walker, Nakamura, & Hodgson, 2010). Research in humans showed that prenatal exposure to maternal cortisol and psychosocial stress predicted cortisol response and rate of behavioral recovery in the infant after a heel‐stick blood draw (Davis, Glynn, Waffarn, & Sandman, 2011). Higher exposure to maternal cortisol and psychosocial stress were also associated with higher anxiety in preadolescent children (Davis & Sandman, 2012). Large epidemiological studies showed that the offspring of mothers with depressive, anxious and stress symptomatology during pregnancy (Betts, Williams, Najman, & Alati, 2015) and the offspring of mothers who experienced severe stress during pregnancy (Park et al., 2014) had higher risk for psychopathology in adulthood.
Neuroimaging studies reported associations between prenatal stress and alterations in gray‐matter volume, including lower volume of prefrontal (Buss et al., 2012a; Mareckova et al., 2018; Sandman, Buss, Head, & Davis, 2015) and anterior cingulate cortex (Mareckova et al., 2018), and higher volume of amygdala (Buss et al., 2012a, 2012b). The offspring of mothers with more depressive symptoms during pregnancy had stronger functional connectivity of the amygdala with several cortical regions at the age of 6 months (Qiu et al., 2015). Literature on the association between prenatal stress and white‐matter properties is rather scarce. In rats, maternal restraint stress during gestation appears to be associated with “hypermyelination” in the offspring brain (Wiggins & Gottesfeld, 1986). In humans, prenatal stress was associated with lower fractional anisotropy in a number of brain regions (Rifkin‐Graboi et al., 2015). Further research focused on the uncinate fasciculus, a white‐matter tract containing fibers running between the amygdala and prefrontal cortex; this work found an association between greater experience of stressful life events in the pregnant mother and higher fractional anisotropy in the right and lower perpendicular diffusivity in the left uncinate fasciculus (Sarkar et al., 2014). Jensen et al. (2017) provided evidence for independent effects of prenatal and postnatal stress on white‐matter properties in the corpus callosum during distinct neurodevelopmental periods. The fact that a majority of these studies considered only a particular hypothesized tract (Jensen et al., 2017; Sarkar et al., 2014) calls for further research that would take a whole‐brain approach.
We conducted a neuroimaging follow‐up of a prenatal birth cohort to assess the developmental origins of the depression‐related white‐matter properties in young adulthood. We took a whole‐brain approach and focused on two periods critical for brain development—the prenatal period and adolescence (Giedd, 1999; UNICEF, 2017)—and assessed (a) the impact of prenatal stress, defined as stressful life events experienced by the mother during the first half of pregnancy, and (b) the impact of depressive symptoms experienced at 15 and 19 years of age on the depression‐related white‐matter properties. We hypothesized that prenatal stress and depression during adolescence will be associated with lower fractional anisotropy across the depression‐related white‐matter tracts. Since previous research reported sex differences in the offspring outcomes following exposure to maternal stress during pregnancy (Buss et al., 2012a, 2012b) as well as sex differences in white‐matter microstructure in adults (Szeszko et al., 2003), we also evaluated the potential interaction between prenatal stress and sex.
2. METHODS
2.1. Participants
Typically developing young adults from the European Longitudinal Study of Pregnancy and Childhood, the Czech Republic (ELSPAC–CZ; Golding, 1989; Piler et al., 2016), a prenatal cohort from the Czech Republic whose members were born between 1991 and 1992, were invited to participate in a neuroimaging study Biomarkers and underlying mechanisms of vulnerability to depression (VULDE; FP7‐IEF‐2013) at the Central European Institute of Technology at Masaryk University. DTI data were collected for a total of 128 individuals (60 males, 68 females). Using 80% power, p = .05 and two‐tailed tests, this sample size would allow us to detect medium (Cohen's d = 0.25) and higher effects. All participants were 23 or 24 years old and of White Caucasian background. After the procedures had been fully explained, all participants provided written informed consent including the agreement to merge data from VULDE with their historic data from ELSPAC‐CZ. The whole VULDE study was conducted in accordance with the Declaration of Helsinki and was approved by ELSPAC Ethics Committee.
2.2. Procedures
All 128 participants were scanned using a 3 T Siemens Prisma MRI scanner and answered the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) in 2015. Questionnaires on stressful life events during the first half of pregnancy (40 questions on stressful events such as break up or divorce with the partner, consideration of abortion, violence, serious illness or death in the family or financial difficulties answered by a 5‐point Likert scale; see Supporting Information) were answered by a subsample of the mothers of our participants (n = 93; 40 men, 53 women) at the time of their pregnancy (20th week of gestation) between 1990 and 1992. Questionnaires on self‐reported depressive symptomatology during adolescence (15 questions answered on a 3‐point Likert scale; see Supporting Information) were assessed repeatedly in a subset of these participants at the age of 15 (n = 59; 28 men, 31 women) and 19 (n = 38; 12 men, 26 women). Using 80% power, p = .05 and two‐tailed tests, this sample size would allow us to detect medium (Cohen's d = 0.3 or higher) effects of prenatal stress, medium (Cohen's d = 0.35 or higher) effects of depression at 15, and large (Cohen's d = 0.45 or higher) effects of depression at 19. The timeline of the study is presented in Figure 1 .
Figure 1.
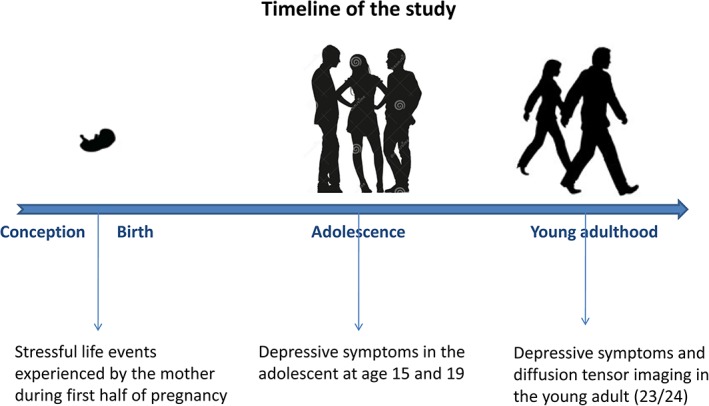
Study timeline [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. MRI acquisition
Diffusion tensor images, which allow to measure diffusion of water and reflect the microstructural organization of white matter, were acquired on 3 T Siemens Prisma MRI scanner with 64 channel head/neck coil and the following acquisition parameters: 64 diffusion directions, voxel size 2 × 2 × 2 mm, field of view read 256 mm, number of slices = 73, slice thickness = 2 mm, TR = 7,800 ms, TE = 69 ms, acceleration factor PE = 2, ref. lines PE = 38, b = 1,000.
2.4. Analyses
Fiber tractography was conducted in Exlore DTI, version 4.8.3 (Leemans & Jones, 2009) for MATLAB and the following corrections to the diffusion‐weighted MRI were applied: head motion, eddy current distortions, and EPI deformations (Leemans & Jones, 2009). Diffusion tensors were estimated with the REKINDLE approach [kappa = 6; Tax, Otte, Viergever, Dijkhuizen, & Leemans, 2015] using an iteratively reweighted linear least squares approach (Veraart, Sijbers, Sunaert, Leemans, & Jeurissen, 2013). Next, we constructed probabilistic maps of the following white‐matter tracts: cingulum (CG), corticospinal tract (CST), fornix (FX), genu of corpus callosum (GCC), inferior fronto‐occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), splenium of corpus callosum (SCC), superior fronto‐occipital fasciculus (SFO), superior longitudinal fasciculus (SLF), uncinate fasciculus (UNC). These tracts were selected according to a priori information on tract location, as the anatomy of these tracts is well known (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008; Wakana et al., 2007). Similarly to Jensen et al. (2017), these probabilistic maps were thresholded at 50%, binarized, and used as masks to calculate the mean fractional anisotropy (FA) values for each tract in each individual by projecting the probabilistic maps back to the native space of each individual to avoid interpolation artifacts (i.e., the transformation matrix consisted of the concatenation of nonlinear transformations from atlas space to native T1W image and then from T1W to FA space). The FA values describe the degree of anisotropy of the diffusion process and lower FA reflects lower movement of water in a single direction (Paus, 2016). This has implications for potential white‐matter tract disruptions because lack of water movement in a single direction was associated with axonal or myelin damage (Aung, Mar, & Benzinger, 2013).
All statistical analyses were done in JMP version 10.0.0 (SAS Institute Inc., Cary, NC). First, we log‐transformed the questionnaire‐based variables to follow a normal distribution (see the means and standard deviations for the raw variables in Supporting Information Table S1). Next, a full factorial general linear model assessed the relationship between the current depressive symptoms (a single BDI score), sex (2), and white‐matter tract (18) on fractional anisotropy in the 128 young adults. Further analyses assessed the developmental origins of the depression‐related white‐matter properties identified in Step 1. As detailed in the Procedures, the effect of prenatal stress could have been assessed in a sample of 93 participants, the effects of depression at the age of 15 in 59, and the effects of depression at the age of 19 in 38 participants. Therefore, we first performed separate linear regressions assessing the effects of prenatal stress and depression during adolescence on fractional anisotropy in the four depression‐related tracts. Sex was again considered as a potential moderator of these relationships. A final multiple regression then assessed the independence of these effects.
3. RESULTS
3.1. Demographic characteristics of the sample
Demographic characteristics of the full sample as well as the sub‐sample for whom prenatal stress data were available are provided in Supporting Information Tables S2 and S3, respectively. There was no relationship between the demographic variables (handedness, BMI, education, birth weight, maternal age at birth, and maternal education) and the prenatal stress, depressive symptomatology or fractional anisotropy in young adulthood (Supporting Information Table S4). Despite the narrow age range in our study (all 23 or 24 years old), there was an interaction between age and the type of white‐matter tract on fractional anisotropy (F [17,2,142] = 0.49, p = .0007). Posthoc analyses revealed that the effect of age on FA was significant in the genu (beta = −0.20, p = .02) and the splenium of the corpus callosum (beta = −0.19, p = .03) and the right uncinate fasciculus (beta = −0.18, p = .046) but not the remaining tracts.
3.2. White‐matter properties and depressive symptoms in young adulthood
The general linear model revealed an interaction between depressive symptoms and the type of white‐matter tract (F [17, 2,108] = 3.09, p < .0001) and no interaction with sex (F [17,2,108] = 0.50, p = .95), pointing out that the associations between depressive symptoms and FA significantly differ in the different white‐matter tracts but that sex does not moderate these effects. The interaction between depressive symptoms and the type of white‐matter tract remained significant (F [17,2091] = 2.69, p = .0002) and the interaction with sex nonsignificant (F[17,2091] = 0.59, p = .91) even when including age as a covariate in this general linear model. Posthoc linear regressions revealed that more depressive symptoms predicted lower FA in the left (beta = −0.22, p = .015, R 2 = 0.05; Figure 2a) and right (beta = −0.22, p = .015, R 2 = 0.05; Figure 2b) cingulum and higher FA in the right corticospinal tract (beta = 0.18, p = .047, R 2 = 0.03; Figure 2c) and superior longitudinal fasciculus (beta = 0.17, p = .048, R 2 = 0.03; Figure 2d). Refer to Table 1 for the effects of depressive symptoms on fractional anisotropy in all the tracts.
Figure 2.
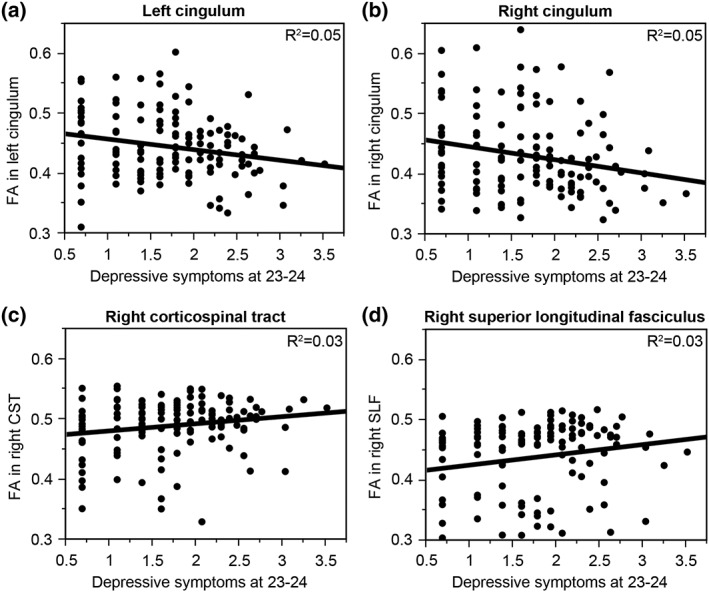
Depression‐related white‐matter tracts. Depressive symptomatology was associated with less FA in the left (a; beta = −0.22, p = .015, R 2 = 0.05) and right (b; beta = −0.22, p = .015, R 2 = 0.05) cingulum and more FA in the right corticospinal tract (c; beta = 0.18, p = .047, R 2 = 0.03) and superior longitudinal fasciculus (d; beta = 0.17, p = .048, R 2 = 0.03)
Table 1.
Effects of depressive symptoms on fractional anisotropy in all the white matter tracts in the 128 young adults
| Tract | Left hemisphere | Right hemisphere |
|---|---|---|
| Cingulum | Beta = −0.22, p = .01, R 2 = 0.05 | Beta = −0.22, p = .01, R 2 = 0.05 |
| Corticospinal tract | Beta = 0.09, p = .29 | Beta = 0.18, p = .04, R 2 = 0.03 |
| Fornix | Beta = 0.16, p = .06 | Beta = 0.17, p = .06 |
| Inferior longitudinal fasciculus | Beta = 0.13, p = .14 | Beta = 0.12, p = .19 |
| Superior longitudinal fasciculus | Beta = 0.15, p = .10 | Beta = 0.17, p = .04, R 2 = 0.03 |
| Inferior fronto‐occipital fasciculus | Beta = 0.17, p = .06 | Beta = 0.12, p = .18 |
| Superior fronto‐occipital fasciculus | Beta = −0.14, p = .11 | Beta = −0.12, p = .19 |
| Uncinate fasciculus | Beta = 0.16, p = .08 | Beta = 0.13, p = .02 |
| Genu of corpus callosum | Beta = 0.07, p = .42 | |
| Splenium of corpus callosum | Beta = 0.09, p = .30 | |
3.3. Developmental origins of depression‐related white‐matter properties
Further analyses focused on the four depression‐related tracts and assessed whether prenatal stress or depressive symptomatology during adolescence might predict the altered white‐matter properties in young adulthood. Prenatal stress was associated with lower FA in the left cingulum (beta = −0.21, p = .04, R 2 = 0.05; Figure 3a) and there was no interactions with sex (beta = −0.07, p = .47). This effect of prenatal stress on FA in left cingulum remained significant even after correcting for mother's age and stressful life events experienced by the mother after birth (at 6–18 months of the offspring; beta = −0.27, p = .04, R 2 = 0.05). Moreover, the effect of prenatal stress on FA in the cingulum remained significant and independent of sex, maternal age, and stressful life events experienced by the mother after birth even when excluding several health problem/obstetric complication‐related questions in the prenatal stress questionnaire (see Supporting Information Results). Effects of prenatal stress on fractional anisotropy in the other three depression‐related tracts (left cingulum, right corticospinal tract, and right superior longitudinal fasciculus) were not significant (Table 2) and there were also no interactions with sex (p > 11).
Figure 3.
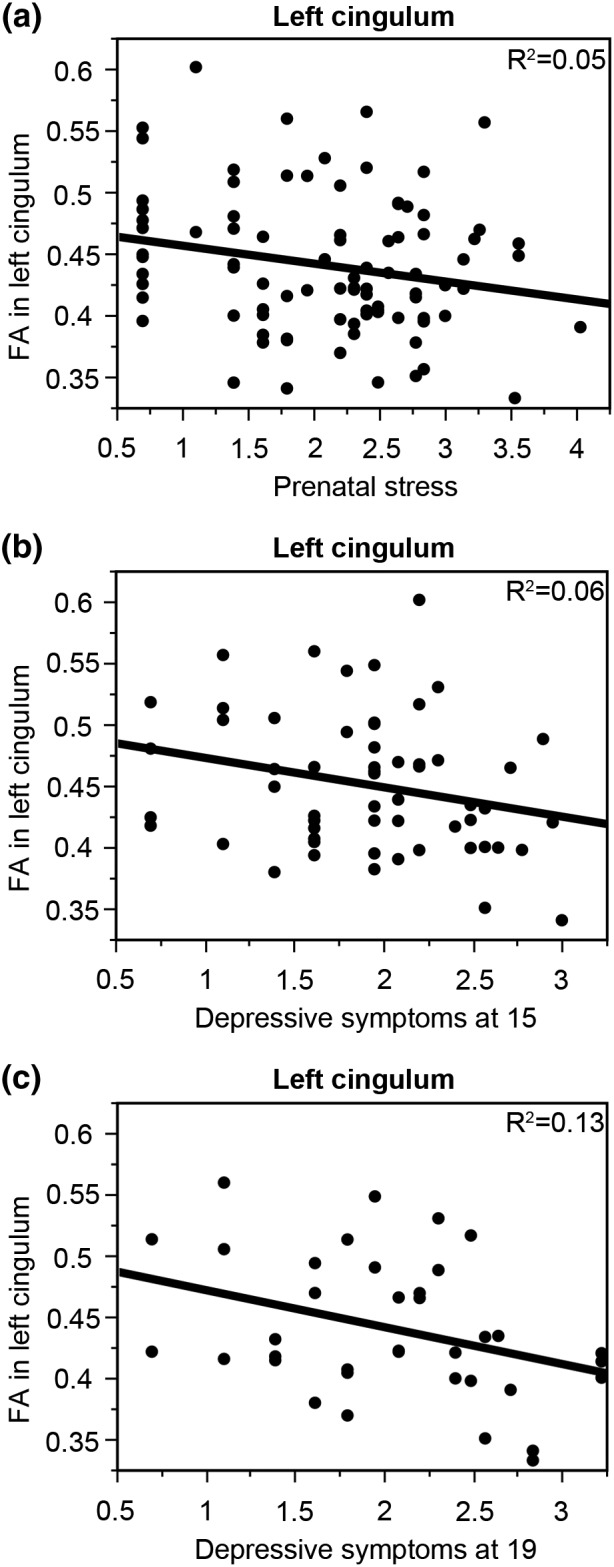
Developmental origins of altered white‐matter properties in left cingulum. Low FA in the left cingulum was associated with greater experience of prenatal stress (a; beta = −0.21, p = .04, R 2 = 0.05), and more depressive symptomatology at age 15 (b; beta = −0.25, p = .05, R 2 = 0.06) and 19 (c; beta = −0.37, p = .02, R 2 = 0.13)
Table 2.
Effects of prenatal stress and depressive symptoms during adolescence on fractional anisotropy in the four depression‐related tracts
| Tract | Prenatal stress | Depressive symptoms at 15 years of age | Depressive symptoms at 19 years of age |
|---|---|---|---|
| Left cingulum | Beta = −0.21, p = .04, R 2 = 0.04 | Beta = −0.25, p = .05, R 2 = 0.06 | Beta = −0.37, p = .02, R 2 = 0.13 |
| Right cingulum | Beta = −0.11, p = .31 | Beta = −0.12, p = .35 | Beta = −0.26, p = .11 |
| Right corticospinal tract | Beta = −0.09, p = .39 | Beta = −0.65, p = .06 | Beta = −0.03, p = .84 |
| Right superior longitudinal fasciculus | Beta = 0.12, p = .26 | Beta = 0.16, p = .23 | Beta = 0.13, p = .44 |
The effects of prenatal stress were tested in a sample of 93 young adults, the effects of depressive symptoms at age 15 in a sample of 59 young adults, and the effects of depressive symptoms at age 19 in a sample of 38 young adults
More depressive symptomatology at age 15 (beta = −0.25, p = .05, R 2 = 0.06; Figure 3b) and 19 (beta = −0.37, p = .02, R 2 = 0.13; Figure 3c) was also associated with lower FA in the left cingulum but not FA in the other three depression‐related tracts (Table 2 ). Again, there were no significant interactions with sex (p > .13). Finally, a multiple regression analysis revealed that the effects of prenatal stress (beta = −0.33, p = .048) and depression at the age of 15 (beta = −0.34, p = .04) were independent predictors of FA in the left cingulum, surviving the correction for maternal age (beta = 0.05, p = .76) and stressful life events experienced by the mother after birth (at 6–18 months of the offspring; beta = 0.18, p = .27). Correlation matrix of the key questionnaire‐based variables is provided in Supporting Information Table S5.
4. DISCUSSION
We studied white‐matter alterations in members of a birth cohort presenting subthreshold forms of depression and demonstrated that tract‐specific alterations in white‐matter properties are present already in previously undiagnosed and hence treatment‐naive young adults with mild depressive symptoms assessed with the Beck Depression Inventory. Using a whole‐brain approach, we showed that the depression‐related white‐matter alterations are not global but regional. This is consistent with volumetric studies in mood disorders that also report regional rather than whole‐brain effects (Konarski et al., 2018).
More depressive symptoms were associated with lower fractional anisotropy in the left and right cingulum and higher fractional anisotropy in the right corticospinal tract and superior longitudinal fasciculus. The cingulum bundle is a fiber bundle that connects regions associated with executive function, decision‐making, and emotion (Heilbronner & Haber, 2014a, 2014b). Altered microstructure of the cingulum bundle has been demonstrated in both adolescent (Henderson et al., 2013) and adult (De Diego‐Adelino et al., 2014) depression. During intraoperative deep brain stimulation in patients with treatment‐resistant depression, the cingulum, and particularly the left cingulum bundle, has been identified as a “depression switch” whose stimulation resulted in transient behavior changes (Choi, Riva‐Posse, Gross, & Mayberg, 2015). Further research also showed lower fractional anisotropy of the cingulum in individuals with subclinical anhedonia or family history of depression (Huang, Fan, Williamson, & Rao, 2011; Keedwell et al., 2012). It has been suggested also that microstructure of the cingulum bundle, the major white‐matter pathway linking cortical regions implicated in cognitive control with limbic regions involved in emotion (Heilbronner & Haber, 2014a, 2014b), is functionally relevant for regulating attentional bias toward negative interpersonal stimuli (Keedwell et al., 2016).
The relationship between depressive symptomatology and higher FA in the right corticospinal tract and superior longitudinal fasciculus are rather unexpected since generally, depression has been associated with lower FA in a number of white‐matter tracts (Murphy & Frodl, 2011). A meta‐analysis of diffusion tensor imaging studies in MDD patients vs. Controls (Murphy & Frodl, 2011) reported higher FA in one white‐matter tract only—the inferior fronto‐occipital fasciculus. Still, our results are consistent with another study (Blood et al., 2010) that also reported higher FA in the right corticospinal tract in depression.
Longitudinal data from our prenatal birth cohort then suggest that prenatal stress might have induced long‐lasting alterations in white‐matter properties in later life. We showed that young adult offspring of mothers who experienced more stressful life events during pregnancy had lower fractional anisotropy in the left cingulum in young adulthood. Our findings are in agreement with previous research in rats, which demonstrated an association between maternal restraint stress applied during gestation and altered myelination in the brain of their offspring (Wiggins & Gottesfeld, 1986), and research in sheep, which demonstrated an association between prenatal exposure to synthetic glucocorticoids and reduced diameter of myelinated axons (Antonow‐Schlorke, Schwab, Li, & Nathanielsz, 2003; Huang, Harper, Evans, Newnham, & Dunlop, 2001). The possible impact of prenatal stress on development of white matter in the cingulum, known as the regulator of attentional bias toward negative interpersonal stimuli (Keedwell et al., 2016) and as the “depression switch” (Choi et al., 2015), supports the behavioral study (Betts et al., 2015), which reported an association between maternal anxiety during pregnancy and self‐reported depressive symptoms in the adolescent offspring, as well as the prenatal stress model of altered emotional development and long‐term psychiatric vulnerability (Goldstein et al., 2014). The fact that the associations between prenatal stress and FA in the cingulum remained significant even when correcting for postnatal stress and maternal age suggests that the cingulum development might be particularly sensitive to stress during the prenatal period.
In contrast to previous research (Buss et al., 2012a, 2012b; Szeszko et al., 2003), we did not find any main or moderating effects of sex on white‐matter microstructure in adults. We also did not find any moderating effects of sex on the relationship between prenatal stress and FA in the depression‐related white‐matter tracts. As explained in our previous work (Mareckova et al., 2018), this might be related to the timing of prenatal exposure to stress. Sexual differentiation of the brain happens mainly during the second and third trimester (Goldstein et al., 2014) but the current study assessed the impact of stressful life events during the first half of pregnancy.
Our study has several strengths. First, it was designed as a neuroimaging follow‐up of a prenatal birth cohort consisting of individuals of a very narrow age range (23 or 24 years old) and from a very similar background (all White Caucasians, typically developing, growing up in the same area, and born in families with very similar socioeconomic status). Second, the longitudinal character of the study eliminates any possible false memories or recall bias. Third, while past research studied the effects of prenatal stress only in single hypothesized white‐matter tracts—Jensen et al. (2017) in corpus callosum, Sarkar et al. (2014) in uncinate fasciculus—our research took a whole‐brain approach and studied the effects of prenatal stress on white‐matter properties in all major tracts.
The DTI‐based FA measure limits our ability to make conclusions about the exact neurobiology underlying variations in white‐matter properties. Lower FA might be related to (a) abnormal neurogenesis/apoptosis, (b) abnormal myelination, or (c) abnormal radial growth of axons (Jensen et al., 2017). Since previous research associated maternal stress with reduced neurogenesis and enhanced apoptosis in the hippocampus in rodents (Lemaire, Koehl, Le Moal, & Abrous, 2000; Lemaire, Lamarque, Le Moal, Piazza, & Abrous, 2006; Van den Hove et al., 2006) and our previous research in this prenatal birth cohort showed an association between higher prenatal stress and lower gray‐matter volume (Mareckova et al., 2018), it might be that also the effect of prenatal stress on lower FA reported in the current study might be, at least in part, related to abnormal neurogenesis/apoptosis. Future research might incorporate the assessment of myelination using new myelin mapping techniques (Deoni et al., 2011) or consider the use of histological data to explain the neurobiological origins of the depression‐related changes in white‐matter properties. Another important limitation of our study is the fact that the findings from the follow‐up analyses on developmental origins (prenatal stress, depression during adolescence) of depression‐related variations in the properties of white matter in the four specific fiber tracts did not survive correction for multiple comparisons. Future research should verify the existence of these tract‐specific relationships.
Overall, we have demonstrated that typically developing young adults with more depressive symptoms already exhibit altered white‐matter properties and that these alterations are not global but regional. Our findings also suggest that the experience of prenatal stress and depressive symptoms in adolescence might contribute to the depression‐related white‐matter properties in the cingulum, thus offering a relevant insight into the developmental origins of the disease. Our study contributes to better understanding of the neural correlates underlying depressive symptomatology in young adulthood and its developmental origins and points out to the importance of early intervention.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Supporting information
Supporting Information
Supplementary Figure 1 – Recruitment flow diagram. Since the ELSPAC study officially ended when the participants were 20 years old, the recruitment of the VULDE neuroimaging follow‐up of ELSPAC participants (done at the age 23–24) was done as follows: All ELSPAC participants with an up‐to‐date email address from the age of 19 (n = 984) received a Thank you Christmas card for their long‐term participation in the study and a link to the ELSPAC website where they could sign‐up for the neuroimaging follow‐up. The neuroimaging follow‐up was done in all individuals from the ELSPAC birth cohort who signed‐up for this follow‐up through the ELSPAC website in 2015 (n = 131). We did not continue with the recruitment beyond 2015 because the Marie Curie project VULDE called for recruitment of 120 individuals only. The DTI data were successfully collected in 128 out of the 131 individuals.
ACKNOWLEDGMENTS
This work was supported by the European Union (Marie Curie Intra‐European Fellowship for Career Development FP7‐IEF‐2013), the Ministry of Education, Youth and Sports of the Czech Republic/MEYS (CEITEC 2020, LQ1601, the RECETOX research infrastructure: LM2015051 and CZ.02.1.01/0.0/0.0/16_013/0001761), and Canadian Institutes of Health Research (MOP125892 to T.P.). The authors thank the core facility MAFIL of CEITEC supported by the Czech‐BioImaging large RI project (LM2015062 funded by MEYS CR) for their support with obtaining the scientific data presented in this article.
Marečková K, Klasnja A, Andrýsková L, Brázdil M, Paus T. Developmental origins of depression‐related white matter properties: Findings from a prenatal birth cohort. Hum Brain Mapp. 2019;40:1155–1163. 10.1002/hbm.24435
Funding information: Canadian Institutes of Health Research , Grant/Award Number: MOP125892; FP7‐IEFPEOPLE‐2013 (Marie Curie Intra‐European Fellowship for Career Development), Grant/Award Number: 6485124 ; Ministry of Education, Youth and Sports of the Czech Republic, Grant/Award Number: CEITEC 2020CZ.02.1.01/0.0/0.0/16_013/0001761LM2015051LM2015062LQ1601
REFERENCES
- Antonow‐Schlorke, I. , Schwab, M. , Li, C. , & Nathanielsz, P. W. (2003). Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. The Journal of Physiology, 547(1), 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung, W. Y. , Mar, S. , & Benzinger, T. L. S. (2013). Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging in Medicine, 5(5), 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babri, S. , Doosti, M. H. , & Salari, A. A. (2014). Strain‐dependent effects of prenatal maternal immune activation on anxiety‐ and depression‐like behaviors in offspring. Brain, Behavior, and Immunity, 37, 164–176. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Ward, C. H. , Mendelson, M. , Mock, J. , & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Betts, K. S. , Williams, G. M. , Najman, J. M. , & Alati, R. (2015). The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioural and emotional problems. Depression and Anxiety, 32(2), 82–90. [DOI] [PubMed] [Google Scholar]
- Bilbo, S. D. , & Schwarz, J. M. (2009). Early‐life programming of later‐life brain and behavior: A critical role for the immune system. Frontiers in Behavioral Neuroscience, 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood, A. J. , Iosifescu, D. V. , Makris, N. , Perlis, R. H. , Kennedy, D. N. , & Dougherty, D. D. (2010). Phenotype genotype project on addiction and mood disorders: Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One, 5, e13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, J. , Rether, K. , Groger, N. , Xie, L. , & Braun, K. (2014). Perinatal programming of emotional brain circuits: An integrative view from systems to molecules. Frontiers in Neuroscience, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C. , Davis, E. P. , Shahbaba, B. , Pruessner, J. C. , Head, K. , & Sandman, C. A. (2012a). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America, 109(20), E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C. , Davis, E. P. , Shahbaba, B. , Pruessner, J. C. , Head, K. , & Sandman, C. A. (2012b). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America, 109, E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Guo, Y. , Zhu, H. , Kuang, W. , Bi, F. , Ai, H. , … Gong, Q. (2017). Intrinsic disruption of white matter microarchitecture in first‐episode, drug‐naive major depressive disorder: A voxel‐based meta‐analysis of diffusion tensor imaging. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 76, 179–187. [DOI] [PubMed] [Google Scholar]
- Chen, G. , Hu, X. , Li, L. , Huang, X. , Lui, S. , Kuang, W. , … Gong, Q. (2016). Disorganization of white matter architecture in major depressive disorder: A meta‐analysis of diffusion tensor imaging with tract‐based spatial statistics. Scientific Reports, 6, 21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K. S. , Riva‐Posse, P. , Gross, R. E. , & Mayberg, H. S. (2015). Mapping the “depression switch”during intraoperative testing of subcallosal cingulated deep brain stimulation. JAMA Neurology, 72(11), 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey, C. E. , Figiel, G. S. , Djang, W. T. , Saunders, W. B. , & Weiner, R. D. (1989). White matter hyperintensity on magnetic resonance imaging: clinical and neuroanatomic correlates in the depressed elderly. The Journal of Neuropsychiatry and Clinical Neurosciences, 1(2), 135–144. [DOI] [PubMed] [Google Scholar]
- Coffey, C. E. , Wilkinson, W. E. , Weiner, R. D. , Parashos, I. A. , Djang, W. T. , Webb, M. C. , … Spritzer, C. E. (1993). Quantitative cerebral anatomy in depression: A controlled magnetic resonance imaging study. Archives of General Psychiatry, 50, 7–16. [DOI] [PubMed] [Google Scholar]
- Copeland, W. E. , Wolke, D. , Shanahan, L. , & Costello, E. J. (2015). Adult functional outcomes of common childhood psychiatric problems: A prospective, longitudinal study. JAMA Psychiatry, 72(9), 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, E. P. , Glynn, L. M. , Waffarn, F. , & Sandman, C. A. (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, 52(2), 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, E. P. , & Sandman, C. A. (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology, 37(8), 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego‐Adelino, J. , Pires, P. , Gómez‐Ansón, B. , Serra‐Blasco, M. , Vives‐Gilabert, Y. , Puigdemont, D. , … Portella, M. J. (2014). Microstructural white matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychological Medicine, 44(6), 1171–1182. [DOI] [PubMed] [Google Scholar]
- de Groot, J. C. , de Leeuw, F. E. , Oudkerk, M. , Hofman, A. , Jolles, J. , & Breteler, M. M. (2000). Cerebral white matter lesions and depressive symptoms in elderly adults. Archives of General Psychiatry, 57, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Deoni, S. C. L. , Mercure, E. , Blasi, A. , Gasston, D. , Thomson, A. , Johnson, M. , … Murphy, D. G. M. (2011). Mapping infant brain myelination with magnetic resonance imaging. The Journal of Neuroscience, 31, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati, M. , Solati, J. , Hosseini, M. H. , Shahi, H. R. , Saki, G. , & Salari, A. A. (2012). Maternal infection during late pregnancy increases anxiety‐ and depression‐like behaviors with increasing age in male offspring. Brain Research Bulletin, 87, 295–302. [DOI] [PubMed] [Google Scholar]
- Entringer, S. , Kumsta, R. , Hellhammer, D. H. , Wadhwa, P. D. , & Wust, S. (2009). Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Hormones and Behavior, 55(2), 292–298. [DOI] [PubMed] [Google Scholar]
- Ghazi Sherbaf, F. , Same, K. , Ashraf‐Ganjouei, A. , & Aarabi, M. H. (2018). Altered white matter microstructure associated with mild and moderate depressive symptoms in young adults, a diffusion tensor imaging study. Neuroreport, 29(8), 685–689. 10.1097/WNR.0000000000001017 [DOI] [PubMed] [Google Scholar]
- Giedd, J. (1999). Brain development, IX: Human brain growth. The American Journal of Psychiatry, 156, 4. [DOI] [PubMed] [Google Scholar]
- Golding, J. (1989). European longitudinal study of pregnancy and childhood (ELSPAC). Paediatric and Perinatal Epidemiology, 3(4), 460–469. [DOI] [PubMed] [Google Scholar]
- Goldstein, J. M. , Handa, R. J. , & Tobet, S. A. (2014). Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Frontiers in Neuroendocrinology, 35, 140–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon, F. M. , Walton, M. , Cheng, J. , Acuna, J. , Klimstra, S. , Zimmerman, M. E. , … Alexopoulos, G. S. (2010). MRI signal hyperintensities and treatment remission of geriatric depression. Journal of Affective Disorders, 126, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner, S. R. , & Haber, S. N. (2014a). Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. The Journal of Neuroscience, 34(30), 10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner, S. R. , & Haber, S. N. (2014b). Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. The Journal of Neuroscience, 34(30), 10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, S. E. , Johnson, A. R. , Vallejo, A. I. , Katz, L. , Wong, E. , & Gabbay, V. (2013). A preliminary study of white matter in adolescent depression: Relationships with illness severity, anhedonia, and irritability. Frontiers in Psychiatry, 4, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom, W. S. , Perlis, R. H. , Smoller, J. W. , Zeng‐Treitler, Q. , Gainer, V. S. , Murphy, S. N. , … Iosifescu, D. V. (2014). Limbic system white matter microstructure and long‐term treatment outcome in major depressive disorder: A diffusion tensor imaging study using legacy data. The World Journal of Biological Psychiatry, 15, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, R. J. , Beats, B. , Forstl, H. , Graves, P. , Bingham, J. , & Levy, R. (1993). White matter changes in late onset depression: A magnetic resonance imaging study. International Journal of Geriatric Psychiatry, 8, 183–185. [Google Scholar]
- Huang, H. , Fan, X. , Williamson, D. E. , & Rao, U. (2011). White matter changes in healthy adolescents at familial risk for unipolar depression: A diffusion tensor imaging study. Neuropsychopharmacology, 36(3), 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. L. , Harper, C. G. , Evans, S. F. , Newnham, J. P. , & Dunlop, S. A. (2001). Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. International Journal of Developmental Neuroscience, 19(5), 487–493. [DOI] [PubMed] [Google Scholar]
- Iosifescu, D. V. , Renshaw, P. F. , Lyoo, I. K. , Lee, H. K. , Perlis, R. H. , Papakostas, G. I. , … Fava, M. (2006). Brain white‐matter hyperintensities and treatment outcome in major depressive disorder. The British Journal of Psychiatry, 188, 180–185. [DOI] [PubMed] [Google Scholar]
- Jensen, S. K. G. , Pangelinan, M. , Björnholm, L. , Klasnja, A. , Leemans, A. , Drakesmith, M. , … Paus, T. (2017). Associations between prenatal, childhood, and adolescent stress and variations in white matter properties in young men. Neuroimage, 182, 389–397. 10.1016/j.neuroimage.2017.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell, P. A. , Chapman, R. , Christiansen, K. , Richardson, H. , Evans, J. , & Jones, D. K. (2012). Cingulum white matter in young women at risk of depression: The effect of family history and anhedonia. Biological Psychiatry, 72(4), 296–302. [DOI] [PubMed] [Google Scholar]
- Keedwell, P. A. , Doidge, A. N. , Meyer, M. , Lawrence, N. , Lawrence, A. D. , & Jones, D. K. (2016). Subgenual cingulum microstructure supports control of emotional conflict. Cerebral Cortex, 26(6), 2850–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler, K. S. , Karkowski, L. M. , & Prescott, C. A. (1999). Causal relationship between stressful life events and the onset of major depression. Psychiatry: Interpersonal and Biological Processes, 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Khan, D. , Fernando, P. , Cicvaric, A. , Berger, A. , Pollak, A. , Monje, F. J. , & Pollak, D. D. (2014). Long‐term effects of maternal immune activation on depression‐like behaviour in the mouse. Translational Psychiatry, 4, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseppa, T. , Eerola, M. , Mäntylä, R. , Neuvonen, T. , Poutanen, V. P. , Luoma, K. , … Isometsä, E. (2010). Major depressive disorder and white matter abnormalities: A diffusion tensor imaging study with tract‐based spatial statistics. Journal of Affective Disorders, 120, 240–244. [DOI] [PubMed] [Google Scholar]
- Konarski, J. Z. , McIntyre, R. S. , Kennedy, S. H. , Rafi–Tari, S. , Soczynska, J. K. , & Ketter, T. A. (2018). Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Bipolar Disord, 10, 1–37. [DOI] [PubMed] [Google Scholar]
- Korgaonkar, M. S. , Grieve, S. M. , Koslow, S. H. , Gabrieli, J. D. , Gordon, E. , & Williams, L. M. (2011). Loss of white matter integrity in major depressive disorder: Evidence using tract‐based spatial statistical analysis of diffusion tensor imaging. Human Brain Mapping, 32(12), 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Walker, L. , Leemans, A. , Phillips, L. , & Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 40(3), 1044–1055. [DOI] [PubMed] [Google Scholar]
- Leemans, A. , & Jones, D. K. (2009). The B‐matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. [DOI] [PubMed] [Google Scholar]
- Lemaire, V. , Koehl, M. , Le Moal, M. , & Abrous, D. N. (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire, V. , Lamarque, S. , Le Moal, M. , Piazza, P. V. , & Abrous, D. N. (2006). Postnatal stimulation of the pups counteracts prenatal stress‐induced deficits in hippocampal neurogenesis. Biological Psychiatry, 59(9), 786–792. [DOI] [PubMed] [Google Scholar]
- Lenze, E. , Cross, D. , McKeel, D. , Neuman, R. J. , & Sheline, Y. I. (1999). White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. The American Journal of Psychiatry, 156(10), 1602–1607. [DOI] [PubMed] [Google Scholar]
- Liao, Y. , Huang, X. , Wu, Q. , Yang, C. , Kuang, W. , du, M. , … Gong, Q. (2013). Is depression a disconnection syndrome? Meta‐analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience, 38(1), 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. L. , & Wang, S. (2014). Prenatal lipopolysaccharide exposure increases depression‐like behaviors and reduces hippocampal neurogenesis in adult rats. Behavioural Brain Research, 259, 24–34. [DOI] [PubMed] [Google Scholar]
- Ma, N. , Li, L. , Shu, N. , Liu, J. , Gong, G. , He, Z. , … Jiang, T. (2007). White matter abnormalities in first‐episode, treatment‐naive young adults with major depressive disorder. The American Journal of Psychiatry, 164, 823–826. [DOI] [PubMed] [Google Scholar]
- Mareckova, K. , Klasnja, A. , Bencurova, P. , Andryskova, L. , Brazdil, M. , & Paus, T. (2018). Prenatal stress, mood, and gray matter volume in young adulthood. Cerebral Cortex. 10.1093/cercor/bhy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham, J. A. , & Koenig, J. I. (2011). Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology, 214, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan, S. , Maes, M. , Wray, N. R. , & Berk, M. (2013). The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Molecular Psychiatry, 18(5), 595–606. [DOI] [PubMed] [Google Scholar]
- Murphy, M. L. , & Frodl, T. (2011). Meta‐analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biology of Mood & Anxiety Disorders, 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. , Kim, B. N. , Kim, J. W. , Shin, M. S. , Yoo, H. J. , Lee, J. , & Cho, S. C. (2014). Associations between maternal stress during pregnancy and offspring internalizing and externalizing problems in childhood. International Journal of Mental Health Systems, 8(1), 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus, T. (2016). Chapter 2 – Population neuroscience In Handbook of clinical neurology. Elsevier, (Vol. 138, pp. 17–37). 10.1016/B978-0-12-802973-2.00002-1. [DOI] [PubMed] [Google Scholar]
- Piler, P. , Kandrnal, V. , Kukla, L. , Andrýsková, L. , Švancara, J. , Jarkovský, J. , … Klánová, J. (2016). Cohort profile: European longitudinal study of pregnancy and childhood (ELSPAC) in the Czech Republic. International Journal of Epidemiology, 46(5), 1379–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, A. , Anh, T. T. , Li, Y. , Chen, H. , Rifkin‐Graboi, A. , Broekman, B. F. P. , … Meaney, M. J. (2015). Prenatal maternal depression alters amygdala functional connectivity in 6‐month‐old infants. Translational Psychiatry, 5, e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins, P. V. , Pearlson, G. D. , Aylward, E. , Kumar, A. J. , & Dowell, K. (1991). Cortical magnetic resonance imaging changes in elderly inpatients with major depression. The American Journal of Psychiatry, 148(5), 617–620. [DOI] [PubMed] [Google Scholar]
- Rifkin‐Graboi, A. , Meaney, M. J. , Chen, H. , Bai, J. , Hameed, W. B.’. , Tint, M. T. , … Qiu, A. (2015). Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 313–321. [DOI] [PubMed] [Google Scholar]
- Sandman, C. A. , Buss, C. , Head, K. , & Davis, E. P. (2015). Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry, 77, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, S. , Craig, M. C. , Dell'Acqua, F. , O'Connor, T. G. , Catani, M. , Deeley, Q. , … Murphy, D. G. M. (2014). Prenatal stress and limbic‐prefrontal white matter microstructure in children aged 6‐9 years: A preliminary diffusion tensor imaging study. The World Journal of Biological Psychiatry, 15(4), 346–352. [DOI] [PubMed] [Google Scholar]
- Shimony, J. S. , Sheline, Y. I. , D'Angelo, G. , Epstein, A. A. , Benzinger, T. L. S. , Mintun, M. A. , … Snyder, A. Z. (2009). Diffuse microstructural abnormalities of normal‐appearing white matter in late life depression: A diffusion tensor imaging study. Biological Psychiatry, 66, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko, P. R. , Vogel, J. , Ashtari, M. , Malhotra, A. K. , Bates, J. , Kane, J. M. , … Lim, K. (2003). Sex differences in frontal lobe white matter microstructure: A DTI study. Neuroreport, 14, 2469–2473. [DOI] [PubMed] [Google Scholar]
- Tax, C. M. W. , Otte, W. M. , Viergever, M. A. , Dijkhuizen, R. M. , & Leemans, A. (2015). REKINDLE: Robust extraction of kurtosis INDices with linear estimation. Magnetic Resonance in Medicine, 73(2), 794–808. [DOI] [PubMed] [Google Scholar]
- Taylor, W. D. , Payne, M. E. , Krishnan, K. R. R. , Wagner, H. R. , Provenzale, J. M. , Steffens, D. C. , & MacFall, J. R. (2001). Evidence of white matter tract disruption in MRI hyperintensities. Biological Psychiatry, 50, 179–183. [DOI] [PubMed] [Google Scholar]
- UNICEF . (2017). The adolescent brain: A second window of opportunity. UNICEF Office of Research–Innocenti, Florence, Italy. Retrieved from https://www.unicef-irc.org/publications/pdf/adolescent_brain_a_second_window_of_opportunity_a_compendium.pdf
- Ustun, T. B. , Ayuso‐Mateos, J. L. , Chatterji, S. , Mathers, C. , & Murray, C. J. L. (2004). Global burden of depressive disorders in the year 2000. British Journal of Psychiatry, 184, 386–392. [DOI] [PubMed] [Google Scholar]
- Van den Hove, D. L. A. , Steinbusch, H. W. , Scheepens, A. , Van de Berg, W. D. , Kooiman, L. A. , Boosten, B. J. , … Blanco, C. E. (2006). Prenatal stress and neonatal rat brain development. Neuroscience, 137(1), 145–155. [DOI] [PubMed] [Google Scholar]
- Veraart, J. , Sijbers, J. , Sunaert, S. , Leemans, A. , & Jeurissen, B. (2013). Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. NeuroImage, 81, 335–346. [DOI] [PubMed] [Google Scholar]
- Wakana, S. , Caprihan, A. , Panzenboeck, M. M. , Fallon, J. H. , Perry, M. , Gollub, R. L. , … Mori, S. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage, 36(3), 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. K. , Nakamura, T. , & Hodgson, D. M. (2010). Neonatal lipopolysaccha‐ ride exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress, 13(6), 506–515. [DOI] [PubMed] [Google Scholar]
- Weinstock, M. (2008). The long‐term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews, 32, 1073–1086. [DOI] [PubMed] [Google Scholar]
- Wiggins, R. C. , & Gottesfeld, Z. (1986). Restraint stress during late pregnancy in rats elicits early hypermyelination in the offspring. Metabolic Brain Disease, 1, 197–203. [DOI] [PubMed] [Google Scholar]
- Wu, F. , Tang, Y. , Xu, K. , Kong, L. , Sun, W. , & Wang, F. (2011). White matter abnormalities in medication‐naive subjects with a single short duration episode of major depressive disorder. Psychiatry Research, 191, 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Ping, E. , Laplante, D. P. , Elgbeili, G. , Hillerer, K. M. , Brunet, A. , O'Hara, M. W. , & King, S. (2015). Prenatal maternal stress predicts stress reactivity at 2.5 years of age: The Iowa flood study. Psychoneuroendocrinology, 56, 62–78. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Wang, X. , Xiao, J. , Zhong, M. , Liao, J. , & Yao, S. (2011). Altered white matter integrity in first‐episode, treatment‐naive young adults with major depressive disorder: A tract‐based spatial statistics study. Brain Research, 1369, 223–229. [DOI] [PubMed] [Google Scholar]
- Zou, K. , Huang, X. , Li, T. , Gong, Q. , Li, Z. , Ou‐yang, L. , … Sun, X. (2008). Alterations of white matter integrity in adults with major depressive disorder: A magnetic resonance imaging study. Journal of Psychiatry & Neuroscience, 33(6), 525–530. [PMC free article] [PubMed] [Google Scholar]
- Zubenko, G. S. , Sullivan, P. , Nelson, J. P. , Belle, S. H. , Huff, F. J. , & Wolf, G. L. (1990). Brain imaging abnormalities in mental disorders of late life. Archives of Neurology, 47(10), 1107–1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplementary Figure 1 – Recruitment flow diagram. Since the ELSPAC study officially ended when the participants were 20 years old, the recruitment of the VULDE neuroimaging follow‐up of ELSPAC participants (done at the age 23–24) was done as follows: All ELSPAC participants with an up‐to‐date email address from the age of 19 (n = 984) received a Thank you Christmas card for their long‐term participation in the study and a link to the ELSPAC website where they could sign‐up for the neuroimaging follow‐up. The neuroimaging follow‐up was done in all individuals from the ELSPAC birth cohort who signed‐up for this follow‐up through the ELSPAC website in 2015 (n = 131). We did not continue with the recruitment beyond 2015 because the Marie Curie project VULDE called for recruitment of 120 individuals only. The DTI data were successfully collected in 128 out of the 131 individuals.


