Abstract
Decline of verbal fluency (VF) performance is one of the most systematically reported neuropsychological adverse effects after subthalamic nucleus deep brain stimulation (STN‐DBS). It has been suggested that this worsening of VF may be related to a microlesion due to the electrode trajectories. We describe the disruption of surrounding white matter tracts following electrode implantation in Parkinson's disease (PD) patients with STN‐DBS and assess whether damage of fiber pathways is associated with VF impairment after surgery. We retrospectively analyzed 48 PD patients undergoing bilateral STN DBS. The lesion mask along the electrode trajectory transformed into the MNI 152 coordinate system, was compared with white matter tract atlas in Tractotron software, which provides a probability and proportion of fibers disconnection. Combining tract‐ and atlas‐based analysis reveals that the trajectory of the electrodes intersected successively with the frontal aslant tract, anterior segment of arcuate tract, the long segment of arcuate tract, the inferior longitudinal fasciculus, the superior longitudinal fasciculus, the anterior thalamic radiation, and the fronto striatal tract. We found no association between the proportion fiber disconnection and the severity of VF impairment 6 months after surgery. Our findings demonstrated that microstructural injury associated with electrode trajectories involved white matter bundles implicated in VF networks.
Keywords: deep brain stimulation, microlesion, Parkinson's disease, subthalamic nucleus, verbal fluency, white matter tracts
1. INTRODUCTION
Subthalamic nucleus deep brain stimulation (STN‐DBS) is a well‐established and effective treatment for selected patients with advanced levodopa responsive form of Parkinson's disease (PD) (Deuschl et al., 2006). While STN‐DBS can significantly improve quality of life, and severe disabling motors fluctuations in PD patients, adverse cognitive effects have also been described (Witt et al., 2008). In particular, decline of verbal fluency (VF) performance, both in phonemic and semantic tasks, is one of the most systematically reported neuropsychological adverse effects (Lefaucheur et al., 2012; Okun et al., 2009; Parsons, Rogers, Braaten, Woods, & Troster, 2006; Witt et al., 2008; Wu, Han, Sun, Hu, & Wang, 2014). Previous neuropsychological investigations conducted from 1 to 6 months after STN‐DBS, in the on‐state phase (on‐stimulation/on‐treatment) showed a worsening of VF. Although the exact mechanism underlying this worsening of VF is not well understood, it has been suggested that it may be related to a microlesion due to the electrode trajectories (Okun et al., 2009; Witt et al., 2008). This hypothesis seems supported by the description of VF decline right after surgery in the absence of STN stimulation (Lefaucheur et al., 2012). Based on the topography of its cortico‐subthalamic projections, the STN has been subdivided into motor, limbic and associative functional regions (Haynes & Haber, 2013). Accordingly, studies suggest that postoperative VF decline may also reflect a direct microlesion of the electrode lead placed through the associative portion of the STN (Lefaucheur et al., 2012; Mikos et al., 2011; Okun et al., 2012). On the other hand, previous studies have shown that lead trajectories intersecting with caudate nuclei increased the risk of global cognitive decline and working memory performance after STN implantation (Witt et al., 2013). In a previous study, we assessed the relationship between the VF decline after STN‐DBS and the stereotactic lead trajectory (Le Goff et al., 2015). We found that PD patients who decline in semantic VF have a left trajectory with a more anterior cortical entry point. Therefore, we assumed that disruption of fibers within the penetrated region may concern cortical areas and subcortical structures involved in VF networks.
Recent neuroimaging and neuropsychological studies suggest that widely distributed, separate and shared brain regions are involved in semantic and phonemic VF (Schwartz, Baldo, Graves, & Brugger, 2003). Thus, underlying cortical neural systems commonly identified in VF performances include the left inferior frontal gyrus, anterior cingulate gyrus, and supramarginal gyrus; semantic fluency involving more temporal networks, and phonemic fluency more left frontal regions (Hirshorn & Thompson‐Schill, 2006; Paulesu et al., 1997). Further studies, focusing on white‐matter networks associated with deficits of semantic and/or phonemic fluency in different patients have determined five left anatomical fiber bundles that are responsible for VF processing. Hence, semantic fluency was found to be supported by the left inferior fronto‐occipital fasciculus and anterior thalamic radiation, whereas phonemic fluency was supported by the left superior longitudinal fasciculus and left frontal aslant tract (Almairac, Herbet, Moritz‐Gasser, de Champfleur, & Duffau, 2015; Catani et al., 2013; Li et al., 2017). The left uncinate fasciculus, the fronto striatal tract, and arcuate fasciculus were associated with both semantic and phonemic fluencies (Kinoshita et al., 2015; Papagno et al., 2011).
Advances in imaging equipment and technique, especially diffusion tensor imaging (DTI), offer powerful methods to the study of brain connectivity by noninvasively tracing connections in the living tissue. However, very few tractography and DTI studies have been conducted in PD patients with DBS, as for example to better understand the pathways involved in the therapeutic action. The paucity of studies is mainly due to technical and safety restrictions. In fact, the electrode‐induced artifact which overestimates the real lesion and the potential intersection may compromise the accurate assessment of disruption of surrounding white matter tracts (Saleh, 2010). Additionally, it has been described that MRI could induce tissue damage due to DBS lead heating (Henderson et al., 2005), prompting recommendations by the supplier (http://www.medtronic.com/physician/activa/downloadablefiles/196813_a_004.pdf) and specified by governmental organizations (e.g., the French health products safety agency).
To the best of our knowledge, no studies have investigated the relationship between white matter fiber bundles lesion and the worsening of VF after STN‐DBS. In this study, we used software based on white matter tract atlas in PD patients with STN‐DBS to describe the disruption of surrounding white matter tracts following electrode implantation. Furthermore, we assessed whether damage of fiber pathways is associated with the severity of VF impairment after surgery.
2. MATERIALS AND METHODS
2.1. Participants
We retrospectively analyzed data from patients with PD undergoing bilateral STN DBS in our center between 2007 and 2013. Patients selected for DBS were clinically diagnosed with a PD, had severe levodopa‐related complications despite optimal adjustment medication, no surgical contraindications, and no dementia or major ongoing psychiatric illness. Within this cohort, subjects were excluded if they were missing any baseline or postoperative measures, especially neuropsychological and/or imaging data. All participants were native French speakers. All the surgical procedures were performed by the same neurosurgeon (SD) as previously described (Le Goff et al., 2015).
2.2. Neuropsychological evaluation
All the patients underwent standardized cognitive assessments, that is, VF tasks, both 1 month before surgery (baseline) in the on‐drug condition, and after surgery at 6 months in the on‐drug/ on‐stimulation condition. Phonemic VF was determined by the number of words beginning with letter “p” produced in 2 min. Semantic VF was determined by the number of animal names produced in 2 min.
All dopaminergic drugs, expressed in dopa‐equivalent daily dose, were recorded before and after surgery.
2.3. Image analysis
All patients had a pre‐operative MRI (Magneton Symphony 1.5 Telsa, Siemens, Erlangen, Germany; T1, T2 spin echo and (CISS) and a postoperative CT‐scan (Light speed, General electric, Milwaukee, Minn) within 72 hr after surgery. Original DICOM of the postoperative CT scans were reviewed and postprocessed with spatial normalization through CT normalize tool of SPM software, to have brain images in the same 3D space, defined by the Montreal Neurological Institute (MNI Space). Regions of interest (ROI) were drawn manually for each patient using MRIcron tool to segment trajectory of electrodes from the cortical surface to the NST target by an experienced neuroimager (YC). The target was determined with the most inferior slice showing the tip of the electrode, for both sides. The entry point was determined by the intersection of the electrode with the cortex. The surgical pathway was then traced between the target and the cortical entry point, referring consistently to the electrode pathway. Then, the lesion mask along the electrode trajectory transformed into the MNI 152 coordinate system was compared with white matter tract atlas in Tractotron software, which provides a probability and proportion of fibers disconnection. A tract with a probability of >50% value is considered as disconnected in accordance with tractotron method paper (Foulon et al., 2018). The proportion fibers disconnection was calculated by dividing the number of damaged voxels in the ROI by the total volume of the tract.
We focused on tracts previously described as being involved in executive and language functions, that is, frontal superior and inferior, fronto‐insular and fronto‐striatal tracts for executive functions, arcuate fasciculi tract for the phonemic task, and uncinate inferior fronto‐temporal tracts for semantic task (Almairac et al., 2015; Catani et al., 2013; Kinoshita et al., 2015; Li et al., 2017; Papagno et al., 2011).
These analyses were performed separately for each hemisphere.
2.4. Statistical analysis
The different VF scores changes were expressed as mean value. Comparison of scores obtained before surgery (baseline on‐state) and respectively at 6 month after surgery used a Wilcoxon test.
Tractotron software provides a file containing probability and proportion of any tract, ready to be analyzed with statistical software. We fixed 50% of probability to consider a tract as disconnected, as previously used in literature (Foulon et al., 2018). Then, we calculated the percentage of subjects with a disconnected tract. We also expressed the probability of each tract to be disconnected with IC 95%. These analyses were performed separately for each tract. The proportion of fibers disconnected was expressed as a mean value and the ranges of IC 95% for all subjects.
Then, we assessed whether VF impairment after surgery is associated with: (a) proportion fibers disconnection; (b) number of tract disconnected; (c) lesion of a specific tract, using a logistic regression. These statistical analyses were performed separately for each hemisphere (dominant and nondominant).
p < 0.05 was considered statistically significant. Statistical analysis was performed with R.3.4.2 software.
3. RESULTS
Forty‐eight patients (mean age, 61.3 ± 7.5 years old; range, 40–73, years; mean disease duration, 16.2 ± 4.9 years; range 7–30 years) were included. Six months after DBS‐STN, the number of total words in the semantic fluency was significantly reduced by 15% in the on drug/on stimulation conditions in the global population (p < 0,001). Meanwhile the number of total words in the phonemic fluency was significantly reduced by 17% in the same condition (p < 0.001). Dopaminergic drugs, expressed in dopa‐equivalents, were reduced by 48% (range, 9.6–100%), from 1,150 ± 727 mg/day preoperatively to 450 ± 263 mg/day at 6 month (p < 0.001) (Table 1).
Table 1.
Characteristics of the population
| Baseline | 6 months | |
|---|---|---|
| Number | 48 | |
| Age at surgery (y) | 61.3 ± 7.5 | |
| PD duration before surgery (y) | 16.2 ± 4.9 | |
| Dopaminergic drugs | 1,150 ± 727 | 450 ± 263* |
| Verbal fluency | ||
| Semantic fluency | 27.4 ± 7.4 | 23.4 ± 8.2* |
| Phonemic fluency | 20.6 ± 7.7 | 16.4 ± 6.6* |
Note. PD, Parkinson disease; y, years. Dopaminergic drugs are expressed in dopa‐equivalent daily dose (mg/day). Values are given as mean ± standard deviation.
Comparison of scores obtained before surgery and respectively 6 months after surgery used a Wilcoxon signed‐rank test (*p < 0.001).
3.1. Probability and proportion white matter tracts crossed by electrode trajectories
Atlas‐based analysis reveals that the trajectory of the electrodes intersected successively with the frontal aslant tract, anterior segment of arcuate tract, the long segment of arcuate tract, the inferior longitudinal fasciculus, the superior longitudinal fasciculus, the anterior thalamic radiation, and the fronto striatal tract (Table 2). None of the patients had a probability >50% to have fronto insular, uncinate or fronto‐occipital fasciculus tracts lesioned. The proportion fibers disconnection is listed in Table 3. Inferior longitudinal fasciculus and frontal aslant tract are the most affected tracts.
Table 2.
Number of patients with a tract disconnected
| White‐matter tract | Number of patients (% of patients) | IC 95% probability of lesion |
|---|---|---|
| Ant thalamic projections left | 48 (100) | [92.7–100] |
| Ant thalamic projections right | 48 (100) | [92.7–100] |
| Arcuate ant segment left | 0 (0) | [0–7.4] |
| Arcuate ant segment right | 42 (87.5) | [74.8–95.3] |
| Arcuate long segment left | 1 (2.1) | [0.1–11.1] |
| Arcuate long segment right | 34 (70.9) | [56–83.1] |
| Frontal aslant left | 48 (100) | [92.7–100] |
| Frontal aslant right | 48 (100) | [92.7–100] |
| Frontal Inf longitudinal left | 41 (85.4) | [72.2–93.9] |
| Frontal Inf longitudinal right | 47 (97.9) | [88.9–99.9] |
| Fronto striatal left | 48 (100) | [92.7–100] |
| Fronto striatal right | 48 (100) | [92.7–100] |
| Superior longitudinal fasciculus I left | 33 (68.8) | [53.8–81.4] |
| Superior longitudinal fasciculus I right | 24 (50) | [35.3–64.8] |
| Superior longitudinal fasciculus II left | 47 (98) | [89–100] |
| Superior longitudinal fasciculus II right | 48 (100) | [92.7–100] |
| Superior longitudinal fasciculus III left | 43 (89.6) | [77.4–96.6] |
| Superior longitudinal fasciculus III right | 48 (100) | [92.7–100] |
Note. Values are given as number of subjects (%) and IC 95% of probabilities for all subjects.
Table 3.
Proportion fibers disconnection
| White‐matter tract | Proportion | IC 95% |
|---|---|---|
| Anterior Thalamic_Projections left | 2.46.10−3 | [0.15–5.07].10−3 |
| Anterior thalamic projections right | 1.77.10−3 | [0.02–4.12].10−3 |
| Arcuate anterior Segment_Right | 1.26.10−3 | [0.09–4.13].10−3 |
| Arcuate long segment right | 1.82.10−3 | [0.07–8.25].10−3 |
| Arcuate anterior segment left | 0 | [0–0] |
| Arcuate long segment left | 0 | [0–0] |
| Frontal aslant tract left | 3.89.10−3 | [0.9–10.67].10−3 |
| Frontal aslant tract right | 3.54.10−3 | [1.53–10.47].10−3 |
| Frontal commissural | 1.29.10−3 | [0.63–3.31].10−3 |
| Frontal inferior longitudinal left | 6.25.10−3 | [0.23–19.41].10−3 |
| Frontal inferior longitudinal right | 5.96.10−3 | [5.41–10.63].10−3 |
| Fronto striatal left | 2.57.10−3 | [1.05–7.66].10−3 |
| Fronto striatal right | 2.76.10−3 | [1.23–7.96].10−3 |
| Superior longitudinal fasciculus I left | 0.45.10−3 | [0.01–1.29].10−3 |
| Superior longitudinal fasciculus I right | 0.74.10−3 | [0.05–2.21].10−3 |
| Superior longitudinal fasciculus II left | 2.87.10−3 | [0.02–6.51].10−3 |
| Superior longitudinal fasciculus II right | 2.77.10−3 | [0.53–5.69].10−3 |
| Superior longitudinal fasciculus III left | 1.36.10−3 | [0.17–5.91].10−3 |
| Superior longitudinal fasciculus III right | 0.94.10−3 | [0.01–3.07].10−3 |
Note. Values are given as mean and range of proportion for all subjects.
Proportion fibers disconnection was calculated by dividing the number of damaged voxels within the ROI by the total volume of the tract.
3.2. Number of damaged tracts
All subjects had at least 13 tracts disconnected, and the majority (N = 19) had 14 tracts disconnected.
3.3. Association between damage of fiber pathways and VF impairment after surgery
We found no association between VF decline after surgery and (a) proportion fibers disconnection; (b) number of tract disconnected; (c) lesion of a specific tract. We obtained similar results for both brain hemispheres assessed together and separately.
4. DISCUSSION
The primary objective of this study was to describe the damage of https://www.sciencedirect.com/topics/neuroscience/white-matter fiber tracts following electrode implantation for STN‐DBS in PD patients. Thus, we observed the lesion of six well‐known bundles involved in VF tasks (i.e., frontal aslant tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, arcuate tract, fronto striatal tracts, and the anterior thalamic radiation) along the electrode trajectories. Furthermore, we attempted to relate structural brain damage to speech fluency and found no association between the damage of fiber pathways and the severity of VF impairment 6 months after surgery.
Growing evidence suggests that the integrity of white matter tracts constitutes a critical factor related to preservation of VF in some neurological disorders. Both VF semantic and phonemic tasks depend on shared cognitive processes (i.e., executive function, attention, language) and distinct ones (e.g., semantic vs. phonemic memory) (Biesbroek et al., 2016; Ruff, Light, Parker, & Levin, 1997; Unsworth, Spillers, & Brewer, 2011). Regions shared by semantic and phonemic fluency are localized in the left https://www.sciencedirect.com/topics/neuroscience/frontal-lobe (Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001; Robinson, Shallice, Bozzali, & Cipolotti, 2012), https://www.sciencedirect.com/topics/neuroscience/parietal-lobe and https://www.sciencedirect.com/topics/neuroscience/thalamus (Birn et al., 2010; Wagner, Sebastian, Lieb, Tüscher, & Tadić, 2014; Whitney et al., 2009). Studies that have investigated the relationship between VF and brain activity following STN‐DBS in PD patients suggested that the decline in performance may reflect dysfunction in frontal lobe‐related cognitive functions (Cilia et al., 2007; Kalbe et al., 2009; Schroeder et al., 2003).
In the present study, the atlas‐based analysis showed that the proximal trajectory of the electrode crossed several frontal tracts, among which one of the most significantly damaged was the left frontal aslant tract (Figure 1). As it is a direct pathway connecting posterior Broca's region with the anterior cingulate and pre‐supplementary motor area, left frontal aslant tract has been described as playing a major role in VF. In fact, Catani et al. (2013) demonstrated that decline of VF in primary progressive aphasia subjects was associated with degeneration of frontal aslant tract. Furthermore, it has been reported that patients with periventricular white matter frontal lesions had impaired VF which could be explained by frontal aslant tract damage. Likewise, resection of glioma close to the left frontal aslant tract was associated with transient speech initiation disorders (Kinoshita et al., 2015).
Figure 1.
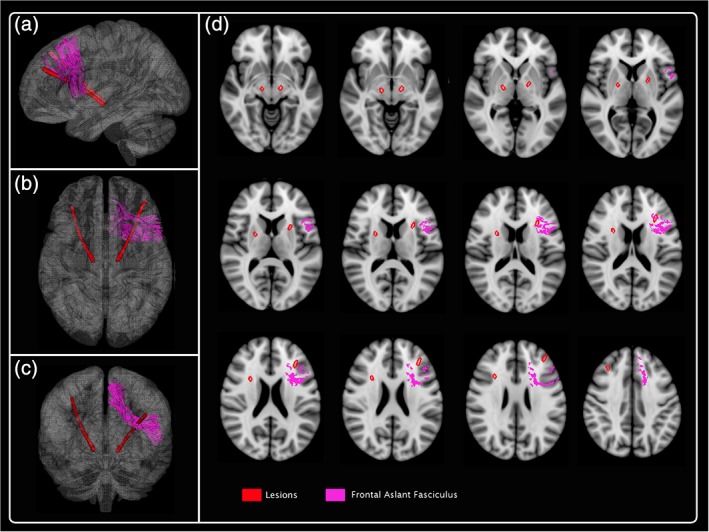
3D reconstruction of one subject: frontal aslant fasciculus. The lesion mask along the electrode trajectory transformed into the MNI 152 coordinate system was compared with white matter tract atlas in Tractotron software. The left frontal aslant tract is one of the most significantly damaged tract
Additionally, we found that the electrode impacted the three components of the superior longitudinal fasciculus which combine U‐shaped tracts connecting motor, premotor and prefrontal regions (Figure 2). Especially, the most posterior component, SLF I, connects the superior and medial parietal cortex to the dorsal and medial cortex of the frontal lobe, particularly pars opercularis of Broca's area, and the supplementary motor cortex. SLF II links caudal part of the inferior parietal lobule and various dorsolateral and ventrolateral frontal regions whereas the more ventral component, SLF III, connects the supramarginal gyrus with the ventral premotor and prefrontal cortex (Schmahmann et al., 2007). This tract has been found to be associated with language articulation (Han et al., 2016; Johnson et al., 2015), processing speed (Kerchner et al., 2012), and working memory (Vestergaard et al., 2011). For instance, the superior longitudinal fasciculus has been identified to contribute to VF performance in schizophrenia (Peters et al., 2012) since reduced integrity of the superior longitudinal fasciculus correlated with poorer VF. Similarly, it has been reported that VF performance can be predicted by the degree of white matter damage to the left superior longitudinal fasciculus, with poorer performance associated with larger lesions (Cristofori et al., 2015). More interestingly, evidence from recent studies in patients with newly diagnosed PD indicate that degeneration of central white matter tracts occurs early in PD and may underlie early cognitive dysfunction (Duncan et al., 2016). Specifically, degeneration of frontal white matter tracts including superior longitudinal fasciculus seems correlated with performance on the semantic VF.
Figure 2.
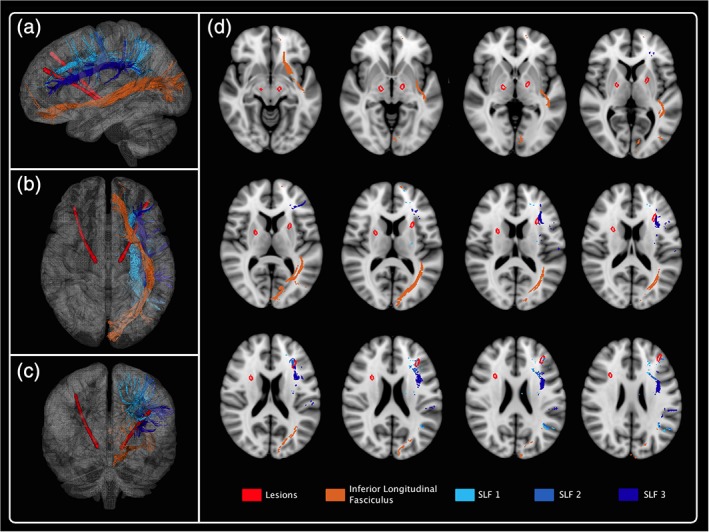
3D reconstruction of one subject: superior longitudinal fasciculus I, II, and III and inferior longitudinal fasciculus. The electrode impacted the three components of the superior longitudinal fasciculus and crossed the inferior longitudinal fasciculus [Color figure can be viewed at http://wileyonlinelibrary.com]
Further along the trajectory, we found that the electrodes crossed the inferior longitudinal fasciculus which connects the occipital lobe with the anterior part of the temporal lobe (Figure 2). It has also been suggested that the inferior longitudinal fasciculus plays a role in semantic processing in PD patients (Duncan et al., 2016). Nevertheless, other studies suggested that damage to this tract may not be necessary or sufficient to lead to a semantic deficit (Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007).
Thereafter, the most posterior track of the electrodes damaged the arcuate fasciculus, a large white matter bundle that arches around the lateral sulcus and connected the two main language fields, Broca's and Wernicke's areas (Figure 3). Indeed, this pathway links superior temporal gyrus to inferior frontal gyrus, but a larger subset of connections within this fasciculus connect the middle and inferior temporal gyri to the inferior precentral and inferior frontal gyrus (Catani et al., 2008). It thus provides a physical connection between regions critically involved in phonological or linguistic processing. Disruption of the arcuate fasciculus has been consistently linked with conduction aphasia. Additionally, studies reported syntactic deficits in patients with primary progressive aphasia after damage of arcuate fasciculus suggesting that the role of this tract is not limited to single word processing (Wilson, Galantucci, Tartaglia, & Gorno‐Tempini, 2012). More recently, studies suggested a possible correlation between right hemisphere arcuate fasciculus abnormalities and deficit in VF in psychosis (Kenney et al., 2017).
Figure 3.
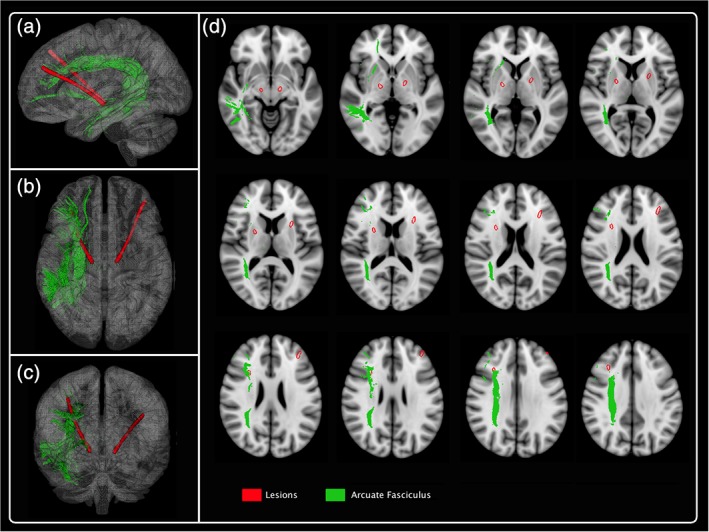
3D reconstruction of one subject: arcuate fasciculus. The most posterior track of the electrodes damaged the arcuate fasciculus [Color figure can be viewed at http://wileyonlinelibrary.com]
Downwards, electrode trajectories intersected the fronto striatal fasciculus (Figure 4). This white matter tract, neighboring the lateral angle of the anterior horn of lateral ventricle connects cingulate gyrus and SMA to the caudate nucleus. Fibers arising from the supero‐medial part of the head of the caudate nucleus have been found to be associated with selection/inhibition of language (Gil Robles, Gatignol, Capelle, Mitchell, & Duffau, 2005). Accordingly, previous studies argued that extensive lesion of the fronto striatal fasciculus may explain limitation in the initiation and preparation of spontaneous speech. Notably, nonfluency aphasia occurred when a stroke is located in the most medial portion of the fronto striatal fasciculus (Naeser, Palumbo, Helm‐Estabrooks, Stiassny‐Eder, & Albert, 1989). In addition, Kinoshita et al. (2015) showed that resection of glioma close to the left fronto striatal fasciculus was associated with both semantic and phonemic VF disorders. More recent studies combining intraoperative direct stimulation mapping and DTI demonstrated the involvement of the right and left fronto striatal fasciculus in motor speech control (Duffau, 2015).
Figure 4.

3D reconstruction of one subject: fronto striatal fasciculus and anterior thalamic radiation. The distal portion of the electrode crossed successively the fronto striatal fasciculus and the anterior thalamic radiation [Color figure can be viewed at http://wileyonlinelibrary.com]
Finally, the distal portion of the electrode crossed the anterior thalamic radiation (Figure 4). This white matter fascicule links the anterior and midline nuclear groups of the thalamus to the ipsilateral pre‐frontal cortex, with reciprocal connections. Several evidences have shown that the left anterior thalamic radiation plays a causal role in semantic processing (Mirman et al., 2015), working memory (Mamah et al., 2010) and executive function (Van der Werf et al., 2003). This different cognitive process may reflect both the prefrontal lobe‐ and thalamus‐related functions. Indeed, neuroimaging studies of aphasic patients with subcortical lesions have described reduced VF after damage to thalamic nuclei (Nadeau & Crosson, 1997; Radanovic & Scaff, 2003). Similarly, VF deficits have been described after vascular lesions of the anterior thalamus (Schmahmann, 2003). Accordingly, recent evidence from stereotactic surgery reported that electrical inhibition of the more anterior part of the thalamus influence word output dynamics during VF tasks on the level of word clustering (Ehlen et al., 2017).
To find https://www.sciencedirect.com/topics/neuroscience/white-matter pathways disruption that support VF decline after STN‐DBS, we separately assess the association between the damage of the major tracts and the performances of semantic and phonemic VF after surgery. Nevertheless, we failed to demonstrate the association between the microlesion of one specific tract and the severity of postoperative VF decline. Since we found that there are a number of functionally and anatomically distinct white matter bundles impaired by electrode implantation we then postulated that disconnection of several main tracts of the whole brain rather than only a limited damage may serve as an explanation for the VF impairment. Once more we did not find association. Several factors may explain these discrepancies.
First, it is crucial to note that all the patients underwent postoperative VF assessments 6 months after surgery. Indeed, we previously reported that VF performances deeply decrease immediately after STN implantation and remain worse 6 month after surgery compared with baseline on‐state. (Lefaucheur et al., 2012). This acute impairment, synonymous with microlesion, may reflect posttraumatic tissue reaction and perifocal oedema along and in the vicinity of definitive electrodes. Accordingly, previous studies have shown that impairment in frontal executive function and phonemic fluency tasks is transient (Zangaglia et al., 2009). Therefore, we assumed that other brain structures within the neural network for VF may compensate later after surgery the functional defect due to the acute cognitive microlesion effect. Moreover, all the patients underwent VF assessment 6 months after surgery in the on‐drug/ on‐stimulation condition. Then, the VF performance may be in part influenced by the electrical stimulation as previously described (Mikos et al., 2011; Schroeder et al., 2003). Altogether, these comments suggest that the damage of the major tracts may correlate better with acute VF decline.
The second methodological limitation of the study is the lack of an accurate quantification of the severity of the fiber tracts disconnection. Notably, we fixed 50% of probability to consider a tract as disconnected, as previously used in literature (Foulon et al., 2018). Moreover, we used as a surrogate measure of tract damage the proportion of the tract that was intersected by the electrodes trajectory. This value can provide an approximate estimate of the overall involvement of the fiber bundle. Nevertheless, it does not indicate whether the lesion affected critical fibers. Additionally, using an atlas based analysis each tract lesioned may not precisely match the exact individual anatomy of the 48 patients. Consequently, this may explain the lack of association between the proportion fiber disconnection and VF decline after surgery.
Third, it may be questioned whether white matter tract atlas in Tractotron software constitute a relevant quantitative imaging techniques to detect microlesion following electrodes trajectories. Few histopathological studies reported the pathological findings in brain of a PD patient treated by DBS (DiLorenzo, Jankovic, Simpson, Takei, & Powell, 2014). Mostly, authors concluded that surgical procedure causes minimal damage such as mild neuronal loss, mild to severe gliosis and spongiosis around the electrode tracts (Capparos‐Lefebvre, Ruchoux, Blond, Petit, & Percheron, 1994; Haberler et al., 2000; Jarraya et al., 2003). We nevertheless consider that even white matter microstructural abnormalities located in a key area could lead to significant impairment of cognitive function.
Fourth, we did not study the short length tracts surrounding the subthalamic nucleus area. Indeed, the connections separating the dorsal STN from the caudal aspect of the thalamus, that is, zona incerta, has been reported to be involved in VF decline after DBS (Fytagoridis et al., 2013). Furthermore, we focused on white matter tracts without considering that VF decline could be the consequence of the lesion of the ventral associative region of the STN itself (Yelnik, 2002).
Finally, the consequences of the white matter tracts disruption on cognitive performances may also depend upon numerous other factors, among which cortical thickness (Gerrits et al., 2016), disease duration and age (Brabo et al., 2014). In fact, previous studies have suggested that advanced age, low levodopa response and higher levodopa equivalent dose at baseline were associated with cognitive decline after DBS (Daniels et al., 2010; Smeding, Speelman, Huizenga, Schuurman, & Schmand, 2011). Nevertheless, meta‐analyses reported that none of the following factors, that is, age, disease duration, stimulation parameters, LED at baseline, LED change after surgery and UPDRS score off medication at baseline, were related to VF worsening after DBS (Combs et al., 2015; Parsons et al., 2006). This suggests that postoperative VF worsening may be due, above all, to microlesion and electrical stimulation.
5. CONCLUSION
Our study provides for the first time a neuroanatomical description of white matter tract disruption along the trajectories of electrode implanted for STN‐DBS in PD. Further studies are needed to determinate more specifically the role of each tract and its causal link with VF decline. Moreover, it would be interesting to know whether damage of fiber pathways correlates with other neuropsychological changes after subthalamic stimulation.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Costentin G, Derrey S, Gérardin E, et al. White matter tracts lesions and decline of verbal fluency after deep brain stimulation in Parkinson's disease. Hum Brain Mapp. 2019;40:2561–2570. 10.1002/hbm.24544
REFERENCES
- Almairac, F. , Herbet, G. , Moritz‐Gasser, S. , de Champfleur, N. M. , & Duffau, H. (2015). The left inferior fronto‐occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Structure and Function, 220(4), 1983–1995. 10.1007/s00429-014-0773-1 [DOI] [PubMed] [Google Scholar]
- Baldo, J. V. , Shimamura, A. P. , Delis, D. C. , Kramer, J. , & Kaplan, E. (2001). Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society, 7(5), 586–596. 10.1017/S1355617701755063 [DOI] [PubMed] [Google Scholar]
- Biesbroek, J. M. , van Zandvoort, M. J. , Kappelle, L. J. , Velthuis, B. K. , Biessels, G. J. , & Postma, A. (2016). Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion‐symptom mapping in patients with ischemic stroke. Brain Structure and Function, 221(4), 2123–2134. 10.1007/s00429-015-1033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn, R. M. , Kenworthy, L. , Case, L. , Caravella, R. , Jones, T. B. , Bandettini, P. A. , & Martin, A. (2010). Neural systems supporting lexical search guided by letter and semantic category cues: A self‐paced overt response fMRI study of verbal fluency. Neuroimage, 49(1), 1099–1107. 10.1016/j.neuroimage.2009.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabo, N. C. , Minett, T. S. , & Ortiz, K. Z. (2014). Fluency in Parkinson's disease: disease duration, cognitive status and age. Arquivos de Neuro‐Psiquiatria, 72(5), 349–355. 10.1590/0004-282X2014001 [DOI] [PubMed] [Google Scholar]
- Capparos‐Lefebvre, D. , Ruchoux, M. M. , Blond, S. , Petit, H. , & Percheron, G. (1994). Longterm thalamic stimulation in Parkinson's disease: Post‐mortem anatomoclinical study. Neurology, 44(10), 1856–1860. 10.1212/WNL.44.10.1856 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Mesulam, M. M. , Jakobsen, E. , Malik, F. , Martersteck, A. , Wieneke, C. , … Rogalski, E. (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136(Pt 8), 2619–2628. 10.1093/brain/awt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , & Mesulam, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex; a journal devoted to the study of the nervous system and behavior, 44(8), 953–961. 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia, R. , Siri, C. , Marotta, G. , De Gaspari, D. , Landi, A. , Mariani, C. B. , … Antonini, A. (2007). Brain networks underlining verbal fluency decline during STN‐DBS in Parkinson's disease: An ECD‐SPECT study. Parkinsonism Related Disorders, 13(5), 290–294. 10.1016/j.parkreldis.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Combs, H. L. , Folley, B. S. , Berry, D. T. R. , Segerstrom, S. C. , Han, D. Y. , Anderson‐Mooney, A. J. , … Van Horne, C. (2015). Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson's disease: A meta‐analysis. Neuropsychology Review, 25(4), 439–454. 10.1007/s11065-015-9302-0 [DOI] [PubMed] [Google Scholar]
- Cristofori, I. , Zhong, W. , Chau, A. , Solomon, J. , Krueger, F. , & Grafman, J. (2015). White and gray matter contributions to executive function recovery after traumatic brain injury. Neurology, 84(14), 1394–1401. 10.1212/WNL.0000000000001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, C. , Krack, P. , Volkmann, J. , Pinsker, M. O. , Krause, M. , Tronnier, V. , … Witt, K. (2010). Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson's disease. Movement Disorders, 25(11), 1583–1589. 10.1002/mds.23078 [DOI] [PubMed] [Google Scholar]
- Deuschl, G. , Schade‐Brittinger, C. , Krack, P. , Volkmann, J. , Schafer, H. , Botzel, K. , … Voges, J. (2006). A randomized trial of deep‐brain stimulation for Parkinson's disease. New England Journal of Medicine, 355(9), 896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]
- DiLorenzo, D. J. , Jankovic, J. , Simpson, R. K. , Takei, H. , & Powell, S. Z. (2014). Neurohistopathological findings at the electrode‐tissue interface in long‐term deep brain stimulation: Systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation, 17(5), 405–418. 10.1111/ner.12192 [DOI] [PubMed] [Google Scholar]
- Duffau, H. (2015). Stimulation mapping of white matter tracts to study brain functional connectivity. Nature Reviews Neurology, 11(5), 255–265. 10.1038/nrneurol.2015.51 [DOI] [PubMed] [Google Scholar]
- Duncan, G. W. , Firbank, M. J. , Yarnall, A. J. , Khoo, T. K. , Brooks, D. J. , Barker, R. A. , … O'Brien, J. T. (2016). Gray and white matter imaging: A biomarker for cognitive impairment in early Parkinson's disease? Movement Disorders, 31(1), 103–110. 10.1002/mds.26312 [DOI] [PubMed] [Google Scholar]
- Ehlen, F. , Vonberg, I. , Tiedt, H. O. , Horn, A. , Fromm, O. , Kühn, A. A. , & Klostermann, F. (2017). Thalamic deep brain stimulation decelerates automatic lexical activation. Brain and Cognition, 111, 34–43. 10.1016/j.bandc.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Foulon, C. , Cerliani, L. , Kinkingnéhun, S. , Levy, R. , Rosso, C. , Urbanski, M. , … Thiebaut de Schotten, M. (2018). Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience, 7(3), 1–17. 10.1093/gigascience/giy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytagoridis, A. , Sjöberg, R. L. , Åström, M. , Fredricks, A. , Nyberg, L. , & Blomstedt, P. (2013). Effects of deep brain stimulation in the caudal zona incerta on verbal fluency. Stereotactic and Functional Neurosurgery, 91(1), 24–29. 10.1159/000342497 [DOI] [PubMed] [Google Scholar]
- Gerrits, N. J. , van Loenhoud, A. C. , van den Berg, S. F. , Berendse, H. W. , Foncke, E. M. , Klein, M. , … van den Heuvel, O. A. (2016). Cortical thickness, surface area and subcortical volume differentially contribute to cognitive heterogeneity in Parkinson's disease. PLoS One, 26, 11(2) 10.1371/journal.pone.0148852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Robles, S. , Gatignol, P. , Capelle, L. , Mitchell, M. C. , & Duffau, H. (2005). The role of dominant striatum in language: A study using intraoperative electrical stimulations. Journal of Neurology, Neurosurgery and Psychiatry, 76(7), 940–946. 10.1136/jnnp.2004.045948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberler, C. , Alesch, F. , Mazal, P. R. , Pilz, P. , Jellinger, K. , Pinter, M. M. , … Budka, H. (2000). No tissue damage by chronic deep brain stimulation in Parkinson's disease. Annals of Neurology, 48(3), 372–376. [DOI] [PubMed] [Google Scholar]
- Han, Z. , Ma, Y. , Gong, G. , Huang, R. , Song, L. , & Bi, Y. (2016). White matter pathway supporting phonological encoding in speech production: A multi‐modal imaging study of brain damage patients. Brain Structure and Function, 221(1), 577–589. 10.1007/s00429-014-0926-2 [DOI] [PubMed] [Google Scholar]
- Haynes, W. I. , & Haber, S. N. (2013). The organization of prefrontal‐subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. Journal of Neuroscience, 33(11), 4804–4814. 10.1523/JNEUROSCI.4674-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, J. M. , Tkach, J. , Phillips, M. , Baker, K. , Shellock, F. G. , & Rezai, A. R. (2005). Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson's disease: Case report. Neurosurgery, 57(5), E1063 10.1227/01.NEU.0000180810.16964.3E [DOI] [PubMed] [Google Scholar]
- Hirshorn, E. A. , & Thompson‐Schill, S. L. (2006). Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia, 44(12), 2547–2557. 10.1016/j.neuropsychologia.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Jarraya, B. , Bonnet, A. M. , Duyckaerts, C. , Houeto, J. L. , Cornu, P. , Hauw, J. J. , & Agid, Y. (2003). Parkinson's disease, subthalamic stimulation, and selection of candidates: A pathological study. Movement Disorders, 18(12), 1517–1520. 10.1002/mds.10607 [DOI] [PubMed] [Google Scholar]
- Johnson, C. P. , Juranek, J. , Swank, P. R. , Kramer, L. , Cox, C. S., Jr. , & Ewing‐Cobbs, L. (2015). White matter and reading deficits after pediatric traumatic brain injury: A diffusion tensor imaging study. Neuroimage Clinical, 19(9), 668–677. 10.1016/j.nicl.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe, E. , Voges, J. , Weber, T. , Haarer, M. , Baudrexel, S. , Klein, J. C. , … Hilker, R. (2009). Frontal FDG‐PET activity correlates with cognitive outcome after STN‐DBS in Parkinson disease. Neurology, 72(1), 42–49. 10.1212/01.wnl.0000338536.31388.f0 [DOI] [PubMed] [Google Scholar]
- Kenney, J. P. M. , McPhilemy, G. , Scanlon, C. , Najt, P. , McInerney, S. , Arndt, S. , … Cannon, D. M. (2017). The arcuate fasciculus network and verbal deficits in psychosis. Translational Neuroscience, 2(8), 117–126. 10.1515/tnsci-2017-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner, G. A. , Racine, C. A. , Hale, S. , Wilheim, R. , Laluz, V. , Miller, B. L. , & Kramer, J. H. (2012). Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS One, 7(11), e50425 10.1371/journal.pone.0050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, M. , de Champfleur, N. M. , Deverdun, J. , Moritz‐Gasser, S. , Herbet, G. , & Duffau, H. (2015). Role of fronto‐striatal tract and frontal aslant tract in movement and speech: An axonal mapping study. Brain Structure and Function, 220(6), 3399–3412. 10.1007/s00429-014-0863-0 [DOI] [PubMed] [Google Scholar]
- Le Goff, F. , Derrey, S. , Lefaucheur, R. , Borden, A. , Fetter, D. , Jan, M. , … Maltête, D. (2015). Decline in verbal fluency after subthalamic nucleus deep brain stimulation in Parkinson's disease: A microlesion effect of the electrode trajectory? Journal of Parkinson's Disease, 5(1), 95–104. 10.3233/JPD-140443 [DOI] [PubMed] [Google Scholar]
- Lefaucheur, R. , Derrey, S. , Martinaud, O. , Wallon, D. , Chastan, N. , Gerardin, E. , … Maltête, D. (2012). Early verbal fluency decline after STN implantation: Is it a cognitive microlesion effect? Journal of the Neurological Sciences, 321(1–2), 96–99. 10.1016/j.jns.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Li, M. , Zhang, Y. , Song, L. , Huang, R. , Ding, J. , Fang, Y. , … Han, Z. (2017). Structural connectivity subserving verbal fluency revealed by lesion‐behavior mapping in stroke patients. Neuropsychologia, 101, 85–96. 10.1016/j.neuropsychologia.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Mamah, D. , Conturo, T. E. , Harms, M. P. , Akbudak, E. , Wang, L. , McMichael, A. R. , … Csernansky, J. G. (2010). Anterior thalamic radiation integrity in schizophrenia: A diffusion‐tensor imaging study. Psychiatry Research, 183(2), 144–150. 10.1016/j.pscychresns.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet, E. , Nouet, A. , Gatignol, P. , Capelle, L. , & Duffau, H. (2007). Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain, 130(Pt 3), 623–629. 10.1093/brain/awl361 [DOI] [PubMed] [Google Scholar]
- Mikos, A. , Bowers, D. , Noecker, A. M. , McIntyre, C. C. , Won, M. , Chaturvedi, A. , … Okun, M. S. (2011). Patient‐specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. NeuroImage, 54, S238–S246. 10.1016/j.neuroimage.2010.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman, D. , Chen, Q. , Zhang, Y. , Wang, Z. , Faseyitan, O. K. , Coslett, H. B. , & Schwartz, M. F. (2015). Neural organization of spoken language revealed by lesion‐symptom mapping. Nature Communications, 16(6), 6762 10.1038/ncomms7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, S. E. , & Crosson, B. (1997). Subcortical aphasia. Brain and Language, 58(3), 355–402; discussion 418‐23. 10.1006/brln.1997.1707 [DOI] [PubMed] [Google Scholar]
- Naeser, M. A. , Palumbo, C. L. , Helm‐Estabrooks, N. , Stiassny‐Eder, D. , & Albert, M. L. (1989). Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain, 112(Pt 1), 1–38 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2917272 [DOI] [PubMed] [Google Scholar]
- Okun, M. S. , Fernandez, H. H. , Wu, S. S. , Kirsch‐Darrow, L. , Bowers, D. , Bova, F. , … Foote, K. D. (2009). Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE trial. Annals of Neurology, 65(5), 586–595. 10.1002/ana.21596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun, M. S. , Gallo, B. V. , Mandybur, G. , Jagid, J. , Foote, K. D. , Revilla, F. J. , … Tagliati, M. (2012). Subthalamic deep brain stimulation with a constant‐current device in Parkinson's disease: An open‐label randomised controlled trial. Lancet Neurology, 11(2), 140–149. 10.1016/S1474-4422(11)70308-8 [DOI] [PubMed] [Google Scholar]
- Papagno, C. , Miracapillo, C. , Casarotti, A. , Romero Lauro, L. J. , Castellano, A. , Falini, A. , … Bello, L. (2011). What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain, 134(Pt 2), 405–414. 10.1093/brain/awq283 [DOI] [PubMed] [Google Scholar]
- Parsons, T. D. , Rogers, S. A. , Braaten, A. J. , Woods, S. P. , & Troster, A. I. (2006). Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: A meta‐analysis. Lancet Neurology, 5(7), 578–588. 10.1016/S1474-4422(06)70475-6 [DOI] [PubMed] [Google Scholar]
- Paulesu, E. , Goldacre, B. , Scifo, P. , Cappa, S. F. , Gilardi, M. C. , Castiglioni, I. , … Fazio, F. (1997). Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport, 8(8), 2011–2017. 10.1097/00001756-199705260-00042 [DOI] [PubMed] [Google Scholar]
- Peters, B. D. , Szeszko, P. R. , Radua, J. , Ikuta, T. , Gruner, P. , DeRosse, P. , … Malhotra, A. K. (2012). White matter development in adolescence: Diffusion tensor imaging and meta‐analytic results. Schizophrenia Bulletin, 38(6), 1308–1317. 10.1093/schbul/sbs054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanovic, M. , & Scaff, M. (2003). Speech and language disturbances due to subcortical lesions. Brain and Language, 84(3), 337–352. 10.1016/S0093-934X(02)00554-0 [DOI] [PubMed] [Google Scholar]
- Robinson, G. , Shallice, T. , Bozzali, M. , & Cipolotti, L. (2012). The differing roles of the frontal cortex in fluency tests. Brain, 135(Pt 7), 2202–2214. 10.1093/brain/aws142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, R. M. , Light, R. H. , Parker, S. B. , & Levin, H. S. (1997). The psychological construct of word fluency. Brain and Language, 57(3), 394–405. 10.1006/brln.1997.1755 [DOI] [PubMed] [Google Scholar]
- Saleh, C. (2010). Knowing the limitations of applied deep brain stimulation technology for a clearer understanding of clinical outcomes. Journal of the Neurological Sciences, 292(1–2), 119 10.1016/j.jns.2010.01.027 [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. (2003). Vascular syndromes of the thalamus. Stroke, 34(9), 2264–2278. 10.1161/01.STR.0000087786.38997.9E [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Pandya, D. N. , Wang, R. , Dai, G. , D'Arceuil, H. E. , de Crespigny, A. J. , & Wedeen, V. J. (2007). Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain, 130(Pt 3), 630–653. 10.1093/brain/awl359 [DOI] [PubMed] [Google Scholar]
- Schroeder, U. , Kuehler, A. , Lange, K. W. , Haslinger, B. , Tronnier, V. M. , Krause, M. , … Ceballos‐Baumann, A. O. (2003). Subthalamic nucleus stimulation affects a frontotemporal network: A PET study. Annals of Neurology, 54(4), 445–450. 10.1002/ana.10683 [DOI] [PubMed] [Google Scholar]
- Schwartz, S. , Baldo, J. , Graves, R. E. , & Brugger, P. (2003). Pervasive influence of semantics in letter and category fluency: A multidimensional approach. Brain and Language, 87(3), 400–401. 10.1016/S0093-934X(03)00141-X [DOI] [PubMed] [Google Scholar]
- Smeding, H. M. M. , Speelman, J. D. , Huizenga, H. M. , Schuurman, P. R. , & Schmand, B. (2011). Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry, 82(7), 754–760. 10.1136/jnnp.2007.140012 [DOI] [PubMed] [Google Scholar]
- Unsworth, N. , Spillers, G. J. , & Brewer, G. A. (2011). Variation in verbal fluency: A latent variable analysis of clustering, switching, and overall performance. Quarterly Journal of Experimental Psychology (Hove), 64(3), 447–466. 10.1080/17470218.2010.505292 [DOI] [PubMed] [Google Scholar]
- Van der Werf, Y. D. , Scheltens, P. , Lindeboom, J. , Witter, M. P. , Uylings, H. B. , & Jolles, J. (2003). Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia, 41(10), 1330–1344. 10.1016/S0028-3932(03)00059-9 [DOI] [PubMed] [Google Scholar]
- Vestergaard, M. , Madsen, K. S. , Baaré, W. F. , Skimminge, A. , Ejersbo, L. R. , Ramsøy, T. Z. , … Jernigan, T. L. (2011). White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. Journal of Cognitive Neuroscience, 23(9), 2135–2146. 10.1162/jocn.2010.21592 [DOI] [PubMed] [Google Scholar]
- Wagner, S. , Sebastian, A. , Lieb, K. , Tüscher, O. , & Tadić, A. (2014). A coordinate‐based ALE functional MRI meta‐analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neuroscience, 24(15), 19 10.1186/1471-2202-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney, C. , Weis, S. , Krings, T. , Huber, W. , Grossman, M. , & Kircher, T. (2009). Task‐dependent modulations of prefrontal and hippocampal activity during intrinsic word production. Journal of Cognitive Neuroscience, 21(4), 697–712. 10.1162/jocn.2009.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. M. , Galantucci, S. , Tartaglia, M. C. , & Gorno‐Tempini, M. L. (2012). The neural basis of syntactic deficits in primary progressive aphasia. Brain and Language, 122(3), 190–198. 10.1016/j.bandl.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt, K. , Daniels, C. , Reiff, J. , Krack, P. , Volkmann, J. , Pinsker, M. O. , … Deuschl, G. (2008). Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: A randomised, multicentre study. Lancet Neurology, 7(7), 605–614. 10.1016/S1474-4422(08)70114-5 [DOI] [PubMed] [Google Scholar]
- Witt, K. , Granert, O. , Daniels, C. , Volkmann, J. , Falk, D. , Van Eimeren, T. , & Deuschl, G. (2013). Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain, 136(7), 2109–2119. 10.1093/brain/awt151 [DOI] [PubMed] [Google Scholar]
- Wu, B. , Han, L. , Sun, B. M. , Hu, X. W. , & Wang, X. P. (2014). Influence of deep brain stimulation of the subthalamic nucleus on cognitive function in patients with Parkinson's disease. Neuroscience Bulletin, 30(1), 153–161. 10.1007/s12264-013-1389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik, J. (2002). Functional anatomy of the basal ganglia. Movement Disorders, 17(Suppl. 3), S15–S21. 10.1002/mds.10138 [DOI] [PubMed] [Google Scholar]
- Zangaglia, R. , Pacchetti, C. , Pasotti, C. , Mancini, F. , Servello, D. , Sinforiani, E. , … Nappi, G. (2009). Deep brain stimulation and cognitive functions in Parkinson's disease: A three‐year controlled study. Movement Disorders, 24(11), 1621–1628. 10.1002/mds.22603 [DOI] [PubMed] [Google Scholar]


