Abstract
Periodontitis is one of the most common chronic oral inflammatory conditions worldwide and is associated with a risk of developing oral squamous cell carcinoma (OSCC). Porphyromonas gingivalis is a major pathogen in periodontitis, and its lipopolysaccharide (LPS) promotes the expression of cyclooxygenase-2 (COX-2) in OSCC both in vivo and in vitro. Celecoxib is a selective COX-2 inhibitor; however, its antitumor effects on P. gingivalis LPS-stimulated OSCC and the underlying molecular mechanism remain unclear. To elucidate the association between periodontitis and OSCC, the effect of P. gingivalis-derived LPS on OSCC cell proliferation was examined both in vitro and in vivo in the present study. The expression levels of COX-2 and p53 in OSCC cells with/without celecoxib treatment were determined via western blotting. The therapeutic potential of celecoxib in LPS-stimulated OSCC was evaluated by staining for Ki-67 and p21, as well as with terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling staining. LPS treatment significantly increased OSCC cell proliferation in vitro, and celecoxib significantly inhibited cell proliferation with/without LPS treatment. Celecoxib treatment of OSCC cells downregulated the protein expression levels of COX-2 compared with untreated cells, but there was little change in p53 expression. In the mouse xenograft model, oral administration of celecoxib significantly suppressed tumor growth, reduced the expression of Ki-67, increased the apoptosis index and induced p21 expression with/without LPS treatment. The results from the present study demonstrate that P. gingivalis' LPS can stimulate tumor growth by interacting with OSCC cells. In conclusion, these results suggest that celecoxib could be used for the effective prevention and treatment of LPS-stimulated OSCC.
Keywords: oral squamous cell carcinoma, periodontal disease, lipopolysaccharide, Porphyromonas gingivalis, celecoxib
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common malignant epithelial tumors that arises in the oral cavity (estimated 263,900 new cases in 2008 worldwide) (1). Although combination treatments employing surgical resection and adjuvant treatments such as radiotherapy and/or chemotherapy have improved, the disease-free and overall survival rates in advanced OSCC have not improved (2,3).
Periodontitis is a common chronic oral inflammatory disease that causes destruction of periodontal tissues (4). Recent studies have demonstrated that patients with periodontal disease have a 2–5-fold greater risk of developing OSCC (4). One of the major pathogens in periodontal disease is the gram-negative, anaerobic bacterium Porphyromonas gingivalis. It can adhere to both epithelial cells and gingival fibroblasts, and induce the expression of pro-inflammatory cytokines involved in the progression of periodontitis. Its primary virulence factor is lipopolysaccharide (LPS) (5), which has recently been revealed to play an important role in the migration, invasion, lymphangiogenesis and metastasis of various types of malignant tumor, including OSCC (6,7).
The induction of cyclooxygenase-2 (COX-2), an enzyme that promotes cell proliferation and invasion, and suppresses apoptosis (8), is considered to be one of the key mechanisms by which LPS affects OSCC cells (9,10). Upregulated COX-2 is frequently observed in head and neck squamous cell carcinoma (HNSCC) (11), where it is associated with a lower survival rate in patients with this disease (12). Inhibition of COX-2 thus represents a potential approach for OSCC therapy. Celecoxib is a selective COX-2 inhibitor that has recently been demonstrated to possess antitumor effects in HNSCC (13,14); however, its method of administration in OSCC has not yet been established.
In the present study, to elucidate the association between chronic periodontitis and OSCC progression, the effect of P. gingivalis-derived LPS on OSCC cell proliferation was examined both in vitro and in vivo. The antitumor effects of celecoxib in an LPS-stimulated OSCC xenograft were evaluated by assessing cell proliferation, apoptosis and the cell cycle. Finally, the present study discusses the molecular mechanisms underlying chronic periodontitis and OSCC, and the therapeutic potential of celecoxib.
Materials and methods
Cell culture
The human OSCC cell line HSC-3 (Japanese Cancer Research Resources Bank) was maintained in α-minimum essential medium (α-MEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Biowest), 100 IU/ml penicillin and 100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2 atmosphere.
Cell viability assay
The effect of celecoxib (Combi-Blocks, Inc.) or P. gingivalis' LPS (InvivoGen, Inc.) on cell viability was assessed using an MTS assay. Celecoxib was dissolved in dimethyl sulfoxide (DMSO; Wako Pure Chemical Industries, Ltd.). The levels of DMSO were minimized to avoid any potential DMSO-associated toxic effects in the MTS assay. In all cases, the final DMSO concentration was <0.1% in the cell cultures. HSC-3 cells were plated at a density of 5,000 cells/well in 96-well plates and incubated for 48 h at 37°C. Cell cultures were exposed to celecoxib (0, 100 and 200 µM), LPS (10 µg/ml), or a combination of the two, for 24 or 48 h. Following this, 20 µl of CellTiter 96 AQueous One Solution Reagent (Promega Corporation) was added to each well and incubated for 2 h at 37°C. The absorbance at 490 nm in each well was then determined (SpectraMax M5; Molecular Devices, LLC).
Western blot analyses of COX-2 and p53
HSC-3 cells were lysed in RIPA lysis buffer (Santa Cruz Biotechnology, Inc.) containing 2 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate and 2% protease inhibitor cocktail. The protein concentration was quantified by DC Protein Assay (Bio-Rad Laboratories, Inc.). Protein samples (10 µg/lane) were separated using 10–20% gradient gels and electro-transferred to Immun-Blot PVDF Membranes (Bio-Rad Laboratories, Inc.). Membranes were blocked using EzBlock Chemi (1:5; ATTO Corporation) for 1 h at room temperature and then probed using the appropriate primary antibodies, including anti-COX-2 (1:1,000; catalog no. ab15191; Abcam), anti-p53 (1:1,000; catalog no. ab1101; Abcam) and anti β-actin (1:1,000; catalog no. 8H10D10; Cell Signaling Technology, Inc.) overnight at 4°C. After washing with Tris-buffered saline with Tween 20 (TBS-T) three times, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-mouse IgG antibody (1:10,000; catalog no. 115-035-072; Jackson ImmunoResearch Laboratories, Inc.) for anti-p53 and anti β-actin or an HRP-conjugated AffiniPure goat anti-rabbit IgG antibody (1:10,000; catalog no. 111-035-144; Jackson ImmunoResearch Laboratories, Inc.) for anti-COX-2 for 1 h at room temperature. Chemiluminescent signals were developed with Western Lightning ECL Pro (PerkinElmer, Inc.) and detected using a cooled charge-coupled device camera (LAS 4000 Mini; GE Healthcare Life Sciences).
Nude mouse tumor model
A total of 32 5-week-old female nude BALB/c nu/nu mice (21.4±1.2 g; CLEA Japan, Inc.) were maintained at 21–25°C and 40–70% humidity in a 12-h dark/light cycle, with continuous free access to food and water. For stimulation, HSC-3 cells were exposed to 10 µg/ml LPS for 48 h before implantation. HSC-3 and LPS-stimulated HSC-3 cells (5×106 cells in 50 µl α-MEM) were then mixed with an equal volume of Matrigel (BD Biosciences) and injected into the flanks of mice during a short period of anesthesia with 2% isoflurane (Abbott Pharmaceutical Co., 4 Ltd.). Tumor-bearing mice were then randomly divided into four groups (n=8 in each group): i) A control group; ii) an LPS-treated group; iii) a celecoxib-treated group; and iv) an LPS + celecoxib-treated group. In all cases, tumors were allowed to grow to ~60 mm3 prior to the treatment. The celecoxib-treated group and LPS + celecoxib-treated group were fed powder feed containing 1,500 ppm celecoxib, as previously described (15); the control and LPS-treated groups were fed common powder feed alone. Mice were assessed twice a week for 28 days, and tumor growth was determined by estimating tumor volume, using the formula 0.5 × length × width2, as previously described (16). Each cage contained four mice; the dietary intake of powdered feed per day in the cage was measured to calculate an estimate of the dietary intake for each mouse. The limitation associated with this method of drug administration was that dietary intake was not an actual dose, but estimated dose. One mouse was euthanized when bleeding from the ulceration of the tumor was observed. On day 28 post-administration, the mice were euthanized using carbon dioxide gas (20%/min gradual displacement) and monitored for 5 min to confirm cardiac arrest, and the tumors were removed along with the surrounding tissue and overlying skin (2 mm from the tumor) for subsequent histological examination as previously described (16). The specimens were fixed immediately with 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin. All animal experiments were approved by the Animal Ethics Committee of the University of Fukui (no. 29105) and performed in accordance with the Guide for the Care and Use of Laboratory Animals (published by the National Institutes of Health) (17).
Immunohistochemistry
The paraffin-embedded tissues were sliced into 4 µm-thick sections. Ki-67 and p21 were stained using indirect immunoperoxidase staining (ImmPRESS Reagent kit; Vector Laboratories, Ltd.). Tissue sections were deparaffinized in Clear Plus (Falma, Co., Ltd.), dehydrated in 100% ethanol for 15 min at room temperature and autoclaved in 1 mM EDTA 2Na and 10 mM Tris buffer (pH 9.0) at 95°C for 30 min for antigen retrieval. Endogenous peroxide activity was eliminated by treatment with 0.3% H2O2 in methanol for 30 min at room temperature. The ImmPRESS reagent with 2.5% normal horse serum (undiluted; Vector Laboratories, Ltd.) was used to block non-specific immunoreactions for 10 min at room temperature. After incubation with a primary monoclonal rabbit anti-human antibody against either Ki-67 (1:100; catalog no. ab16667) or p21 (1:100; catalog no. ab109520) (both from Abcam) for 2 h at room temperature, the sections were incubated with ImmPRESS polymer anti-rabbit IgG reagent (undiluted; cat. no. MP-7800; Vector Laboratories, Ltd.) for 30 min at room temperature. Immunoreactivity was visualized using 3,3′-diaminobenzidine (Dojindo Molecular Technologies, Inc.) for 5 min at room temperature. The sections were also counterstained with hematoxylin for 5 min at room temperature. The sections were rinsed in PBS between all steps. Ki-67-stained or p21-stained tissue sections were observed under an Olympus AX80 light microscope (Olympus Corporation) at ×400 magnification, and the number of Ki-67- or p21-positive cells were counted in each individual microscopic field. At least five microscopic fields per section were used for the subsequent analysis.
Cell death assay
The terminal deoxynucleotidyl-transferase- mediated dUTP nick end labeling (TUNEL) method was used to evaluate apoptosis using the in situ Apoptosis Detection kit (Takara Bio, Inc.). Briefly, the sections were deparaffinized for 15 min, dehydrated in 100% ethanol for 15 min and permeabilized using 10 µg/ml proteinase K (Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min at room temperature. Endogenous peroxide activity was blocked with 3% H2O2 for 5 min at room temperature. The sections were incubated with 50 µl labeling reaction mixture (consisting of TdT Enzyme 5 µl + Labeling Safe Buffer 45 µl) for 90 min at 37°C and reacted with 70 µl anti-FITC horseradish peroxidase (undiluted; cat no. MK503; Takara Bio, Inc.) for 30 min at 37°C. Immunoreactivity was visualized using 3,3′-diaminobenzidine (Dojindo Molecular Technologies, Inc.). The sections were counterstained with hematoxylin for 5 min at room temperature. The apoptosis index was determined by calculating the ratio of the number of TUNEL-positive cells to the total number of tumor cells (avoiding necrotic tumor areas) from a minimum of five randomly selected microscopic fields in each individual section using an Olympus AX80 light microscope (Olympus Corporation) at ×400 magnification as previously described (16).
Statistical analysis
All experiments were independently repeated at least three times. Numerical values are expressed as the mean ± standard deviation. Differences between experimental groups were analyzed using one-way analysis of variance followed by Dunnett's test (cell viability at 24 and 48 h after celecoxib treatment), Turkey-Kramer multiple comparison test (cell viability, tumor volumes, mouse body weights, the expression levels of Ki-67 and p21 and apoptosis index in response to celecoxib with/without LPS treatment) and unpaired Student's t-test (cell viability 24 and 48 h after LPS treatment and the COX-2/β-actin ratio). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of celecoxib on HSC-3 cells in vitro
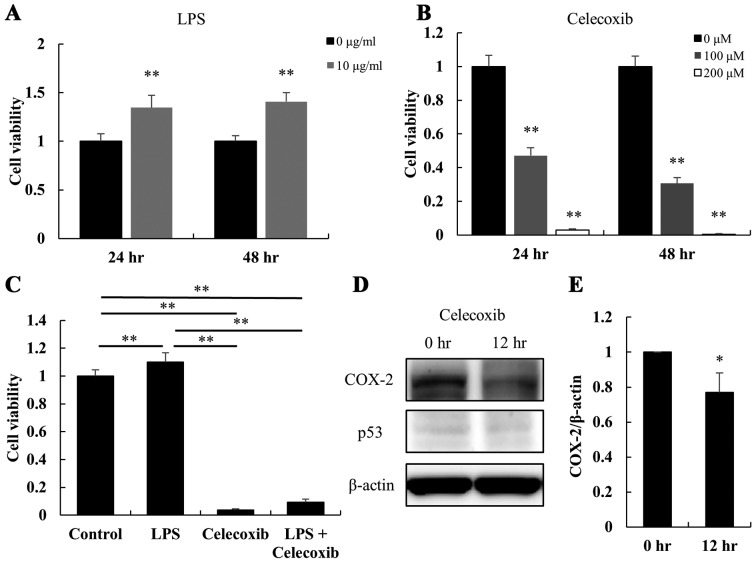
LPS treatment for 24 and 48 h increased the viability of HSC-3 cells (P<0.01; Fig. 1A); whereas, celecoxib decreased cell viability in a dose- and time-dependent manner (P<0.01; Fig. 1B), indicating that the cells were sensitive to celecoxib. The proliferation of LPS-treated HSC-3 cells was significantly inhibited by treatment with celecoxib (100 µM for 48 h) (P<0.01; Fig. 1C). The protein expression levels of COX-2 and p53 with/without celecoxib treatment were also examined in HSC-3 cells via western blotting. Compared with untreated cells, treatment of HSC-3 cells with 100 µM celecoxib downregulated the protein expression levels of COX-2 after 12 h, but there was little change in p53 expression levels (Fig. 1D). The COX-2/β-actin ratios in the HSC-3 cells were significantly decreased by the celecoxib treatment (Fig. 1E).
Figure 1.
Effect of LPS and celecoxib on the viability of HSC-3 cells. The viability of HSC-3 cells was determined using an MTS assay. (A) Cell viability 24 and 48 h after P. gingivalis' LPS treatment (10 µg/ml). (B) Cell viability at 24 and 48 h after treatment with celecoxib (100 and 200 µM). (C) Cell viability 48 h after treatment with celecoxib (100 µM), LPS (10 µg/ml) or the combination of these two agents. (D) The expression levels of COX-2 and p53 with/without celecoxib treatment were determined by western blotting after 0 or 12 h of treatment with 100 µM celecoxib. (E) The COX-2/β-actin ratio was calculated based on the intensity of the bands in the HSC-3 cell lines. Columns represent the mean ± standard deviation. Each experiment was performed at least in triplicate. *P<0.05 and **P<0.01 vs. untreated cells. LPS, lipopolysaccharide; COX-2, cyclooxygenase-2.
Effect of celecoxib on OSCC tumor growth in vivo
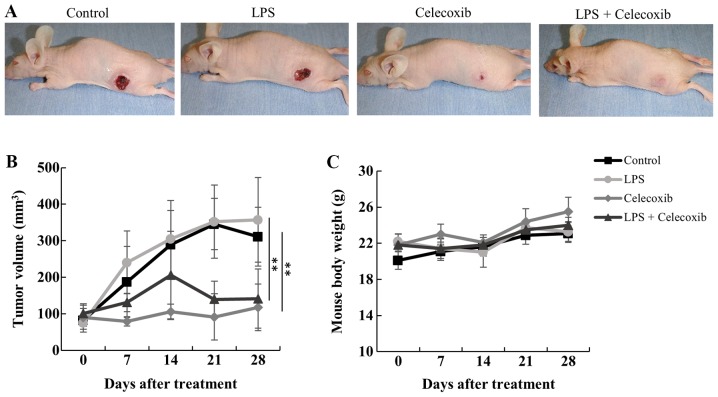
The antitumor effects of celecoxib were examined in control and LPS-treated OSCC tumors in nude mice. After 28 days of treatment, tumor volumes were significantly decreased in both celecoxib-treated and LPS + celecoxib-treated mice compared with control and LPS-treated mice (P<0.01; Fig. 2A and B). There were no significant differences body weight in the four treatment groups (Fig. 2C). Dietary intake throughout the experimental period (3.7–4.6 g/day) is presented in Table I.
Figure 2.
Effect of celecoxib on oral squamous cell carcinoma tumor growth with/without LPS-treated HSC-3 ×enografts. (A) Representative tumor images 28 days after treatment in each group. (B) Mean tumor volumes ± standard deviation. The tumor volumes decreased in the celecoxib-treated and LPS + celecoxib-treated groups compared with the control and LPS-treated groups. (C) Mouse body weights. There were no significant differences in body weight between the four treatment groups. **P<0.01 vs. control or LPS-treated group. LPS, lipopolysaccharide.
Table I.
Estimated amount of dietary intake of powdered feed with/without celecoxib.
| Estimated quantity of dietary intake per day for each mouse, g/day | ||||
|---|---|---|---|---|
| Group | 0–7 days | 8–14 days | 15–21 days | 22–28 days |
| Control | 3.8 | 4.1 | 4.5 | 4.1 |
| LPS | 4.0 | 4.1 | 4.6 | 4.6 |
| Celecoxib | 4.2 | 4.5 | 4.3 | 4.5 |
| LPS + celecoxib | 3.8 | 3.7 | 4.3 | 4.1 |
LPS, lipopolysaccharide.
Effect of celecoxib on OSCC cells in vivo
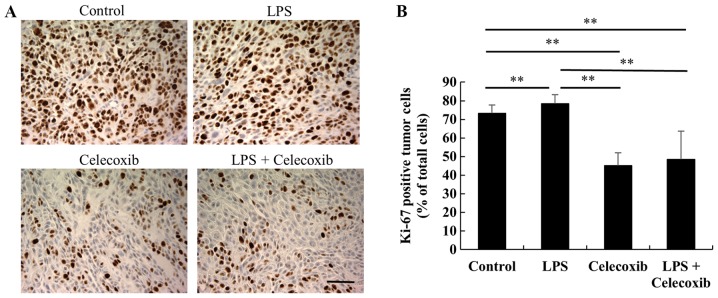
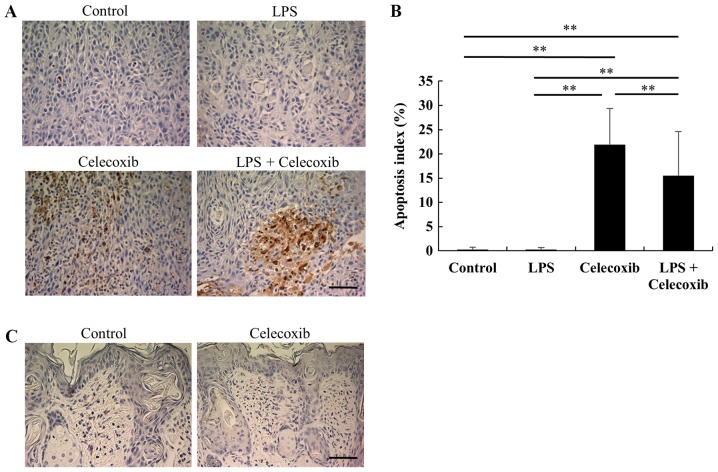
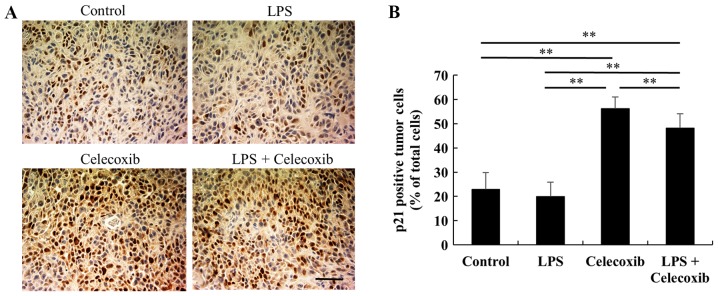
Ki-67 expression levels were significantly decreased in celecoxib-treated and LPS + celecoxib-treated groups when compared with the control and LPS-treated groups (P<0.01) and increased in the LPS-treated group compared with the control group (P<0.01), indicating that cell proliferation was decreased (Fig. 3A and B). Furthermore, TUNEL staining revealed that the apoptotic indices in the celecoxib-treated and LPS + celecoxib-treated groups were significantly higher than in the control and LPS-treated groups (P<0.01), suggesting that celecoxib induced apoptosis in the control and LPS-treated OSCC xenografts (Fig. 4A and B). The apoptotic indices in the LPS + celecoxib-treated group were significantly lower compared with those in the celecoxib-treated group (P<0.01; Fig. 4A and B). Apoptosis was not induced in non-tumorous skin and mucosa tissues surrounding the tumors following celecoxib treatment (Fig. 4C). Furthermore, there were no significant changes in p21 expression levels between the control and LPS-treated groups (Fig. 5A and B), whereas the celecoxib-treated and LPS + celecoxib-treated groups had significantly increased levels of p21 compared with the control and LPS-treated groups (P<0.01), indicating that celecoxib upregulates p21 expression in OSCC xenografts (Fig. 5A and B). The levels of p21 in the LPS + celecoxib-treated groups were significantly decreased compared in the celecoxib-treated groups (Fig. 5A and B).
Figure 3.
Immunohistochemical assessment of Ki-67 in HSC-3 ×enografts in response to celecoxib with/without LPS-treatment. (A) Representative microphotographs of Ki-67-immunostained tumor sections in each group. Scale bar, 50 µm. (B) Percentage of Ki-67-positive tumor cells. Each column represents the mean number of Ki-67-positive cells ± standard deviation. The expression levels of Ki-67 were significantly lower in the celecoxib-treated and LPS + celecoxib-treated group compared with the control and LPS-treated groups and higher in the LPS-treated group compared with the control group. **P<0.01 vs. control or LPS-treated group. LPS, lipopolysaccharide.
Figure 4.
Apoptosis assay in tumor xenografts in response to celecoxib with/without LPS-treatment. (A) Representative microphotographs of TUNEL staining in each group. Scale bar, 50 µm. (B) Apoptosis index by TUNEL staining. Each bar represents the mean ratio of the number of TUNEL-positive cells to the total number of tumor cells ± standard deviation. The apoptosis indices in the celecoxib-treated and LPS + celecoxib-treated group were significantly higher than those in the control and LPS-treated group, whereas the apoptosis indices in the LPS + celecoxib-treated group were significantly lower compared with those in the celecoxib-treated group. (C) Representative microphotographs of non-tumor tissues in TUNEL staining. Apoptosis was not induced in non-tumor tissues exposed to celecoxib treatment. Scale bar, 50 µm. **P<0.01 vs. control or LPS-treated group. LPS, lipopolysaccharide; TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling.
Figure 5.
Immunohistochemical assessment of p21 in HSC-3 ×enografts in response to celecoxib with/without LPS-treatment. (A) Representative microphotographs of p21-immunostained tumor sections in each group. Scale bar, 50 µm. (B) Percentage of p21-positive tumor cells. Each column represents the mean number of p21-positive cells ± standard deviation. The celecoxib-treated and LPS + celecoxib-treated groups had significantly increased levels of p21 compared with the control and LPS-treated groups. The levels of p21 in the LPS + celecoxib-treated group were significantly decreased compared with those in the celecoxib-treated group. **P<0.01 vs. control or LPS-treated group. LPS, lipopolysaccharide.
Discussion
In the present study, P. gingivalis-derived LPS was used to investigate the molecular mechanisms underlying periodontitis in the Toll-like receptor (TLR) 4-expressing OSCC cell line HSC-3 (7). LPS binds directly to the TLR4/myeloid differentiation factor 2 receptor complex (18) activating the myeloid differentiation factor 88 signaling pathway, which in turn activates mitogen-activated protein kinase (MAPK) and the transcription factor nuclear factor-κB, which play an important role in cell proliferation (19). Previous reports have demonstrated that LPS stimulation of TLR4 promotes breast cancer growth in nude mice (6), and, consistently, LPS caused a significant increase in OSCC cell proliferation in vitro in the present study.
To assess whether COX-2 plays a role in LPS-induced proliferation of OSCC cells, the antitumor effect of the COX-2 inhibitor celecoxib was investigated. Celecoxib significantly decreased the proliferation of OSCC cells and inhibited tumor growth in a xenograft model. The expression of Ki-67 was also decreased, and apoptosis was significantly increased in the celecoxib-treated group. This coincides with previous reports that celecoxib significantly decreases the viability of OSCC cells by inhibiting phosphorylated protein kinase B, cyclin D1 or the epithelial-to-mesenchymal transition, as well as by inhibiting tumor growth in OSCC xenografts (14,20).
In the present study, celecoxib treatment downregulated the protein expression levels of COX-2 in OSCC cells. This finding is supported by previous reports that celecoxib attenuates COX-2 expression and inhibits the growth of OSCC cells (21,22). On the other hand, celecoxib has been indicated to inhibit the cell survival of both COX-2-expressing and non-expressing colon carcinoma cells (23), indicating that the effects of celecoxib are independent of COX-2, and there could be other targets/effects that have yet to be defined.
The concentrations of celecoxib used in the present study were much higher than those used in oral administration for humans (24), so the results obtained from the present study may not be directly extrapolatable to humans. However, the antitumor effects of celecoxib have previously been established in epidemiological studies and in clinical trials (24), indicating that celecoxib may be effective even at lower concentrations in humans. Furthermore, no significant weight loss was observed in celecoxib-treated mice in the present study, suggesting that celecoxib did not exhibit toxicity or cause adverse effects. Nonetheless, other studies have suggested that adverse effects may be decreased and controlled using the oral administration of antitumor drugs (25,26), and that celecoxib may be eligible for such application as well. Furthermore, a recent study has revealed that, in HNSCC, a combined metronomic oral celecoxib treatment is advantageous when compared with intravenous single-agent therapy (24); thus, this approach may also improve future results using celecoxib therapy.
In the LPS-stimulated OSCC xenografts, celecoxib upregulated the expression of p21 which induces cell cycle arrest and inhibits tumor development (27). Celecoxib has been demonstrated to inhibit the G0/G1-to-S-phase transition by increasing the expression of p21, thereby decreasing tumor growth in colon cancer (23). In the present study, the expression levels of p53 exhibited little change in the OSCC cells exposed to celecoxib. Since it is known that p21 expression is upregulated by both p53-dependent and independent pathways (27), celecoxib may exhibit its antitumor effect in LPS-stimulated OSCC xenografts by upregulating p21 expression and halting the cell cycle through a p53-independent pathway.
In the present study, HSC-3 cells were stimulated with LPS for 48 h before implantation. This protocol has been supported by reports that describe the pre-treatment of carcinoma cells with LPS prior to implantation in nude mouse xenograft models (6,28). Stimulation of breast carcinoma cells with LPS for 48 h before implantation increased tumor volumes and weights in a xenograft model of breast carcinoma (6). Similarly, stimulation of cluster of differentiation 133+ hepatoma cells with LPS for 1 week before implantation caused an increase in the growth of tumors in nude mice compared with those without LPS (28). Although the duration of LPS stimulations prior to implantation varied between these two reports, both clearly indicate that pre-treatment with LPS has growth-promoting effects in vivo. In the present study, LPS-induced tumor growth was not observed in vivo, although LPS treatment significantly increased the proliferation of OSCC cells in vitro. The limitation of the method of LPS administration used in the present study is that this was a one-time LPS treatment in vivo. In a murine breast cancer model, LPS was intraperitoneally injected for 3 consecutive days and it was observed that this promoted lung metastasis (29). Therefore, intraperitoneal injection of LPS following implantation may improve the experimental model used in the present study.
Understanding the mortality and clinical abnormalities caused by celecoxib is important for chemotherapy. One study reported the adverse effects of celecoxib in patients with locoregionally advanced nasopharyngeal carcinoma (30). According to this report, administration of celecoxib concurrently with nasopharyngeal radiotherapy caused toxicities such as mucositis, weight loss, dermatitis and otitis, although no episodes of toxic mortality occurred with the treatment (30). Furthermore, a regimen of celecoxib combined with radiation was well tolerated in patients with brain metastases, and celecoxib-associated toxicity was limited to a mild skin reaction (31). These reports suggest that celecoxib can be safely administrated to patients with carcinomas such as lung or breast carcinoma or melanoma, including those with metastases.
A limitation of the present study is that it utilizes a single cell line, HSC-3, for both the in vitro and in vivo experiments. In a previous study, an HSC-3-bearing nude mouse model was successfully established and utilized (16). For the in vitro experiments, previous studies support the data from the present study that celecoxib inhibits the growth of OSCC cells (14,20).
The clinical implications of the present study are important. First, P. gingivalis-derived LPS can stimulate tumor growth by interacting with OSCC cells. Thus, it poses a risk for developing OSCC (4). Secondly, celecoxib could be used for the effective prevention and treatment of LPS-stimulated OSCC. Further experiments are required for the future clinical development of celecoxib as an OSCC treatment.
In conclusion, the data from the present study revealed that P. gingivalis-derived LPS can stimulate the growth of OSCC tumors. Celecoxib suppressed the proliferation of LPS-stimulated OSCC cells and inhibited tumor growth by increasing p21 expression levels and inducing apoptosis in OSCC xenografts. These results suggest that celecoxib administration could provide an effective prevention and treatment strategy for LPS-stimulated OSCC.
Acknowledgements
Not applicable.
Funding
The present study was funded in part by the Grant-in-Aid for Young Scientists (grant no. 18K17196) and the Grant-in-Aid for Scientific Research (grant no. 18K09719) from the Japan Society for the Promotion of Science.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HisY, HitY and KS designed the experiments. HisY, HitY, SM, SY, KO, HI and KS performed the experiments. HisY, HitY, MO, TR, TK and MK analyzed the data. HisY, HitY and KS wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of the University of Fukui (approval no. 29105).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 3.Marta GN, Riera R, Bossi P, Zhong LP, Licitra L, Macedo CR, de Castro Junior G, Carvalho AL, William WN, Jr, Kowalski LP. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: Systematic review and meta-analysis. Eur J Cancer. 2015;51:2596–2603. doi: 10.1016/j.ejca.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit Rev Oncol Hematol. 2016;97:197–205. doi: 10.1016/j.critrevonc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000. 2010;54:53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Wang B, Wang T, Xu L, He C, Wen H, Yan J, Su H, Zhu X. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One. 2014;9:e109980. doi: 10.1371/journal.pone.0109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Z, Deng R, Huang X, Ni Y, Yang X, Wang Z, Hu Q. Lipopolysaccharide enhances OSCC migration by promoting epithelial-mesenchymal transition. J Oral Pathol Med. 2015;44:685–692. doi: 10.1111/jop.12285. [DOI] [PubMed] [Google Scholar]
- 8.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Morisaki T, Izuhara K, Uchiyama A, Matsunari Y, Katano M, Tanaka M. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-kappa B activation. Oncogene. 2000;19:1225–1231. doi: 10.1038/sj.onc.1203427. [DOI] [PubMed] [Google Scholar]
- 10.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, Chiarugi V, Masini E. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 12.Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol. 2002;33:708–714. doi: 10.1053/hupa.2002.125376. [DOI] [PubMed] [Google Scholar]
- 13.Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, Müller S, Lewis M, Sica G, Kono S, et al. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: Preclinical and clinical studies. Clin Cancer Res. 2013;19:1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang SL, Velmurugan BK, Chung C, Lin SH, Wang ZH, Hua CH, Tsai MH, Kuo TM, Yeh KT, Chang PY, et al. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci Rep. 2017;7:6235. doi: 10.1038/s41598-017-06673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu XF, Xie CG, Wang XP, Liu J, Yu YC, Hu HL, Guo CY. Selective inhibition of cyclooxygenase-2 suppresses the growth of pancreatic cancer cells in vitro and in vivo. Tohoku J Exp Med. 2008;215:149–157. doi: 10.1620/tjem.215.149. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H, Yoshimura H, Matsuda S, Ryoke T, Kiyoshima T, Kobayashi M, Sano K. Effects of peritumoral bevacizumab injection against oral squamous cell carcinoma in a nude mouse xenograft model: A preliminary study. Oncol Lett. 2018;15:8627–8634. doi: 10.3892/ol.2018.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council, corp-author. 8th. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 18.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Abrahão AC, Giudice FS, Sperandio FF, Pinto Junior Ddos S. Effects of celecoxib treatment over the AKT pathway in head and neck squamous cell carcinoma. J Oral Pathol Med. 2013;42:793–798. doi: 10.1111/jop.12081. [DOI] [PubMed] [Google Scholar]
- 21.Kwak YE, Jeon NK, Kim J, Lee EJ. The cyclooxygenase-2 selective inhibitor celecoxib suppresses proliferation and invasiveness in the human oral squamous carcinoma. Ann N Y Acad Sci. 2007;1095:99–112. doi: 10.1196/annals.1397.014. [DOI] [PubMed] [Google Scholar]
- 22.Li WZ, Wang XY, Li ZG, Zhang JH, Ding YQ. Celecoxib enhances the inhibitory effect of cisplatin on Tca8113 cells in human tongue squamous cell carcinoma in vivo and in vitro. J Oral Pathol Med. 2010;39:579–584. doi: 10.1111/j.1600-0714.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 23.Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 24.Patil VM, Noronha V, Joshi A, Muddu VK, Dhumal S, Bhosale B, Arya S, Juvekar S, Banavali S, D'Cruz A, et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol. 2015;51:279–286. doi: 10.1016/j.oraloncology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol. 2004;22:262–268. doi: 10.1200/JCO.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Kawano S, Zheng Y, Oobu K, Matsubara R, Goto Y, Chikui T, Yoshitake T, Kiyoshima T, Jinno T, Maruse Y, et al. Clinicopathological evaluation of pre-operative chemoradiotherapy with S-1 as a treatment for locally advanced oral squamous cell carcinoma. Oncol Lett. 2016;11:3369–3376. doi: 10.3892/ol.2016.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 28.Lai FB, Liu WT, Jing YY, Yu GF, Han ZP, Yang X, Zeng JX, Zhang HJ, Shi RY, Li XY, et al. Lipopolysaccharide supports maintaining the stemness of CD133(+) hepatoma cells through activation of the NF-κB/HIF-1α pathway. Cancer Lett. 2016;378:131–141. doi: 10.1016/j.canlet.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Xu X, Jiang M, Bi Y, Xu J, Han M. Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway. Mol Med Rep. 2015;11:4454–4462. doi: 10.3892/mmr.2015.3258. [DOI] [PubMed] [Google Scholar]
- 30.Xue WP, Bai SM, Luo M, Bi ZF, Liu YM, Wu SK. Phase I clinical trial of nasopharyngeal radiotherapy and concurrent celecoxib for patients with locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2011;47:753–757. doi: 10.1016/j.oraloncology.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Cerchietti LC, Bonomi MR, Navigante AH, Castro MA, Cabalar ME, Roth BM. Phase I/II study of selective cyclooxygenase-2 inhibitor celecoxib as a radiation sensitizer in patients with unresectable brain metastases. J Neurooncol. 2005;71:73–81. doi: 10.1007/s11060-004-9179-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.