Abstract
Patients with epilepsy are often able to predict seizure occurrence subsequent to an acute stress experience. However, neuroimaging investigations into the neural basis of this relationship or the potential influence of perceived life stress are limited. The current study assessed the relationship between perceived stress and the neurobehavioral response to stress in patients with left temporal lobe epilepsy (LTLE) and healthy controls (HCs) using heart rate, salivary cortisol level, and functional magnetic resonance imaging and compared these effects between HCs and LTLE. Matched on perceived stress levels, groups of 36 patients with LTLE and 36 HCs completed the Montreal Imaging Stress Task, with control and stress math task conditions. Among LTLEs, 27 reported that prior (acute) stress affected their seizures (LTLES+), while nine did not (LTLES−). The results revealed that increased perceived stress was associated with seizure frequency in LTLE. Further, cortisol secretion was greater in LTLE, but did not vary with perceived stress as observed in HCs. A linear mixed‐effects analysis revealed that as perceived stress increased, activation in the hippocampal complex (parahippocampal gyrus and hippocampus) decreased during stressful math in the LTLES+, increased in HCs, but did not vary in the LTLES−. Task‐based functional connectivity analyses revealed LTLE differences in hippocampal functional connectivity with sensory cortex specific to stressor modalities. We argue that the current study demonstrates an inhibitory hippocampal mechanism underlying differences in resilience to stress between HCs and LTLE, as well as LTLE patients who report stress as a precipitant of seizures.
Keywords: cortisol, fMRI, MIST, psychological stress, psychophysiology, temporal lobe epilepsy
1. INTRODUCTION
The prevalence of epilepsy is estimated at 1.2% with approximately 30% of those affected having treatment resistant epilepsy (TRE) (Chen, Brodie, Liew, & Kwan, 2018). Temporal lobe epilepsy (TLE), frequently resistant to treatment (Engel Jr., 1996; Semah et al., 1998), is associated with alterations in emotional function and disposition (Gilliam, 2015; Kanner, 2007; Meletti et al., 2009; Monti & Meletti, 2015; Walpole, Isaac, & Reynders, 2008). Although many behavioral studies have investigated changes in emotion processes in TLE, alterations in physiological and neural functions that underlie the association between treatment resistance in epilepsy and emotion dysfunction remain poorly understood. Understanding the impact of emotional processes on seizure control in individuals with TLE may help identify potential neural mechanisms mediating the relationship between treatment resistance and emotional function.
TRE is associated with disruption in emotional processes (Bonora et al., 2011), and in particular, dysfunction associated with acute stress experience. Patients with TRE can frequently predict subsequent seizure occurrence immediately after an acute stress experience (Haut, Hall, Masur, & Lipton, 2007; Nakken et al., 2005; Privitera et al., 2014). Behavioral stress management techniques, including progressive relaxation, biofeedback, and cognitive‐behavioral therapy, have been proposed as adjunct to anti‐seizure drugs (ASDs) in TRE (Polak, Privitera, Lipton, & Haut, 2012; Ramaratnam, Baker, & Goldstein, 2003). Clinical trials examining stress management interventions showed that these techniques may outperform comparable nonstress targeted interventions in reducing seizure frequency (Lundgren, Dahl, Yardi, & Melin, 2008; Nagai, Goldstein, Fenwick, & Trimble, 2004; Puskarich et al., 1992; Tang, Poon, & Kwan, 2015). However, similar reductions in seizure frequency are observed in behavioral interventions targeting attentional focus (Haut et al., 2018) or in the absence of any additional behavioral intervention (Ridsdale et al., 2018). Stress management interventions may not significantly reduce perceived stress symptoms below comparable nonstress targeted approaches. Recent studies demonstrate that control groups did not differ overall from treatment groups in perceived life stress (Haut et al., 2018; Ridsdale et al., 2018). Thus, the role of stress in triggering seizures, as well as the clinical utility of stress management as a compliment to ASDs, remains debated. Important questions remain regarding the potential role of changes in perceived life stress on stress reactivity associated with TRE. Better understanding of the neurobiological differences in the processing of stress‐related information associated with TRE will inform approaches to behavioral interventions that target specific stress management techniques.
One approach to investigating the neurobiological basis of stress‐related processing is functional magnetic resonance imaging (fMRI) during the Montreal Imaging Stress Task (MIST; (Dedovic et al., 2005)). MIST is designed to acutely induce mild‐to‐moderate psychosocial stress and to assess the neural response to stress by comparing the differential fMRI response to stressful (SMT) and control (CMT) math tasks (Allendorfer et al., 2014; Goodman et al., 2016; Pruessner et al., 2008; Wheelock et al., 2016). Changes in fMRI signal can be compared to hypothalamic–pituitary–adrenal (HPA) axis reactivity (i.e., cortisol secretion), autonomic arousal (i.e., heart rate [HR]), or other measures of emotional disposition (i.e., perceived stress) to assess the neural mechanisms underlying both, emotional behavior and dispositions (Allendorfer et al., 2014; Pruessner et al., 2008; Wheelock et al., 2016). For example, cortisol assessments during the MIST have implicated an inhibitory hippocampal mechanism for HPA activation. Specifically, HPA activation is disinhibited by decreased activation within hippocampal region during stressful math performance (Khalili‐Mahani, Dedovic, Engert, Pruessner, & Pruessner, 2010; Pruessner et al., 2008). These findings are consistent with hippocampal lesion and stimulation effects on HPA activation (Ulrich‐Lai & Herman, 2009). Furthermore, Allendorfer et al., 2014 assessed the neural response to extrinsic negative evaluations by comparing positive and negative auditory feedback during the CMT and SMT conditions. Alternatively, others assessed inhibitory neural mechanisms related to performance demands by comparing arithmetic performance during CMT and SMT conditions (Goodman et al., 2016; Pruessner et al., 2008; Wheelock et al., 2016).
Neuroimaging studies have also investigated changes in neural function underlying stress reactivity in patients with TRE. For example, prior work has shown that differences in the neural response to acute psychosocial stress and facial processing are linked to seizure control (Allendorfer et al., 2014; Szaflarski et al., 2014). Specifically, negative feedback in patients with treatment‐resistant TLE lead to decreased dorsal anterior cingulate activity compared to controls (Allendorfer et al., 2014). Alterations in emotional processes in left TLE (LTLE) patients with poor seizure control have been linked to changes in hippocampal complex (i.e., parahippocampal gyrus and hippocampus) activation during facial processing (Szaflarski et al., 2014). This prior work implicates several key regions that may underlie differences in the emotion processing of stressful stimuli among patients with treatment‐resistant LTLE. However, these prior findings do not assess differences in emotional dispositions that may differ between healthy controls (HCs) and LTLE. Thus, direct investigation of the interaction between perceived stress, seizure control, and the neural and physiological response to stress is currently lacking in the epilepsy literature. The objective of the current study was to investigate the neural response to acute psychosocial stress in LTLE and HCs as a function of perceived life stress and seizure status. The guiding hypothesis for this work was that the neural responses in the emotion processing circuits to stressful stimuli in LTLE would be different from those of HCs. We also hypothesized that the neural response to acute stress would be modulated by perceived stress and associated with differences in connectivity within brain regions activated by stress task conditions.
2. METHODS
2.1. Participants
Thirty‐six patients with LTLE were recruited from the University of Cincinnati Epilepsy Center (UCEC; N = 27) and the University of Alabama at Birmingham Epilepsy Center (Table 1); a subset of these subjects were included in previous reports (Allendorfer et al., 2014; Szaflarski et al., 2014; Szaflarski et al., 2018). In order to maximize homogeneity among LTLE patients in the sample, inclusion criteria were implemented for the LTLE group that included a normal MRI and the absence of cortical atrophy or lesions beyond medial temporal lobe sclerosis. Diagnosis of LTLE was confirmed by clinical history reports and electroencephalogram (EEG) readings. Of the 36 LTLE, 18 experienced seizures in the prior 3 months and 18 did not (“controlled”). Thirty‐six age‐, sex‐, and perceived stress matched HCs were also recruited (N = 24 from UCEC; Table 1). LTLEs and HCs were matched for perceived stress using a short‐form Perceived Stress Scale (PSS‐4, Cohen, Kamarck, & Mermelstein, 1983) administered during initial screening. Twenty‐seven LTLE patients reported that prior stress affected their seizures (LTLES+) and nine did not (LTLES−). Chi‐squared and independent samples t tests were used to test for group differences in demographic variables. All participants provided written informed consent based on procedures approved by both the University of Cincinnati and University of Alabama at Birmingham Institutional Review Boards (IRB). The informed consent document provided as much details about the study as possible without revealing the true nature of the study (e.g., that the subjects would receive $40 or $100 for participation, depending on performance). Following completion of participation in the study, as per IRB requirement, participants were debriefed with a full explanation of the rationale for the study design and methods used for the study and received $100 for their participation (regardless of their performance).
Table 1.
Demographics, psychological, CMT, SMT, and cortisol by groups
| HCs vs. LTLEs | LTLES+ vs. LTLES− | |||||||
|---|---|---|---|---|---|---|---|---|
| HCs | LTLEs | Stat | p | LTLES+ | LTLES− | Stat | p | |
| Demographics | ||||||||
| Sample size | n = 36 | n = 36 | – | – | n = 27 | n = 9 | – | – |
| Age | 38.6 (11) | 40.0 (12.1) | t = −.53 | .60 | 38. 7 (12.3) | 44.0 (10.9) | t = −1.16 | .26 |
| Sex (female) | n = 27 | n = 28 | χ 2 = .08 | .78 | n = 22 | n = 6 | χ 2 = .85 | .35 |
| Years education | 15.0 (3.0) | 14.7 (2.4) | t = .44 | .67 | 14.4 (2.4) | 15.61 (2.2) | t = −1.37 | .18 |
| Seizure frequency (past 3 months) | – | 8.3 (16.1) | – | – | 11.0 (17.9) | 0.2 (0.1) | t = 1.79 | .08 |
| Duration of epilepsy (years) | – | 12.9 (9.9) | – | – | 12.7 (10.9) | 13.7 (6.9) | t = −.25 | .81 |
| Age of seizure onset | – | 27.0 (14.0) | – | – | 26.0 (15. 8) | 30.3 (8.4) | t = −.79 | .44 |
| Psychological indices | ||||||||
| BDI‐II | 10.7 (9.5) | 9.3 (8.8) | t = .66 | .51 | 11.3 (9.0) | 3.2 (4.0) | t = 2.56 | <.05* |
| PSS‐10 | 19.7 (7.3) | 18.1 (7.8) | t = .94 | .35 | 19.6 (7.4) | 13.6 (7.6) | t = 1.68 | .10 |
| CMT | ||||||||
| Math accuracy (% correct) | 94.3 (6.0) | 91.3 (9.7) | t = 1.52 | .13 | 89.6 (10.6) | 96.1 (4.2) | t = −1.76 | .09 |
| Response time (ms) | 2,372.8 (464.7) | 2,534.5 (384.4) | t = −1.57 | .12 | 2,625.1 (376.4) | 2,282.8 (295.8) | t = 2.46 | <.05* |
| Tone accuracy (% correct) | 98.9 (3.6) | 98.9 (6.4) | t = .03 | .98 | 98.5 (7.5) | 100 (0) | t = −.59 | .56 |
| Response time (ms) | 906.9 (333.2) | 1,111.6 (238.3) | t = −2.93 | <.01* | 1,124.7 (251.5) | 1,075 (206.1) | t = .53 | .60 |
| HR (BPM) | 69.37 (10.12) | 69.90 (10.66) | t = −.21 | .83 | 68.79 (8.80) | 73.23 (15.13) | t = −1.09 | .29 |
| SMT | ||||||||
| Math accuracy (% correct) | 56.7 (18.8) | 49.4 (15.6) | t = 1.75 | .08 | 47.6 (15.9) | 54.3 (14.4) | t = 1.12 | .27 |
| Response time (ms) | 3,346.1 (344.1) | 3,255.5 (566.7) | t = .81 | .95 | 3,216.7 (605.3) | 3,363.2 (456) | t = −.66 | .51 |
| Tone accuracy (% correct) | 95.0 (12.6) | 95.2 (13.8) | t = −.07 | .95 | 94 (15.8) | 98.6 (4.2) | t = −.86 | .40 |
| Response time (ms) | 916.0 (488.3) | 969.8 (241.1) | t = −.58 | .57 | 962.5 (247.1) | 989.9 (236.7) | t = −.29 | .78 |
| HR (BPM) | 78.76 (12.09) | 77.91 (13.54) | t = .28 | .78 | 76.79 (11.40) | 81.25 (19.07) | t = −.85 | .40 |
| Cortisol | ||||||||
| Recovery to baseline (CortΔ) | 0.18 (.47) | 0.75 (1.05) | t = −2.95 | <.01* | 0.65 (.90) | 1.04 (1.43) | t = −.95 | .35 |
| t1 time (HH:MM) | 12:52 (02:00) | 12:55 (02:01) | t = .11 | .92 | 12:47 (02:22) | 13:19 (01:55) | t = .57 | .57 |
| Participants t1 < noon | n = 13 | n = 10 | χ 2 = .57 | .45 | n = 10 | n = 3 | χ 2 = .04 | .84 |
Note. Data for HCs versus LTLE patients, who reported stress as a precipitate (LTLES+) versus those who did not (LTLES−), reported as mean (SD) except for sample size, sex, and Participants t1 < noon which are reported as counts (n). Statistical comparisons for counts were carried out using a Chi‐squared test (χ 2) and tested the null hypothesis that the proportion of females to males did not differ between groups. All other comparisons were tested using an independent samples t test (t). Results of these comparisons, including the test statistic (Stat) and p‐value (p) are presented in the adjacent columns to right of the descriptive mean and count comparisons for HCs versus LTLEs and LTLES+ versus LTLES−. * indicates a comparison that reached statistical significance (α = .05, two‐tailed).
Abbreviations: BDI‐II, Beck Depression Inventory, 2nd edition; BPM, beats per minute; CMT, control math task; gPPI, generalized psychophysiological interaction; HC, healthy control; HR, heart rate; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task.
2.2. Psychological measures
Prior to fMRI, all participants completed the 10‐item version of the PSS‐10 (Cohen et al., 1983) to quantify individual differences in perceived stress. The PSS‐10 is a self‐report measure consisting of 10 questions related to stress perception during the month prior to the experimental session and scored on a zero (never) to five (very often) Likert scale. PSS‐10 scores were computed as a sum, ranging from 0 (little or no stress) to 40 (extreme or high stress), that reflected the degree to which participants found situations or life experiences stressful. Single sample t tests compared PSS‐10 scores for both HCs and LTLE to age‐matched normative values. Independent samples t test compared psychological variables between groups. A Spearman rank correlation test compared composite PSS‐10 scores and seizure frequency in LTLE patients.
2.3. Stress tasks for fMRI
During MRI scanning, all participants completed an experimental task based on MIST (Allendorfer et al., 2014) developed in E‐Prime (V. 1.1; Psychology Software Tools, Inc., Pittsburgh, PA). Briefly, participants were familiarized with the math tasks by completing a computer administered set of practice problems prior to entering the MRI. All instructions were scripted to promote uniform administration of the practice and experimental tasks. Once entering the MRI, participants completed a volume control task during a multiecho reference scan designed to calibrate audio volume during the stress tasks. Next, during blood‐oxygen‐level‐dependent (BOLD) echo‐planar imaging (EPI), participants performed CMT and SMT that were adapted to include prerecorded evaluative auditory feedback, regardless of performance in the tasks (Allendorfer et al., 2014). Adding verbal feedback permitted our analysis to distinguish between neural function underlying regulatory processes during challenging arithmetic performance and psychosocial threat processing during verbal recordings that conveyed increased social demands. Each of the stress task scans contained series of unique math trials and eight prerecorded auditory feedback messages. During the CMT, participants completed 34 different subtraction problems. Trials were 5 s in duration separated by 1.5 s intertrial intervals. During each trial, a unique math problem (e.g., “36–14?”) and 2‐item multiple choice alternatives (e.g., “1.22” and “2.30”) appeared on the screen. Participants selected the correct answer to the math problem via pressing either the “1” or “2” button on an MR button box (Current Designs, Philadelphia, PA). Prerecorded positive auditory feedback (e.g., “You're doing great, so keep it up”) was presented at eight fixed points during the CMT scan. Additionally, auditory recordings of tones were presented at eight separate fixed points in which subjects were asked to press “1” or “2” on the button box. Tone events were designed to ensure participants were attentive to the task, regardless of their performance. Following completion of the CMT, participants received instructions and began the SMT. The SMT was identical to the CMT with four exceptions designed to increase participant's stress to a mild–moderate level comparable to everyday stress. First, during the instructions and prior to the SMT scan, participants were told that “researchers” would be evaluating their performance and providing feedback based on how they were doing. Participants were told they had a variable response window between 1 and 5 s in order for their answer to count, and if they wanted to receive full compensation, they must achieve an unspecified number of correct answers commensurate with their level of education. Second, participants were presented with an additional answer choice (3‐item multiple choice alternatives) to each math problem during the SMT. Third, participants were presented with eight prerecorded negative auditory feedback messages (e.g., “You will have to do much better in the remaining questions. If you do not improve, we can only give you $40 for your participation.”) at eight separate fixed points during the SMT scan. Finally, the total number of subtraction problems was increased to 63 trials during the SMT. Independent samples t tests compared behavioral MIST performance between groups.
2.4. Physiological measures
Salivary cortisol was assessed at seven unique time points throughout the experimental session to evaluate the HPA‐axis response, a measure of stress reactivity. At each time point, participants provided 1 mL of saliva via passive drool into plastic tubes. Two participants (1 LTLE and 1 HC) did not provide saliva and were excluded from salivary cortisol analysis. Two samples were collected after consenting during prescan assessments at 30 min (t1) and 15 min prior (t2) to entering the MRI environment. A third sample was collected immediately after completion of MRI scanning (t3). Four samples were collected at 15 min (t4), 30 min (t5), 45 min (t6), and 60 min (t7) following completion of MRI scanning. Samples were stored on ice until being transferred to a freezer following the experimental session. Salivary cortisol levels (mg/μL) were assessed using standard assay kits (Salimetrics, LLC State College, PA) in duplicate and averaged at the Cincinnati Veteran's Administration Hospital. Cortisol reactivity (CortΔ) was calculated by computing the percent change between t3 and t7 using the formula CortΔ = (t3–t7)/t7 (Allendorfer et al., 2014). Greater percentages reflect larger HPA‐axis activation in response to acute psychosocial stress, adjusted for individual differences in baseline cortisol (t7). Independent samples t test assessed whether HPA‐axis activation differed between groups. Additional independent samples t tests compared initial saliva sample time (t1) between LTLEs and HCs, and between LTLES+ and LTLES− to assess potential group differences in time of day on cortisol measures. Additionally, the proportion of participants with t1 times recorded before noon between the groups was assessed using Chi‐squared tests and Pearson's r correlation test assessed whether t1 varied with CortΔ. Spearman rank correlation assessed whether PSS‐10 varied with CortΔ measures overall or within each of the groups.
HR was recorded in beats per minute (BPM) via attachment of a photoplethysmograph to the index finger of the left hand. The current HR appearing on the physiological display of the scanner console at the termination of each positive and negative feedback message was recorded and then averaged across the eight feedback statements separately for the CMT and SMT, respectively. In order to assess whether cardiac reactivity was greater during the SMT than the CMT, and whether this difference varied as a function of LTLE and HC groups, a 2 × 2 mixed model ANOVA compared the main effects of condition (CMT, SMT) and group (LTLE, HC) on HR. Individual differences in cardiac reactivity were computed using the formula HRΔ = (SMT–CMT). Greater differences reflect a larger cardiac response to acute psychosocial stress.
2.5. Magnetic resonance imaging acquisition and analysis
Head‐first supine MRI scans were completed on both a 4T Varian scanner (Varian NMR Instruments, Palo Alto, CA) at the Center for Imaging Research at the University of Cincinnati (n = 50) and a 3T Siemens Allegra scanner (Siemens Medical Solutions USA Inc., Malvern, PA) at the Civitan International Neuroimaging Laboratory at the University of Alabama at Birmingham (n = 28). Participants were fitted with an MR compatible button‐box (right hand) and MR compatible headphones and goggles (Resonance Technologies, Inc., North Ridge, CA) in the Varian scanner, or a mirror affixed to the head coil that reflected a video monitor (Integrated Functional Imaging System (IFIS); InVivo Corp., Gainesville, FL) in the Siemens scanner. The duration of scanning sessions lasted approximately 60 min.
High resolution T1‐weighted anatomical scans were collected in the sagittal plane (Varian: [3D Modified Equilibrium Driven Fourier Transform (MDEFT), Repetition Time (TR) = 13 ms, T[MD] = 1.1 s, Echo Time (TE) = 5.3 ms, flip angle = 22°, Field of View (FOV) = 25.6 × 25.6 × 19.2 cm3, matrix = 256 × 192 × 96 slice thickness = 1 mm); Siemens: (Magnetization Prepared Rapid Gradient Echo (MPRAGE), TR = 2,300 ms, TE = 2.17 ms, TI = 900 ms, flip angle = 9°, FOV = 25.6 × 25.6 × 19.2 cm3, matrix = 256 × 256, slice thickness = 1 mm)]. The task scans began approximately 45 min from the start of scanning sessions. During task scans, BOLD fMRI signal was measured with a gradient‐echo echo‐planar pulse sequence (both Varian and Siemens: TR = 3,000 ms, TE = 25 ms, flip angle = 85°, FOV = 25.6 × 25.6 cm2, matrix = 64 × 64 slice thickness = 3 mm with 1 mm interslice gap) in an axial oblique orientation (20° transverse‐to‐coronal from AC‐PC line).
Analysis of all MRI data was completed using analysis of functional neuroimaging (AFNI; (Cox, 1996)). EPI time‐series data were slice‐time corrected, corrected for head motion, spatially smoothed with a 7.2 mm full width at half maximum Gaussian filter, and coregistered with the structural image. Noise occurring outside of the brain was removed using binary masking. Anatomical and functional data were normalized to the stereotaxic coordinate system (Talairach & Tournoux, 1988) and resampled to a 3 mm3 isotropic resolution. fMRI signal time series from both math tasks were concatenated and then modeled with a gamma variate hemodynamic response function using individual reference waveforms for task events including math trials, audio feedback, and tones for the CMT and SMT (3dDeconvolve in AFNI). The six parameters of participants' head motion were modeled as regressors of no interest. Percent signal change was used as an index of the amplitude of the fMRI signal response to task events. Although responses to tone events were included in first‐level modeling, these data were not submitted for further analysis in the current study.
Two separate linear mixed‐effects analyses (3dLME in AFNI) assessed the neural response to stressful math trials and negative auditory feedback. The first 3dLME analysis identified voxels with a linear relationship between the neural response to stressful math performance (SMT ‐CMT) and perceived stress (PSS‐10) that varied by group (LTLE, HC) and the main effects or interactions for these variables. The second 3dLME analysis identified voxels with a linear relationship between the neural response to negative auditory feedback (SMT–CMT) and perceived stress (PSS‐10) that varied by group (LTLE, HC) and the main effects or interactions for these variables. Because fMRI data were collected from two different scanners, scanner type was included as a covariate in the model. For the initial exploratory whole brain analysis, a gray matter mask restricted the analysis to these regions across the whole brain. Monte Carlo simulations (3dClustSim with ‐acf option) determined the corrected significance threshold with an uncorrected significance threshold of p < .001. Smoothness was averaged across subjects based on spherical autocorrelation function parameters (3dFWHMx) derived from residual volumes from the first level analysis (Cox, Chen, Glen, Reynolds, & Taylor, 2017). The results of this simulation yielded a critical cluster‐extent volume threshold of 384 mm3. A second Monte Carlo simulation was carried out for the hippocampal complex (hippocampus and parahippocampal gyrus) using a small volume correction based on a priori hypotheses for activation in these regions (Goodman et al., 2016; Khalili‐Mahani et al., 2010; Pruessner et al., 2008). For the small volume simulation, an uncorrected significance threshold of p < .01 was used based on the reduced risks of family wise error inherent to restricting the number of comparisons to an a priori region of interest (ROI). The results of this small volume simulation yielded a critical cluster‐extent volume threshold of 362 mm3 for activation within the bilateral hippocampal complex. Cluster volume thresholds for each ROI were determined by the results of the Monte Carlo simulations corresponding to AFNI clusterize options for nearest neighbor 1 and two‐sided criteria.
A second series of follow‐up analyses was implemented to assess group differences in functional connectivity during the SMT within clusters identified by the 3dLMEs. Generalized psychophysiological interaction (gPPI; (Cisler, Bush, & Steele, 2014; McLaren, Ries, Xu, & Johnson, 2012)) analysis was carried out on the SMT fMRI data for all subjects. A 4 mm radius sphere was generated (3dcalc) around the center of mass voxel, producing a functionally defined seed ROI. For each subject, the fMRI time series was averaged within the seed ROI (3dmaskave), detrended (3dDetrend) and deconvolved with a gamma variate hemodynamic response function (3dTfitter). The times series was then upsampled to 1 s in order to match TRs with stimulus timings. Separate interaction terms were then generated for both math and auditory feedback events (1deval and waver). fMRI signal time series from SMT was then modeled with a gamma variate hemodynamic response function identical to our initial deconvolution, with the addition of individual reference waveforms for the seed and interaction time series generated for math trials and audio feedback (3dDeconvolve). This deconvolution produced voxel‐wise stimulus event coefficients and gPPI coefficients of connectivity for the seed region for each subject. The resulting gPPI coefficient maps for math and auditory events were compared between groups (HC vs. LTLE; 3dttest++) using a cluster extend threshold of 945 mm3 (p < .005, uncorrected, p < .05 corrected) based on Monte Carlo simulations (3dClustsim).
2.6. Data availability
The deidentified data that support the findings of this study are available from the corresponding author, upon reasonable request.
3. RESULTS
3.1. Demographic, psychological, behavioral, and physiological results
Chi‐squared test confirmed that the proportion of HC and LTLE participants did not differ between sites (χ 2[1, N = 72] = 1.05, p = .31). The results of all group comparisons are presented in Table 1. HCs did not differ from LTLEs in age, gender, years of education, perceived stress, or depression (BDI‐II, Beck et al., 1996). Further, LTLES+ and LTLES− groups did not differ in age, gender, years of education, perceived stress, or the frequency, age of onset, and duration of seizures. The symptoms of depression reported by the LTLES+ group were significantly higher (t[34] = 2.56, p < .05) when compared to the LTLES− group. PSS‐10 scores for both HCs (t[35] = 5.56, p < .001) and LTLE (t[35] = 3.90, p < .001) were greater than the age‐matched normative value (M = 13.0, age range: 30–44 years). The short‐form PSS‐4, administered during the initial screening and used to match LTLEs and HCs for perceived stress, demonstrated a strong positive correlation to the PSS‐10 assessment, r = .56, p < .001. For LTLEs, the distribution for 3‐month seizure frequency was skewed positively and was therefore binned into quartiles to assess the relationship between perceived stress and seizure frequency. 3‐month seizure frequency was lower for LTLES− compared to LTLES+ group (mean diff = 10.8); however, this group difference failed to reach statistical significance, t(34) = 1.79, p = .08. As PSS‐10 scores increased, 3‐month seizure frequency increased in LTLE patients, (r s = .45, p < .01; Figure 1a). Accuracy measures did not differ between groups during both the CMT and SMT. CortΔ HPA‐axis responses increased in LTLEs compared to HCs. The t1 saliva collection times and proportion of participants who arrived before noon between the LTLEs and HCs or LTLES+ and LTLES− did not differ between groups. There was no significant relationship between CortΔ and t1 times (r = .10, p = .43). As PSS‐10 scores increased, CortΔ demonstrated a corresponding decrease for all participants (r s = −.28, p < .05). There was a similar inverse relationship between PSS‐10 and CortΔ for HCs (r s = −.37, p < .05), but no relationship for LTLE (r s = −.16, p = .36). There was no linear relationship between PSS‐10 and CortΔ for LTLES+ (r s = −.26, p = .20) or the LTLES− (r s = −.31, p = .43). There was a significant increase in HR between CMT and SMT (F[1,70] = 74.57, p < .001) but no group differences or interactions for LTLEs and HCs (Figure 1c).
Figure 1.
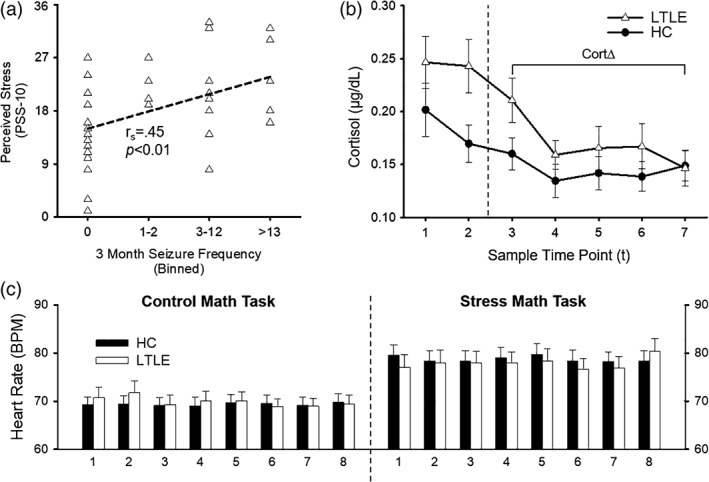
Psychological and physiological results for the healthy control (HC) and left temporal lobe epilepsy (LTLE) groups. The top left panel (a) shows the Spearman rank correlation between seizure frequency (binned by quartiles) in the past 3 months and Perceived Stress Scale (PSS‐10) within the LTLE group. The top right panel (b) shows the group comparison of cortisol secretion during the experimental session. Sample time points reflect collection at 30 min (t1) and 15 min (t2) prior to MRI scanning and 0 (t3), 15 min (t4), 30 min (t5), 45 min (t6), and 60 min (t7) following completion of MRI scanning. Cortisol reactivity (CortΔ = (t3–t7)/t7) was calculated by comparing poststress peak (t3) to recovery to baseline (t7) based on prior literature (Allendorfer et al., 2014). The bottom panel (c) shows the groups comparison of heart rate (HR; beats per minute [BPM]) between control math task and the stress math task (SMT). Each time point reflects the sequence of auditory feedback events during the tasks. There was a significant increase in HR between control and SMTs
3.2. MRI results
For the initial 3dLME to assess math performance, there was a significant main effect of condition (SMT–CMT), a two‐way interaction between condition and group (HC vs. LTLE), and a three‐way interaction between condition, group, and perceived stress on math performance (PSS‐10; Figure 2, Table 2). Clusters of activation for the main effect of condition were located within the superior temporal gyrus and Prefrontal Cortex (PFC) regions. Clusters of activation for the two‐way interaction between condition and group were located within parietal, cingulate, and PFC regions and for the two‐way interaction between condition and perceived stress within the middle temporal gyrus. Clusters of activation for the three‐way interaction between condition, group, and perceived stress were located within hippocampal complex (parahippocampal gyrus and hippocampus, Figure 3) and middle temporal regions. All remaining clusters of activations from the main effects of condition, group, and perceived stress, and their interactions, failed to survive the cluster‐corrected thresholds.
Figure 2.
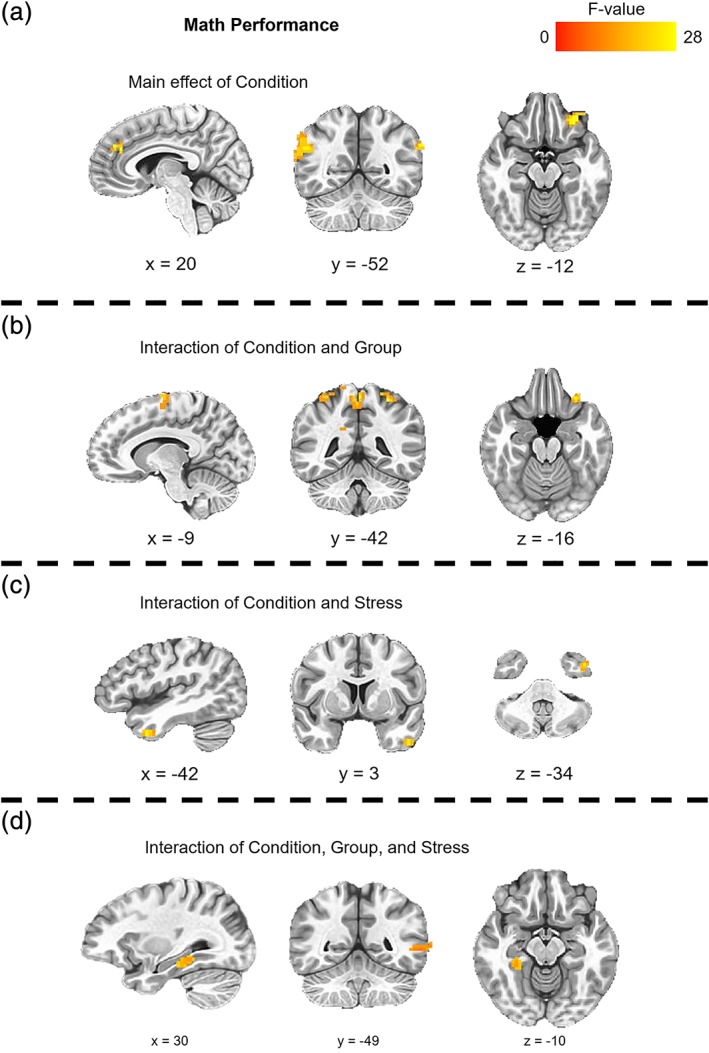
Clusters of significant activation during math performance for the main effect of condition (CMT vs. SMT), interaction of condition and group (HC vs. LTLE), and the interaction of condition, group, and perceived stress (PSS‐10). CMT, control math task; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task
Table 2.
Regions showing condition (CMT vs. SMT) effect, condition × group (HC vs. LTLE) interaction, and condition × group × perceived stress (PSS‐10) interaction during math performance
| Cluster # | Region | Hemisphere | Vol (mm3) | Talairach (x, y, z) | F‐statistic |
|---|---|---|---|---|---|
| Main effect of condition | |||||
| 1 | Superior temporal gyrus | R | 1,269 | 62, −49, 20 | 20.09 |
| 2 | Ventrolateral PFC | L | 999 | −28, 35, −13 | 23.23 |
| 3 | Parahippocampal gyrus | L | 729 | −19, −40, −7 | 12.50 |
| 4 | Dorsomedial PFC | L/R | 459 | 5, 35, 26 | 19.01 |
| Condition × group interaction | |||||
| 1 | Superior parietal lobule | R | 1,701 | 20, −52, 62 | 27.48 |
| 2 | Inferior parietal lobule | L | 1,512 | −28, −46, 53 | 25.00 |
| 3 | Precentral gyrus | L | 972 | −43, −22, 35 | 16.71 |
| 4 | Paracentral lobule | L/R | 891 | −1, −43, 57 | 22.46 |
| 5 | Dorsomedial PFC | L | 864 | −10, −10, 65 | 21.63 |
| 6 | Cingulate gyrus | R | 459 | 14, −34, 38 | 16.12 |
| 7 | Ventrolateral PFC | L | 432 | −28, 29, −16 | 22.79 |
| Condition × stress interaction | |||||
| 1 | Middle temporal Gyrus | L | 513 | −43, 5, −34 | 25.75 |
| Condition × group × stress interaction | |||||
| 1 | Middle temporal gyrus | L | 675 | −55, −49, 2 | 18.82 |
| 2 | Parahippocampal gyrus | R | 540 | 29, −34, −10 | 15.09 |
Note. Cluster #, location, hemisphere, volumes, coordinates from Talairach and Tournoux (1988), and F‐statistic for the peak voxel of significant clusters. All clusters were significant at p < .05 (corrected).
Abbreviations: CMT, control math task; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task.
Figure 3.
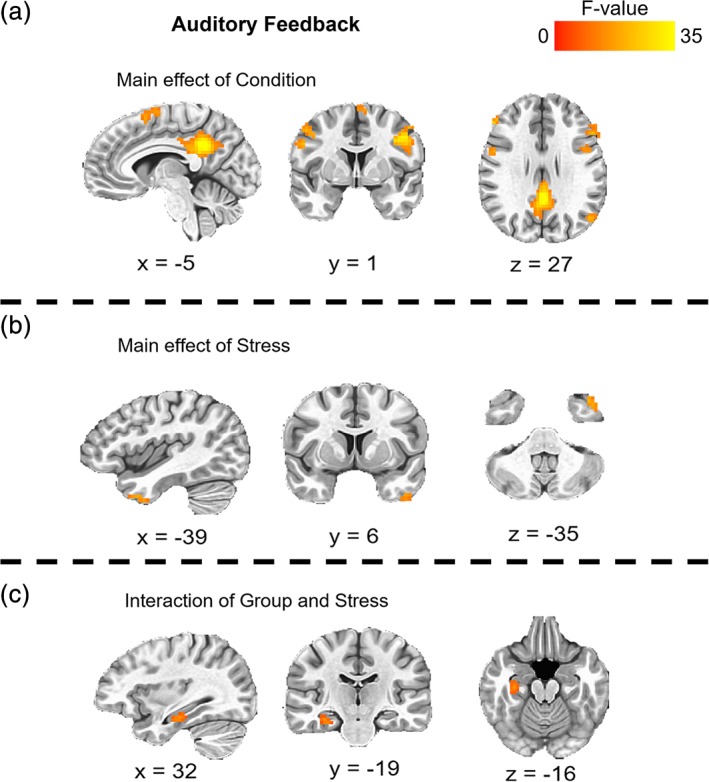
Clusters of significant activation during auditory feedback for the main effect of condition (CMT vs. SMT), the main effect perceived stress (PSS‐10), and the interaction of group (HC vs. LTLE) and perceived stress. CMT, control math task; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task
For the second 3dLME to assess auditory feedback, there was a significant main effect of condition (SMT–CMT), a main effect of perceived stress (PSS‐10), and a two‐way interaction between group (HC vs. LTLE) and perceived stress (Figure 3, Table 3). Specifically, clusters of activation for the main effect of condition were located within cingulate, PFC, middle temporal, parietal, and insula regions. A cluster of activation for the main effect of perceived stress was located within the superior temporal gyrus. A cluster of activation for the two‐way interaction between group and perceived stress was located within the hippocampal complex (parahippocampal gyrus and hippocampus). All remaining clusters of activations from the main effects of condition, group, and perceived stress, and their interactions, failed to survive the cluster‐corrected thresholds.
Table 3.
Regions showing condition (CMT vs. SMT) effect, perceived stress (PSS‐10) effect, and group (HC vs. LTLE) × perceived stress (PSS‐10) interaction during auditory feedback
| Cluster # | Region | Hemisphere | Vol (mm3) | Talairach (x, y, z) | F‐statistic |
|---|---|---|---|---|---|
| Main effect of condition | |||||
| 1 | Cingulate gyrus | L/R | 7,830 | −4, −43, 29 | 41.94 |
| 2 | Dorsolateral PFC | L | 3,348 | −40, 5, 32 | 33.81 |
| 3 | Superior parietal lobule | L | 1,971 | −22, −64, 44 | 20.92 |
| 4 | Dorsomedial PFC | L/R | 1,782 | 2, 11, 50 | 21.79 |
| 5 | Middle temporal gyrus | L | 1,377 | −51, −65, 26 | 19.26 |
| 6 | Dorsolateral PFC | L | 1,161 | −49, 20, 26 | 22.41 |
| 7 | Superior parietal lobule | R | 1,053 | 29, −61, 44 | 22.89 |
| 8 | Inferior parietal lobule | L | 702 | −37, −46, 41 | 16.14 |
| 9 | Dorsolateral PFC | R | 648 | 47, 2, 41 | 20.81 |
| 10 | Dorsolateral PFC | R | 621 | 29, −7, 65 | 18.25 |
| 11 | Dorsolateral PFC | R | 594 | 50, 32, 23 | 25.82 |
| 12 | Dorsolateral PFC | L | 540 | −46, 44, 14 | 20.12 |
| 13 | Precentral gyrus | R | 540 | 50, 2, 29 | 21.37 |
| 14 | Precentral gyrus | R | 459 | 44, −4, 56 | 19.33 |
| 15 | Insula | R | 432 | 38, 14, 8 | 20.12 |
| Main effect of stress | |||||
| 1 | Superior temporal gyrus | L | 486 | −37, 11, −34 | 20.30 |
| Group × stress interaction | |||||
| 1 | Parahippocampal gyrus | R | 945 | 32, −19, −16 | 15.23 |
Note. Cluster #, location, hemisphere, volumes, coordinates from Talairach and Tournoux (1988), and F‐statistic for the peak voxel of significant clusters. All clusters were significant at p < .05 (corrected).
Abbreviations: CMT, control math task; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task.
Because of our a priori hypotheses regarding hippocampal activation during a psychosocial stress task, the hippocampal complex clusters of activation identified by the three‐way interaction during math performance and the two‐way interaction during auditory feedback were submitted to follow‐up analyses to further explore these interactions. A signal extraction for the peak voxel (Talairach coordinates: x = 29, y = −34, z = −10) of the math performance cluster and the peak voxel (Talairach coordinates: x = 32, y = −19, z = −16) of the auditory feedback cluster were performed using 3dROIstats. BOLD signal during math performance during the CMT and SMT were compared for each subject (SMT–CMT). BOLD signal during auditory feedback during the CMT and SMT were averaged for each subject. Correlations compared this differential signal during math performance and perceived stress (PSS‐10 scores) for each group (HCs and LTLE) and within LTLE (LTLES+ and LTLES−; Figure 4). Correlations also compared average signal during auditory feedback ([SMT–CMT]/2) and perceived stress (PSS‐10 scores) for each group (HCs and LTLE) and within LTLE (LTLES+ and LTLES−). The results of these analyses revealed that the three‐way interaction of condition, group, and perceived stress was driven by differential activation (SMT–CMT) increasing with perceived stress in HCs (r = .37, p < .05) and decreasing for LTLE (r = −.40, p < .05). Within LTLE, there was a negative linear relationship between differential activation during math performance and perceived stress for LTLES+ (r = −.51, p < .01), but no relationship for the LTLES− (p = .08) groups. The two‐way interaction of group and perceived stress during auditory feedback was driven by hippocampal activation that decreased as perceived stress increased in HCs (r = −.42, p < .01), but not in LTLEs (r = .02, p = .93). Within LTLE, there was no relationship between hippocampal activation and perceived stress for the LTLES+ or LTLES− groups (both r s < .36, p s > .34).
Figure 4.
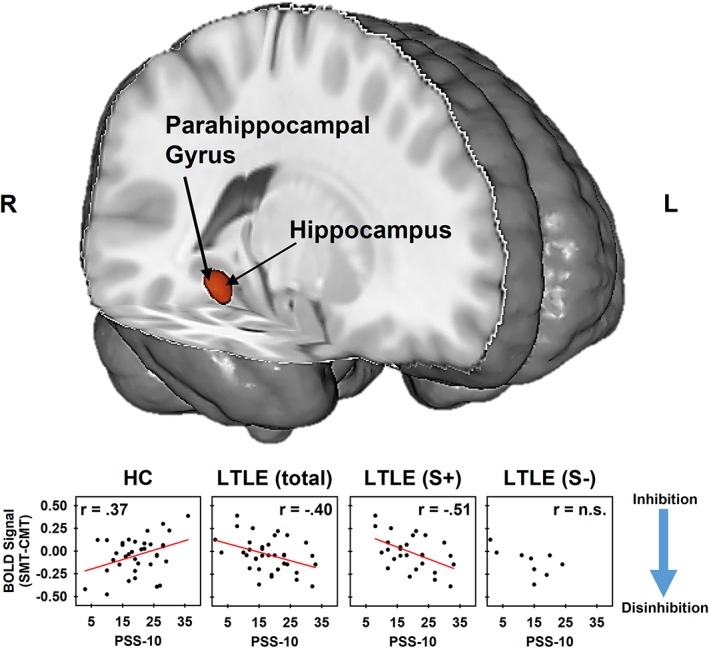
Cluster of activation (red) identified for the interaction of condition, group, and perceived stress in the right hippocampal complex (including parahippocampal gyrus and hippocampus regions). Signal extractions (bottom panel) separated by group reflect differences in the linear relationship between the neural response to stress and perceived stress between HC, LTLE, LTLE who report stress as a precipitate (LTLES+), and LTLE who report stress is not a precipitate (LTLES−) groups. Differences in the correlations between signal extractions and perceived stress reveal that the three‐way interaction condition, group, and perceived stress was due to a positive relationship in HCs and a negative relationship in LTLEs between differential (SMT–CMT) hippocampal activation and perceived stress (PSS‐10). Hippocampal activation serves an inhibitory role of in the emotional response to stress (blue arrow). Psychosocial stress decreases hippocampal activity and ultimately leads to disinhibition that results in the subsequent increase in arousal that is characteristic of the stress response (Dedovic et al., 2005; Goodman et al., 2016; Khalili‐Mahani et al., 2010; Pruessner et al., 2008). Alternatively, increased hippocampal activity has been linked to inhibition of emotional arousal in response to stress (Dedovic et al., 2009). CMT, control math task; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task
To assess group differences in functional connectivity during the SMT, a functionally defined seed ROI was generated based on the overlapping volume of the clusters within the right hippocampal region identified by the 3dLMEs. The COM voxel (Talairach coordinates: x = 30, y = −23, z = −15) was located for the region of overlap between the right hippocampus and parahippocampal gyrus clusters arising from the three‐way interaction during math performance and the two‐way interaction of auditory feedback (Figure 5a). Voxel‐wise stimulus event coefficients and gPPI coefficients of connectivity with hippocampal seed region maps were produced for each subject (Figure 5b). For the gPPI group comparison during math performance, there was increased positive connectivity within the right precentral gyrus (peak voxel: 32, −19, 53, t value = −3.49, vol = 1,323 mm3), extending into the postcentral gyrus, for LTLE versus HCs (Figure 5c, left panel). For the gPPI group comparison during auditory feedback, there was increased positive connectivity within the left superior temporal gyrus (peak voxel: −58, −10, −1, t value = −3.47, vol = 972 mm3), including Wernicke's area, for LTLE versus HCs (Figure 5c, right panel). All remaining clusters of connectivity with the hippocampal seed ROI that resulted from group comparisons of math performance and auditory feedback failed to survive the cluster‐corrected threshold.
Figure 5.
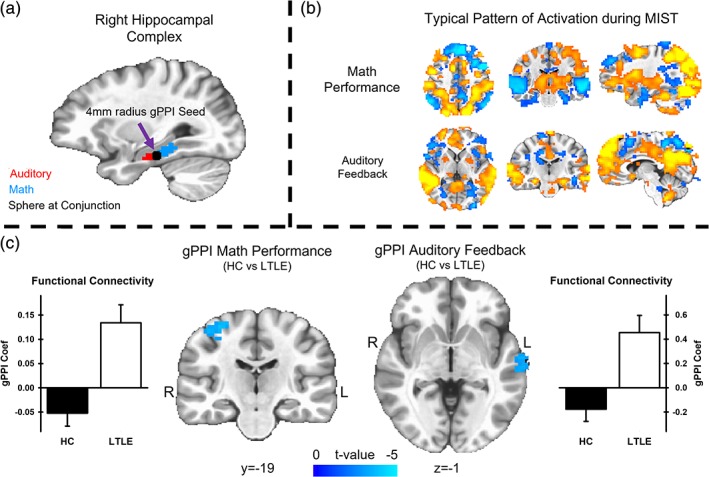
gPPI results comparing functional connectivity with the hippocampal seed region between HC and LTLE groups. The top left panel (a) depicts the conjunction between clusters activation resulting from the three‐way interaction of condition, group, and perceived stress during math performance (blue) and the two‐way interaction of group and perceived stress during auditory feedback (red). A 4 mm radius sphere (black) was constructed with a center voxel derived from the COM for the conjunction of the two interaction clusters. The top right panel (b) depicts the first‐level modeling results of a representative subject during math performance (top) and auditory feedback (bottom) during the stressful math task. The bottom panel shows the results of the gPPI analysis comparing HC and LTLE groups during stressful math performance and auditory feedback. During both math performance and auditory feedback events, the HC group demonstrated negative correlations, while the LTLE group demonstrated significantly increased positive correlations with the hippocampal seed regions during the SMT. COM, center‐of‐mass; CMT, control math task; gPPI, generalized psychophysiological interaction; HC, healthy control; LTLE, left temporal lobe epilepsy; PSS, Perceived Stress Scale; SMT, stress math task
4. DISCUSSION
In the context of perceived life stress, we compared differences between HCs and LTLEs in physiological and neural responses to mild‐to‐moderate stressor and, in LTLE, we assessed the effects of seizure control on these neurobehavioral responses. We hypothesized that the relationship between perceived life stress (PSS‐10) and MIST physiological and neural performance would differ between groups. The analyses revealed that the significant relationship between responses to stress and perceived stress varied between LTLE and HCs. These findings suggest a hippocampal mechanism underlying differences in the hormonal response to stress between HCs and LTLE. Further, our findings suggest an important role for the hippocampus in LTLE patients who report stress as a precipitant of seizures.
4.1. Perceived stress and seizures
Our primary goal was to compare the effects of increased life stress on emotional function between HCs and LTLE, while controlling for group differences in stress that may arise from comparing clinical and healthy populations. Due to matching HCs to LTLE, we noted that the perceived stress levels in our cohort were greater than the values for age‐matched controls (Cohen, 1994). These findings are consistent with prior reports that people with epilepsy endorse mood and anxiety‐related symptoms to a greater degree than typical HCs (Tellez‐Zenteno et al., 2007). Further, while patients with TLE most frequently report stress as a precipitant of seizures (Frucht, Quigg, Schwaner, & Fountain, 2000), to date, this claim lacked a clear biological correlate. We found that perceived stress levels increased with seizure frequency. These findings suggest our sample is well suited for assessing the relationship between perceived stress, physiological responses, and stress‐related neural activation to further elucidate the interaction between these variables in TRE patients.
4.2. Perceived stress and physiological responses
Cortisol, the end result of hippocampus–amygdala–HPA‐axis cortex release, is a gold standard for assessing the emotional response to stress in humans (Gossett et al., 2018; Jankord & Herman, 2008). Although cortisol release, increased HR, and other physiological changes are a necessary adaptive response to acute stress, a chronic maladaptive physiological stress response can have detrimental effects on health. For example, cortisol hypersecretion has negative effects on the body that can contribute to stress‐related disorders (Chrousos, 2009; Myers, McKlveen, & Herman, 2014). By comparing cortisol levels between groups at poststress and return‐to‐baseline time points, we noted a significant increase in cortisol secretion during a mild stressor in LTLE compared to HCs, regardless of perceived stress levels. However, there was an inverse relationship between these variables, regardless of group. Because there was an increase in cortisol secretion in LTLE compared to HCs, a follow‐up correlation analysis revealed that the inverse relationship between perceived stress and cortisol secretion was present only in HCs. Prior literature has suggested that HPA‐axis “burnout” that accompanies chronic stress can lead to blunted cortisol responses (Miller, Chen, & Zhou, 2007; Pruessner, Hellhammer, & Kirschbaum, 1999). Our finding that patients with LTLE do not show cortisol reduction with increases in perceived stress suggests a maladaptive hormonal stress response that may result from failure to develop (or maintain) similar resilience under chronic stress conditions compared to the HCs.
In addition to cortisol assessment, cardiac reactivity measures provided measurement of autonomic emotional responses during MIST. The time‐lag nature of cortisol assessment precludes the temporal resolution to compare stress and control conditions of the math tasks, yet HR can be measured and compared across these task conditions (Gossett et al., 2018). However, there were no significant differences in cardiac responses to stress between LTLE and HCs. The experimental manipulation was successful in inducing a cardiac stress response based on HR increases during the SMT, regardless of group. The finding that cortisol secretion was greater in patients with LTLE is consistent with prior reports of disruptions in endocrine responses to acute stressors among epilepsy populations (Zobel et al., 2004). Thus, hormonal, but not cardiac, reactivity may be an important mechanism underlying disruption in emotional function and disposition observed in LTLE.
4.3. Perceived stress and the neural response to stress
The current study assessed both, the neural response to stressful math performance and negative evaluative feedback while accounting for perceived stress differences between LTLEs and HCs. There were differences in the neural responses to math trials between experimental conditions within superior temporal and PFC regions, regardless of group. These findings are consistent with prior work characterizing typical neural responses to psychosocial stress during math performance (Dedovic et al., 2009; Wheelock et al., 2016). The differences in the neural responses to auditory feedback between our experimental conditions within dlPFC, dmPFC, precentral gyrus, middle temporal, and insula regions, regardless of group, are also consistent with prior findings. Prior work investigating the neural response to negative evaluations appearing as text during the MIST reported differential activation in dlPFC, dorsomedial prefrontal cortex (dmPFC), precentral gyrus, cingulate, middle temporal gyrus, and insula regions (Dedovic et al., 2009; Dedovic et al., 2014). Thus, these brain regions may underlie the behavioral response to social evaluative threat in a similar manner, regardless of method of threat delivery.
LTLE group differences in the neural responses to the current study's experimental conditions are consistent with group differences in cortisol responders reported in prior studies. Specifically, cortisol responders demonstrated greater differential activity between control and stressful task conditions within dmPFC (Dedovic et al., 2009), as well as, precuneus, posterior cingulate, and cingulate gyrus regions (Wheelock et al., 2016). In the current study, LTLEs had greater overall cortisol secretion compared to HCs. Taken together with prior literature on neural correlates of cortisol responses, the converging results from the current study suggests that activation differences within the PFC, cingulate, and precuneus may underlie the group differences in increased cortisol responses we observed in LTLE. Accordingly, the current results suggest a dissociation in neuroendocrine function and underlying brain function that differs for LTLE, while accounting for differences in perceived stress. These findings may point to alterations in PFC, cingulate, and parietal function associated with LTLE, which may arise as a complication that further exacerbates stress reactivity as a trigger for seizures.
Differences in the neural response to stress between LTLE and HCs, while controlling for perceived stress, provide new insight into how brain function may differ between the groups that are unrelated to overall differences in perceived life stress. This assessment addressed a key issue of collinearity of perceived life stress and group comparisons of LTLEs and HCs that were a limitation of prior investigations (Haut et al., 2018; Ridsdale et al., 2018). However, our primary aim was to assess whether the modulatory role of perceived life stress on the neurobehavioral responses to acute stress is altered in LTLE. In the current study, LTLE was associated with differences in the linear relationship between perceived life stress and activation during stressful math performance within the insula, hippocampal complex, and middle temporal areas. Interestingly, at lower levels of perceived stress, HCs demonstrated reduced hippocampal activation during the SMT relative to the CMT. Alternatively, LTLE demonstrated reduced hippocampal activation during SMT relative to CMT at higher levels of perceived stress. These effects were not observed for LTLES−. The linear relationship between perceived life stress and neural responses to auditory feedback (positive and negative) also varied between groups within a similar portion of the hippocampal region. However, there was a positive relationship with perceived stress for HCs, but no relationship for LTLES+ and LTLES−. These separate analyses demonstrate unique dissociations in the functional relationship between inhibitory activation within in the hippocampus and surrounding cortex and perceived stress that differs in LTLE. Specifically, lower levels of perceived life stress were associated with greater decreases in hippocampal activity only in HCs during stressful math performance. The opposite relationship was observed during evaluative feedback, in which lower levels of hippocampal activity were associated with higher perceived life stress, regardless of whether the feedback was positive or negative. Alternatively, LTLEs+ had greater changes in hippocampal activity during stressful math performance at higher levels of perceived life stress. Thus, altered hippocampal activity may play an important role in the emotion dysfunction associated with LTLE.
The results of our follow‐up gPPI analysis suggest that these group differences in hippocampal responses to acute stress may also involve connectivity with sensory processing areas specific to the stressor modality itself. Specifically, hippocampal connectivity with sensorimotor cortex (i.e., precentral and postcentral gyrus) differed between HC and LTLEs during stressful math performance. Somatosensory and motor cortex activity during math performance have been previously reported in the MIST literature (Dedovic et al., 2005; Wheelock et al., 2016). Since math performance requires rapid and accurate behavioral responses on a button box, the somatosensory and motor cortex connectivity differences observed in LTLE may reflect differences in the reaction to performance demands that are closely linked to the motor activity and sensory feedback of behavioral responses themselves. Likewise, hippocampal connectivity within language comprehension regions of the superior temporal gyrus (i.e., Wernicke's area) during stressful auditory feedback differed between HC and LTLE in the current study. While activation in language comprehension areas was an expected outcome for verbal auditory feedback during the MIST, differences in connectivity between HCs and LTLE suggest that the relationship between the neural activity underlying comprehension of negative auditory feedback and hippocampal activity is altered in LTLE. These findings suggest that projections from cortical sensory processing areas known to mediate the hippocampal stress response (Ulrich‐Lai & Herman, 2009), differ in LTLE. Accordingly, alterations of these modality‐specific sensory cortex‐hippocampal‐HPA axis neural circuits may be important mechanisms triggering seizures following acute stress. However, given that gPPI analysis measures nondirectional functional connectivity, it remains unclear whether the changes in hippocampal activity are causally influenced by sensory processing areas. Future studies may elucidate such directional influences between the regions by implementing effective connectivity assessment (i.e., Grainger causality analysis).
In general, hippocampal stimulation inhibits HPA‐axis activity, while hippocampal damage leads to increases in HPA‐axis activity (Herman et al., 2016). Decreased activation in hippocampal regions during stressful math performance serves as a disinhibitory neural mechanism, triggering HPA‐axis activation, and the release of cortisol in response to stress (Dedovic et al., 2009; Khalili‐Mahani et al., 2010; Pruessner et al., 2008). In addition to this role in the endocrine response to stress, hippocampal activity is related to psychological vulnerability and psychosocial stress (Pruessner et al., 2010). In the current study, we assessed hippocampal disinhibition by calculating decreases in differential activation (SMT–CMT) during math performance. Accordingly, HCs hippocampal activity was consistent with increased inhibitory emotion regulation at higher perceived stress. In these same participants, HCs had lower cortisol responses at higher perceived stress. This pattern was not present in the LTLE group.
These findings may reflect healthy adaptations to greater amounts of life stress, or resilience that is altered in LTLE. This claim is further bolstered by our finding that greater perceived life stress was associated with greater seizure frequency in LTLE (however, this could also be interpreted that greater seizure frequency was associated with greater perceived life stress). LTLE seizures triggered by stress may arise from disruption in healthy stress resilience processes that develop as a typical response to greater amounts of life stress. Further, unlike HCs that had decreased peripheral endocrine reactivity and increased hippocampal inhibition at higher levels of perceived stress, LTLEs had increased HPA‐axis reactivity and increased hippocampal disinhibition at higher level of perceived stress. Diurnal cortisol levels have also been shown to uniquely disrupt neuronal excitability for patients who report stress as a seizure precipitant. For example, patients with focal epilepsy who report stress as a seizure precipitant have an increased incidence of epileptiform discharges and decreased functional connectivity (assessed via EEG) between global brain regions in response to increasing waking cortisol levels (den Heijer et al., 2018; van Campen et al., 2016). Differences in the relationship between cortisol and neuronal excitability between patients who do and do not report stress as a seizure precipitant may be due to alterations in stress hormone receptors within brain regions. Specifically, one pathophysiological basis for stress‐associated seizures may be that cortisol uniquely affects amygdala and hippocampal excitability, leading to increased HPA activation, because of an altered distribution of glucocorticoid receptors within these brain regions for this population (van Campen et al., 2016). The unique interaction between hippocampal activation and perceived stress in LTLES+ in the current study is consistent with these prior findings of alterations in neural excitability and synchrony in patients with stress‐associated seizures. Specifically, perceived stress was negatively related to cortisol responses in HCs, but not LTLE, LTLES+, or LTLES−. Thus, changes in distribution of glucocorticoid receptors within hippocampal regions may act as a pathophysiological mechanism underlying the unique inverse relationship between perceived stress and hippocampal activity for patients who report stress as a precipitant that was observed in the present study.
4.4. Limitations
The current study found differences in hippocampal complex function within the hemisphere contralateral to seizure onset. Given that the current study included only LTLEs and HCs, it remains unclear whether parallel changes would be present in right temporal lobe epilepsy (RTLE). The rationale for inclusion of only unilateral LTLE patients was to achieve as large and uniform cohort as possible to address the stress‐seizure conundrum. However, this also resulted in a limitation that constitutes interesting remaining questions for future investigations. Likewise, the relatively low sample size of LTLES− (n = 9) compared to LTLES+ (n = 27) should be considered in the interpretation these findings. LTLE sample size was based on power calculation for sufficient group comparisons in fMRI (N = 12; Desmond & Glover, 2002); however, difficulty in recruitment of LTLES− patients limited our sample size below this enrollment target. Therefore, the achieved sample sizes provided sufficient power for voxel‐wise group‐level comparisons between LTLEs and HCs (n = 36 in each group), but not LTLES+ and LTLES−. Further, the LTLES− group experienced fewer seizures over the last 3 months (mean diff = 10.8 seizures). While this difference is not significantly different, the result of our comparison did trend to significance (p = .08). Given the positive correlation between seizure frequency and perceived stress, some differences in the neural response to stress between LTLES+ and LTLES− may arise from differences in seizure control between the obtained samples. Poor seizure control and increased depression scores in LTLES+ may also contribute to the trend toward significance for decreased accuracy and the increased behavioral response time compared to LTLES−. Thus, future investigation of the dynamic interaction between seizure control, depressive symptoms, and task performance may provide additional understanding of differences between TLE patients that report stress as a seizure precipitant and patients that do not.
All participants were asked to refrain from consuming food at least 1 h prior to sessions and scheduled to arrive at the experimental session in the early afternoon (12:00 p.m.–2:00 p.m.). Experimental sessions where scheduled during this window in order to reduce extraneous variability in cortisol measures that were unrelated to our stress manipulation. Specifically, the typical diurnal cortisol pattern is characterized by relatively large decreases in daily concentrations that occur within 3 h of waking (Pruessner et al., 1997) and subsequent to noon meals (Follenius, Brandenberger, & Hietter, 1982). Although a limited number of volunteers were unable to arrive before or during this session window (see Table 1), time of day for the initiation of experimental sessions (t1) and the proportion of participants with t1 times recorded before noon did not vary between groups. Further, we found no evidence that t1 varied with CortΔ. Thus, although variability in the time of day participants submitted saliva samples should be considered a limitation in the current study, these differences did not covary with group comparisons or CortΔ calculations.
Based on a visual inspection of cortisol measures between groups for all time points (t1–t7), a separate post hoc assessment of t2 cortisol levels revealed a significant increase in salivary cortisol 15 min prior to scanning for LTLEs compared to HCs, t(68) = 2.38, p < .05. Prior work has shown that preparation for an fMRI task elicits an anticipatory cortisol response before volunteers are exposed to the stress task itself (Gossett et al., 2018). Given that our current study claims increased cortisol reactivity to stressors in LTLE compared to HCs, this increased cortisol response prior to the task is consistent with the conclusions of the current study. However, the time course of experimental events and the reliance on CortΔ calculation to quantify individual differences in stress reactivity precludes the likelihood of lingering anticipatory cortisol responses prior to the stress task driving the group differences reported in LTLE and HCs. Specifically, CortΔ = (t3–t7)/t7 (Allendorfer et al., 2014) is derived from cortisol measurement time points that followed MRI scanning and began after sufficient time elapsed for a return to baseline salivary cortisol concentrations following the onset of MRI scanning preparation (≥90 min; for discussion, see Gossett et al., 2018). Thus, variability in CortΔ is unlikely due to group differences in prescanning cortisol levels and more likely due to reactivity during the stress task. Despite these likelihoods, anticipatory cortisol responses to fMRI tasks represent an inherent challenge to all investigations of stress reactivity during MR scanning.
Finally, the current study acquired neuroimaging data from two separate MRI scanner sites with different magnet strengths (3T vs. 4T). Although scanner was included as a covariate in our model, different numbers of HCs and LTLEs assessed at the two different sites should be considered a limitation of the current study. Likewise, cardiac reactivity, measured in BPM, was calculated and recorded on two different photoplethysmograph systems. Thus, our approach to assess cardiac reactivity contains two potential limitations that should be considered when interpreting comparisons in the current study. First, our calculation of BPM provided an overall index of autonomic arousal on the scale of an entire scan. However, HR variability is an alternative approach to index the autonomic stress response which assesses variability in the interbeat intervals and would provide a more ideal measure compared to BPM. Second, HRΔ was a calculation of differential responses to feedback (SMT–CMT) that is controlled for individual differences in baseline, which may have varied between scanners. Regardless of this possibility, the expected increase in HR during the SMT was present in both groups. However, we did not find group differences in HRΔ. Thus, the calculation of HRΔ using BPM and potential differences in HR between scanners should also be considered limitations of the current study.
4.5. Conclusion
The current study provides novel insight on the neural mechanisms that may mediate the relationship between disruptions in emotional brain function and seizure control in LTLE and extends prior literature by assessing the role of perceived life stress, which typically differs in patients with LTLE compared to HCs. The findings suggest that inhibitory hippocampal activity and connectivity are important mechanisms underlying differences in resilience to stress between HCs and LTLE. Given that LTLE in our sample demonstrated decreased seizure control as perceived stress increased, hippocampal inhibition during acute stress experiences may play an important role in seizure control.
ACKNOWLEDGMENTS
The Charles Shor Foundation for Epilepsy Research and the UAB Epilepsy Center supported this study. The authors thank Lucy Mendoza and Nancy Cohen for their contributions to data collection and physicians in both Epilepsy Centers for patient referrals.
Goodman AM, Allendorfer JB, Heyse H, et al. Neural response to stress and perceived stress differ in patients with left temporal lobe epilepsy. Hum Brain Mapp. 2019;40:3415–3430. 10.1002/hbm.24606
Funding information The Charles Shor Foundation for Epilepsy Research; UAB Epilepsy Division
Present address Judd M. Storrs, Department of Radiology, University of Mississippi Medical Center, Jackson, MS.
REFERENCES
- Allendorfer, J. B. , Heyse, H. , Mendoza, L. , Nelson, E. B. , Eliassen, J. C. , Storrs, J. M. , & Szaflarski, J. P. (2014). Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy—A pilot cross‐sectional fMRI study. Epilepsy & Behavior, 36, 115–123. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck depression inventory‐II. San Antonio, 78(2), 490–498. [Google Scholar]
- Bonora, A. , Benuzzi, F. , Monti, G. , Mirandola, L. , Pugnaghi, M. , Nichelli, P. , & Meletti, S. (2011). Recognition of emotions from faces and voices in medial temporal lobe epilepsy. Epilepsy & Behavior, 20, 648–654. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Brodie, M. J. , Liew, D. , & Kwan, P. (2018). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30‐year longitudinal cohort study. JAMA Neurology, 75, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos, G. P. (2009). Stress and disorders of the stress system. Nature Reviews. Endocrinology, 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Cisler, J. M. , Bush, K. , & Steele, J. S. (2014). A comparison of statistical methods for detecting context‐modulated functional connectivity in fMRI. NeuroImage, 84, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. (1994). Perceived Stress Scale. Redwood City, CA: Mind Garden. [Google Scholar]
- Cohen, S. , Kamarck, T. , & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox, R. W. , Chen, G. , Glen, D. R. , Reynolds, R. C. , & Taylor, P. A. (2017). FMRI clustering in AFNI: False‐positive rates Redux. Brain Connectivity, 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic, K. , Duchesne, A. , Engert, V. , Lue, S. D. , Andrews, J. , Efanov, S. I. , … Pruessner, J. C. (2014). Psychological, endocrine and neural responses to social evaluation in subclinical depression. Social Cognitive and Affective Neuroscience, 9, 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic, K. , Renwick, R. , Mahani, N. K. , Engert, V. , Lupien, S. J. , & Pruessner, J. C. (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry & Neuroscience, 30, 319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic, K. , Rexroth, M. , Wolff, E. , Duchesne, A. , Scherling, C. , Beaudry, T. , … Pruessner, J. C. (2009). Neural correlates of processing stressful information: An event‐related fMRI study. Brain Research, 1293, 49–60. [DOI] [PubMed] [Google Scholar]
- den Heijer, J. M. , Otte, W. M. , van Diessen, E. , van Campen, J. S. , Lorraine Hompe, E. , Jansen, F. E. , … Zijlmans, M. (2018). The relation between cortisol and functional connectivity in people with and without stress‐sensitive epilepsy. Epilepsia, 59, 179–189. [DOI] [PubMed] [Google Scholar]
- Desmond, J. E. , & Glover, G. H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. Journal of Neuroscience Methods, 118, 115–128. [DOI] [PubMed] [Google Scholar]
- Engel, J., Jr. (1996). Introduction to temporal lobe epilepsy. Epilepsy Research, 26, 141–150. [DOI] [PubMed] [Google Scholar]
- Follenius, M. , Brandenberger, G. , & Hietter, B. (1982). Diurnal cortisol peaks and their relationships to meals. The Journal of Clinical Endocrinology and Metabolism, 55, 757–761. [DOI] [PubMed] [Google Scholar]
- Frucht, M. M. , Quigg, M. , Schwaner, C. , & Fountain, N. B. (2000). Distribution of seizure precipitants among epilepsy syndromes. Epilepsia, 41, 1534–1539. [DOI] [PubMed] [Google Scholar]
- Gilliam, F. (2015). Social cognition and epilepsy: Understanding the neurobiology of empathy and emotion. Epilepsy Currents, 15, 118–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, A. M. , Wheelock, M. D. , Harnett, N. G. , Mrug, S. , Granger, D. A. , & Knight, D. C. (2016). The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience, 339, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett, E. W. , Wheelock, M. D. , Goodman, A. M. , Orem, T. R. , Harnett, N. G. , Wood, K. H. , … Knight, D. C. (2018). Anticipatory stress associated with functional magnetic resonance imaging: Implications for psychosocial stress research. International Journal of Psychophysiology, 125, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut, S. R. , Hall, C. B. , Masur, J. , & Lipton, R. B. (2007). Seizure occurrence: Precipitants and prediction. Neurology, 69, 1905–1910. [DOI] [PubMed] [Google Scholar]
- Haut, S. R. , Lipton, R. B. , Cornes, S. , Dwivedi, A. K. , Wasson, R. , Cotton, S. , … Privitera, M. (2018). Behavioral interventions as a treatment for epilepsy: A multicenter randomized controlled trial. Neurology, 90, e963–e970. [DOI] [PubMed] [Google Scholar]
- Herman, J. P. , McKlveen, J. , Ghosal, S. , Kopp, B. , Wulsin, A. , Makinson, R. , … Myers, B. (2016). Regulation of the hypothalamic‐pituitary‐adrenocortical stress response. Comprehensive Physiology, 6, 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord, R. , & Herman, J. P. (2008). Limbic regulation of hypothalamo‐pituitary‐adrenocortical function during acute and chronic stress. Annals of the New York Academy of Sciences, 1148, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner, A. M. (2007). Epilepsy and mood disorders. Epilepsia, 48 Suppl. 9, 20–22. [DOI] [PubMed] [Google Scholar]
- Khalili‐Mahani, N. , Dedovic, K. , Engert, V. , Pruessner, M. , & Pruessner, J. C. (2010). Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus, 20, 323–334. [DOI] [PubMed] [Google Scholar]
- Lundgren, T. , Dahl, J. A. , Yardi, N. , & Melin, L. (2008). Acceptance and commitment therapy and yoga for drug‐refractory epilepsy: A randomized controlled trial. Epilepsy & Behavior, 13, 102–108. [DOI] [PubMed] [Google Scholar]
- McLaren, D. G. , Ries, M. L. , Xu, G. , & Johnson, S. C. (2012). A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletti, S. , Benuzzi, F. , Cantalupo, G. , Rubboli, G. , Tassinari, C. A. , & Nichelli, P. (2009). Facial emotion recognition impairment in chronic temporal lobe epilepsy. Epilepsia, 50, 1547–1559. [DOI] [PubMed] [Google Scholar]
- Miller, G. E. , Chen, E. , & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic‐pituitary‐adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. [DOI] [PubMed] [Google Scholar]
- Monti, G. , & Meletti, S. (2015). Emotion recognition in temporal lobe epilepsy: A systematic review. Neuroscience and Biobehavioral Reviews, 55, 280–293. [DOI] [PubMed] [Google Scholar]
- Myers, B. , McKlveen, J. M. , & Herman, J. P. (2014). Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Frontiers in Neuroendocrinology, 35, 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, Y. , Goldstein, L. H. , Fenwick, P. B. C. , & Trimble, M. R. (2004). Clinical efficacy of galvanic skin response biofeedback training in reducing seizures in adult epilepsy: A preliminary randomized controlled study. Epilepsy & Behavior, 5, 216–223. [DOI] [PubMed] [Google Scholar]
- Nakken, K. O. , Solaas, M. H. , Kjeldsen, M. J. , Friis, M. L. , Pellock, J. M. , & Corey, L. A. (2005). Which seizure‐precipitating factors do patients with epilepsy most frequently report? Epilepsy & Behavior, 6, 85–89. [DOI] [PubMed] [Google Scholar]
- Polak, E. L. , Privitera, M. D. , Lipton, R. B. , & Haut, S. R. (2012). Behavioral intervention as an add‐on therapy in epilepsy: Designing a clinical trial. Epilepsy & Behavior, 25, 505–510. [DOI] [PubMed] [Google Scholar]
- Privitera, M. , Walters, M. , Lee, I. , Polak, E. , Fleck, A. , Schwieterman, D. , & Haut, S. R. (2014). Characteristics of people with self‐reported stress‐precipitated seizures. Epilepsy & Behavior, 41, 74–77. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C. , Dedovic, K. , Khalili‐Mahani, N. , Engert, V. , Pruessner, M. , Buss, C. , … Lupien, S. (2008). Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63, 234–240. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C. , Dedovic, K. , Pruessner, M. , Lord, C. , Buss, C. , Collins, L. , … Lupien, S. J. (2010). Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology, 35, 179–191. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C. , Hellhammer, D. H. , & Kirschbaum, C. (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine, 61, 197–204. [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C. , Wolf, O. T. , Hellhammer, D. H. , Buske‐Kirschbaum, A. , von Auer, K. , Jobst, S. , … Kirschbaum, C. (1997). Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences, 61, 2539–2549. [DOI] [PubMed] [Google Scholar]
- Puskarich, C. A. , Whitman, S. , Dell, J. , Hughes, J. R. , Rosen, A. J. , & Hermann, B. P. (1992). Controlled examination of effects of progressive relaxation training on seizure reduction. Epilepsia, 33, 675–680. [DOI] [PubMed] [Google Scholar]
- Ramaratnam, S. , Baker, G. A. , & Goldstein, L. (2003). Psychological treatments for epilepsy. The Cochrane database of systematic reviews, 4, CD002029. [DOI] [PubMed] [Google Scholar]
- Ridsdale, L. , Wojewodka, G. , Robinson, E. J. , Noble, A. J. , Morgan, M. , Taylor, S. J. C. , … Goldstein, L. H. (2018). The effectiveness of a group self‐management education course for adults with poorly controlled epilepsy, SMILE (UK): A randomized controlled trial. Epilepsia, 59, 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semah, F. , Picot, M. C. , Adam, C. , Broglin, D. , Arzimanoglou, A. , Bazin, B. , … Baulac, M. (1998). Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology, 51, 1256–1262. [DOI] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Allendorfer, J. B. , Heyse, H. , Mendoza, L. , Szaflarski, B. A. , & Cohen, N. (2014). Functional MRI of facial emotion processing in left temporal lobe epilepsy. Epilepsy & Behavior, 32, 92–99. [DOI] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Allendorfer, J. B. , Nenert, R. , LaFrance, W. C., Jr. , Barkan, H. I. , DeWolfe, J. , … ver Hoef, L. (2018). Facial emotion processing in patients with seizure disorders. Epilepsy & Behavior, 79, 193–204. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain : An approach to medical cerebral imaging. New York, NY: Thieme Medical Publishers. [Google Scholar]
- Tang, V. , Poon, W. S. , & Kwan, P. (2015). Mindfulness‐based therapy for drug‐resistant epilepsy: An assessor‐blinded randomized trial. Neurology, 85, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Tellez‐Zenteno, J. F. , Patten, S. B. , Jetté, N. , Williams, J. , & Wiebe, S. (2007). Psychiatric comorbidity in epilepsy: a population‐based analysis. Epilepsia, 48(12), 2336–2344. [DOI] [PubMed] [Google Scholar]
- Ulrich‐Lai, Y. M. , & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews. Neuroscience, 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen, J. S. , Hompe, E. L. , Jansen, F. E. , Velis, D. N. , Otte, W. M. , van de Berg, F. , … Zijlmans, M. (2016). Cortisol fluctuations relate to interictal epileptiform discharges in stress sensitive epilepsy. Brain, 139, 1673–1679. [DOI] [PubMed] [Google Scholar]
- Walpole, P. , Isaac, C. L. , & Reynders, H. J. (2008). A comparison of emotional and cognitive intelligence in people with and without temporal lobe epilepsy. Epilepsia, 49, 1470–1474. [DOI] [PubMed] [Google Scholar]
- Wheelock, M. D. , Harnett, N. G. , Wood, K. H. , Orem, T. R. , Granger, D. A. , Mrug, S. , & Knight, D. C. (2016). Prefrontal cortex activity is associated with biobehavioral components of the stress response. Frontiers in Human Neuroscience, 10, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel, A. , Wellmer, J. , Schulze‐Rauschenbach, S. , Pfeiffer, U. , Schnell, S. , Elger, C. , & Maier, W. (2004). Impairment of inhibitory control of the hypothalamic pituitary adrenocortical system in epilepsy. European Archives of Psychiatry and Clinical Neuroscience, 254, 303–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified data that support the findings of this study are available from the corresponding author, upon reasonable request.


