Abstract
Gray matter (GM) atrophy is frequently detected in persons living with HIV, even in the era of combination antiretroviral therapy (cART), but the specificity of regions affected remains elusive. For instance, which regions are consistently affected in HIV? In addition, atrophy at which regions is frequently associated with neurocognitive impairment in HIV? Resolving these questions can potentially help to establish the possible neural profiles of HIV‐associated neurocognitive disorders (HAND) severity, which currently is solely defined by neurobehavioral assessments. Here, we addressed these questions using a novel meta‐analysis technique, the colocalization‐likelihood estimation (CLE) technique, to quantitatively synthesize the findings of GM atrophy in HIV+ adults. Twenty‐one of 386 studies published between 1988 and November 2017 and identified in PubMed were selected, plus four identified in other resources. In the end, 25 studies (1,370 HIV+ adults, 889 HIV− controls) were included in the meta‐analysis. This technique revealed that GM atrophy in HIV+ adults was dominated by two distinct but nonexclusive profiles: frontal (including anterior cingulate cortex, [ACC]) atrophy, which was associated withHIV‐disease and consistently differentiated HIV+ adults from HIV− controls; and caudate/striatum atrophy, which was associated with neurocognitive impairment. The critical role of caudate/striatum atrophy in neurocognitive impairment was further supported by a separate data analysis, which examined the findings of correlation analyses between GM and neurocognitive performance. These results suggest that the frontal lobe and the striatum play critical but differential roles in HAND. A neural model of HAND severity was proposed with several testable predictions.
Keywords: atrophy, caudate, frontal, gray matter, HIV, meta‐analysis, striatum
1. INTRODUCTION
The brain is recognized as a persistent reservoir of HIV despite successful viral suppression with modern antiretroviral treatment in the era of combination antiretroviral therapy (cART) (Churchill & Nath, 2013; Hellmuth, Valcour, & Spudich, 2015; Spudich & Ances, 2017), and HIV‐associated neurocognitive disorders (HAND) remain highly prevalent (Heaton et al., 2010; Sacktor et al., 2016). Neural injury to the frontostriatal circuits has been recognized as a key component of HAND (Ellis, Langford, & Masliah, 2007), but the precise mechanisms remain to be elucidated (Saylor et al., 2016), especially in the context of neurocognitive impairment. For instance, within the frontostriatal circuits and associated brain networks, which regions are most consistently affected in HIV (i.e., comparing HIV+ adults to HIV− controls), and neural injury to which regions is most consistently associated with neurocognitive impairment in HIV (i.e., comparing cognitively impaired HIV+ adults to cognitively normal HIV+ adults)? Addressing these questions might help to develop a “neurally‐based” model of HAND stages/severity that may have the potential to assist with HAND diagnosis, monitoring, and treatment (Ellis & Letendre, 2016). Currently the standard model of HAND is solely based on neurobehavioral assessment obtained with standard neuropsychology tests (Antinori et al., 2007; Heaton et al., 2010).
Brain imaging techniques such as magnetic resonance imaging (MRI) offer a noninvasive way to investigate alterations in brain structure, neuronal function, and neurochemistry that may contribute to HAND and other types of dementia in vivo. In particular, structural MRI is increasingly becoming a standard procedure to detect and assess brain atrophy in both clinical practice and basic research. In HIV, a number of studies have used structural MRI techniques to investigate brain atrophy due to HIV‐disease and the relationship between brain atrophy and neurocognitive performance. These studies have revealed a wide distribution of brain atrophy in individuals with HIV‐disease, including frontal, subcortical, temporal, parietal, occipital, and cerebellum. One of the most consistent findings is subcortical atrophy in HIV, even in the era of cART (Becker et al., 2011). Studies have linked atrophy in basal ganglia to neurocognitive impairment (Kumar, Ownby, Waldrop‐Valverde, Fernandez, & Kumar, 2011), disrupted cerebral metabolite levels (Cohen et al., 2010; Hua et al., 2013), HIV‐disease duration (Ances, Ortega, Vaida, Heaps, & Paul, 2012; Becker et al., 2011), history of immune dysfunction (Tesic et al., 2018), immune recovery (Fennema‐Notestine et al., 2013), and viral suppression (Guha et al., 2016; Kallianpur et al., 2013; Krivine et al., 1999). Studies have even suggested that the size of caudate nucleus might serve as a potential biomarker of HAND (Dal Pan et al., 1992; Hestad et al., 1993; Kieburtz et al., 1996). Meanwhile, there are also many inconsistent findings across studies. For instance, while many studies revealed reduced amygdala volume in HIV+ adults (Ances et al., 2012; Kallianpur et al., 2013; Spies, Ahmed‐Leitao, Fennema‐Notestine, Cherner, & Seedat, 2016), several other studies found no difference between HIV+ adults and HIV− controls (Jernigan et al., 2005; Ragin et al., 2012; Wade et al., 2015), and a final study found increased amygdala volume in HIV+ adults (Clark et al., 2015).
These inconsistent and somewhat conflicting reports can be attributed to many probable factors. First, the sample size of individual studies is usually small and likely underpowered, which could confound the study results in two directions: on the one hand, the small sample size could lead to missed findings due to a lack of statistical power; but on the other hand, the small sample size could push researchers toward using less stringent approaches/criteria, which could lead to an increase in false positives. Second, different approaches in data analysis (i.e., voxel‐based vs regions‐of‐interest [ROI] based) could also lead to different results, even in the same study. Third, with the probable heterogeneities among HIV‐communities, the difference between subject groups in different studies could potentially contribute to some inconsistent findings. The small sample size of individual studies could also increase the chance of sampling bias.
To quantitatively consolidate the inconsistencies and to synthesize the consistent findings across different studies, we performed a meta‐analysis on structural MRI studies published in the cART era and with HIV+ adults, with a focus on gray matter (GM). Studies with children, mainly perinatally‐infected children, were not included due to differences in neuropathogenesis. Conventionally there are two well‐developed and widely used approaches in MRI meta‐analysis: image‐based meta‐analyses, which take advantage of the full statistical maps from the original studies (Salimi‐Khorshidi, Smith, Keltner, Wager, & Nichols, 2009); and coordinate‐based meta‐analyses, which use the reported coordinates of peak locations from published works (Turkeltaub, Eden, Jones, & Zeffiro, 2002). However, both approaches are unsuitable for structural MRI studies in HIV, mainly due to: (a) full statistical maps were generally unavailable; (b) many studies used a methodology that would not produce coordinates needed for a coordinate‐based meta‐analysis, that is, coordinates were not available with an ROI‐based approach that focused on the GM volume of a subset of ROIs based on prior knowledge (e.g., striatum and/or its subregions); and (a) even for studies using voxel‐based (or similar) approaches, the coordinates of peak locations were only reported in a handful of studies.
To overcome these limitations, we adopted a novel colocalization‐likelihood estimation (CLE) technique recently developed (Turkeltaub, Pullman, Pierpont, & Ullman, 2012). Briefly, in this technique, instead of using coordinates or statistical maps to synchronize findings across studies, an ROI‐based approach along with a permutation technique was used to quantify the prevalence of atrophy at different regions. Using this novel CLE technique, here we provide quantitative evidence suggesting that GM atrophy in HIV+ adults in the cART era is dominated by two distinct but nonexclusive profiles: frontal (including anterior cingulate cortex, [ACC]) atrophy, which is associated with HIV‐disease and consistently differentiated HIV+ adults from HIV− controls; and caudate/striatum atrophy, which is associated with neurocognitive decline in HIV. The critical role of caudate/striatum atrophy in neurocognitive impairment in HIV was further supported by a separate qualitative data analysis focusing on the correlations between GM volume/cortical thickness and neurocognitive performance. A neurally‐based model of HAND based on GM atrophy was proposed. The CLE technique was compared to and validated with an established coordinate‐based MRI meta‐analysis technique, the activation likelihood estimation (ALE) technique (Eickhoff et al., 2009; Turkeltaub et al., 2002).
2. MATERIALS AND METHODS
2.1. Literature search and study selection
2.1.1. Literature search
A total of 386 studies published between 1988 and November 2017 were identified through an online search of PubMed using the following search parameters: “(HIV[Title/Abstract] or HIV+[Title/Abstract] or HIV‐1[Title/Abstract]) and (atrophy[Title/Abstract] or thickness[Title/Abstract] or thinning[Title/Abstract] or volume[Title/Abstract] or volumetric[Title/Abstract] or shape[Title/Abstract] or density[Title/Abstract] or VBM[Title/Abstract]) and (MRI OR magnetic resonance imaging)”. Additional papers were searched and reviewed through Web of Science, Google Scholar, and literature review (Figure 1).
Figure 1.
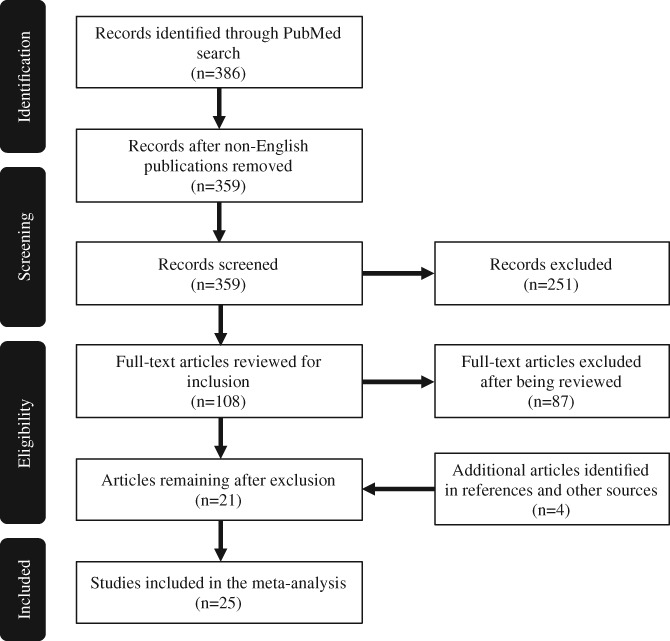
The flow diagram of selecting studies for the colocalization‐likelihood estimation (CLE) meta‐analysis
2.1.2. Initial screening
Articles that met one or more of following exclusion criteria were excluded at the initial screening stage without reviewing the full‐text: not originally published in English (n = 27); studies conducted during the pre‐cART era (n = 62); no HIV+ group (n = 55); not using structural brain imaging (n = 28); no reports of brain structure (n = 55); not original studies, that is, reviews or meta‐analysis (n = 6); not using human adults as subjects (n = 35); sample size too small (less than 10 per group) (n = 8); and postmortem studies (n = 2). Some articles met multiple exclusion criteria, but were only counted once here based on the order of exclusion criteria described above (similarly during the next step of selection shown below).
2.1.3. Final study selection
After this initial screening, 108 articles remained and the full‐text were obtained and reviewed for additional exclusion criteria, including: no results on regional GM volume/thickness (n = 16) as we limited our analysis to the findings of reduced GM volume and/or cortex thickness (both presumably are due to synapto‐dendritic injury and/or neuronal loss), while excluding findings of change in shapes (mainly subcortical regions) and of white matter and ventricles; participants with multiple comorbid health concerns (e.g., neurosyphilis, diabetes, vascular disease) (n = 6); studies that did not include the effects of HIV‐status or neurocognitive performance, that is, focusing solely on the outcome of a clinical trial, a particular psychiatric disorder, and so forth. (n = 18); insufficient information about the data analyses performed or missing results (n = 7).
A total of 61 studies met all the initial inclusion criteria. At the next step, we excluded articles with the same subject cohorts or from the same lab or authors (n = 40). This step is necessary to avoid certain subject groups that have been examined by multiple studies being over‐represented in the meta‐analysis. When deciding between two or more publications from the same lab/group or cohort, studies were favored in the following order: study with whole brain analyses (versus ROI‐based analyses); if both studies used ROI‐based analyses, the study that examined a greater number of ROIs was chosen; study with a larger sample size; study published more recently.
Twenty‐one articles of the 386 articles were selected after the final study selection. In addition, four more articles were identified from other sources and were added to the meta‐analysis. The whole selection process was also shown in Figure 1. In the end, 25 articles were selected and included in the CLE analysis (Table 1). For HIV+ adults included in these studies, except those with primary HIV infection (n = 197), reportedly 79.4% were on cART, and 78.3% with controlled viral load (different studies might have used different criteria to define controlled viral load).
Table 1.
The list of studies included in the colocalization‐likelihood estimation (CLE) meta‐analysis
| Study | Participants (HIV+, HIV‐) | Cognitive performancea | Percentage taking ART | Percentage with suppressed VLb | Analysesc |
|---|---|---|---|---|---|
| (Castillo, Ernst, Cunningham, & Chang, 2018) | 35, 31 | N/A | 97.1% | Unknown | 2 |
| (Sanford et al., 2017) | 125, 62 | HIV+ impaired | 90% | 75% | 2, 3 |
| (Zhou et al., 2017) | 22, 22 | HIV+ unimpaired | 0% | Unknown | 1, 2 |
| (Underwood et al., 2017) | 134, 79 | HIV+ impaired | 100% | 100% | 2, 3 |
| (Clifford et al., 2017) | 38, 24 | 15 CI; 23 CN | 100% | 100% | 1, 2, 3, 4 |
| (Shin et al., 2017) | 22, 11 | 10 CI; 12 CN | 100% | 100% | 1, 2, 3, 4 |
| (Thames et al., 2017) | 48, 29 | HIV+ impaired | 100% | 50% | 2, 3 |
| (Paul et al., 2017) | 146, 34 | N/A | 12.7% | 2.75% | 2 |
| (Kuhn et al., 2017) | 59, 22 | N/A | 93.8% | 78% | 2 |
| (Wang et al., 2016) | 26, 26 | N/A | 34.6% | Unknown | 2 |
| (Corrêa et al., 2016) | 47, 19 | 34 CI; 13 CN | 100% | 95.7% | 1, 2, 3, 4 |
| (Spies et al., 2016) | 62, 62 | HIV+ impaired | 48.4% | 0% | 2, 3 |
| (Wilson et al., 2015) | 17, 17 | HIV+ impaired | 100% | 94.1% | 2, 3 |
| (Wade et al., 2015) | 63, 31 | HIV+ impaired | Unknownd | 61.9% | 2, 3 |
| (Heaps et al., 2015) | 74, 29 | 37 CI; 37 CN | 0% | Unknown | 1, 2, 3, 4 |
| (Clark et al., 2015) | 44, 44 | HIV+ unimpaired | 86.4% | 79.4% | 1, 2 |
| (Li, Li, Gao, Yuan, & Zhao, 2014) | 36, 33 | HIV+ unimpaired | 30.6% | Unknown | 1, 2 |
| (Pfefferbaum et al., 2014) | 51, 65 | HIV+ impaired | 80.4% | Unknown | 2, 3 |
| (Kallianpur et al., 2013) | 35, 12 | N/A | 100% | 28.6% | 2 |
| (Towgood et al., 2012) | 40, 42 | HIV+ unimpaired | 100% | 100% | 1, 2 |
| (Becker et al., 2012) | 81, 67 | HIV+ impaired | Unknowne | Unknown | 1, 2 |
| (Ragin et al., 2012) | 43, 21 | HIV+ impaired | 46.5% | Unknown | 2, 3 |
| (Küper et al., 2011) | 48, 48 | 28 CI; 20 CN | 93.8% | Unknown | 1, 3, 4 |
| (Castelo, Courtney, Melrose, & Stern, 2007) | 22, 22 | HIV+ impaired | 90.9% | 63.6% | 2, 3 |
| (Jernigan et al., 2005) | 52, 37 | HIV+ impaired | 69.2% | 100% | 2, 3 |
Cognitive Performance: N/A: the study did not include neurocognitive data; HIV+ Impaired: HIV+ adults met the HIV‐associated neurocognitive disorders (HAND) definition if the study used the Frascati criteria to define neurocognitive status, or HIV+ adults performed significantly worse than controls in at least one neurocognitive test in the study that did not use the Frascati criteria to define neurocognitive status; HIV+ Unimpaired: HIV+ adults did not meet the HAND definition if the study used the Frascati criteria to define neurocognitive status, or HIV+ adults performed comparable to controls in all neurocognitive tests administered in the study that did not use the Frascati criteria to define neurocognitive status; CN and CI: the study used neurocognitive data to divide HIV+ adults into at least two groups, cognitively “normal” (CN), and cognitively “impaired” (CI). Different criteria might be used in different studies.
Different studies might have different definitions of viral load suppression. To avoid further complications, we simply used the same numbers provided in these studies regardless of how viral load suppression was defined in each individual study.
Analyses. This indicates whether a study was included in one of the four analyses: 1, HIV‐ controls versus cognitively “normal” HIV+ adults; 2, HIV‐ controls versus HIV+ adults (including cognitively “normal” HIV+ adults, cognitively “impaired” HIV+ adults, and those without neurocognitive data); 3, HIV‐ controls versus cognitively “impaired” HIV+ adults; 4, Cognitively “normal” HIV+ adults versus cognitively “impaired” HIV+ adults.
The average disease duration (estimated) was 20.4 years.
The subjects in this study were part of the MACS cohort, and the profile of the entire cohort can be found elsewhere (Becker et al., 2015).
To investigate the relationship between atrophy and neurocognitive performance, we divided the HIV+ adults in these studies into three subgroups (see below). The definition of subgroups was solely based on the data available in each individual study. That is, the standard Frascati diagnostic criteria (Antinori et al., 2007) was used for studies that also used the criteria in defining its HIV+ adults (i.e., HAND vs cognitively normal), otherwise the HIV+ subgroup definition was based on the performance of all neuropsychology tests administered in each of the original studies. The three HIV+ subgroups were:
Cognitively “normal,” which included HIV+ adults who did not meet the HAND criteria if the original study used the Frascati diagnostic criteria to assess neurocognitive performance; and HIV+ adults who performed comparable to HIV− controls on all tests (i.e., no significant difference on any tests that were administered in the study) if the original study did not use the Frascati diagnostic criteria to assess neurocognitive performance.
Cognitively “impaired,” which included HIV+ adults who met the HAND criteria if the original study used the Frascati diagnostic criteria to assess neurocognitive performance; and HIV+ adults who performed significantly worse than controls on at least one neurocognitive test administered in the study that did not use the Frascati diagnostic criteria to assess neurocognitive performance (the reason to use a conservative approach for these studies was due to the fact that they tended to rely on a less comprehensive neuropsychological test battery, or instruments less sensitive to mild cognitive deficits).
HIV+ adults (regardless of neurocognitive performance or presence of neurocognitive evaluation), which included HIV+ adults from 1) and 2) listed above, as well as HIV+ adults from studies that did not report/assess neurocognitive performance.
The strategy we adopted in defining HIV+ subgroups is a necessary compromise. First, this is a meta‐analysis, which is limited by the data available from original studies that have used a wide range of approaches/criteria in assessing neurocognitive performance (and some of them did not include neurocognitive performance data in the publications). Second, this approach has certain limitations. For instance, HIV+ adults from a study with a less comprehensive neuropsychological test battery, or a study with instruments less sensitive to mild cognitive deficits, may inadvertently classify a participant as cognitively “normal” even though some of them might meet HAND criteria (Antinori et al., 2007) if examined with a comprehensive battery or with mores sensitive neuropsychological tests, and vice versa. Third, for the four analyses detailed below, Analysis I and III would be affected by how we defined HIV+ subgroups, but Analysis II and IV would not. Interestingly, the results from Analysis II and IV were in a general agreement with the results from Analysis I and III (see Results and Figure 2), providing evidence supporting our strategy as a reasonable and valid approach.
Figure 2.
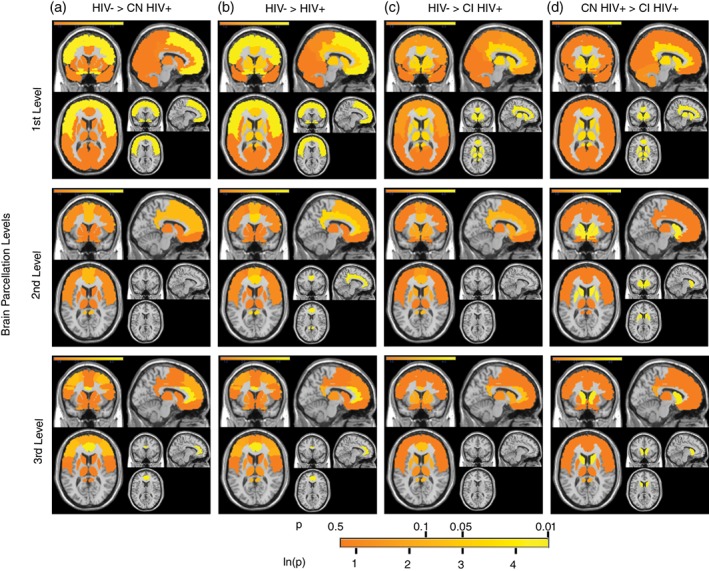
The colocalization‐likelihood estimation (CLE) results of four group comparisons at three different brain parcellation levels. The four group comparisons included: (a) HIV− controls versus cognitively normal HIV+ adults; (b) HIV− controls versus HIV+ adults in general (including HIV+ adults with normal cognitive function (CN), HIV+ adults with cognitive impairment (CI), and those without neurocognitive data (i.e., the neurocognitive data was not reported in the original study); (c) HIV− controls versus cognitively impaired HIV+ adults; and (d) cognitively normal HIV+ adults versus cognitively impaired HIV+ adults. The three levels of brain parcellation included: (a) the first level, which was a coarse parcellation of the entire brain, including frontal, limbic/subcortical, parietal, temporal, and so forth; (b) the second level, which was a parcellation of brain networks within the frontal‐limbic‐subcortical system, such as striatum, cingulate, thalamus, medial temporal lobe, inferior frontal cortex, and so forth; and (c) the third level, which was a more fine parcellation of the subregions within the frontal‐limbic‐subcortical network, including caudate, anterior cingulate cortex, hippocampus, and so forth, (see methods for details). The significance level at each region was illustrated via a color scale, from 0.5 to 0.01 (for the ‐ln[p], the range was from 0.69 to 4.6). For each comparison at each parcellation level, the brain regions that demonstrated significant group difference were shown at the bottom‐right of each subplot. CI HIV+: cognitively impaired HIV+ adults; CN HIV+: cognitively normal HIV+ adults; HIV+: HIV+ adults regardless of neurocognitive function; HIV−: HIV− controls
Following the group definition, the 25 articles were carefully reviewed and included in one or more of the four meta‐analyses detailed below (Analysis I, Analysis II, Analysis III, and Analysis IV) (Table 1):
HIV− controls versus cognitively “normal” HIV+ adults (10 studies with 328 HIV+ and 339 HIV− adults). Inclusion criteria: neurocognitive function was assessed with at least one neuropsychology test in both controls and HIV+ adults; neurocognitive data was reported; HIV+ adults (or a sub‐group of HIV+ adults) performed comparable to controls on all tests (i.e., no significant difference on any tests that were administered in the study) if the original study did not use the standard Frascati criteria in assessing neurocognitive performance, or HIV+ adults who did not meet the HAND definition if the original study used the Frascati criteria; and controls were compared to HIV+ adults. If a study divided HIV+ adults into two groups, cognitively “normal” versus cognitively “impaired”, only the results of controls versus cognitively “normal” HIV+ adults were included in this analysis.
HIV− controls versus HIV+ adults, regardless of neurocognitive performance (24 studies with 1,304 HIV+ and 836 HIV− adults). Inclusion criteria: controls were compared to HIV+ adults (including cognitively “normal” HIV+ adults, cognitively “impaired” HIV+ adults, and those without neurocognitive data in the study). Therefore, the strategy in defining HIV+ subgroups did not interfere with this analysis (Analysis II), which included all HIV+ subjects.
HIV− controls versus cognitively “impaired” HIV+ adults (16 studies with 793 HIV+ and 581 HIV− adults). Inclusion criteria: neurocognitive function was assessed with at least one neuropsychology test in both controls and HIV+ adults; neurocognitive data was reported; HIV+ adults (or a sub‐group of HIV+ adults) performed significantly worse than controls in at least one neurocognitive test if the original study did not use the standard Frascati criteria in assessing neurocognitive performance, or HIV+ adults who met the HAND definition if the original study used the Frascati criteria; and controls were compared to HIV+ adults. If a study divided HIV+ adults into two groups, cognitively “normal” and cognitively “impaired”, only the results of controls versus cognitively “impaired” HIV+ adults were included in this analysis (in contrast to Analysis I).
Cognitively “normal” HIV+ adults versus cognitively “impaired” HIV+ adults (five studies with 124 cognitively “impaired” HIV+ and 105 cognitively “normal” HIV+ adults). Inclusion criteria: neurocognitive function was examined with at least one neuropsychology test for HIV+ adults; neurocognitive data were reported in the article; HIV+ adults were divided into at least two groups based on neurocognitive data, cognitively “normal” versus cognitively “impaired”; and the cognitively “normal” and cognitive “impaired” groups were explicitly compared to each other (i.e., if a study investigated the correlations between neurocognitive performance and GM volume/cortical thickness but did not explicitly compare the two groups, then this study would not be included in this analysis). It is worthwhile to note that, for studies included in this analysis (Analysis IV), all except one (Küper et al., 2011) of these studies have defined the HIV+ subgroups using the standard Frascati diagnostic criteria (Antinori et al., 2007). That is, HIV+ subjects classified as cognitively “impaired” in this analysis do meet the HAND criteria, and vice versa. Excluding the Küper et al. (2011) study (which used the HIV dementia scale [HDS] to define HIV+ subgroups) from the meta‐analysis produced nearly identical results.
If a study conducted more than one group‐wise comparison, that is, comparing cognitively “normal” HIV+ adults against controls, and comparing cognitively “impaired” HIV+ adults against controls, the study would be included in two or more analyses (i.e., I and III for the given example), and the findings from each of the group‐wise comparisons (as well as the number of subjects in each group) were entered into the corresponding analyses.
2.2. Parcellation of brain structures
For the CLE meta‐analysis, findings from each individual study need to be converted and assigned to a corresponding brain structure/label that is based on the brain parcellation scheme. In the present study, three levels of parcellation scheme were used, following the Automated Anatomical Labeling (AAL) atlas (Tzourio‐Mazoyer et al., 2002), with homologous regions from left and right hemisphere combined into one brain structure (i.e., the striatum included the left and the right striatum).
At the first level, the brain was parcellated into seven structures: frontal lobes, temporal lobes, parietal lobes, occipital lobes, the limbic/subcortical system, the insula, and the cerebellum. The limbic/subcortical system included the basal ganglia, the diencephalon/thalamus, the amygdala, the cingulate, and the medial temporal lobe (MTL, including hippocampus, parahippocampus, and entorhinal cortex).
At the second level, the limbic/subcortical system and the frontal cortex was parcellated into following nine subregions based on the AAL atlas: inferior frontal, middle frontal (excluding the orbital part), superior frontal (excluding the orbital part), orbital frontal, motor (medial superior frontal, supplementary motor area, and precentral), cingulate, middle temporal lobe (including amygdala), striatum, and thalamus. Based on previous findings in the literature (Ellis et al., 2007; Plessis et al., 2014) and the results from the analysis of the first‐level brain parcellation in the present study (see below), here at the second and third levels of brain parcellation (see below), we focused on the subregions within the frontal‐limbic‐subcortical system (n = 9 at the second level) while excluding data from other regions.
At the third level, the limbic/subcortical system and the frontal cortex was further parcellated into the following 24 subregions based on the AAL atlas: caudate (including the nucleus accumbens), globus pallidus, putamen, thalamus, ACC, mid‐cingulate cortex (MCC), posterior cingulate cortex (PCC) (including the retrosplenial cortex), amygdala, hippocampus (including the entorhinal cortex), parahippocampus, medial orbitofrontal, pars opercularis (inferior frontal gyrus, IFG), pars orbitalis (IFG), pars triangularis (IFG), orbital part of the middle frontal, middle frontal, superior medial frontal, orbital part of the superior frontal, superior frontal (excluding orbital and medial portions), precentral, supplementary motor area, straight gyrus, olfactory, and the frontal operculum. The nucleus accumbens was combined with the caudate due to its large overlap with the caudate in the AAL atlas (Tzourio‐Mazoyer et al., 2002). At this level, again we excluded data from other regions and only focused on these subregions of the frontal‐limbic‐subcortical system (n = 24 at the third level).
2.3. Coding for atrophy at individual brain structures
For a study included in the meta‐analysis (i.e., the ith study), based on the current brain parcellation scheme (see above), we first coded whether a brain structure s was examined in the ith study as E si:
E si = 1; yes, the brain structure s was examined in the ith study.
E si = 0; no, the brain structure s was not examined in the ith study.
To assign E si = 1 to a brain structure s in the ith study, it had to meet at least one of these three criteria: (a) the ith study examined the whole brain using a voxel‐based (or similar) approach; (b) it was explicitly mentioned that the structure s was examined in the ith study; or (c) at the least one subregion of the brain structure s was examined in the ith study. For instance, the striatum was coded as examined in a study if this study examined the striatum, or one of its subregions (e.g., the caudate nucleus), or the entire brain using a voxel‐wise (or similar approach). However, when a parent region was examined, it had no implications on whether any of its child‐regions were examined or not. For instance, if a study examined total subcortical volume, it did not have any implications on whether the striatum was examined in the same study; or if a study examined total cortical volume, it did not have any implications on whether the frontal lobe was examined in the same study.
Then we coded the status of atrophy for a brain structure s in the ith study as A si:
A si = 1; a reduced volume or a thinner cortex in HIV+ adults (vs controls) or in cognitively “impaired” HIV+ adults (vs cognitively “normal” HIV+ adults).
A si = −1; an increased volume or a thicker cortex in HIV+ adults (vs controls) in cognitively “impaired” HIV+ adults (vs cognitively “normal” HIV+ adults).
A si = 0; no significant group difference, or this structure s was not examined in the study i.
The value of A si was determined using the following steps:
If E si = 0, then the structure s was not examined in the ith study, so A si was set to 0.
If E si = 1, by definition, there were three possible scenarios, that is, the ith study used a voxel‐wise (or similar) approach, or the ith study used a ROI‐approach and explicitly examined the brain structure s, or the ith study used a ROI‐approach and examined at least one or more sub‐regions of the brain structure s. For the first two scenarios, assigning a value to A si was straightforward. By contrast, in the last scenario, as the brain structure s was not explicitly examined in the study, a similar upward but not downward propagation system was applied to determine the value of A si. That is, the value of A si was decided by the sum of its subregions that were explicitly examined. For example, a reduced volume in the cingulate did not have any implications on the coding of the ACC. By contrast, if the entire cingulate was not explicitly examined in the study, the coding of the cingulate was decided by the sum of the coding of its sub‐regions, including the ACC, MCC, and PCC. There were two possible scenarios: if only one of its subregions (i.e., ACC) was examined, the coding of atrophy for the entire cingulate would be the same as this single subregion; whereas if more than one of its subregions (i.e., ACC and PCC) were examined, the coding of atrophy for the cingulate would be the sum of atrophy coding of these sub‐regions (a sum greater or less than 0 was converted to 1 or −1, respectively). In total, we only assigned −1 to A si to three regions in three studies (one in each study): two studies for the comparison of HIV+ adults versus controls, including one for the putamen (Castelo et al., 2007) and the other for the amygdala (Clark et al., 2015); and one study for the comparison of cognitively “impaired” HIV+ adults versus cognitively “normal” HIV+ adults, at the occipital lobe (Shin et al., 2017). Assigning 0 to the three regions in the three studies yielded nearly identical results.
To control for potential confounds/ambiguity, we adopted the following rules when assigning values to E si (to encode whether structure s was examined in the ith study) and A si (to encode the status of atrophy for structure s in the ith study). First, when a study used more than one technique to analyze the structural MRI data, the results of GM volume/thickness from different approaches/techniques were combined. Second, non‐AAL regions were assigned to corresponding AAL regions (one or more) based on its overlap with nearby AAL regions. For example, the nucleus accumbens was coded to be part of the caudate, as the caudate contains approximately 80% or more of the nucleus accumbens structure in AAL parcellation. Third, when two or more (sub)regions were explicitly implicated in a study, all of these (sub)regions were coded. For example, the sensorimotor cortex was coded as precentral and postcentral gyrus (Sanford et al., 2017; Shin et al., 2017). Lastly, one study reported atrophy in the Sylvian fissures (Pfefferbaum et al., 2014), but atrophy in the Sylvian fissures could be due to atrophy in frontal, parietal, and/or temporal cortex, so we decided not to include the finding with Sylvian fissures in any analysis. Similarly, any findings with total GM volume, or total cortical volume, or total subcortical volume were not included due to a lack of specificity.
2.4. The “standard” CLE analysis
The CLE analysis was then conducted with set of E si and A si obtained from the previous step.
First, for a given brain structure at a given parcellation level, s, a weighted proportion of studies that identified atrophy was defined as
where t was the total number of studies, i was the ith study, and w i was the weight of the ith study. And w i was defined as
where N ia was the number of subjects for one group (Group a) in the ith study, and N ib was the number of subjects for another group (Group b). So N ia and N ib were the number of HIV+ adults and controls (Analysis I, II, and III), or number of cognitively “impaired” HIV+ adults and cognitively “normal” HIV+ adults (Analysis IV), respectively. The effect sizes were not included in the equation calculating the weights, because (a) they were not always reported; and (b) it is difficult (and practically impossible) to normalize the effect sizes obtained with different MRI data analysis approaches (i.e., ROI‐based vs voxel‐based).
The wPA s thus provides a quantitative measurement of the probability of atrophy in a given brain structure s, at the current parcellation level, with a range from 0 to 1 as E si ≥ A si for any study i. However, by definition, for a given brain structure s, the value of wPA s depends on two factors: (a) the number of studies that examined s and (b) the sensitivity and specificity of those studies. That is, wPA s can be biased by studies with high sensitivity and low specificity, that is, studies that only examined several pre‐selected brain regions based on prior knowledge or research hypothesis. For an extreme case, if only one study examined the thalamus and found reduced volume in HIV+ adults (compared to controls), wPA thalamus would be one, but this should not be used to support the hypothesis that HIV was always associated with reduced thalamus volume (even though wPA thalamus = 1).
To overcome this problem and to quantitatively measure the probability of a brain structure s affected by HIV‐infection or HAND, we adopted a Monte Carlo‐style permutation method that was recently developed to determine null distributions for wPA s (Turkeltaub et al., 2012). This method takes into considerations the number of studies examining a given structure and their sensitivity and specificity. Basically, in each permutation, the value of each E si was kept consistent and unchanged, while the values of A si were shuffled randomly to generate a new set of A' si. To control for differences in number of studies and the sensitivity and specificity of these studies, for any individual study (i.e., study i), two constraints were enforced while shuffling A si:
= (where m is the number of brain structures examined in the current parcellation scheme); that is, the sum of A' si equals the sum of the raw A si,
A' si = A si = 0 if E si = 0; that is, for any brain structure (s) that was not examined in the study i, the A si value was not shuffled and the A' si value was always set to 0.
After shuffling for every study, the wPA' s was calculated for each brain structure s using the new A' si.
A total of 1,000,000 permutations were run to generate a list of null‐hypothesis wPA' s values. Then for a given brain structure s, the probability of obtaining a given raw wPA s value by chance was defined as the proportion of randomly generated wPA' s values that equals or exceeds the raw wPA s value,
This analysis detected and identified brain regions that were consistently affected in HIV (when comparing HIV+ adults to controls) or in cognitively “impaired” HIV+ adults (when comparing cognitively “impaired” HIV+ adults to cognitively “normal” HIV+ adults). The results were shown in Figure 2.
It is worthwhile to note that by randomly shuffling the same number of atrophies detected in the real data among all structures, it tests the null hypothesis that atrophies are distributed randomly among brain structures. Thus, probA s is a relative assessment of the prevalence of atrophy in structure s compared to all other structures. Therefore, a nonsignificant probA s assessment could indicate that: (a) the atrophy in structure s is highly sparse; or (b) the atrophy in structure s is often detected, but not significantly more so than other region(s). Relatedly, a lack of significant probA s for any regions examined in an analysis could be due to: (a) an absence of consistent atrophy in all structures; or (b) a presence of consistent atrophy in many structures. By contrast, a significant probA s would indicate a high prevalence of atrophy in structure s compared to other structures. For instance, a significant probA s for the frontal lobe in the comparison of HIV+ adults versus controls would suggest that in HIV+ adults, frontal atrophy was relatively more prevalent than many other regions. For reference purpose, we also included the p values for any statistical analyses with a trend for significance.
2.5. Two control simulation analyses
One of the rules in the “standard” CLE analysis is that, for a structure s in a study i, if E si was 0, then A si was always set to 0, including during the permutation. For example, under an extreme case, if a study only examined one structure (e.g., thalamus) and found the presence of thalamus atrophy, the other brain structures were not examined and would be excluded from the permutation (i.e., both E si and A si were set to 0). One consequence with this approach was that, even though this study found thalamus atrophy, it would make zero contribution to the CLE analysis. To investigate the potential limitations of the standard CLE meta‐analysis, we conducted two additional control simulation analyses using different approaches to estimate/simulate the status of potential atrophy in regions that were not examined in a study. In both approaches, any structure that was not examined in a study was re‐coded as examined, that is, E si = 0 ➔ E si = 1, thus findings from a study that only examined a small set of ROIs could influence the CLE analysis results. The status of atrophy of the unexamined regions (A si) was assigned to specific values using the algorithms detailed below. Then we re‐ran the CLE analysis with the simulated E si and A si values, and compared them against the standard CLE approach described in the previous section.
In the first simulation analysis, a single value was chosen (see below) and assigned to A si for all unexamined regions in all studies (i.e., the values were the same across regions and studies). A set of 11 different values (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 08, 0.9, and 1.0) were tested (one at a time), with 0 representing the assumption of no atrophy in any of the unexamined regions in any study, 1 representing the assumption of the presence of atrophy in all unexamined regions in all studies, and other values (from 0.1 to 0.9) representing the assumption of some degree of atrophy (greater than 0 but less than 1) in all unexamined regions in all studies. The simulation results were shown in Supporting Information Figure S1A.
In the second simulation analysis, we made an assumption that, in each individual study, some unexamined regions would meet the atrophy criteria used in the study, while the other unexamined ones would not. In this simulation, we randomly assigned a certain portion (i.e., 20%) of the unexamined regions as regions with atrophy (E si = 1 and A si = 1), and the rest (i.e., 80%) of the unexamined regions as regions without atrophy (E si = 1 and A si = 0). This step was repeated 11 times, with the portion of the unexamined regions being re‐coded as regions with atrophy increasing from 0 to 100% (with a 10% increase at each step), thus the opposite was true for the portion of the unexamined regions being re‐coded as regions without atrophy (i.e., from 100 to 0%, with a 10% decrease at each step). At each of the 11 steps, the coding of A si for each of the unexamined regions was randomly assigned (0 or 1), while keeping the portions to the total number of unexamined regions consistent (i.e., 20% with A si = 1 vs 80% with A si = 0 at the third step). At each step, this randomization of A si was repeated 100 times (i.e., 100 shuffling), and after each shuffling/randomization, the randomly generated E si and A si values were entered into the CLE analysis, and a set of probA s values were obtained. In the end, a total of 100 sets of probA s were obtained at each of the 11 steps, and the final probA s of each step was calculated as the mean of the 100 sets. The simulation results were shown in Suppoting Materials Figure S1B.
The two approaches should produce identical results at the lowest level of atrophy assumption (i.e., 0.0 vs 0%), as well as at the high level of atrophy assumption (i.e., 1.0 vs 100%). Both simulations suggested the standard CLE technique is a sensitive yet balanced approach to synchronize MRI results across studies, especially in the context that many studies in HIV only examined a subset of regions using a ROI‐based approach. In addition, to avoid further complications in both simulations, A si with the value of −1 was always reset to 0 (which did not affect main outcomes as detailed in the previous section).
2.6. The CLE versus the ALE
To validate the novel CLE technique, we conducted additional analyses to compare it against an established coordinate‐based MRI meta‐analysis technique, the Activation Likelihood Estimation (ALE) technique (Eickhoff et al., 2009; Turkeltaub et al., 2002). Five of the 25 studies were identified using following criteria: VBM techniques were used to examine gray matter volume; HIV+ adults (regardless of neurocognitive performance) were compared to HIV− controls; and the peak coordinates were reported (Küper et al., 2011; Li et al., 2014; Wang et al., 2016; Wilson et al., 2015; Zhou et al., 2017).
The software package Ginger ALE version 2.3.6 (http://www.brainmap.org/ale/) was used for the ALE analysis, with a threshold of p < 0.05 (FWE‐corrected), 1,000 permutations, and a cluster size of 200mm3. For a comparison with the CLE technique (see below), we also included the findings with a lower threshold (p < 0.001, uncorrected, cluster size, 200mm3).
The CLE analyses were also conducted on the five studies, following the procedures detailed above. In addition to the three parcellation levels described, a fourth‐level parcellation was introduced, in which the ROIs from the left and right hemisphere were examined separately, that is, the left and the right ACC were examined separately, but again only in the frontal‐limbic‐subcortical regions.
Overall, the results from the CLE and the conventional ALE techniques were in general agreement with each other, supporting the novel CLE technique as a useful tool in investigating GM atrophy (see Supporting Information).
2.7. Correlations between neurocognitive performance and GM atrophy
In addition, we investigated the GM (volume or thickness) at which brain regions consistently correlated with neurocognitive performance in HIV+ adults. In this analysis, we first identified studies that explicitly investigated the relationship between GM volume/cortical thickness and neurocognitive performance (assessed with any neuropsychology test) using a correlation analysis. Then for each individual region, we summarized how many studies found a significant correlation between this region (volume or thickness) and neurocognitive performance. A qualitative rather than a quantitative approach was used, due to the following constraints: (a) different neurocognitive tests were used in different studies; (b) even in the same study, correlations might be done with different neurocognitive tests; (c) effect sizes were not available in many studies.
In this qualitative analysis, two rules were followed: (a) we used the same upward propagation as in the CLE meta‐analysis to assign findings to a brain region of current parcellation scheme, that is, a significant correlation between a neurocognitive test score and caudate implicated a significant correlation between neurocognitive performance and striatum, but not the opposite; (b) we did not differentiate correlation results obtained with different neurocognitive tests due to a lack of consistency across studies and a lack of power to differentiate neurocognitive tests, instead we combined results across neurocognitive tests, that is, if a study found a significant correlation between thalamus volume and motor function, and between hippocampus volume and memory, we simply coded that both thalamus and hippocampus were found to correlate with neurocognitive function in this study. In the end, for each brain region, we counted how many studies found a correlation between GM in this region and neurocognitive performance (measured with any neuropsychological test).
This qualitative analysis should treated as a secondary analysis and be taken with caution due to the constraints/limitations listed above, mainly: (a) a wide range of neurocognitive tests with different sensitivity and specificity levels were used in different studies to correlate with MRI; (b) even in the same study, multiple correlation analyses were usually done with different neurocognitive tests; (c) different neurocognitive tests likely had different neural underpins (i.e., linked to atrophy at different brain regions).
3. RESULTS
3.1. First level brain parcellation
At the first level, the brain was parcellated into seven structures: frontal lobes, temporal lobes, parietal lobes, occipital lobes, the limbic/subcortical system, the insula, and the cerebellum (see Methods).
Compared to HIV− controls, there was consistent frontal atrophy in cognitively “normal” HIV+ adults (p = 0.014);
Compared to HIV− controls, there was consistent frontal atrophy (p = 0.010) and marginal limbic/subcortical atrophy (p = 0.053) in HIV+ adults (regardless of neurocognitive performance);
Compared to HIV− controls, there was consistent limbic/subcortical atrophy in cognitively “impaired” HIV+ adults (p = 0.034);
Compared to cognitively “normal” HIV+ adults, there was consistent limbic/subcortical atrophy in cognitively “impaired” HIV+ adults (p = 0.027).
As shown in Figure 2 (top row), the frontal and the limbic/subcortical atrophy were the two most prevalent atrophies in HIV+ adults (compared to controls). But there was a clear difference between the two regions: compared to controls, the frontal was the most affected region in cognitively “normal” HIV+ adults, while the limbic/subcortical was the most affected region in cognitively “impaired” HIV+ adults. The limbic/subcortical atrophy also consistently differentiated cognitively “impaired” HIV+ adults from cognitively “normal” HIV+ adults, suggesting a potential critical role of limbic/subcortical atrophy in HAND development.
To further localize the atrophy at the subregions within the frontal‐limbic‐subcortical system, we conducted additional analyses at two finer scale parcellation schemes (the second and the third level) while limiting the data analysis to the frontal‐limbic‐subcortical system. The lack of significant results for other regions should not be interpreted as an absence of atrophy in these regions, but rather suggested that atrophy in these regions was less consistent/prevalent than the frontal and limbic/subcortical system in HIV (or HAND) (see Methods).
3.2. Second level brain parcellation
At the second level, the limbic/subcortical system and the frontal cortex was parcellated into following nine subregions: inferior frontal, middle frontal (excluding the orbital part), superior frontal (excluding the orbital part), orbital frontal, motor (medial superior frontal, supplementary motor area, and precentral), cingulate, middle temporal lobe (including amygdala), striatum, and thalamus.
Compared to HIV− controls, there was a weak trend for consistent cingulate atrophy in cognitively “normal” HIV+ adults (p = 0.079);
Compared to HIV− controls, there was consistent cingulate atrophy in HIV+ adults in general (regardless of cognitive performance) (p = 0.032);
For HIV− controls versus cognitively “impaired” HIV+ adults, there were no significant results with the CLE technique, probably due to wide spread atrophy within the frontal‐limbic‐subcortical system (see Methods);
Compared to cognitively “normal” HIV+ adults, there was consistent striatal atrophy in cognitively “impaired” HIV+ adults (p = 0.012).
As shown in Figure 2 (middle row), within the frontal‐limbic‐subcortical system, the cingulate and striatal atrophy were the two most prevalent atrophies in HIV+ adults (compared to controls), with the striatal atrophy consistently linked to neurocognitive impairment.
3.3. Third level brain parcellation
At the third level, the limbic/subcortical system and the frontal cortex was further parcellated into 24 subregions, including caudate, globus pallidus, putamen, thalamus, anterior cingulate cortex (ACC), hippocampus, and so forth (see Methods for a complete list).
Compared to HIV‐ controls, there was consistent atrophy in the ACC in the cognitively “normal” HIV+ adults (p = 0.010);
Compared to HIV− controls, there was consistent atrophy in the ACC in HIV+ adults in general (regardless of cognitive performance) (p = 0.005);
Compared to HIV− controls, there was a weak trend for consistent atrophy in the ACC in cognitively “impaired” HIV+ adults (p = 0.065);
Compared to cognitively “normal” HIV+ adults, there was consistent caudate atrophy in cognitively “impaired” HIV+ adults (p = 0.002);
As shown in Figure 2 (bottom row), at a fine parcellation level and within the frontal‐limbic‐subcortical system, the ACC was the most consistently affected region in HIV+ adults (compared to controls), especially in cognitively “normal” HIV+ adults. By contrast, the caudate atrophy consistently differentiated cognitively “impaired” HIV+ adults from cognitively “normal” HIV+ adults.
Across the three parcellation levels (Figure 2), from cognitively “normal” HIV+ adults to cognitively “impaired” HIV+ adults, there was a shift in regions affected by GM atrophy: the frontal/ACC atrophy was more prominent/prevalent in cognitively “normal” HIV+ adults, whereas the caudate/striatum atrophy was more prominent/prevalent in cognitively “impaired” HIV+ adults.
3.4. Correlations between neurocognitive performance and GM atrophy
Consistent with the findings from the quantitative CLE meta‐analysis, this qualitative analysis revealed the limbic/subcortical system (especially the caudate/striatum) as the region(s) most consistently associated with neurocognitive performance in HIV in the cART era, providing further evidence supporting a critical role of the caudate/striatum in neurocognitive impairment in HIV (Figure 3). However, we did not observe consistent links between deficits in specific neurocognitive domains and corresponding brain regions due to limited sample size and high heterogeneity in neurocognitive assessment across studies.
Figure 3.
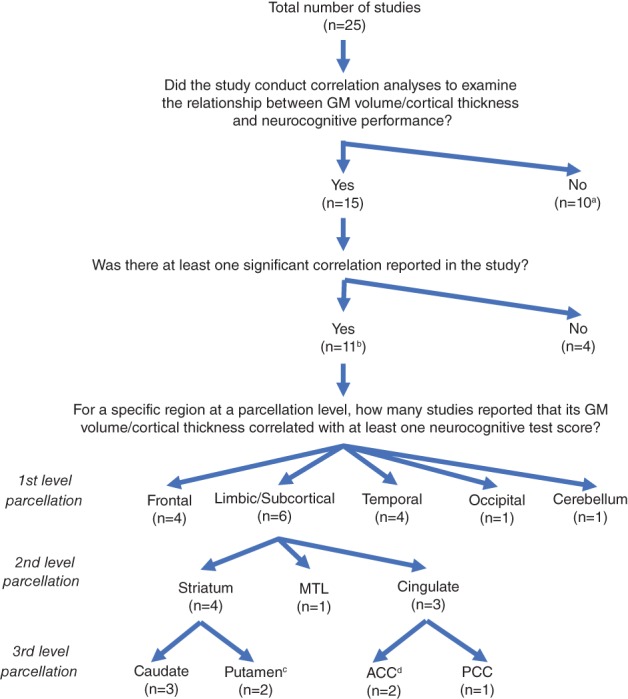
The summary of correlation analyses between GM volume/thickness and neurocognitive performance. Note. aOne study (Pfefferbaum et al., 2014) that only examined the correlation with the trajectory of changes in neurocognitive performance was included in the list of studies that did not examine the correlations. bThe list of 11 studies included one study (Clark et al., 2015) that examined emotion recognition, and another study (Ragin et al., 2012) that correlated neurocognitive performance with total GM volume and total cortical GM volume. cThis includes a study (Castelo et al., 2007) that detected a negative correlation between right putamen volume and motor speed performance. dThe list included a study (Clark et al., 2015) that found a correlation between ACC volume and emotion recognition. ACC: anterior cingulate cortex; GM: gray matter; MTL: medial temporal lobe; PCC: posterior cingulate cortex [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In the present study, using a novel meta‐analysis technique (CLE), we provide quantitative evidence suggesting that, compared to HIV− controls, frontal atrophy is consistently detected in HIV+ adults, including cognitively “normal” HIV+ adults. By contrast, limbic/subcortical atrophy is consistently detected in cognitively “impaired” HIV+ adults (compared to HIV− controls or to cognitively “normal” HIV+ adults). Within the frontal‐limbic‐subcortical system, the ACC atrophy is consistently detected in HIV+ adults (compared to controls), whereas the caudate/striatum atrophy consistently differentiated cognitively “impaired” HIV+ adults from cognitively “normal” HIV+ adults (Figure 2). These results not only provide further evidence supporting a critical role of frontostriatal injury in HAND, but also suggest that the frontal (including ACC) and the striatal (especially caudate) atrophy might play differential roles at different stages of HAND severity. The critical role of the caudate/striatum in HAND is also supported by a separate qualitative analysis (Figure 3). The CLE technique is then compared to the ALE technique, an established coordinate‐based MRI meta‐analysis technique, and both techniques produce similar results.
4.1. The frontal/ACC atrophy
The frontal lobe has long been considered as the essential hub of brain networks involved in executive function (Stuss & Alexander, 2000). In the present study, the CLE meta‐analysis technique reveal evidence suggesting that in the cART era, the frontal cortex may be the most consistently affected brain region in HIV+ adults (compared to HIV− controls), especially in cognitively “normal” HIV+ individuals, suggesting that the frontal cortex might be affected early on in HIV‐disease, in line with previous studies using different imaging techniques that have identified frontal injury shortly after seroconversion (Peluso et al., 2013; Young et al., 2014). The high prevalence of frontal atrophy may directly contribute to the highly prevalent executive dysfunction in the cART era (Heaton et al., 2011), and may underlie early and more subtle neurocognitive impairment in HIV+ adults (Kamat et al., 2016; Prakash et al., 2017). However, within the frontal lobe, we do not detect a single subregion that is significantly more likely to be affected than other subregions, suggesting that GM atrophy in the frontal lobe might be widespread and diffuse, which in turn implicate that the impacts of frontal atrophy on neurocognitive performance might be diverse, and probably subtle (i.e., not always easily detectable).
In addition to the frontal lobe, the ACC has also been proposed to be a critical region in executive function, as well as an important region in regulating emotional processing (Shenhav, Botvinick, & Cohen, 2013). In the present study, the CLE technique reveals evidence suggesting that the ACC might be the most consistently affected sub‐region within the frontal‐limbic‐subcortical system in HIV+ adults, even in those who performed comparable to HIV− controls on varying neurocognitive tests. Interestingly, using different imaging techniques such as functional MRI (fMRI) and positron emission tomography (PET), we (Jiang, Barasky, Olsen, Riesenhuber, & Magnus, 2016) and others (Garvey et al., 2014), respectively, have also found ACC dysfunction/injury in cognitively “normal” HIV+ adults who perform comparable to controls on standard neurocognitive tests, and the degree of injury correlate with performance. Studies using other techniques such as magnetic resonance spectroscopy (MRS) have also identified injury to ACC during primary HIV infection (Peluso et al., 2013). Together the findings from the present study and those previous studies suggest that ACC injury might be present in some HIV+ adults who do not meet the criteria of HAND diagnosis (i.e., at pre‐asymptomatic neurocognitive impairment, pre‐ANI, stage).
The hypothesis of early ACC dysfunction in HIV‐disease is also supported by the high prevalence of apathy in HIV (Paul et al., 2005; Walker & Brown, 2017), even in those with acute infection (Kamat et al., 2016). Neuropsychology studies (Cummings, 1993) have revealed a direct link between apathy and lesions to the ACC, suggesting the high prevalence of apathy in HIV might be associated with the highly prevalence of ACC injury. Apathy also correlates with neurocognitive performance in HIV (Paul et al., 2005; Shapiro, Mahoney, Zingman, Pogge, & Verghese, 2013), especially when examined with more demanding tasks (Castellon, Hinkin, & Myers, 2000), suggesting apathy and subtle decline in executive function might share some common neural mechanisms, that is, ACC neural injury (including associated white matter (Hoare et al., 2010)).
The high prevalence of frontal/ACC atrophy in HIV+ adults is likely due to multiple pathophysiological factors, including injury to the dopaminergic system. The frontal/ACC (especially the ACC) receives rich dopaminergic innervation as part of the mesocortical pathway that originates from dopaminergic neurons in the ventral tegmental area (Nolan & Gaskill, 2019), and studies have suggested that the mesocortical dopamine fibers projection to the prefrontal cortex and ACC is important for executive function, working memory, motivation, and emotion [Nolan & Gaskill, 2019]. Here we hypothesized that the injury to the dopaminergic system in HIV (Chang et al., 2008; Kumar et al., 2009; Nath et al., 2000) might have contributed to the high prevalence of frontal/ACC atrophy, as well as executive dysfunction (Heaton et al., 2010) and apathy (Paul et al., 2005; Walker & Brown, 2017) in HIV+ adults. Future studies with multimodal neuroimaging techniques along with comprehensive neuropsychology tests are needed to test this hypothesis.
However, directly relating early injury in the frontal lobe and ACC to HIV‐infection per se is complicated by the fact that common comorbidities in HIV+ adults such as substance abuse (Taylor, Alhassoon, Schweinsburg, Videen, & Grant, 2000) and depression (Drevets, Price, & Furey, 2008) can also have a detrimental effect on the frontal/ACC. For instance, using the MRS technique, Taylor, Grant, and colleagues have found that substance abuse and HIV‐disease have an additive impact on ACC neuronal function (Taylor et al., 2000), suggesting this presumably less severe stage of HAND (i.e., ANI or pre‐ANI) might be heavily influenced by comorbidities other than HIV‐disease. Future studies are needed to systematically investigate the potential interactions of HIV‐infection, depression, substance abuse, and other factors (such as early life stress (Spies et al., 2016)) on the frontal/ACC dysfunction.
4.2. The caudate/ striatum atrophy
Neural injury to the striatum and other subcortical regions in HIV has been well‐documented, including during acute infection (Heaps et al., 2015; Ragin et al., 2015; Wright et al., 2016). Neural injury to the striatum has been consistently linked to neurocognitive impairment (Moore et al., 2006; Rottenberg et al., 1996). In line with these early findings, both the quantitative CLE analysis and the qualitative correlation analysis in the present study suggest that striatal atrophy is consistently associated with cognitive impairment in HIV+ adults, providing further evidence supporting a critical role of the striatum in HAND (Ellis et al., 2007). In the discussion below we will focus on the caudate nucleus, a sub‐region of the striatum, but most of the discussion is applicable to the striatum as well (indeed the caudate and nucleus accumbens are defined as one single region in the present study, see Methods).
At a finer scale, the CLE analysis suggest the caudate nucleus is the most consistently affected brain region in cognitively “impaired” HIV+ adults (compared to cognitively “normal” HIV+ adults) within the frontal‐limbic‐subcortical system. Interestingly, during the pre‐cART era, the caudate nucleus had been identified as the most commonly affected brain region in individuals with HIV‐disease (Paul, Cohen, Navia, & Tashima, 2002), and the caudate atrophy had been linked to neurocognitive impairment (especially HIV‐associated dementia) (Dal Pan et al., 1992; Hestad et al., 1993; Kieburtz et al., 1996) and AIDS disease stage (Di Sclafani et al., 1997; Stout et al., 1998), as well as low CD4+ cell count (Stout et al., 1998) and high viral load in the cerebrospinal fluid (CSF) (Krivine et al., 1999). In line with the findings in the pre‐cART era, a recent diffusion tensor imaging (DTI) study provided evidence suggesting that the caudate neural injury might happen days after seroconversion and injury to the caudate correlates with CD4+ cell count, a measure of immune suppression (Ragin et al., 2015). In the present study, the CLE meta‐analysis technique reveal the caudate as the brain region most consistently associated with neurocognitive impairment (HAND). The persistent association between caudate atrophy and neurocognitive impairment in the cART era is intriguingly in line with findings in the pre‐cART era (Paul et al., 2002) and may be due to several distinct but nonexclusive factors.
First, this might be due to a legacy effect of medical history, especially in the survivors of early HIV‐epidemics. Due to the lack of effective treatment in the pre‐cART era, significant and somewhat irreversible neural injury in the caudate might have occurred. Indeed, earlier studies have revealed that the caudate injury correlated with low CD4+ cell count (Stout et al., 1998) as well in a recent study with acute infection patients (Ragin et al., 2015), and high CSF viral load (Krivine et al., 1999). This early injury might be a contributing factor to the caudate atrophy, and to HAND in general. Studies have found that medical history, especially past immunosuppression (nadir CD4+), is a strong predictor of neurocognitive performance (Ellis et al., 2011).
Second, the caudate nuclei might be one of the key HIV reservoirs in the brain in the post‐cART era. The presumably ongoing viral replication in the caudate nuclei, along with the associated neurotoxicity due to neuroinflammation (and/or neurotoxic side effects of antiretroviral agents), might result in growing neuronal injury in the caudate (Chang et al., 2008; Wang et al., 2004), which may eventually lead to detectable reduction in caudate volume and neurocognitive performance. Our hypothesis of this ongoing caudate injury model is also supported by findings from other studies: (a) Studies with postmortem brains and SIV models have suggested a high concentration of virus in the caudate nuclei (Kumar, Borodowsky, Fernandez, Gonzalez, & Kumar, 2007; Perez et al., 2018) and the basal ganglia in general, and the viral load in the caudate (along with the frontal cortex and the globus pallidus) correlates with neurocognitive impairment (Kumar et al., 2011); (b) Studies have found that the viral load in plasma and/or CSF correlates with caudate volume or neuronal injury in the caudate (Dewey et al., 2010; Krivine et al., 1999; Wang et al., 2004); (c) Studies have suggested a correlation between caudate volume and the estimated duration of HIV‐disease (Ances et al., 2012; Becker et al., 2011), implicating a gradual and ongoing decline in caudate volume after seroconversion, despite being on cART; (d) A recent longitudinal study with HIV+ older adults have found that not only the annualized rate of atrophy is higher in the caudate (0.74%) than any other brain regions in HIV (including the frontal lobe (0.48%) and the globus pallidus (0.73%)), but also the difference in the annualized rates of atrophy between HIV+ older adults and age‐matched controls is the largest in the caudate (ln [(1–0.0003)/(1–0.0074)] = ln (0.9997)−ln [0.9926] = 0.0071), followed by total cortical GM (0.0049) and the frontal lobe (0.0047), and is “twice” more than the globus pallidus (0.0034) (Clifford et al., 2017), suggesting that the caudate remains one of the most vulnerable and affected brain regions in HIV in the cART era; (e) Another longitudinal study also found that HIV+ adults with chronic infection have smaller caudate, putamen, and other subcortical regions than HIV+ adults with acute infection (Sanford et al., 2018), suggesting an ongoing neural injury to the caudate/striatum.
4.3. A hypothesized neural model of HAND severity
Based on the findings from the CLE analysis of GM atrophy in HIV+ adults (Figure 2) and previous neuroimaging and animal studies of HIV, here we propose a neural model of HAND severity in the cART era, with a focus on injury to key brain regions. The current standard model of HAND is solely based on neurobehavioral assessment with standard neuropsychology tests (Antinori et al., 2007; Heaton et al., 2010). The neural model proposed here was highly simplified and represented the first‐step toward this direction. It is expected to have limitations and likewise may contain inaccurate assumptions, and therefore should be treated with caution (especially with the technical limitations of the present study, as detailed in the next section). Our intention with this neural model is to stimulate more research in this direction rather than to make a conclusion.
As shown in Figure 4, we are proposing a neurally‐defined multistage HAND model, corresponding to the standard HAND model, with the addition of a pre‐ANI stage while excluding the HIV‐associated dementia (HAD) stage, as the HAD stage likely involves significant and widespread injury to the entire brain. This model is inspired by work in Alzheimer's disease research (Jack & Holtzman, 2013), and basically include two stages: a frontal/ACC stage, and a caudate/striatum stage.
Figure 4.
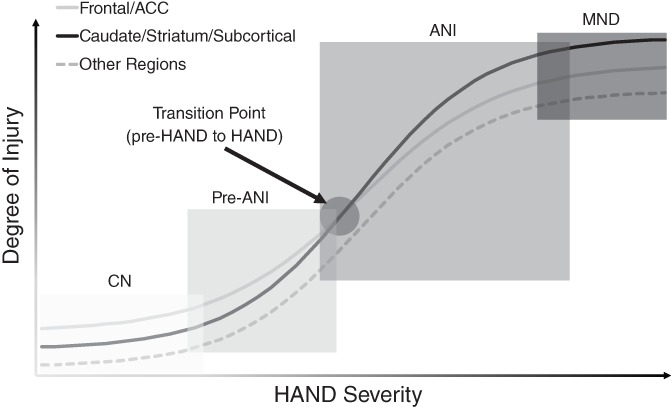
A neural model of the HIV‐associated neurocognitive disorders (HAND) severity. This model was inspired by research in Alzheimer's disease (Jack & Holtzman, 2013). As in the Alzheimer's disease model, the degree of neural injury was very hypothetical and was used to illustrate the potentially different degree of injury to different regions. The potential overlaps between different HAND stages were shown. One novel and specific prediction of this model is the transition from pre‐ANI to ANI that happens when the injury to the caudate/striatum/subcortical becomes prominent (i.e., overweighs the injury to the frontal/ACC). It is worthwhile to note that injury to other key regions such as the hippocampus and cerebellum might be prominent as well, probably even more than the frontal at more advanced stages of HAND, such as HIV‐associated dementia (HAD) (Moore et al., 2006). ACC: anterior cingulate cortex; ANI: asymptomatic neurocognitive impairment; CN: cognitively normal; MND: mild neurocognitive disorder
First, a frontal/ACC stage, which is characterized by neural injury to the frontal/ACC. At this stage, the frontal/ACC injury is highly prevalent in HIV+ adults, including those who performed “normally” on standard neurocognitive tests. Neural injury to other regions is likely to be present as well, but the frontal/ACC injury is more prominent than other brain regions (including the caudate/striatum) at this stage. The most likely affected domains at this stage are those linked to the frontal/ACC, including executive function, learning (but not delayed recall), and apathy. The neurocognitive impairment is likely subtle, and probably even undetectable with standard neuropsychology tests. For its relationship with the standard model, this frontal/ACC stage might include: (a) cognitively “normal” HIV+ adults, that is, those who perform normally on neuropsychology tests; (b) pre‐ANI HIV+ adults, that is, those who perform worse than controls but do not meet the criteria of ANI yet (e.g., two standard deviations [SD] below normal in only one neurocognitive domain); and (c) a small portion of HIV+ adults who meet the definition of ANI. Comorbidities such as substance abuse and depression probably play an important role at this stage.
Second, a caudate/striatum stage, which is characterized by neural injury to the caudate/striatum and other subcortical regions. At this stage, there is widespread neural injury in many regions, but caudate/striatum (and subcortical in general) injury might be more prominent than other regions (including frontal/ACC). It is worthwhile to note the frontal/ACC injury and the caudate/striatum injury do not exclude each other, but rather coexist with each other, that is, the frontal/ACC injury still persists at the caudate/striatum stage, but is outweighed by the caudate/striatum injury. The most likely affected domains at this stage are those linked to caudate/striatum and other subcortical regions (in addition to frontal/ACC). The neurocognitive impairment is mild, but always detectable with standard neuropsychology tests. For its relationship with the standard model, this caudate/striatum stage likely includes everyone who meets the definition of mild neurocognitive disorder (MND), plus the majority of those who meet the definition of ANI.
Comorbidities such as substance abuse, depression, or early life stress, and age might play an independent or interactive role, but probably more so in the frontal/ACC stage, while the caudate/striatum stage may be primarily driven by ongoing viral replication and/or associated neurotoxicity (such as neuroinflammation, the toxic effect of antiretroviral drugs, etc.). To fully address these questions (including the presumable interactions between neural injury to the frontal/ACC and to the caudate/striatum (du Plessis et al., 2016) as they are part of integrated neural circuits that are known to be affected in HIV (Ellis et al., 2007)), future studies that can synthesize and combine findings from other modalities (such as white matter injury, shift in neurochemistry, and altered neuronal activity) are definitely needed.
Nevertheless, this novel neural model can make several concrete and testable predictions. First, cognitively “normal” HIV+ adults might struggle with a task that heavily involves the ACC, such as a cognitive control task (Jiang et al., 2016); Second, a ratio of caudate (or striatum) volume versus frontal volume might be more sensitive to detect the presence of ANI/MND than the traditionally used ratio of caudate versus total brain volume (Dal Pan et al., 1992; Hestad et al., 1993; Kieburtz et al., 1996); Third, learning is affected at both stages, but delayed recall might be affected more at the caudate/striatum stage; Fourth, previous studies have suggested a high concentration of virus in the subcortical regions (Kumar et al., 2007; Perez et al., 2018), therefore, a rebound in viral load is likely to be more associated with injury to the caudate/striatum than to the frontal/ACC.
As mentioned above, the model is highly limited and simplified, but the model itself and the findings from the CLE technique might have meaningful theoretical and practical implications in HAND clinical diagnosis, management, treatment, and future research. Integrating this neurally‐based model with the standard behavioral model of HAND might help better characterize and diagnose HAND, which in turn might help develop better targeted and likewise more efficient treatment. In particular, it will be of great interest for future studies to explicitly test the model's predictions (including those listed above and beyond).
4.4. Limitations and future directions
There are some obvious limitations of the present study. First, this is a meta‐analysis that is based on, as well as limited by, available data in the literature. One of the main limitations is that the coordinates of peak locations were not available in most studies, thus we could not conduct a meta‐analysis using a more established technique (Turkeltaub et al., 2002). This also limited the capability of detecting atrophy in small brain structures, such as the nucleus accumbens, which is classified as part of the caudate nucleus in the AAL atlas. The functions of the caudate and the nucleus accumbens are markedly different, although both are part of the striatum, which is shown to be associated with neurocognitive impairment in HIV in the present study and previous studies. Second, the definition of cognitively “normal” versus cognitively “impaired” HIV+ adults is solely based on neurobehavioral data within each individual study. A wide range of neurocognitive tests have been used in these studies, and some tests are likely to be less sensitive than others, and therefore it is possible that the HIV+ adults grouped as cognitively “normal” in one study could be identified as cognitively “impaired” if they were examined with more stringent neuropsychology tests from another study. However, this should not interfere with the results from Analysis II, HIV− controls versus HIV+ adults, which included all HIV+ adults, regardless of neurocognitive performance; and the results from Analysis IV, cognitively “normal” HIV+ adults versus cognitively “impaired” HIV+ adults, as all except one studies (Küper et al., 2011) have used the standard Frascati diagnostic criteria to define neurocognitive status. Third, due to a lack of power, we could not examine potential links between GM atrophy and other important clinical measurements, including medical history (such as nadir CD4 count, viral load, and duration of disease). For instance, few studies have identified a significant effect of viral load on gray matter volume (Kallianpur et al., 2013). Future studies with new techniques other than CLE might be able to address these questions. Fourth, the nature of the study did not allow us to investigate the profiles of white matter injury, which has been shown to be highly prevalent in HIV+ adults and has been proposed to be an important contributing factor of HAND (Ragin et al., 2015; Wu et al., 2018). Fifth, detectable brain atrophy usually happens at a relatively late stage of neural injury, and is likely to be preceded by changes in other modalities such as a shift in neurochemistry and/or alterations in neuronal activity. While the results of the CLE meta‐analysis on GM atrophy are in line with the findings of other techniques (see discussion above), future studies with new techniques that can integrate and directly relate findings from different modalities are needed. Last but not the least, the CLE technique has its own methodological limitations, as it did not consider the different size of each region, and the statistical significance of reported atrophy from different studies. Both were difficult to control due to different approaches and statistical analyses were used in different studies. Future methodological development is necessary to address these concerns. However, despite its limitations, this kind of technique is highly desired and necessary in HIV study, as the presence of highly heterogeneous approaches made it impossible to use established meta‐analysis technique. In addition, the two additional simulation analyses helped to elevate some of these concerns, and a direct comparison with the ALE technique helped to validate the novel CLE technique. However, future studies with improved methodologies, along with the capability to integrate findings from different modalities, such as gray matter atrophy, disrupted white matter integrity, shifts in neurochemistry, alterations in neuronal activity, and neurobehavioral performance, are necessary to develop a more comprehensive and more accurate neurally‐based model of HAND in the cART era.
5. CONCLUSIONS
In summary, this novel CLE technique reveal evidence suggesting that GM atrophy in HIV is characterized by frontal/ACC atrophy and caudate/striatum atrophy, with the former associated with HIV‐disease and the latter associated with neurocognitive impairment. These results suggest the frontal lobe (including ACC) and the caudate/striatum may play critical but differential roles in HAND development. Integrating findings from the present study and previous studies, we propose a neural model of HAND severity with several testable predictions and discuss the model and the meta‐analysis results in the context of the standard behaviorally‐based HAND model.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
We thank Michael Ullman for suggestions on brain parcellation and atrophy encoding, and Beau Ances for his inspirations for Figure 4. This research was supported in part by 1R01MH108466 (X.J.).
Israel SM, Hassanzadeh‐Behbahani S, Turkeltaub PE, Moore DJ, Ellis RJ, Jiang X. Different roles of frontal versus striatal atrophy in HIV‐associated neurocognitive disorders. Hum Brain Mapp. 2019;40:3010–3026. 10.1002/hbm.24577
REFERENCES
- Ances, B. M. , Ortega, M. , Vaida, F. , Heaps, J. , & Paul, R. (2012). Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndromes 1999, 59, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori, A. , Arendt, G. , Becker, J. T. , Brew, B. J. , Byrd, D. A. , Cherner, M. , … Wojna, V. E. (2007). Updated research nosology for HIV‐associated neurocognitive disorders. Neurology, 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. T. , Kingsley, L. A. , Molsberry, S. , Reynolds, S. , Aronow, A. , Levine, A. J. , … Selnes, O. A. (2015). Cohort profile: Recruitment cohorts in the neuropsychological substudy of the multicenter AIDS cohort study. International Journal of Epidemiology, 44, 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. T. , Maruca, V. , Kingsley, L. A. , Sanders, J. M. , Alger, J. R. , Barker, P. B. , … Multicenter AIDS Cohort Study . (2012). Factors affecting brain structure in men with HIV disease in the post‐HAART era. Neuroradiology, 54, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. T. , Sanders, J. , Madsen, S. K. , Ragin, A. , Kingsley, L. , Maruca, V. , … Multicenter AIDS Cohort Study . (2011). Subcortical brain atrophy persists even in HAART‐regulated HIV disease. Brain Imaging and Behavior, 5, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon, S. A. , Hinkin, C. H. , & Myers, H. F. (2000). Neuropsychiatric disturbance is associated with executive dysfunction in HIV‐1 infection. Journal of the International Neuropsychological Society, 6, 336–347. [DOI] [PubMed] [Google Scholar]
- Castelo, J. M. B. , Courtney, M. G. , Melrose, R. J. , & Stern, C. E. (2007). Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Archives of Neurology, 64, 1275–1280. [DOI] [PubMed] [Google Scholar]
- Castillo, D. , Ernst, T. , Cunningham, E. , & Chang, L. (2018). Altered associations between pain symptoms and brain morphometry in the pain matrix of HIV‐seropositive individuals. Journal of Neuroimmune Pharmacology, 13, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L. , Wang, G.‐J. , Volkow, N. D. , Ernst, T. , Telang, F. , Logan, J. , & Fowler, J. S. (2008). Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage, 42, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, M. , & Nath, A. (2013). Where does HIV hide? A focus on the central nervous system. Current Opinion in HIV and AIDS, 8, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, U. S. , Walker, K. A. , Cohen, R. A. , Devlin, K. N. , Folkers, A. M. , Pina, M. J. , & Tashima, K. T. (2015). Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia, 70, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford, K. M. , Samboju, V. , Cobigo, Y. , Milanini, B. , Marx, G. A. , Hellmuth, J. M. , … Valcour, V. G. (2017). Progressive brain atrophy despite persistent viral suppression in HIV patients older than 60 years. Journal of Acquired Immune Deficiency Syndromes, 76, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, R. A. , Harezlak, J. , Gongvatana, A. , Buchthal, S. , Schifitto, G. , Clark, U. , … Navia, B. (2010). Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. Journal of Neurovirology, 16, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa, D. G. , Zimmermann, N. , Netto, T. M. , Tukamoto, G. , Ventura, N. , de Castro Bellini Leite, S. , … Gasparetto, E. L. (2016). Regional cerebral gray matter volume in HIV‐positive patients with executive function deficits. Journal of Neuroimaging, 26, 450–457. [DOI] [PubMed] [Google Scholar]
- Cummings, J. L. (1993). Frontal‐subcortical circuits and human behavior. Archives of Neurology, 50, 873–880. [DOI] [PubMed] [Google Scholar]
- Dal Pan, G. J. , McArthur, J. H. , Aylward, E. , Selnes, O. A. , Nance‐Sproson, T. E. , Kumar, A. J. , … McArthur, J. C. (1992). Patterns of cerebral atrophy in HIV‐1‐infected individuals: Results of a quantitative MRI analysis. Neurology, 42, 2125–2130. [DOI] [PubMed] [Google Scholar]
- Dewey, J. , Hana, G. , Russell, T. , Price, J. , McCaffrey, D. , Harezlak, J. , … HIV Neuroimaging Consortium . (2010). Reliability and validity of MRI‐based automated volumetry software relative to auto‐assisted manual measurement of subcortical structures in HIV‐infected patients from a multisite study. NeuroImage, 51, 1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sclafani, V. , Mackay, R. D. , Meyerhoff, D. J. , Norman, D. , Weiner, M. W. , & Fein, G. (1997). Brain atrophy in HIV infection is more strongly associated with CDC clinical stage than with cognitive impairment. Journal of the International Neuropsychological Society, 3, 276–287. [PMC free article] [PubMed] [Google Scholar]
- Drevets, W. C. , Price, J. L. , & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function, 213, 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis, S. , Vink, M. , Joska, J. A. , Koutsilieri, E. , Bagadia, A. , Stein, D. J. , & Emsley, R. (2016). Prefrontal cortical thinning in HIV infection is associated with impaired striatal functioning. Journal of Neural Transmission, 123, 643–651. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, R. , Langford, D. , & Masliah, E. (2007). HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nature Reviews Neuroscience, 8, 33–44. [DOI] [PubMed] [Google Scholar]
- Ellis, R. , & Letendre, S. L. (2016). Update and new directions in therapeutics for neurological complications of HIV infections. Neurotherapeutics, 13, 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, R. J. , Badiee, J. , Vaida, F. , Letendre, S. , Heaton, R. K. , Clifford, D. , … Grant I, CHARTER Group . (2011). CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS (London, England), 25, 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema‐Notestine, C. , Ellis, R. J. , Archibald, S. L. , Jernigan, T. L. , Letendre, S. L. , Notestine, R. J. , … Grant I, Group C . (2013). Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. Journal of Neurovirology, 19, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey, L. J. , Pavese, N. , Politis, M. , Ramlackhansingh, A. , Brooks, D. J. , Taylor‐Robinson, S. D. , & Winston, A. (2014). Increased microglia activation in neurologically asymptomatic HIV‐infected patients receiving effective ART. AIDS (London, England), 28, 67–72. [DOI] [PubMed] [Google Scholar]
- Guha, A. , Brier, M. R. , Ortega, M. , Westerhaus, E. , Nelson, B. , & Ances, B. M. (2016). Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. Journal of Acquired Immune Deficiency Syndromes, 73, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaps, J. M. , Sithinamsuwan, P. , Paul, R. , Lerdlum, S. , Pothisri, M. , Clifford, D. , … SEARCH 007/011 study groups . (2015). Association between brain volumes and HAND in cART‐naïve HIV+ individuals from Thailand. Journal of Neurovirology, 21, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K. , Clifford, D. B. , Franklin, D. R. , Woods, S. P. , Ake, C. , Vaida, F. , … Grant I, CHARTER Group . (2010). HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology, 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K. , Franklin, D. R. , Ellis, R. J. , McCutchan, J. A. , Letendre, S. L. , Leblanc, S. , … CHARTER Group, HNRC Group . (2011). HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology, 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth, J. , Valcour, V. , & Spudich, S. (2015). CNS reservoirs for HIV: Implications for eradication. Journal of Virus Eradication, 1, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad, K. , McArthur, J. H. , Dal Pan, G. J. , Selnes, O. A. , Nance‐Sproson, T. E. , Aylward, E. , … McArthur, J. C. (1993). Regional brain atrophy in HIV‐1 infection: Association with specific neuropsychological test performance. Acta Neurologica Scandinavica, 88, 112–118. [DOI] [PubMed] [Google Scholar]
- Hoare, J. , Fouche, J.‐P. , Spottiswoode, B. , Joska, J. A. , Schoeman, R. , Stein, D. J. , & Carey, P. D. (2010). White matter correlates of apathy in HIV‐positive subjects: A diffusion tensor imaging study. The Journal of Neuropsychiatry and Clinical Neurosciences, 22, 313–320. [DOI] [PubMed] [Google Scholar]
- Hua, X. , Boyle, C. P. , Harezlak, J. , Tate, D. F. , Yiannoutsos, C. T. , Cohen, R. , … HIV Neuroimaging Consortium . (2013). Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV‐infected patients on stable treatment. NeuroImage: Clinical, 3, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R. , & Holtzman, D. M. (2013). Biomarker modeling of Alzheimer's disease. Neuron, 80, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan, T. L. , Gamst, A. C. , Archibald, S. L. , Fennema‐Notestine, C. , Mindt, M. R. , Marcotte, T. D. , … Grant, I. (2005). Effects of methamphetamine dependence and HIV infection on cerebral morphology. The American Journal of Psychiatry, 162, 1461–1472. [DOI] [PubMed] [Google Scholar]
- Jiang, X. , Barasky, R. , Olsen, H. , Riesenhuber, M. , & Magnus, M. (2016). Behavioral and neuroimaging evidence for impaired executive function in “cognitively normal” older HIV‐infected adults. AIDS Care, 28, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur, K. J. , Shikuma, C. , Kirk, G. R. , Shiramizu, B. , Valcour, V. , Chow, D. , … Sailasuta, N. (2013). Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology, 80, 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat, R. , Doyle, K. L. , Iudicello, J. E. , Morgan, E. E. , Morris, S. , Smith, D. M. , … Translational Methamphetamine AIDS Research Center (TMARC) Group . (2016). Neurobehavioral disturbances during acute and early HIV infection. Cognitive and Behavioral Neurology, 29, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieburtz, K. , Ketonen, L. , Cox, C. , Grossman, H. , Holloway, R. , Booth, H. , … Caine, E. D. (1996). Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology, 53, 155–158. [DOI] [PubMed] [Google Scholar]
- Krivine, A. , Force, G. , Servan, J. , Cabée, A. , Rozenberg, F. , Dighiero, L. , … Lebon, P. (1999). Measuring HIV‐1 RNA and interferon‐alpha in the cerebrospinal fluid of AIDS patients: Insights into the pathogenesis of AIDS dementia complex. Journal of Neurovirology, 5, 500–506. [DOI] [PubMed] [Google Scholar]
- Kuhn, T. , Schonfeld, D. , Sayegh, P. , Arentoft, A. , Jones, J. D. , Hinkin, C. H. , … Thames, A. D. (2017). The effects of HIV and aging on subcortical shape alterations: A 3D morphometric study. Human Brain Mapping, 38, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. M. , Borodowsky, I. , Fernandez, B. , Gonzalez, L. , & Kumar, M. (2007). Human immunodeficiency virus type 1 RNA levels in different regions of human brain: Quantification using real‐time reverse transcriptase‐polymerase chain reaction. Journal of Neurovirology, 13, 210–224. [DOI] [PubMed] [Google Scholar]
- Kumar, A. M. , Fernandez, J. B. , Singer, E. J. , Commins, D. , Waldrop‐Valverde, D. , Ownby, R. L. , & Kumar, M. (2009). Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of Neurovirology, 15, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. M. , Ownby, R. L. , Waldrop‐Valverde, D. , Fernandez, B. , & Kumar, M. (2011). Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. Journal of Neurovirology, 17, 26–40. [DOI] [PubMed] [Google Scholar]
- Küper, M. , Rabe, K. , Esser, S. , Gizewski, E. R. , Husstedt, I. W. , Maschke, M. , & Obermann, M. (2011). Structural gray and white matter changes in patients with HIV. Journal of Neurology, 258, 1066–1075. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Li, H. , Gao, Q. , Yuan, D. , & Zhao, J. (2014). Structural gray matter change early in male patients with HIV. International Journal of Clinical and Experimental Medicine, 7, 3362–3369. [PMC free article] [PubMed] [Google Scholar]
- Moore, D. J. , Masliah, E. , Rippeth, J. D. , Gonzalez, R. , Carey, C. L. , Cherner, M. , … Grant I, HNRC Group . (2006). Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS (London, England), 20, 879–887. [DOI] [PubMed] [Google Scholar]
- Nath, A. , Anderson, C. , Jones, M. , Maragos, W. , Booze, R. , Mactutus, C. , … Mattson, M. (2000). Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. Journal of Psychopharmacology, 14, 222–227. [DOI] [PubMed] [Google Scholar]
- Nolan, R. , & Gaskill, P. J. (2019). The role of catecholamines in HIV neuropathogenesis. Brain Research, 1702, 54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R. , Cohen, R. , Navia, B. , & Tashima, K. (2002). Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type‐1. Neuroscience and Biobehavioral Reviews, 26, 353–359. [DOI] [PubMed] [Google Scholar]
- Paul, R. , Flanigan, T. P. , Tashima, K. , Cohen, R. , Lawrence, J. , Alt, E. , … Hinkin, C. (2005). Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. The Journal of Neuropsychiatry and Clinical Neurosciences, 17, 114–118. [DOI] [PubMed] [Google Scholar]
- Paul, R. H. , Phillips, S. , Hoare, J. , Laidlaw, D. H. , Cabeen, R. , Olbricht, G. R. , … Joska, J. (2017). Neuroimaging abnormalities in clade C HIV are independent of tat genetic diversity. Journal of Neurovirology, 23, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso, M. J. , Meyerhoff, D. J. , Price, R. W. , Peterson, J. , Lee, E. , Young, A. C. , … Spudich, S. (2013). Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. The Journal of Infectious Diseases, 207, 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, S. , Johnson, A.‐M. , Xiang, S.‐H. , Li, J. , Foley, B. T. , Doyle‐Meyers, L. , … Ling, B. (2018). Persistence of SIV in the brain of SIV‐infected Chinese rhesus macaques with or without antiretroviral therapy. Journal of Neurovirology, 24, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum, A. , Rogosa, D. A. , Rosenbloom, M. J. , Chu, W. , Sassoon, S. A. , Kemper, C. A. , … Sullivan, E. V. (2014). Accelerated aging of selective brain structures in human immunodeficiency virus infection: A controlled, longitudinal magnetic resonance imaging study. Neurobiology of Aging, 35, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis, S. D. , Vink, M. , Joska, J. A. , Koutsilieri, E. , Stein, D. J. , & Emsley, R. (2014). HIV infection and the fronto‐striatal system: A systematic review and meta‐analysis of fMRI studies. AIDS (London, England), 28, 803–811. [DOI] [PubMed] [Google Scholar]
- Prakash, A. , Hou, J. , Liu, L. , Gao, Y. , Kettering, C. , & Ragin, A. B. (2017). Cognitive function in early HIV infection. Journal of Neurovirology, 23, 273–282. [DOI] [PubMed] [Google Scholar]
- Ragin, A. B. , Du, H. , Ochs, R. , Wu, Y. , Sammet, C. L. , Shoukry, A. , & Epstein, L. G. (2012). Structural brain alterations can be detected early in HIV infection. Neurology, 79, 2328–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin, A. B. , Wu, Y. , Gao, Y. , Keating, S. , Du, H. , Sammet, C. , … Epstein, L. G. (2015). Brain alterations within the first 100 days of HIV infection. Annals of Clinical Translational Neurology, 2, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg, D. A. , Sidtis, J. J. , Strother, S. C. , Schaper, K. A. , Anderson, J. R. , Nelson, M. J. , & Price, R. W. (1996). Abnormal cerebral glucose metabolism in HIV‐1 seropositive subjects with and without dementia. Journal of Nuclear Medicine, 37, 1133–1141. [PubMed] [Google Scholar]
- Sacktor, N. , Skolasky, R. L. , Seaberg, E. , Munro, C. , Becker, J. T. , Martin, E. , … Miller, E. (2016). Prevalence of HIV‐associated neurocognitive disorders in the multicenter AIDS cohort study. Neurology, 86, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi‐Khorshidi, G. , Smith, S. M. , Keltner, J. R. , Wager, T. D. , & Nichols, T. E. (2009). Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. NeuroImage, 45, 810–823. [DOI] [PubMed] [Google Scholar]
- Sanford, R. , Ances, B. M. , Meyerhoff, D. J. , Price, R. W. , Fuchs, D. , Zetterberg, H. , … Collins, D. L. (2018). Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary HIV infection. Clinical Infectious Diseases, 67(11), 1697–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, R. , Fernandez Cruz, A. L. , Scott, S. C. , Mayo, N. E. , Fellows, L. K. , Ances, B. M. , & Collins, D. L. (2017). Regionally specific brain volumetric and cortical thickness changes in HIV‐infected patients in the HAART era. Journal of Acquired Immune Deficiency Syndromes, 74, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor, D. , Dickens, A. M. , Sacktor, N. , Haughey, N. , Slusher, B. , Pletnikov, M. , … McArthur, J. C. (2016). HIV‐associated neurocognitive disorder ‐ pathogenesis and prospects for treatment. Nature Reviews Neurology, 12, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, M. E. , Mahoney, J. R. , Zingman, B. S. , Pogge, D. L. , & Verghese, J. (2013). Apathy correlates with cognitive performance, functional disability, and HIV RNA plasma levels in HIV‐positive individuals. Journal of Clinical and Experimental Neuropsychology, 35, 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav, A. , Botvinick, M. M. , & Cohen, J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79, 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, N.‐Y. , Hong, J. , Choi, J. Y. , Lee, S.‐K. , Lim, S. M. , & Yoon, U. (2017). Retrosplenial cortical thinning as a possible major contributor for cognitive impairment in HIV patients. European Radiology, 27, 4721–4729. [DOI] [PubMed] [Google Scholar]
- Spies, G. , Ahmed‐Leitao, F. , Fennema‐Notestine, C. , Cherner, M. , & Seedat, S. (2016). Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. Journal of Neurovirology, 22, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich, S. S. , & Ances, B. M. (2017). CROI 2017: Neurologic complications of HIV infection. Topics in Antiviral Medicine, 25, 69–76. [PMC free article] [PubMed] [Google Scholar]
- Stout, J. C. , Ellis, R. J. , Jernigan, T. L. , Archibald, S. L. , Abramson, I. , Wolfson, T. , … Grant, I. (1998). Progressive cerebral volume loss in human immunodeficiency virus infection: A longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Archives of Neurology, 55, 161–168. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T. , & Alexander, M. P. (2000). Executive functions and the frontal lobes: A conceptual view. Psychological Research, 63, 289–298. [DOI] [PubMed] [Google Scholar]
- Taylor, M. J. , Alhassoon, O. M. , Schweinsburg, B. C. , Videen, J. S. , & Grant, I. (2000). MR spectroscopy in HIV and stimulant dependence HNRC group. HIV neurobehavioral research center. Journal of the International Neuropsychological Society, 6, 83–85. [DOI] [PubMed] [Google Scholar]
- Tesic, T. , Boban, J. , Bjelan, M. , Todorovic, A. , Kozic, D. , & Brkic, S. (2018). Basal ganglia shrinkage without remarkable hippocampal atrophy in chronic aviremic HIV‐positive patients. Journal of Neurovirology, 24, 478–487. [DOI] [PubMed] [Google Scholar]
- Thames, A. D. , Kuhn, T. P. , Williamson, T. J. , Jones, J. D. , Mahmood, Z. , & Hammond, A. (2017). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV‐ adults. Drug and Alcohol Dependence, 170, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood, K. J. , Pitkanen, M. , Kulasegaram, R. , Fradera, A. , Kumar, A. , Soni, S. , … Kopelman, M. D. (2012). Mapping the brain in younger and older asymptomatic HIV‐1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 48, 230–241. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. , Pullman, M. , Pierpont, E. , & Ullman, M. (2012). A novel meta‐analytic technique reveals the neuroanatomy of specific language impairment. Procedia ‐ Social and Behavioral Sciences, 61, 34. [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Underwood, J. , Cole, J. H. , Caan, M. , De Francesco, D. , Leech, R. , Van Zoest, R. A. , … Collaboration C in R to A (COBRA) . (2017). Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clinical Infectious Diseases, 65(3), 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, B. S. C. , Valcour, V. , Busovaca, E. , Esmaeili‐Firidouni, P. , Joshi, S. H. , Wang, Y. , & Thompson, P. M. (2015). Subcortical shape and volume abnormalities in an elderly HIV+ cohort. Proceedings of SPIE ‐ The International Society for Optical Engineering, 94171S. https://www.ncbi.nlm.nih.gov/pubmed/25844123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, K. A. , & Brown, G. G. (2017). HIV‐associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta‐analysis. Journal of Clinical and Experimental Neuropsychology, 40(4), 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Liu, Z. , Liu, J. , Tang, Z. , Li, H. , & Tian, J. (2016). Gray and white matter alterations in early HIV‐infected patients: Combined voxel‐based morphometry and tract‐based spatial statistics. Journal of Magnetic Resonance Imaging, 43, 1474–1483. [DOI] [PubMed] [Google Scholar]
- Wang, G.‐J. , Chang, L. , Volkow, N. D. , Telang, F. , Logan, J. , Ernst, T. , & Fowler, J. S. (2004). Decreased brain dopaminergic transporters in HIV‐associated dementia patients. Brain: A Journal of Neurology, 127, 2452–2458. [DOI] [PubMed] [Google Scholar]
- Wilson, T. W. , Heinrichs‐Graham, E. , Becker, K. M. , Aloi, J. , Robertson, K. R. , Sandkovsky, U. , … Swindells, S. (2015). Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV‐infected older adults. Human Brain Mapping, 36, 897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, P. W. , Pyakurel, A. , Vaida, F. F. , Price, R. W. , Lee, E. , Peterson, J. , … Ances, B. M. (2016). Putamen volume and its clinical and neurological correlates in primary HIV infection. AIDS (London, England), 30, 1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Fatukasi, O. , Yang, S. , Alger, J. , Barker, P. B. , Hetherington, H. , … Neuropsychology Working Group of the Multicenter AIDS Cohort Study . (2018). HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS (London, England), 32, 1803–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, A. C. , Yiannoutsos, C. T. , Hegde, M. , Lee, E. , Peterson, J. , Walter, R. , … Spudich, S. (2014). Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology, 83, 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Li, R. , Wang, X. , Miao, H. , Wei, Y. , Ali, R. , … Li, H. (2017). Motor‐related brain abnormalities in HIV‐infected patients: A multimodal MRI study. Neuroradiology, 59, 1133–1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


