Abstract
Recently, graph theoretical approaches applied to neuroimaging data have advanced understanding of the human brain connectome and its abnormalities in psychiatric disorders. However, little is known about the topological organization of brain white matter networks in posttraumatic stress disorder (PTSD). Seventy‐six patients with PTSD and 76 age, gender, and years of education‐matched trauma‐exposed controls were studied after the 2008 Sichuan earthquake using diffusion tensor imaging and graph theoretical approaches. Topological properties of brain networks including global and nodal measurements and modularity were analyzed. At the global level, patients showed lower clustering coefficient (p = .016) and normalized characteristic path length (p = .035) compared with controls. At the nodal level, increased nodal centralities in left middle frontal gyrus, superior and inferior temporal gyrus and right inferior occipital gyrus were observed (p < .05, corrected for false‐discovery rate). Modularity analysis revealed that PTSD patients had significantly increased inter‐modular connections in the fronto‐parietal module, fronto‐striato‐temporal module, and visual and default mode modules. These findings indicate a PTSD‐related shift of white matter network topology toward randomization. This pattern was characterized by an increased global network integration, reflected by increased inter‐modular connections with increased nodal centralities involving fronto‐temporo‐occipital regions. This study suggests that extremely stressful life experiences, when they lead to PTSD, are associated with large‐scale brain white matter network topological reconfiguration at global, nodal, and modular levels.
Keywords: connectome, graph theory, modularity, posttraumatic stress disorder, psychoradiology, white matter
1. INTRODUCTION
Posttraumatic stress disorder (PTSD) is a trauma‐related psychiatric disorder characterized by four symptom clusters: re‐experience, avoidance, negative cognitions and mood, and arousal (American Psychiatric Association, 2013). It develops in about 6% of the general population following exposure to traumatic life events, and can cause persistent psychological distress and compromised quality of life (Goldstein et al., 2016). The neural substrates of PTSD are not yet fully elucidated. Neuroimaging studies of PTSD have demonstrated abnormal functional and structural alterations in focal brain regions (Daniels, Lamke, Gaebler, Walter, & Scheel, 2013; Li et al., 2014; Li et al., 2016; Meng et al., 2014; O'Doherty et al., 2018; Patel, Spreng, Shin, & Girard, 2012; Siehl, King, Burgess, Flor, & Nees, 2018; Yin et al., 2012). Analyzing the whole brain network in an integrated fashion might provide a more comprehensive understanding of brain network abnormalities that might be imperfectly detected by traditional regional or voxel‐based analysis (Li et al., 2018).
Recent advances in psychoradiology (https://radiopaedia.org/articles/psychoradiology), allow the direct noninvasive characterization of brain network topology in neuropsychiatric patients. Graph theoretical analysis characterizes brain anatomy as a complex network of nodes and edges from which graph metrics are calculated to describe network attributes (Craddock et al., 2013). Categories of topological properties include functional segregation (how optimized the network is for specialized processing) and functional integration (how well the network can combine specialized information across distributed regions; Rubinov & Sporns, 2010). The small‐world model is of special interest when describing human brain networks, because it supports efficient information segregation and integration with low energy and wiring costs (Liao, Vasilakos, & He, 2017). Network studies have accelerated the process of mapping the human brain connectome in healthy and disease states (Liao, Vasilakos, & He, 2017). Several studies of PTSD have reported topological alterations in the whole brain functional and gray matter structural connectome (Cisler et al., 2016; Lei et al., 2015; Niu et al., 2018a, 2018b; Qi et al., 2018; Suo et al., 2015).
Graph theory analysis of diffusion tensor imaging (DTI) data can be used to explore white matter network topology. Studying brain white matter in PTSD is a promising research direction, as multiple studies in nonhuman primates and rodents have reported that exposure to stress such as maternal deprivation, maltreatment, and other adverse conditions can impair white matter integrity (Coplan et al., 2010, 2016; Ding et al., 2013; Howell et al., 2013; Sarabdjitsingh, Loi, Joels, Dijkhuizen, & van der Toorn, 2017). We have previously characterized white matter networks in pediatric PTSD, showing a disrupted topological organization (Suo et al., 2017). In a study of white matter networks in adult PTSD, increased global integration reflected by shorter path length was reported (Long et al., 2013). However, this study compared adult PTSD patients with nontraumatized healthy controls, making it difficult to determine whether observed effects were correlated with PTSD per se or represented a general consequence of traumatic stress exposure. Further, potential effects of prior drug therapies may have influenced study findings. A modularity analysis of the functional brain network in adult PTSD revealed a spared functional connectivity in the posterior default mode network (DMN) (Akiki et al., 2018). However, this study did not exclude participants with psychiatric comorbidities and psychotropic medications. While graph theory analyses of the brain connectome in PTSD have been informative, the nature of whole brain structural network and modularity alterations early in the course of illness before drug treatment remain to be established in adult noncomorbid PTSD. Analysis of whole brain white matter connectome may provide important insights into the alterations of brain structural networks in PTSD.
The present study used graph theoretical analysis methods of DTI data to compare the topological properties of brain white matter networks in adult PTSD patients and trauma‐exposed non‐PTSD controls at global, nodal, and modular levels. All participants had no psychiatric comorbidity and had received no prior treatment with psychiatric medications. Based on previous evidence of increased segregation in functional networks (Akiki et al., 2018; Lei et al., 2015) and increased integration of white matter networks in adult PTSD after traffic accidents trauma (Long et al., 2013), we hypothesized that similar disruptions would characterize white matter networks in adult PTSD patients. We also hypothesized that PTSD patients would show abnormal modular characterization of white matter networks, for example, in DMN. Finally, we predicted the white matter network alterations would be related to PTSD symptom severity.
2. MATERIALS AND METHODS
2.1. Participants
Individuals who survived a severe earthquake in Sichuan province of China in 2008 were recruited between January 2009 and August 2009, and selected through a large‐scale survey of 4,200 survivors using the PTSD checklist, a 17‐item self‐report measure (PCL; Weathers, Litz, Herman, Huska, & Keane, 1994). At follow‐up visits carried out over the following 8–15 months post‐earthquake, the presence/absence of a diagnosis of PTSD in survivors was confirmed using the Clinician‐Administered PTSD Scale (CAPS) (Blake et al., 1995) and the Structured Clinical Interview for the DSM‐IV Diagnosis (SCID) (First, Spitzer, Gibbon, & Williams, 1994). For full details of inclusion and exclusion criteria used to select this sample, see Supporting Information. In short, survivors with PCL score ≥35 and CAPS score ≥50 were eligible for inclusion in the PTSD group if a diagnosis of PTSD was confirmed by SCID interview; those scoring <35 on the PCL and without diagnosis of PTSD following SCID interviews were included as non‐PTSD controls. Finally, 76 first‐episode drug‐naive PTSD patients and 76 demographically‐matched stressed controls who did not develop PTSD participated in the study. This recruitment strategy ensured that the survivors with and those without PTSD had similar demographic characteristics and earthquake experiences. The acquisition of magnetic resonance imaging (MRI) scans and clinical data from survivors took place between 9 and 15 months after the earthquake, by which time most individuals who would develop PTSD after the earthquake would have done so (Jin et al., 2014). All subjects provided written informed consent before participation, and this study was approved by the university research ethics committee.
2.2. Data acquisition
All subjects were scanned using a 3‐T MRI system (EXCITE; General Electric) with an eight‐channel phased array head coil. The head was stabilized with foam padding and ear plugs were used. High‐resolution T1‐weighted images were acquired using a three‐dimensional inversion recovery spoiled gradient recalled sequence with the following parameters: repetition time (TR), 8.5 ms; echo time (TE), 3.4 ms; inversion time (TI), 400 ms; section thickness, 1 mm without gap; 156 axial sections; matrix, 256 × 256; field of view (FOV), 240 × 240 mm2; flip angle, 12°. DTI images were acquired with 15 noncollinear directions (b = 1,000 s/mm2), as well as a reference image without diffusion weighting (b = 0) with the following parameters: TR 12000 ms, TE 70.8 ms, number of excitations 2, 3 mm slice without gap, matrix 128 × 128, FOV 240 × 240 mm2. A neuroradiologist verified image quality and the absence of visually observable brain abnormalities.
2.3. Data Preprocessing and structural network construction
Data preprocessing and brain white matter network construction were conducted using PANDA software (http://www.nitrc.org/projects/panda/; a pipeline tool for diffusion MRI analysis; Cui, Zhong, Xu, He, & Gong, 2013). Patient and control samples did not differ in head motion during scans (see Supporting Information). Preprocessing steps included: skull removal with BET; correction of eddy current distortion; building diffusion tensor models and obtaining fractional anisotropy (FA) maps with DTIFIT; and registration to Montreal Neurological Institute (MNI) space with voxel size of 2 × 2 × 2 mm3. Considering the limitation of the diffusion sequence, we chose the relative simple deterministic fiber‐tracking algorithm which was more suitable for current scenario. Deterministic fiber tractography proceeded until either it turned an angle greater than 45° or the FA was less than 0.2 using the Fiber Assignment by Continuous Tracking algorithm to reconstruct white matter tracts (Mori et al., 2002; Mori, Crain, Chacko, & van Zijl, 1999).
To develop brain graphs, we followed the approach used previously to construct whole brain anatomical networks (Gong, He, et al., 2009). First, to define the nodes of the network, the automated anatomical labeling (AAL) atlas was used to divide the whole brain into 90 cortical and subcortical regions (Tzourio‐Mazoyer et al., 2002). This parcellation procedure has been discussed and applied previously (Gong, He, et al., 2009; Gong, Rosa‐Neto, et al., 2009). Each individual T1‐weighted image was co‐registered to their b0 images in native diffusion space using a linear transformation, and then nonlinearly mapped to MNI space. The derived transformation parameters were inverted and used to warp the AAL atlas from MNI space to the native diffusion space in which the discrete labeling values were preserved by using a nearest neighbor interpolation method. The transformed AAL atlas and the individual b0 image were then visualized together in the individual space and checked by two of the authors (Xueling Suo and Wenbin Li) for each participant to ensure there was no obvious mismatching error. Next, to define the edges of the network, the averaged FA of linking fibers was calculated. Lastly, a weighted and undirected symmetrical anatomical 90 × 90 matrix for each subject was obtained.
2.4. Global and nodal network analysis
We applied a network sparsity parameter, S, to provide each network with the same number of edges. Consistent with previous studies (Suo et al., 2017), we selected a range of S thresholds for the white matter connectivity network based on the following criteria: (1) the averaged degree over all nodes of each thresholded network was larger than 2 × log (90); and (2) the small‐worldness σ of the thresholded networks was larger than 1.1 for all participants. Based on these criteria, we defined S ranging from 0.1 to 0.34. For each network, the area under the curve (AUC; Figure S1) was calculated over the range of S values with an interval step of 0.01, which provides a summarized scalar for the topological characterization of brain networks to limit potential bias of any single threshold.
Graph theoretical analysis was carried out on individual participant's white matter network using GRETNA software (http://www.nitrc.org/projects/gretna/; Wang et al., 2015). Global metrics included: small‐world parameters (clustering coefficient C p, characteristic path length L p, normalized clustering coefficient γ, normalized characteristic path length λ, and small‐worldness σ) and network efficiency parameters (local efficiency E loc and global efficiency E glob). To estimate small‐world properties, the C p and L p of the network were compared with those of random network, where C random and L random are the mean C p and L p of 100 matched random networks that preserve the same number of nodes, edges, and degree distribution as the real network (Gong, He, et al., 2009). A small‐world network was defined with small worldness σ = γ/λ > 1, which fulfilled the conditions of γ (normalized C p = C p/C random) > 1 and λ (normalized L p = L p/L random) ≈ 1 (Watts & Strogatz, 1998). The nodal metrics examined included the following three nodal centrality metrics: nodal degree (Rubinov & Sporns, 2010), nodal efficiency (Achard & Bullmore, 2007), and nodal betweenness (Freeman, 1977). Global and nodal metrics can be used to characterize the topology of networks. Global metrics measure the architecture of the whole brain network, while nodal metrics measure the topology of single network nodes individually. High C p, γ, and E loc reflect network segregation, which is the ability for specialized neuronal processing carried out among densely interconnected regions; Low L p, λ, and high E glob show the network integration in the brain, which is the ability for global information communication or distributed network integration; σ characterizes an optimized balance between network segregation and integration (Dai et al., 2019; Zhao, Xu, & He, 2019).
2.5. Modular analysis
A module can be generally defined as a subset of nodes in the graph that are more densely connected to nodes in the same module than to nodes outside the module. It quantifies the degree to which the network can be optimally partitioned into distinct subcommunities. Modules are nonoverlapping, with each node assigned to only one module. We computed a modularity measure (Newman's Q) to evaluate the degree to which a brain connectivity network is subdivided into specific modules (Newman, 2006). The modularity Q(p) for a given partition p of a weighted network is defined as where N M is the number of modules, W is the total weight of the network, w s is the sum of the connectional weights between all nodes in module S, and W s is the sum of degrees of nodes in module S. A modified greedy optimization algorithm that was implemented in the GRETNA was used to find the optimal modular structure in the resultant representative brain structural networks, which was constructed by averaging all participants' white matter connectivity networks (Li et al., 2018). This approach considers the heterogeneity of module size as occurs in real networks. Then, the modular characteristic differences (intra‐ and inter‐modular connectivity) were calculated after applying the modular architecture derived from all the participants. Intra‐modular connectivity of particular modules was defined as the average of all connectional weights within the module to represent the significance of the module within the brain network. Inter‐modular connectivity assesses the connection between two modules, computed as the average of connectional weights between the modules.
2.6. Statistical analysis
Two‐tailed independent‐sample t tests and chi‐square tests were used to compare quantitative and qualitative variables, respectively. A nonparametric permutation test was performed to test for between‐group differences in the AUC of global and nodal network metrics, modularity (Q), and modular characteristics (intra‐ and inter‐modular connectivity; Zhang et al., 2011). For nodal metric analysis, a False Discovery Rate (FDR) correction for multiple comparisons (i.e., 90 brain regions in the current study) was performed to maintain a significance level of .05 (Genovese, Lazar, & Nichols, 2002). The permutation test was repeated 10,000 times. For the network metrics with significant between‐group differences, correlational analyses were performed to assess the relationships between these metrics and CAPS scores that reflect PTSD symptom severity (p < .05, FDR corrected). When controlling for age in these analyses, our main findings were maintained (see Table E2).
3. RESULTS
3.1. Demographic and clinical comparisons
Demographic and clinical characteristics of the 76 PTSD patients and 76 trauma‐exposed non‐PTSD controls participating in the current study are summarized in Table 1. No significant differences were identified between the two groups in age, gender, years of education or time since trauma (p > .05).
Table 1.
Demographic and clinical characteristics of study participantsa
| Variables | Non‐PTSD (n = 76) | PTSD (n = 76) | p value |
|---|---|---|---|
| Age (years)b | 43.6 ± 10.0 (20–65) | 43.0 ± 10.8 (19–67) | .71c |
| Gender (male/female) | 21/55 | 25/51 | .59d |
| Years of educationb | 6.8 ± 3.3 (0–12) | 6.8 ± 3.2 (0–16) | .99c |
| Time since trauma (months)b | 11.4 ± 2.3 (8–15) | 11.0 ± 2.1 (8–15) | .25c |
| PTSD checklist | 28.2 ± 7.0 (18–54) | 47.8 ± 13.3 (21–80) | <.001c |
| CAPS | 22.7 ± 11.7 (3–48) | 63.3 ± 9.5 (51–95) | <.001c |
Abbreviations: CAPS, Clinician‐administered PTSD scale; PTSD, posttraumatic stress disorder.
Data are presented as mean ± SD (minimum‐maximum) unless noted.
Age, years of education, and time since trauma were defined relative to the time of MRI scanning.
The p value was obtained by using a two‐sample two‐tailed t test.
The p value was calculated by using a two‐tailed Chi‐squared‐test.
3.2. Global and nodal topological alterations of the white matter networks
Both PTSD patients and trauma‐exposed non‐PTSD controls showed a small‐world organization of white matter networks expressed by a γ > 1 and λ ≈ 1 (Figure 1). However, the PTSD group showed significantly lower C p (p = .016) and λ (p = .035). No significant differences were identified in L p (p = .093), γ (p = .176), σ (p = .271), E glob (p = .079), or E loc (p = .303; Figure 2, Table E1).
Figure 1.

The key small‐world parameters of the white matter networks as a function of sparsity threshold. Graphs show that in the defined threshold range, both the PTSD and trauma‐exposed non‐PTSD control groups exhibited γ substantially larger than 1 and λ approximately equal to 1, which indicates that both groups exhibited the typical features of small‐world topology. PTSD, posttraumatic stress disorder; γ, normalized clustering coefficient; λ, normalized characteristic path length [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.

Differences in global properties of the brain white matter networks between PTSD patients and trauma‐exposed non‐PTSD controls. C p (p = .016) and λ (p = .035) were significantly different between the two groups (nonparametric permutation test, p < .05). Box plots present in ascending order the minimum value, the first quartile, the median, the third quartile and the maximum value. PTSD, posttraumatic stress disorder; C p, clustering coefficient; γ, normalized clustering coefficient; L p, characteristic path length; λ, normalized characteristic path length; E loc, local efficiency; E glob, global efficiency; σ, small‐worldness [Color figure can be viewed at http://wileyonlinelibrary.com]
We identifed the brain regions that exhibited significant between‐group differences in at least one nodal metric. Compared with the non‐PTSD controls, the PTSD patients exhibited increased nodal centralities in the left middle frontal gyrus, superior and inferior temporal gyrus, and right inferior occipital gyrus (p < .05, FDR corrected; Table 2).
Table 2.
Regions showing higher AUC values of nodal centralities in the PTSD patients compared with the trauma‐exposed non‐PTSD controls
| p value | |||
|---|---|---|---|
| Brain regions | Nodal degree | Nodal efficiency | Nodal betweenness |
| Left middle frontal gyrus | .0002 | .0016 | .1070 |
| Right inferior occipital gyrus | .0016 | .0012 | .0272 |
| Left superior temporal gyrus | .0006 | .0012 | .2755 |
| Left inferior temporal gyrus | .0896 | .0008 | .0828 |
Regions were considered abnormal in PTSD patients if they exhibited significant between‐group differences (p < .05, FDR corrected) in at least one of the three nodal centralities (shown in bold font).
Abbreviations: AUC, area under the curve; PTSD, posttraumatic stress disorder.
3.3. Modular structure of white matter networks
Derived from all participants, identified modules included a left fronto‐parietal module (module I); right fronto‐parietal module (module II); left fronto‐striato‐temporal module (module III); right fronto‐striato‐temporal module (module IV); posterior components of the DMN and visual module (module V; details in Supporting Information; Figure 3).
Figure 3.
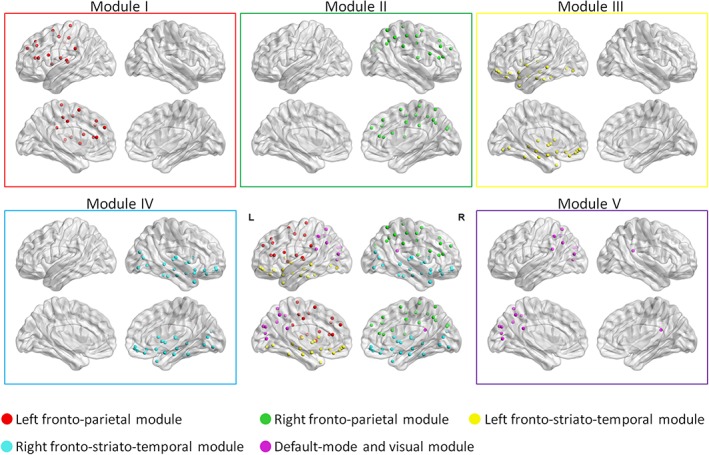
Modular organization of the white matter network as derived from all participants. The five identified modular structures include a left fronto‐parietal module (module I); right fronto‐parietal module (module II); left fronto‐striato‐temporal module (module III); right fronto‐striato‐temporal module (module IV); posterior components of the default mode network and visual module (module V; details in Supporting Information). The results were visualized using the BrainNet Viewer package (http://www.nitrc.org/projects/bnv; Xia, Wang, & He, 2013). L, left; R, right
While modularity Q decreased with increasing white matter connectivity cost (Figure 4a), the AUC did not differ between the PTSD patients and non‐PTSD controls. Relative to non‐PTSD controls, PTSD patients demonstrated increased intra‐modular connectivity for module V (p = .032; Figure 4b). There were no significant differences between patients and controls in intra‐modular connectivity for module I (p = .118), II (p = .813), III (p = .556), or IV (p = .429). Increased inter‐modular connections relative to controls were found in PTSD patients in modules II–III (p = .004) and IV–V (p = .003; Figure 4c). There were no significant differences in the inter‐modular connection for modules I–II (p = .182), I–III (p = .054), I–IV (p = .330), I–V (p = .055), II–IV (p = .895), II–V (p = .894), III–IV (p = .709), or III–V (p = .600).
Figure 4.
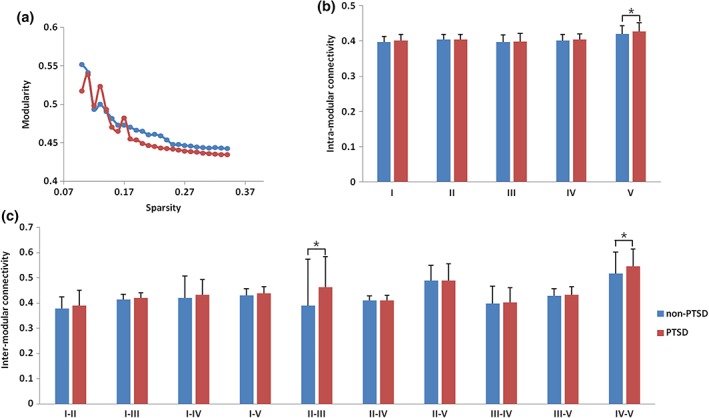
Modular characteristics of white matter networks in PTSD patients and non‐PTSD controls. Group differences were observed in network modularity Q (a), intra‐modular connectivity (b), and inter‐modular connectivity (c). Relative to non‐PTSD controls, PTSD patients demonstrated higher intra‐modular connections for module V and higher inter‐modular connections between modules II–III and IV–V (p < .05). The bars represent the mean values and the error bars represent standard deviations. PTSD, posttraumatic stress disorder [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Relationships between topological properties and clinical variables
As all study participants had experienced major psychological trauma, with PTSD determined by psychological symptom persistence and severity, the two participant groups were pooled to examine associations of MRI findings with PTSD symptom severity. As shown in Figure 5, CAPS scores were negatively correlated with C p (r = −.229, p = .011) and λ (r = −.199, p = .019), as well as positively with the nodal efficiency of the left middle frontal gyrus (r = .220, p = .012), right inferior occipital gyrus (r = .204, p = .018), left superior temporal gyrus (r = .262, p = .006), and left inferior temporal gyrus (r = .235, p = .009), and positively with the nodal degree of the left middle frontal gyrus (r = .270, p = .005), right inferior occipital gyrus (r = .242, p = .009), and left superior temporal gyrus (r = .299, p < .001).
Figure 5.
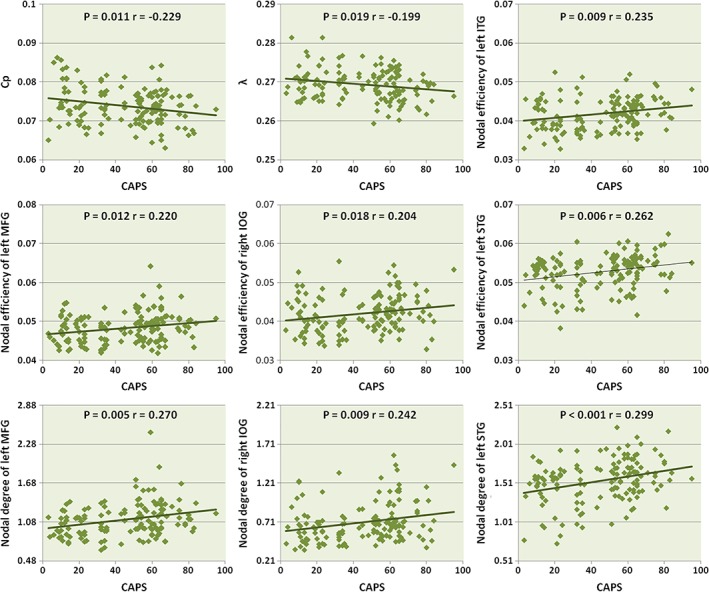
Scatter plots of C p, λ, nodal centralities of left ITG, MFG, STG, and right IOG in relation to CAPS scores in PTSD and non‐PTSD controls. PTSD, posttraumatic stress disorder; CAPS, clinician‐administered PTSD scale; C p, clustering coefficient; λ, normalized characteristic path length; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus; IOG, inferior occipital gyrus [Color figure can be viewed at http://wileyonlinelibrary.com]
As shown in Figure S3, the nodal efficiency of left inferior temporal gyrus was positively correlated with CAPS scores in the PTSD group (r = .35, p = .002). There were no significant relationships between other measures examined and CAPS scores in PTSD or non‐PTSD group (p > .05).
3.5. Relation between white matter, functional, and morphological networks parameters
We have previously published a functional network and a gray matter morphology network analysis of PTSD in this sample (Lei et al., 2015; Niu et al., 2018b). We thus conducted secondary analyses to investigate the relationship between white matter findings in the present study with functional/morphological network properties from the same patients, imaging acquisition system and network analysis software. This analysis is described in detail in Supporting Information. Both global and nodal network parameters including C p, L p, γ, λ, σ, E loc, E glob, nodal degree, nodal efficiency, and nodal betweenness in PTSD group were used to examine the relation between white matter and functional and morphological network characteristics. There was a negative correlation of nodal degree and nodal efficiency of left superior temporal gyrus between white matter and functional network in the PTSD group (r = −.30, p = .020; r = −.27, p = .036; Figure S4 ). A trend toward a negative correlation between global C p of white matter and functional networks was also observed (r = −.22, p = .058). No other significant correlations were found in the relationship of white matter network features with functional or morphological networks.
4. DISCUSSION
The present study examined brain white matter networks in adult PTSD patients compared with traumatized non‐PTSD controls. We have found significant brain structural network topological architecture changes in different observation levels in PTSD patients. At the global level, PTSD patients had decreased segregation and increased integration reflected by lower C p and λ of whole‐brain white matter networks. Modularity analysis indicated that PTSD patients had greater inter‐ and intra‐modular connectivity than controls. At the nodal level, several frontal, temporal, and occipital regions exhibited increased nodal centralities in PTSD patients, which were positively correlated with symptom severity.
A small‐world organization reflects an optimal balance between network segregation (reflected by C p, γ, or E loc) and network integration (reflected by L p, λ, or E glob) of information processing. Despite having an overall small‐world architecture similar to non‐PTSD controls, PTSD patients showed lower C p and λ. These alterations were negatively correlated with symptom severity, indicating a clinical significance to the decreased network segregation and increased network integration. These observations suggest that brain white matter networks of drug‐naïve PTSD patients exhibit a shift toward “randomization” (in which the network transforms from a small‐world to a relative random network; Suo et al., 2018), consistent with a previous white matter networks study (Long et al., 2013). The randomization reported by Long et al. was primarily a result of increased network integration (lower L p and λ) in PTSD with comorbid depression compared with nontraumatized healthy controls. Adults with PTSD have higher rates of depression than trauma exposed adults without PTSD (Breslau, Davis, Peterson, & Schultz, 2000). The neural mechanisms of PTSD and depression might overlap (Kroes, Rugg, Whalley, & Brewin, 2011), and caution is required when interpreting findings to be related to either factor. Our findings indicate that the shift toward randomization can be seen in PTSD without depression or other psychiatric comorbidity even in comparison with similarly stressed individuals who did not go on to develop PTSD, indicating that this shift was related to PTSD per se rather than to traumatic stress exposure or comorbidity, regardless of the type of trauma. This randomization of global properties has been previously reported in other psychiatric disorders including major depressive disorder, schizophrenia, and bipolar disorder (Li, Zhou, Yang, Wang, & Zhong, 2017; Xia et al., 2019). The randomization in the current study might contribute to impairments in emotion processing in patients with PTSD, as suggested by correlations between the altered global properties and symptom severity scores.
Our recent pediatric PTSD research found decreased segregation (lower E loc) and decreased integration (lower E glob and higher L p) in the white matter networks using the same DTI analysis procedure (Suo et al., 2017). There were similarities and differences of global alterations in our recent pediatric PTSD study and the current adult PTSD study. Both studies showed decreased network segregation in PTSD patients compared with traumatized non‐PTSD controls. However, the pediatric patients showed decreased network functional integration in white matter network organization while increased network integration was observed in the current study of PTSD in adults. During development, brain structural networks are fine‐tuned as reflected in increased integration (Cao, Huang, Peng, Dong, & He, 2016). This aspect of typical developmental trajectory may contribute to the different large‐scale brain network alterations observed in pediatric and adult PTSD patients.
The different global network alterations characterized by increased network segregation and increased network integration in adult PTSD has been observed previously in recent functional networks studies of PTSD (Lei et al., 2015) and gray matter structural networks analyses (Qi et al., 2018). The primary difference relative to previous study findings in the current results was in regard to network segregation. Several possible explanations may account for this difference. First, the edge definition varies across imaging modalities. Alterations in synchronized brain activity at a point in time measured by functional MRI and the correlation between the cortical thicknesses of every pair of brain regions could exist in the absence of direct abnormalities in white matter tracts. Second, the alteration of network segregation reflected by lower C p in the present white matter study was in the opposite direction from that seen in our previous functional study with a highly overlapping patient sample (Lei et al., 2015), and there was a trend for a negative correlation between C p in white matter networks with C p in functional networks. Because disruptions of white matter might lead to compensatory increases in functional connectivity due to a reduction in efficient information transfer, the trend for an inverse relation between C p of functional and white matter networks suggests that the functional change might represent a compensatory response to the decreased segregation of information processing in white matter networks. Caution is required when interpreting and comparing network analysis results from different imaging modalities, as the imaging modalities are measuring different aspects of brain connectivity whose differences themselves can have important biological meaning. The very modest level of association between similar measures of gray matter, white matter, and functional networks highlights the independence of these network features in PTSD. Another potential factor that may contribute to differences in findings across studies is the use of different control groups, for example, the similarly stressed non‐PTSD controls in the current study and nontraumatized healthy controls by Qi et al. (2018).
We conducted a module analysis to explore which modularity alterations contributed to the observed global abnormalities in white matter networks. There was a decreased trend of modularity in PTSD patients relative to non‐PTSD controls, suggesting a loss of intra‐ relative to inter‐modular connections and supporting the finding of decreased network segregation reflected by lower C p in patients. PTSD patients showed increased inter‐modular connections between a right fronto‐parietal module and a left fronto‐striato‐temporal module, and between a right fronto‐striato‐temporal module and the DMN‐visual module. A decrease in the λ in small‐worldness was a reflection of increased inter‐modular connection. Previous studies have found that functional connectivity of the fronto‐parietal network was increased and associated with impairment of attention control in PTSD patients (Zhang et al., 2016), and connectivity of the fronto‐temporal network with DMN has been related to dissociative symptoms (Tursich et al., 2015). In addition, we also found increased intra‐modular connections of DMN‐visual module in patients. Our previous functional connectome study on an overlapping patient sample has shown abnormal DMN in PTSD (Lei et al., 2015). The coupled structural‐functional association suggests that the functional interactions in DMN are more directly related to the underlying anatomical connection in PTSD. Results from the present study replicate and extend reports of increased white matter connections in PTSD, which may provide a potential structural basis for the alterations of brain function and contribute significantly to global network alterations in PTSD.
We also explored the role of individual nodes in white matter networks. Increased nodal centralities in PTSD patients were found in the left middle frontal gyrus, superior and inferior temporal gyrus, and right inferior occipital gyrus. Our recent studies of gray matter networks and functional networks on an overlapping patient sample have also observed increased nodal centralities in the similar frontal, temporal, and occipital areas in PTSD (Lei et al., 2015; Niu et al., 2018b). Consistent with previous findings, the current results suggest an enhanced capacity for emotional and visual processing of these areas, which might be relevant for hyperarousal (Daniels, Frewen, Theberge, & Lanius, 2016) and persistent visual flashback experiences (Bourne, Mackay, & Holmes, 2013) in PTSD patients. Further, our observation that the nodal centralities of frontal, temporal, and occipital areas were positively related to CAPS scores suggests that these nodal impairments may be related to illness severity. Most increased nodal centralities were located in the DMN. In contrast, a recent white matter study with motor vehicle accident survivors with PTSD found increases in nodal centrality mainly in the salience network (Long et al., 2013). Given previous findings showing the recruitment of different neural networks according to different traumatic events (Boccia et al., 2016), discrepancies of nodal centralities across studies may stem from differences in the type of trauma (i.e., natural disasters versus personal injury). Such differences may be important in understanding how different types of stress impact brain network organization.
Overall, global, modular, and nodal level measures provide convergent results indicating that PTSD is a disorder of network integration disruption related to increased inter‐ and intra‐modular connections, and increased nodal centralities in widespread neocortical regions. The network attribute of integration highlights the efficiency of information processing at the global level. High integration indicates that white matter pathways have increased capacity to transfer information within and between brain modules (Sporns, 2011). Increased nodal centralities in frontal, temporal, and occipital regions are informative about not only how individual regions are altered but also how regional changes impact the overall network. The current study expands understanding of the neurobiology of PTSD by comparing drug‐naïve adult PTSD patients with similarly stressed non‐PTSD controls and analyzing modular properties, and demonstrating that impaired brain network organization at multiple levels is related to the clinical severity of PTSD above and beyond acute stress effects as experienced by the non‐PTSD controls.
Our study has several limitations that need to be considered. First, given that we acquired a limited number of diffusion directions, acquiring data for this study before newer diffusion‐weighted schemes were available for more accurate fiber tractography, and future research with newer DTI protocols might refine our conclusions. Second, there is no widely accepted optimal approach for defining nodes and edges. We used the AAL 90 template regions as nodes and mean FA values of fibers as the weighting factor in the construction of graphs. Other measures such as Harvard‐Oxford atlas and the number or averaged length of linking fibers as weighting factors could also be considered for calculating network metrics (Cui, Zhong, Xu, He, & Gong, 2013). Third, this study was cross‐sectional; how the white matter network architecture associated with PTSD evolves dynamically and predicts future PTSD conversion after major life stress remain to be clarified in longitudinal studies. Fourth, there were relatively modest correlations between CAPS ratings of PTSD symptom severity and altered topological properties. Approaches measuring other aspects of PTSD functional impairment might yield more compelling findings. Fifth, our study aim was to identify brain features that discriminate stressed individuals who develop PTSD patients from similarly stressed individuals who did not later develop PTSD. Without a parallel group of matched controls, we cannot determine how both of these groups differ from healthy individuals to examine general stress effects. Sixth, studying people after a single type of trauma increased our sample's homogeneity, but this leaves open the question of whether our findings generalize to other causes of PTSD. Seventh, according to a recent meta‐analysis (Siehl, King, Burgess, Flor, & Nees, 2018), adult PTSD patients may differ depending on whether traumatic experiences occurred in adulthood (aa‐PTSD) or childhood (ac‐PTSD). In our study, all of our patients were aa‐PTSD, and thus the relevance of our findings for ac‐PTSD remains to be determined.
5. CONCLUSION
In summary, our results provide novel insights into disrupted brain white matter network organization in adult PTSD patients at multiple levels. The finding of increased global integration indicates a shift toward “randomization” in PTSD, with increased inter‐ and intra‐modular connections and increased nodal centralities mainly involving frontal‐temporal‐occipital regions that was correlated with symptom severity. Our results suggest that PTSD is associated with large‐scale brain white matter network disruptions at different levels, providing a potential structural basis for the alterations of brain function and behavior in PTSD.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
Data S1: Supporting Information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation (Grant Nos. 81621003, 81761128023, 81220108013, 81227002, and 81030027); the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant IRT16R52) of China; the Changjiang Scholar Professorship Award (Award No. T2014190) of China; and the CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education. X. Suo is supported by the Graduate Student's Research and Innovation Fund of Sichuan University (No. 2018YJSY099).
Suo X, Lei D, Li W, et al. Large‐scale white matter network reorganization in posttraumatic stress disorder. Hum Brain Mapp. 2019;40:4801–4812. 10.1002/hbm.24738
Xueling Suo and Du Lei authors contributed equally to this work.
Funding information Changjiang Scholar Professorship Award of China, Grant/Award Number: T2014190; CMB Distinguished Professorship Award administered by the Institute of International Education, Grant/Award Number: F510000/G16916411; Graduate Student's Research and Innovation Fund of Sichuan University, Grant/Award Number: 2018YJSY099; National Natural Science Foundation of China, Grant/Award Numbers: 81030027, 81220108013, 81227002, 81621003, 81761128023; Program for Changjiang Scholars and Innovative Research Team in University of China, Grant/Award Number: IRT16R52
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki, T. J. , Averill, C. L. , Wrocklage, K. M. , Scott, J. C. , Averill, L. A. , Schweinsburg, B. , … Abdallah, C. G. (2018). Default mode network abnormalities in posttraumatic stress disorder: A novel network‐restricted topology approach. NeuroImage, 176, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders. DSM‐5 (5th ed.). Arlington, VA: Author. [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a clinician‐administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Boccia, M. , D'Amico, S. , Bianchini, F. , Marano, A. , Giannini, A. M. , & Piccardi, L. (2016). Different neural modifications underpin PTSD after different traumatic events: An fMRI meta‐analytic study. Brain Imaging and Behavior, 10, 226–237. [DOI] [PubMed] [Google Scholar]
- Bourne, C. , Mackay, C. E. , & Holmes, E. A. (2013). The neural basis of flashback formation: The impact of viewing trauma. Psychological Medicine, 43, 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau, N. , Davis, G. C. , Peterson, E. L. , & Schultz, L. R. (2000). A second look at comorbidity in victims of trauma: The posttraumatic stress disorder‐major depression connection. Biological Psychiatry, 48, 902–909. [DOI] [PubMed] [Google Scholar]
- Cao, M. , Huang, H. , Peng, Y. , Dong, Q. , & He, Y. (2016). Toward developmental connectomics of the human brain. Frontiers in Neuroanatomy, 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M. , Sigel, B. A. , Kramer, T. L. , Smitherman, S. , Vanderzee, K. , Pemberton, J. , & Kilts, C. D. (2016). Modes of large‐scale brain network organization during threat processing and posttraumatic stress disorder symptom reduction during TF‐CBT among adolescent girls. PLoS One, 11, e0159620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan, J. D. , Abdallah, C. G. , Tang, C. Y. , Mathew, S. J. , Martinez, J. , Hof, P. R. , … Gorman, J. M. (2010). The role of early life stress in development of the anterior limb of the internal capsule in nonhuman primates. Neuroscience Letters, 480, 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan, J. D. , Kolavennu, V. , Abdallah, C. G. , Mathew, S. J. , Perera, T. D. , Pantol, G. , … Tang, C. (2016). Patterns of anterior versus posterior white matter fractional anistotropy concordance in adult nonhuman primates: Effects of early life stress. Journal of Affective Disorders, 192, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock, R. C. , Jbabdi, S. , Yan, C. G. , Vogelstein, J. T. , Castellanos, F. X. , Di Martino, A. , … Milham, M. P. (2013). Imaging human connectomes at the macroscale. Nature Methods, 10, 524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Z. , Zhong, S. , Xu, P. , He, Y. , & Gong, G. (2013). PANDA: A pipeline toolbox for analyzing brain diffusion images. Frontiers in Human Neuroscience, 7, 42 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, Z. , Lin, Q. , Li, T. , Wang, X. , Yuan, H. , Yu, X. , … Wang, H. (2019). Disrupted structural and functional brain networks in Alzheimer's disease. Neurobiology of Aging, 75, 71–82. [DOI] [PubMed] [Google Scholar]
- Daniels, J. K. , Frewen, P. , Theberge, J. , & Lanius, R. A. (2016). Structural brain aberrations associated with the dissociative subtype of post‐traumatic stress disorder. Acta Psychiatrica Scandinavica, 133, 232–240. [DOI] [PubMed] [Google Scholar]
- Daniels, J. K. , Lamke, J. P. , Gaebler, M. , Walter, H. , & Scheel, M. (2013). White matter integrity and its relationship to PTSD and childhood trauma––A systematic review and meta‐analysis. Depression and Anxiety, 30, 207–216. [DOI] [PubMed] [Google Scholar]
- Ding, A. Y. , Li, Q. , Zhou, I. Y. , Ma, S. J. , Tong, G. , McAlonan, G. M. , & Wu, E. X. (2013). MR diffusion tensor imaging detects rapid microstructural changes in amygdala and hippocampus following fear conditioning in mice. PLoS One, 8, e51704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. (1994). Structured clinical interview for Axis I DSM‐IV disorders. New York: Biometrics Research. [Google Scholar]
- Freeman, L. C. (1977). A set of measures of centrality based on betweenness. Sociometry, 40, 35–41. [Google Scholar]
- Genovese, C. R. , Lazar, N. A. , & Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage, 15, 870–878. [DOI] [PubMed] [Google Scholar]
- Goldstein, R. B. , Smith, S. M. , Chou, S. P. , Saha, T. D. , Jung, J. , Zhang, H. , … Grant, B. F. (2016). The epidemiology of DSM‐5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on alcohol and related conditions‐III. Social Psychiatry and Psychiatric Epidemiology, 51, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, G. , He, Y. , Concha, L. , Lebel, C. , Gross, D. W. , Evans, A. C. , & Beaulieu, C. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cerebral Cortex, 19, 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, G. , Rosa‐Neto, P. , Carbonell, F. , Chen, Z. J. , He, Y. , & Evans, A. C. (2009). Age‐ and gender‐related differences in the cortical anatomical network. The Journal of Neuroscience, 29, 15684–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, B. R. , McCormack, K. M. , Grand, A. P. , Sawyer, N. T. , Zhang, X. , Maestripieri, D. , … Sanchez, M. M. (2013). Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: Associations with high cortisol during infancy. Biology of Mood & Anxiety Disorders, 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C. , Qi, R. , Yin, Y. , Hu, X. , Duan, L. , Xu, Q. , … Li, L. (2014). Abnormalities in whole‐brain functional connectivity observed in treatment‐naive post‐traumatic stress disorder patients following an earthquake. Psychological Medicine, 44, 1927–1936. [DOI] [PubMed] [Google Scholar]
- Kroes, M. C. , Rugg, M. D. , Whalley, M. G. , & Brewin, C. R. (2011). Structural brain abnormalities common to posttraumatic stress disorder and depression. Journal of Psychiatry & Neuroscience, 36, 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, D. , Li, K. , Li, L. , Chen, F. , Huang, X. , Lui, S. , … Gong, Q. (2015). Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology, 276, 818–827. [DOI] [PubMed] [Google Scholar]
- Li, F. , Lui, S. , Yao, L. , Ji, G. J. , Liao, W. , Sweeney, J. A. , & Gong, Q. (2018). Altered white matter connectivity within and between networks in antipsychotic‐naive first‐episode schizophrenia. Schizophrenia Bulletin, 44, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Zhou, H. , Yang, Y. , Wang, H. , & Zhong, N. (2017). More randomized and resilient in the topological properties of functional brain networks in patients with major depressive disorder. Journal of Clinical Neuroscience, 44, 274–278. [DOI] [PubMed] [Google Scholar]
- Li, L. , Lei, D. , Li, L. , Huang, X. , Suo, X. , Xiao, F. , … Gong, Q. (2016). White matter abnormalities in post‐traumatic stress disorder following a specific traumatic event. eBioMedicine, 4, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Wu, M. , Liao, Y. , Ouyang, L. , Du, M. , Lei, D. , … Gong, Q. (2014). Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neuroscience and Biobehavioral Reviews, 43, 163–172. [DOI] [PubMed] [Google Scholar]
- Liao, X. , Vasilakos, A. V. , & He, Y. (2017). Small‐world human brain networks: Perspectives and challenges. Neuroscience and Biobehavioral Reviews, 77, 286–300. [DOI] [PubMed] [Google Scholar]
- Long, Z. , Duan, X. , Xie, B. , Du, H. , Li, R. , Xu, Q. , … Chen, H. (2013). Altered brain structural connectivity in post‐traumatic stress disorder: A diffusion tensor imaging tractography study. Journal of Affective Disorders, 150, 798–806. [DOI] [PubMed] [Google Scholar]
- Meng, Y. , Qiu, C. , Zhu, H. , Lama, S. , Lui, S. , Gong, Q. , & Zhang, W. (2014). Anatomical deficits in adult posttraumatic stress disorder: A meta‐analysis of voxel‐based morphometry studies. Behavioural Brain Research, 270, 307–315. [DOI] [PubMed] [Google Scholar]
- Mori, S. , Crain, B. J. , Chacko, V. P. , & van Zijl, P. C. (1999). Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45, 265–269. [DOI] [PubMed] [Google Scholar]
- Mori, S. , Kaufmann, W. E. , Davatzikos, C. , Stieltjes, B. , Amodei, L. , Fredericksen, K. , … van Zijl, P. C. (2002). Imaging cortical association tracts in the human brain using diffusion‐tensor‐based axonal tracking. Magnetic Resonance in Medicine, 47, 215–223. [DOI] [PubMed] [Google Scholar]
- Newman, M. E. (2006). Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America, 103, 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, R. , Lei, D. , Chen, F. , Chen, Y. , Suo, X. , Li, L. , … Gong, Q. (2018a). Disrupted grey matter network morphology in pediatric posttraumatic stress disorder. NeuroImage: Clinical, 18, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, R. , Lei, D. , Chen, F. , Chen, Y. , Suo, X. , Li, L. , … Gong, Q. (2018b). Reduced local segregation of single‐subject gray matter networks in adult PTSD. Human Brain Mapping, 39, 4884–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, D. C. M. , Ryder, W. , Paquola, C. , Tickell, A. , Chan, C. , Hermens, D. F. , … Lagopoulos, J. (2018). White matter integrity alterations in post‐traumatic stress disorder. Human Brain Mapping, 39, 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , Spreng, R. N. , Shin, L. M. , & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36, 2130–2142. [DOI] [PubMed] [Google Scholar]
- Qi, S. , Mu, Y. F. , Cui, L. B. , Zhang, J. , Guo, F. , Tan, Q. R. , … Yin, H. (2018). Anomalous gray matter structural networks in recent onset post‐traumatic stress disorder. Brain Imaging and Behavior, 12, 390–401. [DOI] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh, R. A. , Loi, M. , Joels, M. , Dijkhuizen, R. M. , & van der Toorn, A. (2017). Early life stress‐induced alterations in rat brain structures measured with high resolution MRI. PLoS One, 12, e0185061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl, S. , King, J. A. , Burgess, N. , Flor, H. , & Nees, F. (2018). Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta‐analysis. NeuroImage: Clinical, 19, 581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2011). The human connectome: A complex network. Annals of the New York Academy of Sciences, 1224, 109–125. [DOI] [PubMed] [Google Scholar]
- Suo, X. , Lei, D. , Chen, F. , Wu, M. , Li, L. , Sun, L. , … Gong, Q. (2017). Anatomic insights into disrupted small‐world networks in pediatric posttraumatic stress disorder. Radiology, 282, 826–834. [DOI] [PubMed] [Google Scholar]
- Suo, X. , Lei, D. , Li, K. , Chen, F. , Li, F. , Li, L. , … Gong, Q. (2015). Disrupted brain network topology in pediatric posttraumatic stress disorder: A resting‐state fMRI study. Human Brain Mapping, 36, 3677–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo, X. , Lei, D. , Li, L. , Li, W. , Dai, J. , Wang, S. , … Gong, Q. (2018). Psychoradiological patterns of small‐world properties and a systematic review of connectome studies of patients with 6 major psychiatric disorders. Journal of Psychiatry & Neuroscience, 43, 170214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich, M. , Ros, T. , Frewen, P. A. , Kluetsch, R. C. , Calhoun, V. D. , & Lanius, R. A. (2015). Distinct intrinsic network connectivity patterns of post‐traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132, 29–38. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, X. , Xia, M. , Liao, X. , Evans, A. , & He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9, 386 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, D. J. , & Strogatz, S. H. (1998). Collective dynamics of 'small‐world' networks. Nature, 393, 440–442. [DOI] [PubMed] [Google Scholar]
- Weathers, F. W. , Litz, B. T. , Herman, D. , Huska, J. , & Keane, T. (1994). The PTSD checklist‐civilian version (PCL‐C). Boston, MA: National Center for PTSD. [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, M. , Womer, F. Y. , Chang, M. , Zhu, Y. , Zhou, Q. , Edmiston, E. K. , … Wang, F. (2019). Shared and distinct functional architectures of brain networks across psychiatric disorders. Schizophrenia Bulletin, 45, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y. , Jin, C. , Eyler, L. T. , Jin, H. , Hu, X. , Duan, L. , … Li, L. (2012). Altered regional homogeneity in post‐traumatic stress disorder: A resting‐state functional magnetic resonance imaging study. Neuroscience Bulletin, 28, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Wang, J. , Wu, Q. , Kuang, W. , Huang, X. , He, Y. , & Gong, Q. (2011). Disrupted brain connectivity networks in drug‐naive, first‐episode major depressive disorder. Biological Psychiatry, 70, 334–342. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xie, B. , Chen, H. , Li, M. , Liu, F. , & Chen, H. (2016). Abnormal functional connectivity density in post‐traumatic stress disorder. Brain Topography, 29, 405–411. [DOI] [PubMed] [Google Scholar]
- Zhao, T. , Xu, Y. , & He, Y. (2019). Graph theoretical modeling of baby brain networks. NeuroImage, 185, 711–727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


