Key Points
Question
Is excess body weight associated with long-term outcomes in hypertrophic cardiomyopathy?
Findings
In this international cohort study that included 3282 patients, a body mass index (calculated as weight in kilograms divided by height in meters squared) greater than 30 was independently associated with atrial fibrillation, disease progression, and heart failure onset irrespective of age, sex, outflow tract obstruction, and genotype.
Meaning
Strategies aimed at controlling obesity via proactive counselling should be part of daily clinical practice and risk factor control.
This cohort study describes the association of body mass index with long-term outcomes in patients with hypertrophic cardiomyopathy in terms of overall disease progression, heart failure symptoms, and arrhythmias.
Abstract
Importance
Patients with hypertrophic cardiomyopathy (HCM) are prone to body weight increase and obesity. Whether this predisposes these individuals to long-term adverse outcomes is still unresolved.
Objective
To describe the association of body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) with long-term outcomes in patients with HCM in terms of overall disease progression, heart failure symptoms, and arrhythmias.
Design, Setting, and Participants
In this cohort study, retrospective data were analyzed from the ongoing prospective Sarcomeric Human Cardiomyopathy Registry, an international database created by 8 high-volume HCM centers that includes more than 6000 patients who have been observed longitudinally for decades. Records from database inception up to the first quarter of 2018 were analyzed. Patients were divided into 3 groups according to BMI class (normal weight group, <25; preobesity group, 25-30; and obesity group, >30). Patients with 1 or more follow-up visits were included in the analysis. Data were analyzed from April to October 2018.
Exposures
Association of baseline BMI with outcome was assessed.
Main Outcome and Measures
Outcome was measured against overall and cardiovascular mortality, a heart failure outcome (ejection fraction less than 35%, New York Heart Association class III/IV symptoms, cardiac transplant, or assist device implantation), a ventricular arrhythmic outcome (sudden cardiac death, resuscitated cardiac arrest, or appropriate implantable cardioverter-defibrillator therapy), and an overall composite outcome (first occurrence of any component of the ventricular arrhythmic or heart failure composite end point, all-cause mortality, atrial fibrillation, or stroke).
Results
Of the 3282 included patients, 2019 (61.5%) were male, and the mean (SD) age at diagnosis was 47 (15) years. These patients were observed for a median (interquartile range) of 6.8 (3.3-13.3) years. There were 962 patients in the normal weight group (29.3%), 1280 patients in the preobesity group (39.0%), and 1040 patients in the obesity group (31.7%). Patients with obesity were more symptomatic (New York Heart Association class of III/IV: normal weight, 87 [9.0%]; preobesity, 138 [10.8%]; obesity, 215 [20.7%]; P < .001) and more often had obstructive physiology (normal weight, 201 [20.9%]; preobesity, 327 [25.5%]; obesity, 337 [32.4%]; P < .001). At follow-up, obesity was independently associated with the HCM-related overall composite outcome (preobesity vs normal weight: hazard ratio [HR], 1.102; 95% CI, 0.920-1.322; P = .29; obesity vs normal weight: HR, 1.634; 95% CI, 1.332-1.919; P < .001) and the heart failure composite outcome (preobesity vs normal weight: HR, 1.192; 95% CI, 0.930-1.1530; P = .20; obesity vs normal weight: HR, 1.885; 95% CI, 1.485-2.393; P < .001) irrespective of age, sex, left atrium diameter, obstruction, and genetic status. Obesity increased the likelihood of atrial fibrillation but not of life-threatening ventricular arrhythmias.
Conclusions and Relevance
Obesity is highly prevalent among patients with HCM and is associated with increased likelihood of obstructive physiology and adverse outcomes. Strategies aimed at preventing obesity and weight increase may play an important role in management and prevention of disease-related complications.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic disorder of the myocardium.1 Signs and symptoms of the disease are highly variable. A complex and incompletely understood interaction of genotype and environmental factors ultimately determines phenotype and clinical course.2,3 In this context, obesity, a highly prevalent risk factor for cardiovascular disease, has been shown to be significantly associated with left ventricular (LV) mass and symptom severity.4 However, whether body weight is associated with disease course and outcomes in patients with HCM remains unclear.
Recently, results from the international Sarcomeric Human Cardiomyopathy Registry (SHARE) provided in-depth insights into novel aspects of the natural history and genotype-phenotype correlations of HCM based on thousands of patients enrolled and actively observed at specialized centers worldwide.5 Therefore, we chose to address the association of body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) with the long-term prognosis of patients with HCM in SHARE. Specifically, we aimed to assess whether excess body weight was a determinant of adverse outcomes in terms of HCM-related events ranging from heart failure (HF) to life-threatening arrhythmias.
Methods
SHARE and Participating Sites
The Sarcomeric Human Cardiomyopathy Registry is an international database generated by the clinical activity of 8 high-volume HCM centers, updated quarterly. For the purpose of this analysis, records from database inception up to the end of March 2018 were analyzed. Baseline clinical features and natural history of the SHARE HCM population have been published recently.5 The study conforms to the principles of the Declaration of Helsinki,6 and the local institutional review boards approved the study protocol. All study participants provided oral informed consent to participate in SHARE, and deidentified data were used in this study.
Study Population
We retrospectively reviewed the clinical records of all patients in SHARE who met the following inclusion criteria: (1) site-designated diagnosis of HCM, defined by the presence of increased asymmetric LV wall thickness of 13 mm or greater in the absence of abnormal loading conditions, in patients 18 years and older1; (2) availability of BMI4 at first evaluation; and (3) presence of at least 2 SHARE site contacts at least 1 year apart.
Clinical records included sex, age at diagnosis, age at clinical evaluation, baseline New York Heart Association (NYHA) class, 12-lead electrocardiography, 24-hour monitoring, and echocardiography. The eFigure in the Supplement summarizes the patient selection process. Among the 4317 patients with HCM enrolled in SHARE 18 years and older with weight and height data available, 3282 (76.0%) met the inclusion criteria and constitute the study population. Patients were classified into 3 groups according to BMI at first visit: a normal weight group (BMI <25), a preobesity group (BMI of 25 to 30), and an obesity group (BMI >30).
Definition of Study Outcomes
All-cause and cardiovascular mortality (defined as sudden cardiac death, HF-related death, aborted cardiac arrest, or heart transplant) were the primary outcomes. Three prespecified composite end points, which had been previously identified and published separately,5 were defined as follows:
Overall composite outcome: first occurrence of any component of the ventricular arrhythmic or HF composite end point (without inclusion of LV ejection fraction), all-cause mortality, atrial fibrillation (AF), and stroke.
Heart failure composite outcome: LV ejection fraction less than 35%, NYHA class III/IV symptoms, cardiac transplant, or LV assist device implantation.
Ventricular arrhythmic composite outcome: first occurrence of sudden cardiac death, resuscitated cardiac arrest, or appropriate implantable cardioverter-defibrillator therapy.
Statistical Analysis
Continuous variables, reported as means with standard deviations or as medians with interquartile ranges for nonnormal distributions, were compared between groups with t tests or nonparametric tests, as appropriate. Categorical variables, reported as counts and percentages, were compared between groups with χ2 tests or Fisher exact tests when any expected cell count was less than 5.
All time-to-event analyses were performed to the time that a patient first fulfilled any of the components of the outcome of relevance (the overall, HF, or ventricular arrhythmia composite outcome). Patients who met the end points prior or at the time of initial evaluation were excluded from survival analyses.
Survival analyses were performed on patients who had follow-up data for 1 year or more. A specific ancillary analysis was limited to patients with positive results on genetic testing (ie, with a pathogenic/likely pathogenic [P/LP] variant). In addition, we assessed the association of BMI with the overall composite end point against genetic status (P/LP sarcomeric variants vs genotype negative). Cox multivariable regression analysis (variable selection method with backward stepwise elimination) was performed including all candidate variables (P < .10 at univariate analysis). A 2-sided P value less than .05 was considered statistically significant. All analyses were performed using SPSS Statistics for Macintosh version 25.0 (IBM).
Results
Prevalence of Preobesity and Obesity in HCM
Of the 3282 included patients, 2019 (61.5%) were male, and the mean (SD) age at diagnosis was 47 (15) years. There were 962 patients in the normal weight group (29.3%), 1280 patients in the preobesity group (39.0%), and 1040 patients in the obesity group (31.7%). Patients with normal weight were younger at diagnosis and were more often women (Table 1). A total of 757 patients with obesity (72.8%) were enrolled in US SHARE sites, which showed a 42.3% prevalence of obesity (757 of 1788) compared with 18.9% for the non-US sites (283 of 1494) (P < .001). Patients in the preobesity and obesity groups were more often symptomatic (NYHA class III/IV symptoms: normal weight, 87 [9.0%]; preobesity, 138 [10.8%]; obesity, 215 [20.7%]; P < .001) and had a higher prevalence of hypertension and diabetes (Table 1).
Table 1. Baseline Clinical Characteristics of Patients With 1 Year or More of Follow-up by Body Mass Index (BMI) Category.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Overall (N = 3282) | BMI Categorya | ||||
| Normal Weight (n = 962) | Preobesity (n = 1280) | Obesity (n = 1040) | |||
| US SHARE site | 1788 (54.7) | 379 (39.4) | 652 (50.9) | 757 (72.8) | <.001 |
| Age at diagnosis, mean (SD), y | 47 (15) | 45 (15) | 48 (15) | 47 (14) | <.001 |
| BMI, mean (SD) | 28 (6) | 23 (2) | 27 (1) | 35 (5) | <.001 |
| Male | 2019 (61.5) | 497 (51.7) | 893 (69.7) | 629 (60.5) | <.001 |
| Race | <.001 | ||||
| White | 2642 (80.5) | 785 (81.6) | 1052 (82.2) | 805 (77.4) | |
| Black | 128 (3.9) | 25 (2.6) | 31 (2.4) | 72 (6.9) | |
| Asian | 85 (2.6) | 40 (4.2) | 33 (2.6) | 12 (1.2) | |
| Other | 427 (13.0) | 112 (11.6) | 164 (12.8) | 151 (14.5) | |
| NYHA class III/IV symptoms | 440 (13.4) | 87 (9.0) | 138 (10.8) | 215 (20.7) | <.001 |
| Hypertension | 1279 (38.9) | 260 (27.0) | 483 (37.7) | 536 (51.5) | <.001 |
| Diabetes | 306 (9.3) | 44 (4.6) | 95 (7.4) | 167 (16.1) | <.001 |
| Genetic testing | 2260 (68.9) | 668 (69.4) | 884 (69.1) | 708 (68.1) | .27 |
| P/LP variant | 1035 (45.7) | 358 (53.5) | 418 (47.2) | 259 (36.5) | <.001 |
| Variant of unknown significance | 199 (8.8) | 62 (9.2) | 67 (7.6) | 70 (9.9) | |
| SCD family history | 727 (22.2) | 212 (22.0) | 283 (22.1) | 232 (22.3) | >.99 |
| Echocardiographic parameters | |||||
| LAD, mean (SD), mm | 45 (10) | 42 (10) | 45 (10) | 48 (10) | <.001 |
| Maximum LVWT, mean (SD), mm | 18 (5) | 17 (5) | 18 (5) | 18 (5) | .01 |
| LVEF, mean (SD), % | 65 (9) | 65 (9) | 65 (8) | 66 (9) | .11 |
| LVOT obstruction, median (IQR), mm Hg | |||||
| At rest | 16 (7-39) | 13 (6-26) | 14 (7-36) | 21 (9-51) | <.001 |
| Valsalva maneuver | 34 (10-75) | 20 (8-59) | 30 (10-73) | 54 (17-86) | <.001 |
| Obstructive | 865 (26.4) | 201 (20.9) | 327 (25.5) | 337 (32.4) | <.001 |
Abbreviations: IQR, interquartile range; LAD, left atrial diameter; LVEF, left ventricle ejection fraction; LVOT, left ventricle outflow tract; LVWT, left ventricle wall thickness; NYHA, New York Heart Association; P/LP, pathogenic/likely pathogenic; SCD, sudden cardiac death; SHARE, Sarcomeric Human Cardiomyopathy Registry.
Calculated as weight in kilograms divided by height in meters squared. Normal weight defined as a BMI less than 25; preobesity, a BMI from 25 to 30; and obesity, a BMI greater than 30.
Genetic testing was performed in 2260 patients (68.9%). Overall, the prevalence of P/LP variants was 45.8% (1035 of 2260), and the prevalence of variants of unknown significance was 8.8% (199 of 2260), respectively. Baseline clinical characteristics of patients with P/LP variants were similar to the overall HCM cohort (eTable 1 in the Supplement). The prevalence of P/LP variants was significantly greater among patients with normal weight compared with those with preobesity and obesity (Table 1).
Association of BMI With Dynamic Outflow Tract Obstruction
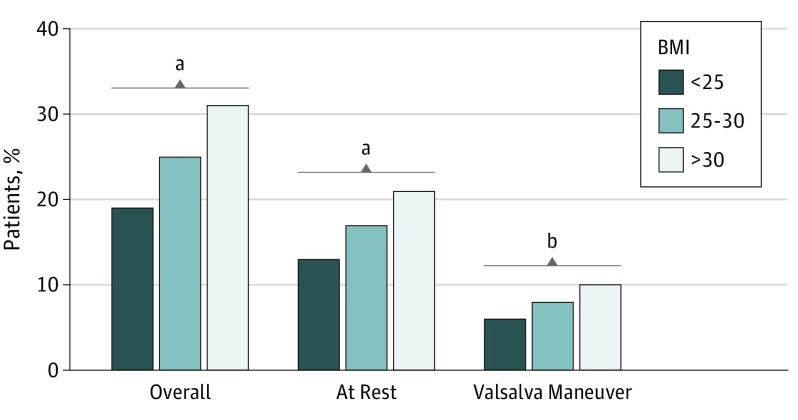
At echocardiographic evaluation, patients with obesity more often had LV outflow tract (LVOT) obstruction, both at rest and with Valsalva maneuver (Table 1) (Figure 1). Overall, the prevalence of resting or inducible LVOT obstruction was 20.9% (201 of 962) in the normal weight group, 25.5% (327 of 1280) in the preobesity group, and 32.4% (337 of 1040) in the obesity group (P < .001). Left atrial diameter and LV septal thickness were greater with increasing BMI class, whereas LV ejection fraction was comparable across groups.
Figure 1. Prevalence of Left Ventricular Outflow Tract Obstruction at Baseline by Body Mass Index (BMI) Class.
Body mass index calculated as weight in kilograms divided by height in meters squared.
aP < .001.
bP < .05.
Consistent with higher prevalence of LVOT obstruction, referral to septal reduction therapies (ie, surgical myectomy, alcohol septal ablation, or a combination of both) was higher in patients in the preobesity and obesity groups (normal weight, 157 [16.3%]; preobesity, 278 [21.7%]; obesity, 330 [31.7%]; P = .001). Symptomatic benefit at last SHARE site contact following these procedures was similar (NYHA class I/II symptoms: normal weight, 122 of 157 [77.7%]; preobesity, 223 of 278 [80.2%]; obesity, 254 of 330 [77.0%]; P = .62).
Association of Obesity With Outcome
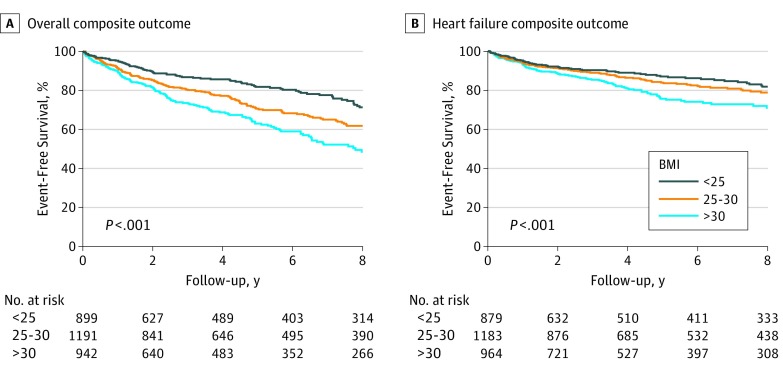
Patients were observed for a median (interquartile range) of 6.8 (3.3-13.3) years. At last evaluation, patients with obesity were more frequently symptomatic (NYHA class III/IV symptoms: normal weight, 100 [10.4%]; preobesity, 139 [10.9%]; obesity, 166 [16.0%]; P = .001). The overall composite and the HF composite end points occurred more frequently in patients with obesity (Table 2) (Figure 2), reflecting greater disease burden linked to obesity (overall composite outcome: normal weight, 402 [41.8%]; preobesity, 580 [45.3%]; obesity, 574 [55.2%]; P < .001; HF composite outcome: normal weight, 178 [18.5%]; preobesity, 254 [19.8%]; obesity, 311 [29.9%]; P < .001). On Cox multivariable analysis (Table 3), BMI class was independently associated with the overall composite end point after adjustment for age at diagnosis, sex, LVOT obstruction, and left atrial size (preobesity vs normal weight: hazard ratio [HR], 1.102; 95% CI, 0.920-1.322; P = .29; obesity vs normal weight: HR, 1.634; 95% CI, 1.332-1.919; P < .001). Body mass index was also associated with the HF composite outcome (preobesity vs normal weight: HR, 1.192; 95% CI, 0.930-1.530; P = .20; obesity vs normal weight: HR, 1.885; 95% CI, 1.485-2.393; P < .001). Conversely, occurrence of the ventricular arrhythmic end point was similar among BMI subgroups (Table 3). Of note, similar outcomes were captured when patients with P/LP variants were analyzed separately (eTables 2 and 3 in the Supplement), and mutational status overall did not affect the association of obesity with outcome (eTable 4 in the Supplement).
Table 2. Clinical Outcomes of Patients With 1 Year or More of Follow-up by Body Mass Index (BMI) Category.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Overall (N = 3282) | BMI Categorya | ||||
| Normal Weight (n = 962) | Preobesity (n = 1280) | Obesity (n = 1040) | |||
| Age at last visit, mean (SD), y | 55 (15) | 53 (17) | 56 (15) | 55 (13) | <.001 |
| Length of follow-up, median (IQR), y | 6.8 (3.3-13.3) | 6.9 (3.3-13.5) | 7.1 (3.2-12.6) | 5.3 (2.8-10.1) | .002 |
| NYHA class III/IV symptomsb | 405 (12.3) | 100 (10.4) | 139 (10.9) | 166 (16.0) | <.001 |
| SRTs | 765 (23.3) | 157 (16.3) | 278 (21.7) | 330 (31.7) | <.001 |
| NYHA class III/IV symptomsb | 166 (21.7) | 35 (22.3) | 55 (19.8) | 76 (23.0) | .62 |
| ICD | 757 (23.1) | 203 (21.1) | 274 (21.4) | 280 (26.9) | .001 |
| Appropriate discharge | 86 (11.4) | 26 (12.8) | 33 (12.0) | 27 (9.6) | .26 |
| Clinical outcomes | |||||
| Atrial fibrillation | 717 (21.9) | 186 (19.3) | 281 (21.9) | 250 (24.2) | .03 |
| Syncope | 295 (9.0) | 109 (11.3) | 104 (8.1) | 82 (7.8) | .008 |
| TIA/stroke | 200 (6.1) | 52 (5.4) | 82 (6.4) | 66 (6.4) | .54 |
| Death | 206 (6.3) | 71 (7.4) | 76 (5.9) | 59 (5.7) | .15 |
| CVD death | 90 (2.7) | 28 (2.9) | 33 (2.6) | 29 (2.8) | .88 |
| Composite outcome | |||||
| HF | 743 (22.6) | 178 (18.6) | 254 (19.8) | 311 (30.0) | <.001 |
| Overall | 1556 (47.4) | 402 (41.8) | 580 (45.3) | 574 (55.2) | <.001 |
| Arrhythmia | 177 (5.4) | 56 (5.8) | 64 (5.0) | 57 (5.5) | .70 |
Abbreviations: CVD, cardiovascular disease; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; NYHA, New York Heart Association; SRT, septal reduction therapy; TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared. Normal weight defined as a BMI less than 25; preobesity, a BMI from 25 to 30; and obesity, a BMI greater than 30.
Data taken from last contact.
Figure 2. Kaplan-Meier Analysis of the Overall Composite and Heart Failure Composite Outcomes.
Body mass index (BMI) calculated as weight in kilograms divided by height in meters squared. Analysis of nonsignificant differences in ventricular arrhythmias composite end point was not plotted.
Table 3. Cox Multivariable Regression Models for Patients With 1 Year or More of Follow-up.
| Variable | Overall Composite Outcome | HF Composite Outcome | Atrial Fibrillation | Ventricular Arrhythmia Composite Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| BMIa | ||||||||
| Preobesity vs normal weight | 1.102 (0.920-1.322) | <.001 | 1.192 (0.930-1.530) | <.001 | 1.067 (0.846-1.345) | .006 | NA | NA |
| Obesity vs normal weight | 1.634 (1.332-1.919) | 1.885 (1.485-2.393) | 1.416 (1.115-1.798) | NA | NA | |||
| Age at diagnosis (per y increase) | 1.038 (1.032-1.043) | <.001 | 1.034 (1.027-1.041) | <.001 | 1.039 (1.031-1.046) | <.001 | 1.019 (1.001-1.038) | .04 |
| Sex (men vs women) | 0.808 (0.700-0.931) | .003 | 0.609 (0.506-0.732) | <.001 | NA | NA | NA | NA |
| Race (white vs nonwhite) | 0.927 (0.771-1.115) | .42 | NA | NA | NA | NA | NA | NA |
| LAD | 1.021 (1.014-1.027) | <.001 | 1.024 (1.016-1.032) | <.001 | 1.026 (1.018-1.034) | <.001 | 1.032 (1.011-1.053) | .003 |
| Obstruction (yes vs no)b | 1.554 (1.345-1.795) | <.001 | 1.894 (1.548-2.224) | <.001 | 1.286 (1.060-1.560) | .01 | NA | NA |
Abbreviations: BMI, body mass index; HF, heart failure; HR, hazard ratio; LAD, left atrial diameter; NA, not applicable.
Calculated as weight in kilograms divided by height in meters squared. Normal weight defined as a BMI less than 25; preobesity, a BMI from 25 to 30; and obesity, a BMI greater than 30.
Defined as a left ventricular outflow tract gradient greater than 30 mm Hg.
When assessing the individual causes of death, no difference was found in all-cause mortality, cardiovascular mortality, or the ventricular arrhythmic composite end point based on BMI class (Table 2) (Figure 2). Implantable cardioverter-defibrillator use was higher in patients with obesity, but there was no difference in the rate of appropriate shocks across BMI classes (Table 2).
Patients in the preobesity and obesity groups had a higher prevalence of AF (Table 2), although when the analysis was confined to genotyped patients with P/LP variants, prevalence of AF did not differ across BMI classes (normal weight, 102 of 358 [28.5%]; preobesity, 120 of 418 [28.7%]; obesity, 89 of 259 [34.4%]; P = .14). On multivariable analysis, obesity was independently associated with AF (preobesity vs normal weight: HR, 1.067; 95% CI, 0.846-1.345; P = .58; obesity vs normal weight: HR, 1.416; 95% CI, 1.115-1.798; P = .004). By contrast, syncope was inversely associated with BMI, being more prevalent in patients with normal weight (normal weight, 109 [11.3%]; preobesity, 104 [8.1%]; obesity, 82 [7.9%]; P = .008).
Discussion
In this multicenter HCM study from SHARE, the prevalence of patients with preobesity and obesity reached 70%, comparable with previously reported HCM cohorts7 and populations with HF with preserved ejection fraction (such as the Irbesartan in Heart Failure With Preserved Systolic Function study8 or the Get With The Guidelines–Heart Failure registry9). This trend showed a clear predominance in US-based SHARE sites, where frank obesity reached 42.3%, clearly in excess compared with the estimates for the general US population.10 Of further concern, this population was younger than previous cohorts at the time of diagnosis, with a mean (SD) age of 47 (15) years, suggesting an acceleration of this trend.
Patients with a BMI greater than 25 were not only more symptomatic, consistent with prior evidence,4 but also manifested higher rates of hypertension, diabetes, AF (often a turning point in the natural history of the disease), and LVOT obstruction. These findings were similar in the subgroup of patients with HCM carrying P/LP mutations compared with genotype-negative individuals, suggesting little influence of genetic background on HCM phenotype response to obesity. Most importantly, over a median follow-up of 6.8 years, patients with HCM with a BMI greater than 25 were more likely to incur adverse outcomes, with more than 50% meeting the overall composite end point and almost one-third developing HF-related complications.
The association of weight with prognosis was confirmed on multivariable analysis, where obesity was independently associated with overall disease progression, irrespective of other well-known factors, such as age, sex, left atrial size, LVOT obstruction, and genotype. By contrast, preobesity and obesity were not associated with increased likelihood of hard end points, such as appropriate implantable cardioverter-defibrillator discharge, cardiovascular death, or all-cause mortality. However, far from being reassuring, failure to identify excess mortality likely reflects low event rates in HCM populations and the need for longitudinal studies with longer follow-up.5
Only with specific regard to syncope, we observed an apparently counterintuitive decrease in rate among patients with greater BMI. However, this finding is not unexpected when one considers that the most common cause of syncope, even in patients with HCM, is neuromediated rather than cardiogenic. Obesity may well exert a protective effect from the former owing to an associated hypervolemic state, sympathetic and neurohormonal activation, and high blood pressure levels.11
Causes of Obesity in HCM
The 70% prevalence of patients with excess body weight, including 39% with preobesity and 32% with obesity, is of serious concern. Although obesity represents a universally increasing problem in developed countries, patients with HCM appear to be disproportionally affected compared with the general population across the whole spectrum of the disease and irrespective of genotype. The excess prevalence in US SHARE sites compared with non-US SHARE sites likely emphasizes the relevance of an environmental milieu, including dietary and lifestyle issues. Notably, there are established differences in lifestyle practices between patients with HCM and the general population.7 Patients interviewed in a 2013 survey derived by the National Health and Nutrition Examination Survey7 were found to spend less time in recreational activity or engaging in work than the average individual—and even when they did engage in exercise, the reported duration and frequency was significantly lower. Psychological issues related to the diagnosis of genetic heart disease are also likely to play a role; although self-imposed restrictions in physical exercise negatively influenced patients’ emotional health, almost two-thirds of respondents felt they were necessary after a diagnosis of HCM, especially with a family history of sudden cardiac death.7
These lifestyle modifications following HCM diagnosis understandably reflect the need to adapt to a long-term cardiac disease. However, the potential consequences of an unhealthy lifestyle are equally important and include increased prevalence of cardiovascular risk factors, such as obesity, diabetes, hypertension, and dyslipidemia. Notably, patients with HCM often fail to undertake even basic recommended levels of physical activity because of perceived barriers regarding their personal health12 as well as restrictions often recommended by their health care professionals.
Implications for Management
Overall, our observations strongly emphasize the need for specific exercise programs and lifestyle interventions in patients with HCM, often quite capable of exercising regularly and safely.13 The culture of physical fitness and cardiovascular prevention often is considered a low priority in patients with HCM because of greater focus on other management issues and concerns over sports-associated hazards. Our study, combined with recent literature, suggests that it is high time to change this way of thinking in the pursuit of a global health management for patients with HCM.
As a case in point, in 2017, moderate-intensity aerobic training was shown to increase peak oxygen consumption in patients with HCM without signals for harm.13 Therefore, low-intensity to moderate-intensity physical activity should be encouraged as a means to improve physical functioning as well as weight control.14,15 Strategies aimed at prevention and treatment of comorbidities, such as hypertension, diabetes, and coronary artery disease, are also critical.2 Specialized centers should advocate an individually tailored approach whereby newly diagnosed patients with HCM receive counselling regarding lifestyle modification to achieve an optimal control of modifiable cardiovascular risk factors and psychosocial adjustment. Strategies involving novel pharmacological possibilities16,17 and, in selected patients with HCM with morbid obesity, aggressive treatment, even with bariatric surgery, should be considered to reduce the risk of disease-related complications.18
Association of Obesity With LVOT Obstruction and AF
The prevalence of LVOT obstruction was greater in patients with preobesity and obesity, both at rest and during Valsalva maneuver. Consistently, referral to septal reduction therapies (ie, surgical myectomy and alcohol septal ablation) was greater in patients with obesity and associated with favorable results. Although obesity is associated with increased total blood volume, which, in theory, should decrease LVOT obstruction (and may explain the lower prevalence of syncope in individuals with obesity), this aspect is likely outweighed by the augmented sympathetic tone and elevated abdominal and thoracic pressures associated with excess body weight.2,19,20 It is tempting to hypothesize that significant weight loss may reduce the prevalence of symptoms related to obstruction and the need for invasive therapies and exposure to interventional risk.
The increased incidence of AF observed in patients with obesity with HCM likely follows a similar pathophysiological mechanism; increase in LV wall stress and hypertrophy are known causes of LV diastolic dysfunction, resulting in increased left atrial pressure and volume.21 Longitudinal population studies have shown that BMI may be a key risk factor for progressive left atrial enlargement over time.22,23 Therefore, our data support the pursuit of strategies aimed at preventing obesity as a therapeutic target in the long-term management of HCM,24 including the control of inducible obstruction and prevention of arrhythmias.
Limitations
This study has limitations. This is a retrospective registry-based observational study where causal relationships between BMI and prognosis cannot be inferred. The registry unavoidably reflects variations in management owing to different practices and protocols. Nevertheless, we aimed to minimize such biases by adopting a standard follow-up protocol at each center with regular visits at 6 months to 1 year, which have been prospectively uploaded to the registry since its inception.
In this cohort, we observed a prevalence of obstruction of 26%, consistent with most previous reports based on real-world cohorts in which patients with HCM have not been systematically referred for exercise echocardiography.25 Therefore, the true prevalence of provocable gradients may have been underestimated.
Conclusions
Obesity is highly prevalent among patients with HCM and is associated with increased likelihood of LVOT obstruction and adverse outcomes, particularly owing to HF and AF. Strategies aimed at fostering healthier body weight, such as low-intensity to moderate-intensity exercise programs and nutritional counselling tailored for the individual patient, are warranted for prevention of disease-related complications.
eTable 1. Baseline clinical characteristics of patients with 1 year or more of follow-up by BMI category with pathogenic/likely pathogenic variants.
eTable 2. Clinical outcomes of patients with 1 year or more of follow-up by BMI category.
eTable 3. Cox multivariable regression models for patients with pathogenic/likely pathogenic variants with 1 or more years of follow-up.
eTable 4. Cox multivariable regression model for the overall composite end point for patients who performed a genetic test and had a single pathogenic/likely pathogenic mutation or were genotype negative with 1 year or more of follow-up.
eFigure. Flow diagram summarizing patient selection in SHARE (March 2018 registry update).
References
- 1.Elliott PM, Anastasakis A, Borger MA, et al. ; Authors/Task Force members . 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 2.Finocchiaro G, Magavern E, Sinagra G, et al. Impact of demographic features, lifestyle, and comorbidities on the clinical expression of hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;6(12):e007161. doi: 10.1161/JAHA.117.007161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749-770. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivotto I, Maron BJ, Tomberli B, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62(5):449-457. doi: 10.1016/j.jacc.2013.03.062 [DOI] [PubMed] [Google Scholar]
- 5.Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHARE). Circulation. 2018;138(14):1387-1398. doi: 10.1161/CIRCULATIONAHA.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 7.Reineck E, Rolston B, Bragg-Gresham JL, et al. Physical activity and other health behaviors in adults with hypertrophic cardiomyopathy. Am J Cardiol. 2013;111(7):1034-1039. doi: 10.1016/j.amjcard.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 8.Dalos D, Mascherbauer J, Zotter-Tufaro C, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68(2):189-199. doi: 10.1016/j.jacc.2016.04.052 [DOI] [PubMed] [Google Scholar]
- 9.Powell-Wiley TM, Ngwa J, Kebede S, et al. Impact of body mass index on heart failure by race/ethnicity from the Get With The Guidelines-Heart Failure (GWTG-HF) Registry. JACC Heart Fail. 2018;6(3):233-242. doi: 10.1016/j.jchf.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991-1006. doi: 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeting J, Ingles J, Timperio A, Patterson J, Ball K, Semsarian C. Physical activity in hypertrophic cardiomyopathy: prevalence of inactivity and perceived barriers. Open Heart. 2016;3(2):e000484. doi: 10.1136/openhrt-2016-000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA. 2017;317(13):1349-1357. doi: 10.1001/jama.2017.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saberi S, Day SM. Exercise and hypertrophic cardiomyopathy: time for a change of heart. Circulation. 2018;137(5):419-421. doi: 10.1161/CIRCULATIONAHA.117.029989 [DOI] [PubMed] [Google Scholar]
- 15.Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019;40(1):19-33. doi: 10.1093/eurheartj/ehy730 [DOI] [PubMed] [Google Scholar]
- 16.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 17.Pabel S, Wagner S, Bollenberg H, et al. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail. 2018;20(12):1690-1700. doi: 10.1002/ejhf.1328 [DOI] [PubMed] [Google Scholar]
- 18.Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. 2017;135(17):1577-1585. doi: 10.1161/CIRCULATIONAHA.116.025629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canepa M, Sorensen LL, Pozios I, et al. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112(8):1182-1189. doi: 10.1016/j.amjcard.2013.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. doi: 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 21.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70(16):2022-2035. doi: 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 22.McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121(5):667-674. doi: 10.1161/CIRCULATIONAHA.109.885806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471-2477. doi: 10.1001/jama.292.20.2471 [DOI] [PubMed] [Google Scholar]
- 24.Olivotto I, Marchionni N. No heart is an island: hypertrophic cardiomyopathy, diabetes, and the test of time. Eur Heart J. 2019;40(21):1678-1680. doi: 10.1093/eurheartj/ehy732 [DOI] [PubMed] [Google Scholar]
- 25.Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232-2239. doi: 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline clinical characteristics of patients with 1 year or more of follow-up by BMI category with pathogenic/likely pathogenic variants.
eTable 2. Clinical outcomes of patients with 1 year or more of follow-up by BMI category.
eTable 3. Cox multivariable regression models for patients with pathogenic/likely pathogenic variants with 1 or more years of follow-up.
eTable 4. Cox multivariable regression model for the overall composite end point for patients who performed a genetic test and had a single pathogenic/likely pathogenic mutation or were genotype negative with 1 year or more of follow-up.
eFigure. Flow diagram summarizing patient selection in SHARE (March 2018 registry update).