Abstract
In patients with gliomas, changes in hemispheric specialization for language determined by magnetoencephalography (MEG) were analyzed to elucidate the impact of treatment and tumor recurrence on language networks. Demonstration of reorganization of language networks in these patients has significant implications on the prevention of postoperative functional loss and recovery. Whole‐brain activity during an auditory verb generation task was estimated from MEG recordings in a group of 73 patients with recurrent gliomas. Hemisphere of language dominance was estimated using the language laterality index (LI), a measure derived from the task. The initial scan was performed prior to resection; patients subsequently underwent surgery and adjuvant treatment. A second scan was performed upon recurrence prior to repeat resection. The relationship between the shift in LI between scans and demographics, anatomic location, pathology, and adjuvant treatment was analyzed. Laterality shifts were observed between scans; the median percent change was 29.1% across all patients. Laterality shift magnitude and relative direction were associated with the initial position of language dominance; patients with increased lateralization experienced greater shifts than those presenting more bilateral representation. A change in LI from left or right to bilateral (or vice versa) occurred in 23.3% of patients; complete switch occurred in 5.5% of patients. Patients with tumors within the language‐dominant hemisphere experienced significantly greater shifts than those with contralateral tumors. The majority of patients with glioma experience shifts in language network organization over time which correlate with the relative position of language lateralization and tumor location.
Keywords: brain tumor, glioma, language dominance, language laterality index, language lateralization, magnetoencephalography, neurosurgery, recurrence, verb generation
Abbreviations
- fMRI
functional magnetic resonance imaging
- IAP
intracarotid amobarbital procedure
- IES
intraoperative electrical stimulation
- LI
laterality index
- meg
magnetoencephalography
- MRI
magnetic resonance imaging
- MSI
magnetic source imaging
- nTMS
navigated transcranial magnetic stimulation
- Pseudo‐F
pseudo‐F‐value; statistic expressed in logarithmic values (dB)
- TMS
transcranial magnetic stimulation
- VOI
volume of interest
1. INTRODUCTION
The guiding principle of glioma surgery is to maximize tumor resection while preserving function (Bowyer et al., 2005; Tarapore et al., 2012). In patients with lesions in presumed‐eloquent language cortex, safe tumor removal relies on distinguishing critical functional regions from noncritical surrounding tissue. Using this process, known as language mapping, it becomes possible to remove infiltrating tumors from within anatomic language cortex without causing new deficits. It is sometimes the case that residual tumor is knowingly left behind to preserve neurological function. In these patients, as well as in those with recurrent tumor, it has been widely reported that, over time, the neural circuitry supporting language function can reorganize and the location of critical cortical sites can change, often referred to as functional plasticity (Duffau, Bauchet, Lehéricy, & Capelle, 2001; Duffau, Denvil, & Capelle, 2002).
Currently, the extent to which language reorganization takes place and the factors that influence it are not well understood. In this study, we focused on shifts in language laterality, a calculated, unitless, global measure of hemispheric language dominance that incorporates measures of activation for both receptive and expressive language circuitry. The lateralization techniques used during this study were based on magnetoencephalography (MEG) recordings and were developed to correlate most closely with the Wada test, or intracarotid amobarbital procedure (IAP), outcomes, as these represent the accepted gold standard.
To determine language laterality via MEG, a verb generation task was conducted during the scan. Neural activations were recorded and characterized using a language laterality index (LI), a measure that incorporates activations in brain regions associated with expressive and receptive language functions and establishes the hemisphere of language dominance. The language LI has been previously validated against the IAP (Findlay et al., 2012; Hirata et al., 2004), and was found to produce optimal results when both receptive and expressive regions, known as volumes of interest (VOI), and localization relative to receptive (stimulus‐locked) and expressive (response‐locked) portions of the task were taken into account (Findlay et al., 2012). Because our language lateralization algorithm is MEG‐based, it has the advantage of being noninvasive (and thus not subject to the complications of the IAP; Wada, 1949). In most cases, the language LI can replace the IAP entirely, and is frequently incorporated into the standard presurgical imaging battery (Breier, Billingsley‐Marshall, Pataraia, Castillo, & Papanicolaou, 2006; Doss, Zhang, Risse, & Dickens, 2009; Frye, Rezaie, & Papanicolaou, 2009; Pang, Wang, Malone, Kadis, & Donner, 2011; Passaro et al., 2011). By repeating the same verb generation task at each scan, we are able to compare subsequent scans to identify changes in language laterality, over time, on a patient‐by‐patient basis.
In this study, our primary objective was to assess how language laterality changes over time in patients with glioma. We predicted that tumors ipsilateral to language dominance would drive language to a more bilateral representation. Our secondary objective was to identify factors (demographic, pathologic, anatomic, and so forth) that influence changes in language laterality. To the best of our knowledge, this is the first and largest study to examine the evolution of language laterality utilizing MEG in patients with glioma.
2. METHODS
2.1. Study design
All patients with primary brain tumor who were referred for MEG imaging at the University of California, San Francisco (UCSF) Biomagnetic Imaging Lab between June 2005 and October 2015 were considered for this study. All selected patients received a subsequent craniotomy with intraoperative electrical stimulation (IES) mapping and tumor resection by a single neurosurgeon (M.S.B.). The experimental protocol of this study was approved by the Committee on Human Research of the University of California, San Francisco, registration number 11‐05249, and all research was conducted according to the Declaration of Helsinki. Written, informed consent was received from all patients.
2.2. Patient characteristics
From the aforementioned patient group, a population of 99 patients was retrospectively assembled to include patients with recurrent glioma and at least two serial MEG scans. From this subgroup, patients were identified who presented with primary gliomas located within language, motor, or sensory cortices. Each patient completed their first scan within 2–3 days of initial resection. Subsequent scans were also conducted within 2–3 days of repeat resections in case of recurrence. The anatomic region of tumor involvement was determined based upon the first scan results. MEG scanning, as a part of a preoperative assessment of functionality, was routinely scheduled 1–3 days before tumor resection. Patients were primarily excluded on criteria related to their MEG scan: 14 patients were discounted as a result of missing or incomplete available data; 11 patients were removed due to the presence of significant artifact in MEG recordings of verb generation task, or low quality, incomplete, or absent MEG‐based language mapping; one patient was excluded for the presence of a bilateral tumor. After exclusion, a final patient population of 73 patients was identified. For a subset of the analysis comparing relative tumor and lateralization position to LI shift, designated the relative position analysis, an additional eight patients were excluded due to presentation of bilateral language function (as indicated by the laterality index), refining the patient population to 65 patients in total, for this section only. All patients experienced tumor recurrence and underwent at least two preoperative MEG scans.
Data was extracted from a total of 86 patients from APeX, the current EPIC based, electronic medical and health record system used by UCSF. Patient information gathered included gender, tumor side, handedness, dominance of tumor hemisphere, tumor location, tumor entity, patient's language deficit, and status postradiation or chemotherapy. Tumor size and location were revalidated by an experienced neurosurgeon using magnetic resonance imaging (MRI) files on a PACS radiological workstation. For the purposes of this study, tumor side, location, and pathology were determined according to the initial presentation. A history of radiation therapy or chemotherapy was considered positive if utilized at any time in the patient's history. Handedness was determined according to patient self‐reporting. Tumor entity was used to determine tumor grade, defined as low for grade I and II, and high for grade III and IV entities (WHO designations). Language dominant hemisphere was defined using the language laterality index. The terms language “dominance” and “lateralization” will be used interchangeably for the duration of this article.
At the first MEG scan, patients ranged in age from 18 to 69 years (mean age 42.5 ± 12.4 years) and from 22 to 70 years (mean age 45.4 ± 12.2 years) at the second. Time between MEG scans ranged from 63 and 3,018 days (mean days 1,057 ± 711 days), or, on average, a little under 3 years.
2.3. Data acquisition
2.3.1. Magnetic resonance imaging
Preoperative structural imaging was performed for each patient using MRI at 1.5 or 3 Tesla. MRI protocol routinely included two sequences: a T1‐weighted, three‐dimensional spoiled gradient‐recalled echo, steady‐state sequence with 34 ms TR, 3–8 ms TE, and flip angle of 30°, and a T2‐weighted, three‐dimensional fast spin‐echo sequence with 3,000 ms TR and 105 ms TE. Scan characteristics include slice thicknesses recorded at 1.5 mm, a matrix of 256 × 256 × (108–140), and a field view of 260 × 260 mm. Both sequences utilized skin‐to‐skin coverage to include anatomical landmarks including the nasion and preauricular points for later coregistration with MEG scans.
Each patient's MRI was normalized to a standard MNI template using SPM2 (Tzourio‐Mazoyer et al., 2002; http://www.fil.ion.co.uk/spm2). Normalization was performed using either the patient's high‐resolution T1 or T2 series. Normalization was visualized using SPM and checked by eye for proper normalization. We have found that normalization does fail for some tumors using the T1 series; in these cases, the T2 series can almost always be normalized satisfactorily. Anatomically based VOIs used for laterality analysis that is identical in size across patients were selected in the normalized brain. These VOIs were subsequently transformed back into patients' native space for LI analysis.
2.3.2. Magnetoencephalographic imaging (MEG recordings)
Magnetoencephalography (MEG) imaging was performed in a magnetically shielded room with a 275‐channel whole‐head CTF Omega 2000 system (CTF MEG International Services LP, Coquitlam, British Columbia, Canada), using a sampling rate of 1,200 Hz. MEG scans were recorded during resting state, while patients were awake with eyes closed, and active state periods, while patients were performing a task. Prior studies have described MEG imaging and recordings in detail (Babiloni, Pizzella, Gratta, Ferretti, & Romani, 2009; Baillet, 2017; Salmelin, 2007).
Head position, relative to the sensor array, was recorded before and after each scan using three fiducial coils placed on the nasion and 1 cm rostral to the left and right preauricular points in the direction of the nasion. Coregistration with a structural MRI was later completed using these points and a head shape was generated. All scans were completed with patients lying in a supine position having been instructed to remain awake with their eyes closed during resting state recordings and to perform an auditory verb generation task or other type task during active state recordings, with their eyes closed when possible.
MEG scans were recorded in resting state and during language, auditory, somatosensory, and motor tasks to locate respective brain functions. To detect language laterality, the patients performed an auditory verb generation language semantic association task. This task was designed to activate both the receptive and expressive language functions to account for the bilateral characteristics of language processing, and therefore prevent false determination of lateralization (Findlay et al., 2012; Papanicolaou et al., 2006). The general protocol of the verb generation task consisted of 100 nouns presented to the patient at 4 s intervals. Upon hearing the prompter nouns, patients were instructed to think of either a verb or “action word” associated with the noun and to respond vocally into a microphone located in the MEG chamber, as depicted in Figure 1. Analysis of the verb generation task data was completed by an MEG technologist under the guidance of a neuroscientist using adaptive spatial filtering (Dalal et al., 2008; Findlay et al., 2012; Hinkley, Nagarajan, Dalal, Guggisberg, & Disbrow, 2011).
Figure 1.
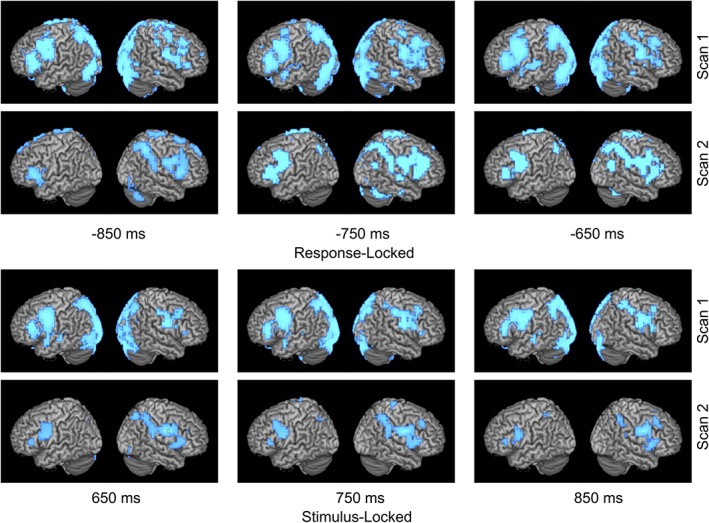
Left to right shift in language laterality. Time course of language network activation over two scans for patient with a left frontal glioma who experienced a rightward shift in LI of −0.416 (0.116 to −0.3) over 1 year. Activity is time‐locked to either stimulus or response onset. Combined LI score is calculated with both stimulation and response activations. At second scan for both response and stimulus locked conditions, larger area of activation (in blue) can be observed on right hemisphere compared to less area of activation on left hemisphere [Color figure can be viewed at http://wileyonlinelibrary.com]
The MEG data obtained during the language task was high pass filtered between 1 and 12 Hz before, and for the purpose of, artifact detection in cases where artifact was present across most trials. Artifact detection was completed through a visual examination of each individual trial. Trials with an eye blink, EMG artifact, interictal spiking, other obvious artifact, or speaking within 300 ms of initiation of the auditory stimulus presentation were removed from the analysis (Findlay et al., 2012).
2.3.3. Language laterality index
The present study located language function using a language laterality index (LI) derived from an MEG recorded during an auditory verb generation task, and subsequent imaging analysis of oscillatory activity (Findlay et al., 2012). CTF synthetic aperture magnetometry (SAM) analysis between 12 and 30 Hz, synchronized on either the auditory noun stimulus (stimulus‐locked) or the spoken verbal response (response‐locked), was performed over multiple 300 ms, active time windows to generate two pseudo‐F‐image time courses. The control period used was the 300 ms time window prior to noun stimulus (Findlay et al., 2012). The MEG imaging activations are reconstructed across the entire brain volume regardless of the presence of anatomic abnormalities. It is particularly important to incorporate the lesional area within the volume of interest (VOI) because, in these low‐grade lesions, infiltrated tissue often remains functional. Magnetic permeability is very similar between the brain, lesion, CSF, and skull; as a result, magnetic field distortion is very low across these tissues. A stimulus‐locked LI and response‐locked LI were calculated using the time points for each VOI that had previously been shown to most significantly indicating laterality as compared to the Wada test, and a comprehensive “combined” LI was calculated by averaging the stimulus and response‐locked LIs. The combined LI was used for analysis in the present study.
The LI was determined for each time‐frequency window for both analysis conditions using the average pseudo‐F‐value of each VOI, and was calculated using the following formula:
where “L” and “R” represent the average left VOI pseudo‐F‐value and average right VOI pseudo‐F‐value, respectively. An LI value of +1.0 indicates a relative beta band power decrease in the left hemisphere, and −1.0 indicates a relative beta band power decrease in the right hemisphere. The LI was derived assuming that a power decrease in the beta band frequency is an indicator for cortical activation and uneven hemispheric power decrease is a marker for function lateralization (Findlay et al., 2012; Hirata et al., 2004). Beta band analysis is well validated for language lateralization with MEG, as demonstrated previously by our group, as well as others (Findlay et al., 2012; Hinkley et al., 2016; Hirata et al., 2004).
Figures 1, 2, 3 demonstrate the time course of average verb generation beta‐power change for the stimulus‐ and response‐locked conditions used in determining language dominance for certain patients. Each image represents the average of every 300 ms epoch, with 100 ms steps, that overlaps with the labeled timepoint. For example, for the response‐locked analysis, “−850” consists of the average of three 300 ms epochs (−1,000 to −700, −900 to −600, −800 to −500). For a detailed discussion, refer to Dalal et al., 2008, and Hinkley et al., 2011. Although Figures 1, 2, 3 display whole brain activity, the VOI used in calculation of laterality index was more restricted. The VOI used for stimulus‐locked analysis included superior temporal gyrus and supramarginal gyrus; the VOI used for response‐locked included precentral, middle and inferior frontal gyri. An empirically determined combined LI threshold of ±0.1 was used to classify MEG results as left or right lateralized (Hirata et al., 2004). Therefore, an LI was categorized as right for LI < −0.1 or left for LI > +0.1. LIs were categorized as bilateral, or indeterminate, when LI fell between −0.1 and + 0.1. Percent changes in LI across scans were calculated as the absolute value of the LI shift between scans, divided by LI at first scan.
Figure 2.
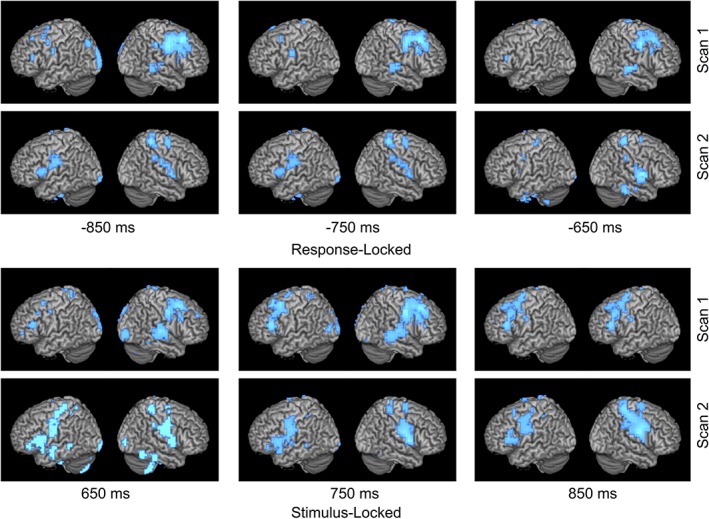
Right to left shift in language laterality. Time course of language network activation over two scans for patient with a left temporal glioma who experienced a leftward shift in LI of +0.67 (−0.557 to 0.113) over 5 years. Activity is time‐locked to either stimulus or response onset. Combined LI score is calculated with both stimulation and response activations. At second scan for both response and stimulus locked conditions, larger area of activation (in blue) can be observed on left hemisphere compared to less area of activation on right hemisphere [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
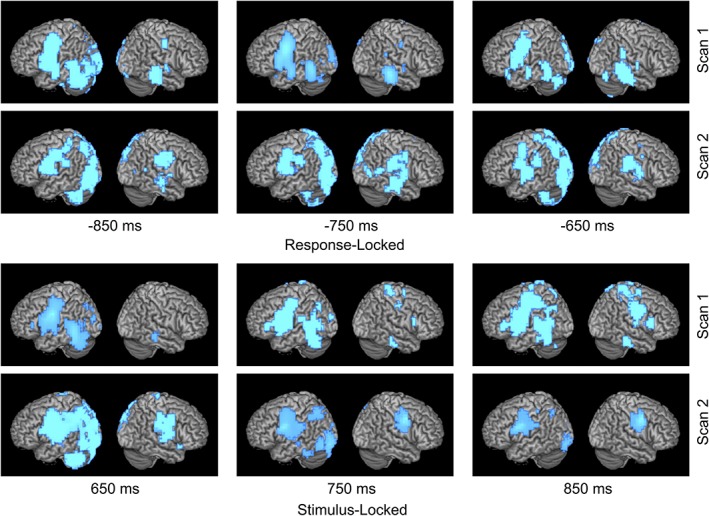
Left to bilateral shift in language laterality. Time course of language network activation over two scans for patient with a right insular glioma who experienced a bilateral (left to both hemispheres) shift in LI of −0.275 (0.34–0.065) over 3 years. Activity is time‐locked to either stimulus or response onset. Combined LI score is calculated with both stimulation and response activations. At second scan for both response and stimulus locked conditions, large areas of activation (in blue) can be observed on both left and right hemispheres [Color figure can be viewed at http://wileyonlinelibrary.com]
Activation images during the verb generation task for both the stimulus and response conditions were created for three patients; one who experienced a left to right shift (Figure 1), one with a right to left shift (Figure 2), and one with a left to bilateral shift (Figure 3). For these figures, power change display was thresholded at half of the absolute maximum pseudo‐F‐value over the shown time course. For the stimulus‐locked condition, time windows from 650 to 850 ms for VOI‐TP (STG, supramarginal gyrus) were used in determining language laterality. For the response‐locked condition, power change display was thresholded at half of the absolute maximum pseudo‐F‐value over the shown time course, and time windows from −850 to −650 ms for VOI‐F (IFG, MFG, precentral gyrus) were used in determining language laterality.
2.4. Statistical testing
Significance testing was completed for all variables relevant to the study. Independent variables included tumor location and side, tumor grade, language lateralization, age at first scan, time between scans, gender, and history of adjuvant chemotherapy or radiation. Derived independent variables included laterality shift between scans, laterality flip (indicates classification change between left, right, or bilateral lateralization between scans), relative tumor position (defined as tumor ipsilateral or contralateral to language dominance), and speed of plasticity (defined as laterality shift divided by time between scans). Date of MEG scan was also included as an independent covariate to account for evolution in the MEG scanning protocol. Furthermore, the relative value of the laterality shift (after correcting for right lateralized patients) was used to homogenize the experimental cohort to investigate the effect of relative tumor position and language lateralization on LI shift during the relative position analysis. In addition, eight patients who presented bilateral language function (defined by the laterality index) were excluded from the observational population, resulting in a total patient population of 65 patients. The data set was then reorganized to represent tumor location as either ipsi‐ or contralateral to language dominance (as opposed to left or right hemispheric), and to redefine relative laterality shift as either toward or away from tumor location (by setting the four right lateralized patients' values to their corresponding left lateralized values and defining negative LI shifts as “away from” and positive LI shifts as “toward” the region containing the tumor and language dominance). This process was only completed for the investigation of the impact of relative tumor location and initial language dominance on LI shift between scans (relative position analysis).
The independent variable of interest was laterality shift as measured by the language LI. t tests (independent samples or paired samples) were calculated dependent on the correlation between the two variables. A linear regression model was used to determine the correlation between the quantitative variables and laterality shift. Independent samples t tests were used to determine the relationship between tumor side and laterality shift, as well as relative tumor location (tumor ipsi‐ or contralateral to language location) and laterality shift. Percent changes in LI across scans were calculated as the absolute value of the LI shift between scans, divided by LI at first scan. Potential collinearity between independent variables was determined to be insignificant using a multiple regression model. A univariate regression model was then generated to analyze each of the independent variables to determine significance. A significance level of p < .05 was used. Statistical testing was conducted using SPSS Statistics (IBM SPSS Statistics, 64‐bit edition 2015, Version 23.0.0.0, Armonk, NY).
3. RESULTS
3.1. Patient characteristics
Retrospective analysis of our prospectively collected MEG database identified 99 patients who underwent repeat language testing with MEG between 2005 and 2015. The demographic, clinical, and pathological characteristics of the patients are outlined in Tables 1 and 2. Twenty‐six patients were excluded from the analysis due to unavailability or lack of data, inability to complete the verb generation task at each MEG, the presence of significant MEG artifact, or the presence of bilateral tumors. A more specific breakdown based on location of tumor as ipsilateral or contralateral to language dominance is displayed in Table 2. For the comparison of tumors, ipsi‐ and contralateral to lateralization and laterality shift, an additional eight patients were excluded for the presence of a bilateral LI, and resulted in a population of 65 patients for this relative position analysis only. The workflow behind patient selection and categorization is detailed in the Supporting Information Table S1. Further details regarding patient characteristics related to hemispheric glioma location, initial language lateralization, and vector of LI shift are available in the Supporting Information Table S2. The overall resulting population of 73 patients consisted of 32 women and 41 men, with a mean age of 42.5 years (range 18.9–69.6 years). All patients received a minimum of two MEG scans; 12 patients received 3 (16.4%). The median time between scans was 116 weeks (range 9–431 weeks, ±102 weeks), approximately just under 3 years. Of the 73 patients, 61 (83.6%) expressed left hemisphere language LI values, 4 (5.5%) expressed right hemisphere LI values, and 8 (11.0%) expressed a bilateral (or indeterminate) LI at the first MEG. This finding is consistent with past studies observing the majority of patients to present with left‐lateralized language function (Strauss & Wada, 1983). Patients self‐reported predominantly as right handed (82.2%); there were 11 left handed (15.1%) and two ambidextrous (2.7%) patients. Discounting the bilateral patients, tumors presented ipsilaterally to the hemisphere of initial language dominance for 61.5% (40 of 65) of patients and contralateral for 38.5% (25 of 65) patients. Sixty‐nine (94.5%) patients were observed to have only a single lesion; only two patients had multiple lesions (2.7%). Data on lesion count was unavailable for the remaining two patients. Of the 73 patients, 5 (6.8%) were reported to exhibit some level of language deficit prior to the first MEG scan, and 11 (15.1%) prior to the second; 60 (82.2%) patients had no recorded history of language deficit at either scan. All patients completed the auditory verb generation task sufficiently for language lateralization, a necessary inclusion criterion for the present study. Fifty‐one (69.9%) patients were noted to have received chemotherapy at any point during treatment.
Table 1.
Patient characteristics
| Number of patients | ||
|---|---|---|
| Total | n = 73 | |
| Gender | Female | 32 (44%) |
| Male | 41 (56%) | |
| Tumor side | Left | 48 (66%) |
| Right | 25 (34%) | |
| Handedness | Left | 11 (15%) |
| Right | 60 (82%) | |
| Ambidextrous | 2 (3%) | |
| Language dominant hemisphere (at first scan) | Left | 61 (84%) |
| Right | 4 (5%) | |
| Bilateral | 8 (11%) | |
| Tumor in relation to language dominance | Ipsilateral | 40 (61%) |
| Contralateral | 25 (38%) | |
| Tumor location within lobes | Frontal | 20 (28%) |
| Frontal‐insular | 7 (10%) | |
| Frontal‐insular‐temporal | 27 (37%) | |
| Frontal–parietal | 1 (1%) | |
| Parietal | 8 (11%) | |
| Temporal | 6 (8%) | |
| Temporal‐insular‐parietal | 3 (4%) | |
| Temporal‐insular | 1 (1%) | |
| Entity | WHO°II glioma | 41 (56%) |
| WHO°III glioma | 18 (25%) | |
| WHO°IV glioma | 14 (19%) | |
| Language deficit | No deficit | 60 (82%) |
| Reported at first scan | 5 (7%) | |
| Reported at second scan | 11 (15%) | |
| Radiation therapy | No history of | 50 (69%) |
| History of | 23 (31%) | |
| Chemotherapy | No history of | 15 (21%) |
| History of | 58 (79%) |
Table 2.
Patient characteristics related to language lateralization and position of glioma
| Full cohort analysis | Relative position analysisa | ||||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Total | |||
| Population size | N = 73 | N = 40 | N = 25 | N = 65 | |
| Age (years) | 42.5 | 40.9 | 44.2 | 42.1 | |
| Time between scans (weeks) | 151 (2.9 years) | 153 (2.9 years) | 146 (2.8 years) | 150 (2.9 years) | |
| Gender | Female | 32 (43.8%) | 18 (45%) | 11 (44%) | 29 (44.6%) |
| Male | 41 (56.2%) | 22 (55%) | 14 (56%) | 36 (55.4%) | |
| Tumor grade | Low | 31 (42.5%) | 18 (45%) | 13 (52%) | 31 (47.7%) |
| High | 42 (57.5%) | 22 (55%) | 12 (48%) | 34 (52.3%) | |
| Tumor location | Left | 48 (65.8%) | 39 (97.5%) | 22 (88%) | 61 (93.8%) |
| Right | 25 (34.2%) | 1 (2.5%) | 3 (12%) | 4 (6.2%) | |
| Language laterality index | Left | 61 (83.6%) | 39 (97.5%) | 22 (88%) | 61 (93.8%) |
| Bilateral | 8 (11.0%) | – | – | – | |
| Right | 4 (5.5%) | 1 (2.5%) | 3 (12%) | 4 (6.2%) | |
No results are available for the bilateral language LI section of the relative position analysis because all patients with bilateral LI values were excluded.
Low tumor grade = grade I or grade II gliomas; high tumor grade = grade III or grade IV (Glioblastoma) tumors.
For the relative position analysis (tumors ipsi‐ and contralateral to lateralization compared to laterality shift), an additional eight patients were excluded for the presence of bilateral LI values.
3.2. Tumor characteristics
Forty‐eight of 73 patients (65.8%) presented with left hemisphere tumors and 25 patients (34.2%) had right hemisphere tumors. Twenty tumors (27.4%) were located in the frontal lobe (two in the supplementary motor area), six tumors (8.2%) were located in the temporal lobe, eight tumors (11.0%) were located in the parietal lobe, and 39 tumors (53.4%) were located in multiple lobes. Of the tumors composed of multiple lobes, 27 tumors (37.0%) were determined to be in the frontal, temporal, and insular lobes, seven tumors (9.6%) were located in the frontal and insular lobes, three tumors (4.1%) were located in the temporal, insular, and parietal lobes, one tumor (1.4%) was located in the temporal and insular lobes, and one tumor (1.4%) was located in the frontal and parietal lobes. No occipital tumors were included in the present study. Histopathological analysis reported low‐grade glioma (WHO grade I or II) in 31 cases (42.5%) and high‐grade glioma (WHO grade III or IV) in 42 cases (57.5%). Of the high‐grade gliomas, 27 cases exhibited anaplastic glioma (64.3%), and 15 exhibited glioblastoma (35.7%).
3.3. Functional plasticity outcomes
The presence and extent of functional plasticity in the cohort was assessed using the full patient population of 73 patients. Evidence of functional plasticity was observed; the hemispheric location of language lateralization shifted by a median percent change of 29.1% across all patients. Forty‐six of 73 patients (63.0%) presented with an LI shift of greater than 10.0% change between scans. Interestingly, 21 (28.8%) patients experienced a shift in LI classification between scans. Seventeen (23.3%) of 21 patients presented with a shift in LI classification from left or right to bilateral, or vice versa, and in 4 (5.5%), a complete switch in lateralization between left and right hemispheres was recorded. Forty‐one of 73 patients (56.2%) exhibited a change of greater than ±0.1 and 54 (74.0%) were noted to express a nonzero shift. The percent LI changes for all patients are depicted in Figure 4a. Percent LI changes are presented separately for left and right hemispheric glioma patients in the Supporting Information Figure S1.
Figure 4.
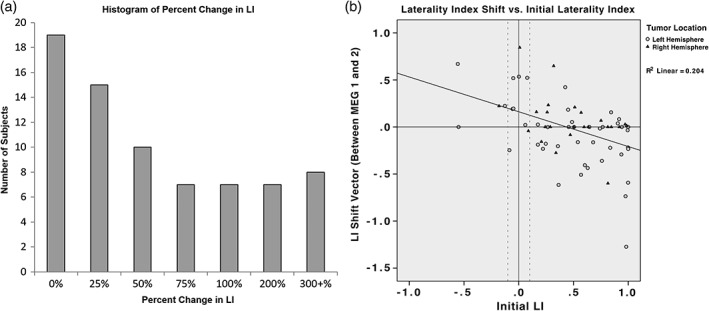
(a) Histogram of percent change in LI. Patients are grouped according to their percent change in LI, defined as the absolute value of the change in LI divided by the initial LI. Patients are binned by quartiles from 0% to 100%, then by 100% increments up to 300%, and finally by 300+%. (b) Association between laterality shifts and initial laterality presentation. Patients with increasing left LI values shift more rightward, and patients with increasing right LI values shift leftward. There is an association between greater initial left lateralization and increasing rightwards LI shift, and vice versa. Points are grouped based on tumor location. Positive values indicate left hemispheric initial position (horizontal axis) and leftward LI movement between scans (vertical axis). Negative values represent right hemispheric initial position (horizontal axis) and rightward LI movement between scans (vertical axis)
The relationship between initial language lateralization and LI shift was investigated using the same cohort (Figure 4b). This trend is presented for left and right hemispheric glioma patients separately in the Supporting Information Figure S2. The position of initial language lateralization and the direction and magnitude of the resultant LI shift were observed to be linearly correlated with a coefficient of determination (R 2) of 0.204 (p = .000). Patients initially presenting with farther right language laterality exhibited greater LI shifts leftwards (positive ΔLI), while those exhibiting initial left laterality displayed increasingly rightwards (negative ΔLI) shift values.
Examination of the resultant LI shift, observed between MEG scans, based on relative tumor location and language laterality showed significance (Figure 5). The LI shift observed between MEG scans was found to be greater when language function was initially positioned ipsilateral to the tumor; the average resultant LI shift for ipsilateral tumors (−0.148 ± 0.048 SE) was significantly greater than contralateral tumors (−0.003 ± 0.052 SE). With equal variances not assumed, an independent samples t test comparing these means was significant with a p value of .045 (using p < .05). The combined LI values at the first and second MEG scan were shown to be significantly different (p = .013) using a paired‐samples t test with equal variances not assumed.
Figure 5.
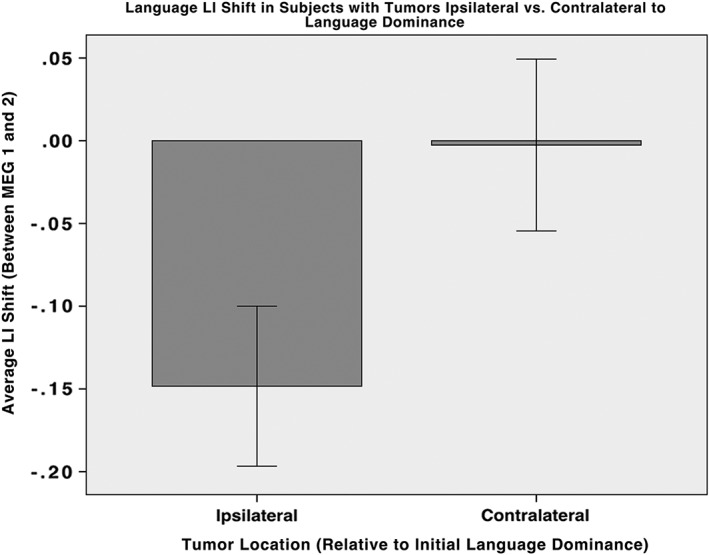
Observed average LI shift in patients with tumors ipsilateral or contralateral relative to the hemisphere of initial language dominance. Positive LI shift values indicate movement away from the affected hemisphere. Patients with tumors positioned ipsilaterally to language dominance present a significantly greater shift in LI away from the tumor location between scans than those with contralateral pathologies, on average. Patients with tumors ipsilateral to language function experience greater LI index shifts on average away from affected hemisphere, and therefore away from language dominant hemisphere. Most patients with tumors contralateral to language dominance experience no shift, while some experience a shift toward the affected hemisphere, but away from language. Error bars drawn using ±1 SE
No significant relationship was found between changes in LI and tumor grade (low [grade I or II] or high [grade III or IV]), or time between scans. Using an independent samples t test, with equal variances not assumed, the relationship was determined to be insignificant for LI changes across scans (functional plasticity) and tumor grade (p = .874), time between scans (p = .497) and gender (p = .326). No significant relationship (p = .212) was found between dominant or nondominant tumors and the presence (Yes vs. No) of a complete laterality flip (L to R, R to L, or Bilateral to L or R), using an independent samples t test, with equal variances not assumed. Date at first MEG was not predictive of LI shift (p = .608). In addition, the administration of adjuvant chemotherapy was not associated with LI shift (p = .591) but adjuvant radiation therapy was associated with shift (p = .031). Age at first scan (p = .050) was noted to be predictive of LI shift across scans.
4. DISCUSSION
This study documents longitudinal changes in the organization of language networks in patients with glioma. We found evidence that a glioma ipsilateral to the dominant language hemisphere induces a shift of language away from that hemisphere. In addition, we report plasticity expressed as functional reorganization in the location of language laterality in these patients over time. Significant correlation of LI relative to tumor location was observed, potentially indicating a causal relationship.
We selected our patient population to be maximally homogenous and worked to attribute observations to our hypothesis by excluding patients with potentially confounding conditions. For the present study, we observed only primary brain tumor patients and removed those with bilateral tumors and significant loss of function. As previously mentioned, the observed majority of patients presenting with left‐lateralized language function is consistent with past studies (Strauss & Wada, 1983). However, comparisons of LI classification distribution with prior publications from our and other groups are limited by sampling bias toward patients with probable bilateral/right‐sided language (i.e., left‐handed patients). In a previous article published by our group (Findlay et al., 2012), bihemispheric activation was noted in the verb generation language task, consistent with the findings of related published research (Edwards et al., 2010; Hickok et al., 2008; Hickok & Poeppel, 2007), as well as the existence of maximally lateralized function during time‐windows in cortical regions for receptive and expressive language (Findlay et al., 2012). Mounting evidence has demonstrated the dynamic expression of brain activity during language processing and its dispersion across multiple regions of presently known language cortex (Bastiaansen & Hagoort, 2006; Hickok et al., 2008; Papanicolaou et al., 2006). This phenomenon is directly observable through bilateral spectral power changes localized in the cortical regions subserving language during an MEG verb generation task and is dependent on the character of the language task employed (Findlay et al., 2012).
The laterality index used for this study is a viable metric of language dominance determination, in concordance with prior studies. MEG scans are a well‐established method of determining language lateralization (Findlay et al., 2012; Hirata et al., 2004; Tanaka et al., 2013). In addition, MEG language laterality indices can be compiled from both complex and simple language tasks with accuracy when compared to the traditionally used Wada test (Kim & Chung, 2008). The combined LI analysis (averaged receptive stimulus‐ and response‐locked recordings) used in the present study was found to be highly correlated with the Wada test (IAP) results in 97% of the patient population in a previous study by our group (Findlay et al., 2012). Compared to the IAP results, the combined LI exhibited a test sensitivity and specificity of 100 and 96%, respectively, using a LI threshold of ±0.1 (Findlay et al., 2012). These results are aligned with previous studies and further establish MEG based lateralization techniques as valid methods of language dominance determination (Castillo et al., 2001; Frye et al., 2009; Pang et al., 2011; Passaro et al., 2011; Salmelin, 2007).
4.1. The majority of patients experienced a functional shift
In the present study, nearly two‐thirds of the patients were observed to exhibit an LI shift of greater than 10.0% between scans, and the majority exhibited a nonzero shift between scans. The median percent change in LI shift of 29.1% was relatively large in magnitude, considering 20.5% of patients were demonstrated to shift by a percent change of greater than 100.0%. While the importance of the magnitude of LI has yet to be validated, these findings suggest that patients with glioma have a capacity for substantial reorganization (Mbwana et al., 2009; McCormack, McLeod, Harrison, & McAllister, 2010). Indeed, over one‐quarter of patients experienced a shift in language LI classification between scans. Most commonly, a change between unihemispheric and bihemispheric classification was reported. However, a small number of patients experienced a complete switch in language lateralization from one hemisphere to the other, providing compelling evidence for the presence of functional plasticity in these patients. As examples of this phenomenon, whole brain activation from the verb generation task is displayed for a left to right shift in Figure 2a, and a right to left shift in Figure 2b. A more common situation is displayed in Figure 2c, with a left to bilateral shift in language.
4.2. The initial location of language dominance is associated with the vector of the resultant LI shift
The initial hemispheric language dominance was related to the magnitude and direction of the laterality shift (for cases where a laterality shift occurred; Figure 3). Patients with increasing left LI values were observed to shift more rightward, and patients with increasing right LI values leftward. Figure 4 displays the association between the location of initial language dominance and vector of the corresponding language LI shift, providing evidence of their correlation and suggesting the likelihood of nonrandom functional plasticity of language cortex in the setting of glioma. In addition, this finding may demonstrate that functional shifts can occur independently of the removal of dominant side tissue, suggesting that the glioma itself, in conjunction with relative language lateralization, induces reorganization more than the surgical resection. As expected, postsurgical radiation treatment was associated with shift in the LI. Given that postsurgical radiation is standard of care in all high‐grade gliomas, this finding is likely reflective of the shift in LI that we saw in the population overall.
4.3. Relative location of tumor and initial language dominance is associated with the magnitude of LI shift
In the present series, tumor location relative to language dominance had a significant correlation with the magnitude of the LI shift. Figure 5 demonstrates the observation that patients presenting with tumors located ipsilateral to the side of initial language dominance (LI at first MEG) displayed greater laterality shifts away from tumor location than in patients with tumors contralateral to language dominance. The average resultant LI shift for ipsilateral tumors was significantly greater than contralateral tumors. These results provide compelling evidence that tumor location and subsequent management of the tumor (including surgery) have a significant impact on the magnitude of functional reorganization. In addition, these findings validate the stability of the LI measure over time because, when tumors occur contralateral to the hemisphere of language dominance, the change in LI between visits is minimal.
4.4. Limitations
The findings in this article should not be regarded without first considering some limitations. The primary limitation of this study is its retrospective design; as a result, the data set is limited by the typical challenges of incomplete medical records, poor documentation, and patient attrition. Certain clinical variables, such as adjuvant chemotherapy and radiation, were often absent from the medical record due to the patients receiving postoperative care at their home institutions. Given that both these adjuvant modalities are standard of care in glioma management, there are few cases where one or the other is utilized alone. Therefore, differences in their contributions to LI shift are more likely a result of incomplete clinical data than a true effect.
A secondary limitation is the relatively new adoption of the language laterality index as a reliable measure of hemispheric dominance determination. We correct for this limitation by presenting evidence from multiple studies that clearly presents the MEG imaging (Breier et al., 1999, 2006; Doss et al., 2009; Frye et al., 2009; Passaro et al., 2011; Tanaka et al., 2013), as well as the VOI pseudo‐F‐value derived language laterality index (Findlay et al., 2012; Hirata et al., 2004), as functionally similar to the “gold standard” preoperative language determination test the IAP, or Wada test. Furthermore, we have other research currently in press that provides additional validation of language dominance mapping for this protocol using the auditory verb generation and two other language tasks.
In addition, an unavoidable limitation is the inherent population bias created by the constraints of our observational methods. The patient population may be skewed toward preserved language function and the exhibition of plasticity because an essential requirement for inclusion in this study is the ability of each patient to complete the verb generation task at each MEG. Patients unable to complete this task were necessarily excluded from the experimental population as the characteristics of their presurgical and postsurgical language function and LI could not be determined. Also, surgery may have not been an option for patients who would have likely suffered a severe loss of language function. Other factors may include self‐reporting of various factors used during the statistical analysis and definition of shift versus nonshift in language LI. Furthermore, distribution of language laterality in our study may not generalize to the population as a whole as our patient cohort reflects bias in referral patterns; many of these patients were referred for MEG language testing because of a lesion within or close to eloquent language cortex.
Finally, it is possible the observed reorganization may not be solely driven by disease, as there exist other factors that may affect language network reorganization, and our study is unable to distinguish between tumor and surgical resection as the primary driver of reorganization. One must also consider the possibility that chemotherapy, adjuvant radiotherapy, or some other factor, such as anatomical constraint (van Geemen, Herbet, Moritz‐Gasser, & Duffau, 2014), play a role in inducing or modulating plasticity. Further study of this issue would require an observational, noninterventional study design which is unlikely to be conducted due to ethical concerns, as surgical intervention is the standard‐of‐care in the management of these lesions. The effects of these factors on observed functional plasticity were determined to be statistically insignificant to the maximal feasible extent.
4.5. Future directions
Future studies should further investigate functional plasticity associated with surgical events by implementing routine postoperative neuroimaging (Duffau et al., 2001). In addition, imaging using both MEG and fMRI techniques in conjunction may provide even greater accuracy of language lateralization determination during presurgical mapping, and should be considered in future examinations of language function (Grummich, Nimsky, Pauli, Buchfelder, & Ganslandt, 2006; Liljeström, Hultén, Parkkonen, & Salmelin, 2009). Further refinement of the LI is also of importance: future studies will assess the benefit of implementing additional tasks to verb generation such as picture naming and nonword repetition. These expansions may allow language testing to be performed even in impaired patients who have difficulty with language processing. Furthermore, aggregation of a sample size of sufficient magnitude will validate the volumetric analysis of patients presenting with tumors within the same grade (low‐ or high‐grade gliomas) and should be pursued.
The use of TMS and nTMS technology to identify areas of interest has been used for presurgical mapping of motor function previously (Bulubas et al., 2016), and may be worth further analysis to establish practicality of implementation in confirming language lateralization. The specific effects of tumors on language function should be the focus of supplementary research by conducting a volumetric analysis of language eloquent tumors, as well as the relationship between the size and mass of the tumor, and the presence and magnitude of the resultant language LI shift.
5. CONCLUSION
Changes in language lateralization are observed when MEG scans are compared over time, showing the plasticity of language function and subsequent reorganizational trends. Functional reorganization of language function is demonstrated in a significant portion of patients and is influenced by the relative position (ipsilateral vs. contralateral) of the tumor and initial language hemispheric dominance.
CONFLICT OF INTEREST
The study was financed by institutional grants from our university's department for neurological surgery and the department of radiology. TT was not funded for his research at UCSF. The authors report no conflict of interest concerning the materials or methods used in this study, or the findings specified in this article. This manuscript was not intended for financial gain, or any purpose other than research.
Supporting information
Figure S1 Supporting information
Figure S2 Supporting information
Table S1 The workflow behind patient selection and categorization for this study.
Table S2 Patient characteristics related to tumor hemisphere, language lateralization, and LI shift direction
ACKNOWLEDGMENTS
TT acknowledges all members of the UCSF Biomagnetic Imaging Laboratory, as well as Dr. Mitchel Berger and the departments of neurosurgery and radiology, and Dr. Henry Mahncke.
Traut T, Sardesh N, Bulubas L, et al. MEG imaging of recurrent gliomas reveals functional plasticity of hemispheric language specialization. Hum Brain Mapp. 2019;40:1082–1092. 10.1002/hbm.24430
Ethics Committee Registration Number: 10‐02027
REFERENCES
- Babiloni, C. , Pizzella, V. , Gratta, C. D. , Ferretti, A. , & Romani, G. L. (2009). Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. International Review of Neurobiology, 86, 67–80. [DOI] [PubMed] [Google Scholar]
- Baillet, S. (2017). Magnetoencephalography for brain electrophysiology and imaging. Nature Neuroscience, 20(3), 327–339. [DOI] [PubMed] [Google Scholar]
- Bastiaansen, M. , & Hagoort, P. (2006). Oscillatory neuronal dynamics during language comprehension. Progress in Brain Research, 159, 179–196. [DOI] [PubMed] [Google Scholar]
- Bowyer, S. M. , Moran, J. E. , Weiland, B. J. , Mason, K. M. , Greenwald, M. L. , Smith, B. J. , … Tepley, N. (2005). Language laterality determined by MEG mapping with MR‐FOCUSS. Epilepsy & Behavior, 6(2), 235–241. [DOI] [PubMed] [Google Scholar]
- Breier, J. I. , Billingsley‐Marshall, R. , Pataraia, E. , Castillo, E. M. , & Papanicolaou, A. C. (2006). Magnetoencephalographic studies of language reorganization after cerebral insult. Archives of Physical Medicine and Rehabilitation, 87(12 Suppl 2), S77–S83. [DOI] [PubMed] [Google Scholar]
- Breier, J. I. , Simos, P. G. , Zouridakis, G. , Wheless, J. W. , Willmore, L. J. , Constantinou, J. E. , … Papanicolaou, A. C. (1999). Language dominance determined by magnetic source imaging: A comparison with the Wada procedure. Neurology, 53(5), 938–945. [DOI] [PubMed] [Google Scholar]
- Bulubas, L. , Sabih, J. , Wohlschlaeger, A. , Sollmann, N. , Hauck, T. , Ille, S. , … Krieg, S. M. (2016). Motor areas of the frontal cortex in patients with motor eloquent brain lesions. Journal of Neurosurgery, 125(6), 1431–1442. [DOI] [PubMed] [Google Scholar]
- Castillo, E. M. , Simos, P. G. , Venkataraman, V. , Breier, J. I. , Wheless, J. W. , & Papanicolaou, A. C. (2001). Mapping of expressive language cortex using magnetic source imaging. Neurocase, 7(5), 419–422. [DOI] [PubMed] [Google Scholar]
- Dalal, S. S. , Guggisberg, A. G. , Edwards, E. , Sekihara, K. , Findlay, A. M. , Canolty, R. T. , … Nagarajan, S. S. (2008). Five‐dimensional neuroimaging: Localization of the time‐frequency dynamics of cortical activity. NeuroImage, 40(4), 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss, R. C. , Zhang, W. , Risse, G. L. , & Dickens, D. L. (2009). Lateralizing language with magnetic source imaging: Validation based on the Wada test. Epilepsia, 50(10), 2242–2248. [DOI] [PubMed] [Google Scholar]
- Duffau, H. , Bauchet, L. , Lehéricy, S. , & Capelle, L. (2001). Functional compensation of the left dominant insula for language. NeuroReport, 12(10), 2159–2163. [DOI] [PubMed] [Google Scholar]
- Duffau, H. , Denvil, D. , & Capelle, L. (2002). Long term reshaping of language, sensory, and motor maps after glioma resection: A new parameter to integrate in the surgical strategy. Journal of Neurology, Neurosurgery, and Psychiatry, 72(4), 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, E. , Nagarajan, S. S. , Dalal, S. S. , Canolty, R. T. , Kirsch, H. E. , Barbaro, N. M. , & Knight, R. T. (2010). Spatiotemporal imaging of cortical activation during verb generation and picture naming. NeuroImage, 50, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay, A. M. , Ambrose, J. B. , Cahn‐Weiner, D. A. , Houde, J. F. , Honma, S. , Hinkley, L. B. , … Kirsch, H. E. (2012). Dynamics of hemispheric dominance for language assessed by magnetoencephalographic imaging. Annals of Neurology, 71(5), 668–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, R. E. , Rezaie, R. , & Papanicolaou, A. C. (2009). Functional neuroimaging of language using Magnetoencephalography. Physics of Life Reviews, 6(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummich, P. , Nimsky, C. , Pauli, E. , Buchfelder, M. , & Ganslandt, O. (2006). Combining fMRI and MEG increases the reliability of presurgical language localization: A clinical study on the difference between and congruence of both modalities. NeuroImage, 32(4), 1793–1803. [DOI] [PubMed] [Google Scholar]
- Hickok, G. , Okada, K. , Barr, W. , Pa, J. , Rogalsky, C. , Donnelly, K. , … Grant, A. (2008). Bilateral capacity for speech sound processing in auditory comprehension: Evidence from Wada procedures. Brain and Language, 107, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Hinkley, L. B. , Marco, E. J. , Brown, E. G. , Bukshpun, P. , Gold, J. , Hill, S. , … Nagarajan, S. S. (2016). The contribution of the corpus callosum to language lateralization. The Journal of Neuroscience, 36(16), 4522–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley, L. B. , Nagarajan, S. S. , Dalal, S. S. , Guggisberg, A. G. , & Disbrow, E. A. (2011). Cortical temporal dynamics of visually guided behavior. Cerebral Cortex, 21(3), 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, M. , Kato, A. , Taniguchi, M. , Saitoh, Y. , Ninomiya, H. , Ihara, A. , … Yoshimine, T. (2004). Determination of language dominance with synthetic aperture magnetometry: Comparison with the Wada test. NeuroImage, 23, 46–53. [DOI] [PubMed] [Google Scholar]
- Kim, J. S. , & Chung, C. K. (2008). Language lateralization using MEG beta frequency desynchronization during auditory oddball stimulation with one‐syllable words. NeuroImage, 42(4), 1499–1507. [DOI] [PubMed] [Google Scholar]
- Liljeström, M. , Hultén, A. , Parkkonen, L. , & Salmelin, R. (2009). Comparing MEG and fMRI views to naming actions and objects. Human Brain Mapping, 30(6), 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbwana, J. , Berl, M. M. , Ritzl, E. K. , Rosenberger, L. , Mayo, J. , Weinstein, S. , … Gaillard, W. D. (2009). Limitations to plasticity of language network reorganization in localization related epilepsy. Brain, 132(Pt 2), 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack, J. , McLeod, S. , Harrison, L. J. , & McAllister, L. (2010). The impact of speech impairment in early childhood: Investigating parents' and speech‐language pathologists' perspectives using the ICF‐CY. Journal of Communication Disorders, 43(5), 378–396. [DOI] [PubMed] [Google Scholar]
- Pang, E. W. , Wang, F. , Malone, M. , Kadis, D. S. , & Donner, E. J. (2011). Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI. Neuroscience Letters, 490(3), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou, A. C. , Pazo‐Alvarez, P. , Castillo, E. M. , Billingsley‐Marshall, R. L. , Breier, J. I. , Swank, P. R. , … Passaro, A. D. (2006). Functional neuroimaging with MEG: Normative language profiles. NeuroImage, 33(1), 326–342. [DOI] [PubMed] [Google Scholar]
- Passaro, A. D. , Rezaie, R. , Moser, D. C. , Li, Z. , Dias, N. , & Papanicolaou, A. C. (2011). Optimizing estimation of hemispheric dominance for language using magnetic source imaging. Brain Research, 1416, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin, R. (2007). Clinical neurophysiology of language: The MEG approach. Clinical Neurophysiology, 118(2), 237–254. [DOI] [PubMed] [Google Scholar]
- Strauss, E. , & Wada, J. (1983). Lateral preferences and cerebral speech dominance. Cortex, 19(2), 165–177. [DOI] [PubMed] [Google Scholar]
- Tanaka, N. , Liu, H. , Reinsberger, C. , Madsen, J. R. , Bourgeois, B. F. , Dworetzky, B. A. , … Stufflebeam, S. M. (2013). Language lateralization represented by spatiotemporal mapping of magnetoencephalography. American Journal of Neuroradiology, 34(3), 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore, P. E. , Martino, J. , Guggisberg, A. G. , Owen, J. , Honma, S. M. , Findlay, A. , … Nagarajan, S. S. (2012). Magnetoencephalographic imaging of resting‐state functional connectivity predicts postsurgical neurological outcome in brain gliomas. Neurosurgery, 71(5), 1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- van Geemen, K. , Herbet, G. , Moritz‐Gasser, S. , & Duffau, H. (2014). Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients. Human Brain Mapping, 35(4), 1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, J. (1949). A new method for determination of the side of cerebral speech dominance: A preliminary report on the intracarotid injection of sodium amytal in man. Iqakaa te Seibutzuqaki, 14, 221–222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supporting information
Figure S2 Supporting information
Table S1 The workflow behind patient selection and categorization for this study.
Table S2 Patient characteristics related to tumor hemisphere, language lateralization, and LI shift direction


