Abstract
Individuals with schizophrenia and bipolar disorder show alterations in functional neural connectivity during rest. However, resting‐state network (RSN) disruptions have not been systematically compared between the two disorders. Further, the impact of RSN disruptions on social cognition, a key determinant of functional outcome, has not been studied. Forty‐eight individuals with schizophrenia, 46 with bipolar disorder, and 48 healthy controls completed resting‐state functional magnetic resonance imaging. An atlas‐based approach was used to examine functional connectivity within nine RSNs across the cortex. RSN connectivity was assessed via nonparametric permutation testing, and associations with performance on emotion perception, mentalizing, and emotion management tasks were examined. Group differences were observed in the medial and lateral visual networks and the sensorimotor network. Individuals with schizophrenia demonstrated reduced connectivity relative to healthy controls in all three networks. Individuals with bipolar disorder demonstrated reduced connectivity relative to controls in the medial visual network and connectivity within this network was significantly positively correlated with emotion management. In healthy controls, connectivity within the medial and lateral visual networks positively correlated with mentalizing. No significant correlations were found for either visual network in schizophrenia. Results highlight the role of altered early visual processing in social cognitive deficits in both schizophrenia and bipolar disorder. However, individuals with bipolar disorder appear to compensate for disrupted visual network connectivity on social cognitive tasks, whereas those with schizophrenia do not. The current study adds clarity on the neurophysiology underlying social cognitive deficits that result in impaired functioning in serious mental illness.
Keywords: bipolar disorder, resting‐state fMRI, schizophrenia, social cognition
1. INTRODUCTION
Deficits in social cognition are well established in schizophrenia and are linked to problems in daily social functioning (Green, Horan, & Lee, 2015). Similarly, individuals with bipolar disorder have impairments in social cognition. While social cognition has not been studied in bipolar disorder as extensively as schizophrenia, extant findings indicate significant deficits, albeit at attenuated levels compared to schizophrenia (e.g., Lee et al., 2013; Vlad, Raucher‐chéné, Henry, & Kaladjian, 2018).
The underlying neurobiology contributing to social cognitive deficits in schizophrenia and bipolar disorder remains poorly understood. Advances in social neuroscience indicate that functionally interconnected brain areas (e.g., networks) are critical to social cognitive abilities (Lieberman, 2007). Functional connectivity analysis of resting‐state functional magnetic resonance imaging (rsfMRI) offers insight into the intrinsic activity of these networks, and allows for examination of their relationship to social cognitive performance (Doruyter, Dupont, Stein, & Warwick, 2017).
Substantial work has examined regional activation and, more recently, functional connectivity, associated with social cognitive processes in healthy adults, mostly utilizing task‐based fMRI. For recent topical reviews, see (e.g., Eisenberger, 2013; Jankowski & Takahashi, 2014; Mahy, Moses, & Pfeifer, 2014; Rotge et al., 2015; Santos, Almeida, Oliveiros, & Castelo‐Branco, 2016). Previous fMRI studies of social cognition often focus on specific regions of interest, including dorsal and ventral aspects of medial prefrontal cortex (mPFC), anterior cingulate cortex, and posterior cingulate cortex, collectively known as cortical midline structures, as well as the temporal–parietal junction, temporal pole, and anterior insula (e.g., Bolling et al., 2012; Mitchell, Banaji, & Macrae, 2005; Morawetz et al., 2016; Murray, Debbané, Fox, Bzdok, & Eickhoff, 2015; Schilbach, Eickhoff, Rotarska‐Jagiela, Fink, & Vogeley, 2008; Schmälzle et al., 2017; Strombach et al., 2015; Szekely, Silton, Heller, Miller, & Mohanty, 2017; Zaki, Ochsner, Hanelin, Wager, & Mackey, 2007). Together, these regions constitute a broad social cognition network, which is thought to interact with other systems, including sensorimotor and affective networks, to facilitate social understanding (Molnar‐Szakacs & Uddin, 2013). Of note, many of these regions, particularly those along the cortical midline, largely overlap with the default mode network (DMN; Mars et al., 2012), which is associated with mental activities that support introspection and internal states (Buckner, Andrews‐Hanna, & Schacter, 2008; Frith & Frith, 2006; Schilbach et al., 2008). Thus, much of the work linking rsfMRI and social cognition has focused on the DMN (Amft, Bzdok, Laird, & Eickhoff, 2015; Andrews‐Hanna, 2012; Laird et al., 2009; Moran, Kelley, & Heatherton, 2013; Schilbach et al., 2012; Takeuchi et al., 2014).
Although social neuroscience approaches have been increasingly applied to understand social cognition in schizophrenia and bipolar disorder (Fujiwara, Yassin, & Murai, 2015; Green et al., 2015; Green, Horan, & Lee, 2019), relatively few studies have examined functional connectivity related to social cognition using rsfMRI (Karbasforoushan & Woodward, 2012; Mukherjee et al., 2012, 2014; Wang, Metzak, & Woodward, 2011). Studies of the DMN in schizophrenia have shown both hypoconnectivity and hyperconnectivity within the network compared to controls (Fornito, Zalesky, Pantelis, & Bullmore, 2012; Narr & Leaver, 2015; Sheffield & Barch, 2016). DMN connectivity with sensorimotor and affective networks is also reduced (Berman et al., 2016; Martino et al., 2018). Hypoconnectivity of resting‐state networks (RSNs) in schizophrenia is not limited to DMN; however, it has also been found in the dorsal attention and executive control networks (Woodward, Rogers, & Heckers, 2011).
rsfMRI studies of individuals with bipolar disorder also reveal alterations in regions associated with social cognition. Regions within DMN showed hyperconnectivity compared to healthy controls that were associated with enhanced emotional awareness (Das, Calhoun, & Malhi, 2014). Both hypoconnectivity and hyperconnectivity between amygdala and sensory‐motor regions (e.g., supplementary motor area) has been observed ((Brady, Margolis, Masters, Keshavan, & Öngür, 2017; Li, Liu, Andari, Zhang, & Zhang, 2018); see also (Wang et al., 2016)) which may be associated with motor and cognitive inhibition. Finally, hyperconnectivity (Torrisi et al., 2013) and reduced anticorrelation (Chepenik et al., 2010) between amygdala and ventral PFC has been found in bipolar patients, which is thought to be associated with emotion regulation difficulties in the disorder.
A few studies have investigated direct comparisons of RSN disruptions between bipolar and schizophrenia, revealing both similarities and differences (e.g., Mamah, Barch, & Repovš, 2013). For example, Liu et al. (2014) found reduced amygdala‐dorsal lateral PFC connectivity in the schizophrenia group, perhaps reflecting difficulty in higher order emotional and cognitive integration, whereas amygdala‐ventral PFC connectivity was reduced in the bipolar disorder group, reflecting disrupted emotion regulation and impaired inhibitory control. Similarly, Ongür et al. (2010) found reduced connectivity within the mPFC region of the DMN in both schizophrenia and bipolar disorder. However, the alterations were specific to dorsal mPFC in individuals with schizophrenia and ventral mPFC in individuals with bipolar disorder.
Taken together, substantial empirical support, as well as strong theoretical links to social cognition (Mars et al., 2012), suggests that alterations in DMN connectivity are associated with social cognitive deficits in schizophrenia and bipolar disorder. DMN has thus been a logical starting point toward understanding intrinsic neural activity underlying social cognitive deficits. However, other networks might also be involved, particularly given associations between early auditory and visual processing and social cognition in serious mental illness. These linkages have not yet been fully explored.
In summary, the links between social cognition and rsfMRI in schizophrenia and bipolar disorder have so far been indirect and speculative. As a result, several critical gaps in knowledge exist, including whether abnormalities in specific RSNs map onto distinct domains of social cognition, or whether the associations are generalized across networks or domains. Additionally, it is not known whether associations between RSN abnormalities and social cognitive deficits are shared or distinct between schizophrenia and bipolar disorder. The current study aimed to address these gaps in the literature by examining functional connectivity in established RSNs across the brain in three groups: individuals with schizophrenia, those with bipolar disorder, and healthy comparison subjects. First, we evaluated functional connectivity in RSNs and assessed for group differences. Based on previous findings and theoretical connections to social cognition, we hypothesized that the two patient groups would show altered connectivity in DMN (either hypoconnectivity or hyperconnectivity). Next, we examined any network that showed group differences for within‐group associations (i.e., individual differences) with social cognitive performance on tasks of emotion perception, mentalizing, and emotion management. We expected DMN to be correlated with social cognitive performance across groups. Finally, we conducted exploratory analyses of all RSNs (regardless of group differences) within each group to examine associations between individual differences on functional connectivity and each of the three social cognitive domains. Overall, the goal of the current study was to evaluate directly any links between abnormal resting‐state functional connectivity and impaired social cognition.
2. METHODS
The study protocol was reviewed and approved by the Institutional Review Boards of the VA Greater Los Angeles Healthcare System (GLA) and the University of California, Los Angeles (UCLA). All participants had the capacity to give informed consent and provided written informed consent prior to participation.
2.1. Participants
Forty‐nine individuals with schizophrenia, 49 individuals with bipolar disorder, and 52 healthy comparison participants completed resting‐state fMRI as part of a large National Institue of Mental Health‐sponsored study of visual processing in major mental illness. Patients were recruited from community outpatient treatment facilities in the Los Angeles area, and outpatient treatment clinics at GLA and UCLA. Healthy controls were recruited through internet postings. Selection criteria for all subjects included: (a) age 18–65 years, (b) sufficiently fluent in English to consent and understand testing procedures, (c) no known contraindications for MRI scanning, (d) vision/corrected vision of at least 20/30, (e) no evidence of IQ <70 or developmental disability based on chart review, (f) no clinically significant neurological disease determined by medical history (e.g., epilepsy), (g) no history of serious head injury (i.e., loss of consciousness >1 hr, neuropsychological sequelae, cognitive rehabilitation posthead injury), (h) no sedatives or benzodiazepines within 12 hr of testing, (i) no positive urine toxicology screening on day of assessment, (j) no history of a mood episode in the past 2 months, and (k) no substance or alcohol dependence in the past 3 months; no evidence of substance or alcohol abuse in past month.
Selection criteria for patient participants included: (a) a diagnosis of schizophrenia or bipolar disorder I or II based on the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I) (First, Spitzer, Gibbon, & Williams, 1997), and (b) clinically stable (i.e., no inpatient hospitalizations for 3 months prior to enrollment, no changes in psychoactive medication in the 4 weeks prior to enrollment). Additional selection criteria for healthy controls included: (a) no history of psychotic disorder, bipolar spectrum disorder, or other major mood disorder based on SCID‐I interview or of avoidant, paranoid, schizotypal, schizoid, or borderline personality disorders based on the SCID‐II (First, Gibbon, & Spitzer, 1997), and (b) no family history of a psychotic disorder or bipolar disorder in first‐degree relatives, based on participant report.
All SCID interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center to a minimum k of 0.75 for key psychotic and mood items (Ventura, Liberman, Green, Shaner, & Mintz, 1998). When available, medical records and reports from treating clinicians were used to corroborate retrospective self‐reported information for patient diagnoses. Clinical symptoms were characterized for the patient participants using the Brief Psychiatric Rating Scale (BPRS) (Ventura, Nuechterlein, Subotnik, & Gilbert, 1995), Clinical Assessment Interview for Negative Symptoms (CAINS) (Kring, Gur, Blanchard, Horan, & Reise, 2013), Young Mania Rating Scale (YMRS) (Young, Briggs, Ziegler, & Meyer, 1978), and Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960).
2.2. Procedures
2.2.1. Social cognitive assessment
Three measures were used that correspond to three domains of social cognition: emotion perception, mentalizing, and emotion management.
The Emotion in Biological Motion task (Heberlein, Adolphs, Tranel, & Damasio, 2004; Kern et al., 2013) is an emotion perception task that uses 30 point‐light walker video clips 5–10 s in length that capture a range of commonly displayed emotions. Immediately following presentation of each clip on a computer screen, five emotional states (fear, anger, happiness, sadness, or neutral) are presented on the computer screen and the participant is asked to choose which emotion best described the movement of the walker. Accuracy is measured as percent correct.
The Awareness of Social Inference Test (TASIT)—Part 3 (social inference—enriched) (Mcdonald, Flanagan, Rollins, & Kinch, 2003) is a videotape measure of mentalizing. The task assesses the ability to use contextual knowledge (visual and verbal), in addition to voice and face cues, to derive meaning from a conversation. It contains 16 scenes with two or three method actors appearing in each one. In each scene there is an untrue comment presented as either sarcasm or as a lie. After presentation of each scene, subjects respond verbally to questions about (a) the characters' communicative intentions, (b) whether they want the literal or nonliteral meaning of their message to be believed, (c) their beliefs and knowledge about the situation, and (d) their emotional state. Responses are summed to create a composite score.
The social cognition domain of the MATRICS Consensus Cognitive Battery (MCCB) is the managing emotions (ME) component of the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) (Mayer, Salovey, & Caruso, 2002; Mayer, Salovey, Caruso, & Sitarenios, 2003). The MSCEIT‐ME examines emotion regulation in oneself and in relationships with others. It includes vignettes of various situations, along with ways to cope with the emotions depicted in the vignettes. Subjects are required to indicate the effectiveness of each solution, ranging from one (very ineffective) to five (very effective). Total scores are age and gender corrected.
2.2.2. Nonsocial cognitive assessment
The MCCB (Nuechterlein & Green, 2006) was used to assess general cognitive performance. An age‐ and gender‐corrected Neurocognitive Composite score was derived from six domains of neurocognition: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving.
2.2.3. MRI data acquisition and preprocessing
Scanning was performed on a Siemens Tim Trio 3 T MRI scanner equipped with a 12‐channel head coil (Siemens Medical Solutions, Erlangen, Germany) at the UCLA Staglin Center for Cognitive Neuroscience. Two sets of structural images were acquired for registration purposes: a T1‐weighted magnetization prepared rapid‐acquisition gradient echo (MPRAGE) sequence (repetition time, TR = 1,900 ms; echo time, TE = 3.43 ms; flip angle = 9°; slice thickness = 1 mm; voxel size = 1 × 1 × 1 mm3; field of view, FOV = 256 mm; acquisition matrix = 256 × 256; 160 slices), and a T2‐weighted matched‐bandwidth high resolution sequence with the same slice prescription as the functional image (TR = 6,000 ms; TE = 66 ms; flip angle = 90°; slice thickness = 3.3 mm; voxel size = 1.5 × 1.5 × 3.3; FOV = 192 mm; matrix = 64 × 64; 38 slices). A 5‐min resting‐state functional MRI image was acquired using a T2*‐weighted echo planar imaging gradient‐echo pulse sequence (TR = 2,500 ms; TE = 35 ms; flip angle = 75°; slice thickness = 3.3 mm; voxel size = 3 × 3 × 3.3; FOV = 192 mm; matrix = 96 × 96; 38 slices). Slices were oriented parallel to the anterior commissure‐posterior commissure axis of the brain. The first two volumes of the functional scan were automatically discarded before data collection began to allow for T1 equilibrium.
Image analyses were performed using the FMRIB Software Library (FSL v5.0.9; Analysis Group, Oxford, UK). Prior to any preprocessing, Power framewise displacement (FD) (average of rotation and translation parameter differences using weighted scaling (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012)) and Jenkinson FD (average of rotation and translation parameter differences using matrix RMS formulation (Jenkinson, 1999)) were calculated using fsl_motion_outliers. The corresponding mean FD values of each participant were used to assess differences in motion. Analyses did not show any differences between the groups (see Table S1a, Supporting Information). Preprocessing steps included skull stripping using Brain Extraction Tool (Smith, 2002), spatial smoothing using a Gaussian kernel of 5 mm full width at half maximum, and motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Independent component analysis‐based Automatic Removal of Motion Artifacts (ICA‐AROMA) (Pruim, Mennes, Buitelaar, & Beckmann, 2015) was then used for denoising. Components identified as head motion were removed from each individual's resting‐state data in native space by means of linear regression (nonaggressive denoising) using the function fsl_regfilt. To further control for effects due to motion inside the scanner, participants with too few signal components (less than two standard deviations from the mean) identified by ICA‐AROMA were excluded. Group comparison of motion parameters identified by ICA‐AROMA is listed in Table S1b, Supporting Information. Registration was carried out using FMRIB's Linear Image Registration Tool (FLIRT) (Jenkinson & Smith, 2001). The preprocessed functional resting‐state data was registered first to the coplanar matched‐bandwidth T2‐weighted image (affine transformation; 6 degrees of freedom), then to the T1‐weighted MPRAGE (Boundary‐Based Registration) (Greve & Fischl, 2009), then to Montreal Neurological Institute standard space (affine transformation, 12 degrees of freedom) using 4 × 4 × 4 mm3 resolution. High‐pass temporal filtering was performed using a .01 Hz threshold.
2.2.4. Identification of RSNs
Rather than relying on the potentially idiosyncratic properties of the current sample to obtain functional connectivity RSNs, we used a well‐validated whole‐brain RSN atlas (Smith et al., 2009) (Smith atlas) as a template from which to derive RSNs for each participant (see Figure 1). The set of 10 RSN spatial maps from the Smith atlas was used to generate subject‐specific versions of each map and associated time series using dual regression (Filippini et al., 2009; Nickerson, Smith, Öngür, & Beckmann, 2017). For subsequent analyses, we excluded examination of cerebellum (RSN 5 from the Smith atlas), because we did not achieve adequate coverage of cerebellum during the scan.
Figure 1.
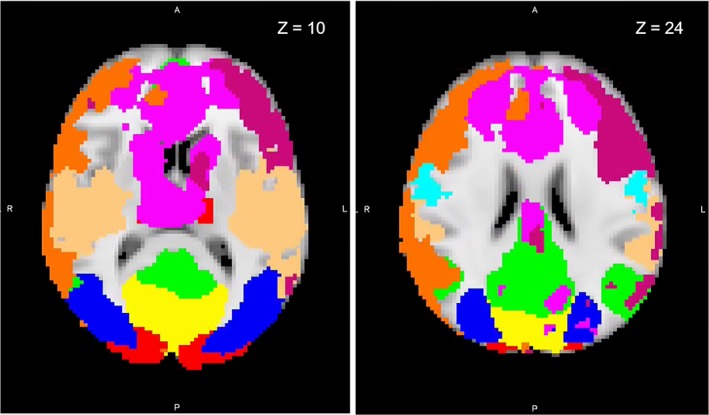
Resting‐state functional magnetic resonance imaging network maps identified by Smith and Nichols (2009) overlaid onto a standard brain in Montreal Neurological Institute (MNI) space. Axial slices displayed at coordinate shown. Yellow, medial visual; red, visual occipital pole; blue, lateral visual; green, default mode; not shown, cerebellum; light blue, sensorimotor; light orange, auditory; purple, executive control; orange, right frontoparietal; dark pink, left frontoparietal [Color figure can be viewed at http://wileyonlinelibrary.com]
To validate the current findings, we ran parallel analyses using a different published atlas (Gordon et al., 2016) (Gordon atlas). Results are included in Table S2.
2.3. Statistical analyses
Differences in demographic and clinical characteristics as well as performance on the social and nonsocial cognition tests among the groups were examined using chi‐square tests for categorical variables and one‐way analysis of variance tests for continuous variables, with pairwise chi‐square/Tukey's post hoc comparisons in case of statistical significance.
Within‐group averages and between‐group differences for each of the nine RSNs of interest were assessed using FSL's randomize nonparametric permutation‐testing tool (Smith & Nichols, 2009). The threshold free cluster enhancement algorithm was used with 5,000 permutations (Li, Nickerson, Nichols, & Gao, 2017). An omnibus F test was performed to identify any differences between the three groups. Pairwise between‐group comparisons were assessed if an initial omnibus F test was significant at a familywise error rate p value (p‐FWE) <.05.
Associations between strength of functional connectivity within any RSNs showing between group differences and behavioral measures of social cognition were examined. For each RSN, outliers were identified as individuals with values more than three times the interquartile range and excluded for that analysis. Bivariate correlations were then conducted between mean beta values extracted from each RSN and behavioral performance on the Emotion in Biological Motion task (emotion perception), TASIT task (mentalizing), and the MSCEIT‐ME (emotion management) within each group separately. Correlations with the Neurocognitive Composite Score of the MCCB were also included for comparison purposes. Correction for multiple comparisons was conducted by means of the Benjamini–Hochberg false discovery rate procedure (Benjamini & Hochberg, 1995).
3. RESULTS
A total of eight participants (one schizophrenia, three bipolar, and four healthy controls) were excluded from analysis: One healthy control participant did not complete the full resting‐state fMRI scan, which resulted in an insufficient duration of the scan for analysis. One individual with bipolar disorder was excluded because resting‐state data could not be processed and was likely corrupted. Six participants (one schizophrenia, two bipolar, three healthy controls) had excessive movement (motion parameters outlined in Section 2.2.3). Finally, 48 individuals with schizophrenia, 46 individuals with bipolar disorder, and 48 healthy comparison participants were eligible for analysis. Demographics and results are presented for these subjects.
3.1. Demographic and behavioral data
Table 1 provides participant demographic and clinical information. There were no differences between groups in terms of age, sex, ethnicity, or parental education. Patients were medicated and exhibited mild to moderate symptoms. Mean daily doses for antipsychotic medication reported in Table 1 were calculated in chlorpromazine equivalents (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). Schizophrenia and bipolar disorder groups did not differ in terms of age of onset, number of hospitalizations, HAMD total, or YMRS total. Schizophrenia patients had higher psychiatric symptom scores as measured by the BPRS and CAINS. In the bipolar disorder group, all participants were out of mood episode at the time of assessment. Forty out of the 46 bipolar patients were euthymic as defined by an HAMD score <15 and a YMRS score <12 (Pizzagalli, Goetz, Ostacher, Iosifescu, & Perlis, 2008).
Table 1.
Demographic and clinical data
| Characteristic | SCZ (n = 48) | BD (n = 46) | HC (n = 48) | Group comparison |
|---|---|---|---|---|
| Sex | 31M 17F | 23M 23F | 21M 27F | x 2 (2) = 4.37, p = .11 |
| Handedness | 41R 7L | 41R 4L | 41R 7L | x 2 (2) = 0.89, p = .64 |
| Ethnicity | ||||
| % Hispanic | 25.0 | 26.1 | 18.8 | x 2 (2) = 0.83, p = .66 |
| Race | ||||
| % Caucasian | 60.4 | 71.1 | 47.9 | x 2 (6) = 7.39, p = .29 |
| % African American | 25.0 | 13.3 | 31.3 | |
| % Asian | 4.2 | 2.2 | 8.3 | |
| % other | 10.4 | 13.3 | 12.5 | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|---|---|---|---|---|
| Age | 46.02 (11.47) | 43.98 (12.56) | 47.27 (8.10) | F(2,139) = 1.10, p = .34 |
| Personal education | 12.94 (2.13) | 14.13 (2.31) | 14.35 (1.77) |
F(2,138) = 6.43, p = .002 SCZ < HC, p = .003 SCZ < BD, p = .02 BD = HC |
| Parental education | 12.98 (2.85) | 14.02 (2.64) | 13.59 (2.89) | F(2,130) = 1.56, p = .22 |
| Age of onset | 22.40 (7.68) | 20.66 (9.16) | t(86) = 0.97, p = .33 | |
| No. hospitalizations | 6.67 (6.44) | 5.28 (7.52) | t(86) = 0.94, p = .35 | |
| CPZ equivalent | 350.52 (253.34) | 303.165 (208.67) | t(59) = 0.76, p = .45 | |
| BPRS total | 39.29 (10.43) | 33.25 (5.33) | t(91) = 3.48, p = .001 | |
| CAINS | ||||
| Motivation | 1.62 (0.66) | 1.06 (0.65) | t(92) = 4.14, p < .001 | |
| Expressive | 1.12 (0.88) | 0.45 (0.56) | t(92) = 4.36, p < .001 | |
| YMRS total | 4.58 (4.05) | 3.39 (4.43) | t(92) = 1.36, p = .18 | |
| HAMD (21‐item total) | 5.94 (4.97) | 6.24 (4.55) | t(92) = −0.31, p = .76 | |
Abbreviations: BD, bipolar disorder; BPRS, Brief Psychiatric Rating Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; CPZ equivalent, chlorpromazine equivalent; F, female; HAMD, Hamilton Depression Rating Scale; HC, healthy controls; L, left; M, male; R, right; SCZ, schizophrenia; SD, standard deviation; YMRS, Young Mania Rating Scale.
Behavioral performance on the social and nonsocial cognition task battery is listed in Table 2. Significant group effects were found on three of the measures and a trend toward significance was observed for the fourth measure. All results followed the same pattern, with reduced performance in the schizophrenia group compared to the bipolar disorder group and/or healthy controls.
Table 2.
Behavioral performance data
| Characteristic | SCZ (n = 48) | BD (n = 46) | HC (n = 48) | Group comparison |
|---|---|---|---|---|
| MCCB neurocognitive composite | 40.19 (11.50) | 45.29 (12.99) | 50.40 (10.78) |
F(2,138) = 9.03, p ≤ .001 SCZ < HC, p < .001 SCZ < BD, p < .05 BD < HC, p < .05 |
| Emotion in biological motion (emotion perception) | 0.74 (.12) | 0.79 (.10) | 0.77 (.09) |
F(2,135) = 2.86, p = .06 SCZ < BD, p < .05 |
| TASIT (mentalizing) | 47.60 (6.35) | 50.91 (5.56) | 52.68 (4.52) |
F(2,138) = 10.33, p ≤ .001 SCZ < HC, p < .001 SCZ < BD, p < .01 |
| MSCEIT‐ME (emotion management) | 39.25 (13.00) | 48.13 (10.30) | 48.98 (11.55) |
F(2,138) = 10.11, p ≤ .001 SCZ < HC, p < .001 SCZ < BD, p < .001 |
Abbreviations: BD, bipolar disorder; HC, healthy controls; MCCB, MATRICS Consensus Cognitive Battery; MSCEIT‐ME, Mayer–Salovey–Caruso Emotional Intelligence Test, Managing Emotions component; SCZ, schizophrenia; TASIT, The Awareness of Social Inference Test, Part 3.
3.2. RSN group comparison
Results from the between‐group analyses for each of the nine RSNs provide p values for the F tests (across groups) as well as p values for the paired group comparisons (see Table 3). Significant differences in functional connectivity across the three groups were observed in three out of the nine networks examined: the medial visual network (MVN; p‐FWE = .01), lateral visual network (LVN; p‐FWE = .01), and the sensorimotor network (p‐FWE = .05). Follow‐up pairwise comparisons revealed that in the MVN, individuals with schizophrenia (p‐FWE = .02) and individuals with bipolar disorder (p‐FWE = .03) had reduced functional connectivity compared to healthy controls. Individuals with schizophrenia also showed significantly reduced functional connectivity within the LVN (p‐FWE = .01) and sensorimotor network (p‐FWE = .02) compared to healthy controls. The schizophrenia and bipolar groups were not significantly different from each other in the MVN, LVN, or sensorimotor network.
Table 3.
RSN group comparison
| Component | RSN | F test (p value) | Pairwise comparisons |
|---|---|---|---|
| IC0000 | Medial visual | .01 |
SCZ < HC, p = .02 BD < HC, p = .03 SCZ = BD |
| IC0001 | Visual occipital pole | .48 | N/A |
| IC0002 | Lateral visual | .01 |
SCZ < HC, p = .01 BD = HC SCZ = BD |
| IC0003 | DMN | .79 | N/A |
| IC0004 | Cerebellum | N/A | N/A |
| IC0005 | Sensorimotor | .05 |
SCZ < HC, p = .02 BD = HC SCZ = BD |
| IC0006 | Auditory | .28 | N/A |
| IC0007 | Executive control | .64 | N/A |
| IC0008 | Frontoparietal R | .96 | N/A |
| IC0009 | Frontoparietal L | .61 | N/A |
Abbreviations: BD, bipolar disorder; DMN, default mode network; HC, healthy controls; L, left; R, right; RSN, resting‐state network; SCZ, schizophrenia.
We cross validated the findings from the Smith atlas (Smith et al., 2009) using RSNs that were derived from the Gordon atlas (Gordon et al., 2016). Similar to the initial findings, we found significant differences using the Gordon atlas in functional connectivity across the three groups in the visual network (p‐FWE = .01) and a subcomponent of the sensorimotor network labeled SMhand (p‐FWE = .01). Note that the Gordon atlas does not subdivide the visual network into subcomponents. Pairwise comparisons revealed that both of these effects were driven by reduced functional connectivity in the schizophrenia group compared to the healthy control group (p‐FWE = .02 and p‐FWE = .01, respectively). We found no significant bipolar—healthy control differences using the Gordon atlas. Hence, the differences between schizophrenia and controls were highly consistent between the two atlases, but the differences between bipolar and healthy controls became attenuated. These findings are detailed in Table S2.
3.3. RSN external correlates
We examined correlations within each group between functional connectivity within the MVN, LVN, and sensorimotor network, and the behavioral measures of social and nonsocial cognition. Several correlations were significant after correction for multiple comparisons (see Figure 2) (Benjamini & Hochberg, 1995). Among healthy controls, functional connectivity within MVN and LVN was significantly positively correlated with mentalizing (both r's = .39, p corr = .03), and functional connectivity within the sensorimotor network was significantly positively correlated with nonsocial cognition (r = .40, p corr = .03). For individuals with bipolar disorder, functional connectivity within MVN was significantly positively correlated with emotion management (r = .44, p corr = .03). No significant correlations (after correction for multiple comparisons) were found for either visual network or the sensorimotor network in the schizophrenia group. Complete results are listed in Table 4.
Figure 2.
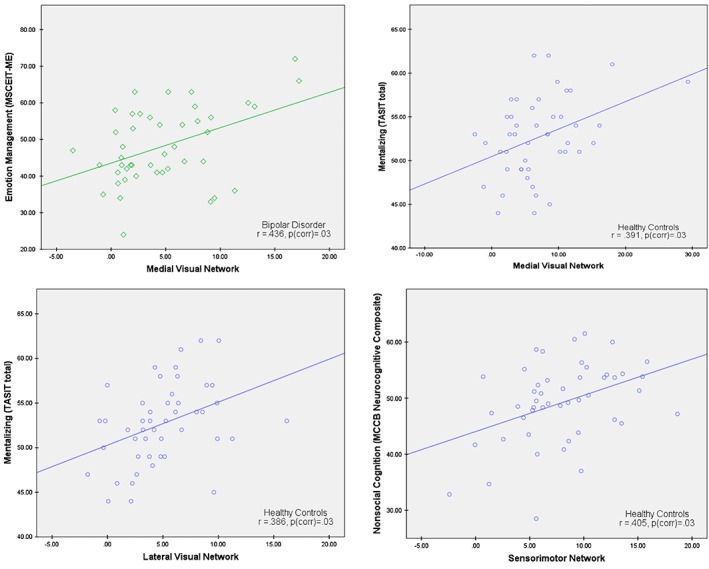
Scatter plots depicting significant within‐group correlations (corrected for multiple comparisons) between functional connectivity within the medial visual network (MVN), lateral visual network (LVN), and sensorimotor network, and the behavioral measures of social and nonsocial cognition [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Within‐group correlations between functional connectivity within the MVN, LVN, and sensorimotor network, and the behavioral measures of social and nonsocial cognition
| SCZ | BD | HC | ||||
|---|---|---|---|---|---|---|
| r value | p value | r value | p value | r value | p value | |
| IC0000: Medial visual network | ||||||
| MCCB neurocognitive composite | −.052 | .292 | .292* | |||
| Emotion in biological motion (emotion perception) | −.264 | .319* | .262 | |||
| TASIT (mentalizing) | −.156 | .072 | .391** | .029 | ||
| MSCEIT‐ME (emotion management) | −.016 | .436** | .033 | .091 | ||
| IC0002: lateral visual network | ||||||
| MCCB neurocognitive composite | .009 | .169 | .258 | |||
| Emotion in biological motion (emotion perception) | −.192 | .268 | .235 | |||
| TASIT (mentalizing) | −.093 | .082 | .386** | .029 | ||
| MSCEIT‐ME (emotion management) | .210 | .148 | .055 | |||
| IC0005: sensorimotor network | ||||||
| MCCB neurocognitive composite | .035 | .091 | .405** | .029 | ||
| Emotion in biological motion (emotion perception) | .173 | .202 | .024 | |||
| TASIT (mentalizing) | .213 | .197 | .245 | |||
| MSCEIT‐ME (emotion management) | .080 | .333* | .302* | |||
Note. Showing significant p values corrected for multiple comparisons.
Abbreviations: BD, bipolar disorder; HC, healthy controls; MCCB, MATRICS Consensus Cognitive Battery; LVN, lateral visual network; MSCEIT‐ME, Mayer–Salovey–Caruso Emotional Intelligence Test, Managing Emotions component; MVN, medial visual network; SCZ, schizophrenia; TASIT, The Awareness of Social Inference Test, Part 3.
In follow‐up analyses, we assessed the influence of clinical symptoms on the relationships between RSN connectivity within the MVN, LVN, and sensorimotor network and social cognitive performance. Partial correlations, controlling for scores on the BPRS (positive subscale), CAINS (total score), YMRS, and HAMD, were performed within each patient group. For individuals with bipolar disorder, functional connectivity within MVN and sensorimotor network was significantly positively correlated with emotion management (r = .51, p corr = .01 and r = .45, p corr = .02, respectively). No significant correlations (after correction for multiple comparisons) were found for either visual network or the sensorimotor network in the schizophrenia group.
For the exploratory analyses, we examined correlations between the remaining RSNs and behavioral measures. We found several additional significant (uncorrected) correlations within the bipolar group, including between DMN and emotion management, as well as between the auditory network and all three measures of social cognition. Healthy controls showed additional significant correlations between the left frontoparietal network and nonsocial cognition and emotion perception. The schizophrenia group did not show any significant correlations with any RSN. Findings are detailed in Table S3.
4. DISCUSSION
The current study aimed to address several critical gaps in knowledge regarding the links between social cognition and intrinsic network activity in schizophrenia and bipolar disorder. We found both similarities and differences between the two patient groups in terms of RSN connectivity compared to controls. Individuals with schizophrenia showed reduced functional connectivity in three RSNs: MVN, LVN, and the sensorimotor network. Individuals with bipolar disorder showed reduced functional connectivity in only one network compared with controls, MVN. We examined whether functional connectivity in these three RSNs related to behavioral performance in distinct domains of social cognition and found significant correlations between two networks and mentalizing in healthy controls, between MVN and emotion management in individuals with bipolar disorder, and no associations in individuals with schizophrenia. Exploratory analyses further revealed that, within the bipolar group, DMN connectivity was positively correlated with emotion management, and auditory network connectivity was positively correlated with all three measures of social cognition. Within the healthy control group, left frontoparietal network connectivity was positively correlated with nonsocial cognition and emotion perception. No additional correlations were found within the schizophrenia group for any RSN.
Our findings of altered functional connectivity within visual RSNs in serious mental illness are consistent with prior work. Visual processing deficits are well established in schizophrenia (e.g., Butler, Silverstein, & Dakin, 2008; Silverstein & Keane, 2011) and have been proposed as a potential mechanism underlying difficulties not just with perceptual abnormalities and tasks in the visual domain, but also more downstream cognitive abnormalities (Green, Hellemann, Horan, Lee, & Wynn, 2012). In schizophrenia, our group and others have demonstrated associations between regional activation abnormalities in early and midlevel visual processing areas (e.g., striate and extrastriate cortex; lateral occipital complex) and performance on specific visual tasks (Anderson et al., 2017; Green et al., 2009; Martinez et al., 2008; Silverstein et al., 2015). Areas within the MVN and LVN correspond to several of these previously explored visual processing regions. The atlas we used included a third network involved with visual processing (i.e., the occipital pole), but this network did not show group differences.
Aberrant visual processing may also exist in bipolar disorder (e.g., Bestelmeyer et al., 2006; Jahshan et al., 2014). Some previous studies found visual perceptual deficits in bipolar disorder at similar levels to schizophrenia (e.g., Lee et al., 2018), other found intermediate deficits, between those seen in schizophrenia versus healthy controls (e.g., Macqueen, Young, Galway, & Joffe, 2001); see also Reavis et al., 2017), while still others did not show deficits at all (e.g., Sponheim, Sass, Noukki, & Hegeman, 2013). Given that overall the findings are mixed, more work is needed to clarify the precise nature of abnormal visual perception and its neural correlates in bipolar disorder.
We also found reduced functional connectivity of the sensorimotor network in schizophrenia compared to healthy controls, similar to prior studies (e.g., Berman et al., 2016). Sensorimotor regions are implicated in low‐level shared representations of action perception, or mirroring (e.g., Caspers, Zilles, Laird, & Eickhoff, 2010; Iacoboni, 2009). Interaction between the sensorimotor network and regions of DMN (underlying higher level, inference‐based mentalizing processes) is thus thought to be critical to social understanding (Lombardo et al., 2010; Molnar‐Szakacs & Uddin, 2013). We did not, however, find any significant correlations between functional connectivity within the sensorimotor network and the measures of social cognition we assessed, in any group. This highlights the need for further work to understand the role of sensorimotor functional connectivity deficits in social cognition.
We did not find DMN functional connectivity differences in either of our patient samples compared with controls. This was contrary to our hypothesis and previous findings of DMN abnormalities in both clinical samples (e.g., Das et al., 2014; Karbasforoushan & Woodward, 2012). However, the results for schizophrenia vary in terms of the direction of the effect, and some do not show any differences between patients and controls (e.g., Fox et al., 2017; Wolf et al., 2011), consistent with our current findings. For bipolar disorder, the lack of DMN functional connectivity differences from healthy controls is consistent with other studies that showed no differences, particularly during clinical remission (e.g., Syan et al., 2018).
Apart from this consistency with prior findings, the lack of DMN differences in the current study might be attributed to the use of an atlas‐based approach to identify the DMN, in contrast to ICA‐ or seed‐based approaches in most previous studies. For example, using the latter two approaches, DMN differences were found in schizophrenia which included areas that are usually not considered part of the DMN (e.g., lateral temporal lobe) (Mannell et al., 2010; Woodward et al., 2011). Such regions are not included in the DMN defined by the Smith (Smith et al., 2009) and Gordon (Gordon et al., 2016) atlases.
Many studies tend to utilize group ICA to identify resting‐state networks in a given subject sample. These methods can produce robust and reliable RSNs, but also might reflect the idiosyncratic nature of a particular subject sample and thus produce findings that are difficult to replicate. To increase the generalizability of our findings and reduce potential for spurious results, we chose to identify RSNs based on a well validated, commonly used, atlas (Smith et al., 2009). We then validated the findings on a separate published atlas (Gordon et al., 2016). The findings replicated very well across the two atlases, with one exception. We did not see the reduced visual network functional connectivity in individuals with bipolar disorder compared to healthy controls using the Gordon atlas, perhaps because the Gordon atlas does not divide into visual subcomponents (i.e., the MVN, LVN, and visual occipital pole network in the Smith atlas). Larger network volumes result in a larger probability of finding a cluster by chance. Therefore, the cluster found in the Smith atlas for a specific visual network subcomponent (MVN) might not have passed the cluster‐forming threshold/critical cluster size for the single visual network in the Gordon atlas. Results for the schizophrenia group are much more robust in that regard because we saw the same result in both atlases. Overall, the atlas‐based approach used in this study strengthens confidence in the findings and invites future studies to provide replication.
Despite the DMN's theoretical connections to social cognition, we found only one significant correlation (uncorrected) between DMN functional connectivity and a measure of social cognition (i.e., emotion management), and only in the bipolar disorder group. This may be due to the fact that the empirical link between DMN and social cognition is mainly based on task‐based fMRI studies (e.g., Mahy et al., 2014; Santos et al., 2016; Schilbach et al., 2008), as opposed to resting state as used here. Although task‐evoked activity generally shows high concordance with resting‐state activity, there still may be key differences in the patterns of functional connectivity associated with each, as well as their external correlates (Lynch et al., 2018). This lack of concordance between the two imaging methods might be especially true for patients with schizophrenia (Ebisch et al., 2018). Thus, the lack of connection between resting‐state activity and task‐evoked activity might explain the lack of correlations with performance in the current study.
An additional aim of the study was to compare schizophrenia to bipolar disorder. Specifically, we wanted to examine whether the pattern of alterations in RSN functional connectivity was similar between groups, and whether those alterations correlate with social cognitive deficits in a similar manner. We found only one RSN, the MVN, which was similarly disrupted in both groups. Further, MVN functional connectivity was correlated to performance on the emotion management task in bipolar disorder, but not schizophrenia. However, performance on the emotion management task was intact in the bipolar disorder group, whereas it was impaired in schizophrenia. This finding suggests that individuals with bipolar disorder may be able to compensate for disrupted visual network connectivity on social cognitive tasks, whereas those with schizophrenia cannot. More work is needed to understand the mechanisms underlying impaired social functioning and poor outcomes in bipolar disorder (Huxley & Baldessarini, 2007; Sanchez‐Moreno et al., 2009).
A limitation of the current study is that our patient sample was comprised of individuals with chronic schizophrenia and individuals with bipolar disorder out of mood episode; all patients were medicated as clinically indicated. Therefore, we do not know whether similar patterns of functional connectivity deficits would be observed in recent‐onset, manic, or unmedicated individuals. In addition, our analysis of the relationship between RSN connectivity and behavioral performance was limited to correlations between mean beta values extracted from within networks and behavioral measures. While important, this link is only a first step in understanding this complex relationship. Future work could examine, for example, more complex features of resting‐state network architecture, perhaps from a graph theoretical perspective, and in even larger samples. Finally, we acknowledge a general limitation of social cognitive measures commonly used in experimental settings, including the measures utilized in this study. These measures have limited ecological validity and, unfortunately, there is currently a paucity of measures that capture real world ability in real time. Whether network connectivity during or related to experimental measures is different than what we might find with more ecologically valid measures is an open empirical question.
5. CONCLUSION
Given that two of the three networks that showed group differences were involved in visual processing, the current study highlights the role of altered early visual processing in social cognitive deficits in both schizophrenia and bipolar disorder. The findings suggest that visual processing is directly associated with social cognition in healthy controls, and to some extent in bipolar disorder. However, it appears that in schizophrenia, the reliance upon networks within the visual system to perform social cognitive tasks breaks down. In contrast, individuals with bipolar disorder may be able to compensate for early visual system disruptions to maintain performance. The findings provide a nuanced understanding of the neurophysiology underlying social cognitive deficits that result in impaired functioning in serious mental illness.
CONFLICT OF INTERESTS
J.L. has served as a consultant for Takeda. M.F.G. has been a consultant for AiCure, Biogen, Takeda, and Lundbeck, a member of the Scientific Board of Cadent, and has received unrelated research support from Forum. He is an officer within MATRICS Assessment, Inc., the publisher of the MCCB, but does not receive any financial remuneration for this role. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
Supporting information
Table S1 A. Group comparison of motion parameters as identified by fsl_motion_outliers
B. Group comparison of motion parameters as identified by ICA‐AROMA
Table S2. Parallel analysis of resting state network group comparison using the Gordon atlas.
Table S3. Exploratory within‐group correlations between functional connectivity of each resting state network and the behavioral measures of social and non‐social cognition. Showing p values uncorrected for multiple comparisons.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Mental Health R01MH095878 to M.F.G. The funder had no role in the study design, data collection, management, analysis and interpretation, decision to publish, or the preparation, review or approval of the manuscript. The authors would like to thank Ana Ceci Myers, Julio Iglesias, and the rest of the laboratory staff for assistance in data collection, and Michelle J. Dolinsky for assistance with participant recruitment. The authors would also like to thank One Mind—Center for Cognitive Neuroscience at UCLA for their generous support to the CCN scanner. fMRI data analysis for this study used computational and storage services associated with the Hoffman2 Shared Cluster provided by UCLA Institute for Digital Research and Education's Research Technology Group.
Jimenez AM, Riedel P, Lee J, Reavis EA, Green MF. Linking resting‐state networks and social cognition in schizophrenia and bipolar disorder. Hum Brain Mapp. 2019;40:4703–4715. 10.1002/hbm.24731
Funding information National Institute of Mental Health, Grant/Award Number: R01MH095878
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Amft, M. , Bzdok, D. , Laird, A. R. , & Eickhoff, S. B. (2015). Definition and characterization of an extended social‐affective default network. Brain Structure and Function, 220, 1031–1049. 10.1007/s00429-013-0698-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, E. J. , Tibber, M. S. , Schwarzkopf, D. S. , Sukhwinder, S. S. , Fernandez‐Egea, E. , Rees, G. , & Dakin, S. C. (2017). Visual population receptive fields in people with schizophrenia have reduced inhibitory surrounds. The Journal of Neuroscience, 37(6), 1546–1556. 10.1523/JNEUROSCI.3620-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, N. C. , Pressler, M. , Nopoulos, P. , Miller, D. , & Ho, B. (2010). Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–262. 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. (2012). The brain's default network and its adaptive role in internal mentation. The Neuroscientist, 18(3), 251–270. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B, 57(1), 289–300. [Google Scholar]
- Berman, R. A. , Gotts, S. J. , Mcadams, H. M. , Greenstein, D. , Lalonde, F. , Clasen, L. , … Rapoport, J. (2016). Disrupted sensorimotor and social – cognitive networks underlie symptoms in childhood‐onset schizophrenia. Brain, 139, 276–291. 10.1093/awv330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestelmeyer, P. E. G. , Tatler, B. W. , Phillips, L. H. , Fraser, G. , Benson, P. J. , & St, D. (2006). Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophrenia Research, 87, 212–222. 10.1016/j.schres.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Bolling, D. Z. , Pitskel, N. B. , Deen, B. , Crowley, M. J. , McPartland, J. C. , Mayes, L. C. , & Pelphrey, K. A. (2012). Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage, 54(3), 2462–2471. 10.1016/j.neuroimage.2010.10.049.Dissociable [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, R. O. , Margolis, A. , Masters, G. A. , Keshavan, M. , & Öngür, D. (2017). Bipolar mood state reflected in cortico‐amygdala resting state connectivity: A cohort and longitudinal study. Journal of Affective Disorders, 217, 205–209. 10.1016/j.jad.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Butler, P. D. , Silverstein, S. M. , & Dakin, S. C. (2008). Visual perception and its impairment in schizophrenia. Biological Psychiatry, 64, 40–47. 10.1016/j.biopsych.2008.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers, S. , Zilles, K. , Laird, A. R. , & Eickhoff, S. B. (2010). ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–1167. 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik, L. G. , Raffo, M. , Hampson, M. , Lacadie, C. , Wang, F. , Jones, M. M. , … Blumberg, H. P. (2010). Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Research: Neuroimaging, 182(3), 207–210. 10.1016/j.pscychresns.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, P. , Calhoun, V. , & Malhi, G. S. (2014). Bipolar and borderline patients display differential patterns of functional connectivity among resting state networks. NeuroImage, 98, 73–81. 10.1016/j.neuroimage.2014.04.062 [DOI] [PubMed] [Google Scholar]
- Doruyter, A. , Dupont, P. , Stein, D. J. , & Warwick, J. M. (2017). Resting‐state fMRI and social cognition: An opportunity to connect. Human Psychopharmacology: Clinical and Experimental, 32, 4–7. 10.1002/hup.2627 [DOI] [PubMed] [Google Scholar]
- Ebisch, S. J. H. , Gallese, V. , Salone, A. , Martinotti, G. , Mantini, D. , Gianni, M. , … Northoff, G. (2018). Disrupted relationship between “resting state ” connectivity and task‐evoked activity during social perception in schizophrenia. Schizophrenia Research, 193, 370–376. 10.1016/j.schres.2017.07.020 [DOI] [PubMed] [Google Scholar]
- Eisenberger, N. I. (2013). An empirical review of the neural underpinnings of receiving and giving social support: Implications for health. Psychosomatic Medicine, 75(6), 545–556. 10.1097/PSY.0b013e31829de2e7.An [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini, N. , Macintosh, B. J. , Hough, M. G. , Goodwin, G. M. , Frisoni, G. B. , Smith, S. M. , … Mackay, C. E. (2009). Distinct patterns of brain activity in young carriers of the APOE‐e4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106(17), 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. , Gibbon, M. , & Spitzer, R. (1997). Structured clinical interview for DSM‐IV Axis II Personality Disorders (SCID‐II). Washington, DC: American Psychiatric Press. [Google Scholar]
- First, M. , Spitzer, R. , Gibbon, M. , & Williams, J. (1997). Structured clinical interview for DSM‐IV Axis I Disorders‐Patient Edition (SCID‐I/P). New York, NY: American Psychiatric Press. [Google Scholar]
- Fornito, A. , Zalesky, A. , Pantelis, C. , & Bullmore, E. T. (2012). Schizophrenia, neuroimaging and connectomics. NeuroImage, 62(4), 2296–2314. 10.1016/j.neuroimage.2011.12.090 [DOI] [PubMed] [Google Scholar]
- Fox, J. M. , Abram, S. V. , Reilly, J. L. , Eack, S. , Goldman, M. B. , Csernansky, J. G. , … Smith, M. J. (2017). Default mode functional connectivity is associated with social functioning in schizophrenia. Journal of Abnormal Psychology, 126(4), 392–405. 10.1037/abn0000253.Default [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith, C. D. , & Frith, U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534. 10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Fujiwara, H. , Yassin, W. , & Murai, T. (2015). Neuroimaging studies of social cognition in schizophrenia. Psychiatry and Clinical Neurosciences, 69, 259–267. 10.1111/pcn.12258 [DOI] [PubMed] [Google Scholar]
- Gordon, E. M. , Laumann, T. O. , Adeyemo, B. , Huckins, J. F. , Kelley, W. M. , & Petersen, S. E. (2016). Generation and evaluation of a cortical area parcellation from resting‐state correlations. Cerebral Cortex, 26(1), 288–303. 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. F. , Hellemann, G. , Horan, W. P. , Lee, J. , & Wynn, J. K. (2012). From perception to functional outcome in schizophrenia. Archives of General Psychiatry, 69(12), 1216–1224. 10.1001/archgenpsychiatry.2012.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. F. , Horan, W. P. , & Lee, J. (2015). Social cognition in schizophrenia. Nature Reviews Neuroscience, 16, 620–631. 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- Green, M. F. , Horan, W. P. , & Lee, J. (2019). Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry, 18, 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. F. , Lee, J. , Cohen, M. S. , Engel, S. A. , Korb, A. S. , Nuechterlein, K. H. , … Glahn, D. C. (2009). Functional neuroanatomy of visual masking deficits in schizophrenia. Archives of General Psychiatry, 66(12), 1295–1303. 10.1001/archgenpsychiatry.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. NeuroImage, 48(1), 63–72. 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein, A. S. , Adolphs, R. , Tranel, D. , & Damasio, H. (2004). Cortical regions for judgments of emotions and personality traits from point‐light walkers. Journal of Cognitive Neuroscience, 16(7), 1143–1158. 10.1162/0898929041920423 [DOI] [PubMed] [Google Scholar]
- Huxley, N. , & Baldessarini, R. J. (2007). Disability and its treatment in bipolar disorder patients. Bipolar Disorders, 9(5), 183–196. 10.1111/j.1399-5618.2007.00430.x [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. (2009). Imitation, empathy, and mirror neurons. Annual Review of Psychology, 60(0066–4308, 0066–4308, 653–670. 10.1146/annurev.psych.60.110707.163604 [DOI] [PubMed] [Google Scholar]
- Jahshan, C. , Wynn, J. K. , Mccleery, A. , Glahn, D. C. , Altshuler, L. L. , & Green, M. F. (2014). Cross‐diagnostic comparison of visual processing in bipolar disorder and schizophrenia. Journal of Psychiatric Research, 51, 42–48. 10.1016/j.jpsychires.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski, K. F. , & Takahashi, H. (2014). Cognitive neuroscience of social emotions and implications for psychopathology: Examining embarrassment, guilt, envy, and schadenfreude. Psychiatry and Clinical Neurosciences, 68, 319–336. 10.1111/pcn.12182 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. (1999). Measuring transformation error by RMS deviation. Internal Technical Report TR99MJ1. Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. 10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Karbasforoushan, H. , & Woodward, N. (2012). Resting‐state networks in schizophrenia. Current Topics in Medicinal Chemistry, 12, 2404–2414. 10.2174/1568026611212210011 [DOI] [PubMed] [Google Scholar]
- Kern, R. S. , Penn, D. L. , Lee, J. , Horan, W. P. , Reise, S. P. , Ochsner, K. N. , … Green, M. F. (2013). Adapting social neuroscience measures for schizophrenia clinical trials, part 2: Trolling the depths of psychometric properties. Schizophrenia Bulletin, 39(6), 1201–1210. 10.1093/schbul/sbt127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring, A. M. , Gur, R. E. , Blanchard, J. J. , Horan, W. P. , & Reise, S. P. (2013). The clinical assessment interview for negative symptoms (CAINS): Final development and validation. American Journal of Psychiatry, 170, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Robin, D. A. , Fox, P. T. , Glahn, D. C. , Eickhoff, S. B. , & Li, K. (2009). Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. Journal of Neuroscience, 29(46), 14496–14505. 10.1523/jneurosci.4004-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Ph, D. , Altshuler, L. , Glahn, D. C. , Ph, D. , Miklowitz, D. J. , … Ph, D. (2013). Social and nonsocial cognition in bipolar disorder and schizophrenia: Relative levels of impairment. American Journal of Psychiatry, 170, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Reavis, E. A. , Engel, S. A. , Altshuler, L. L. , Cohen, M. S. , Glahn, D. C. , … Green, M. F. (2019). fMRI evidence of aberrantneural adaptation for objects in schizophrenia and bipolar disorder. Human Brain Mapping, 40, 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Liu, P. , Andari, E. , Zhang, A. , & Zhang, K. (2018). The role of amygdala in patients with euthymic bipolar disorder during resting state. Frontiers in Psychiatry, 9, 19–24. 10.3389/fpsyt.2018.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Nickerson, L. D. , Nichols, T. E. , & Gao, J. (2017). Comparison of a non‐stationary Voxelation‐corrected cluster‐size test with TFCE for group‐level MRI inference. Human Brain Mapping, 38, 1269–1280. 10.1002/hbm.23453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M. D. (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Tang, Y. , Womer, F. , Fan, G. , Lu, T. , Driesen, N. , … Wang, F. (2014). Differentiating patterns of amygdala‐frontal functional connectivity in schizophrenia and bipolar disorder. Schizophrenia Bulletin, 40(2), 469–477. 10.1093/schbul/sbt044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo, M. V. , Chakrabarti, B. , Bullmore, E. T. , Wheelwright, S. J. , Sadek, S. A. , Suckling, J. , & Baron‐Cohen, S. (2010). Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, 22(7), 1623–1635. 10.1162/jocn.2009.21287 [DOI] [PubMed] [Google Scholar]
- Lynch, L. K. , Lu, K. H. , Wen, H. , Zhang, Y. , Saykin, A. J. , & Liu, Z. (2018). Task‐evoked functional connectivity does not explain functional connectivity differences between rest and task conditions. Human Brain Mapping, 39(12), 4939–4948. 10.1002/hbm.24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macqueen, G. M. , Young, L. T. , Galway, T. M. , & Joffe, R. T. (2001). Backward masking task performance in stable, euthymic out‐patients with bipolar disorder. Psychological Medicine, 31(7), 1269–1277. 10.1017/S0033291701004597 [DOI] [PubMed] [Google Scholar]
- Mahy, C. E. V. , Moses, L. J. , & Pfeifer, J. H. (2014). Developmental cognitive neuroscience how and where: Theory‐of‐mind in the brain. Accident Analysis and Prevention, 9, 68–81. 10.1016/j.dcn.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah, D. , Barch, D. M. , & Repovš, G. (2013). Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. Journal of Affective Disorders, 150(2), 601–609. 10.1016/j.jad.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannell, M. V. , Franco, A. R. , Calhoun, V. D. , Cañive, J. M. , Thoma, J. , & Mayer, A. R. (2010). Resting state and task‐induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Human Brain Mapping, 31(3), 424–437. 10.1002/hbm.20876.Resting [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars, R. B. , Neubert, F.‐X. , Noonan, M. P. , Sallet, J. , Toni, I. , & Rushworth, M. F. S. (2012). On the relationship between the “default mode network ” and the “social brain”. Frontiers in Human Neuroscience, 6(189), 1–9. 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, A. , Hillyard, S. A. , Dias, E. C. , Hagler, D. J. , Butler, P. D. , Guilfoyle, D. N. , … Javitt, D. C. (2008). Magnocellular pathway impairment in schizophrenia: Evidence from functional magnetic resonance imaging. The Journal of Neuroscience, 28(30), 7492–7500. 10.1523/JNEUROSCI.1852-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, M. , Magioncalda, P. , Yu, H. , Li, X. , Wang, Q. , Meng, Y. , … Li, T. (2018). Abnormal resting‐state connectivity in a substantia nigra‐related striato‐thalamo‐cortical network in a large sample of First‐episode drug‐Naïve patients with schizophrenia. Schizophrenia Bulletin, 44(2), 419–431. 10.1093/schbul/sbx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, J. , Salovey, P. , & Caruso, D. (2002). Mayer‐Salovey‐Caruso Emotional Intelligence Test (MSCEIT) User's manual. Toronto, Canada: Multi‐Health System Publishers. [Google Scholar]
- Mayer, J. D. , Salovey, P. , Caruso, D. R. , & Sitarenios, G. (2003). Measuring emotional intelligence with the MSCEIT V2.0. Emotion, 3(1), 97–105. 10.1037/1528-3542.3.1.97 [DOI] [PubMed] [Google Scholar]
- Mcdonald, S. , Flanagan, S. , Rollins, J. , & Kinch, J. (2003). TASIT: A new clinical tool for assessing social perception after traumatic brain injury. Journal of Head Trauma Rehabilitation, 18(3), 219–238. [DOI] [PubMed] [Google Scholar]
- Mitchell, J. P. , Banaji, M. R. , & Macrae, C. N. (2005). The link between social cognition and self‐referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience, 17(8), 1306–1315. 10.1162/0898929055002418 [DOI] [PubMed] [Google Scholar]
- Molnar‐Szakacs, I. , & Uddin, L. Q. (2013). Self‐processing and the default mode network: Interactions with the mirror neuron system. Frontiers in Human Neuroscience, 7(571), 1–11. 10.3389/fnhum.2013.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, J. M. , Kelley, W. M. , & Heatherton, T. F. (2013). What can the organization of the brain's default mode network tell us about self‐knowledge? Frontiers in Human Neuroscience, 7, 1–6. 10.3389/fnhum.2013.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Kellermann, T. , Kogler, L. , Radke, S. , Blechert, J. , & Derntl, B. (2016). Intrinsic functional connectivity underlying successful emotion regulation of angry faces, 11 1980–1991 Social Cognitive and Affective Neuroscience. 10.1093/scan/nsw107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, P. , Whalley, H. C. , McKirdy, J. W. , McIntosh, A. M. , Johnstone, E. C. , Lawrie, S. M. , & Hall, J. (2012). Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophrenia Research, 134(2–3), 118–124. 10.1016/j.schres.2011.09.033 [DOI] [PubMed] [Google Scholar]
- Mukherjee, P. , Whalley, H. C. , McKirdy, J. W. , Sprengelmeyer, R. , Young, A. W. , McIntosh, A. M. , … Hall, J. (2014). Altered amygdala connectivity within the social brain in schizophrenia. Schizophrenia Bulletin, 40(1), 152–160. 10.1093/schbul/sbt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, R. J. , Debbané, M. , Fox, P. T. , Bzdok, D. , & Eickhoff, S. B. (2015). Functional connectivity mapping of regions associated with self‐ and other‐processing. Human Brain Mapping, 36(4), 1304–1324. 10.1002/hbm.22703.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr, K. L. , & Leaver, A. M. (2015). Connectome and schizophrenia. Current Opinion in Psychiatry, 28, 229–235. 10.1097/YCO.0000000000000157 [DOI] [PubMed] [Google Scholar]
- Nickerson, L. D. , Smith, S. M. , Öngür, D. , & Beckmann, C. F. (2017). Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Frontiers in Neuroscience, 11, 1–18. 10.3389/fnins.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein, K. H. , & Green, M. F. (2006). MATRICS consensus cognitive battery manual. Los Angeles, CA: MATRICS Assessment Inc. [Google Scholar]
- Ongür, D. , Lundy, M. , Greenhouse, I. , Shinn, A. K. , Menon, V. , Cohen, B. M. , & Renshaw, P. F. (2010). Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Research, 183(1), 59–68. 10.1016/j.pscychresns.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Goetz, E. , Ostacher, M. , Iosifescu, D. V. , & Perlis, R. H. (2008). Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biological Psychiatry, 64(2), 162–168. 10.1016/j.biopsych.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. H. R. , Mennes, M. , Buitelaar, J. K. , & Beckmann, C. F. (2015). Evaluation of ICA‐AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage, 112, 278–287. 10.1016/j.neuroimage.2015.02.063 [DOI] [PubMed] [Google Scholar]
- Reavis, E. A. , Lee, J. , Wynn, J. K. , Engel, S. A. , Jimenez, A. M. , & Green, M. F. (2017). Cortical thickness of functionally defined visual areas in schizophrenia and bipolar disorder. Cerebral Cortex, 27, 2984–2993. 10.1093/cercor/bhw151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge, J. , Lemogne, C. , Hinfray, S. , Huguet, P. , Grynszpan, O. , Tartour, E. , … Fossati, P. (2015). A meta‐analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, 10, 19–27. 10.1093/scan/nsu110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Moreno, J. , Martinez‐Aran, A. , Tabarés‐Seisdedos, R. , Torrent, C. , Vieta, E. , & Ayuso‐Mateos, J. L. (2009). Functioning and disability in bipolar disorder: An extensive review. Psychotherapy and Psychosomatics, 78, 285–297. 10.1159/000228249 [DOI] [PubMed] [Google Scholar]
- Santos, S. , Almeida, I. , Oliveiros, B. , & Castelo‐Branco, M. (2016). The role of the amygdala in facial trustworthiness processing: A systematic review and meta‐analyses of fMRI studies. PLoS One, 11(11), 1–28. 10.1371/journal.pone.0167276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach, L. , Bzdok, D. , Timmermans, B. , Fox, P. T. , Laird, A. R. , Vogeley, K. , & Eickhoff, S. B. (2012). Introspective minds: Using ALE meta‐analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One, 7(2), e30920 10.1371/journal.pone.0030920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach, L. , Eickhoff, S. B. , Rotarska‐Jagiela, A. , Fink, G. R. , & Vogeley, K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17(2), 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Schmälzle, R. , Brook, M. , Donnell, O. , Garcia, J. O. , Cascio, C. N. , Bayer, J. , … Falk, E. B. (2017). Brain connectivity dynamics during social interaction reflect social network structure. Proceedings of the National Academy of Sciences of the United States of America, 114(20), 5153–5158. 10.1073/pnas.1616130114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, J. M. , & Barch, D. M. (2016). Neuroscience and biobehavioral reviews cognition and resting‐state functional connectivity in schizophrenia. Neuroscience and Biobehavioral Reviews, 61, 108–120. 10.1016/j.neubiorev.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, S. M. , Harms, M. P. , Carter, C. S. , Gold, J. M. , Keane, B. P. , Macdonald, A. , … Barch, D. M. (2015). Neuropsychologia cortical contributions to impaired contour integration in schizophrenia. Neuropsychologia, 75, 469–480. 10.1016/j.neuropsychologia.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, S. M. , & Keane, B. P. (2011). Perceptual organization impairment in schizophrenia and associated brain mechanisms: Review of research from 2005 to 2010. Schizophrenia Bulletin, 37(4), 690–699. 10.1093/schbul/sbr052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing , threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Sponheim, S. R. , Sass, S. M. , Noukki, A. L. , & Hegeman, B. M. (2013). Fragile early visual percepts mark genetic liability specific to schizophrenia. Schizophrenia Bulletin, 39(4), 839–847. 10.1093/schbul/sbs041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strombach, T. , Weber, B. , Hangebrauk, Z. , Kenning, P. , Karipidis, I. I. , Tobler, P. N. , & Kalenscher, T. (2015). Social discounting involves modulation of neural value signals by temporoparietal junction. Proceedings of the National Academy of Sciences of the United States of America, 112(5), 1619–1624. 10.1073/pnas.1414715112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syan, S. K. , Smith, M. , Frey, B. N. , Remtulla, R. , Kapczinski, F. , Hall, G. B. C. , & Minuzzi, L. (2018). Resting‐state functional connectivity in individuals with bipolar disorder during clinical remission: A systematic review. Journal of Psychiatry & Neuroscience, 43(5), 298–316. 10.1503/jpn.170175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely, A. , Silton, R. L. , Heller, W. , Miller, G. A. , & Mohanty, A. (2017). Differential functional connectivity of rostral anterior cingulate cortex during emotional interference. Social Cognitive and Affective Neuroscience, 12(3), 476–486. 10.1093/scan/nsw137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Nouchi, R. , Sekiguchi, A. , Hashizume, H. , Sassa, Y. , … Kawashima, R. (2014). Association between resting‐state functional connectivity and empathizing/systemizing. NeuroImage, 99, 312–322. 10.1016/j.neuroimage.2014.05.031 [DOI] [PubMed] [Google Scholar]
- Torrisi, S. , Moody, T. D. , Vizueta, N. , Thomason, M. E. , Monti, M. M. , Townsend, J. D. , … Altshuler, L. L. (2013). Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disorders, 15, 156–166. 10.1111/bdi.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura, J. , Liberman, R. P. , Green, M. F. , Shaner, A. , & Mintz, J. (1998). Training and quality assurance with the Structured Clinical Interview for DSM‐IV (SCID‐I/P). Psychiatry Research, 79(2), 163–173. 10.1016/S0165-1781(98)00038-9 [DOI] [PubMed] [Google Scholar]
- Ventura, J. , Nuechterlein, K. H. , Subotnik, K. , & Gilbert, E. (1995). Symptom dimensions in recent‐onset schizophrenia: The 24‐item expanded BPRS. Schizophrenia Research, 15(1), 22. [DOI] [PubMed] [Google Scholar]
- Vlad, M. , Raucher‐chéné, D. , Henry, A. , & Kaladjian, A. (2018). Functional outcome and social cognition in bipolar disorder: Is there a connection ? European Psychiatry, 52, 116–125. 10.1016/j.eurpsy.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Metzak, P. D. , & Woodward, T. S. (2011). Aberrant connectivity during self‐other source monitoring in schizophrenia. Schizophrenia Research, 125(2–3), 136–142. 10.1016/j.schres.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Zhong, S. , Jia, Y. , Sun, Y. , Wang, B. , Liu, T. , … Huang, L. (2016). Disrupted resting‐state functional connectivity in nonmedicated bipolar disorder. Radiology, 280(2), 529–536. [DOI] [PubMed] [Google Scholar]
- Wolf, N. D. , Sambataro, F. , Vasic, N. , Frasch, K. , Schmid, M. , Schönfeldt‐Lecuona, C. , … Wolf, R. C. (2011). Dysconnectivity of multiple resting‐state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. Journal of Psychiatry and Neuroscience, 36(6), 366–374. 10.1503/jpn.110008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, N. D. , Rogers, B. , & Heckers, S. (2011). Functional resting‐state networks are differentially affected in schizophrenia. Schizophrenia Research, 130(1–3), 86–93. 10.1016/j.schres.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R. , Briggs, J. , Ziegler, V. , & Meyer, D. (1978). A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry, 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zaki, J. , Ochsner, K. N. , Hanelin, J. , Wager, T. D. , & Mackey, S. C. (2007). Different circuits for different pain: Patterns of functional connectivity reveal distinct networks for processing pain in self and others. Social Neuroscience, 2(3–4), 276–291. 10.1080/17470910701401973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 A. Group comparison of motion parameters as identified by fsl_motion_outliers
B. Group comparison of motion parameters as identified by ICA‐AROMA
Table S2. Parallel analysis of resting state network group comparison using the Gordon atlas.
Table S3. Exploratory within‐group correlations between functional connectivity of each resting state network and the behavioral measures of social and non‐social cognition. Showing p values uncorrected for multiple comparisons.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


