Abstract
Helicobacter pylori (H. pylori) is a gram-negative pathogen that colonizes gastric epithelial cells. The drug resistance rates of H. pylori have dramatically increased, causing persistent infections. Chronic infection by H. pylori is a critical cause of gastritis, peptic ulcers and even gastric cancer. In host cells, autophagy is stimulated to maintain cellular homeostasis following intracellular pathogen recognition by the innate immune defense system. However, H. pylori-induced autophagy is not consistent during acute and chronic infection. Therefore, a deeper understanding of the association between H. pylori infection and autophagy in gastric epithelial cells could aid the understanding of the mechanisms of persistent infection and the identification of autophagy-associated therapeutic targets for H. pylori infection. The present review describes the role of H. pylori and associated virulence factors in the induction of autophagy by different signaling pathways during acute infection. Additionally, the inhibition of autophagy in gastric epithelial cells during chronic infection was discussed. The present review summarized H. pylori-mediated autophagy and provided insights into its mechanism of action, suggesting the induction of autophagy as a novel therapeutic target for persistent H. pylori infection.
Keywords: Helicobacter pylori, autophagy, stomach neoplasms, vacuolating cytotoxin, cytotoxin-associated gene A
1. Introduction
Helicobacter pylori (H. pylori) is a gram-negative, spiral, flagellated, microaerophilic bacterium that was identified in 1983 (1). Among the global population, ~50% are chronically infected with H. pylori, resulting in various symptoms, including gastritis, peptic ulcer and increased neoplastic disease, as well as adenocarcinoma and mucosal-associated lymphoid tissue lymphoma (2). In addition, the World Health Organization defines H. pylori as a class 1 carcinogen (3). Eradication of H. pylori may reduce the incidence of gastric cancer by ~3-fold (4,5).
In the past years, the efficacy of conventional therapy for H. pylori has decreased (6). H. pylori is highly resistant to metronidazole (76.3%), and moderately resistant to clarithromycin (44.9%) and dual clarithromycin and metronidazole (33.3%) (7). In the USA, the resistance rates for metronidazole, clarithromycin and ciprofloxacin have been estimated to be 79.4, 70.6 and 42.9%, respectively (8). The mechanisms underlying H. pylori multi-drug resistance include gene mutation, virulence genes and host immunologic tolerance (9,10). Additionally, the presence of different types of virulence factors, notably vacuolating cytotoxin (VacA) and cytotoxin-associated gene A (CagA), can result in gastric cancer carcinogenesis (11,12). Several mechanisms have been attributed for H. pylori resistance considering various associated processes and factors, including autophagy, apoptosis, reactive oxygen species (ROS) and proinflammatory responses (13–15).
Autophagy is upregulated to maintain cytosolic homeostasis when the innate immune defence recognizes invasive bacterial pathogens (16). However, autophagy can be upregulated or downregulated in gastric epithelial cells during H. pylori infection (17,18). The present review focused on the molecular mechanisms currently considered to be associated with H. pylori-mediated autophagy. The hypothesis that the induction of autophagy can be a novel therapeutic target for persistent H. pylori infection was presented.
2. Autophagy
The 2016 Nobel Prize in Physiology or Medicine was awarded to Yoshinori Ohsumi, who first illustrated that nutrient deficiency induced extensive autophagy in yeast cells in 1992 (19). Autophagy is defined as the segregation of organelles and cellular components within double membrane vacuoles called autophagosomes (20). The fusion of autophagosomes and lysosomes generates autophagolysosomes, which degrade cytoplasmic contents (20). Therefore, autophagy can be stimulated as an intracellular defence mechanism to eliminate pathogens following their recognition by the innate immune system (21). Autophagosomes can deliver pathogens to lysosomes. Furthermore, autophagolysosomes can degrade pathogens for cellular homeostasis (20,21).
Autophagy is classified into canonical and non-canonical autophagy (22). The process of autophagy is divided into several steps, including signal induction, membrane nucleation, cargo targeting, phagophore elongation, autophagosome formation, fusion with the lysosome, cargo degradation and nutrient recycling (23). The Unc-51-like kinase 1 (ULK) complex [ULK1, ULK2, autophagy related (ATG)13, ATG101 and RB1 inducible coiled-coil 1] is essential for initiation during canonical autophagy (24). The ULK complex recruits the PI3K complex (ATG14L, phosphatidylinositol 3-kinase catalytic subunit type 3, beclin-1 and phosphoinositide-3-kinase regulatory subunit 4) to produce phosphatidylinositol 3-phosphate [PI(3)P] for the phagophore membrane nucleation step (25). Subsequently, PI(3)P binds to WD repeat domain phosphoinositide-interacting (WIPI)1, WIPI2, ATG5, ATG12 and autophagy related 16 like 1 (ATG16L1) to elongate the phagophore (23). The ATG5-ATG12-ATG16L1 complex conjugates microtubule-associated protein light chain 3-phosphatidylethanolamine for autophagosome formation (26). Finally, autophagosomes fuse with lysosomes to degrade cytoplasmic components (27).
Non-canonical autophagy is another type of ATG7- and ATG3-independent autophagy, which has been described during the development of the Drosophila midgut (28). Non-canonical autophagy has also reported as an ATG5-independent signaling pathway of autophagy (29). The non-canonical process of autophagy does not occur from a double-membrane autophagosome and is called LC3-associated phagocytosis (LAP) (22,30). LAP promotes phagosome maturation and lysosomal fusion (31).
Autophagy not only eradicates pathogens, but also serves a dual role in carcinogenesis. In 1980, a study demonstrated that the process of autophagy could be induced in leukemic cells following treatment with an antiproliferative drug (32). Our previous studies indicated that matrine had potent antitumour activity against gastric cancer cells (33,34). Autophagy is upregulated in gastric cancer cells during this antitumour process, and autophagy acts as a cytoprotective mechanism to overcome lethal stress (33). Additionally, combination treatment with matrine and autophagy inhibitors can enhance the antitumour effect of matrine in gastric cancer (34). Our previous study further demonstrated that matrine exhibited antitumour activity and induced autophagy in hepatocellular carcinoma cells (35). The extensive activation of autophagy induces autophagic cell death (35).
3. Bacteria and autophagy
Numerous pathogens can be degraded by autophagy, including bacteria, such as Mycobacterium tuberculosis (M. tuberculosis), Listeria monocytogenes (L. monocytogenes), Francisella tularensis (F. tularensis), Legionella pneumophila (L. pneumophila), Coxiella burnetii (C. burnetii), Yersinia pseudotuberculosis (Y. pseudotuberculosis), Brucella abortus (B. abortus) and Salmonella Typhimurium (S. Typhimurium) (36–43). Specific bacteria, including M. tuberculosis and L. monocytogenes, can induce the process of canonical autophagy (36,37). LRG-47 has been proposed as the only agent specifically active against M. tuberculosis (44). LRG-47 is involved in interferon-dependent autophagy, which can suppress intracellular survival of M. tuberculosis (36). L. monocytogenes interacts with the protein internalin K (InlK), a member of the internalin family of proteins specific to L. monocytogenes that interact with the major vault protein (MVP) (37). MVP recruitment prevents the autophagic recognition of intracellular bacteria, leading to an increased survival rate of InlK-overexpressing bacteria (37). Canonical autophagy can restrain the growth of intracellular bacterial species. These intracellular bacterial pathogens evade intracellular defence mechanisms of host cells by escaping from the autophagosome and by modulating canonical autophagy (45). Specific bacteria can generate the process of non-canonical autophagy, including F. tularensis, L. pneumophila, C. burnetii, Y. pseudotuberculosis, B. abortus and S. Typhimurium (38–43). F. tularensis can induce ATG5-independent autophagy, which provides nutrients that support bacterial proliferation (38). B. abortus ensures its persistent survival by forming the Brucella-containing vacuole (BCV) (46). BCV formation is independent of the autophagy proteins, namely ATG5, ATG16L1, autophagy related 4B cysteine peptidase, ATG7 and protein light chain 3B (42). Non-canonical autophagy may be beneficial to the infectivity and growth of intercellular bacteria (38). Although H. pylori is an extracellular pathogen, it can also reside and grow in gastric epithelial cells, causing persistent infection (47). The first observation of autophagy was reported for a cytotoxin of H. pylori in 1992 (48). Subsequently, it has been verified that H. pylori invasion of the gastric mucosa can trigger canonical rather than non-canonical autophagy (17,49,50).
4. Acute infection of H. pylori can induce autophagy
A physiological mechanism of outer membrane vesicles (OMVs) from bacteria can deliver peptidoglycans into the host cell cytosol and induce an immune response in vivo (51). OMVs from H. pylori can induce autophagy, which is essential for proinflammatory chemokine production (52). OMVs rely on the nucleotide-binding oligomerization domain-1-receptor interacting serine/threonine kinase 2 signaling pathway, which is essential for the induction of autophagy and the production of interleukin 8 (52,53). In addition, H. pylori OMVs induce autophagosome formation, which is not dependent on VacA (52). H. pylori secretes HP0175, which has been identified as an inducer of apoptosis in gastric epithelial cells (54). HP0175 can also upregulate the expression of autophagy-associated genes independent of functional VacA during acute infection (17).
VacA is a critical virulence factor involved in the pathogenesis of peptic ulceration and gastric cancer (55). The toxins of VacA can induce a series of intracellular alterations, including cell vacuolation, membrane channel formation, disruption of endosomal/lysosomal function, apoptosis and immunomodulation (56). VacA localizes in the mitochondria and induces their dysfunction (57). VacA relies on the inhibition of rapamycin complex 1 (mTORC1), which coordinates nutrients and energy stress signals in order to promote metabolic homeostasis (58). In VacA-intoxicated cells, the VacA-dependent inhibition of mTORC1 signaling results in the activation of cellular autophagy via the ULK1 complex (59). Low-density lipoprotein receptor-related protein-1 (LRP1) is the receptor for VacA-induced autophagy (60). VacA forms LRP1 conjugates in order to regulate the formation of autophagosomes and autolysosomes (60). Additionally, VacA can induce autophagy via endoplasmic reticulum (ER) stress (61). Inhibition of autophagy can decrease VacA-induced cell death in AGS cells (61). Tribble pseudokinase 3 (TRIB3) serves an important role in ER stress-induced autophagy (61,62). VacA can trigger ER stress and increase the expression of TRIB3 in AGS cells (61). Knockdown of the ER stress effector protein can significantly decrease the formation of autolysosomes and cell death (61). Therefore, VacA causes autophagic cell death via ER stress in gastric epithelial cells. Additionally, VacA-induced autophagy can degrade the toxins and limit host cell damage, leading to the maintenance of cellular homeostasis (63). VacA-induced autophagy does not affect the formation of VacA-large vacuoles (49).
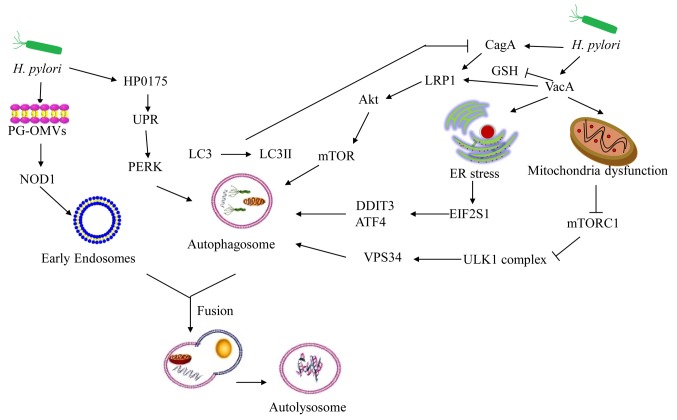
The CagA protein is the fourth most abundant protein of H. pylori (64). This bacterium uses the Cag type IV secretion system to release CagA into host cells (65). CagA can induce multiple cellular activities, such as cytosolic vacuolation, mitochondrial dysfunction, ER stress, and endosomal stress, resulting in tissue inflammation (66). Intracellular CagA does not persist in the AGS cell line (67). VacA can reduce glutathione levels and bind to LRP1 to enhance Akt phosphorylation and activate autophagy, leading to CagA protein degradation (60,68). Intracellular CagA is degraded by autophagy induction caused by the accumulation of ROS, suggesting that CagA may not promote carcinogenesis (68). Due to the resistance of ROS, CD44-positive gastric cancer stem-like cells increase the expression levels of CagA by inhibiting autophagy (68,69). Sulfasalazine can prevent the accumulation of CagA in CD44-positive cells by upregulating autophagy, suggesting a prophylactic effect of this compound on CagA-dependent gastric cancer development (68). Overall, H. pylori may disturb homeostasis in host cells during acute infection. This effect has been noted in the VacA+ or Cag+ H. pylori strains (60,61,68). Autophagy, which targets intracellular bacteria to restrict their growth and survival, is an important defence mechanism for gastric epithelial cells (Fig. 1).
Figure 1.
Acute infection of H. pylori can induce autophagy. H. pylori-OMVs stimulate NOD1, thereby triggering an autophagic response. H. pylori secretes HP0175, which upregulates UPR-dependent autophagy. VacA inhibits mTORC1, subsequently activating cellular autophagy via the ULK1 complex. VacA forms conjugates with LRP1 to regulate the formation of autophagosomes and autolysosomes. VacA can also induce autophagy via induction of ER stress. VacA can reduce GSH levels to bind LRP1 and subsequently enhance Akt phosphorylation to activate autophagy, causing CagA degradation. VacA, vacuolating cytotoxin; CagA, cytotoxin-associated gene A; LRP1, low-density lipoprotein receptor-related protein-1; ER, endoplasmic reticulum; EIF2S1, eukaryotic translation initiation factor 2 subunit 1; DDIT3, DNA damage-inducible transcript 3; ATF4, activating transcription factor 4; ULK1, Unc-51-like kinase 1; UPR, unfolded protein response; PERK, PKR-like ER kinase; PG-OMVs, peptidoglycan-outer membrane vesicles; NOD1, nucleotide-binding oligomerization domain-1; LC3, microtubule-associated protein light chain 3; GSH, glutathione; H. pylori, Helicobacter pylori; mTORC1, mTOR complex 1; VPS34, phosphatidylinositol 3-kinase catalytic subunit type 3.
5. Chronic infection of H. pylori can inhibit autophagy
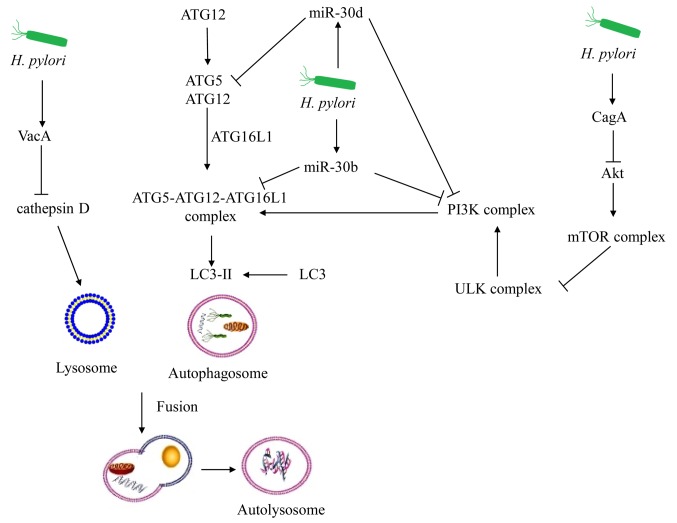
The mechanisms of chronic infections of H. pylori are not the same as those described for acute infection. MicroRNA (miR)-30b is upregulated during chronic H. pylori infection (70). Beclin-1 (BECN1) and ATG12 are targets of miR-30b, and inhibit autophagosome formation (71). Compromised autophagy promotes persistent infection of H. pylori (71). Additionally, ATG2B, ATG5, ATG12, BECN1 and BCL2 interacting protein 3 like are targets of miR-30d, leading to the repression of autophagy (72). miR-30d further promotes the intracellular survival of H. pylori during chronic infection (72). Furthermore, H. pylori infection induces methylation silencing of microtubule associated protein 1 light chain 3α variant 1, which may impair autophagy and facilitate gastric carcinogenesis (73). With regard to chronic infection and H. pylori-induced autophagy in vitro and in vivo, a consensus has been reached. A total of 28 autophagic genes are significantly downregulated in H. pylori GC026-challenged AGS cells (74). Previous findings derived from the human autophagy database and published microarray data demonstrated that the core autophagic genes (ATG16L1, ATG5, ATG4D and ATG9A) are downregulated in patients with chronic H. pylori infection with mild dyspeptic symptoms (75).
Exposure to VacA for prolonged periods may mimic the chronic infection model of VacA+ H. pylori strains (76). Autophagy is disrupted by the prolonged co-culture of VacA, since cathepsin D expression is inhibited in autophagosomes (18). Therefore, VacA can further inhibit autophagy in gastric epithelial cells during chronic infection of H. pylori.
In addition, CagA+ H. pylori strains can persistently reside in gastric mucosal tissues (77). The expression levels of autophagic proteins are downregulated by the c-Met/Akt signaling pathway, whereas the production of the inflammatory cytokines is upregulated in the CagA+ H. pylori patients (78). Therefore, several signaling pathways can induce the downregulation of autophagy as a result of chronic infection of H. pylori (Fig. 2). To the best of our knowledge, the role of inflammation in H. pylori-induced autophagy dysfunction remains largely unknown. Luo et al (79) demonstrated that autophagy is required for hepatitis B virus X protein-induced NF-κB activation, and for pro-inflammatory cytokine production. Dysregulated autophagy causes activation of NF-κB, which can stimulate the inflammatory response (80). This process is manifested by upregulation of cytokines and chemokines and by the inflammatory cell infiltration of the pancreas (81). Notably, the combination of sialic acid and catechins can upregulate autophagy and downregulate apoptosis in order to protect against H. pylori-induced gastric injury (15,82). Additionally, rapamycin can increase the clearance of H. pylori by upregulating autophagy (83). The inducers of autophagy may be novel therapeutic antibiotics that can be used for the treatment of chronic H. pylori infection.
Figure 2.
Chronic infection of H. pylori can inhibit autophagy. Autophagy-associated proteins are the targets of miR-30d and miR-30b, inhibiting autophagosome formation. Autophagy is disrupted by the prolonged co-culture of VacA since cathepsin D expression is inhibited in autophagosomes. The expression of the autophagic proteins is downregulated by the Akt signaling pathway during chronic CagA+ H. pylori infection. VacA, vacuolating cytotoxin; CagA, cytotoxin-associated gene A; ULK, Unc-51-like kinase 1; LC3, microtubule-associated protein light chain 3; ATG5, autophagy related 5; ATG12, autophagy related 12; ATG16L1, autophagy related 16 like 1; mir-30b, microRNA-30b; mir-30d, microRNA-30d.
6. Conclusions
Our previous studies demonstrated that autophagy exhibited a cytoprotective function in cancer cells and that it could induce autophagic cell death at different stages of cancer formation (33–35). H. pylori may disturb homeostasis in host cells during acute infection, notably via the secretion of virulence factors. Autophagy is an important defence mechanism that can restrict bacterial survival and growth. Gastric epithelial cells can induce canonical autophagy in order to maintain homeostasis during acute infection with H. pylori. Chronic infections with H. pylori can cause the dysfunction of autophagy-associated proteins. The inhibition of autophagy can lead to persistent infection. H. pylori can resist antibiotic treatment, and, as a consequence, the chronic infection of this bacterial strain has become a global health issue. During infection, the induction of autophagy, which maintains cellular homeostasis, is inhibited. By upregulating autophagy-associated proteins in gastric epithelial cells, H. pylori can be eliminated. This strategy can be applied with the use of autophagy inducers as novel therapeutic agents. Although the mechanism of multi-drug-resistance acquired by H. pylori relies on associated virulence factors to cause downregulation of autophagy and maintenance of persistent infection, the precise identification of the proteins involved in this signaling pathway remains unclear.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 31770537).
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
YL contributed to the planning and design of the study. FZ, CC, JH, RS, JZ, ZH, HC and YL were responsible for the literature search and the writing of the manuscript. FZ and YL performed revisions of the manuscript. All authors have read and approved the final manuscript for publication.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Blaser MJ. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987;93:371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- 2.Strugatsky D, McNulty R, Munson K, Chen CK, Soltis SM, Sachs G, Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2013;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schistosomes, liver flukes and Helicobacter pylori, corp-author. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC monographs on the evaluation of carcinogenic risks to humans. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M, Japan Gast Study Group Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: A meta-analysis. Helicobacter. 2014;19:243–248. doi: 10.1111/hel.12146. [DOI] [PubMed] [Google Scholar]
- 6.Lin TF, Hsu PI. Second-line rescue treatment of Helicobacter pylori infection: Where are we now? World J Gastroenterol. 2018;24:4548–4553. doi: 10.3748/wjg.v24.i40.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z, Zhou L, Zhang J, He L, Bai P, Xue Y. Hybrid therapy as first-line regimen for Helicobacter pylori eradication in populations with high antibiotic resistance rates. Helicobacter. 2016;21:382–388. doi: 10.1111/hel.12294. [DOI] [PubMed] [Google Scholar]
- 8.Tan B, Yang JC, Young CL, Bishu S, Owyang SY, El-Zaatari M, Zhang M, Grasberger H, Qian JM, Kao JY. Helicobacter pylori Antimicrobial susceptibility testing-guided salvage therapy in the USA: A real life experience. Dig Dis Sci. 2018;63:437–445. doi: 10.1007/s10620-017-4880-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Kim N, Nam RH, Park JH, Kim JM, Jung HC, Song IS. Mutations of Helicobacter pylori associated with fluoroquinolone resistance in Korea. Helicobacter. 2011;16:301–310. doi: 10.1111/j.1523-5378.2011.00840.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalach N, Bontems P, Raymond J. Helicobacter pylori infection in children. Helicobacter. 2017;22(Suppl 1) doi: 10.1111/hel.12414. doi: 10.1111/hel.12414. [DOI] [PubMed] [Google Scholar]
- 11.Ranjbar R, Khamesipour F, Jonaidi-Jafari N, Rahimi E. Helicobacter pylori in bottled mineral water: Genotyping and antimicrobial resistance properties. BMC Microbiol. 2016;16:40. doi: 10.1186/s12866-016-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taneike I, Nami A, O'Connor A, Fitzgerald N, Murphy P, Qasim A, O'Connor H, O'Morain C. Analysis of drug resistance and virulence-factor genotype of Irish Helicobacter pylori strains: Is there any relationship between resistance to metronidazole and cagA status? Aliment Pharmacol Ther. 2009;30:784–790. doi: 10.1111/j.1365-2036.2009.04095.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–C457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Marusawa H, Watanabe N, Chiba T. Molecular pathogenesis of Helicobacter pylori-related gastric cancer. Gastroenterol Clin North Am. 2015;44:625–638. doi: 10.1016/j.gtc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Yang JC, Chien CT. A new approach for the prevention and treatment of Helicobacter pylori infection via upregulation of autophagy and downregulation of apoptosis. Autophagy. 2009;5:413–414. doi: 10.4161/auto.5.3.7826. [DOI] [PubMed] [Google Scholar]
- 16.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder P, Datta C, Kumar R, Sharma AK, Basu J, Kundu M. The secreted antigen, HP0175, of Helicobacter pylori links the unfolded protein response (UPR) to autophagy in gastric epithelial cells. Cell Microbiol. 2015;17:714–729. doi: 10.1111/cmi.12396. [DOI] [PubMed] [Google Scholar]
- 18.Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A, Romero-Gallo J, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–1171. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randow F, Youle RJ. Self and nonself: How autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–411. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Brumell JH. Bacteria-autophagy interplay: A battle for survival. Nat Rev Microbiol. 2014;12:101–114. doi: 10.1038/nrmicro3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scambler T, Feeley C, McDermott MF. Protection against lupus-like inflammatory disease is in the LAP of non-canonical autophagy. Ann Transl Med. 2016;4:513. doi: 10.21037/atm.2016.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parzych KR, Klionsky DJ. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura T, Mizushima N. The ULK complex initiates autophagosome formation at phosphatidylinositol synthase-enriched ER subdomains. Autophagy. 2017;13:1795–1796. doi: 10.1080/15548627.2017.1358344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leidal AM, Levine B, Debnath J. Autophagy and the cell biology of age-related disease. Nat Cell Biol. 2018;20:1338–1348. doi: 10.1038/s41556-018-0235-8. [DOI] [PubMed] [Google Scholar]
- 27.Krokowski S, Mostowy S. Interactions between Shigella flexneri and the Autophagy Machinery. Front Cell Infect Microbiol. 2016;6:17. doi: 10.3389/fcimb.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang TK, Shravage BV, Hayes SD, Powers CM, Simin RT, Wade Harper J, Baehrecke EH. Uba1 functions in Atg7- and Atg3-independent autophagy. Nat Cell Biol. 2013;15:1067–1078. doi: 10.1038/ncb2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindqvist LM, Simon AK, Baehrecke EH. Current questions and possible controversies in autophagy. Cell Death Discov. 2015;1(pii):15036. doi: 10.1038/cddiscovery.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai SC, Devenish RJ. LC3-associated phagocytosis (LAP): Connections with host autophagy. Cells. 2012;1:396–408. doi: 10.3390/cells1030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nature Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Mikles-Robertson F, Dave C, Porter CW. Apparent autophagocytosis of mitochondria in L1210 leukemia cells treated in vitro with 4,4′-diacetyl-diphenylurea-bis(guanylhydrazone) Cancer Res. 1980;40:1054–1061. [PubMed] [Google Scholar]
- 33.Li Y, Zhang J, Ma H, Chen X, Liu T, Jiao Z, He W, Wang F, Liu X, Zeng X. Protective role of autophagy in matrineinduced gastric cancer cell death. Int J Oncol. 2013;42:1417–1426. doi: 10.3892/ijo.2013.1817. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Li Y, Chen X, Liu T, Chen Y, He W, Zhang Q, Liu S. Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells. Oncol Rep. 2011;26:115–124. doi: 10.3892/or.2011.1277. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JQ, Li YM, Liu T, He WT, Chen YT, Chen XH, Li X, Zhou WC, Yi JF, Ren ZJ. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J Gastroenterol. 2010;16:4281–4290. doi: 10.3748/wjg.v16.i34.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Dortet L, Mostowy S, Samba-Louaka A, Gouin E, Nahori MA, Wiemer EA, Dussurget O, Cossart P. Recruitment of the major vault protein by InlK: A Listeria monocytogenes strategy to avoid autophagy. PLoS Pathog. 2011;7:e1002168. doi: 10.1371/annotation/a70544fc-6d8b-4549-921a-9e86557b0ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele S, Brunton J, Ziehr B, Taft-Benz S, Moorman N, Kawula T. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog. 2013;9:e1003562. doi: 10.1371/journal.ppat.1003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choy A, Dancourt J, Mugo B, O'Connor TJ, Isberg RR, Melia TJ, Roy CR. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, Roy CR. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ligeon LA, Moreau K, Barois N, Bongiovanni A, Lacorre DA, Werkmeister E, Proux-Gillardeaux V, Galli T, Lafont F. Role of VAMP3 and VAMP7 in the commitment of Yersinia pseudotuberculosis to LC3-associated pathways involving single- or double-membrane vacuoles. Autophagy. 2014;10:1588–1602. doi: 10.4161/auto.29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, López-Otin C, Virgin HW, Celli J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu HB, Croxen MA, Marchiando AM, Ferreira RB, Cadwell K, Foster LJ, Finlay BB. Autophagy facilitates Salmonella replication in HeLa cells. MBio. 2014;5:e00865–14. doi: 10.1128/mBio.00865-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 45.Kimmey JM, Stallings CL. Bacterial pathogens versus autophagy: Implications for therapeutic interventions. Trends Mol Med. 2016;22:1060–1076. doi: 10.1016/j.molmed.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 47.Terebiznik MR, Vazquez CL, Torbicki K, Banks D, Wang T, Hong W, Blanke SR, Colombo MI, Jones NL. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun. 2006;74:6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catrenich CE, Chestnut MH. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- 49.Raju D, Jones NL. Methods to monitor autophagy in H. pylori vacuolating cytotoxin A (VacA)-treated cells. Autophagy. 2010;6:138–143. doi: 10.4161/auto.6.1.10222. [DOI] [PubMed] [Google Scholar]
- 50.Wang YH, Wu JJ, Lei HY. When Helicobacter pylori invades and replicates in the cells. Autophagy. 2009;5:540–542. doi: 10.4161/auto.5.4.8167. [DOI] [PubMed] [Google Scholar]
- 51.Parker H, Keenan JI. Composition and function of Helicobacter pylori outer membrane vesicles. Microbes Infect. 2012;14:9–16. doi: 10.1016/j.micinf.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner LJ, Thomas BJ, Malosse C, Gantier MP, Casillas LN, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe. 2014;15:623–635. doi: 10.1016/j.chom.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 54.Basak C, Pathak SK, Bhattacharyya A, Pathak S, Basu J, Kundu M. The secreted peptidyl prolyl cis, trans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J Immunol. 2005;174:5672–5680. doi: 10.4049/jimmunol.174.9.5672. [DOI] [PubMed] [Google Scholar]
- 55.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isomoto H, Moss J, Hirayama T. Pleiotropic actions of Helicobacter pylori vacuolating cytotoxin, VacA. Tohoku J Exp Med. 2010;220:3–14. doi: 10.1620/tjem.220.3. [DOI] [PubMed] [Google Scholar]
- 57.Willhite DC, Cover TL, Blanke SR. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 58.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim IJ, Lee J, Oh SJ, Yoon MS, Jang SS, Holland RL, Reno ML, Hamad MN, Maeda T, Chung HJ, et al. Helicobacter pylori Infection modulates host cell metabolism through VacA-dependent inhibition of mTORC1. Cell Host Microbe. 2018;23:583–593.e8. doi: 10.1016/j.chom.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahiro K, Satoh M, Nakano M, Hisatsune J, Isomoto H, Sap J, Suzuki H, Nomura F, Noda M, Moss J, Hirayama T. Low-density lipoprotein receptor-related protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. J Biol Chem. 2012;287:31104–31115. doi: 10.1074/jbc.M112.387498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu P, Xue J, Zhang ZJ, Jia YP, Tong YN, Han D, Li Q, Xiang Y, Mao XH, Tang B. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis. 2017;8:3207. doi: 10.1038/s41419-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terebiznik MR, Raju D, Vázquez CL, Torbricki K, Kulkarni R, Blanke SR, Yoshimori T, Colombo MI, Jones NL. Effect of Helicobacter pylori's vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5:370–379. doi: 10.4161/auto.5.3.7663. [DOI] [PubMed] [Google Scholar]
- 64.Jungblut PR, Bumann D, Haas G, Zimny-Arndt U, Holland P, Lamer S, Siejak F, Aebischer A, Meyer TF. Comparative proteome analysis of Helicobacter pylori. Mol Microbiol. 2000;36:710–725. doi: 10.1046/j.1365-2958.2000.01896.x. [DOI] [PubMed] [Google Scholar]
- 65.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 66.Kumar S, Dhiman M. Inflammasome activation and regulation during Helicobacter pylori pathogenesis. Microb Pathog. 2018;125:468–474. doi: 10.1016/j.micpath.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa S, Ohta T, Hatakeyama M. Stability of Helicobacter pylori CagA oncoprotein in human gastric epithelial cells. FEBS Lett. 2009;583:2414–2418. doi: 10.1016/j.febslet.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 68.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Fan X, Long A, Goggins M, Fan X, Keeling PW, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut. 1996;38:507–512. doi: 10.1136/gut.38.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD, et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916–925. doi: 10.1086/605443. [DOI] [PubMed] [Google Scholar]
- 71.Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang HG, Fang Y, Yu B, Zhang JY, Xie QH, et al. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy. 2012;8:1045–1057. doi: 10.4161/auto.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang XJ, Si RH, Liang YH, Ma BQ, Jiang ZB, Wang B, Gao P. Mir-30d increases intracellular survival of Helicobacter pylori through inhibition of autophagy pathway. World J Gastroenterol. 2016;22:3978–3991. doi: 10.3748/wjg.v22.i15.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muhammad JS, Nanjo S, Ando T, Yamashita S, Maekita T, Ushijima T, Tabuchi Y, Sugiyama T. Autophagy impairment by Helicobacter pylori-induced methylation silencing of MAP1LC3Av1 promotes gastric carcinogenesis. Int J Cancer. 2017;140:2272–2283. doi: 10.1002/ijc.30657. [DOI] [PubMed] [Google Scholar]
- 74.Castano-Rodriguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. Autophagy in Helicobacter pylori infection and related gastric cancer. Helicobacter. 2015;20:353–369. doi: 10.1111/hel.12211. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka S, Nagashima H, Uotani T, Graham DY, Yamaoka Y. Autophagy-related genes in Helicobacter pylori infection. Helicobacter. 2017:22. doi: 10.1111/hel.12376. doi: 10.1111/hel.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raju D, Hussey S, Jones NL. Crohn disease ATG16L1 polymorphism increases susceptibility to infection with Helicobacter pylori in humans. Autophagy. 2012;8:1387–1388. doi: 10.4161/auto.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 78.Li N, Tang B, Jia YP, Zhu P, Zhuang Y, Fang Y, Li Q, Wang K, Zhang WJ, Guo G, et al. Helicobacter pylori CagA protein negatively regulates autophagy and promotes inflammatory response via c-Met-PI3K/Akt-mTOR signaling pathway. Front Cell Infect Microbiol. 2017;7:417. doi: 10.3389/fcimb.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo MX, Wong SH, Chan MT, Yu L, Yu SS, Wu F, Xiao Z, Wang X, Zhang L, Cheng AS, et al. Autophagy mediates HBx-induced nuclear Factor-kappaB activation and release of IL-6, IL-8, and CXCL2 in hepatocytes. J Cell Physiol. 2015;230:2382–2389. doi: 10.1002/jcp.24967. [DOI] [PubMed] [Google Scholar]
- 80.Li N, Wu X, Holzer RG, Lee JH, Todoric J, Park EJ, Ogata H, Gukovskaya AS, Gukovsky I, Pizzo DP, et al. Loss of acinar cell IKKa triggers spontaneous pancreatitis in mice. J Clin Invest. 2013;123:2231–2243. doi: 10.1172/JCI64498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gukovskaya AS, Gukovsky I, Algul H, Habtezion A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153:1212–1226. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang JC, Shun CT, Chien CT, Wang TH. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J Nutr. 2008;138:2084–2090. doi: 10.3945/jn.108.090985. [DOI] [PubMed] [Google Scholar]
- 83.Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157–4165. doi: 10.1128/IAI.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.